Abstract
Previously, it has been shown that the exposure of Semliki Forest virus (SFV) to a mildly acidic environment induces a rapid and complete loss of the ability of the virus to bind and fuse to target membranes added subsequently. In the present study, incubation of SFV at low pH followed by a specific reneutralization step resulted in a partial reversion of this loss of viral fusion capacity, as assessed in a liposomal model system. Also, the ability of the viral E1 fusion protein to undergo liposome-stimulated trimerization was restored. Furthermore, acid-treated and neutralized SFV largely retained infectivity. Exposure of SFV to low pH induced dissociation of the E1/E2 heterodimer, which was not reversed upon neutralization. It is concluded that the SFV E1 fusion protein, after acid-induced dissociation from E2, rapidly adopts an intermediate, nontrimeric conformation in which it is no longer able to interact with target membrane lipids. Neutralization restores the ability of E1 to interact with membranes. This interaction, however, remains strictly dependent on low pH.
Semliki Forest virus (SFV) is an enveloped positive-strand RNA virus belonging to the genus Alphavirus of the family Togaviridae. It is well established that SFV enters its host cell through receptor-mediated endocytosis via the clathrin-coated pit pathway, fusing subsequently from within acidic endosomes (14, 20). Through this fusion reaction, the viral genome gains access to the host cell cytosol and initiates the infection process. The low-pH-induced fusion process of SFV has been studied extensively in cell-free model systems involving liposomes as receptor-free target membranes (3, 16, 25, 37, 39, 40). These studies have demonstrated that low pH is the sole trigger for membrane fusion of SFV and that receptor interaction is not required for the induction of the process. In addition, these studies have revealed a striking dependence of SFV fusion on the presence of both cholesterol (Chol) and sphingolipids in the target membrane (3, 5, 15, 16, 24, 25, 36, 37, 39, 40). Studies conducted with another prototype alphavirus, Sindbis virus, have led to similar conclusions (30, 31, 32, 34).
Membrane fusion of alphaviruses is mediated by the E1 component of the heterodimeric E1/E2 envelope glycoprotein (6, 37). Recent X-ray crystallographic analyses of the structure of the alphavirus membrane fusion protein (19) have revealed that it has striking similarities with the structure of membrane fusion protein E of flaviviruses (22, 27) and major differences with the structure of the fusion protein hemagglutinin (HA) of influenza virus (29, 41) and other HA-related viral membrane fusion proteins. This has led to the definition of class I (HA and related proteins) and class II (alphavirus and flavivirus proteins) viral fusion proteins. Interestingly, very recent structural analyses of the postfusion structures of two class II proteins (2, 10, 11, 23) suggest that there may well be mechanistic similarities between the fusion reactions mediated by the structurally distinct class I and class II viral fusion proteins.
Membrane fusion of alphaviruses involves a low-pH-induced dissociation of the E1/E2 heterodimer (12, 38) and formation of a highly stable homotrimer of E1 (37). Heterodimer dissociation exposes the fusion peptide region of E1 (between residues 83 and 100) (6, 19) located at the tip of domain II of the molecule (13). Subsequent trimerization is strongly stimulated by the interaction of E1 with cholesterol- and sphingolipid-containing target membranes (1, 18), suggesting that binding of the virus to target membranes kinetically precedes E1 trimer formation. Indeed, studies using Zn2+ as an inhibitor of E1 trimer formation (4) or a mutant of E1 blocked in trimer formation (17) have shown that acid-induced E1/E2 heterodimer dissociation suffices for efficient binding of SFV to target liposomes containing cholesterol and sphingolipids. It would thus appear that the trimerization of E1, while in association with target membranes, represents a key step in the fusion mechanism of alphaviruses.
In apparent agreement with the picture described above, it has been shown that prior exposure to low pH of SFV alone results in a rapid and complete loss of the ability of the virus to subsequently bind to and fuse with liposomes (3, 37). Exposure of the fusion peptide region of E1 in the absence of target membranes would rapidly lead to a conformation of the protein unable thereafter to interact with membranes. Interestingly, under these conditions, E1 also undergoes a limited degree of trimerization (37). However, this E1 trimer appears to be inactive, lacking the ability to interact with liposomes.
In this paper, we show that, in agreement with earlier observations, exposure of SFV to low pH in the absence of target membranes results in a rapid and complete loss of viral membrane fusion activity. However, when SFV was exposed to low pH followed by a specific reneutralization step, the capacity of the virus to subsequently fuse with liposomes at low pH was restored to a significant extent.
Fusion of SFV with liposomes was measured by using pyrene-labeled virus, essentially as described before (3, 25, 33, 37). Briefly, SFV was grown on baby hamster kidney cells (BHK-21) cultured beforehand in the presence of 1-pyrenehexadecanoic acid (Molecular Probes, Leiden, The Netherlands). Liposomes (large unilamellar vesicles [LUVs]) were prepared by the dispersion of lipid mixtures, dried from chloroform, in 5.0 mM HEPES, 150 mM NaCl, and 0.1 mM EDTA, pH 7.4 (HNE buffer), in five cycles of freeze-thawing, with subsequent extrusion of the dispersions through Unipore polycarbonate filters with pore sizes of 0.2 μm by use of a mini-extruder (LiposoFast, Avestin, Ottawa, Canada). Liposomes consisted of egg phosphatidylcholine (PC), phosphatidylethanolamine (PE) generated from egg PC, bovine brain sphingomyelin (SPM), and Chol in a molar ratio of 1:1:1:1.5. Phospholipids and Chol were from Avanti Polar Lipids (Alabaster, AL).
