Abstract
Purpose
Gestational obesity (GO) presents a multifaceted challenge to maternal and fetal health, with an escalating prevalence and far-reaching consequences extending beyond pregnancy. This perspective statement by the Italian Society of Obesity (SIO) provides current insights into the diagnosis, maternal and fetal impacts, and treatment strategies for managing this pressing condition.
Methods
This article provides a comprehensive review of the maternal and fetal effects of GO and provides suggestions on strategies for management. Comprehensive review was carried out using the MEDLINE/PubMed, CINAHL, EMBASE, and Cochrane Library databases.
Results
The diagnosis of GO primarily relies on pre-pregnancy body mass index (BMI), although standardized criteria remain contentious. Anthropometric measures and body composition assessments offer valuable insights into the metabolic implications of GO. Women with GO are predisposed to several health complications, which are attributed to mechanisms such as inflammation and insulin resistance. Offspring of women with GO face heightened risks of perinatal complications and long-term metabolic disorders, indicating intergenerational transmission of obesity-related effects. While nutritional interventions are a cornerstone of management, their efficacy in mitigating complications warrants further investigation. Additionally, while pharmacological interventions have been explored in other contexts, evidence on their safety and efficacy specifically for GO remains lacking, necessitating further investigation.
Conclusion
GO significantly impacts maternal and fetal health, contributing to both immediate and long-term complications. Effective management requires a multifaceted approach, including precise diagnostic criteria, personalized nutritional interventions, and potential pharmacological treatments. These findings underscore the need for individualized care strategies and further research to optimize outcomes for mothers and their offspring are needed. Enhanced understanding and management of GO can help mitigate its intergenerational effects, improving public health outcomes.
Level of evidence: Level V narrative review.
Keywords: Pregnancy, Obesity, Gestational obesity, Gestational weight gain, Gestational diabetes mellitus, Pregnancy, Nutrition, Diet
Introduction
Gestational obesity (GO) is a complex condition that poses maternal and fetal health at significant risks [1]. Its prevalence is steadily increasing, with consequences that extend beyond the gestational period, affecting the long-term health of both mother and child.
The adoption of standard cut-offs to define obesity in pregnancy is still debated, with several organizations proposing varying criteria [2]. Anthropometric assessment is critical to detecting GO, but an analysis of body composition could provide additional information on the severity and metabolic implications of this condition [3].
Women with GO are at risk of developing a number of complications, including gestational diabetes (GDM), gestational hypertension, preeclampsia, and difficulties during labor and delivery [1]. In particular, studies suggest an association between GO and an increased risk of short- and long-term complications for the mother, with mechanisms involving chronic inflammation, insulin resistance, and metabolic dysfunction [4]. In addition, infants born to mothers with GO face a significant risk of perinatal complications, including macrosomia, neonatal hypoglycemia, and an increased risk of birth defects [1]. Long-term effects include an increased risk of childhood obesity, type 2 diabetes, and cardiometabolic diseases, suggesting intergenerational transmission of the metabolic consequences of GO [5].
Nutritional interventions are a key component of GO management, with an emphasis on a balanced diet, calorie intake control, and body weight regulation [6]. However, the effectiveness of such interventions in reducing GO-related complications is still subject to evaluation. Finally, pharmacological approaches for obesity, although promising, are not currently available for women with GO, requiring further study to determine their efficacy and safety in this vulnerable population.
Thus, this position statement of the Italian Society of Obesity (SIO) aimed to provide a comprehensive overview of the current knowledge on GO, focusing on diagnosis, maternal and fetal effects, and available therapeutic interventions. Moving from these evidence, the main purpose of this position statement was to inform healthcare professionals, including physicians, midwives, dietitians, and researchers, about the clinical implications and best practices to be adopted in the management of this critical condition.
Diagnosis of gestational obesity
Pre-pregnancy obesity
Excess adiposity is commonly measured through anthropometric methods and expressed as body mass index (BMI), obtained from the ratio of body weight in kilograms to height in square meters (kg/m2). In the pre-pregnancy period, as in other life course phases, the diagnosis of obesity is based on the calculation of BMI with reference to the internationally used World Health Organization classification [7].
Pre-pregnancy BMI is an important predictor of maternal and neonatal health outcomes. A high BMI at the beginning of the pregnancy can affect the course of gestation and the outcomes of childbirth. Since the resulting GO seems to negatively affect both the health of the mother and the health of the fetus, international guidelines agree that optimal control of obesity should begin before pregnancy [2, 6, 8–11]
In many countries, including Italy, epidemiological data on pre-pregnancy BMI are scarce. In Italy, statistics report a constant increase in weight excess in adult female population (age range: 18–69) and suggest that around 22% of women in the reproductive years (18–44 years of age) are likely to have a BMI above the normal range (18.5–24.9 kg/m2) [10, 12]. Recently, a study conducted in the Friuli Venezia Giulia region of Italy between 2017 and 2018 found that a significant portion of pregnant women faced issues related to overweight and obesity [13]. At the beginning of pregnancy, 17.7% and 8.2% of women were classified as overweight or with obesity, respectively [13].
Pre-pregnancy weight loss has proven to be an effective intervention to improve medical comorbidities [14, 15] and it should be encouraged, especially in view of the fact that not only large weight reductions, as in the case of bariatric surgery [16], but even small pre-pregnancy weight reductions in women with obesity may be associated with improved pregnancy outcomes [8].
Anthropometric assessment
The most common anthropometric measures used during pre-conception and pregnancy to evaluate maternal body composition and changes throughout pregnancy are BMI (most often pre-pregnancy) and gestational weight gain (GWG) [17]. A meta-analysis about maternal anthropometry and pregnancy outcomes conducted by the WHO confirmed the inherent value of maternal weight, height, arm circumference, and BMI as a predictor of specific infant and maternal outcomes [17].
In particular, GWG has been related not only to fetal growth and newborn weight [18] but also to the risk of intrauterine growth retardation, low birth weight, and prematurity [12]. GWG is defined as the weight gained during pregnancy and up to the pre-delivery visit compared to the weight documented at the first prenatal visit that occurs due to several modifications, including the enlargement of the uterus, fetal development, the formation of amniotic fluid and the placenta, the increase in intra- and extravascular fluids, the breast enlargement, and the fat store [19]. Placenta, fetus, and amniotic fluid generally represents approximately 35% of the total GWG [19].
Currently, recommendations for GWG are based on pre-pregnancy BMI [19] (see Table 1). Specifically, it is recommended a total GWG range of 7.0–11.5 kg for women with overweight and a total GWG range of 5.0–9.0 kg for women with obesity (BMI > 30 kg/m2) regardless of obesity class. Given the limitation of evidence by class of obesity, the general IOM recommendation for women with BMI > 30 kg/m2 tries to balance the risk of large- vs. small-for-gestational-age infants and high postpartum weight maintenance vs. preterm delivery [19]. The lack of specific guidance on GWG for classes of obesity is a much-debated topic among specialists in the field, and the literature data provide contrasting results [18, 20, 21]. The literature is unanimous in stating that GWG above the IOM recommendations is associated with an increased risk of adverse maternal and infant outcomes and a long-term effect on maternal health, with an increased risk of mortality for cardiometabolic diseases [18, 20, 21]. On the contrary, the debated question is whether the recommended 5- to 9-kg GWG for women with BMI > 30 kg/m2 should require an additional restriction for women with BMI greater than 35 kg/m2 and weight maintenance or even weight loss for women with BMI greater than 40 kg/m2 [2].
Table 1.
Recommendations on gestational weight gain according to pre-pregnancy weight class and BMI
| Pre-pregnancy weight class and BMI (kg/m2) | IOM Recommendation 2009 [19] | Voerman E et al. 2019a [12] | Devlieger R et al. 2020b [22] | Kiel DW et al. 2007c [23] | |
|---|---|---|---|---|---|
| Recommended WG range (kg) | Recommended WG in 2° and 3° trimester range (kg/wk) | Suggested WG range (kg) | Suggested WG (kg) | Suggested WG range (kg) | |
|
Underweight (< 18.5 kg/m2) |
12.5–18.0 |
0.44–0.58 (0.51) |
14–16 | 21 | - |
|
Normal weight (18.5–24.9 kg/m2) |
11.5–16.0 |
0.35–0.50 (0.42) |
10–18 | 14 | - |
|
Overweight (25.0–29.9 kg/m2) |
7.0–11.5 |
0.23–0.33 0.28 |
2–16 | 8 | |
|
Obesity (≥ 30 kg/m2) |
5.0–9.0 |
0.17–0.27 (0.22) |
|||
| Obesity Class 1 (30.0–34.9 kg/m2) | – | – | 2–6 | 0 | 4.5–11.4 |
| Obesity Class 2 (35.0–39.9 kg/m2) | – | – | 0–4 | Loss-4 | 0–4.1 |
| Obesity Class 3 (≥ 40 kg/m2) | – | – | 0–6 | Loss-5 | Loss 0–4.1 |
BMI, body mass index; IOM, Institute of Medicine; WG, weight gain
aWG based on risk reduction in any adverse outcome
bWG associated with the lowest risk of pregnancy complications
cWG based on minimal risk of all outcomes
Body composition assessment
GWG and BMI have been proposed as screening methods for identifying pregnancies with abnormal progression that might be at risk of adverse perinatal outcomes. Nonetheless, they provide very limited information regarding changes in women’s body compositions throughout their pregnancies. Moreover, fetal growth may be influenced by more specific maternal tissue changes (fat or fat-free mass) than by total GWG or BMI [24].
Maternal fat mass (FM) is the most variable component of GWG, and its changes are positively correlated with GWG and inversely related to pre-pregnancy BMI [3]. Based on serial measurements in pregnant women, most of the FM deposited during pregnancy is subcutaneous, with a preferential accumulation over the hips, back, and upper thighs up to about thirty weeks’ gestation. During pregnancy, a large and variable accumulation of water contributes to GWG and determines an increase in the hydration of fat-free mass (FFM) [3]. In the second trimester, GWG is mainly due to FM accumulation, while in the third trimester, it is mainly due to FFM accumulation and fetal growth at the expenses of fat accumulation suggesting that the second trimester is a more opportune window for the assessment of GO because it is characterized by fat accumulation [25].
Several methods could be used for evaluating maternal body composition and to assess changes in FM and FFM before, during, and after pregnancy, and several studies have been performed to compare the link of the measures assessed by different techniques with maternal and child outcomes [26]. Methods used to assess body composition include skinfold thickness (SFT), bioimpedance analysis (BIA), air displacement plethysmography (BOD POD), underwater weighing, and isotope dilution. Simple and cost-effective techniques such as SFT and BIA, a more accurate but expensive method like BOD POD, are all influenced by the hydration of FFM [26].
Equations exist to estimate the percentage of body fat using SFT in order to adjust the data for fluid shifts; however, they are specific to certain gestational stages [27]. Despite these limitations, SFT has shown a high correlation with the percentage of body fat obtained through other techniques (densitometry, DEXA, or dilutional methods) [26]. Thus, this method is useful to describe normal body fat changes throughout gestation, to identify women with unusually small or large changes in body fat during pregnancy, and to estimate initial body fat content [24].
BIA is used to assess the body composition and hydration status. This technique represents a non-invasive, reliable, and fast clinical approach, that is well tolerated and widely accepted by patients. BIA measurements should not be taken when participants are dehydrated, within 4 h of consuming food and drink, or within 12 h of intense exercise [28]. However, the main limit of BIA is that it cannot distinguish between the maternal and fetal tissues [27]. However, despite the advantages of BIA, the abnormal fluid distribution during pregnancy renders different BIA methods either inappropriate or in need of further validation [29]. The whole-body impedance is mainly predicted by the impedance in the limbs [30]; however, during pregnancy, a large amount of water is in the trunk [29]. Thus, a segmental impedance measurement might be advantageous for pregnant women, particularly in late pregnancy [29, 30] (see Table 2).
Table 2.
