Abstract
Background
This retrospective study analyzed the incidence of subsequent brain metastases after palliative radiotherapy or chemoradiation in patients with stage II/III non-small cell lung cancer (NSCLC). Risk factors for brain metastases development and survival after diagnosis were evaluated.
Methods
Different baseline parameters including but not limited to age, stage and target volume size were assessed. Outcomes were abstracted from electronic health records. Uni- and multivariate tests were performed.
Results
The study included 102 patients and found an actuarial risk of brain metastases of 15% (standard error ± 4) at one year and 20% (± 5) at two years. The maximum time interval was 15 months from start of radiation treatment. A non-significant survival difference was observed (median 12 months without versus 8.3 months with brain metastases, p = 0.21). Incidence was higher in patients with N2/3 stage, larger planning target volume size, and younger age (univariately significant factors). Trends were seen for stage III and adenocarcinoma histology. The multivariate analysis confirmed age as the most important risk factor.
Conclusion
The risk of brain metastases development was comparable to that reported in studies of curative chemoradiation. All events occurred within 15 months of follow-up, suggesting that long-term surveillance imaging may not be warranted. Patients younger than 60 years had a very high risk of brain metastases development.
Keywords: Cerebral metastases, Radiation therapy, Palliative treatment, Predictive factors, Hypofractionation
Introduction
Patients with non-small cell lung cancer (NSCLC) are often diagnosed with incurable, clinically symptomatic stage IV disease [1, 2]. At first diagnosis, 10% were found to harbor brain metastases [3], depending on the presence of certain genomic alterations [4]. Even in lower, potentially curable stages (I-III), whose incidence may become larger due to implementation of screening programs, a proportion of patients does not proceed to curative treatment, either because of patient preference or treatment providers’ reluctance. The latter may be caused by safety concerns due to a mismatch between organ function and, in case of radiotherapy, achievable dose distribution and organ-at-risk doses, among others [5, 6]. Therefore, alternatives to standard chemoradiation (platinum doublet, 60–66 Gy in 30–33 fractions) have been developed [7–11], including but not limited to the Norwegian CONRAD regimen (42 Gy in 15 fractions) [12]. This regimen typically contains carboplatin/vinorelbine, but can also be modified to include other drugs. Stand-alone radiotherapy, possibly to lower doses such as 30–39 Gy in 10–13 fractions, represents an option for older and/or less fit patients [13], commonly resulting in symptom improvement, temporary tumor growth inhibition and moderately prolonged survival [14]. Very frail patients may benefit from extreme hypofractionation.
As repeatedly reported in studies of curative standard chemoradiation, isolated development of distant metastases, especially in the brain, is a clinically relevant scenario, together with thoracic disease relapse and mixed types of cancer progression [15–17]. Several studies reported that 15–20% of patients developed brain metastases and some of these were not amenable to efficacious therapy. In a large US study, patients with brain metastases had a 1.56 times greater risk of death versus those with no brain metastases [18]. However, survival after diagnosis of brain metastases depends on treatment approach/efficacy and several well-established prognostic factors [19]. In patients with brain metastases and adverse prognostic features such as reduced performance status and simultaneous extracranial metastases, controversy exists about the preferred management approach [20]. In general, stereotactic radiotherapy represents an effective option. Given its large clinical impact and the fact that previous studies almost exclusively focused on curative/radical primary treatment, we analyzed brain metastases development in patients treated with lower doses of radiation with or without concomitant systemic therapy.
