Abstract
This study aims to investigate the potential relationships between the systemic immune-inflammation index (SII) and body mass index (BMI), waist circumference, and the prevalence of obesity. A cross-sectional analysis was conducted on 7,645 individuals aged 20 and above from the NHANES 2017–2020. Multivariate linear regression analyses were conducted to evaluate the association of the logarithmically transformed SII (lgSII) with BMI and waist circumference. Additionally, multivariable logistic regression was utilized to explore the relationship between lgSII and the prevalence of obesity. Fitted smoothing curves and threshold-effect analysis were applied to elucidate nonlinear relationships. In the fully adjusted model, a positive relationship was observed between lgSII and BMI, waist circumference, and obesity prevalence (β = 3.13, 95% CI 2.10–4.16; β = 7.81, 95% CI 5.50–10.13; OR = 1.44, 95% CI 1.12–1.86). The variables of gender, age, race, education, marital status, poverty income ratio (PIR), energy intake, sleep disorder, smoking status, and alcohol use did not significantly modify the positive association between lgSII and obesity. However, physical activity appeared to influence the positive correlation between lgSII and obesity. Using a two-segment linear regression model, an inverted U-shaped relationship was observed between lgSII and both BMI and waist circumference. Furthermore, lgSII demonstrated a linear positive correlation with obesity prevalence. When stratified by physical activity, lgSII showed a non-significant negative correlation with obesity in the physically active group. Our findings underscore a robust association between the logarithmically transformed SII and BMI, waist circumference, and the prevalence of obesity.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73659-3.
Subject terms: Diseases, Health care, Medical research, Risk factors
Introduction
Obesity represents a significant public health challenge globally, contributing to reduced life expectancy, heightened disease risk, and increased healthcare costs worldwide1. The global prevalence of obesity is projected to rise from 14% in 2020 to 24% by 2035, affecting approximately 2 billion adults, children, and adolescents2. The primary comorbidities and complications associated with obesity encompass type 2 diabetes mellitus (T2DM)3, cardiovascular disease4,5, sleep apnea6, chronic kidney disease7, gastrointestinal disorders1, and an elevated risk of various malignancies8–10. Obesity results from a prolonged imbalance between energy intake and expenditure and is a multifactorial condition influenced by genetic, epigenetic, physiological, behavioural, sociocultural and environmental factors1.
Inflammation is thought to be an important mechanism in the pathogenesis of obesity and its associated diseases, and drugs or diets may reduce obesity by alleviating inflammation11–16. Wang et al. found that Toll-like receptor 9 (TLR9) deficiency in B cells leads to obesity by promoting inflammation and gut dysbiosis11. Some researchers have also suggested that targeting the Nod-like receptor protein 3 (NLRP3) inflammasome may be a chief instigator of obesity17. Several blockers targeting the NLRP3 inflammasome have been effective in treating obesity and obesity-induced insulin resistance18. Many anti-obesity drugs have been found to have anti-inflammatory effects that start even before meaningful weight loss is achieved, the anti-inflammatory action might help to boost their effectiveness against obesity14. For example, selegiline has been found to reduce obesity by inhibiting oxidative stress and inflammation19. In addition, it has also been proposed that anti-inflammatory diets play an essential role in the management of obesity by affecting the intestinal flora and controlling inflammatory responses15. Inflammation also plays a crucial role in the pathogenesis of various obesity-related complications, including fatty liver, cardiovascular disease, insulin resistance in T2DM, asthma, neurodegeneration, and certain cancers20,21. Consequently, reducing inflammation is vital for improving obesity and associated metabolic dysfunctions. However, population-based research exploring the correlation between inflammation and obesity remains limited.
The systemic immune-inflammation index (SII), introduced by Hu et al. in 2014, is a novel and integrated immunoinflammatory biomarker. Based on platelet, neutrophil, and lymphocyte counts, it effectively reflects both local immune response and systemic inflammation22,23. Initially, SII was widely used to assess prognosis in various cancer types, including hepatocellular carcinoma22, non-small cell lung cancer24, gastric cancer25, rectal cancer26, and pancreatic cancer27. It has also been demonstrated to have significant prognostic value in cardiovascular diseases28–30. Moreover, several studies utilizing the NHANES database have identified correlations between SII and metabolic diseases such as hyperlipidemia31, diabetic nephropathy32, hepatic steatosis23, and metabolic syndrome33. A population-based study involving 223 adult obese individuals revealed an increase in SII with body mass index (BMI)34, whereas a study in obese children did not show a corresponding increase in SII with rising BMI35. There is a notable absence of nationwide population-based studies confirming the relationship between SII and obesity prevalence.
Therefore, this study was conducted to determine the association between SII and BMI, waist circumference, and obesity prevalence in adult participants from the National Health and Nutrition Examination Survey (NHANES).
Methods
Data sources and study population
This study employed a cross-sectional design to analyse data from the National Health and Nutrition Examination Survey (NHANES) collected between 2017 and March 2020, prior to the pandemic. NHANES, a national survey conducted by the Centers for Disease Control and Prevention’s National Center for Health Statistics (NCHS), aims to evaluate the health and nutritional status of individuals residing in the United States36. The NHANES investigation protocol was approved by the NCHS Ethics Review Committee, and informed consent was obtained in writing from each participant. As the NHANES database is publicly accessible, the present study did not require additional ethical approval. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cross-sectional studies37. Comprehensive information on NHANES study designs and data is available at http://www.cdc.gov/nchs/nhanes/.
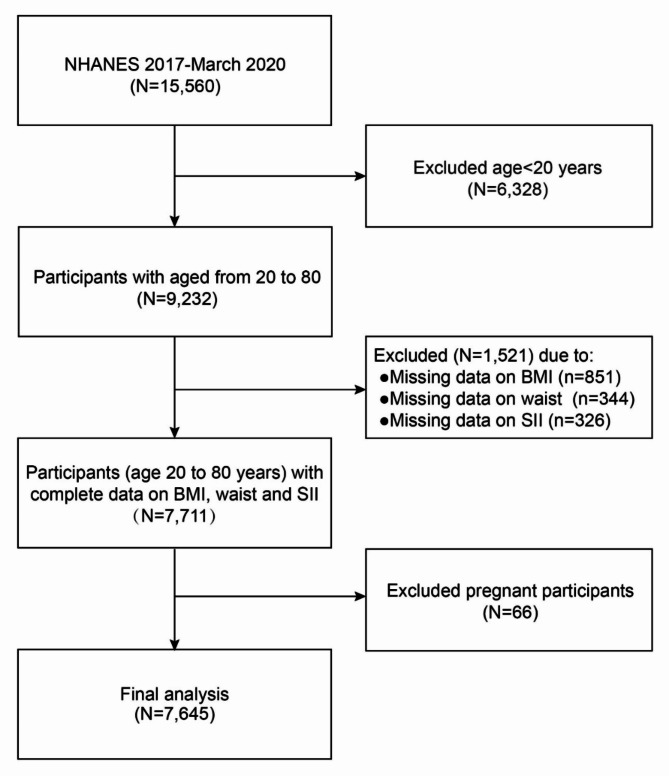
From the NHANES database, 15,560 participants were initially considered for the 2017–March 2020 survey cycle. The study excluded 6,328 participants under 20 years of age, 851 participants with missing BMI data, 344 with incomplete waist circumference data, 326 with insufficient SII data, and 66 pregnant women. Consequently, 7,645 participants were included in this study. Figure 1 illustrates the flowchart of sample selection.
