Abstract
The increase in the construction of mega dams in tropical basins is considered a threat to freshwater fish diversity. Although difficult to detect in conventional monitoring programs, rheophilic species and those reliant on shallow habitats comprise a large proportion of fish diversity in tropical basins and are among the most sensitive species to hydropower impacts. We used Baited Remote Underwater Video (BRUV), an innovative, non-invasive sampling technique, to record the impacts caused by Belo Monte, the third largest hydropower project in the world, on fishes inhabiting fast waters in the Xingu River. BRUV were set in a river stretch of ~ 240 km for 7 years, 2 before and 5 after the Belo Monte operation. We explored the spatial and temporal variation in fish diversity (α, β, and γ) and abundance (MaxN) using generalized additive models. We also investigated the variation of environmental variables and tested how much information we gained by including them in the diversity and abundance models. Belo Monte altered the flow regime, water characteristics, and fishery yield in the Xingu, resulting in changes in the fish community structure. Temporally, we observed sharp declines in α diversity and abundance, far exceeding those from a previous study conducted with more conventional sampling methods (i.e., catch-based) in the region. γ-diversity was also significantly reduced, but we observed a non-expected increase in β diversity over time. The latter may be associated with a reduction in river connectivity and an increase in environmental heterogeneity among river sectors. Unexpected signs of recovery in diversity metrics were observed in the last years of monitoring, which may be associated with the maintenance of flow levels higher than those previously planned. These results showed that BRUV can be a useful and sensitive tool to monitor the impacts of dams and other enterprises on fish fauna from clear-water rivers. Moreover, this study enhances our comprehension of the temporal variations in freshwater fish diversity metrics and discusses the prevalent assumption that a linear continuum in fish-structure damage associated with dam impoundments may exhibit temporal non-linearity.
Subject terms: Biodiversity, Ecology
Introduction
Electricity demand is rapidly rising, and hydropower has emerged as the predominant renewable energy source, attracting unprecedented investments1,2. Particularly in developing countries across Africa, Asia, and South America, there has been a notable surge in dam construction3. Apart from its large potential and reduced greenhouse gas emissions, hydropower can create several economic and social benefits, including low operation costs and potential to foster regional development,4. However, dams come with significant ecological up- and downstream impacts on free-flowing rivers affecting their aquatic biota, particularly fishes as well as numerous social impacts, such as displacing riverside populations and disrupting livelihoods dependent on fishing5,6.
Dams reduce river connectivity, constrain fish migration, and dispersal of species7. From a river sectoring perspective, the reservoir created by dams imposes a strong environmental filter as the transition from lotic to lentic conditions, which favors few generalist species pre-adapted to the latter8. Downstream to the dams, the river flow is regulated and generally reduced, decreasing the connectivity of the river channel with lateral habitats (e.g., riparian vegetation, floodplain lakes) that are important nursery and feeding grounds for many fish species9. Dams also tend to reduce downstream aquatic productivity due to sediment retention10 and the decline in water quality conditions11, facilitate the establishment of exotic species due to novel environmental conditions12–14, and increase deforestation to build associated infrastructure15. At the drainage level, large or cascading hydroelectric dams may either reduce between-sites diversity (β) by homogenizing the environmental conditions16,17 or increase it due to habitat isolation18. Total diversity (γ) generally decreases after river impoundment due to the disappearance of migratory and rheophilic species (i.e., species that inhabit fast‐moving, oxygen‐rich waters)16,19,20. Exploring diversity indices beyond total diversity (γ), such as α and β diversity, offer a nuanced understanding of spatial–temporal modifications in freshwater fish communities in response to dam impoundments17. However, dam impacts on fish communities may take decades to unfold and changes are often non-linear4,11. Indeed, fish productivity and richness tend to peak right after dam installation due to a temporary upsurge of nutrients from flooded vegetation8,21. In addition, recent literature reviews indicate that the impact of dams on fish communities may depend on the type of biome (e.g., tropical, temperate) where the dam is installed22 and the presence of exotic species23.
The scientific and managerial debate regarding the impacts of dams on fish fauna stems from complex issues, ranging from the formation of barriers for migrating fish to habitat loss and modifications for other species1. It is important to note that not only do large dams contribute to this threat; the cumulative impact of numerous small barriers on fish can often be even more significant than the impact of a few large barriers110,111. In response to these concerns, while there has been extensive exploration of the potential for large hydropower dams in countries such as China, Russia, and the United States, there is a notable shift in the trend, particularly in temperate regions with high topographic gradient streams, towards the construction of small dams24–26. In contrast, emerging economies seeking energy independence, notably in the tropics, are experiencing a construction boom of large dams in large, diverse river systems1,27–29. One such system is the Amazon River basin, which harbors the highest freshwater biodiversity in the world (> 3000 valid species of fish;30,31) and holds the highest latent potential for hydro-energy among tropical rivers, with 18% of the planet’s freshwater flow in a catchment area of nearly 7 million square kilometers32,33. Over 300 new dams are currently planned or under construction in the Amazon basin, including 16 large dams (> 30 MW installed capacity)28,32,34,35.
An increasing number of studies have indicated that mega-dams recently installed in large, highly diverse Amazonian tributaries have already impacted fish communities. For example, in the Madeira River, two large run-of-river dams (Santo Antônio and Jirau, each with more than 3.6 GW installed capacity) operating since 2016 led to a reduction in the income of local fishers due to declines in the abundance of economic, highly-valuable migratory species (e.g., dourada Brachyplatystoma rousseauxii and curimatã Prochilodus nigricans)34,36. Patterns of species’ response to river impoundment have shown to be sensitive to underlying environmental gradients, such as hydrological seasons and habitats37,38. In this context, analyses evaluating the impact of dams must account for potential confounding effects stemming from environmental drivers. These include various types of habitats where fish species are closely associated, as well as temporal variations in hydrology and climate, which are known to influence fish dynamics. It is equally important to explore new methodological approaches that can be standardized to accommodate the heterogeneity of river habitats, enhance sampling coverage, and improve species detectability. In this regard, the Baited Remote Underwater Video (BRUV) method emerges as a promising alternative, particularly in conditions with adequate water visibility.
Rheophilic fish species are among the most imperiled organisms by dam projects28. However, these species are difficult to detect in monitoring programs, especially in rivers with remote access39 and with extensive and potentially dangerous fast-flowing rapids areas that cannot be sampled with traditional fishing gear, such as gillnets and seine nets. One alternative is the use of video techniques to survey fish assemblages, which is becoming increasingly widespread for fisheries assessments and monitoring, as well as fish ecology studies40. Thus, the BRUV, which was developed and employed primarily in marine systems41,42, is gradually replacing traditional underwater visual census (UVC) techniques. This cost and time-efficient, non-destructive sampling method has been applied in recent years to study fishes in rivers of clear waters43,44, including one Amazon tributary45. BRUV have proven to produce less biased and more robust species richness and abundance estimates data than underwater visual census 46, and bypass methodological trade-offs of fishing-derived inconsistencies as encountered by Ref4, for instance. Furthermore, the use of bait additionally reduces zero counts due to the active attraction of fish and detects large and mobile species47.
In light of these video monitoring advancements, our study provides a strong and innovative approach using a non-destructive BRUV method to investigate hydropower impacts on fishes in clearwater rivers, offering a potential solution to methodological limitations. A recent previous study indicated that the diversity and abundance of fishes after the Belo Monte project operation have declined in several habitats, including lotic environments38. However, authors used gillnets and local divers to capture fishes, which were deemed relatively inefficient for capturing small fishes from shallow water on sandbanks and rheophilic benthic fishes38; these form a relevant proportion of the river’s diversity. Consequently, the utilization of BRUVs could offer an advantageous alternative to catch-based methods. Here, we assessed the impact of the installation and operation of the Belo Monte Hydropower Project (herein, Belo Monte project), the third largest hydropower project in the world, on fish community attributes (abundance, α, β, and γ diversity) using BRUV systems. The Belo Monte project is located in the Xingu River, the largest clearwater tributary of the Amazon River and harbors formidable fish diversity (~ 450 known species) and endemism (~ 10% of all species)48–50. We hypothesize that construction of the dam will lead to alterations in fish abundance and diversity, with varying responses in different habitats and sections of the drainage basin, based on their unique hydrological characteristics. Specifically, we expect that sections directly impacted by changes in flow regime due to damming and those inundated by flooding will experience more pronounced declines in fish community descriptors. These changes are expected to manifest over time and be observed along a longitudinal gradient, considering the two primary lotic habitats present in the river: rocky rapids and submerged shallow sandy beaches. Quantifying changes in fish structure by dams require identifying which range fragments are more affected because they prone disturbs to support viable species populations1. By considering these regional contexts and hypothesizing differential responses based on hydrological changes, we also explored a range of environmental and fishery variables, including physical–chemical parameters, hydrology, climate, and fishery yields to provide a comprehensive understanding of the ecological impacts of dam construction on neotropical freshwater systems.
Results
A total of 97 species, from 22 taxonomic families and 7 orders, were recorded during the 7 years of monitoring in the Middle Xingu River. Around 44% of all fish recorded were small characids, such as Moenkhausia spp, Creagrutus spp., and Hemigrammus spp. Other abundant families include Iguanodectidae (Lizard bite tetras) and Serrasalmidae (pacus and piranhas), each comprising ~ 14% of all fishes recorded. We recorded 2.6 times more species in the rapids (52 spp.) than in the beach habitats (20 spp.).
