Abstract
Human cytomegalovirus (CMV) infection is dependent on the functions of structural glycoproteins at multiple stages of the viral life cycle. These proteins mediate the initial attachment and fusion events that occur between the viral envelope and a host cell membrane, as well as virion-independent cell-cell spread of the infection. Here we have utilized a cell-based fusion assay to identify the fusogenic glycoproteins of CMV. To deliver the glycoprotein genes to various cell lines, we constructed recombinant retroviruses encoding gB, gH, gL, and gO. Cells expressing individual CMV glycoproteins did not form multinucleated syncytia. Conversely, cells expressing gH/gL showed pronounced syncytium formation, although expression of gH or gL alone had no effect. Anti-gH neutralizing antibodies prevented syncytium formation. Coexpression of gB and/or gO with gH/gL did not yield detectably increased numbers of syncytia. For verification, these results were recapitulated in several cell lines. Additionally, we found that fusion was cell line dependent, as nonimmortalized fibroblast strains did not fuse under any conditions. Thus, the CMV gH/gL complex has inherent fusogenic activity that can be measured in certain cell lines; however, fusion in fibroblast strains may involve a more complex mechanism involving additional viral and/or cellular factors.
Human cytomegalovirus (CMV) is a significant human pathogen that causes substantial disease throughout the world. CMV is the leading viral cause of congenital birth defects in the United States and is a significant factor in the success of allograft transplantations (1). A hallmark of CMV, and of all members of the Herpesviridae, is that infections are never fully cleared by the immune system. As such, development of immunoincompetence at any time throughout the life of an infected individual can result in potentially serious consequences, including, but not limited to, organ failure, retinitis, or pneumonitis, upon CMV reactivation (20).
Once an individual has contracted CMV, the infection spreads throughout the body, where it can infect a variety of cell types encompassing nearly every organ system. Surprisingly, this broad cellular tropism does not lead to the production of extracellular viral particles in the circulatory system during an acute infection. In fact, very little extracellular virus is detected in the plasma, and lateral transmission by blood transfusion can be inhibited by removing peripheral leukocytes (34, 51). These data, along with others, has lead CMV biologists to hypothesize that the majority of viral dissemination within an infected individual can be attributed to cell-cell transmission events between infected leukocytes and uninfected tissues (50).
The mechanisms by which CMV-infected cells transmit the virus to uninfected tissues are not well understood. One proposal is that interactions between viral glycoproteins on the surface of infected cells and cellular receptors on adjacent cells mediate cell-cell transmission (11). In support of this hypothesis, it has been shown that direct contact between plasma membranes of CMV-infected and uninfected cells is required for cell-cell spread of the infection in vitro (16).
Cell-cell fusion assays have been used as a tool to identify the fusion machinery of several other herpesviruses. In herpes simplex virus type 1 (HSV-1) and HSV-2, Epstein-Barr virus (EBV), and Kaposi's sarcoma-associated virus (KSHV), homologs of glycoprotein B (gB), glycoprotein H (gH), and glycoprotein L (gL) were required to induce syncytium formation between glycoprotein-expressing cells and receptor-positive cells (19, 37, 41, 55). HSV-1 and HSV-2 require coexpression of an additional receptor binding protein, gD. This is also true for EBV in certain cell types. In contrast, varicella-zoster virus (VZV) encodes two complexes, gH/gL and gB/gE, that are independently capable of inducing cell-cell fusion (12). Therefore, as no consistent paradigm exists for all herpesvirus fusion machines, it remains important to identify the glycoproteins that mediate fusion of each specific herpesvirus independently.
During infection, CMV glycoproteins are expressed and displayed on the cell surface (9, 24). CMV gB (UL55) is a type 1 transmembrane protein that exists as a proteolytically processed dimer on the surface of infected cells and the viral envelope. A soluble form of gB displays biphasic binding to fibroblast cells, attaching to heparan sulfate proteoglycans and additional receptor sites in a dose-dependent manner (7). Several laboratories have shown that separate monoclonal antibodies to gB inhibit infection at different steps during viral entry (17, 38, 39). For example, antibodies that potently inhibit virus attachment fail to inhibit viral entry after attachment has occurred. Conversely, antibodies that do not hinder attachment inhibit infectivity at a postattachment step and inhibit cell-to-cell spread (38). These observations imply that gB is functionally important for attaching the viral particle to the surface of host cells and plays an undefined role in membrane fusion.
All herpesviruses examined to date encode a heterodimeric envelope complex consisting of gH and gL. During CMV infection, type 1 transmembrane gH (UL75) and gL (UL115) associate cotranslationally (24). In addition, correct folding of gH and surface presentation of gH/gL is strictly dependent on coexpression of both proteins (24, 49). Our laboratory and others determined that a third glycoprotein associated with the gH/gL heterodimer to form a unique, high-molecular-weight, disulfide-bonded complex (23, 33). We subsequently mapped the gene encoding the third component to the UL74 locus and designated the protein gO (23). In contrast to gH/gL knockouts, which are lethal to the viral life cycle, gO-null viruses remain viable in cell culture, albeit severely attenuated (21). Viruses containing a gO knockout also display a small-plaque phenotype. Thus, although gO is not strictly required for replication in tissue culture, gO likely serves an accessory role in CMV pathogenesis and cell-to-cell spread.
Pretreatment either of viral particles with monoclonal antibodies to gH or of permissive cells with an anti-idiotypic antibody that mimics gH does not hinder attachment of the virus to host cells. These antibodies do, however, potently inhibit viral penetration and cell-to-cell spread (26, 44). Taken together, these results imply that the gH/gL heterodimer is essential for membrane fusion.
Reconstitution of cell-cell fusion in herpesviruses has led to an emerging paradigm that coexpression of multiple glycoproteins is required to induce membrane fusion. Thus, the goal of this study was twofold. We first aimed to determine whether expression of individual CMV glycoproteins was sufficient to induce cell-cell fusion. We expressed gB (UL55), gH (UL75), gL (UL115), and gO (UL74) in several model cell lines. Individual- or multiple-glycoprotein-expressing cells were then assayed for the formation of multinucleated syncytia. As shown here, we were unable to induce the formation of multinucleated cells over background levels in any cell type when individual glycoproteins were expressed.
Consequently, the second objective was to induce cell-cell fusion by coexpression of multiple CMV glycoproteins. Taking into consideration the glycoproteins implicated in cell-cell fusion for several other herpesviruses, we hypothesized that coexpression of CMV gB and gH/gL would be necessary and sufficient to induce cell-cell fusion. To our surprise, however, we found that coexpression of the genes encoding gH and gL was sufficient to induce syncytium formation in certain cell lines. Moreover, formation of syncytia was inhibited when cells expressing gH/gL were treated with anti-gH/gL neutralizing monoclonal antibodies. Finally, we showed by immunofluorescence that CMV glycoproteins specifically localized to multinucleated syncytia, confirming that glycoprotein expression was responsible for cell-cell fusion. By contrast, these results were not recapitulated in nonimmortalized secondary fibroblast strains. Ultimately these findings show that the CMV gH/gL complex has inherent fusogenic activity that can be measured in model cell lines. Moreover, cell-cell fusion and therefore cell-cell transmission in nonimmortalized cell strains and primary cells may utilize a more complex mechanism involving other viral and/or cellular factors.
