Abstract
The Epstein-Barr virus (EBV) genome is highly methylated in latently infected cells. We recently reported that the EBV immediate-early (IE) protein BZLF1 (Z) preferentially binds to and activates transcription from the methylated form of the BRLF1 IE gene promoter (Rp). We now report that serine residue 186 in the Z DNA-binding domain plays an important role in the ability of Z to bind to and activate methylated Rp. A Z mutant containing an alanine residue at position 186 [Z(S186A)] was significantly defective in binding to methylated, as well as unmethylated, ZREs (Z-responsive elements) in Rp and was unable to activate lytic EBV gene transcription from the methylated or demethylated form of the viral genome. A Z mutant containing threonine at residue 186 [Z(S186T)] bound only to the methylated form of the ZRE-2 site in Rp and induced lytic EBV gene transcription from the methylated, but not demethylated, form of the viral genome. The defect in both of these mutants was primarily due to an inability to activate the Rp in the context of the viral genome. Finally, a Z mutant containing an aspartic acid at position 186 [Z(S186D)] did not bind to either the consensus AP-1 site or to the methylated or unmethylated Rp ZRE-2 site and did not induce lytic gene transcription. These results indicate that replacement of serine with threonine at residue 186 in the Z DNA-binding domain differentially affects its ability to reactivate the unmethylated, versus methylated, viral genome.
Epstein-Barr virus (EBV) is the causative agent of infectious mononucleosis and is associated with B-cell lymphomas, nasopharyngeal carcinoma, gastric carcinomas, and other malignancies (44, 56). EBV primarily infects two cell types, epithelial cells and B cells (44, 50). In normal oral epithelial cells, the virus exists in a lytic state (34, 37, 44, 51), whereas EBV infection of B cells usually results in a latent type of infection (34, 44). However, in a small percentage of B cells, the virus periodically reactivates and replicates in the lytic manner. This reactivation is initiated by expression of the two immediate-early genes, BZLF1 and BRLF1 (8, 13, 15, 33, 46, 47, 51, 55), which encode the transcriptional activators Z and R, respectively.
Z is a bZip protein homologous to the cellular proteins c-Fos and c-Jun and binds directly to AP-1 and AP-1-like motifs (known as Z-responsive elements [ZREs]) present in many lytic viral promoters (12, 19, 21). Z expression results in activation of lytic EBV gene expression in essentially all EBV-positive cell lines. Z initially activates expression of the BRLF1 immediate-early promoter (Rp) by binding directly to three somewhat atypical ZRE sites in Rp, referred to as ZRE-1, ZRE-2, and the recently identified ZRE-3 (6, 41). Z and R together then activate the expression of the entire complement of lytic EBV genes, thus inducing lytic EBV infection.
The EBV genome in latently infected cells is extensively methylated (18, 39). Methylation likely suppresses the expression of lytic viral genes, thus helping to maintain latent infection. In addition, methylation of viral promoters driving LMP-1 and EBNA-2 expression is associated with the most stringent form of viral latency (type I) (2, 18, 28, 30, 38, 52), in which LMP-1 and EBNA-2 are not expressed. It has been proposed that DNA methylation causes transcriptional repression by multiple different mechanisms, including modification of the histone acetylation state and prevention of transcription factor binding to DNA (5, 7, 9, 10, 14, 16, 26, 29, 31, 32, 35, 36, 40, 48, 49, 54). However, we have recently shown that Z unexpectedly preferentially binds to and activates transcription from the methylated form of Rp (6). In addition, Z preferentially activates lytic EBV gene transcription from the methylated form of the viral genome in latently infected cells (6).
Two of the three known ZRE sites in Rp (ZRE-2 and ZRE-3) contain CpG motifs and hence could be methylated in latently infected cells. We recently showed that Z preferentially binds to the methylated form of the Rp ZRE-2 site (TGAGCGA) and binds only to the methylated form of the Rp ZRE-3 site (TTCGCGA) (6). The Rp ZRE-1 site (TGAGCCA) cannot be methylated. The crystal structure of a Fos-Jun heterodimer bound to a consensus AP-1 site indicates that the residues in the basic DNA-binding domain of c-Fos and c-Jun which make direct contact with DNA are analogous to Z residues 182, 185, 186, 189, and 190 and that Z residue 186 would directly contact the methylated cytosine in the Rp ZRE-2 motif (TGAGCGA) (25). Interestingly, the residues that directly contact DNA are identical in the c-Fos, c-Jun, and Z proteins, with the exception of the Z serine residue at position 186, which is an alanine at the analogous position in c-Fos and c-Jun (23, 25).
A mutant Z protein in which serine 186 is switched to an alanine, Z(S186A), efficiently activates promoters containing the consensus AP-1 site in reporter gene assays. Nevertheless, Z(S186A) cannot activate BRLF1 transcription from the viral genome in latently infected cells (1, 23) and, hence, cannot activate early gene transcription, which requires both the Z and R proteins in the context of the intact viral genome. Z(S186A) binds at least as well as wild-type Z to the consensus AP-1 site but binds with reduced affinity in comparison to wild-type Z to the unmethylated form of the Rp ZRE-2 site as well as the Rp ZRE-1 site (1, 24). A mutant Z containing a threonine at position 186, Z(S186T), was previously reported to be only slightly reduced in comparison to wild-type Z in its ability to activate BRLF1 transcription (as well as early gene transcription) from the latent viral genome in Raji cells (24), but its binding affinity to the Rp ZRE-1, ZRE-2, and ZRE-3 sites has not been previously reported.
In this paper, we have examined the effect of CpG methylation on Z(S186A) and Z(S186T) binding to the ZRE-2 and ZRE-3 sites in Rp as well as the effect of viral genome methylation on the ability of these Z mutants to disrupt viral latency. In addition, we have examined the phenotype of the Z(S186D) mutant, which has not been previously described in detail. We show that the Z(S186D) mutant does not bind to either the consensus AP-1 site or the methylated or unmethylated forms of Rp ZRE-2 and cannot activate lytic gene transcription. The Z(S186A) mutant, which binds as well as wild-type Z to the AP-1 site, cannot bind efficiently to any of the three Rp ZRE sites, regardless of their methylation status, and cannot activate BRLF1 transcription (or early gene transcription) from the intact viral genome in either the methylated or unmethylated form. The Z(S186T) mutant, similar to wild-type Z, binds only to the methylated form of the Rp ZRE-3 site. Z(S186T) binds efficiently to the methylated form of the ZRE-2 site, but in contrast to wild-type Z, has essentially lost the ability to bind to the unmethylated form of ZRE-2. Consistent with this DNA-binding profile, the Z(S186T) mutant can activate BRLF1 as well as early gene transcription from the methylated but not unmethylated form of the viral genome. These results indicate that residue 186 in the Z DNA-binding domain plays a critical role in regulating the affinity of Z binding to methylated as well as unmethylated Rp ZRE sites.
MATERIALS AND METHODS
Cell lines.
The 293/EBV-ZKO cell line was derived by infecting human embryonic kidney 293 cells with a BZLF1-deleted (green fluorescent protein [GFP]-containing) EBV genome as previously described (20). 293T is a derivative of the 293 cell line which expresses the simian virus 40 (SV40) large T antigen. DG75 is an EBV-negative Burkitt's lymphoma cell line. The AGS-EBV cell line was derived by infecting the AGS gastric carcinoma line with wild-type (GFP-containing) B95-8 virus. The GFP gene was inserted into the wild-type and BZLF1-deleted versions of the B95-8 genome at the site of the right hand (dispensable) copy of oriLyt as previously described (20). D98/HE-R1 cells are a fusion line created with HeLa cells and the Burkitt lymphoma P3HR1 line. LCL-1 is a lymphoblastoid cell line derived using wild-type (GFP-containing) B95-8 virus. Raji is an EBV-positive Burkitt lymphoma line.
Plasmids.
