Abstract
For centuries, cannabinoids have been utilized for their medicinal properties, particularly in Asian and South-Asian countries. Cannabis plants, known for their psychoactive and non-psychoactive potential, were historically used for spiritual and remedial healing. However, as cannabis became predominantly a recreational drug, it faced prohibition. Recently, the therapeutic potential of cannabinoids has sparked renewed research interest, extending their use to various medical conditions, including cancer. This review aims to highlight current data on the involvement of cannabinoids in cancer signaling pathways, emphasizing their potential in cancer therapy and the need for further investigation into the underlying mechanisms. A comprehensive literature review was conducted using databases such as PubMed/MedLine, Google Scholar, Web of Science, Scopus, and Embase. The search focused on peer-reviewed articles, review articles, and clinical trials discussing the anticancer properties of cannabinoids. Inclusion criteria included studies in English on the mechanisms of action and clinical efficacy of cannabinoids in cancer. Cannabinoids, including Δ9-THC, CBD, and CBG, exhibit significant anticancer activities such as apoptosis induction, autophagy stimulation, cell cycle arrest, anti-proliferation, anti-angiogenesis, and metastasis inhibition. Clinical trials have demonstrated cannabinoids’ efficacy in tumor regression and health improvement in palliative care. However, challenges such as variability in cannabinoid composition, psychoactive effects, regulatory barriers, and lack of standardized dosing remain. Cannabinoids show promising potential as anticancer agents through various mechanisms. Further large-scale, randomized controlled trials are essential to validate these findings and establish standardized therapeutic protocols. Future research should focus on elucidating detailed mechanisms, optimizing dosing, and exploring cannabinoids as primary chemotherapeutic agents.
Keywords: Anticancer effects, Apoptosis, Cannabinoids, CBD, Δ9-THC, Metastasis, Pharmacological mechanisms
Introduction
Cannabinoids are the organic compounds that belongs to the endocannabinoid system having psychoactive as well as non-psychoactive activities. Cannabis plants have known history of medicinal purpose along with its wide use on the religious or spiritual basis as in Chinese and Indian populations. Natural bioactive compounds, including cannabinoids, polyphenols, flavonoids, and terpenes, have demonstrated beneficial effects in treating chronic diseases, including cancer, by modulating key signaling pathways and enhancing the efficacy of conventional therapies [1–4] Cannabis Sativa and Cannabis Indica are two paramount species of Cannabis plant that vary in their role on the basis of the variation of different factors including climatic, geological and the ratio of the pharmacological compounds present [5]. Cannabis Sativa sp. are mainly associated with the production of pharmacological significant compounds “Cannabinoids” that have valuable role in different ailments including neurological disorders as epilepsy, multiple sclerosis, insomnia, spasticity, vomiting, nausea and the pain that are critically associated with chemotherapy [6, 7]. Cannabinoids are considered as potentially therapeutic or pharmacological before their discovery being a psychoactive compound. Cannabis plants produces hundreds of therapeutically important compounds and hence considered as “Store house” for such compounds. Figure 1 illustrate different compounds that are produced by cannabis plants. Biological activity of these varying compounds depends upon the presence of different concentrations of the pharmacological compounds [8, 9]. The therapeutic effects of cannabinoids extend to lethal diseases like cancer as well which has been made evident through studies ranging from in vitro lab experiments, animal models, and clinical trials [10]. With nausea and vomiting being the most common complications associated with chemotherapy, cannabinoids have shown their antiemetic potential in clinical trials with patients preferring cannabinoids over conventional drugs [11–13]. Furthermore, cannabinoids also show promise in stimulating appetite in cancer patients [14, 15] and eliciting analgesic responses by inhibiting the nociceptive receptors [16–18].
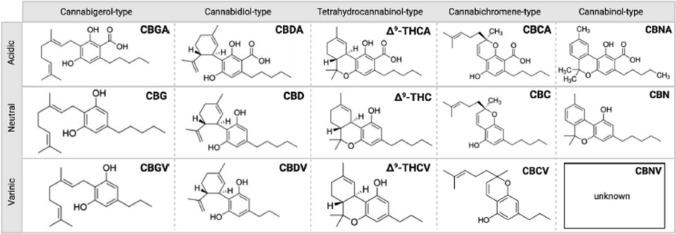
Fig. 1.
Different typed of Cannabinoids synthesized by cannabis plant [8, 19]
The anticancer potencies of cannabinoids have been under extensive research for an extended period of time now. Although now we know heaps more about the undeniable, albeit slightly conflicted, anti-cancerous properties of cannabinoids, there is arguably far more detail that needs to be uncovered about the exact mechanism of action of cannabinoids in different cancer cells. In this review, we’ve aimed to cover all the information obtained thus far about cannabinoids, the endocannabinoid system, the anticancer characteristics of cannabinoids, and the manner in which those characteristics are implemented in cells.
Methodology
A comprehensive literature search was conducted to analyze the anticancer properties of cannabinoids, utilizing five major databases: PubMed/MedLine, Google Scholar, Web of Science, Scopus, and Embase. The search strategy incorporated specific Medical Subject Headings (MeSH) terms and keywords, employing Boolean operators to refine the search. The primary MeSH terms and keywords included “Cannabinoids,” “Cannabinoid Receptors,” “Cannabinoids and Cancer,” “Phytocannabinoids,” “Synthetic Cannabinoids,” “Endocannabinoids,” “Cannabinoids Therapeutic Use,” “Antineoplastic Agents,” “Cancer Cell Signaling,” and “Cannabinoids Mechanism of Action.” Boolean operators such as AND, OR, and NOT were employed to combine and exclude terms as necessary, enhancing the search specificity and relevance. The inclusion criteria were set to encompass peer-reviewed articles published in English that focused on the anticancer effects of cannabinoids. This included research articles, review articles, and meta-analyses that involved in vitro, in vivo, and clinical trials. Exclusion criteria were applied to eliminate articles not in English, studies concentrating solely on the recreational use of cannabinoids, case reports, editorials, commentaries, and studies lacking substantial data or presenting inconclusive results. The taxonomy of the cannabis plants discussed in the selected studies was validated using the World Flora Online (WFO) database, ensuring botanical accuracy [20]. The chemical structures of the cannabinoids were cross-referenced and validated using PubChem, guaranteeing the chemical data’s integrity [21]. Data extraction focused on synthesizing relevant information into comprehensive tables and figures, highlighting key findings, mechanisms of action, therapeutic potentials, and clinical outcomes. The most critical data are summarized in these visual aids to provide a clear and effective presentation of the current research landscape regarding cannabinoids and cancer.
Cannabinoids: general aspects
Classification, roles and therapeutic potential of cannabinoids
Cannabinoids are classified into three major divisions based on their origins, as evident by their names, as endogenous or endocannabinoids, phytocannabinoids and synthetic cannabinoids (Fig. 2).
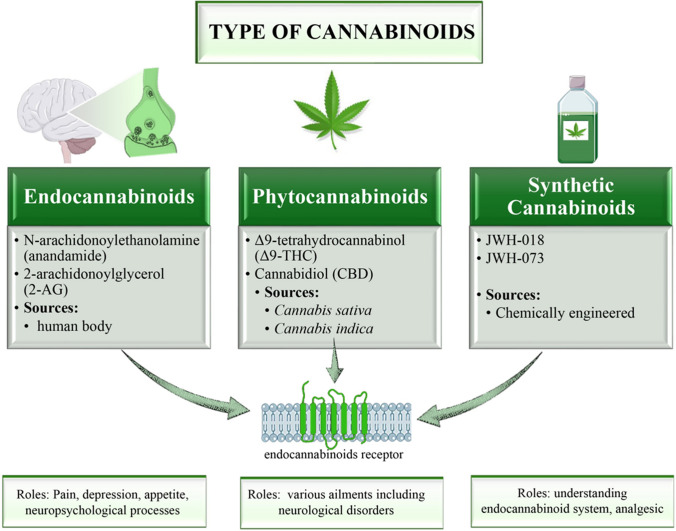
Fig. 2.
Classification and roles of cannabinoids. The figure illustrates the three primary classifications of cannabinoids based on their origins: endocannabinoids, phytocannabinoids, and synthetic cannabinoids. Endocannabinoids, synthesized within the human body, include N-arachidonoylethanolamine (anandamide) and 2-arachidonoylglycerol (2-AG). They regulate pain, depression, appetite, and various neuropsychological processes. Phytocannabinoids, derived from Cannabis sativa and Cannabis indica plants, include Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD), and are used therapeutically for neurological disorders. Synthetic cannabinoids, such as JWH-018 and JWH-073, are chemically engineered for research and analgesic purposes. The diagram shows the interaction of these cannabinoids with endocannabinoid receptors and their respective roles in medical and therapeutic contexts
Cannabinoids synthesized within the human body, and that act on cannabinoid receptors eliciting a cannabinomimetic response, are termed as endocannabinoids. Two prominent molecules in this group, N-arachidonoylethanolamine (anandamide), first discovered in porcine brain extract [22], and 2-arachidonoylglycerol (2-AG) [23, 24], have become the target of extensive neurological research due to their inherent role in pain, stress, depression, appetite, and other neuropsychological processes [25]. Taking into account the effect of the endocannabinoid system on the immune system, cellular reproduction, survival, and death [26], their molecular involvement in cancer biology is being explored at length [27, 28]. Among cannabis species worldwide, Cannabis sativa and Cannabis indica are regarded as the most prevalent and ubiquitous sources of pythocannabinoids [29]. These plants are known for their abundant production of therapeutic phytocannabinoids, including Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD). These, and an additional > 110 cannabinoid compounds found in cannabis plants [30], administer their effects via the mammalian endocannabinoid system, by binding to the CB1 and CB2 cannabinoid receptors [31]. Synthetic cannabinoids, as the name suggests, are chemically engineered mainly to achieve the goal of understanding the endocannabinoid system while simultaneously avoiding the complications and restrictions associated with using cannabis and endocannabinoids [32]. Continued research on synthetic cannabinoids leads to recurrent discovery of new synthetic cannabinoids, hence their classification becomes a tricky task. Most recently, they’ve been classified according to their structural relevance to naturally occurring cannabinoids, namely classical cannabinoids, nonclassical cannabinoids, hybrid cannabinoids, aminoalkylindoles, eicosanoids and miscellaneous cannabinoids [33]. JWH-018, a synthetically manufactured analgesic, JWH-073 [34], and many others are examples of synthetic cannabinoids. Over the years, research based on novel cannabinergic, cannabimimetic, and cannabinoid-based therapeutic drugs targeting the endocannabinoid system (ECS) has brought about a wide range of targetable diseases including psychological disorders [35, 36], psychophysiological disorders [37, 38], neurodegenerative disorders [39, 40], autoimmune disorders [41–43], dermatological diseases [44–47], cardiovascular disorders [48], diabetes [49], and a number of cancers [50–56] among others. The ability of cannabimimetic and cannabinoid-derived drugs to elicit a response within the body is possible solely due to the presence of the innate endocannabinoid system in mammals [57]. Till date, research on the involvement of this system in gastrointestinal (GI) function [58], appetite and metabolism [59, 60], sensation of pain [16, 61], memory [62], movement [63], immunity [64], and inflammation [65] has been reaffirmed. Ligands of the cannabinoid receptors are becoming the promising factors for research now as they are considered as their greatest role in the therapeutics. Dibenzopyran derivatives (delta-8- and delta-9-tetrahydrocannabinols), THC (synthetically manufactured dicyclic compounds having analgesic effect) which are the most influential cannabinoids, anti-inflammatory amino-alkyl-indole structure agonists and pro and anti-apoptotic endogenous compounds are the four classes respectively of CBR agonists categorised by extensive research [66].
Legal and therapeutic status of cannabinoid-derived drugs
Despite the increasing number of cannabinoid-extracted drugs still under clinical trials, reflecting the need for extensive research to identify the therapeutic use of cannabinoids unhindered by legal restrictions, the range of therapeutic potential offered by said drugs necessitates their approval for use at the national level by one or more governing agencies [67]. In some countries, like the US and in Europe, the Food and Drug Administration (FDA) and European Medicines Agency (EMA) have approved the use of a few cannabinoid-derived drugs for medical purposes as safe and accurate for treatment. Although still labelled as Schedule I (highly restricted) substances, most states in America have authorized and taken part in the cultivation, production, safety assessment, and supply of an assortment of these cannabis-derived substances [68]. Where state laws permit the use of cannabis and cannabinoid products, their use in medicine, research, and recreation remains illegal by federal legislation [69]. The regulatory landscape surrounding cannabinoid-derived drugs remains complex and inconsistent despite growing acceptance and legalization for both medical and recreational use in various jurisdictions [70]. In certain regions, specific cannabinoid products, such as cannabidiol (CBD), have gained approval for medical applications, notably in the treatment of epilepsy, with products like Epidiolex®; however, products containing tetrahydrocannabinol (THC), which possess psychoactive properties, continue to face stricter regulations, limiting their broader therapeutic use [71]. This fragmented regulatory framework presents significant challenges for patients seeking access to cannabinoid therapies, healthcare providers prescribing them, and researchers conducting studies [72]. The variability in legal status across regions also complicates the standardization of treatment protocols, potentially leading to inconsistent therapeutic outcomes. For instance, inconsistent access and legal restrictions hinder the ability to conduct large-scale, robust clinical trials that could clarify the safety and efficacy profiles of these therapies [72, 73]. Recent research has emphasized the importance of standardizing cannabinoid products to ensure consistent therapeutic outcomes. Studies have shown that variability in cannabinoid concentrations and quality across different products can lead to inconsistent treatment effects, particularly in cancer therapy [74]. In addition, the lack of rigorous clinical trials limits the development of clear dosing guidelines and increases the risk of adverse drug interactions [75]. To address these challenges, ongoing research is fundamental to establish standardized protocols for the cultivation, extraction, and formulation of cannabinoid products. Furthermore, educating healthcare providers on the latest developments in cannabinoid pharmacology is essential to ensure patient safety and optimize therapeutic outcomes Such education will help healthcare providers navigate the complex legal landscape and make informed decisions regarding cannabinoid-based treatments [75].
The approved drugs, along with their therapeutic uses, side effects, and methods of administration, are summarized in Table 1.
Table 1.