Patients and methods
We evaluated an established single-institution database (2009–2022) [11], after updates for survival and brain metastases development in January 2024. The retrospective analysis included 102 consecutive patients with stage II-III managed with standard palliative external beam radiotherapy regimens with doses ranging from 10 fractions of 3 Gy to 15 fractions of 2.8 or 3 Gy. Radiotherapy fractionation was at the discretion of the treating oncologist. However, a multidisciplinary lung cancer board (MDTB) provided general recommendations about treatment intention and additional systemic therapy. Interrupted or permanently discontinued radiotherapy series were included to comply with the intention-to-treat principle. Staging did often, but not uniformly include positron emission tomography (PET) scans (18-FDG) and brain magnetic resonance imaging (sometimes computed tomography). Staging according to the TNM system [21] was provided by the MDTB. Follow-up was scheduled every 3–4 months for 2 years and every 6 months afterwards. However, many patients received additional systemic therapy for relapses/metastases and were seen more frequently. Surveillance imaging of the brain was not performed. Scans were taken only in case of clinical symptoms. The Department of Oncology and Palliative Medicine is responsible for all oncology care (radiation, drugs etc.) and utilizes the hospital’s electronic health records that formed the basis of the present study.
Standard descriptive analyses were employed. Overall survival (time to death) from the first day of radiotherapy was calculated employing the Kaplan–Meier method (SPSS 28, IBM Corp., Armonk, NY, USA). In 20 surviving patients, survival was censored after a median follow-up of 35 months. Time to development of brain metastases was also analyzed by Kaplan–Meier curves, which were compared by means of log-rank tests. In this context, deceased patients were censored at the time of death, and patients in continued follow-up and free from brain metastases were censored at the time of last contact. After univariate log-rank tests, a multivariate forward stepwise Cox regression analysis was performed. P-values ≤ 0.05 were considered statistically significant.
All procedures performed in the study patients were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was performed as a retrospective analysis in the context of our already institutional review board (IRB)-approved longitudinal monitoring of NSCLC management. Additional approval from the Regional Committee for Medical and Health Research Ethics was not necessary for this project, which already had exempt status. This research project was carried out according to our institutions’ guidelines and with permission to access the patients’ data. Written informed consent was received from all patients.
Results
The cohort was dominated by elderly patients with stage III disease and squamous cell histology (Table 1). Molecular targets for systemic therapy such as epidermal growth factor receptor (EGFR) were tested only in case of non-squamous histology and absent in all patients. The programmed death ligand 1 (PD-L1) status was known in a minority only and therefore excluded from further analysis. Thirteen patients (13%) developed brain metastases after thoracic radiotherapy. The maximum time interval was 15 months from start of radiation treatment. We calculated an actuarial risk of 15% (standard error ± 4) at one year and 20% (± 5) at two years (Kaplan–Meier method, censoring as described in Patients and Methods). Median survival was 11 months (2-year estimate 25%). A numerical difference by brain metastases status was observed (12 months without versus 8.3 months with brain metastases, p = 0.21, i.e. not statistically significant). Typically, a limited number of brain metastases were detected (1–3 in 9 patients, 4–9 in three, ≥ 10 in one). Most patients received local treatment (neurosurgical resection and/or radiotherapy, n = 11), while two were unable to proceed to active treatment, i.e. received best supportive care. Both had severely reduced performance status and extracranial metastases at the time of brain metastases development.
Table 1.