Plasmid DNA was purified through QIAGEN columns as described by the manufacturer. Rp-CAT contains the immediate-early BRLF1 promoter sequences from −962 to +5 linked to the chloramphenicol acetyltransferase (CAT) gene. The Z expression vector (pSG5-Z) contains genomic Z downstream of the SV40 promoter (a gift from S. Diane Hayward) in the pSG5 vector (Stratagene). The Z(S186A) expression vector (a gift from Erik Flemington) contains a point mutation at Z residue 186 which converts serine to alanine; the Z(S186T) expression vector (a gift from George Miller) contains a point mutation at amino acid 186 which converts serine to threonine. The Z cDNA (a gift From Paul Farrell) was cloned into the SG5 vector to create SG5-ZcDNA. This vector was used to in vitro translate the Z protein. Z(S186A) and Z(S186T) mutations in the SG5-ZcDNA vector were constructed using the QuikChange site-directed mutagenesis kit (Stratagene) to allow in vitro translation of the mutant proteins. A Z(S186D) mutant was also constructed in the context of the SG5-ZcDNA vector and used to in vitro translate this mutant as well as express it in transfected cells. The R expression plasmid contains genomic R sequences downstream of the SV40 promoter in the pSG5 vector (a gift from S. Diane Hayward). The GST-Z vector was constructed as previously described (43) and contains the BZLF1 protein fused in frame to the glutathione S-transferase (GST) protein.
Determining the methylation status of the Epstein-Barr virus BRLF1 promoter.
Total genomic DNA was isolated from cultured EBV-positive cells using the DNeasy tissue kit (QIAGEN). Bisulfite modification of genomic DNA was performed as previously described (27). Briefly, 1 μg of genomic DNA was denatured by the addition of 5 μl 2 N NaOH followed by incubation at 37°C for 10 min. Modification of C residues was accomplished by the addition of 30 μl of 10 mM hydroquinone (Sigma) and 520 μl of 3 M sodium bisulfite, pH 5.0 (Aldrich), followed by incubation at 50°C for 16 h. Modified DNA was purified using a Wizard DNA cleanup kit (Promega) and eluted from the resin with 50 μl H2O preheated to 70°C. The final desulfonation was accomplished by the addition of 2 N NaOH to 0.3 N (8.9 μl) followed by incubation at room temperature for 5 min. The DNA was ethanol precipitated, and the final pellet was resuspended in water. Bisulfite-modified EBV genomic DNA was amplified using Taq polymerase (Promega) in 1× buffer, 1.5 mM MgCl2, 0.2 mM concentrations of deoxynucleoside triphosphates, and 10 pmol of each primer. Primary PCR was performed using the EBV R promoter primers 5′-TGTGTAGTGAGGTGTTGTGTTTTG-3′ and 5′-TTTTAACTACAATATTTCCTCCAAAAA-3′. Nested PCR was performed using the primers 5′-TGTGTAGTGAGGTGTTGTGTTTTG-3′ and 5′-TGAGGTGTTGTGTTTTGTATGGT-3′. Thermal cycler conditions for the primary and nested PCR were as follows: 1 cycle at 94°C for 2 min followed by 35 cycles of 94°C for 30 seconds, 58°C for 30 seconds, 72°C for 30 seconds, and a final cycle of 72°C for 7 min. PCR products were agarose gel purified with a QIAGEN gel extraction kit and then cloned into the pCR 2.1-TOPO vector (TOPO TA cloning kit; Invitrogen) according to the manufacturer's instructions. For each cell line, 10 clones were sequenced using ABI prism (UNC-CH Automated DNA Sequencing Facility) to determine the methylation status of the BRLF1 promoter.
Probes for EMSA.
The oligonucleotides listed below were synthesized with or without methylated cytosines at the CpG motif(s) within potential ZRE sites (MWG Biotech and Oligos, Etc., Inc.). The sequences of the oligonucleotides used in electrophoretic mobility shift assays (EMSAs) are shown below. Positions of the oligonucleotides in the EBV genome relative to the BRLF1 start site are shown in parentheses. ZRE sequences are underlined. The oligonucleotides were either unmethylated or methylated at the cytosine shown in boldface type. Synthetic oligonucleotides containing the Rp ZRE-1 site, or the AP-1 site in the BMRF1 promoter, were made as previously described (1). Synthetic double-stranded oligonucleotides were 5′ end labeled with 32P using the Klenow reaction. The oligonucleotides and sequences are as follows: ZRE-2 (−184 to −203), 5′-GATCAAGCTTATGAGCGATTTTAT-3′ and 5′-GATCATAAAATCGCTCATAAGCTT-3′; ZRE-3 (−245 to −264), 5′-GATCTCAAAATTCGCGATGCTATA-3′ and 5′-GATCTATAGCATCGCGAATTTTGA-3′.
EMSA.
Labeled probes were incubated either with in vitro-translated wild-type Z, Z(S186A), or Z(S186T) from reticulocyte lysate (or the untranslated lysate) or whole-cell extracts made from DG75 cells electroporated with either the empty pSG5 vector or plasmid expressing wild-type Z, Z(S186A), Z(S186T), or Z(S186D). In vitro-translated wild-type and mutant Z proteins were made using the TNT T7 quick-coupled transcription/translation system (Promega) according to the manufacturer's instructions. DG75 extracts were made 48 h after transfection by suspending the cells in 200 μl of a buffer containing 50 mM HEPES (pH 7), 250 mM NaCl, 0.1% NP-40, 5 mM EDTA, 5 mM dithiothreitol, 1× Complete protease inhibitors (Roche), and 15% glycerol, followed by freeze-thawing twice and centrifugation. The supernatant was used for EMSAs. Z binding reactions were performed in a buffer consisting of 100 mM KCl, 20 mM HEPES (pH 7.3), 10% glycerol, 0.2 mM EDTA, and 4 mM dithiothreitol with 2 μg of poly(dI-dC)/poly(dI-dC) (Pharmacia). Bovine serum albumin (10 μg; Promega) was added to EMSA mixtures with purified GST-Z. Five micrograms of DG75 whole-cell extracts or 2 μl of in vitro-translated protein was added to each reaction mixture and incubated at 4°C for 10 min before the addition of labeled probe (10,000 cpm). For competition EMSAs, a 10-fold, 50-fold, or 250-fold excess of cold competitor DNA was also added to the reaction mixture prior to the addition of labeled probe. The reaction mixtures were incubated further for 30 min at 4°C after the addition of labeled probe. The reaction mixtures were then loaded onto a 4% polyacrylamide gel and run in 0.5× Tris-borate-EDTA buffer at room temperature. The binding was quantitated using a phosphorimager (Molecular Dynamics) or NIH Image (National Institutes of Health).
Expression and purification of GST-Z protein.
Saturated bacterial culture (250 μl) was diluted in 10 ml of LB plus ampicillin and incubated at 37°C to an A600 of 0.6. Protein expression was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and incubation at 30°C for 2 h. Bacteria were pelleted, resuspended in 500 μl of 1× phosphate-buffered saline (PBS), and then sonicated on ice. Fifty microliters of 1× PBS plus 10% Triton X-100 was added, and the mixture was then centrifuged for 10 min at 13,000 rpm at 4°C. A mixture of 100 μl of extract, 50 μl of 50% slurry of glutathione-agarose beads, and 500 μl of buffer C (50 mM Tris [pH 7.5], 100 mM NaCl, 5 mM MgCl2, 0.1% NP-40, 10% glycerol, 1× Complete protease inhibitors [Roche]) was incubated at 4°C for 2 h on an orbital mixer (Adams Nutator). Beads were pelleted, and the supernatant was removed, resuspended in 500 μl of buffer C, and then incubated for 5 min at 4°C on a nutator. Beads were repelleted and washed three times with buffer C. The bead pellet was then resuspended in 100 μl of 50 mM Tris (pH 7.5) and 20 mM reduced glutathione and incubated on a nutator for 5 min at room temperature. Beads were pelleted for 1 min at 13,000 rpm, and the supernatant was removed to a microcentrifuge tube. This step was repeated. The combined supernatant was dialyzed with a 3,500-molecular weight cutoff Slide-A-Lyzor mini dialysis unit (Pierce) against 50 mM Tris (pH 8.0) and 10% glycerol for 2 h at 4°C.
CAT assays.
CAT vectors were mock methylated or methylated in vitro using SssI CpG methylase (New England Biolabs), and methylation was confirmed by complete resistance to HpaII cutting. Methylated plasmids were subsequently cleaned by phenol-chloroform extraction and ethanol precipitation prior to transfection into cells. Cell extracts were prepared 2 days posttransfection and incubated at 37°C with [14C]chloramphenicol in the presence of acetyl coenzyme A. The percent acetylation was determined by thin-layer chromatography followed by phosphorimager screening (Molecular Dynamics).
DNA transfection.
