Abstract
Rabies virus (RV) of the Rhabdoviridae family grows in alpha/beta interferon (IFN)-competent cells, suggesting the existence of viral mechanisms preventing IFN gene expression. We here identify the viral phosphoprotein P as the responsible IFN antagonist. The critical involvement of P was first suggested by the observation that an RV expressing an enhanced green fluorescent protein (eGFP)-P fusion protein (SAD eGFP-P) (S. Finke, K. Brzózka, and K. K. Conzelmann, J. Virol. 78:12333-12343, 2004) was eliminated in IFN-competent HEp-2 cell cultures, in contrast to wild-type (wt) RV or an RV replicon lacking the genes for matrix protein and glycoprotein. SAD eGFP-P induced transcription of the IFN-β gene and expression of the IFN-responsive MxA and STAT-1 genes. Similarly, an RV expressing low levels of P, which was generated by moving the P gene to a promoter-distal gene position (SAD ΔPLP), lost the ability to prevent IFN induction. The analysis of RV mutants lacking expression of truncated P proteins P2, P3, or P4, which are expressed from internal AUG codons of the wt RV P open reading frame, further showed that full-length P is competent in suppressing IFN-β gene expression. In contrast to wt RV, the IFN-inducing SAD ΔPLP caused S386 phosphorylation, dimerization, and transcriptional activity of IFN regulatory factor 3 (IRF-3). Phosphorylation of IRF-3 by TANK-binding kinase-1 expressed from transfected plasmids was abolished in wt RV-infected cells or by cotransfection of P-encoding plasmids. Thus, RV P is necessary and sufficient to prevent a critical IFN response in virus-infected cells by targeting activation of IRF-3 by an upstream kinase.
The alpha/beta interferon (IFN) system, comprising IFN-β and the IFN-α family, represents a crucial defense element of higher organisms that activates both innate and adaptive immunity (for reviews, see references 2, 3, 26, and 35). IFN expression is tightly controlled by latent transcription factors, which are activated upon recognition of intruding viruses by cytoplasmic receptors that sense viral double-stranded RNA (dsRNA) such as retinoic acid-inducible gene I (RIG-I) (58) or through Toll-like receptors sensing exogenous ligands (2). The key factor for initiating an IFN response is interferon regulatory factor 3 (IRF-3), which is constitutively expressed in the cytoplasm of most cell types. Latent IRF-3 is activated by phosphorylation at C-terminal serine residues and then can form dimers that are recruited to the IFN-β enhancer as part of a protein complex that includes the transcription factors ATF-2/c-Jun and NF-κB and coactivators p300/CBP (38, 57, 59). As shown recently, the critical IRF-3 phosphorylation step is executed by kinases of the IKK family, i.e., TANK-binding kinase 1 (TBK-1) which is constitutively expressed, and the inducible IKK-i (22, 30, 41, 49).
Binding of the secreted early IFNs, predominantly IFN-β, to the IFN-α receptor in an autocrine or paracrine fashion activates JAK/STAT-mediated signal transduction pathways that culminate in the expression of a huge set of IFN-stimulated genes (ISGs). Among these is IRF-7, which is activated like IRF-3 and which allows transcription of the “late” IFN-α genes. Several of the ISGs encode enzymes with antiviral function, such as Mx proteins, 2′-5′ oligoadenylate synthetase, PKR, or factors that inhibit cell growth and promote apoptosis, thereby restricting viral spread. Moreover, IFN signaling stimulates mechanisms of the adaptive immune response, including expression of major histocompatibility complex, activation of NK cells, maturation of dendritic cells, and promotion of the T-helper cell response toward the Th1 type.
For members of most virus groups, including negative-strand RNA viruses, specific antagonists of IFN have been identified during the past years. These may interfere with IFN gene induction, IFN signaling, or the activity of ISGs (for recent reviews, see references 3 and 24). Members of the Paramyxovirinae subfamily encode small nonessential proteins, such as V and C, which are expressed from the phosphoprotein (P) gene through alternative translation initiation and mRNA editing. V and C proteins interfere with IFN JAK/STAT signaling, rendering the viruses IFN resistant. IFN resistance of respiratory syncytial virus (RSV) of the Pneumovirinae subfamily of paramyxoviruses, genus Pneumovirus, is mediated by the concerted action of two nonstructural proteins, NS1 and NS2. In this case, however, IFN signaling and expression of IFN-stimulated genes are not significantly affected (7, 47). Intriguingly, both the NS proteins of RSV (8, 50) and the V proteins of Paramyxovirinae (44) were recently also shown to be involved in inhibiting IFN induction, but with different targets, since NS proteins prevent activation of IRF-3 (8), whereas V proteins affect activation of NF-κB and IRF-3 (44).
In contrast to paramyxoviruses, members of the Rhabdoviridae such as rabies virus (RV) or vesicular stomatitis virus (VSV) are sensitive to exogenous IFN and therefore must prevent IFN induction. For VSV, the lack of IFN induction was correlated with the activity of the viral matrix (M) protein, which causes a general shutoff of host-directed gene expression (1, 18). Compared to VSV, RV is more slowly growing and much less cytopathic, and a general cell shutoff is not observed, allowing expression of host cell genes throughout infection. We therefore reasoned that RV must encode specific mechanisms for preventing IFN induction.
The RV genome comprises five genes, encoding nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and polymerase (L) in the order 3′-N-P-M-G-L-5′. The M and G proteins are predominantly involved in formation of the viral envelope. The N, P, and L proteins are components of the viral ribonucleoprotein, and each of them is essential for accomplishing RNA synthesis (13). The P protein is phosphorylated by cellular kinases (27) and is present in homo-oligomers. It associates both with the L protein to function as a noncatalytic cofactor for RNA polymerization and with the N protein to support specific and proper RNA encapsidation in a chaperone-like way (11, 12, 33). In addition to full-length P (P1), amino-terminally truncated products (P2, P3, and P4) which are translated from internal in-frame AUG initiation codons by a leaky scanning mechanism have been found in infected cells and in purified RV (10). The RV P gene contains a second open reading frame (ORF), termed C in analogy to similar ORFs in VSV P genes. Whereas VSV C proteins have been shown to be expressed (51), the existence of an RV C ORF product is unclear.
We here provide evidence that the P protein of RV, in addition to its above-described functions in RNA synthesis, is responsible for inhibition of IFN induction in RV-infected cells. Replicating RV and full-length P1 expressed from transfected plasmids interfered with phosphorylation of IRF-3 by the upstream kinase TBK-1 such that dimerization of IRF-3 and transcriptional activity was prevented. Although P is crucially required for viral RNA synthesis, it was possible to generate replicating infectious RV that could not prevent IFN induction and that therefore was not viable in IFN-competent cells and tissues. This was achieved by reducing the expression of P to levels sufficient for virus replication but insufficient for blocking IRF-3 activation. Such viruses are particularly interesting for development of live vaccines and oncolytic virus vectors.
MATERIALS AND METHODS
Cells and viruses.
Vero (African green monkey kidney) and HEp-2 (human laryngeal epidermoid carcinoma) cells were maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum and antibiotics. HEK 293 (human embryonic kidney), 2fTGH, and U3A cells (40) were propagated in Dulbecco's modified Eagle's medium with 10% fetal calf serum, l-glutamine, and antibiotics. U3A cells were incubated in the presence of 250 μg/ml hygromycin. The BSR T7/5 cell clone, which constitutively expresses bacteriophage T7 polymerase (9), was used for RV rescue from cDNA as described previously (20).