Fig. 1.
The flowchart of participants selection.
Exposure and outcome definition
In this analysis, the systemic immune-inflammation index (SII) was regarded as the exposure variable. SII is calculated by multiplying the neutrophil-to-lymphocyte ratio (N/L) with the total peripheral platelet count (SII = N/L × P)22. Neutrophil, lymphocyte, and platelet counts were measured using the Beckman Coulter DxH 800 instrument at the NHANES mobile examination center (MEC) and are presented as ×10³ cells/µl. Body mass index (BMI), waist circumference, and obesity were designated as outcome variables. The body measures data were collected by trained health technicians in accordance with the NHANES Anthropometry Procedures Manual in the MEC. Height, weight and waist circumference were measured using a stadiometer, a digital weight scale and a steel measuring tape, respectively. BMI was calculated by dividing the weight in kilograms by the square of the height in meters. Obesity was defined as having a BMI of 30 kg/m² or higher, in conjunction with a waist circumference exceeding 88 cm for women and 102 cm for men38.
Selection of covariates
In this study, we considered several covariates as potential confounders in our statistical model: demographic factors (age, gender, race, marital status, educational level, and income), and lifestyle variables (smoking status, alcohol consumption, physical activity, sleep disorders, and energy intake). These covariates are elaborated in Table 1. The basis for selecting covariates is described in Table S1 in the supplementary material. Race was categorised into Mexican American, non-Hispanic white, non-Hispanic black, and other. Household income was defined using the poverty income ratio (PIR) and categorised as less than 1.3, 1.3 to 3.5, and more than 3.539. Educational attainment was classified as less than high school, high school, and more than high school. Marital status was grouped as married/living with partner, widowed/divorced/separated, and never married. Energy intake was assessed by a 24-hour dietary recall interview, and the average of day 1 and day 2 energy intake was taken for this study. Smoking status was categorised into three groups: never smoker (fewer than 100 cigarettes prior to the survey), former smoker (100 or more cigarettes prior to the survey but ceased smoking before the survey), and current smoker (100 or more cigarettes prior to the survey and smoked during the survey period)40. Participants who consumed 12 or more drinks (A drink means 12 oz beer, 5 oz wine, or 1.5 oz liquor) in a year were classified as alcohol users41. Physical activity levels were categorised according to guidelines recommending more than 75 min of vigorous-intensity exercise or more than 150 min of moderate-intensity physical activity per week42. The categories were defined as active (above the recommended level), less active (below the recommended level), and inactive (no physical activity). Sleep disorder was measured by asking participants: “Have you ever told doctor had trouble sleeping”. Responses to this question was “yes” or “no”43.
Table 1.
Characteristics of SII quartile-based among adults in NHANES 2017–2020. Mean ± SD for continuous variables: the P value was calculated by the weighted linear regression model. (%) for categorical variables: the P value was calculated by the weighted chi-square test. SII: systemic immune-inflammation index; BMI: body mass index; PIR: poverty-to-income ratio.
| Variables | Overall | Quartiles of SII | P-value | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Age (years) | 48.43 ± 17.21 | 47.46 ± 16.98 | 47.33 ± 17.07 | 48.51 ± 17.38 | 50.18 ± 17.21 | < 0.0001 |
| Gender (%) | < 0.0001 | |||||
| Male | 48.68 | 57.03 | 51.25 | 44.57 | 43.69 | |
| Female | 51.32 | 42.97 | 48.75 | 55.43 | 56.31 | |
| Race (%) | < 0.0001 | |||||
| Mexican American | 8.34 | 8.19 | 9.57 | 8.46 | 7.16 | |
| Non-hispanic White | 63.69 | 52.46 | 63.67 | 65.95 | 70.28 | |
| Non-Hispanic black | 10.82 | 19.89 | 10.07 | 8.64 | 6.56 | |
| Other races | 17.15 | 19.47 | 16.69 | 16.95 | 15.99 | |
| Educational level (%) | 0.1604 | |||||
| < High school | 10.49 | 9.89 | 10.48 | 10.78 | 10.70 | |
| High school | 27.28 | 26.88 | 25.46 | 27.35 | 29.27 | |
| > High school | 62.23 | 63.23 | 64.06 | 61.87 | 60.03 | |
| Marital status (%) | 0.0084 | |||||
| Married/living with partner | 62.32 | 61.24 | 62.98 | 64.11 | 60.81 | |
| Widowed/divorced/separated | 18.47 | 18.07 | 16.66 | 18.79 | 20.20 | |
| Never married | 19.21 | 20.70 | 20.37 | 17.10 | 18.99 | |
| PIR (%) | 0.0013 | |||||
| < 1.3 | 18.28 | 20.02 | 17.31 | 16.76 | 19.33 | |
| 1.3–3.5 | 35.01 | 32.72 | 33.41 | 35.78 | 37.61 | |
| > 3.5 | 46.71 | 47.26 | 49.27 | 47.45 | 43.06 | |
| Energy intake (1000 kcal/day) | 2.06 ± 0.82 | 2.02 ± 0.83 | 2.06 ± 0.79 | 2.05 ± 0.80 | 2.09 ± 0.86 | 0.1607 |
| BMI (kg/m2) | 30.08 ± 7.50 | 29.62 ± 7.38 | 29.59 ± 6.86 | 30.39 ± 7.42 | 30.59 ± 8.18 | < 0.0001 |
| Waist (cm) | 101.29 ± 17.34 | 99.95 ± 17.16 | 100.09 ± 16.35 | 102.10 ± 17.14 | 102.72 ± 18.40 | < 0.0001 |
| SII | 534.16 ± 342.95 | 239.95 ± 54.86 | 380.89 ± 37.65 | 529.70 ± 52.74 | 915.06 ± 441.03 | < 0.0001 |
| Physical activity (%) | < 0.0001 | |||||
| Inactive | 48.58 | 40.60 | 47.96 | 49.50 | 54.63 | |
| Less active | 28.46 | 30.38 | 27.29 | 28.57 | 27.96 | |
| Active | 22.96 | 29.01 | 24.75 | 21.93 | 17.41 | |
| Sleep disorder (%) | 0.0383 | |||||
| Yes | 30.57 | 28.93 | 30.08 | 29.87 | 32.99 | |
| No | 69.43 | 71.07 | 69.92 | 70.13 | 67.01 | |