DNA (1 μg of the CAT construct plasmids and 4 μg of the protein expression plasmids) was transfected into DG75 cells resuspended in RPMI 1640 medium (Gibco BRL) by electroporation with a Zapper electroporation unit (Medical Electronics Shop, University of Wisconsin) at 1,500 V. Lipofectamine 2000 (Gibco BRL) was used to transfect DNA into EBV-positive 293 cells (or 293T cells) according to the manufacturer's instructions. A DNA/Lipofectamine 2000 ratio of 1:2 (mass/volume) was used.
Viral genome methylation influence on disruption of viral latency.
293 cells latently infected with a Z-deleted EBV genome (293/EBV-ZKO) were treated for 5 days in the presence or absence of 5-aza-2′-deoxycytidine (5-aza-2′-dc, 1 μM; Sigma). 5-Aza-2′-dc was removed during the lipofection procedure and then replaced after 6 h. Expression of lytic EBV proteins was quantitated by immunoblot analysis 2 days after transfection.
Immunoblot analysis.
Cell extracts harvested as described for the CAT assays or cell extracts made using a buffer containing 150 mM sodium chloride, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris (pH 8.0), 50 mM sodium fluoride, 50 mM β-glycerophosphate, 2 mM sodium vanadate, and 1× Complete protease inhibitor (Roche) were electrophoresed on a 7% sodium dodecyl sulfate-polyacrylamide gel electrophoresis denaturing gel. The proteins were transferred onto a nitrocellulose membrane (Protran), blocked in 1× PBS-5% milk-0.1% Tween 20, and incubated with primary antibody for 1 h at room temperature using anti-EBV BZLF1 (Z, 1:100; Argene), anti-BRLF1 (R, 1:100; Argene), anti-EBV EA-D (BMRF1, 1:100; Capricorn), or anti-β-actin (1:5,000; Sigma) antibody. The membrane was washed with 1× PBS-0.1% Tween 20, incubated with secondary antibody (goat anti-mouse κ-horseradish peroxidase, 1:10,000; Southern Biotechnology) at room temperature for 1 h, and washed. The results were visualized with the ECL chemiluminescent kit (Amersham) according to the manufacturer's instructions.
RESULTS
The BRLF1 promoter is highly methylated in cells with latent EBV infection.
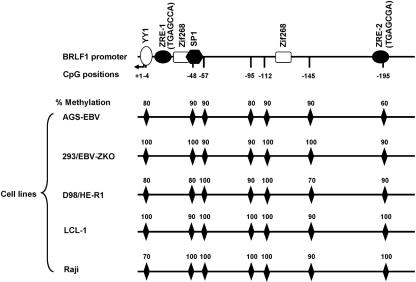
To determine the extent of BRLF1 promoter (Rp) methylation in latently infected cells, we examined its methylation status in five different EBV-infected cell lines using sodium bisulfite/PCR analysis. The cell lines examined included two epithelial cell lines (EBV-positive gastric carcinoma [AGS] cells and EBV-positive 293 cells), a fusion line created with HeLa cells and the Burkitt lymphoma P3HR1 line (D98/HE-R1 cells), a lymphoblastoid cell line (LCL-1), and a Burkitt lymphoma line (Raji). Following bisulfite treatment of the DNA, the BRLF1 promoter sequence was cloned and 10 independent clones were sequenced for each cell line.
The Rp sequence was highly methylated in each of the cell lines analyzed, whether epithelial or B-cell derived (Fig. 1). The CpG motif contained within the Rp ZRE-2 site (TGAGCGA) was almost completely methylated in every cell line examined, with the exception of the AGS-EBV cells, in which this site was methylated in 60% of the clones sequenced. Interestingly, the AGS-EBV cells have a small amount of detectable constitutive BRLF1 gene expression, whereas the other cell lines are essentially completely latent (data not shown), suggesting that activation of Rp by cellular transcription factors may preferentially occur when the promoter is less methylated. The methylation status of the Rp ZRE-3 site was also examined in EBV-positive 293 cells; the ZRE-3 site was fully methylated in this cell line (data not shown). As Z efficiently induces BRLF1 expression in 293 cells (as well as the other lines examined), these results indicate that Z activates BRLF1 transcription in latently infected cells even when the Rp ZRE-2 and ZRE-3 sites are methylated.
FIG. 1.
The BRLF1 IE promoter (Rp) is highly methylated in latently infected cells. The methylation status of CpG sites in Rp in 5 EBV-positive cell lines (indicated on the left) was determined by sodium bisulfite treatment followed by PCR and sequencing. The position of each CpG site, represented by a diamond, with respect to the R transcription start site (+1) is indicated at the top. The percentage of clones with methylated CpG at each position is indicated on top of the diamond representing that CpG for each cell line analyzed; 10 clones for each cell were sequenced.
Z residue 186 regulates binding to the methylated and unmethylated forms of the Rp ZREs.
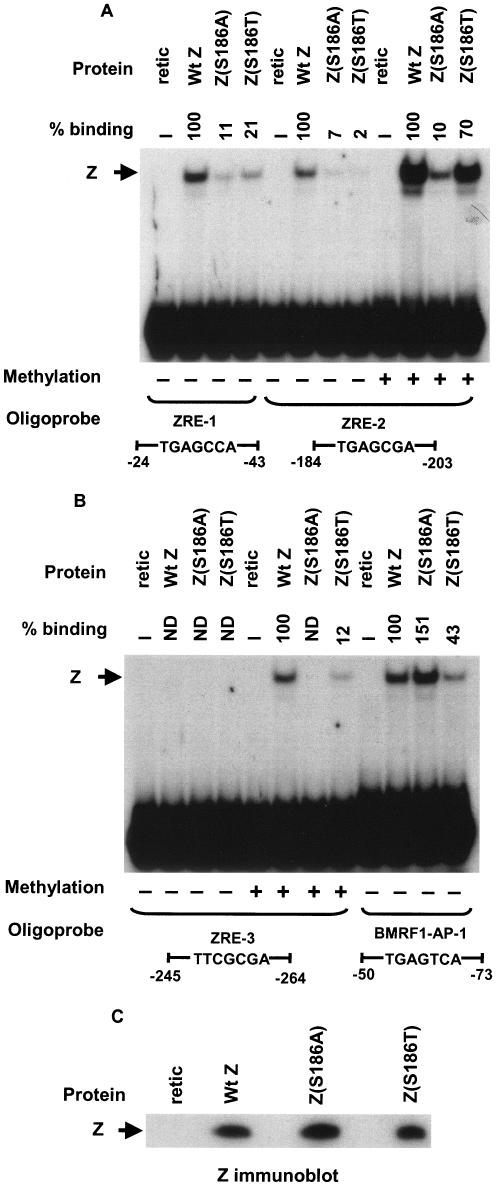
We recently showed that wild-type Z preferentially binds to and activates transcription from the methylated form of Rp (6). To determine whether Z residue 186 regulates binding to methylated ZRE sites, we performed EMSAs to compare the ability of in vitro-translated wild-type Z, Z(S186A), and Z(S186T) to bind to labeled oligonucleotide probes containing the ZRE-1, ZRE-2, or ZRE-3 sites in Rp or the consensus AP-1 site from the BMRF1 early viral promoter. The ZRE-2 and ZRE-3 probes were synthesized so that they contained either unmethylated or methylated cytosine at the CpG motifs.