Cannabinoid based approved drugs
| Drug | Trade name | Composition | Administration | Treatment | Side effects | References |
|---|---|---|---|---|---|---|
| Dronabinol | Marinol® | (Δ9-THC)Δ9-tetrahydrocannabinol produced synthetically | Oral administration | Anorexia, weight loss in patients, AIDS, Nausea and vomiting induced by chemotherapy | Abdominal pain, asthenia, amnesia heart palpitations, rare depersonalization | [76] |
| Nabilone | Cesamet™ | Synthetic cannabinoid (having structure similar to Δ9-THC | Oral administration | Nausea and vomiting, psychoactivity | Drowsiness/vertigo, orthostatic hypotension, dry mouth, dyspnea, euphoria, headache and rare but serious psychosis | [77] |
| Nabiximols | Sativex® | Extracted from plants having equal quantity of CBD and Δ9-THC | Oromucosal spray | Spasticity | Blurred vision, dizziness, fatigue, constipation, vertigo, changes in appetite, depression. Rare but serious hallucination, changes in B.P and palpitation | [78] |
| Cannabidiol | Epidiolex® | Pure (98%) plant derived solution of CBD | Oral administration | Lennox-Gastaut syndrome, Dravet syndrome | Hepatocellular toxicity, fatigue decreased appetite, and diarrhea, drowsiness | [79] |
Rimonabant (Acomplia®) was also approved by European market from 2006 to 2009 as an antagonist to CB1 receptor. It is produced synthetically and involved in the treatment of weight management, type II diabetes and dyslipidemia. But due to adverse side effects like upper respiratory infection, nausea, suicidal thoughts and depression, it was withdrawn by EMA in 2009 from market [80].
Cannabinoid receptors
Roles and distribution in the human body
Cannabinoids perform their role in the human body by interaction and modulation of the most critical component of the endocannabinoid system, i.e. the cannabinoid receptors, that are naturally occurring receptors in the body [81]. The endocannabinoid system consist of endogenous cannabinoids, enzymes that synthesize endocannabinoids and most importantly the Cannabinoid receptors [82]. Attempts to understand the mechanism of phytocannabinoid action prompted researchers to focus their attention on the endocannabinoid system. This led to the discovery of two G protein-coupled receptors (GPCRs), namely cannabinoid receptors type 1 (CB1R) and cannabinoid receptors type 2 (CB2R) [83–85]. Each cannabinoid receptor exerts its effect differently on the basis of their mode of action and site of expression in the body as represented graphically in Fig. 3.
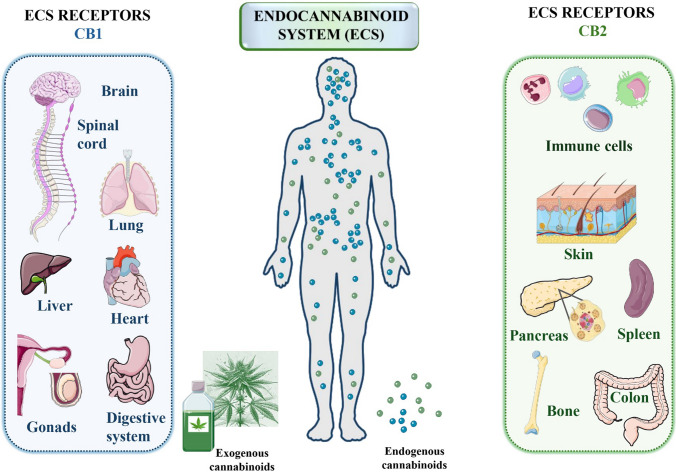
Fig. 3.
Distribution and roles of cannabinoid receptors CB1R and CB2R in the human body. The diagram illustrates the two main types of G-protein coupled cannabinoid receptors: cannabinoid receptor 1 (CB1R) and cannabinoid receptor 2 (CB2R). CB1R are predominantly found in the brain and spinal cord, where they play vital roles in neurological functions such as synaptic remodeling, neurogenesis, neuron migration, axonal targeting, and synaptogenesis. These receptors are distributed across various regions of the central nervous system, including the hypothalamus, hippocampus, basal ganglia, amygdala, cortex, and cerebellum. In contrast, CB2R are primarily located in the cells and tissues of the immune system, where they significantly modulate immune responses. They are present in immune cells like macrophages in the spleen and tonsils, aiding the immunosuppressive functions of the endocannabinoid system. The diagram emphasizes the specific locations and functions of CB1R and CB2R, highlighting their critical roles in both neurological and immune system processes
CB1 receptors (CB1R) are highly concentrated in the brain and spinal cord, where they play a fundamental role in various processes during brain development [86]. These processes include the remodeling of neuronal synapses during learning, neurogenesis, neuronal migration, axonal targeting, and synaptogenesis. CB1R are widely distributed throughout the central nervous system, including regions such as the hypothalamus, hippocampus, basal ganglia, amygdala, cortex, and especially the cerebellum. In addition to their presence in the central nervous system, CB1R are also found in smaller but significant amounts in peripheral tissues, including the liver, adipose tissue, vascular and cardiac tissues, reproductive organs, bone, and skin [87, 88]. Further research has confirmed the presence of CB1R on both neuronal and immune cells. However, detailed investigations of peripheral tissues with immune functions, such as macrophages in the spleen and tonsils, led to the discovery of a related cannabinoid receptor, CB2R. This receptor, known as cannabinoid receptor type 2 (CB2R), is primarily expressed in immune system tissues and cells, where it plays a key role in modulating the immune response and supporting the endocannabinoid system through immunosuppression [87–89].
Molecular structure and regulation of cannabinoid receptors
By now we are fully cognizant of the value of the human endocannabinoid system. The diverse range of physiological processes regulated by this system reinforce the years long belief of scientists to explore and understand it. To accomplish this, studies to elucidate the detailed structure of cannabinoid receptors became favorable. By targeting the receptors as focus of study, instead of their intrinsic and extrinsic ligands, researchers aimed to provide a more lucid and coherent model regarding the interactions between agonists and antagonists of the cannabinoid system and their subsequent downstream signaling. As such, molecular modeling studies of GPCRs have been using the structure of bacteriorhodopsine (BR) as template structures [90], while the ground state of Rhodopsin has been established at 2.8 Å resolution by X-ray crystallography [91] and NMR [92, 93]. These readings allowed for reliable modeling of GPCRs and subsequently enabled building of 3D models of both CB1R and CB2R. CNR1 gene, located on chromosome 6q14-q15 [94, 95], is responsible for the production of CB1R protein which is 472 amino acids in length [96]. Initially found in the intron leading up to the exon coding CB1R [94], several polymorphisms of the CB1 receptor have been identified till date. On the other hand, CB2R is encoded by the CNR2 gene, containing a single coding exon, located on chromosome 1p36 [97]. This protein is a single polypeptide chain consisting of 360 amino acids in length [98]. The main differences between the two receptors lies not only in amino acid number, but also their sequence, especially in the extracellular N-terminal sequences [99]. Both CB1R and CB2R belong to the class A (rhodopsin-like) GPCRs. The general structure of the class A GPCR cannabinoid receptors (depicted in Fig. 4) encompass seven transmembrane α-helices (TMHs) positioned as closed bundles, loops that connect the TMHs extending intra- and extracellularly, an extracellular N-terminus, and an intracellular C-terminus which has a short helical segment (Helix 8) at the beginning aligned side by side to the membrane surface [100]. Studies have shown that, among the G protein families, CB1R binds predominantly to Gi/o protein and, under specific conditions, binds to Gs and Gq as well [101, 102], while CB2R binds to Gi/o protein exclusively. Analysis of the interactive physiology of cannabinoid ligands to cannabinoid receptors was carried out through site-specific mutation studies on Rhodopsin subfamily of receptors. The inference showed that the ligand binding site is located in the TMH core within the crevice formed by TM3, TM5, TM6, and TM7 [91, 92] (Fig. 4).
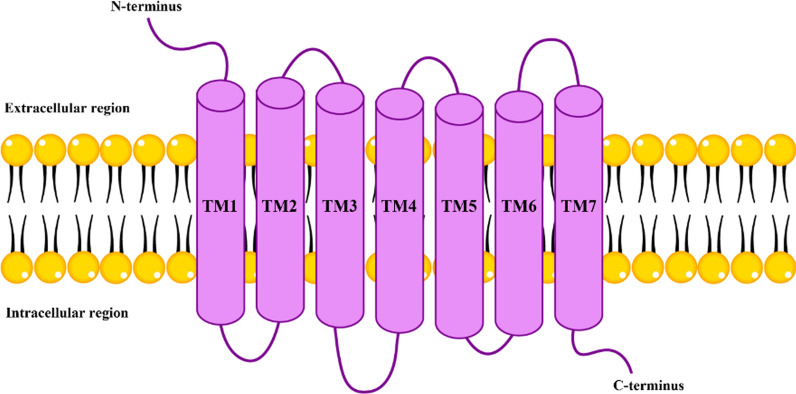
Fig. 4.
Structural model of cannabinoid receptors. This image depicts the structural model of cannabinoid receptors, specifically highlighting the seven transmembrane α-helices (TM1 to TM7). These helices span the cell membrane, forming a bundle that is essential for the receptor’s function. The loops connecting these transmembrane segments extend into both the intracellular and extracellular environments. The receptor features an extracellular N-terminus and an intracellular C-terminus, which includes a short helical segment known as Helix 8. This structural configuration is typical of class A G-protein coupled receptors (GPCRs), including cannabinoid receptors CB1R and CB2R. These receptors are pivotal in various physiological processes, mediating the effects of endogenous and exogenous cannabinoids by interacting with G proteins and triggering intracellular signaling pathways
Along with Gi/o-mediated signaling, CB1R and CB2R undergo phosphorylation by G protein receptor kinases (GRKs) which paves way for subsequent regulation by β-arrestin1 and β-arrestin2 [103, 104]. Cannabinoid receptors perform their function in the body by regulating variety of systems including regulation of calcium and potassium channels, MAPK pathway regulation, regulating the activity of adenylyl cyclase and variety of intracellular pathways as ERK, JNK and p38 etc. [105, 106].
Anticancer effects of cannabinoids
Effects on various cancer types
One of the most notorious diseases known and feared throughout the world is cancer, made distinctive by its specific hallmarks, pre-eminently the rapid and uncontrolled proliferation of abnormal cells that surpass their usual extent. The multi-phase transformation of tumor begins with damage to DNA, that causes mutations, cell cycle defects, and the inhibition of apoptosis [107]. Recent studies have detailed different molecular techniques that were used by different researchers to indicate the expression or over expression of cannabinoid receptors in various types of cancers [108, 109]. Cannabinoids are reported to be involved in various types of cancers including glioma, breast cancer, colorectal cancer, leukemia, prostate, esophageal, lung cancer, ovarian cancer, blood cancer etc.[110] (Table 2). Through in vitro, in vivo, and a few clinical trials, psychoactive THC and non-psychoactive cannabidiol (CBD) are observed to have the most anticancer activity in a number of cancers such as listed below.
Table 2.
Anticancer activity of THC and CBD in different cancer types
| Cancer type | Cannabinoid | Effect on cancer cells | Mechanism | References |
|---|---|---|---|---|
| Breast cancer | THC | ↓ Cell proliferation | Inhibition of estradiol-induced proliferation via ERα inhibition | [111–114] |
| THC |
↑ HER2 expression ↓ Tumor growth |
Increased HER2 expression and tumor cell growth | [115, 116] | |
| CBD |
↑ Apoptosis ↑ Autophagy ↓ Metastasis |
↑ ROS production ↓EGF, ↓ EMT |
[117, 119, 120] | |
| Glioma | THC | Dose-specific effects | Variable effects including inhibition of tumor growth and apoptosis induction | [121–126] |
| CBD |
↑ Apoptosis ↓Tumor cell migration |
ROS generation | [122, 127–130] | |
| THC + CBD | Synergistic anti-cancer effects | Combined treatment enhanced therapeutic efficacy | [122, 132] | |
| Leukemia | THC + CBD | Dose-dependent effects | Sensitization to chemotherapy, ↑ Cell death | [133, 134] |
| Lung cancer | THC | ↑ Proliferation, ↓ Immunogenicity |
↓Epidermal growth factor (EGF) ↓EGFR |
[126, 135] |
| CBD |
↑ Apoptosis, ↓ Invasion, ↓ Metastasis ↓tumor growth |
↑ COX2 ↑PPARγ ↑Immune cell susceptibility |
[136, 137, 140, 144] | |
| Melanoma | THC, THC + CBD |
↓ Cell viability ↓ Proliferation ↓ Metastasis ↓ Angiogenesis |
↑Autophagy ↑Apoptosis |
[145–147] |
| Myeloma | THC, CBD | [149] | ||
| Hepatocellular carcinoma | THC, CBD | [150] | ||
| Pancreatic cancer | THC, CBD | [151, 152] | ||
| Colon cancer | THC, CBD |
↑ Apoptosis ↑ Necrosis |
[153, 154] | |
| Endometrial and Cervical cancers | THC, CBD |
↓ Cell viability ↓ Proliferation ↓ Metastasis ↓ Angiogenesis |
[155] | |
| Oral cancer | THC, CBD | [156] | ||
| Prostate cancer | THC | ↑ Cannabinoid receptor expression, ↑ Apoptosis ↑ Necrosis | increased expression in cancer cells, apoptosis induction | [158] |
| CBD | Protective factor in mouse models | protective effects in colon cancer | [159] | |
| Melanoma (CB2R) | CBD | Protective and therapeutic agent | suppression of tumor growth, modification of immune cell roles | [159] |
Abbrevi ations: AKT Protein kinase B, CBD Cannabidiol, CB1R Cannabinoid receptor type 1, CB2R Cannabinoid receptor type 2, COX2 Cyclooxygenase 2, EGF Epidermal growth factor, EGFR Epidermal growth factor receptor, EMT Epithelial-mesenchymal transition, ERK Extracellular signal-regulated kinase, HER2 Human epidermal growth factor receptor 2, HSP Heat shock proteins, PPARγ Peroxisome proliferator-activated receptor gamma, pERK Phosphorylated extracellular signal-regulated kinase, ROS Reactive oxygen species, THC Δ9-Tetrahydrocannabinol
Breast cancer
THC, in a controlled concentration, can inhibit overall cell proliferation and growth of breast cancer cells [111]. A number of studies monitored the exposure of THC on breast cancer cells and have reported the inhibition of estradiol-induced cell proliferation via estrogen receptor α inhibition [112], antagonization of 17β-estradiol-induced proliferation [113], induction of apoptosis by targeting the ErbB2 [114], and more. In contradiction, a number of studies have also reported the exact opposite effect of THC on breast cancer cells by increasing Humna Epidermal Growth Factor 2 (HER2) expression [115], and increase in tumor cell growth [116]. Cannabidiol, on the other hand, demonstrated apoptosis and autophagy [111] by increasing production of reactive oxygen species (ROS) [117], inhibition of epidermal growth factor (EGF) [118], inhibition of epithelial-mesenchymal transition (EMT) [119], and has even been reported to mitigate advanced-stage breast cancer metastasis [120].
