Abstract
Abstract
This is the first consensus statement of the Joint Committee on Renal Denervation of the Japanese Society of Hypertension (JSH)/Japanese Association of Cardiovascular Intervention and Therapeutics (CVIT)/Japanese Circulation Society (JCS). The consensus is that the indication for renal denervation (RDN) is resistant hypertension or “conditioned” uncontrolled hypertension, with high office and out-of-office blood pressure (BP) readings despite appropriate lifestyle modification and antihypertensive drug therapy. “Conditioned” uncontrolled hypertension is defined as having one of the following: 1) inability to up-titrate antihypertensive medication due to side effects, the presence of complications, or reduced quality of life. This includes patients who are intolerant of antihypertensive drugs; or 2) comorbidity at high cardiovascular risk due to increased sympathetic nerve activity, such as orthostatic hypertension, morning hypertension, nocturnal hypertension, or sleep apnea (unable to use continuous positive airway pressure), atrial fibrillation, ventricular arrythmia, or heart failure. RDN should be performed by the multidisciplinary Hypertension Renal Denervation Treatment (HRT) team, led by specialists in hypertension, cardiovascular intervention and cardiology, in specialized centers validated by JSH, CVIT, and JCS. The HRT team reviews lifestyle modifications and medication, and the patient profile, then determines the presence of an indication of RDN based on shared decision making with each patient. Once approval for real-world clinical use in Japan, however, the joint RDN committee will update the indication and treatment implementation guidance as appropriate (annually if necessary) based on future real-world evidence.
Graphical Abstract
Keywords: Hypertension, Renal denervation, Resistant hypertension, Consensus statement
Introduction
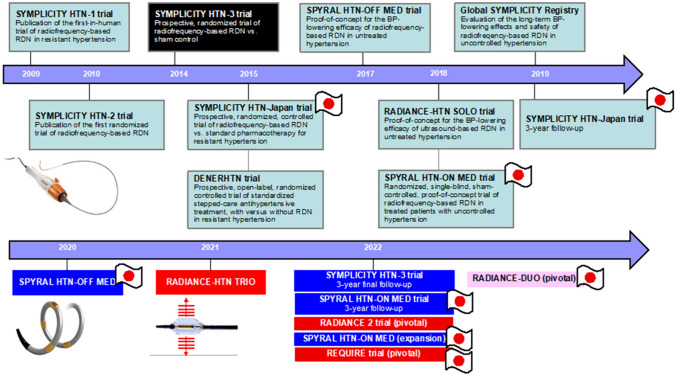
Renal denervation (RDN) is an antihypertensive treatment that has a novel mechanism of action, acting on the central nervous system by denervating sympathetic afferent pathways [1–3]. The first “proof-of-principle” clinical trial of transcatheter RDN showed that treatment was associated with a marked reduction in blood pressure (BP) in patients with resistant hypertension [4]. While the results of the first pivotal trial of the first generation of radiofrequency-based RDN (SYMPLICITY HTN-2) were positive, this was an open-label trial with no sham control group [5]. Subsequently, the first sham-controlled trial of radiofrequency RDN, SYMPLICITY HTN-3, failed to document a significant difference in systolic BP (SBP) reduction between RDN and sham groups at 6 months after the procedure in patients with resistant hypertension [6]. Since then, there have been many other sham-controlled trials of both the second generation of radiofrequency- and ultrasound-based RDN in a variety of hypertensive patient populations (Fig. 1) [7–12]. Many of these have reported positive findings, with significantly greater reductions in BP in the RDN versus control group [7–9, 11]. Japan contributed to several of the key clinical trials, both with [8, 10, 12] and without [13, 14] a sham control group.
Fig. 1.
History of clinical trials investigating renal denervation (adapted and updated from Kario et al. Cardio Discov 2021;1:112–127)
Based on the available data from the above trials, the US Food and Drug administration approved (FDA) approved both the SYMPLICITY SPYRAL radiofrequency RDN system and the PARADISE ultrasound RDN system for the adjunctive treatment of hypertension in patients with hypertension for whom lifestyle modifications and antihypertensive drug therapy do not adequately control BP.
The 2023 European Society of Hypertension (ESH) guidelines make a class II recommendation for the use of RDN in patients with uncontrolled hypertension [15], and consensus statements about RDN have been published by several societies and working groups [16–19]. This article details the Joint Consensus Statement on Renal Denervation Therapy in Japan 2024, developed by the Japanese Society of Hypertension (JSH), the Japanese Association of Cardiovascular Intervention and Therapeutics (CVIT), and the Japanese Circulation Society (JCS).
Process and pre-procedure management
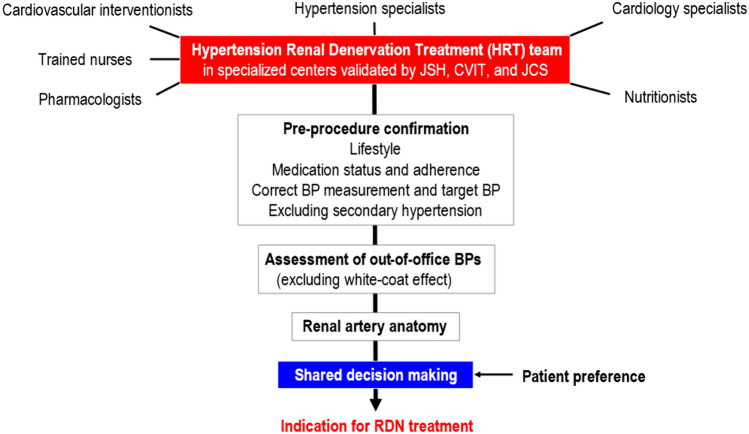
A multidisciplinary team approach to the management of individuals undergoing RDN is recommended [18–20]. In specialized Japanese centers validated by academic societies, the Hypertension Renal Denervation Treatment (HRT) team should consist of specialists in hypertension, cardiovascular intervention, and cardiology, along with nurses, pharmacologists, and nutritionists. This team should review lifestyle modifications, medication status and drug adherence. Out-of-office BP monitoring is required to exclude white-coat hypertension, and potential causes of secondary hypertension also need to be excluded. It is important to ensure that the patient’s renal artery anatomy is suitable for RDN. Also, a shared decision-making process between the HRT team and patients is important (Fig. 2).