Recombinant RV SAD L16 (48), comprising the sequence of the attenuated vaccine strain SAD B19 (14), was used as wild-type (wt) RV. SAD eGFP-P is a recombinant virus encoding an eGFP-P fusion protein (enhanced green fluorescent protein [eGFP] fused to the N terminus of P) instead of the authentic P (19). SAD VB GFP is a recombinant virus containing an extra eGFP gene between the G and L genes. NPgrL virus is a recombinant RV in which the matrix (M)- and glycoprotein (G)-encoding genes are replaced by reporter genes encoding eGFP and DsRed (red fluorescent protein from Discosoma sp.) (21).
cDNA constructs.
Mutations in the P protein-coding sequences were introduced into the P expression plasmid pTIT-P (20) by using the Chameleon mutagenesis kit (Stratagene) according to the suppliers instructions.
For replacement of the P ORF of the full-length cDNA clone pSAD L16 (48), a pSADΔPsap subclone was generated, in which the coding region of the P gene was replaced by a linker comprising restriction enzyme sites in the following order: SapI-KpnI-NheI-KpnI-SapI. After SapI digestion, ATG and TAA overhangs at the stop and start codons of P allow insertion of SapI-digested PCR products amplified from mutated P-encoding plasmids (primers SADPatg [5′-GACAGAGCTCTTCGATGAGCAAGATCTTTGTCAA-3′] and SADPtaa [5′-TCTCTCGCTCTTCATTAGCAAGATGTATAGCGATCC-3′]) without causing alterations outside the P ORF. In a second cloning step, the plasmids were cut in the N and M genes, and a DNA fragment containing the modified P ORF was inserted in the full-length pSAD L16.
A cDNA full-length clone encoding an additional copy of the RV P gene downstream of the L gene (pSAD PLP; gene order, 3′-N-P-M-G-L-P-5′) was generated by insertion of an N/P gene border copy downstream of the L ORF, followed by a copy of the P-coding sequence. From pSAD PLP, the original P ORF was deleted by replacement of a region spanning from the N to the L gene with the corresponding DNA fragment from pSADΔPsap to generate SAD ΔPLP (gene order, 3′-N-M-G-L-P-5′). The sequences of all constructs are available from the authors upon request. Vaccinia virus-free rescue of recombinant RV from the cDNAs was performed as described previously (20) by transfection of 10 μg of full-length cDNA and plasmids pTIT-N (5 μg), pTIT-P (2.5 μg), and pTIT-L (2.5 μg) in 106 BSR T7/5 cells grown in 8-cm2 culture dishes.
To generate a cytomegalovirus (CMV) promoter-controlled P expression plasmid, pCR3-P, the P ORF was amplified from pTIT-P by PCR (primers P1Acc65I [5′-TATAGGTACCATGAGCAAGATCTTT-3′] and P1NotI3 [5′-ATATGCGGCCGCTTAGCAAGATGTATAGCGATTCAA-3′]) and was inserted in the plasmid pCR3 (Invitrogen). The P gene of bovine respiratory syncytial virus (BRSV) was excised from pTIT PRSV (9) and was also inserted in pCR3, resulting in pCR3-PRSV.
Transfection.
For reporter gene assays with infected cells, 1 × 106 Vero cells were seeded in 21-cm2 cell culture dishes. After 16 h, 8 μg of the reporter plasmid p125luc, p55C1Bluc, p55A2luc (60) (kindly provided by Takashi Fujita, Tokyo), or pAP1luc (Stratagene) was transfected using Lipofectamine 2000 transfection reagent (Invitrogen). As an internal control, 20 ng of pCMV-RL (renilla luciferase) was cotransfected in all experiments. After 6 h the transfected cells were split and infected with viruses at a multiplicity of infection (MOI) of 3 or stimulated with 5 × 103 units of tumor necrosis factor alpha (Sigma) when indicated. After a further 48 h, cell extracts were prepared and subjected to the reporter gene assay. For transfection of HEK293 cells, the calcium phosphate precipitation method (mammalian transfection kit; Stratagene) was used. Cells were seeded into 12-well plates at 4 × 105 cells/well and were transfected after 16 h with 1 μg of p125luc or p55C1Bluc, 2 μg of fl-TBK-1, 0.1 μg of pCMV-RL, and 2 μg of pCR3 vector. For Western blot analyses, HEK293 cells were transfected in six-well plates (1.2 × 106 cells/well) with 3 μg of fl-TBK-1, 6 μg of pCR3-RVP, or empty vector as indicated. After 24 h, cell extracts were prepared and were analyzed by nondenaturing polyacrylamide gel electrophoresis (PAGE) or sodium dodecyl sulfate (SDS)-PAGE and Western blotting.
Luciferase assay.
Cell lysates were prepared at the indicated time points and subjected to reporter gene assay using the dual luciferase reporter system (Promega). Luciferase activity was measured in a luminometer (Berthold) according to the supplier's instructions.
Western blots and antibodies.
For native PAGE, cell extracts from infected or transfected cell cultures were prepared as described previously (31), and 1.5 × 106 cells were lysed in 100 μl lysis buffer. After centrifugation (14,000 rpm, 4°C, 10 min), 10 μl of the lysate was loaded on the gel. For SDS-PAGE, cells were lysed in cell lysis buffer (62.5 mM Tris, 2% SDS, 10% glycerol, 6 M urea, 5% β-mercaptoethanol, 0.01% bromophenol blue, 0.01% phenol red) at the indicated time points and loaded onto an SDS-10% polyacrylamide gel. Proteins were transferred to a nitrocellulose membrane (Schleicher & Schuell) with a semidry transfer apparatus (OWL Scientific). After incubation with blocking solution (2.5% dry milk and 0.05% Tween 20 in phosphate-buffered saline) at room temperature for 1 h, membranes were incubated overnight with the respective antibodies. The MxA antibody M134 (23) and the mouse polyclonal anti-P serum were kindly provided by Otto Haller and Georg Kochs, Freiburg, and Danielle Blondel, Gif-sur-Yvette, respectively. Primary antibodies to STAT-1 (Cell Signaling), actin (Sigma), IRF-3 (Santa Cruz Biotechnology), IRF-3 Ser386 (phosphorylated) (IBL) were purchased. Secondary antibodies were conjugated with peroxidase, and Western Lightning chemiluminescence reagent (Perkin-Elmer) was used for detection.
RNA analysis.
Total RNA from infected cells was isolated with the RNeasy minikit (QIAGEN) according to the manufacturer's instructions. In RNA preparations for reverse transcription-PCR (RT-PCR) analysis, an additional on column DNase digestion was performed according to the supplier's instructions. Northern blotting and hybridizations with [α-32P]dCTP-labeled cDNAs were performed as described previously (15). Hybridization signals were quantitated by phosphorimaging (Molecular Dynamics Storm). IFN-β-specific RT-PCR was performed on 1 μg RNA isolated after 18 h postinfection (p.i.) with the primers hIFN-β+ (5′-CTCCTCCAAATTGCTCTCCTGTTGTG-3′) and hIFN-β− (5′-AAGATGTTCTGGAGCATCTCATAGATG-3′). As a control, β-actin-specific RT-PCR was performed using the primers βactin+ (5′-GGCATCGTGATGGACTCC-3′) and βactin2− (5′ CCGCCAGACAGCACTGTGTTGGCGTA-3′).
RESULTS
An RV expressing an eGFP-P fusion protein induces IFN.