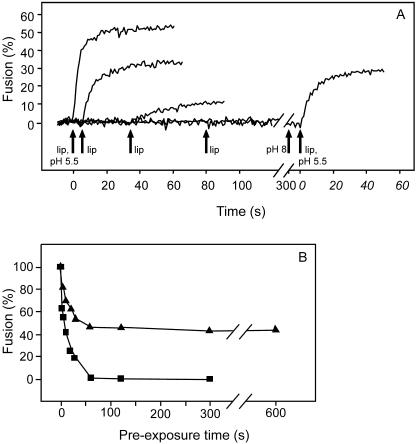
Pyrene-labeled SFV was incubated in HNE buffer at pH 5.5 with continuous stirring in a quartz cuvette of an SLM-Aminco AB2 fluorimeter, maintained at 37°C, in the absence of target liposomes. At different time intervals postacidification with the pH maintained at 5.5, liposomes were added, and the fusion process of the virus with the liposomes was monitored continuously. As a control, a mixture of SFV and liposomes was acidified. Figure 1A shows the results. Exposure of SFV to pH 5.5 in the presence of liposomes resulted in fast and extensive fusion. However, when liposomes were added at different time intervals after the acidification of the virus, the rate and extent of fusion rapidly diminished. There was no detectable fusion when liposomes were added 80 s or longer after the initial acidification of the virus.
FIG. 1.
Effects of acidification, neutralization, and reacidification on SFV fusion with LUVs. Fusion of pyrene-labeled SFV with LUVs was measured on-line as the decrease of the pyrene-excimer fluorescence at 480 nm (33). The fusion scale was set such that the initial excimer fluorescence at 480 nm represented 0% fusion and the residual fluorescence, after addition of the detergent octaethyleneglycol-monododecyl ether to a final concentration of 10 mM (representing infinite dilution of the fluorophore), corresponded to 100% fusion. Panel A presents a compilation of multiple measurements in which virus (1 μM), in a continuously stirred final volume of 0.7 ml HNE buffer (pH 7.4) in a thermostated quartz cuvette (37°C), was acidified to pH 5.5 by the injection of a small, pretitrated volume of 0.1 M MES (morpholinoethanesulfonic acid) and 0.2 M acetic acid at 0 s. Subsequently, PC-PE-Chol-SPM (1:1:1.5:1) LUVs (100 μM) were added at 5 s, 35 s, and 80 s (arrows). For a control, a mixture of virus and LUVs was acidified to pH 5.5 at 0 s (first curve on the left). At 300 s, the pH of the virus suspension was neutralized to pH 8 by the injection of a small volume of 0.1 M NaOH (second arrow from the right), after which LUVs were added, and the mixture was acidified again to pH 5.5 with a pretitrated volume of 0.1 M MES and 0.2 M acetic acid (outer arrow on the right). lip, liposomes (LUVs). For panel B, virus was acidified as described for panel A, and at the indicated time points, PC-PE-Chol-SPM LUVs were added (squares) or the mixture was neutralized first, after which LUVs were added and the mixture was reacidified to determined the recovery of fusion activity of the virus (triangles).
To determine if this fusion inactivation was reversible, we exposed pyrene-labeled SFV to low pH for 5 min and then took the pH back to 8.0 by the addition of a small, predetermined volume of 0.1 M NaOH. Subsequently, liposomes were added while the pH was kept at 8.0, and then the virus-liposome mixture was reacidified to pH 5.5. Figure 1A shows that under these conditions, a significant fraction of the virus fused with the liposomes, indicating that the neutralization step had induced a partial reversion of the loss of viral membrane fusion capacity.
Figure 1B shows the kinetics of fusion inactivation as determined in the liposomal model system with and without neutralization. Clearly, without subsequent neutralization, exposure of SFV to pH 5.5 resulted in a very fast and complete loss of viral fusion capacity. Neutralization rescued about 45% of the initial viral fusion capacity, which was assessed after the subsequent addition of target liposomes and reacidification. Even when the virus was exposed to pH 5.5 for up to 10 min, neutralization restored approximately 45% of the original viral membrane fusion capacity.