Baseline characteristics for 102 patients, risk factors for brain metastases
| Parameter | n* | Risk factor, univariate analyses |
|---|---|---|
| Sex | ||
| Female | 45 | Not significant |
| Male | 57 | |
| Tumor stage | ||
| II | 14 | Not significant |
| III | 88 | |
| Primary tumor (T) stage | ||
| T1 or absent after surgical removal | 16 | Not significant |
| T2 | 18 | |
| T3 | 37 | |
| T4 | 31 | |
| Thoracic lymph node metastases (N) stage | ||
| N0 or 1 | 25 | p = 0.02 |
| N2 or 3 | 77 | |
| Histology | ||
| Adenocarcinoma | 36 | Not significant |
| Squamous cell carcinoma | 50 | |
| Large cell carcinoma | 5 | |
| Unspecified/mixed/others | 11 | |
| Site | ||
| Left lung | 37 | Not significant |
| Right lung | 53 | |
| Both lungs | 1 | |
| Mediastinum only | 11 | |
| Smoking history | ||
| Never | 23 | Not significant |
| Previous or active | 79 | |
| Treatment | ||
| Concurrent chemoradiotherapy | 42 | Not significant |
| Systemic non-concurrent treatment | 15 | |
| Within 4 weeks before radiotherapy | 9 | |
| Earlier than 4 weeks before radiotherapy | 6 | |
| No preceding treatment | 45 | |
| Radiotherapy dose category | ||
| Low such as 10 fractions of 3 Gy | 16 | Not significant |
| Intermediate such as 12 fractions of 3 Gy | 20 | |
| High such as 15 fractions of 2.8–3 Gy | 66 | |
| Radiotherapy fraction number | ||
| < 10 | 3 | Not significant |
| 10 | 22 | |
| > 10 | 77 | |
| Radiotherapy dose per fraction | ||
| < 3 Gy | 55 | Not significant |
| 3 Gy | 39 | |
| > 3 Gy | 8 | |
| Planning target volume size | ||
| Median size, range (ccm) | 395, 23–1272 | p = 0.03 |
| Age | ||
| Median age, range (years) | 72.5, 47–89 | p < 0.001 |
*Identical to % with this particular sample size
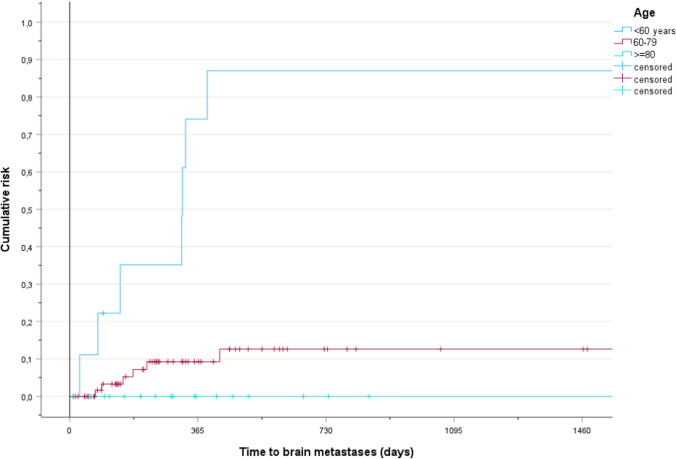
Risk factors for brain metastases were identified (Table 1). The planning target volume (PTV) size was statistically significant when analyzed by median, p = 0.03. All events occurred in patients with PTV size ≥ 155 ccm (Fig. 1, p = 0.14). N stage was statistically significant. All events occurred in patients with N2-3 disease (Fig. 2, p = 0.02). Age was statistically significant. Brain metastases were not diagnosed in patients aged 80 years or older (Fig. 3, p < 0.001). Median age was 59 years (brain metastases) and 74 years (no brain metastases), respectively. Non-significant trends were observed for two parameters. Patients with stage III disease had higher rates than their peers in stage II. Those with adenocarcinoma had higher rates than patients with squamous cell carcinoma. The Cox regression analysis showed that age was the only independent predictor of risk, p < 0.001 both as continuous and three-tiered variable. PTV size, N stage, stage and histology had p-values of 0.2 or higher.
Fig. 1.
Time to development of brain metastases in patients with smaller or larger planning target volumes, p = 0.14
Fig. 2.
Time to development of brain metastases in patients with lower or higher N stage, p = 0.02
Fig. 3.
Time to development of brain metastases in patients in different age groups, p < 0.001
Discussion
To the best of our knowledge, this is the only contemporary study examining the risk of brain metastases development in patients with NSCLC who received lower doses of radiation than the current curative standard. Aside from old age and large PTV size, which were major reasons for choosing a non-curative approach, this patient cohort is comparable to those curatively treated by other groups (discussion below). The actuarial risk of brain metastases was in the expected range (up to 23%, also discussed below) and in most cases limited spread was found, i.e. 1–3 lesions, despite lack of surveillance imaging. All events appeared early during follow-up (within 15 months; the majority in the first year), suggesting that initially undetectable micrometastases represent the main source of brain relapse, in contrast to continuous seeding from persistent thoracic disease (in particular nodal N2-3 disease), which in theory might occur after non-curative locoregional therapy. In our experience, thoracic disease progression often occurs in the second year, when relatively few patients developed brain metastases. The early appearance of brain metastases suggests that long-term brain imaging surveillance might not be warranted.