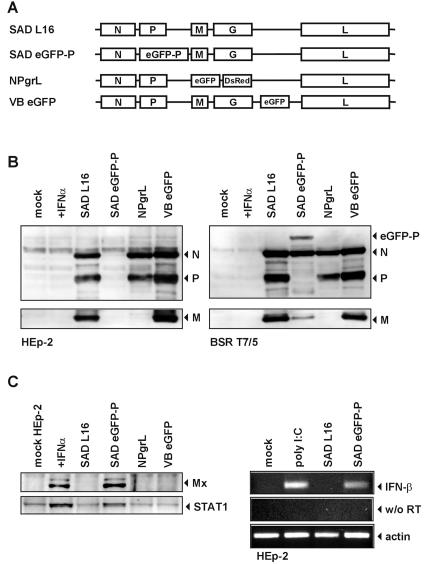
Infection of interferon-competent cell lines such as HEp-2 with the RV SAD L16 does not result in interferon induction. To identify the responsible viral factors, we screened a series of recombinant RV mutants derived from strain SAD L16 (48) for their ability to suppress IFN induction in HEp-2 cells. Due to the sensitivity of SAD L16 to IFN (47), a more severe attenuation of growth in HEp-2 cells compared to IFN-incompetent BSR T7/5 cells served as a first criterion for the possibility of IFN production. An RV in which eGFP was fused to the N terminus of the P protein, SAD eGFP-P (19) (Fig. 1A), fulfilled this criterion, whereas another RV mutant, expressing eGFP from an extra gene inserted between G and L, did not. In BSR cells, SAD eGFP-P reached titers of 106 focus-forming units/ml after 3 days of infection at an MOI of 0,01, whereas HEp-2 cells virtually did not support virus amplification. Microscopic examination revealed initial eGFP-P expression in SAD eGFP-P-infected HEp-2 cells; however, accumulation of viral N and eGFP-P proteins was observed only in BSR cell cultures (Fig. 1B), suggesting induction of IFN by SAD eGFP-P. Notably, infection of HEp-2 cells with an RV lacking both the M and G genes, SAD NPgrL, (21) led to effective expression of the viral N and P proteins (Fig. 1B), demonstrating that the M protein is not required for virus replication in HEp-2 cells.
FIG. 1.
SAD eGFP-P induces IFN-β gene expression. (A) Genome organization of wt SAD L16; SAD eGFP-P, expressing a P protein with an N-terminal eGFP-moiety; NPgrL virus, with M and G genes replaced with eGFP and DsRed genes; and SAD VB eGFP, containing an extra eGFP gene. (B) Whole-cell extracts of BSR T7/5 and HEp-2 cells were harvested at 48 h p.i. (MOI of 1) and analyzed by Western blotting for expression of the viral N, P, and M proteins. In contrast to infection of BSR cells (right panel), SAD eGFP-P produced low levels of N and eGFP-P protein in HEp-2 cells (left panel). (C) SAD eGFP-P infection of HEp-2 cells induces transcription of IFN-β mRNA as shown by RT-PCR (right panel). and up-regulation of MxA and STAT1 proteins as shown by Western blotting (left panel).
To confirm that growth restriction of SAD eGFP-P in HEp-2 cells is correlated to production of IFN, we checked the presence of IFN-β mRNAs by RT-PCR and the biological effects of IFN by demonstrating expression of ISGs. IFN-β RNA was detectable in SAD eGFP-P-infected cells but not in SAD L16-infected cells at 24 h p.i. (Fig. 1C). In accordance with this finding, expression of the IFN-inducible MxA protein and an up-regulation of STAT-1 levels were observed only in SAD eGFP-infected HEp-2 cell cultures and not in cultures infected with wt RV or NPgrL (Fig. 1C). From these results it appeared that RV P gene products are important in preventing IFN production in RV-infected cells, whereas the viral M protein is not required.
Induction of IFN by an RV expressing low levels of P.
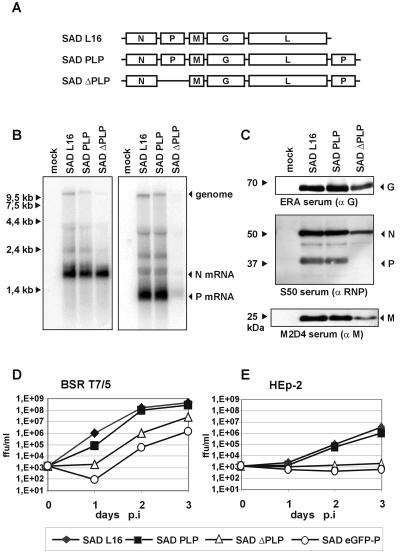
To further support the notion that RV P is critical in preventing IFN production in virus-infected cells, a recombinant RV was engineered to express low levels of P. To this end, we took advantage of the typical stop-start mechanism of nonsegmented negative-strand RNA viruses yielding a transcription gradient. In SAD ΔPLP, the viral genome organization was changed such that the P gene was moved from the second to the most promoter-distal fifth position (genome organization NMGLP) (Fig. 2A). As a control, SAD PLP (gene order, NPMGLP) was generated, retaining the P gene in its original position but containing an extra P copy downstream of L like in SAD ΔPLP. Viable virus was rescued from both cDNA constructs. As revealed by Northern hybridization, much less P mRNA was transcribed in SAD ΔPLP-infected cells than in SAD L16- or SAD PLP-infected cells. In contrast, the amounts of N mRNAs were more similar for all viruses (Fig. 2B). Also, the amount of P protein was greatly lower in SAD ΔPLP-infected cells than in cells infected with the control viruses, as shown by Western blot analyses (Fig. 2B). A decelerated accumulation of other virus proteins such as N, M, and G and of viral RNAs further indicated that the reduced levels of P protein were limiting RNA synthesis of SAD ΔPLP.
FIG. 2.
The level of P protein is crucial for growth of RV in IFN-competent cells. (A) Genome organization of recombinant RV. SAD PLP carries an extra copy of the P gene downstream of L (gene order, 3′-N-P-M-G-L-P-5′). In SAD ΔPLP, the P gene downstream of N in SAD PLP was deleted, resulting in a virus with a single P gene copy downstream of L (gene order, 3′-N-M-G-L-P-5′). (B) Northern blot hybridization with N and P gene-specific cDNA of RNA from BSR T7/5 cells infected for 24 h at an MOI of 1 with the indicated viruses. SAD ΔPLP virus produces lower levels of P mRNA than SAD L16. (C) Expression of RV N, P, M, and G proteins was analyzed in Western blots of cell extracts from BSR T7/5 at 24 h p.i. at an MOI of 1. (D and E) Single-step growth curves were performed on BSR T7/5 cells (D) and on HEp-2 cells (E) after infection with the indicated viruses at an MOI of 0.01. ffu, focus-forming units.
Concordantly, growth of SAD ΔPLP in BSR cell cultures lagged behind that of SAD L16 and SAD PLP; however, final infectious titers of 107 focus-forming units/ml at 3 days p.i. were only 10-fold lower than those of wt RV (Fig. 2D). Compared to SAD eGFP-P, however, SAD ΔPLP yielded 10-fold-higher titers, indicating an RNA synthesis more severely affected by the eGFP-P fusion protein.
