Abstract
Body odor is considered a diagnostic indicator of various infectious and chronic diseases. But, few studies have examined the odor markers for various toxic effects in the mammalian system. This study attempted to identify the novel diagnostic odor biomarkers for chemical-induced hepatotoxicity in animals. The changes in the concentration of odors were analyzed in the urine of Sprague Dawley (SD) rats treated with two dosages (100 or 200 mg/kg) of 1,2,3-trichloropropane (TCP) using gas chromatography-mass spectrometry (GC–MS). The TCP treatment induced significant toxicity, including a decrease in body weight, an increase in serum biochemical factors, and histopathological changes in the liver of SD rats. During this hepatotoxicity, the concentrations of six odors (ethyl alcohol, acrolein (2-propenal), methanesulfonyl chloride, methyl ethyl ketone, cyclotrisiloxane, and 2-heptanone) in urine changed significantly after the TCP treatment. Among them, acrolein, an acrid and pungent compound, showed the highest rate of increase in the TCP-treated group compared to the Vehicle-treated group. In addition, this increase in acrolein was accompanied by enhanced spermine oxidase (SMOX) expression, an acrolein metabolic enzyme, and the increased level of IL-6 transcription as a regulator factor that induces SMOX production. The correlation between acrolein and other parameters was conformed using correlagram analyses. These results provide scientific evidence that acrolein have potential as a novel diagnostic odor biomarker for TCP-induced hepatotoxicity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s43188-024-00253-0.
Keywords: 1,2,3-Trichloropropane (TCP); Hepatotoxicity; Odor; Acrolein; Spermine oxidase
Introduction
Body odors result from a combination of various volatile organic compounds (VOCs) secreted from many body tissues through metabolic pathways [1, 2]. Several secretions, including sweat, breath, stools, urine, and vaginal secretions, and some tissues, such as blood and skin, are considered the primary sources of these compounds [1, 3]. Body odors are influenced by various factors, such as age, sex, genetic background, diet, and physiological condition, because of these characteristics of VOCs [4]. Therefore, body odors are even considered novel types of fingerprints that can distinguish individuals [5, 6]. In addition, body odors provide diagnostic clues because the production of new VOCs and changes in the VOC concentrations can influence various pathological processes [1, 7]. Among them, several VOCs, including dimethyl disulfide and p-menth-1-en-8-ol, were detected in stools and damaged areas of cholera, advanced breast cancer, and gynecological tumors [8–11]. Furthermore, some inherited metabolic disorders caused by enzyme deficiencies or transport defects are linked to the accumulation of specific VOCs. Phenylacetic acid, isovaleric acid, α-hydroxybutyric acid, and trimethylamine were detected in phenylketonuria, isovaleric acidemia, methionine malabsorption syndrome, and trimethylaminuria, respectively [12–15]. Moreover, other different disease types, including diabetes, lung cancer, and asthma, are associated with changes in the concentrations of acetone, alkanes, pentane, ethane, and 8-isoprostane [16–18]. Only a few body odors have been reported after poisoning with several toxins. Bitter-almond odors were measured in the breath of people who ingested cyanide, while garlic-like odors were detected after the ingestion of arsenic, thallium, or organic phosphate insecticides [1]. In addition, the odor of the violet flower has been detected in the urine of individuals poisoned by turpentine [1]. Therefore, novel odor markers for toxic effects have attracted considerable attention because they allow toxicity evaluations without sacrificing animals.
1,2,3-Trichloropropane (TCP) is considered as primary products produced from chlorpyrifos (CPF) and CPF-methyl through in vivo metabolism and environmental degradation [19]. It uses primarily in manufacturing industrial and agricultural products as a paint or varnish remover [20]. Generally, TCP is widely distributed and accumulated in soil and water based on its long half-life in soil and great solubility in water [21]. During their commercial use, humans are exposed to this compound and parent molecules (CFP and CFP-methyl) through various routes, including oral, percutaneous, abdominal, skin, and inhalation [22]. They induce a wide range of toxicity, such as cytotoxicity, acute toxicity, genotoxicity, reproductive toxicity, and immunotoxicity against human and experimental animals, and is a strong carcinogen although very few reports have focused on the toxicity of TCP [23–25]. In an early study, a large amount of TCP administered orally is observed in the muscles, blood, skin and adipose tissue of rats. They were completely eliminated within 8 days after oral administration [26]. But, subsequent studies show that the liver can be considered as one of important targets in the toxicity of TCP. The highest concentrations of DNA adducts were detected in the liver of male Fischer-344 rats treated with 30 mg/kg of TCP among nine major target organs [27]. Also, the high concentration of TCP was measured in gastrointestinal tract, adipose, kidney and liver of rats at 6 h after TCP oral administration, while highest accumulation were founded in liver, kidney and forestomach of rats at 60 h after the same treatment [28]. Within these organs, TCP may covalently bind to various macromolecules and remain as non-extractable metabolites for long periods of time [28]. Furthermore, hepatocellular necrosis, karyomegaly, and biliary hyperplasia have been evaluated as the principal toxic lesions related to the administration of TCP to rats [29]. However, there has been little research on the diagnostic odor biomarker for TCP-induced hepatotoxicity because there is insufficient information on the TCP concentration in the human body, including tissues, fluids, urine, and breath.
Meanwhile, the unpleasant body odors can be associated with sign for wide range of liver diseases. Several volatiles containing sulfur such as mercaptan, dimethyl-sulfide, hydrogen sulfide, carbonyl sulfide, carbon disulfide and isoprene are increased in patients with liver disease [30–33]. The levels of ethanol and acetone are elevated during hepatic steatosis and nonalcoholic steatohepatitis [34]. Also, the alcoholic fatty liver disease, nonalcoholic fatty liver disease, cirrhosis and cancer are correlated to alterations of acetaldehyde and isoprene gas [35]. The patients with cirrhosis show a different pattern of VOC that is the increased levels of dimethyl-sulfide, acetone, 2-butanone and 2-pentanone as well as the decreased concentrations of dimethyl selenide and indole [36]. Furthermore, the alcoholic hepatotoxicity is correlated to increase of pentane and ethane volatiles [37]. During these analyses, urine can be considered as one of major sources for measuring odor because the breath gases is difficult to measure in individual animals, but urine can be easily collected without contamination. Moreover, several odor markers have been reported in urine samples previously. Fish odor syndrome was detected as abnormal excretion of trimethylamine (TMA) in the breath, urine, sweat, saliva and vaginal secretions [38, 39]. Also, novel odor marker associated with aging was identified in the urine of 10-month-old ICR mice [40]. But, no studies have been attempted on the correlation between odor in urine and TCP-induced hepatotoxicity. Especially, the most of liver disease related odor were focused in the breath gas of patients, but have rarely been tried in experimental animals because this method can be easily applied to human.
In this study, we hypothesized that various odor biomarkers could be changed during TCP-induced hepatotoxicity of SD rats. To achieve this, the changes in the concentration of odors in the urine samples of SD rats with TCP-induced hepatotoxicity were analyzed, and one candidate was selected among them. The metabolic mechanism of this odor was investigated further in the liver tissue of the same rats to confirm its action. These results provide scientific evidences for novel odor diagnostic biomarkers during TCP-induced hepatoxicity.
Materials and methods
Animal care and experimental design
The Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC, Approval Number PNU-2022-0097) reviewed and approved the animal protocol for this study. All SD rats were purchased from SamTako Biokorea Co. (Osan, Korea) and provided with a standard irradiated chow diet (Altromin Spezialfutter GmbH & Co. KG, Lage, Germany) ad libitum. The nutritional value of this diet consisted of crude protein (22.6%), crude fat (5.0%), crude fiber (4.5%), crude ash (7.1%), moisture (11.0%) and nitrogen free extract (49.8%). The diet had the metabolized energy including fat (463 kcal/kg), protein (901 kcal/kg) and carbohydrate (1,976 kcal/kg). These rats were maintained in a specific pathogen-free state (SPF) under a strict light cycle (lights on at 08:00 h and off at 20:00 h), at 22 ± 2 °C, and with a relative humidity of 50 ± 10%. The experimental procedures on animals were performed at the Pusan National University-Laboratory Animal Resources Center, which is accredited by the Korea Food and Drug Administration (KFDA) (Accredited Unit Number: 000231) and AAALAC International (Accredited Unit Number: 001525).