MATERIALS AND METHODS
Cell maintenance and CMV preparations.
Chinese hamster ovary (CHO) cells were maintained in F12 medium in the presence of 10% fetal bovine serum, 0.1% penicillin-streptomycin-amphotericin B (Fungizone), and 0.3% glutamine (Harlan Biosciences). U373 MG glioblastoma cells, HeLa cells, immortalized fibroblasts, and secondary cell strain normal human dermal fibroblast (NHDF) cells were cultivated in Dulbecco's modified Eagle's medium supplemented with 10% bovine calf serum (HyClone), 1% penicillin-streptomycin-amphotericin B, and 0.3% glutamine (Harlan Biosciences). CMV strain AD169 was propagated in NHDF cells, and titers were determined as previously described on NHDF cell monolayers (13).
Antibodies.
Monoclonal antibodies AP86, 27-78, 27-39, 7-17, and 14-4b were provided by W. Britt and have been described previously (3, 8, 10, 22, 56). Polyclonal 6824 and anti-UL74 have also been described previously (22, 23, 32). 6394 is a polyclonal antipeptide antibody to glycoprotein L (designated anti-gL). Hydrophilicity plots of the gene product of AD169 UL115 indicated a putative antigenic area of 20 charged amino acids at the carboxy terminus. Peptide GlyL-260 (CTRVNLPAHSRYGPQAVDAR) corresponding to this area was synthesized, conjugated to keyhole limpet hemocyanin, and injected into rabbits (Alpha Diagnostic). Polyclonal antiserum was harvested biweekly and purified over a GlyL-260 affinity column. Briefly, 5 μg of GlyL-260 was conjugated to cyanogen bromide-activated Sepharose and packed into a column (Amersham Pharmacia). Antiserum was repeatedly passed over the column at 4°C overnight using a peristaltic pump. Anti-gL antibodies were eluted by successive washes with 100 mM glycine (pH 2.5) and 100 mM triethylamine (pH 11.5), concentrated by ammonium sulfate precipitation, and desalted by dialysis against phosphate-buffered saline (PBS). Purified immunoglobulin G (IgG) of 14.4b and 27-39 acites fluid was obtained by utilizing an ImmunoPure (G) IgG purification kit according to the manufacturer's instructions (Pierce). Concentrations of 27-39 and 14-4b were obtained by Bio-Rad protein assay using bovine IgG as a standard.
Plasmids and retrovirus production.
pCAGGS.gH, pCAGGS.gL, and pCAGGS.gO have been described previously (32, 52). pCAGGS.gB was produced by removing the entire UL55 open reading frame from a cosmid library by EagI/EcoRV (NEB) digestion. The resulting fragment was gel purified and cloned into pBluescript II (pBS) (Stratagene). UL55 was then removed from pBS by SacI/XhoI (NEB) digestion and subcloned into pCAGGS (32). pCMMP.UL55.IRES.GFP, pCMMP.UL75.IRES.GFP, pCMMP.UL115.IRES.GFP, and pCMMP.UL74.IRES.GFP were constructed by PCR amplification of the genes encoding gB, gH, gL, and gO, respectively, from a genomic template of laboratory CMV strain AD169 with primer sets 5′-ACT GCG GCC GCC ACC ATG GAA TCC AGG ATC TGG-3′ and 5′-ACT CTC GAG CAG ACG TTC TCTT CTT CG-3′, 5′-ACT GCG GCC GCC ACC ATG CGG CCC GGC CTC CCC-3′ and 5′-ACT CTC GAG TCA GCA TGT CTT GAG CAT G-3′, 5′-ACT GCG GCC GCC ACC ATG TGC CGC CGC CCG GAT-3′ and 5′-ACT CTC GAG TTA GCG AGC ATC CAC TGC-3′, and 5′-ACT GCG GCC GCC ACC ATG GGG AGA AAA GAG ATG-3′ and 5′-ACT CTC GAG TTA CTG CAA CCA CCA CCA-3′, incorporating restriction sites NotI at the 5′ end and XhoI at the 3′ end. Amplified fragments were digested (NEB), gel purified (QIAGEN), and cloned into the retroviral transfer vector pCMMP.MCS.IRES.GFP (a murine leukemia virus [MLV] vector, gift of the W. Sugden laboratory [University of Wisconsin, Madison]) (Fig. 1A). Constructs were confirmed by sequencing (University of Wisconsin Biotechnology Center). pMD.old.gagpol (encoding MLV Gag and Gag-Pol proteins) was a gift of R. C. Mulligan (Whitehead Institute). pCAGGS.MCS.VSV-G (encoding vesicular stomatitis virus glycoprotein G) was a gift from Y. Kawaoka (University of Wisconsin, Madison). Recombinant retrovirus was generated as described elsewhere, substituting pCAGGS.MCS.VSV-G for pMDtet.G (4, 40).
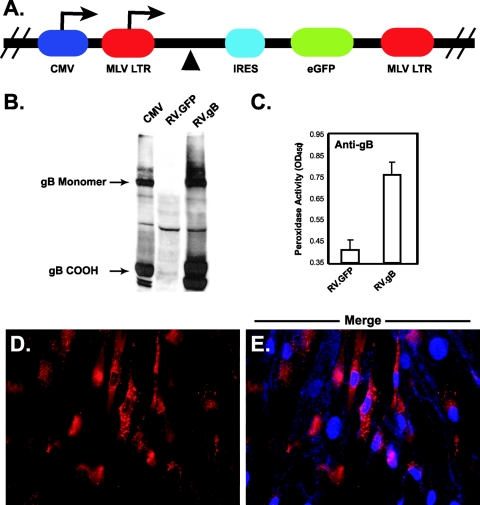
FIG. 1.
Retroviral expression of gB in CHO and NHDF cells. (A) The genes encoding CMV glycoproteins were cloned into plasmid pCMMP.MCS.IRES.GFP at the multiple cloning site (▴). Transcripts are driven by either the CMV promoter or the MLV long terminal repeat (LTR) after transfection or transduction, respectively. In each case, transcripts are bicistronic, carrying both the transgene and the gene encoding GFP (eGFP) under translational control of an IRES. Recombinant retrovirus was generated as described in Materials and Methods. (B) CHO cells were transduced with a recombinant retrovirus encoding gB (RV.gB) or an empty retrovirus encoding only GFP (RV.GFP), and glycoprotein expression was confirmed by reducing 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by immunoblotting with monoclonal antibody 27-78 (anti-gB), recognizing the carboxy-terminal region of gB. Mouse antibodies were identified with goat anti-rabbit-HRP and detected by enhanced chemiluminescence. Retrovirally transduced cells express both monomeric and cleaved (COOH) gB. In all figures, CMV represents NHDF cell lysates infected with strain AD169 at a multiplicity of infection of 1 and harvested at 6 days postinfection, RV.GFP represents CHO cells transduced with an empty retrovirus, and RV.gB represents cells transduced with a retrovirus encoding gB. (C) Cell surface expression was examined by CELISA with anti-gB antibodies on gB-expressing cells. Mouse antibodies were identified with goat anti-mouse-HRP, followed by peroxidase detection with TMB substrate. Error bars indicate standard deviations. (D and E) To visualize gB expression, NHDF cells expressing gB were stained with anti-gB antibodies and goat anti-mouse-Alexa Fluor 594. (E) In a merged photograph, blue staining represents DAPI-stained nuclei. OD450, optical density at 450 nm.