Fig. 2.
Process to determine the presence of an indication for renal denervation. BP blood pressure, CVIT Japanese Association of Cardiovascular Intervention and Therapeutics, JCS Japanese circulation society, JSH Japanese society of hypertension, RDN renal denervation
Data from Germany suggest that around one-third of individuals with hypertension would choose RDN over lifelong antihypertensive drug therapy [21]. In Japan, 32% of the 2392 patients with hypertension surveyed said that they had a preference for RDN, compared to antihypertensive drug therapy. The preference rates were higher in males versus females, in younger versus older patients, in those with higher rather than lower blood pressure, in patients who were less adherent versus more adherent to antihypertensive drug therapy, and in those who did versus did not have antihypertensive drug-related side effects [22]. These data highlight the importance of taking patient preference into account when determining an indication for RDN.
According to the JSH 2019 hypertension guidelines [23], the following factors need to be carefully considered both before and after RDN:
Lifestyle: Check whether adequate lifestyle modifications such as salt reduction, weight loss, smoking cessation, appropriate exercise, adequate fluid intake, good sleep, and stress management are being implemented.
Medication adherence status: If an individual is found to have poor adherence to antihypertensive drug therapy, attention should be paid to whether a partnership with the patient is being established, and appropriate action should be taken based on the hypertension treatment guidelines and targeted to the cause(s) of nonadherence. Given that polypharmacy reduces medication adherence rates [24], consider switching to a higher dose of a single agent or (preferably) a single pill combination containing multiple antihypertensive agents with different mechanisms of action [25, 26].
Prescription of antihypertensive medications: Similar to the above point, antihypertensive drug therapy should be critically evaluated, with consideration given to titrating the dosage of current antihypertensive agents or adding antihypertensives that have a complementary mechanism of action. However, it is important to note that increasing the number of antihypertensive agents does not necessarily improve the rate of BP control [27].
Correct BP measurement, and target BP: Ensure that BP measurements, including out-of-office BP, are being taken correctly and consistently, as specified by current recommendations [15, 23, 28–34], and that the chosen target BP is appropriate.
Exclude causes of secondary hypertension: Careful examination should be performed to exclude the presence of secondary hypertension, including drug-induced hypertension (e.g., associated with the use of glycyrrhizinic acid, non-steroidal anti-inflammatory drugs, and health foods). Exclusion of possible primary aldosteronism is essential to identify individuals who will respond to RDN [10].
Indication
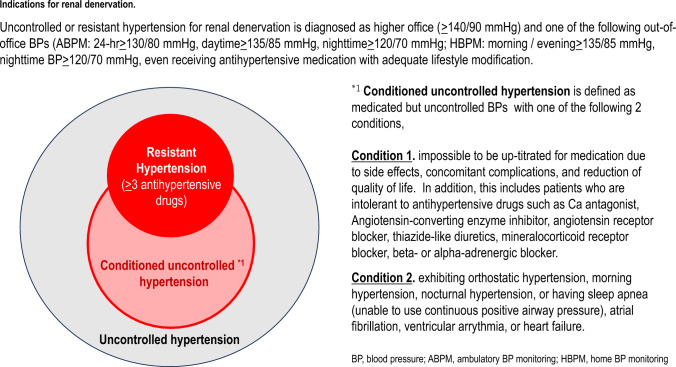
Transcatheter RDN is an effective BP-lowering treatment for resistant hypertension and “conditioned” uncontrolled hypertension despite appropriate treatment such as lifestyle modification and antihypertensive drug therapy (Graphical abstract).
After exclusion of the white-coat effect and secondary hypertension, especially primary aldosteronism, resistant or uncontrolled hypertension is defined as follows; office BP (≥ 140/90 mmHg) and/or out-of-office BP (24 h ambulatory BP ≥ 130/80 mmHg, daytime ambulatory BP ≥ 135/85 mmHg, nighttime ambulatory BP ≥ 120/70 mmHg, morning/evening home BP ≥ 135/85 mmHg, or nighttime home BP ≥ 120/70 mmHg) (Table 1) despite adequate lifestyle modification and treatment with maximum tolerated dosages of three or more antihypertensive agents from different classes, including a diuretic (except where there is a contraindication for use of diuretics) [23].
Table 1.