Seven-week-old male SD rats (n = 30) were randomly divided into one of three experimental groups (10 rats/group): Vehicle-treated group, 100 mg/kg TCP-treated group (LoTCP treated group) and 200 mg/kg TCP-treated group (HiTCP treated group). Three TCP-treated groups were orally administered 100 and 200 mg/kg of TCP (Thermo Fisher Scientific Inc., Waltham, MA, USA) twice within 48 h (Fig. 1A). The vehicle-treated group was orally administered the same volume of corn oil (Sigma, St Louis, MO, USA) twice within 48 h. Urine samples were collected from the metabolic cage at 24 h, including fasting for 12 h after the final administration. The SD rats were euthanized by CO2 gas with a minimum purity of 99.0% based on the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals. A cage containing rats was placed in the chamber, and 99.0% CO2 gas was introduced into the chamber without pre‑charging, with a fill rate of ~ 50% of the chamber volume per minute. After confirming the death of the SD rats, the whole blood and liver samples were collected from the subset group for histopathological analyses and molecular assay. Among total ten TCP administrated mice, five to seven mice were used for the physiological and histological analysis, while three to five mice were used for the molecular biological analysis.
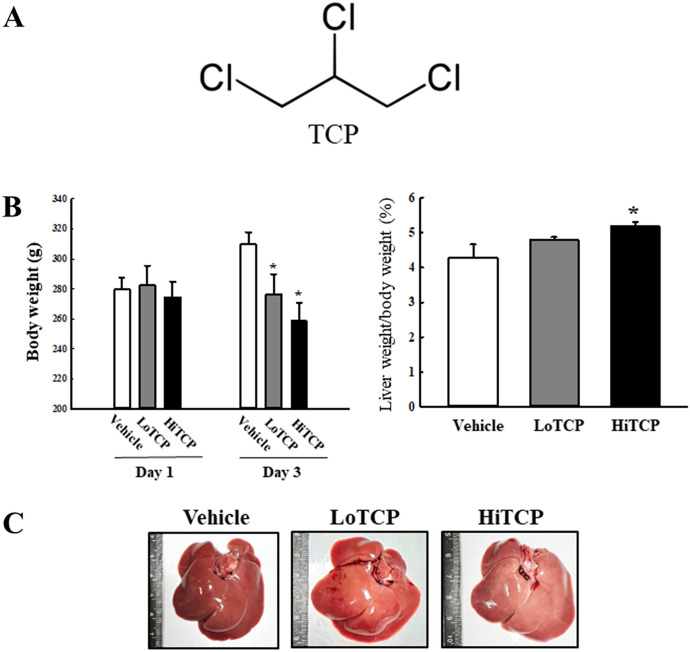
Fig. 1.
Body and liver weight in the TCP-treated SD rats. (a) Chemical structure of TCP. (b) Analyses of the body weight and liver weight/body weight rate. The body weight was measured on days one and three during the experimental period. The liver organ was collected from TCP-treated SD rats and weighed after removing some debris. (c) Morphological image of the liver organs. The body and liver weight were measured in five to seven rats per group, and each measurement was performed twice for each rat and tissue. The data are reported as the mean ± SD. *p < 0.0.5 compared to the Vehicle-treated group. TCP 1,2,3-Trichloropropane, LoTCP Low concentration of TCP, HiTCP High concentration of TCP
Measurement of body and liver weight
The body and liver weight of SD rats in each group were measured using an electronic balance (Mettler Toledo, Greifensee, Switzerland) before and after the TCP treatment. The morphological features of the livers were observed under a stereomicroscope, and images were taken using a digital camera.
Gas chromatography–mass spectrometry (GC–MS) analysis
The odor compounds were identified in the urine samples using GC–MS analysis, which was performed using a slight modification of the methods described elsewhere [41]. Five drops of urine were harvested from each mouse, and a supernatant was collected from the centrifugated sample. The urine sample (250 μL) which the debris had been removed were dried at 60 °C for 30 min. The samples were absorbed into the tube at 100 mL per minute for five minutes. These tubes were analyzed by GC–MS (QP-2010A, Shimadzu, Japan) equipped with an automatic thermal desorption apparatus (ATD 400, Perkin Elmer, UK). The AT-1 capillary column was 60 m × 0.32 mm × 1.0 μm, and the mass range was 20 − 350 m/z. The temperature ramp was in a programmed mode with an initial temperature of 35 °C held for 10 min, and then increased linearly from 35 °C to 120 °C at 8 °C/10 min, from 120 °C to 180 °C at 12 °C/min, from 180 °C to 230 °C at 15 °C/min, and held at 230 °C for 10 min. The experimental mass spectra were compared with the data stored in the Wiley229, Nist21, and Nist107 Library to identify the GC traces of the odorant compounds in urine metabolites. Duplicate samples were collected during each sampling process to assess the precision and reliability of the analytical method. In addition, laboratory blanks and pump calibrations were conducted before every sampling. The flow rate of the sampling pumps was calibrated using a mass flow meter before each sampling to ensure that the flow deviation was less than 5%. The calibration curve of toluene as the standard was obtained by fitting the eight different concentration points (range of 0.003−0.2 μg) with R2 > 0.99. the quantitative comparisons of the emitted odors were semi-quantified as peak areas because few calibration gases for most of individual odors [42].
Serum biochemical analysis
Blood samples collected from an abdominal vein of SD rats were incubated briefly in the serum-separating tubes (BD Containers, Franklin Lakes, NJ, USA) for 30 min at room temperature. Subsequently, the total serum was obtained by centrifugation at 1500 × g for 15 min. The concentrations of alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and blood urea nitrogen (BUN) were analyzed in the serum using the BS-120 Automatic Chemical Analyzer (Mindray, Shenzhen, China). All assays were conducted in duplicate using fresh serum.
Complete blood count
The fresh whole bloods were collected from the abdominal veins using a 1 mL syringe (26 SWG). The levels of the following 10 blood factors were analyzed using an automated cell counter (Beckman-Coulter, Inc.) with the Vetscan HM5 Reagents Pack (cat. no. 89126-098; Abaxis, Inc.; Zoetis Services LLC) according to the manufacturer's protocols: white blood cells (WBC), lymphocytes (LYM), neutrophils (NEU), red blood cells (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelet (PLT), and mean platelet volume (MPV). These assays were measured in duplicate for each blood sample.
Histopathological analysis
The liver tissue collected from SD rats of all group were fixed with 10% neutral buffered formaldehyde for 12 h, embedded in paraffin wax. Subsequently, liver sections (4 μm) were cut from the paraffin-embedded tissues using a Leica microtome (DM500, Leica Microsystems, Bannockburn, IL, USA). The sections were stained with hematoxylin and eosin (H&E, Sigma-Aldrich Co.). The morphological features of these sections were observed using the Leica Application Suite (Leica Microsystems, Herbrugg, Switzerland).
Quantitative real-time (qRT)-PCR analysis
The total RNA was purified from each liver tissue sample using an RNA Bee solution (Tet-Test Inc., Friendswood, TX, USA) according to the manufacturer’s instructions. After homogenization using a Polytron PT-MR 3100 D Homogenizer (Kinematica AG, Lusern, Switzerland), the total RNAs were harvested from tissue lysates by centrifugation at 10,000 × g for 15 min. The RNA concentration was determined using a Nano-300 Micro-Spectrophotometer (Allsheng Instruments Co. Ltd., Hangzhou, China). The total complementary DNA (cDNA) was synthesized against mRNA (4 μg) using 200 units of Superscript II reverse transcriptase (Thermo Scientific, Wilmington, DE, USA). The cDNA for three acrolein metabolism-related enzymes including spermine oxidase (SMOX), myeloperoxidase (MPO), gamma-glutamyltransferase (γ-GTP) and two cytokines such as interlukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were amplified from the mixture solution containing the cDNA template (1 μL), along with 2 × Power SYBR Green (6 μL; Toyobo Life Science, Osaka, Japan) and specific primers (Supplementary Table S1) using qRT-PCR. The cycle quantification value (Cq) was calculated using the method described elsewhere [43].
