Abstract
To investigate long-term treatment outcomes of polypoidal choroidal vasculopathy (PCV) with classic type leakage and to compare the outcomes with those of PCV without classic type leakage. This retrospective study included 153 patients diagnosed with PCV and treated with anti-vascular endothelial growth factor (VEGF). Patients showing classic type leakage on fluorescein angiography were included in the classic type leakage group (N = 40, 26.1%), and those without classic type leakage were included in the occult group (N = 113, 73.9%). The best-corrected visual acuity (BCVA) at baseline and 24 months, changes in BCVA, incidence of fibrosis, and lesion reactivation after initial loading injections were compared between the two groups. There was no significant difference in the baseline BCVA between the classic type leakage group (mean logarithm of minimal angle of resolution 0.67 ± 0.53[Snellen equivalents = 20/93]) and the occult group (0.55 ± 0.49[20/70])(P = 0.639). In addition, the BCVA at 24 months (0.44 ± 0.53[20/55] vs. 0.38 ± 0.41[20/47])(P = 1.000), changes in BCVA (0.22 ± 0.42 improvement[2.2 lines] vs. 0.16 ± 0.36 improvement[1.6 lines]) (P = 0.366), and lesion reactivation (P = 0.787) did not differ between the two groups. The incidence of fibrosis was higher in the classic type leakage group (37.5%) than in the occult group (14.2%) (P = 0.002). Although the incidence of fibrosis was higher in PCVs with classic type leakage, the overall treatments were not significantly different between PCVs with and without classic type leakage. In addition, substantial visual improvement was noted at 24 months, suggesting that PCVs with classic type leakage can be effectively treated with anti-VEGF therapy.
Keywords: Age-related macular degeneration, Anti-vascular endothelial growth factor, Classic type leakage, Polypoidal choroidal vasculopathy, Outcomes
Subject terms: Eyelid diseases, Retinal diseases
Introduction
Polypoidal choroidal vasculopathy (PCV) is a subtype of choroidal neovascularization characterized by polypoidal lesions and branching vascular networks on indocyanine green angiography (ICGA)1,2. In general, PCV lesions are located beneath the RPE layer and are thus considered a variant of type 1 macular neovascularization (MNV)3. In addition, the fluorescein angiography (FA) findings of PCV often exhibit occult choroidal neovascularization (CNV) pattern2. However, previous studies have demonstrated that some eyes diagnosed with PCV exhibit classic type leakage on fluorescein angiography4–7, demonstrating that sub-retinal lesions can develop in PCV.
Type 2 MNV generally responds well to anti-VEGF therapy, accompanying significant visual gain8,9. However, when compared to type 1 MNV, long-term visual outcomes are relatively inferior in type 2 MNV8,10,11. Type 2 MNV often exhibits classic type leakage on fluorescein angiography (FA). Therefore, the presence of classic type leakage, which may indicate the presence of type 2 MNV in some patients, could influence the treatment outcomes of PCV.
In fact, previous studies have indicated a potential difference in treatment outcomes between PCV with and without classic type leakage. In a study by Tamura et al., patients showed generally poor visual outcomes after photodynamic therapy (PDT)5. In a more recent study by Izumi et al., vascular subretinal hyperreflective material (SHRM) in PCV with classic CNV type leakage was found to be more susceptible to developing fibrosis than avascular SHRM following short-term anti-vascular endothelial growth factor (VEGF) therapy7.
Anti-VEGF therapy is effective in treating PCV12 and is currently considered the mainstay of treatment. However, it is difficult to expect a complete cure for PCV, necessitating long-term treatment. The long-term treatment outcomes of anti-VEGF for patients with PCV with classic type leakage have not yet been fully elucidated. In the present study, we aimed to address this topic. We additionally investigated whether there is any difference in the outcomes between PCVs with and without classic type leakage.
Materials and methods
This retrospective observational study was conducted at a single center (Kim’s Eye Hospital, Seoul, South Korea). The study was approved by the Institutional Review Board of Kim’s Eye Hospital and was conducted in accordance with the tenets of the Declaration of Helsinki. Owing to the retrospective nature of this study, the need for informed consent was waived (Kim’s Eye Hospital IRB, Seoul, South Korea).
Study participants and treatment
The inclusion criteria for the present study were as follows: (1) treatment-naïve patients diagnosed with PCV between January 2020 and December 2020; (2) patients who were initially received three loading injections of ranibizumab (0.5 mg/0.05 mL of Lucentis; Genentech, South San Francisco, CA, USA) or aflibercept (2.0 mg/0.05 mL of Eylea; Regeneron, Tarrytown, NY, USA); (3) patients who underwent further treatment on an as-needed basis after the initial injections.; (4) myopia lesser than − 6.0 diopters. Patients who met any of the following exclusion criteria were not included: (1) extensive macular hemorrhage that hinders lesion classification; (2) previous history of glaucoma surgery or vitreoretinal surgery; and (3) follow-up period of less than 24 months after the initial diagnosis were excluded.
