Abstract
Precis:
We found significant differences in macular vascular microcirculation between normal and glaucomatous eyes using optical coherence tomography angiography (OCTA). Macular vascular microcirculation changes also showed significant correlations with visual field (VF) severity classification systems.
Purpose:
To correlate VF severity defined by different classification systems and macular vascular microcirculation in eyes with glaucoma using OCTA.
Patients and Methods:
Twenty normal and 58 open-angle glaucoma (OAG) eyes were scanned using a swept-source OCTA (Plex Elite 9000) and macular vascular microcirculation was measured by calculating the overall blood flux index (BFI) and vessel area density (VAD) over the entire 6×6 mm area excluding the big retinal vessels. Glaucomatous eyes were staged into severity groups based on 4 VF severity classifications: Hodapp-Parrish-Anderson scale, Glaucoma Severity Staging system, ICD-10 glaucoma staging definitions, and VF mean deviation. Central 10-degree VF mean sensitivity (CMS) was calculated based on 24–2 VF. One-way analysis of variance was used to analyze the differences and correlation between macular vascular microcirculation and other clinical parameters.
Results:
Glaucomatous eyes had significantly lower ganglion cell and inner plexiform layer BFI and VAD (P < 0.0001) compared with normal eyes. In OAG patients, BFI and VAD were significantly higher in mild OAG compared with severe OAG with all VF disease severity classification systems (P < 0.001). Glaucoma Severity Staging had the highest correlation with changes in macular vascular microcirculation metrics (r = 0.734 for BFI; r = 0.647 for VAD) and VF CMS had highest correlation with macular vascular microcirculation metrics (r = 0.887 for BFI; r = 0.903 for VAD).
Conclusion:
Macular vascular microcirculation metrics detected by OCTA correlate with disease severity in glaucomatous eyes. VF CMS, calculated from only 12 tested central 10-degree points, correlated best with macular OCTA.
Keywords: optical coherence tomography angiography, open-angle glaucoma, macular perfusion, visual field
Glaucoma is the leading causes of irreversible blindness worldwide, and the number of people with bilateral blindness resulting from glaucoma is expected to exceed 11 million by 2020.1 The disease is characterized by the degeneration of retinal ganglion cells (RGCs), optic neuropathy, characteristic changes of the optic nerve head, retinal nerve fiber layer (RNFL) and associated visual field (VF) defects. Approximately 50% of the RGCs are within the macular region, and macular damage has been clearly demonstrated in early glaucoma.2
Although elevated intraocular pressure remains the only modifiable risk factor,3,4 disturbed ocular blood flow has emerged as a risk factor for glaucoma progression. Several studies have shown that indirect measurements of blood flow such as aging, systemic blood pressure, nocturnal hypotension, ocular perfusion pressure, migraine, disc hemorrhage, and diabetes mellitus are related to open-angle glaucoma (OAG) progression.5–7 However, it remains unclear whether ischemia is secondary to defective autoregulation of ocular blood flow or if a primary vascular component promotes damage to the optic nerve and RGCs in OAG patients.
Optical coherence tomography angiography (OCTA) can generate 3-dimensional (3D) information of both the blood flow and structural information in the retina and choroid without dye injection. Earlier studies using OCTA in glaucoma have demonstrated that blood flow metrics in the optic disc, peripapillary retina, and macula are associated with the severity of glaucomatous VF damage.8–16
Automated static perimetry is the standard test for assessing visual function in glaucoma. The severity of the VF defect helps physicians establish the rate and the risk of progression of each subtype of glaucomatous VF loss, which is crucial to optimize treatment. In addition, it is an easy way for patients with glaucoma to understand their disease severity. Numerous standard automated perimetry staging systems have been proposed, however, few publications compare the different systems.17–19
The purposes of the present study are (1) to correlate VF severity and macular vascular microcirculation in eyes with glaucoma, and (2) to identify which VF disease severity classification system has the strongest correlation with changes in macular vascular microcirculation metrics. We hypothesized that macular microcirculation detected by OCTA correlates with disease severity in glaucomatous eyes and determining the strongest correlation between blood flow metrics and VF severity has the potential of helping guide clinicians when selecting a severity classification system.
PATIENTS AND METHODS
Subjects
This study was approved by the Institutional Review Board of the University of Washington (UW) and informed consent was obtained from all subjects before imaging. This study followed the tenets of the Declaration of Helsinki and was conducted in compliance with the Health Insurance Portability and Accountability Act.