Body composition assessment methods in pregnancy
| Method | Measures | Strengths | Weaknesses |
|---|---|---|---|
| Skinfold thickness |
Estimate of FM Use of skinfold thickness measurements themselves rather than estimates of FM and FFM are often preferred |
Simple, inexpensive | Equations to estimate total BF, taking into account the change in hydration of the FFM, only available for certain gestational ages |
| Bioelectrical impedance analysis | TBW, FM, FFM, ECW, ICW | Simple, inexpensive | Equations to estimate total BF only available for certain gestational ages; unable to disentangle the maternal–fetal unit |
| Underwater weighing | Body volume (estimate FM and FFM) | Accurate | Method requiring complex equipment. Unable to disentangle the maternal–fetal unit. Results are influenced by changes in hydration of FFM during pregnancy |
|
Air displacement plethysmography (ADP/BOD POD) |
Body volume (estimate FM and FFM) | Accurate | Expensive and requiring complex equipment. Unable to disentangle the maternal–fetal unit. Result influenced by changes in hydration of FFM during pregnancy |
| Isotopic dilution | TBW (estimate FFM) | Accurate | Require frequent urine collection. For research only. It is expensive and requires complex equipment and experienced technicians |
| Whole-body potassium counting | BCM, FFM | Accurate | It is expensive and requires complex equipment; less accurate during pregnancy due to variation in postassi content in the FFM |
| Magnetic resonance imaging | FM, FFM, Ectopic FAT, FAT distribution | Accurate | For research only. It is expensive and requires complex equipment and experienced technicians |
| Dual energy X-ray absorptiometry | FM, FFM, Bone density | Contraindicated during pregnancy due to the radiation exposure | |
BF: body fat; TBW total body water; FM fat mass; FFM fat-free mass; ECW extracellular water; ICW intracellular water; BCM body cell mass
However, due to the dynamic state of pregnancy, there is no agreement on the optimal method for quantitatively assessing body fat in pregnant women. The validation of algorithms that regulate the hydration changes observed in the various gestational stages would be necessary.
In addition to body composition, changes in adipose tissue distribution throughout pregnancy could have a differential impact on metabolic health and adverse outcomes [31, 32]. Imaging technologies such as magnetic resonance imaging (MRI) are accurate but expensive. Less expensive is the ultrasound technique that permits the measurement of subcutaneous (SAT), visceral (VAT), and total adipose tissue depth. In this contest, VAT and total adipose tissue at 11–14 weeks of pregnancy have been found to be associated with GDM at 24–28 weeks’ gestation [31]. In addition, simple and inexpensive anthropometric measures such as neck circumference, waist circumference, hip circumference, arm circumference, and waist-to-hip ratio were related to the risk of GDM [32, 33].
Maternal effects of gestational obesity
Short-term effects
Obesity during pregnancy represents a risk factor for spontaneous abortion and for complications through the gestational period and delivery [34]. Maternal overnutrition and obesity are associated with multiple modifications that can harm the health of mothers, inducing hypertensive disorders, GDM, and affecting labor and delivery [34].
Based on wide cohort studies, the incidence of any maternal complication increases with BMI and with GWG; in particular, more than 60% of women with grade III obesity develop at least one pregnancy complication, regardless of weight gain [12]. This evidence is related to the definition of optimal ranges of GWG according to pre-conception weight status [19]. Interestingly, a recent systematic review emphasizes the superiority of adiposity markers, in particular waist circumference and waist-to-hip ratio, as strong predictors of adverse maternal health outcomes [33].
GDM represents one of the most frequent complications in mothers with obesity, and it predisposes to large for gestational age, fetal macrosomia, and cesarean delivery [35]. Worldwide, it has been estimated that the prevalence of GDM is about 18.3% and more when considering the female population with obesity, which has been associated with a three-fold risk of GDM [36, 37]. Many epidemiological studies indicated overweight and obesity as main determinants in GDM development, in addition to non-white ethnicity, maternal age, family or personal history of GDM, type 2 diabetes mellitus, subfertility, and infertility [38–40]. Moreover, obesity predisposes to early onset of GDM compared to healthy women; therefore, Italian guidelines for diabetes care included BMI ≥ 30 kg/m2 within high-risk conditions for GDM justifying anticipated screening with a 75 g oral glucose tolerance test (OGTT) at the 16–18th week [41]. If negative, the test must be repeated at the 24–28th week [41].
Obesity and GMD are frequently associated with hypertensive disorders. Further, gestational hypertension and preeclampsia are the leading causes of both maternal and fetal mortality worldwide and have been associated with the development of premature cardiovascular diseases and mortality in the offspring [42, 43]. It has been demonstrated that women with abnormal echocardiograms are at an increased risk of hypertensive disorders, in particular severe preeclampsia [44].
When considering delivery, GO and consequent fetal macrosomia have been associated with a longer first stage of labor (usually independent of fetal size), an increased risk of prolonged inductions of labor and induction failure, and shoulder dystocia, resulting in higher rates of cesarean birth (ranging from 18.7 to 43.9% in different cohort studies) [45, 46]. Besides, during puerperium, obesity predisposes not only to genital and wound infections [47, 48], deep venous thrombosis, and pulmonary embolism [49, 50] but also to mental health problems [51].
The pathogenic role of obesity during pregnancy is not completely clarified; however, accumulating evidence has demonstrated its capacity to induce inadequate adaptation of the organism during pregnancy, thus promoting and anticipating the onset of short- and long-term complications.
Pregnancy is associated with GWG, owing to fat accumulation, increased circulating blood volume, and extracellular fluid expansion; moreover, it involves cardiovascular and metabolic modifications to sustain energy delivery for fetus development and growth [52, 53]. Hemodynamic adaptations include an increase in heart rate, stroke volume, and cardiac output, cardiac hypertrophy, increase in plasma volume, decrease in arterial and peripheral vascular resistance, and decrease in blood pressure levels [54]. Metabolic change differentiates during pregnancy: the first half of pregnancy is characterized by higher insulin sensitivity, an anabolic state, and increased lipid storage; during the second half, insulin sensitivity reduces and cortisol, prolactin, human placental lactogen levels rise, leading to a catabolic state with an increased blood concentration of glucose, amino acids, and lipids available for the fetus. The development of insulin resistance is usually balanced by an increased rate of insulin secretion due to β-cell hyperplasia, which contributes to the maintenance of normal maternal glucose levels [54]. In this scenario, maternal central adiposity, insulin resistance, and inflammation appear to play central roles in the pathogenesis of pregnancy and delivery complications.
In particular, VAT accumulation is associated with worsening of insulin resistance and derangements of insulin signaling, disturbance of lipid metabolism, modulation of the inflammatory and immune responses, pro-thrombotic state, and induction and activation of the angiotensin I–II system [55, 56]. VAT acts like an endocrine organ, producing a great amount of adipokines with pro-inflammatory activity (leptin, visfatin, and resistin) and tumor necrosis factor-alpha (TNF-α), while anti-inflammatory adiponectin is reduced [57]. This is responsible for the increase of specific cytokines, such as interleukin (IL)-1β, IL-2, and IL-6, promoting the expression of maternal antiangiogenic factors and supporting the development of insulin resistance and systemic inflammation [58]. Furthermore, hyperleptinemia has been directly involved in placental development and function; it reduces cytotrophoblast proliferation and placental growth factor (P1GF), contributing to placental ischemia and early preeclampsia [59].
In mothers with visceral obesity, insulin resistance is compounded compared to healthy women and β-cell secretory function is insufficient to tackle the enhanced hormonal demand [60]. Moreover, low dietary intake of fiber and a sedentary lifestyle, as well as the pre-existence of obesity comorbidities, such as polycystic ovary syndrome, can contribute to worsening glucose metabolism during pregnancy. The overall results are progressively growing glucose and advanced glycation end products (AGEs) levels in mothers, while the development of macrosomia, fat deposition, insulin resistance, and neonatal hypoglycemia in babies [60]. Recent studies conducted with lipidomic techniques in pregnant women confirm that insulin resistance is associated with altered lipid metabolism toward lipogenesis, resulting in increased free fatty acids and cholesterol flow [61], a reduction of sphingomyelin, and polyunsaturated fatty acid-rich lipids [62]. Free fatty acids contribute to induce endothelial dysfunction and pro-thrombotic state (reduction of nitric oxide and prostacyclin, increase of reactive oxygen species, thromboxane A2, and endothelin 1), which overlap with vessel modification induced by hyperinsulinemia (vessel wall hypertrophy, narrowing of the vessel lumen, and increased peripheral resistance), collectively leading to hypertension [62]. High low-density lipoprotein (LDL) cholesterol and low high-density lipoprotein (HDL) cholesterol levels have been associated with the deposition of atherosclerotic plaques in the placental vasculature and coronary system, contributing to the increased cardiovascular risk in mothers [63]. Finally, hyperinsulinemia influences innate immune system function involved in the pathogenesis of preeclampsia. The complement system is activated by lipid (primarily non-esterified fatty acids) accumulation in the placenta [64]. Further, hyperinsulinemia increases the expression of M1 macrophages and modulates the mammalian target of rapamycin complex 1 (mTORC1) pathway, thus inducing an imbalance between pro-inflammatory (increased) and anti-inflammatory (reduced) T-lymphocyte populations [65], overall contributing to heightening both systemic and placental inflammatory states; furthermore, it acts locally at the maternal–fetal interface, reducing the number of decidual natural killer cells (dNKs) and vascular endothelial growth factor (VEGF) production while increasing TNF-α [58].
In conclusion, the data highlight the negative impact of visceral obesity in the progression of pregnancy and, thereafter, on women’s and babies’ health. In particular, the presence of visceral adiposity seems to be pivotal in inducing systemic and local inflammation, immune system derangement, oxidative stress, insulin resistance, antiangiogenic effects, and endothelial damage, all of which are associated with clinical maternal and fetal manifestations. Based on these observations, it is crucial to put in place strategies for prevention, diagnosis, monitoring, and treatment of obesity complications, starting from the pre-conception period, as endorsed by the International Federation of Gynecology and Obstetrics guidelines [11, 66].
Long-term effects
Breastfeeding
Obesity and overweight are associated with low rates and shorter durations of breastfeeding [67, 68] compared with women of normal weight, and this can further contribute to later adverse health outcomes that are associated with obesity, including breast and endometrial cancer [69, 70] in the menopausal period and type 2 diabetes [71, 72] as well as postpartum weight retention [73]. Delayed onset of lactation, due to high rates of cesarean section [74] and elevated progesterone levels (which prevent lactogenesis), is implicated [75], but also physical discomfort due to heavy breasts and postnatal depression may be potential contributory factors [76]. Breastfeeding should be encouraged because it may exert protective effects against later obesity-related adverse outcomes for the mother and offspring.
Postpartum weight retention
Pregnancy has been recognized as an independent risk factor for both persistent and new-onset obesity in women [77]. Most women gain rather than lose weight between pregnancies [78–80] and GWG is the main predictor of postpartum weight retention [81–83] as well as an important contributor to the increasing prevalence of obesity in women of reproductive age [84]. Indeed, women with excessive GWG have a 2–4.5-fold higher risk of being affected by overweight or obesity at 21 years postpartum, respectively [82]. Independent of GWG, pre-conceptional BMI seems to impact postpartum weight retention [85, 86] with a 0.10 kg increase in weight retention per unit of baseline BMI at the beginning of a second pregnancy [87]. In addition, there is some evidence suggesting regional differences in fat accumulation during postpartum, with a preferentially central rather than peripheral distribution in women with obesity compared to their normal weight counterparts [88, 89], with important implications for the development of later obesity complications. Finally, it should acknowledge that regardless of GWG, trajectories of weight gain over life span reveal greater gains among women with children compared to women with no births [90, 91].