| Smoke status (%) | 0.0028 | |||||
| Never smoker | 57.09 | 58.20 | 58.71 | 57.29 | 54.48 | |
| Former smoker | 25.98 | 26.46 | 25.04 | 26.87 | 25.66 | |
| Current smoker | 16.93 | 15.34 | 16.25 | 15.85 | 19.86 | |
| Alcohol use (%) | 0.1090 | |||||
| Yes | 53.67 | 53.65 | 55.67 | 51.55 | 53.84 | |
| No | 46.33 | 46.35 | 44.33 | 48.45 | 46.16 | |
| Obesity (%) | < 0.0001 | |||||
| Yes | 42.76 | 39.50 | 40.21 | 46.36 | 44.26 | |
| No | 57.24 | 60.50 | 59.79 | 53.64 | 55.74 | |
Statistical analysis
Statistical analyses adhered to CDC guidelines. NHANES sample weights were applied, and the complex, multistage, subgroup survey design was accounted for. Continuous variables were presented as means ± Standard Deviations (SDs), while categorical variables were expressed as proportions. Participants were grouped into quartiles based on their SII values, and differences were analysed using weighted linear regression for continuous variables and weighted chi-square tests for categorical variables. Beta values and 95% confidence intervals were calculated using multivariate linear regression to analyse the relationship between SII and both BMI and waist circumference. Odds Ratios (ORs) and 95% confidence intervals were derived from multivariate logistic regression to explore the relationship between SII and obesity. The multivariate model was constructed in three stages: model 1 without adjustment; model 2 adjusted for gender, age, and race; and model 3 adjusted for all covariates. In order to assess the effects of different covariates on the association between SII and obesity, to determine whether associations between SII and obesity were consistent across subgroups, and to help increase power in testing for the overall effect of SII on obesity, subgroup analyses and interaction tests stratified by gender, age, race, educational level, marital status, PIR, energy intake, physical activity, sleep disorders, smoking status, and alcohol consumption were conducted. Smoothed curve fits were employed to explore the relationship between SII and BMI, waist circumference, and obesity prevalence. A threshold effect analysis model was utilized to investigate the relationship and inflection point between SII and both BMI and waist circumference. Identical statistical methods were applied to the physical activity subgroups. It is noteworthy that due to the skewed distribution of SII, the logarithmically transformed SII (lgSII) was used in regression analyses. All analyses were performed using EmpowerStats (version 2.0), with a p-value of < 0.05 considered statistically significant.
Results
Baseline characteristics of participants
This study analysed data from 7,645 participants, with an average age of 48.43 ± 17.21 years, ranging from 20 to 80 years. The cohort comprised 48.68% males and 51.32% females. Participants were categorised into quartiles based on their systemic immune-inflammation index (SII) values: quartile 1 (Q1) represented the lowest SII group (10.73 ≤ SII < 314.46); quartile 2 (Q2) the relatively low SII group (314.46 ≤ SII < 446.54); quartile 3 (Q3) the relatively high SII group (446.54 ≤ SII < 630.00); and quartile 4 (Q4) the highest SII group (630.00 ≤ SII ≤ 9828). The prevalence of obesity in these groups was 39.50% in Q1, 40.21% in Q2, 46.36% in Q3, and 44.26% in Q4, respectively. Statistically significant differences were observed among the SII quartiles in terms of age, gender, race, marital status, poverty income ratio (PIR), physical activity, sleep disorder, smoking status, body mass index (BMI), waist circumference, obesity, and SII (all P-values < 0.05). No significant differences were found in educational level, energy intake, and alcohol use (all P-values > 0.05). Table 1 details the baseline characteristics of the participants across SII quartiles.
Association between SII and obesity
The outcomes of the multivariate regression analysis are displayed in Table 2. In the unadjusted model, the logarithmically transformed SII (lgSII) showed a positive association with BMI [β = 1.43, 95% CI: (0.68, 2.18)] and waist circumference [β = 4.17, 95% CI: (2.44, 5.89)]. After adjustment for age, gender, and race, the results were β = 1.41, 95% CI: (0.65, 2.17) for BMI and β = 4.28, 95% CI: (2.53, 6.03) for waist circumference in the second multivariate linear regression model. With full covariate adjustment, the findings were β = 3.13, 95% CI: (2.10, 4.16) for BMI and β = 7.81, 95% CI: (5.50, 10.13) for waist circumference in the third model. This indicates that each unit increase in lgSII score is associated with a 3.13 kg/m² increase in BMI and a 7.81 cm increase in waist circumference. Additionally, a positive relationship was observed between lgSII and obesity status, with odds ratios (OR) of 1.35, 95% CI: (1.12, 1.64) in the first logistic regression model, 1.41, 95% CI: (1.16, 1.72) in the second model, and 1.44, 95% CI: (1.12, 1.86) in the third model. This suggests that each unit increase in lgSII score is associated with a 44% increase in the likelihood of obesity after adjusting for all covariates. In a sensitivity analysis, lgSII was converted from a continuous to a categorical variable (quartiles). Participants in the highest lgSII quartile (Q4) exhibited a 2.04 kg/m² increase in BMI, a 4.87 cm increase in waist circumference, and a 34% higher obesity prevalence compared to those in the lowest lgSII quartile (Q1), with all differences being statistically significant.
Table 2.