The treatment and follow-up schedules resembled those employed in our prior studies13,14. In this retrospective study, there were no specific criteria for selecting anti-VEGF agents or treatment methods. These decisions were based on the preferences of the treating physicians. In re-treatment after initial loading injections, bevacizumab (1.25 mg/0.05 mL of Avastin; Genentech, South San Francisco, CA, USA) or brolucizumab (6.0 mg/0.05 ml of Beovu; Novartis, Basel, Switzerland) was also used. During the initial year, patients underwent regular follow-up assessments, typically scheduled every 1–2 months. For certain patients, the follow-up interval was extended to three months. In the subsequent year, the follow-up interval was extended to 4 months based on the judgment of the treating physicians. When the treating physician deemed a more effective treatment necessary, the treatment approach was switched to a treat-and-extend regimen. There were no specific criteria for selecting different anti-VEGF agents or adjusting follow-up schedules based on the presence or absence of a classic type leakage.
Outcome measures
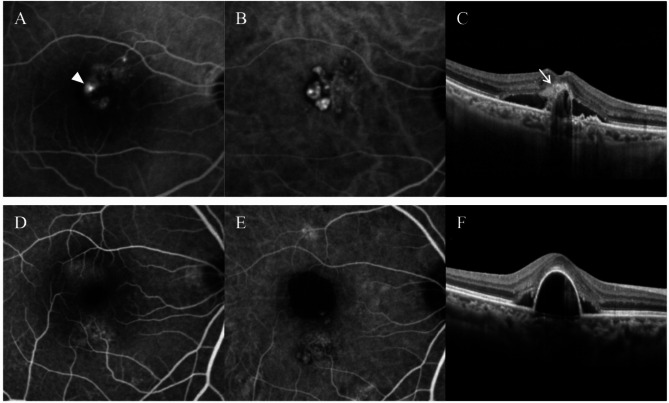
Patients were classified into two groups, according to the FA characteristics: classic type leakage group = patients exhibiting classic type leakage on FA (Fig. 1A–C) vs. occult group = patients without classic type leakage on FA (Fig. 1D–F). A classic type leakage was diagnosed when intense bright fluorescence was observed in the early phases of the angiogram, which leaked in the late phases15.
Fig. 1.
Representative cases showing polypoidal choroidal vasculopathy with (A-C) and without (D-F) classic type leakage. Classic type leakage was observed on fluorescein angiography (A, arrowhead), and subretinal hyper-reflective material was noted at the site of classic leakage on optical coherence tomography (C, arrow). (A,D) = fluorescein angiography; (B,E) = indocyanine-green angiography; (C,F) = optical coherence tomography.
The following characteristics were compared between the two groups: age, sex, hypertension, diabetes mellitus, central retinal thickness, presence of ≥ 1 disc area extent of subretinal hemorrhage, type of anti-VEGF agent used for initial loading injections, the total number of anti-VEGF injections during the 24 months follow-up period.
The best-corrected visual acuities (BCVA) were measured at diagnosis, three months (one month after the initial loading injections), and 24 months. Change in BCVA during 24 months, incidence of fibrosis, and lesion reactivation after initial loading injections were compared between the two groups. The BCVA at diagnosis, at three months and 24 months, were compared between patients with and without fibrosis.
Fibrosis was diagnosed based on the criteria described by Daniel et al.16 Fibrosis was defined as obvious white or yellow mounds of fibrous-appearing tissue that were well-defined in shape and appeared solid on color fundus photography images. The diagnosis of fibrosis was further confirmed by the presence of subretinal hyperreflective lesions on OCT imaging17. Although Daniel et al. used both fundus photographs and FAG to define fibrosis16, in the current study we relied solely on fundus photographs for diagnosis.
Lesion reactivation was defined as the recurrence of subretinal or intraretinal fluid detected on OCT examination, or new-onset retinal hemorrhage in the macular area. Recurrence or an increase in the height of pigment epithelial detachment was not regarded as lesion reactivation. We did not specifically evaluate whether lesion reactivation originated from classic lesions.
Patients who met the eligibility criteria but were not included in the study owing to loss to follow-up before 24 months were compared with included patients in terms of age, sex, the proportion of eyes exhibiting a classic type leakage, BCVA, CRT, ≥ 1 disc area extent of subretinal hemorrhage, and the type of anti-VEGF agent used for initial loading injections. In the classic type leakage group, 24-month BCVA was compared between eyes with or without foveal fibrosis.
In the classic type leakage group, the degree of improvement in the BCVA during the 24-month follow-up period and the incidence of fovea-involving fibrosis were compared between eyes that exhibited more than one classic type leakage and those that did not. The number of injections and the degree of improvement in BCVA were compared between eyes with or without submacular hemorrhage. To identify the factors associated with the development of fibrosis, multivariate analysis was performed using the following factors: age, sex, hypertension, diabetes mellitus, presence of classic type leakage, baseline CRT and BCVA, presence of subretinal hemorrhage, type of anti-VEGF agent used for the initial loading injections, and number of anti-VEGF injections.