Glioma
The effect of THC on glioma cells is particularly dose-specific. Different concentrations of THC have produced divergent results [121–125], while some have documented, as in the case of breast cancer cells, an increase in glioma cell growth [126].
The inhibition of tumor growth and induction of apoptosis has been a more consistent outcome of cannabidiol in glioma cells [122, 127–130]. One particular study highlighted the increased expression of heat shock proteins (HSP) through cannabidiol-induced ROS generation in glioma cells treated with cannabidiol [131]. Cannabidiol also exhibited positive results when used in the treatment of neuroblastoma cells by inducing apoptosis and decreasing tumor cell migration [129]. Administrating a combination of THC and cannabidiol used in glioblastoma therapy improved overall treatment as both drugs combined presented synergistic anti-cancer effects [122, 132].
Leukemia
The effect of cannabinoids in leukemia models are being examined thoroughly. Dose-dependency plays a fundamental role in leukemia treatment as well [133]. A combination of both cannabinoids also produced vague results, with the sequence of administration, that is to say administering cannabinoids after chemotherapy, resulted in increased cell death. The sensitization of leukemia cells to chemotherapeutic drugs was documented to have increased most probably as a result of decreased expression of phosphorylated ERK by THC [134].
Lung cancer
Conflicting results dominate the research carried out to identify the role of THC in lung cancer treatment. While some studies published an increase in cellular proliferation [126, 135] and a decrease in tumor immunogenicity [136, 137] by THC administration, others proclaim reduced expression of EGFR, chemotaxis, and tumor invasion [138, 139]. Records on the application of cannabidiol in lung cancer therapy show the activation of cyclooxygenase 2 (COX2) and PPARγ, leading to apoptosis [140], decrease in invasion and metastasis through various signaling pathways [141, 142], increase immune cell susceptibility of tumor cells [143], and has favorably lessened tumor growth and metastasis in in vivo mouse models of lung cancer [140, 144].
Melanoma
The cannabinoid receptors are known to be expressed by human melanomas. Stimulation of these cells by THC and THC-cannabidiol combination have shown positive results such as decreased melanoma cell viability, proliferation, metastasis, angiogenesis, induction of autophagy and apoptosis [145–147], and these results are consistent in in vivo tests as well [145, 148]. Similar results have been observed in studies done on myeloma [149], hepatocellular carcinoma [150], pancreatic cancer [151, 152], colon cancer [153, 154], endometrial and cervical cancers [155], and oral cancer [156]. Cannabinoid receptor CB2R when studied on the various cell lines of breast cancer shows antiproliferative activity. Initiation of apoptosis and blocking the cell cycle by the activation of CB2R by THC leads to the reduction of breast cancer [157]. Cannabinoid receptors also perform as prognostic markers. Comparison of cell lines of prostate cancer and normal epithelial cells of prostate indicated an increased expression of cannabinoid receptors in cancer cells than normal cells. Apoptosis and necrosis are the main indicators in the reduction of prostate cancer by targeting CB1 receptor [158]. In a recent study on mouse model, CB2 receptor is recognized as protective factor. Experimental studies of this paper indicated that polymorphism in the gene (CNR2) of CB2R might be the reason for colon cancer. In mice, absence of CB2R correlated with more severe symptoms of colon cancer [159]. CB2 receptor behaves as a protective and therapeutic agent in the case of melanoma skin cancer as well. Activation of CB2 alternates the role of myeloid cells and T cells along with modification of propagation of keratinocyte, in order to suppress tumor growth in case of melanoma [160].
Various in vivo and in vitro studies demonstrated that cannabinoid receptors are involved in a variety of processes including necrosis, apoptosis, autophagy, cell cycle arrest by targeting diverse pathways and different cancer cell lines [161, 162]. Three different types of mechanisms involved in the treatment of cancer and act as antitumor agent. It involves mainly autophagy process leading to cell death, autophagy leading to apoptosis and apoptosis followed by signaling pathway. Secondly it involves tumor angiogenesis inhibition, metastasis and invasiveness and thirdly the regulation of immune response as antitumor agent [163]. Cannabinoids are also significant in the palliative care as it helps in appetite regulation, regulation of pain along with antiemetic role [164].
Synergistic effects of cannabinoids and chemotherapy in cancer treatment
Recent studies have increasingly explored the synergistic potential of cannabinoids combined with traditional chemotherapeutic agents in cancer treatment.
Research suggests that cannabinoids may enhance the cytotoxic effects of chemotherapy through several mechanisms. For instance, cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC) have been shown to induce apoptosis and reduce cell proliferation when used in conjunction with chemotherapeutic agents such as cisplatin, gemcitabine, and paclitaxel [165]. These cannabinoids modulate key pathways involved in cell cycle regulation and apoptosis, thereby increasing the susceptibility of cancer cells to chemotherapy-induced death [165]
In gastric cancer models, the endogenous cannabinoid anandamide (AEA) has been found to synergistically enhance the effects of paclitaxel by promoting apoptosis through the activation of caspase enzymes. This combination therapy significantly improved the cytotoxicity of paclitaxel in cancer cells, suggesting that cannabinoids may serve as effective adjuncts in chemotherapy regimens [166]. The combination of CBD and dasatinib has shown a synergistic effect in treating lung cancer by targeting the SRC/PI3K/AKT and Bax/Bcl-2/caspase-3 pathways. This combination induces significant apoptosis and reduces dasatinib-related side effects, offering a more effective and safer therapeutic option for lung cancer treatment [167].
A recent study explored the synergistic effects of cannabidiol (CBD) with chemotherapeutic drugs (docetaxel, doxorubicin, paclitaxel, vinorelbine, and SN − 38) in MCF7 breast cancer cells [168]. Strong synergy was observed, particularly with SN − 38 and vinorelbine at a CBD-to-drug molar ratio of 636:1. Enhanced apoptosis was seen in all combinations compared to monotherapies. Proteomics analysis revealed 121 dysregulated proteins in CBD-treated cells and 91 dysregulated proteins in the CBD-SN − 38 combination, impacting pathways like telomerase, EGFR1, TP53, and death receptor signaling. The study suggests CBD could be an effective adjuvant therapy, warranting further in vivo and clinical studies [168]. Another study showed that Cannflavin A, a flavonoid from Cannabis sativa, demonstrated significant synergistic anticancer effects when combined with cannabinoids like Δ9-tetrahydrocannabinol, cannabidiol, cannabichromene, and cannabivarin in bladder cancer cells. These combinations enhanced apoptosis through caspase 3 activation and reduced cancer cell viability and invasion more effectively than when cannflavin A or cannabinoids were used alone. This synergy suggests that cannflavin A could potentiate the anticancer effects of cannabinoids, making them more effective as a combined therapy in bladder cancer treatment [169]
Action mechanism of Cannabinoids in cancer
Cannabis plant produces hundreds of different cannabinoids, among those WIN-552122, AEA, CBDA, Met-F-AEA, 2-AG, Δ9-THC, CBD, HU120, AME121, R-(+)-MET and JWH-133 are cannabinoids having anticancer characteristics in different types of cancers including prostate cancer, lung cancer, glioblastomas, colon, testicular, leukemia etc. Cannabinoids perform their function by regulating different signaling pathways which control the growth, differentiation and other physiological characteristics of cells, mainly being involved in the AMPK /AKT/ PI3K/mTOR/ or the RAS/MAPK pathways [10, 170, 171]. While cannabinoids have been shown to modulate key signaling pathways, including the PI3K/Akt/mTOR and MAPK pathways, the precise molecular mechanisms underlying these effects remain an area of active investigation. For instance, studies have demonstrated that cannabinoids can inhibit the PI3K/Akt/mTOR pathway, leading to decreased cell proliferation and increased apoptosis in cancer cells [172]. However, the exact molecular interactions through which cannabinoids exert these effects are still not fully understood. Similarly, the role of cannabinoids in the MAPK pathway, which regulates cell growth and differentiation, has been explored, but more detailed studies are needed to clarify how these interactions vary across different cancer types [145]. Recent research has begun to identify potential biomarkers that may predict the response of cancer cells to cannabinoid treatment, providing insights into which signaling pathways are most relevant for specific contexts [173]
Just as the signaling pathways inside a cell are a complicated and complex mechanism to understand, the effects of cannabinoids on these pathways, especially in the case of cancer cells, depend deeply on the frame of reference [174].
The signaling pathways involved in cancer are highly intricate and interconnected, presenting significant challenges in therapeutic targeting [175]. Cannabinoids, due to their ability to interact with multiple receptors and modulate various signaling pathways, can have wide-ranging effects on cancer biology [174]. This includes influences on cell proliferation, apoptosis, angiogenesis, and metastasis. However, the non-specificity of cannabinoids in targeting single pathways can lead to unpredictable outcomes. For instance, while cannabinoids may inhibit tumor growth through the suppression of specific pro-tumorigenic pathways, they may simultaneously activate compensatory mechanisms in other pathways, potentially undermining the therapeutic efficacy [173]. Furthermore, the complexity of these signaling networks means that the effects of cannabinoids can vary significantly depending on the cancer type, the molecular characteristics of the tumor, and the specific cannabinoid receptor subtypes involved; this complexity underscores the need for more detailed studies to elucidate the precise molecular mechanisms by which cannabinoids interact with these signaling pathways and to identify biomarkers that can predict treatment response [173]. The effects of cannabinoids are highly context-dependent, varying significantly based on the type of cancer, specific genetic mutations, and the tumor microenvironment [176]. This variability complicates the identification of consistent signaling pathways that can be reliably targeted across different cancer types; for example, in some cancers, cannabinoids may inhibit tumor growth, while in others, they may have minimal effects or even promote tumor progression depending on the specific molecular context [177]. As a result, a more nuanced understanding of these context-dependent effects is essential for developing effective cannabinoid-based therapies. This highlights the importance of personalized approaches in cancer treatment that consider the unique characteristics of each tumor. Cannabinoid receptors play significant roles in targeting cancer hallmarks, including anti-proliferative effects, apoptosis stimulation, and inhibition of tumor invasiveness, metastasis, and angiogenesis [178]. Cannabinoids primarily exert their effects through CB1 and CB2 receptors; however, they also interact with other receptors and signaling molecules, including TRPV1 and GPR55 [179]. This receptor diversity contributes to the complexity of cannabinoid signaling, leading to divergent outcomes depending on the cellular context. The activation of receptors like TRPV1 can modulate pain perception and inflammation, while GPR55 has been implicated in cancer cell proliferation [180]. These interactions make it challenging to pinpoint which pathways are most relevant for therapeutic targeting, highlighting the need for further research to understand the full scope of cannabinoid activity in different cancer types.
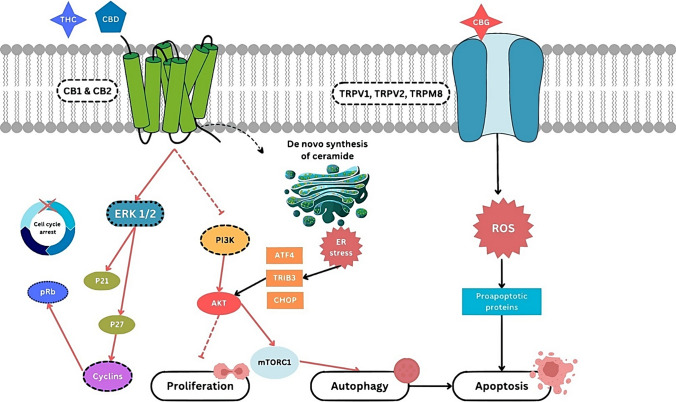
Figure 5 highlights the action mechanism of cannabinoid receptors.
Fig. 5.
Molecular pathways mediated by cannabinoid receptors in cancer cells. This figure illustrates the molecular pathways mediated by cannabinoid receptors CB1 and CB2, as well as TRPV1, TRPV2, and TRPM8, in cancer cells. The interaction of cannabinoids, such as THC, CBD, and CBG, with these receptors initiates various intracellular signaling cascades that influence cell fate decisions. Activation of CB1 and CB2 receptors by THC and CBD can lead to the modulation of several signaling pathways. The activation of the ERK1/2 pathway results in the upregulation of cyclin-dependent kinase inhibitors p21 and p27, leading to cell cycle arrest by preventing the phosphorylation of the retinoblastoma protein (pRb). This process halts the progression of the cell cycle. Additionally, the activation of the PI3K/AKT pathway influences cell proliferation and autophagy through mTORC1. Prolonged activation of the PI3K/AKT pathway can lead to endoplasmic reticulum (ER) stress and the activation of ATF4, TRIB3, and CHOP, which are markers of cellular stress and can trigger apoptosis. CBG interacts with TRPV1, TRPV2, and TRPM8 receptors, leading to the production of reactive oxygen species (ROS). The accumulation of ROS induces the activation of proapoptotic proteins, promoting programmed cell death (apoptosis). Abbreviations: AKT Protein kinase B, ATF4 Activating transcription factor 4, CB1R Cannabinoid receptor type 1, CB2R Cannabinoid receptor type 2, CBD Cannabidiol, CBG Cannabigerol, CHOP C/EBP homologous protein, ER Endoplasmic reticulum, ERK1/2 Extracellular signal-regulated kinases 1 and 2, mTORC1 Mechanistic target of rapamycin complex 1, PI3K Phosphoinositide 3-kinase, pRb Retinoblastoma protein, ROS Reactive oxygen species, THC Δ9-tetrahydrocannabinol, TRIB3 Tribbles pseudokinase 3, TRPM8 Transient receptor potential cation channel subfamily M member 8, TRPV1 Transient receptor potential vanilloid 1, TRPV2 Transient receptor potential vanilloid 2
Autophagy
Cannabinoids play role in autophagy by inducing the mTOR/P13K/Akt pathways. Autophagy plays dual role as induction as well as inhibition of apoptosis. Cannabinoid perform its action by downregulation of the AKT, decreased AKT level stimulates different pathways as p21 induced cell cycle inhibition, autophagy induced by mTOR pathway and Caspase 3 and 9 activation leading towards the apoptosis [163, 181]. Δ9-THC [182] and WIN-55,212-2 [56] have shown to stimulate autophagy in glioma cells and mantle cell lymphoma respectively. It is believed that autophagy occurs as a result of CB1R and CB2R activation setting serine palmitoyltransferase enzyme in motion, thereupon prompting de novo ceramide synthesis [183]. De novo synthesis of ceramide regulated the process of autophagy as synthesis of ceramide carried in endoplasmic reticulum, in which different kinds of enzymes participate in conversion of dihydroceramides to ceramide. Production of ceramide in returns activate TRIB3 and stress protein p8/Nupr1 leading to upregulation of CHOP and ATF4 causing reduction of Akt and in return mTORC [184].