Statistical analysis
Statistical significance was evaluated using a one-way analysis of variance (ANOVA, SPSS for Windows, Release 10.10, Standard Version, Chicago, IL, USA) followed by a Tukey multiple comparison test. Correlogram data was processed using R version 4.3.3. Correlogram was drawn using the package corrplot from R. Correlation between concentration of acrolein and other parameters was performed using Pearson’s bivariate correlation coefficient in R, and their results were represented as scatterplot for the two variables. All values are reported as the mean ± SD. A p-value < 0.05 was considered significant.
Results
Induction of hepatotoxicity in TCP-treated SD rats
First, the body and liver weight, histopathological structure, and serum biochemical factors in TCP-treated SD rats were analyzed to determine if acute TCP treatment induced hepatotoxicity. The body weight was lower in the LoTCP- and HiTCP-treated groups than in the Vehicle-treated group, while the liver weight remained constant (Fig. 1B). On the other hand, the surface color of the liver was changed remarkably from dark red to light red, even though the overall shape maintained its structure (Fig. 1C). In addition, the hepatotoxicity was detected in serum biochemical factors. Among them, ALT and AST concentrations were significantly higher in the TCP-treated group than in the vehicle-treated group (Table 1). Furthermore, in blood cell analyses, the TCP treatment induced an increase in the NEU number and HCT level and a decrease in LYM number (Supplementary Table S2). Significant toxicity was observed in the histopathological structure of liver tissue of TCP-treated SD rats. The number of granule-contained, binucleated, and inflammatory cells increased after the TCP treatment (Fig. 2). Therefore, the acute TCP treatment successfully induced hepatotoxicity in SD rats, making them suitable for identifying novel odor markers.
Table 1.
Serum biochemical analyses of TCP–treated SD rats
| Category | Vehicle | LoTCP | HiTCP |
|---|---|---|---|
| ALT (U/L) | 60.7 8.36 | 87.2 14.7 | 120.3 23.28* |
| AST (U/L) | 78.1 8.50 | 100 15.4 | 150.4 58.05* |
| ALP (g/mL) | 511.9 82.03 | 685.56 108.69 | 647 110.20 |
| BUN (mg/dL) | 19.86 1.73 | 20.48 2.03 | 22.26 3.12 |
Serum biochemical analysis for the hepatotoxicity indicators. The ALT, AST, ALP, and BUN concentrations in the serum were analyzed using a serum biochemical analyzer. The whole blood samples were prepared from five to seven rats per group, and serum biochemical analyses were performed twice for each sample. The data are reported as the mean ± SD
TCP 1,2,3-Trichloropropane, LoTCP Low concentration of TCP, HiTCP High concentration of TCP, AST aspartate aminotransferase, ALP Alkaline phosphatase, BUN Blood urea nitrogen
*p < 0.05 compared with the Vehicle-treated group
Fig. 2.
Histopathological structure of the liver in TCP-treated SD rats. After H&E staining of the liver section, the histopathological structure of the liver tissue was observed at 200 × and 400 × magnification. The stained liver tissue section was prepared from five to seven rats per group, and the histological structure was observed twice for each sample. TCP 1,2,3-Trichloropropane, LoTCP Low concentration of TCP, HiTCP High concentration of TCP, H&E Hematoxylin & Eosin, G Granulation, B Binucleated cells, I Inflammatory cells
Identification of novel odor biomarkers associated with hepatotoxicity in the urine of TCP-treated SD rats
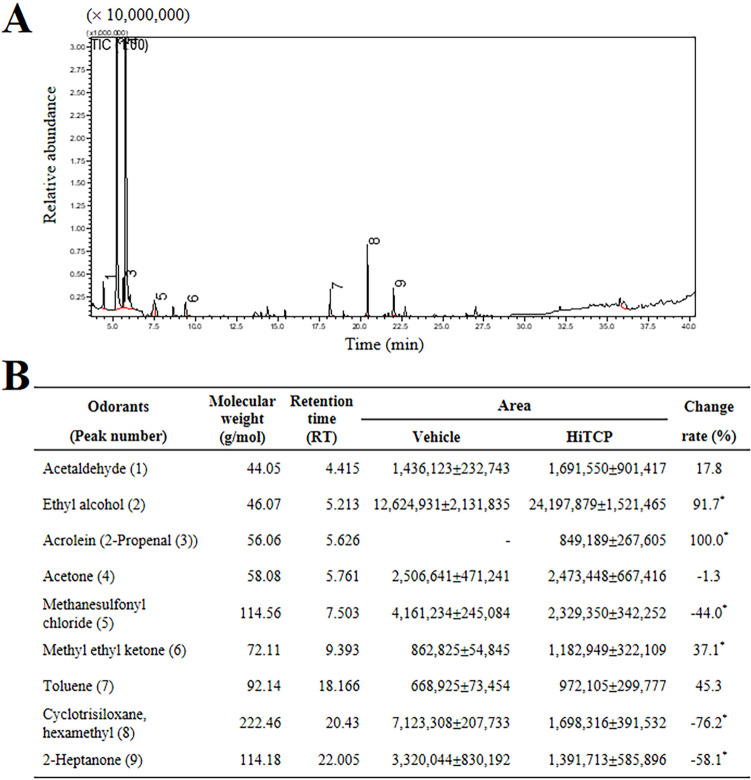
The novel odor biomarkers associated with TCP-induced hepatotoxicity were identified by measuring the changes in the concentration of odors in urine obtained from HiTCP-treated rats using comparative GC–MS analysis. Nine peaks were identified in GC–MS chromatograms, as shown in Fig. 3A. These peaks were verified using GCMS Postrun Analysis (admin) program (Supplementary Fig. S1). Only six odor compounds showed a significant change in the concentrations between the Vehicle-treated and HiTCP-treated groups. After the acute TCP treatment, the concentrations of acrolein, ethyl alcohol, and methyl ethyl ketone were remarkably higher than the Vehicle-treated group. In contrast, the concentrations of other compounds, including methanesulfonyl chloride, cyclotrisiloxane, and 2-heptanone, decreased in the same group. In particular, the acrolein concentration in urine was 100-fold higher in the HiTCP-treated SD rats than in the Vehicle-treated rats (Fig. 3B). Therefore, acrolein was selected as a candidate for novel odor biomarkers for TCP-induced hepatotoxicity based on the present results of TCP-induced acrolein upregulation, and the previous studies reported as acrid and pungent odorous compounds with sensory irritating effects [44, 45].
Fig. 3.