Subjects with the diagnosis of OAG or normal subjects without glaucoma were prospectively enrolled at the UW Medicine Eye Institute. Inclusion criteria were best-corrected visual acuity of 20/40 or better, and refractive error between −6.0 and +3.0D spherical equivalent. Exclusion criteria were significant media opacity preventing high-quality imaging, any ocular disease other than glaucoma or cataract, and previous intraocular surgeries other than uncomplicated glaucoma or cataract surgery. Normal subjects with a previous diagnosis of migraine were also excluded as reduction in RNFL thickness has been reported in migraine patients without glaucoma.20
The diagnosis of OAG was based on (1) optic disc rim defect (thinning or notching) or RNFL defect visible on slit-lamp biomicroscopy or on optic coherence tomography (OCT) scans; and (2) consistent glaucomatous VF loss. All subjects underwent a comprehensive ophthalmologic examination at time of enrollment, and glaucoma subjects received a VF examination to determine mean deviation (MD) and pattern standard deviation (PSD). All VFs were performed on a Humphrey Field Analyzer II (Carl Zeiss Meditec, Dublin, CA), and only reliable tests were included (<20% for fixation loss and <15% for false-positive response rates). VF central mean sensitivity (VF CMS) (1/Lambert, L) was calculated by averaging the anti-log absolute sensitivity values within the central 10 degrees (12 tested points) area.21 One eye from each subject was included in this study. A single eye was selected and imaged if both were eligible.
Blood pressure (BP) was measured in a seated position using the Welch Allyn (Model LXI #4700–60; Welch Allyn, Skaneateles Falls, New York) automatic BP monitor. The BP was measured once at the same visit after the OCTA scan to calculate mean ocular perfusion pressure (MOPP). MOPP was defined as 2/3 (mean arterial pressure−intraocular pressure), where mean arterial pressure=diastolic BP +1/3(systolic BP−diastolic BP).
Sample size was calculated using G*Power 3.122; with P significant at 0.05 and 90% power, the total sample size of 38 was estimated to achieve the 0.5 correlation between 2 variables.
Severity Classification Systems
Each VF was staged with the 4 different VF classification systems: Hodapp-Parrish-Anderson (HPA) System, Glaucoma Severity Staging (GSS), International Classification of Disease and Related Health Problems (ICD-10) staging, and VF MD.
HPA System
The HPA classification system23 has a total of 3 severity stages (early defect, moderate defect, and severe defect) based on the overall extent of damage using both MD value and the number of defective points in the Humphrey pattern deviation probability map as well as the depth of the defect(s) and the proximity of defect(s) to fixation.
GSS System
The GSS,24 a modified version of the HPA system,23 is based on MD, the location and number of points depressed on the pattern deviation plot at P < 0.01 and P < 0.05, the Glaucoma Hemifield Test (GHT), and visual acuity. GSS has a total of 6 stages: (i) fields with no defect are categorized into stage 0; (ii) fields with early defects are categorized into stage 1; (iii) fields with moderate defects are categorized into stage 2; (iv) fields with advanced defects are categorized into stage 3; (v) fields with severe defect are categorized into stage 4; (vi) and fields with end-stage disease are categorized into stage 5.
International Classification of Disease and Related Health Problems (ICD-10) Staging System
The ICD-10 system25 has a total of 3 severity stages (mild or early-stage glaucoma, moderate-stage glaucoma, and advanced, late, severe stage glaucoma). Mild or early stage is defined as optic nerve abnormalities consistent with glaucoma but no VF abnormalities on any VF test or abnormalities present only on short-wavelength automated perimetry or frequency doubling perimetry. Moderate stage is defined as optic nerve abnormalities consistent with glaucoma, and glaucomatous VF abnormalities in one hemifield not within 5 degrees of fixation. Severe stage is defined as optic nerve abnormalities consistent with glaucoma and glaucomatous VF abnormalities in both hemifields and/or loss within 5 degrees of fixation in at least 1 hemifield.
VF MD System
The VF MD system was based on HPA system23 and divided patients into 3 severity stages (mild, moderate, and severe) based solely on a continuous global index, the Zeiss MD value. Mild stage has an MD no worse than −6.00 dB; moderate stage has an MD worse than −6.00 dB but no worse than −12.00 dB; severe stage has MD worse than −12.00 dB.
Image Acquisition and Scanning Protocol
All the subjects were scanned centered at the foveola using swept-source OCTA (Plex Elite 9000; Zeiss, Dublin, CA), characterized by a central wavelength of 1050 nm, a bandwidth of 100 nm, and a 100 kHz scanning rate. Each scan consisted of 500 A-scans within 1 B-scan and 500 B-scan clusters (2 repeats at each transverse location) covering a 6 mm by 6 mm scanning area. The scanning depth was 3.0 mm in tissue with 1536 sampling points. Blood flow signals were extracted using a complex optical microangiography method and exported from the Plex Elite device. A semiautomatic retinal layer segmentation program26 was applied to the structural OCT images to precisely separate the ganglion cell and inner plexiform layer (GCIPL) (from the outer boundary of nerve fiber layer to outer boundary of inner plexiform layer). Macular vascular en face images were generated using blood flow signals by detecting the highest flow intensity along the axial direction within the GCIPL. Macular vascular microcirculation was then measured by calculating the overall blood flux index (BFI) (the mean flow signal intensity) and vessel area density (VAD) (percentage of the detected vessels) over the entire 6×6 mm area excluding large retinal vessels and compared among groups. The method for large retinal vessels removal has been described previously.8 Scans with an OCT signal strength <7 (as recommended by the manufacturer) were excluded from analysis.