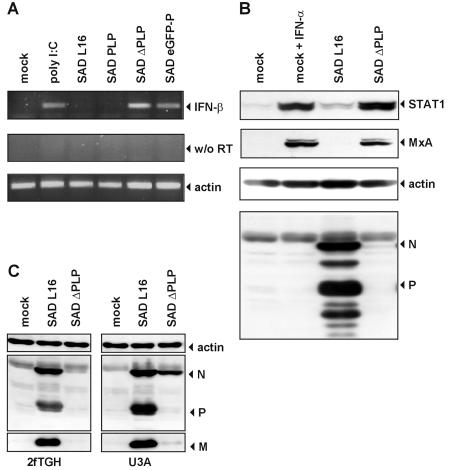
To investigate the effect of reduced P levels on the production of IFN, HEp-2 cells were infected in parallel with SAD L16, SAD PLP, SAD ΔPLP, and SAD eGFP-P as a positive control for IFN induction. Whereas SAD L16 and SAD PLP rapidly amplified to titers of greater than 106 after 3 days of infection, SAD ΔPLP and SAD eGFP-P were not able to productively grow in HEp-2 cells (Fig. 2E). RT-PCR and Western blot analyses of ISGs confirmed that this failure was correlated with the presence of IFN-β mRNAs (Fig. 3A) and with stimulation of MxA and STAT-1 expression. In contrast to those of SAD L16, proteins of SAD ΔPLP were hardly detectable in HEp-2 cells at 48 h p.i. (Fig. 3B). The inability of SAD ΔPLP to inhibit IFN expression is therefore correlated to a reduction of P levels below a critical threshold. To further demonstrate that the IFN-induced establishment of an antiviral state is responsible for the observed growth restriction of SAD ΔPLP, 2fTGH cells and a mutant of 2fTGH, U3A, which lack STAT-1 and therefore do not respond to IFN (40), were infected in parallel. Whereas SAD L16 replicated effectively in both cell lines, accumulation of SAD ΔPLP proteins was observed only in the nonresponding U3A cells (Fig. 3C).
FIG. 3.
The level of P protein is crucial for IFN-β gene expression. (A) SAD ΔPLP effectively induces transcription of IFN-β mRNA in HEp-2 cells as shown by RT-PCR. Mock-infected cells were stimulated by poly(I · C) transfection. RNA was isolated 20 h after infection at an MOI of 1. For RT-PCR, primers specific for IFN-β or β-actin (as a loading control) were used. (B) SAD ΔPLP infection induces an antiviral response and up-regulates expression of MxA and STAT1 as shown by Western blotting at 48 h p.i. Only in wt SAD L16-infected cells were abundant amounts of RV N and P proteins detected.(C) Infection (MOI of 1) with SAD ΔPLP of U3A cells lacking STAT-1 leads to accumulation of viral proteins, in contrast to the case for the parental STAT-1-containing 2fTGH cells.
IFN-antagonistic activity of full-length P (P1).
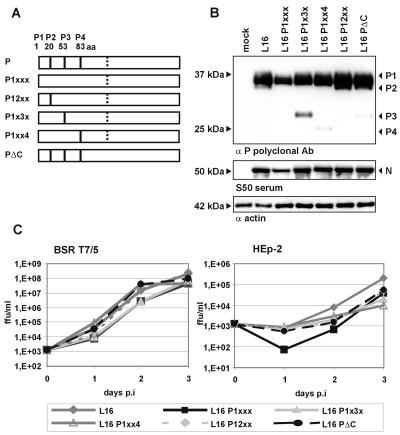
In addition to full-length P encoded by the P ORF (P1), N-terminally truncated forms of the P protein can be expressed from the RV P gene, due to ribosomal leaky scanning and internal translation initiation (10). Since the IFN-inducing phenotype of SAD eGFP-P could be due to the lack of truncated P products from the eGFP-P fusion gene, it was important to show IFN-antagonistic activity of the full-length P1. To generate an RV encoding only full-length P1, SAD P1xxx, the first three internal methionine codons of the SAD P ORF (codons 20, 53, and 83), which could serve for translation of P2, P3, and P4, respectively, were changed to isoleucine codons by site-directed mutagenesis. In addition, constructs expressing P1 along with one of the truncated P products (SAD P12xx, P1x3x, and P1xx4) were generated. In another construct, SAD PΔC, the methionine initiation codon of the internal C ORF of the SAD P gene was destroyed by a T384C base pair exchange, leaving the P protein sequence unchanged (Fig. 4A).
FIG. 4.
Mutation of internal AUG start codons does not abrogate IFN-antagonistic activity of P. (A) Schematic representation of mutations introduced into the P protein. Solid vertical bars represent authentic methionine codons. Lack of the bar indicates substitution with isoleucine codons. Dotted vertical bars represent the AUG codon of the hypothetical C of P ORF + 1. In SAD PΔC, the AUG codon was mutated to ACG. (B) Protein synthesis of RV encoding mutated P proteins. BSR T7/5 cells were infected with the indicated viruses at an MOI of 1. Cell extracts were analyzed by Western blotting at 24 h p.i. P and N proteins were detected by mouse polyclonal anti-P serum and rabbit polyclonal serum S50, respectively. (C) Single-step growth curves of RVs lacking short forms of P protein on BSR T7/5 and HEp-2 cells infected at an MOI of 0.01. RV SAD P1xxx, lacking P2, P3, and P4, grew productively in HEp-2 cells.
All mutant cDNAs could be rescued into viable virus displaying the predicted protein expression pattern. Only full-length P1 was detectable in cells infected with SAD P1xxx, and P1 along with P2 was detectable in SAD P12xx-infected cells. A slightly increased expression of P3 and P4 was observed for SAD P1x3x and SAD P1xx4, respectively, consistent with increased translation initiation at downstream methionine codons when upstream initiation codons are missing (Fig. 4B). The growth kinetics of the mutants in BSR cells, including SAD PΔC, did not greatly differ from that of SAD L16, although the triple mutant SAD P1xxx tended to lag behind at early time points of infection. This indicates some attenuating effect of the mutations introduced into P. However, the virus reached comparable infectious titers at 3 days p.i. (Fig. 4C). Most importantly, all mutants were able to grow productively in the IFN-competent HEp-2 cells. Some lag phase was observed again for SAD P1xxx; however, the virus caught up and was amplified to titers similar to those of other P mutants. Thus, the full-length P1 expressed from SAD P1xxx is active in preventing IFN induction to an extent that is sufficient to permit virus growth in HEp-2 cells.
RV prevents phosphorylation of IRF-3 by TBK-1.
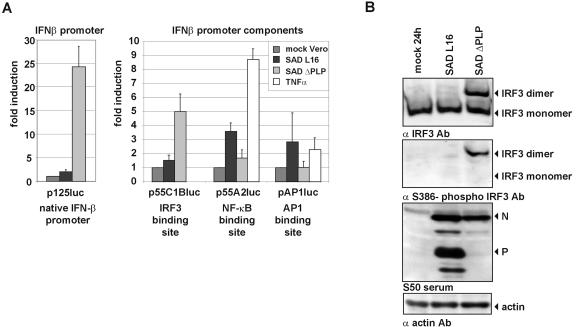
Induction of the IFN-β promoter is known to require the binding of transcription factors NF-κB, IRF-3, and AP-1 (dimers of ATF-2 or heterodimers of ATF-2/c-Jun), which are activated in response to virus infection. To address the mechanisms utilized by RV to prevent IFN induction, we first made use of reporter gene assays in which a plasmid-encoded luciferase gene is controlled by the entire IFN-β promoter (p125luc) or by individual regulatory elements responsive to AP-1 (pAP1luc), NF-κB (p55A2luc), or IRF-3 (p55C1Bluc). Infection of Vero cells with SAD L16 had very little effect on luciferase expression from p125luc- and p55C1Bluc-driven reporter plasmids, containing the native IFN-β promoter and the IRF-3-responsive element, respectively, compared to mock infection of cells. However, infection with SAD ΔPLP resulted in 25- and 5-fold increases of luciferase activity from p125luc and p55C1Bluc, respectively. This indicated that RV P is able to inhibit transcriptional activity of IRF-3. In contrast, an inhibition of NF-κB or AP-1 was not apparent. Whereas SAD L16 caused similar three- to fourfold increases of luciferase activity from p55A2luc and pAP1luc, an even less effective stimulation was observed in SAD ΔPLP-infected cells (Fig. 5A).