Changes in the concentrations of odor components in the urine of TCP-treated SD rats. (a) Chromatograms for urine samples. Each peak refers to the compounds mentioned in the bottom table. The odor compounds were identified by comparing the MS spectrum and RIs of the components in EHF with authentic standards available in the NIST Library (2005). (b) Retention time and peak area of nine compounds in the urine samples of TCP-treated SD rats. The urine sample was prepared from three to five SD rats per group, and GC–MS analyses were performed twice for each sample. The data are reported as the means ± SD. *p < 0.05 compared with the vehicle-treated group. TCP 1,2,3-Trichloropropane, HiTCP High concentration of TCP, GC–MS Gas chromatography-mass spectrometry, RIs retention indices, EHF extremely high frequency, NIST National Institute of Standards and Technology
Changes in the expression level of metabolic enzymes of acrolein during TCP-induced hepatotoxicity
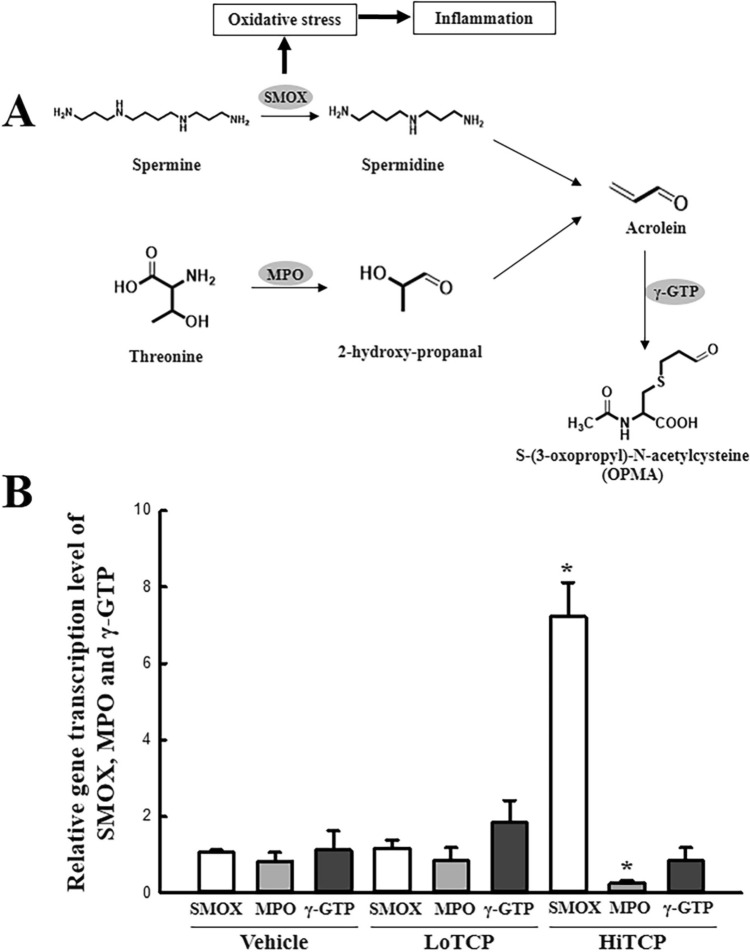
The transcription levels of three enzymes (SMOX, MPO, and γ-GTP) in the liver tissue of TCP-treated SD rats were analyzed to determine if the upregulation of acrolein is accompanied by changes in the expression levels of its metabolic enzymes. Among them, the transcription levels of only two genes (SMOX and MPO) changed significantly after the TCP treatment; one gene (γ-GTP) had a constant expression level. In particular, the SMOX level increased by 680% in the HiTCP-treated group compared to the vehicle-treated group. In contrast, the transcription of MPO decreased by 69.5% after the HiTCP treatment (Fig. 4). Furthermore, this study examined whether the upregulation of SMOX mRNA was accompanied by the enhancement of inflammatory cytokines in the liver tissue. The change in SMOX transcription was reflected in the transcription of the IL-6 gene although LoTCP-treated group showed the decrease level. The level in the HiTCP-treated group was higher than the vehicle-treated group, while the transcription of TNF-α decreased in the same group (Fig. 5). These results suggest that the increased acrolein concentration is linked to the enhancement of SMOX and IL-6 transcripts in TCP-treated SD rats.
Fig. 4.
Transcription level of the genes for the acrolein metabolism-related enzymes. (a) Metabolic pathway of acrolein. Three key enzymes, including SMOX, MPO, and γ-GTP, mediated the production and conversation of acrolein. (b) Transcription levels of SMOX, MPO, and γ-GTP. The transcription levels of these three genes were detected in the total mRNA of the liver tissue by qRT-PCR analysis using the specific primers. The total RNA sample in liver tissue was prepared from three to five SD rats per group, and qRT-PCR analysis was performed twice for each sample. The data are reported as the means ± SD. *p < 0.05 compared to the Vehicle-treated group. TCP 1,2,3-Trichloropropane, LoTCP Low concentration of TCP, HiTCP High concentration of TCP, SMOX Spermine oxidase, MPO Myeloperoxidase, γ-GTP Gamma-Glutamyltransferase, qRT-PCR Quantitative real-time reverse transcription
Fig. 5.
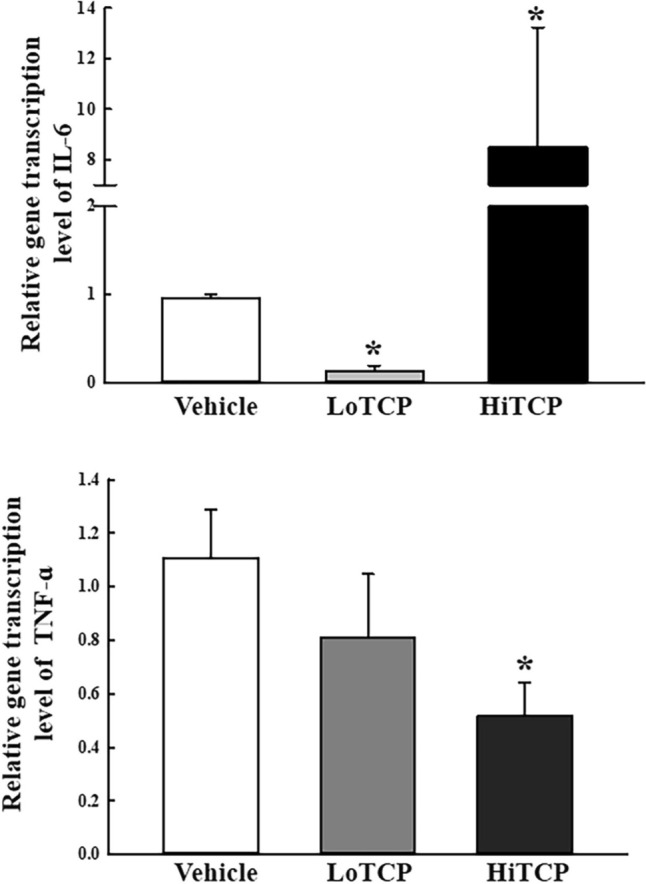
Transcription level of the inflammatory cytokines. The levels of IL-6 and TNF-ɑ transcription were detected in the total mRNA of the liver tissue by qRT-PCR analysis using the specific primers. Total RNA sample in the liver tissue was prepared from three to five SD rats per group, and qRT-PCR analyses were performed twice for each sample. The data are reported as the means ± SD. *p < 0.05 compared to the Vehicle-treated group. SMOX Spermine oxidase, IL-6 Interlukin-6, TNF-α Tumor necrosis factor-α, TCP 1,2,3-Trichloropropane, LoTCP Low concentration of TCP, HiTCP High concentration of TCP, qRT-PCR Quantitative real-time reverse transcription
Correlation between acrolein concentration and toxicological parameters
Finally, we analyzed the correlation between acrolein concentration and other toxicological parameters to verify the potential for novel diagnostic odor during TCP-induced hepatotoxicity. As shown Fig. 6, eight parameters have strong relationship with acrolein concentration. Among them, four including ALT concentration (0.97), AST concentration (0.98), liver wight (0.81) and SMOX transcription (0.65) showed the positive correlations with acrolein concentration. Other four parameter such as ALP concentration (− 0.22), body weight (− 0.6), MPO transcription (− 0.56) and γ-GTP transcription (− 0.25) were negatively correlated with acrolein concentration. Therefore, these results provide that alteration of acrolein concentration may tightly link to key parameters during TCP induced hepatotoxicity although they contain both positive and negative relationships.
Fig. 6.
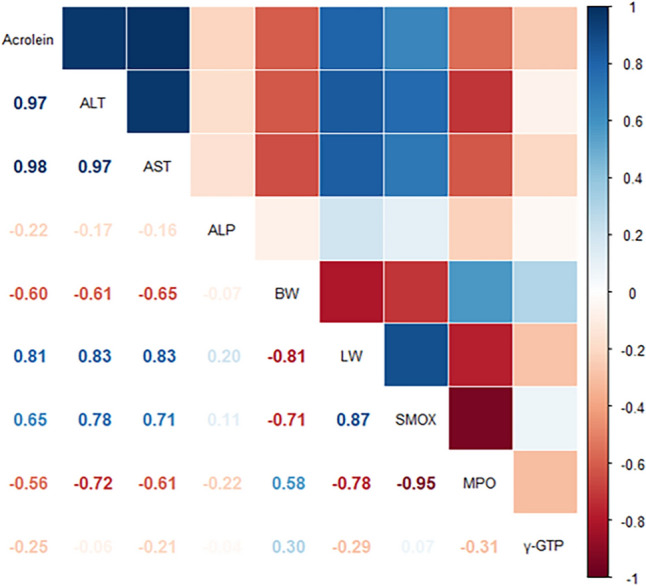
Correlogram drawn in Corrplot for the significance of correlative relationships between acrolein concentration and other parameters. P-values corresponding to the Pearson correlation coefficients were represented numbers. The coloring degree is scaled from strongly positive (dark red) to strongly negative (dark red). Numbers and colors represent the same values in a symmetrical structure based on the factors located on the diagonal. ALT Alanine transaminsase, AST Aspartate transaminase, ALP Alkaline phosphatase, LW Liver weight, BW Body weight, SMOX Spermine oxidase, MPO Myeloperoxidase, r-GTP gamma-glutamyl transpeptidase
Discussion
Various characteristic odors in body fluids are closely related to the conditions of various diseases, including diabetic ketosis, foetor hepaticus, typhoid, smallpox, scurvy, bronchiectasis, mental hospitals, and schizophrenia [46, 47]. They are caused by the accumulation of certain metabolites in specific target organs and tissues because of a dysfunction of metabolic enzymes during the pathogenesis of diseases [1]. Hence, body odors are candidate diagnostic biomarkers for several diseases [48]. This study attempted to identify a novel diagnostic marker for hepatotoxicity induced by chemical toxins. The changes in the concentration of odors were measured in the urine sample of the TCP-treated SD rats. Six odors (ethyl alcohol, acrolein, methanesulfonyl chloride, methyl ethyl ketone, cyclotrisiloxane, and 2-heptanone) changed significantly after the acute treatment of TCP. In particular, these findings suggest the potential of acrolein as an important diagnostic odor biomarker for TCP-induced hepatotoxicity.