Statistical Analysis
Two-sample, independent t tests were used to compare the macular BFI and VAD between normal and glaucoma eyes. Linear regression models were further used to investigate the correlation between macular BFI, VAD, RNFL thickness, cup-to-disc ratio (CDR), and VF indices. One-way analyses of variance were performed to analyze the macular vascular microcirculation differences among groups. Pearson correlation was used to determine the correlation coefficient between blood flow metrics, VF CMS, and VF severity classification systems. A P-value <0.05 was considered statistically significant.
RESULTS
Twenty eyes from 20 normal subjects and 58 eyes from 58 OAG subjects were enrolled (Table S1, Supplemental Digital Content 1, http://links.lww.com/IJG/A285). There was no significant difference in age, sex, or the proportion of subjects with diabetes mellitus or systemic hypertension between normal and glaucoma groups. In addition, no significant differences were detected in systolic BP, diastolic BP, and MOPP between normal and glaucoma groups (P ≥ 0.437). The average VF MD, VF PSD, and VF CMS of glaucoma subjects was −6.91 ± 6.10, 7.32 ± 4.88, and 650.51 ± 262.38 (1/L), respectively. Ten glaucoma subjects (17.2%) had VF loss confined to a single hemifield; of those, 8 patients had severe VF loss and 2 had moderate VF loss as per ICD-10 system classification. In addition, 10 glaucoma patients (17.2%) had a history of prior trabeculectomy; all patients had surgery at least 1 year before OCTA. For the OCTA parameters of macular GCIPL, glaucoma eyes showed significantly thinner GCIPL thickness, and lower GCIPL blood flow metrics (BFI and VAD), when compared with normal eyes (P < 0.0001).
Figure 1 presents examples of the microvasculature in the GCIPL in the macular region in normal eyes and eyes with OAG with disease severity graded according to VF MD classification. The top layer displays the vascular en face images in the macular region and the bottom layer displays the detected microcirculation. By comparing the blood flux in the GCIPL in the macular region among normal and eyes with mild, moderate and severe glaucoma, a reduction in the microcirculation in glaucomatous eyes is seen when compared with normal, with severe glaucoma showing a much more severe reduction.
FIGURE 1.
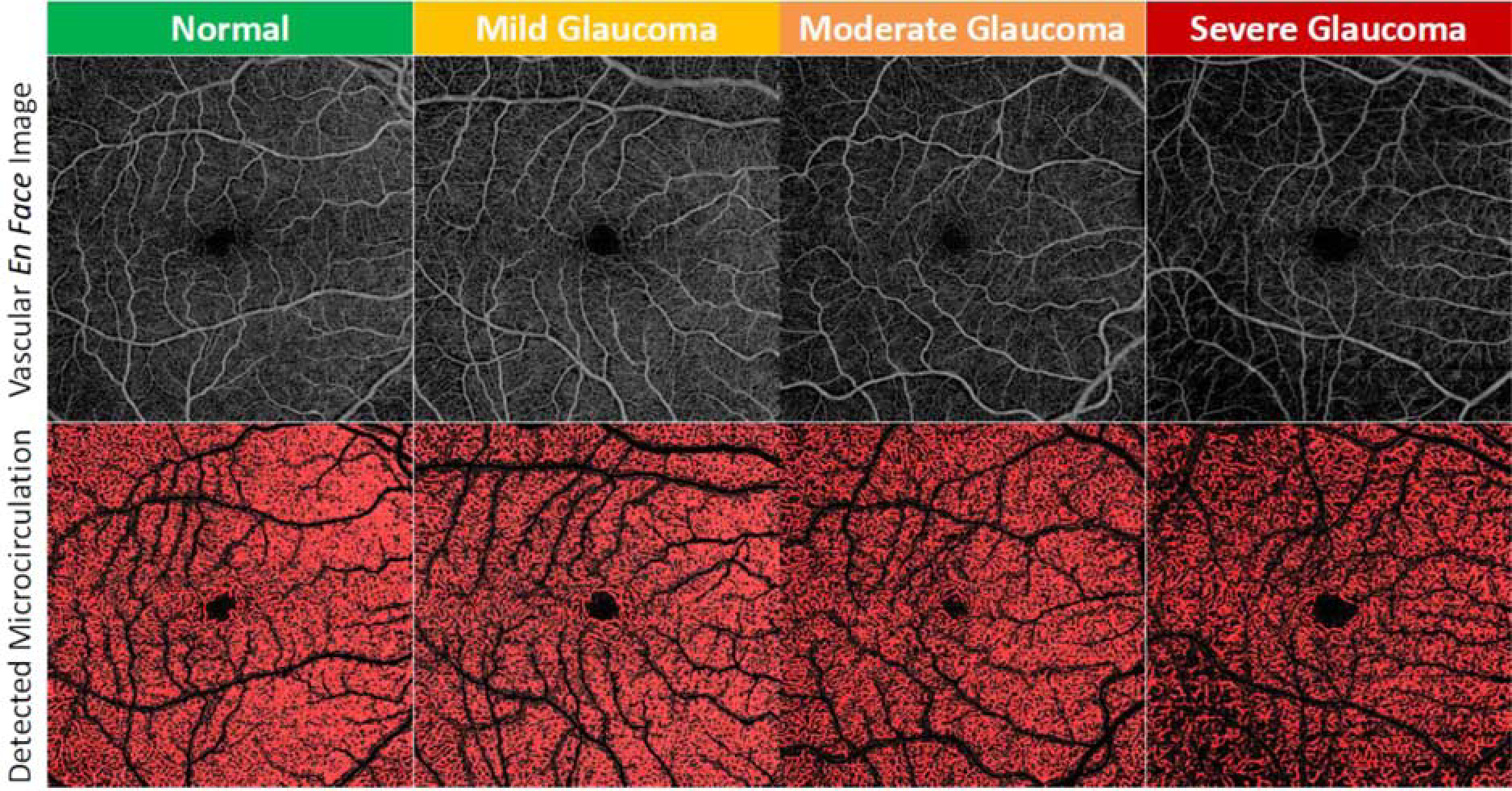
Microvasculature in the ganglion cell and inner plexiform layer in the macular region in normal eyes and eyes with glaucoma with various disease severity (disease severity in this figure based on the visual field mean deviation system). Top layer: vascular en face images; bottom layer: vascular en face images with detected flow signal presented in red.
