Abstract
Fear reactivity is an early emerging temperament trait that predicts longer-term behavioral and health outcomes. The current analysis tests the hypothesis, an extension of prior research on maternal immune activation (MIA), that the prenatal maternal immune system is a reliable predictor of observed fear reactivity in infancy. The analysis is based on a prospective longitudinal cohort study that collected data from the first trimester and conducted observational assessments of temperament at approximately 12 months of age (n=281 infants). MIA was assessed from immune biomarkers measured in maternal blood at each trimester; infant temperament was assessed using the Laboratory Temperament Assessment Battery (lab-TAB) assessment at 12 months; covariates included family and sociodemographic factors. Patterns of inflammatory markers across gestation reliably predicted observed temperament: elevated prenatal maternal immune activation was associated with high fear reactivity to novel stimuli. The findings provide novel evidence of prenatal origins of fear reactivity and suggest developmental mechanisms that may underlie early emerging individual differences in child temperament.
Keywords: Temperament, Fear reactivity, Maternal Immune Activation, Immune markers, Cytokines, Neurodevelopment
Introduction
The study of early-emerging and stable individual differences in behavior poses essential questions for a developmental understanding of brain and behavior and offers practical applications for clinical and educational settings. Furthermore – and particularly relevant to the current special issue – it is also one of Jerome Kagan’s hallmark contributions to developmental psychology. Key features of his approach include the use of observational methods over reliance on parent questionnaires; emphasis on early-emerging patterns of inhibited versus uninhibited temperament (as particularly stable, early-emerging phenotypes); and consideration of biological bases of fear reactivity and inhibited behavior (Fox et al., 2015; Hirshfeld et al., 1992; Kagan et al., 1988). Incorporating these features, we extend existing research by testing the hypothesis that prenatal maternal immune activation predicts early-emerging fear reactivity.
There is growing interest in the prenatal developmental origins of individual differences in infant and child temperament. These lines of research include multiple exposures and, perhaps more importantly, competing developmental models. Specifically, one line of research, derived from a teratology or toxicology model, posits that prenatal exposures to xenobiotics or toxicants such as metals (Ames et al., 2023) or alcohol exposure (Molteno et al., 2014) have direct and adverse effects on the fetus, sometimes leading to severe and persisting impairment. A competing model based on developmental programming or predictive adaptive response suggests that prenatal exposures induce adaptive responses in the fetus, with long-term implications on behavior and biology (Barker, 1993; Gluckman et al., 2005). Initially focused on prenatal nutritional environment and offspring cardio-metabolic health, this framework has informed a large program of study linking prenatal stress and stress physiology to child outcomes (Entringer et al., 2010; Glover et al., 2010; O’Connor et al., 2013), including temperament (Bergman et al., 2008; Davis et al., 2005; Huizink et al., 2002; Zhang et al., 2018).
An alternative model, and the focus of the current study, is the “Maternal Immune Activation” (MIA) model; it is distinguished by a focus on the prenatal maternal immune system (as the exposure) and offspring neurodevelopment (as the outcome of primary interest). The maternal immune system is a natural target in studies of prenatal exposure because: (1) pregnancy is an immune-altered state (i.e., modifications of the maternal immune system are required for a successful pregnancy); and (2) the maternal immune system is responsive to many common conditions and exposures, including stress, obesity, illness, and environmental chemicals, that may be associated with child outcomes. A strength of the model is its reliance on a foundation of mechanistic evidence suggesting that (fetal) neural development is altered in response to (maternal) inflammatory molecules (Jonakait, 2007), and wide-spread demonstration of these effects on normal neurogenesis as shown in gene expression studies (Baines et al., 2020; Smith et al., 2007) and implied by studies using brain imaging techniques in humans (Rasmussen et al., 2021; Spann et al., 2018).
The MIA model has garnered considerable empirical support in animal and human research on neurodevelopment (Bilbo et al., 2005; Brown & Meyer, 2018; Careaga et al., 2017; Estes & McAllister, 2016; Gilman et al., 2017; Han et al., 2021; O'Connor et al., 2022), but basic questions remain. The current study was designed to extend research in three important ways. The first is the consideration of fear reactivity as a phenotype associated with prenatal maternal immune activation. Much of the research on MIA is dominated by two significant clinical phenotypes, schizophrenia and autism (Brown & Meyer, 2018; Careaga et al., 2017; Han et al., 2021; Lyall et al., 2021); very limited research considers other neurodevelopmental phenotypes and especially with respect to variation within the normal range (Camerota et al., 2022; Nazzari & Frigerio, 2020; O'Connor et al., 2022). Evidence of that kind is needed to determine if the MIA model has significance outside of a clinical focus and to assess broader developmental and clinical health implications. There are several reasons why temperamental fear reactivity may be positively associated with MIA – most notably the viability of a mechanistic link. For example, animal models suggest that the brain’s limbic system is responsive to signals from the peripheral immune system and that amygdala connections in the brain may coordinate autonomic nervous system responses (Engler et al., 2011). A second extension of the MIA model in the current paper is our focus on early-onset evidence of possible effect. Congruent with Kagan and others’ focus on temperament in infancy (Braungart-Rieker et al., 2010; Willoughby et al., 2017), we reasoned that, if maternal immune activation is a bona fide predictor of fear reactivity (as implied above), then evidence of this would be available from infancy, when it can be reliably assessed. Accordingly, the current study’s focus on early-onset child behavioral outcomes offers a particularly important and novel test that the MIA model of neurodevelopment.
A third innovation of the current analysis concerns the measurement of maternal immune activation. In contrast to previous studies that have relied on a very limited immune assessment or only indirect measures (e.g., illness reports), the current study capitalized on recent methodological innovations to assess a panel of immune markers from blood samples collected at three points across gestation. We focus on a broad subset of immune markers that have been implicated in MIA research in animals and humans and provide a novel analytic approach for assessing observed temperament using the leading observational measure (Goldsmith, 1999; Planalp et al., 2017).
Methods
Study Overview and Sample
The current analysis is based on data from a prospective longitudinal cohort study of prenatal influences on child health outcomes based in Rochester, NY (Understanding Pregnancy Signals and Infant Development, “UPSIDE”; O’Connor et al., 2021); the cohort is part of the NIH Environmental influences on Child Health Outcomes program (Knapp et al., 2023). Between December 2015 and April 2019, women were recruited in their first trimester of pregnancy from outpatient obstetric clinics affiliated with the University of Rochester. Eligibility criteria were: age 18 or older, singleton pregnancy, no known substance abuse problems or a history of psychotic illness, ability to communicate in English; not greater than normal medical risk. Women with significant endocrine disorders (e.g., polycystic ovary syndrome) or obstetric problems were excluded. Of the 326 women who were initially enrolled, we subsequently excluded n=18 participants (e.g., multiple pregnancy, miscarriage, medical screen failure). Supplementary Figure 1 details participant flow and the current analysis follows STROBE guidelines for observational studies. The study was approved by the University of Rochester Research Subjects Review Board; all participants provided written informed consent. Participants were compensated for each research visit and were provided transportation if needed. For postnatal follow-up, additional inclusion criteria were: gestational age at birth of 37 weeks or greater, no known significant medical/genetic problem as determined by a medical staff. Perinatal data indicated that the children in the study were well within the normal range on key indicators, including birth weight (M = 3,352.8 g, SD = 295.0 g), birth length (M = 51.1 cm, SD = 3.1 cm), and gestational age (M = 39.5 weeks, SD = 1.6 weeks). Analyses were restricted to those families who had available inflammatory marker and/or temperament data (see data analysis section below). The current analyses focus on the temperament assessment conducted at 12 months. Table 1 reports descriptive data on the infants who were enrolled and eligible at the 1-month postnatal assessment; Supplementary Figure 1 details participant flow and the current analysis follows STROBE guidelines for observational studies and Supplementary Table 1 presents data on those with observed temperament data or not at the 12-month assessment. The study was approved by the local Institutional Review Board; participants provided written informed consent.