Apoptosis induction
Apoptotic action is facilitated as induction of ER stress and increased ROS is result of TRPM8 activation leading to apoptosis. MAPK/ERK pathway is activated through stimulation of GPR55 through Gαq subunit of LP1 leading to release of DAG and Ca2 + from PLC production. These pathways leading to transcription of gene by NF-κß and CREB transcription factors. PLC, p38/ATF2 and actin cytoskeleton are regulated by Gα12/13 subunit induced RhoA/ROCK pathway. ROS production is controlled by action of ATF2/p38 which inhibits antiapoptotic proteins along with increased Vps34 and Beclin-1 interaction [185, 186]. Cannabinoids perform its action in cell cycle arrest as cannabinoids activate CB1 and CB2 receptors which initiation the ERK1/2 signaling leading to activation of p21and p27 causing increased pRb which decreases the cdc2, cdk2 and cyclin E and D leading to arrest of cell cycle. One notable molecule involved in apoptosis initiation and cell cycle arrest is ceramide, formed by the breakdown of sphingomyelin [187]. By regulating sphingomyelin metabolism, cannabinoids have been able to cause apoptosis in cancer cells [9] through cannabinoid receptor-dependent pathway [9], or via de novo ceramide synthesis by activating the cannabinoid receptor [205, 206, 237] . The increase in ceramide levels leads to the activation of Raf1-MEK-ERK pathway, and inhibits the AKT signaling pathways [205, 206]. Cell proliferation takes place upon ERK activation; however, prolonged activation causes cell cycle arrest and even cell death. Moreover, studies have noted that cannabinoids exert their proapoptotic effects through ceramide levels [9, 172]. The ceramides produce anti-tumorigenic effects via the activation of p8 by elF2α following the tribble homolog 3/AKT/rapamycin complex 1 pathway according to one study [188], whereas another study proposes the TRB3 pathway activation through ceramide-dependent p8 upregulation [189]. The role of ceramide in signaling apoptosis is being investigated to achieve a route to regulate cancer cell apoptosis [117], especially since its relation with CB1R and CB2R has been reported to stimulate apoptosis [190].
Key signaling pathways targeted by cannabinoids
Experimental evidence shows that cannabinoids can regulate cell signaling pathways that decide the fate of the cell. Here we highlight a few important signaling pathways and the role of cannabinoids reported in them.
PI3K/AKT Pathway
Both RAS-MAPK and PI3K-AKT pathways are prominent cell survival mechanisms [191, 192], both of which are often aberrantly involved in multiple cancer types. Cell proliferation is inhibited by decrease in Akt due to inhibition of P13K pathway.
Recurringly, the hyperactivation of PI3K-AKT pathway is regarded as one of the most common molecular hallmarks of human cancers [193]. A particularly well known, cannabis-plant derived cannabinoid, THC, has been reported to inhibit the RAS-MAPK and PI3K-AKT signaling pathways via the CB1 receptor. This eventually leads to the downstream activation of the proapoptotic BCL2 family member BAD, thus resulting in colorectal cancer cell death [194]. Another study showed a synthetic cannabinoid, WIN 55,212-2, had the same response in glioma cells [195]. In contrast, the introduction of CB2R agonists caused the activation of AKT/PKB pathway which subsequently promoted the inhibition of GSK3β, provoking cancerous cells to become more aggressive [196]. However, a more recent study suggests that treatment with chemotherapeutic drugs increases CB2R expression which benefits breast cancer treatment [197].
Ras/Raf pathway
The Ras-activated Raf kinases target the MEK/ERK downstream cascades, essentially regulating the growth, survival, and differentiation of a cell [198]. As their primary role would suggest, any defects in their signaling pathway can result in cancerous manifestation [199]. A study on cultured rat dorsal root ganglion neurons demonstrated the activation of TRPV2 protein by cannabidiol which led to inhibition of the Ras/Raf/MEK/ERK pathway increasing cancer cell drug intake and sensitivity to chemotherapeutic agents [200]. The activation of CB1R, but not CB2R, by THC leading to ERK and AKT downregulation [194], ERK1/2 pathway inhibition [201], ultimately leading to apoptosis and retardation of cancer cell proliferation.
Anti- proliferative effect
Different types of cancer indicated the anti-proliferative role of the cannabinoids by inhibiting the cell cycle. Anti-proliferation is induced in a way that different cancer cells are restricted to the G1 and inhibits the cells to move into the S or synthesis phase by regulating the CB2 receptor. It induces the expression of p27 and p21 that regulate Cdks, cdc and cyclin proteins to inhibit cell cycle and ultimately cell proliferation [202, 203]. The endocannabinoid system and its role in preventing cancer cell growth and proliferation is being researched extensively considering the complicated results from various experiments performed. The cannabinoid receptors, particularly CB2R, produce conflicting results [116, 204–208] depending upon the cancer tissue, cannabinoid used, receptor activated, and signaling pathway stimulated.
Anti-angiogenesis
VEGF (vascular endothelial growth factor) are the major inducers of the angiogenesis. VEGF, Ang-2 and PLGF are major factors of the angiogenesis inhibited by the cannabinoid. CB1R and CB2R agonists, Δ9-THC and WIN-55,212-2, inhibit the VEGF receptor and 2 major angiogenic factors, PIGF and Ang-2 [209, 210]. Cannabinoids through CB1 and CB2 receptors mainly targeted the cascade of pathways like hoA/Src/FAK that are significant in angiogenesis. Cannabinoids inhibits metastasis and angiogenesis by expression inhibition of metalloproteinase MMP9 and MMP2 and TIMP. MMP9 and MMP2 inhibition via p8 pathway/ceramide is studied as an important antitumor targeted therapy [163, 186]
Metastasis inhibition
The survival of tumor cells is strengthened by the migration, invasion, and neoplasm of pre-existing tumor cells. This gives term to a process notoriously noted as metastasis and is a hallmark of cancer that is more often than not very difficult to treat [211]. One of the main outcomes of metastasis is the degradation of matrix metalloproteinases (MMP) in the extracellular matrix [212]. The administration of cannabidiol, and other cannabinoids, has shown very promising results in suppressing tumor migration and metastasis in a number of cancers including colon carcinoma cells [213], glioma cells [214], breast cancer [215], along with others. Experimentation demonstrates that the cannabinoid receptors involved in the regulation of cannabinoid-induced migration inhibition of cancer cells [216].
Table 3 summarizes the anticancer mechanisms of various cannabinoids and their targets, highlighting their roles in apoptosis induction, autophagy stimulation, cell cycle arrest, anti-proliferation, anti-angiogenesis, and metastasis inhibition across different cancer types.
Table 3.
Anticancer mechanisms of cannabinoids and their molecular targets
| Anticancer mechanisms | Cannabinoid(s) | Cancer type(s) | Pathways and targets | References |
|---|---|---|---|---|
| Apoptosis induction | Δ9-THC, CBD, CBG | Glioma, Breast, Prostate, Colorectal | Induction of ER stress and ROS production; Activation of CB1/CB2 receptors leading to ceramide synthesis | [10, 189] |
| Autophagy stimulation | Δ9-THC, WIN-55,212-2 | Glioma, Lymphoma | Modulation of mTOR/PI3K/AKT pathways; Induction via ceramide synthesis through CB1/CB2 receptor activation | [182] |
| Cell cycle arrest | WIN-55,212-2, R-(+)-MET | Various Cancers | Upregulation of p21 and p27; Inhibition of cdc2, cdk2, cyclin E and D | [217] |
| Anti-proliferation | Δ9-THC, CBD | Colorectal, Glioma | Inhibition of RAS/MAPK and PI3K/AKT pathways; Modulation via CB2 receptor leading to p21 and p27 expression | [116, 204–208] |
| Anti-angiogenesis | Δ9-THC, WIN-55,212-2 | Various Cancers | Inhibition of VEGF, Ang-2, and PLGF; Suppression of MMP9 and MMP2 via ceramide pathway | [163, 186] |
| Metastasis inhibition | CBD, CBG | Colon, Breast, Glioma | Suppression of tumor cell migration and invasion; Regulation of cannabinoid-induced migration inhibition | [211, 215, 216] |
Abbreviations: 2-AG 2-Arachidonoylglycerol, AEA Anandamide, AKT Protein Kinase B, Ang-2 Angiopoietin-2, ATF4 Activating Transcription Factor 4, CB1R Cannabinoid Receptor Type 1, CB2R Cannabinoid Receptor Type 2, CBD Cannabidiol, CBDA Cannabidiolic Acid, CBG Cannabigerol, cdk Cyclin-Dependent Kinase, CHOP C/EBP Homologous Protein, DAG Diacylglycerol, ER Endoplasmic Reticulum, ERK1/2 Extracellular Signal-Regulated Kinases 1 and 2, GSK3β Glycogen Synthase Kinase 3 Beta, MAPK Mitogen-Activated Protein Kinase, mTOR Mechanistic Target of Rapamycin, MMP Matrix Metalloproteinase, PI3K Phosphoinositide 3-Kinase, PLGF Placental Growth Factor, pRb Retinoblastoma Protein, ROS Reactive Oxygen Species, THC Δ9-Tetrahydrocannabinol, TRIB3 Tribbles Pseudokinase 3, TRPM8 Transient Receptor Potential Cation Channel Subfamily M Member 8, TRPV1 Transient Receptor Potential Vanilloid 1, TRPV2 Transient Receptor Potential Vanilloid 2, VEGF Vascular Endothelial Growth Factor
Clinical efficacy of cannabinoids in cancer therapy
A few cannabinoids have reached the point of clinical trials for treatment of a number of diseases including cancer [218, 219]. Reports emerging from a few completed clinical trials communicate achieving tumor remission in a terminal acute lymphoblastic leukemia patient using Cannabis sativa oil (with a normally higher THC content than other cannabinoids) [220], suppression of glioblastoma multiforme tumor cell proliferation and reduction in tumor cell Ki67 immunostaining [217], and decrease in VEGF levels and VEGF-2 receptor activation [221]. Two pilocytic astrocytoma patients consuming cannabis via inhalation revealed tumor regression in over a 3-year period suggests cannabis was potentially responsible [222]. Patients in palliative care were given daily THC doses where approximately 50% experienced overall health improvement [223]. Dronabinol, an FDA approved cannabinoid, is being tested in multiple clinical trials, however its use in CNS cancers is limited due to risk of adverse effects. One study published the abated side effects in primary brain tumor patients, however, the low sample size and low dosage make this study less acceptable [224]. A long term study, from 2000 to 2010, aimed to ascertain the antiemetic effects of dronabinol in pediatric cancer patients receiving chemotherapy found 60% of patients had a positive feedback [225]. Dronabinol has also been effective in inhibiting differentiation blockage in two leukemia patients [226]. A phase I clinical trial conducted by Guzmán et al. investigated the safety and efficacy of intracranial administration of THC in patients with recurrent glioblastoma multiforme. The study enrolled nine patients, all of whom had failed standard treatments. The results demonstrated that THC administration was safe, with no significant adverse effects observed. Moreover, some patients exhibited a reduction in tumor size, suggesting potential antitumor activity of THC. The median survival time was 24 weeks, with two patients surviving for more than one year, indicating a possible survival benefit [227]. Another study by McAllister et al. focused on the effects of cannabidiol (CBD) on breast cancer. This preclinical investigation was followed by a small clinical study involving patients with aggressive breast cancer. The results revealed that CBD significantly reduced the proliferation of both estrogen receptor-positive and estrogen receptor-negative breast cancer cells. Additionally, CBD treatment led to a decrease in the expression of Id-1, a key regulator of tumor aggressiveness, in patient-derived breast cancer cells. These findings suggest that CBD may offer a novel, non-toxic therapeutic option for breast cancer patients [228]. These clinical studies and trials highlight the promising role of cannabinoids as anticancer agents. The evidence suggests that cannabinoids can exert antitumor effects through various mechanisms, including induction of apoptosis, inhibition of proliferation, and reduction of metastasis. However, the limited sample sizes and early-phase nature of these studies necessitate further large-scale, randomized controlled trials to validate these findings and establish standardized therapeutic protocols for the use of cannabinoids in oncology.
Palliative role of cannabinoids in cancer
Cannabinoids, the bioactive compounds found in Cannabis sativa, have gained significant attention for their potential therapeutic benefits in managing cancer-related symptoms. In palliative care, cannabinoids are being increasingly recognized for their ability to alleviate multiple distressing symptoms, thereby improving the quality of life for cancer patients [229]. Cancer-related pain is one of the most challenging symptoms to manage, often requiring complex multimodal approaches. Cannabinoids have emerged as a promising option for pain relief in cancer patients. THC and cannabidiol (CBD) interact with the endocannabinoid system to modulate pain perception, showing significant analgesic properties [230]. For example, in a clinical trial by Blake et al., patients with advanced cancers who received cannabinoid therapy for pain management exhibited stabilized disease progression, possibly due to cannabinoids' anti-inflammatory and immune-modulating effects [231]. This highlights that cannabinoids' pain-relief properties may not only improve patient comfort but also potentially influence cancer outcomes by reducing inflammation and immune suppression, which are often linked to cancer progression [231].