Table 1 presents the findings of correlation and univariate regression analysis between BFI, VAD, and functional and structural measurements for the OAG group. Global BFI and global VAD were significantly correlated with VF MD, VF PSD, VF CMS, CDR, RNFL thickness, and GCIPL thickness (P ≤ 0.004). Additional subanalysis including only eyes that did not have prior glaucoma surgery (N = 48) revealed that the blood flow metrics remained significantly correlated with the structural and functional measurements (P ≤ 0.003).
TABLE 1.
Summary of Correlation and Univariate Regression Analyses Results Between Blood Flux Index and Vessel Area Density, and Other Functional and Structural Clinical Measurements for the Glaucoma Group (N= 58)
| Global GCIPL Blood Flux Index |
Global GCIPL Vessel Area Density |
|||
|---|---|---|---|---|
| Variables | Correlation [r (R2)] | P | Correlation [r (R2)] | P |
|
| ||||
| RNFL thickness (μm) | 0.515 (0.265) | < 0.001 | 0.376 (0.141) | < 0.004 |
| Cup-to-disc ratio | −0.597 (0.356) | < 0.001 | −0.619 (0.383) | < 0.001 |
| VF MD (dB) | 0.677 (0.458) | < 0.001 | 0.664 (0.441) | < 0.001 |
| VF PSD (dB) | −0.616 (0.379) | < 0.001 | −0.587 (0.345) | < 0.001 |
| VF CMS parafoveal area (1/L) | 0.721 (0.519) | < 0.001 | 0.762 (0.581) | < 0.001 |
| GCIPL Thickness (μm) | 0.622 (0.387) | < 0.001 | 0.504 (0.254) | < 0.001 |
Bold values indicate statistically significant (P<0.05).
CMS indicates central mean sensitivity; GCIPL, ganglion cell and inner plexiform layer; MD, mean deviation; PSD, pattern standard deviation; RNFL, retinal nerve fiber layer; VF, visual field.
Blood flow metrics were correlated to changes in macular vascular microcirculation metrics using all VF disease severity classification systems (Supplemental Tables 2–5, Supplemental Digital Content 1, http://links.lww.com/IJG/A285). With the HPA system, the BFI was significantly lower between mild and severe glaucoma (P < 0.001). VAD was significantly different between both mild and severe glaucoma and moderate and severe glaucoma (P < 0.001). For the GSS system, the BFI was significantly lower between stage 0 and stage 1, stage 2, stage 3, and stage 4 (P < 0.001). VAD was significantly different between stage 0 and stage 3, and stage 4 (P < 0.001). For the ICD-10 system, the BFI was significantly lower between mild, moderate and severe glaucoma (P < 0.001). VAD was significantly different between both mild and severe glaucoma and moderate and severe glaucoma (P = 0.002). For the VF MD system, BFI and VAD were significantly different between both mild and severe glaucoma and moderate and severe glaucoma (P < 0.001).