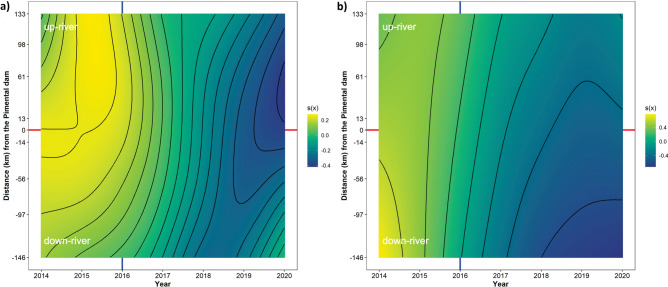
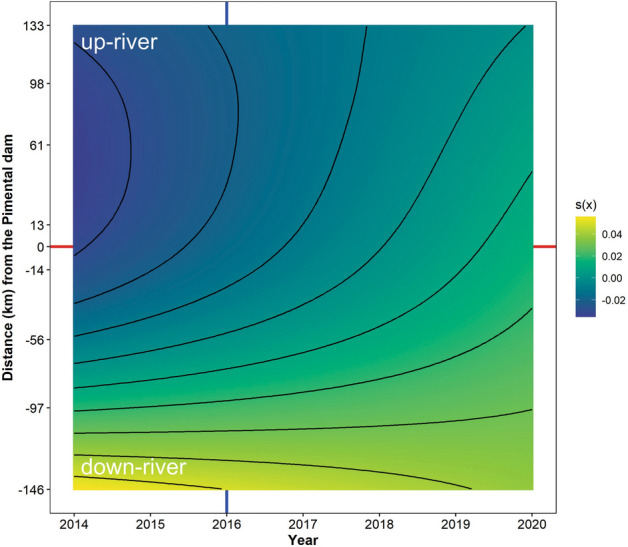
The spatial–temporal models of α-diversity (richness) and MaxN (a proxy for abundance) were significant (P < 0.001; Table S4) and explained a relevant proportion of the data (Adj. R2 = 0.60 and 0.61, respectively). There was an overall decline in both α-diversity and MaxN during our study period (Fig. 1a, b, Fig. S1). The models indicated that whereas MaxN had a progressive decline over the years, α-diversity reduced after 2016, the year when the Belo Monte project started to operate. The magnitude of the reduction in richness and abundance also varied according to the location along the river and was usually stronger in rapids, the habitat with the highest α-diversity and MaxN (Fig. S1a and c). River sectors that experienced a reduction in flow responded differently compared to those that were flooded. The flooded sectors exhibited lasting declines in α-diversity and MaxN, whereas the sectors with reduced flow showed a recovery in fish metrics by the end of the temporal series (Figs. S2 and S3).
Fig. 1.
Predicted α-diversity (richness) (a) and MaxN (a proxy for abundance) (b) variation along time (year) and space (distance from the Pimental dam). Vertical blue lines indicate the year when the Belo Monte project started its operations. Horizontal red lines show the location of the Pimental dam.
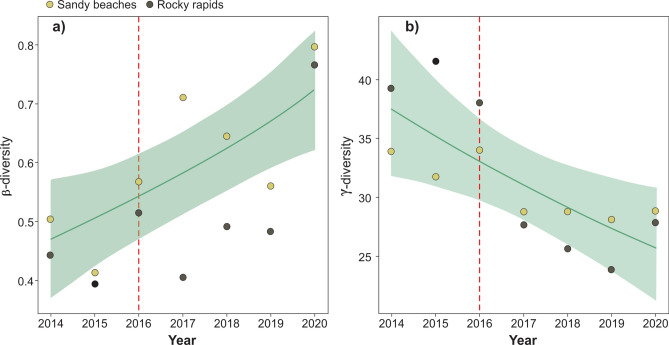
There was also a significant decline in γ-diversity (the total number of species) (Ref. DF = 1, Chi.sq = 6.5, P = 0.01; Fig. 2b, Table S4). On the other hand, β-diversity (between-sites diversity) increased, indicating that sites become more dissimilar over the years (Ref. DF = 1.48, F = 9.04, P = 0.01; Fig. 2a, Table S4). This agrees with LCBD (local contribution to β-diversity), which changed significantly over the years (Ref. DF = 5.77, F = 3.36; P < 0.01; Fig. 3, Table S4). Before the dam operation, high LCBD values were mainly associated with downstream sites. As time progressed, LCBD became more homogenous across the river, indicating that sites became more divergent from β-diversity (Fig. 3, Table S4). β-diversity was mainly associated with species replacement (0.58 ± 0.1 SD), which was more or less constant throughout the study period (Ref. DF = 1, F = 1.82, P = 0.2; Table S4).
Fig. 2.
Variation in ꞵ- (a) and γ-diversity (b) along the studied period. Vertical dotted red lines indicate the year when the Belo Monte project started its operations.
Fig. 3.
Predicted variation in local contribution to β-diversity (LCBD) through time (year) and space (distance from the Pimental dam). The vertical blue line indicates the year when the Belo Monte project started its operations. The horizontal red line shows the location of the Pimental dam.
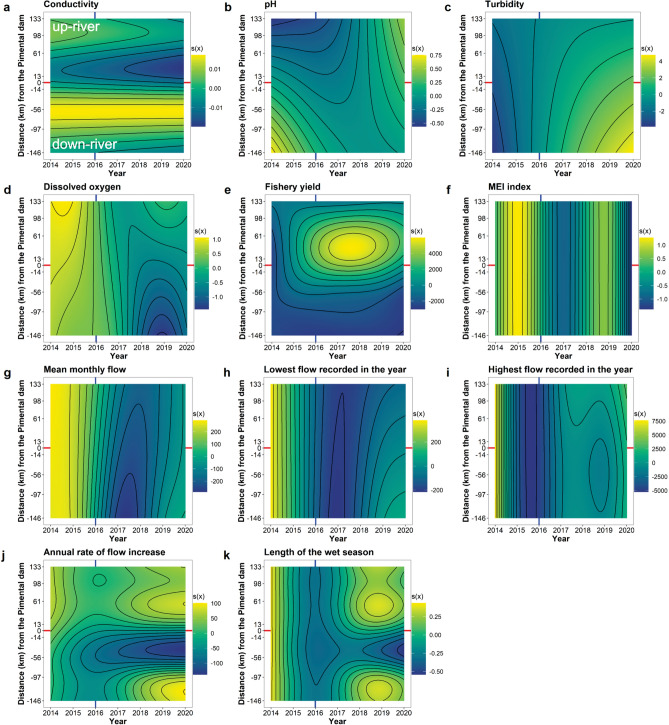
Our spatial–temporal models explained up to 85% (Adj. R2) of the environmental variation. We found a strong conductivity gradient along the river, with higher values associated with the reduced flow sector and lower values with the main reservoir sector (Ref. DF = 10.18, F = 2.55, P = 0.01; Fig. 4a, S4). pH varied significantly during the study period, but with a small magnitude and without a clear spatial–temporal pattern (Ref. df = 5.74, F = 3.23, P = 0.01; Fig. 4b, S4). Turbidity tended to increase during the study period, especially in downstream sectors (Ref. df = 3.91, F = 2.30, P = 0.06; Figs. 4c, S4). Dissolved oxygen was higher before dam operation, especially in upstream sectors (Ref. df = 8.48, F = 10.2, P < 0.001; Figs. 4d, S4). Conversely, we found a strong increase in fishery yield in the main reservoir sector after dam operation (Ref. df = 19.55, F = 6.53, P < 0.001; Figs. 4e, S5). The MEI index, which measures climate anomalies, had significant peaks in 2015 and late 2018/early 2019 (Ref. df = 8.99, F = 16.05, P < 0.001; Figs. 4f, S5). Regarding hydrological variables, we found consistent declines in mean monthly flow (Ref. df = 10.12, F = 15.26, P < 0.001; Fig. 4g, S5), lowest (Ref. df = 11.25, F = 9.59, P < 0.001; Figs. 4h, S5), and highest flow (Ref. df = 8.77, F = 34.24, P < 0.001; Figs. 4i, S6) with the start of the Belo Monte project operation throughout the river. On the other hand, the annual rate of flow increased (Ref. df = 22.82, F = 5.59, P < 0.001; Figs. 4j, S6) and the length of the wet season (Ref. df = 28.3, Chi.sq = 482, P < 0.001; Figs. 4k, S6) decreased consistently only in the reduced flow sector.
Fig. 4.
Predicted variation in conductivity (a), pH (b), turbidity (c), dissolved oxygen (d), fishery yield (e), MEI (Multivariate El Niño Southern Oscillation) index (f), mean monthly flow (g), lowest (h), (i) highest flow recorded in the year, annual rate of flow increase (j), and length of the wet season (k) along time (year) and space (distance from the Pimental dam). Vertical blue lines indicate the year when the Belo Monte project started its operations. Horizontal red lines show the location of the Pimental dam.
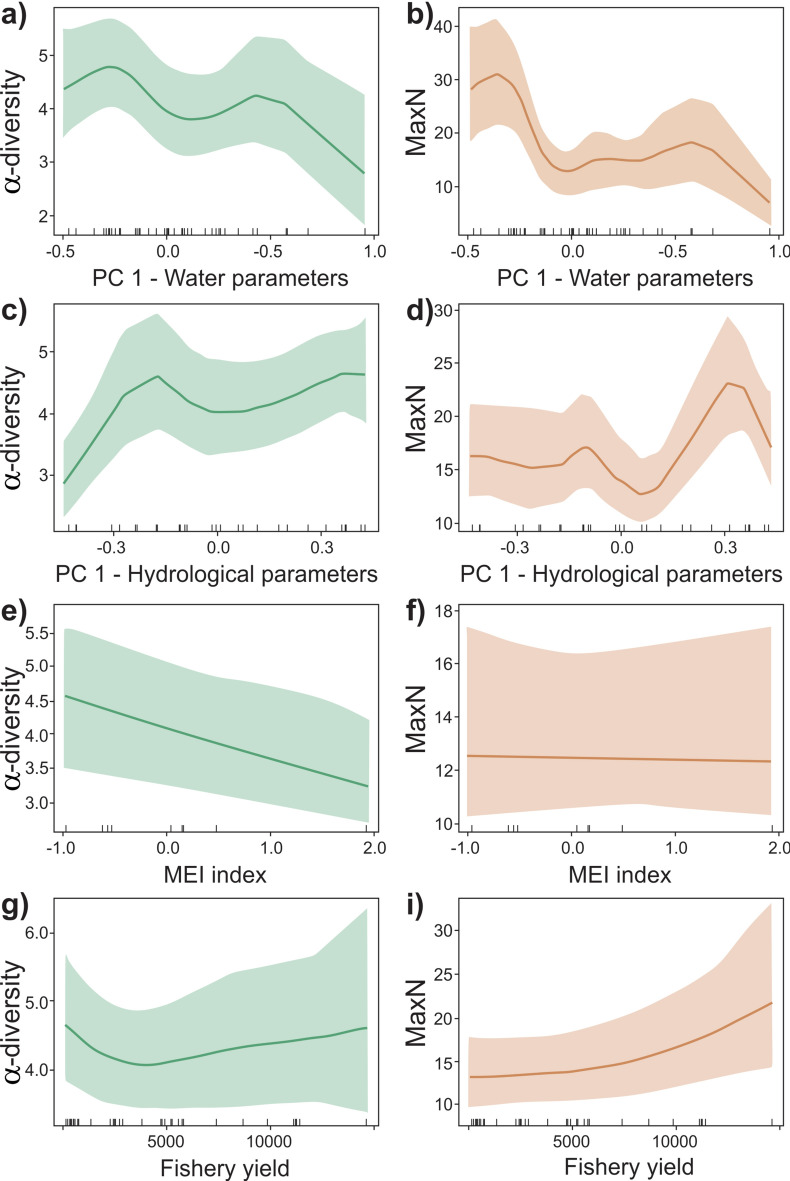
Fig. 6.