Meanwhile, alterations on the weights of body and several internal organs is considered as some primary indicators for toxicity of various chemicals during the toxicological evaluation process [49, 50]. Among these internal organs, the liver are major target organs on the toxic response because the most enzymes and metabolic pathways for xenobiotic excretion are accumulated in them [51]. Also, four serum enzymes including ALT, AST, ALP and LDH are well known as other indicators for hepatotoxicity although there are some differences in their tissue distribution [50, 52]. These indicators are released into the blood when liver cells are disrupted by various factors including fatty liver disease, alcohol, viral agents, some herbal medicines, toxic agents and genetic disorders [53]. Furthermore, these indicators were completely reflected on the histopathological changes such as necrosis, vacuolization, hemosiderosis, binucleation and hyperplasia [54, 55]. Meanwhile, alterations on these indicators for hepatotoxicity were detected in animals after TCP treatment although only a few studies have been carried out. In SD rats orally administrated with 0.4 μmol kg−1/day−1 for 10 days and 0.4 μmol kg−1/day−1, the body weight was significantly decreased with 29–33% for 10 days and 24–16% for 90 days, while the liver weight showed similar pattern in TCP treated group. Also, a significant increase after TCP treatment was detected in the concentration of ALT and AST [56]. The SD rats exposed with drinking water containing TCP for 90 days show the decrease of body weight and increase of liver weight [57]. Also, similar responses were detected in F344/N rats treated with less than 125 mg/kg per day. They show the decrease of body weight and the increase of liver weight although decrease rate was varied. The concentration of ALT was significantly enhanced, while the concentration of AST was decreased after TCP treatment. Furthermore, the necrosis of hepatocytes was clearly detected in the liver section of these rats after H&E staining analyses [29]. In the present study, we analyzed the body weight, liver weight and serum biochemical indicators and histopathological structure after twice treatment within 48 h. The decrease of body weight and the increase of liver weight/body weight rate were exhibited in SD rats treated with 100 mg/kg and 200 mg/kg of TCP. A marginal enhancement was detected in ALT, AST and ALP concentration, while severe histopathological injuries including necrosis, vacuolization and infiltration of inflammatory cells were observed in the liver section. There is some inconsistency between changes on the concentration of serum biochemical indicators and alterations on the histopathological structure of liver tissue after TCP treatment. This difference may think to be due to high concentration of TCP, insufficient response time, and administration routs. Furthermore, most of our results tend to be very similar to the results of previous studies although periods and dosage for TCP treatment are different. In particular, our findings differed from the reduction of AST concertation in F344/N rats treated with 63 mg/kg of TCP for 17 weeks [29]. This difference may be majorly caused by the period of TCP treatment.
The changes in body odor after exposure to toxic substances have attracted limited attention [47, 58]. A bitter almond odor caused by benzaldehyde was detected in the breath of individuals who ingested cyanide. In addition, a garlic-like odor caused by allicin (diallyl thiosulfinate) was observed in individuals who ingested arsenic, thallium, or organic phosphate insecticides [1]. In addition, a person poisoned with turpentine exhibited a violet odor caused by the combination of α-ionone, β-ionone, and dihydro-β-ionone in urine [1]. In the present study, six odor compounds (ethyl alcohol, acrolein, methanesulfonyl chloride, methyl ethyl ketone, cyclotrisiloxane, and 2-heptanone) were first detected in the urine samples of DS rats during TCP-induced hepatotoxicity. The methanesulfonyl chloride widely used various field including the pharmaceutical, photographic, fiber, dye, and agricultural industries, but it can be considered as one of major cause severe ocular, skin, and mucous membrane irritation [59]. It causes 10% and 90% mortality when exposed for 4 h at 20 ppm and 28 ppm [60]. Also, methyl ethyl ketone is known as volatile, sweet odor, mint-like odor and a characteristic odor resembling that of acetone. It has a rage of odor threshold from 0.25 to 0.025 ppm [61]. The cyclotrisiloxane is applied as an intermediates, solvent and emollient for the manufacture of cosmetics, and adhesives and sealants [62]. The lowest observed adverse effect level (LOAEL) and no observed adverse effect level (NOAEL) of this odorant are determined to be 1000 mg/kg bw/day in 28-day rat gavage study and 24.5 mg/m3 in 90-day rat inhalation study [63]. Furthermore, 2-heptenone is permitted by FDA as food additive for human consumption because it has a banana-like, fruity and sweat odor [64]. But, this chemical was detected in urine of human exposed to heptane as well as plasticizers in shoe and tire company [65, 66]. Therefore, the results of the present study provide novel scientific evidence that several odors can be candidates for novel diagnostic odor markers for TCP-induced hepatotoxicity. Nevertheless, further studies will be needed to elucidate the mechanism of the accumulation and production of each odor identified from TCP-treated liver tissue.
Meanwhile, acrolein is a reactive aldehyde with an acrid, and pungent odor that is produced when organic matter is heated or burned [44, 67]. In addition, it has strong irritating effects against the mucous membranes of the eyes, skin and nasal passages, and is used as a biocide to control fungi and algae [44, 45, 68]. In biological systems, acrolein can be produced as a chemotherapy and endogenous metabolite. This odor is produced in the urinary bladder after the cyclophosphamide and ifosfamide treatment, while it is produced as a component of reuterin produced by the gut microbes when glycerol is present [69, 70]. Also, the metabolism of acrolein can be controlled by three enzymes including SMOX, MPO and γ-GTP [71–73]. Especially, SMOX catalyze the spermine to spermidine, hydrogen peroxides and 3-aminopropanal, and is upregulated by two inflammatory cytokines, IL-6 and TNF-α [74]. The transcripts of this gene are abundantly founded as two distinct splice variants (SMOα and μ) in various tissue [75]. The transcription levels of SMOX are upregulated by various physiological condition including redox status [76], several cancers [77] and apoptosis [78]. MPO is well known as a lysosomal enzyme present distributed in the neutrophils and monocytes of human and play an important role in the oxygen-dependent killing of infected pathogens [79]. The alteration level of this enzyme can be caused by several conditions such as infection, inflammation and cancer [80, 81]. On the other hand, acrolein has never been considered a diagnostic indicator of the toxic effects of chemical substances until now. The present study attempted to identify novel diagnostic odors for TCP-induced hepatotoxicity in animal systems. Six odor compounds showed significant changes in concentration after the TCP treatment. Among them, acrolein was identified as one of the candidates for hepatotoxicity because the highest rate of change was detected in this compound. Therefore, this odor can be considered a strong candidate for indicating hepatotoxicity induced by a TCP treatment. Furthermore, the present study analyzed the transcription level of three enzymes mediating acrolein to fine the molecular mechanism of them. A significant change was detected on the transcription level of SMOX and MPO in HiTCP-treated group. However, the decrease of SMOX in LoTCP-treated group are need further studies based on the regulation of transcriptional level. In addition, our study suggest that TCP treatment can be considered as a new cause for alteration of SMOX and MPO expression level in liver tissue although further studies of the molecular mechanism of their action are needed.