Table 1.
Descriptive Statistics of the Analytic Sample
| Variable | Mean (SD) | N (%) |
|---|---|---|
| Age (years) | 29.1 (4.6) | |
| Race/Ethnicity | ||
| Non-Hispanic White | 165 (58.5) | |
| Non-Hispanic Black | 67 (23.8) | |
| Asian | 11 (3.9) | |
| Hispanic | 27 (9.6) | |
| Other Races/ Ethnicities* | 11 (4.3) | |
| Education | ||
| Less than high school | 12 (4.3) | |
| High School | 89 (31.6) | |
| Some College | 40 (14.2) | |
| College Degree | 70 (24.8) | |
| Post College Degree | 70 (24.8) | |
| Employed | 204 (72.3) | |
| Married/Living as Married | 170 (60.2) | |
| Infant Sex | ||
| Male | 140 (49.6) | |
| Female | 141 (50.4) |
Note. n=281* Other Races/ Ethnicities includes American Indian/Alaska Native and individuals self-reporting as ‘other’.
Measures
Maternal Immune Activity
A blood sample from venipuncture was obtained from participants at each trimester. Blood collection was not conducted if there was sign of acute illness; also as noted, an immunological or endocrinological disorder (as judged by the medical team) was an exclusion criterion, which would also exclude individuals taking confounding medication. Blood samples were processed typically within an hour of collection and stored at −80⁰ C for later assay.
Maternal immune markers were measured using a MILLIPLEX Human High Sensitivity T Cell Magnetic Bead Panel, a 96-well plate assay. Standard protocols for preparation of plasma samples were followed. Reagents for the immunoassay were also prepared according to standard protocols, including the preparation of antibody-immobilized beads, serum matrix, quality controls, wash buffer, and human high sensitivity T cell standards. A total of five panels were conducted; the current analyses focus on the subset of markers that were identified a priori as markers of maternal inflammation and/or included in previous research on maternal immune activation and child/adolescent/adult behavioral or brain imaging results (Beversdorf et al., 2018; Canetta et al., 2014; Carter et al., 2022; Lombardo et al., 2018): C-Reactive Protein (CRP), IFNg, IL-10, IL-17A, IL-1b, IL-2, IL-4, IL-6, IL-8, and TGF-Beta. Supplementary Table 2 provides the rationale behind the markers used for this study. Immune markers were stable over gestation (ICC’s > .5) and no substantial mean changes across gestation were observed. Accordingly, each immune measure was loaded onto a latent factor to reflect exposure across gestation.
Observational Measure of Temperament
When participating children were approximately 12 months of age we administered three tasks from the Laboratory Temperament Assessment Battery (Lab-TAB), the leading observational measure of childhood temperament (Goldsmith, 1999; Planalp et al., 2017), according to procedures described in the Lab-TAB manual. Two fear reactivity tasks (Mechanical Toy and Spider) and a frustration task (Arm Restraint) were administered for up to three trials in accordance with procedures described in the manual. Trained raters blind to identifying information coded behaviors from videotaped recordings. For each task and on each trial, child behavior was assessed using the scoring provided in the manual. For the fear tasks, ratings of facial fear, bodily fear, escape behavior, distress vocalizations, and latency (inverse scored) were completed for each available trial. Each observed behavioral score was averaged across the three trials. Average inter-rater reliability across tasks was ICC = .88. Additional steps were taken to provide quality assurance. All videotapes were independently reviewed (blinded to other child data) and rated for quality (e.g., regarding child on-task behavior, integrity of administration, or external interference); based on consensus ratings, two participants whose trials that did not meet administration quality were removed from the analysis dataset.
Parent Reported Temperament
At the same visit as the Lab-TAB administration, parents (in approximately 90% of cases, the mother) completed the Infant Behavior Questionnaire-Revised (IBQ-R), a 191-item questionnaire that assesses 14 dimensions of temperament (Putnam et al., 2014). We focused analyses on subscales most closely associated with fear and approach because of our interest in fearful and inhibited behavior; the activity scale was included as a sensitivity or control measure of parent-reported temperament. The 16-item Fear subscale assessed an infant’s distress to sudden changes or novel objects. The Approach scale is comprised of 12 items that assesses how much an infant gets excited by and moves toward novel objects. The 15-item Activity scale assesses an infant’s overall gross motor activity. Cronbach alphas for the Fear, Approach, and Activity scales were .91, .74, and .80, respectively.
Covariates
Aside from child sex, there is little consistency in covariate selection in analyses of infant temperament. We considered inclusion of sociodemographic covariates including highest level of maternal education, income and Medicaid status (see Table 1); self-reported race and ethnicity (measured categorically as Hispanic, Non-Hispanic White, Non-Hispanic Black, Asian, and Other, including more than one race) was included as a social construct and proxy for potential exposure to systemic racism. Obstetric and perinatal factors were also considered as possible covariates, including birth weight and gestational age (this was a medically healthy sample, e.g., individuals with significant gestational diabetes or preeclampsia as judged by our medical team were excluded). Maternal anxiety based on the Penn State Worry Questionnaire (PSWQ) was included as a prenatal and postnatal covariate. The Penn State Worry scale is a 16-item inventory designed to assess trait-level worry in adults with considerable reliability and validity (Meyer et al., 1990). Mothers completed the PSWQ at each trimester. Scores were highly correlated across trimester (r’s = .75 - .86) and so prenatal scores were averaged across trimesters to create a mean score over the course of pregnancy; the postnatal measure of PSWQ at 12 months was used as a covariate in additional analyses.
Data Analysis
Data were cleaned and prepared for analyses using SPSS version 28.0.0 (IMB Corporation, 2021). Descriptive statistics and correlations between study variables were calculated using the base package in R version 4.2.2 (R Core Team, 2021). Child sex, race/ethnicity, maternal education, and maternal anxiety were included as a priori covariates in analysis. None of the other covariates mentioned above were reliably associated with temperament measures and so were not considered further. Structural equation models were fitted using the ‘lavaan’ package version 0.6-16 in R (Rosseel, 2012). Models were estimated using maximum likelihood estimation with robust standard errors. Model fit was assessed based on model X2, root mean square error of approximation (RMSEA), and Comparative Fit Index (CFI).