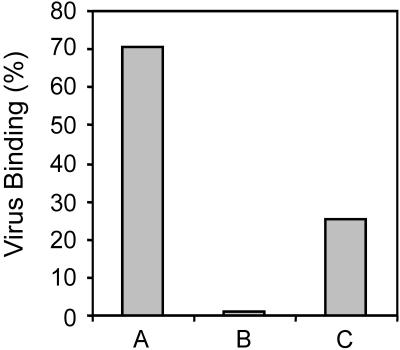
The loss of membrane fusion activity during incubation of SFV at low pH appeared to be due to a loss of ability of the virus to bind to the liposomes. This was determined in a direct binding assay. Radioactively labeled SFV, prepared by infection of BHK-21 cells in the presence of [35S]methionine as described before (3, 25, 33, 37), was exposed to pH 5.5 in the absence of liposomes for 2 min. Subsequently, liposomes were added, and the incubation was continued for 3 min, after which the mixture was neutralized for analysis. Alternatively, the virus, after exposure to pH 5.5 for 5 min, was first neutralized to pH 8.0 as described above, liposomes were added, and the mixture was reacidified to pH 5.5 for 1 min and neutralized for analysis. As a control, a mixture of virus and liposomes was exposed to low pH for 5 min and subsequently neutralized for analysis. Binding of the virus to the liposomes was assessed by flotation analysis on a sucrose density gradient as described before (3, 25, 33, 37). Figure 2 shows the results. Clearly, exposure of a virus-liposome mixture to pH 5.5 resulted in extensive (70%) irreversible binding of the virus to the liposomes (Fig. 2, bar A). Under these conditions the virus also fused to the liposomes (Fig. 1A). On the other hand, after preexposure to low pH for 2 min, the virus had completely lost its ability to bind to liposomes (Fig. 2, bar B). Neutralization, however, partially restored the ability of the virus to bind to liposomes upon subsequent reacidification (Fig. 2, bar C). Approximately 25% of the virus floated with the liposomes to the top of the gradient under these conditions, corresponding to a restoration of 36% of the original virus binding capacity. We note that while the recovery of fusion activity after reacidification of low-pH-exposed and neutralized virus in the presence of liposomes was about 45%, the rescue of binding activity under the same conditions was somewhat lower (36%). Although we do not know what the reason for this difference is, we think that it may due to the conditions used in the binding assay, possibly causing a limited degree of dissociation of bound virus from the liposomes.
FIG. 2.
Effects of acidification, neutralization, and reacidification on SFV binding to LUVs. Binding of SFV to PC-PE-Chol-SPM (1:1:1.5:1) LUVs was determined by coflotation of virus with LUVs on sucrose density gradients (33). Radioactively labeled SFV (105 to 106 cpm) in HNE buffer in a glass tube was continuously stirred at 37°C. Bar A, LUVs in HNE buffer (200 μM, pH 7.4) were added, and subsequently, the mixture was acidified to pH 5.5 by the injection of a small, pretitrated volume of 0.1 M MES and 0.2 M acetic acid. After 5 min, the mixture was neutralized with a pretitrated volume of 0.1 M NaOH and placed on ice. Bar B, the virus was first acidified to pH 5.5, and, after 2 min, LUVs in HNE buffer (pH 5.5) were added, and 3 min later, the mixture was neutralized and placed on ice. Bar C, the virus was first acidified to pH 5.5, and, after 5 min, the pH of the mixture was raised to pH 8. Then, LUVs were added, and the mixture was reacidified to pH 5.5; 1 min later, the mixture was neutralized and placed on ice. From the samples, 0.1 ml was mixed with 1.4 ml of 50% (wt/vol) sucrose in HNE buffer and placed on the bottom of a Beckman SW50 ultracentrifuge tube. On top of this, 2.0 ml of 20% (wt/vol) sucrose in HNE buffer and 1.0 ml of 5% (wt/vol) sucrose in HNE buffer were layered. After centrifugation in a Beckman SW50 rotor at 150,000 × g for 2 h at 4°C, the gradient was fractionated in 10 samples, starting from the top. The distribution of the viral radioactivity was quantified by liquid scintillation analysis. The radioactivity in the top four fractions, relative to the total amount of radioactivity, was taken as a measure for virus-LUV binding.
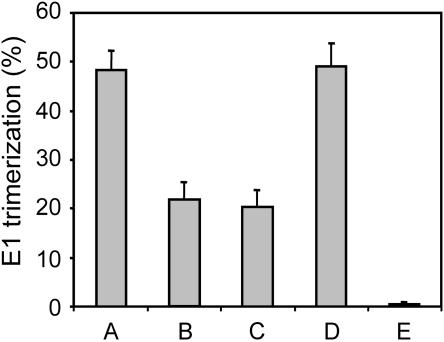
It is well established that upon exposure to low pH, the E1/E2 heterodimeric spike protein dissociates and that three E1 monomers subsequently form a stable homotrimer (3, 15, 18, 37). As indicated above, there is good evidence to indicate that efficient E1 trimer formation occurs only after the interaction of acid-activated virus with cholesterol- and sphingolipid-containing target membranes. Therefore, it was of interest to examine whether SFV, during exposure to low pH, in the absence of LUVs, loses the ability to form E1 trimers in the presence of subsequently added liposomes, and, if so, to see whether this ability is restored upon neutralization. The formation of NP-40-resistant E1 homotrimers was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, using [35S]methionine-labeled SFV, essentially as described before (3, 37), and quantified by phosphorimaging analysis using Image Quant 3.3 software (Molecular Dynamics, Sunnyvale, CA). The results are shown in Fig. 3. Exposure of SFV to pH 5.5 in the presence of liposomes resulted in the conversion of almost 50% of the viral E1 to homotrimers (bar A). In the absence of liposomes, the extent of E1 trimer formation was significantly lower (22%, bar B). The addition of liposomes to acid-exposed SFV at pH 5.5 did not stimulate E1 trimer formation (bar C), indicating that E1 had lost its ability to interact with cholesterol and sphingolipids in target liposomes. Neutralization of acid-exposed SFV to pH 8.0 and subsequent reacidification of the virus in the presence of liposomes, however, restored the ability of E1 to respond to the presence of the liposomes by extensive trimer formation (bar D). There was no trimer formation in a control incubation in which SFV was not acidified in the first place (bar E).