Finally, this study attempted to identify the diagnostic odor markers for TCP-induced hepatotoxicity in urine. These results provide novel scientific evidence that TCP-induced hepatotoxicity is closely related to six odors alternated in the urine of SD rats. In particular, acrolein can be considered an important marker for TCP-induced hepatotoxicity based on the correlagram analyses. Nevertheless, further studies will be needed to improve the understanding of the metabolic pathway of acrolein production and accumulation during TCP-induced hepatotoxicity. The present study provides limited information on the correlation between the odors and TCP-induced hepatotoxicity because the metabolic pathway for the other five odors, was not analyzed in TCP-treated SD rats, requiring further confirmation. Also, our finding suggests that acroleine should be considered only as an odor biomarker in hepatotoxicity since we did not investigate the toxicity of TCP to other organs including kidney, respiratory tract, blood, heart and eyeball.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Jin Hyang Hwang, the animal technician, for directing animal use and care at the Laboratory Animal Resources Center at Pusan National University.
Abbreviations
- ALP
Alkaline phosphatase
- ALT
Alanine transaminase
- ANOVA
One-way analysis of variance
- AST
Aspartate transaminase
- AVMA
American Veterinary Medical Association
- BUN
Blood urea nitrogen
- CPF
Chlorpyrifos
- GC/MS
Gas chromatography-Mass spectrometry
- HCT
Hematocrit
- H&E
Hematoxylin & eosin
- HGB
Hemoglobin
- KFDA
Korea Food and Drug Administration
- LYM
Lymphocytes
- LOAEL
Lowest observed adverse effect level
- MCH
Mean corpuscular hemoglobin
- MCHC
Mean corpuscular hemoglobin concentration
- MCV
Mean corpuscular volume
- MPV
Mean platelet volume
- NEU
Neutrophils
- NOAEL
No observed adverse effect level
- PLT
Platelet
- PNU-IACUC
Pusan National University-Institutional Animal Care and Use Committee
- qRT
Quantitative Real-Time
- RBC
Red blood cells
- RDW
Red cell distribution width
- SD
Sprague Dawley
- SMOX
Spermine oxidase
- SPF
Specific pathogen-free state
- TCP
1,2,3-Trichloropropane
- TMA
Trimethylamine
- VOCs
Volatile organic compounds
- WBC
White blood cells
Author contributions
JEK, TRK, HJS, YJR, AS, KHP, ESP, and KSM participated in the study design, sample preparation, animal experiments, and data analyses. K-BK, SJK, and YSJ helped with data analysis and manuscript preparation. DYH designed the experiment, wrote and corrected the original manuscript, and managed the general research and drafting. All authors read and approved the final manuscript.
Funding
This research was supported by a grant (20183MFDS410) from the Ministry of Food and Drug Safety in 2020 and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. RS-2023-00211033). These funders had no role in the design of this study, nor did they have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Ji Eun Kim and Tae Ryeol Kim contributed equally to this study.
References
- 1.Shirasu M, Touhara K (2011) The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem 150:257–266. 10.1093/jb/mvr090 [DOI] [PubMed] [Google Scholar]
- 2.Hakim M, Broza YY, Barash O, Peled N, Phillips M, Amann A, Haick H (2012) Volatile organic compounds of lung cancer and possible biochemical pathways. Chem Rev 112:5949–5966. 10.1021/cr300174a [DOI] [PubMed] [Google Scholar]
- 3.Sharma A, Kumar R, Varadwaj P (2023) Smelling the disease: diagnostic potential of breath analysis. Mol Diag Ther 27:321–347. 10.1007/s40291-023-00640-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broza YY, Vishinkin R, Barash O, Nakhleh MK, Haick H (2018) Synergy between nanomaterials and volatile organic compounds for non-invasive medical evaluation. Chem Soc Rev 47:4781–4859. 10.1039/c8cs00317c [DOI] [PubMed] [Google Scholar]
- 5.Penn DJ, Oberzaucher E, Grammer K, Fischer G, Soini HA, Wiesler D, Novotny MV, Dixon SJ, Xu Y, Brereton RG (2007) Individual and gender fingerprints in human body odour. J R Soc Interface 4:331–340. 10.1098/rsif.2006.0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smeets MAM, Rosing EAE, Jacobs DM, van Velzen E, Koek JH, Blonk C, Gortemaker I, Eidhof MB, Markovitch B, de Groot J, Semin GR (2020) Chemical fingerprints of emotional body odor. Metabolites 10:84. 10.3390/metabo10030084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzatenta A, Giulio CD, Pokorski M (2013) Pathologies currently identified by exhaled biomarkers. Respir Physiol Neurobiol 187:128–134. 10.1016/j.resp.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 8.Garner CE, Smith S, Bardhan PK, Ratcliffe NM, Probert CS (2009) A pilot study of faecal volatile organic compounds in faeces from cholera patients in Bangladesh to determine their utility in disease diagnosis. Trans R Soc Trop Med Hyg 103:1171–1173. 10.1016/j.trstmh.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 9.Shirasu M, Nagai S, Hayashi R, Ochiai A, Touhara K (2009) Dimethyl trisulfide as a characteristic odor associated with fungating cancer wounds. Biosci Biotechnol Biochem 73:2117–2120. 10.1271/bbb.90229 [DOI] [PubMed] [Google Scholar]
- 10.Dankert J, Holloway Y, Bouma J, van der Werf J, Wolthers BG (1981) Metronidazole in smelly gynaecological tumours. Lancet 2:1295. 10.1016/s0140-6736(81)91539-7 [DOI] [PubMed] [Google Scholar]
- 11.Pavlou AK, Turner AP (2000) Sniffing out the truth: clinical diagnosis using the electronic nose. Clin Chem Lab Med 38:99–112. 10.1515/CCLM.2000.016 [DOI] [PubMed] [Google Scholar]
- 12.Burke DG, Halpern B, Malegan D, McCairns E, Danks D, Schlesinger P, Wilken B (1983) Profiles of urinary volatiles from metabolic disorders characterized by unusual odors. Clin Chem 29:1834–1838 [PubMed] [Google Scholar]
- 13.Centerwall SA, Centerwall WR (2000) The discovery of phenylketonuria: the story of a young couple, two retarded children, and a scientist. Pediatrics 105:89–103. 10.1542/peds.105.1.89 [DOI] [PubMed] [Google Scholar]
- 14.Smith AJ, Strang LB (1958) An inborn error of metabolism with the urinary excretion of alpha-hydroxy-butyric acid and phenylpyruvic acid. Arch Dis Child 33:1091–1310. 10.1136/adc.33.168.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humbert JA, Hammond KB, Hathaway WE (1970) Trimethylaminuria: the fish-odour syndrome. Lancet 2:770771. 10.1016/S0140-6736(70)90241-2 [DOI] [PubMed] [Google Scholar]
- 16.Liebich HM (1983) Analysis of acidic metabolites by capillary column GC and GC/MS. J High Resolut Chromatogr Chromatogr Commun 6:640–650. 10.1002/jhrc.1240061202 [Google Scholar]
- 17.Phillips M, Gleeson K, Hughes JM, Greenberg J, Cataneo RN, Baker L, McVay WP (1999) Volatile organic compounds in breath as markers of lung cancer: a cross-sectional study. Lancet 353:1930–1933. 10.1016/S0140-6736(98)07552-7 [DOI] [PubMed] [Google Scholar]
- 18.Olopade CO, Zakkar M, Swedler WI, Rubinstein I (1997) Exhaled pentane levels in acute asthma. Chest 111:862–865. 10.1378/chest.111.4.862 [DOI] [PubMed] [Google Scholar]
- 19.Smith JN, Wang J, Lin YH, Klohe EM, Timchalk C (2012) Pharmacokinetics and pharmacodynamics of chlorpyrifos and 3,5,6-trichloro-2-pyridinol in rat saliva after chlorpyrifos administration. Toxicol Sci 130:245–256. 10.1093/toxsci/kfs251 [DOI] [PubMed] [Google Scholar]