Transfections and retrovirus transductions.
Transfections were performed with Lipofectamine or Lipofectamine 2000 (Promega) in six-well plates (Corning) according to the manufacturer's protocol. In brief, polyethylene glycol-purified DNA (45) encoding each glycoprotein was diluted in 200 μl of Opti-MEM (Promega). Concomitantly, Lipofectamine was diluted in 200 μl Opti-MEM and added to the plasmid-containing tubes. DNA/Lipofectamine complexes were allowed to form for 30 min and then added to 90% confluent cells in a total volume of 2 ml Opti-MEM. After 6 h, cells were washed with PBS and allowed to recover overnight in complete medium. For retroviral delivery of the genes encoding CMV proteins, recombinant retrovirus was incubated with cells in the presence of 5 μg/ml Polybrene in a minimal amount of medium. At 6 hours, retrovirus was removed and replaced with 10% serum-containing medium. To minimize cytotoxicity associated with infection with multiple retroviruses simultaneously, cells were allowed to recover for 18 to 20 h before undergoing subsequent rounds of transduction.
Immunoblots and CELISA assays.
Immunoblotting identifying gB, gH, and gL was performed as previously described (22, 23). For cell enzyme-linked immunosorbent assay (CELISA) experiments, 1 × 104 glycoprotein-expressing cells were plated in 96-well plates. At 24 h, cells were fixed in 3% paraformaldehyde (PFA). Cells were then blocked with PBS plus 20% normal goat serum (NGS) (Pierce) for 2 h. Primary antibody 27-78, AP86, or 6394 was diluted in PBS plus 2% NGS and incubated on cells for 1 h. Subsequently, goat anti-mouse or goat anti-rabbit secondary antibodies conjugated to horseradish peroxidase (HRP) (Pierce) were diluted 1:5,000 in PBS plus 2% NGS and allowed to attach for 1 h. Antibody binding was quantified by the addition of 3,3′,5,5′ tetramethyl benzidine (TMB) substrate (Pierce) followed by reading the optical density of TMB at 450 nm in a Spectramax 190 spectrophotometer (Molecular Devices).
Cell-cell fusion assays.
Glycoprotein-expressing cells were transduced as described above with individual or multiple retroviruses as indicated in the figure legends. Glycoprotein-expressing cells were then trypsinized, counted, and plated with untransduced cells at a ratio of 1:1. Syncytia were allowed to form for 24 to 30 h, and formation was halted by PFA fixation. Plates were photographed for green fluorescent protein (GFP) fluorescence and subsequently stained with Giemsa stain (Sigma) to view individual nuclei. To quantify syncytium formation, equal numbers of glycoprotein-expressing and nonexpressing cells were plated in triplicate on 12-well plates and allowed to form syncytia for 24 h. Plates were then fixed and stained with Giemsa. In each well, an area equivalent to 1 × 104 cells was counted and assayed for syncytium formation. Syncytium numbers from each cell type were subsequently normalized to represent the number of syncytia formed per 2 × 104 cells. The average number of syncytia and standard deviation were calculated and graphed. We determined the average number of cells involved in fusion by two separate methods. First, the number of nuclei per syncytium was calculated by determining the average number of nuclei within syncytial cell populations. This number was then multiplied by the average number of syncytia formed (see Fig. 6A) to yield an average number of cells involved in syncytia formation. Second, we employed the histogram function of Adobe Photoshop (2). Using representative photographs of each experimental sample, we were able to select areas involved in syncytium formation. These areas were then compared to the total area analyzed, and the percentage of cells that form syncytia was calculated.
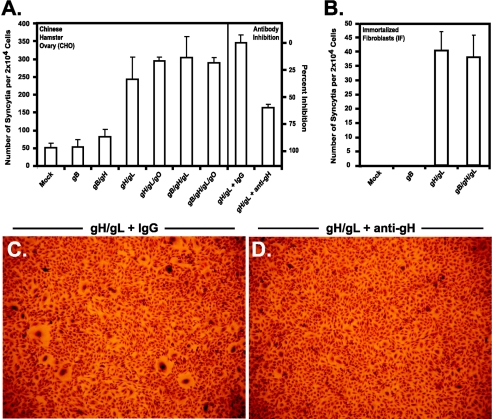
FIG. 6.
Quantification of the numbers of syncytia formed in glycoprotein-expressing CHO cells. CHO cells (panel A) and immortalized fibroblasts (panel B) were transduced with the genes encoding CMV glycoproteins as indicated and allowed to form syncytia. Fusogenicity was then calculated as the number of syncytia formed per 2 × 104 cells, where at least five nuclei were required to be considered a syncytium. Syncytium formation was inhibited when gH/gL-expressing cells were treated with neutralizing antibody 14-4b (anti-gH) but not control IgG (panel A). CHO cells expressing gH/gL formed larger syncytia when treated with control IgG (panel C) than in the presence of anti-gH antibodies (panel D).
Antibody inhibition of syncytium formation.
gH/gL-expressing CHO cells were plated in triplicate as described above. After cellular adherence to the plate, the medium was removed and overlaid with either control neutralizing antibodies 7-17 and 27-39, recognizing gB, or neutralizing antibody 14-4b, recognizing gH. Throughout syncytium formation, antibody-containing plates were rocked periodically to promote antibody binding. At 24 h postplating, glycoprotein-expressing cells were fixed in PFA and syncytium formation was quantified as described above.
Imaging and immunofluorescence.
Photographs of Giemsa-stained cells were taken on an inverted microscope with a digital camera. For immunofluorescence imaging of CMV glycoproteins within syncytia, transfected or transduced cells were trypsinized at 24 h posttransfection and replated on glass coverslips with untransfected cells at a ratio of 1:1. Fusion was allowed to proceed for an additional 24 h and then halted by PFA fixation. All immunofluorescence staining and imaging were performed as previously described (32). Images were subsequently edited and assembled using Adobe Photoshop 6.0.1. Mouse monoclonal antibodies were stained with Alexa Fluor 488, Alexa Fluor 594, or Alexa Fluor 350 goat anti-mouse secondary antibodies, while rabbit polyclonal antibodies were stained with Alexa Fluor 594 goat anti-rabbit secondary antibodies (Molecular Probes). In merged panels, yellow coloring represents coexpression of individual genes in representative syncytia. When Alexa Fluor 350 (which is blue) was not used, nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (Molecular Probes).
RESULTS
Retrovirus-mediated expression of CMV glycoproteins.
To date, coexpression of multiple gene products has been found to be required to induce cell-cell fusion by herpesviruses. The composition of proteins needed to drive fusion by human cytomegalovirus has not been determined. Due to variables involved with transfecting multiple plasmids into an individual cell, we sought an alternative method to deliver candidate CMV genes to a population of cells. Retrovirus-mediated gene delivery is widely used to efficiently transduce up to 100% of a population of mammalian cells in vitro. Thus, we constructed replication-defective recombinant retroviruses to effectively deliver individual CMV glycoproteins to cells. We employed a bicistronic retroviral construct that encodes gB (UL55), gH (UL75), gL (UL115), or gO (UL74) and an internal ribosome entry site (IRES) followed by the gene encoding GFP (Fig. 1A). Importantly, as both the gene of interest and GFP are present on the same mRNA transcript, levels of glycoprotein expression directly correlate to levels of GFP fluorescence within retrovirally infected (transduced) cells.
