Abstract
Strategies are needed for human immunodeficiency virus type 1 vaccine development that improves the neutralizing antibody response against primary isolates of the virus. Here we examined recombinant DNA priming followed by subunit protein boosting as a strategy to generate neutralizing antibodies. Both plasmid-based and recombinant protein envelope (Env) glycoprotein immunogens were derived from a primary viral isolate, JR-FL. Serum from rabbits immunized with either gp120 or gp140 DNA vaccines delivered by gene gun inoculation followed by recombinant gp120 protein boosting was capable of neutralizing JR-FL. Neither the DNA vaccines alone nor the gp120 protein alone generated a detectable neutralizing antibody response against this virus. Neutralizing antibody responses using gp120 DNA and gp140 DNA for priming were similar. The results suggest that Env DNA priming followed by gp120 protein boosting provides an advantage over either approach alone for generating a detectable neutralizing antibody response against primary isolates that are not easily neutralized.
Neutralizing antibodies are considered critical immune components for effective vaccination against human immunodeficiency virus type 1 (HIV-1) infection and disease (5, 10, 16, 19). The HIV-1 envelope glycoproteins (Env) are the primary viral antigens targeted by neutralizing antibodies. However, efforts to develop an Env-based immunogen that elicits an effective neutralizing antibody response are hampered by the high mutation rate of the virus in infected individuals (23) and the resulting genetic heterogeneity and structural complexity exhibited by Env (24). Thus, an effective HIV-1 vaccine will need to target a plethora of genetic and antigenic variants of the virus.
A variety of candidate HIV-1 vaccines have included Env for the purpose of generating a neutralizing antibody response (8, 14, 20). Among these, DNA vaccines have proven to be poor inducers of neutralizing antibodies on their own but nonetheless prime for a detectable neutralizing antibody response after Env protein boosting (1, 9, 11, 13, 21, 22). Unfortunately, the neutralizing antibodies generated in these studies have primarily targeted T-cell-line-adapted strains and a small fraction of primary isolates of HIV-1 that are unusually sensitive to neutralization. Most primary isolates of HIV-1 are substantially less sensitive to neutralization and more difficult to target with vaccines (2-4, 15).
It has not been clear whether the DNA prime and protein boost strategy affords an advantage over Env protein immunization alone with respect to the elicitation of a neutralizing antibody response that targets typical primary HIV-1 isolates that are not easily neutralized. In this regard, the JR-FL strain of HIV-1 exhibits such a neutralization phenotype (6) and therefore represents a relevant viral target upon which different vaccine strategies can be evaluated and compared. In the present study, we investigated the ability of the JR-FL gp120 protein to generate a neutralizing antibody response with and without prior priming with recombinant DNA vaccines expressing soluble secreted forms of either JR-FL gp120 or JR-FL gp140.
Two versions of JR-FL Env DNA vaccines were constructed by subcloning codon-optimized JR-FL Env gene sequences (7) into the pJW4303 DNA vaccine vector (12). The gp120 DNA vaccine codes for the ectodomain of the JR-FL Env protein. The gp140 DNA vaccine encodes gp120 plus the extracellular region of gp41, with the cleavage site between gp120 and gp41 left intact. Results of a previous study suggested that both the gp120 and gp140 forms of Env DNA vaccines were able to overcome the low immunogenicity of a full-length Env DNA vaccine, insofar as high-level anti-Env antibody responses were elicited by the gp120 and gp140 DNA vaccines compared to a gp160 DNA vaccine (13). The expression of JR-FL gp120 and gp140 by the DNA vaccines was confirmed by Western blotting using supernatants from 293T cells transiently transfected with either of the two DNA plasmids.
In the present study, Env DNA priming followed by gp120 protein boosting was compared to gp120 protein immunization alone. New Zealand White rabbits received either a gp120 DNA vaccine (RJ001 and RJ002) or a gp140 DNA vaccine (RJ003 and RJ004) by gene gun inoculation at weeks 0, 4, 8, and 12. The DNA dose at each immunization was 36 μg. The recombinant JR-FL gp120 protein consisted of 100 μg of gp120 mixed with incomplete Freund's adjuvant; the gp120 protein was administered by intramuscular injection at weeks 16 and 20. One control group (rabbits RJ005 and RJ006) was inoculated with empty DNA vector at weeks 0, 4, 8, and 12 followed by two gp120 protein boosts at weeks 16 and 20. A second control group (RJ007 to RJ009) was inoculated with gp120 protein at weeks 2, 4, 8, and 30 (Fig. 1).