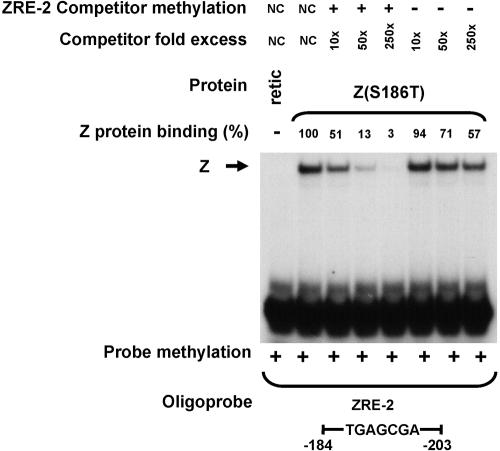
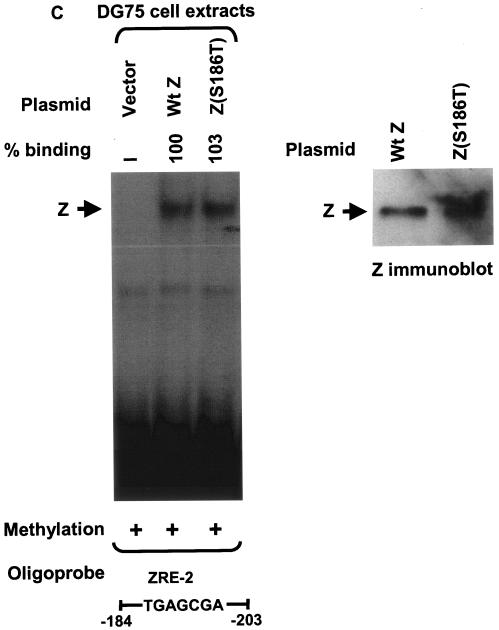
The in vitro-translated Z(S186A) mutant was impaired in comparison to wild-type Z for binding to the ZRE-1 site (Fig. 2A), the unmethylated as well as methylated forms of the ZRE-2 site (Fig. 2A), and the methylated ZRE-3 site (Fig. 2B), although as previously reported (1, 24), it bound at least as well as wild-type Z to the consensus AP-1 site (Fig. 2B). The in vitro-translated Z(S186T) mutant was partially compromised for binding to the Rp ZRE-1 site (Fig. 2A), the consensus AP-1 site (Fig. 2B), and the methylated Rp ZRE-3 site (Fig. 2B). Most interestingly, however, while binding of the Z(S186T) mutant to the unmethylated form of the Rp ZRE-2 site was severely impaired (only 2% binding compared to the wild-type protein), it bound almost as well as the wild-type Z to the methylated form of Rp ZRE-2 (Fig. 2A). Competition analysis using the methylated versus unmethylated forms of ZRE-2 as cold competitor DNA confirmed that the Z(S186T) mutant bound almost exclusively to the methylated form of ZRE-2 (Fig. 3). These results indicate that Z residue 186 regulates binding to both methylated and unmethylated ZRE sites in the BRLF1 promoter.
FIG. 2.
Mutation of Z residue 186 affects binding to the three Rp ZRE sites. Binding of in vitro-translated wild-type Z (Wt Z), Z(S186A), and Z(S186T) to oligonucleotide probes containing the Rp ZRE-1 or ZRE-2 sites (A) or the Rp ZRE-3 site or the AP-1 site in the early EBV BMRF1 gene promoter (BMRF1-AP-1) (B) was compared. The sequences and methylation status of each probe are indicated. Rabbit reticulocyte lysate (retic) was used as a negative control for each probe. The binding abilities of mutant Z proteins to each probe were measured relative to wild-type Z, which was set at 100% for each probe. ND, nondetectable binding; +, present; −, absent. (C) The expression level of each in vitro-translated Z protein was analyzed by immunoblotting.
FIG. 3.
Z(S186T) binds almost exclusively to the methylated form of the Rp ZRE-2 site. Increasing amounts of methylated versus unmethylated ZRE-2 cold competitor oligoprobe were used to compete binding of Z(S186T) to a methylated 32P-labeled ZRE-2 probe. Z binding complexes were quantitated, and the percent Z binding (relative to the amount in the absence of specific competitor DNA, set at 100%) is indicated. +, present; −, absent; retic, rabbit reticulocyte lysate; NC, no competitor.
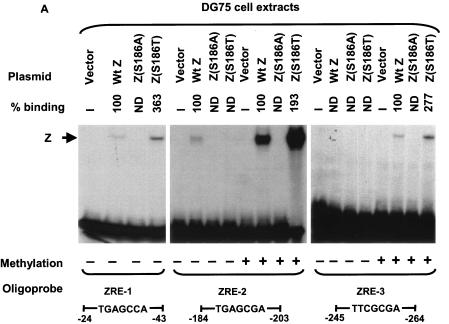
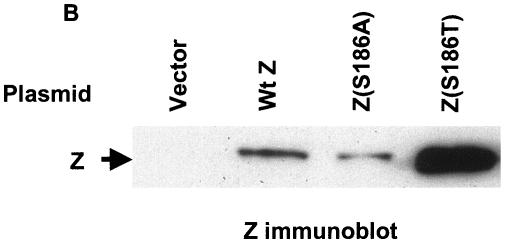
EMSA was also performed using whole-cell extracts of DG75 cells transfected with expression vectors for wild-type Z, Z(S186A), or Z(S186T) (Fig. 4A). When transfected into DG75 cells, the Z(S186A) mutant was even more impaired in its binding (relative to wild-type Z) than when expressed as an in vitro-translated protein. In DG75 cells, Z(S186A) bound extremely poorly to the unmethylated and methylated forms of all three Rp ZRE sites (Fig. 4A), although binding to the AP-1 site was observed (data not shown). In the case of the Z(S186T) mutant, although its expression level was much higher than wild-type Z in DG75 cells (Fig. 4B), Z(S186T) binding to the unmethylated form of ZRE-2 was almost undetectable, while this mutant clearly bound to the methylated form of this site (Fig. 4A). When the expression levels of wild-type Z and Z(S186T) in DG75 cells were normalized, the two proteins bound to methylated Rp ZRE-2 with similar efficiency (Fig. 4C). The partial defects of in vitro-translated Z(S186T) binding to the ZRE-1 (Fig. 2A) and ZRE-3 (Fig. 2B) sites were not apparent when this mutant was expressed in DG75 cells at a high level (Fig. 4A).
FIG. 4.
Comparison of wild-type Z, Z(S186A), and Z(S186T) binding in transfected DG75 cells extracts. (A) EMSA was performed using either unmethylated or methylated (as indicated) 32P-labeled oligonucleotide probes containing ZRE-1, ZRE-2, or ZRE-3 motifs and whole-cell extracts harvested from DG75 cells transfected with vector DNA or wild-type Z (Wt Z), Z(S186A), or Z(S186T). The binding abilities of mutant Z proteins to each probe were measured relative to wild-type Z, which was set at 100% for each probe. (B) Immunoblot analysis of the whole-cell extracts made from DG75 cells transfected with an empty vector (Vector) or a plasmid expressing wild-type Z, Z(S186A), or Z(S186T) was performed using a monoclonal antibody directed against Z. (C) EMSA was performed using DG75 whole-cell extracts containing normalized levels of wild-type Z and Z(S186T) (as shown in the immunoblot) and 32P-labeled oligonucleotide probe containing methylated ZRE-2. ND, nondetectable binding; +, present; −, absent.
Z residue 186 plays a critical role in activating methylated Rp.
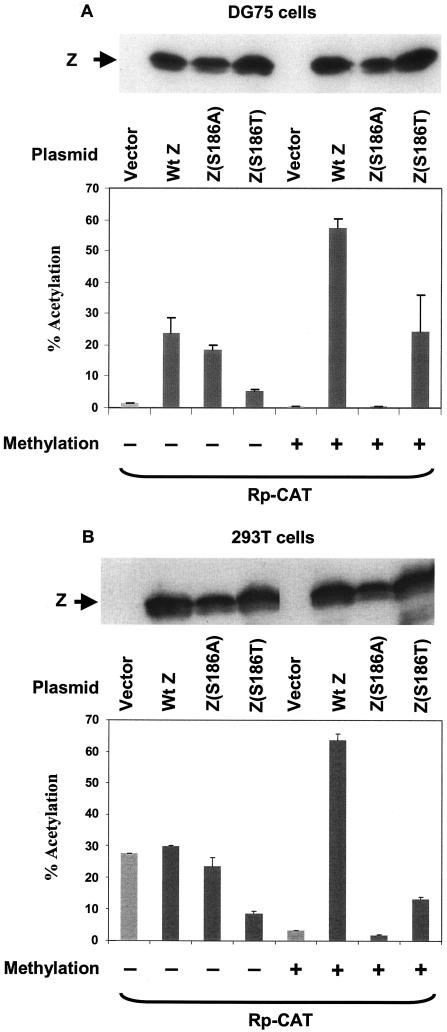
To determine whether Z serine residue 186 is required for activation of methylated Rp, we compared the ability of wild-type Z, the Z(S186A) mutant, and the Z(S186T) mutant to activate the methylated or unmethylated Rp-CAT construct in DG75 (Fig. 5A) or 293T (Fig. 5B) cells. Prior to transfection, the CAT vectors were methylated (or mock methylated) with SssI. Methylation was confirmed by resistance to HpaII cutting (data not shown). The mock-methylated or methylated Rp-CAT construct was cotransfected with a control vector or vectors expressing either wild-type Z or one of the Z mutants.