- 20.Agency for Toxic Substances and Disease Registry (1992) Toxicological profile for 1,2,3-trichloropropane (report). U.S. CDC. https://semspub.epa.gov/work/09/1117960.pdf [PubMed]
- 21.Liu YH, Shen DY, Zhong DL, Mo RH, Ni ZL, Tang FB (2014) Time-dependent movement and distribution of chlorpyrifos and its metabolism in bamboo forest under soil surface mulching. J Agric Food Chem 62:6565–6570. 10.1021/jf501540e [DOI] [PubMed] [Google Scholar]
- 22.Ur Rahman HU, Asghar W, Nazir W, Sandhu MA, Ahmed A, Khalid N (2021) A comprehensive review on chlorpyrifos toxicity with special reference to endocrine disruption: evidence of mechanisms, exposures and mitigation strategies. Sci Total Environ 755:142649. 10.1016/j.scitotenv.2020.142649 [DOI] [PubMed] [Google Scholar]
- 23.Cooke M (2009) Emerging contaminant-1,2,3-trichloropropane (TCP) (report). United States EPA. https://nepis.epa.gov/Exe/ZyNET.exe
- 24.Liu X, Qiu Z, Shen W, Pemg X (2012) The clinical analysis of 18 cases with acute trichloropropane poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 30:307–309 [PubMed] [Google Scholar]
- 25.Irwin RD, Haseman JK, Eustis SL (1995) 1,2,3-Trichloropropane: a multisite carcinogen in rats and mice. Fundam Appl Toxicol 25:241–252. 10.1006/faat.1995.1060 [DOI] [PubMed] [Google Scholar]
- 26.Volp RF, Sipes IG, Falcoz C, Carter DE, Gross JF (1984) Disposition of 1,2,3-trichloropropane in the fischer 344 rat: conventional and physiological pharmacokinetics. Toxicol Appl Pharmacol 75:8–17. 10.1016/0041-008x(84)90070-x [DOI] [PubMed] [Google Scholar]
- 27.La DK, Schoonhoven R, Ito N, Swenberg JA (1996) The effects of exposure route on DNA adduct formation and cellular proliferation by 1,2,3-trichloropropane. Toxicol Appl Pharmacol 140:108–114. 10.1006/taap.1996.0203 [DOI] [PubMed] [Google Scholar]
- 28.Mahmood NA, Overstreet D, Burka LT (1991) Comparative disposition and metabolism of 1,2,3-trichloropropane in rats and mice. Drug Metab Dispos 19:411–418 [PubMed] [Google Scholar]
- 29.National Toxicology Program (1993) NTP toxicology and carcinogenesis of 1,2,3-Trichloropropane (CAS No. 96–18–4) in F344/N rats and B6C3F1 mice (Gavage Studies). Natl Toxicol Program Tech Rep Ser 384:1–348. https://ntp.niehs.nih.gov/go/tr384abs [PubMed]
- 30.Chen S, Mahadevan V, Zieve L (1970) Volatile fatty acids in the breath of patients with cirrhosis of the liver. J Lab Clin Med 75:622–627 [PubMed] [Google Scholar]
- 31.Hisamura M (1979) Quantitative analysis of methyl mercaptan and dimethyl sulfide in human expired alveolar gas and its clinical application: study in normal subjects and patients with liver diseases (author’s transl). Nippon Naika Gakkai Zasshi 68:1284–1292. 10.2169/naika.68.1284 [PubMed] [Google Scholar]
- 32.Kaji H, Hisamura M, Saito N, Murao M (1978) Evaluation of volatile sulfur compounds in the expired alveolar gas in patients with liver cirrhosis. Clin Chim Acta 85:279–284. 10.1016/0009-8981(78)90305-4 [DOI] [PubMed] [Google Scholar]
- 33.Sehnert SS, Jiang L, Burdick JF (2002) Risby TH (2002) Breath biomarkers for detection of human liver diseases: preliminary study. Biomarkers 7:174–187. 10.1080/13547500110118184 [DOI] [PubMed] [Google Scholar]
- 34.Solga SF, Alkhuraishe A, Cope K, Tabech A, Clark JM, Torbenson M, Schwartz P, Magnuson T, Diehl AM, Risby TH (2006) Breath biomarkers and non-alcoholic fatty liver disease: preliminary observations. Biomarkers 11:174–183. 10.1080/13547500500421070 [DOI] [PubMed] [Google Scholar]
- 35.Netzer M, Millonig G, Osl M, Pfeifer B, Praun S, Villinger J, Vogel W, Baumgartner C (2009) A new ensemble-based algorithm for identifying breath gas marker candidates in liver disease using ion molecule reaction mass spectrometry. Bioinformatics 25:941–947. 10.1093/bioinformatics/btp093 [DOI] [PubMed] [Google Scholar]
- 36.Dadamio J, Van den Velde S, Laleman W, Hee PV, Coucke W, Nevens F, Quirynen M (2012) Breath biomarkers of liver cirrhosis. J Chromatogr B Anal Technol Biomed Life Sci 905:17–22. 10.1016/j.jchromb.2012.07.025 [DOI] [PubMed] [Google Scholar]
- 37.Letteron P, Duchatelle V, Berson A, Fromenty B, Fisch C, Degott C, Benhamou JP, Pressayre D (1993) Increased ethane exhalation, an in vivo index of lipid peroxidation, in alcohol-abusers. Gut 34:409–414. 10.1136/gut.34.3.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehman HU (1999) Fish odor syndrome. Postgrad Med J 75:451–452. 10.1136/pgmj.75.886.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cashman JR, Camp K, Fakharzadeh SS, Fennessey PV, Hines RN, Mamer OA, Mitchell SC, Nguyen GP, Schlenk D, Smith RL, Tjoa SS, Williams DE, Yannicelli S (2003) Biochemical and clinical aspects of the human flavin-containing monooxygenase form 3 (FMO3) related to trimethylaminuria. Curr Drug Metab 4:151–170. 10.2174/1389200033489505 [DOI] [PubMed] [Google Scholar]
- 40.Kim JE, Choi YJ, Lee SJ, Gong JE, Seong JE, Park SH, Hwang DY (2022) Evaluation of deodorizing effects of Saccharina japonica in 10-month-mld ICR mice using a novel odor marker associated with aging. Evid Based Complement Alternat Med 2022:1410144. 10.1155/2022/1410144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JE, Choi YJ, Lee SJ, Gong JE, Lee YJ, Sung JE, Jung YS, Lee HS, Hong JT, Hwang DY (2021) Antioxidant activity and laxative effects of tannin-enriched extract of Ecklonia cava in loperamide-induced constipation of SD rats. PLoS One 16:e0246363. 10.1371/journal.pone.0246363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brodzik K, Faber J, Gotda-Kopek A, Łomankiewicz D (2016) Impact of multisource VOC emission on in-vehicle air quality: test chamber simulation. IOP Conf Ser Mater Sci Eng 148:012033. 10.1088/1757-899x/148/1/012033 [Google Scholar]
- 43.Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 44.Beauchamp RO Jr, Andjelkovich DA, Kligerman AD, Morgan KT, Heck HD (1985) A critical review of the literature on acrolein toxicity. Crit Rev Toxicol 14:309–380. 10.3109/10408448509037461 [DOI] [PubMed] [Google Scholar]
- 45.Ernstgård L, Dwivedi AM, Lundström JN, Johanson G (2017) Measures of odor and lateralization thresholds of acrolein, crotonaldehyde, and hexanal using a novel vapor delivery technique. PLoS One 12:e0185479. 10.1371/journal.pone.0185479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liddell K (1976) Smell as a diagnostic marker. Postgrad Med J 52:136–138. 10.1136/pgmj.52.605.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenblatt Y, Phan P, Desandre P, Lobon L, Hsu C (2000) Diagnostic odor recognition. Acad Emerg Med 7:1168–1169 [PubMed] [Google Scholar]
- 48.Penn D, Potts WK (1998) Chemical signals and parasite-mediated sexual selection. Trends Ecol Evol 13:391–396. 10.1016/s0169-5347(98)01473-6 [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J, Li Y, Jiao F, Zhao Y, Chai Z (2007) Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168:176–185. 10.1016/j.toxlet.2006.12.001 [DOI] [PubMed] [Google Scholar]
- 50.Bergmeyer HU (1974) Methods of enzymatic analysis, 2nd edn. Academic Press, Weinheim, p 20 [Google Scholar]
- 51.Yun TK, Lee YS, Kwon HY, Choi KJ (1996) Saponin contents and anticarcinogenic effects of ginseng depending on types and ages in mice. Zhongguo Yao Li Xue Bao 17:293–298 [PubMed] [Google Scholar]