Marginal effect of water parameters (a, b), hydrological parameters (c, d), MEI (e, f), and fishery yield (g, h) on α-diversity (richness) and MaxN. Black lines are the average predictions and the gray ribbons are the standard errors. Tick marks represent the position of the sampling units according to the x variable.
The addition of environmental variables into HGAMs (Hierarchical Generalized Additive Models) led to a relevant increase in explanation power (Table 1). The PCA1 of water parameters was the most relevant variable among the environmental variables (Fig. 5) and was negatively associated with both α-diversity and MaxN (Table S4; Fig. 6a, b). This indicates that higher richness and abundance are found in locations with low turbidity and conductivity and high dissolved oxygen and pH. The PCA1 of hydrological parameters was the second most important variable for both MaxN and α-diversity (Table 1, Fig. 5). The combination of water and hydrological parameters, along with fishery yields, exhibited the highest explanatory power for MaxN (Table S4, Fig. 6c, d), while water and hydrological parameters, coupled with MEI, demonstrated greater significance for α-diversity (Table 1). α-diversity tended to be low during El Niño years (high MEI values for 2015 and late 2018/early 2019; Table S4, Fig. 6e, f) and fishery yield was linked with high MaxN (Table S4, Fig. 6g, i).
Table 1.
List of models created to describe the variation in α-diversity (richness) and MaxN (a proxy for abundance) of fishes from the Middle Xingu river.
| Response | Fixed parameters | Covariables | Random effects | AICc | Delta | Weight | Adj. R2 |
|---|---|---|---|---|---|---|---|
| α-diversity | Water.PC1 + Hidrol.PC1 + MEI | Dist.dam + Year | Habitat | 2010.17 | 0.00 | 0.36 | 0.49 |
| Water.PC1 + Hidrol.PC1 + MEI + Fish.yield | Dist.dam + Year | Habitat | 2011.22 | 1.04 | 0.22 | 0.50 | |
| Water.PC1 + Hidrol.PC1 + Fish.yield | Dist.dam + Year | Habitat | 2012.65 | 2.47 | 0.11 | 0.49 | |
| Water.PC1 + MEI + Fish.yield | Dist.dam + Year | Habitat | 2012.68 | 2.51 | 0.10 | 0.49 | |
| Water.PC1 + MEI | Dist.dam + Year | Habitat | 2013.30 | 3.13 | 0.08 | 0.48 | |
| MaxN | Water.PC1 + Hidrol.PC1 + Fish.yield | Dist.dam + Year | Habitat | 3897.39 | 0.00 | 0.70 | 0.43 |
| Water.PC1 + Hidrol.PC1 + MEI + Fish.yield | Dist.dam + Year | Habitat | 3899.16 | 1.77 | 0.29 | 0.43 | |
| Water.PC1 + Hidrol.PC1 | Dist.dam + Year | Habitat | 3906.54 | 9.16 | 0.01 | 0.40 | |
| Water.PC1 + Hidrol.PC1 + MEI | Dist.dam + Year | Habitat | 3907.30 | 9.92 | 0.00 | 0.40 | |
| Water.PC1 + Fish.yield | Dist.dam + Year | Habitat | 3909.03 | 11.64 | 0.00 | 0.39 |
Models are ranked according to their AICc value. Water.PC1 = first PCA axis of the physical–chemical variables, Hidrol.PC1 = first PCA axis of the hydrological variables, Fish.yield = Fishery yield, Dist.dam = Distance to the Pimental dam.
Fig. 5.
Relative importance of environmental variables to α-diversity (richness) and MaxN (a proxy for abundance). We calculated the relative importance by summing all Akaike weights of the models that contain the variable of interest103. The relative importance varies from 0 (worse variable) to 1 (best variable).
Discussion
Overview of dam impacts on fish assemblage structure
Dams persist in exerting a multifaceted influence on freshwater fishes and the surrounding ecosystems. These impacts encompass alterations in hydrological regimes, changes in sediment transport patterns, modifications in water characteristics, and disruptions to the natural flow dynamics of rivers51,52. While numerous studies have demonstrated the effects of dam impoundments on fish fauna, particularly concerning species replacement and changes in assemblage structure53,54, a nuanced perspective utilizing diversity metrics may provide a more detailed understanding of such changes. Our results indicated that fish abundance (MaxN) and local richness (α-diversity) in lotic habitats of the Middle Xingu River have suffered sharp declines over the years, following the environmental changes associated with the Belo Monte project operation. Variables such as the MEI and fishery yields also significantly contributed to the reduction in MaxN and α-diversity (see Table 1). We also found a significant reduction in the total number of fish species (γ-diversity), and an increase (~ 48%) in community dissimilarity between rivers sites (β-diversity). These early impacts caused by the Belo Monte operation and recorded with BRUV systems are more intense than suggested by previous studies conducted with more traditional sampling methods in the region (e.g., gillnets;38). These effects also are in line with the magnitude of early impacts observed in most dam projects worldwide22,23, including Neotropical dams (e.g., Ref16,21,55,56). We argue that investigating such changes, particularly in terms of α-diversity and considering different habitats and river sectors experiencing localized changes, could provide more nuanced evidence of these alterations.
Exploring temporal changes
From a temporal perspective, the reduction of α-diversity was most pronounced soon after the completion of the dam and filling of the intermediate reservoir in 2016, which was a major step of the Belo Monte project and involved a series of alterations in the environmental conditions that could have triggered the observed changes. These include reductions in dissolved oxygen and alterations in river flow, which were correlated with fish α-diversity and are known to degrade water quality, disrupt cues for fish spawning, and restrict fish movement across the landscape; all potentially impacting fish populations by increasing the rates of emigration, mortality, and reducing recruitment57,58. On the other hand, signs of MaxN reduction and environmental changes (e.g., turbidity, lowest and highest flow recorded in the year, and length of the wet season) were seen as early as 2015, which may reflect ongoing environmental transformations induced during the initial stages of the Belo Monte project in 2012. These include the construction of associated infrastructure, partial obstruction of the river channel, and increases in boat traffic, light/sound pollution, and soil erosion59. For example, in the Mekong River dams, disturbed sites were distinguished by a limited seasonal variability in flow that manifested unpredictably, resulting in distinct fish assemblage structures compared to earlier impoundment scenarios54. It is plausible that the early impacts on the flow regime, occurring even before the complete filling of the dam (i.e., Belo Monte project), contributed to the reduction of MaxN (see Fig. S1e and Fig. S2). In addition, there was a strong El-Niño event in 2015, which lead to record-breaking warmth and drought in the region, impacting river conditions and fish communities60. Collectively, these factors provide a rationale for the observed decline in fish abundance from 2015.
Delving spatial changes along the river
From a macro spatial perspective, it was evident that pronounced declines in α-diversity and MaxN radiated from the dam axis. Steeper declines in α-diversity were observed near the Pimental dam, including both the main reservoir and the reduced flow sector. This agrees with previous studies and indicates that the original fish community structure may persist in distant sectors of the river (e.g., free-flowing tributaries, upstream reaches) that have environmental conditions (e.g., river flow) less altered by the dam21,38,61. On the other hand, the decline in fish MaxN was more pronounced downstream to the Pimental dam, including the reduced flow sector and the downstream sector. This underscores the dam axis as the epicenter driving heightened more intense changes in fish structure. The MaxN decline in the reduced flow sector and the downstream sector are likely associated with a reduction in connectivity to important nursery and foraging habitats, such as flood forests and rocky beds, impacting secondary production62–64. Declines in the abundance of fish in this section of the river have long been a concern of riverine and indigenous populations that rely on this natural resource for food security49,65. In addition, it is important to mention that MaxN was influenced mostly by species of small body sizes that are less relevant to local fisheries. Nonetheless, such strong reductions in the abundance of small-bodied fishes may trigger bottom-up effects, leading to the reduction of large predatory fish. These larger predators primarily feed on small fishes and play a crucial role in sustaining local fisheries. Furthermore, the observed decline in abundance, noted in both this study and a prior investigation38, was, to some extent, offset by a substantial increase in fishery yield within the main reservoir post river impoundment. This boost in fishery yield in the main reservoir is likely attributable to a temporary upsurge of nutrients linked to its formation66 or possibly a spillover effect from the intermediate reservoir, a newly established aquatic system where fisheries are prohibited. Evidence of fish spillover into marine protected areas (MPAs) is well-documented, showcasing the conservation benefits for fish fauna by nurturing impoverished areas through migration from protected areas. Similar considerations can be applied to freshwater protect areas (FPAs)113. Indeed, the next steps within our monitoring study involve a thorough investigation into the contribution of the spillover effect from the intermediate reservoir, which holds promise as a conservation measure within this context.