Statistical methods
The data are presented as the mean ± standard deviation or No. (%) where applicable. The statistical analyses were performed using a commercially available software package (SPSS, ver. 12.0 for Windows; IBM Corporation, Armonk, NY, USA). Comparisons of characteristics between the classic type leakage and occult groups were performed using independent samples t-test, chi-square test, or Fisher’s exact test. The difference in BCVA between the two groups at each time point was analyzed using independent samples t-test with a Bonferroni’s correction. The difference in lesion reactivation between the two groups was analyzed using Kaplan-Meier survival analysis. Multivariate analysis was performed using binary logistic regression. Comparison of BCVA between eyes with or without foveal fibrosis in the classic type leakage group was performed using the Mann-Whitney U test. Comparisons between eyes with or without more than one classic type leakage were performed using the Mann-Whitney U test and Fisher’s exact test. Comparisons between eyes with or without submacular hemorrhage were performed using an independent sample t-test. Binary logistic regression analysis was performed to identify the factors associated with fibrosis. P-values less than 0.05 were considered significant.
Results
A total of 153 patients (119 men and 34 women) were included (Table 1).
Table 1.
Baseline characteristics of the included patients (n = 153).
| Characteristics | Values |
|---|---|
| Age | 68.2 ± 8.4 |
| Sex | |
| Men | 119 (77.8%) |
| Women | 34 (22.2%) |
| Hypertension | 68 (44.4%) |
| Diabetes mellitus | 31 (20.3%) |
| Angiographic characteristics | |
| Classic type leakage (+) | 40 (26.1%) |
| Classic type leakage (-) | 113 (73.9%) |
| Central retinal thickness, µm | 446.9 ± 154.3 |
| ≥ 1 disc area extent of subretinal hemorrhage | 44 (28.8%) |
| Type of anti-VEGF agent used for initial loading injections | |
| Ranibizumab | 17 (11.1%) |
| Aflibercept | 136 (88.9%) |
Data are presented as mean ± standard deviation or number (%) where applicable.
Abbreviations: VEGF, vascular endothelial growth factor.
The number of patients excluded based on the exclusion criteria was as follows: (1) extensive macular hemorrhage that hinders lesion classification, 9 eyes; (2) previous history of glaucoma surgery or vitreoretinal surgery, 5 eyes; and (3) follow-up period of less than 24 months after the initial diagnosis, 32 eyes.
On FA occult type leakage was noted in all 153 patients. The classic type leakage was noted in 40 patients (26.1%), and these patients were included in the classic type leakage group. The remaining 113 patients (73.9%) were included in the occult group. In the classic type leakage group, subretinal hyper-reflective material (SHRM) was noted in all 40 patients at the location of classic type leakage on FA.
Table 2 summarizes the results of comparisons between the classic type leakage group and the occult group.
Table 2.
Comparison of characteristics between the classic type leakage group and the occult group.
| Characteristics | Classic type leakage group (n = 40) | Occult group (n = 113) |
p-value |
|---|---|---|---|
| Age | 68.9 ± 9.5 | 67.9 ± 8.0 | 0.562* |
| Sex | 0.694† | ||
| Men | 32 (80.0%) | 87 (76.9%) | |
| Women | 8 (20.0%) | 26 (23.0%) | |
| Hypertension | 18 (45.0%) | 50 (44.2%) | 0.934† |
| Diabetes mellitus | 6 (15.0%) | 25 (22.1%) | 0.335† |
| Central retinal thickness, µm | 460.9 ± 128.2 | 442.1 ± 162.7 | 0.510* |
| ≥ 1 disc area extent of subretinal hemorrhage | 9 (22.5%) | 35 (30.9%) | 0.309† |
| Type of anti-VEGF agent used for initial loading injections | 0.149‡ | ||
| Ranibizumab | 7 (17.5%) | 10 (8.8%) | |
| Aflibercept | 33 (82.5%) | 103 (91.2%) | |
| No. of VEGF injections | 6.2 ± 2.9 | 6.2 ± 3.0 | 0.872* |
Data are presented as mean ± standard deviation or No. (%) where applicable.
Abbreviations: VEGF, vascular endothelial growth factor.
*Statistical analysis with independent samples t-test.
†Statistical analysis using the chi-square test.
‡Statistical analysis using Fisher’s exact test.
There was no difference in age (P = 0.562), sex (P = 0.694), hypertension (P = 0.934), diabetes mellitus (P = 0.335), central retinal thickness (P = 0.510), the incidence of ≥ 1 disc area extent of subretinal hemorrhage (P = 0.309), type of anti-VEGF agent used for initial loading injections (P = 0.149), and the total number of anti-VEGF injections during 24 months (P = 0.872).