To examine CMV protein expression in transduced cells, CHO or NHDF cells were infected with individual retroviruses and analyzed by several methods. First, cell lysates were analyzed by immunoblotting. Transduced CHO cells expressed forms of gB similar to those seen in CMV-infected cells (Fig. 1B). Next, we measured gB at the cell surface by a CELISA assay on transduced cells with antibodies to gB. As shown in Fig. 1C, gB was detected on the cell surface in transduced CHO cells. Finally, to visualize gB expression, we performed immunofluorescence on transduced NHDF cells. As shown in Fig. 1D and E, gB was detected in a prominent vesicular pattern consistent with patterns observed in CMV-infected cells. From these experiments, we conclude that retroviral delivery of the gene encoding gB is an efficient delivery method for expression of gB in multiple cell types.
Expression of gH and gL was measured in similar experiments. As shown by immunoblot analysis, transduced CHO cells expressed both gH and gL (Fig. 2A). Other investigations have shown that correct processing and cell surface presentation of gH are dependent on gL coexpression (25, 49). Consistent with these results and the idea that misfolded proteins are quickly degraded, we consistently detected lower levels of gH in the absence of gL (Fig. 2A). By treating the surface of transduced cells with antibodies to both gH and gL, we confirmed that gH is present at the plasma membrane only when cells are transduced with both gH and gL (Fig. 2B). Conversely, in the absence of gH, gL was transported to the cell surface. These results were also reflected in the imaging experiments. Intracellular gH localization appears to be consistent with an endoplasmic reticulum staining pattern (Fig. 2C and D), while gL appears to be concentrated within perinuclear compartments, indicative of Golgi staining (Fig. 2E and F). Taken together, we have demonstrated by several independent methods that retroviral delivery of the genes encoding gB, gH, and gL leads to CMV glycoprotein expression in both CHO and NHDF cells.
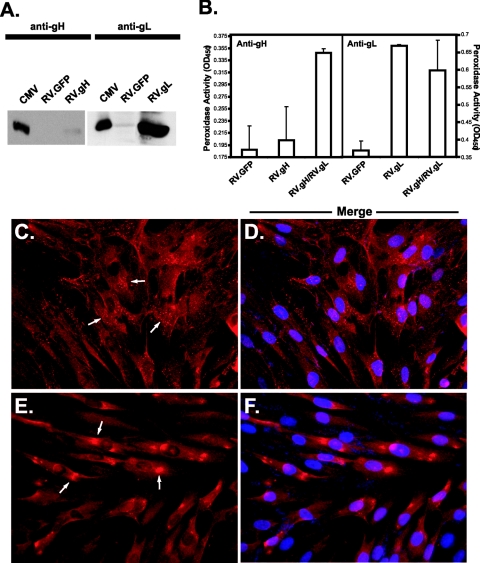
FIG. 2.
Retroviral expression of gH and gL in CHO and NHDF cells. (A) CHO cells were transduced with a retrovirus encoding gH (RV.gH) or gL (RV.gL), and expression was confirmed by reducing 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by immunoblotting with polyclonal antibody 6824 (anti-gH) or 6394 (anti-gL). Rabbit antibodies were identified with goat anti-rabbit-HRP and detected by enhanced chemiluminescence. (B) Cell surface gH/gL expression was examined by CELISA with AP86 (anti-gH) or anti-gL antibodies. Mouse antibodies were detected with goat anti-mouse-HRP, while rabbit antibodies were detected with goat anti-rabbit-HRP followed by peroxidase detection with TMB substrate. Error bars indicate standard deviations. (C to F) Glycoprotein expression was visualized by immunofluorescence with AP86 (anti-gH, panels C and D) or anti-gL (panels E and F). In each case, primary antibodies were detected with Alexa Fluor 594-conjugated secondary antibodies. Merged photographs (D and F) show panels C and E stained with DAPI, which appears blue. Arrows illustrate diffuse staining representative of endoplasmic reticulum retention (C) or concentrated perinuclear staining representative of Golgi apparatus localization (E). OD450, optical density at 450 nm.
Expression of individual CMV glycoproteins is not sufficient to induce syncytium formation.
We next tested the ability of these proteins to induce fusion in cell lines that have frequently been used in the literature to measure herpesvirus-induced fusion. CHO cells in particular have been used by several research groups due to their low tendency to spontaneously form multinucleated syncytia (19, 35, 41, 42). Cells were transduced with each indicated retrovirus, allowed to express CMV glycoproteins, fixed, microscopically photographed for GFP expression, and subsequently stained with Giemsa stain to visualize nuclei. Expression of gB in CHO cells did not result in the formation of multinucleated syncytia (Fig. 3A, E, and I). Importantly, this was not due to low numbers of cells expressing gB, as retroviral transduction effectively promotes expression in nearly 100% of transduced cell populations as measured by GFP expression (Fig. 3A). We also assayed for syncytium formation by immunofluorescence with gB-specific antibodies on gB-expressing cell monolayers. Without exception, gB-expressing cells were not multinucleated (data not shown).
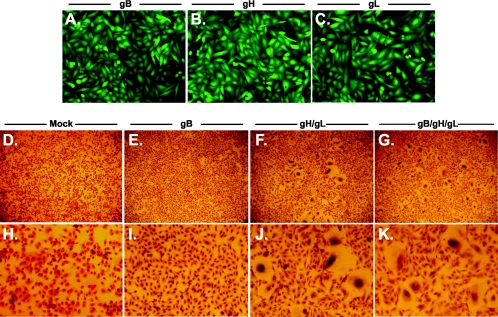
FIG. 3.
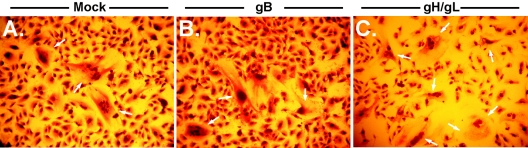
Coexpression of gH/gL is sufficient to induce syncytium formation. CHO cells were transduced with individual or multiple retroviruses as described in Materials and Methods. Nearly 100% of CHO cells transduced with gB (panel A), gH (panel B), or gL (panel C) express GFP and the glycoprotein indicated (magnification, ×160). CHO cells mock transduced (panels D and H), or transduced with gB (panels E and I), gH and gL (panels F and J), or gB, gH, and gL (panels G and K) were plated with untransduced cells, allowed to form syncytia, fixed, and stained with Giemsa stain to visualize multinucleated cells (magnification, ×32 in panels D to G). To specifically show syncytial bodies, the magnification was increased to ×150 in panels H to K.