Data analyses were carried out in three steps. First, measurement models for each immune cytokine (CRP, IFNg, IL-10, IL-17A, IL-1b, IL-2, IL-4, IL-6, IL-8, and TGF-Beta) were calculated using the measured cytokine concentrations at each trimester as indicators. We then extracted the estimated latent variable scores for each participant using the ‘lavPredict’ function and tested correlations between the latent immune markers and measured aspects of temperament for each lab-Tab task (e.g., facial fear, bodily fear, etc.).
Second, we fitted a series of measurement models for each temperament task (Spider, Mechanical Toy, Frustration) using the measured aspects of temperament (e.g., facial fear, bodily fear, etc.). In models of observed temperament, latent variables for the fear reactivity (Spider and Mechanical Toy) and frustration tasks were calculated based on the summary scores averaged across trial. Specifically, the measured behaviors (e.g., facial fear, bodily fear, escape behavior, distress vocalizations for the Spider task) were loaded onto a single factor. Latency was not included in the spider or mechanical toy latent factors due to a low factor loading with the other measured components of the spider and toy tasks. Similar to the immune markers, we extracted latent scores for each Lab-Tab task and estimated correlations between the latent immune marker scores calculated in the first step and the estimated lab-Tab task scores. This provides an estimate for the unadjusted association between the immune markers and latent temperament scores.
Third, we fitted a series of adjusted models regressing the latent temperament scores onto the latent immune marker scores. Latent immune and temperament factors were estimated in each model (i.e., these models did not use the extracted factor scores) and scores on the Spider, Mechanical Toy, and Frustration tasks were included simultaneously as outcome variables in each model. Scores on the Spider, Mechanical Toy, and Frustration tasks were allowed to covary. See Figure 1 for a sample path diagram with CRP as the predictor. Separate models were fitted for each immune marker as there was a high degree of multicollinearity among several of the immune markers and because each immune marker was selected a priori from the existing human literature on MIA. Similarly, a series of models were fit with measured parent-reported temperament (Fear, Approach, Activity) as outcome variables. We applied a Benjamini-Hochberg correction to correct the alpha level for multiple testing (Benjamini & Hochberg, 1995). Because of the novel and exploratory nature of the study, a false discovery rate (FDR) was set at 0.25, which is consistent with previous exploratory research (e.g. Koskinen et al., 2023). In all models, missing data were handled using full information maximum likelihood estimation under the assumption that data were missing at random (Enders & Bandalos, 2001).
Figure 1.
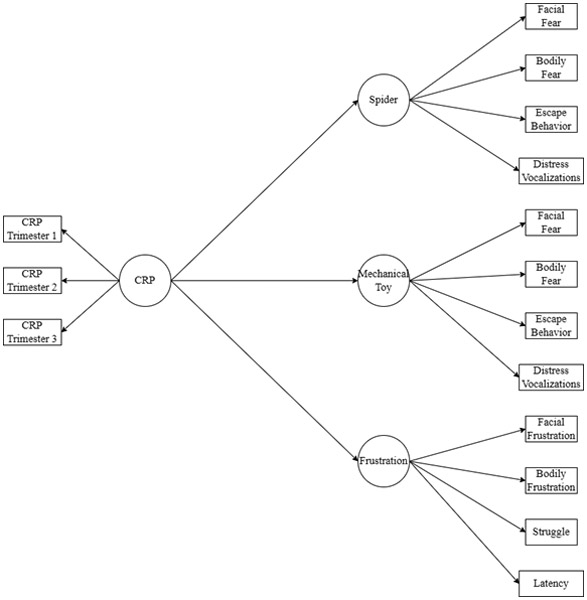
Sample Path Diagram of Associations between Immune Cytokines and Observed Temperament
Results
Descriptive Statistics and Bivariate Correlations
Descriptive statistics for the analytic sample (from pregnancy for maternal data) are provided in Table 1. The sample was diverse and included a comparatively high percentage of mothers with a high school education or less. Supplementary Table 1 provides information on sample characteristics according to the available of the lab-TAB measure at 12 months; missing lab-TAB scores were typically due to because of loss to contact, moving from the area, or inability to conduct the assessment during the research visit; notably, the visits occurred before the SARS-CoV-2 pandemic halted in-person research visits.
There was a modest association between rater scores of fear reactivity in the Spider and Mechanical Toy task (r = .143); fear behavior in these tasks was weakly associated with frustration scores on the Arm Restraint task (r’s = −.05 for mechanical toy and −.026 for spider). Consistent with previous research; there was not a sex difference in behavior observed in the two fear reactivity and frustration tasks (p’s > .20 for the difference in the means between males and females).
Measured aspects of the Spider task were positively associated with several inflammatory markers (see Table 2). Specifically, greater vocal fear during the spider task was associated with higher concentrations of IL-4, IL-6, IL-8, IL-10, and IL-1beta (r’s = .16 - .23). Bodily fear during the Spider Task was positively correlated with concentrations of IL-6, IL-8, and IL-10 (r’s .17-.19). Escape behavior during the Spider Task were positively correlated with IL-2, IL-4, IL-8, IL-10, IL-17a, and IL-1beta (r’s = .17 - .27). Facial fear during the Spider Task was not significantly associated with any of the inflammatory markers. Vocal fear and escape behaviors during the mechanical toy task were positively associated with concentrations of IL-4, IL-6, and IL-8 (r’s = .17 - .22; see Supplementary Table 3). Scores on the components of the frustration task did not significantly correlate with immune markers (see Supplementary Table 4). None of the latent lab-Tab scores correlated significantly with any of the scales on the IBQ-R (see Supplementary Table 5).
Table 2.
Bivariate Correlations between Spider Task and Inflammatory Markers
| Spider Facial Fear r (p-value) |
Spider Vocal Fear r (p-value) |
Spider Bodily Fear r (p-value) |
Spider Escape r (p-value) |
|
|---|---|---|---|---|
| CRP | .096 (.263) | .068 (.415) | −.064 (.448) | −.039 (.639) |
| IL.10 | .130 (.129) | .206* (.013) | .174* (.037) | .267** (.001) |
| IL.4 | .072 (.417) | .175* (.041) | .076 (.382) | .183* (.033) |
| IL.6 | .058 (.512) | .183* (.032) | .169* (.047) | .130 (.130) |
| IL.2 | .010 (.910) | .092 (.273) | .069 (.408) | .172* (.039) |
| TGF.beta | −.029 (.737) | .014 (.869) | .003 (.976) | .045 (.594) |
| IFNg | .006 (.946) | .090 (.283) | .139 (.096) | .140 (.093) |
| IL1Beta | .067(.436) | .163* (.050) | .090 (.282) | .235** (.005) |
| IL.8 | .145 (.099) | .232** (.006) | .191* (.025) | .227** (.008) |
| IL.17A | .044 (.607) | .108 (.199) | .147 (.079) | .176* (.034) |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
Note. N’s range from 130-145. All values presented are Pearson correlation coefficients
Measurement Models
Standardized factor loadings for the late immune and lab-Tab factors are present in Supplementary Tables 6-7, respectively. Factor loadings were within the acceptable range for all latent factors; with the exception of the trimester 1 loading on TGF-beta (which was 0.39), all standardized loadings were greater than or equal to 0.40. Correlations among the latent immune marker scores revealed significant overlap between several immune markers (see Table 3). Latent Spider Task scores were positively correlated with IL-8 and IL-1-beta (r’s = .17 and .18, respectively, p < .05). There were no significant associations between any of the immune markers and latent Mechanical Toy or Frustration task scores.