Long-term morbidity
Whether postpartum weight retention negatively influences the risk of long-term complications of GO and the maternal and fetal outcomes in the next pregnancy, the complications of obesity during pregnancy represent a further threat to the long-term health of women [92, 93]. Indeed, women with GDM [92], raised HbA1c, or fasting glucose concentrations [94] are at increased risk not only for recurrent GDM [95] but also for developing type 2 diabetes after delivery, with a sevenfold higher incidence of type 2 diabetes in the first decade compared with non-complicated pregnancies [96], as well as BMI ≥ 25 kg/m2, family history of diabetes, non-white ethnicity, multiparity, and older maternal age [94]. Maternal derangements of plasma glucose are an increasingly recognized risk factor even for future cardiovascular disease, with a 13% higher risk of cardiovascular disease for every 1 mmol/L increment in the glucose challenge test even in the non-diabetic range [97]. Similarly, after preeclampsia, a fourfold higher risk of hypertension and a doubled risk of ischemic heart disease and cerebrovascular disease have been reported [98]. A pathogenetic interplay between pre-pregnancy heightened cardiovascular risk and cardiometabolic effects of hypertensive disorders of pregnancy such as endothelial dysfunction, inflammation, dyslipidemia, and angiogenic changes leading to accelerated atherogenesis that may sustain in the postpartum period has been hypothesized [76].
The postpartum increase in central adiposity predisposes women with obesity to hypertension and alterations in serum lipids, emerging as early as 1 year after delivery and persisting for more than 10 years [99, 100].
Women with GO developing pregnancy complications should undergo postpartum follow‐up for prevention, diagnosis, and timely management of chronic diseases.
Fetal effects of gestational obesity
Short-term effects
Perinatal complications
An increased BMI in pregnancy is associated with an increased risk of maternal morbidity and mortality in the perinatal period, with a dose–response relationship [101]. A retrospective population-based study on a large cohort of women from Washington State found a progressive higher risk of severe maternal complications, from class I to class III pre-pregnancy obesity compared with a normal BMI. Overall, women with an elevated BMI had a significantly higher risk of antepartum or postpartum hemorrhage, sepsis, thromboembolism, cardiovascular or respiratory morbidity, acute renal failure, eclampsia, complications of obstetric intervention, and maternal death. Additionally, women with obesity also had a higher rate of previous infant death, preterm birth, labor induction, delivery complications, and cesarean delivery [102]. As for delivery complications, in addition to the increased risk of hemorrhage and sepsis post vaginal birth, GO also predisposes to prolonged labor, an increased requirement and dosage of oxytocin, and higher rates of anesthetic complications, including both epidural and general anesthesia. These complications may negatively impact the newborn, inhibiting the initiation of breastfeeding, a known protective factor for a child’s health [101]. Maternal perinatal death in women with obesity is mainly due to cardiovascular complications [103]. Moreover, obesity has been described as one of the most frequent comorbidities for mortality related to COVID-19 infection, in pregnant women [104]. Furthermore, women with overweight or obesity have a significantly higher risk of developing hypertension and GDM [105], as well as sleep-disordered breathing [106], compared to women with normal weight, and these pathological conditions are associated with adverse impacts on delivery and newborn health. Finally, it has been reported that there is an association between GO and perinatal depression, a condition that includes depressive symptoms both during pregnancy and/or in the postpartum period, which can have a negative influence on newborn care following birth. Although the mechanisms supporting this association are not clear, it has been hypothesized that hypovitaminosis D, or body image disturbance, may play a role [107].
Fetal complications
GO during pregnancy has a negative impact on the fetus and represents an independent risk factor for various fetal complications during pregnancy, delivery, and throughout the offspring’s life [101]. GO at conception and the related metabolically unfavorable ovarian follicular environment [108] could negatively influence early embryonic development through metabolic, cellular, and epigenetic mechanisms, since the preimplantation stage, with negative consequences for offspring’s health over the lifetime [109]. The periconceptional environment could also affect babies born as a result of assisted reproductive treatments. In a retrospective observational study investigating supernumerary embryos from women with a BMI > 25 kg/m2 undergoing fertility treatment, morphologic and metabolic alterations indicative of a poorer potential in the resulting blastocysts have been described [110]. As regards fetus development, the placenta plays a central role in the exchange of materials between fetus and mother. In the case of GO, an altered adipokine pattern and impaired angiogenesis could compromise placental function, leading to a negative impact on nutrient transport capacity and placental blood flow [111]. This, in turn, can adversely affect fetal growth and health throughout life [112]. On this concern, several pre-clinical and clinical studies support the hypothesis that an inadequate in-utero environment represents a favorable factor in the development of various complications in the offspring [113]. Concerning fetal early complications, perinatal death, congenital fetal abnormalities, and fetal macrosomia are more likely to occur among mothers with obesity compared to those with a normal weight [76]. A systematic review and meta-analysis reported that even a slight increase in maternal BMI was associated with an elevated risk of stillbirth, fetal, perinatal, and neonatal death [114]. Moreover, it has been reported that overweight and obesity are the major modifiable risk factors for stillbirth in high-income countries [115]. A recent population-based study, carried out in a large sample of Swedish women, found a double risk of stillbirth among women with overweight and a fourfold risk among those with obesity, compared with women of normal weight [116], in line with the results of a previous large population-based cohort study [117]. In addition, pregnancy complications associated with GO, such as hypertension, preeclampsia, fetal anomalies, placental diseases, or umbilical cord abnormalities, are predisposing factors to an increased risk for stillbirth as well as for having small-for-gestational-age (SGA) babies [117]. SGA birthweight is a condition strongly associated with neonatal morbidity and mortality, but its relationship with GO is unclear, also depending on the SGA classification method used. In this regard, in a large cohort study, SGA, classified using intrauterine references, was associated with a five-fold increased risk of stillbirth among women with obesity, after excluding those with diabetes or hypertension. However, in the same cohort, women with overweight or obesity had a lower risk of SGA than those with a normal weight [118]. On the other hand, a cohort study in a Chinese population, reported a higher SGA risk among women with pre-pregnancy obesity compared to those with normal weight, hypothesizing a possible role of placental inflammation in obesity [119]. Although the pathogenetic mechanisms are still unclear, it has been suggested that the higher risk of stillbirth among women with obesity could be related to metabolic alterations, inflammatory status, or placental dysfunction associated with obesity [120]. A case–control study comparing the placental characteristics in cases of stillbirth and live-born to women with obesity found an association between stillbirth and umbilical cord alterations [121]. GO, particularly severe obesity, is also associated with a higher risk of spontaneous abortion or recurrent miscarriage [122], cesarean section and subsequent risk of infections [123], preterm delivery, both spontaneous and induced [124], transfer to neonatal intensive care units [125], and a low Apgar score at five minutes after birth [116].
With regard to congenital malformations, a meta-analysis reported an increased risk of various structural anomalies, mainly neurological, cardiac, and limb anomalies, among women with obesity compared to women with a recommended BMI [126]. More recently, a large population-based cohort study found that the risk of major congenital anomalies progressively increases with maternal BMI, rising from overweight to class III obesity [127]. Among neurological malformations, fetal spina bifida is the most common, affecting approximately 1 in 3000 births [128]. The prevalence of this congenital anomaly has been reported to be ten times higher among the offspring of women with overweight or obesity compared to those of women with normal weight [129]. Moreover, obesity presents a substantial clinical concern given that the Management of Myelomeningocele Study eligibility criteria preclude prenatal surgery, considered the optimal treatment for fetal spina bifida, for women with a BMI > 35 kg/m2 due to safety issues [130]. However, a recent preliminary single-center study that included women with a BMI > 35 kg/m2 in the fetal spina bifida surgery cohort did not find any relevant difference in clinical outcomes compared to women with a lower BMI [131]. Other neurodevelopmental outcomes, such as attention deficit-hyperactivity disorder, autism spectrum disorder, developmental delay, and emotional and behavioral problems, are observed more frequently among infants born to mothers with GO [132]. The inflammatory status, redox imbalance, high levels of oxidative stress, nutritional deficiencies, changes in the maternal gut microbiota, and epigenetic alterations associated with GO could potentially represent causal factors for the abnormalities observed in neural development among newborn of women with obesity [133]. Although there is still little evidence in the human population, pre-clinical studies have shown that chronic inflammation, metabolic and hormonal changes, including elevated insulin and leptin levels, and alterations in the serotonin and dopamine systems associated with obesity can impact brain development and subsequent functioning, leading to neurological and psychological disorders [132, 134]. Congenital heart disease (CHD), characterized by defects in cardiac development within the first six weeks of pregnancy, is the most common congenital abnormality, with an increasing global birth prevalence and a strong clinical and social impact [135]. Among the various factors contributing to CHD susceptibility, a meta-analysis, incorporating 23 studies that assessed the relationship between CHD risk and maternal BMI, found a significant association between GO and elevated CHD risk in offspring [134]. Possible pathogenetic mechanisms include impaired folate and homocysteine levels, as well as metabolic, hormonal, or inflammatory alterations that may adversely affect the intrauterine environment [134]. Moreover, a recent register cohort study conducted in Finland highlighted that gestational overweight or obesity was specifically associated with an increased risk for complex cardiac defects and outflow tract obstruction defects, suggesting the existence of specific teratogenic mechanisms, different from those linked with GDM, for these obesity-related CHD subtypes [136]. Aside from glycemic dysregulation, other possible mechanisms that may contribute to an increased CHD risk in the offspring of women with GO include an abnormal maternal lipid profile, increased oxidative stress, endothelial dysfunction, and endocardial dysfunction [136]. Other studies have observed a positive association between GO and congenital abnormalities of the kidney and urinary tract in offspring [29, 137]. This group of malformations exposes affected children to a high risk of morbidity and mortality [29, 137]. Additionally, GO has been linked to genital malformations, specifically hypospadias and cryptorchidism, in male offspring, although the underlying mechanisms remain unknown [138]. Fetal macrosomia is defined as a birth weight above the 90th percentile for gestational age, or > 4000 g, and has a prevalence ranging from 0.9 to 12% of all pregnancies. Macrosomia increases the risk of various short-term complications, including preterm birth, postpartum hemorrhage, maternal birth canal trauma, cesarean delivery, shoulder dystocia, fetal asphyxia, and neonatal hypoglycemia. Additionally, it increases the risk for long-term complications such as obesity, type 2 diabetes, metabolic syndrome, and in general, non-communicable diseases [139]. Among the predisposing factors, GO, encompassing both pre-pregnancy obesity and excessive GWG shows a strong association with fetal macrosomia [140]. Maternal insulin resistance and subsequent hyperglycemia associated with obesity are major determinants of fetal macrosomia, leading to increased placental glucose transport and fetal insulin hypersecretion. Insulin, through its anabolic effects, promotes excess fetal growth [141]. Moreover, GO is associated with an increased placental weight compared to women with normal weight [142] and placental weight is associated with birth weight [143]. Finally, it has been reported that there is a higher incidence of viral and bacterial infections among newborns of women with obesity, suggesting a detrimental effect of GO on the fetal immune system [144].
Long-term effects
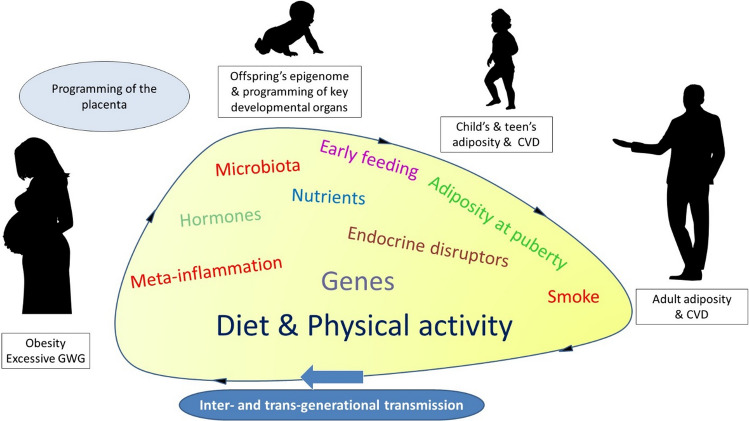
Beyond their immediate implications for maternal and offspring health in the short term, GO and inadequate GWG during pregnancy pose significant risks for offspring health in the long term. Extensive experimental literature in animal models, especially non-human primates [145], and studies in humans have shown that offspring born to mothers with obesity compared to offspring of mothers with a healthy weight are more likely to develop obesity, insulin resistance, cardiovascular diseases, type 2 diabetes [146], and also metabolically associated steatotic liver disease (MASLD) later in life [147]. Additionally, GO has been linked to an increased risk of neurodevelopmental, disorders including autism spectrum disorders [148], poorer cognitive performance [149], and allergic disease in offspring [150].