Notwithstanding, it is possible that gB fusogenicity is cell type specific. Previous investigations have shown that gB expression is sufficient to induce cell-cell fusion of adjacent U373 glioblastoma cells (5, 38, 53, 54). To confirm this result, U373 cells were transduced as described above with retroviruses encoding gB, gH, and gL. As measured by this experimental system, U373 cells display an extremely high propensity to fuse in the absence of any viral proteins (Fig. 4A). Therefore, we were unable to reliably measure gB-mediated fusogeneic activity in this cell type. One possible explanation for this discrepancy is that gB-induced fusion of U373 cells is dependent on very high levels of expression of gB that are unobtainable by retroviral transduction. Overall, however, the high background levels of syncytium formation in U373 cells rendered them unsuitable for fusion measurements, and they were not further analyzed.
FIG. 4.
Expression of CMV gB and gH/gL in U373 cells. U373 glioblastoma cells were either mock transduced (panel A), transduced with a retrovirus encoding gB (panel B), or transduced with retroviruses encoding gH and gL (panel C). Transduced cells were allowed to form syncytia, fixed, and stained with Giemsa stain to visualize multinucleated cells. Photographs show representative fields of each experimental group (magnification, ×200). Arrows call attention to multinucleated syncytia within each panel.
These results show that gB is not fusogenic when expressed alone in the cell lines we tested. We obtained similar results when gH and gL were individually expressed (Fig. 3B and C, respectively). Although the majority of transduced cells expressed either gH or gL, none formed multinucleated syncytia. Thus, neither gB, gH, nor gL has inherent fusogenic activity in the cell types we tested.
Expression of the gH/gL heterodimer induces syncytium formation.
To determine which glycoprotein complexes are fusogenic, we next expressed CMV glycoproteins in combination. CHO cells were infected with individual glycoprotein-encoding retroviruses and allowed to recover for 18 to 20 h. They were subsequently infected with a second or third retrovirus as indicated in the figure legends. As shown in Fig. 3F and J, CHO cells expressing the gH/gL heterodimer formed readily detectable syncytia throughout the cell monolayer. These results were surprising, as HSV, EBV, and KSHV require coexpression of gB with gH/gL to induce cell-cell fusion. Although unexpected, this result is not novel. VZV also requires only gH/gL expression to induce cell-cell fusion. By this assay, coexpression of gB with gH/gL had no apparent effect on the formation of syncytia in CHO cells (Fig. 3G, K). These findings were replicated in HeLa cells under identical experimental conditions (data not shown).
Syncytium formation in permissive cells.
We next wanted to examine the effects of expressing CMV glycoproteins in cells permissive for viral replication. Although we showed that NHDF cells express CMV glycoproteins (Fig. 1D and E and 2C to F), we were unable to induce the formation of multinucleated cells in this secondary cell strain under any experimental condition (Fig. 5A to D). These results were not unexpected, since infection of NHDF cells in vitro rarely results in the formation of multinucleated syncytia.
FIG. 5.
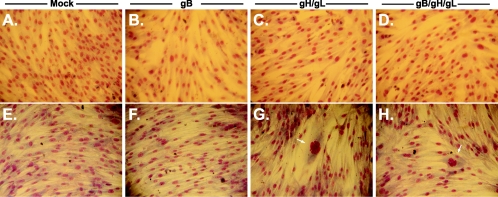
Expression of CMV glycoproteins in permissive cells. NHDF cells (panels A to D) and immortalized fibroblasts (panels E to H) were mock transduced (panels A and E) or transduced with gB (panels B and F), gH and gL (panels C and G), or gB, gH, and gL (panels D and H). Cells were then fixed and stained with Giemsa stain to view individual nuclei (magnification, ×150). Arrows identify syncytia in panels G and H.
As an alternative approach, we expressed CMV glycoproteins in telomerase-immortalized fibroblasts (13). As seen with other model cell lines, immortalized fibroblasts expressing gB did not form multinucleated syncytia (Fig. 5F). Conversely, immortalized fibroblasts expressing the gH/gL heterodimer readily formed syncytia (Fig. 5G). Coexpression of gB with gH/gL did not appear to modulate syncytium formation as determined by an increased number of syncytia per well. These results are similar to those observed with other herpesviruses fusion proteins. Analysis of these proteins has shown strict cell type specificity during cell-cell fusion. For example, KSHV glycoprotein-expressing cells readily fuse with 293T and BJAB cells but not CHO or Vero cells (41). VZV-infected cells clearly show differential levels of syncytium formation between human epidermal cells and NHDF cells (12). Very little is known about the mechanistic differences of fusion seen between any of these cell lines. One possibility is that levels of viral protein synthesis and cell surface localization are considerably different between cell types.
Coexpression of gB and gO with gH/gL neither enhances nor inhibits syncytium formation.
To address potential differences in cell-cell fusion between gH/gL-expressing cells and cells coexpressing other CMV glycoproteins, we wanted to directly quantify syncytium formation. We compared CHO cells expressing different combinations of CMV glycoproteins by several methods. First, the average number of syncytia formed was calculated. Here, expression of neither gB nor gB/gH resulted in formation of syncytia above background levels (Fig. 6A). Expression of the gH/gL heterodimer induced the formation of five- to eightfold more syncytia over background levels. Moreover, coexpression of gB and/or gO with gH/gL had no significant effect on the number of syncytia formed. Fusion was also quantified in immortalized fibroblasts. In contrast to CHO cells, these cells do not form background syncytia under any conditions (Fig. 6B). Importantly, immortalized fibroblast cells expressing gH/gL and expressing gH/gL with gB readily formed syncytia. As no syncytia are formed in the absence of fusogenic glycoproteins, immortalized fibroblasts may more accurately reflect bona fide fusion events and may prove useful in future studies of CMV-induced fusion.
We next asked whether coexpression of gB and/or gO with gH/gL could increase the size of syncytia formed in CHO cells. These data are summarized in Table 1. First, we counted the average number of nuclei contained within glycoprotein-expressing syncytial cells. On average, cells expressing gH/gL incorporate over two times more nuclei than gB- or mock-expressing cells (Table 1). When combined with the data in Fig. 6A, these numbers suggest that of 2 × 104 cells, fewer than 400 cells are involved in background syncytium formation while greater than 4,000 cells are involved in gH/gL-induced formation of syncytia (Table 1). To confirm these observations, we employed a quantitative method similar to that published by Avitabile et al. (2). By this assay, the area of cells involved in syncytium formation is measured by the histogram function of Adobe Photoshop. This area is then compared to the total area assayed, and the number of cells involved in formation of syncytia can be calculated. Here, 2% of mock-transduced CHO cells formed background syncytia, whereas gH/gL-expressing cells formed syncytia that occupied 25% of representative experiments (Table 1). These numbers closely correlate with the previous data, showing that gH/gL-expressing cells form over 10 times more syncytia than mock-transduced cells. Similar numbers were obtained for gH/gL-expressing cells in combination with gB and/or gO, suggesting that neither gB nor gO inhibits or promotes syncytium formation in CHO cells.
TABLE 1.
Quantification of the numbers of cells involved in syncytium formation in glycoprotein-expressing cells
| Glycoprotein expression | Avg no. of nuclei per syncytiuma | No. of cells involved syncytiab | % of cells involved in syncytiac |
|---|---|---|---|
| Mock | 6 | 350 ± 50 | 2 |
| gB | 6 | 350 ± 50 | 2 |
| gH/gL | 15 | 4,375 ± 625 | 25 |
| gH/gL/gO | 14 | 4,300 ± 100 | 22 |
| gB/gH/gL | 14 | 4,500 ± 300 | 24 |
| gB/gH/gL/gO | 14 | 4,400 ± 200 | 23 |
Transduced CHO cells were allowed to form syncytia for 24 hours and stained with Giemsa stain to view individual nuclei, and the average number of nuclei within individual syncytial cell bodies was determined. As in Fig. 5, at least five nuclei were required to be considered syncytia.