In a recent study of 310 radically treated stage III patients, 52 (16.8%) developed brain metastases [22]. Three clinical variables (age, histology, and nodal gross tumor volume (GTVn)) and five radiomics features were significantly associated with brain metastases. Radiomic features measuring tumor heterogeneity were the most relevant. The clinical model identified three significant factors associated with brain metastases: a higher age (> 60 years) was protective, while non-squamous histology, and a larger GTVn were associated with an increased risk. These findings are basically compatible with the present ones. However, GTVn was not routinely contoured in our palliatively treated patients. The incidence reported by Taugner et al. (16%) was comparable to the Zeng et al. study and the median time interval was 5 months in those who developed brain metastases [17].
Xu et al. studied 134 patients (stage II or III, definitive radiotherapy) and brain metastases occurred in 25 (18.7%) [23]. The 1-year and 3-year cumulative incidence were 10.5% and 20%, respectively. Patients with brain metastases had worse overall survival than those without. According to multivariate analysis, non-squamous histology (p < 0.001), biologically effective radiation dose (BED) < 72 Gy (p = 0.017), and PTV > 157.7 ccm (p = 0.043) were independent risk factors for brain metastases.
Farris et al. studied 219 patients (stage II or III, definitive chemoradiotherapy) and 39 (17.8%) developed brain metastases [24]. Ninety percent of these occurred within 2 years. Alhusaini et al. studied 279 patients (stage III, curative intent), yet only 160 with adequate records were eligible for analysis [25]. After treatment, 23 patients (14%) received planned surveillance brain scans, initially after 6, followed by 12 and 24 months. The 2-year cumulative incidence of brain metastases was 17% and eventually 23% developed brain metastases (37 of 160). Patients with adenocarcinoma were at increased risk, compared to those with squamous cell carcinoma. Even if not statistically significant, a numerically higher 2-year incidence was found in patients who received regular surveillance brain imaging relative to those without planned scans. Both studies discussed in this paragraph indicated that a small proportion of brain metastases may appear after more than 2 years, i.e. later than in our study.
In a larger study, 838 patients (stage III, chemoradiotherapy) were included and 18.2% developed brain metastases [26]. Younger age, female sex, more advanced N-stage and adenocarcinoma histology were significant risk factors. The chemotherapy setting (concomitant versus sequential) had no influence on brain metastases development. According to Mitra et al. (n = 255, stage II or III, curative intent), age younger than 65 years, N3 nodal status and EGFR mutation were risk factors for brain metastases in univariate analyses [27]. The multivariate analysis confirmed the effect of EGFR mutation and advanced nodal stage, which were strongly associated with brain metastases development, while age was borderline significant (p = 0.05).
Finally, Chen et al. included 43 studies with more than 11,000 patients in a meta-analysis [28]. The following factors were significantly associated with an elevated risk of brain metastases in NSCLC patients (p < 0.05): female sex (odds ratio (OR) 1.32, 95% confidence interval (CI) 1.17–1.49, p < 0.00001), adenocarcinoma (OR 2.34, 95% CI: 1.76–3.11, p < 0.00001), higher overall cancer stage (OR 1.48, 95% CI 1.01–2.17, p = 0.04), N stage (OR 2.19, 95% CI 1.39–3.45, p = 0.0007), kirsten rat sarcoma viral oncogene (KRAS) mutation (OR 2.99, 95% CI 1.82–4.91, p < 0.00001), EGFR mutation (OR 1.88, 95% CI 1.26–2.80, p = 0.002), and higher serum levels of tumor markers such as carcinoembryonic antigen (CEA), which are not part of standard work-up in many countries, especially not in stage II and III.