CB1R and µ-opioid are present in the peripheral nervous system. CB1 receptor regulates the calcium influx to modulate neurotransmitters to regulate pain and also it works in collaboration with opioid to relieve the pain [87]. A randomized controlled trial demonstrated that cannabis extract, containing both THC and CBD, provided effective pain relief in cancer patients unresponsive to opioids, with a statistically significant reduction in pain scores compared to placebo [87]. Chemotherapy-induced nausea and vomiting (CINV) significantly impair the quality of life in cancer patients. Emesis is the factor induced due to chemotherapy and controlled by the endocannabinoid system by opposite effect of CB1 and 5-HT3 on neuromediators that are present neurons (GABA-ergic) [185, 232]. Cannabinoids, particularly THC, have been shown to be effective antiemetics. A meta-analysis of 30 randomized controlled trials reported that cannabinoids were superior to conventional antiemetics in controlling CINV [233]. The antiemetic effects of cannabinoids are primarily mediated through CB1 receptors in the central nervous system, which play a fundamental role in the regulation of nausea and vomiting [233].
Cancer cachexia is a multifactorial syndrome characterized by severe weight loss and muscle wasting, leading to significant morbidity and mortality [234]. THC has demonstrated orexigenic effects, enhancing appetite and caloric intake in cancer patients. A clinical trial investigating the use of THC in patients with advanced cancer and anorexia reported a significant increase in appetite and a stabilization of body weight [235]. By stimulating appetite, cannabinoids can mitigate the effects of cachexia, thereby improving nutritional status and overall well-being [236].
Challenges and clinical barriers in utilizing cannabinoids as therapeutic agents in oncology
Although cannabinoids have shown potential as therapeutic agents in cancer treatment, several limitations and clinical gaps must be addressed before they can be widely adopted in clinical practice.
-
i.
Variability in cannabinoid composition: the therapeutic potential of cannabinoids in oncology is hindered by significant variability in their composition. This variability arises from differences in the source of the cannabis plants, the methods of extraction, and the formulations used. Such inconsistency can lead to variable therapeutic outcomes, making it challenging to establish standardized treatment protocols. This inconsistency complicates the reproducibility of clinical trial results and poses a significant barrier to the widespread clinical adoption of cannabinoid-based therapies.
-
ii.
Psychoactive effects: THC, a primary cannabinoid, has psychoactive properties that can cause adverse effects such as anxiety, paranoia, and cognitive impairment. These psychoactive effects limit the therapeutic window of THC, particularly in patients with a history of psychiatric disorders. The psychoactivity of THC necessitates cautious dosing and monitoring, reducing its attractiveness as a therapeutic agent despite its potential anticancer effects.
-
iii.
Regulatory and legal barriers: the legal status of cannabis and cannabinoids varies widely across different regions and countries, with many jurisdictions imposing strict regulatory controls or outright prohibition. These barriers significantly impede the ability to conduct large-scale clinical trials and hinder the development of standardized cannabinoid-based therapies. Furthermore, the legal complexities create challenges for patients seeking access to these treatments, limiting their availability and potential benefits in clinical practice. Addressing these regulatory hurdles is essential for advancing research and ensuring that patients can access safe and effective cannabinoid-based therapies.
-
iv.
Lack of standardized dosing guidelines: there is currently no consensus on the optimal dosing regimen for cannabinoids in cancer therapy. Clinical trials to date have employed a wide range of dosages, leading to inconsistent results and difficulties in determining the most effective and safe doses for patients. This lack of standardized dosing guidelines hampers the ability to provide consistent and reliable cannabinoid-based treatments in clinical practice.
-
v.
Lack of robust clinical data: the preclinical studies have provided valuable insights into the effects of cannabinoids on various signaling pathways, but there is a significant gap in robust clinical data that validates these findings in human patients. The translation of preclinical results into clinical practice remains challenging due to the complexity of human biology and the variability in patient responses. Rigorous clinical trials are essential to confirm the relevance of specific pathways targeted by cannabinoids and to establish effective treatment protocols. Without such data, it remains difficult to fully understand the therapeutic potential of cannabinoids in cancer treatment and other chronic diseases These trials should focus on evaluating the safety, efficacy, and long-term outcomes of cannabinoid-based therapies to ensure their appropriate use in clinical settings.
-
vi.
Adverse effects and drug interactions: annabinoids have been associated with various side effects, such as dizziness, dry mouth, euphoria, and cognitive impairment. Additionally, cannabinoids can interact with other medications frequently prescribed to cancer patients, which may lead to adverse drug interactions. These interactions can affect the metabolism of other drugs, potentially enhancing or diminishing their effects. Moreover, cannabinoids may interact with other drugs that target similar signaling pathways, such as PI3K/Akt and MAPK, potentially altering their efficacy or increasing toxicity. For example, combining cannabinoids with chemotherapeutic agents could either amplify therapeutic effects or increase adverse reactions depending on the specific pathways involved. Understanding these interactions is crucial for developing safe and effective combination therapies. Due to these concerns, careful patient monitoring is essential when using cannabinoids as part of a cancer treatment regimen. These safety considerations may limit the broader application of cannabinoids in cancer therapy, emphasizing the need for further research to better understand and manage these risks
-
vii.
Limited large-scale clinical trials: most clinical research on cannabinoids in oncology consists of small-scale studies or preclinical investigations. Large, randomized controlled trials (RCT) are limited, which restricts the robustness of the evidence supporting the use of cannabinoids in cancer therapy. The paucity of large-scale RCT makes it difficult to draw definitive conclusions about the efficacy and safety of cannabinoids in treating various cancers.
-
viii.
Mechanistic understanding: while preclinical studies have elucidated some mechanisms by which cannabinoids exert antitumor effects, the precise molecular pathways in human cancer remain incompletely understood. A more comprehensive understanding of these mechanisms is essential for optimizing cannabinoid-based therapies and identifying biomarkers for patient stratification.
-
ix.
Long-term safety data: there is limited data on the long-term safety and efficacy of cannabinoid use in cancer patients. Most studies have focused on short-term outcomes, leaving a gap in understanding the chronic effects of cannabinoid therapy. This lack of long-term safety data poses a challenge in assessing the risk–benefit ratio of prolonged cannabinoid use in cancer treatment.
-
x.
Patient selection criteria: clear criteria for identifying which cancer patients are most likely to benefit from cannabinoid therapy are lacking. Without well-defined patient selection criteria, it is challenging to tailor treatments to individual patients, potentially leading to suboptimal therapeutic outcomes. This gap highlights the need for further research to establish biomarkers and other indicators of cannabinoid responsiveness.
-
xi.
Combination with conventional therapies: more research is needed to understand how cannabinoids interact with standard cancer treatments such as chemotherapy, radiotherapy, and targeted therapies. The lack of detailed studies on these interactions limits the ability to develop effective combination treatment protocols that maximize therapeutic efficacy and minimize adverse effects.
-
xii.
Pharmacokinetics and pharmacodynamics: detailed studies on the pharmacokinetics (absorption, distribution, metabolism, and excretion) and pharmacodynamics (biological effects and mechanisms of action) of cannabinoids in cancer patients are lacking. This information is critical for optimizing dosing regimens, predicting therapeutic outcomes, and ensuring the safe and effective use of cannabinoids in oncology.
-
xiii.
One significant challenge in cancer treatment is the ability of cancer cells to develop resistance to therapies, including those that target specific signaling pathways. When cannabinoids are used to modulate particular pathways, there is a risk that cancer cells may adapt by activating alternative signaling mechanisms, thereby bypassing the effects of treatment [174]. This phenomenon has been observed in other targeted therapies, where cancer cells utilize redundant or compensatory pathways to maintain their survival and proliferation For instance, while cannabinoids may inhibit the PI3K/Akt pathway, cancer cells could upregulate the MAPK pathway as an alternative survival mechanism [177]. Therefore, a comprehensive understanding of the potential for resistance is essential in developing effective combination therapies that can mitigate this risk.
-
xiv.
Variability in cannabinoid composition and therapeutic outcomes: the therapeutic effects of cannabinoids can vary significantly depending on the specific composition of the cannabinoid compounds used, such as THC, CBD, and CBG. Each of these compounds interacts with signaling pathways differently, leading to distinct biological effects. For example, THC primarily interacts with CB1 receptors and has psychoactive properties, while CBD has a broader range of interactions, including effects on CB2 receptors and non-cannabinoid receptors like TRPV1. This variability in cannabinoid composition across different products can result in inconsistent therapeutic outcomes, complicating efforts to establish standardized treatment protocols. Furthermore, the lack of regulatory oversight in some markets exacerbates these challenges, making it difficult to ensure consistent quality and dosage in cannabinoid-based therapies.
Conclusion and future perspectives
Cannabinoids, derived from plants, synthesized naturally in the human body, or produced artificially in laboratories, have demonstrated considerable therapeutic potential. Historically, cannabis plants have shown promising results both as psychoactive agents and therapeutics. Various studies have indicated the role of cannabinoids and their receptors in cancer treatment and targeted therapy. Despite the evident anti-cancer properties of cannabinoids from numerous experimental results, the exact mechanisms of action still require extensive research. The involvement of cannabinoid receptors in cancer has been documented, showing both pro-tumor and anti-tumor effects. These contradictory outcomes may be attributed to the specific cannabinoid receptors involved, the ligands binding to these receptors, the type of cancer, and other environmental factors. For example, THC has produced more conflicting results compared to cannabidiol (CBD), which has been more consistent in its anti-tumor and pro-apoptotic effects. Several cannabinoid-derived drugs have been approved in various parts of the world, with many still undergoing clinical trials. However, further investigation is needed to fully understand the effects and consequences of these drugs. Despite the positive outcomes of using cannabinoids in cancer therapy, there remain significant gaps in knowledge regarding their modes of action, effects on the tumor microenvironment, and the physiology of the signaling pathways they affect. Cannabinoids have demonstrated antiemetic and antinociceptive effects in patients receiving chemotherapy, which necessitates further investigation into their synergistic and antagonistic properties. Animal model studies have shown promise in the antimetastatic and antiangiogenic properties of cannabinoids. Clinical trials have also indicated improvements in patient health when cannabinoids are administered in combination with chemotherapeutic drugs. These data support the role of cannabinoids as adjuvants in chemotherapy. Future studies should focus not only on the adjuvant role of cannabinoids but also on their potential as primary chemotherapeutic agents. By expanding our understanding of cannabinoid mechanisms and their interactions with cancer cells, we can better harness their therapeutic potential in oncology.
Acknowledgements
The authors would like to express their gratitude to Dr. Irina Zamfir, RCP London, Basildon University Hospital UK, for providing professional English editing of this manuscript and for editorial support.
Abbreviations
- 2-AG
2-Arachidonoylglycerol
- AEA
N-arachidonoylethanolamine
- AKT
Protein kinase B
- ATF4
Activating transcription factor 4
- BAD
Bcl-2-associated death promoter
- BCL2
B-cell lymphoma 2
- CB1R
Cannabinoid receptor type 1
- CB2R
Cannabinoid receptor type 2
- CBD
Cannabidiol
- CBG
Cannabigerol
- CHOP
C/EBP homologous protein
- COX2
Cyclooxygenase 2
- DAG
Diacylglycerol
- EGF
Epidermal growth factor
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial-mesenchymal transition
- ER
Endoplasmic reticulum
- ERK
Extracellular signal-regulated kinase
- GABA
Gamma-aminobutyric acid
- GSK3β
Glycogen synthase kinase 3 beta
- HER2
Human epidermal growth factor receptor 2
- HSP
Heat shock proteins
- MAPK
Mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- MMP
Matrix metalloproteinase
- mTORC1
Mechanistic target of rapamycin complex 1
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- p27
Cyclin-dependent kinase inhibitor 1B
- p21
Cyclin-dependent kinase inhibitor 1A
- PPARγ
Peroxisome proliferator-activated receptor gamma
- pERK
Phosphorylated extracellular signal-regulated kinase
- PI3K
Phosphoinositide 3-kinase
- PLC
Phospholipase C
- pRb
Retinoblastoma protein
- ROS
Reactive oxygen species
- SPT
Serine palmitoyltransferase
- THC
Δ9-Tetrahydrocannabinol
- TRB3
Tribbles pseudokinase 3
- TRPM8
Transient receptor potential cation channel subfamily M member 8
- TRPV1
Transient receptor potential vanilloid 1
- TRPV2
Transient receptor potential vanilloid 2
- VEGF
Vascular endothelial growth factor
Author contributions
MBF, FN, MI, MAA, LM.E, DAA, AMA, DC, KK, JS-R made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas that is, revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and confirming to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Data availablity
Not Applicable.
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daniela Calina, Email: calinadaniela@gmail.com.
Khushbukhat Khan, Email: khushi-khan2011@hotmail.com.
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
References
- 1.Amin R, Thalluri C, Docea AO, et al. Therapeutic potential of cranberry for kidney health and diseases. eFood. 2022;3(5):e33–e33. [Google Scholar]
- 2.Sharifi-Rad J, Quispe C, Durazzo A, et al. Resveratrol' biotechnological applications: enlightening its antimicrobial and antioxidant properties. J Herbal Med. 2022;32.
- 3.Javed Z, Khan K, Herrera-Bravo J, et al. Myricetin: targeting signaling networks in cancer and its implication in chemotherapy. Cancer Cell Int. 2022;22(1). [DOI] [PMC free article] [PubMed]
- 4.Sharma E, Tewari M, Sati P, et al. Serving up health: how phytochemicals transform food into medicine in the battle against cancer. Food Front. n/a(n/a).