Although GSS showed the highest correlation with changes in macular vascular microcirculation metrics (r = 0.734 for BFI; r = 0.647 for VAD, P < 0.001) (Table 2), there was no significant difference compared with the other correlation coefficients (P ≥ 0.0548). VF MD system showed the highest correlation between VF CMS and changes in macular vascular microcirculation metrics (r = 0.887 for BFI; r = 0.903 for VAD; P < 0.001); the correlation coefficients for VF MD system were significantly different from those with ICD-10 system (P = 0.007 for BFI comparison and P = 0.035 for VAD comparison) and with HPA system (P = 0.037 for BFI comparison). VAD correlated the best with VF CMS versus blood flow metrics for all VF severity criteria (Table 3).
TABLE 2.
Correlation Coefficient Between Blood Flow Metrics and the Different Visual Field Severity Classification Systems
| ICD-10 |
Hodapp-Parrish-Anderson |
Visual Field Mean Deviation |
Glaucoma Severity System |
|||||
|---|---|---|---|---|---|---|---|---|
| Variables | r | P | r | P | r | r | P | |
|
| ||||||||
| Global GCIPL blood flux index | 0.681 | < 0.001 | 0.649 | < 0.001 | 0.678 | < 0.001 | 0.734 | < 0.001 |
| Global GCIPL vessel area density | 0.434 | < 0.001 | 0.542 | < 0.001 | 0.630 | < 0.001 | 0.647 | < 0.001 |
Bold values indicate statistically significant (P<0.05).
GCIPL indicates ganglion cell and inner plexiform layer.
TABLE 3.
Correlation Coefficient Between Blood Flow Metrics and Visual Field (VF) Central Mean Sensitivity According to VF Severity Classification Systems
| ICD-10 |
Hodapp-Parrish-Anderson |
Visual Field Mean Deviation |
Glaucoma Severity System |
|||||
|---|---|---|---|---|---|---|---|---|
| Variables | r | P | r | P | r | P | r | P |
|
| ||||||||
| Global GCIPL blood flux index | 0.734 | < 0.001 | 0.788 | < 0.001 | 0.887 | < 0.001 | 0.835 | < 0.001 |
| Global GCIPL vessel area density | 0.815 | < 0.001 | 0.832 | < 0.001 | 0.903 | < 0.001 | 0.872 | < 0.001 |
Bold values indicate statistically significant (P<0.05).
GCIPL indicates ganglion cell and inner plexiform layer.
DISCUSSION
In this study, we investigated macular vascular microcirculation among normal eyes and OAG eyes with different VF disease severities using OCTA and different severity grading methods. A proprietary semiautomatic segmentation software enabled isolation of the GCIPL of the macula, providing a better understanding of the regions affected by glaucoma. Significant reductions in retinal blood flow metrics, as measured by BFI and VAD, were detected in OAG eyes compared with age-matched normal controls. In addition, BFI and VAD were significantly correlated with VF MD, VF PSD, VF CMS, CDR, GCIPL thickness, and RNFL thickness (P < 0.001 for all BFI comparison; P ≤ 0.004 for all VAD comparison).
Other authors have investigated structure and function in the macula using OCTA. Penteado et al16 studied the superficial retinal capillary plexuses (blood vessels located within a volume between the RNFL and the IPL) vessel density (VD) from 3×3 mm2 macula scans centered at the fovea of 185 eyes from 38 healthy participants, 31 glaucoma suspects, 72 mild glaucoma patients, and 44 moderate/severe glaucoma patients (classified based on VF MD system) and found that mean VD was significantly higher in healthy eyes and glaucoma suspect eyes compared with glaucoma eyes with mild and moderate/severe disease and significantly associated with central 10–2 VF MS. Yarmohammadi et al15 also used 3×3 mm2 scans centered on the fovea to study the RNFL-GCIPL perfusion in 58 glaucoma patients with VF loss confined to a single hemifield and demonstrated that OCTA VD in the macula was strongly associated with functional VF measures [VF mean sensitivity and perifoveal VD (r = 0.615) in the corresponding hemiretinae]. Similar to our study, Takusagawa et al27 also studied 6×6 mm2 macular scan areas; however, they included the RNFL in both the structural and perfusion measures of the macula in 30 glaucoma patients; they reported significant associations between functional and structural measures of glaucoma (r = 0.444 between VF sensitivity and superficial vascular complex VD and r = 0.804 for GCC thickness and superficial vascular complex VD). Rao et al28 used an optical microangiography system and studied 6×6 mm2 macular scan areas in 39 eyes of 26 OAG patients. They analyzed angiographic images of the superficial retinal slab (internal limiting membrane to GCIPL) and reported that OCTA parameters were similarly associated with GCIPL thickness and visual sensitivity measurements, at least in the inferior macular sector (r = 0.56 for GCIPL thickness and VD and r = 0.54 for GCIPL thickness and perfusion density; r = 0.53 for visual sensitivity and VD and r = 0.49 for visual sensitivity and perfusion density). Richter et al29 recently reported that although OCTA measures of perfusion were significantly associated with functional measures of glaucoma in 34 patients, they were not significantly associated with structural measures of glaucoma (GCIPL thickness). They postulated that the lower association was related to the fact that previous studies had included RNFL in both the structural and perfusion measures, which would increase the total anatomic structures and corresponding microvasculature being assessed, that are affected by glaucoma.
