Abstract
Look-through mutagenesis (LTM) is a multidimensional mutagenesis method that simultaneously assesses and optimizes combinatorial mutations of selected amino acids. The process focuses on a precise distribution within one or more complementarity determining region (CDR) domains and explores the synergistic contribution of amino acid side-chain chemistry. LTM was applied to an anti-TNF-α antibody, D2E7, which is a challenging test case, because D2E7 was highly optimized (Kd = 1 nM) by others. We selected and incorporated nine amino acids, representative of the major chemical functionalities, individually at every position in each CDR and across all six CDRs (57 aa). Synthetic oligonucleotides, each introducing one amino acid mutation throughout the six CDRs, were pooled to generate segregated libraries containing single mutations in one, two, and/or three CDRs for each VH and VL domain. Corresponding antibody libraries were displayed on the cell surface of yeast. After positive binding selection, 38 substitutions in 21 CDR positions were identified that resulted in higher affinity binding to TNF-α. These beneficial mutations in both VH and VL were represented in two combinatorial beneficial mutagenesis libraries and selected by FACS to produce a convergence of variants that exhibit between 500- and 870-fold higher affinities. Importantly, these enhanced affinities translate to a 15- to 30-fold improvement in in vitro TNF-α neutralization in an L929 bioassay. Thus, this LTM/combinatorial beneficial mutagenesis strategy generates a comprehensive energetic map of the antibody-binding site in a facile and rapid manner and should be broadly applicable to the affinity maturation of antibodies and other proteins.
Keywords: look-through mutagenesis, maturation, mutagenesis, TNF-α
Nowadays one can create antibodies in vitro by methods that duplicate most aspects of the natural immune system (1, 2). For instance, combinatorial antibody libraries allow one to obtain numbers of antibodies that greatly exceed the diversity of the natural repertoire and are not limited by the phenomenon of immune tolerance. In addition, multiple display technologies are available to link recognition and replication such that improved antigen-binding clones can be enriched, recovered, and retrieved. One significant aspect of the natural immune system not yet duplicated by current methods is the use of the powerful evolutionary principle of mutation and selection to achieve affinity maturation. However, as we can now create very large numbers of antibodies in vitro, it should be possible to duplicate or even improve on nature's affinity maturation process by the use of a precise selection process based on chemical principles that optimize the interactions between antibody and antigen. Here, we describe a method where such a chemical approach is used to dramatically increase the affinity of an antibody. To demonstrate the substantial power of this method, we chose an antibody whose affinity had already been optimized by several orders of magnitude.
Current antibody affinity maturation methods belong to two mutagenesis categories: stochastic and nonstochastic. Errorprone PCR, mutator bacterial strains (3), and saturation mutagenesis (4–7) are typical examples of stochastic mutagenesis methods. When applied to multiple positions within multiple complementarity determining regions (CDRs) these stochastic strategies fail to provide practical comprehensive mutagenic coverage because the size of any resulting complete library typically exceeds the limitations of all current physical display systems. Nonstochastic techniques often use alanine-scanning or site-directed mutagenesis to generate limited collections of specific variants.
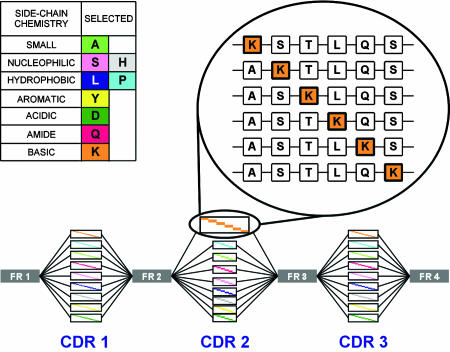
Look-through mutagenesis (LTM) was developed to provide a comprehensive optimization map of the antibody-binding site in a facile and rapid manner. For LTM, nine amino acids, representative of the major side-chain chemistries provided by the 20 natural amino acids, were selected (Fig. 1) to dissect the functional side-chain contributions to binding at every position in all six CDRs of the antibody (Fig. 1). LTM generates a positional series of single mutations within a CDR where each “wild type” residue is systematically substituted by one of nine selected amino acids. Mutated CDRs are combined to generate combinatorial single-chain variable fragment (scFv) libraries of increasing complexity and size without becoming prohibitive to the quantitative display of all variants. After positive selection, clones with improved binding were sequenced, and those beneficial mutations were mapped. To identify synergistic mutations for improved binding, combinatorial libraries (combinatorial beneficial mutations, CBMs) expressing all beneficial permutations were produced by mixed DNA probes, positively selected, and analyzed to identify a panel of optimized scFVs candidates.