- 5.Shah SA, Gupta AS, Kumar P. Emerging role of cannabinoids and synthetic cannabinoid receptor 1/cannabinoid receptor 2 receptor agonists in cancer treatment and chemotherapy-associated cancer management. J Cancer Res Ther. 2021;17(1):1–9. [DOI] [PubMed] [Google Scholar]
- 6.Allan GM, Finley CR, Ton J, et al. Systematic review of systematic reviews for medical cannabinoids: pain, nausea and vomiting, spasticity, and harms. Can Fam Physician. 2018;64(2):e78–94. [PMC free article] [PubMed] [Google Scholar]
- 7.Falasca M and Maccarrone MJC. Cannabinoids and Cancer. 2021, MDPI. p. 4458. [DOI] [PMC free article] [PubMed]
- 8.Nahar L, Uddin SJ, Alam MA, et al. Extraction of naturally occurring cannabinoids: an update. Phytochem Anal. 2021;32(3):228–41. [DOI] [PubMed] [Google Scholar]
- 9.Mangal N, Erridge S, Habib N, et al. Cannabinoids in the landscape of cancer. J Cancer Res Clin Oncol. 2021;147:2507–34. 10.1007/s00432-021-03710-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzman M. Cannabinoids: potential anticancer agents. Nat Rev Cancer. 2003;3(10):745–55. [DOI] [PubMed] [Google Scholar]
- 11.Robson P. Therapeutic aspects of cannabis and cannabinoids. Br J Psychiatry. 2001;178(2):107–15. [DOI] [PubMed] [Google Scholar]
- 12.Walsh D, Nelson KA, Mahmoud F. Established and potential therapeutic applications of cannabinoids in oncology. Support Care Cancer. 2003;11:137–43. [DOI] [PubMed] [Google Scholar]
- 13.Tramèr MR, Carroll D, Campbell FA, et al. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323(7303):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Project A.W.H.O.D. A phase II study of delta-9-tetrahydrocannabinol for appetite stimulation in cancer-associated anorexia. J Palliative Care 1994;10(1):14–18. [PubMed]
- 15.Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20(2):567–73. [DOI] [PubMed] [Google Scholar]
- 16.Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63(5):569–611. [DOI] [PubMed] [Google Scholar]
- 17.Walker JM, Huang SM. Cannabinoid analgesia. Pharmacol Ther. 2002;95(2):127–35. [DOI] [PubMed] [Google Scholar]
- 18.Calignano A, Rana GL, Giuffrida A, et al. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394(6690):277–81. [DOI] [PubMed] [Google Scholar]
- 19.Franco R, Rivas-Santisteban R, Reyes-Resina I, et al. Pharmacological potential of varinic-, minor-, and acidic phytocannabinoids. Pharmacol Res. 2020;158: 104801. [DOI] [PubMed] [Google Scholar]
- 20.WFO. An Online Flora of All Known Plants. 2023. Available from: https://www.worldfloraonline.org/.
- 21.PubChem. Available from: https://pubchem.ncbi.nlm.nih.gov/.
- 22.Devane WA, Hanuš L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–9. [DOI] [PubMed] [Google Scholar]
- 23.Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90. [DOI] [PubMed] [Google Scholar]
- 24.Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylgylcerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215(1):89–97. [DOI] [PubMed] [Google Scholar]
- 25.Huang WJ, Chen WW, Zhang X. Endocannabinoid system: role in depression, reward and pain control (Review). Mol Med Rep. 2016;14(4):2899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fonseca B, Teixeira N, Correia-da-Silva G. Cannabinoids as modulators of cell death: clinical applications and future directions. Rev Physiol Biochem Pharmacol. 2017;173:63–88. [DOI] [PubMed] [Google Scholar]
- 27.McAllister SD, Abood ME, Califano J, et al. Cannabinoid cancer biology and prevention. JNCI Monographs. 2021;2021(58):99–106. [DOI] [PubMed] [Google Scholar]
- 28.Dobovišek L, Krstanović F, Borštnar S, et al. Cannabinoids and hormone receptor-positive breast cancer treatment. Cancers. 2020;12(3):525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maccarrone M. Phytocannabinoids and endocannabinoids: different in nature. Rendiconti Lincei Scienze Fisiche e Naturali. 2020;31:931–8. [Google Scholar]
- 30.Calvi L, Pentimalli D, Panseri S, et al. Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC-MS and LC-HRMS (q-exactive orbitrap®) approach. J Pharm Biomed Anal. 2018;150:208–19. [DOI] [PubMed] [Google Scholar]
- 31.Lu H-C, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiat. 2016;79(7):516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Boisselier R, Alexandre J, Lelong-Boulouard V, et al. Focus on cannabinoids and synthetic cannabinoids. Clin Pharmacol Ther. 2017;101(2):220–9. [DOI] [PubMed] [Google Scholar]
- 33.Alves VL, Gonçalves JL, Aguiar J, et al. The synthetic cannabinoids phenomenon: from structure to toxicological properties. A review. Crit Rev Toxicol. 2020;50(5):359–82. [DOI] [PubMed] [Google Scholar]
- 34.Brents LK, Zimmerman SM, Saffell AR, et al. Differential drug–drug interactions of the synthetic cannabinoids JWH-018 and JWH-073: implications for drug abuse liability and pain therapy. J Pharmacol Exp Ther. 2013;346(3):350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blessing EM, Steenkamp MM, Manzanares J, et al. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. 2015;12(4):825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergamaschi MM, Queiroz RHC, Chagas MHN, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology. 2011;36(6):1219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirkham TC, Williams CM, Fezza F, et al. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136(4):550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costiniuk CT, Mills E, Cooper CL. Evaluation of oral cannabinoid-containing medications for the management of interferon and ribavirin-induced anorexia, nausea and weight loss in patients treated for chronic hepatitis C virus. Can J Gastroenterol Hepatol. 2008;22:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopelli E, Samara M, Siargkas A, et al. The role of cannabidiol oil in schizophrenia treatment. A systematic review and meta-analysis. Psychiatry Res. 2020;291: 113246. [DOI] [PubMed] [Google Scholar]
- 40.von Wrede R, Helmstaedter C, Surges R. Cannabidiol in the Treatment of Epilepsy. Clin Drug Investig. 2021;41:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagarkatti P, Pandey R, Rieder SA, et al. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1(7):1333–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katchan V, David P, Shoenfeld Y. Cannabinoids and autoimmune diseases: a systematic review. Autoimmun Rev. 2016;15(6):513–28. [DOI] [PubMed] [Google Scholar]
- 43.Klein TW, Newton CA, Friedman H. Cannabinoids and the immune system. Pain Res Manage. 2001;6:95–101. [DOI] [PubMed] [Google Scholar]
- 44.Zákány N, Oláh A, Markovics A, et al. Endocannabinoid tone regulates human sebocyte biology. J Investig Dermatol. 2018;138(8):1699–706. [DOI] [PubMed] [Google Scholar]
- 45.Dobrosi N, Tóth BI, Nagy G, et al. Endocannabinoids enhance lipid synthesis and apoptosis of human sebocytes via cannabinoid receptor-2-mediated signaling. FASEB J. 2008;22(10):3685–95. [DOI] [PubMed] [Google Scholar]
- 46.Maccarrone M, Di Rienzo M, Battista N, et al. The endocannabinoid system in human keratinocytes: evidence that anandamide inhibits epidermal differentiation through CB1 receptor-dependent inhibition of protein kinase C, activating protein-1, and transglutaminase. J Biol Chem. 2003;278(36):33896–903. [DOI] [PubMed] [Google Scholar]
- 47.Del Río C, Navarrete C, Collado JA, et al. The cannabinoid quinol VCE-004.8 alleviates bleomycin-induced scleroderma and exerts potent antifibrotic effects through peroxisome proliferator-activated receptor-γ and CB2 pathways. Sci Reports. 2016;6(1):21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanley CP, Hind WH, O’Sullivan SE. Is the cardiovascular system a therapeutic target for cannabidiol? Br J Clin Pharmacol. 2013;75(2):313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abioye A, Ayodele O, Marinkovic A, et al. Δ9-Tetrahydrocannabivarin (THCV): a commentary on potential therapeutic benefit for the management of obesity and diabetes. J Cannabis Res. 2020;2(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramer R, Hinz B. Cannabinoids as anticancer drugs. Adv Pharmacol. 2017;80:397–436. [DOI] [PubMed] [Google Scholar]
- 51.Massi P, Solinas M, Cinquina V, et al. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75(2):303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bifulco M, Malfitano AM, Pisanti S, et al. Endocannabinoids in endocrine and related tumours. Endocr Relat Cancer. 2008;15(2):391. [DOI] [PubMed] [Google Scholar]
- 53.Diaz-Laviada I. The endocannabinoid system in prostate cancer. Nat Rev Urol. 2011;8(10):553–61. [DOI] [PubMed] [Google Scholar]
- 54.Sarfaraz S, Adhami VM, Syed DN, et al. Cannabinoids for cancer treatment: progress and promise. Can Res. 2008;68(2):339–42. [DOI] [PubMed] [Google Scholar]
- 55.Solinas M, Massi P, Cantelmo A, et al. Cannabidiol inhibits angiogenesis by multiple mechanisms. Br J Pharmacol. 2012;167(6):1218–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Velasco G, Sánchez C, Guzmán M. Towards the use of cannabinoids as antitumour agents. Nat Rev Cancer. 2012;12(6):436–44. [DOI] [PubMed] [Google Scholar]
- 57.Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discovery. 2008;7(5):438–55. [DOI] [PubMed] [Google Scholar]
- 58.Izzo AA, Mascolo N, Capasso F. The gastrointestinal pharmacology of cannabinoids. Curr Opin Pharmacol. 2001;1(6):597–603. [DOI] [PubMed] [Google Scholar]
- 59.Gómez R, Navarro M, Ferrer B, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22(21):9612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Marzo V, Melck D, Bisogno T, et al. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21(12):521–8. [DOI] [PubMed] [Google Scholar]
- 61.Calignano A, La Rana G, Loubet-Lescoulié P, et al. A role for the endogenous cannabinoid system in the peripheal control of pain initiation. 2000. [DOI] [PubMed]
- 62.Castellano C, Rossi-Arnaud C, Cestari V, et al. Cannabinoids and memory; animal studies. Curr Drug Targets-CNS Neurol Disord. 2003;2(6):389–402. [DOI] [PubMed] [Google Scholar]
- 63.de Fonseca FR, Del Arco I, Martín-Calderón JL, et al. Role of the endogenous cannabinoid system in the regulation of motor activity. Neurobiol Dis. 1998;5(6):483–501. [DOI] [PubMed] [Google Scholar]
- 64.Cabral GA. Marijuana and cannabinoids: effects on infections, immunity, and AIDS. Journal of Cannabis Therapeutics. 2001;1(3–4):61–85. [Google Scholar]
- 65.Robson P. Therapeutic potential of cannabinoid medicines. Drug Test Anal. 2014;6(1–2):24–30. [DOI] [PubMed] [Google Scholar]
- 66.Śmiarowska M, Białecka M and Machoy-Mokrzyńska AJNNP. Cannabis and cannabinoids: pharmacology and therapeutic potential. 2022;56(1):4–13 [DOI] [PubMed]
- 67.Lowe H, Toyang N, Steele B, et al. The endocannabinoid system: a potential target for the treatment of various diseases. Int J Mol Sci. 2021;22(17):9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trautman LJ, Seaborn P, Sulkowski A, et al. Cannabis at the crossroads: a transdisciplinary analysis and policy prescription. Okla City UL Rev. 2020;45:125. [Google Scholar]
- 69.Marcu J. The legalization of cannabinoid products and standardizing cannabis-drug development in the United States: a brief report. Dialogues Clin Neurosci. 2020;22(3):289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jameson LE, Conrow KD, Pinkhasova DV, et al. Comparison of state-level regulations for cannabis contaminants and implications for public health. Environ Health Perspect. 2022;130(9):97001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orenstein DG, Glantz SA. Cannabis legalization in state legislatures: public health opportunity and risk. Marquette Law Rev. 2020;103(4):1313–400. [PMC free article] [PubMed] [Google Scholar]
- 72.Cooper ZD, Abrams DI, Gust S, et al. Challenges for clinical cannabis and cannabinoid research in the United States. J Natl Cancer Inst Monogr. 2021;2021(58):114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Connor JP, Stjepanović D, Le Foll B, et al. Cannabis use and cannabis use disorder. Nat Rev Dis Primers. 2021;7(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leinen ZJ, Mohan R, Premadasa LS, et al. Therapeutic potential of cannabis: a comprehensive review of current and future applications. Biomedicines. 2023;11(10). [DOI] [PMC free article] [PubMed]
- 75.Bell AD, MacCallum C, Margolese S, et al. Clinical practice guidelines for cannabis and cannabinoid-based medicines in the management of chronic pain and co-occurring conditions. Cannabis Cannabinoid Res. 2023;9(2):669–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pressman P, Hayes AW, Hoeng J et al. Δ(9)-Tetrahydrocannabinol (THC): a critical overview of recent clinical trials and suggested guidelines for future research. J Clin Med. 2024;13(6). [DOI] [PMC free article] [PubMed]
- 77.Harrison NJ, Simpson H. Nabilone (Cesamet). In: Cannabinoids and pain. Springer; 2021. p. 109–12. [Google Scholar]
- 78.Sastre-Garriga J, Vila C, Clissold S, et al. THC and CBD oromucosal spray (Sativex®) in the management of spasticity associated with multiple sclerosis. Expert Rev Neurother. 2011;11(5):627–37. [DOI] [PubMed] [Google Scholar]
- 79.O’Sullivan SE, Jensen SS, Nikolajsen GN, et al. The therapeutic potential of purified cannabidiol. J Cannabis Res. 2023;5(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Legare CA, Raup-Konsavage WM, and Vrana KEJP. Therapeutic potential of cannabis, cannabidiol, and cannabinoid-based pharmaceuticals. 2022;107(3–4):131–49. [DOI] [PubMed]
- 81.An D, Peigneur S, Hendrickx LA, et al. Targeting cannabinoid receptors: current status and prospects of natural products. Int J Mol Sci. 2020;21(14):5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Basavarajappa BS, Subbanna S. Molecular Insights into epigenetics and cannabinoid receptors. Biomolecules. 2022;12(11):1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–5. [DOI] [PubMed] [Google Scholar]
- 84.Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–4. [DOI] [PubMed] [Google Scholar]
- 85.Devane WA, Dysarz FR, Johnson MR, et al. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34(5):605–13. [PubMed] [Google Scholar]
- 86.Rosado-Franco JJ, Ellison AL, White CJ, et al. Roadmap for the expression of canonical and extended endocannabinoid system receptors and metabolic enzymes in peripheral organs of preclinical animal models. Physiol Rep. 2024;12(4): e15947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zou S and Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3). [DOI] [PMC free article] [PubMed]