Abnormal thresholds office and out-of-office blood pressure
| Blood pressure metric | Threshold* |
|---|---|
| Office BP | SBP ≥ 140 mmHg or DBP ≥ 90 mmHg |
| Out-of-office BP | |
| Ambulatory BP | |
| 24 h | SBP ≥ 130 mmHg or DBP ≥ 80 mmHg |
| Daytime | SBP ≥ 135 mmHg or DBP ≥ 85 mmHg |
| Nighttime | SBP ≥ 120 mmHg or DBP ≥ 70 mmHg |
| Home BP | |
| Morning | SBP ≥ 135 mmHg or DBP ≥ 85 mmHg |
| Evening | SBP ≥ 135 mmHg or DBP ≥ 85 mmHg |
| Nighttime | SBP ≥ 120 mmHg or DBP ≥ 70 mmHg |
*Indication for renal denervation is office blood pressure above the defined threshold plus at least one out-of-office BP measurement above the defined thresholds
BP blood pressure, DBP diastolic blood pressure, SBP systolic blood pressure
“Conditioned” uncontrolled hypertension is defined as inability to up-titrate antihypertensive medication due to side effects, the presence of complications, or reduced quality of life despite adequate lifestyle modifications. This includes patients who are intolerant of antihypertensive drugs (i.e., calcium channel blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, thiazide-like diuretics, mineralocorticoid receptor blockers, and beta- or alpha-adrenergic blockers), or those with orthostatic hypertension [35, 36], morning hypertension [16, 37–41], nocturnal hypertension [16, 37–41], or sleep apnea (unable to use continuous positive airway pressure) [16, 37, 42, 43], atrial fibrillation [2, 44, 45], ventricular arrythmia [2, 46], or heart failure [2, 47]. Individuals with any of these conditions are at high cardiovascular risk due to increased sympathetic activity. However, at the present, the level of evidence for RDN in patients with these conditions is low because there is an absence of data from randomized controlled trials with a sham control group.
Individuals with renal aneurysm, renal artery stenosis or unsuitable renal artery anatomy (based on contrast-enhanced computed tomography evaluation), and those with an estimated glomerular filtration rate of < 30 ml/min/1.73m2 should not undergo RDN. In Japan, it has been estimated that a substantial proportion of individuals with hypertension would potentially be eligible for RDN [48].
Evidence for BP-lowering effects
The design and findings of seven sham-controlled clinical trials of RDN that had a sample size of > 100 patients are summarized in Table 2 [6–12]. All of the trials used ambulatory BP-monitoring metrics as the primary endpoint, apart from SYMPLICITY HTN-3 which used office SBP. The three OFF MED trials showed that RDN significantly reduced ambulatory BP compared with the sham control [7, 8, 11], indicating that RDN significantly lowered BP throughout the 24 h period. In the ON MED trials, RDN significantly reduced daytime or 24 h ambulatory BP from baseline (by 6.5 to 8.0 mmHg) [9, 10, 12]. However, in two of these trials (REQUIRE and SPYRAL HTN-ON MED expansion [10, 12]) there was also a significant reduction from baseline in ambulatory BP in the sham control group, meaning that there was no significant between-group difference in the BP reduction. In contrast, the RADIANCE TRIO study documented a significant difference in daytime BP reduction from baseline between the RDN and sham control groups [9].
Table 2.
Summary of seven sham-controlled, randomized trials of radiofrequency or ultrasound renal denervation that had a sample size of ≥ 100 patients
| Author, date | Study | Device | Population | Renal function, eGFR in ml/min/1.73m2 | Renal artery site treated | N (RDN/sham) | Primary endpoint | BP reduction, mmHg | Between-group p-value |
|---|---|---|---|---|---|---|---|---|---|
| Bhatt et al. [6] | SYMPLICITY HTN-3 | SYMPLICITY FLEX |
Resistant HTN (≥ 3 meds) |
≥ 45 | main | 364/171 | Office SBP at 6 months |
RDN: 14.1 Sham: 11.7 |
0.26 |
| Azizi et al. [7] | RADIANCE-HTN SOLO | PARADISE | Untreated HTN | ≥ 40 | main | 74/72 | Daytime SBP at 2 months |
RDN: 8.5 Sham: 2.2 |
< 0.001 |
| Bohm et al. [8] | SPYRAL HTN-OFF MED pivotal | SYMPLICITY SPYRAL | Untreated HTN | ≥ 45 | main + branch | 166/165 | 24 h SBP at 3 months |
RDN: 4.7 Sham: 0.6 |
< 0.001 |
| Azizi et al. [9] | RADIANCE-HTN TRIO | PARADISE |
Resistant HTN (≥ 3 meds) |
≥ 40 | main | 69/67 | Daytime SBP at 2 months |
RDN: 8.0 Sham: 3.0 |
0.022 |
| Kario et al. [10] | REQUIRE | PARADISE |
Resistant HTN (≥ 3 meds) |
≥ 40 | main | 69/67 | 24 h SBP at 3 months |
RDN: 6.6 Sham: 6.5 |
0.971 |
| Azizi et al. [11] | RADIANCE-II | PARADISE | Untreated HTN | ≥ 40 | main | 150/74 | Daytime SBP at 2 months |
RDN: 7.9 Sham: 1.8 |
< 0.00001 |
| Kandzari et al. [12] | SPYRAL HTN-ON MED expansion | SYMPLICITY SPYRAL |
Resistant/uncontrolled HTN (1–3 meds) |
≥ 45 | main + branch | 206/131 | 24 h SBP at 6 months |
RDN: 6.5 Sham: 4.5 |
0.12 |
BP blood pressure, HTN hypertension; meds antihypertensive medications, RDN renal denervation, SBP systolic blood pressure
An additional analysis of REQUIRE, exhibited no significant inter-group difference in the whole patients [10], showed that the sham group had poor adherence to antihypertensive drug therapy at baseline, which improved after the RDN procedure [49]. This likely contributed to the marked post-procedure reduction in BP in the sham group. A similar pharmacologic dilution of the RDN treatment effect was seen in the SPYRAL HTN-ON MED expansion, where there was also an unexpectedly large reduction in BP in the sham group [12]. The number of antihypertensives being used at 3 and 6 months after RDN was greater in the sham versus RDN group. In contrast, minimal difference in the changes in antihypertensive medication between treatment groups during follow-up meant that RDN was significantly more effective than sham control with respect to reductions in office and 24 h BP in the SPYRAL HTN-ON MED trial [50].