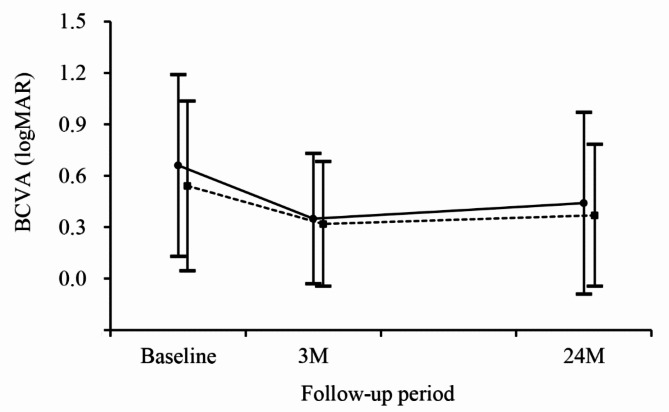
In the classic type leakage group, the mean BCVA was 0.67 ± 0.53 (Snellen equivalents = 20/93) at baseline, 0.35 ± 0.38 (20/44) at three months, and 0.44 ± 0.53 (20/55) at 24 months (Fig. 2).
Fig. 2.
Changes in the logarithm of minimal angle of resolution (logMAR) best-corrected visual acuity (BCVA) in the classic type leakage group (solid group, N = 40) and the occult group (dotted line, N = 113), according to the follow-up period.
In the occult group, the BCVA was 0.55 ± 0.49 (20/70) at baseline, 0.33 ± 0.36 (20/42) at three months, and 0.38 ± 0.41 (20/47) at 24 months. There was no difference in the BCVA at baseline (P = 0.639), at three months (P = 1.000), and at 24 months (P = 1.000) between the two groups. The degree of visual improvement during a 24-month follow-up period was 0.22 ± 0.42 (2.2 lines) in the classic type leakage group and 0.16 ± 0.36 (1.6 lines) in the occult group (P = 0.366).
During the 24-month follow-up period, fibrosis were noted in 31 patients (20.3%), and fovea was involved in 20 (64.5%). In patients with fibrosis, the mean BCVA was 1.07 ± 0.59 (20/234) at baseline, 0.61 ± 0.47 (20/81) at three months, and 0.78 ± 0.61 (20/120) at 24 months. The values were 0.45 ± 0.39 (20/56) at baseline, 0.26 ± 0.29 (20/36) at three months, and 0.30 ± 0.33 (20/39) at 24 months for patients without the fibrosis. There was a significant difference in BCVA at baseline (P < 0.001), at three months (P < 0.001), and at 24 months (P < 0.001) between the two groups. The incidence of fibrosis was 37.5% (15 of 40 patients) in the classic type leakage group and 14.2% (16 of 113 patients) in the occult group (P = 0.002). Among the fibrosis cases, the incidence of the fovea-involving fibrosis was 66.7% (10 of 15 cases) in the classic type leakage group and 62.5% (10 of 26 cases) in the occult group. Figure 3 shows a representative case of fibrosis in the classic type leakage group.
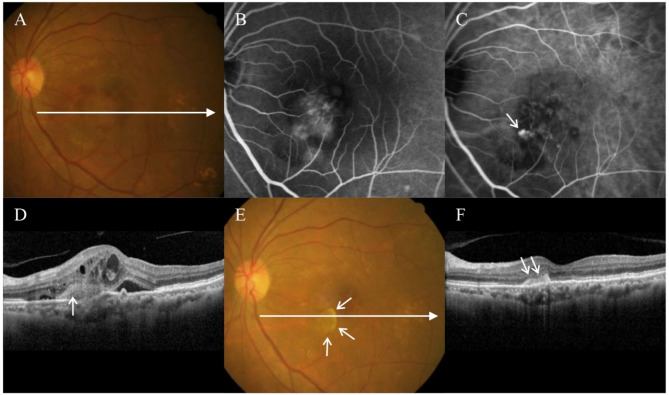
Fig. 3.
A 71-year-old patient was diagnosed with polypoidal choroidal vasculopathy. At diagnosis (A-C), classic type leakage was noted on fluorescein angiography (B), and polypoidal lesions were noted on indocyanine-green angiography (C, arrow). On optical coherence tomography (D), subretinal hyper-reflective material was observed at the site of classic leakage (arrow). The best-corrected visual acuity was measured as 20/100. The patient was treated using three intravitreal aflibercept injections. At three months (E,F), the BCVA has improved to 20/50 with the complete resolution of retinal fluid. However, a fovea-sparing fibrosis has been noted (E,F, short arrows). (A,E) = fundus photography; (B) = fluorescein angiography; (C) = indocyanine-green angiography; (D,F) = optical coherence tomography. Long horizontal arrows on Figures (A) and (E) indicate the optical coherence tomography scanning line for Figures (D) and (F), respectively.
In the classic type leakage group, the BCVA at 24 months was 0.90 ± 0.84 in eyes with a foveal fibrosis (n = 10) and 0.28 ± 0.26 in those without (n = 30). BCVA was significantly worse in eyes with fibrosis than in those without (P = 0.002).
