Abstract
Adenoviruses (Ad) are efficient vehicles for gene delivery in vitro and in vivo. Therefore, they are a promising tool in gene therapy, particularly in the treatment of cancer and cardiovascular diseases. However, preclinical and clinical studies undertaken during the last decade have revealed a series of problems that limit both the safety and efficacy of Ad vectors, specifically after intravenous application. Major obstacles to clinical use include innate toxicity and Ad sequestration by nontarget tissues. The factors and mechanisms underlying these processes are poorly understood. The majority of intravenously injected Ad particles are sequestered by the liver, which in turn causes an inflammatory response characterized by acute transaminitis and vascular damage. Here, we describe a novel pathway that is used by Ad for infection of hepatocytes and Kupffer cells upon intravenous virus application in mice. We found that blood factors play a major role in targeting Ad vectors to hepatic cells. We demonstrated that coagulation factor IX and complement component C4-binding protein can bind the Ad fiber knob domain and provide a bridge for virus uptake through cell surface heparan sulfate proteoglycans and low-density lipoprotein receptor-related protein. An Ad vector, Ad5mut, which contained mutations in the fiber knob domain ablating blood factor binding, demonstrated significantly reduced infection of liver cells and liver toxicity in vivo. This study contributes to a better understanding of adenovirus-host interactions for intravenously applied vectors. It also provides a rationale for novel strategies to target adenovirus vector to specific tissues and to reduce virus-associated toxicity after systemic application.
Adenoviruses (Ad) are nonenveloped viruses possessing a linear, double-stranded DNA genome of about 35 kb. So far, 51 different serotypes of human Ads have been identified, and they have been classified into six subgenera (groups A through F). Early virological studies focused on C-group serotypes 2 and 5 (9, 17). Ad2 and Ad5 genomes have been modified and corresponding recombinant viruses have been used for in vitro and in vivo gene transfer for decades (for a review, see reference 33). First-generation Ad vectors have the viral E1 and E3 genes deleted. Newer generation vectors are devoid of all viral genes (8, 25). Currently, adenovirus vectors are being used in about one quarter of all gene therapy trials, predominantly for the treatment of cancer (55). Despite the widespread clinical application of Ads, the mechanisms governing Ad tropism in vivo are poorly understood. In vitro studies suggest that direct binding of the adenovirus fiber protein to a cellular receptor(s) is the first and most critical step in virus infection (50). Ads belonging to all subgroups except subgroup B utilize the coxsackie-adenovirus receptor (CAR) as a primary attachment receptor for in vitro infection (42). However, in vivo, when Ad5 vectors are administered intravenously, CAR expression levels do not correlate with the virus tissue distribution (14). Moreover, the introduction of mutations that abrogate CAR binding does not significantly impact the level and pattern of Ad infectivity in vivo (2, 13). Specifically, transduction of the liver, which takes up the majority of intravenously applied Ad5 vectors in mice (56) and in nonhuman primates (39), was as efficient with Ads ablated for CAR binding as with unmodified vectors. These data indicate that the presence of CAR is not a critical factor in determining susceptibility of tissues to Ad infection in vivo. The lack of understanding of the mechanisms underlying Ad in vivo tropism poses significant risks for systemic application of Ad vectors and complicates strategies for cell type-specific Ad targeting through capsid modification.
One of the greatest obstacles to the clinical application of Ad vectors is the innate immune response towards the vector (for a review, see reference 31). It occurs within 24 h of virus administration and depends entirely on virus capsid interactions with host cells (31). This phase is therefore seen for both first-generation and helper-dependent Ad vectors (7). The innate response is dose dependent and is characterized by complement activation, production of proinflammatory cytokines/chemokines (such as interleukin 6 [IL-6], IL-10, IL-8, tumor necrosis factor alpha, gamma interferon [IFN-γ], and macrophage inflammatory proteins 1 and 2) that can lead to vascular damage, mobilization of tissue factors, and deadly systemic inflammatory responses in animals (32, 36, 40, 43) and humans (3, 11, 35, 41). It is thought that the uptake of Ad particles into hepatic macrophages, Kupffer cells, and (to a lesser degree) hepatocytes is causatively associated with innate Ad toxicity (27, 28, 37). Although we showed recently that Ad uptake into Kupffer cells is CAR independent (45), the precise mechanisms for this process remained elusive.