The average number of cells involved in syncytia was calculated according to the equation [(average number of nuclei per syncytium) × (average number of syncytia formed) + (percentage of cells involved in syncytia) × (2 × 104 cells)]/2; the average number of syncytia formed is from Fig. 6A. Error values were determined by subtracting the average from the larger of the two cell counts.
The area of cells involved in syncytium formation was determined with the histogram function of Adobe Photoshop and compared to the total area.
Antibodies to gH/gL inhibit formation of syncytia.
To independently confirm that gH/gL expression was indeed responsible for syncytium formation, glycoprotein-expressing cells were subjected to antibody-mediated inhibition of cell-cell fusion. CHO cells expressing gH/gL were plated in the presence of a gH-specific neutralizing antibody and compared to cells treated with a control antibody. Although the control antibody had no effect on the formation of syncytia, anti-gH/gL antibodies decreased syncytium formation by greater than 50% (Fig. 6A). Furthermore, when syncytial size was compared, syncytia produced in the presence of anti-gH were considerably smaller than the control (Fig. 6C and D). Taken in sum, these results strongly support the conclusion that the gH/gL heterodimer is inherently fusogenic.
Localization of CMV glycoproteins within an individual syncytium.
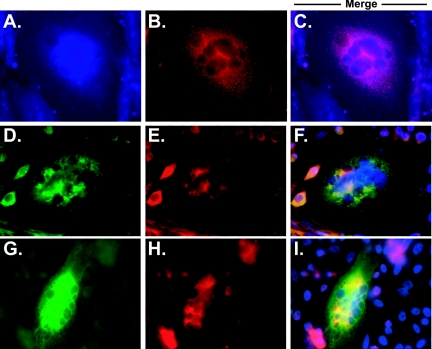
To confirm that the expressed glycoproteins were localized to syncytia, we performed a series of immunofluorescence experiments on multinucleated cells. Retrovirally transduced cells express large amounts of GFP, thereby forcing the use of blue secondary antibodies. Unfortunately, technical limitations did not allow us to clearly visualize blue wavelengths (Fig. 7A to C). As an alternative, we utilized plasmid transfection to visualize glycoproteins within multinucleated cells. By transfection, gB, gO, and gH were readily identified within representative syncytia (Fig. 7D to I). We were unable to visualize more than two CMV proteins at once within syncytia, as our antibodies were produced in mice and rabbits; however, since neither gB nor gO induces syncytium formation alone, we can assume that each syncytium shown is positive for gH/gL as well as gB and/or gO. From these results, we made two conclusions. First, like transduced cells, transfected cells readily form syncytia. Second, all four CMV glycoproteins that we analyzed were readily identified in multinucleated cells.
FIG. 7.
Expression of CMV glycoproteins within syncytia. Representative syncytia formed from transduced (panels A to C) or transfected (panels D to I) cells are shown. CHO cells were stained with antibodies to gH (panels A and H), gL (panel B), gO (panel E), or gB (panels D and G). Cells transduced with gH- and gL-encoding retroviruses express large amounts of GFP; therefore, Alexa Fluor 350 (panel A) was used to detect mouse antibodies, while rabbit antibodies were visualized with Alexa Fluor 594 (panel B). Technical limitations with clear imaging of blue wavelengths forced the use of an alternative expression method. Thus, CHO cells were transfected with plasmids encoding gH (pCAGGS.gH), gL (pCAGGS.gL), gO (gCAGGS.gO), and gB (gCAGGS.gB). Alexa Fluor 488 was subsequently used to detect mouse antibodies (panels D and G), while Alexa Fluor 594 was used to detect rabbit antibodies (panels E and H). In merged photographs (panels F and I), blue staining represents DAPI-stained nuclei. In each case, CMV glycoprotein expression was readily identified in each syncytium.
DISCUSSION
Virally regulated fusion between adjacent membranes is a fundamental process that is required for the vitality of all enveloped viral pathogens. Although the viral determinants of cell-cell fusion have been defined for several herpesviruses, the requirements for CMV-induced cell-cell fusion remained undefined. Consequently, we set out to identify the fusogenic glycoproteins of CMV. We hypothesized that coexpression of several glycoproteins would be required to induce cell-cell fusion; therefore, we employed retroviral delivery to efficiently express multiple gene products in a population of cells. The genes encoding gB, gH, gL, and gO were cloned into a retroviral transfer vector, and expression was confirmed in both CHO and NHDF cells (Fig. 1 and 2). Our results showed that expression of any one glycoprotein gene was not sufficient to induce syncytium formation. By contrast, when gH and gL were coexpressed, we detected multinucleated cells within transduced CHO and immortalized fibroblast cell monolayers (Fig. 3). Since gB was previously reported to be fusogenic in U373 cells, we repeated our experiments with this cell line. We found that U373 cells exhibited very high background formation of syncytia, and these cells were therefore excluded from further experimentation (Fig. 4). By our methods, we did not detect significant differences when gB and/or gO was coexpressed with gH/gL (Fig. 6; Table 1). We confirmed that glycoprotein expression was responsible for syncytium formation by inhibiting glycoprotein-induced fusion with neutralizing antibodies to gH/gL (Fig. 6). Additionally, we showed glycoprotein localization within syncytial bodies by immunofluorescence utilizing antibodies to all four CMV glycoproteins (Fig. 7).
Perhaps the most intriguing finding from this report is that CMV gH/gL is inherently fusogenic in certain cell lines. For other herpesviruses, coexpression of a receptor binding protein is required to induce syncytium formation. These observations have led to a receptor-mediated fusion hypothesis where receptor binding is required to induce membrane fusion. Several studies have suggested that the gH/gL heterodimer binds to specific cellular receptors during herpesvirus entry. The most significant evidence has come from laboratories utilizing human herpesvirus 6 (HHV-6) to study viral entry. Santoro et al. showed that HHV-6 utilizes a specific cellular receptor, CD46, for viral entry and fusion (47). HHV-6 gH was subsequently identified as the viral ligand for CD46 (36, 46). Furthermore, expression of HSV gH in CHO cells prior to infection with HSV dramatically inhibits virus entry (48). Although the mechanism of inhibition is not known, further analysis may show that expression of gH leads to intracellular receptor binding and therefore receptor downregulation or so-called interference. Last, EBV utilizes gH/gL as a receptor binding protein during virus entry into epithelial cells (6). These lines of evidence strongly suggest that gH/gL binds to specific cellular receptors and support the model that ligand-receptor interactions drive membrane fusion.