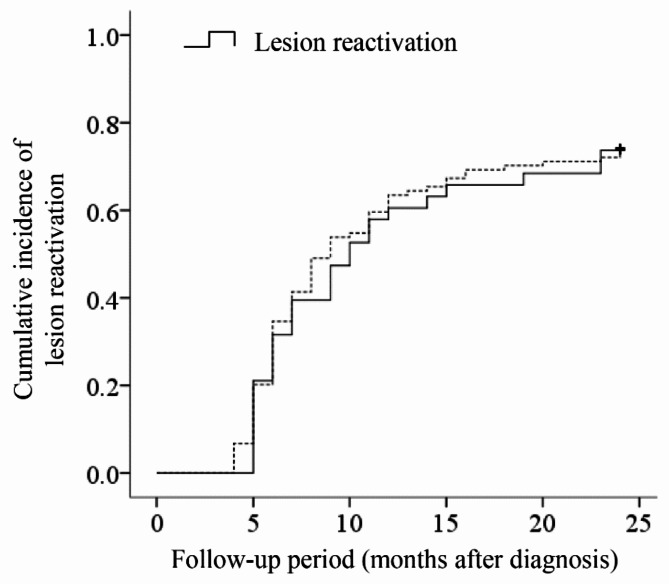
After initial loading injections, residual intraretinal subretinal fluid was noted in 3 (7.5%) in the classic type leakage group and 9 (7.9%) in the occult group (P = 1.000). Figure 4 shows the Kaplan-Meier graph showing lesion reactivation in 141 patients who exhibited complete fluid resolution after initial treatment.
Fig. 4.
A Kaplan-Meier curve showing the time-dependent cumulative incidence of lesion reactivation in the classic type leakage group (solid group, N = 40) and the occult group (dotted line, N = 113), according to the follow-up period.
The lesion reactivation was noted in 28 of 37 (75.7%) in the classic type leakage group and 77 of 104 (74.0%) in the occult group. There was no difference in lesion reactivation between the two groups (P = 0.787).
Thirty-two patients (32 eyes) met the eligibility criteria but were excluded from the outcome analysis owing to loss to follow-up within 24 months. The average age of these 32 patients was 70.7 ± 8.4 years, with a male-to-female ratio of 20:12. The proportion of eyes showing a classic type leakage was 31.3%, with a mean BCVA of 0.56 ± 0.39, a mean CRT of 446.7 ± 146.5 μm, and 18.8% exhibited ≥ 1 disc area extent of subretinal hemorrhage. For the initial loading injections, ranibizumab was used in 2 eyes and aflibercept in 30 eyes. When comparing the baseline characteristics of these 32 eyes (not included in the analysis) with the 153 eyes that were included, there were no significant differences in age (P = 0.135), sex (P = 0.275), the proportion of eyes exhibiting a classic type leakage (P = 0.554), BCVA (P = 0.996), CRT (P = 0.791), ≥ 1 disc area extent of subretinal hemorrhage (P = 0.246), or the type of anti-VEGF agent used for initial loading injections (P = 0.536).
In the classic type leakage group, more than one classic type leakage was noted in 8 eyes (20.0%). The amount of improvement in BCVA during the 24-month follow-up period was significantly greater in these 8 eyes (mean 0.46 ± 0.36) than in the remaining 32 eyes with only one classic type leakage (0.17 ± 0.42)(P = 0.017). There was no significant difference in the incidence of fovea-involving fibrosis between the two groups (25.0% vs. 28.1%, P = 1.000). The number of anti-VEGF injections in 44 eyes with submacular hemorrhage (mean 5.2 ± 2.7) was significantly lower than that in 109 eyes without (mean 6.6 ± 3.0)(P = 0.005). In addition, the amount of improvement of BCVA during the 24-month follow-up period was significantly greater in eyes with submacular hemorrhage (mean 0.32 ± 0.47) than those without (mean 0.12 ± 0.31)(P = 0.003).
In multivariate analysis, presence of classic type leakage (P = 0.002, β = 5.788, 95% confidence interval = 1.911 –17.533) and baseline BCVA (P < 0.001, β = 0.083, 95% confidence interval = 0.022 –0.311) were found to be significantly associated with the development of fibrosis. Other factors, including age (P = 0.461), sex (P = 0.313), hypertension (P = 0.252), diabetes mellitus (P = 0.207), baseline CRT (P = 0.718), presence of subretinal hemorrhage (P = 0.056), type of anti-VEGF agent used for initial loading injections (P = 0.186), and number of anti-VEGF injections (P = 0.085), were not associated with fibrosis.
Discussion
The histopathological findings of PCV differ from those of typical neovascular AMD. The histopathology of PCV is generally characterized by dilated choroidal vessels18–20, sub-RPE or sub/intra Bruch’s membrane neovascular lesions18–21, and Bruch’s membrane schisis21. In some cases, subretinal neovascular tissue is also observed in patients diagnosed with PCV18,22. However, not all PCV cases showing a classic type leakage on FAG are accompanied by subretinal neovascular tissue. PCV, which is a classic type leakage, often accompanies subretinal hyper-reflective material on OCT7. These features may result from subretinal neovascularization or the invasion of polypoidal lesions into the subretinal space by eroding the RPE7. However, this may also be caused by the presence of fibrinous materials without subretinal neovascularization (pseudo-classic CNV)4,8. Sia et al. reported that PCV lesions may exist above the level of the RPE (naked polyp) owing to discontinuity of the RPE and Bruch’s membrane23.