Fig. 1.
Triple LTM example. Nine amino acids, representative of the 20, were selected for the LTM process based on their side-chain chemical functionalities (Inset Left). Discrete CDR oligonucleotides are synthesized to produce a mutagenized CDR with one target amino acid mutation at each CDR position (Inset Right). In the triple CDR library, all combinations of CDR1, CDR2, and CDR3 oligonucleotides are combined to produce libraries with three simultaneously mutagenized CDRs.
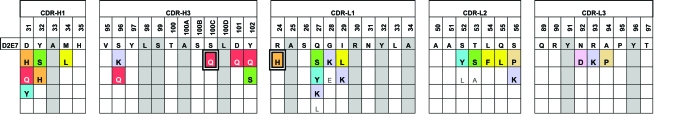
The model system used to test the LTM affinity maturation technology was the anti-TNF-α antibody D2E7 (8). Six LTM libraries with single mutations in each individual CDR, 15 LTM libraries with single mutations in two CDRs, and 20 LTM libraries with single mutations in three CDRs were generated for the combined VH and VL domains and screened for improved affinity. In this first broad iteration, 38 mutations identified in 21 CDR positions (comprising five of the six CDRs) yielded higher-affinity binders (Table 1). These individual beneficial mutations were combined to generate two sets of CBM libraries (VH and VL libraries independently) that were subsequently screened, combined, and further selected for improved binding. Ultimately, 42 clones with dramatically improved binding were characterized and sequenced (Table 2). Among these selected CBM library variants were antibodies that exhibit up to 870-fold enhancement in binding, resulting in low picomolar affinities for TNF-α. Equally important, through the process of generating and examining optimized sequences from the LTM diversity libraries a comprehensive map of the D2E7 binding site was quickly delineated.
Table 1. LTM mutations that yield higher affinity for TNF-α.
In gray are amino positions where no LTM substitutions are observed. In color are amino acid positions that were combined in the CBM process. Highlighted positions are derived from magnetic bead selections. Amino acid positions are numbered according to Kabat et al. (34).
Table 2. Sequence of CBM clones displaying higher affinity for TNF-α.
Materials and Methods
Reagents. Synthetic oligonucleotides were obtained from Syngen (San Carlos, CA). Restriction enzymes were obtained from New England Biolabs. Polymerases and yeast strain EBY100 were obtained from Invitrogen. DNA purification kits were obtained from Qiagen (Valencia, CA). dNTPs were obtained from Fermentas (Hanover, MD). TNF-α was obtained from PeproTech (Rocky Hill, NJ), and streptavidin (SA)–phycoerytherin (PE), anti-His-FITC, and Biotin-XX were from Molecular Probes. Endotoxin QCL-1000 kits were purchased from Cambrex (East Rutherford, NJ). All media were prepared according to the vendor's instructions (Invitrogen).
D2E7 scFv Construction and Display. The D2E7 scFv construct was assembled by overlap PCR. Briefly, an equimolar mixture of 30 oligonucleotides (final 0.4 μM) was PCR-assembled by using 0.5 μl of Pfx DNA polymerase (2.5 units per μl) and 5 μl of Pfx buffer (Invitrogen). A second PCR step with oligonucleotide primers to incorporate 5′ BamHI and 3′ NotI restriction sites was used for directional subcloning into modified yeast display vector pYD1 (Invitrogen). The modified construct ultimately incorporated a C-terminal hexahistidine and c-myc epitope tag for purification and detection, respectively. After sequence verification the construct served as the template for subsequent LTM libraries.
TNF-α Biotinylation. Briefly, 300 μl of TNF-α (1 mg/ml) was added to 30 μl of 1 M sodium bicarbonate buffer (pH 8.3) and 5.8 μl of Biotin-XX (20 mg/ml in DMSO). The mixture was incubated for 1 h at room temperature. Unincorporated biotin was removed by microconcentration (Microcon, Millipore), and buffer was exchanged with four volumes of PBS washes. Protein concentration was determined by OD280.
scFv Expression and Display. The D2E7 construct and LTM/CBM variants were transformed into EBY100 yeast and selected in complete supplement mixture, minus tryptophan media. Cultures were then induced by 20% galactose select media for 48 h at 20°C. After induction, cells were washed twice with PBS and resuspended in PBS/0.5% BSA.