The alterations due to impoundment were also observed at the habitat level. With rocky rapids environments exhibiting more pronounced negative changes in fish fauna structure than sandy beaches, our study also delves into the specific findings of these alterations in the predominant habitats of the riverscape. This likely reflects the reduction and fragmentation of rocky rapids after the Belo Monte operation. Habitat loss and fragmentation are one of the main causes of population declines and species extinction worldwide67 and a major concern in dam projects (e.g., Ref68,69). While the impoundment caused by the Belo Monte project submerged rocky habitats in the reservoir section, it affected rocky rapids in the reduced flow sector, particularly during the wet season. However, during the dry season, the flow regime remained similar to pre-dam conditions. This contrasting scenario of habitat loss, driven by different causal mechanisms, had discernible impacts on fish fauna adapted to these environments over years, as evidenced by a reduction in γ-diversity (see Fig. 4). Nevertheless, rocky rapids harbored a larger variety of species, including a larger proportion of rheophilic fishes that are sensitive to river impoundment29. These include large catfishes (e.g., Pseudoplatystoma punctifer, Pinirampus pirinampu) that undergo long migrations and depend on free-flowing rivers to spawn70, as well as loricariids (e.g., Baryancistrus spp.) and pacus (e.g., Myloplus schomburgkii) that feed algae and macrophytes from rocky habitats and rely on well-oxygenated water50,63.
The diversity reduction observed at local scales propagated to the regional scale, reducing γ-diversity during the monitoring period. Indeed, the total number of species recorded declined from 62 to an average of 51 after the Belo Monte operation (see Table S5). The decline in diversity occurred in both rocky rapids and sandy beaches, and may only be partially attributed to the reduction in BRUV system deployment following the Belo Monte operation. This decrease is likely associated with habitat degradation in certain sections of the river, notably in the main reservoir section. However, even after controlling for sampling size effort across years, the declining trend remained consistent, with signs of recovery in the later year (i.e., 2020), particularly for rocky rapids (see Fig. S7). The decline in species detection by BRUV systems was not restricted to a single group, but rather involved species from different taxonomic families (e.g., Anostomidae, Serrasalmidae, Cynodontidae, and Pimelodidae) (Table S5). This result is likely associated with the high heterogeneity of the Middle Xingu River49,50, and the complex responses of fish communities to dam operation impacts, which vary according to the river sector and species traits29,38. In addition, impoundments can alter food availability for fish, leading to shifts in ecosystem and community properties71.
Riverscape connectivity and hydrology management remarks
Fish communities at different sites become more dissimilar (i.e., higher β-diversity) after the Belo Monte operation. In early years, the downstream sites were more distinct (i.e., higher LCBD) than all the other sites, which reflected mainly the barriers imposed by the rocky rapids in the Big Bend area upstream and also the connectivity with the Amazon River downstream48. The increase in dissimilarity between sites could be linked to a reduction in river connectivity associated with the construction of the Pimental dam, which isolated the Big Bend area (i.e., reduced flow sector) from the upstream sites. Reduction in riverscape connectivity leads to more isolated populations and communities, reducing mass and rescue effects that increase metacommunity homogeneity72,73. In addition, the Belo Monte project increased the environmental heterogeneity (e.g., water flow, depth, and velocity) between the river sectors38, likely exacerbating environmental filtering and species-sorting processes that are essential drivers of species turnover and nestedness between sites72,73. In the context of environmental filtering caused by dam impoundments, understanding fish species turnover and nestedness is crucial. High turnover rates may indicate significant alterations in habitat suitability and resources availability, highlighting areas of potential ecological concern, whereas nestedness can indicate the loss of species rich-habitats and the concentration of species in remaining suitable habitats114. Nestedness patterns can help identify areas that are particularly important for conservation efforts114. Overall, investigating fish species turnover and nestedness provides valuable insights into the ecological consequences of dam impoundments and can inform management strategies aimed at mitigating their impacts on freshwater ecosystems.
The results presented here can be considered the early impacts of the Belo Monte project on the rheophilic species of the Middle Xingu River. Both richness and abundance are expected to decline as the peak water flow is reduced to 8000 m3 s−1 in the reduced flow sector59,74, representing a 35% decrease from the minimum peak flow recorded since the initiation of the Belo Monte operation38. However, there have been signs of partial recovery of diversity metrics, especially for downstream sites (see Fig. S2). This is corroborated by preliminary monitoring data from 2021 and 2023 (Personal observation; TG and FWK).
Limitations of underwater video sampling
When utilizing underwater video systems in freshwater environments affected by dam impoundments to studying fish diversity metrics over multiple years, it is important to acknowledge certain limitations. Firstly, the variable conditions in water visibility can significantly impacts the effectiveness of BRUVs115. Fluctuations in water clarity due to factors such as sedimentation, turbidity, and seasonal changes, which can suffer influence from hydropower plant operation can hinder the visibility of fish and reduce the accuracy of species identification. Additionally, BRUVs may have reduced capability in identifying cryptic, herbivorous, and nocturnal species. Cryptic species, which possess camouflage or mimicry adaptations, may remain undetected by the cameras. Herbivores, which often feed on vegetation and algae, may be less attracted to baited rigs and therefore underrepresented in BRUV surveys. Similarly, nocturnal species, which are active during nighttime, may not be adequately sampled during daytime deployments of BRUVs. These limitations underscore the importance of complementing BRUV surveys with other sampling techniques and considering the potential biases when interpreting long-term trends in fish diversity metrics.
Conclusions
Dams have been disrupting rivers and changing environmental conditions and fish communities worldwide, including highly diverse tropical basins, such as the Mekong, Congo, and Amazon22,28,75. Here, we used BRUV systems to quantify how strong these impacts can be in fast-flowing clear-water tropical rivers, which are hard to sample effectively using traditional methods. We detected nearly 100 species spanning various functional groups (e.g., pelagic and benthic fishes) and clades (e.g., characids, loricariids, and cichlids) during our study period. This diverse range included endemic (e.g., Anostomoides passionis, Baryancistrus xanthellus, Cichla melaniae, Potamotrygon leopoldi) and endangered species (Paratrygon aiereba), which are challenging to record through conventional catch methods such as gillnets. Additionally, our findings align with previous evidence38, indicating a decline in species within the Middle Xingu River following the initiation of the Belo Monte operation. There have been few studies utilizing BRUV to investigate fish fauna in rivers43,45,76, and to our knowledge, none have employed it for such an extended period to monitor the impacts of dams and other human enterprises. However, BRUV systems may revolutionize the way fish monitoring is done in clear-water rivers since it is a cost-effective, non-invasive method to detect a wide range of species45,46. In addition, the exponential advancements in technology in the past decades, including data storage, processing, transferring, and analysis, and camera power77, are proving a unique opportunity to expand the study of fish in freshwaters. In conclusion, institutions responsible for permitting and financing hydropower dam development should prioritize adherence to diverse best practice frameworks in dam monitoring biota throughout their operation. This is crucial for accurately assessing the extensive impacts that may go unnoticed when relying on traditional and outdated sampling methods. Failure to do so may perpetuate the adverse effects on key natural resources, such as fish, which are vital for both ecosystem services (e.g., fisheries) and biodiversity preservation.
Methods
Study area
The Xingu River has a basin area of approximately 450,000 km2 and a length of over 1900 km, crossing the border of the States of Mato Grosso, where its headwaters are located, and Pará50,78. The Xingu River drains through ancient, mainly Precambrian, strongly eroded crystalline bedrock of the Brazilian Shield49,78,79, explaining its low-sediment-carrying clearwater characteristics, with water visibilities that have exceeded 4 m during the low-water period (September to November) in years before the hydropower dam80. Natural river flow fluctuates seasonally, with average flow values varying from ~ 8000 m3 s−1 (rainy season, December to May) to ~ 2000 m3 s−1 (dry season, June to November)81.
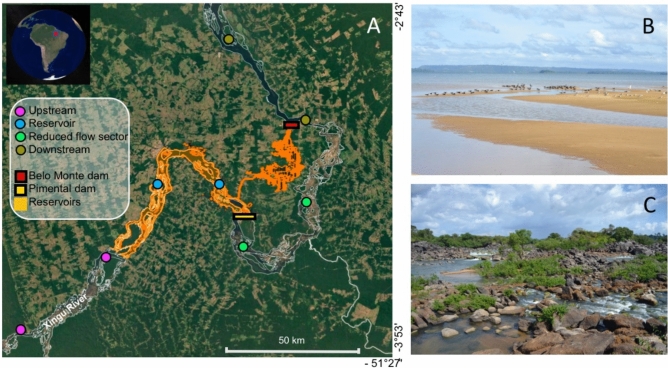
The complex morphology of the Middle Xingu’s fluvial landscape and the extreme hydrological seasonality formed a very diverse array of aquatic habitats, presenting strong variations of hydrochemical compositions, notably during the dry season when the main channel splits into countless small channels, creeks, and isolated ponds48–50. These characteristics and conditions were major drivers that favored the magnificent speciation and adaption found in the Xingu’s fish biodiversity48,49,82. Volta Grande (Big Bend), a stretch of rapids located downstream of the Pimental Dam (Fig. 7A), is an important fish diversity hotspot, harboring almost half of the 63 endemic rheophilic fish species known in the Xingu River49,50.
Fig. 7.
Map (A) shows the location of the sampling units along the Middle Xingu River and the location of the Belo Monte project, which is composed of two main dams: The Belo Monte and the Pimental. Map generated from GOOGLE EARTH. Pictures of the two lotic habitats sampled, submerged shallow sandy beaches (B) and rocky rapids (C), can be found on the right panels. Credit Images: Authors.