Our study also included only the macular ganglion cell layer and IPL; we found a significant association between blood flow metrics and functional measures, and also a significant association with GCIPL thickness and RNFL thickness. It is possible that differences between the previous studies are due to location of VF defects because the macular region would be more affected if VF damage included the paracentral points. We attempted to evaluate the differences by including the VF CMS from the central 10 degrees of the 24–2 humphrey visual field (HVF) and notably observed significant correlation between functional measures and changes in macular vascular microcirculation (r = 0.721 for BFI and r = 0.762 for VAD; Table 1). Even though the VF CMS is calculated from only the 12 central points of the 24–2 VF, it provided the strongest correlation with macular OCTA metrics.
Staging glaucomatous damage into broad categories such as mild, moderate, and advanced enhances patient management. An ideal system should be objective, reproducible, and user friendly. In addition, it should provide a classification that is consistent with structural damage data and be able to monitor changes in functional loss over time. Having a common severity staging system would be desirable, as it would entitle researchers and clinicians to directly compare treatment and diagnostic devices, however, that is not currently available. We chose to examine the relationship between macular vascular microcirculation as detected by OCTA and VF severity using 4 different VF disease severity classification systems—HPA system, the GSS system, the ICD-10 system, and the VF MD system—as a potential tool to help guide clinicians when selecting a severity classification system.
The HPA scoring system23 is a commonly used criteria to stage glaucoma. It uses both MD value and the number of defective points in the HVF, and, in addition, it considers the depth of the defect and the proximity of defect(s) to fixation. In our study, blood flow index was significantly different between mild and severe glaucoma, but not between mild and moderate, though we had few moderate-stage patients by HPA criteria. In addition, although the HPA system was associated with changes in macular vascular microcirculation (P < 0.001 for BFI and VAD), among all 4 disease severity classification systems it had the weakest correlation with the blood flow index (r = 0.649). We could speculate that those differences are likely because, in HPA, the VF defect is characterized into three relatively coarse stages (mild, moderate, and severe) which may be inappropriate for a fine categorization of VF defects, meaning that some VF may be classified under the same stage but have different severity and possibly different prognosis (for instance, in HPA even a single point in central 5 degrees with 0 dB sensitivity will be classified as a severe defect).
In 2006, Mills et al24 proposed a new system, GSS, which is an extended version of the HPA scoring system and incorporates several parameters, including MD value, the location and number of points depressed on the pattern deviation plot at P < 0.01 and P < 0.05, the GHT, and visual acuity. It has 1 normal (stage 0) and 5 abnormal stages (stages 1 to 5) providing finer distinctions than HPA during disease progression; 2 additional stages beyond the HPA severe stage are added. However, similar to the HPA system, calculating the GSS is time-consuming, which makes it less helpful for day-to-day clinical use. In our study, GSS had the highest correlation with changes in macular vascular microcirculation metrics, however, that was not statically significant from the other classification system correlation coefficients. In addition, GSS had a significant correlation with the central 10 degrees VF MS (r = 0.835 between VF sensitivity and BFI; r = 0.872 between VF sensitivity and VAD). Takusagawa et al27 also used GSS to stage glaucoma patients and similar to our results, reported a significant association between functional and structural measures of glaucoma (r = 0.444 between VF sensitivity and superficial vascular complex VD and r = 0.804 for GCC thickness and superficial vascular complex VD).