Tamura et al. speculated on the actual presence of type 2 MNV in cases of PCV displaying classic CNV features and exhibiting limited response to photodynamic therapy5. Izumi et al. demonstrated the usefulness of OCT-angiography in determining actual type 2 MNV from subretinal fibrin accumulation in PCV7. In the present study, OCT-angiography was not routinely performed. Therefore, it is not certain whether the classic type leakage observed in our study results from the presence of subretinal neovascular lesions or merely an exudation from lesions located under the RPE level.
A fibrosis is an infrequent finding in PCV, with the incidence of macular fibrosis reported to be approximately 5% after treatment24,25. In particular, submacular hemorrhage was associated with a high risk of fibrosis25. The incidence of fibrosis in our patients was relatively higher than in previous studies24,25. In the present study, subretinal hemorrhage, known to be associated with the high risk of fibrosis25,26, was noted in 28.8% of the cases included. In addition, while previous studies conducted short-term follow-ups ranging from 6 to 12 months, our study implemented a long-term 24-month follow-up observation. Moreover, we identified all the fibrosis within the macular area, irrespective of foveal involvement. We postulate that these factors could be attributed to the higher incidence of fibrosis in our patients.
In our study, there was a higher incidence of fibrosis in patients exhibiting classic type leakage compared to those who did not show it. This aligns with a previous study suggesting an association between a classic type leakage in PCV and the high risk of fibrosis7. Furthermore, this observation also aligns with the high risk of fibrosis in classic CNV reported in previous studies16. In the present study, patients with fibrosis showed worse visual outcomes than those without fibrosis. In our study, visual acuity measured at 24 months was slightly worse in the classic type leakage group than in the occult group. The difference in the incidence of fibrosis likely contributes to the difference in visual outcomes. Although no significant difference in visual acuity outcome was observed between the two groups, the possibility that significant results were not obtained owing to inadequate sample size cannot be excluded.
When focusing on the change in visual acuity, the classic type leakage group demonstrated a slightly greater degree of visual improvement than the occult group. Specifically, more than three lines of noticeable visual improvement were observed during the initial loading phase. While there was a decline in visual acuity over the course of the follow-up period, the mean final visual acuity was still more than two lines better than the baseline value.
In a study by Tamura et al., the visual acuity changed from mean logMAR 0.68 –0.81 to 0.35 –0.84 at mean 14.0 months after photodynamic therapy5. Three lines or greater of visual improvement was noted in 12 of 38 eyes (31.6%)27. In a study by Izumi et al., were patients underwent anti-VEGF therapy, the BCVA changed from mean logMAR 0.25–0.32 to 0.20–0.31 after a mean 3.0 months of treatment period7. In that study, the number of anti-VEGF antibodies used varied from one to three7. The visual outcomes in our patients were relatively superior to that of the previous studies5,7, with mean logMAR 0.32 improvement at 3 months and 0.23 improvement at 24 months. In our study, we administered three loading injections at one-month intervals immediately after diagnosis. Such intensive treatment is likely to have influenced the favorable visual outcomes.
Among the fibrosis observed in the classic type leakage group, 33.3% did not involve the fovea. In such cases, the occurrence of fibrosis might not have significantly impacted visual deterioration, suggesting that this aspect could have had some influence on long-term visual improvement in our patients.
In the present study, the classic type leakage group had a 37.5% incidence of fibrosis. In a study by Izumi et al., SHRM was noted on OCT in all the patients with PCV exhibiting classic CNV on FA7. Among them, 30% showed vascular SHRM on OCT-angiography, and the remaining 70% showed avascular SHRM. After anti-VEGF therapy, a fibrosis was observed in all eyes with vascular SHRM, while no fibrosis occurred in eyes with avascular SHRM7. In a study by Tamura et al., the outcome of PDT for PCV with actual type 2 MNV was poorer than those with pure fibrinous tissue without MNV5. Ida T. also emphasized the clinical significance of the findings by Tamura et al. for patient management28. Similar to the previous study7, SHRM was noted in all our patients with classic type leakage. However, since OCT-angiography was not routinely performed in the present study, the incidence of vascular and avascular SHRM could not be separately identified.
Although several investigators have attempted to investigate the potential difference in lesion reactivation among the different types of MNV9, no studies have analyzed differences in lesion reactivation based on the presence or absence of classic type leakage in PCV. In the present study, there was no difference in the baseline characteristics and lesion reactivation rate based on the presence or absence of the classic type leakage. Furthermore, the two groups had no difference in the total number of anti-VEGF injections during the follow-up period. These findings suggest that similar treatment approaches can be employed regardless of the presence of the classic type leakage in PCV.