FIG. 5.
Rabies virus targets the IRF-3 activation pathway. (A) Vero cells were transfected with reporter plasmid p125luc, p55C1Bluc, p55A2luc, or pAP1luc and infected at an MOI of 3 with SAD L16 or SAD ΔPLP. Cell lysates were analyzed using the dual luciferase reporter system (Promega) for luciferase activity at 48 h p.i. In contrast to SAD ΔPLP, SAD L16 is able to prevent expression of firefly luciferase from plasmids containing the IFN-β promoter (p125luc) and the IRF-3 binding site (p55C1Bluc). In contrast to IRF-3, the activities of NF-κB and AP-1 are not further stimulated in SAD ΔPLP-infected cells compared to SAD L16-infected cells. Incubation of cells with tumor necrosis factor alpha was used as a positive control. Error bars indicate standard deviations. (B) Dimerization and Ser386 phosphorylation of IRF-3 in SAD ΔPLP-infected cells. HEp-2 cells were infected at an MOI of 1 and cell extracts were analyzed at 24 h p.i. by native PAGE and Western blotting. In contrast to SAD ΔPLP, wt SAD L16 prevents IRF-3 dimerization and phosphorylation on Ser386. The same cell lysates were analyzed by SDS-PAGE for expression of viral N and P proteins.
To further address the failure of IRF-3-dependent transcription in RV-infected cells, we analyzed the activation status of IRF-3. C-terminal phosphorylation of IRF-3 by TBK-1 or the inducible IKK-i is a prerequisite for IRF-3 dimerization, nuclear import, association with CBP/p300 coactivators, and activation of IFN-β gene transcription. The IRF-3 status in extracts from virus-infected HEp-2 cells was analyzed by native PAGE. In contrast to mock- and SAD L16-infected cells, in which only monomeric IRF-3 was detectable, a prominent band of IRF-3 dimers appeared in cells infected with SAD ΔPLP (Fig. 5B). Western blotting with a serum specific for S386-phospho-IRF-3 confirmed that only the IRF-3 dimers were phosphorylated at S386, which is known as a target residue for TBK-1 phosphorylation (42). Thus, RV has the ability to selectively block the IRF-3 phosphorylation whereas SAD ΔPLP does not, because of insufficient expression of P protein.
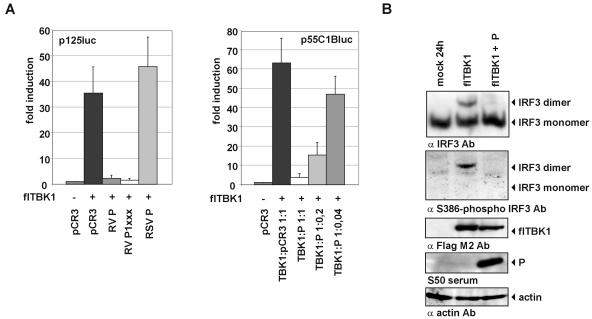
To verify that RV P is able to directly inhibit TBK-1-mediated IRF-3 phosphorylation, we expressed the authentic P gene and P1xxx from transfected plasmids and investigated the effect on TBK-1-stimulated expression of luciferase from the IFN-β promoter in p125luc. As a control for the specificity of inhibition, a plasmid encoding the P protein of BRSV was used. Transfection of a TBK-1-encoding plasmid into cells transfected in addition with vector lacking an insert, or with the BRSV P-encoding plasmid, stimulated luciferase activity 40- to 50-fold. Cotransfection of pRV-P and pRV-P1xxx, however, abolished luciferase activity from p125 almost completely (Fig. 6A, left panel). Similarly, transfection of P abolished TBK-1-mediated expression of luciferase from p55C1Bluc containing only the IRF-3-responsive elements. This occurred in a dose-dependent manner, as evidenced by cotransfection of TBK-1 with decreasing amounts of P-encoding plasmids (Fig. 6A, right panel). The inability of TBK-1 to stimulate luciferase expression from p125luc and p55C1Bluc in the presence of RV P was correlated with the lack of IRF-3 phosphorylation, as visualized in Western blot experiments with cell extracts from transfected 293 cells (Fig. 6B). Whereas expression of TBK-1 resulted in appearance of S386-phosphorylated IRF-3 dimers, phosphorylation and dimerization of IRF-3 were not detectable in cells cotransfected with TBK-1- and P-encoding plasmids. In summary, these data show that RV P prevents IFN induction in virus-infected cells by obstructing phosphorylation of IRF-3 through its kinase, TBK-1.
FIG. 6.
Expression of P is sufficient to prevent TBK-1-mediated IFN-β induction. (A) HEK 293 cells were transfected with expression plasmids encoding the indicated genes and reporter plasmids harboring the firefly luciferase gene under control of the IFN-β promoter and renilla luciferase controlled by the CMV promoter. Luciferase activities were determined at 48 h posttransfection. Cotransfection of P or of P1xxx with TBK-1 inhibited activation of the IFN-β promoter almost completely. The P protein of BRSV (RSV P) was used as a control that is not able to inhibit TBK-1-mediated activation of the p125luc reporter plasmid (left panel). The inhibition of TBK-1-mediated activation of IRF-3 by RV P is dose dependent as revealed by luciferase expression from p55C1Bluc (right panel). Error bars indicate standard deviations. (B) Expression of RV P inhibits TBK-1-mediated dimerization of endogenous IRF-3 and phosphorylation of IRF-3 at Ser386. Cell extracts were harvested at 24 h posttransfection of TBK-1 or TBK-1 and P-encoding plasmids and were analyzed by native PAGE and Western blotting. Expression of Flag-tagged TBK-1 (fl-TBK1), RV P, and β-actin was confirmed by SDS-PAGE and Western blot analysis of the same lysates.
DISCUSSION
In this study we present evidence that the phosphoprotein P of RV is an IFN antagonist preventing transcription of IFN-β in virus-infected cells. Viruses with reduced P expression such as SAD ΔPLP cannot sufficiently interfere with IFN-β transcription in HEp-2 cells. A critical IFN response is therefore activated, which prohibits productive virus replication. A substantial contribution of the M protein to IFN escape of RV, as observed for VSV (1), could be excluded, since RV replicons encoding N, P, and L did not induce an IFN response. IFN induction by SAD ΔPLP was correlated with the activation of the critical transcription factor IRF-3, whereas the activities of NF-κB and AP-1 were unchanged, demonstrating that the IRF-3 activation pathway is specifically blocked in RV-infected cells.
Activation of IRF-3 in virus-infected cells depends on the recognition of viruses by cytosolic receptors and on signal transduction pathways leading from recognition to activation of IRF-3. Recently, the RNA helicase RIG-I was identified as key in sensing viral dsRNA of Newcastle disease virus and in subsequent activation of IRF-3 and NF-κB (58). Downstream signaling by RIG-I required an active helicase function and caspase recruitment domains (58), suggesting the involvement of caspase recruitment domain-containing adapter proteins that relay signals to the IRF-3 kinase TBK-1 or IKK-i.