The effects of GO and GWG on offspring health are believed to be influenced by multiple mechanisms that include mainly shifts in epigenetics such as changes in DNA methylation, histone modifications, and microRNA expression [146, 151]. Indeed, exposure to various maternal factors during pregnancy has been demonstrated to induce permanent alterations in fetal metabolic control processes, serotonin and dopamine pathways, lipid peroxidation, and corticosteroid receptor expression [152] through epigenetic modulation. These alterations encompass, but are not limited to, the hypothalamic response to leptin and subsequent regulation of appetite, as well as pancreatic beta cell physiology [153]. The underlying mechanisms are likely multifaceted and may involve maternal metabolic changes such as variations in glucose and fatty acids [154], modifications in maternal hypothalamic–pituitary–adrenal axis activity [155], inflammation, and alterations in hormonal milieu and placental function. Additionally, other factors contributing to persistent metabolic changes over time include modifications in the offspring gut microbiome, resulting in reduced microbial diversity [156] and the production of short-chain fatty acid metabolites, mirroring the mother’s gut microbiota features [157]. The aberrant gut microbiome, known to be associated with GO, provides a key mechanism to explain not only the immune and metabolic consequences [156] on the developing fetus but also the association between GO and the risk of neurodevelopmental disorders in the offspring [151].
Both greater maternal pre-pregnancy BMI, spanning the entire spectrum, and GWG correlate with increased childhood adiposity and unfavorable body fat distribution [53, 158].
A study of 2432 Australians found that greater maternal GWG was associated with higher offspring BMI at age 21, independent of maternal pre-pregnancy BMI [159]. Similarly, a study in Israel involving 1400 mother–offspring pairs showed that higher maternal pre-pregnancy BMI correlated with increased offspring BMI at age 30, with associations of maternal BMI with cardiovascular risk explained by adult BMI [160]. The Helsinki Birth Cohort Study (HBCS) also indicated a positive association between maternal BMI and offspring BMI at age 60, with higher maternal BMI linked to less favorable body fat distribution in female offspring at around age 62 [161].
Research on registries in Finland and birth records in the UK underscores the role of GO during pregnancy in offspring cardiovascular health [162, 163]. Higher maternal BMI has been linked to increased risks of premature mortality and cardiovascular hospitalizations in adult offspring, independent of socioeconomic status [163]. The HBCS further confirms these findings, showing elevated rates of cardiovascular diseases, coronary heart disease, type 2 diabetes, and stroke among offspring of mothers living with obesity [162].
Studies have sought to determine crucial periods of GWG and their impact on childhood outcomes [146, 164, 165]. Among 5,000 UK mother–offspring pairs, GWG in the first 14 weeks was linked to increased offspring adiposity at age 9 [164]. Similarly, a study with 6,000 Dutch mother–offspring pairs found that early-pregnancy weight gain was associated with an adverse cardiometabolic profile in childhood [165], independent of pre-pregnancy weight gain or later pregnancy weight gain. Godfrey et al. speculate that weight gain in early pregnancy, when maternal fat accumulation is a significant component of total weight gain, may be a critical period for childhood cardiovascular risk [146].
As to MASLD, maternal GWG but not pre-pregnancy BMI has been associated with a risk of hepatic steatosis in carriers of 148 M patatin-like phospholipase domain-containing 3 polymorphisms [166].
GO is associated with a rise in childhood asthma and allergic diseases [167, 168]. A meta-analysis involving 108,321 mother–child pairs found that gestational overweight or obesity during pregnancy increased the risks of childhood asthma or wheezing ever and current asthma or wheezing, independent of offspring BMI [167]. Higher maternal GWG was also linked to increased odds of current asthma or wheezing in offspring. However, GO mainly impacts asthma and wheezing, not eczema, sensitization, or hay fever, suggesting tissue-specific effects [168].
While both maternal pre-pregnancy obesity and excessive GWG appear to correlate with elevated blood pressure, unfavorable lipid profiles, and insulin resistance in childhood, there is some indication that these connections are largely influenced by childhood BMI [165, 169]. In fact, despite the potential for an adverse prenatal environment to heighten the risk of non-communicable diseases from childhood onward, its effects can still be mitigated and reversed through intervention, particularly during childhood and adolescence. These life stages present crucial opportunities for modifying and potentially reversing the risk of adult-onset diseases.
In human studies, a significant hurdle is disentangling intrauterine effects from lingering confounding factors, such as shared genetic variants between mother and offspring, poor dietary choices, sedentary behaviors, and metabolic irregularities, all of which can directly influence fetal development (Fig. 1). Maternal hyperglycemia and excessive GWG are notably impactful risk factors for unfavorable pregnancy outcomes and the health of offspring. Moreover, socioeconomic disparities, including limited healthcare access and insufficient prenatal care, can amplify the impact of GO on offspring health. Postnatal influences on diet/lifestyle behaviors and microbiome-related mechanisms represent additional confounders. Methodological variations, sampling biases, and discrepancies in defining obesity and its outcomes further compound the challenge, often leading to conflicting findings.
Fig. 1.
Long-term effects of GO on offspring health
Nutritional intervention for gestational obesity
Nutritional treatment for women with GO is of paramount importance in managing GWG and reducing associated risks. Meta-analyses have found that diet and lifestyle interventions during pregnancy have only a limited effect on reducing GWG [170], but methodological differences between studies in design, dietary regimen, intensity, and measures of compliance make comparison of results difficult. This lack of consensus limits the ability to develop clinical guidelines and apply the evidence in clinical practice.
One of the main weight intervention strategies involves calorie control. Caloric goals typically range from 1200 to 1800 kcal/day for pregnant women with obesity and are adjusted based on factors such as body weight, gestational age, and level of physical activity [171]. For instance, Renualt et al. prescribed a calorie range of 1200–1675 kcal/day for women with GO [172], while Wolff et al. modified calorie goals based on basal metabolic rate and physical activity level [173]. Both interventions demonstrated significant reductions in GWG without adverse effects on maternal or fetal outcomes [172, 173].
Studies have shown that implementing personalized diets tailored to the needs of pregnant women can effectively limit GWG without compromising the health of the mother or fetus [170]. Using maternal and neonatal data on 275,708 full term and singleton cases, Beyerlein et al. concluded that women with GO were safe to have weight loss during pregnancy; their conclusions were based on the association of GWG and a ≤ 20% predicted risk of having small-for-gestational-age and large-for-gestational-age infants [174]. Additionally, the LARN (Reference Intake Levels of Nutrients and Energy for the Italian population) recommend an increase of 350 and 460 kcal per day during the second and third trimesters for pregnant women [175]. Jebeile et al. found that the energy intake of pregnant women remained the same in early and late pregnancy, and therefore, they recommended that additional energy intake be avoided to prevent excessive GWG [174]. By closely monitoring caloric intake, it is possible to prevent excessive GWG and reduce the likelihood of related health complications. However, it is important to ensure that the diet provides adequate nutrients to support the growth and development of the fetus.
In addition to calorie control, optimizing macronutrient composition is essential to managing GO. Studies have examined the effects of different macronutrient ratios on GWG and maternal health outcomes [176, 177]. Macronutrient goals typically include a balance of protein, fats, and carbohydrates, with specific percentages tailored to individual needs. For example, Petrella et al. set macronutrient goals of 20% protein, 25% fat (6% saturated fat), and 55% carbohydrate for women with GO [176]. Similarly, Thornton et al. aimed for a macronutrient distribution of 30% protein, 30% fat, and 40% carbohydrate [177]. These interventions demonstrated significant reductions in GWG among participants who adhered to the prescribed macronutrient goals [176, 177].
Furthermore, promoting healthy eating patterns and behaviors is crucial to managing GO. Recently, high‐quality large‐scale randomized controlled trials have reported that lifestyle interventions during pregnancy that include diet and exercise advice and behavior change support can reduce excessive GWG and the frequency of large‐for‐gestational‐age babies (LIMIT, UPBEAT, PEARS trials) [178–180]. Lifestyle interventions during pregnancy that include diet and physical activity have also been shown to reduce the risk of pregnancy‐induced hypertension, cesarean delivery, and respiratory distress in neonates [181].
In addition to general nutritional strategies, some specific approaches have emerged that can be used in the nutritional treatment of pregnant women with obesity. For example, a study investigated the effects of a high-protein low-glycemic index (HPLGI) diet on GWG, birth weight, and the risk of gestational complications in women with GO [182]. A total of 279 women with GO were randomly assigned to either a HPLGI or a moderate-protein, moderate-glycemic index (MPMGI) diet. The results showed that women in the HPLGI group had a significantly lower GWG compared to those in the MPMGI group, without significant differences in birth weight. The HPLGI group also experienced a lower incidence of pregnancy complications, including a lower incidence of cesarean delivery [182]. Then, a secondary analysis of the same study mentioned above, aimed to evaluate the association between increased animal protein intake and blood pressure changes in pregnant women with overweight or obesity, indicated that increased animal protein intake, along with a low-glycemic index, was not associated with changes in blood pressure [183].
A study investigated the effects of whole blueberry and soluble fiber supplementation on cardiometabolic profiles in women with GO at high risk of developing GDM [184]. The study included 34 women who were randomly assigned to either the intervention group or the control group. The intervention group received daily supplementation of whole blueberries and soluble fiber in addition to standard prenatal care. The results showed that the dietary intervention group had significantly lower GWG compared to the control group. Additionally, markers of inflammation and blood glucose levels were also lower in the intervention group. However, there were no significant differences between the two groups in terms of conventional lipids or infant birth weight [184].
Finally, in GO, managing glycemic control is critical to preventing both hypoglycemia and hyperglycemia [185]. Nutraceuticals like inositol and probiotics have shown promise in this context [185]. Myo-inositol (MI) and D-chiro-inositol (DCI) have insulin-mimetic properties that may reduce GDM risk, especially when taken early in pregnancy [186]. In women with GO, MI (2000 mg twice daily) has shown potential in stabilizing postprandial glucose and reducing glycemic variability, though more extensive research is needed before widespread recommendations can be made [185]. Probiotics may improve insulin sensitivity by modulating gut microbiota, which is particularly relevant in GO. However, evidence is mixed regarding their impact on fasting glucose and GDM prevention, with varying outcomes depending on the strains, dosage, and timing used [185]. While promising, consistent guidelines for probiotic use in this population remain undeveloped.
In conclusion, setting both calorie and macronutrient goals may facilitate healthy eating behaviors and, consequently, better gestational weight management. Specific approaches, such as high-protein, low-glycemic index diets or supplementation with whole blueberries, soluble fiber, and MI, can be used to achieve positive outcomes in gestational weight control and improve maternal and fetal health outcomes. However, although the collective evidence from previous research indicates that healthy eating strategies can help pregnant women with obesity limit excessive GWG, the details of the most appropriate nutritional intervention for these types of women have yet to be studied.
Pharmacological intervention for gestational obesity
Anti-obesity medications
All the currently approved anti-obesity medications are not recommended during pregnancy or breastfeeding. Indeed, the lack of adequate data on reproductive outcomes does not allow for definitive statements about their safety in human pregnancy.
Animal studies have suggested teratogenic effects for naltrexone/bupropion, liraglutide, semaglutide, and tirzepatide, except for orlistat, that has shown no embryotoxicity or teratogenicity in rats and rabbits even at supratherapeutic doses [187].
In studies on pregnancy outcomes after inadvertent exposure to anti-obesity medications during unintended pregnancies, the use of orlistat for weight reduction seems not to increase birth defects [188], whereas a positive association between early-pregnancy bupropion use and left outflow tract heart defects [189] and fetal cardiac arrhythmia [190] has been reported. No clinical data on liraglutide and semaglutide exposure in human pregnancies or breastfed infants are available. Due to its long half-life, semaglutide should be discontinued at least 2 months before a planned pregnancy [191] while there is no official recommendation for when to stop liraglutide before pregnancy [192].