All currently published data, with the exception of the REQUIRE study, found a significant difference between RDN and sham control with respect to reductions in nighttime BP [7–9, 11, 12, 37], which is an important target for cardiovascular risk reduction [51–56]. Finally, based on currently available data, there does not seem to be any relevant differences in the magnitude of BP-lowering effects after radiofrequency RDN and ultrasound RDN [57].
Place in hypertension management
It seems reasonable that RDN can be used in combination with antihypertensive drug therapy for the treatment of “true” uncontrolled or resistant hypertension with high office and out-of-office BP because achieving control of nocturnal and morning hypertension is difficult using drug treatment alone. Most current antihypertensive agents, even those with a longer half-life, have a limited 24 h BP-lowering effect. The HI-JAMP study, which used the same “all-in-one” BP-monitoring device to measure office, home and ambulatory BPs, found that about one-third of individuals had uncontrolled office and daytime ambulatory BP, and rates of uncontrolled morning hypertension and nocturnal hypertension were around 45% and 55%, respectively, even in those being treated with two or more antihypertensive agents [27]. This is relevant because uncontrolled nocturnal and morning hypertension, and a riser pattern of nocturnal BP are associated with increased risk for cardiovascular events, including heart failure [15, 23, 51–53, 58, 59]. Therefore, it seems reasonable to infer that the long-term “always on”, 24 h BP-lowering effect achieved after RDN could contribute to a reduction in cardiovascular disease events [41, 54, 60].
Japan Renal Denervation (J-RED) Registry
All individuals who undergo RDN in Japan have to be registered in the J-RED registry and have follow-up of office, ambulatory, and home BPs for 2 years. This all-case registration study will provide us the real-world data on the BP-lowering effects and its characteristics by RDN. Besides, the optional 10-year follow-up after RDN may give an insight to the impact of RDN on cardiovascular outcomes.(Table 3). These longer-term follow-up data can then be compared with historical cohorts evaluated using ambulatory or home BP monitoring, such as those enrolled in the J-HOP, JAMP, and HI-JAMP studies [27, 51, 52, 58, 61]. This should allow evaluation of the clinical benefit of RDN compared with antihypertensive drug therapy only. In addition, along with global registries [62, 63], J-RED will provide important data in Japanese individuals, allowing any potential ethnic differences in the effects of RDN on BP and cardiovascular outcomes to be determined.
Table 3.
Follow-up assessments in the Japan REnal Denervation (J-RED) registry
| Before RDN | At RDN | Just after RDN | Mandatory 2-year follow-up after RDN | Optional 10-year follow-up after RDN | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 mo | 6 mo | 12 mo | 24 mo | 3 yr | 4 yr | 5 yr | 6 yr | 7 yr | 8 yr | 9 yr | 10 yr | ||||
| Patient characteristics (disease history, height, etc.) | x | ||||||||||||||
| Body weight | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| RDN procedure | x | ||||||||||||||
| Office BP (trough) | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| 24 h ABPM | x | x | x | x | x | x | x | x | x | x | x | x | x | ||
| Home BP measurement (5 days) | x | x | x | x | x | x | x | x | x | x | x | x | x | ||
| Blood tests | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| Creatinine | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| NT-proBNP | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| HbA1c | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| LDL cholesterol | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| Proteinuria | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| ECG, LVH, SV1 + RV5 mm | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| Atrial fibrillation | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |
| Renal artery assessment | |||||||||||||||
| Angiography | x | ||||||||||||||
| Computed tomography | x | x | |||||||||||||
| Clinical outcomes (ASCVD, HF, diabetes, CKD, AF, hypotension, CV death, all-cause death) & complications | x | x | x | x | x | x | x | x | x | x | x | x | |||
| Antihypertensives (number of classes & dose) | x | x | x | x | x | x | x | x | x | x | x | x | x | ||
ABPM ambulatory blood pressure monitoring, AF atrial fibrillation, ASCVD atherosclerotic cardiovascular disease, BP blood pressure, CKD chronic kidney disease, CV cardiovascular, ECG electrocardiogram, HbA1c glycosylated haemoglobin, HF heart failure, LDL low-density lipoprotein, LVH left ventricular hypertrophy, mo months, NT-proBNP N-terminal pro B-type natriuretic peptide, RDN renal denervation, SV1 + RV5 electrocardiogram criteria used in the diagnosis of left ventricular hypertrophy, yr years
The Joint RDN Committee of the JSH/CVIT/JCS from Japan will also ensure that the J-RED registry captures important safety data relating to the RDN procedure, and will make sure that facilities enroll all individuals undergoing RDN into the registry. The aim is to have RDN performed at appropriately qualified, high-quality facilities. In addition, it is important to provide continuing education for all members of multidisciplinary treatment teams.