FIG. 3.
Effects of acidification, neutralization, and reacidification on E1 trimerization. Trimerization of E1 was determined under the same experimental conditions as in the fusion and binding experiments. Radioactively labeled SFV (105 to 106 cpm) in HNE buffer in a glass tube was continuously stirred at 37°C. Bar A represents SFV that was acidified to pH 5.5 by the injection of a small, pretitrated volume of 0.1 M MES and 0.2 M acetic acid in the presence of PC-PE-Chol-SPM (1:1:1.5:1) LUVs. After 5 min, the mixture was neutralized by the addition of a small amount of 0.1 M NaOH and placed on ice. Bar B represents SFV alone that was acidified and, 5 min later, neutralized and placed on ice. Bar C represents SFV alone that was acidified and to which, at 2 min, PC-PE-Chol-SPM LUVs were added. At 5 min, the mixture was neutralized and placed on ice. Bar D represents SFV alone that was acidified and for which, 5 min later, the pH of the mixture was raised to pH 8. Subsequently, the LUVs were added, and the mixture was reacidified to pH 5.5. After 1 min, the mixture was neutralized and placed on ice. Bar E represents SFV alone placed on ice. The samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. E1 trimerization was determined by autoradiography and phosphorimaging analysis. The numbers of counts in the trimer band are shown as percentages of the total amount of E1 present.
The results described above suggest that SFV exposed to low pH might, in fact, retain significant infectivity after neutralization. In order to determine the effect of low pH on viral infectivity, we performed a titration on acid-treated virus. SFV, at a total protein concentration of 1.75 μg/ml in HNE buffer, was incubated at pH 5.5 or 7.4 for 10 min at 37°C and subsequently neutralized with a predetermined volume of 0.1 M NaOH. Subsequently, an infection assay was carried out on BHK-21 cells in 96-well plates by use of serial dilutions of virus. Infection of the wells was scored in an all-or-none fashion, and virus titers were determined from the dilution causing infection in 50% of the wells (26). While the control virus had a titer of 1.9 × 109 IU/ml, SFV exposed to pH 5.5 and reneutralized had a titer of 1.6 × 109 IU/ml. This result shows that preexposure of SFV to low pH had an only minor effect on infectivity under the conditions of the experiment, in agreement with the results described above, showing significant retention of membrane fusion capacity of acid-exposed and reneutralized virus. In a previous study, we have also shown that SFV exposed to low pH and reneutralized retained a considerable fraction of its initial infectivity, while a less stable mutant, SFVΔ6K, did not (21).
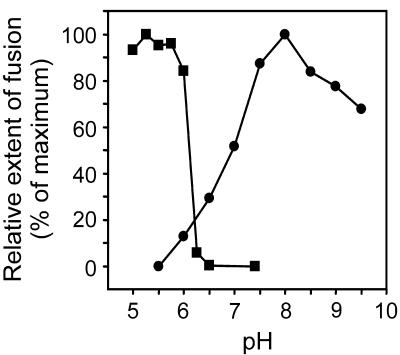
The results described above indicate that the neutralization of acid-exposed SFV restores the capacity of the virus to subsequently bind to and fuse with target membranes upon reacidification. This suggests that there is a certain degree of reversibility in the acid-induced conformational changes in the viral spike complex involved in the binding and fusion processes. With this perspective, we next studied the detailed pH dependence of the restoration of viral membrane fusion activity after preexposure of the virus to low pH. SFV was incubated at pH 5.5 for 5 min and neutralized to different pH values as indicated, liposomes were added, and the virus-liposome mixtures were reacidified to pH 5.5. For reasons of comparison, we determined, in the same experiment, the detailed pH dependence of the initial acid-induced conformational change by assessment of fusion in the presence of liposomes. The results are shown in Fig. 4. Clearly, in the presence of liposomes, the fusion of SFV was activated at a threshold pH of 6.2 and reached almost optimal extents at pH 5.8. This indicates that the conformational changes in the viral spike mediating the fusion process occur in a narrow pH range. Remarkably, the pH dependence of fusion restoration upon neutralization of virus preexposed to low pH was much less narrow and no mirror image of the pH dependence of fusion activation. For example, for optimal rescue of fusion activity, acid-exposed virus had to be taken up to pH 8.0, whereas with decreasing pH, fusion activation in the presence of liposomes started around pH 6.2. This suggests that the restoration of fusion capacity of the viral spike upon neutralization is not due to a simple completely reversible event with low activation energy.