LTM Library Construction. Individual oligonucleotides were synthesized to encode each amino acid substitution for each CDR position and provide sufficient overlap for PCR priming from the D2E7 template. Briefly, PCRs containing LTM oligonucleotide mixtures corresponding to individual CDRs were used to amplify LTM-substituted CDR fragments. Next, these PCR products were gel-purified, and equimolar aliquots were combined for megaprime PCR to regenerate full-length scFv. To generate combinatorial LTM libraries for multiple CDRs we followed the protocol described above, except instead of using the parental D2E7 construct as PCR template a previously generated single or double CDR LTM library was used instead.
Magnetic Bead LTM Library Selection. Briefly, 1 × 107 cells were incubated for 2 h at room temperature with biotinylated TNF-α (final concentrations of 50, 0.5, and 0.1 nM), pelleted, and bound to 1 × 108 SA beads (Spherotech, Libertyville, IL). The magnet was applied, and cells were washed with PBS/0.5% BSA. The beads were directly resuspended into 2 ml of glucose selective media and grown for 2 days at 30°C, shaking at 300 rpm.
FACS Sorting of scFv Libraries. After scFv induction, cells were incubated with 400 nM biotin–TNF-α for 3 h at room temperature under shaking. Cells were washed with two volumes of PBS, then resuspended in PBS/BSA containing 1 μM TNF-α, and incubated at 25°C for an additional 24–70 h. After this chase, the cells were washed twice with PBS/BSA buffer, then labeled with SA–PE (2 mg/ml) and anti-His–FITC (25 nM) for 30 min on ice, washed, and resuspended as described. The D2E7 bearing yeast were used to set a threshold for sorting improved clones from the LTM library. Cells expressing the scFv fusion (FITC-positive) that displayed greater binding to biotin–TNF-α (PE signal) were bulk-sorted. After collecting these clones, we performed a postsort flow cytometry analysis to confirm enrichment. Bulk-sorted clones were then propagated in 10% glucose select media at 30°C for 48 h and then plated on solid media for clonal segregation.
CBM Library Construction. Twenty nine selected beneficial D2E7 CDR mutations obtained through the LTM screen were used to combinatorially construct CBM libraries by the use of degenerate CDR oligonucleotides. They were CDR H1 (ctgggttcacctttgac BAK YMT GCT MTG CAT tgggtccgacaagcgccag), CDR H3 (gtatattactgtgcaaag GTG MRY TAC TTA TCA ACA GCT TCT MRK CTA SAK YMK tgggggcaaggcactctag), CDR L1 (gacagagtaacaataacgtgt CRT GCA TCT HRK RRA MWA AGA AAT TAT CTC GCA tggtatcaacagaagccg), CDR L2 (cacctaagctgttaatttat GCC GCC TMT WCT TTW CWA MVK ggtgtgccttctaggtttag), and CDR L3 (gacgttgcaacatattactgt CAA AGA TAC RAT ARA SCT CCA TAT ACA ttcggtcaaggtactaaagtc). Degenerated oligos were used to separately produce heavy- and light-chain CBM libraries by PCR (VH CBM-VL parental and VH parental-VL CBM). These two scFv libraries were subjected to a single round of dissociation-kinetics FACS selection, yielding two enriched populations (VH CBM-rd1-VL parental and VH parental-VL CBM-rd1) The CBM-rd1 heavy and light chains were then PCR-amplified and used to generate three additional libraries (VH CBM-rd1-VL CBM, VH CBM-VL CBM-rd1, and VH CMB-rd1-VL CBM-rd1). Those three libraries were finally pooled and subjected to two additional rounds of FACS-based selection.
Expression and Purification of D2E7 Variants. All scFvs were subcloned into pBAD (Invitrogen) and secreted into the Escherichia coli periplasm of LMG194 cells (Invitrogen) with a C-terminal 6×His-Myc epitope tag. Individual colonies were grown at 37°C to OD600 1–2 in RM media (M9 salts/2% Casamino acids/0.2% glucose/1 mM MgCl2), then diluted 100-fold into LB media and grown at 37°C to OD600 0.5 and then induced with arabinose (final concentration of 0.0002%) overnight at room temperature. Subsequently, bacteria were harvested by centrifugation, hypertonically lysed by resuspending the cell pellet in 1/25th volume sucrose buffer (20% sucrose/30 mM Tris, pH 8.0/1 mM EDTA), and incubated for 1 h on ice. Cells were then gently collected by centrifugation, resuspended (equal in volume to sucrose buffer) in BBS buffer (200 mM boric acid/160 mM NaCl/10 mM EDTA), and incubated overnight at 4°C. EDTA was then removed from the sucrose and BBS buffers by dialysis or through chelation with four mole equivalents of MgSO4 and then purified by nickel chelate chromatography (Qiagen) and eluted with PBS containing 250 mM imidazole. Samples were then concentrated by using ultra-free concentrators (Amicon) and exchanged into citrate buffer (20 mM sodium citrate, pH 5.5) by using a PD-10 size exclusion column (Amersham Pharmacia). The resulting eluate was purified to homogeneity by using cation exchange chromatography on a mono S column (Amersham Pharmacia). After cation exchange, the resulting protein was buffer-exchanged into PBS by using a PD-10 column. For cell-based assays, endotoxin was removed by reisolation from an endotoxin-free PBS-equilibrated superdex 75 column that had been previously treated with 1 M NaOH. Resulting endotoxin levels were <2.5 units per mg for all samples, as assayed by an endotoxin kit (Cambrex).