To circumvent IFN, viruses must either avoid their recognition and eliciting of an alert or target steps of the excited signaling pathways. Recognition of influenza A virus RNA and thus activation of IRF-3 are prevented by the viral NS1 protein, which has a pronounced dsRNA binding activity and thereby makes the critical virus pattern inaccessible to dsRNA receptors (16, 53). Although no direct RNA binding activity has been reported for RV P, it is an important structural component of the RV RNP and serves as a chaperone for proper encapsidation of RNA into N protein. Accordingly, it could be speculated that a critical level of RV P may be important for proper masking of viral RNAs in the RNA complex. Although it is formally not excluded that P may improve such camouflage, the fact that P is active at a downstream step of the IRF-3 activation pathway (see below) argues in favor of recognition of cytoplasmic RV RNPs by sensing molecules such as RIG-I, activation of the pathway, and a downstream inhibition at the step of IRF-3 phosphorylation. Indeed, activation of IRF-3 by replicating RNP complexes of VSV has been demonstrated recently (54).
Signaling pathways triggered by recognition of cytosolic virus and of dsRNA and lipopolysaccharide by Toll-like receptors 3 and 4, respectively, lead to activation of IRF-3 by two IKK-related kinases, TBK-1 and IKK-i (22, 30, 41, 49). The constitutively expressed TBK-1 may be the more important kinase for IRF-3 activation, since the IFN response of TBK-1 knockout mice is severely affected (30, 41). Activation of cytoplasmic IRF-3 involves phosphorylation of multiple serine residues at the C terminus of the protein which allow dimerization of IRF-3, accumulation in the nucleus, and binding of DNA in a complex including transcription factors ATF-2/c-Jun (AP-1) and NF-κB and coactivators CBP/p300 (38, 39, 45, 52, 57, 59). Evidence is accumulating that the phosphorylation status of the Ser386 residue critically determines the transcriptional activity of IRF-3, since TBK-1 can phosphorylate Ser386 and IRF-3 phosphorylated at Ser386 is observed exclusively within dimers. Moreover, mutation at Ser386 abolishes the dimerization potential of IRF-3 (42). In RV-infected cells, IRF-3 is not activated, as demonstrated by reporter gene analyses using plasmids controlled by the IFN-β promoter (p125luc) or the IRF-3 binding part of the IFN-β promoter (p55C1Bluc). The lack of transcriptional activity correlates with the absence of Ser386-phosphorylated IRF-3 and of IRF-3 dimers. In contrast, in SAD ΔPLP-infected cells, IRF-3-dependent expression of reporter genes was possible, and Ser386-phosphorylated IRF-3 was readily detectable (Fig. 5). As predicted, S386 phosphorylation was observed only in IRF-3 dimers and not in monomers.
How the activity of TBK-1 by TRIF or by putative adapters of the cytosolic IFN-inducing pathway is regulated is not known. However, overexpression of TBK-1 in cells is sufficient for IRF-3 activation (22). We have utilized this feature to demonstrate that the IFN-inhibitory activity of RV involves a downstream target of the IRF-3 activation pathway. In addition, this assay revealed that RV P alone is sufficient for inhibiting IRF-3 activation. As a control, the phosphoprotein P of BRSV was used, which has comparable functions in RNA synthesis and encapsidation of viral RNA. As we have shown previously, the BRSV nonstructural proteins NS1 and NS2 are critical for preventing IRF-3 activation (8), and therefore a corresponding activity of the RSV P protein appeared unlikely. In contrast to BRSV P, or empty vector, transfection of expression plasmids comprising the SAD L16 P ORF led to a drastic reduction of TBK-1-mediated transcriptional activity. The same effect was observed after expression of pP1xxx, in which the initiation codons of P2, P3, and P4 were deleted, confirming activity of full-length P. Further evidence that P alone is responsible for the phenotype observed in RV-infected cells was obtained by investigating the IRF-3 phosphorylation and dimerization status after overexpression of TBK-1 in the presence of P. These experiments confirmed that P precludes the critical S386 phosphorylation of IRF-3 by TBK-1 such that dimerization and transcriptional activity are prevented. Further experiments are needed to reveal the molecular mechanisms involved in blocking IRF-3 phosphorylation. From preliminary experiments it appears that a direct and strong interaction of P with either TBK-1 or IRF-3 is unlikely, since coimmunoprecipitations have been unsuccessful so far. Distinct colocalization of P and TBK-1 or IRF-3 in virus-infected cells has also not been observed, suggesting indirect mechanisms.
Activation of IRF-3 is a key step in initiating an antiviral innate and adaptive immune response and is apparently nonredundant in most tissues. The identification of proteins from negative-strand RNA viruses that interfere with IRF-3 activation is therefore not surprising. In most cases, small accessory proteins which are not strictly required for virus replication and which have “luxury functions” (34) are active as IFN antagonists. As first shown for influenza A virus, the NS1 protein hides viral dsRNA that could serve as a trigger for IRF-3 activation and IFN induction (53). However, RNA binding-deficient mutants still have some IRF-3-inhibiting capacity, suggesting additional activities (16). For the influenza B virus NS1 C terminus, an activity independent of RNA binding has been demonstrated, supporting the idea that influenza virus NS1 holoproteins combine multiple activities in inhibiting IRF-3 (17). Pneumovirus NS1 and NS2 proteins, which are encoded by two extra genes, and Paramyxovirinae V and C proteins, which are encoded on P genes, are active in preventing both IFN response (reviewed in reference 24) and IFN induction (29, 36, 44). Whereas the RSV NS proteins are involved in targeting the IRF-3 pathway (8), V and C proteins appear to prevent activation of both IRF-3 and NF-κB (29, 36, 44), suggesting that they target different steps of the pathway.
The present work identifies an essential rhabdovirus protein as a specific IFN antagonist blocking activation of IRF-3. Interestingly, the Ebola virus VP35, which corresponds to the P protein of other Mononegavirales with respect to RNA synthesis and RNA encapsidation, has previously been shown to interfere with IFN induction and IRF-3 activation when expressed from transfected plasmids (4, 5, 28). This suggests that Filoviridae target a similar or more upstream target of the pathway.
Our data further indicate that the full-length RV P is active in preventing IFN induction. A recombinant virus expressing a P gene in which three internal methionine codons that in the wt P serve for internal translation initiation were exchanged for isoleucine codons (SAD P1xxx) was viable in BSR cells. Therefore, the N-terminally truncated P products are not essential for virus replication, as indicated previously by the viability of SAD eGFP-P (19). A slower growth of SAD P1xxx compared to wt RV may indicate that the functions of the P1xxx protein in RNA synthesis and assembly are somehow affected by the three Met-Ile mutations. Importantly, SAD P1xxx was able to grow in HEp-2 cells, showing that expression of P1xxx is sufficient to counteract IFN in virus-infected cells. On the other hand, this does not mean that the truncated P products may not aid P in blocking IRF-3 activation. The major difference from full-length P is that they do not possess the N-terminal L binding site (12), but oligomerization and binding to N should be possible (11). In addition, the binding domains for dynein light chain-1 (LC-8) (32, 43, 46) and the IFN-induced promyelocytic leukemia protein are retained (6). Expression experiments are under way to determine the activity of curtailed P proteins fragments to identify the minimal domains of P able to counteract IRF-3 activation.