Overall, combined evidence suggests that brain metastases are more common in patients with adenocarcinoma and higher N stage. The latter impacts overall stage as well as radiotherapy target volume size, i.e. parameters that sometimes were identified as additional predictors. Younger age was repeatedly but not uniformly reported to increase the risk of brain metastases. Tumor mutation status also impacts on development of brain metastases, but was not included in our study due to absence of relevant mutations, in particular EGFR. KRAS mutation analysis was not routinely performed in this cohort.
Furthermore, combined evidence suggests that brain metastases often shorten survival despite excellent local treatment options and more efficacious systemic therapy than in previous decades [29–31]. In this context, one has to be aware of the fact that brain metastases often are part of general dissemination, rather than isolated oligometastases. Attempts to establish prophylactic cranial irradiation (PCI; brain metastases prevention) have so far not resulted in a new standard of care [32, 33]. Both neurocognitive impairment after PCI and development of simultaneous extracranial metastases limiting survival may reduce the overall gain.
The present study has all the typical limitations of retrospective analyses, such as potential selection bias, non-standardized imaging for staging and variable follow-up intensity. It was performed in a geographical region where EGFR mutations and other targetable alterations are less common than elsewhere. Detailed mutation analyses and assessment of PD-L1 status were available in very few patients. Furthermore, data on development of extracranial metastases was not available. Collection of these data may be interesting in the present era of local treatment options for limited spread or oligoprogression. The size of the cohort and consequently number of events was limited, impacting on the ability to confirm statistically significant predictors in multivariate analysis. Nevertheless, relevant data from a previously understudied population was acquired, suggesting that brain metastases development may shorten survival even in patients not amenable to primary curative treatment. We have previously reported that performance status and N stage were significantly associated with overall survival after palliative radiotherapy in multivariate analyses, thereby guiding the choice of fractionation [34]. Appropriate selection is important, given that higher doses such as the CONRAD regimen may translate into survival well beyond 2 years, as already stated in the Results section. Additional studies in populations with defined PD-L1 and targetable mutation status appear warranted, especially if the patients receive systemic therapies that may diminish the risk of brain metastases.
Conclusions
The risk of brain metastases development was comparable to that reported in studies of curative chemoradiation. All events occurred within 15 months of follow-up, suggesting that long-term surveillance imaging may not be warranted. Patients younger than 60 years had a very high risk of brain metastases development.
Acknowledgements
Not applicable.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by C.N. The first draft of the manuscript was written by C.N. and S.G.A., L.S. & B.M. commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by UiT The Arctic University of Norway (incl University Hospital of North Norway).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. As a retrospective quality of care analysis, no approval from the Regional Committee for Medical Research Ethics Northern Norway (REK North, Tromsø, Norway) was necessary. The study was performed in the context of our already institutional review board (IRB)-approved longitudinal monitoring of NSCLC management. This research project was carried out according to our institutions’ guidelines and with permission to access the patients’ data. Written informed consent was received from all patients.
Consent for publication
Not applicable.