BIAcore Assay. Binding affinities (KD = kd/ka = koff/kon) of the scFv antibodies were measured by using a BIAcore 3000 surface plasmon resonance system (BIAcore, Neuchatel, Switzerland). TNF-α was immobilized on a CM5 chip according to the manufacturer's instructions. scFv samples in concentrations between 30 pM and 1 nM were injected over the TNF surfaces with a 10-min association phase followed by a 3-h dissociation phase. All experiments were carried out at 25°C. Binding data were fit to a simple 1:1 interaction model to extract binding constants by using clamp (9). For the TNF-β cross-reactivity experiment, TNF-α and TNF-β were immobilized to ≈1,000 response units as above, and the chips were subjected to a solution containing 100 nM scFv in running buffer.
Cell Culture. L929 cells were propagated in Eagle's minimal essential medium, supplemented with 2 mM l-glutamine, and Earle's balanced salt solution adjusted to contain 1.5 g/liter sodium bicarbonate, 0.1 mM nonessential amino acids, 1.0 mM sodium pyruvate, 10% FBS, and 50 μg/ml gentamycin. Cells were maintained throughout the study at 37°C and 5% CO2.
TNF-α Neutralization Assay. A total of 35,000 L929 cells in complete growth media were added to each well of a 96-well plate and grown overnight. For inhibition studies scFvs were preincubated with TNF-α (350 pg/ml) at room temperature for 30 min. Just before TNF-α treatment, growth media were removed and replaced with 0.5 volume Eagle's minimal essential medium containing 10% FBS and 1 μg/ml actinomycin D. The TNF-α–scFvs complexes were next added to the plate immediately after changing the media so that the cells were exposed to actinomycin D for no longer than 15 min. Next, the L929 cells were incubated for 20–24 h at 37°C. The next day, 20 μl of WST-1 was added to each well and incubated for an additional 4 h at 37°C, and OD450 were then read. IC50s were determined from these readings by using prism 3.02 software (GraphPad, San Diego). Each measurement was performed in quadruplicate, and each experiment was performed a minimum of two times.
Results
Design and Yeast Display of D2E7 scFv. The D2E7 scFv was designed starting from a published sequence by using codons optimized for both Saccharomyces cerevisiae and E. coli usage (10) and constructed by assembly PCR. In the D2E7 scFv the C terminus of VH was fused to the N terminus of VL by a (Gly4–Ser)3 linker. The scFv was displayed as a fusion to the C terminus of the yeast extracellular protein Aga2p. We found the displayed scFv fusion bound TNF-α with an EC50 of 13.5 nM (data not shown).
LTM Library Construction. In LTM, only one wild-type amino acid is substituted per CDR with a selected amino acid (Fig. 1). In total, nine selected amino acids were chosen to produce individual CDR LTM libraries. The individual CDR libraries were then combined to generate 15 double (two CDRs simultaneously mutated per clone) and 20 triple (three CDRs mutated) LTM libraries (Fig. 2). By design, the most diverse triple LTM library contained 1.4 × 106 distinct scFv mutants. As the number of mutants for four, five, and six mutated CDRs would exceed the practical limits of yeast display, we restricted the complexity of the LTM libraries to any three of the light- and heavy-chain CDRs. The final LTM library thus is made of 6 single, 15 double, and 20 triple LTM libraries. To assess library integrity and diversity we sequenced and analyzed ≈200 random clones (data not shown). In general, we found that the libraries were highly diverse with an extremely low number of contaminating wild-type clones. We did notice increased error rates that correlated to the increasing complexity of each library. This finding is not surprising as the aggregation of errors in template libraries is cumulatively propagated in the generation of subsequent more complex libraries.
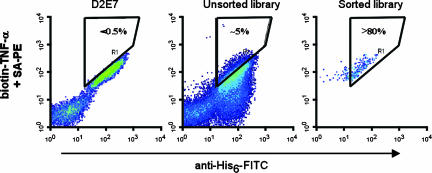
Fig. 2.