Since the planning phase in the 1980s, the Belo Monte project has experienced strong resistance from indigenous and riverine populations, expected to be severely affected by the combined social and environmental impacts. Brazilian and international conservationists as well as the scientific community have continuously raised major concerns regarding the irreversible ecological consequences of this project83–85. The project with its run-of-the-river design includes two dams (Fig. 7): (i) the Pimental dam, located downstream to the city of Altamira, which created the main reservoir; and (ii) the Belo Monte dam, downstream of the intermediate reservoir49,79. In December 2015, the filling of these reservoirs began and was completed in February 2016, by deviating water from the Big Bend area. To reach the maximum power production capacity, a water deviation of up to 80% from this ecologically important river section was planned32,49,79. An extensive environmental impact monitoring program was established in 2010 by the hydropower company and specific fish fauna monitoring is being carried out since the year 2012 by the Federal University of Pará86, through which the fish assemblage data for this study was collected.
Fish sampling
The Middle Xingu River was sampled during the low water season (September–November) from 2014 to 2020, corresponding to two previous (2014–2015) and five posterior years (2016–2020) of the Belo Monte project completion, reservoir filling, and operation (energy production). BRUV systems were deployed on a river stretch of approximately 240 km, including (i) the upstream sector, which was supposedly not hydrologically affected by the dams; (ii) the main reservoir, which was formed by the Pimental dam construction; (iii) the reduced flow sector of the “Volta Grande” (Big Bend) area, downstream to the Pimental dam; and (iv) the downstream sector, located downstream of the Belo Monte dam.
BRUV samplings occurred in two sites per section and two habitats at each site, one formed by the shallow sandy beaches (Fig. 7B) and the other by the rocky rapids (Fig. 7C). Five BRUV samples were acquired per site (10 per sector) and year for sandy beaches (SB) habitats. In the spatially predominant rocky rapids (RO) habitats, it was possible to acquire up to 15 replicate samples in each site (30 per sector) and year. After the dam and reservoir-filling completion in 2016, rocky rapids and sandy beaches became much reduced or inexistent in the main reservoir, being completely submerged and transformed into muddy-bottom habitats. This impaired us from using the BRUV system in several sites of the main reservoir after 2015. For more details about the sampling design and effort, see Table S1.
We used a previously established BRUV sampling protocol for Amazonian clearwater rivers45. BRUV systems consisted of a galvanized iron frame holding a single digital high-definition GoPro Hero 3 + model camera (www.gopro.com), an attached bait arm with a plastic mesh bait bag, filled with 400 g of defrosted, cubed, and crushed ungutted sardine (Sardinella brasiliensis). While it is known that there are fish species in the Amazon that are specialized for nocturnal activity, these do not constitute a distinct guild and are often associated with specific and/or predator avoidance behaviors112. Therefore, to avoid biases from nocturnal and crepuscular species, the BRUV systems were deployed during the daytime period (8:30–17:00 h). After 60 min underwater, the BRUV systems were recovered by boat with the help of signaling buoys attached by floating Polypropylene ropes.
BRUVs were dropped randomly in both habitat types (SB and RO) (Fig. 8) with a minimal lateral distance of 200 m and never positioning any system downstream from another, avoiding potential bait plume overlap45. Video samples were downloaded to external hard disks after each sampling day and underwent visual quality control. In rare cases, samples with deployment or technical (camera or memory card) problems were repeated whenever possible within the tight logistical schedule of the fieldwork campaigns.
Fig. 8.
Visualization of Baited Remote Underwater video (BRUV) deployment across two distinct habitat types along the Middle Xingu River. Credit Images: Authors.
The experimental protocol did not involve handling live vertebrates; only underwater images were acquired, and there was no direct human contact with the fish. In accordance with Brazilian National environmental guidelines, licenses and ethical approvals are deemed necessary solely in cases involving the capture of fauna.
Video analysis
All BRUV samples were analyzed using the free software VLC media player www.videolan.org in the facilities of the Aquatic Ecology Group (GEA) at the Federal University of Pará. The sampling time was standardized to 60 min, counted from the moment the BRUV has been positioned on the substrate45. In the lab, trained personnel identified visually all fishes entering the camera fields to the lowest taxonomic level possible. Water visibility and its variations between sites and/or years were never limiting factors in any of the analyzed video samples. This can be attributed to the fact the sampling periods coincided with clear water conditions in the watershed. The horizontal water visibility consistently ranged between 1.5 and 2 m, enabling the accurate identification and quantification of numerous morphologically similar fish species solely within a relatively short distance between the individuals and the camera (Fig. S8). This procedure ensured that environmental turbidity variations within the typical visibility range did not interfere with abundance estimates. The number of species detected by each BRUV system and the relative abundance (MaxN), corresponding to the maximum number of individuals per species within a paused still frame at any time of the 60 min video footage, were recorded for analysis41,45,87. We used MaxN as a conservative metric of abundance as done in previous BRUV studies (e.g., Ref88,89) to avoid the repeated count of individuals reentering the field of view. Taxa not identified at the species level were described as morphotypes within their genus or family. This was mainly the case for some small species from the Characidae family, such as Hemigrammus spp.
Environmental variables and fishery yield
Three measures of conductivity (mS/cm), turbidity (unt), dissolved oxygen (mg/L), and pH values were randomly taken for each site and sampling year during the BRUV deployments using a multiparameter YSI®. These values were later averaged for statistical analyses to describe the variation in physical–chemical parameters in each site and year. In addition, we obtained water flow measurements (m3 s−1) from water stations located in all four sectors of the Middle Xingu River during all years of our study. These water flow measurements were utilized to calculate five hydrological metrics through the Indicators of Hydrologic Alteration software (IHA;90): (i) Mean monthly flow; (ii) lowest, and (iii) highest flow recorded in the year, which were calculated as the median of 7 days; (iv) annual rate of flow increase, which was estimated as the average of all positive differences between consecutive daily values, and (v) length of the wet season, which was estimated as the number of days in the year that the flow was above 8000 m3s−1. This flow threshold corresponds to the minimum value of river flow observed throughout the entire wet season.
To track potential seasonal and interannual variation caused by large-scale climate effects, we extracted the Multivariate El Niño Southern Oscillation (ENSO) Index Version 2 (herein, MEI) from NOAA’s website91. The index is an expansion of MEI index92 and was produced from an empirical orthogonal function of five oceanic and atmospheric variables (outgoing longwave radiation, surface meridional winds, surface zonal winds, sea surface temperature, and sea level pressure) from the tropical Pacific (30° S–30° N and 100° E–70° W;91). ENSO is known to cause extreme interannual changes in climate in the Amazon Basin and is considered a confounding variable during the study period38,60.
The fishery yield, defined as the total fish landing (in kilograms), of commercial small-scale fisheries was monitored throughout the study period for each sampled sector and sampling year. This monitoring was conducted through a fishery monitoring program funded by Ref93. The monitoring program entailed inspecting a significant proportion of fishing boats arriving at landing ports along the Middle Xingu River. From the fishing monitoring dataset, we specifically extracted data tailored to our sector-based sample design. This encompassed crucial fishery effort metrics, including the number of boats engaged in fishing, fishing days, and fishery yields. These meticulously weighted metrics were then integrated into our model output for comprehensive analysis. Fishery yield reflects, to some extent, fishing effort at each sampled sector, which is known to potentially impact the diversity and total abundance of fish communities in the Amazon basin94–96.
Data analysis
Fish diversity
We analyzed α-, β-, and γ-diversity across the study period to investigate the impacts of the Belo Monte project on fish communities. The species richness of each BRUV sample was considered our measurement of α-diversity. γ-diversity was estimated for each habitat type as the total species richness at each sampling year across all sampling sites. β-diversity was estimated for each habitat type as the dissimilarity between sampling sites in each sampling year. We preferred to use the dissimilarity between sites (i.e., all samples from the same sites were pooled together) rather than samples because a relevant number of samples had a low number of species observed (19% of the samples had up to 2 species), which difficult β-diversity estimates. The dissimilarity was calculated using the Ružička index97 which is equivalent to calculating the Jaccard index for abundance data (in this case, MaxN). MaxN was Hellinger-transformed for β-diversity calculation to avoid issues related to the species abundance paradox98. Finally, we decomposed β-diversity into replacement (i.e., nestedness) and richness difference components (i.e., turnover) according to99. We also calculated local contribution to β-diversity (LCBD) according to100 to better understand the ecological uniqueness of each site along the river.
Spatial–temporal models
We used Hierarchical Generalized Additive Models (HGAM)101 to explore the spatial–temporal variation of fish and environmental attributes. More specifically, fish abundance (MaxN), fish diversity (α, β, γ), and the environmental variables (conductivity, pH, turbidity, dissolved oxygen, fishery yield, MEI, mean monthly flow, lowest and highest flow recorded in the year, annual rate of flow increase, and length of the wet season) were set as response variables in separate HGLM univariate models. For fish abundance (MaxN) and α-diversity (richness), we used BRUV samples as our sampling units. In this case, we used a tensor product (equivalent to an interaction between smooth terms) between year and distance from the Pimental dam as our fixed variables. This tensor product had a single common smoother plus group-level smoothers for different habitat levels (Beach-Rapids), each with different wiggliness (i.e., model GI; Table S2;102). In this sense, habitat was treated as a random effect. We opted to use tensor products rather than separate effects because it is expected that sites along the river will respond differently to the effect of the dams (e.g., upstream sites may be less affected than sites located downstream of the dam). Since α-diversity and MaxN are count variables, we used Poisson and Negative Binomial distributions as our probability distributions, respectively. We had to use a Negative Binomial distribution rather than the Poisson distribution for MaxN to avoid a problem of overdispersion.
For γ- and β-diversity (as well as its components: turnover and nestedness), we used year as our exploratory variable with a single global smoother plus group-level smoothers for different habitat levels. To investigate the spatial–temporal variation in LCBD, once again we used a tensor product between year and distance from the Pimental dam with a single common smoother plus group-level smoothers for different habitat levels. However, in this case, we assumed the same wiggliness between habitats to avoid overfitting the data, which was composed of a lower number of sampling units. Gaussian distribution was used as the probability distribution of the response variables in all cases, except γ-diversity, which was based on Poisson distribution (Table S2).