After intravenous application, most of Ad particles are sequestered by the liver (56). Once the reticuloendothelial system of the liver is saturated, Ad transduces other cell types (56). In animal models and humans, transgene expression after systemic Ad5 vector administration is predominantly found in the hepatocytes (10). While this might be beneficial for treatment of acquired or inherited liver or metabolic diseases, detargeting Ad tropism from hepatocytes is desirable for other gene therapy applications such as treatment of disseminated cancer or cardiovascular disease. To date, the attempts undertaken to modify in vivo Ad tropism by abolishing CAR and/or integrin interactions have not been successful (2, 26, 34, 51, 53).
In this study, we demonstrate that blood factors play a major role in targeting Ad vectors to hepatic cells in vivo. We also constructed a prototype vector, Ad5mut, which is ablated for blood factor binding. Intravenous application of this vector in mice demonstrated significantly reduced liver cell infection and toxicity. Our study provides a rationale for novel strategies aimed towards construction of safe and efficient adenovirus vectors targeted to diseased tissues after intravascular administration.
MATERIALS AND METHODS
Cells and viruses.
293 cells were from Microbix (Toronto, Canada). CHO-K1 (heparan sulfate proteoglycan [HSPG] expressing; ATCC CCL-61), CHO-pgsA745 (HSPG negative; ATCC CCL-61), and human hepatoma HepG2 cells were from the American Type Culture Collection. Plated primary human hepatocytes were from BioWhittaker (Walkersville, MD). Mouse embryo fibroblasts (MEF) lrp+/+ and MEF lrp−/− cells were kindly provided by Michael Gotthardt (Max-Delbrück-Centrum für Molekulare Medizin [MDC], Berlin, Germany). All cell lines were grown on Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum. 293-DH26 cells were obtained by stable transfection of 293 cells with plasmid pDH.2 expressing the membrane-anchored scFv, recognizing a six-His tag (12). Primary mouse hepatocytes were isolated by collagenase perfusion (29) and cultured in Primaria dishes (Corning Corp., Acton, MA) in William's E medium supplemented with 10% fetal bovine serum. Ad5L1 and Ad5*F express luciferase and green fluorescent protein (GFP); and Ad5L2 and Ad5/35L express β-galactosidase from identical expression cassettes. Ad5L1 and Ad5*F are described elsewhere as Ad5GFPLuc and AdGFPLucY477Ax6H, respectively (2). Ad5*F contains a mutation that compromises CAR binding. The Ad5mut virus contains the following mutations: the Y477A mutation (2), a deletion of amino acids 489 to 492 (TAYT) in the FG loop, a peptide insertion (SKCDCRGECFCD) into position 547 of the HI loop, and a C-terminal six-histidine tag as described for Ad5*F (2). All viruses were propagated on 293 or 293-DH26 cells and purified; titers for genomes and PFU were determined as described elsewhere (48).
Ad infection in vitro.
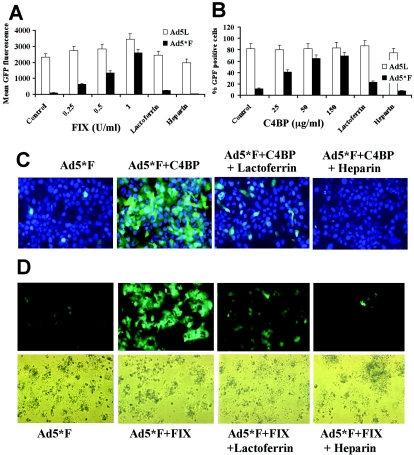
293 and 293-DH26 cells (2.5 × 105) were infected with Ad5*F and Ad5mut viruses at multiplicities of infection (MOIs) of 1,000 virus particles per cell for 2 h in the presence or absence of coagulation factor IX (FIX) or complement component C4-binding protein (C4BP). Twenty-four hours postinfection (p.i.), cell transduction was assessed by GFP reporter gene expression by flow cytometry. 2.5 × 105 CHO-K1, CHO-pgsA745, MEF lrp+/+, and MEF lrp−/− cells were infected at an MOI of 1,000 virus particles/cell with or without FIX (3 U/ml) in 300 μl saline. Two hours later, the virus-containing saline was replaced by growth medium. Reporter gene activity was analyzed 48 h later. HepG2 and DH-26 cells and primary human or mouse hepatocytes were infected with indicated Ads at an MOI of 1,000 virus particles/cell with or without C4BP (150 μg/ml) or FIX (1 U/ml) in 300 μl saline. Two hours later, the virus-containing saline was replaced by growth medium. Reporter gene activity was analyzed 48 h later. In competition studies, human lactoferrin (0.5 mg/ml) or heparin (10 U/ml) was used.
Ad infection in vivo.