Dot plot of yeast displayed anti-TNF-α scFv. (Left) D2E7 staining was used to thresholds for improved binders. (Center) The LTM libraries. Shown is FACS profile for the unsorted library. (Right) The dot plot profile for the sorted library.
Isolation of Improved Binding Clones from LTM Libraries. Initially, an equilibrium magnetic bead-based method was used and only resulted in a modest 2-fold increased affinity. Further improvements by this selection strategy were inherently limited by low signal-to-noise ratios as equilibrium selection methods require antigen levels in the range of the antibody KD (<1 nM).
However, because most affinity maturation techniques yield optimization of the dissociation rate constant, koff (5, 11, 12), a pulse–chase strategy was used to select for clones that display slower dissociation kinetics than the parent molecule. We accomplished this selection by labeling the yeast cell surface-displayed libraries with biotin-TNF-α and chasing with unlabeled TNF-α. Subsequent FACS sorting enabled the enrichment of slower dissociating clones. As expected, a bulk of the library displayed unproductive low-expressing, fast-dissociating constituents. However, a significant number of mutants displayed improved binding compared with D2E7 (Fig. 2).
After three rounds of sorting the single, double, and triple libraries separately, we combined these enriched libraries and conducted an additional two rounds of selections. Finally, 210 colonies were analyzed for dissociation kinetics and sequenced. Thirty-two unique clones (Table 1) displayed slower dissociation kinetics (dissociation profiles for some of these are shown in Fig. 3). In all, we found 14 positions in the CDRs (one in CDRH1, three in CDRH2, four in CDRH3, four in CDRL1, and two in CDRL3) that were never mutated and were termed as the primary binding architecture. Mutations conferring higher affinity were characterized as beneficial mutations and mutations that did not alter affinity were characterized as neutral mutations.
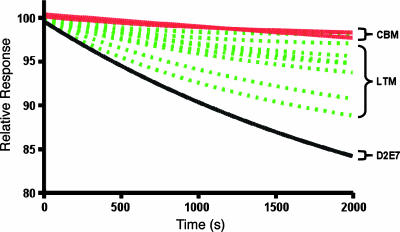
Fig. 3.
BIAcore dissociation profiles of D2E7, LTM (broken lines), and CBM clones.
Generating and Screening CBM Libraries. An amino acid map of all clones that confer higher affinity (or slower dissociation rates) was compiled for each of the CDR sequences (Table 1). Mutations observed in multiple clones were selected and combined into CBM libraries to explore the synergistic potential among these individual mutations. Degenerate oligonucleotides encoding the selected amino acid mutations, in addition to the wild-type amino acid, were synthesized and assembled to produce these libraries. The use of degenerate nucleotides not only reduces the number of oligonucleotides necessary to introduce the selected mutations, but also allows the introduction of yet another aspect of natural evolution into the process (Table 4, which is published as supporting information on the PNAS web site). Because of the practical display limitations of S. cerevisiae, scFv libraries were built separately for the VH (diversity of 2 × 105) and VL (diversity of 6 × 105) domains. After three rounds of selection the VH and VL libraries were combined by PCR, and the resulting libraries were pooled for two rounds of additional selection. From 68 selected mutants, we found a total of 58 mutants that displayed slower dissociation rates than D2E7. A total of 14 positions in CDRH1, CDRH2, CDRH3, CDRL1, and CDRL3 were mutated. From sequence analysis of the 42 unique clones we discovered a dramatic substitution pattern (Table 2). Only seven positions in VH CDRs are mutated with little convergence in type of mutation. However, in VL CDRs there is remarkable convergence of mutations. Four positions in the CDR L1 were mutated repeatedly with a strict requirement for His-24, two positively charged residues Lys-27 and Arg-28 in the middle, followed by a conservative change in hydrophobic Ile-29 to Leu-29. Three mutations in CDR L2 were also detected in the majority of the clones. The first change is a conservative change from Thr-53 to Ser-53 followed by a less conservative Gln-55 to Leu-55. There is little convergence in the type of mutation in position 56 in the CDR, suggesting a less stringent requirement in this position. Two positions (92 and 94) in the middle of CDRL3 were mutated in almost all instances, whereas an Arg-93 to Lys-93 conservative mutation was observed in only some of the clones. Interestingly, the selection of a Pro-94–Pro-95 sequence in this CDR suggests dramatic changes in secondary structure in this region.