We used a similar model structure for modeling the environmental variables, but, in this case, without the group-level smoothers for different habitat levels (Table S2). We did not include habitat as a random effect in these models because environmental measurements were not taken for each habitat individually. For all environmental variables, except the length of the wet season (count data—Poisson distribution), the probability distribution used was Gaussian.
Across all models, the thin plate regression spline (TPRS) was used as our smoothing basis. We adjust the number of basis functions (K) across the models to balance computation time and the necessity of having enough degrees of freedom to represent the underlying trends101. More details about model structures can be found in Table S2.
Adding environmental variables to spatial–temporal models
To investigate the importance of environmental variables to explain α-diversity (richness) and MaxN, we conducted additional HGAM models with all possible combinations of physical–chemical variables, hydrological variables, fishery yield, and the MEI. Since both physical–chemical variables and hydrological variables showed a considerable level of autocorrelation, we conducted a principal component analysis (PCA) to reduce each group of variables into a single principal component (PC1) before including them in HGAM models. The first PCA axis of the physical–chemical variables explained 41% of the data, being positively correlated with conductivity and turbidity, and negatively associated with DO and pH (Table S3). Conversely, the first PCA axis of the hydrological variables explained 73% of the data and was negatively associated with all hydrological parameters (Table S3).
Overall, 16 alternative HGAM models were created (Table S4). In all models, we included two fixed co-variables, year and distance from the Pimental dam, and one random effect, habitat. This was necessary to (1) investigate the gain of information by including environmental variables in spatial–temporal models; (2) control for spatial–temporal autocorrelation and meet the residual independence assumption101; and (3) deal with richness and MaxN differences between habitats. All fixed effects included in the models had both a single global smoother. We did not include tensor products and group-level smoothers in the models to avoid data overfitting. Smoothing basis and probability distribution followed the same structure detailed in the section above (Spatial–temporal models).
Model selection was based on the second-order variant of Akaike’s Information Criterion suited for small samples (AICc), which is more conservative than AIC due to its extra penalty term for the number of parameters103. Under this statistical framework, the best model is the simplest model within two AICc units of the lowest AICc value (ΔAICc < 2;101). Because the differences between the best model and other model candidates were usually small, we also estimated the relative importance of our environmental variables by summing all Akaike weights over all models that include each environmental predictor103. The relative importance varies from 0 to 1 and the larger the value of the relative importance of a predictor, the more important it is compared to the others.
All analyses were conducted in R104. β-diversity and its decomposition were carried out in the adespatial package105. HGAMs were conducted in the mgcv package101. We also used MuMIn106 for model selection procedures, and mgcViz107, sjPlot108, and ggplot2109 for models’ visualization.
Supplementary Information
Acknowledgements
Our thanks to the editor and reviewers for the constructive comments and recommendations, which improved quality of the paper. Authors thank grants provided by the National Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Education Personnel, and the Research Development and Support Foundation (Fadesp). This study was financed in part by the Coordenação de Aperfeiçoamento do Pessoal de Nível Superior - Brasil (CAPES) – Finance Code 001 (K.S.). Simone Franceschini and Tommaso Russo contributed within the activity of the Go For It Project “Progetto di conservazione dell’ittiofauna del fiume Xingu nell’area di influenza della diga idroelettrica di Belo Monte”, funded by the Ministero dell'Università e della Ricerca (MUR, Italy), in collaboration between the University of Rome Tor Vergata and the Federal University of Pará. JAR-F received financial support post-doctoral fellowship from FADESP (#339020/2022). FWK would like to thank the support provided by CNPq (grant number 150773/2024-2), FADESP, and Federal University of Rio Grande do Norte (UFRN). TG is funded by the Brazilian National Council for Scientific and Technological Development, CNPq (#308528/2022-0). Publication support was guaranteed from PAPQ/PROPESP 02/2023 program of Universidade Federal do Pará.
Author contributions
K.S. Conceptualization, Methodology, Investigation, Data Curation, Writing—Original Draft, Writing—Review & Editing. F.W.K. Formal Analysis, Visualization, Writing—Original Draft, Writing—Review & Editing. S.F. Formal Analysis, Writing—Review & Editing. J.B. Writing—Review & Editing, Supervision. F.R.M.S. Methodology, Investigation, and Review. J.H.S.S. Methodology, Investigation, and Review. T.R. Writing—Review & Editing, Supervision. T.G. Conceptualization, Methodology, Investigation, Funding acquisition, Project administration, Supervision, Writing—Review & Editing. J.A.R.F. Methodology, Investigation, Writing—Review & Editing. All authors reviewed the manuscript.
Funding
This study was funded by Norte Energia S.A. (P&D-02-2020) and Fundação de Amparo e Desenvolvimento da Pesquisa (FADESP) (PR-C-006/2020). The funders had no role in the design, execution, or analyses of this study.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kurt Schmid and Friedrich Wolfgang Keppeler.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-70636-8.
References
- 1.Keijzer, T. et al. Threats of dams to persistence of the word’s freshwater fishes. Glob. Change Biol.10.1111/gcb.17166 (2024). [Google Scholar]
- 2.Lange, K. et al. Basin-scale effects of small hydropower on biodiversity dynamics. Front. Ecol. Environ.16, 397–404 (2018). [Google Scholar]
- 3.Zhang, A. T. & Gu, V. X. Global dam tracker: A database of more than 35,000 dams with location, catchment, and attribute information. Sci. Data10, 111. 10.1038/s41597-023-02008-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao, X. et al. Regime shift in fish assemblage structure in the Yangtze River following construction of the Three Gorges dam. Sci. Rep.9, 4212 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arthington, A. H., Dulvy, N. K., Gladstone, W. & Winfield, I. J. Fish conservation in freshwater and marine realms: Status, threats and management. Aquat. Conserv. Mar. Freshw. Ecosyst.26, 838–857. 10.1002/aqc.2712 (2016). [Google Scholar]
- 6.Fearnside, P. M. Impacts of Brazil’s Madeira River dams: Unlearned lessons for hydroelectric development in Amazonia. Environ. Sci. Policy38, 164–172 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Barbarossa, V. et al. Impacts of current and future large dams on the geographic range connectivity of freshwater fish worldwide. Proc. Natl. Acad. Sci.117, 3648–3655. 10.1073/pnas.1912776117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agostinho, A. A., Gomes, L. C., Santos, N. C., Ortega, J. C. & Pelicice, F. M. Fish assemblages in Neotropical reservoirs: Colonization patterns, impacts and management. Fish. Res.173, 26–36 (2016). [Google Scholar]
- 9.Winemiller, K. O. & Jepsen, D. B. Effects of seasonality and fish movement on tropical river food webs. J. Fish Biol.53, 267–296 (1998). [Google Scholar]
- 10.Maavara, T., Lauerwald, R., Regnier, P. & Van Cappellen, P. Global perturbation of organic carbon cycling by river damming. Nat. Commun.8, 15347. 10.1038/ncomms15347 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winton, R. S., Calamita, E. & Wehrli, B. Reviews and syntheses: Dams, water quality and tropical reservoir stratification. Biogeosciences16, 1657–1671. 10.5194/bg-16-1657-2019 (2019). [Google Scholar]
- 12.Havel, J. E., Lee, C. E. & Zanden, J. M. V. Do reservoirs facilitate invasions into landscapes?. Bioscience55, 518–525. 10.1641/0006-3568(2005)055[0518:DRFIIL]2.0.CO;2 (2005). [Google Scholar]
- 13.Johnson, P. T., Olden, J. D. & Zanden, J. M. V. Dam invaders: Impoundments facilitate biological invasions into freshwaters. Front. Ecol. Environ.6, 357–363. 10.1890/070156 (2008). [Google Scholar]
- 14.Caiola, N., Ibáñez, C., Verdú, J. & Munné, A. Effects of flow regulation on the establishment of alien fish species: A community structure approach to biological validation of environmental flows. Ecol. Indic.45, 598–604. 10.1016/j.ecolind.2014.05.012 (2014). [Google Scholar]
- 15.Laurance, W. F., Goosem, M. & Laurance, S. G. W. Impacts of roads and linear clearings on tropical forests. Trends Ecol. Evol.24, 659–669. 10.1016/j.tree.2009.06.009 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Petesse, M. L. & Petrere, M. Jr. Tendency towards homogenization in fish assemblages in the cascade reservoir system of the Tietê river basin, Brazil. Ecol. Eng.48, 109–116 (2012). [Google Scholar]
- 17.Liu, X., Olden, J. D., Wu, R., Ouyang, S. & Wu, X. Dam construction impacts fish biodiversity in a subtropical river network, China. Diversity14, 476. 10.3390/d14060476 (2022). [Google Scholar]
- 18.Ganassin, M. J. M. et al. Effects of reservoir cascades on diversity, distribution, and abundance of fish assemblages in three Neotropical basins. Sci. Total Environ.778, 146246 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Hoeinghaus, D. J. et al. Effects of river impoundment on ecosystem services of large tropical rivers: Embodied energy and market value of artisanal fisheries. Conserv. Biol.23, 1222–1231 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Santos, N. C. L. et al. Environmental filters predict the trait composition of fish communities in reservoir cascades. Hydrobiologia802, 245–253 (2017). [Google Scholar]