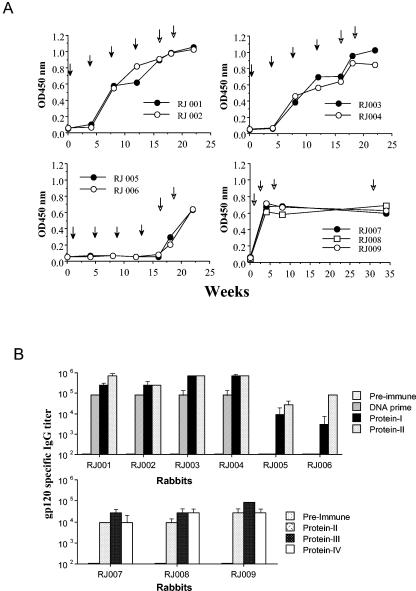
FIG. 1.
HIV-1 JR-FL Env-specific IgG responses in New Zealand White rabbits as measured by ELISA. (A) Temporal serum anti-gp120 IgG responses were monitored for rabbits immunized with either the JR-FL gp120 DNA vaccines (RJ001 and RJ002), the JR-FL gp140 DNA vaccines (RJ003 and RJ004), or the control vector DNA plasmid (RJ005 and RJ006) followed by gp120 protein boosts. Rabbits in the second control group (RJ007 to RJ009) received four JR-FL gp120 protein immunizations without a DNA prime. Black arrows indicate the times of DNA immunizations, and open arrows indicate the times of protein immunizations. Equal amounts of the JR-FL gp120 antigens (0.1 μg/well) were added to plates precoated with concanavalin A (5 μg/well). Detection of gp120-specific IgG was done as previously reported (13) using biotinylated anti-rabbit IgG and horseradish peroxidase-conjugated streptavidin (Vector Lab). (B) The end titration titers of the above rabbit sera were determined when the optical density readings at the highest serum dilution were twofold above the optical density reading of the negative control wells with the normal rabbit sera. Geometric means of the anti-gp120 IgG titers are shown with standard deviations for sera collected before the start of immunization (Pre-immune), 2 weeks after the fourth DNA immunization (DNA prime), or 2 weeks after one or more protein boosts (as indicated).
JR-FL Env-specific immunoglobulin G (IgG) responses were examined by enzyme-linked immunosorbent assay (ELISA) (Fig. 1). Priming with either gp120 DNA or gp140 DNA and boosting with gp120 protein generated anti-Env antibody responses higher than those in control animals that either received protein alone or were primed with empty DNA vector and boosted with protein. Little difference was seen in the magnitude of the response between the gp120 DNA-primed and gp140 DNA-primed animals. Repeated boosting with either protein alone or after empty vector DNA priming was less effective, in that peak titers of anti-Env IgG were approximately 1 log lower than the peak titers generated by Env DNA priming plus protein boosting (Fig. 1B).
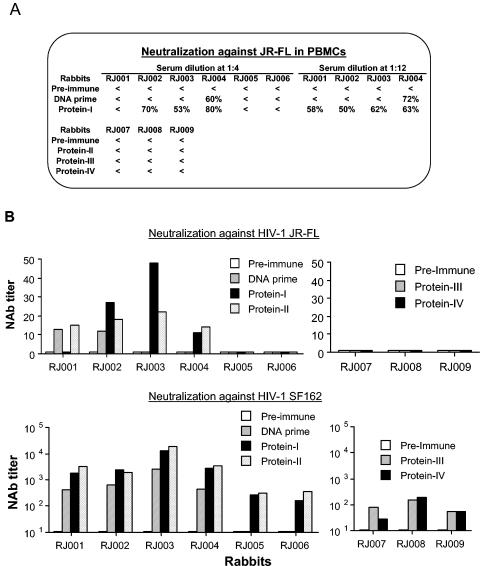
The serum samples were next assessed for neutralizing activity against the vaccine strain, JR-FL, and a highly neutralization-sensitive R5 strain, SF162 (Fig. 2). Neutralization was measured as either reductions in p24 Gag antigen synthesis in peripheral blood mononuclear cells (18) or reductions in Tat-responsive luciferase reporter gene expression in 5.25.EGFP.Luc.M7 cells (17). Antibodies capable of neutralizing JR-FL were detected only in animals that were primed with Env DNA and boosted with protein. In these animals, sporadic neutralization of JR-FL was detected after DNA priming. Neutralizing activity was much more consistent after protein boosting, as detected in both assays. Titers of neutralizing antibodies against JR-FL were between 1:12 and 1:48 (Fig. 2B). There were no clear differences in the magnitude of this response in animals primed with gp120 DNA and those primed with gp140 DNA.