Conclusion and perspectives
This Japanese consensus statement has a strong focus on the effectiveness and safety of RDN. RDN effectively reduces BP throughout the 24 h period. The BP-lowering effect of RDN is not impacted by adherence and overcomes several limitations of antihypertensive drug therapy, such as effective, long-term control of early morning and nocturnal BP. Knowledge about the impact of RDN on hard cardiovascular clinical outcomes, such as rates of stroke, myocardial infarction, heart failure and aortic dissection, will grow as clinical evidence accumulates. In addition, future advances in technology may improve the effectiveness of RDN. Therefore, Joint RDN Committee will contribute to reviewing the indications for RDN and facility accreditation annually, and update information on guidance as necessary to ensure optimum use of the RDN procedure to reduce BP in patients with hypertension.
Acknowledgements
We are grateful to Dr Michael Weber (USA), Dr. Felix Mahfoud (Europe), and Dr.Tzung-Dau Wang (Asia) for evaluation of this statement as international experts. Medical writing support was provided by Nicola Ryan, independent medical writer, and by Ayako Okura, English publication coordinator, funded by Jichi Medical University.
Data Availability
Not applicable.
Declarations
Conflict of interest
Kario K receives grants from A&D, Omron Healthcare, Fukuda Denshi, CureApp, Sanwa Kagaku Kenkyusho; Honoraria from Daichi Sankyo, Viatris, Novartis, Otsuka Pharmaceuticals, Medtronic, Otsuka Medical device, Omron Healthcare. Kai H receives honoraria from Daiichi Sankyo, Novartis and Otsuka Medical Device. Rakugi H receives honoraria from Daiichi Sankyo Co., Ltd., Novartis Pharma K.K., and Otsuka Pharmaceutical Co., Ltd.; research funding from Novartis Pharma K.K.; scholarship from Daiichi Sankyo Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd. Takeda Pharmaceutical Company Ltd., and Sumitomo Pharma, Co., Ltd. Hoshide S receives honoraria from Novartis. Node K receives research grants from Otsuka Medical Devices, Medtronic, Astellas, Bayer Yakuhin, Boehringer Ingelheim Japan, Fujiyakuhin, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Novartis Pharma; Scholarship from Abbott, Boehringer Ingelheim Japan, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Teijin Pharma; Honoraria from AstraZeneca, Bayer Yakuhin, Boehringer Ingelheim Japan, Daiichi Sankyo, Eli Lilly Japan, Kowa, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, MSD, Novartis Pharma, Novo Nordisk Pharma, Ono Pharmaceutical, Otsuka, Tsumura & Co. Maekawa Y receives Scholarship funds or Donations Scholarship funds from Abbott Medical Japan LLC, and BIOTRONIK JAPAN. Tsutsui H receives consultancy from Boehringer Ingelheim Co., Ltd., Bayer Yakuhin, Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Astra Zeneca Co., Ltd.; Remuneration from MSD K.K., Astellas Pharma Inc., Pfizer Japan Inc., Bristol-Myers Squibb Company, Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Boehringer Ingelheim Co., Ltd., Takeda Pharmaceutical Company Limited, Bayer Yakuhin, Ltd., Novartis Pharma K.K., Kowa Pharmaceutical Co. Ltd., Teijin Pharma Ltd.; Manuscript fees from Medical View, Nippon Rinsho; Research funding from Actelion Pharmaceuticals Japan Ltd., Japan Tobacco Inc., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo Co., Ltd., IQVIA Services Japan, Omron Healthcare; Scholarship funds from Astellas Pharma Inc., Novartis Pharma K.K., Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Teijin Pharma Ltd., MSD K.K. Sakata Y receives grants from Abbott Medical Japan, Otsuka Pharmaceutical, Nippon Boehringer Ingelheim, Boston Scientific Japan, BIOTRONIK JAPAN; Honoraria from AstraZeneca, Otsuka Pharmaceutical, Nippon Boehringer Ingelheim, Novartis Pharma and Bayer Yakuhin. Aoki J receives Honoraria from Medtronic, Otsuka Medical Device, Otsuka Pharmaceutical, and Daiichi Sankyo. Nanto S receives Honoraria from Medtronic, Otsuka Medical device. Yokoi H receives Honoraria from Daiichi Sankyo, Medtronic, Otsuka Medical device. Role of clinical trials: Kario K is an executive committee principal investigator for the Spyral OFF MED, the Spyral ON MED, the DUO and the REQUIRE; a coordinating investigator for the TCD-16164 study; a site principal investigator for the HTN-J, the Spyral OFF MED, the Spyral ON MED, the DUO, the REQUIRE and the TCD-16164 study. Kai H is a site sub-investigator for the HTN-J; a medical expert for the DUO and the REQUIRE. Rakugi H is a site sub-investigator for the HTN-J, the DUO and the REQUIRE. Hoshide S is a site sub-investigator for the HTN-J, the Spyral OFF MED, the Spyral ON MED, the DUO, the REQUIRE and the TCD-16164 study.; a medical expert for the REQUIRE. Node K is a site sub investigator for the REQUIRE. Maekawa Y is a site principal investigator for the DUO. Sakata Y is a principal investigator for the DUO and the REQUIRE; a site sub-investigator for the HTN-J. Aoki J is a site sub investigator for the HTN-J; a site principal investigator for the Spyral OFF MED, the Spyral ON MED studies, the DUO and the REQUIRE. Nanto S is an executive committee principal investigator for the DUO and the REQUIRE; a member of the advisory team for ablation site for the REQUIRE; a site principal investigator for the REQUIRE. Yokoi H is a site principal investigator for the DUO, the REQUIRE and the TCD-16164 study;. an advisor on renal artery access for the REQUIRE.