Determining Affinities of Improved Clones. Several selected clones from the LTM and CBM libraries were expressed as scFvs in E. coli. Affinity measurements for these clones were determined by surface plasmon resonance (Fig. 3; values for kinetic and equilibrium parameters are in Table 3). Remarkably, in the CBM clones there is dramatic improvement in both kon and koff. In fact, the highest affinity clone displays a 20-fold improvement in kon and 10-fold in koff.
Table 3. BIAcore parameters for WT, LTM, and CBM clones.
| scFv | kon (× 106 M-1·s-1) | Error | koff (× 10-5 s-1) | Error | kD, pM | Relative KD improvement |
|---|---|---|---|---|---|---|
| D2E7 | 1.12 | 0.02 | 107.000 | 0.1 | 955 ± 17 | |
| A1 | 11.1 | 0.3 | 2.086 | 0.004 | 1.88 ± 0.05 | 500 |
| cb2-44 | 5.8 | 0.4 | 0.637 | 0.01 | 1.10 ± 0.08 | 870 |
| cb1-3 | 7.1 | 0.1 | 0.770 | 0.02 | 1.08 ± 0.02 | 870 |
| cb2-6 | 4.0 | 0.9 | 0.0454 | 0.02 | 1.1 ± 0.3 | 870 |
A1 sequence has D2E7 mutations: CDRH1:D31Q, CDRH3:S99P, CDRL1:G28E.
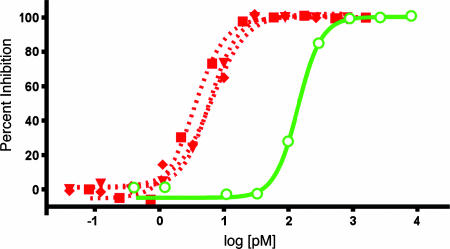
L929 in Vitro Neutralization Assay. Next, we tested the neutralization activity of the affinity-enhanced CBM clones by using a TNF-α-responsive L929 cell-based bioassay. By this assay we found the bioactivity of the parent D2E7 (105 pM) consistent with previous reports (8), whereas the IC50 values for our CBM-optimized clones (3–6 pM) demonstrated 15- to 30-fold enhancement in neutralization (Fig. 4).
Fig. 4.
Affinity-matured anti-TNF-α antibodies have improved neutralizing abilities. L929 cells were treated with actinomycin and TNF-α, as indicated for 24 h, in the absence and presence of purified anti-TNF-α scFv antibodies. WST-1 was added and incubated for an additional 4 h, and OD450 was recorded. Fifty percent neutralizing dose values were 141 pM for D2E7 (open green circle), 3.5 pM for A1 (red square), 6.1 pM for cb1-3 (red triangle), and 6.5 pM for cb2-6 (red diamond). Data represent mean ± SEM with each measurement performed in quadruplicate.
Discussion
Antibodies have become an increasingly important class of therapeutic molecules for numerous indications, including cancer, organ rejection, antiviral prophylaxis, rheumatoid arthritis, psoriasis, and Crohn's disease (13). High affinity and specificity are critical parameters to their therapeutic utility. To generate Abs of clinical utility with the highest affinity possible, a facile and comprehensive affinity maturation method is needed.
Numerous protein engineering and mutagenesis strategies have been used for the enhancement of antibody affinity (3, 5, 6, 11, 14–29). For small regions (<8–10 aa), saturation mutagenesis is typically used to produce all combinations of the 20 naturally occurring amino acids. However, this process becomes impractical beyond eight to 10 positions because of library display capacities (30). Affinity maturation of antibodies involving 60 positions (six CDR loops) would yield unmanageably large libraries (>1078) that are impossible to produce and screen with current expression-display systems. As a result, some researchers have limited saturation mutagenesis to one or two CDRs, such as CDR L3 and H3, because a majority of binding energy is thought to be contributed by these two CDR loops (19). Reduced codon complexity to limit library diversity has also been proposed (15, 20, 31). Alternatively, specific residues among the six CDRs are selected based on their propensity for somatic hypermutation (6, 29). Although these strategies can result in significant affinity enhancement, none comprehensively interrogate all six CDR positions in a precise and informative fashion. Other stochastic methods used for maturation (21, 23) are also limited because of the inherent randomness in such mutagenesis strategies. Although improved properties might be obtained through these methods, a directed, rational, and informative method based on chemical principles should yield vastly improved variants and informative results about the binding site architecture.