FIG. 5.
Mutation of Z residue 186 affects its ability to activate methylated Rp in CAT reporter gene assays. A vector containing the CAT gene linked to the BRLF1 promoter (Rp-CAT), either methylated or mock-methylated in vitro, was transfected into DG75 cells (A) or 293T cells (B) in combination with either empty vector (vector) or vector expressing wild-type Z (Wt Z), Z(S186A), or Z(S186T), as indicated. CAT activity was measured as the percentage of acetylation. The methylation status of each promoter construct is indicated. +, present; −, absent.
We have previously shown that all three Rp ZRE sites are important for activation of the unmethylated BRLF1 promoter by wild-type Z, whereas only the ZRE-2 and ZRE-3 sites (which can be methylated) are required for activation of the methylated promoter (6). Consistent with its inability to bind to either the methylated ZRE-2 site or the methylated ZRE-3 site in vivo (Fig. 4A), the Z(S186A) mutant was unable to activate the methylated form of Rp-CAT in either DG75 cells (Fig. 5A) or 293T cells (Fig. 5B). Somewhat surprisingly, given its poor binding in vivo to each of the unmethylated Rp ZRE sites (Fig. 4A), the Z(S186A) mutant could activate the unmethylated form of Rp-CAT in DG75 cells (Fig. 5A). This result suggests that Z(S186A) may activate the unmethylated BRLF1 promoter in plasmid-based reporter gene assays through a mechanism not involving direct DNA binding.
The Z(S186T) mutant, which binds to the methylated but not unmethylated form of ZRE-2 and is partially defective for binding to the ZRE-1 and ZRE-3 sites, was defective for activation of the unmethylated form of Rp-CAT (in DG75 cells) (Fig. 5A) and partially defective for activation of the methylated form of Rp-CAT in DG75 (Fig. 5A) and 293T cells (Fig. 5B). These results suggest that Z residue 186 is important for activating methylated Rp in vivo.
Z residue 186 regulates the ability of Z to induce lytic EBV gene transcription in latently infected cells with a methylated viral genome.
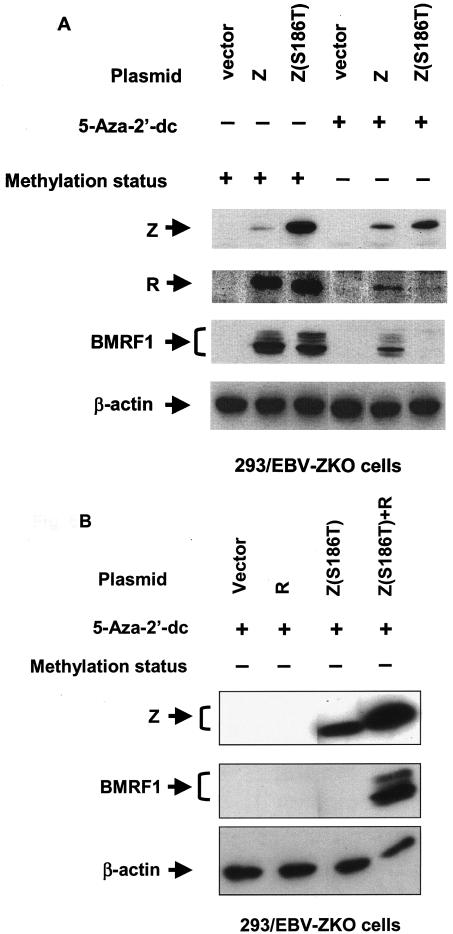
To determine whether the methylation status of the latent EBV genome differentially affects the ability of wild-type Z, Z(S186A), and Z(S186T) to disrupt viral latency, 293 cells latently infected with BZLF1-deleted EBV (293/EBV-ZKO) (20) were treated with the DNA demethylating agent 5-aza-2′-dc prior to transfection with a control vector or expression vectors for wild-type Z, Z(S186A), or Z(S186T). The sensitivity of the Rp region to cutting by the methylation-sensitive HpaII restriction enzyme was examined by Southern blot analysis before and after 5-aza-2′-dc treatment; the results confirmed that the Rp region of the EBV genome was efficiently demethylated following treatment (6). Cellular extracts were analyzed 2 days posttransfection for the expression of Z, R, BMRF1 (an early viral protein), or β-actin by immunoblotting.
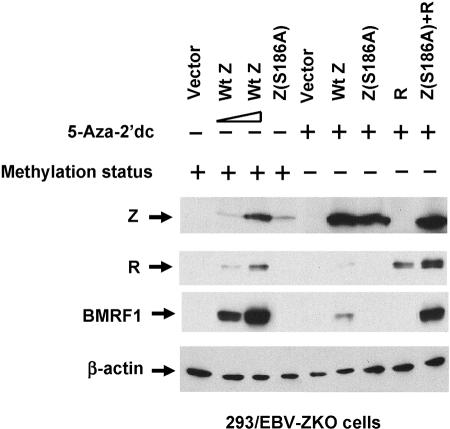
As previously reported (6), prior demethylation of the viral genome significantly reduced, but did not completely abolish, the ability of wild-type Z to activate transcription of either the immediate-early (IE) BRLF1 gene or the early BMRF1 gene (Fig. 6A). In contrast, the Z(S186T) mutant, which binds only to the methylated form of the Rp ZRE-2 motif, only induced lytic EBV gene transcription from the methylated viral genome (Fig. 6A). Furthermore, the defect in the Z(S186T) mutant was limited to its inability to activate the Rp, since the defect in activating BMRF1 transcription from the unmethylated viral genome could be rescued by cotransfection with an R expression vector (Fig. 6B). The Z(S186A) mutant, as expected, could not induce lytic gene transcription from either the methylated or demethylated viral genome (Fig. 7), although in both cases this defect was rescued by cotransfection with the R expression vector (Fig. 7 and data not shown). These results suggest that the serine residue at position 186 in Z plays an important role in inducing lytic viral infection from both the unmethylated and methylated viral genomes.
FIG. 6.
Changing Z residue 186 from serine to threonine affects the ability of Z to induce lytic EBV infection in a methylation-dependent manner. (A) 293/EBV-ZKO cells (20) were treated with 5-aza-2′-dc or not treated, as shown, prior to transfection with either empty vector (Vector) or a vector expressing wild-type Z (Wt Z) or Z(S186T). The methylation status of the EBV genome after the 5-aza-2′-dc treatment is indicated. Immunoblots were performed on cell extracts 2 days after transfection with monoclonal antibody directed against Z, BMRF1 (an early lytic EBV protein), R, or β-actin, as indicated. (B) 293/EBV-ZKO cells pretreated with 5-aza-2′-dc were transfected with empty vector or a vector expressing R, Z(S186T), or both R and Z(S186T). The methylation status of the EBV genome is indicated. Immunoblotting was performed for Z, BMRF1, or β-actin, as indicated. +, present; −, absent.
FIG. 7.
The Z(S186A) mutant is defective for inducing lytic EBV gene expression regardless of the methylation state of the EBV genome. 293/EBV-ZKO cells (untreated or pretreated with 5-aza-2′-dc, as indicated) were transfected with empty vector (vector) or a vector expressing wild-type Z (Wt Z, 0.5 μg in lane 2 and 1 μg in lane 3), Z(S186A), R, or both Z(S186A) and R, and immunoblots were performed for Z, R, BMRF1, or β-actin, as indicated. The methylation status of the EBV genome is indicated. +, present; −, absent.
Phosphorylation of the serine residue at position 186 is not required for the ability of Z to bind preferentially to the methylated Rp ZRE-2.