Keay and others have reported in a series of papers that CMV gH/gL binds to a 92.5-kDa cellular receptor on the surface of permissive cells (27, 30). Even though the identity of this protein remains unknown, interactions of gH/gL with this receptor induces measurable consequences for cell physiology, including protein phosphorylation and calcium ion flux (28, 29). In view of the fact that little is known about the precise interactions of gH/gL with this molecule, future studies will undoubtedly focus on this cellular protein and its involvement in viral entry and cell-cell fusion. Expression of the gH/gL heterodimer induces substantial formation of syncytia in the cell lines we tested, including immortalized fibroblasts, but not secondary fibroblast strains. One possibility for this difference is that levels of retrovirally expressed proteins may be substantially higher in transformed cell lines. Alternatively, transformed cells may express larger amounts of cellular receptors required for cell-cell fusion. Another possibility is that additional CMV-encoded glycoproteins are required for efficient fusion in nontransformed cells. Ultimately the reason for this difference is not known; however, there is substantial evidence that primary cells negatively regulate fusion. Multinucleated cells are rarely observed in the context of infection even though plaque formation indicates that cell-to-cell transmission occurs. Gerna et al. have shown that transmission of CMV from infected leukocytes to uninfected fibroblasts involves transient microfusion events that do not result in the formation of multinucleated syncytia (16). Taken together, these observations suggest that cell-cell transmission in primary cells is a tightly regulated and perhaps short-lived event that is quickly reversed by an unknown mechanism. Certainly, it will be interesting to establish the mechanism by which more physiologically relevant cell strains remain refractory to syncytium formation, as this level of control may prove beneficial to understanding viral dissemination in vivo.
Cell-cell and virus-cell fusion have been hypothesized to be related, but their mechanisms may not be identical. We show here that expression of gH/gL is sufficient to induce cell-cell fusion, while several lines of evidence suggest that gB is required for virus-cell fusion. Accurately determining the precise number of steps required for virus entry has been complicated by the fact that gB associates with a number of cell surface molecules during virus entry. gB reportedly interacts with heparan sulfate proteoglycans, annexin II, epidermal growth factor receptor, cellular integrins, and Toll-like receptor 2 (13-15, 18, 43, 57). Clearly, a more in-depth understanding of the physical interactions that occur between these molecules and gB will be instrumental to further dissect CMV entry and the mechanistic role gB plays during virus-cell fusion.
This study provides the groundwork for an in-depth analysis of the fusion machine of CMV. Understanding the molecular mechanisms by which herpesviruses induce membrane fusion will lead to the development of new antiviral therapies. Given the success of inhibitors to human immunodeficiency virus gp41-induced membrane fusion, drugs directed against CMV-mediated fusion may effectively inhibit viral dissemination within an infected individual (31).
Acknowledgments
This work was supported by U.S. Public Health Service grants RO1 A144203 and RO1 A134998. E.R.K was supported by Predoctoral Training in Experimental Oncology grant T32 CA09135.
E.R.K. thanks S. Perry for the use of his digital camera, M. Guererro for assistance with sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the members of the Compton lab for critical review and discussion during preparation of the manuscript.
REFERENCES
- 1.Alford, C. A., and W. J. Britt. 1993. Cytomegalovirus, p. 227-255. In B. Roizman, R. J. Whitley, and C. Lopez (ed.), The human herpesviruses. Raven Press, Ltd., New York, N.Y.
- 2.Avitabile, E., G. Lombardi, T. Gianni, M. Capri, and G. Campadelli-Fiume. 2004. Coexpression of UL20p and gK inhibits cell-cell fusion mediated by herpes simplex virus glycoproteins gD, gH-gL, and wild-type gB or an endocytosis-defective gB mutant and downmodulates their cell surface expression. J. Virol. 78:8015-8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billstrom, M. A., and W. J. Britt. 1995. Postoligomerization folding of human cytomegalovirus glycoprotein B: identification of folding intermediates and importance of disulfide bonding. J. Virol. 69:7015-7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boerger, A. L., S. Snitkovsky, and J. A. Young. 1999. Retroviral vectors preloaded with a viral receptor-ligand bridge protein are targeted to specific cell types. Proc. Natl. Acad. Sci. USA 96:9867-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bold, S., M. Ohlin, W. Garten, and K. Radsak. 1996. Structural domains involved in human cytomegalovirus glycoprotein B-mediated cell-cell fusion. J. Gen. Virol. 77:2297-2302. [DOI] [PubMed] [Google Scholar]
- 6.Borza, C. M., A. J. Morgan, S. M. Turk, and L. M. Hutt-Fletcher. 2004. Use of gHgL for attachment of Epstein-Barr virus to epithelial cells compromises infection. J. Virol. 78:5007-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle, K. A., and T. Compton. 1998. Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein B. J. Virol. 72:1826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britt, W. J. 1984. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology 135:369-378. [DOI] [PubMed] [Google Scholar]
- 9.Britt, W. J., L. Vugler, E. J. Butfiloski, and E. B. Stephens. 1990. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J. Virol. 64:1079-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Britt, W. J., and L. G. Vugler. 1992. Oligomerization of the human cytomegalovirus major envelope glycoprotein complex gB (gp55-116). J. Virol. 66:6747-6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocchi, F., L. Menotti, P. Dubreuil, M. Lopez, and G. Campadelli-Fiume. 2000. Cell-to-cell spread of wild-type herpes simplex virus type 1, but not of syncytial strains, is mediated by the immunoglobulin-like receptors that mediate virion entry, nectin1 (PRR1/HveC/HIgR) and nectin2 (PRR2/HveB). J. Virol. 74:3909-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole, N. L., and C. Grose. 2003. Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus. Rev. Med Virol. 13:207-222. [DOI] [PubMed] [Google Scholar]
- 13.Compton, T. 1993. An immortalized human fibroblast cell line is permissive for human cytomegalovirus infection. J. Virol. 67:3644-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feire, A., Koss, H., and Compton, T. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA 101:15470-15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerna, G., E. Percivalle, F. Baldanti, S. Sozzani, P. Lanzarini, E. Genini, D. Lilleri, and M. G. Revello. 2000. Human cytomegalovirus replicates abortively in polymorphonuclear leukocytes after transfer from infected endothelial cells via transient microfusion events. J. Virol. 74:5629-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gicklhorn, D., M. Eickmann, G. Meyer, M. Ohlin, and K. Radsak. 2003. Differential effects of glycoprotein B epitope-specific antibodies on human cytomegalovirus-induced cell-cell fusion. J. Gen. Virol. 84:1859-1862. [DOI] [PubMed] [Google Scholar]
- 18.Guerrero, M., and T. Compton. Unpublished data.
- 19.Haan, K. M., S. K. Lee, and R. Longnecker. 2001. Different functional domains in the cytoplasmic tail of glycoprotein B are involved in Epstein-Barr virus-induced membrane fusion. Virology 290:106-114. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch, M. 1998. Cytomegalovirus and human herpesvirus types 6, 7, and 8, p. 195-238. In Harrison's principles of internal medicine. McGraw-Hill Book Co., New York, N.Y.