In 2015, the implementation of the ICD-10 codes greatly expanded the codes and diagnoses used by physicians in clinical practice. ICD-1025 glaucoma staging definitions include 3 severity stages (mild or early-stage glaucoma, moderate stage glaucoma and advanced, late, severe stage glaucoma) and is based on location of VF abnormality (1 vs. 2 hemispheres) and location of loss (within 5 degrees of fixation or not). Staging using ICD-10 criteria has the advantage of being a relatively easy system to apply clinically. In our study, staging of the abnormal VFs was skewed more to the severe stage with ICD-10 compared with HPA, GSS, and VF MD. In addition, BFI was statistically significantly different between mild, moderate and severe glaucoma. ICD-10 had second highest association with BFI (r = 0.681) but had the weakest association with VAD (r = 0.434, P < 0.001). Richter et al29 used the ICD-9 glaucoma staging definition, which uses the same criteria as ICD-10 staging and reported a similar association between global VF MD and GCIPL VAD (r = 0.452, P = 0.0060).
The VF MD system is loosely based on HPA23 and classified patients into mild, moderate and severe glaucoma based solely on HVF MD value. In our study, BFI and VAD were statistically significant differences between both mild and severe glaucoma and moderate and severe glaucoma, but not between mild and moderate glaucoma. In addition, it showed statistically significant correlation with BFI (r = 0.678) and VAD (r = 0.630). When we compared with central VF MS, the VF MD severity system showed the highest association between VF CMS and changes in macular vascular microcirculation (r = 0.887 between VF CMS and BFI; r = 0.903 between VF CMS and VAD) and the correlation coefficients were significantly different from ICD-10 system (P = 0.007 for BFI comparison and P = 0.035 for VAD comparison) and HPA system (P = 0.037 for BFI comparison). The Collaborative Initial Glaucoma Treatment Study (CIGTS) group compared the CIGTS VF score and the MD reported on the HVF printout and reported that the systems correlated highly (r = −0.93) with the advantage of MD being more reproducible than the CIGTS score when comparing the first and second baseline measures.30 The MD system may provide better correlation with macular OCTA because subtle loss of retinal sensitivity, which may not trigger a change in scoring in systems such as HPA, GSS, or ICD-10, will still contribute to MD. Also, systems such as HPA and GSS incorporate P-values from the pattern deviation plot, but this may sacrifice dynamic range given the small number of P-values categorized. In addition, the MD system is practical and has been previously validated.
Our study has some limitations. First, there is no gold standard that is universally used for glaucoma severity classification. Therefore, the severity classification systems were compared with each other based on which system correlates best with the changes in macular vascular microcirculation as detected by OCTA. Second, we used results from 24–2 HVF testing to assess the central VF MS. Although 24–2 VF testing is routinely used in glaucoma patients, the VF 10–2 test could have increased the sensitivity to detect parafoveal VF defects.31 Third, there is no gold standard for OCTA parameters and our results represent the findings for a specific device (Plex Elite 9000; Zeiss). Fourth, the inclusion of glaucoma patient with prior glaucoma surgery may affect our results, as glaucoma surgery likely affects ocular blood flow; however, we performed a subanalysis of only eyes without prior glaucoma surgery, and still found that the OCTA blood flow metrics remained significantly correlated with the structural and functional measurements (P ≤ 0.003). Fifth, we chose to report global blood flow metrics, which does not allow for comparison of regional blood flow differences within the 6×6 scan area; this could affect our results as we included eyes with hemifield defects and some VF severity criteria take into account the location of the VF defect. Lastly, we did not obtain VF testing in our normal subjects, though all normal subjects underwent comprehensive ocular examination and were found to have healthy optic nerves, had statistically normal peripapillary RNFL thickness, and normal optic disc measures using OCT.
In conclusion, we found significant correlations between blood flow metrics in the macular vascular microcirculation and structural and functional changes related to glaucoma. All 4 disease severity classification systems used in this study were correlated with changes in macular vascular microcirculation with increasing disease severity. To be best of our knowledge, this is the first study to compare results to different VF severity classification systems using same scanning patterns and OCTA device and our results should encourage other researchers to report multiple VF classification systems when reporting their work to confirm our findings.
Supplementary Material
Acknowledgments
Supported in part by National Institutes of Health contract NEI R01-EY024158, Carl Zeiss Meditec Inc. and Research to Prevent Blindness (New York, NY).