FIG. 4.
Effect of neutralization pH on the extent of fusion. Fusion was measured as described in the legend to Fig. 1. Solid circles represent SFV in HNE buffer that was acidified to pH 5.5 by injection of a small, pretitrated volume of 0.1 M MES and 0.2 M acetic acid. After 5 min, the samples were neutralized to the indicated pH values by the addition of a small, pretitrated volume of 0.1 M NaOH. Subsequently, PC-PE-Chol-SPM (1:1:1.5:1) LUVs were added, and the mixtures were reacidified to pH 5.5. Squares represent mixtures of SFV and PC-PE-Chol-SPM (1:1:1.5:1) LUVs in HNE buffer acidified to the indicated pH values as described above.
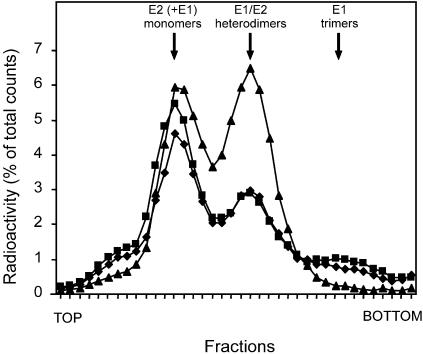
As indicated above, upon exposure to low pH, the SFV E1/E2 heterodimeric spike first dissociates, after which the virus binds to cholesterol- and sphingolipid-containing target membranes. Since the results described above suggested that the restoration of binding and fusion capacity of acid-exposed and reneutralized virus does not involve a simple reversible conformational change, we were interested to see whether the dissociation of the E1/E2 heterodimer was reversible. E1/E2 dissociation was determined by solubilization of [35S]methionine-labeled SFV in 1% NP-40 in HNE buffer, followed by sedimentation analysis on a 5 to 20% (wt/wt) sucrose density gradient, essentially as described before (37, 38). In this gradient, the larger E1 trimers move faster to the bottom of the gradient than heterodimers, which in turn move faster than E1 or E2 monomers. SFV was exposed to pH 5.5 and solubilized in NP-40 at acidic pH. Alternatively, SFV was exposed to pH 5.5, neutralized to pH 8.0, and then solubilized in NP-40. As a control, untreated SFV was solubilized in NP-40 and analyzed. The results are shown in Fig. 5. A major peak corresponding to heterodimers was recovered from untreated SFV. In acid-exposed SFV, as expected, the amount of E1/E2 heterodimers was significantly reduced, while a distinct band appeared at the bottom of the gradient corresponding to E1 homotrimers (see also references 37 and 38). Neutralization of acid-exposed SFV to pH 8.0 did not result in any detectable reformation of E1/E2 heterodimers. Clearly, the restoration of binding and fusion activity of acid-exposed and reneutralized SFV does not appear to involve reconstitution of the original E1/E2 heterodimer. It should be noted that the apparent background E1/E2 dissociation under the conditions of the experiment was relatively high, with a significant fraction of E1 and E2 in untreated virus being observed as monomers. This implies that reformation of comparatively unstable E1/E2 heterodimers upon pH neutralization may have gone unnoticed in the assay. Yet the results presented in Fig. 5 do suggest that reformation of the original stable E1/E2 heterodimer does not occur.
FIG. 5.
Effects of acidification and neutralization on E1/E2 heterodimer dissociation. Dissociation of the E1/E2 heterodimer was determined under the same experimental conditions as in the fusion and binding experiments. Radioactively labeled SFV (105 cpm) in HNE buffer (pH 7.4) in a glass tube was continuously stirred at 37°C. Squares represent SFV acidified for 5 min by addition of a small titrated volume 0.1 M MES and 0.2 M acetic acid. Subsequently, the virus was solubilized by addition of NP-40 (to a 1% final volume), and the mixture was neutralized (to a final concentration of 1%) by addition of a small, pretitrated volume of 0.1 M NaOH. Diamonds represent SFV acidified as described above. After 5 min, the pH of the sample was raised to pH 8 by the addition of a small, pretitrated volume of 0.1 M NaOH. Subsequently, NP-40 (1%) was added for solubilization of the virus. Triangles represent SFV in HNE buffer (pH 7.4) solubilized by the addition of NP-40 (1%). The samples were layered on top of a 5 to 20% (wt/wt) continuous sucrose gradient in HNE buffer (pH 7.4) and 0.1% NP-40 in a Beckman SW50 centrifuge tube. After centrifugation in a Beckman SW50 rotor at 192,000 × g for 16 h at 4°C, the gradients were fractionated in 38 fractions from the top. The radioactivity in each fraction was determined by liquid scintillation analysis.