- 88.Lazarini-Lopes W, da Silva-Júnior RMP, Servilha-Menezes G et al. Cannabinoid Receptor Type 1 (CB1R) expression in limbic brain structures after acute and chronic seizures in a genetic model of epilepsy. Front Behav Neurosci. 2020;14. [DOI] [PMC free article] [PubMed]
- 89.Zhang Q, Zhao Y, Wu J, et al. The progress of small molecules against cannabinoid 2 receptor (CB(2)R). Bioorg Chem. 2024;144: 107075. [DOI] [PubMed] [Google Scholar]
- 90.Montero C, Campillo NE, Goya P, et al. Homology models of the cannabinoid CB1 and CB2 receptors. A docking analysis study. Eur J Med Chem. 2005;40(1):75–83. [DOI] [PubMed] [Google Scholar]
- 91.Palczewski K, Kumasaka T, Hori T, et al. Crystal structure of rhodopsin: AG protein-coupled receptor. Science. 2000;289(5480):739–45. [DOI] [PubMed] [Google Scholar]
- 92.Yeagle PL, Choi G, Albert AD. Studies on the structure of the G-protein-coupled receptor rhodopsin including the putative G-protein binding site in unactivated and activated forms. Biochemistry. 2001;40(39):11932–7. [DOI] [PubMed] [Google Scholar]
- 93.Teller DC, Okada T, Behnke CA, et al. Advances in determination of a high-resolution three-dimensional structure of rhodopsin, a model of G-protein-coupled receptors (GPCRs). Biochemistry. 2001;40(26):7761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caenazzo L, Hoehe M, Hsieh W-T, et al. HindIII identifies a two allele DNA polymorphism of the human cannabinoid receptor gene (CNR). Nucleic Acids Res. 1991;19(17):4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoehe M, Caenazzo L, Martinez M, et al. Genetic and physical mapping of the human cannabinoid receptor gene to chromosome 6q14-q15. New Biol. 1991;3(9):880–5. [PubMed] [Google Scholar]
- 96.Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bonner T. Molecular biology of cannabinoid receptors. J Neuroimmunol. 1996;69(1–2):15–7. [Google Scholar]
- 98.Xie XQ, Chen JZ, Billings EM. 3D structural model of the G-protein-coupled cannabinoid CB2 receptor. Proteins. 2003;53(2):307–19. [DOI] [PubMed] [Google Scholar]
- 99.Feng W, Song Z. Functional roles of the tyrosine within the NP (X) n Y motif and the cysteines in the C-terminal juxtamembrane region of the CB2 cannabinoid receptor. FEBS Lett. 2001;501(2–3):166–70. [DOI] [PubMed] [Google Scholar]
- 100.Lynch DL, Hurst DP, Reggio PH. The nucleotide-free state of the cannabinoid CB2/Gi complex. Cell. 2020;180(4):603–4. [DOI] [PubMed] [Google Scholar]
- 101.Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17(14):5327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lauckner JE, Hille B, Mackie K. The cannabinoid agonist WIN55, 212–2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc Natl Acad Sci. 2005;102(52):19144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Breivogel CS, Puri V, Lambert JM, et al. The influence of beta-arrestin2 on cannabinoid CB1 receptor coupling to G-proteins and subcellular localization and relative levels of beta-arrestin1 and 2 in mouse brain. J Recept Signal Transduc. 2013;33(6):367–79. [DOI] [PubMed] [Google Scholar]
- 104.Chen X, Zheng C, Qian J, et al. Involvement of β-arrestin-2 and clathrin in agonist-mediated internalization of the human cannabinoid CB2 receptor. Curr Mol Pharmacol. 2014;7(1):67–80. [DOI] [PubMed] [Google Scholar]
- 105.Almogi-Hazan O, Or R. Cannabis, the Endocannabinoid System and Immunity—the Journey from the Bedside to the Bench and Back. Int J Mol Sci. 2020;21(12):4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Greiner B, Sommerfeld M, Kintscher U et al. Differential regulation of MMPs, apoptosis and cell proliferation by the cannabinoid receptors CB1 and CB2 in vascular smooth muscle cells and cardiac myocytes. 2022;10(12):3271. [DOI] [PMC free article] [PubMed]
- 107.Senga SS, Grose RP. Hallmarks of cancer—the new testament. Open Biol. 2021;11(1): 200358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hashemi M, Bashi S, Zali A. The expression level of cannabinoid receptors type 1 and 2 in the different types of astrocytomas. Mol Biol Rep. 2020;47(7):5461–7. [DOI] [PubMed] [Google Scholar]
- 109.Cherkasova V, Wang B, Gerasymchuk M, et al. Use of cannabis and cannabinoids for treatment of cancer. Cancers. 2022;14(20):5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vecera L, Gabrhelik T, Prasil P, et al. The role of cannabinoids in the treatment of cancer. Bratisl Lek Listy. 2020;121(1):79–95. [DOI] [PubMed] [Google Scholar]
- 111.Ligresti A, Moriello AS, Starowicz K, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318(3):1375–87. [DOI] [PubMed] [Google Scholar]
- 112.Takeda S, Yoshida K, Nishimura H, et al. Δ9-Tetrahydrocannabinol disrupts estrogen-signaling through up-regulation of estrogen receptor β (ERβ). Chem Res Toxicol. 2013;26(7):1073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Von Bueren A, Schlumpf M, Lichtensteiger W. Delta (9)-tetrahydrocannabinol inhibits 17β-estradiol-induced proliferation and fails to activate androgen and estrogen receptors in MCF7 human breast cancer cells. Anticancer Res. 2008;28(1A):85–9. [PubMed] [Google Scholar]
- 114.Caffarel MM, Andradas C, Mira E, et al. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol Cancer. 2010;9(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takeda S, Yamaori S, Motoya E, et al. Δ9-Tetrahydrocannabinol enhances MCF-7 cell proliferation via cannabinoid receptor-independent signaling. Toxicology. 2008;245(1–2):141–6. [DOI] [PubMed] [Google Scholar]
- 116.McKallip RJ, Nagarkatti M, Nagarkatti PS. Δ-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol. 2005;174(6):3281–9. [DOI] [PubMed] [Google Scholar]
- 117.Shrivastava A, Kuzontkoski PM, Groopman JE, et al. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol Cancer Ther. 2011;10(7):1161–72. [DOI] [PubMed] [Google Scholar]
- 118.Elbaz M, Nasser MW, Ravi J, et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol Oncol. 2015;9(4):906–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.García-Morales L, Castillo AM, Tapia Ramírez J, et al. CBD reverts the mesenchymal invasive phenotype of breast cancer cells induced by the inflammatory cytokine IL-1β. Int J Mol Sci. 2020;21(7):2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Murase R, Kawamura R, Singer E, et al. Targeting multiple cannabinoid anti-tumour pathways with a resorcinol derivative leads to inhibition of advanced stages of breast cancer. Br J Pharmacol. 2014;171(19):4464–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Allister SDM, Chan C, Taft RJ, et al. Cannabinoids selectively inhibit proliferation and induce death of cultured human glioblastoma multiforme cells. J Neurooncol. 2005;74:31–40. [DOI] [PubMed] [Google Scholar]
- 122.Marcu JP, Christian RT, Lau D, et al. Cannabidiol enhances the inhibitory effects of Δ9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol Cancer Ther. 2010;9(1):180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jacobsson SO, Rongård E, Stridh M, et al. Serum-dependent effects of tamoxifen and cannabinoids upon C6 glioma cell viability. Biochem Pharmacol. 2000;60(12):1807–13. [DOI] [PubMed] [Google Scholar]
- 124.Goncharov I, Weiner L, Vogel Z. Δ9-tetrahydrocannabinol increases C6 glioma cell death produced by oxidative stress. Neuroscience. 2005;134(2):567–74. [DOI] [PubMed] [Google Scholar]
- 125.Widmer M, Hanemann C, Zajicek J. High concentrations of cannabinoids activate apoptosis in human U373MG glioma cells. J Neurosci Res. 2008;86(14):3212–20. [DOI] [PubMed] [Google Scholar]
- 126.Hart S, Fischer OM, Ullrich A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor α-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Can Res. 2004;64(6):1943–50. [DOI] [PubMed] [Google Scholar]
- 127.Nabissi M, Morelli MB, Santoni M, et al. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis. 2013;34(1):48–57. [DOI] [PubMed] [Google Scholar]
- 128.Massi P, Vaccani A, Ceruti S, et al. Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J Pharmacol Exp Ther. 2004;308(3):838–45. [DOI] [PubMed] [Google Scholar]
- 129.Alharris E, Singh NP, Nagarkatti PS, et al. Role of miRNA in the regulation of cannabidiol-mediated apoptosis in neuroblastoma cells. Oncotarget. 2019;10(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Singer E, Judkins J, Salomonis N, et al. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015;6(1):e1601–e1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Scott KA, Dennis JL, Dalgleish AG, et al. Inhibiting heat shock proteins can potentiate the cytotoxic effect of cannabidiol in human glioma cells. Anticancer Res. 2015;35(11):5827–37. [PubMed] [Google Scholar]
- 132.Scott KA, Dalgleish AG, Liu WM. The combination of cannabidiol and Δ9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol Cancer Ther. 2014;13(12):2955–67. [DOI] [PubMed] [Google Scholar]
- 133.Scott KA, Dalgleish AG, Liu WM. Anticancer effects of phytocannabinoids used with chemotherapy in leukaemia cells can be improved by altering the sequence of their administration. Int J Oncol. 2017;51(1):369–77. [DOI] [PubMed] [Google Scholar]
- 134.Liu WM, Scott KA, Shamash J, et al. Enhancing the in vitro cytotoxic activity of Δ9-tetrahydrocannabinol in leukemic cells through a combinatorial approach. Leuk Lymphoma. 2008;49(9):1800–9. [DOI] [PubMed] [Google Scholar]
- 135.Baram L, Peled E, Berman P, et al. The heterogeneity and complexity of Cannabis extracts as antitumor agents. Oncotarget. 2019;10(41):4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu LX, Sharma S, Stolina M, et al. Δ-9-Tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J Immunol. 2000;165(1):373–80. [DOI] [PubMed] [Google Scholar]
- 137.Burnette-Curley D, Cabral G. Differential Inhibition of RAW264. 7 Macrophage tumoricidal activity by δ 9 tetrahydrocannabinol. Proc Soc Exp Biol Med. 1995;210(1):64–76. [DOI] [PubMed] [Google Scholar]
- 138.Preet A, Ganju R, Groopman J. Δ9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene. 2008;27(3):339–46. [DOI] [PubMed] [Google Scholar]
- 139.Milian L, Mata M, Alcacer J, et al. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE. 2020;15(2): e0228909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ramer R, Heinemann K, Merkord J, et al. COX-2 and PPAR-γ confer cannabidiol-induced apoptosis of human lung cancer cells. Mol Cancer Ther. 2013;12(1):69–82. [DOI] [PubMed] [Google Scholar]
- 141.Ramer R, Rohde A, Merkord J, et al. Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm Res. 2010;27:2162–74. [DOI] [PubMed] [Google Scholar]
- 142.McMahon GA, Petitclerc E, Stefansson S, et al. Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J Biol Chem. 2001;276(36):33964–8. [DOI] [PubMed] [Google Scholar]
- 143.Haustein M, Ramer R, Linnebacher M, et al. Cannabinoids increase lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1. Biochem Pharmacol. 2014;92(2):312–25. [DOI] [PubMed] [Google Scholar]
- 144.Ramer R, Merkord J, Rohde H, et al. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem Pharmacol. 2010;79(7):955–66. [DOI] [PubMed] [Google Scholar]
- 145.Blázquez C, Carracedo A, Barrado L, et al. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006;20(14):2633–5. [DOI] [PubMed] [Google Scholar]
- 146.Armstrong JL, Hill DS, McKee CS, et al. Exploiting cannabinoid-induced cytotoxic autophagy to drive melanoma cell death. J Investig Dermatol. 2015;135(6):1629–37. [DOI] [PubMed] [Google Scholar]
- 147.Verykiou S, Alexander M, Edwards N, et al. Harnessing autophagy to overcome mitogen-activated protein kinase kinase inhibitor-induced resistance in metastatic melanoma. Br J Dermatol. 2019;180(2):346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Glodde N, Jakobs M, Bald T, et al. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35–40. [DOI] [PubMed] [Google Scholar]
- 149.Nabissi M, Morelli MB, Offidani M, et al. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget. 2016;7(47):77543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Prester L, Mikolić A, Jurič A, et al. Effects of Δ9-tetrahydrocannabinol on irinotecan-induced clinical effects in rats. Chem Biol Interact. 2018;294:128–34. [DOI] [PubMed] [Google Scholar]
- 151.Carracedo A, Gironella M, Lorente M, et al. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress–related genes. Can Res. 2006;66(13):6748–55. [DOI] [PubMed] [Google Scholar]
- 152.Kosgodage US, Mould R, Henley AB, et al. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front Pharmacol. 2018;9:889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Honarmand M, Namazi F, Mohammadi A, et al. Can cannabidiol inhibit angiogenesis in colon cancer? Comp Clin Pathol. 2019;28:165–72. [Google Scholar]
- 154.Jeong S, Yun HK, Jeong YA, et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019;447:12–23. [DOI] [PubMed] [Google Scholar]
- 155.Zhang Y, Zheng W, Shen K et al. Δ9‑tetrahydrocannabinol inhibits epithelial‑mesenchymal transition and metastasis by targeting matrix metalloproteinase‑9 in endometrial cancer. Oncol Lett. 2018;15(6). [DOI] [PMC free article] [PubMed]
- 156.Whyte DA, Al-Hammadi S, Balhaj G, et al. Cannabinoids inhibit cellular respiration of human oral cancer cells. Pharmacology. 2010;85(6):328–35. [DOI] [PubMed] [Google Scholar]
- 157.Morin-Buote J, Ennour-Idrissi K, Poirier É, et al. Association of breast tumour expression of cannabinoid receptors CBR1 and CBR2 with prognostic factors and survival in breast cancer patients. J Personaliz Med. 2021;11(9):852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pennant NM and Hinton CV. The evolution of cannabinoid receptors in cancer. WIREs Mech Dis. 2023; e1602. [DOI] [PMC free article] [PubMed]
- 159.Iden JA, Raphael-Mizrahi B, Awida Z, et al. The anti-tumorigenic role of cannabinoid receptor 2 in colon cancer: a study in mice and humans. Int J Mol Sci. 2023;24(4):4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Iden JA, Raphael-Mizrahi B, Naim A, et al. The ANTI-TUMORIGENIC ROLE OF CANNABINOID RECEPTOR 2 IN NON-MELANOMA SKIN CANCEr. Int J Mol Sci. 2023;24(9):7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lowe H, Toyang N, Steele B, et al. The endocannabinoid system: a potential target for the treatment of various diseases. 2021;22(17):9472. [DOI] [PMC free article] [PubMed]