- 21.Agostinho, A. A., Pelicice, F. M. & Gomes, L. C. Dams and the fish fauna of the Neotropical region: Impacts and management related to diversity and fisheries. Braz. J. Biol.68, 1119–1132 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Turgeon, K., Turpin, C. & Gregory-Eaves, I. Dams have varying impacts on fish communities across latitudes: A quantitative synthesis. Ecol. Lett.22, 1501–1516. 10.1111/ele.13283 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Liew, J. H., Tan, H. H. & Yeo, D. C. J. Dammed rivers: Impoundments facilitate fish invasions. Freshw. Biol.61, 1421–1429. 10.1111/fwb.12781 (2016). [Google Scholar]
- 24.Anderson, E. P., Pringle, C. M. & Rojas, M. Transforming tropical rivers: An environmental perspective on hydropower development in Costa Rica. Aqua. Conserv. Mar. Freshw. Ecosyst.16, 679–693 (2006). [Google Scholar]
- 25.Bilotta, G. S., Burnside, N. G., Gray, J. C. & Orr, H. G. The effects of run-of-river hydroelectric power schemes on fish community composition in temperate streams and rivers. PloS One11, e0154271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lange, K. et al. Small hydropower goes unchecked. Front. Ecol. Environ.17, 256–258. 10.1002/fee.2049 (2019). [Google Scholar]
- 27.Fearnside, P. M. Environmental and social impacts of hydroelectric dams in Brazilian Amazonia: Implications for the aluminum industry. World Dev.77, 48–65 (2016). [Google Scholar]
- 28.Winemiller, K. O. et al. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science351, 128–129. 10.1126/science.aac7082 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Arantes, C. C., Fitzgerald, D. B., Hoeinghaus, D. J. & Winemiller, K. O. Impacts of hydroelectric dams on fishes and fisheries in tropical rivers through the lens of functional traits. Curr. Opin. Environ. Sustain.37, 28–40 (2019). [Google Scholar]
- 30.Tisseuil, C. et al. Global diversity patterns and cross-taxa convergence in freshwater systems. J. Anim. Ecol.82, 365–376 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Val, A. L. Fishes of the Amazon: Diversity and beyond. Anais da Academia Brasileira de Ciências91, 1–9. 10.1590/0001-3765201920190260 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Lees, A. C., Peres, C. A., Fearnside, P. M., Schneider, M. & Zuanon, J. A. Hydropower and the future of Amazonian biodiversity. Biodivers. Conserv.25, 451–466 (2016). [Google Scholar]
- 33.Latrubesse, E. M. et al. Damming the rivers of the Amazon basin. Nature546, 363–369 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Lima, M. A. L., Carvalho, A. R., Nunes, M. A., Angelini, R. & Doria, C. R. C. Declining fisheries and increasing prices: The economic cost of tropical rivers impoundment. Fish. Res.221, 105399 (2020). [Google Scholar]
- 35.Flecker, A. S. et al. Reducing adverse impacts of Amazon hydropower expansion. Science375, 753–760. 10.1126/science.abj4017 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Arantes, C. C. et al. Functional responses of fisheries to hydropower dams in the Amazonian Floodplain of the Madeira River. J. Appl. Ecol.59, 680–692. 10.1111/1365-2664.14082 (2021). [Google Scholar]
- 37.Mendes, Y. A. et al. Sedentary fish as indicators of changes in the river flow rate after impoundment. Ecol. Indic.125, 107466. 10.1016/j.ecolind.2021.107466 (2021). [Google Scholar]
- 38.Keppeler, F. W. et al. Early impacts of the largest Amazonian hydropower project on fish communities. Sci. Total Environ.838, 155951. 10.1016/j.scitotenv.2022.155951 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Townsend, S., C. et al. Monitoring river health in the wet–dry tropics: strategic considerations, community participation and indicators. TRaCK publication, Darwin. http://www.nespnorthern.edu.au/wp-content/uploads/2016/02/TRaCK_Monitoring-River-Health_FINAL.pdf. (2012).
- 40.Sward, D., Monk, J. & Barrett, N. A systematic review of remotely operated vehicle surveys for visually assessing fish assemblages. Front. Mar. Sci.6, 134. 10.3389/fmars.2019.00134 (2019). [Google Scholar]
- 41.Cappo, M., Speare, P. & De’ath, G. Comparison of baited remote underwater video stations (BRUVS) and prawn (shrimp) trawls for assessments of fish biodiversity in inter-reefal areas of the Great Barrier Reef Marine Park. J. Exp. Mar. Biol. Ecol.302, 123–152 (2004). [Google Scholar]
- 42.Harvey, E. S. et al.The Use of BRUVs as a Tool for Assessing Marine Fisheries and Ecosystems: A Review of the Hurdles and Potential (The University of Western Australia, 2013). [Google Scholar]
- 43.Ebner, B. & Morgan, D. Using remote underwater video to estimate freshwater fish species richness. J. Fish Biol.82, 1592–1612 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Cousins, S., Kennard, M. J. & Ebner, B. C. Corrigendum to: Depth-related composition and structuring of tropical riverine fish assemblages revealed by baited video. Mar. Freshw. Res.68, 1976–1976 (2017). [Google Scholar]
- 45.Schmid, K., Reis-Filho, J. A., Harvey, E. & Giarrizzo, T. Baited remote underwater video as a promising nondestructive tool to assess fish assemblages in clearwater Amazonian rivers: Testing the effect of bait and habitat type. Hydrobiologia784, 93–109 (2017). [Google Scholar]
- 46.Watson, D. L., Harvey, E. S., Anderson, M. J. & Kendrick, G. A. A. Comparison of temperate reef fish assemblages recorded by three underwater stereo-video techniques. Mar. Biol.148, 415–425 (2005). [Google Scholar]
- 47.Sherman, C. S. et al. Repeatability of baited remote underwater video station (BRUVS) results within and between seasons. PLoS ONE15, e0244154. 10.1371/journal.pone.0244154 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Camargo, M., Giarrizzo, T. & Isaac, V. Review of the geographic distribution of fish fauna of the Xingu River basin, Brazil. Ecotropica10, 123–147 (2004). [Google Scholar]
- 49.Pérez, M. S. Where the Xingu bends and will soon break. Am. Sci.103, 395–403 (2015). [Google Scholar]
- 50.Fitzgerald, D. B. et al. Diversity and community structure of rapids-dwelling fishes of the Xingu River: Implications for conservation amid large-scale hydroelectric development. Biol. Conserv.222, 104–112. 10.1016/j.biocon.2018.04.002 (2018). [Google Scholar]
- 51.Sofi, M. S., Bhat, S. U., Rashid, I. & Kuniyal, J. C. The natural flow regime: A master variable for maintaining river ecosystem health. Ecohydrology13(8), e2247. 10.1002/eco.2247 (2020). [Google Scholar]
- 52.Grill, G. et al. Mapping the world’s free-flowing rivers. Nature569, 215–221 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Fukushima, M., Jutagate, T., Grudpan, C., Phomikong, P. & Nohara, S. Potential effects of hydroelectric dam development in the Mekong River basin on the migration of Siamese mud carp (Henicorhynchus siamensis and H. lobatus) elucidated by otolith microchemistru. PLoS One9, e103722. 10.1371/journal.pone.0103722 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ngor, P. B., Legendre, P., Oberdorff, T. & Lek, S. Flow alterations by dams shaped fish assemblage dynamics in the complex Mekong-S river system. Ecol. Indic.88, 103–114 (2018). [Google Scholar]
- 55.Agostinho, A. et al. Patterns of colonization in neotropical reservoirs, and prognoses on aging. In Theoretical Reservoir Ecology and Its Applications (eds Tundisi, J. G. & Straskraba, M.) 227–265 (Backhuys Publishers, 1999). [Google Scholar]
- 56.Cella-Ribeiro, A., Costa Doria, C. R., Dutka-Gianelli, J., Alves, H. & Torrente-Vilara, G. Temporal fish community responses to two cascade run-of-river dams in the Madeira River, Amazon basin. Ecohydrology10, e188 (2017). [Google Scholar]
- 57.Welcomme, R.L. & Halls, A. Dependence of tropical river fisheries on flow. In: Proceedings of the Second International Symposium on the Management of Large Rivers for Fisheries (Volume II): Sustaining Livelihoods and Biodiversity in the New Millennium. Food and Agriculture Organization of the United Nations (RAP Publication 2004/16), Phnom Penh. pp. 267–283 (2004).
- 58.Agostinho, A. A. et al. Fish die-off in river and reservoir: A review on anoxia and gas supersaturation. Neotrop. Icthyology19, e210037 (2021). [Google Scholar]
- 59.Rima (Relatório de Impacto Ambiental). Aproveitamento hidrelétrico Belo Monte, accessed 1 June 2021; https://eletrobras.com/pt/Paginas/Belo-Monte.aspx (2009).
- 60.Jiménez-Muñoz, J. C. et al. Record-breaking warming and extreme drought in the Amazon rainforest during the course of El Niño 2015–2016. Sci. Rep.6, 33130. 10.1038/srep33130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Araújo, E. S. et al. Changes in distance decay relationships after river regulation: Similarity among fish assemblages in a large Amazonian River. Ecol. Freshw. Fish22, 543–552. 10.1111/eff.12054 (2013). [Google Scholar]
- 62.Junk, W. J., Bayley, P. B. & Sparks, R. E. The flood pulse conception in river-floodplain systems. Can. Spec. Public. Fish. Aqua. Sci.106, 110–127 (1989). [Google Scholar]
- 63.Andrade, M. C., Fitzgerald, D. B., Winemiller, K. O., Barbosa, P. S. & Giarrizzo, T. Trophic niche segregation among herbivorous serrasalmids from rapids of the Lower Xingu River, Brazilian Amazon. Hydrobiologia829, 265–280. 10.1007/s10750-018-3838-y (2019). [Google Scholar]
- 64.Bower, L. M. et al. Effects of hydrology on fish diversity and assemblage structure in a Texan coastal plains river. Trans. Am. Fish. Soc.148, 207–218. 10.1002/tafs.10129 (2019). [Google Scholar]
- 65.Isaac, V. J., Almeida, M. C., Cruz, R. E. A. & Nunes, L. G. Artisanal fisheries of the Xingu River basin in Brazilian Amazon. Braz. J. Biol.75, 125–137 (2015). [DOI] [PubMed] [Google Scholar]
- 66.McCartney, M., Funge-Smith, S. & Kura, Y. Enhancing fisheries productivity through improved management of reservoirs, dams and other water control structures. Penang, Malaysia: CGIARResearch Program on Fish Agri-Food Systems. Program Brief: FISH-2018-11 (2018).