FIG. 2.
Neutralizing activities of rabbit sera. Neutralization was measured as either reductions in p24 Gag antigen synthesis in peripheral blood mononuclear cells (A) (18) or reductions in Tat-responsive luciferase reporter gene expression in 5.25.EGFP.Luc.M7 cells (B) (17). The actual percent reductions of p24 Gag antigen at rabbit serum dilutions of 1:4 or 1:12 were used for panel A. Any reduction of p24 less than 50% is indicated by <. Neutralization titers are the dilution at which relative luminescence units (RLU) were reduced by 50% compared to virus control wells after subtraction of background RLU (B). Sera collected before the start of immunization (Pre-immune), 2 weeks after the last DNA immunization (DNA prime), or 2 weeks after one or more protein immunizations (as indicated) were used in both types of neutralization studies. The starting serum dilution was 1:20 for assay against SF162 and 1:10 for assay against JR-FL. NAb, neutralizing antibody.
Env DNA alone and gp120 protein alone were both capable of generating antibodies that neutralized SF162. This response rose in magnitude after gp120 protein boosting in Env DNA-primed animals, at which time the titers surpassed those in animals immunized with empty vector DNA and boosted with gp120 protein (Fig. 2B). As with JR-FL, no clear difference was seen between gp120 DNA priming and gp140 DNA priming for generating antibodies that neutralize SF162. The differential neutralization of SF162 and JR-FL suggests that recombinant gp120 alone was able to elicit an antibody response against certain shared determinants but was not effective in eliciting antibodies recognizing specific antigenic conformations associated with JR-FL neutralization. It appears that priming with Env DNA and boosting with gp120 protein was able to circumvent this problem.
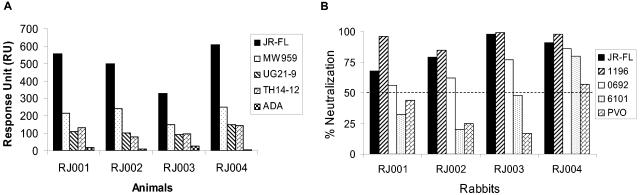
We further studied the breadth of the anti-Env antibody responses after gp120 protein boosting in Env DNA-primed rabbits by using highly sensitive surface plasmon resonance analysis. Biacore chips were coated individually with a panel of five recombinant gp120 antigens produced from CHO cells. The rabbit sera showed high specificity against the autologous JR-FL gp120 antigen, moderate reactivity against gp120 from primary HIV-1 isolates 92MW959 (subgroup C), 92UG21-9 (subgroup A), and 92TH14-12 (subgroup B), and poor reactivity against gp120 from ADA (subgroup B) (Fig. 3A). These data indicated that serum samples from rabbits immunized with JR-FL gp120 (delivered by DNA prime and protein boost) had a preferred reactivity against the autologous JR-FL gp120 antigen.
FIG. 3.
Breadth of rabbit anti-gp120 IgG responses in rabbit sera showed positive neutralizing antibody responses against JR-FL. (A) Cross-reactivity of antibodies specific for HIV-1 gp120s in rabbit sera 2 weeks after the second protein boost was assessed using a Biacore 3000 biosensor. Each serum sample was diluted 1/100 in HBT-CMD running buffer (HEPES buffer, pH 7.4, 150 mM NaCl, 0.1% Tween 20, 0.5% soluble carboxymethyl dextran) and passed at a flow rate of 5 μl/min for 20 min over surfaces to which the indicated gp120s had been covalently coupled to a density of approximately 750 relative units (RU). A reference flow cell was coated with alcohol dehydrogenase to approximately the same density to control for nonspecific surface effects. The response obtained from each sensorgram 5 s prior to the end of the association phase was determined. The response from preimmune serum of each rabbit was subtracted to control for nonspecific binding of serum proteins to the sensor surface. (B) The same rabbit serum samples at 2 weeks after the second protein boost were analyzed for neutralizing activities against four primary HIV-1 isolates (1196, 0692, 6101, and PVO) in addition to the autologous JR-FL strain. All of the viruses were grown in peripheral blood mononuclear cells, and the assays were conducted in 5.25.EGFP.Luc.M7 cells (17) with rabbit serum at a 1:10 dilution. Neutralizing activities are presented as the percent reduction of RLU compared to virus control wells after subtraction of background RLU. Preimmune sera for all four rabbits did not show any positive neutralizing activities (data not shown).