The results in this paper demonstrate that, upon exposure of SFV to low pH, the E1 fusion protein of the virus rapidly adopts a conformation in which it can no longer interact with cholesterol- and sphingolipid-containing liposomes added after the low-pH trigger. Neutralization of acid-exposed SFV induces a substantial restoration of the ability of the virus to interact with target membranes. Remarkably, however, under these conditions, virus-liposome interaction still requires an acidic pH, even though after the initial acid treatment and neutralization, the original E1/E2 heterodimer does not appear to be reformed. If, indeed, the heterodimer remains dissociated upon neutralization with a concomitant continued exposure of the E1 fusion peptide (13), these results suggest that peptide exposure in itself is not sufficient for stable interaction of E1 with target membranes. An acidic pH would appear to remain an additional essential requirement.
During the course of this work, Gibbons and coworkers published a paper on the role of low pH in the interaction of SFV E1 with target membranes (9). Using a monoclonal antibody against the fusion peptide, the authors showed that, in the intact virus, exposure of the E1 fusion peptide was dependent on low pH, consistent with the notion that the fusion peptide is shielded in the native virus by E2 and becomes exposed only after low-pH-induced E1/E2 dissociation. Importantly, exposure of the peptide was detected after pH neutralization, in agreement with our suggestion above that neutralization does not result in reformation of the original E1/E2 heterodimer in which the E1 fusion peptide would be shielded by E2. The authors also studied interaction of the fusion peptide-specific monoclonal antibody with isolated E1 ectodomains (E1*). Consistent with the notion that, in the intact E1/E2 heterodimer, it is the E2 subunit that shields the E1 fusion peptide, they found that the fusion peptide in E1* is fully exposed irrespective of the pH. Yet, in agreement with previous studies (1, 18), E1* interacted with liposomes at low pH only, indicating a direct pH control in this interaction. Our present results are in complete agreement with these observations and support the idea that the interaction of E1 with cholesterol- and sphingolipid-containing membranes in itself is controlled by low pH, independent of the initial low-pH-induced E1/E2 heterodimer dissociation. Thus, there would appear to be multiple levels at which low pH controls the membrane interaction of SFV.
Gibbons et al. (9) also demonstrated that E1* does not become irreversibly inactivated when incubated at low pH alone. While under these conditions the ectodomain did not trimerize, subsequent neutralization, followed by the addition of liposomes and reacidification, resulted in target membrane binding and efficient formation of E1 trimers. Again, our present results with whole virus are in complete agreement with this observation.
The most intriguing aspect of the present study relates to the conformation that the majority of E1 adopts soon after exposure of SFV to low pH in the absence of liposomes. Without neutralization and reacidification, this conformation lacks the ability to interact with target membranes. It is unlikely that this nonreactive conformation of E1, which is susceptible to functional reactivation upon pH neutralization, is a stable homotrimer. While there is a limited extent of E1 trimer formation in low-pH-exposed virus (Fig. 3), the well-documented stability of the E1 trimer (8, 37) makes it unlikely that any of this E1 is involved in the subsequent interaction with target membranes after neutralization and reacidification of the virus. Therefore, after low-pH-induced E1/E2 heterodimer dissociation, it is probably a monomeric form of E1 that rapidly adopts a nonreactive conformation. It would appear that under these conditions, the E1 fusion peptide remains fully exposed (9, 13) yet is unable to interact with target liposomes despite the fact that the pH is maintained at 5.5. It is possible that regions of E1 other than the fusion peptide are involved in the control of the interaction of the protein with cholesterol-containing membranes (9, 19). For example, a loop region around E1 position 226 has been proposed to act as a sensor for cholesterol in the target membrane (9), since mutations at this position affect the cholesterol dependence of the SFV infection process (35). Perhaps this region becomes rapidly shielded upon exposure of SFV alone to low pH. In this regard, it would be interesting to know whether the E1 ectodomain exhibits a similar loss of reactivity towards target membranes when the pH is maintained at 5.5 during the addition of liposomes; this is not clear from the study of Gibbons et al. (9).
Upon pH neutralization, acid-exposed SFV regains the ability to interact with target membranes when reexposed to low pH. As argued above, we cannot formally exclude the possibility that, under these conditions, E1 reunites with E2 to form an unstable E1/E2 heterodimer. However, the original stable E1/E2 heterodimer does not appear to be reformed (Fig. 5). The results of Gibbons et al. (9) also support the notion that the original E1/E2 heterodimer is not reformed upon neutralization of virus preexposed to low pH. One possibility is that neutralization of acid-exposed virus brings the putative cholesterol-sensing loop around position 226 in monomeric E1 back in a reactive position. In any case, subsequent reacidification remains an absolute requirement for functional binding and fusion of the virus to target membranes due to the strict pH control of the lipid interaction of the E1 fusion peptide, as discussed above.