Table 3.
Correlations Among Latent Immune Cytokine Factors and Temperament Factors
| CRP | IL-1- Beta |
IL-2 | IL-4 | IL-6 | IL-8 | IL-10 | IL- 17A |
TGF- Beta |
IFN-G | Spider | Mechanical Toy |
Frustration | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP | - | ||||||||||||
| IL-1-Beta | .05 | - | |||||||||||
| IL-2 | .01 | .82* | - | ||||||||||
| IL-4 | .02 | .50* | .50* | - | |||||||||
| IL-6 | .09 | .38* | .36* | .72* | - | ||||||||
| IL-8 | .02 | .39* | .35* | .71* | .87* | - | |||||||
| IL-10 | .06 | .75* | .70* | .50* | .44* | .42* | - | ||||||
| IL-17A | .06 | .75* | .77* | .48* | .42* | .44* | .69* | - | |||||
| TGF-beta | .05 | .35* | .17* | .23* | .12* | .12* | .27* | .18* | - | ||||
| IFN-G | .04 | .65* | .72* | .51* | .43* | .43* | .66* | .85* | .17* | - | |||
| Spider | .14 | .18* | .14 | .10 | .12 | .17* | .16 | .14 | −.02 | .08 | - | ||
| Mechanical Toy | .02 | .08 | .00 | .02 | .02 | −.05 | .04 | .02 | .02 | .04 | .33* | - | |
| Frustration | −.07 | .06 | −.04 | .05 | .10 | .10 | .06 | −.04 | .02 | −.00 | .16 | .02 | - |
Note. Estimated values for each immune marker and temperament task were first estimated from a measurement model before correlations were calculated.
indicates a correlation that is significant at p < .05.
Associations between Immune Markers and Observed Temperament
The fit of all of the models was acceptable based on the X2, RMSEA, and CFI statistics (see Supplementary Table 8). Associations between each latent immune marker and the latent temperament scores are presented in Table 4 and suggest a broad and positive set of associations for fear behavior in the spider task, after adjusting for covariates. Higher fear scores on the spider task were associated with higher maternal prenatal concentrations of IL-1 beta (B = 0.60, SE = 0.26, p = .023) and higher concentrations of IL-10 (B = 0.27, SE = 0.10, p = .007). After applying the Benjamini-Hochberg adjustment to the alpha level, the association between IL-10 and the Spider score remained statistically significant (see Supplementary Table 9). Each 1 SD increase in prenatal IL-10 was associated with about a quarter SD increase in the Spider Task score. Notably, there was not reliable evidence that prenatal maternal immune markers predicted child behavior in the unfamiliar mechanical toy or frustration tasks.
Table 4.
Associations between Maternal Prenatal Immune Cytokine Concentrations and Observed Temperament at 12 Months
| Spider Task | Mechanical Toy Task | Frustration Task | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | p-value | B | SE | β |
p- value |
B | SE | β | p-value | |
| CRP | 0.09 | 0.06 | 0.18 | .131 | 0.03 | 0.05 | 0.06 | .447 | −0.02 | 0.06 | −0.03 | .785 |
| IL-1-Beta | 0.60 | 0.26 | 0.23 | .023 | 0.38 | 0.34 | 0.13 | .274 | 0.30 | 0.33 | 0.12 | .352 |
| IL-2 | 0.28 | 0.15 | 0.17 | .071 | 0.04 | 0.16 | −0.02 | .807 | 0.04 | 0.20 | 0.03 | .844 |
| IL-4 | 0.12 | 0.08 | 0.13 | .143 | 0.01 | 0.07 | 0.01 | .942 | 0.10 | 0.09 | 0.11 | .240 |
| IL-6 | 0.17 | 0.12 | 0.13 | .152 | 0.05 | 0.10 | 0.03 | .617 | 0.19 | 0.13 | 0.15 | .143 |
| IL-8 | 0.26 | 0.14 | 0.20 | .058 | −0.03 | 0.11 | −0.02 | .818 | 0.21 | 0.12 | 0.17 | .077 |
| IL-10 | 0.27 | 0.10 | 0.21 | .007 | 0.06 | 0.17 | 0.04 | .727 | 0.15 | 0.14 | 0.12 | .274 |
| IL-17A | 0.26 | 0.17 | 0.16 | .157 | 0.11 | 0.20 | 0.06 | .586 | 0.00 | 0.17 | 0.00 | .976 |
| TGF-beta | −0.04 | 0.23 | −0.02 | .854 | 0.09 | 0.26 | 0.03 | .734 | 0.00 | 0.20 | 0.00 | .983 |
| IFN-G | 0.15 | 0.18 | 0.08 | .353 | −0.06 | 0.18 | −0.03 | .755 | 0.05 | 0.18 | 0.03 | .776 |
Note. Models were fit separately for each immune marker. Latent immune marker and temperament scores were estimated within the model. All models were adjusted for maternal education (high school or less = 0, some college or more = 1), infant race (nonwhite = 0, white = 1), infant sex (0 = male, 1 = female), and maternal prenatal anxiety. Findings significant at p < .05 are bolded for clarity. B indicates an unstandardized beta estimate and β indicates a standardized beta. SE stands for standard error.
Associations between Immune Markers and Parent-Reported Temperament
All of the models fit the data acceptably based on the X2, RMSEA, and CFI statistics (see Supplementary Table 10). See Table 5 for associations between each latent immune marker and parent-reported temperament. There was consistent evidence for parent-reported Approach, which was positively associated with prenatal maternal IL-4 (B = 0.16, SE = 0.05, p = .003), IL6 (B = 0.26, SE = 0.08, p = .001), and IL-8 (B = 0.29, SE = 0.07, p < .001). After adjusting the alpha levels for multiple comparisons, the associations between IL-4, IL-6, and IL-8 and parent-reported approach remained significant (see Supplementary Table 11). There was not reliable evidence that prenatal immune activation was associated with the fear and activity subscales.
Table 5.