A more demanding task will be the dissociation of P functions and the identification of mutations that eliminate the IFN-antagonistic function of P without significantly impairing its essential roles in virus RNA synthesis and assembly. The IFN-inducing SAD ΔPLP virus still expresses the authentic P gene, although at much lower levels. The low levels of P do not greatly limit gene expression, replication, and growth in BSR cells (Fig. 2D), but they are not sufficient to inhibit induction of an IFN response. In case of SAD eGFP-P, both reduced levels of the P fusion protein (Fig. 1B) and the impediment of P functions by the N-terminal eGFP moiety may be responsible for the limited growth and the inability to prevent IFN induction. Indeed, from previous experiments involving complementation of P-deficient RV, it appears that the eGFP-P fusion protein has severe defects in mRNA synthesis, while virus formation is well supported. In contrast, a protein in which eGFP was fused to the C terminus of P, P-eGFP, supported transcription well but had defects in virus assembly (19).
The possibility of generating recombinant RVs that are not able to prevent an IFN response in infected hosts, such as SAD ΔPLP, holds promise for the development of safe, attenuated live vaccines. Since alpha/beta IFN is able to foster the development of an adaptive immune response (for a review, see reference 55), such viruses should induce a potent adaptive immune response. Indeed, a potent adjuvant function of virus-induced early IFN in vivo is suggested by the high immunogenicity of viruses lacking critical IFN antagonists, such as influenza virus (25) or BRSV (56). Another approach in which the identification and knockdown of rhabdovirus IFN antagonists are important is oncolytic virotherapy (for a recent review, see reference 37). In contrast to nonmalignant tissue, many tumors are nonresponsive to IFN. Viruses that both induce the production of IFN and are susceptible to its antiviral effects should therefore preferentially replicate in malignant tissue and may serve as a basis for development of promising therapeutics.
Acknowledgments
We thank Nadin Hagendorf for perfect technical assistance and S. Marozin for TBK-1 cDNA. The reporter plasmids p125luc, p55C1Bluc, and p55A2luc were a generous gift of Takashi Fujita, Tokyo, Japan. The MxA antibody M134 and the mouse polyclonal anti-P serum were kindly provided by Otto Haller and Georg Kochs, Freiburg, and Danielle Blondel, Gif-sur-Yvette, respectively.
This work was supported by the Deutsche Forschungsgemeinschaft through SFB 455, “Viral functions and immune modulation” project A3, and FI 941/1-1.
REFERENCES
- 1.Ahmed, M., M. O. McKenzie, S. Puckett, M. Hojnacki, L. Poliquin, and D. S. Lyles. 2003. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 77:4646-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 3.Basler, C. F., and A. Garcia-Sastre. 2002. Viruses and the type I interferon antiviral system: induction and evasion. Int. Rev. Immunol. 21:305-337. [DOI] [PubMed] [Google Scholar]
- 4.Basler, C. F., A. Mikulasova, L. Martinez-Sobrido, J. Paragas, E. Muhlberger, M. Bray, H. D. Klenk, P. Palese, and A. Garcia-Sastre. 2003. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J. Virol. 77:7945-7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basler, C. F., X. Wang, E. Muhlberger, V. Volchkov, J. Paragas, H. D. Klenk, A. Garcia-Sastre, and P. Palese. 2000. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl. Acad. Sci. USA 97:12289-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blondel, D., T. Regad, N. Poisson, B. Pavie, F. Harper, P. P. Pandolfi, H. De The, and M. K. Chelbi-Alix. 2002. Rabies virus P and small P products interact directly with PML and reorganize PML nuclear bodies. Oncogene 21:7957-7970. [DOI] [PubMed] [Google Scholar]
- 7.Bossert, B., and K. K. Conzelmann. 2002. Respiratory syncytial virus (RSV) nonstructural (NS) proteins as host range determinants: a chimeric bovine RSV with NS genes from human RSV is attenuated in interferon-competent bovine cells. J. Virol. 76:4287-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bossert, B., S. Marozin, and K. K. Conzelmann. 2003. Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J. Virol. 77:8661-8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chenik, M., K. Chebli, and D. Blondel. 1995. Translation initiation at alternate in-frame AUG codons in the rabies virus phosphoprotein mRNA is mediated by a ribosomal leaky scanning mechanism. J. Virol. 69:707-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chenik, M., K. Chebli, Y. Gaudin, and D. Blondel. 1994. In vivo interaction of rabies virus phosphoprotein (P) and nucleoprotein (N): existence of two N-binding sites on P protein. J. Gen. Virol. 75:2889-2896. [DOI] [PubMed] [Google Scholar]
- 12.Chenik, M., M. Schnell, K. K. Conzelmann, and D. Blondel. 1998. Mapping the interacting domains between the rabies virus polymerase and phosphoprotein. J. Virol. 72:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conzelmann, K. K. 1998. Nonsegmented negative-strand RNA viruses: genetics and manipulation of viral genomes. Annu. Rev. Genet. 32:123-162. [DOI] [PubMed] [Google Scholar]
- 14.Conzelmann, K. K., J. H. Cox, L. G. Schneider, and H. J. Thiel. 1990. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology 175:485-499. [DOI] [PubMed] [Google Scholar]
- 15.Conzelmann, K. K., J. H. Cox, and H. J. Thiel. 1991. An L (polymerase)-deficient rabies virus defective interfering particle RNA is replicated and transcribed by heterologous helper virus L proteins. Virology 184:655-663. [DOI] [PubMed] [Google Scholar]
- 16.Donelan, N. R., C. F. Basler, and A. Garcia-Sastre. 2003. A recombinant influenza A virus expressing an RNA-binding-defective NS1 protein induces high levels of beta interferon and is attenuated in mice. J. Virol. 77:13257-13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donelan, N. R., B. Dauber, X. Wang, C. F. Basler, T. Wolff, and A. Garcia-Sastre. 2004. The N- and C-terminal domains of the NS1 protein of influenza B virus can independently inhibit IRF-3 and beta interferon promoter activation. J. Virol. 78:11574-11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferran, M. C., and J. M. Lucas-Lenard. 1997. The vesicular stomatitis virus matrix protein inhibits transcription from the human beta interferon promoter. J. Virol. 71:371-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finke, S., K. Brzózka, and K. K. Conzelmann. 2004. Tracking fluorescence-labeled rabies virus: enhanced green fluorescent protein-tagged phosphoprotein p supports virus gene expression and formation of infectious particles. J. Virol. 78:12333-12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finke, S., and K. K. Conzelmann. 1999. Virus promoters determine interference by defective RNAs: selective amplification of mini-RNA vectors and rescue from cDNA by a 3′ copy-back ambisense rabies virus. J. Virol. 73:3818-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finke, S., R. Mueller-Waldeck, and K. K. Conzelmann. 2003. Rabies virus matrix protein regulates the balance of virus transcription and replication. J. Gen. Virol. 84:1613-1621. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald, K. A., S. M. McWhirter, K. L. Faia, D. C. Rowe, E. Latz, D. T. Golenbock, A. J. Coyle, S. M. Liao, and T. Maniatis. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491-496. [DOI] [PubMed] [Google Scholar]