It is interesting that reversible pH-induced conformational changes in viral envelope glycoproteins have been observed before, notably in the case of rhabdoviruses, such as rabies virus (7, 28) and vesicular stomatitis virus (42). The trimeric rhabdovirus G envelope glycoprotein assumes three distinct conformations. Besides the neutral-pH native state (N), there appear to be a low-pH-activated state (A) which initiates the membrane fusion process and a fusion-inactive conformation (I) which is observed after prolonged incubation of the virus at low pH (28), which are apparently similar to the conformations of the alphavirus E1/E2 glycoprotein observed in the present study. Yet the reversibility of the pH-induced conformational change of the G protein appears to be different from that of the alphavirus E1/E2. Whereas for G there is a genuine thermodynamic equilibrium between the N, A, and I states (28), the reversibility of the low-pH-induced conformational change in the alphavirus envelope glycoprotein is primarily a functional phenomenon without reformation of the exact same native structure of the spike upon pH neutralization.
Acknowledgments
This work was supported by The Netherlands Organization for Scientific Research (NWO) under the auspices of the Chemical Foundation (CW), and by the U.S. National Institutes of Health (grant HL16660).
REFERENCES
- 1.Ahn, A., D. L. Gibbons, and M. Kielian. 2002. The fusion peptide of Semliki Forest virus associates with sterol-rich membrane domains. J. Virol. 76:3267-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bressanelli, S., K. Stiasny, S. L. Allison, E. A. Stura, S. Duquerroy, J. Lescar, F. X. Heinz, F. A. Rey. 2004. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 23:728-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bron, R., J. M. Wahlberg, H. Garoff, and J. Wilschut. 1993. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 12:693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corver, J., R. Bron, H. Snippe, C. Kraaijeveld, and J. Wilschut. 1997. Membrane fusion activity of Semliki Forest virus in a liposomal model system: specific inhibition by Zn2+ ions. Virology 238:14-21. [DOI] [PubMed] [Google Scholar]
- 5.Corver, J., L. Moesby, R. K. Erukulla, K. C. Reddy, R. Bittman, and J. Wilschut. 1995. Sphingolipid-dependent fusion of Semliki Forest virus with cholesterol-containing liposomes requires both the 3-hydroxyl group and the double bond of the sphingolipid backbone. J. Virol. 69:3220-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garoff, H., A. M. Frischauf, K. Simons, H. Lehrach, and H. Delius. 1980. Nucleotide sequence of cDNA coding for Semliki Forest virus membrane glycoproteins. Nature 288:236-241. [DOI] [PubMed] [Google Scholar]
- 7.Gaudin, Y. 2000. Reversibility in fusion protein conformational changes. The intriguing case of rhabdovirus-induced membrane fusion. Subcell. Biochem. 34:379-408. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons, D. L., A. Ahn, P. K. Chatterjee, and M. Kielian. 2000. Formation and characterization of the trimeric form of the fusion protein of Semliki Forest virus. J. Virol. 74:7772-7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbons, D. L., A. Ahn, M. Liao, L. Hammar, R. H. Cheng, and M. Kielian. 2004. Multistep regulation of membrane insertion of the fusion peptide of Semliki Forest virus. J. Virol. 78:3312-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbons, D. L., I. Erk, B. Reilly, J. Navaza, M. Kielian, F. A. Rey, and J. Lepault. 2003. Visualization of the target-membrane-inserted fusion protein of Semliki Forest virus by combined electron microscopy and crystallography. Cell 114:573-583. [DOI] [PubMed] [Google Scholar]
- 11.Gibbons, D. L., M.-C. Vaney, A. Roussel, A. Vigouroux, B. Reilly, J. Lepault, M. Kielian, and F. A. Rey. 2004. Conformational change and protein-protein interactions of the fusion protein of Semliki Forest virus. Nature 427:320-325. [DOI] [PubMed] [Google Scholar]
- 12.Haag, L., H. Garoff, L. Xing, L. Hammar, S.-T. Kan, and R. H. Cheng. 2002. Acid-induced movements in the glycoprotein shell of an alphavirus turn the spikes into membrane fusion mode. EMBO J. 21:4402-4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammar, L., S. Markarian, L. Haag, H. Lankinen, A. Salmi, R. H. Cheng. 2003. Prefusion rearrangements resulting in fusion peptide exposure in Semliki Forest virus. J. Biol. Chem. 278:7189-7198. [DOI] [PubMed] [Google Scholar]
- 14.Helenius, A., J. Kartenbeck, K. Simons, and E. Fries. 1980. On the entry of Semliki forest virus into BHK-21 cells. J. Cell Biol. 84:404-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kielian, M., P. K. Chatterjee, D. L. Gibbons, and Y. E. Lu. 2000. Specific roles for lipids in virus fusion and exit. Examples from the alphaviruses. Subcell. Biochem. 34:409-455. [DOI] [PubMed] [Google Scholar]