- 67.Rands, M. R. W. et al. Biodiversity conservation: Challenges beyond 2010. Science329, 1298–1303 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Anderson, E. P. et al. Fragmentation of Andes-to-Amazon connectivity by hydropower dams. Sci. Adv.4, 1642 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cutler, J. S., Olivos, A., Sidlauskas, B. & Arismendi, I. Habitat loss due to dam development may affect the distribution of marine-associated fishes in Gabon, Africa. Ecosphere11, e03024. 10.1002/ecs2.3024 (2020). [Google Scholar]
- 70.Barthem, R.B., de Brito Ribeiro, M.C.L. & Petrere, M. Life strategies of some long-distance migratory catfish in relation to hydroelectric dams in the Amazon Basin. Biological Conservation55, 339–345 (1991).
- 71.McLaughlin, R. L. et al. Effects of lowhead barriers on stream fishes: Taxonomic affiliations and morphological correlates of sensitive species. Can. J. Fish. Aqua. Sci.63, 766–779 (2006). [Google Scholar]
- 72.Leibold, M. A. et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett.7, 601–613. 10.1111/j.1461-0248.2004.00608.x (2004). [Google Scholar]
- 73.Chase, J. M. & Leibold, M. A. Metacommunity Ecology (Princeton University Press, 2017). [Google Scholar]
- 74.IBAMA (Brazilian Institute of Environment and Renewable Natural). Resolução 911, de 07 de julho de 2014. https://www.ibama.gov.br/component/legislacao/?view=legislacao&legislacao=133281 (2014)
- 75.Poff, N. L., Olden, J. D., Merritt, D. M. & Pepin, D. M. Homogenization of regional river dynamics by dams and global biodiversity implications. Proc. Natl. Acad. Sci.104, 5732–5737. 10.1073/pnas.0609812104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ebner, B. R. et al. Filming and snorkelling as visual techniques to survey fauna in difficult to access tropical rainforest streams. Mar. Freshw. Res.66, 120 (2014). [Google Scholar]
- 77.Allan, B. M. et al. Futurecasting ecological research: The rise of technoecology. Ecosphere9, e02163. 10.1002/ecs2.2163 (2018). [Google Scholar]
- 78.Sioli, H. Amazônia: Fundamentos da Ecologia da Maior Região de Florestas Tropicais. Vozes, Petrópolis (1985).
- 79.Hahn, L. et al. Biotelemetry reveals migratory behaviour of large catfish in the Xingu River, Eastern Amazon. Sci. Rep.9, 464. 10.1038/s41598-019-44869-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barthem, R. B. & Fabré, N. N. Biologia e diversidade dos recursos pesqueiros da Amazônia. A pesca e os recursos pesqueiros na Amazônia brasileira1, 17–62 (2004). [Google Scholar]
- 81.Camargo, M. & Ghilardi, J.R.R. Entre a Terra, as Águas e os Pescadores do Médio Rio Xingu - Uma Abordagem Ecológica. Mauricio Camargo, Belém (2009).
- 82.Zuanon, J.A.S. História Natural da Ictiofauna de Corredeiras do Rio Xingu, na Região de Altamira, Pará. PhD dissertation, Universidade Estadual de Campinas, Campinas (1999).
- 83.Villas-Bôas, A., Garzón, B.R., Reis, C., Amorim, L. & Leite, L. Dossiê Belo Monte: Não Há Condições para a Licença de Operação. Instituto Socioambiental (ISA), Brasília. http://t.co/zjnVPhPecW (2015).
- 84.Fearnside, P. M. Belo Monte: actors and arguments in the struggle over Brazil’s most controversial Amazonian dam. DIE ERDE J. Geograph. Soc. Berlin148, 14–26 (2017). [Google Scholar]
- 85.Fearnside, P. M. Brazil’s Belo Monte dam: Lessons of an Amazonian resource struggle. DIE ERDE J. Geograph. Soc. Berlin148, 167–184 (2017). [Google Scholar]
- 86.Norte Energia. Projeto Básico Ambiental da Usina Hidrelétrica Belo Monte. Planos, programas e projetos. Norte Energia, Brasília (2010).
- 87.Harvey, E. S., Cappo, M., Butler, J. J., Hall, N. & Kendrick, G. A. Bait attraction affects the performance of remote underwater video stations in assessment of demersal fish community structure. Mar. Ecol. Progr. Ser.350, 245–254. 10.3354/meps07192 (2007). [Google Scholar]
- 88.Ellis, D. & DeMartini, E. Evaluation of video camera technique for indexing abundances of juvenile pink snapper, Pristipomoides filamentosus, and other Hawaiian insular shelf fishes. Fish. Bull.93, 67–77 (1995). [Google Scholar]
- 89.Willis, T. J. & Babcock, R. C. A baited underwater video system for the determination of relative density of carnivorous reef fish. Mar. Freshw. Res.51, 755–763 (2000). [Google Scholar]
- 90.The Nature Conservancy. Indicators of hydrologic alteration (Version 7.1). https://www.conservationgateway.org/ConservationPractices/Freshwater/EnvironmentalFlows/MethodsandTools/IndicatorsofhydrologicAlteration/Pages/indicators-hydrologicalt.aspx (2009).
- 91.NOAA (National Oceanic and Atmospheric Administration), accessed 1 June 2021. Multivariate ENSO index version 2 (MEI.v2). https://psl.noaa.gov/enso/mei/ (2021).
- 92.Wolter, K. & Timlin, M. S. El Niño/southern oscillation behaviour since 1871 as diagnosed in an extended multivariate ENSO index (MEI.ext). Int. J. Climatol.31, 1074–1087. 10.1002/joc.2336 (2011). [Google Scholar]
- 93.Norte Energia. 19o Relatório Final Consolidado de Andamento do PBA e do Atendimento de Condicionantes. Norte Energia, Brasília (2021).
- 94.Castello, L. et al. The vulnerability of Amazon freshwater ecosystems. Conserv. Lett.6, 217–229. 10.1111/conl.12008 (2013). [Google Scholar]
- 95.Silvano, R. A. M. et al. Co-management and spatial features contribute to secure fish abundance and fishing yields in tropical floodplain lakes. Ecosystems17, 271–285. 10.1007/s10021-013-9722-8 (2014). [Google Scholar]
- 96.Keppeler, F. W. et al. Ecological influences of human population size and distance to urban centres on fish communities in tropical lakes. Aqua. Conserv. Mar. Freshw. Ecosyst.28, 1030–1043. 10.1002/aqc.2910 (2018). [Google Scholar]
- 97.Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr.23, 1324–1334. 10.1111/geb.12207 (2014). [Google Scholar]
- 98.Legendre, P. & Gallagher, E. D. Ecologically meaningful transformations for ordination of species data. Oecologia129, 271–280. 10.1007/s004420100716 (2001). [DOI] [PubMed] [Google Scholar]
- 99.Podani, J., Ricotta, C. & Schmera, D. A general framework for analyzing beta diversity, nestedness and related community-level phenomena based on abundance data. Ecol. Complex.15, 52–61. 10.1016/j.ecocom.2013.03.002 (2013). [Google Scholar]
- 100.Legendre, P. & De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett.16, 951–963. 10.1111/ele.12141 (2013). [DOI] [PubMed] [Google Scholar]
- 101.Wood, S. N. Generalized Additive Models: An Introduction with R (Chapman and Hall/CRC, 2017). [Google Scholar]
- 102.Pedersen, E. J., Miller, D. L., Simpson, G. L. & Ross, N. Hierarchical generalized additive models in ecology: An introduction with mgcv. PeerJ7, e6876. 10.7717/peerj.6876 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: An Information Theoretic Approach (Springer, 2002). [Google Scholar]
- 104.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/ (2021).
- 105.Dray, S. et al. adespatial: multivariate multiscale spatial analysis. R package version 0.3–14. https://CRAN.R-project.org/package=adespatial (2021).
- 106.Barton, K. MuMIn: multi-model inference. R package version 1.43.17. https://CRAN.R-project.org/package=MuMIn (2020).
- 107.Fasiolo, M., Nedellec, R., Goude, Y. & Wood, S. N. Scalable visualisation methods for modern generalized additive models. J. Comput. Graph. Stat.29, 78–86. 10.1080/10618600.2019.1629942 (2020). [Google Scholar]
- 108.Lüdecke, D. sjPlot: data visualization for statistics in social science. R package version 2.8.9. https://CRAN.R-project.org/package=sjPlot (2021).
- 109.Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, 2016). [Google Scholar]
- 110.Consuegra, S. et al. Impacts of large and small barriers on fish assemblage composition assessed using environmental DNA metabarcoding. Sci. Total Environ.790, 148054. 10.1016/j.scitotenv.2021.148054 (2021). [DOI] [PubMed] [Google Scholar]
- 111.Reis-Filho, J. A. & Leduc, A. O. H. C. Balancing renewable energy and river conservation: effect of hydropeaking from small hydroelectric power plants on fish stranding in small Brazilian rivers. Aqua. Ecol.10.1007/s10452-024-10090-w (2024). [Google Scholar]
- 112.Sazima, I., Carvalho, L. N., Mendonça, F. P. & Zuanon, J. Fallen leaves on the water-bed: Diurnal camouflage of three night active fish species in an Amazonian streamlet. Neotrop. Ichthyology4, 119–122 (2006). [Google Scholar]
- 113.Zolderdo, A. J. et al. Evidence of fish spillover from freshwater protected areas in lakes of eastern Ontario. Aqu. Conserv. Mar. Freshw. Ecosyst.10.1002/aqc.3155 (2019). [Google Scholar]
- 114.Li, Q. et al. Effects of low-head dams on fish assemblages in subtropical streams: Context dependence on local habitat and landscape conditions. Ecol. Indic.121, 107190. 10.1016/j.ecolind.2020.107190 (2021). [Google Scholar]
- 115.Tweedie, J. B., Cockburn, J. M. H. & Villard, P. V. The potential use of remote underwater video (RUV) to evaluate small-bodied fish assemblages. Hydrobiology2, 507–520 (2023). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.