Competing interests
S.G.A. has received lecture fees from Merck, BMS, Astra Zeneca and Pfizer. B.M. has received lecture fees from BMS, Astra Zeneca, Gilead, Roche, Novartis and Pfizer. L.S. has received lecture fees from Pfizer, MSD, Janssen and Ipsen. LS har received consulting fees from MSD. C.N.: no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Su CC, Wu JT, Choi E, Myall NJ, Neal JW, Kurian AW, Stehr H, Wood D, Henry SM, Backhus LM, Leung AN, Wakelee HA, Han SS. Overall survival among patients with de novo stage IV metastatic and distant metastatic recurrent non-small cell lung cancer. JAMA Netw Open. 2023;6(9): e2335813. 10.1001/jamanetworkopen.2023.35813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregoire J. Guiding principles in the management of synchronous and metachronous primary non-small cell lung cancer. Thorac Surg Clin. 2021;31(3):237–54. 10.1016/j.thorsurg.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Miccio JA, Tian Z, Mahase SS, Lin C, Choi S, Zacharia BE, Sheehan JP, Brown PD, Trifiletti DM, Palmer JD, Wang M, Zaorsky NG. Estimating the risk of brain metastasis for patients newly diagnosed with cancer. Commun Med (Lond). 2024;4(1):27. 10.1038/s43856-024-00445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillespie CS, Mustafa MA, Richardson GE, Alam AM, Lee KS, Hughes DM, Escriu C, Zakaria R. Genomic alterations and the incidence of brain metastases in advanced and metastatic NSCLC: a systematic review and meta-analysis. J Thorac Oncol. 2023;18(12):1703–13. 10.1016/j.jtho.2023.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Hoppen L, Sarria GR, Kwok CS, Boda-Heggemann J, Buergy D, Ehmann M, Giordano FA, Fleckenstein J. Dosimetric benefits of adaptive radiation therapy for patients with stage III non-small cell lung cancer. Radiat Oncol. 2023;18(1):34. 10.1186/s13014-023-02222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wurstbauer K, Kazil M, Meinschad M, Pinter R, De Vries C, Clemens P, Kreuter C, Hernler T, Hitzl W, Cerkl P, Künzler T, De Vries A. Locally advanced NSCLC: a plea for sparing the ipsilateral normal lung-prospective, clinical trial with DART-bid (dose-differentiated accelerated radiation therapy, 1.8 Gy twice daily) by VMAT. Radiat Oncol. 2022;17(1):120. 10.1186/s13014-022-02083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simone CB 2nd, Bradley J, Chen AB, Daly ME, Louie AV, Robinson CG, Videtic GMM, Rodrigues G. ASTRO radiation therapy summary of the ASCO guideline on management of stage III non-small cell lung cancer. Pract Radiat Oncol. 2023;13(3):195–202. 10.1016/j.prro.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 8.King J, Patel K, Woolf D, Hatton MQ. The use of palliative radiotherapy in the treatment of lung cancer. Clin Oncol (R Coll Radiol). 2022;34(11):761–70. 10.1016/j.clon.2022.08.032. [DOI] [PubMed] [Google Scholar]
- 9.Lewis TS, Kennedy JA, Price GJ, Mee T, Woolf DK, Bayman NA, Chan C, Coote JH, Faivre-Finn C, Harris MA, Hudson AM, Pemberton LS, Salem A, Sheikh HY, Mistry HB, Cobben DCP. Palliative lung radiotherapy: higher dose leads to improved survival? Clin Oncol (R Coll Radiol). 2020;32(10):674–84. 10.1016/j.clon.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aires F, Rodrigues E, Marques M, Pinto M. Factors affecting survival after palliative radiotherapy in patients with lung cancer. Rep Pract Oncol Radiother. 2021;26(5):674–82. 10.5603/RPOR.a2021.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieder C, Imingen KS, Mannsaker B, Yobuta R. Palliative thoracic radiotherapy for non-small cell lung cancer: Is there any impact of target volume size on survival? Anticancer Res. 2021;41(1):355–8. 10.21873/anticanres.14783. [DOI] [PubMed] [Google Scholar]