Footnotes
Kazuomi Kario Chairman and Hiroyoshi Yokoi Co-chairman of the Japan Renal Denervation Joint Committee of Japanese Society of Hypertension (JSH), Japanese Association of Cardiovascular Intervention and Therapeutics (CVIT), and the Japanese Circulation Society (JCS).
This article is co-published in the journals Cardiovascular Intervention and Therapeutics, Hypertension Research, and Circulation Journal. 10.1007/s12928-024-01017-1, 10.1038/s41440-024-01700-z, or 10.1253/circj.CJ-66-0225.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weber MA, Mahfoud F, Schmieder RE, Kandzari DE, Tsioufis KP, Townsend RR, et al. Renal denervation for treating hypertension: current scientific and clinical evidence. JACC Cardiovasc Interv. 2019;12(12):1095–105. [DOI] [PubMed] [Google Scholar]
- 2.Lauder L, Mahfoud F, Azizi M, Bhatt DL, Ewen S, Kario K, et al. Hypertension management in patients with cardiovascular comorbidities. Eur Heart J. 2023;44(23):2066–77. [DOI] [PubMed] [Google Scholar]
- 3.Katsurada K, Shinohara K, Aoki J, Nanto S, Kario K. Renal denervation: basic and clinical evidence. Hypertens Res. 2022;45(2):198–209. [DOI] [PubMed] [Google Scholar]
- 4.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–81. [DOI] [PubMed] [Google Scholar]
- 5.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–9. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–401. [DOI] [PubMed] [Google Scholar]
- 7.Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391(10137):2335–45. [DOI] [PubMed] [Google Scholar]
- 8.Bohm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395(10234):1444–51. [DOI] [PubMed] [Google Scholar]
- 9.Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy T, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397(10293):2476–86. [DOI] [PubMed] [Google Scholar]
- 10.Kario K, Yokoi Y, Okamura K, Fujihara M, Ogoyama Y, Yamamoto E, et al. Catheter-based ultrasound renal denervation in patients with resistant hypertension: the randomized, controlled REQUIRE trial. Hypertens Res. 2022;45(2):221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azizi M, Saxena M, Wang Y, Jenkins JS, Devireddy C, Rader F, et al. Endovascular ultrasound renal denervation to treat hypertension: the RADIANCE II randomized clinical trial. JAMA. 2023;329(8):651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandzari DE, Townsend RR, Kario K, Mahfoud F, Weber MA, Schmieder RE, et al. Safety and efficacy of renal denervation in patients taking antihypertensive medications. J Am Coll Cardiol. 2023;82(19):1809–23. [DOI] [PubMed] [Google Scholar]
- 13.Kario K, Ogawa H, Okumura K, Okura T, Saito S, Ueno T, et al. SYMPLICITY HTN-Japan - first randomized controlled trial of catheter-based renal denervation in asian patients. Circ J. 2015;79(6):1222–9. [DOI] [PubMed] [Google Scholar]
- 14.Kario K, Yamamoto E, Tomita H, Okura T, Saito S, Ueno T, et al. Sufficient and persistent blood pressure reduction in the final long-term results from SYMPLICITY HTN-Japan - safety and efficacy of renal denervation at 3 years. Circ J. 2019;83(3):622–9. [DOI] [PubMed] [Google Scholar]
- 15.Mancia G, Kreutz R, Brunstrom M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH buidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European society of hypertension: endorsed by the international society of hypertension (ISH) and the European renal association (ERA). J Hypertens. 2023;41(12):1874–2071. [DOI] [PubMed] [Google Scholar]
- 16.Kario K, Kim BK, Aoki J, Wong AY, Lee YH, Wongpraparut N, et al. Renal denervation in Asia: consensus statement of the Asia renal denervation consortium. Hypertension. 2020;75(3):590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang TD, Lee YH, Chang SS, Tung YC, Yeh CF, Lin YH, et al. 2019 consensus statement of the Taiwan hypertension society and the Taiwan society of cardiology on renal denervation for the management of arterial hypertension. Acta Cardiol Sin. 2019;35(3):199–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmieder RE, Mahfoud F, Mancia G, Azizi M, Bohm M, Dimitriadis K, et al. European Society of hypertension position paper on renal denervation 2021. J Hypertens. 2021;39(9):1733–41. [DOI] [PubMed] [Google Scholar]
- 19.Chia YC, Wan Ahmad WA, Fong AYY, Rosman A, Abdul Rahman AR, Choo GH, et al. 2022 Malaysian working group consensus statement on renal denervation for management of arterial hypertension. Hypertens Res. 2022;45(7):1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbato E, Azizi M, Schmieder RE, Lauder L, Bohm M, Brouwers S, et al. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2023;44(15):1313–30. [DOI] [PubMed] [Google Scholar]
- 21.Schmieder RE, Hogerl K, Jung S, Bramlage P, Veelken R, Ott C. Patient preference for therapies in hypertension: a cross-sectional survey of German patients. Clin Res Cardiol. 2019;108(12):1331–42. [DOI] [PubMed] [Google Scholar]
- 22.Kario K, Kagitani H, Hayashi S, Hanamura S, Ozawa K, Kanegae H. A Japan nationwide web-based survey of patient preference for renal denervation for hypertension treatment. Hypertens Res. 2022;45(2):232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235–481. [DOI] [PubMed] [Google Scholar]