Off-label obesity pharmacotherapy
Metformin has not been officially approved as an anti-obesity medications because its effect on body weight remains inconsistent, although it is often prescribed to improve insulin resistance in non-diabetic subjects with obesity [193, 194]. In contrast to anti-obesity medications, there are relatively few studies on the use of metformin during human pregnancy and breastfeeding, with different results regarding its safety. Metformin readily crosses the placenta, and its use during the first trimester was associated with an increased risk of many birth defects [195], although other studies have not confirmed these associations. Metformin has been reported to reduce GWG and promote weight loss after delivery [193], but it does not appear to reduce the incidence of GDM or improve other direct markers of maternal outcomes [193, 195]. With regard to offspring outcomes, in a recent meta-analysis, metformin exposure resulted in smaller neonates with an acceleration of postnatal growth and a higher BMI in childhood, so guidelines do not recommend metformin as the first-line treatment for GDM [196] and suggest caution, especially in pregnant women with hypertensive disorders or at risk for intrauterine growth restriction [197, 198]. Metformin is detectable in breastmilk, but prospective studies have shown no adverse effects on breastfed infants, although prudence is recommended in mothers with premature infants or with renal impairment [199].
Conclusion
GO is a threat to both the mother's and fetus’ health and could predispose to the onset of short-term and long-term complications. In order to reduce the risk of developing GO, it is advisable to maintain a healthy diet, exercise, reach a normal weight, and keep GWG within the allowed ranges during pregnancy. Table 3 provides a comprehensive overview of recommendations at each step of pregnancy to manage GO effectively.
Table 3.
Recommendations for pregnancy management in women with GO
| Stage of Pregnancy | Recommendations |
|---|---|
| Before pregnancy |
Risk factors definition: Measure BMI, BP, FBG, and lipids; collect medical history; consider age and ethnicity Counseling: Inform about immediate and long-term risks of obesity; encourage weight reduction (5–10% over 6 months) Treatment: Consider lifestyle intervention, anti-obesity medications, bariatric surgery, hypertension treatment (BP ≥ 135/85 mmHg), and dyslipidemia treatment. Women with BMI ≥ 30 kg/m2 should take folic acid (400 μg or 5 mg/day) starting 1–3 months before conception and continuing through the first trimester |
| During pregnancy |
Counseling: Inform about optimal ranges of GWG based on pre-pregnancy BMI Monitoring (mothers): Frequent control of weight, BP, FBG, urine tests; closer nutritional status monitoring if bariatric surgery was done Monitoring (Fetus): Periodic assessment of Doppler velocimetry, fetal growth, and size via ultrasound Treatment: Manage overt diabetes and hypertension (BP ≥ 140/90 mmHg outpatient, BP ≥ 135/85 mmHg at home) Screening for GDM: OGTT at 16–18 weeks; if negative, repeat at 24–28 weeks. Early screening for preeclampsia: Evaluate additional major risk factors in women with BMI ≥ 30 kg/m2; consider acetylsalicylic acid (100–150 mg/day) and calcium (600 mg/day) by the 16th week; increase fetal growth assessment frequency Labor and Delivery: Multidisciplinary management for women with BMI ≥ 35 kg/m2 |
| After delivery |
Cesarean delivery: Prescribe prophylactic antibiotics at the time of surgery; adjust pharmacologic thromboprophylaxis based on body weight Women with GDM: FBP monitoring soon after delivery. Preeclampsia: Monitor within the first 2 h and continue medical treatment post-discharge |
| After pregnancy |
Breastfeeding: Support initiation and maintenance Screening: Check for postpartum mental health issues (depression, anxiety) Counseling: Emphasize importance of post-pregnancy weight loss to reduce future risks. Monitoring: If GDM occurred, repeat OGTT within 6–12 weeks. For preeclampsia, periodic BP assessments and a medical evaluation after 3 months |
| Postpartum follow-up care |
GDM: Diabetes screening (OGTT, FPG, or HbA1C) from 4 to 12 weeks postpartum and every 1–3 years based on risk factors. OGTT is preferred early postpartum Hypertensive disorders: Physical examination, BP measurements, screening for cardiovascular risk factors (serum lipids, blood glucose) 6–12 weeks after birth and annually thereafter Liver Assessment: For women with MASLD Cancer Screening: For obesity-related cancers Renal Function: Laboratory testing of eGFR, BUN, creatinine, and microalbuminuria at 3 months postpartum and every 3–5 years Obstructive Sleep Apnea: Assessment and treatment Mental Health: Screening for postpartum depression and anxiety |
BMI: body mass index; BP: blood pressure; FBG: fasting blood glucose; GWG: gestational weight gain; GDM: gestational diabetes mellitus; OGTT: oral glucose tolerance test; MASLD: metabolically associated steatotic liver disease; eGFR: estimated glomerular filtration rate; BUN: blood urea nitrogen
Strength and limits
The key strength of this position statement of SIO lies in its comprehensive analysis of GO and the associated maternal and fetal complications. This extensive examination provides detailed insights into the risks and underlying mechanisms of GO. The robustness of the data supports its findings. However, this study is limited by the significant heterogeneity of the included studies. Variations in diagnostic criteria, measurement methods, and population characteristics across studies may impact the comparability and generalizability of the results. Additionally, the retrospective design of many included studies poses challenges for establishing causality.
What is already known on this subject?
Prior to this study, extensive research indicated that gestational GO and excessive GWG are significantly associated with adverse maternal and fetal outcomes, including GDM, hypertensive disorders, preeclampsia, and fetal macrosomia. These adverse outcomes have both short-term and long-term health implications for both mothers and their offspring.
What this study adds?
This study provides a more nuanced understanding of the impacts of GO on maternal and fetal health, emphasizing the increased risks associated with higher classes of obesity. It elucidates the differential effects of various obesity classes, advocating for more precise and individualized GWG recommendations for women with different obesity levels to mitigate adverse outcomes. The findings highlight the critical need for personalized lifestyle interventions, including tailored dietary and physical activity programs, to improve pregnancy outcomes. These results suggest that more individualized prenatal care guidelines and targeted interventions are essential for enhancing the health of pregnant women with GO and their children, potentially informing future policy and clinical practice guidelines.
Author contributions
Giovanna Muscogiuri and Luigi Barrea contributed to the conception and design of the study. All authors contributed to the first draft of the manuscript, and all authors commented on earlier versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barrea L, Vetrani C, Verde L, Frias-Toral E, Garcia-Velasquez E, Ranasinghe P et al (2022) Gestational obesity: An unconventional endocrine disruptor for the fetus. Biochem Pharmacol 198:114974 [DOI] [PubMed] [Google Scholar]
- 2.Simon A, Pratt M, Hutton B, Skidmore B, Fakhraei R, Rybak N et al (2020) Guidelines for the management of pregnant women with obesity: a systematic review. Obes Rev 21(3):e12972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Most J, Marlatt KL, Altazan AD, Redman LM (2018) Advances in assessing body composition during pregnancy. Eur J Clin Nutr 72(5):645–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantham P, Aye IL, Powell TL (2015) Inflammation in maternal obesity and gestational diabetes mellitus. Placenta 36(7):709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichetzeder C (2021) Overweight and obesity in pregnancy: their impact on epigenetics. Eur J Clin Nutr 75(12):1710–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma RCW, Schmidt MI, Tam WH, McIntyre HD, Catalano PM (2016) Clinical management of pregnancy in the obese mother: before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol 4(12):1037–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obesity: preventing and managing the global epidemic. Report of a WHO consultation (2000) World Health Organ Tech Rep Ser 894:i–xii, 1–253 [PubMed]
- 8.Obesity in pregnancy: ACOG practice bulletin, number 230 (2021) Obstet Gynecol 137(6):e128–e44 [DOI] [PubMed]
- 9.Denison FC, Aedla NR, Keag O, Hor K, Reynolds RM, Milne A et al (2019) Care of women with obesity in pregnancy: green-top guideline No. 72. BJOG 126(3):e62–e106 [DOI] [PubMed] [Google Scholar]
- 10.Maxwell C, Gaudet L, Cassir G, Nowik C, McLeod NL, Jacob CE et al (2019) Guideline No. 391-pregnancy and maternal obesity part 1: pre-conception and prenatal care. J Obstet Gynaecol Can 41(11):1623–40 [DOI] [PubMed] [Google Scholar]
- 11.McAuliffe FM, Killeen SL, Jacob CM, Hanson MA, Hadar E, McIntyre HD et al (2020) Management of prepregnancy, pregnancy, and postpartum obesity from the FIGO Pregnancy and Non-Communicable Diseases Committee: A FIGO (International Federation of Gynecology and Obstetrics) guideline. Int J Gynaecol Obstet 151(Suppl 1):16–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group, Voerman E, Santos S, Inskip H, Amiano P et al (2019) Association of gestational weight gain with adverse maternal and infant outcomes. JAMA 321(17):1702–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pani P, Carletti C, Giangreco M, Knowles A, Clagnan E, Gobbato M et al (2023) Monitoring gestational weight gain: setting up a regional surveillance system in Italy. BMC Public Health 23(1):132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price S, Nankervis A, Permezel M, Prendergast L, Sumithran P, Proietto J (2018) Health consequences for mother and baby of substantial pre-conception weight loss in obese women: study protocol for a randomized controlled trial. Trials 19(1):248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto M, Aoki S, Shinoda S, Ishikawa H, Miyagi E (2024) Impact of interpregnancy weight changes and perinatal outcomes: a retrospective study in Japan. PLoS ONE 19(2):e0299794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson K, Cnattingius S, Naslund I, Roos N, Trolle Lagerros Y, Granath F et al (2015) Outcomes of pregnancy after bariatric surgery. N Engl J Med 372(9):814–824 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization (1995) Maternal anthropometry and pregnancy outcomes: a WHO collaborative study. Bull World Health Organ 73(Suppl):1–98 [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH et al (2017) Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA 317(21):2207–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen KM, Yaktine AL (eds) (2009) Weight gain during pregnancy: reexamining the guidelines. The National Academies Collection: Reports funded by National Institutes of Health, Washington (DC) [PubMed] [Google Scholar]
- 20.Hinkle SN, Mumford SL, Grantz KL, Mendola P, Mills JL, Yeung EH et al (2023) Gestational weight change in a diverse pregnancy cohort and mortality over 50 years: a prospective observational cohort study. Lancet 402(10415):1857–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Victor A, de Franca da Silva Teles L, Aires IO, de Carvalho LF, Luzia LA, Artes R et al (2024) The impact of gestational weight gain on fetal and neonatal outcomes: the Araraquara Cohort Study. BMC Pregnancy Childbirth 24(1):320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devlieger R, Ameye L, Nuyts T, Goemaes R, Bogaerts A (2020) Reappraisal of gestational weight gain recommendations in obese pregnant women: a population-based study of 337,590 births. Obes Facts 13(4):333–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL (2007) Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstet Gynecol 110(4):752–758 [DOI] [PubMed] [Google Scholar]
- 24.Committee on Nutritional Status During Pregnancy, Lactation (1990) Nutrition during pregnancy: part I weight gain: part II nutrient supplements. National Academies Press, Washington (DC) [PubMed] [Google Scholar]
- 25.Most J, Altazan AD, Hsia DS, Beyl RA, Redman LM (2020) Body composition during pregnancy differs by obesity class. Obesity 28(2):268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maran A, Atkinson SA, Bertram V, Vanniyasingam T, Thabane L, Mottola MF et al (2022) Exploring comparative assessment of adiposity measures during pregnancy and postpartum. Clin Nutr ESPEN 49:365–371 [DOI] [PubMed] [Google Scholar]
- 27.Widen EM, Gallagher D (2014) Body composition changes in pregnancy: measurement, predictors and outcomes. Eur J Clin Nutr 68(6):643–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai M, Susic D, O’Sullivan AJ, Henry A (2020) Reproducibility of bioelectrical impedance analysis in pregnancy and the association of body composition with the risk of gestational diabetes: a substudy of MUMS cohort. J Obes 2020:3128767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fattah C, Farah N, Barry S, O’Connor N, Stuart B, Turner MJ (2009) The measurement of maternal adiposity. J Obstet Gynaecol 29(8):686–689 [DOI] [PubMed] [Google Scholar]
- 30.Lorenzo AD, Andreoli A (2003) Segmental bioelectrical impedance analysis. Curr Opin Clin Nutr Metab Care 6(5):551–555 [DOI] [PubMed] [Google Scholar]
- 31.De Souza LR, Berger H, Retnakaran R, Maguire JL, Nathens AB, Connelly PW et al (2016) First-trimester maternal abdominal adiposity predicts dysglycemia and gestational diabetes mellitus in midpregnancy. Diabetes Care 39(1):61–64 [DOI] [PubMed] [Google Scholar]
- 32.Rahnemaei FA, Abdi F, Pakzad R, Sharami SH, Mokhtari F, Kazemian E (2022) Association of body composition in early pregnancy with gestational diabetes mellitus: a meta-analysis. PLoS ONE 17(8):e0271068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heslehurst N, Ngongalah L, Bigirumurame T, Nguyen G, Odeniyi A, Flynn A et al (2022) Association between maternal adiposity measures and adverse maternal outcomes of pregnancy: systematic review and meta-analysis. Obes Rev 23(7):e13449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alves FCR, Moreira A, Moutinho O (2024) Maternal and long-term offspring outcomes of obesity during pregnancy. Arch Gynecol Obstet. 10.1007/s00404-023-07349-2 [DOI] [PubMed] [Google Scholar]
- 35.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS et al (2005) Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 352(24):2477–2486 [DOI] [PubMed] [Google Scholar]
- 36.Sadikot S, Purandare CN, Cho NH, Hod M (2018) FIGO-IDF joint statement and declaration on hyperglycemia in pregnancy. Diabetes Res Clin Pract 145:1–4 [DOI] [PubMed] [Google Scholar]
- 37.Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH et al (2004) Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. Am J Obstet Gynecol 190(4):1091–1097 [DOI] [PubMed] [Google Scholar]
- 38.Bidhendi Yarandi R, Vaismoradi M, Panahi MH, Gare Kymre I, Behboudi-Gandevani S (2021) Mild gestational diabetes and adverse pregnancy outcome: a systemic review and meta-analysis. Front Med 8:699412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moon JH, Jang HC (2022) Gestational diabetes mellitus: diagnostic approaches and maternal-offspring complications. Diabetes Metab J 46(1):3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Zheng W, Huang W, Zhang L, Liang X, Li G (2022) Differing risk factors for new onset and recurrent gestational diabetes mellitus in multipara women: a cohort study. BMC Endocr Disord 22(1):3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.AMDA-SIdD (SID) (2018) Standard Italiani per la cura del diabete mellito 2018. https://aemmedi.it/wp-content/uploads/2009/06/AMD-Standard-unico1.pdf.