Associations between Maternal Prenatal Immune Cytokine Concentrations and Parent-Reported Temperament at 12 Months
| Fear | Approach | Activity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | p-value | B | SE | β | p-value | B | SE | β | p-value | |
| CRP | 0.05 | 0.07 | 0.07 | .381 | 0.04 | 0.04 | 0.10 | .324 | 0.01 | 0.04 | 0.01 | .873 |
| IL-1-Beta | −0.23 | 0.32 | −0.07 | .460 | 0.09 | 0.20 | 0.04 | .650 | −0.11 | 0.25 | −0.04 | .655 |
| IL-2 | −0.16 | 0.18 | −0.07 | .397 | 0.10 | 0.11 | 0.08 | .361 | −0.10 | 0.13 | −0.06 | .462 |
| IL-4 | 0.01 | 0.10 | 0.01 | .911 | 0.16 | 0.05 | 0.21 | .003 | −0.00 | 0.07 | −0.00 | .960 |
| IL-6 | −0.14 | 0.14 | −0.08 | .322 | 0.26 | 0.08 | 0.25 | .001 | 0.00 | 0.12 | 0.00 | .981 |
| IL-8 | −0.16 | 0.14 | −0.09 | .247 | 0.29 | 0.07 | 0.28 | .001 | −0.05 | 0.12 | −0.04 | .676 |
| IL-10 | 0.02 | 0.14 | 0.01 | .873 | 0.10 | 0.10 | 0.10 | .318 | 0.03 | 0.11 | 0.03 | .781 |
| IL-17A | −0.15 | 0.17 | −0.07 | .364 | 0.09 | 0.09 | 0.07 | .431 | −0.19 | 0.15 | −0.12 | .219 |
| TGF-beta | 0.23 | 0.25 | 0.07 | .343 | 0.07 | 0.17 | 0.04 | .673 | −0.08 | 0.22 | −0.04 | .702 |
| IFN-G | −0.03 | 0.17 | −0.01 | .867 | 0.12 | 0.12 | 0.08 | .311 | −0.13 | 0.16 | −0.07 | .408 |
Note. Models were fit separately for each immune marker. Latent immune marker and temperament scores were estimated within the model. All models were adjusted for maternal education (high school or less = 0, some college or more = 1), infant race (nonwhite = 0, white = 1), infant sex (0 = male, 1 = female), and maternal prenatal anxiety. Findings significant at p < .05 are bolded for clarity. B indicates an unstandardized beta estimate and β indicates a standardized beta. SE stands for standard error.
Discussion
Fear reactivity and behavioral inhibition are reliably assessed early markers of important behavioral and health outcomes, notably anxiety (Kagan, 2002; Tang et al., 2022). Kagan and others (Kagan et al., 1987, 1988) have added substantially to our understanding of its assessment in early infancy and its biological and environmental origins. Findings from the current study provide an important extension to this work by demonstrating a novel biological exposure for early-emerging fear reactivity: prenatal maternal immune activation. Results indicated that infants whose mothers exhibited greater prenatal immune activation – based on an extensive assessment of multiple immune markers gathered across gestation – showed elevated fear reactivity on an observational task when the children were approximately 12 months of age; predictions were limited to fear reactivity on the more salient fear stimulus – the spider task – and were not observed in relation to parental reports of child fearfulness. The findings enhance research on the developmental origins of temperament and significantly extend research on the MIA hypothesis for child neurodevelopment.
There is considerable evidence linking maternal immune activation to the clinical phenotypes of autism and schizophrenia (Beversdorf et al., 2019; Brown et al., 2004; Goldstein et al., 2014; Nielsen et al., 2021). However, with few exceptions (Camerota et al., 2022; Ghassabian et al., 2018; Kelly et al., 2022; Nazzari & Frigerio, 2020; O'Connor et al., 2022), research has not considered normative variability in cognitive and behavioral outcomes or assessments from young children. These are both arguably significant limitations given the MIA hypothesis, and considerable animal evidence, that maternal immune activation alters neurodevelopment in ways would be expected to be far broader than these clinical phenotypes – and the corollary that such effects could be detectable in young children.
Maternal immune activation was assessed using ten markers selected based on prior studies of immune activation and derived from a larger multiplex panel. We adopted a broad approach to measuring maternal immune activation given the diversity, and inconsistency, in how this construct has been measured in previous studies. There is a clear emphasis in prior studies (Canetta et al., 2014; Carter et al., 2022; Ghassabian et al., 2018), and in the current study, on selection of markers that reflect inflammation and induction of inflammatory processes (e.g., IL-1b, IL-6, IL-17a, CRP). On the other hand, a focus on inflammatory processes in studies of maternal immune activation is almost certainly too limited; of the two markers most strongly associated with observed fear in the current study, one (IL-1b) but not the other (IL-10) is a “classic” pro-inflammatory marker. Additionally, in bivariate correlation models IL-4 and IL-8 (as well as the pro-inflammatory marker IL-6) were associated with fear behaviors during the spider task (vocalizations, escape behaviors). Most markers have known diverse roles within the immune system that might include involvement of the inflammation process but also antibody production or induction of T cells, as in the case of IL-4; other markers included in MIA-related research also have multiple and regulatory functions, e.g., IL-10 has immune stimulating effects (e.g., allergic response) but is also plays an anti-inflammatory role. Interpretation of immune markers in pregnancy and the long-standing model emphasizing a balance of Th1-type and Th2-type cytokines for healthy pregnancy layers additional complication as regards the markers of interest and interpretation of function with respect to fetal/child neurodevelopment. Our approach to selecting immune markers is not intended as a definitive approach to characterizing maternal immune activation; rather, it reflects the more general need to broaden the measurement of immune markers in studies of perinatal and child health outcomes beyond the smaller number of markers that have been previously considered. One general conclusion from the overall pattern of findings is that “activation” is an apt description of the maternal immune system as associated with child neurodevelopment and is likely more biologically accurate than the more restrictive term “inflammation.”
Kagan’s work on the biological basis of fear and inhibited behavior (Kagan et al., 1987), coupled with subsequent studies on MIA (Chen et al., 2022; Engler et al., 2011; Gur et al., 2017; Rasmussen et al., 2021), suggest plausible pathways for our novel finding that prenatal maternal activation may increase fear reactivity in the offspring. Follow-up of the current sample is needed to determine if the prediction to fear reactivity in infancy constitutes a novel finding or, alternatively, if early-emerging fear reactivity is an early-emerging component of neurodevelopmental outcomes already reported. As regards the latter hypothesis, elevated fear reactivity and affective dysregulation has been noted in children with autism spectrum disorder and difficulties in executive function (White et al., 2014; Willoughby et al., 2017). However, we suggest that the former hypothesis is more likely. As a community sample, the children in the study would not be at elevated risk for neurodevelopmental disorders that have dominated research on maternal immune activation, most notably autism spectrum disorder. In addition, evidence from the current study – both the latent profiles and the bivariate correlations – show a dose-response pattern of exposure and outcome within the normal range. A stronger association with the spider stimulus than the unfamiliar mechanical toy stimulus further indicates that the dose-response pattern is stronger with the more salient stimulus; this might explain the general tendency in past studies to find more reliable effects with the more salient spider task, e.g., (Gagne et al., 2011).
Kagan, Goldsmith, and colleagues have provided a strong observational basis for assessing temperament that preempts some of the well-documented problems of parent-report measures. In this context, there is a long history of research showing a lack of association between observational and parent-report measures and a different pattern of findings with these two sources of measurement (Gagne et al., 2011; Seifer et al., 1994). Finding that maternal immune activation predictors of observed fear reactivity and did not strongly overlap with maternal prenatal immune activation predictors of parent-reported fear is therefore not surprising. This may be less a lack of replication and more a reflection that observational measures provide a more sensitive, or different, index of child behavior than parent reports. Although we did not observe any association between maternal immune activation and parent-reported fear, IL-4, IL-6, and IL-8 were positively associated with parent-reported approach behaviors, which reflect excitement in anticipation of pleasurable activities and rapid approach to novel stimuli (Putnam et al., 2014). Higher approach behaviors in infancy and toddlerhood have been linked with attentional difficulties and impulsivity in early childhood (Garthstein et al., 2012). Inter-rater reliability (between parents) has been found to be higher for the Approach scale of the IBQ-R than the Fear scale (Parade & Leerkes, 2008), which may reflect a more general pattern of parents being more reliable reporters of child externalizing behaviors (e.g., impulsivity, aggression) than internalizing behaviors. Our findings are in agreement with a hallmark of Kagan’s work on inhibited and fearful temperament: a reliance on observational measures (Kagan et al., 1988) and the limits of relying only on parent reports.