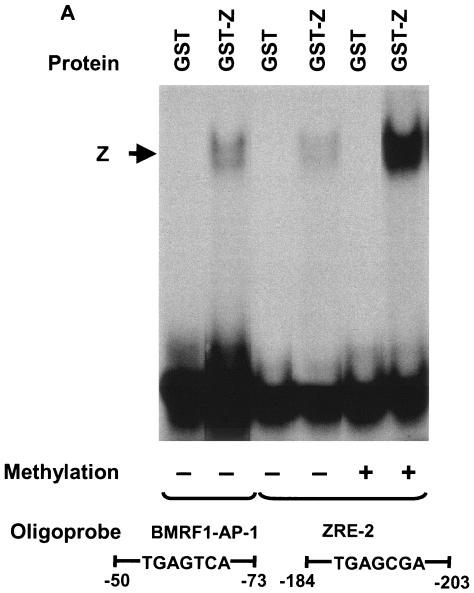
Serine residue 186 is phosphorylated by protein kinase C (PKC) in vitro (4) and thus could potentially be phosphorylated in vivo (as well as in reticulocyte extracts). Previous results by another group showed that in vitro phosphorylation of Z protein by PKC decreased its binding to unmethylated ZRE sites (4). To examine whether Z phosphorylated at serine 186 behaves differently than the unphosphorylated protein in regard to its binding to methylated versus unmethylated ZRE motifs, we performed several different experiments. First, we compared the binding of a bacterially expressed and purified protein containing Z linked in frame to GST (GST-Z) to the methylated versus unmethylated forms of ZRE-2. The GST-Z protein clearly bound more efficiently to the methylated form of the ZRE-2 site (Fig. 8A). Since this bacterially derived protein presumably cannot be phosphorylated, this result indicates that the unphosphorylated form of Z binds more efficiently to the methylated versus unmethylated form of the Rp ZRE-2 site.
FIG. 8.
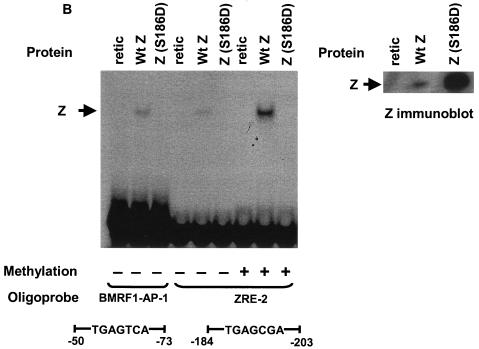
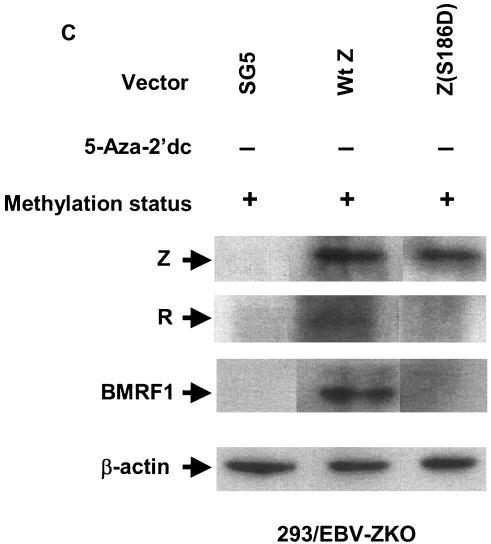
Phosphorylation of Z serine residue 186 is not required for Z binding to methylated Rp ZRE-2. (A) EMSA was performed using bacterially expressed purified GST-Z and 32P-labeled oligonucleotide probes containing the AP-1 site in the BMRF1 promoter and either unmethylated or methylated (as indicated) 32P-labeled oligonucleotide probes containing ZRE-2. (B) EMSA was performed using in vitro-translated wild-type Z (Wt Z) and Z(S186D) (as shown) and 32P-labeled oligonucleotide probes containing the AP-1 site in the BMRF1 promoter and either unmethylated or methylated (as indicated) 32P-labeled oligonucleotide probes containing ZRE-2. (C) 293/EBV-ZKO cells (20) were transfected with either control vector (Vector) or a vector expressing wild-type Z (Wt Z) or Z(S186D). Immunoblots were performed on cell extracts 2 days after transfection with monoclonal antibody directed against Z, R, BMRF1, or β-actin, as indicated. +, present; −, absent; retic, rabbit reticulocyte lysate.
Second, we constructed a Z mutant, Z(S186D), in which the serine residue at position 186 was switched to an aspartic acid residue to mimic the phosphorylated form of Z. Interestingly, the in vitro-translated Z(S186D) mutant did not bind detectably to either the AP-1 site in the BMRF1 promoter or the methylated or unmethylated forms of the Rp ZRE-2 site (Fig. 8B). Consistent with its inability to bind to DNA, the Z(S186D) mutant was also unable to induce R and BMRF1 expression in 293/EBV-ZKO cells (Fig. 8C). This suggests that phosphorylation of Z at position 186 is certainly not required for its ability to bind preferentially to the methylated Rp ZRE-2 site and may instead globally inhibit Z binding to multiple different motifs.
DSCUSSION
DNA methylation plays an important role in regulation of both viral and cellular gene expression. Most models regarding the effect of DNA methylation on gene transcription propose that gene methylation leads to gene silencing either by preventing the binding of transcription factors to promoters or by imposing a restrictive chromatin state. Nevertheless, we recently demonstrated that the EBV Z protein, which mediates the switch between latent and lytic EBV infection, actually requires its immediate downstream target promoter, Rp, to be methylated to activate this promoter efficiently (6). We identified two ZRE sites in Rp (ZRE-2 and ZRE-3) that can be methylated through a CpG motif and showed that Z binding to these atypical ZRE sites, as well as Z activation of Rp, was surprisingly enhanced by methylation. Most importantly, Z induced lytic EBV gene transcription in latently infected cells more efficiently when the viral genome was methylated than when it was demethylated. In contrast, the ability of the R IE protein to disrupt viral latency in 293 cells latently infected with BRLF1-deleted EBV (293/EBV-RKO) cells was enhanced by prior demethylation of the viral genome (6).
In this study, we have examined the effect of serine residue 186 in the Z DNA-binding domain on the ability of Z to efficiently bind to and activate the methylated versus unmethylated forms of Rp. As the crystal structure of a Fos-Jun heterodimer binding to a consensus AP-1 site suggested that Z residue 186 would directly contact the methylated cytosine in the Rp ZRE-2 motif (TGAGCGA) (25), we hypothesized that this residue might be important for mediating Z activation of methylated Rp. Our results indicate that a Z mutant in which residue 186 is switched from a serine to threonine, Z(S186T), binds to the methylated form but not to the unmethylated form of the Rp ZRE-2 site in vitro and induces lytic EBV transcription from the methylated viral genome but not from the demethylated viral genome in vivo. The inability of this mutant to activate early lytic EBV gene transcription from the demethylated viral genome was rescued by R, suggesting that the Z(S186T) defect primarily reflects its inability to induce transcription from unmethylated Rp. In contrast, the Z(S186A) mutant, although able to bind efficiently to the consensus AP-1 site, could not bind efficiently to any of the three Rp ZRE sites in either the methylated or unmethylated form. Consistent with this binding defect, the Z(S186A) mutant could not activate transcription from either the methylated or demethylated form of the EBV viral genome.
The previous description of the Z(S186T) mutant as being close to wild-type Z in its ability to induce lytic EBV infection from the intact viral genome in Raji cells (24) is not particularly surprising given our finding here that Rp is highly methylated in Raji cells (as well as the other cell lines examined). Another group recently examined the methylation state of the BRLF1 and BZLF1 promoters in a series of nasopharyngeal carcinomas (11). Similar to our results here, Rp was found to be highly methylated in latently infected tumors. Whether the Z(S186T) mutant would have a more obvious defect in situations such as primary lytic EBV infection in which the viral genome does not become methylated is as yet unknown.
Serine and threonine are very similar polar amino acids containing a hydroxyl group. This might explain the fact that Z(S186T) retains the ability to bind to and activate methylated Rp and induce lytic EBV infection from the methylated viral genome. On the other hand, the replacement of serine 186 with alanine, a nonpolar amino acid, significantly decreases Z binding to all three Rp ZRE sites, regardless of the ZRE site methylation status, and abolishes the ability of Z to induce lytic gene expression from the intact viral genome regardless of the viral methylation state.