In the present study our approach improved the affinity of an important therapeutic antibody from low nanomolar (1 nM) to low picomolar (1.1 pM). Some of the mutations that we observe that are important to this optimization agree with previous work, in that a significant loss of binding is observed when either Tyr-91 or Tyr-95a in CDRL3 or Leu-98 and Leu-100d in CDRH3 (Table 2) are mutated to alanine (8). However, LTM goes much further in that we identify 10 additional residues as nonenhanceable sites. Although the process of alanine scanning (32, 33) is useful for defining thermodynamic properties of individual positions in the antibody–antigen interface, LTM is better suited for affinity maturation. In LTM, residues important to interaction with antigen and/or overall structure are essentially captured by LTM as a subset of the primary binding architecture, while simultaneously and expeditiously defining mutations that result in improved binding.
The LTM process, in which there is a single amino acid mutation in all positions for each CDR loop, allows the construction of a high-resolution contextual roadmap to rapidly direct further affinity enhancement by combining multiple mutations for synergistic gains. The CBM strategy builds on the LTM process to produce libraries encoding all of the observed beneficial mutations, as well as the parent amino acid sequence, thereby allowing a simultaneous combinatorial “backcross” to occur, thereby eliminating all nonproductive combinations. An advantage of using degenerate oligonucleotides is the incorporation of additional related amino acids during the process, thus allowing further fine-tuning of side-chain interactions. These additional amino acids were allowed and selected in multiple positions, most notably Arg-27 in CDR L1 (see Table 4). As a result, this CBM process produces a convergence of highly optimized activities in the broadest scope of sequence space. The backcross aspect of affinity maturation used here is not part of the process of natural antibody evolution in that, in nature, the recombination event occurs before somatic mutation, and thus further synergy between the two processes is not allowed.
Interestingly, the analysis of the most improved CBM mutants shows a preponderant number of variants in the VL chain as compared with the VH. These results may support the notion that the VH chain has been provided in nature with a more robust optimization strategy than that for the VL chain (i.e., by VDJ joining). A less optimized VL chain could in turn be the focus for an expedited affinity maturation path, especially if multiple chemical amino acid contributions can be assessed simultaneously. Thus, our chemical algorithm that allows for both VH and VL optimization by a concerted process of recombination and mutation may be more powerful than the genetic algorithm of nature.
Acknowledgments
We thank Jennifer Jones, Tonia Buccholz, and Randy Shen for technical contributions, Mark Gilbert and Dick Stovel at the Stanford FACS facility (Stanford, CA) for sorting the yeast libraries, David Myszka at Biosensor Tools (Salt Lake City) for BIAcore measurements, and Laurie Goodman at Marin Biologic Laboratories (Tiburon, CA) for cell assays.
Abbreviations: CDR, complementarity determining region; LTM, look-through mutagenesis; CBM, combinatorial beneficial mutation; SA, streptavidin; PE, phycoerytherin; scFv, single-chain variable fragment.
References
- 1.Huse, W. D., Sastry, L., Iverson, S. A., Kang, A. S., Alting-Mees, M., Burton, D. R., Benkovic, S. J. & Lerner, R. A. (1989) Science 246 1275-1281. [DOI] [PubMed] [Google Scholar]
- 2.McCafferty, J., Griffiths, A. D., Winter, G. & Chiswell, D. J. (1990) Nature 348 552-554. [DOI] [PubMed] [Google Scholar]
- 3.Low, N. M., Holliger, P. H. & Winter, G. (1996) J. Mol. Biol. 260 359-368. [DOI] [PubMed] [Google Scholar]
- 4.Nishimiya, Y., Tsumoto, K., Shiroishi, M., Yutani, K. & Kumagai, I. (2000) J. Biol. Chem. 275 12813-128120. [DOI] [PubMed] [Google Scholar]
- 5.Yang, W. P., Green, K., Pinz-Sweeney, S., Briones, A. T., Burton, D. R. & Barbas, C. F., 3rd (1995) J. Mol. Biol. 254 392-403. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury, P. S. & Pastan, I. (1999) Nat. Biotechnol. 17 568-572. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury, P. S. (2002) Methods Mol. Biol. 178 269-285. [DOI] [PubMed] [Google Scholar]
- 8.Salfeld, J. G., Allen, D. J., Hoogenboom, H. R., Kaymakcalan, Z., Labkovsky, B., Mankovich, J. A., McGuinness, B. T., Roberts, A. J., Sakorafas, P., Schoenhaut, D., et al. (2003) U.S. Patent 6,090,382.