- 12.Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467–75. 10.1038/bjc.2013.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller ED, Fisher JL, Haglund KE, Grecula JC, Xu-Welliver M, Bertino EM, He K, Shields PG, Carbone DP, Williams TM, Otterson GA, Bazan JG. Identifying patterns of care for elderly patients with non-surgically treated stage III non-small cell lung cancer: an analysis of the national cancer database. Radiat Oncol. 2018;13(1):196. 10.1186/s13014-018-1142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bezjak A. Palliative therapy for lung cancer. Semin Surg Oncol. 2003;21(2):138–47. 10.1002/ssu.10031. [DOI] [PubMed] [Google Scholar]
- 15.Kishi N, Matsuo Y, Shintani T, Ogura M, Mitsuyoshi T, Araki N, Fujii K, Okumura S, Nakamatsu K, Kishi T, Atsuta T, Sakamoto T, Ohtsu S, Katagiri T, Narabayashi M, Fujishiro S, Iizuka Y, Ozasa H, Hirai T, Mizowaki T, Kyoto Radiation Oncology Study Group (KROSG). Recurrence patterns and progression-free survival after chemoradiotherapy with or without consolidation durvalumab for stage III non-small cell lung cancer. J Radiat Res. 2023;64(1):142–53. 10.1093/jrr/rrac057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito H, Matsuo Y, Ohtsu S, Nishimura T, Terada Y, Sakamoto T, Mizowaki T. Impact of histology on patterns of failure and clinical outcomes in patients treated with definitive chemoradiotherapy for locally advanced non-small cell lung cancer. Int J Clin Oncol. 2020;25(2):274–81. 10.1007/s10147-019-01566-z. [DOI] [PubMed] [Google Scholar]
- 17.Taugner J, Eze C, Käsmann L, Roengvoraphoj O, Gennen K, Karin M, Petrukhnov O, Tufman A, Belka C, Manapov F. Pattern-of-failure and salvage treatment analysis after chemoradiotherapy for inoperable stage III non-small cell lung cancer. Radiat Oncol. 2020;15(1):148. 10.1186/s13014-020-01590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smyth EN, John J, Tiu RV, Willard MD, Beyrer JK, Bowman L, Sheffield KM, Han Y, Brastianos PK. Clinicogenomic factors and treatment patterns among patients with advanced non-small cell lung cancer with or without brain metastases in the United States. Oncologist. 2023;28(11):e1075–91. 10.1093/oncolo/oyad170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieder C, Hintz M, Oehlke O, Bilger A, Grosu AL. Validation of the graded prognostic assessment for lung cancer with brain metastases using molecular markers (lung-molGPA). Radiat Oncol. 2017;12(1):107. 10.1186/s13014-017-0844-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieder C, Guckenberger M, Gaspar LE, Rusthoven CG, De Ruysscher D, Sahgal A, Nguyen T, Grosu AL, Mehta MP. Management of patients with brain metastases from non-small cell lung cancer and adverse prognostic features: multi-national radiation treatment recommendations are heterogeneous. Radiat Oncol. 2019;14(1):33. 10.1186/s13014-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15(1):4–9. [PubMed] [Google Scholar]
- 22.Zeng H, Tohidinezhad F, De Ruysscher DKM, Willems YCP, Degens JHRJ, van Kampen-van den Boogaart VEM, Pitz C, Cortiula F, Brandts L, Hendriks LEL, Traverso A. The association of gross tumor volume and its radiomics features with brain metastases development in patients with radically treated stage III non-small cell lung cancer. Cancers (Basel). 2023;15(11):3010. 10.3390/cancers15113010. [DOI] [PMC free article] [PubMed]