We observed that a slightly higher proportion of patients in the occult group received initial aflibercept treatment than did those in the classic type leakage group. Both ranibizumab and aflibercept are useful in the treatment of PCV, but aflibercept has a slightly longer duration of action than ranibizumab27, and the recurrence rate after the initial injection is also slightly lower29. Therefore, although the difference in the choice of drugs between the two groups could have some impact on the evaluation of recurrence after initial treatment, considering the minimal difference in recurrence rates (74.0% vs. 75.7%), it is unlikely that it had a significant impact on the study results.
Given the retrospective nature of this study, it was not possible to fully control for various key variables. The patients’ baseline characteristics and choice of anti-VEGF agents were not controlled, and a strict protocol for retreatment after the initial loading injections was not established. Therefore, considering that these limitations could impact the comparison between the classic type leakage and occult groups, we compared the characteristics between the two groups to check for any significant differences that could influence the results.
The present study has the following limitations. First and most importantly, OCT-angiography findings were not assessed. Thus, the presence of actual type 2 MNV in the classic type leakage group could not be identified. Therefore, the results cannot demonstrate the differences in clinical features and treatment outcomes based on the presence of actual type 2 MNV in PCV. Instead, they can only be applied to cases showing classic type leakage on FA. Second, there were no specific criteria for selecting anti-VEGF agents or treatment methods and there were no separate treatment protocols based on the presence or absence of the classic type leakage. Although some patients may have been undertreated owing to the absence of strict treatment protocols, it is unlikely that this aspect significantly influenced the results of our study, which analyzed the treatment outcomes of cases with and without the classic type leakage. Third, in certain cases with subretinal hemorrhage, thick hemorrhage may have obscured the identification of classic type leakage. Fourth, as the study included patients who completed a 24-month follow-up, the potential impact of selection bias on the results could not be entirely disregarded. Fifth, various types of anti-VEGF agents were used in a mixed manner for treatment. Sixth, as all the patients were treated with anti-VEGF monotherapy, the study result may not be valid for patients who underwent PDT. Seventh, a sample size calculation to estimate the appropriate sample size was not performed in this retrospective study. Based on the results from comparing visual acuity at diagnosis between the two groups, setting a significance level of α = 0.05 and a power of β = 0.20 (power = 0.80) would yield a sample size of 284 individuals per group. This indicates that the actual number of patients included in this study was much smaller than the required sample size. Eighth, FAG alone demonstrates a relatively lower performance in accurately identifying the classic type leakage than OCT-angiography7. Therefore, relying solely on FAG for lesion classification is a limitation of this study. Lastly, all the patients were of Korean ethnicity.
In conclusion, the 24-month visual acuity outcomes of PCVs with classic type leakage on FA were slightly inferior yet not significantly different from those without classic type leakage. While the incidence of fibrosis was higher in PCVs with classic type leakage, one-third of these fibroses were outside the foveal region. In addition, the presence of classic type leakage did not affect lesion reactivation following the initial treatment. These findings suggest that anti-VEGF therapy can effectively treat PCV with classic type leakage. The long-term outcomes of this condition warrant further investigation.
Acknowledgements
The treatment and follow-up schedules in this study resembled those employed in previous studies (Kim JH, Kim JW, Kim CG (2023) Influence of lesion location on lesion reactivation after initial treatment in neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 261:3139-3148; Kim JH, Kim JW, Kim CG (2022) Comparison of 24-month treatment outcomes between as-needed treatment and switching to treat-and-extend in type 3 macular neovascularization. Sci Rep 12:22546).
Author contributions
Involved in conception and design (J.H.K.); acquisition of data (J.H.K., S.M.P., C.G.K., J.W.K.); analysis and interpretation (J.H.K., S.M.P., C.G.K., J.W.K.); drafting the article (J.H.K., S.M.P.); revising the article critically for important intellectual content (J. H. K.); and final approval of the article (J.H.K., S.M.P., C.G.K., J.W.K.). J.H.K. and S.M.P. contributed equally to this work and are considered co–first authors.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jae Hui Kim and Sang Min Park.