All experimental procedures were conducted in accordance with the institutional guidelines set forth by the University of Washington. Mice were housed in specific pathogen-free facilities. Ads were injected either into the portal vein through a permanently placed catheter or into the tail vein at a dose of 1011 viral particles/mouse in 200 μl saline. C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and C57BL/6 FIX-knockout mice were kindly provided by Katherine High (Children's Hospital of Philadelphia, Philadelphia, PA).
To analyze the role of blood factors in liver transduction by Ads, in the with-blood setting, liver was flushed with Hanks' balanced salt solution 15 minutes after Ad infusion, followed by collagenase perfusion to isolate and culture hepatocytes for analysis of reporter gene expression. Routinely, cell preparations had <5% contamination with other liver cell types (44). In without-blood settings, the vena porta and vena cava inferior were canulated and blood was flushed from the liver through the portal vein with 20 ml of phosphate-buffered saline using a low-speed peristaltic pump. After 10 min of continuous flushing, the liver color changed from dark brown to light brown-yellow, demonstrating that most of the blood was efficiently removed from the liver. Virus infection studies were started when the liver flowthrough, collected from the vena cava inferior, was free of blood. (Notably, no blood cells were found in liver sections harvested upon flushing with 20 ml phosphate-buffered saline.) Next, virus (2.5 × 1010 viral particles/ml) in 8 ml of saline was infused through the portal vein and the circulation between the vena porta and vena cava was closed, allowing asanguinous, isolated liver perfusion (at 37°C). Thirty minutes after virus application, hepatocytes were isolated by collagenase perfusion. In both settings, reporter gene expression in plated hepatocytes was analyzed 48 h postinfection.
In vivo competition studies were done by preinjecting polymerized bovine serum albumin (pBSA) at 1 mg/mouse, asialofetuin at 0.5 mg/mouse, human low-density lipoprotein (hLDL) at 0.5 mg of protein/mouse, lactoferrin at 1 mg/mouse, or saline (as a control) 5 min before virus administration into the portal vein. The reporter gene in hepatocyte expression was analyzed as described above. Heparinase I (30 U per mouse) was administrated into the tail vein 30 min before virus injection.
Analysis of adenovirus-Kupffer cell interaction in vivo.
To analyze Ad interactions with Kupffer cells, Ads were labeled with fluorophore Cy-3 (45, 47). Fluorophore-labeled Ads (1011 particles) were injected into the tail vein, and 30 min later, livers were flushed with saline via cardiac perfusion, harvested, and immediately frozen in optimal cutting temperature compound. Frozen liver sections either remained unstained or were stained with rat anti-mouse CD45 or F4/80 primary antibody (BD Biosciences, Palo Alto, CA) to detect Kupffer cells. Specific binding of primary antibodies was visualized with secondary anti-rat Alexa Flour 488 antibody (Molecular Probes, Inc., Eugene, OR).
Southern blot analysis.
Seventy-two hours post virus administration into the tail vein, liver DNA (1 μg) was analyzed by Southern blotting to determine the prevalence of Ad genomic DNA in the liver tissue as described previously (46). Equivalent loading was assessed by hybridization of the membranes with a mouse β-glucuronidase-specific probe.
Mass spectrometry analysis.
Recombinant Ad5 and Ad35 fiber knob domains were expressed in Escherichia coli and purified as described previously (49). Fiber knobs were conjugated to Ni-agarose beads via a C-terminal six-His tag and incubated with EDTA-preserved fresh mouse plasma for 1 h at 4°C. Next, beads were pelleted and washed five times with saline, and knob-interacting plasma proteins were eluted with 8 M urea. Eluted proteins were then concentrated using Centricon centrifugation YM3000 filter units (Millipore Corp., Bedford, MA); after digestion with trypsin, the proteins were subjected to tandem mass spectrometry analysis (University of Washington Mass Spectrometry Core Facility). The mass spectrometry analysis data were processed using Mascot search software (http://www.matrixscience.com) and the NCBInr database (revision date, 20040304).
Analysis of levels of proinflammatory cytokines and aminotransferases in mouse plasma.
Plasma levels of proinflammatory cytokines were analyzed 6 h after intravenous Ad administration. Blood samples were collected into heparin-treated Eppendorf tubes, and plasma was obtained and stored at −80°C in small aliquots. To measure cytokine-chemokine concentrations, a Mouse Inflammatory Cytometric Bead Array (BD Biosciences, Palo Alto, CA) was used according to the manufacturer's protocol. For each Ad vector, plasma samples were obtained from at least three mice and were analyzed in duplicate. To measure plasma levels of alanine aminotransferase (ALT), a colorimetric ALT detection kit (TECO Diagnostics, Anaheim, CA) was used according to the manufacturer's protocol. Measurement of ALT levels was performed in duplicate using plasma samples obtained from at least three mice per treatment.