- 23.Xu X, Chen G, Fan S, Zhang Q, Huang W, Chen J, Ji W, Ma J, He J. Risk factors for brain metastases in locally advanced non-small cell lung cancer patients treated with radical radiotherapy. J Thorac Dis. 2024;16(1):479–90. 10.21037/jtd-23-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farris JC, Hughes RT, Razavian NB, Pearce JB, Snavely AC, Chan MD, Steber CR, Leyrer CM, Bunch PM, Willey JS, Farris MK. Brain metastasis incidence and patterns of presentation after definitive treatment of locally advanced non-small cell lung cancer: a potential argument for brain magnetic resonance imaging surveillance. Adv Radiat Oncol. 2022;8(3): 101058. 10.1016/j.adro.2022.101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alhusaini S, Lanman TA, Ko RB, Therkelsen KE, Eyben RV, Diehn M, Soltys SG, Pollom EL, Chin A, Vitzthum L, Wakelee HA, Padda SK, Ramchandran K, Loo BW Jr, Neal JW, Nagpal S. Real-world risk of brain metastases in stage III non-small cell lung cancer in the era of PET and MRI staging. Front Oncol. 2023;13:1139940. 10.3389/fonc.2023.1139940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendriks LEL, Brouns JWM, Amini M, Uyterlinde W, Wijsman R, Bussink J, Biesma B, Oei SB, Stigt JA, Bootsma GP, Belderbos JSA, De Ruysscher DKM, Van den Heuvel MM, Dingemans AC. Development of symptomatic brain metastases after chemoradiotherapy for stage III non-small cell lung cancer: does the type of chemotherapy regimen matter? Lung Cancer. 2016;101:68–75. 10.1016/j.lungcan.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Mitra D, Chen YH, Li R, Hermann G, Atkins K, Kozono D, Baldini EH, Aizer A, Chukwueke U, Mak RH. EGFR mutant locally advanced non-small cell lung cancer is at increased risk of brain metastasis. Clin Transl Radiat Oncol. 2019;18:32–8. 10.1016/j.ctro.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, Hua X, Jia J, Wu Y, Wei S, Xu L, Han S, Zhang H, Zhu X. Risk factors for brain metastases in patients with non-small cell lung cancer: a meta-analysis of 43 studies. Ann Palliat Med. 2021;10(4):3657–72. 10.21037/apm-20-1722. [DOI] [PubMed] [Google Scholar]
- 29.Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, Dunn IF, Gaspar LE, Gatson NTN, Gondi V, Jordan JT, Lassman AB, Maues J, Mohile N, Redjal N, Stevens G, Sulman E, van den Bent M, Wallace HJ, Weinberg JS, Zadeh G, Schiff D. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40(5):492–516. 10.1200/JCO.21.02314.Erratum.In:JClinOncol.2022;40(12):1392. [DOI] [PubMed] [Google Scholar]
- 30.Nieder C, Aanes SG, Haukland E. Primary systemic therapy for patients with brain metastases from lung cancer ineligible for targeted agents. J Cancer Res Clin Oncol. 2022;148(11):3109–16. 10.1007/s00432-022-03919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popat S, Ahn MJ, Ekman S, Leighl NB, Ramalingam SS, Reungwetwattana T, Siva S, Tsuboi M, Wu YL, Yang JC. Osimertinib for EGFR-mutant non-small-cell lung cancer central nervous system metastases: current evidence and future perspectives on therapeutic strategies. Target Oncol. 2023;18(1):9–24. 10.1007/s11523-022-00941-7. Erratum in: Target Oncol. 2023 Feb 8. [DOI] [PubMed]
- 32.Maldonado F, Gonzalez-Ling A, Oñate-Ocaña LF, Cabrera-Miranda LA, Zatarain-Barrón ZL, Turcott JG, Flores-Estrada D, Lozano-Ruiz F, Cacho-Díaz B, Arrieta O. Prophylactic cranial irradiation in patients with high-risk metastatic non-small cell lung cancer: quality of life and neurocognitive analysis of a randomized phase II study. Int J Radiat Oncol Biol Phys. 2021;111(1):81–92. 10.1016/j.ijrobp.2021.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Witlox WJA, Ramaekers BLT, Lacas B, Le Pechoux C, Pignon JP, Sun A, Wang SY, Hu C, Redman M, van der Noort V, Li N, Guckenberger M, van Tinteren H, Groen HJM, Joore MA, De Ruysscher DKM. Individual patient data meta-analysis of prophylactic cranial irradiation in locally advanced non-small cell lung cancer. Radiother Oncol. 2021;158:40–7. 10.1016/j.radonc.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Nieder C, Tollåli T, Haukland E, Reigstad A, Flatøy LR, Dalhaug A. External validation of a prognostic score for patients receiving palliative thoracic radiotherapy for lung cancer. Clin Lung Cancer. 2017;18(4):e297–301. 10.1016/j.cllc.2017.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.