There are several limitations of the study. For example, the panel selected was broader than most studies on the MIA model, but nonetheless offers a limited picture of the dynamic, complex network of immune markers and how they may shape fetal and child development. Second, we did not evaluate intermediatory factors, notably placenta mechanisms or connected biological systems, e.g., neuroendocrine system that may be relevant to consider in this context. Third, although the analytic models can account for missing data, selective attrition from the first trimester through 1 year postnatal is still a limitation of this work. Fourth, the assessment at 12 months provided a reliable index of fear reactivity but further follow-up is needed to determine if the effects reported here have persisting effects on fear, anxiety, and related phenotypes in childhood. These limitations were offset to a considerable degree by several notable strengths of the study, including the prospective design starting in the first trimester, extensive assessment and analysis of prenatal immune markers, and detailed observational measures of temperament.
In summary, the findings add to a growing body of research that examines the impact of normative variation in common prenatal exposures and early emerging temperament (Davis et al., 2011; Mohler et al., 2006). Further research is needed to examine the degree to which the putative mechanism of immune activation suggested in the current study compounds other prenatal mechanisms such as stress physiology. Additional research is also needed to describe the role of placenta mechanisms, the child’s developing immune system, and child autonomic nervous system physiology. Similarly, further research is needed to understand potentially protective or exacerbating roles of postnatal factors such as caregiving and childhood environment, which have been largely overlooked in studies of prenatal exposures (Bergman et al., 2008). Kagan and others inspired by his work (Williams et al., 2009) have demonstrated that developmental trajectories of children with fearful and inhibited temperament can be moderated by caregiving quality. Research of that kind is needed to understand the longer-term behavioral and health outcomes prompted by prenatal exposures.
Supplementary Material
Public Health Significance Statement.
This research examines the prenatal developmental origins of a key aspect of child behavioral development, fear reactivity in infancy. The findings provide the first robust evidence that maternal immune activation in pregnancy is associated with early-emerging fear reactivity. These results extend prior work on prenatal influence on child behavioral development in ways that suggest developmental mechanisms and potential intervention targets for promoting child health and behavioral and social development.
Acknowledgements:
Funding was provided by NIH grants OD023349, HD083369, and The Wynne Center for Family Research; the project described in this publication was supported by the University of Rochester CTSA award number UL1 TR002001 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no conflicts of interest.
References
- Ames JL, Burjak M, Avalos LA, Braun JM, Bulka CM, Croen LA, Dunlop AL, Ferrara A, Fry RC, Hedderson MM, Karagas MR, Liang D, Lin PD, Lyall K, Moore B, Morello-Frosch R, O'Connor TG, Oh J, Padula AM, Woodruff TJ, Zhu Y, Hamra GB, & program collaborators for Environmental influences on Child Health, O. (2023, May 1). Prenatal Exposure to Per- and Polyfluoroalkyl Substances and Childhood Autism-related Outcomes. Epidemiology, 34(3), 450–459. 10.1097/EDE.0000000000001587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asparouhov T, & Muthén B (2014). Variable-specific entropy contribution. Technical appendix. [Google Scholar]
- Baines KJ, Hillier DM, Haddad FL, Rajakumar N, Schmid S, & Renaud SJ (2020). Maternal Immune Activation Alters Fetal Brain Development and Enhances Proliferation of Neural Precursor Cells in Rats. Frontiers in Immunology, 11, 1145. 10.3389/fimmu.2020.01145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ (1993, Mar). Fetal origins of coronary heart disease [Editorial]. British Heart Journal, 69(3), 195–196. http://www.ncbi.nlm.nih.gov/pubmed/8461215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, Glover V, & O'Connor TG (2008, Oct). Quality of child-parent attachment moderates the impact of antenatal stress on child fearfulness. Journal of Child Psychology and Psychiatry, 49(10), 1089–1098. https://doi.org/JCPP1987 [pii] 10.1111/j.14697610.2008.01987.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beversdorf DQ, Stevens HE, Margolis KG, & Van de Water J (2019). Prenatal Stress and Maternal Immune Dysregulation in Autism Spectrum Disorders: Potential Points for Intervention. Current Pharmaceutical Design, 25(41), 4331–4343. 10.2174/1381612825666191119093335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, & Maier SF (2005, Feb). Neonatal infection induces memory impairments following an immune challenge in adulthood [Research Support, U.S. Gov't, P.H.S.]. Behavioral neuroscience, 119(1), 293301. 10.1037/0735-7044.119.1.293 [DOI] [PubMed] [Google Scholar]
- Braungart-Rieker JM, Hill-Soderlund AL, & Karrass J (2010, Jul). Fear and anger reactivity trajectories from 4 to 16 months: the roles of temperament, regulation, and maternal sensitivity. Dev Psychol, 46(4), 791–804. 10.1037/a0019673 [DOI] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, & Susser ES (2004, Aug). Serologic evidence of prenatal influenza in the etiology of schizophrenia. Archives of General Psychiatry, 61(8), 774–780. 10.1001/archpsyc.61.8.774 [DOI] [PubMed] [Google Scholar]
- Brown AS, & Meyer U (2018, Nov 1). Maternal Immune Activation and Neuropsychiatric Illness: A Translational Research Perspective. American Journal of Psychiatry, 175(11), 1073–1083. 10.1176/appi.ajp.2018.17121311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Murai T, & Bauman MD (2017, Mar 1). Maternal Immune Activation and Autism Spectrum Disorder: From Rodents to Nonhuman and Human Primates. Biological Psychiatry, 81(5), 391–401. 10.1016/j.biopsych.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerota M, Wylie A, Goldblum J, Wideman L, Cheatham CL, & Propper CB (2022). Testing a cascade model linking prenatal inflammation to child executive function. Behavioural Brain Research, 431, 113959. 10.1016/j.bbr.2022.113959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZZ, Luo BL, Yang QG, Ni MZ, Wu QT, Li Y, Li XW, & Chen GH (2022). Prenatal exposure to inflammation increases anxiety-like behaviors in F1 and F2 generations: possible links to decreased FABP7 in hippocampus. Frontiers in Behavior Neuroscience, 16, 973069. 10.3389/fnbeh.2022.973069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, & Sandman CA (2005). Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Developmental Neuroscience, 27(5), 299–305. https://doi.org/DNE2005027005299 [pii] 10.1159/000086709 [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, & Sandman CA (2011, Feb). Prenatal maternal stress programs infant stress regulation [Research Support, N.I.H., Extramural]. Journal of child psychology and psychiatry, and allied disciplines, 52(2), 119–129. 10.1111/j.1469-7610.2010.02314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Doenlen R, Engler A, Riether C, Prager G, Niemi MB, Pacheco-Lopez G, Krugel U, & Schedlowski M (2011, Oct). Acute amygdaloid response to systemic inflammation. Brain Behavior and Immunity, 25(7), 1384–1392. 10.1016/j.bbi.2011.04.005 [DOI] [PubMed] [Google Scholar]