- 23.Flohr, F., S. Schneider-Schaulies, O. Haller, and G. Kochs. 1999. The central interactive region of human MxA GTPase is involved in GTPase activation and interaction with viral target structures. FEBS Lett. 463:24-28. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Sastre, A. 2004. Identification and characterization of viral antagonists of type I interferon in negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 283:249-280. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Sastre, A., A. Egorov, D. Matassov, S. Brandt, D. E. Levy, J. E. Durbin, P. Palese, and T. Muster. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324-330. [DOI] [PubMed] [Google Scholar]
- 26.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 27.Gupta, A. K., D. Blondel, S. Choudhary, and A. K. Banerjee. 2000. The phosphoprotein of rabies virus is phosphorylated by a unique cellular protein kinase and specific isomers of protein kinase C. J. Virol. 74:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartman, A. L., J. S. Towner, and S. T. Nichol. 2004. A C-terminal basic amino acid motif of Zaire Ebola virus VP35 is essential for type I interferon antagonism and displays high identity with the RNA-binding domain of another interferon antagonist, the NS1 protein of influenza A virus. Virology 328:177-184. [DOI] [PubMed] [Google Scholar]
- 29.He, B., R. G. Paterson, N. Stock, J. E. Durbin, R. K. Durbin, S. Goodbourn, R. E. Randall, and R. A. Lamb. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology 303:15-32. [DOI] [PubMed] [Google Scholar]
- 30.Hemmi, H., O. Takeuchi, S. Sato, M. Yamamoto, T. Kaisho, H. Sanjo, T. Kawai, K. Hoshino, K. Takeda, and S. Akira. 2004. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J. Exp. Med. 199:1641-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwamura, T., M. Yoneyama, K. Yamaguchi, W. Suhara, W. Mori, K. Shiota, Y. Okabe, H. Namiki, and T. Fujita. 2001. Induction of IRF-3/-7 kinase and NF-kappaB in response to double-stranded RNA and virus infection: common and unique pathways. Genes Cells 6:375-388. [DOI] [PubMed] [Google Scholar]
- 32.Jacob, Y., H. Badrane, P. E. Ceccaldi, and N. Tordo. 2000. Cytoplasmic dynein LC8 interacts with lyssavirus phosphoprotein. J. Virol. 74:10217-10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacob, Y., E. Real, and N. Tordo. 2001. Functional interaction map of lyssavirus phosphoprotein: identification of the minimal transcription domains. J. Virol. 75:9613-9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato, A., K. Kiyotani, Y. Sakai, T. Yoshida, and Y. Nagai. 1997. The paramyxovirus, Sendai virus, V protein encodes a luxury function required for viral pathogenesis. EMBO J. 16:578-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 36.Komatsu, T., K. Takeuchi, J. Yokoo, and B. Gotoh. 2004. C and V proteins of Sendai virus target signaling pathways leading to IRF-3 activation for the negative regulation of interferon-beta production. Virology 325:137-148. [DOI] [PubMed] [Google Scholar]
- 37.Lichty, B. D., A. T. Power, D. F. Stojdl, and J. C. Bell. 2004. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol. Med. 10:210-216. [DOI] [PubMed] [Google Scholar]
- 38.Lin, R., C. Heylbroeck, P. M. Pitha, and J. Hiscott. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18:2986-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin, R., Y. Mamane, and J. Hiscott. 1999. Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol. Cell. Biol. 19:2465-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKendry, R., J. John, D. Flavell, M. Muller, I. M. Kerr, and G. R. Stark. 1991. High-frequency mutagenesis of human cells and characterization of a mutant unresponsive to both alpha and gamma interferons. Proc. Natl. Acad. Sci. USA 88:11455-11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McWhirter, S. M., K. A. Fitzgerald, J. Rosains, D. C. Rowe, D. T. Golenbock, and T. Maniatis. 2004. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl. Acad. Sci. USA 101:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori, M., M. Yoneyama, T. Ito, K. Takahashi, F. Inagaki, and T. Fujita. 2004. Identification of Ser-386 of interferon regulatory factor 3 as critical target for inducible phosphorylation that determines activation. J. Biol. Chem. 279:9698-9702. [DOI] [PubMed] [Google Scholar]
- 43.Poisson, N., E. Real, Y. Gaudin, M. C. Vaney, S. King, Y. Jacob, N. Tordo, and D. Blondel. 2001. Molecular basis for the interaction between rabies virus phosphoprotein P and the dynein light chain LC8: dissociation of dynein-binding properties and transcriptional functionality of P. J. Gen. Virol. 82:2691-2696. [DOI] [PubMed] [Google Scholar]
- 44.Poole, E., B. He, R. A. Lamb, R. E. Randall, and S. Goodbourn. 2002. The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-beta. Virology 303:33-46. [DOI] [PubMed] [Google Scholar]
- 45.Qin, B. Y., C. Liu, S. S. Lam, H. Srinath, R. Delston, J. J. Correia, R. Derynck, and K. Lin. 2003. Crystal structure of IRF-3 reveals mechanism of autoinhibition and virus-induced phosphoactivation. Nat. Struct. Biol. 10:913-921. [DOI] [PubMed] [Google Scholar]
- 46.Raux, H., A. Flamand, and D. Blondel. 2000. Interaction of the rabies virus P protein with the LC8 dynein light chain. J. Virol. 74:10212-10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlender, J., B. Bossert, U. Buchholz, and K. K. Conzelmann. 2000. Bovine respiratory syncytial virus nonstructural proteins NS1 and NS2 cooperatively antagonize alpha/beta interferon-induced antiviral response. J. Virol. 74:8234-8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnell, M. J., T. Mebatsion, and K. K. Conzelmann. 1994. Infectious rabies viruses from cloned cDNA. EMBO J. 13:4195-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 50.Spann, K. M., K. C. Tran, B. Chi, R. L. Rabin, and P. L. Collins. 2004. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 78:4363-4369. (Erratum, 78:6705.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spiropoulou, C. F., and S. T. Nichol. 1993. A small highly basic protein is encoded in overlapping frame within the P gene of vesicular stomatitis virus. J. Virol. 67:3103-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahasi, K., N. N. Suzuki, M. Horiuchi, M. Mori, W. Suhara, Y. Okabe, Y. Fukuhara, H. Terasawa, S. Akira, T. Fujita, and F. Inagaki. 2003. X-ray crystal structure of IRF-3 and its functional implications. Nat. Struct. Biol. 10:922-927. [DOI] [PubMed] [Google Scholar]
- 53.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.tenOever, B. R., S. Sharma, W. Zou, Q. Sun, N. Grandvaux, I. Julkunen, H. Hemmi, M. Yamamoto, S. Akira, W. C. Yeh, R. Lin, and J. Hiscott. 2004. Activation of TBK1 and IKKvarepsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J. Virol. 78:10636-10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tough, D. F. 2004. Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk. Lymphoma 45:257-264. [DOI] [PubMed] [Google Scholar]
- 56.Valarcher, J. F., J. Furze, S. Wyld, R. Cook, K. K. Conzelmann, and G. Taylor. 2003. Role of alpha/beta interferons in the attenuation and immunogenicity of recombinant bovine respiratory syncytial viruses lacking NS proteins. J. Virol. 77:8426-8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wathelet, M. G., C. H. Lin, B. S. Parekh, L. V. Ronco, P. M. Howley, and T. Maniatis. 1998. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol. Cell 1:507-518. [DOI] [PubMed] [Google Scholar]
- 58.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 59.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Sato, K. Ozato, and T. Fujita. 1996. Autocrine amplification of type I interferon gene expression mediated by interferon stimulated gene factor 3 (ISGF3). J. Biochem. (Tokyo) 120:160-169. [DOI] [PubMed] [Google Scholar]