The same serum samples were tested for neutralizing activity against additional heterologous strains of HIV-1 to determine whether there might have been a significant improvement in breadth (Fig. 3). The most potent neutralizing activity was detected against SS1196, a strain of virus that is generally more sensitive to neutralization than JR-FL (D. Montefiori, unpublished observations). Little or no neutralizing activity against other heterologous primary isolates was detected. This limited cross-neutralizing activity indicates that additional improvements in immunogen design, such as a polyvalent Env formulation, will be needed to elicit a neutralizing antibody response of sufficient breadth to benefit a vaccine.
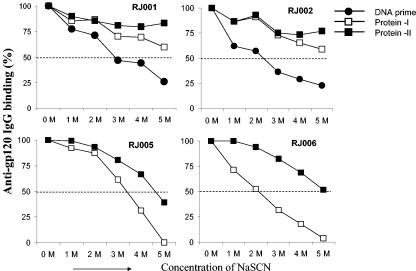
The exact mechanism underlying the advantage of DNA prime plus protein boost is not clear at this point. Results of a previous study suggested that antibody maturation through increased avidity might be responsible for improved neutralizing activity in rabbits immunized by DNA priming and protein boosting (21). We therefore examined avidity in the presence of increasing concentrations of NaSCN. Consistent with previous results mentioned above, avidity increased more rapidly after gp120 boosting in gp120 DNA-primed rabbits than in rabbits that were primed with vector DNA (Fig. 4). However, our results cannot exclude the possibility that the difference in avidity is in part due to the duration and/or the number of immunizations used in this study.
FIG. 4.
Avidity of gp120-specific IgG in rabbits that received recombinant gp120 protein antigens with (RJ001 and RJ002) and without (RJ005 and RJ006) gp120 DNA priming. Avidity was measured by NaSCN displacement ELISA (21). Rabbit sera were first added to the ELISA plate coated with JR-FL gp120 and incubated for 60 min at 23°C. After washing, increasing concentrations of sodium thiocyanate (NaSCN) were added to the plates for 20 min. After another round of washing, bound antibodies were detected by using the regular ELISA procedure as previously described (21). Rabbit sera collected 2 weeks after the last DNA immunization (DNA prime) or 2 weeks after one or two protein boosts (as indicated) were used.
Our results suggest that, in addition to a dose-sparing benefit, the DNA prime and Env protein boost strategy may be superior to Env protein alone when evaluating candidate HIV-1 Env vaccines for optimal neutralizing antibody responses. At this point, we are not aware of any adjuvant that can assist recombinant gp120 to specifically improve the quality of neutralizing antibody response against more resistant primary HIV-1 isolates. Therefore, the potential advantage for neutralizing antibody responses would add to the value of DNA vaccines that also induce HIV-1-specific T-cell responses.
Acknowledgments
We thank Brian Seeds and Eun-Chung Park for codon-optimized JR-FL Env gene and Jim Bradac (Division of AIDS, National Institute of Allergy and Infectious Diseases) for providing recombinant JR-FL gp120 protein. We also thank Xiaoyun Huang and Theodore Giehl for their technical assistance. We thank Susan Fragala for expert administrative assistance.
This project was supported in part by NIH/NIAID grants AI40337 and AI46294 (S.L.) and AI30034 (D.M.).