- 16.Kielian, M., and A. Helenius. 1984. Role of cholesterol in fusion of Semliki Forest virus with membranes. J. Virol. 52:281-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kielian, M., M. R. Klimjack, S. Ghosh, and W. A. Duffus. 1996. Mechanisms of mutations inhibiting fusion and infection by Semliki Forest virus. J. Cell Biol. 134:863-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klimjack, M. R., S. Jeffrey, and M. Kielian. 1994. Membrane and protein interactions of a soluble form of the Semliki Forest virus fusion protein. J. Virol. 68:6940-6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lescar, J., A. Roussel, M. W. Wien, J. Navaza, S. D. Fuller, G. Wengler, G. Wengler, and F. A. Rey. 2001. The Fusion glycoprotein shell of Semliki Forest virus: an icosahedral assembly primed for fusogenic activation at endosomal pH. Cell 105:137-148. [DOI] [PubMed] [Google Scholar]
- 20.Marsh, M., E. Bolzau, and A. Helenius. 1983. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell 32:931-940. [DOI] [PubMed] [Google Scholar]
- 21.McInerney, G. M., J. M. Smit, P. Liljeström, and J. Wilschut. 2004. Semliki Forest virus produced in the absence of the 6K protein has an altered spike structure as revealed by decreased membrane fusion capacity. Virology 325:200-206. [DOI] [PubMed] [Google Scholar]
- 22.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2003. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 100:6986-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2004. Structure of the dengue virus envelope protein after membrane fusion. Nature 427:313-319. [DOI] [PubMed] [Google Scholar]
- 24.Moesby, L., J. Corver, R. K. Erukulla, R. Bittman, and J. Wilschut. 1995. Sphingolipids activate membrane fusion of Semliki Forest virus in a stereospecific manner. Biochemistry 34:10319-10324. [DOI] [PubMed] [Google Scholar]
- 25.Nieva, J. L., R. Bron, J. Corver, and J. Wilschut. 1994. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 13:2797-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 27.Rey, F. A., F. X. Heinz, C. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 28.Roche, S., and Y. Gaudin. 2002. Characterization of the equilibrium between the native and fusion-inactive conformation of rabies virus glycoprotein indicates that the fusion complex is made of several trimers. Virology 297:128-135. [DOI] [PubMed] [Google Scholar]
- 29.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 30.Smit, J. M., R. Bittman, and J. Wilschut. 1999. Low-pH-dependent fusion of Sindbis virus with receptor-free cholesterol- and sphingolipid-containing liposomes. J. Virol. 73:8476-8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smit, J. M., L. Gang, P. Schoen, J. Corver, R. Bittman, K. C. Lin, and J. Wilschut. 2002. Fusion of alphaviruses with liposomes is a non-leaky process. FEBS Lett. 521:62-66. [DOI] [PubMed] [Google Scholar]
- 32.Smit, J. M., W. B. Klimstra, K. Ryman, R. Bittman, R. E. Johnston, and J. Wilschut. 2001. PE2 cleavage mutants of Sindbis virus: correlation between viral infectivity and pH-dependent membrane fusion activation of the spike heterodimer. J. Virol. 75:11196-11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smit, J. M., B.-L. Waarts, R. Bittman, and J. Wilschut. 2003. Liposomes as target membranes in the study of virus receptor interaction and membrane fusion. Methods Enzymol. 372:374-392. [DOI] [PubMed] [Google Scholar]
- 34.Smit, J. M., B.-L. Waarts, K. Kimata, W. B. Klimstra, R. Bittman, and J. Wilschut. 2002. Adaptation of alphaviruses to heparan sulfate: interaction of Sindbis and Semliki Forest viruses with liposomes containing lipid-conjugated heparin. J. Virol. 76:10128-10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vashishtha, M., T. Phalen, M. T. Marquardt, J. S. Ryu, A. C. Ng, and M. Kielian. 1998. A single point mutation controls the cholesterol dependence of Semliki Forest virus entry and exit. J. Cell Biol. 140:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waarts, B.-L., R. Bittman, and J. Wilschut. 2002. Sphingolipid and cholesterol dependence of alphavirus membrane fusion. Lack of correlation with lipid raft formation in target liposomes. J. Biol. Chem. 277:38141-38147. [DOI] [PubMed] [Google Scholar]
- 37.Wahlberg, J. M., R. Bron, J. Wilschut, and H. Garoff. 1992. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J. Virol. 66:7309-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wahlberg, J. M., and H. Garoff. 1992. Membrane fusion process of Semliki Forest virus. I. Low pH-induced rearrangement in spike protein quaternary structure precedes virus penetration into cells. J. Cell Biol. 116:339-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White, J. M., and A. Helenius. 1980. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc. Natl. Acad. Sci. USA 77:3273-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilschut, J., J. Corver, J. L. Nieva, R. Bron, L. Moesby, K. C. Reddy, and R. Bittman. 1995. Fusion of Semliki Forest virus with cholesterol-containing liposomes at low pH: a specific requirement for sphingolipids. Mol. Membr. Biol. 12:143-149. [DOI] [PubMed] [Google Scholar]
- 41.Wilson, I. A., J. J. Skehel, and D. C. Wiley. 1981. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 289:366-373. [DOI] [PubMed] [Google Scholar]
- 42.Yao, Y., K. Ghosh, R. F. Epand, R. M. Epand, and H. P. Gosh. 2003. Membrane fusion activity of vesicular stomatitis virus glycoprotein G is induced by low pH but not by heat or denaturant. Virology 310:319-332. [DOI] [PubMed] [Google Scholar]