- 42.Gaillard R, Jaddoe VWV (2023) Maternal cardiovascular disorders before and during pregnancy and offspring cardiovascular risk across the life course. Nat Rev Cardiol 20(9):617–630 [DOI] [PubMed] [Google Scholar]
- 43.Khedagi AM, Bello NA (2021) Hypertensive Disorders of Pregnancy. Cardiol Clin 39(1):77–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hopkins MK, Levine LD, Koelper NC, Durnwald C (2022) Screening echocardiogram in high-risk women with class III obesity to predict the risk of preeclampsia. Am J Perinatol 39(5):457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ballesta-Castillejos A, Gomez-Salgado J, Rodriguez-Almagro J, Ortiz-Esquinas I, Hernandez-Martinez A (2020) Relationship between maternal body mass index and obstetric and perinatal complications. J Clin Med 9(3):707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez-Plaza E, Bellart J, Martinez-Verdu MA, Arranz A, Lujan-Barroso L, Seguranyes G (2021) Pre-pregnancy overweight and obesity prevalence and relation to maternal and perinatal outcomes. Enferm Clin. 10.1016/j.enfcle.2021.04.006 [DOI] [PubMed] [Google Scholar]
- 47.Robinson HE, O’Connell CM, Joseph KS, McLeod NL (2005) Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol 106(6):1357–1364 [DOI] [PubMed] [Google Scholar]
- 48.Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW et al (2001) Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 25(8):1175–1182 [DOI] [PubMed] [Google Scholar]
- 49.Blondon M, Harrington LB, Boehlen F, Robert-Ebadi H, Righini M, Smith NL (2016) Pre-pregnancy BMI, delivery BMI, gestational weight gain and the risk of postpartum venous thrombosis. Thromb Res 145:151–156 [DOI] [PubMed] [Google Scholar]
- 50.Kevane B, Donnelly J, D’Alton M, Cooley S, Preston RJ, Ni AF (2014) Risk factors for pregnancy-associated venous thromboembolism: a review. J Perinat Med 42(4):417–425 [DOI] [PubMed] [Google Scholar]
- 51.Molyneaux E, Poston L, Ashurst-Williams S, Howard LM (2014) Obesity and mental disorders during pregnancy and postpartum: a systematic review and meta-analysis. Obstet Gynecol 123(4):857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown HL, Warner JJ, Gianos E, Gulati M, Hill AJ, Hollier LM et al (2018) Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation 137(24):e843–e852 [DOI] [PubMed] [Google Scholar]
- 53.Gaillard R (2015) Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur J Epidemiol 30(11):1141–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramlakhan KP, Johnson MR, Roos-Hesselink JW (2020) Pregnancy and cardiovascular disease. Nat Rev Cardiol 17(11):718–731 [DOI] [PubMed] [Google Scholar]
- 55.Poniedzialek-Czajkowska E, Mierzynski R, Leszczynska-Gorzelak B (2023) Preeclampsia and obesity—the preventive role of exercise. Int J Environ Res Public Health 20(2):1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schiavone MJ, Perez MP, Aquieri A, Nosetto D, Pronotti MV, Mazzei M et al (2024) The role of obesity in the development of preeclampsia. Curr Hypertens Rep. 10.1007/s11906-024-01299-z [DOI] [PubMed] [Google Scholar]
- 57.Lopez-Jaramillo P, Barajas J, Rueda-Quijano SM, Lopez-Lopez C, Felix C (2018) Obesity and preeclampsia: common pathophysiological mechanisms. Front Physiol 9:1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rogers BN, Stephens JM, Sones JL (2022) Linking inflammatory adipose tissue to placental abnormalities in obese preeclamptic pregnancies. Physiol Genomics 54(8):319–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spradley FT, Palei AC, Granger JP (2015) Increased risk for the development of preeclampsia in obese pregnancies: weighing in on the mechanisms. Am J Physiol Regul Integr Comp Physiol 309(11):R1326–R1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Makhijani P, Basso PJ, Chan YT, Chen N, Baechle J, Khan S et al (2023) Regulation of the immune system by the insulin receptor in health and disease. Front Endocrinol 14:1128622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee SM, Moon JY, Lim BY, Kim SM, Park CW, Kim BJ et al (2019) Increased biosynthesis and accumulation of cholesterol in maternal plasma, but not amniotic fluid in pre-eclampsia. Sci Rep 9(1):1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White SL, Koulman A, Ozanne SE, Furse S, Poston L, Meek CL (2023) Towards precision medicine in gestational diabetes: pathophysiology and glycemic patterns in pregnant women with obesity. J Clin Endocrinol Metab 108(10):2643–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alston MC, Redman LM, Sones JL (2022) An overview of obesity, cholesterol, and systemic inflammation in preeclampsia. Nutrients 14(10):2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olson KN, Redman LM, Sones JL (2019) Obesity “complements” preeclampsia. Physiol Genomics 51(3):73–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eghbal-Fard S, Yousefi M, Heydarlou H, Ahmadi M, Taghavi S, Movasaghpour A et al (2019) The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia. J Cell Physiol 234(4):5106–5116 [DOI] [PubMed] [Google Scholar]
- 66.AIP (AIPE). I disordini ipertensivi in gravidanza: classificazione, diagnosi e terapia. Raccomandazioni di buona pratica clinica 2020. https://www.sigo.it/wp-content/uploads/2020/11/RaccomandazioniAIPE-Disordini_Ipertensivi_Gravidanza.pdf.
- 67.Amir LH, Donath S (2007) A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy Childbirth 7:9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turcksin R, Bel S, Galjaard S, Devlieger R (2014) Maternal obesity and breastfeeding intention, initiation, intensity and duration: a systematic review. Matern Child Nutr 10(2):166–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colleluori G, Perugini J, Barbatelli G, Cinti S (2021) Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev Endocr Metab Disord 22(2):241–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma X, Zhao LG, Sun JW, Yang Y, Zheng JL, Gao J et al (2018) Association between breastfeeding and risk of endometrial cancer: a meta-analysis of epidemiological studies. Eur J Cancer Prev 27(2):144–151 [DOI] [PubMed] [Google Scholar]
- 71.Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB (2005) Duration of lactation and incidence of type 2 diabetes. JAMA 294(20):2601–2610 [DOI] [PubMed] [Google Scholar]
- 72.Schwarz EB, Brown JS, Creasman JM, Stuebe A, McClure CK, Van Den Eeden SK et al (2010) Lactation and maternal risk of type 2 diabetes: a population-based study. Am J Med 123(9):863.e1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang M, Gao H, Vinyes-Pares G, Yu K, Ma D, Qin X et al (2018) Association between breastfeeding duration and postpartum weight retention of lactating mothers: a meta-analysis of cohort studies. Clin Nutr 37(4):1224–1231 [DOI] [PubMed] [Google Scholar]
- 74.Ahluwalia IB, Li R, Morrow B (2012) Breastfeeding practices: does method of delivery matter? Matern Child Health J 16(Suppl 2):231–237 [DOI] [PubMed] [Google Scholar]
- 75.Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG (2010) Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr 92(3):574–584 [DOI] [PubMed] [Google Scholar]
- 76.Poston L, Caleyachetty R, Cnattingius S, Corvalan C, Uauy R, Herring S et al (2016) Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol 4(12):1025–1036 [DOI] [PubMed] [Google Scholar]
- 77.Gunderson EP (2009) Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin N Am 36(2):317–32, ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cnattingius S, Villamor E (2016) Weight change between successive pregnancies and risks of stillbirth and infant mortality: a nationwide cohort study. Lancet 387(10018):558–565 [DOI] [PubMed] [Google Scholar]
- 79.Jain AP, Gavard JA, Rice JJ, Catanzaro RB, Artal R, Hopkins SA (2013) The impact of interpregnancy weight change on birthweight in obese women. Am J Obstet Gynecol 208(3):205.e1–7 [DOI] [PubMed] [Google Scholar]
- 80.Callegari LS, Sterling LA, Zelek ST, Hawes SE, Reed SD (2014) Interpregnancy body mass index change and success of term vaginal birth after cesarean delivery. Am J Obstet Gynecol 210(4):330.e1-e7 [DOI] [PubMed] [Google Scholar]
- 81.Siega-Riz AM, Herring AH, Carrier K, Evenson KR, Dole N, Deierlein A (2010) Sociodemographic, perinatal, behavioral, and psychosocial predictors of weight retention at 3 and 12 months postpartum. Obesity 18(10):1996–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mamun AA, Kinarivala M, O’Callaghan MJ, Williams GM, Najman JM, Callaway LK (2010) Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: evidence from 21 y postpartum follow-up. Am J Clin Nutr 91(5):1336–1341 [DOI] [PubMed] [Google Scholar]
- 83.Amorim AR, Rossner S, Neovius M, Lourenco PM, Linne Y (2007) Does excess pregnancy weight gain constitute a major risk for increasing long-term BMI? Obesity 15(5):1278–1286 [DOI] [PubMed] [Google Scholar]
- 84.Rasmussen KM, Abrams B, Bodnar LM, Butte NF, Catalano PM, Maria S-R (2010) Recommendations for weight gain during pregnancy in the context of the obesity epidemic. Obstet Gynecol 116(5):1191–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joseph NP, Hunkali KB, Wilson B, Morgan E, Cross M, Freund KM (2008) Pre-pregnancy body mass index among pregnant adolescents: gestational weight gain and long-term post partum weight retention. J Pediatr Adolesc Gynecol 21(4):195–200 [DOI] [PubMed] [Google Scholar]
- 86.Rooney BL, Schauberger CW, Mathiason MA (2005) Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol 106(6):1349–1356 [DOI] [PubMed] [Google Scholar]
- 87.Ostbye T, Krause KM, Swamy GK, Lovelady CA (2010) Effect of breastfeeding on weight retention from one pregnancy to the next: results from the North Carolina WIC program. Prev Med 51(5):368–372 [DOI] [PubMed] [Google Scholar]
- 88.Gunderson EP, Murtaugh MA, Lewis CE, Quesenberry CP, West DS, Sidney S (2004) Excess gains in weight and waist circumference associated with childbearing: the Coronary Artery Risk Development in Young Adults Study (CARDIA). Int J Obes Relat Metabol Disord 28(4):525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soltani H, Fraser RB (2000) A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. Br J Nutr 84(1):95–101 [DOI] [PubMed] [Google Scholar]
- 90.Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE (1994) Longitudinal changes in adiposity associated with pregnancy: the CARDIA study—coronary artery risk development in young adults study. JAMA 271(22):1747–51 [PubMed] [Google Scholar]
- 91.Williamson DF, Madans J, Pamuk E, Flegal KM, Kendrick JS, Serdula MK (1994) A prospective study of childbearing and 10-year weight gain in US white women 25 to 45 years of age. Int J Obes Relat Metabol Disord 18(8):561–569 [PubMed] [Google Scholar]
- 92.Kim C, Newton KM, Knopp RH (2002) Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 25(10):1862–1868 [DOI] [PubMed] [Google Scholar]
- 93.Catalano PM (2010) Obesity, insulin resistance, and pregnancy outcome. Reproduction 140(3):365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S (2016) Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia 59(7):1403–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim C, Berger DK, Chamany S (2007) Recurrence of gestational diabetes mellitus: a systematic review. Diabetes Care 30(5):1314–1319 [DOI] [PubMed] [Google Scholar]
- 96.Bellamy L, Casas JP, Hingorani AD, Williams D (2009) Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373(9677):1773–1779 [DOI] [PubMed] [Google Scholar]