- 21.Hobom, U., W. Brune, M. Messerle, G. Hahn, and U. Koszinowski. 2000. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J. Virol. 74:7720-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber, M. T., and T. Compton. 1997. Characterization of a novel third member of the human cytomegalovirus glycoprotein H-glycoprotein L complex. J. Virol. 71:5391-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber, M. T., and T. Compton. 1998. The human cytomegalvirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 72:8191-8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber, M. T., and T. Compton. 1999. Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus. J. Virol. 73:3886-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaye, J. F., U. A. Gompels, and A. C. Minson. 1992. Glycoprotein H of human cytomegalovirus (HCMV) forms a stable complex with the HCMV UL115 gene product. J. Gen. Virol. 73:2693-2698. [DOI] [PubMed] [Google Scholar]
- 26.Keay, S., and B. Baldwin. 1991. Anti-idiotype antibodies that mimic gp86 of human cytomegalovirus inhibit viral fusion but not attachment. J. Virol. 65:5124-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keay, S., and B. Baldwin. 1992. The human fibroblast receptor for gp86 of human cytomegalovirus is a phosphorylated glycoprotein. J. Virol. 66:4834-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keay, S., and B. R. Baldwin. 1996. Evidence for the role of cell protein phosphorylation in human cytomegalovirus/host cell fusion. J. Gen. Virol. 77:2597-2604. [DOI] [PubMed] [Google Scholar]
- 29.Keay, S., B. R. Baldwin, M. W. Smith, S. S. Wasserman, and W. F. Goldman. 1995. Increases in [Ca2+]i mediated by the 92.5-kDa putative cell membrane receptor for HCMV gp86. Am. J. Physiol. 269:C11-C21. [DOI] [PubMed] [Google Scholar]
- 30.Keay, S., T. C. Merigan, and L. Rasmussen. 1989. Identification of cell surface receptors for the 86-kilodalton glycoprotein of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 86:10100-10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilby, J. M., S. Hopkins, T. M. Venetta, B. DiMassimo, G. A. Cloud, J. Y. Lee, L. Alldredge, E. Hunter, D. Lambert, D. Bolognesi, T. Matthews, M. R. Johnson, M. A. Nowak, G. M. Shaw, and M. S. Saag. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4:1302-1307. [DOI] [PubMed] [Google Scholar]
- 32.Kinzler, E., R. N. Theiler, and T. Compton. 2002. Expression and reconstitution of the gH/gL/gO complex of human cytomegalovirus. J. Clin. Virol. 25(Suppl. 2):S87-S95. [DOI] [PubMed] [Google Scholar]
- 33.Li, L., J. A. Nelson, and W. J. Britt. 1997. Glycoprotein H-related complexes of human cytomegalovirus: identification of a third protein in the gCIII complex. J. Virol. 71:3090-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ljungman, P., K. Larsson, G. Kumlien, J. Aschan, L. Barkholt, A. Gustafsson-Jernberg, I. Lewensohn-Fuchs, and O. Ringden. 2002. Leukocyte depleted, unscreened blood products give a low risk for CMV infection and disease in CMV seronegative allogeneic stem cell transplant recipients with seronegative stem cell donors. Scand. J. Infect. Dis. 34:347-350. [DOI] [PubMed] [Google Scholar]
- 35.Maresova, L., T. J. Pasieka, and C. Grose. 2001. Varicella-zoster virus gB and gE coexpression, but not gB or gE alone, leads to abundant fusion and syncytium formation equivalent to those from gH and gL coexpression. J. Virol. 75:9483-9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mori, Y., X. Yang, P. Akkapaiboon, T. Okuno, and K. Yamanishi. 2003. Human herpesvirus 6 variant A glycoprotein H-glycoprotein L-glycoprotein Q complex associates with human CD46. J. Virol. 77:4992-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muggeridge, M. I. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81:2017-2027. [DOI] [PubMed] [Google Scholar]
- 38.Navarro, D., P. Paz, S. Tugizov, K. Topp, J. Lavail, and L. Pereira. 1993. Glycoprotein-B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology 197:143-158. [DOI] [PubMed] [Google Scholar]
- 39.Ohizumi, Y., H. Suzuki, Y. Matsumoto, Y. Masuho, and Y. Numazaki. 1992. Neutralizing mechanisms of two human monoclonal antibodies against human cytomegalovirus glycoprotein 130/55. J. Gen. Virol. 73:2705-2707. [DOI] [PubMed] [Google Scholar]
- 40.Ory, D. S., B. A. Neugeboren, and R. C. Mulligan. 1996. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. USA 93:11400-11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pertel, P. E. 2002. Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J. Virol. 76:4390-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 43.Pietropaolo, R. L., and T. Compton. 1997. Direct interaction between human cytomegalovirus glycoprotein B and cellular annexin II. J. Virol. 71:9803-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rasmussen, L., R. Nelson, D. Kelsall, and T. Merigan. 1984. Murine monoclonal antibody to a single protein neutralizes the infectivity of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 81:876-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 46.Santoro, F., H. L. Greenstone, A. Insinga, M. K. Liszewski, J. P. Atkinson, P. Lusso, and E. A. Berger. 2003. Interaction of glycoprotein H of human herpesvirus 6 with the cellular receptor CD46. J. Biol. Chem. 278:25964-25969. [DOI] [PubMed] [Google Scholar]
- 47.Santoro, F., P. E. Kennedy, G. Locatelli, M. S. Malnati, E. A. Berger, and P. Lusso. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817-827. [DOI] [PubMed] [Google Scholar]
- 48.Scanlan, P. M., V. Tiwari, S. Bommireddy, and D. Shukla. 2003. Cellular expression of gH confers resistance to herpes simplex virus type-1 entry. Virology 312:14-24. [DOI] [PubMed] [Google Scholar]
- 49.Spaete, R. R., K. Perot, P. I. Scott, J. A. Nelson, M. F. Stinski, and C. Pachl. 1993. Coexpression of truncated human cytomegalovirus gH with the UL115 gene product or the truncated human fibroblast growth factor receptor results in the transport of gH to the cell surface. Virology 193:853-861. [DOI] [PubMed] [Google Scholar]
- 50.Stoddart, C. A., R. D. Cardin, J. M. Boname, W. C. Manning, G. B. Abenes, and E. S. Mocarski. 1994. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J. Virol. 68:6243-6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sweet, C. 1999. The pathogenicity of cytomegalovirus. FEMS Microbiol. Rev. 23:457-482. [DOI] [PubMed] [Google Scholar]
- 52.Theiler, R. N., and T. Compton. 2001. Characterization of the signal peptide processing and membrane association of human cytomegalovirus glycoprotein O. J. Biol. Chem. 276:39226-39231. [DOI] [PubMed] [Google Scholar]
- 53.Tugizov, S., D. Navarro, P. Paz, Y. L. Wang, I. Qadri, and L. Pereira. 1994. Function of human cytomegalovirus glycoprotein B: syncytium formation in cells constitutively expressing gB is blocked by virus-neutralizing antibodies. Virology 201:263-276. [DOI] [PubMed] [Google Scholar]
- 54.Tugizov, S., W. Yilong, I. Qadri, D. Navarro, E. Maidji, and L. Pereira. 1995. Mutated forms of human cytomegalovirus glycoprotein B are impaired in inducing syncytium formation. Virology 209:580-591. [DOI] [PubMed] [Google Scholar]
- 55.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Utz, U., W. Britt, L. Vugler, and M. Mach. 1989. Identification of a neutralizing epitope on glycoprotein gp58 of human cytomegalvirus. J. Virol. 63:1995-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, X., S. M. Huong, M. L. Chiu, N. Raab-Traub, and E. S. Huang. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456-461. [DOI] [PubMed] [Google Scholar]