- 24.Burnier M. Drug adherence in hypertension. Pharmacol Res. 2017;125(Pt B):142–9. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin IJ, Kreutz R, Olsen MH, Schutte AE, Lopez-Jaramillo P, Frieden TR, et al. Fixed-dose combination antihypertensive medications. Lancet. 2019;394(10199):637–8. [DOI] [PubMed] [Google Scholar]
- 26.Salam A, Kanukula R, Atkins E, Wang X, Islam S, Kishore SP, et al. Efficacy and safety of dual combination therapy of blood pressure-lowering drugs as initial treatment for hypertension: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2019;37(9):1768–74. [DOI] [PubMed] [Google Scholar]
- 27.Kario K, Tomitani N, Nishizawa M, Harada N, Kanegae H, Hoshide S. Concept, study design, and baseline blood pressure control status of the nationwide prospective HI-JAMP study using multisensor ABPM. Hypertens Res. 2023;46(2):357–67. [DOI] [PubMed] [Google Scholar]
- 28.Park S, Buranakitjaroen P, Chen CH, Chia YC, Divinagracia R, Hoshide S, et al. Expert panel consensus recommendations for home blood pressure monitoring in Asia: the Hope Asia Network. J Hum Hypertens. 2018;32(4):249–58. [DOI] [PubMed] [Google Scholar]
- 29.Kario K, Park S, Buranakitjaroen P, Chia YC, Chen CH, Divinagracia R, et al. Guidance on home blood pressure monitoring: A statement of the HOPE Asia Network. J Clin Hypertens (Greenwich). 2018;20(3):456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kario K, Park S, Chia YC, Sukonthasarn A, Turana Y, Shin J, et al. 2020 Consensus summary on the management of hypertension in Asia from the HOPE Asia network. J Clin Hypertens (Greenwich). 2020;22(3):351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, et al. European society of hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24(12):779–85. [DOI] [PubMed] [Google Scholar]
- 32.Kario K, Hoshide S, Chia YC, Buranakitjaroen P, Siddique S, Shin J, et al. Guidance on ambulatory blood pressure monitoring: a statement from the HOPE Asia Network. J Clin Hypertens (Greenwich). 2021;23(3):411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cepeda M, Pham P, Shimbo D. Status of ambulatory blood pressure monitoring and home blood pressure monitoring for the diagnosis and management of hypertension in the US: an up-to-date review. Hypertens Res. 2023;46(3):620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71(6):1269–324. [DOI] [PubMed] [Google Scholar]
- 35.Kario K, SPYRAL HTN-OFF MED trial group. Orthostatic hypertension as potential identifier of responders to renal denervation: Post-hoc analysis from the SPYRAL HTN-OFF MED trial. Paper presented at: American Heart Association Scientific Meeting 2019; Nov 16–18, 2019; Philadelphia.
- 36.Kirtane AJ, Sharp ASP, Mahfoud F, Fisher NDL, Schmieder RE, Daemen J, et al. Patient-level pooled analysis of ultrasound renal denervation in the sham-controlled RADIANCE II, RADIANCE-HTN SOLO, and RADIANCE-HTN TRIO trials. JAMA Cardiol. 2023;8(5):464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kario K, Bhatt DL, Brar S, Cohen SA, Fahy M, Bakris GL. Effect of catheter-based renal denervation on morning and nocturnal blood pressure: insights rrom SYMPLICITY HTN-3 and SYMPLICITY HTN-Japan. Hypertension. 2015;66(6):1130–7. [DOI] [PubMed] [Google Scholar]
- 38.Kario K, Bohm M, Mahfoud F, Townsend RR, Weber MA, Patel M, et al. Twenty-four-hour ambulatory blood pressure reduction patterns after renal denervation in the SPYRAL HTN-OFF MED trial. Circulation. 2018;138(15):1602–4. [DOI] [PubMed] [Google Scholar]
- 39.Kario K, Weber MA, Mahfoud F, Kandzari DE, Schmieder RE, Kirtane AJ, et al. Changes in 24-hour patterns of blood pressure in hypertension following renal denervation therapy. Hypertension. 2019;74(2):244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahfoud F, Kandzari DE, Kario K, Townsend RR, Weber MA, Schmieder RE, et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet. 2022;399(10333):1401–10. [DOI] [PubMed] [Google Scholar]
- 41.Kario K, Mahfoud F, Kandzari DE, Townsend RR, Weber MA, Schmieder RE, et al. Long-term reduction in morning and nighttime blood pressure after renal denervation: 36-month results from SPYRAL HTN-ON MED trial. Hypertens Res. 2023;46(1):280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kario K, Hettrick DA, Prejbisz A, Januszewicz A. Obstructive sleep apnea-induced neurogenic nocturnal hypertension: a potential role of renal denervation? Hypertension. 2021;77(4):1047–60. [DOI] [PubMed] [Google Scholar]
- 43.Kario K, Bhatt DL, Kandzari DE, Brar S, Flack JM, Gilbert C, et al. Impact of renal denervation on patients with obstructive sleep apnea and resistant hypertension - insights from the SYMPLICITY HTN-3 trial. Circ J. 2016;80(6):1404–12. [DOI] [PubMed] [Google Scholar]
- 44.Heradien M, Mahfoud F, Greyling C, Lauder L, van der Bijl P, Hettrick DA, et al. Renal denervation prevents subclinical atrial fibrillation in patients with hypertensive heart disease: randomized, sham-controlled trial. Heart Rhythm. 2022;19(11):1765–73. [DOI] [PubMed] [Google Scholar]