- 52.You AS, Jeong MH, Park KH, Kim BS, Lee JB, Choi JH, Kwon OK, Kim JH (2007) Effect on antioxidant function of onion to reduce pesticides toxicity. Korean J Pestic Sci 11:222–229. https://koreascience.kr/article/JAKO200709906340883.pdf
- 53.Losser MR, Payen D (1996) Mechanisms of liver damage. Semin Liver Dis 16:357–367. 10.1055/s-2007-1007249 [DOI] [PubMed] [Google Scholar]
- 54.Jarrar BM, Taib NT (2012) Histological and histochemical alterations in the liver induced by lead chronic toxicity. Saudi J Biol Sci 19:203–210. 10.1016/j.sjbs.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziaolhagh SJ, Ardakanizadeh M, Kaveh A, Yahyaei B (2023) Liver tissue changes induced by biological and chemical silver nanoparticles in trained male Wistar rats. J Trace Elem Med Biol 79:127253. 10.1016/j.jtemb.2023.127253 [DOI] [PubMed] [Google Scholar]
- 56.Merrick BA, Robinson M, Condie LW (1991) Cardiopathic effect of 1,2,3-trichloropropane after subacute and subchronic exposure in rats. J Appl Toxicol 11:179–187. 10.1002/jat.2550110305 [DOI] [PubMed] [Google Scholar]
- 57.Villeneuve DC, Chu I, Secours VE, Cote MG, Plaa GL, Valli VE (1985) Results of a 90-day toxicity study on 1,2,3- and 1,1,2-trichloropropane and administered via the drinking water. Sci Total Environ 47:421–426. 10.1016/0048-9697(85)90346-8 [DOI] [PubMed] [Google Scholar]
- 58.Behrman AD, Goertemoeller S (2009) What is that smell? J Emerg Nurs 35:263–264. 10.1016/j.jen.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 59.Shertzer HG (2001) Organic sulfur compounds. In: Bingham E, Cohrssen B, Powell CH (eds) Patty’s toxicology, vol 7. Wiley, New York, pp 746–747 [Google Scholar]
- 60.Committee on Acute Exposure Guideline Levels (2013) Methanesulfonyl chloride: acute exposure guideline levels (report no. PWT 45/861670). AEGL 14. https://www.ncbi.nlm.nih.gov/books/NBK201481/
- 61.Krasavage WJ, O’Donoghue JL, Di Vincenzo GD (1982) Methyl isobutyl ketone. In: Clayton, Clayton (eds) Patty’s industrial hygiene and toxicology, vol 2e, 4747. John Wily & Son, New York [Google Scholar]
- 62.Wang R, Moody RP, Koniecki D, Zhu J (2009) Low molecular weight cyclic volatile methylsiloxanes in cosmetic products sold in Canada: implication for dermal exposure. Environ Int 35:900–904. 10.1016/j.envint.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 63.Fromme H, Debiak M, Sagunski H, Röhl C, Kraft M, Kolossa-Gehring M (2019) The German approach to regulate indoor air contaminants. Int J Hyg Environ Health 222:347–354. 10.1016/j.ijheh.2018.12.012 [DOI] [PubMed] [Google Scholar]
- 64.Denawaka CJ, Fowlis IA, Dean JR (2016) Source, impact and removal of malodour from soiled clothing. J Chromatogr A 1438:216–225. 10.1016/j.chroma.2016.02.037 [DOI] [PubMed] [Google Scholar]
- 65.Perbellini L, Brugnone F, Cocheo V, De Rosa E, Bartolucci GB (1986) Identification of the n-heptane metabolites in rat and human urine. Arch Toxicol 58:229–234. 10.1007/BF00297111 [DOI] [PubMed] [Google Scholar]
- 66.Walker V, Mills GA (2001) Urine 4-heptanone: a beta-oxidation product of 2-ethylhexanoic acid from plasticisers. Clin Chim Acta 306:51–61. 10.1016/s0009-8981(01)00390-4 [DOI] [PubMed] [Google Scholar]
- 67.Weber-Tschopp A, Fischer T, Gierer R, Grandjean E (1977) Experimentally induced irritating effects of acrolein on men (author’s transl). Int Arch Occup Environ Health 40:117–130. 10.1007/BF00575156 [DOI] [PubMed] [Google Scholar]
- 68.Arntz D, Fischer A, Höpp M, Jacobi S, Sauer J, Ohara T, Sato T, Shimizu N, Schwind H (2007) Acrolein and methacrolein. In: Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. 10.1002/14356007.a01_149.pub2 [Google Scholar]
- 69.Paci A, Rieutord A, Guillaume D, Traore F, Ropenga J, Husson HP, Brion F (2000) Quantitative high-performance liquid chromatography chromatographic determination of acrolein in plasma after derivatization with Luminarin 3. J Chromatogr B Biomed Sci Appl 739:239–246. 10.1016/s0378-4347(99)00485-5 [DOI] [PubMed] [Google Scholar]
- 70.Engels C, Schwab C, Zhang J, Stevens MJA, Bieri C, Ebert MO, McNeill K, Sturla SJ, Lacroix C (2016) Acrolein contributes strongly to antimicrobial and heterocyclic amine transformation activities of reuterin. Sci Rep 6:36246. 10.1038/srep36246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stevens JF, Maier CS (2008) Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res 52:7–25. 10.1002/mnfr.200700412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharmin S, Sakata K, Kashiwagi K, Ueda S, Iwasaki S, Shirahata A, Igarashi K (2001) Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem Biophys Res Commun 282:228–235. 10.1006/bbrc.2001.4569 [DOI] [PubMed] [Google Scholar]
- 73.Anderson MM, Hazen SL, Hsu FF, Heinecke JW (1997) Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein: a mechanism for the generation of highly reactive alpha-hydroxy and alpha, beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest 99:424–432. 10.1172/JCI119176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cervelli M, Bellavia G, Fratini E, Amendola R, Polticelli F, Barba M, Federico R, Signore F, Gucciardo G, Grillo R, Woster PM, Casero RA Jr, Mariottini P (2010) Spermine oxidase (SMO) activity in breast tumor tissues and biochemical analysis of the anticancer spermine analogues BENSpm and CPENSpm. BMC Cancer 10:555. 10.1186/1471-2407-10-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cervelli M, Polticelli F, Federico R, Mariottini P (2003) Heterologous expression and characterization of mouse spermine oxidase. J Biol Chem 278:5271–5276. 10.1074/jbc.M207888200 [DOI] [PubMed] [Google Scholar]
- 76.Ceci R, Duranti G, Leonetti A, Pietropaoli S, Spinozzi F, Marcocci L, Amendola R, Cecconi F, Sabatini S, Mariottini P, Cervelli M (2017) Adaptive responses of heart and skeletal muscle to spermine oxidase overexpression: evaluation of a new transgenic mouse model. Free Radic Biol Med 103:216–225. 10.1016/j.freeradbiomed.2016.12.040 [DOI] [PubMed] [Google Scholar]
- 77.Kim S, Kim D, Roh S, Hong I, Kim H, Ahn TS, Kang DH, Lee MS, Baek MJ, Kwak HJ, Kim CJ, Jeong D (2022) Expression of spermine oxidase is associated with colorectal carcinogenesis and prognosis of patients. Biomedicines 10:626. 10.3390/biomedicines10030626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fratini E, Cervelli M, Mariottini P, Kanamori Y, Amendola R, Agostinelli E (2019) Link between spermine oxidase and apoptosis antagonizing transcription factor: a new pathway in neuroblastoma. Int J Oncol 55:1149–1156. 10.3892/ijo.2019.4878 [DOI] [PubMed] [Google Scholar]
- 79.Nauseef WM (1987) Posttranslational processing of a human myeloid lysosomal protein, myeloperoxidase. Blood 70:1143–1150 [PubMed] [Google Scholar]
- 80.Xie L, Qin WX, He XH, Shu HQ, Yao GF, Wan DF, Gu JR (2004) Differential gene expression in human hepatocellular carcinoma Hep3B cells induced by apoptosis-related gene BNIPL-2. World J Gastroenterol 10:1286–1291. 10.3748/wjg.v10.i9.1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khan AA, Alsahli MA, Rahmani AH (2018) Myeloperoxidase as an active disease biomarker: recent biochemical and pathological perspectives. Med Sci (Basel) 6:33. 10.3390/medsci6020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.