- 97.Retnakaran R, Shah BR (2019) Glucose screening in pregnancy and future risk of cardiovascular disease in women: a retrospective, population-based cohort study. Lancet Diabetes Endocrinol 7(5):378–384 [DOI] [PubMed] [Google Scholar]
- 98.Xu Y, Shen S, Sun L, Yang H, Jin B, Cao X (2014) Metabolic syndrome risk after gestational diabetes: a systematic review and meta-analysis. PLoS ONE 9(1):e87863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gunderson EP, Lewis CE, Murtaugh MA, Quesenberry CP, Smith West D, Sidney S (2004) Long-term plasma lipid changes associated with a first birth: the Coronary Artery Risk Development in Young Adults study. Am J Epidemiol 159(11):1028–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fraser A, Tilling K, Macdonald-Wallis C, Hughes R, Sattar N, Nelson SM et al (2011) Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC). Am J Clin Nutr 93(6):1285–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reed J, Case S, Rijhsinghani A (2023) Maternal obesity: perinatal implications. SAGE Open Med 11:20503121231176130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lisonkova S, Muraca GM, Potts J, Liauw J, Chan WS, Skoll A et al (2017) Association between prepregnancy body mass index and severe maternal morbidity. JAMA 318(18):1777–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Langley-Evans SC, Pearce J, Ellis S (2022) Overweight, obesity and excessive weight gain in pregnancy as risk factors for adverse pregnancy outcomes: a narrative review. J Hum Nutr Diet 35(2):250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.La Verde M, Riemma G, Torella M, Cianci S, Savoia F, Licciardi F et al (2021) Maternal death related to COVID-19: a systematic review and meta-analysis focused on maternal co-morbidities and clinical characteristics. Int J Gynaecol Obstet 154(2):212–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.D’Souza R, Horyn I, Pavalagantharajah S, Zaffar N, Jacob CE (2019) Maternal body mass index and pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol MFM 1(4):100041 [DOI] [PubMed] [Google Scholar]
- 106.Brown NT, Turner JM, Kumar S (2018) The intrapartum and perinatal risks of sleep-disordered breathing in pregnancy: a systematic review and metaanalysis. Am J Obstet Gynecol 219(2):147–61.e1 [DOI] [PubMed] [Google Scholar]
- 107.Pavlik LB, Rosculet K (2020) Maternal obesity and perinatal depression: an updated literature review. Cureus 12(9):e10736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Valckx SD, De Pauw I, De Neubourg D, Inion I, Berth M, Fransen E et al (2012) BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum Reprod 27(12):3531–3539 [DOI] [PubMed] [Google Scholar]
- 109.Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J et al (2018) Origins of lifetime health around the time of conception: causes and consequences. Lancet 391(10132):1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leary C, Leese HJ, Sturmey RG (2015) Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod 30(1):122–132 [DOI] [PubMed] [Google Scholar]
- 111.Howell KR, Powell TL (2017) Effects of maternal obesity on placental function and fetal development. Reproduction 153(3):R97–R108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kelly AC, Powell TL, Jansson T (2020) Placental function in maternal obesity. Clin Sci 134(8):961–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Denizli M, Capitano ML, Kua KL (2022) Maternal obesity and the impact of associated early-life inflammation on long-term health of offspring. Front Cell Infect Microbiol 12:940937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aune D, Saugstad OD, Henriksen T, Tonstad S (2014) Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA 311(15):1536–1546 [DOI] [PubMed] [Google Scholar]
- 115.Flenady V, Koopmans L, Middleton P, Froen JF, Smith GC, Gibbons K et al (2011) Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet 377(9774):1331–1340 [DOI] [PubMed] [Google Scholar]
- 116.Akselsson A, Rossen J, Storck-Lindholm E, Radestad I (2023) Prolonged pregnancy and stillbirth among women with overweight or obesity—a population-based study in Sweden including 64,632 women. BMC Pregnancy Childbirth 23(1):21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eberle A, Czuzoj-Shulman N, Abenhaim HA (2022) Timing of delivery in obese women and risk of stillbirth. J Matern Fetal Neonatal Med 35(25):7771–7777 [DOI] [PubMed] [Google Scholar]
- 118.Hinkle SN, Sjaarda LA, Albert PS, Mendola P, Grantz KL (2016) Comparison of methods for identifying small-for-gestational-age infants at risk of perinatal mortality among obese mothers: a hospital-based cohort study. BJOG 123(12):1983–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen YH, Li L, Chen W, Liu ZB, Ma L, Gao XX et al (2019) Pre-pregnancy underweight and obesity are positively associated with small-for-gestational-age infants in a Chinese population. Sci Rep 9(1):15544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Woolner AM, Bhattacharya S (2015) Obesity and stillbirth. Best Pract Res Clin Obstet Gynaecol 29(3):415–426 [DOI] [PubMed] [Google Scholar]
- 121.Amark H, Westgren M, Sirotkina M, Hulthen Varli I, Persson M, Papadogiannakis N (2021) Maternal obesity and stillbirth at term; placental pathology—a case control study. PLoS ONE 16(4):e0250983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goncalves C, Feitosa BM, Cavalcante BV, Lima A, de Souza CM, Joventino LB et al (2023) Obesity and recurrent miscarriage: the interconnections between adipose tissue and the immune system. Am J Reprod Immunol 90(3):e13757 [DOI] [PubMed] [Google Scholar]
- 123.Krogh LQ, Glavind J, Henriksen TB, Thornton J, Fuglsang J, Boie S (2023) Full-term induction of labor vs expectant management and cesarean delivery in women with obesity: systematic review and meta-analysis. Am J Obstet Gynecol MFM 5(5):100909 [DOI] [PubMed] [Google Scholar]
- 124.Tersigni C, Neri C, D’Ippolito S, Garofalo S, Martino C, Lanzone A et al (2022) Impact of maternal obesity on the risk of preterm delivery: insights into pathogenic mechanisms. J Matern Fetal Neonatal Med 35(16):3216–3221 [DOI] [PubMed] [Google Scholar]
- 125.Liu P, Xu L, Wang Y, Zhang Y, Du Y, Sun Y et al (2016) Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes Rev 17(11):1091–1102 [DOI] [PubMed] [Google Scholar]
- 126.Stothard KJ, Tennant PW, Bell R, Rankin J (2009) Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA 301(6):636–650 [DOI] [PubMed] [Google Scholar]
- 127.Persson M, Cnattingius S, Villamor E, Soderling J, Pasternak B, Stephansson O et al (2017) Risk of major congenital malformations in relation to maternal overweight and obesity severity: cohort study of 1.2 million singletons. BMJ 357:j2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Copp AJ, Adzick NS, Chitty LS, Fletcher JM, Holmbeck GN, Shaw GM (2015) Spina bifida. Nat Rev Dis Primers 1:15007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang L, Zhang Y, Li Z, Ren A, Liu J, Ye R (2021) Maternal periconceptional body mass index and risk for neural tube defects: results from a large cohort study in China. J Matern Fetal Neonatal Med 34(2):274–280 [DOI] [PubMed] [Google Scholar]
- 130.Sacco A, Simpson L, Deprest J, David AL (2018) A study to assess global availability of fetal surgery for myelomeningocele. Prenat Diagn 38(13):1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zepf J, Vonzun L, Ruegg L, Strubing N, Krahenmann F, Meuli M et al (2024) Fetal spina bifida repair in obese mothers: is maternal and fetal safety compromised? Fetal Diagn Ther 51(2):175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sanchez CE, Barry C, Sabhlok A, Russell K, Majors A, Kollins SH et al (2018) Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obes Rev 19(4):464–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Basak S, Das RK, Banerjee A, Paul S, Pathak S, Duttaroy AK (2022) Maternal obesity and gut microbiota are associated with fetal brain development. Nutrients 14(21):4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu L, Li N, Liu Y (2023) Association between maternal factors and risk of congenital heart disease in offspring: a systematic review and meta-analysis. Matern Child Health J 27(1):29–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu Y, Chen S, Zuhlke L, Black GC, Choy MK, Li N et al (2019) Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol 48(2):455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Turunen R, Pulakka A, Metsala J, Vahlberg T, Ojala T, Gissler M et al (2024) Maternal diabetes and overweight and congenital heart defects in offspring. JAMA Netw Open 7(1):e2350579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Macumber I, Schwartz S, Leca N (2017) Maternal obesity is associated with congenital anomalies of the kidney and urinary tract in offspring. Pediatr Nephrol 32(4):635–642 [DOI] [PubMed] [Google Scholar]
- 138.Arendt LH, Ramlau-Hansen CH, Lindhard MS, Henriksen TB, Olsen J, Yu Y et al (2017) Maternal overweight and obesity and genital anomalies in male offspring: a population-based Swedish cohort study. Paediatr Perinat Epidemiol 31(4):317–327 [DOI] [PubMed] [Google Scholar]
- 139.Pereda J, Bove I, Pineyro MM (2020) Excessive maternal weight and diabetes are risk factors for macrosomia: a cross-sectional study of 42,663 pregnancies in Uruguay. Front Endocrinol 11:588443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jenabi E, Salehi AM, Farashi S, Salimi Z (2023) The environmental risk factors associated with fetal macrosomia: an umbrella review. Pediatr Neonatol. 10.1016/j.pedneo.2023.09.007 [DOI] [PubMed] [Google Scholar]
- 141.Ahlsson F, Diderholm B, Jonsson B, Norden-Lindberg S, Olsson R, Ewald U et al (2010) Insulin resistance, a link between maternal overweight and fetal macrosomia in nondiabetic pregnancies. Horm Res Paediatr 74(4):267–274 [DOI] [PubMed] [Google Scholar]
- 142.Swanson LD, Bewtra C (2008) Increase in normal placental weights related to increase in maternal body mass index. J Matern Fetal Neonatal Med 21(2):111–113 [DOI] [PubMed] [Google Scholar]
- 143.Flatley C, Sole-Navais P, Vaudel M, Helgeland O, Modzelewska D, Johansson S et al (2022) Placental weight centiles adjusted for age, parity and fetal sex. Placenta 117:87–94 [DOI] [PubMed] [Google Scholar]
- 144.Yong W, Wang J, Leng Y, Li L, Wang H (2023) Role of obesity in female reproduction. Int J Med Sci 20(3):366–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dunn GA, Mitchell AJ, Selby M, Fair DA, Gustafsson HC, Sullivan EL (2022) Maternal diet and obesity shape offspring central and peripheral inflammatory outcomes in juvenile non-human primates. Brain Behav Immun 102:224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG et al (2017) Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol 5(1):53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hagstrom H, Simon TG, Roelstraete B, Stephansson O, Soderling J, Ludvigsson JF (2021) Maternal obesity increases the risk and severity of NAFLD in offspring. J Hepatol 75(5):1042–1048 [DOI] [PubMed] [Google Scholar]
- 148.Li YM, Ou JJ, Liu L, Zhang D, Zhao JP, Tang SY (2016) Association between maternal obesity and autism spectrum disorder in offspring: a meta-analysis. J Autism Dev Disord 46(1):95–102 [DOI] [PubMed] [Google Scholar]
- 149.Han VX, Patel S, Jones HF, Dale RC (2021) Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat Rev Neurol 17(9):564–579 [DOI] [PubMed] [Google Scholar]