Association of lgSII with BMI, waist circumference and obesity prevalence among adults in NHANES 2017–2020. Model 1: unadjusted. Model 2: adjusted for age, gender, and race. Model 3: adjusted for age, gender, race, educational level, marital status, PIR, energy intake, physical activity, sleep disorder, smoke status, and alcohol use. SII: systemic immune-inflammation index; BMI: body mass index; PIR: poverty-to-income ratio.
| lgSII groups | BMI value | Waist circumference value | Obesity |
|---|---|---|---|
| β (95%CI) | β (95%CI) | OR (95%CI) | |
| Model1 | |||
| Continuous | 1.43 (0.68, 2.18) | 4.17 (2.44, 5.89) | 1.35 (1.12, 1.64) |
| Categories | |||
| Q1 | Reference | Reference | Reference |
| Q2 | − 0.02 (− 0.51, 0.47) | 0.14 (− 0.99, 1.28) | 1.09 (0.96, 1.24) |
| Q3 | 0.78 (0.29, 1.27) | 2.15 (1.02, 3.29) | 1.23 (1.08, 1.40) |
| Q4 | 0.98 (0.49, 1.46) | 2.77 (1.64, 3.90) | 1.27 (1.12, 1.45) |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 |
| Model2 | |||
| Continuous | 1.41 (0.65, 2.17) | 4.28 (2.53, 6.03) | 1.41 (1.16, 1.72) |
| Categories | |||
| Q1 | Reference | Reference | Reference |
| Q2 | − 0.03 (− 0.52, 0.47) | 0.21 (− 0.93, 1.36) | 1.11 (0.97, 1.27) |
| Q3 | 0.77 (0.27, 1.27) | 2.33 (1.19, 3.48) | 1.26 (1.10, 1.44) |
| Q4 | 0.96 (0.47, 1.46) | 2.86 (1.71, 4.00) | 1.32 (1.15, 1.50) |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 |
| Model3 | |||
| Continuous | 3.13 (2.10, 4.16) | 7.81 (5.50, 10.13) | 1.44 (1.12, 1.86) |
| Categories | |||
| Q1 | Reference | Reference | Reference |
| Q2 | 0.46 (− 0.19, 1.11) | 0.77 (− 0.70, 2.23) | 1.09 (0.92, 1.30) |
| Q3 | 1.11 (0.46, 1.76) | 3.06 (1.60, 4.53) | 1.22 (1.03, 1.45) |
| Q4 | 2.04 (1.38, 2.69) | 4.87 (3.40, 6.35) | 1.34 (1.13, 1.60) |
| P for trend | < 0.0001 | < 0.0001 | 0.0004 |
Subgroup analysis
The stratified subgroup analysis in this study revealed inconsistent relationships between lgSII levels and obesity (Table 3). Statistically significant associations were observed only in subgroups of male participants, those aged 60 years or older, non-Hispanic whites, other races, individuals with a high school education, PIR greater than 3.5, low energy intake, physical inactivity, sleep disorders, and alcohol users, when stratified by gender, age, race, education, PIR, energy intake, physical activity, sleep disorder, and alcohol use. Despite a lack of statistical significance (P > 0.05), a positive correlation between lgSII and obesity status was noted in females, individuals younger than 60 years, non-Hispanic blacks, those with less or more than a high school education, PIR below 1.3 or between 1.3 and 3.5, high energy intake, physically less active individuals, those without sleep disorders, and non-alcohol users. The relationship between lgSII and obesity status was consistently positive but not statistically significant in subgroups stratified by marital status and smoking status. Additionally, a negative association between lgSII and obesity status was observed in Mexican Americans [OR = 0.55; 95% CI: (0.24, 1.28)] and physically active individuals [OR = 0.75, 95% CI: (0.43, 1.32)], although these associations were not statistically significant.
Table 3.
Subgroup analysis for the association between lgSII and obesity prevalence among adults in NHANES 2017–2020. Adjusted for age, gender, race, educational level, marital status, PIR, energy intake, physical activity, sleep disorder, smoke status, and alcohol use except the corresponding stratification variable. SII: systemic immune-inflammation index; PIR: poverty-to-income ratio.
| Subgroups | OR (95%CI) | P-interaction |
|---|---|---|
| Stratified by gender | 0.3835 | |
| Male | 1.63 (1.14, 2.34) | |
| Female | 1.30 (0.89, 1.88) | |
| Stratified by age | 0.5476 | |
| Age < 60 years | 1.35 (0.97, 1.87) | |
| Age ≥ 60 years | 1.58 (1.05, 2.36) | |
| Stratified by race | 0.0639 | |
| Mexican American | 0.55 (0.24, 1.28) | |
| Non-hispanic White | 1.60 (1.04, 2.47) | |
| Non-Hispanic black | 1.32 (0.85, 2.07) | |
| Other races | 2.14 (1.21, 3.79) | |
| Stratified by education | 0.2907 | |
| < High school | 1.17 (0.59, 2.31) | |
| High school | 2.03 (1.22, 3.36) | |
| > High school | 1.31 (0.94, 1.82) | |
| Stratified by Marital status | 0.7420 | |
| Married/living with partner | 1.36 (0.97, 1.92) | |
| Widowed/divorced/separated | 1.44 (0.87, 2.38) | |
| Never married | 1.78 (0.99, 3.22) | |
| Stratified by PIR | 0.4468 | |
| < 1.3 | 1.19 (0.75, 1.88) | |
| 1.3–3.5 | 1.41 (0.94, 2.10) | |
| > 3.5 | 1.81 (1.13, 2.92) | |
| Stratified by energy intake | 0.7609 | |
| Low energy intake | 1.49 (1.04, 2.15) | |
| High energy intake | 1.38 (0.96, 1.97) | |
| Stratified by physical activity | 0.0102 | |
| Inactive | 2.00 (1.41, 2.84) | |
| Less active | 1.17 (0.68, 2.02) | |
| Active | 0.75 (0.43, 1.32) | |
| Stratified by sleep disorder | 0.2347 | |
| Yes | 1.79 (1.15, 2.78) | |
| No | 1.30 (0.95, 1.77) | |
| Stratified by smoking status | 0.6967 | |
| Never smoker | 1.29 (0.91, 1.82) | |
| Former smoker | 1.52 (0.89, 2.59) | |
| Current smoker | 1.68 (0.96, 2.95) | |
| Stratified by alcohol use | 0.1174 | |
| Yes | 1.78 (1.23, 2.58) | |
| No | 1.18 (0.82, 1.68) | |
Interaction tests did not reveal significant differences in the correlations between lgSII and obesity status across the variables of gender, age, race, education, marital status, PIR, energy intake, sleep disorder, smoking status, and alcohol use, indicating that these factors did not significantly modify this positive association (all interactions P > 0.05). However, physical activity appeared to influence the positive correlation between lgSII and obesity status (interaction P < 0.05).