- 9.Myszka, D. G. & Morton, T. A. (1998) Trends Biochem. Sci. 23 149-150. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura, Y., Gojobori, T. & Ikemura, T. (2000) Nucleic Acids Res. 28 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson, J., Pope, T., Tung, J. S., Chan, C., Hollis, G., Mark, G. & Johnson, K. S. (1996) J. Mol. Biol. 256 77-88. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins, R. E., Russell, S. J. & Winter, G. (1992) J. Mol. Biol. 226 889-896. [DOI] [PubMed] [Google Scholar]
- 13.Brekke, O. H. & Sandlie, I. (2003) Nat. Rev. Drug Discov. 2 52-62. [DOI] [PubMed] [Google Scholar]
- 14.Gram, H., Marconi, L. A., Barbas, C. F., 3rd, Collet, T. A., Lerner, R. A. & Kang, A. S. (1992) Proc. Natl. Acad. Sci. USA 89 3576-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balint, R. F. & Larrick, J. W. (1993) Gene 137 109-118. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths, A. D., Malmqvist, M., Marks, J. D., Bye, J. M., Embleton, M. J., McCafferty, J., Baier, M., Holliger, K. P., Gorick, B. D., Hughes-Jones, N. C., et al. (1993) EMBO J. 12, 725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Kruif, J., Boel, E. & Logtenberg, T. (1995) J. Mol. Biol. 248 97-105. [DOI] [PubMed] [Google Scholar]
- 18.Crameri, A., Cwirla, S. & Stemmer, W. P. (1996) Nat. Med. 2 100-102. [DOI] [PubMed] [Google Scholar]
- 19.Schier, R., McCall, A., Adams, G. P., Marshall, K. W., Merritt, H., Yim, M., Crawford, R. S., Weiner, L. M., Marks, C. & Marks, J. D. (1996) J. Mol. Biol. 263 551-567. [DOI] [PubMed] [Google Scholar]
- 20.Schier, R., Balint, R. F., McCall, A., Apell, G., Larrick, J. W. & Marks, J. D. (1996) Gene 169 147-155. [DOI] [PubMed] [Google Scholar]
- 21.Wu, H., Beuerlein, G., Nie, Y., Smith, H., Lee, B. A., Hensler, M., Huse, W. D. & Watkins, J. D. (1998) Proc. Natl. Acad. Sci. USA 95 6037-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, Y., Wiesmann, C., Fuh, G., Li, B., Christinger, H. W., McKay, P., de Vos, A. M. & Lowman, H. B. (1999) J. Mol. Biol. 293 865-881. [DOI] [PubMed] [Google Scholar]
- 23.Wu, H., Nie, Y., Huse, W. D. & Watkins, J. D. (1999) J. Mol. Biol. 294 151-162. [DOI] [PubMed] [Google Scholar]
- 24.Boder, E. T., Midelfort, K. S. & Wittrup, K. D. (2000) Proc. Natl. Acad. Sci. USA 97 10701-10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daugherty, P. S., Chen, G., Iverson, B. L. & Georgiou, G. (2000) Proc. Natl. Acad. Sci. USA 97 2029-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maynard, J. A., Maassen, C. B., Leppla, S. H., Brasky, K., Patterson, J. L., Iverson, B. L. & Georgiou, G. (2002) Nat. Biotechnol. 20 597-601. [DOI] [PubMed] [Google Scholar]
- 27.Short, M. K., Krykbaev, R. A., Jeffrey, P. D. & Margolies, M. N. (2002) J. Biol. Chem. 277 16365-16370. [DOI] [PubMed] [Google Scholar]
- 28.Graff, C. P., Chester, K., Begent, R. & Wittrup, K. D. (2004) Protein Eng. Des. Sel. 17 293-304. [DOI] [PubMed] [Google Scholar]
- 29.Ho, M., Kreitman, R. J., Onda, M. & Pastan, I. (2005) J. Biol. Chem. 280 607-617. [DOI] [PubMed] [Google Scholar]
- 30.Smothers, J. F., Henikoff, S. & Carter, P. (2002) Science 298 621-622. [DOI] [PubMed] [Google Scholar]
- 31.Chames, P., Coulon, S. & Baty, D. (1998) J. Immunol. 161 5421-5429. [PubMed] [Google Scholar]
- 32.Jones, J. T., Ballinger, M. D., Pisacane, P. I., Lofgren, J. A., Fitzpatrick, V. D., Fairbrother, W. J., Wells, J. A. & Sliwkowski, M. X. (1998) J. Biol. Chem. 273 11667-11674. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham, B. C. & Wells, J. A. (1989) Science 244 1081-1085. [DOI] [PubMed] [Google Scholar]
- 34.Kabat, E. A., Wu, T. T., Perry, H. M., Gottesmann, K. S. & Foeller, C. (1991) Sequences of Proteins of Immunological Interest (U.S. Department of Health, and Human Services, Bethesda, MD), 5th Ed.