- 150.Dumas O, Varraso R, Gillman MW, Field AE, Camargo CA Jr (2016) Longitudinal study of maternal body mass index, gestational weight gain, and offspring asthma. Allergy 71(9):1295–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Manco M (2012) Gut microbiota and developmental programming of the brain: from evidence in behavioral endophenotypes to novel perspective in obesity. Front Cell Infect Microbiol 2:109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rivera HM, Christiansen KJ, Sullivan EL (2015) The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci 9:194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Patel N, Pasupathy D, Poston L (2015) Determining the consequences of maternal obesity for offspring health. Exp Physiol 100(12):1421–1428 [DOI] [PubMed] [Google Scholar]
- 154.Bianchi M, Alisi A, Fabrizi M, Vallone C, Rava L, Giannico R et al (2019) Maternal intake of n-3 polyunsaturated fatty acids during pregnancy is associated with differential methylation profiles in cord blood white cells. Front Genet 10:1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stirrat LI, O’Reilly JR, Barr SM, Andrew R, Riley SC, Howie AF et al (2016) Decreased maternal hypothalamic-pituitary-adrenal axis activity in very severely obese pregnancy: associations with birthweight and gestation at delivery. Psychoneuroendocrinology 63:135–143 [DOI] [PubMed] [Google Scholar]
- 156.Gohir W, Ratcliffe EM, Sloboda DM (2015) Of the bugs that shape us: maternal obesity, the gut microbiome, and long-term disease risk. Pediatr Res 77(1–2):196–204 [DOI] [PubMed] [Google Scholar]
- 157.Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ et al (2015) Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 6:7320 [DOI] [PubMed] [Google Scholar]
- 158.Robinson SM, Crozier SR, Harvey NC, Barton BD, Law CM, Godfrey KM et al (2015) Modifiable early-life risk factors for childhood adiposity and overweight: an analysis of their combined impact and potential for prevention. Am J Clin Nutr 101(2):368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mamun AA, O’Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA (2009) Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation 119(13):1720–1727 [DOI] [PubMed] [Google Scholar]
- 160.Hochner H, Friedlander Y, Calderon-Margalit R, Meiner V, Sagy Y, Avgil-Tsadok M et al (2012) Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation 125(11):1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Eriksson JG, Sandboge S, Salonen M, Kajantie E, Osmond C (2015) Maternal weight in pregnancy and offspring body composition in late adulthood: findings from the Helsinki Birth Cohort Study (HBCS). Ann Med 47(2):94–99 [DOI] [PubMed] [Google Scholar]
- 162.Eriksson JG, Sandboge S, Salonen MK, Kajantie E, Osmond C (2014) Long-term consequences of maternal overweight in pregnancy on offspring later health: findings from the Helsinki Birth Cohort Study. Ann Med 46(6):434–438 [DOI] [PubMed] [Google Scholar]
- 163.Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC et al (2013) Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ 347:f4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Brion MJ, Benfield L et al (2010) Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation 121(23):2557–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gaillard R, Steegers EA, Franco OH, Hofman A, Jaddoe VW (2015) Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes: the Generation R Study. Int J Obes 39(4):677–85 [DOI] [PubMed] [Google Scholar]
- 166.Bedogni G, De Matteis G, Fabrizi M, Alisi A, Crudele A, Pizzolante F et al (2019) Association of bright liver with the PNPLA3 I148M gene variant in 1-year-old toddlers. J Clin Endocrinol Metab 104(6):2163–2170 [DOI] [PubMed] [Google Scholar]
- 167.Forno E, Young OM, Kumar R, Simhan H, Celedon JC (2014) Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics 134(2):e535–e546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Harpsoe MC, Basit S, Bager P, Wohlfahrt J, Benn CS, Nohr EA et al (2013) Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: a study within the Danish National Birth Cohort. J Allergy Clin Immunol 131(4):1033–1040 [DOI] [PubMed] [Google Scholar]
- 169.Oostvogels AJ, Stronks K, Roseboom TJ, van der Post JA, van Eijsden M, Vrijkotte TG (2014) Maternal prepregnancy BMI, offspring’s early postnatal growth, and metabolic profile at age 5–6 years: the ABCD Study. J Clin Endocrinol Metab 99(10):3845–3854 [DOI] [PubMed] [Google Scholar]
- 170.Flynn AC, Dalrymple K, Barr S, Poston L, Goff LM, Rogozinska E et al (2016) Dietary interventions in overweight and obese pregnant women: a systematic review of the content, delivery, and outcomes of randomized controlled trials. Nutr Rev 74(5):312–328 [DOI] [PubMed] [Google Scholar]
- 171.Guidelines (2013) for managing overweight and obesity in adults. Preface to the Expert Panel Report (comprehensive version which includes systematic evidence review, evidence statements, and recommendations). Obesity. 2014;22 Suppl 2:S40. [DOI] [PubMed]
- 172.Renault KM, Norgaard K, Nilas L, Carlsen EM, Cortes D, Pryds O et al (2014) The Treatment of Obese Pregnant Women (TOP) study: a randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am J Obstet Gynecol 210(2):134.e1–9 [DOI] [PubMed] [Google Scholar]
- 173.Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A (2008) A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes 32(3):495–501 [DOI] [PubMed] [Google Scholar]
- 174.Beyerlein A, Schiessl B, Lack N, von Kries R (2009) Optimal gestational weight gain ranges for the avoidance of adverse birth weight outcomes: a novel approach. Am J Clin Nutr 90(6):1552–1558 [DOI] [PubMed] [Google Scholar]
- 175.SIdNU (SINU). LARN-Livelli di Assunzione di Riferimento di Nutrienti ed energia per la popolazione italiana-IV Revisione. https://sinu.it/tabelle-larn-2014/.
- 176.Petrella E, Malavolti M, Bertarini V, Pignatti L, Neri I, Battistini NC et al (2014) Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. J Matern Fetal Neonatal Med 27(13):1348–1352 [DOI] [PubMed] [Google Scholar]
- 177.Thornton YS, Smarkola C, Kopacz SM, Ishoof SB (2009) Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. J Natl Med Assoc 101(6):569–577 [DOI] [PubMed] [Google Scholar]
- 178.Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN et al (2014) Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 348:g1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kennelly MA, Ainscough K, Lindsay KL, O’Sullivan E, Gibney ER, McCarthy M et al (2018) Pregnancy exercise and nutrition with smartphone application support: a randomized controlled trial. Obstet Gynecol 131(5):818–826 [DOI] [PubMed] [Google Scholar]
- 180.Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L et al (2015) Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 3(10):767–777 [DOI] [PubMed] [Google Scholar]
- 181.Farpour-Lambert NJ, Ells LJ, de Tejada BM, Scott C (2018) Obesity and weight gain in pregnancy and postpartum: an evidence review of lifestyle interventions to inform maternal and child health policies. Front Endocrinol 9:546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Geiker NRW, Magkos F, Zingenberg H, Svare J, Chabanova E, Thomsen HS et al (2022) A high-protein low-glycemic index diet attenuates gestational weight gain in pregnant women with obesity: the “An optimized programming of healthy children” (APPROACH) randomized controlled trial. Am J Clin Nutr 115(3):970–979 [DOI] [PubMed] [Google Scholar]
- 183.Larson EA, Magkos F, Zingenberg H, Svare J, Astrup A, Geiker NRW (2023) A high protein low glycemic index diet has no adverse effect on blood pressure in pregnant women with overweight or obesity: a secondary data analysis of a randomized clinical trial. Front Nutr 10:1289395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Basu A, Feng D, Planinic P, Ebersole JL, Lyons TJ, Alexander JM (2021) Dietary blueberry and soluble fiber supplementation reduces risk of gestational diabetes in women with obesity in a randomized controlled trial. J Nutr 151(5):1128–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.SIdD (SID). Linee di indirizzo sulla nutrizione nella gravidanza fisiologica o complicata da obesità e/o diabete. https://www.siditalia.it/clinica/linee-guida-societari/send/80-linee-guida-documenti-societari/5775-20-12-2023-linee-di-indirizzo-amd-sid-sulla-nutrizione-nella-gravidanza-fisiologica-o-complicata-da-obesita-e-o-diabete.
- 186.Factor PA, Corpuz H (2023) The efficacy and safety of myo-inositol supplementation for the prevention of gestational diabetes mellitus in overweight and obese pregnant women: a systematic review and meta-analysis. J ASEAN Fed Endocr Soc 38(2):102–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nuako A, Tu L, Reyes KJC, Chhabria SM, Stanford FC (2023) Pharmacologic treatment of obesity in reproductive aged women. Curr Obstet Gynecol Rep 12(2):138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kallen BA (2014) Antiobesity drugs in early pregnancy and congenital malformations in the offspring. Obes Res Clin Pract 8(6):e571–e576 [DOI] [PubMed] [Google Scholar]
- 189.Alwan S, Reefhuis J, Botto LD, Rasmussen SA, Correa A, Friedman JM et al (2010) Maternal use of bupropion and risk for congenital heart defects. Am J Obstet Gynecol 203(1):52e1-6 [DOI] [PubMed] [Google Scholar]
- 190.Leventhal K, Byatt N, Lundquist R (2010) Fetal cardiac arrhythmia during bupropion use. Acta Obstet Gynecol Scand 89(7):980–981 [DOI] [PubMed] [Google Scholar]
- 191.https://www.ema.europa.eu/en/documents/product-information/ozempic-epar-product-information_en.pdf.
- 192.https://www.ema.europa.eu/en/documents/product-information/victoza-epar-product-information_en.pdf.
- 193.Lentferink YE, Knibbe CAJ, van der Vorst MMJ (2018) Efficacy of metformin treatment with respect to weight reduction in children and adults with obesity: a systematic review. Drugs 78(18):1887–1901 [DOI] [PubMed] [Google Scholar]
- 194.Pu R, Shi D, Gan T, Ren X, Ba Y, Huo Y et al (2020) Effects of metformin in obesity treatment in different populations: a meta-analysis. Ther Adv Endocrinol Metabol 11:2042018820926000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dukhovny S, Van Bennekom CM, Gagnon DR, Hernandez Diaz S, Parker SE, Anderka M et al (2018) Metformin in the first trimester and risks for specific birth defects in the National Birth Defects Prevention Study. Birth Defects Res 110(7):579–586 [DOI] [PubMed] [Google Scholar]
- 196.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D et al (2023) 15. Management of diabetes in pregnancy: standards of care in diabetes-2023. Diabetes Care 46(Suppl 1):S254–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Barbour LA, Scifres C, Valent AM, Friedman JE, Buchanan TA, Coustan D et al (2018) A cautionary response to SMFM statement: pharmacological treatment of gestational diabetes. Am J Obstet Gynecol 219(4):367e1-e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Barbour LA, Feig DS (2019) Metformin for gestational diabetes mellitus: progeny, perspective, and a personalized approach. Diabetes Care 42(3):396–399 [DOI] [PubMed] [Google Scholar]
- 199.Vanky E, Nordskar JJ, Leithe H, Hjorth-Hansen AK, Martinussen M, Carlsen SM (2012) Breast size increment during pregnancy and breastfeeding in mothers with polycystic ovary syndrome: a follow-up study of a randomised controlled trial on metformin versus placebo. BJOG Int J Obstet Gynaecol 119(11):1403–1409 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.