Statistical analyses.
All statistical analyses were done using unpaired two sided Student's t test on Instat software. The data are expressed as means ± standard deviation. The number of animals used in experiments varied from three to eight and is indicated for each experimental condition in the figure legends.
RESULTS
Infection of liver cells with Ad in vivo and in vitro.
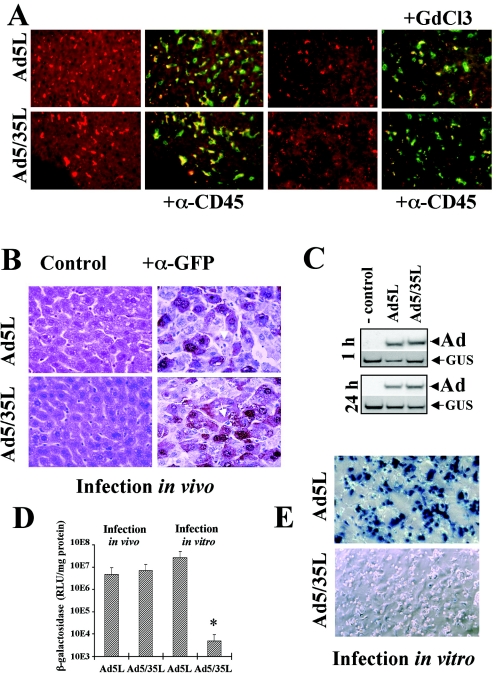
To analyze Ad liver cell infection in vivo, we used Ad5-based vectors containing fiber knob domains derived from Ad5 (Ad5L vector, subgroup C, CAR interacting) (42), and Ad35 (Ad5/35L vector subgroup B, non-CAR interacting) (48). Vectors with Ad35 fiber knob domain recognize CD46 as a primary attachment receptor (16). At 1 h after intravenous Ad administration, the vast majority of both fluorophore Cy-3-labeled Ad5L and Ad5/35L particles were found in association with Kupffer cells. Kupffer cells were identified by positive staining for CD45 (Fig. 1A, left) (58), the macrophage-specific marker F4/80 (data not shown), and their sensitivity to gadolinium chloride (GdCl3) (Fig. 1A, right two panels) (18). Since Cy-3-labeled capsid proteins are degraded over time, virus distribution in normal nontreated animals at day 3 p.i. was assessed based on Ad-mediated transgene expression (Fig. 1B). At this time point, GFP reporter gene expression was detectable only in hepatocytes but not in the Kupffer cells (Fig. 1B, right two panels) or in CD31+ vascular endothelial cells (data not shown). To further analyze Ad liver cell infection in vitro and in vivo, we quantified vector genomic DNA and transgene expression at different times post virus administration. Quantitative Southern blot analysis demonstrated comparable levels of Ad5L and Ad5/35L genomes in the liver at 1 h and 24 h post virus administration (Fig. 1C). Quantification of β-galactosidase reporter gene expression revealed that both Ad5L and Ad5/35L vectors expressed similar levels of the transgene after intravenous application (Fig. 1D, “Infection in vivo” bars).
FIG. 1.
Liver cell transduction with Ads in vivo and in vitro. Indicated Ads were injected into the tail vein of C57BL/6 mice. Livers of mice injected with Cy3-labeled Ad5L and Ad5/35L vectors were recovered 1 h (A) and 3 days (B) after virus administration. (A) Frozen liver sections either remained unstained or were stained with anti-mouse CD45 antibody (green) to detect Kupffer cells. Cy-3-labeled Ad particles appear in red. In settings with GdCl3, two doses of the drug were injected into mice 30 h and 6 h before Ad administration as described earlier (16). Note a significant reduction in virus accumulation within Kupffer cells after treatment of mice with GdCl3 (right). GdCl3 functionally inactivates Kupffer cells. (B) Paraffin sections of mouse livers 3 days after Ad virus administration were stained with hematoxilin-eosin (left) or stained with anti-GFP monoclonal antibody (brown). Kupffer cells are indicated by arrows. Note that GFP expression was detected in hepatocytes but not in Kupffer cells. (C) Southern blot analysis of Ad genomes in liver tissue 1 h and 24 h post virus administration. Mice were injected with saline (control) or Ad5L or Ad5/35L vectors. At the indicated time points, livers were recovered, and total DNA was purified and subjected to Southern blot analysis. For quantitative assessment of the data, equivalent loads of genomic DNA were confirmed by hybridization of the membranes with a probe specific for the mouse β-glucuronidase gene (GUS). After exposure to X-ray film, membranes were stripped and rehybridized with an Ad-specific probe to determine the levels of vector DNA associated with liver cells (Ad). (D) β-Galactosidase reporter gene activity in liver tissue (“Infection in vivo” bars), following intravenous administration of indicated Ad vectors or in purified mouse hepatocytes infected with Ad in vitro (“Infection in vitro” bars). (E) Purified mouse hepatocytes were infected with indicated Ad vectors and 48 h later stained in situ for β-galactosidase activity. Representative fields are shown. Studies shown in panels B through E were done with mice without GdCl3 treatment.