References
- 1.Yannuzzi, L. A., Sorenson, J., Spaide, R. F. & Lipson, B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina10, 1–8 (1990). [PubMed] [Google Scholar]
- 2.Spaide, R. F., Yannuzzi, L. A., Slakter, J. S., Sorenson, J. & Orlach, D. A. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina15, 100–110 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Dansingani, K. K., Gal-Or, O., Sadda, S. R., Yannuzzi, L. A. & Freund, K. B. Understanding aneurysmal type 1 neovascularization (polypoidal choroidal vasculopathy): a lesson in the taxonomy of ‘expanded Spectra’ - a review. Clin. Exp. Ophthalmol.46, 189–200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otsuji, T. et al. Evaluation of cases of polypoidal choroidal vasculopathy showing classic choroidal neovascularization in their natural course. Nippon Ganka Gakkai Zasshi110, 454–461 (2006). [PubMed] [Google Scholar]
- 5.Tamura, H. et al. Polypoidal choroidal vasculopathy appearing as classic choroidal neovascularisation on fluorescein angiography. Br. J. Ophthalmol.91, 1152–1159 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang, S., Shi, X., Rosenfeld, P. J. & Li, X. Type 2 choroidal neovascularisation in polypoidal choroidal vasculopathy: a retrospective case series. Br. J. Ophthalmol.102, 1570–1574 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Izumi, T., Koizumi, H., Maruko, I., Hasegawa, T. & Iida, T. Optical coherence tomography angiography findings of classic choroidal neovascularization in polypoidal choroidal vasculopathy. Retina42, 123–128 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Freund, K. B. et al. Macular neovascularization lesion type and vision outcomes in neovascular age-related macular degeneration: post hoc analysis of HARBOR. Graefes Arch. Clin. Exp. Ophthalmol.260, 2437–2447 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, J. H., Kim, J. W. & Kim, C. G. Difference in lesion reactivation between pure type 2 and mixed type 1 and 2 macular neovascularization and its influence on long-term treatment outcomes. Semin Ophthalmol.38, 358–364 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Chae, B. et al. Baseline predictors for good versus poor visual outcomes in the treatment of neovascular age-related macular degeneration with intravitreal anti-VEGF therapy. Invest. Ophthalmol. Vis. Sci.56, 5040–5047 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Mrejen, S. et al. Long-term visual outcomes for a treat and extend anti-vascular endothelial growth factor regimen in eyes with neovascular age-related macular degeneration. J. Clin. Med.4, 1380–1402 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, W. K. et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET study: a randomized clinical trial. JAMA Ophthalmol.136, 786–793 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, J. H., Kim, J. W. & Kim, C. G. Influence of lesion location on lesion reactivation after initial treatment in neovascular age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol.261, 3139–3148 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Kim, J. H., Kim, J. W. & Kim, C. G. Comparison of 24-month treatment outcomes between as-needed treatment and switching to treat-and-extend in type 3 macular neovascularization. Sci. Rep.12, 22546 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration. guidelines for evaluation and treatment in the macular photocoagulation study. Arch. Ophthalmol.109, 1242–1257 (1991). [PubMed] [Google Scholar]
- 16.Daniel, E. et al. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology121, 656–666 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souied, E. H. et al. Spectral-domain optical coherence tomography analysis of fibrotic lesions in neovascular age-related macular degeneration. Am. J. Ophthalmol.214, 151–171 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Nakajima, M., Yuzawa, M., Shimada, H. & Mori, R. Correlation between indocyanine green angiographic findings and histopathology of polypoidal choroidal vasculopathy. Jpn J. Ophthalmol.48, 249–255 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Kuroiwa, S., Tateiwa, H., Hisatomi, T., Ishibashi, T. & Yoshimura, N. Pathological features of surgically excised polypoidal choroidal vasculopathy membranes. Clin. Exp. Ophthalmol.32, 297–302 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Terasaki, H., Miyake, Y., Suzuki, T., Nakamura, M. & Nagasaka, T. Polypoidal choroidal vasculopathy treated with macular translocation: Clinical pathological correlation. Br. J. Ophthalmol.86, 321–327 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, G. et al. Clinicopathological study of the polypoidal lesions of polypoidal choroidal vasculopathy. Graefes Arch. Clin. Exp. Ophthalmol.260, 2369–2377 (2022). [DOI] [PubMed] [Google Scholar]
- 22.MacCumber, M. W. et al. Clinicopathologic correlation of the multiple recurrent serosanguineous retinal pigment epithelial detachments syndrome. Retina14, 143–152 (1994). [DOI] [PubMed] [Google Scholar]
- 23.Sia, D. I., Ebneter, A., Sinkar, S. & Gilhotra, J. Polypoidal choroidal vasculopathy: naked polyp. Int. Ophthalmol.33, 67–69 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Koh, A. et al. EVEREST study: Efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina32, 1453–1464 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Kim, J. H. et al. Morphologic features associated with fibrotic scarring after anti-vascular endothelial growth factor therapy in polypoidal choroidal vasculopathy. Retina38, 2168–2176 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Daniel, E. et al. Development and course of scars in the comparison of age-related macular degeneration treatments trials. Ophthalmology125, 1037–1046 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niwa, Y., Kakinoki, M., Sawada, T., Wang, X. & Ohji, M. Ranibizumab and aflibercept: Intraocular pharmacokinetics and their effects on aqueous VEGF level in vitrectomized and nonvitrectomized macaque eyes. Invest. Ophthalmol. Vis. Sci.56, 6501–6505 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Iida, T. Polypoidal choroidal vasculopathy with an appearance similar to classic choroidal neovascularisation on fluorescein angiography. Br. J. Ophthalmol.91, 1103–1104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, J. H., Chang, Y. S., Lee, D. W., Kim, C. G. & Kim, J. W. Incidence and timing of the first recurrence in neovascular age-related macular degeneration: Comparison between ranibizumab and aflibercept. J. Ocul Pharmacol. Ther.33, 445–451 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.