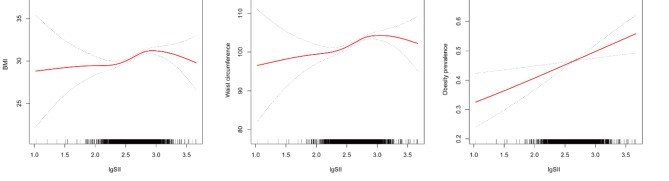
Smoothing curve fitting and threshold effect analysis
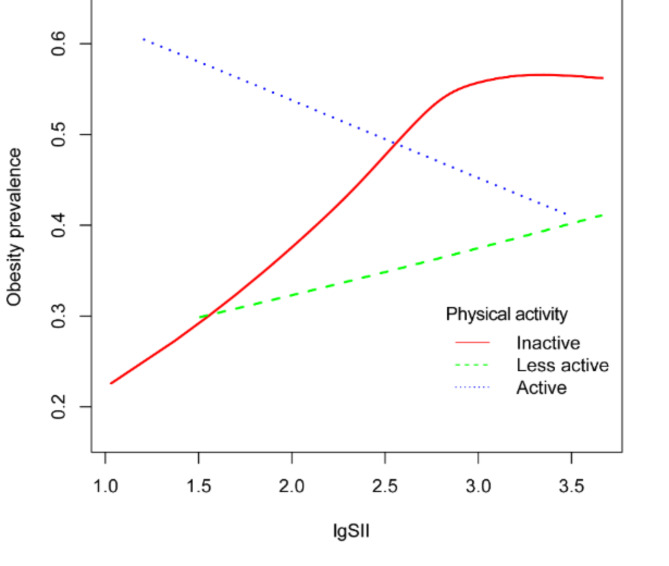
Smooth curve fitting was employed to ascertain the relationship between lgSII and BMI, waist circumference, and the prevalence of obesity. As illustrated in Fig. 2, lgSII exhibited an inverted U-shaped association with increasing BMI and waist circumference across all study participants. Subsequently, we utilized a segmented regression model to pinpoint the threshold effect, as detailed in Table 4. An inflection point of 2.86 was discerned in the correlation between lgSII and BMI. Below this threshold, a positive correlation was evident [β = 3.09, 95% CI: (1.92, 4.25)], while above it, a negative correlation prevailed [β = -1.86, 95% CI: (-5.01, 1.28)]. Similarly, an inflection point of 2.86 was also identified for the relationship between lgSII and waist circumference. To the left of this inflection point, lgSII displayed a positive association with waist circumference [β = 7.47, 95% CI: (4.81, 10.14)], whereas to the right, the association became negative [β = -2.95, 95% CI: (-10.16, 4.25)]. Elevated lgSII exhibited a linear positive correlation with an increasing prevalence of obesity, as depicted in Fig. 2C. Figure 3; Table 5 demonstrate that when stratified by physical activity level, a persistent positive correlation was observed between lgSII and obesity in those who were physically less active [OR = 1.14, 95% CI: (0.66, 1.99)]. Nevertheless, a distinct inflection point was identified within the fitted curve for the inactive group, and this inflection point was determined to be 2.86. To the left of this inflection point, lgSII exhibited a significantly positive association with obesity [OR = 2.88, 95% CI: (1.82, 4.56)], while to the right, the association turned negative [OR = 0.50, 95% CI: (0.16, 1.51)]. It is noteworthy that in the active group, lgSII displayed a linear negative correlation with obesity, although this observation did not attain statistical significance [OR = 0.79, 95% CI: (0.45, 1.38)].
Fig. 2.
Adjusted dose-response relationships between lgSII and BMI (A), waist circumference (B), and obesity prevalence (C) among adults in NHANES 2017–2020. Adjusted for age, gender, race, educational level, marital status, PIR, energy intake, physical activity, sleep disorder, smoke status, and alcohol use.
Table 4.
The threshold effect analysis of lgSII on BMI and waist circumference among adults in NHANES 2017–2020. Adjusted for age, gender, race, educational level, marital status, PIR, energy intake, physical activity, sleep disorder, smoke status, and alcohol use. SII: systemic immune-inflammation index; BMI: body mass index; PIR: poverty-to-income ratio.
| β (95% CI), P-value | |
|---|---|
| BMI | |
| Fitting by the standard linear model | 2.14 (1.22, 3.05) < 0.0001 |
| Fitting by the two-piecewise linear model | |
| Inflection point | 2.86 |
| LgSII < 2.86 | 3.09 (1.92, 4.25) < 0.0001 |
| LgSII > 2.86 | -1.86 (-5.01, 1.28) 0.2461 |
| P for Log-likelihood ratio | 0.009 |
| Waist circumference | |
| Fitting by the standard linear model | 5.47 (3.37, 7.57) < 0.0001 |
| Fitting by the two-piecewise linear model | |
| Inflection point | 2.86 |
| LgSII < 2.86 | 7.47 (4.81, 10.14) < 0.0001 |
| LgSII > 2.86 | -2.95 (-10.16, 4.25) 0.4219 |
| P for Log-likelihood ratio | 0.016 |
Fig. 3.
Adjusted dose-response relationship between lgSII and obesity prevalence stratified by physical activity among adults in NHANES 2017–2020. Adjusted for age, gender, race, educational level, marital status, PIR, energy intake, sleep disorder, smoke status, and alcohol use.
Table 5.
The threshold effect analysis of lgSII and obesity prevalence stratified by physical activity among adults in NHANES 2017–2020. Adjusted for age, gender, race, educational level, marital status, PIR, energy intake, sleep disorder, smoke status, and alcohol use. SII: systemic immune-inflammation index; PIR: poverty-to-income ratio.
| Physical activity | Inactive | Less active | Active |
|---|---|---|---|
| Fitting by the standard linear model, OR (95% CI) | 1.97 (1.38, 2.79) | 1.14 (0.66, 1.99) | 0.79 (0.45, 1.38) |
| Fitting by the two-piecewise linear model, OR (95% CI) | |||
| Inflection point (K) | 2.86 | 2.48 | 2.23 |
| lgSII < K | 2.88 (1.82, 4.56) | 0.75 (0.16, 3.51) | 0.01 (0.00, 1.19) |
| lgSII > K | 0.50 (0.16, 1.51) | 1.33 (0.62, 2.81) | 1.11 (0.59, 2.09) |
| P for Log-likelihood ratio | 0.011 | 0.572 | 0.017 |
Discussion
Considerable attention has been devoted to elucidating the link between chronic inflammation and obesity. This study represents the inaugural attempt to concurrently investigate the relationship between SII and BMI, waist circumference, and the prevalence of obesity within a nationally representative cohort of adult Americans. Our findings reveal an inverted U-shaped correlation between lgSII and both BMI and waist circumference, with identical inflection points at 2.86. Additionally, we observed a consistent positive linear association between lgSII and the prevalence of obesity. Upon stratification by physical activity levels, the less active group continued to exhibit a positive correlation between lgSII and obesity prevalence. Intriguingly, within the inactive group, we also identified an inverted U-shaped connection between the two variables, with an inflection point at 2.86. Notably, in the active group, lgSII demonstrated a non-significant negative correlation with obesity.