Footnotes
Disclosure: R.K.W. receives royalty from intellectual property owned by Oregon Health & Science University and research support from Carl Zeiss Meditec Inc. The remaining authors declare no conflict of interest.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hood DC, Slobodnick A, Raza AS, et al. Early glaucoma involves both deep local, and shallow widespread, retinal nerve fiber damage of the macular region. Invest Ophthalmol Vis Sci. 2014;55:632–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leske MC, Connell AM, Wu SY, et al. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch Ophthalmol. 1995;113:918–924. [DOI] [PubMed] [Google Scholar]
- 4.Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–1095. [DOI] [PubMed] [Google Scholar]
- 5.Heijl A, Leske MC, Bengtsson B, et al. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. [DOI] [PubMed] [Google Scholar]
- 6.Hulsman CA, Vingerling JR, Hofman A, et al. Blood pressure, arterial stiffness and open angle glaucoma. The Rotterdam study. Arch Ophthalmol. 2007;125:805–812. [DOI] [PubMed] [Google Scholar]
- 7.Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: The Egna-Neumarkt Study. Ophthalmology. 2000;107:1287–1293. [DOI] [PubMed] [Google Scholar]
- 8.Chen CL, Bojikian KD, Gupta D, et al. Optic nerve head perfusion in normal eyes and eyes with glaucoma using optical coherence tomography-based microangiography. Quant Imaging Med Surg. 2016;6:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CL, Zhang A, Bojikian KD, et al. Peripapillary retinal nerve fiber layer vascular microcirculation in glaucoma using optical coherence tomography-based microangiography. Invest Ophthalmol Vis Sci. 2016;57:OCT475–OCT485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CL, Bojikian KD, Wen JC, et al. Peripapillary retinal nerve fiber layer vascular microcirculation in eyes with glaucoma and single-hemifield visual field loss. JAMA Ophthalmol. 2017;135:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia Y, Morrison JC, Tokayer J, et al. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express. 2012;3:3127–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Jia Y, Takusagawa HL, et al. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol. 2015;133:1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Jiang C, Ko T, et al. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol. 2015;253:1557–1564. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Jiang C, Wang X, et al. Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Invest Ophthalmol Vis Sci. 2015;56:3212–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Peripapillary and macular vessel density in patients with glaucoma and single-Hemifield visual field defect. Ophthalmology. 2017;124:709e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penteado RC, Zangwill LM, Daga FB, et al. Optical coherence tomography angiography macular vascular density measurements and the central 10–2 visual field in glaucoma. J Glaucoma. 2018;27:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brusini P, Johnson CA. Staging functional damage in glaucoma: review of different classification methods. Surv Ophthalmol. 2007;52:156–179. [DOI] [PubMed] [Google Scholar]
- 18.Katz J, Sommer A, Gaasterland DE, et al. Comparison of analytic algorithms for detecting glaucomatous visual field loss. Arch Ophthalmol. 1991;109:1684–1689. [DOI] [PubMed] [Google Scholar]
- 19.Ng M, Sample PA, Pascual JP, et al. Comparison of visual field severity classification systems for glaucoma. J Glaucoma. 2012;21:551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gipponi S, Scaroni N, Venturelli E. Reduction in retinal nerve fiber layer thickness in migraine patients. Neurol Sci. 2013;34:841–845. [DOI] [PubMed] [Google Scholar]
- 21.Kwon J, Choi J, Shin JW, Lee J, Kook MS. Alterations of the foveal avascular zone measured by optical coherence tomography angiography in glaucoma patients with central visual field defects. Invest Ophthalmol Vis Sci. 2017;58:1637–1645. [DOI] [PubMed] [Google Scholar]
- 22.Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. [DOI] [PubMed] [Google Scholar]
- 23.Hodapp E, Parrish RK II, Anderson DR. Clinical Decisions in Glaucoma, 1st ed. St. Louis: Mosby-Year Book; 1993. [Google Scholar]
- 24.Mills RP, Budenz DL, Lee PP, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol. 2006;141:24–30. [DOI] [PubMed] [Google Scholar]
- 25.American Academy of Ophthalmology practice management.2015. Available at: www.aao.org/practice-management/news-detail/icd-10-glaucoma-staging-definitions. Accessed June 28, 2019.
- 26.Yin X, Chao JR, Wang RK. User-guided segmentation for volumetric retinal optical coherence tomography images. J Biomed Opt. 2014;19:086020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takusagawa HL, Liu L, Ma KN, et al. Projection-resolved optical coherence tomography angiography of macular retinal circulation in glaucoma. Ophthalmology. 2017;124:1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao HL, Riyazuddin M, Dasari S, et al. Relationship of macular thickness and function to optical microangiography measurements in glaucoma. J Glaucoma. 2018;27:210–218. [DOI] [PubMed] [Google Scholar]
- 29.Richter GM, Madi I, Chu Z, et al. Structural and functional associations of macular microcirculation in the ganglion cell-inner plexiform layer in glaucoma using optical coherence tomography angiography. J Glaucoma. 2018;27:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillespie BW, Musch DC, Guire KE, et al. The Collaborative Initial Glaucoma Treatment Study: baseline visual field and test-retest variability. Invest Ophthalmol Vis Sci. 2003;44:2613–2620. [DOI] [PubMed] [Google Scholar]
- 31.Park SC, Kung Y, Su D, et al. Parafoveal scotoma progression in glaucoma: Humphrey 10–2 versus 24–2 visual field analysis. Ophthalmology. 2013;120:1546–1550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