- 162.Pagano C, Navarra G, Coppola L, et al. Molecular mechanism of cannabinoids in cancer progression. Int J Mol Sci. 2021;22(7):3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vecera L, Gabrhelik T, Prasil P et al. The role of cannabinoids in the treatment of cancer. 2020;121(1):79–95. [DOI] [PubMed]
- 164.Pergam SA, Woodfield MC, Lee CM et al. Cannabis use among patients at a comprehensive cancer center in a state with legalized medicinal and recreational use. 2017;123(22): 4488–97. [DOI] [PMC free article] [PubMed]
- 165.Whynot EG, Tomko AM, Dupré DJ. Anticancer properties of cannabidiol and Δ(9)-tetrahydrocannabinol and synergistic effects with gemcitabine and cisplatin in bladder cancer cell lines. J Cannabis Res. 2023;5(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Miyato H, Kitayama J, Yamashita H, et al. Pharmacological synergism between cannabinoids and paclitaxel in gastric cancer cell lines. J Surg Res. 2009;155(1):40–7. [DOI] [PubMed] [Google Scholar]
- 167.Ye Q, Gui C, Jin D, et al. Synergistic effect of cannabidiol with dasatinib on lung cancer by SRC/PI3K/AKT signal pathway. Biomed Pharmacother. 2024;173: 116445. [DOI] [PubMed] [Google Scholar]
- 168.Alsherbiny MA, Bhuyan DJ, Low MN, et al. Synergistic interactions of cannabidiol with chemotherapeutic drugs in MCF7 cells: mode of interaction and proteomics analysis of mechanisms. Int J Mol Sci. 2021;22(18):10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tomko AM, Whynot EG, Dupré DJ. Anti-cancer properties of cannflavin A and potential synergistic effects with gemcitabine, cisplatin, and cannabinoids in bladder cancer. J Cannabis Res. 2022;4(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rueda D, Navarro B, A. Martínez-Serrano, et al. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J Biol Chem. 2002;277(48):46645–50. [DOI] [PubMed] [Google Scholar]
- 171.Del Pulgar TG, de Ceballos ML, Guzmán M, et al. Cannabinoids protect astrocytes from ceramide-induced apoptosis through the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2002;277(39):36527–33. [DOI] [PubMed] [Google Scholar]
- 172.Fu Z, Zhao PY, Yang XP, et al. Cannabidiol regulates apoptosis and autophagy in inflammation and cancer: A review. Front Pharmacol. 2023;14:1094020. [DOI] [PMC free article] [PubMed]
- 173.Dariš B, Tancer Verboten M, Knez Ž, et al. Cannabinoids in cancer treatment: therapeutic potential and legislation. Bosn J Basic Med Sci. 2019;19(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pagano C, Navarra G, Coppola L et al. Molecular mechanism of cannabinoids in cancer progression. Int J Mol Sci. 2021;22(7). [DOI] [PMC free article] [PubMed]
- 175.Saleem A, Khan MU, Zahid T, et al. Biological role and regulation of circular RNA as an emerging biomarker and potential therapeutic target for cancer. Mol Biol Reports. 2024;51(1). [DOI] [PubMed]
- 176.Bakshi HA, Faruck HL, Ravesh Z, et al. Therapeutic potential of cannabinoids on tumor microenvironment: a molecular switch in neoplasia transformation. Integr Cancer Ther. 2022;21:15347354221096766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cherkasova V, Wang B, Gerasymchuk M, et al. Use of cannabis and cannabinoids for treatment of cancer. Cancers (Basel). 2022;14(20). [DOI] [PMC free article] [PubMed]
- 178.Pyszniak M, Tabarkiewicz J, Łuszczki JJJO, et al., Endocannabinoid system as a regulator of tumor cell malignancy–biological pathways and clinical significance. 2016;4323–36. [DOI] [PMC free article] [PubMed]
- 179.Zamith Cunha R, Zannoni A, Salamanca G, et al. Expression of cannabinoid (CB1 and CB2) and cannabinoid-related receptors (TRPV1, GPR55, and PPARα) in the synovial membrane of the horse metacarpophalangeal joint. Front Vet Sci. 2023;10:1045030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Louis-Gray K, Tupal S and Premkumar LS. TRPV1: a common denominator mediating antinociceptive and antiemetic effects of cannabinoids. Int J Mol Sci. 2022;23(17). [DOI] [PMC free article] [PubMed]
- 181.Lee XC, Werner E, and Falasca MJC. Molecular mechanism of autophagy and its regulation by cannabinoids in cancer. 2021;13(6):1211. [DOI] [PMC free article] [PubMed]
- 182.Lorente M, Torres S, Salazar M, et al. Stimulation of the midkine/ALK axis renders glioma cells resistant to cannabinoid antitumoral action. Cell Death Differ. 2011;18(6):959–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. [DOI] [PubMed] [Google Scholar]
- 184.Gomez-Larrauri A, Das Adhikari U, Aramburu-Nuñez M, et al. Ceramide metabolism enzymes-therapeutic targets against cancer. Medicina (Kaunas). 2021;57(7). [DOI] [PMC free article] [PubMed]
- 185.Cherkasova V, Wang B, Gerasymchuk M, et al. Use of cannabis and cannabinoids for treatment of cancer. 2022;14(20): 5142. [DOI] [PMC free article] [PubMed]
- 186.Pagano C, Navarra G, Coppola L, et al. Molecular mechanism of cannabinoids in cancer progression. 2021;22(7):3680. [DOI] [PMC free article] [PubMed]
- 187.Li RZ, Wang XR, Wang J, et al. The key role of sphingolipid metabolism in cancer: New therapeutic targets, diagnostic and prognostic values, and anti-tumor immunotherapy resistance. Front Oncol. 2022;12:941643. [DOI] [PMC free article] [PubMed]
- 188.Salazar M, Carracedo A, Salanueva ÍJ, et al. TRB3 links ER stress to autophagy in cannabinoid anti-tumoral action. 2009. [DOI] [PubMed]
- 189.Carracedo A, Lorente M, Egia A, et al. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 2006;9(4):301–12. [DOI] [PubMed] [Google Scholar]
- 190.Perry DK, Carton J, Shah AK, et al. Serine palmitoyltransferase regulates de novoceramide generation during etoposide-induced apoptosis. J Biol Chem. 2000;275(12):9078–84. [DOI] [PubMed] [Google Scholar]
- 191.Bonni A, Brunet A, West AE, et al. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and-independent mechanisms. Science. 1999;286(5443):1358–62. [DOI] [PubMed] [Google Scholar]
- 192.Tewari D, Patni P, Bishayee A et al. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: a novel therapeutic strategy. In: Seminars in cancer biology. 2022. Elsevier. [DOI] [PubMed]
- 193.Noorolyai S, Shajari N, Baghbani E, et al. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–8. [DOI] [PubMed] [Google Scholar]
- 194.Greenhough A, Patsos HA, Williams AC, et al. The cannabinoid δ9-tetrahydrocannabinol inhibits RAS-MAPK and PI3K-AKT survival signalling and induces BAD-mediated apoptosis in colorectal cancer cells. Int J Cancer. 2007;121(10):2172–80. [DOI] [PubMed] [Google Scholar]
- 195.Ellert-Miklaszewska A, Kaminska B, Konarska L. Cannabinoids down-regulate PI3K/Akt and Erk signalling pathways and activate proapoptotic function of Bad protein. Cell Signal. 2005;17(1):25–37. [DOI] [PubMed] [Google Scholar]
- 196.Martínez-Martínez E, Martín-Ruiz A, Martín P, et al. CB2 cannabinoid receptor activation promotes colon cancer progression via AKT/GSK3β signaling pathway. Oncotarget. 2016;7(42):68781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Song Q, Zhang W, Shi D, et al. Overexpression of cannabinoid receptor 2 is associated with human breast cancer proliferation, apoptosis, chemosensitivity and prognosis via the PI3K/Akt/mTOR signaling pathway. Cancer Med. 2023;12(12):13538–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Degirmenci U, Wang M, Hu J. Targeting aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells. 2020;9(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bahar ME, Kim HJ, Kim DR. Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal Transduct Target Ther. 2023;8(1):455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Qin N, Neeper MP, Liu Y, et al. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28(24):6231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhong N, Li D, Wang B, et al. Cannabinol inhibits cell growth and triggers cell cycle arrest and apoptosis in cancer cells. Biocatal Agric Biotechnol. 2023;48: 102627. [Google Scholar]
- 202.Roberto D, Klotz LH, and Venkateswaran VJTP. Cannabinoid WIN 55,212‐2 induces cell cycle arrest and apoptosis, and inhibits proliferation, migration, invasion, and tumor growth in prostate cancer in a cannabinoid‐receptor 2 dependent manner. 2019;79(2):151–9. [DOI] [PubMed]
- 203.Kisková T, Mungenast F, Suváková M, et al. Future aspects for cannabinoids in breast cancer therapy. 2019;20(7):1673. [DOI] [PMC free article] [PubMed]
- 204.De Petrocellis L, Melck D, Palmisano A, et al. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc Natl Acad Sci. 1998;95(14):8375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pulgar TG, Velasco G, Sánchez C, et al. De novo-synthesized ceramide is involved in cannabinoid-induced apoptosis. Biochem J. 2002;363(1):183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Galve-Roperh I, Sánchez C, Cortés ML, et al. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med. 2000;6(3):313–9. [DOI] [PubMed] [Google Scholar]
- 207.Sarfaraz S, Afaq F, Adhami VM, et al. Cannabinoid receptor as a novel target for the treatment of prostate cancer. Can Res. 2005;65(5):1635–41. [DOI] [PubMed] [Google Scholar]
- 208.Sarnataro D, Pisanti S, Santoro A, et al. The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits human breast cancer cell proliferation through a lipid raft-mediated mechanism. Mol Pharmacol. 2006;70(4):1298–306. [DOI] [PubMed] [Google Scholar]
- 209.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8(4):210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hermanson DJ, Marnett LJ. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev. 2011;30:599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2015;237(3):273–81. [DOI] [PubMed] [Google Scholar]
- 213.Joseph J, Niggemann B, Zaenker KS, et al. Anandamide is an endogenous inhibitor for the migration of tumor cells and T lymphocytes. Cancer Immunol Immunother. 2004;53:723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vaccani A, Massi P, Colombo A, et al. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br J Pharmacol. 2005;144(8):1032–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Grimaldi C, Pisanti S, Laezza C, et al. Anandamide inhibits adhesion and migration of breast cancer cells. Exp Cell Res. 2006;312(4):363–73. [DOI] [PubMed] [Google Scholar]
- 216.Nieder C, Adam M, Molls M, et al. Therapeutic options for recurrent high-grade glioma in adult patients: recent advances. Crit Rev Oncol Hematol. 2006;60(3):181–93. [DOI] [PubMed] [Google Scholar]
- 217.Guzman M, Duarte M, Blazquez C, et al. A pilot clinical study of Δ9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer. 2006;95(2):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Inglet S, Winter B, Yost SE, et al. Clinical data for the use of cannabis-based treatments: a comprehensive review of the literature. Ann Pharmacother. 2020;54(11):1109–43. [DOI] [PubMed] [Google Scholar]
- 219.Afrin F, Chi M, Eamens AL, et al. Can hemp help? Low-THC cannabis and non-THC cannabinoids for the treatment of cancer. Cancers. 2020;12(4):1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Singh Y, Bali C. Cannabis extract treatment for terminal acute lymphoblastic leukemia with a Philadelphia chromosome mutation. Case Reports Oncol. 2013;6(3):585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Blázquez C, González-Feria L, Alvarez L, et al. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Can Res. 2004;64(16):5617–23. [DOI] [PubMed] [Google Scholar]
- 222.Foroughi M, Hendson G, Sargent MA, et al. Spontaneous regression of septum pellucidum/forniceal pilocytic astrocytomas—possible role of Cannabis inhalation. Childs Nerv Syst. 2011;27:671–9. [DOI] [PubMed] [Google Scholar]
- 223.Good PD, Greer RM, Huggett GE, et al. An open-label pilot study testing the feasibility of assessing total symptom burden in trials of cannabinoid medications in palliative care. J Palliat Med. 2020;23(5):650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Allen DH; Dronabinol therapy: central nervous system adverse events in adults with primary brain tumors. Clin J Oncol Nurs. 2019;23(1). [DOI] [PubMed]
- 225.Elder JJ, Knoderer HM. Characterization of dronabinol usage in a pediatric oncology population. J Pediatric Pharmacol Therapeutics. 2015;20(6):462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kampa-Schittenhelm KM, Salitzky O, Akmut F, et al. Dronabinol has preferential antileukemic activity in acute lymphoblastic and myeloid leukemia with lymphoid differentiation patterns. BMC Cancer. 2016;16(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Guzmán M, Duarte MJ, Blázquez C, et al. A pilot clinical study of Δ9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer. 2006;95(2):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.McAllister SD, Christian RT, Horowitz MP, et al. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol Cancer Ther. 2007;6(11):2921–7. [DOI] [PubMed] [Google Scholar]
- 229.Troyer J and Tanco K. Review of the use of medicinal cannabis products in palliative care. Cancers (Basel). 2024;16(7). [DOI] [PMC free article] [PubMed]
- 230.Sexton M, Garcia JM, Jatoi A, et al. The management of cancer symptoms and treatment-induced side effects with cannabis or cannabinoids. 2021;2021(58):86–98. [DOI] [PMC free article] [PubMed]
- 231.Blake A, Wan BA, Malek L, et al. A selective review of medical cannabis in cancer pain management. Ann Palliat Med. 2017;6(Suppl 2):S215-s222. [DOI] [PubMed] [Google Scholar]
- 232.Page R and Blanchard EJJOP. Opioids and cancer pain: patients’ needs and access challenges. 2019, American Society of Clinical Oncology. pp. 229–31. [DOI] [PubMed]
- 233.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–73. [DOI] [PubMed] [Google Scholar]
- 234.Mariean CR, Tiucă OM, Mariean A, et al. Cancer cachexia: new insights and future directions. Cancers (Basel). 2023;15(23). [DOI] [PMC free article] [PubMed]
- 235.Strasser F, Luftner D, Possinger K, et al. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol. 2006;24(21):3394–400. [DOI] [PubMed] [Google Scholar]
- 236.Turcott JG, del Rocío Guillen Núñez M, Flores-Estrada D, et al. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: a randomized, double-blind clinical trial. 2018;26:3029–38. [DOI] [PubMed]
- 237.Sánchez C, Galve-Roperh I, Canova C, Brachet P, Guzmán M. Delta9-tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS letters. 1998;436(1):6–10. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.