Mahemuti and colleagues previously reported a heightened prevalence of hyperlipidemia in association with elevated SII levels within a cross-sectional study involving adult individuals in the United States31. Obesity, a chronic disease characterised by abnormal or excessive accumulation of body fat, exhibits a close connection to hyperlipidemia44. The interplay between obesity and inflammation has attracted considerable attention, prompting the formulation of numerous hypotheses aimed at elucidating the mechanisms responsible for the occurrence of chronic inflammation within adipose tissue during obesity. The initial hypothesis posits that an excess of nutrients triggers the accumulation of misfolded or unfolded proteins within the endoplasmic reticulum. This, in turn, activates the unfolded protein response pathway (UPR), leading to an upsurge in the expression of pro-inflammatory cytokines45,46. The second hypothesis suggests that an excessive burden of adipocytes results in significant macrophage infiltration, subsequently leading to the differentiation and activation of cytotoxic T cells, thereby amplifying local and systemic inflammation47–49. An alternative theory revolves around the expansion of adipose tissue, which compromises perfusion, induces hypoxia, and activates pro-inflammatory signalling pathways. This hypoxia-induced inflammation, in turn, precipitates necrosis and macrophage infiltration within the adipose tissue50. Motivated by the aforementioned studies, our investigation was geared towards exploring the relationship between SII and obesity, with the aim of furnishing robust evidence to support future basic and clinical research.
The primary finding of our study underscores a robust positive association between SII and BMI, waist circumference, and the prevalence of obesity. A population-based study involving 223 obese adults in Turkey unveiled a significant positive linear correlation between SII and BMI34. Similarly, Zhao et al.33 reported that participants with higher SII exhibited elevated waist circumference in a cross-sectional study drawing data from NHANES. Our findings corroborate these observations and further reveal an inverted U-shaped correlation between lgSII and both BMI and waist circumference. It is worth noting that no prior reports have linked SII to obesity prevalence, making our study pioneering in bridging the gap between SII and obesity prevalence. In general, obesity is defined as a BMI ≥ 30.0 kg/m2; however, the BMI has its limitations as it cannot distinguish between lean and fat body mass. Consequently, our study employed additional criteria for defining obesity, namely a BMI ≥ 30 kg/m2 and a waist circumference exceeding 88 cm in women and 102 cm in men. Intriguingly, subgroup analysis has unveiled the potential influence of physical activity on the positive correlation between SII and obesity prevalence. Furthermore, a detailed examination employing smooth curve fitting has illuminated a linear negative association between lgSII and obesity within the active group, although this negative correlation did not reach statistical significance.
Obesity and inflammation are intricately interlinked, exerting mutual influence across various metabolic dimensions. A wealth of data, spanning in vitro, rodent, and human studies, underscores this connection. In addition to the multifaceted hypotheses elucidating chronic adipose tissue inflammation in obesity, the hypothalamus and gut hormones assume pivotal roles in regulating this association. The central regulation of appetite is notably governed by the leptin-melanocortin signalling pathway. Leptin (LEP), a hormone produced by adipose tissue mass, binds to the hypothalamic leptin receptor (LEPR), setting off a cascade of events. This includes the production of pro-opiomelanocortin (POMC), which is subsequently processed into α-melanocyte-stimulating hormone (MSH) and β-MSH, among other peptides. α- and β-MSH, upon binding to the Melanocortin-4 receptor (MC4R), elicit feelings of satiety, thus curbing food intake and altering energy expenditure51. Genetic mutations in the LEP and LEPR genes within this signalling system can lead to hyperphagia, resulting in severe early-onset obesity52. Notably, the constituents of the leptin-melanocortin signalling pathway exert immunomodulatory effects in addition to their role in appetite and satiety regulation. Individuals with diminished LEP and LEPR levels exhibit reduced cytokine production and lymphocyte proliferation, rendering them more susceptible to infections. Evidence suggests an elevated risk of infection-related childhood mortality, particularly in cases of Leptin deficiency52,53. Furthermore, the upregulation of pro-inflammatory proteins such as TNFα, IL-6, and JNK3 has been observed in the hypothalamus of rats subjected to a high-fat diet54. Prolonged consumption of a high-fat diet by rodents is associated with hypothalamic inflammation, leading to leptin resistance and subsequent obesity55–57. Researchers have also demonstrated that Roux-en-Y gastric bypass surgery reduces adipose tissue macrophage infiltration, thereby mitigating inflammation58. This finding implies the potential involvement of gut hormones in mediating obesity-related inflammation.
Physical activity has emerged as an independent anti-inflammatory factor, distinct from its role in promoting weight loss59. In a cross-sectional study involving healthy middle-aged men, a negative correlation was established between leisure-time physical activity and hs-CRP levels, with CRP levels diminishing as the intensity of physical activity increased60. Ding et al. conducted a study that revealed a negative association between leisure-time physical activity and inflammatory biomarkers in both overweight and obese populations. Notably, vigorous leisure-time physical activity demonstrated superior efficacy over moderate activity, particularly in regulating inflammation61. It is important to note that intense and extreme exercise can also elevate inflammatory cytokine levels. Marathon runners, for instance, have been observed to experience increased levels of TNF-α, IL-6, and IL-1β following a race62,63. These findings may shed light on the variations observed in the relationship between SII and obesity prevalence within different physical activity subgroups.
This study boasts several notable strengths. Firstly, it stands as the pioneering investigation explicitly probing the correlation between SII and obesity prevalence, not only confirming the relationship between inflammation and obesity from a clinical perspective, but also providing clinical evidence for further investigation of the pathophysiologic mechanisms underlying the association of SII with obesity in the future. Secondly, the robustness and reliability of our study have been fortified through a substantial sample size and meticulous covariate adjustments. Furthermore, the ample sample size facilitated subgroup analyses, allowing us to explore potential influences of other variables on the link between SII and obesity. Thirdly, the inclusion of sensitivity analysis has served to mitigate the likelihood of spurious findings.
However, certain limitations must be acknowledged. Firstly, the cross-sectional design employed in this study is inherently limited in its capacity to establish causation, and thus the findings may be affected by reverse causality. Prospective investigations with extensive sample sizes are requisite for establishing causal relationships. Additionally, despite our efforts to encompass as many covariates as feasible, certain variables, such as the use of steroids or weight-loss medications, remained unrecorded within the NHANES dataset. Lastly, since our study exclusively encompasses adult participants from the United States, prudence dictates the need for further validation before generalizing our findings to other demographic groups.