One surprising aspect of our results was the lack of correlation between the ability of the Z(S186A) mutant to activate the unmethylated Rp-CAT vector versus its ability to activate BRLF1 transcription from the unmethylated viral genome. The inability of this mutant to activate the BRLF1 transcription from the intact viral genome (in either the methylated or unmethylated state) is consistent with its inability to bind significantly to any of the three Rp ZRE sites in vivo. The unresolved issue, however, is how this mutant activates the unmethylated Rp-CAT vector in reporter gene assays. It is possible that very weak binding of this mutant is sufficient to activate the unmethylated promoter in the context of naked DNA but not in the chromatin context of the intact viral genome. Alternatively, we favor the hypothesis that Z(S186A) activates the unmethylated Rp-CAT construct through an indirect mechanism not requiring direct protein binding to the Rp ZRE sites. Consistent with this, another Z mutant that is globally defective in DNA binding (due to an alteration of residues 178, 179, and 180) was reported to activate certain promoters (including the BZLF1 and BRLF1 promoters) through an indirect mechanism (22). However, this alternative mechanism for Z transcriptional effects is clearly not sufficient to activate lytic gene transcription in the context of the intact viral genome.
Phosphorylation is a common mechanism for regulating the function of proteins, and Z can be phosphorylated in vitro at serine 186 by PKC (4). We speculated that phosphorylation of Z residue 186 in vivo (which could occur on either a serine or threonine) may be required for Z binding to methylated Rp ZRE sites, although PKC-mediated phosphorylation of GST-Z was previously reported by another group to inhibit its binding in vitro to unmethylated ZRE sites (4). However, our results did not support this hypothesis. Bacterially expressed and purified GST-fused Z protein (GST-Z), which is not phosphorylated, also bound preferentially to the methylated form of the Rp ZRE-2 motif. Furthermore, a mutant form of Z in which serine 186 was replaced with aspartic acid to mimic a phosphorylated residue did not bind detectably to either the AP-1 site in the BMRF1 promoter or the unmethylated or methylated form of the Rp ZRE-2 site. Consistent with its DNA-binding defect, Z(S186D) was also unable to induce R and BMRF1 expression in 293/EBV-ZKO cells. Thus, phosphorylation of Z at position 186 is certainly not a prerequisite for its ability to bind preferentially to the methylated form of Rp ZRE-2 and may instead inhibit Z DNA binding globally. Furthermore, the majority of evidence suggests that Z is not constitutively phosphorylated at position 186 in vivo, nor is PKC required for Z disruption of viral latency (17). As there is some evidence suggesting that Z binds to DNA sites using more than one protein conformation (17), another potential explanation for the binding effects of the Z(S186A) and Z(S186T) mutations is that different Z conformations are required for binding to the methylated versus unmethylated forms of the Rp ZRE-2 site and that only wild-type Z can assume the different conformations required to bind to both forms of this site.
Methylation of the EBV genome may protect the host cell by shutting off transcription of potentially transforming latency genes (in particular, LMP1) expressed in type II and III latency and converting the virus to the most stringent, nontransforming, form of latency, type I (42, 45). Nevertheless, EBV genome methylation also promotes persistence of EBV by inhibiting expression of viral latency proteins that are recognized by cytotoxic T cells (42, 45). In addition, methylation of the EBV genome presumably inhibits the well-known ability of unmethylated CpG motifs to activate Toll-like receptor 9 (3). Interestingly, LMP1 was recently shown to activate expression of cellular methyl transferases (53), suggesting that EBV actually promotes its own genome methylation. Although methylation may be beneficial to the virus in terms of promoting viral latency and evading the host immune system, if EBV could not efficiently convert back to the lytic form of infection from a methylated viral genome under appropriate circumstances, it would not have successfully infected the great majority of the human population. The ability of the EBV lytic switch protein Z to activate the methylated form of the IE BRLF1 promoter likely contributes to the long-term success of this virus as both a latent and lytic pathogen.
Acknowledgments
This work was supported by NIH grants P01-CA19014 and R01 CA58853
REFERENCES
- 1.Adamson, A., and S. C. Kenney. 1998. Rescue of the Epstein-Barr virus BZLF1 mutant, Z(S186A), early gene activation defect by the BRLF1 gene product. Virology 251:187-197. [DOI] [PubMed] [Google Scholar]
- 2.Altiok, E., J. Minarovits, L. F. Hu, B. Contreras-Brodin, G. Klein, and I. Ernberg. 1992. Host-cell-phenotype-dependent control of the BCR2/BWR1 promoter complex regulates the expression of Epstein-Barr virus nuclear antigens 2-6. Proc. Natl. Acad. Sci. USA 89:905-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashkar, A. A., and K. L. Rosenthal. 2002. Toll-like receptor 9, CpG DNA and innate immunity. Curr. Mol. Med. 2:545-556. [DOI] [PubMed] [Google Scholar]
- 4.Baumann, M., M. Mischak, S. Dammeier, W. Kolch, O. Gires, D. Pich, R. Zeidler, H.-J. Delecluse, and W. Hammerschmidt. 1998. Activation of the Epstein-Barr virus transcription factor BZLF1 by 12-O-tetradecanoylphorbol-13-acetate-induced phosphorylation. J. Virol. 72:8105-8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bednarik, D. P., J. A. Cook, and P. M. Pitha. 1990. Inactivation of the HIV LTR by DNA CpG methylation: evidence for a role in latency. EMBO J. 9:1157-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhende, P. M., and S. C. Kenney. 2004. The EBV lytic switch protein, Z, preferentially binds to and activates the methylated viral genome. Nat. Genet. 36:1099-1104. [DOI] [PubMed] [Google Scholar]
- 7.Bird, A. P., and A. P. Wolffe. 1999. Methylation-induced repression-belts, braces and chromatin. Cell 99:451-454. [DOI] [PubMed] [Google Scholar]
- 8.Bogedain, C., P. Alliger, F. Schwarzmann, M. Marschall, H. Wolf, and W. Jilg. 1994. Different activation of Epstein-Barr virus immediate-early and early genes in Burkitt lymphoma cells and lymphoblastoid cell lines. J. Virol. 68:1200-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyes, J., and A. P. Bird. 1992. Repression of genes by DNA methylation depends on CpG density and promoter strength: evidence for involvement of a methyl-CpG binding protein. EMBO J. 11:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buschhausen, G., B. Wittig, M. Graessmann, and A. Graessmann. 1987. Chromatin structure is required to block transcription of the methylated herpes simplex virus thymidine kinase gene. Proc. Natl. Acad. Sci. USA 84:1177-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan, A. T. C., Q. Tao, K. D. Robertson, I. W. Flinn, R. B. Mann, B. Klencke, W. H. Kwan, T. W. Leung, P. J. Johnson, and R. F. Ambinder. 2004. Azacitidine induces demethylation of the Epstein-Barr virus genome in tumors. J. Clin. Oncol. 22:1373-1381. [DOI] [PubMed] [Google Scholar]
- 12.Chang, Y., D. Dong, G. Hayward, and S. D. Hayward. 1990. The Epstein-Barr virus Zta transactivator: a member of the bZIP family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J. Virol. 64:3358-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chevallier-Greco, A., E. Manet, P. Chavrier, C. Mosnier, J. Daillie, and A. Sergeant. 1986. Both Epstein-Barr virus (EBV) encoded transacting factors, EB1 and EB2, are required to activate transcription from an early EBV promoter. EMBO J. 5:3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark, S. J., J. Harrison, and P. L. Molloy. 1997. Sp1 binding is inhibited by (m)Cp(m)CpG methylation. Gene 195:67-71. [DOI] [PubMed] [Google Scholar]
- 15.Countryman, J., and G. Miller. 1985. Activation of expression of latent Epstein-Barr virus after gene transfer with a small cloned fragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA 82:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doerfler, W. 1983. DNA methylation and gene activity. Annu. Rev. Biochem. 5:93-124. [DOI] [PubMed] [Google Scholar]
- 17.El-Guindy, A. S., L. Heston, Y. Endo, M.-S. Cho, and G. Miller. 2002. Disruption of Epstein-Barr virus latency in the absence of phosphorylation of zebra by protein kinase C. J. Virol. 76:11199-11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernberg, I., K. Falk, J. Minarovits, P. Busson, T. Tursz, M. G. Masucci, and G. Klein. 1989. The role of methylation in the phenotype-dependent modulation of Epstein-Barr nuclear antigen 2 and latent membrane protein genes in cells latently infected with Epstein-Barr virus. J. Gen. Virol. 70:2989-3002. [DOI] [PubMed] [Google Scholar]