REFERENCES
- 1.Barnett, S. W., S. Rajasekar, H. Legg, B. Doe, D. H. Fuller, J. R. Haynes, C. M. Walker, and K. S. Steimer. 1997. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine 15:869-873. [DOI] [PubMed] [Google Scholar]
- 2.Beddows, S., S. Lister, R. Cheingsong, C. Bruck, and J. Weber. 1999. Comparison of the antibody repertoire generated in healthy volunteers following immunization with a monomeric recombinant gp120 construct derived from a CCR5/CXCR4-using human immunodeficiency virus type 1 isolate with sera from naturally infected individuals. J. Virol. 73:1740-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belshe, R. B., G. J. Gorse, M. J. Mulligan, T. G. Evans, M. C. Keefer, J. L. Excler, A. M. Duliege, J. Tartaglia, W. I. Cox, J. McNamara, K. L. Hwang, A. Bradney, D. Montefiori, K. J. Weinhold, and the NIAID AIDS Vaccine Evaluation Group. 1998. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. AIDS 12:2407-2415. [DOI] [PubMed] [Google Scholar]
- 4.Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 16:2019-2035. [DOI] [PubMed] [Google Scholar]
- 5.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 6.Fouts, T. R., J. M. Binley, A. Trkola, J. E. Robinson, and J. P. Moore. 1997. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J. Virol. 71:2779-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas, J., E. C. Park, and B. Seed. 1996. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr. Biol. 6:315-324. [DOI] [PubMed] [Google Scholar]
- 8.Letvin, N. L., D. H. Barouch, and D. C. Montefiori. 2002. Prospects for vaccine protection against HIV-1 infection and AIDS. Annu. Rev. Immunol. 20:73-99. [DOI] [PubMed] [Google Scholar]
- 9.Letvin, N. L., D. C. Montefiori, Y. Yasutomi, H. C. Perry, M. E. Davies, C. Lekutis, M. Alroy, D. C. Freed, C. I. Lord, L. K. Handt, M. A. Liu, and J. W. Shiver. 1997. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc. Natl. Acad. Sci. USA 94:9378-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letvin, N. L., and B. D. Walker. 2003. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat. Med. 9:861-866. [DOI] [PubMed] [Google Scholar]
- 11.Leung, L., I. K. Srivastava, E. Kan, H. Legg, Y. Sun, C. Greer, D. C. Montefiori, J. zur Megede, and S. W. Barnett. 2004. Immunogenicity of HIV-1 Env and Gag in baboons using a DNA prime/protein boost regimen. AIDS 18:991-1001. [DOI] [PubMed] [Google Scholar]
- 12.Lu, S., S. Manning, and J. Arthos. 1999. Antigen engineering in DNA immunization, p. 355-374. In D. B. Lowrie and R. G. Whalen (ed.), DNA vaccines: methods and protocols. Humana Press Inc., Totowa, N.J. [DOI] [PubMed]
- 13.Lu, S., R. Wyatt, J. F. Richmond, F. Mustafa, S. Wang, J. Weng, D. C. Montefiori, J. Sodroski, and H. L. Robinson. 1998. Immunogenicity of DNA vaccines expressing human immunodeficiency virus type 1 envelope glycoprotein with and without deletions in the V1/2 and V3 regions. AIDS Res. Hum. Retrovir. 14:151-155. [DOI] [PubMed] [Google Scholar]
- 14.Mascola, J. R., and G. J. Nabel. 2001. Vaccines for the prevention of HIV-1 disease. Curr. Opin. Immunol. 13:489-495. [DOI] [PubMed] [Google Scholar]
- 15.Mascola, J. R., S. W. Snyder, O. S. Weislow, S. M. Belay, R. B. Belshe, D. H. Schwartz, M. L. Clements, R. Dolin, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, M. C. Walker, K. F. Wagner, J. G. McNeil, F. E. McCutchan, D. S. Burke, and the National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J. Infect. Dis. 173:340-348. [DOI] [PubMed] [Google Scholar]
- 16.McMichael, A. J., and T. Hanke. 2003. HIV vaccines 1983-2003. Nat. Med. 9:874-880. [DOI] [PubMed] [Google Scholar]
- 17.Montefiori, D. C. 2004. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays, p. 12.11.1-12.11.15. In J. E. Coligan, A. M. Kruisbeek, D. H. Margolis, E. M. Shevach, W. Strober, and R. Coico (ed.), Current protocols in immunology. John Wiley & Sons, New York, N.Y. [DOI] [PubMed]
- 18.Montefiori, D. C., G. Pantaleo, L. M. Fink, J. T. Zhou, J. Y. Zhou, M. Bilska, G. D. Miralles, and A. S. Fauci. 1996. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J. Infect. Dis. 173:60-67. [DOI] [PubMed] [Google Scholar]
- 19.Moore, J. P., and D. R. Burton. 2004. Urgently needed: a filter for the HIV-1 vaccine pipeline. Nat. Med. 10:769-771. [DOI] [PubMed] [Google Scholar]
- 20.Mulligan, J., and J. Weber. 1999. Human trials of HIV-1 vaccines. AIDS 13(Suppl. A):S105-S112. [PubMed] [Google Scholar]
- 21.Richmond, J. F., S. Lu, J. C. Santoro, J. Weng, S. L. Hu, D. C. Montefiori, and H. L. Robinson. 1998. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J. Virol. 72:9092-9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, S., R. Pal, J. R. Mascola, T. W. Chou, I. Mboudjeka, S. Shen, S. Whitney, T. Keen, B. C. Nair, V. S. Kalyanaraman, P. Markham, and S. Lu. Unpublished data.
- 23.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 24.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]