Conclusion
Our findings underscore a robust association between the logarithmically transformed SII and BMI, waist circumference, and the prevalence of obesity, which provide clinical evidence for further investigation of the pathophysiologic mechanisms underlying the association of SII with obesity in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
S.Y.: Investigation, methodology, writing-original draft preparation; S.Z.: Software, writing-original draft preparation; J.R.D. and J.J.X.: Revision; J.Y.Z.: supervision, methodology, revision and funding; E.T.J.: Conceptualization, writing-reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (82174290), 2021 Shenzhen Science and Technology Programme (JCYJ20210324120405016), and Sanming Project of Medicine in Shenzhen (No. SZZYSM202311003).
Data availability
The data used in the present research were obtained from publicly accessible sources. These data could be acces-sible at the following URL: https://www.cdc.gov/nchs/nhanes/.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shuo Yang and Shan Zhang.
Contributor Information
Jianyong Zhang, Email: 13823396319@163.com.
Ertao Jia, Email: sailing1980@126.com.
References
- 1.Bray, G. A., Frühbeck, G., Ryan, D. H. & Wilding, J. P. Management of obesity. Lancet. 387, 1947–1956. 10.1016/s0140-6736(16)00271-3 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Salminen, P. et al. IFSO consensus on definitions and clinical practice guidelines for obesity management-an International Delphi Study. Obes. Surg.34, 30–42. 10.1007/s11695-023-06913-8 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maggio, C. A. & Pi-Sunyer, F. X. Obesity and type 2 diabetes. Endocrinol. Metab. Clin. N. Am.32, 805–822, viii, (2003). 10.1016/s0889-8529(03)00071-9 [DOI] [PubMed]
- 4.Piché, M. E., Tchernof, A. & Després, J. P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circul. Res.126, 1477–1500. 10.1161/circresaha.120.316101 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Powell-Wiley, T. M. et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 143, e984–e1010. 10.1161/cir.0000000000000973 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meurling, I. J., Shea, D. O. & Garvey, J. F. Obesity and sleep: a growing concern. Curr. Opin. Pulm. Med.25, 602–608. 10.1097/mcp.0000000000000627 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Lakkis, J. I. & Weir, M. R. Obesity and kidney disease. Prog. Cardiovasc. Dis.61, 157–167. 10.1016/j.pcad.2018.07.005 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Calle, E. E., Rodriguez, C., Walker-Thurmond, K. & Thun, M. J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med.348, 1625–1638. 10.1056/NEJMoa021423 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Colditz, G. A. & Peterson, L. L. Obesity and cancer: evidence, impact, and future directions. Clin. Chem.64, 154–162. 10.1373/clinchem.2017.277376 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Avgerinos, K. I., Spyrou, N., Mantzoros, C. S. & Dalamaga, M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metab. Clin. Exp.92, 121–135. 10.1016/j.metabol.2018.11.001 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Wang, P. et al. Tlr9 deficiency in B cells leads to obesity by promoting inflammation and gut dysbiosis. Nat. Commun.15, 4232. 10.1038/s41467-024-48611-8 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley, K. P. et al. Inflammation promotes adipocyte lipolysis via IRE1 kinase. J. Biol. Chem.29610.1016/j.jbc.2021.100440 (2021). [DOI] [PMC free article] [PubMed]
- 13.Ajoolabady, A., Lebeaupin, C., Wu, N. N., Kaufman, R. J. & Ren, J. ER stress and inflammation crosstalk in obesity. Med. Res. Rev.43, 5–30. 10.1002/med.21921 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Lenharo, M. Obesity drugs have another superpower: taming inflammation. Nature. 626, 246. 10.1038/d41586-024-00118-4 (2024). [DOI] [PubMed] [Google Scholar]
- 15.Bagheri, S., Zolghadri, S. & Stanek, A. Beneficial effects of anti-inflammatory diet in modulating gut microbiota and controlling obesity. Nutrients. 1410.3390/nu14193985 (2022). [DOI] [PMC free article] [PubMed]
- 16.Li, L. et al. Orm2 deficiency aggravates high-fat diet-induced obesity through gut microbial dysbiosis and intestinal inflammation. Mol. Nutr. Food Res.68, e2300236 10.1002/mnfr.202300236 (2024). [DOI] [PubMed]
- 17.Sehgal, A. et al. Targeting NLRP3 inflammasome as a chief instigator of obesity, contributing to local adipose tissue inflammation and insulin resistance. Environ. Sci. Pollut. Res. Int.28, 43102–43113. 10.1007/s11356-021-14904-4 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Thornton, P. et al. Reversal of high fat diet-Induced obesity, systemic inflammation, and astrogliosis by the NLRP3 inflammasome inhibitors NT-0249 and NT-0796. J. Pharmacol. Exp. Ther.388, 813–826. 10.1124/jpet.123.002013 (2024). [DOI] [PubMed] [Google Scholar]
- 19.Wang, X., Weng, W., Cui, Y. & Zou, C. Sitagliptin alleviates obesity in immature mice by inhibiting oxidative stress and inflammation. Reprod. Sci.. 10.1007/s43032-024-01623-7 (2024). [DOI] [PubMed] [Google Scholar]
- 20.Winer, S. et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med.15, 921–929. 10.1038/nm.2001 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanneganti, T. D. & Dixit, V. D. Immunological complications of obesity. Nat. Immunol.13, 707–712. 10.1038/ni.2343 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Hu, B. et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res.20, 6212–6222. 10.1158/1078-0432.Ccr-14-0442 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Xie, R. et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front. Immunol.1310.3389/fimmu.2022.925690 (2022). [DOI] [PMC free article] [PubMed]
- 24.Yilmaz, M., Baran, A. & Yilmaz, M. K. Predictive significance of inflammatory indexes in metastatic nonsmall cell lung cancer patients treated with platinum-doublet chemotherapy. J. Cancer Res. Ther.18, 220–223. 10.4103/jcrt.jcrt_1902_20 (2022). [DOI] [PubMed] [Google Scholar]
- 25.He, K. et al. Preoperative systemic Immune-inflammation index (SII) as a Superior Predictor of Long-Term Survival Outcome in patients with stage I-II gastric Cancer after radical surgery. Front. Oncol.12, 829689. 10.3389/fonc.2022.829689 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen, J. H. et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J. Gastroenterol.23, 6261–6272. 10.3748/wjg.v23.i34.6261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aziz, M. H. et al. High systemic immune inflammation index is associated with low skeletal muscle quantity in resectable pancreatic ductal adenocarcinoma. Front. Oncol.12, 827755. 10.3389/fonc.2022.827755 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yaşar, E. & Bayramoğlu, A. systemic Immune-inflammation index as a predictor of microvascular dysfunction in patients with cardiac syndrome X. Angiology. 73, 615–621. 10.1177/00033197221087777 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Yavuz, S. & Engin, M. Atherosclerotic burden and systemic immune inflammation index. Echocardiography. 39, 654. 10.1111/echo.15343 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Orhan, A. L. et al. Evaluating the systemic immune-inflammation index for in-hospital and long-term mortality in elderly non-ST-elevation myocardial infarction patients. Aging Clin. Exp. Res.34, 1687–1695. 10.1007/s40520-022-02103-1 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Mahemuti, N. et al. Association between systemic immunity-inflammation index and hyperlipidemia: a population-based study from the NHANES (2015–2020). Nutrients. 1510.3390/nu15051177 (2023). [DOI] [PMC free article] [PubMed]