- Entringer S, Buss C, & Wadhwa PD (2010, Dec). Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings [Research Support, N.I.H., Extramural Review]. Current opinion in endocrinology, diabetes, and obesity, 17(6), 507–516. 10.1097/MED.0b013e3283405921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes ML, & McAllister AK (2016, Aug 19). Maternal immune activation: Implications for neuropsychiatric disorders. Science, 353(6301), 772–777. 10.1126/science.aag3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Snidman N, Haas SA, Degnan KA, & Kagan J (2015, Jan). The relation between reactivity at 4 months and Behavioral Inhibition in the second year: Replication Across Three Independent Samples. Infancy: the official journal of the International Society on Infant Studies, 20(1), 98–114. 10.1111/infa.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JR, Van Hulle CA, Aksan N, Essex MJ, & Goldsmith HH (2011, Jun). Deriving childhood temperament measures from emotion-eliciting behavioral episodes: scale construction and initial validation [Research Support, N.I.H., Extramural]. Psychological Assessment, 23(2), 337–353. 10.1037/a0021746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Putnam SP, & Rothbart MK (2012). Etiology of preschool behavior problems: Contributions of temperament attributes in early childhood. Infant Mental Health Journal, 33(2), 197–211. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, Albert PS, Hornig M, Yeung E, Cherkerzian S, Goldstein RB, Buka SL, Goldstein JM, & Gilman SE (2018, Mar 13). Gestational cytokine concentrations and neurocognitive development at 7 years. Translational Psychiatry, 8(1), 64. 10.1038/s41398-018-0112-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Hornig M, Ghassabian A, Hahn J, Cherkerzian S, Albert PS, Buka SL, & Goldstein JM (2017, Jun 27). Socioeconomic disadvantage, gestational immune activity, and neurodevelopment in early childhood. Proceedings of the National Academy of Sciences U S A, 114(26), 6728–6733. 10.1073/pnas.1617698114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V, O'Connor TG, & O'Donnell K (2010, Sep). Prenatal stress and the programming of the HPA axis. Neuroscience and Biobehavior Reviews, 35(1), 17–22. https://doi.org/S0149-7634(09)00174-2 [pii] 10.1016/j.neubiorev.2009.11.008 [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, & Pinal C (2005, Jul). The developmental origins of adult disease. Maternal and Child Nutrition, 1(3), 130–141. https://doi.org/MCN20 [pii] 10.1111/j.17408709.2005.00020.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HHR, M.K. (1999). Laboratory temperament assessment battery (Lab-TAB) version 3.1. University of Wisconsin. [Google Scholar]
- Goldstein JM, Cherkerzian S, Seidman LJ, Donatelli JA, Remington AG, Tsuang MT, Hornig M, & Buka SL (2014, Nov). Prenatal maternal immune disruption and sex dependent risk for psychoses. Psychological Medicine, 44(15), 3249–3261. 10.1017/S0033291714000683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur TL, Shay L, Palkar AV, Fisher S, Varaljay VA, Dowd S, & Bailey MT(2017, Aug). Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behavior and Immunity, 64, 50–58. 10.1016/j.bbi.2016.12.021 [DOI] [PubMed] [Google Scholar]
- Han VX, Patel S, Jones HF, Nielsen TC, Mohammad SS, Hofer MJ, Gold W, Brilot F, Lain SJ, Nassar N, & Dale RC (2021, Jan 21). Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Translational Psychiatry, 11(1), 71. 10.1038/s41398-021-01198-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N,Reznick JS, & Kagan J (1992, Jan). Stable behavioral inhibition and its association with anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 31(1), 103–111. 10.1097/00004583-199201000-00016 [DOI] [PubMed] [Google Scholar]
- Huizink AC, de Medina PG, Mulder EJ, Visser GH, & Buitelaar JK (2002, Sep). Psychological measures of prenatal stress as predictors of infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry, 41(9), 1078–1085. [DOI] [PubMed] [Google Scholar]
- Jonakait GM (2007, Nov). The effects of maternal inflammation on neuronal development: possible mechanisms. International Journal of Developmental Neuroscience, 25(7), 415–425. 10.1016/j.ijdevneu.2007.08.017 [DOI] [PubMed] [Google Scholar]
- Kagan J. (2002, Sep). Childhood predictors of states of anxiety. Dialogues in Clinical Neuroscience, 4(3), 287–293. 10.31887/DCNS.2002.4.3/jkagan [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, & Snidman N (1987, Dec). The physiology and psychology of behavioral inhibition in children. Child Development, 58(6), 1459–1473. http://www.ncbi.nlm.nih.gov/pubmed/3691195 [PubMed] [Google Scholar]
- Kagan J, Reznick JS, & Snidman N (1988, Apr 8). Biological bases of childhood shyness. Science, 240(4849), 167–171. 10.1126/science.3353713 [DOI] [PubMed] [Google Scholar]
- Kelly RS, Lee-Sarwar K, Chen YC, Laranjo N, Fichorova R, Chu SH, Prince N, Lasky-Su J, Weiss ST, & Litonjua AA (2022, Dec 3). Maternal Inflammatory Biomarkers during Pregnancy and Early Life Neurodevelopment in Offspring: Results from the VDAART Study. International Journal of Molecular Sciences, 23(23). 10.3390/ijms232315249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp EA, Kress AM, Parker CB, Page GP, McArthur K, Gachigi KK, Alshawabkeh AN, Aschner JL, Bastain TM, Breton CV, Bendixsen CG, Brennan PA, Bush NR, Buss C, Camargo CA Jr., Catellier D, Cordero JF, Croen L, Dabelea D, Deoni S, D'Sa V, Duarte CS, Dunlop AL, Elliott AJ, Farzan SF, Ferrara A, Ganiban JM, Gern JE, Giardino AP, Towe-Goodman NR, Gold DR, Habre R, Hamra GB, Hartert T, Herbstman JB, Hertz-Picciotto I, Hipwell AE, Karagas MR, Karr CJ, Keenan K, Kerver JM, Koinis-Mitchell D, Lau B, Lester BM, Leve LD, Leventhal B, LeWinn KZ, Lewis J, Litonjua AA, Lyall K, Madan JC, McEvoy CT, McGrath M, Meeker JD, Miller RL, Morello-Frosch R, Neiderhiser JM, O'Connor TG, Oken E, O'Shea M, Paneth N, Porucznik CA, Sathyanarayana S, Schantz SL, Spindel ER, Stanford JB, Stroustrup A, Teitelbaum SL, Trasande L, Volk H, Wadhwa PD, Weiss ST, Woodruff TJ, Wright RJ, Zhao Q, Jacobson LP (2023, Aug 4). The Environmental Influences on Child Health Outcomes (ECHO)-Wide Cohort. American Journal of Epidemiology, 192(8), 1249–1263. 10.1093/aje/kwad071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen MK, Aatsinki A, Kortesluoma S, Mustonen P, Munukka E, Lukkarinen M, Perasto L, Keskitalo A, Karlsson H, & Karlsson L (2023, Aug). Hair cortisol, cortisone and DHEA concentrations and the composition of microbiota in toddlers. Psychoneuroendocrinology, 154, 106309. 10.1016/j.psyneuen.2023.106309 [DOI] [PubMed] [Google Scholar]