- 19.Farrell, P., D. Rowe, C. Rooney, and T. Kouzarides. 1989. Epstein-Barr virus BZLF1 transactivator specifically binds to consensus Ap1 site and is related to c-fos. EMBO J. 8:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flemington, E., and S. Speck. 1991. Evidence for coiled-coil dimer formation by an Epstein-Barr virus transactivator that lacks a heptad repeat of leucine residues. Proc. Natl. Acad. Sci. USA 87:9459-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flemington, E. K., J. P. Lytle, C. Cayrol, A. M. Borras, and S. H. Speck. 1994. DNA-binding-defective mutants of the Epstein-Barr virus lytic switch activator Zta transactivate with altered specificities. Mol. Cell. Biol. 14:3041-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francis, A. L., L. Gradoville, and G. Miller. 1997. Alteration of a single serine in the basic domain of the Epstein-Barr virus ZEBRA protein separates its functions of transcriptional activation and disruption of latency. J. Virol. 71:3054-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis, A. L., T. Ragoczy, L. Gradoville, L. Heston, A. El-Guindy, Y. Endo, and G. Miller. 1999. Amino acid substitutions reveal distinct functions of serine 186 of the ZEBRA protein in activation of early lytic cycle genes and synergy with the Epstein-Barr virus R transactivator. J. Virol. 73:4543-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glover, J. N. M., and S. C. Harrison. 1995. Crystal structure of the heterodimeric bZIP transcription factor c-Fos-c-Jun bound to DNA. Nature 373:257-261. [DOI] [PubMed] [Google Scholar]
- 26.Hendrich, B., and A. P. Bird. 1998. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol. Cell. Biol. 18:6538-6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman, J. G., J. R. Graff, S. Myohanen, B. D. Nelkin, and S. B. Baylin. 1996. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc. Natl. Acad. Sci. USA 93:9821-9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu, L. F., J. Minarovits, S. L. Cao, B. Contreras-Salazar, L. Rymo, K. Falk, G. Klein, and I. Ernberg. 1991. Variable expression of latent membrane protein in nasopharyngeal carcinoma can be related to methylation status of the Epstein-Barr virus BNLF-1 5′-flanking region. J. Virol. 65:1558-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iguchi-Ariga, S. M. M., and W. Schaffner. 1989. CpG methylation of the cAMP-responsive enhancer/promoter sequence TGACGTCA abolishes specific factor binding as well as transcriptional activation. Genes Dev. 3:612-619. [DOI] [PubMed] [Google Scholar]
- 30.Jansson, A., M. Masucci, and L. Rymo. 1992. Methylation of discrete sites within the enhancer region regulates the activity of the Epstein-Barr virus BamHI W promoter in Burkitt lymphoma lines. J. Virol. 66:62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kass, S. U., J. P. Goddard, and R. L. Adams. 1993. Inactive chromatin spreads from a focus of methylation. Mol. Cell. Biol. 13:7372-7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kass, S. U., N. Landsberger, and A. P. Wolffe. 1997. DNA methylation directs a time-dependent repression of transcription initiation. Curr. Biol. 7:157-165. [DOI] [PubMed] [Google Scholar]
- 33.Kenney, S., J. Kamine, E. Holley-Guthrie, J. C. Lin, E. C. Mar, and J. Pagano. 1989. The Epstein-Barr virus (EBV) BZLF1 immediate-early gene product differentially affects latent versus productive EBV promoters. J. Virol. 63:1729-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieff, E. 1996. Epstein-Barr virus and its replication, p. 2343-2396. In B. N. Fields et al. (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 35.Kovesdi, I., R. Reichel, and J. R. Nevins. 1987. Role of an adenovirus E2 promoter binding factor in E1A-mediated coordinate gene control. Proc. Natl. Acad. Sci. USA 84:2180-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis, J. D., R. R. Meehan, W. J. Henzel, I. Maurer-Fogy, P. Jeppesen, F. Klein, and A. Bird. 1992. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 69:905-914. [DOI] [PubMed] [Google Scholar]
- 37.Li, Q. X., L. S. Young, G. Niedobitek, C. W. Dawson, M. Birkenbach, F. Wang, and A. B. Rickinson. 1992. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature 356:347-350. [DOI] [PubMed] [Google Scholar]
- 38.Masucci, M. G., B. Contreras-Salazar, E. Ragnar, K. Falk, J. Minarovits, I. Ernberg, and G. Klein. 1989. 5-Azacytidine up regulates the expression of Epstein-Barr virus nuclear antigen 2 (EBNA-2) through EBNA-6 and latent membrane protein in the Burkitt's lymphoma line rael. J. Virol. 63:3135-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minarovits, J., S. Minarovits-Kormuta, B. Ehlin-Henriksson, K. Falk, G. Klein, and I. Ernberg. 1991. Host cell phenotype-dependent methylation patterns of Epstein-Barr virus DNA. J. Gen. Virol. 72:1591-1599. [DOI] [PubMed] [Google Scholar]
- 40.Ohtani-Fujita, N., T. Fujita, A. Aoike, N. E. Osifchin, P. D. Robbins, and T. Sakai. 1993. CpG methylation inactivates the promoter activity of the human retinoblastoma tumor-suppressor gene. Oncogene 8:1063-1067. [PubMed] [Google Scholar]
- 41.Packham, G., A. Economou, C. M. Rooney, D. Rowe, and P. J. Farrell. 1990. Structure and function of the Epstein-Barr virus BZLF1 protein. J. Virol. 64:2110-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulson, E. J., and S. H. Speck. 1999. Differential methylation of Epstein-Barr virus latency promoters facilitates viral persistence in healthy seropositive individuals. J. Virol. 73:9959-9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinlivan, E. B., E. A. Holley-Guthrie, M. Norris, D. Gutch, S. L. Bachenheimer, and S. C. Kenney. 1993. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 21:1999-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. N. Fields et al. (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 45.Robertson, K. D., and R. F. Ambinder. 1997. Methylation of the Epstein-Barr virus genome in normal lymphocytes. Blood 90:4480-4484. [PubMed] [Google Scholar]
- 46.Rooney, C., N. Taylor, J. Countryman, H. Jenson, J. Kolman, and G. Miller. 1988. Genome rearrangements activate the Epstein-Barr virus gene whose product disrupts latency. Proc. Natl. Acad. Sci. USA 85:9801-9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rooney, C., D. Rowe, T. Ragot, and P. Farrell. 1989. The spliced BZLF1 gene of Epstein-Barr virus transactivates an early EBV promoter and induces the virus productive cycle. J. Virol. 63:3109-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saggioro, D., M. Forino, and L. Chieco-Bianchi. 1991. Transcriptional block of HTLV-I LTR by sequence-specific methylation. Virology 182:68-75. [DOI] [PubMed] [Google Scholar]
- 49.Schulze-Forster, K., F. Gotz, H. Wagner, H. Kroger, and D. Simon. 1990. Transcription of HIV1 is inhibited by DNA methylation. Biochem. Biophys. Res. Commun. 168:141-147. [DOI] [PubMed] [Google Scholar]
- 50.Sixby, J. W., J. G. Nedrud, N. Raab-Traub, R. A. Hanes, and J. S. Pagano. 1984. Epstein-Barr virus replication in oropharyngeal epithelial cells. N. Engl. J. Med. 310:1225-1230. [DOI] [PubMed] [Google Scholar]
- 51.Takada, K., N. Shimizu, S. Sakuma, and Y. Ono. 1986. Transactivation of the latent Epstein-Barr virus genome after transfection of the EBV DNA fragment. J. Virol. 57:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tao, Q., K. D. Robertson, A. Manns, A. Hildesheim, and R. F. Ambinder. 1998. The Epstein-Barr virus major latent promoter Qp is constitutively active, hypomethylated, and methylation sensitive. J. Virol. 72:7075-7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai, C.-N., C.-L. Tsai, K.-P. Tse, H.-Y. Chang, and Y.-S. Chang. 2002. The Epstein-Barr virus oncogene product, latent membrane protein 1, induces the down-regulation of E-cadherin gene expression via activation of DNA methyltransferases. Proc. Natl. Acad. Sci. USA 99:10084-10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watt, F., and P. L. Molloy. 1988. Cytosine methylation prevents binding to DNA of a HeLa cell transcription factor required for optimal expression of the adenovirus major late promoter. Genes Dev. 2:1136-1143. [DOI] [PubMed] [Google Scholar]
- 55.Zalani, S. E., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zur Hausen, H., H. Schulte-Holthausen, G. Klein, W. Henle, G. Henle, P. Clifford, and L. Santesson. 1970. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature 228:1956-1958. [DOI] [PubMed] [Google Scholar]