- 32.Guo, W. et al. Systemic immune-inflammation index is associated with diabetic kidney disease in type 2 diabetes mellitus patients: evidence from NHANES 2011–2018. Front. Endocrinol.13, 1071465. 10.3389/fendo.2022.1071465 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao, Y. et al. Association between systemic immune-inflammation index and metabolic syndrome and its components: results from the National Health and Nutrition Examination Survey 2011–2016. J. Transl. Med.21, 691. 10.1186/s12967-023-04491-y (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furuncuoğlu, Y. et al. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur. Rev. Med. Pharmacol. Sci.20, 1300–1306 (2016). [PubMed] [Google Scholar]
- 35.Eren, C. & Cecen, S. The relationship between childhood obesity with inflammatory mediators. J. Pak. Med. Assoc.70, 1737–1741. 10.5455/jpma.27772 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Chen, T. C., Clark, J., Riddles, M. K., Mohadjer, L. K. & Fakhouri, T. H. I. National Health and Nutrition Examination Survey, 2015–2018: Sample Design and Estimation Procedures. Vital and health statistics. Series 2, Data evaluation and methods research, 1–35 (2020). [PubMed]
- 37.von Elm, E. et al. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 18, 800–804. 10.1097/EDE.0b013e3181577654 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Dong, W. & Yang, Z. Association of nickel exposure with body mass index, waist circumference and incidence of obesity in US adults. Chemosphere. 338, 139599. 10.1016/j.chemosphere.2023.139599 (2023). [DOI] [PubMed] [Google Scholar]
- 39.Lei, X., Wen, H. & Xu, Z. Higher oxidative balance score is associated with lower kidney stone disease in US adults: a population-based cross-sectional study. World J. Urol.42, 222. 10.1007/s00345-024-04919-0 (2024). [DOI] [PubMed] [Google Scholar]
- 40.Yan, Y. et al. The association between remnant cholesterol and rheumatoid arthritis: insights from a large population study. Lipids Health Dis.2310.1186/s12944-024-02033-z (2024). [DOI] [PMC free article] [PubMed]
- 41.Sun, H., Huang, J., Tang, H. & Wei, B. Association between weight-adjusted-waist index and urge urinary incontinence: a cross-sectional study from NHANES 2013 to 2018. Sci. Rep.14, 478 10.1038/s41598-024-51216-2 (2024). [DOI] [PMC free article] [PubMed]
- 42.Bull, F. C. et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med.54, 1451–1462. 10.1136/bjsports-2020-102955 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, S. et al. Association between vitamin C in serum and trouble sleeping based on NHANES 2017–2018. Sci. Rep.14, 9727. 10.1038/s41598-024-56703-0 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussain, A. et al. Attenuation of obesity-induced hyperlipidemia reduces tumor growth. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 186710.1016/j.bbalip.2022.159124 (2022). [DOI] [PubMed]
- 45.Gregor, M. F. & Hotamisligil, G. S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol.29, 415–445. 10.1146/annurev-immunol-031210-101322 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol.8, 519–529. 10.1038/nrm2199 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Surmi, B. K. & Hasty, A. H. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol.3, 545–556. 10.2217/17460875.3.5.545 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yudkin, J. S. Adipose tissue, insulin action and vascular disease: inflammatory signals. Int. J. Obes. Relat. Metabolic Disorders: J. Int. Association Study Obes.27(Suppl 3), 25–28. 10.1038/sj.ijo.0802496 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Yudkin, J. S., Kumari, M., Humphries, S. E. & Mohamed-Ali, V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 148, 209–214. 10.1016/s0021-9150(99)00463-3 (2000). [DOI] [PubMed] [Google Scholar]
- 50.Hosogai, N. et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 56, 901–911. 10.2337/db06-0911 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Farooqi, I. S. & O’Rahilly, S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat. Clin. Pract. Endocrinol. Metab.4, 569–577. 10.1038/ncpendmet0966 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Montague, C. T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 387, 903–908. 10.1038/43185 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Farooqi, I. S. et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Investig.110, 1093–1103. 10.1172/jci15693 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Souza, C. T. et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 146, 4192–4199. 10.1210/en.2004-1520 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Kreutzer, C. et al. Hypothalamic inflammation in human obesity is mediated by environmental and genetic factors. Diabetes. 66, 2407–2415. 10.2337/db17-0067 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig.122, 153–162. 10.1172/jci59660 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jais, A. & Brüning, J. C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investig.127, 24–32. 10.1172/jci88878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cancello, R. et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 54, 2277–2286. 10.2337/diabetes.54.8.2277 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Nicklas, B. J., You, T. & Pahor, M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ: Can. Med. Association J. = J. De l’Association Medicale Canadienne. 172, 1199–1209. 10.1503/cmaj.1040769 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bergström, G., Behre, C. J. & Schmidt, C. Moderate intensities of leisure-time physical activity are associated with lower levels of high-sensitivity C-reactive protein in healthy middle-aged men. Angiology. 63, 412–415. 10.1177/0003319711423386 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Ding, Y. & Xu, X. Dose-response relationship between leisure-time physical activity and biomarkers of inflammation and oxidative stress in overweight/obese populations. J. Sci. Med. Sport. 26, 616–621. 10.1016/j.jsams.2023.09.010 (2023). [DOI] [PubMed] [Google Scholar]
- 62.Ostrowski, K., Rohde, T., Asp, S., Schjerling, P. & Pedersen, B. K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol.515(Pt 1), 287–291. 10.1111/j.1469-7793.1999.287ad.x (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ostrowski, K., Rohde, T., Zacho, M., Asp, S. & Pedersen, B. K. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol.508(Pt 3), 949–953. 10.1111/j.1469-7793.1998.949bp.x (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in the present research were obtained from publicly accessible sources. These data could be acces-sible at the following URL: https://www.cdc.gov/nchs/nhanes/.