- Lyall K, Ames JL, Pearl M, Traglia M, Weiss LA, Windham GC, Kharrazi M, Yoshida CK, Yolken R, Volk HE, Ashwood P, Van de Water J, & Croen LA (2021, Mar 18). A profile and review of findings from the Early Markers for Autism study: unique contributions from a population-based case-control study in California. Molecular Autism, 12(1), 24. 10.1186/s13229-021-00429-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, & Borkovec TD (1990). Development and validation of the Penn State Worry Questionnaire. Behavior Research and Therapy, 28(6), 487–495. https://doi.org/0005-7967(90)90135-6 [pii] [DOI] [PubMed] [Google Scholar]
- Mohler E, Parzer P, Brunner R, Wiebel A, & Resch F (2006, Nov). Emotional stress in pregnancy predicts human infant reactivity. Early Human Development, 82(11), 731–737. https://doi.org/S0378-3782(06)00085-5 [pii] 10.1016/j.earlhumdev.2006.02.010 [DOI] [PubMed] [Google Scholar]
- Molteno CD, Jacobson JL, Carter RC, Dodge NC, & Jacobson SW (2014, Feb). Infant emotional withdrawal: a precursor of affective and cognitive disturbance in fetal alcohol spectrum disorders. Alcohol Clin Exp Res, 38(2), 479–488. 10.1111/acer.12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzari S, & Frigerio A (2020). The programming role of maternal antenatal inflammation on infants’ early neurodevelopment: A review of human studies. Journal of Affective Disorders, 263, 739–746. 10.1016/j.jad.2019.10.010 [DOI] [PubMed] [Google Scholar]
- Nielsen TC, Nassar N, Shand AW, Jones H, Guastella AJ, Dale RC, & Lain SJ (2021, Mar 1). Association of maternal autoimmune disease with Attention Deficit/Hyperactivity Disorder in children. JAMA Pediatrics, 175(3), e205487. 10.1001/jamapediatrics.2020.5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TG, Ciesla AA, Sefair AV, Thornburg LL, Brown AS, Glover V, & O'Donnell KJ (2022, May). Maternal prenatal infection and anxiety predict neurodevelopmental outcomes in middle childhood. Journal of Psychopathology and Clinical Science, 131(4), 422–434. 10.1037/abn0000746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TG, Winter MA, Hunn J, Carnahan J, Pressman EK, Glover V, Robertson-Blackmore E, Moynihan JA, Lee FE, & Caserta MT (2013, Aug). Prenatal maternal anxiety predicts reduced adaptive immunity in infants [Research Support, N.I.H., Extramural]. Brain, Behavior, and Immunity, 32, 21–28. 10.1016/j.bbi.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parade SH, & Leerkes EM (2008). The reliability and validity of the Infant Behavior Questionnaire-Revised. Infant Behavior and Development, 31(4), 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planalp EM, Van Hulle C, Gagne JR, & Goldsmith HH (2017). The Infant Version of the Laboratory Temperament Assessment Battery (Lab-TAB): Measurement Properties and Implications for Concepts of Temperament. Frontiers in psychology, 8, 846. 10.3389/fpsyg.2017.00846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam SP, Helbig AL, Gartstein MA, Rothbart MK, & Leerkes E (2014). Development and assessment of short and very short forms of the infant behavior questionnaire-revised. Journal of Personality Assessment, 96(4), 445–458. 10.1080/00223891.2013.841171 [DOI] [PubMed] [Google Scholar]
- Rasmussen JM, Graham AM, Gyllenhammer LE, Entringer S, Chow DS, O'Connor TG, Fair DA, Wadhwa PD, & Buss C (2021, Mar 22). Neuroanatomical Correlates Underlying the Association Between Maternal Interleukin-6 Concentration During Pregnancy and Offspring Fluid Reasoning Performance in Early Childhood. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 10.1016/j.bpsc.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifer R, Sameroff AJ, Barrett LC, & Krafchuk E (1994, Oct). Infant temperament measured by multiple observations and mother report. Child Development, 65(5), 1478–1490. http://www.ncbi.nlm.nih.gov/pubmed/7982363 [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, & Patterson PH (2007, Oct 3). Maternal immune activation alters fetal brain development through interleukin-6. Journal of Neuroscience, 27(40), 10695–10702. 10.1523/JNEUROSCI.2178-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann MN, Monk C, Scheinost D, & Peterson BS (2018, Mar 14). Maternal Immune Activation During the Third Trimester Is Associated with Neonatal Functional Connectivity of the Salience Network and Fetal to Toddler Behavior. Journal of Neuroscience, 38(11), 2877–2886. 10.1523/JNEUROSCI.2272-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A, Harrewijn A, Benson B, Haller SP, Guyer AE, Perez-Edgar KE, Stringaris A, Ernst M, Brotman MA, Pine DS, & Fox NA (2022, Dec 1). Striatal Activity to Reward Anticipation as a Moderator of the Association Between Early Behavioral Inhibition and Changes in Anxiety and Depressive Symptoms From Adolescence to Adulthood. JAMA psychiatry, 79(12), 1199–1208. 10.1001/jamapsychiatry.2022.3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Mazefsky CA, Dichter GS, Chiu PH, Richey JA, & Ollendick TH (2014, Dec). Social-cognitive, physiological, and neural mechanisms underlying emotion regulation impairments: understanding anxiety in autism spectrum disorder. Internal Journal of Developmental Neuroscience, 39, 22–36. 10.1016/j.ijdevneu.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LR, Degnan KA, Perez-Edgar KE, Henderson HA, Rubin KH, Pine DS, Steinberg L, & Fox NA (2009, Nov). Impact of behavioral inhibition and parenting style on internalizing and externalizing problems from early childhood through adolescence. Journal of Abnormal Child Psychology, 37(8), 1063–1075. 10.1007/s10802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby MT, Gottfredson NC, Stifter CA, & Family Life Project I (2017, Feb). Observed temperament from ages 6 to 36 months predicts parent- and teacher-reported attention-deficit/hyperactivity disorder symptoms in first grade. Development and Psychopathology, 29(1), 107–120. 10.1017/S0954579415001236 [DOI] [PubMed] [Google Scholar]
- Zhang W, Rajendran K, Ham J, Finik J, Buthmann J, Davey K, Pehme PM, Dana K, Pritchett A, Laws H, & Nomura Y (2018, Jul). Prenatal exposure to disaster-related traumatic stress and developmental trajectories of temperament in early childhood: Superstorm Sandy pregnancy study. Journal of Affective Disorders, 234, 335–345. 10.1016/j.jad.2018.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