- 45.Steinberg JS, Shabanov V, Ponomarev D, Losik D, Ivanickiy E, Kropotkin E, et al. Effect of renal denervation and catheter ablation vs catheter ablation alone on atrial fibrillation recurrence among patients with paroxysmal atrial fibrillation and hypertension: the ERADICATE-AF randomized clinical trial. JAMA. 2020;323(3):248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prado GM, Mahfoud F, Lopes RD, Moreira DAR, Staico R, Damiani LP, et al. Renal denervation for the treatment of ventricular arrhythmias: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2021;32(5):1430–9. [DOI] [PubMed] [Google Scholar]
- 47.Rommel KP, Pagoulatou S, Kresoja KP, Rosch S, Schober AR, von Roeder M, et al. Modulation of pulsatile left ventricular afterload by renal denervation in heart failure with preserved ejection fraction. Circ Heart Fail. 2023;16(10): e010543. [DOI] [PubMed] [Google Scholar]
- 48.Kagitani H, Hayashi S, Hanamura S, Ozawa K, Kobayashi D, Hiki S, et al. A Japan nationwide web-based survey of estimation on patients for renal denervation based on blood pressure level and the number of antihypertensives (J-NEEDs survey). J Clin Hypertens (Greenwich). 2021;23(9):1684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kario K, Kai H, Nanto S, Yokoi H. Anti-hypertensive medication adherence in the REQUIRE trial: post-hoc exploratory evaluation. Hypertens Res. 2023;46(8):2044–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Townsend RF, Kandzari K, Kario D, Mahfoud K, Weber F,Schmieder MA, Pocock R, Tsioufis S, David K, Steigerwalt S, Walton S, Hopper T, Bertolet I, Calhoun B, Sharif W, Lurz F, Fengler P, Patel K, Fahy K, Hettrick M, Brar D, Böhm SM. Impact of antihypertensive medication changes on blood pressure after renal denervation among different patient groups in the SPYRAL HTN-ON MED trial. Hypertension 2024;in press. [DOI] [PMC free article] [PubMed]
- 51.Narita K, Hoshide S, Kario K. Nighttime home blood pressure is associated with the cardiovascular disease events risk in treatment-resistant hypertension. Hypertension. 2022;79(2):e18–20. [DOI] [PubMed] [Google Scholar]
- 52.Kario K, Hoshide S, Mizuno H, Kabutoya T, Nishizawa M, Yoshida T, et al. Nighttime blood pressure phenotype and cardiovascular prognosis: practitioner-based nationwide JAMP study. Circulation. 2020;142(19):1810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kario K, Williams B. Nocturnal hypertension and heart failure: mechanisms, evidence, and new treatments. Hypertension. 2021;78(3):564–77. [DOI] [PubMed] [Google Scholar]
- 54.Staplin N, de la Sierra A, Ruilope LM, Emberson JR, Vinyoles E, Gorostidi M, et al. Relationship between clinic and ambulatory blood pressure and mortality: an observational cohort study in 59 124 patients. Lancet. 2023;401(10393):2041–50. [DOI] [PubMed] [Google Scholar]
- 55.Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370(9594):1219–29. [DOI] [PubMed] [Google Scholar]
- 56.Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111(14):1777–83. [DOI] [PubMed] [Google Scholar]
- 57.Ogoyama Y, Tada K, Abe M, Nanto S, Shibata H, Mukoyama M, et al. Effects of renal denervation on blood pressures in patients with hypertension: a systematic review and meta-analysis of randomized sham-controlled trials. Hypertens Res. 2022;45(2):210–20. [DOI] [PubMed] [Google Scholar]
- 58.Kario K, Hoshide S, Narita K, Okawara Y, Kanegae H. Cardiovascular prognosis in drug-resistant hypertension stratified by 24-hour ambulatory blood pressure: the JAMP study. Hypertension. 2021;78(6):1781–90. [DOI] [PubMed] [Google Scholar]
- 59.Kario K, Saito I, Kushiro T, Teramukai S, Tomono Y, Okuda Y, et al. Morning home blood pressure Is a strong predictor of coronary artery disease: the HONEST study. J Am Coll Cardiol. 2016;67(13):1519–27. [DOI] [PubMed] [Google Scholar]
- 60.Bhatt DL, Vaduganathan M, Kandzari DE, Leon MB, Rocha-Singh K, Townsend RR, et al. Long-term outcomes after catheter-based renal artery denervation for resistant hypertension: final follow-up of the randomised SYMPLICITY HTN-3 Trial. Lancet. 2022;400(10361):1405–16. [DOI] [PubMed] [Google Scholar]
- 61.Hoshide S, Yano Y, Haimoto H, Yamagiwa K, Uchiba K, Nagasaka S, et al. Morning and evening home blood pressure and risks of incident stroke and coronary artery disease in the Japanese general practice population: the Japan morning surge-home blood pressure study. Hypertension. 2016;68(1):54–61. [DOI] [PubMed] [Google Scholar]
- 62.Mahfoud F, Mancia G, Schmieder RE, Ruilope L, Narkiewicz K, Schlaich M, et al. Cardiovascular risk reduction after renal denervation according to time in therapeutic systolic blood pressure range. J Am Coll Cardiol. 2022;80(20):1871–80. [DOI] [PubMed] [Google Scholar]
- 63.Mahfoud F, Azizi M, Daemen J, Sharp ASP, Patak A, Iglesias JF, et al. Real-world experience with ultrasound renal denervation utilizing home blood pressure monitoring: the Global Paradise System registry study design. Clin Res Cardiol. 2023. 10.1007/s00392-00023-02325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.