Surprisingly, although the non-CAR interacting Ad5/35L vector efficiently transduced hepatocytes in vivo (Fig. 1B), it could neither bind to (data not shown) nor transduce primary mouse hepatocytes in vitro (Fig. 1D, “Infection in vitro” bars, and Fig. 1E). This implies the existence of a novel CAR-independent mechanism for Ad infection of hepatocytes in vivo. We hypothesized that a factor(s) present only in vivo, specifically, a blood factor(s), serves as a bridge between the virus and the cell surface, allowing for efficient Ad infection of liver cells in vivo.
Blood factors mediate CAR-independent hepatocyte infection in vivo.
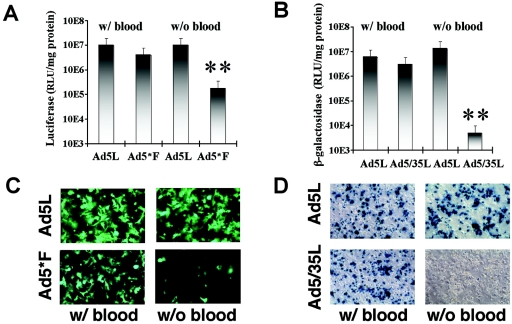
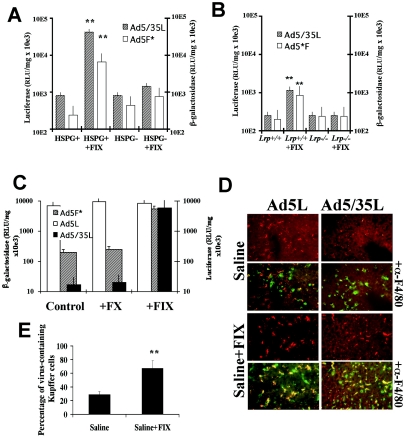
To assess the contribution of blood factors to liver transduction, we employed an in situ perfusion technique that allows for vascular exclusion of the liver and analysis of gene delivery to hepatic cells in the absence of blood (see Materials and Methods for details) (5, 56). In vivo transduction was analyzed for the native CAR-binding Ad5-based vectors, Ad5L1 (expressing GFP and luciferase) and Ad5L2 (expressing β-galactosidase), and compared to transduction of non-CAR-binding vectors, Ad5*F (expressing GFP and luciferase) and Ad5/35L (expressing β-galactosidase). Ad5*F corresponds to Ad5L; however, it has a single point mutation in the fiber knob domain (amino acids 477 Y→ A) that compromises CAR binding (2). As expected from the studies shown in Fig. 1B, upon vector infusion in the presence of blood, reporter gene expression in hepatocytes was comparable for CAR-interacting (Ad5L1/2) and non-CAR-interacting (Ad5*F and Ad5/35L) vectors (Fig. 2A and B). However, liver perfusion in the absence of blood resulted in 2 to 3 orders of magnitude less efficient hepatocyte transduction with non-CAR-interacting vectors than corresponding CAR binding controls (Fig. 2B and C). Ad5L1/2 efficiently transduced hepatocytes in the absence of blood, most likely through interaction with CAR. In conclusion, these data demonstrate that liver uptake of Ad5 vectors occurs through two different mechanisms: via interaction with CAR and by a novel, CAR-independent mechanism mediated by blood factors. In the following studies, we employed the non-CAR-interacting vectors Ad5/35L and Ad5F* as tools to investigate the blood factor-mediated pathway.
FIG. 2.
The role of blood factors in Ad transduction of hepatocytes in vivo. (A) Ad5L1 and Ad5*F express GFP and luciferase as reporters. (B) Ad5L2 and Ad5/35L express β-galactosidase (β-Gal) as a reporter. In the with-blood setting, Ad5L1/2, Ad5/35L, or Ad5*F was injected into the portal vein through a permanently placed catheter. Fifteen minutes later, hepatocytes were isolated for culture and analysis of reporter gene expression. In without-blood settings, the portal vein and vena cava inferior were canulated and blood was flushed from the liver through the portal vein. Virus was infused through the portal vein and the circulation between vena porta and vena cava was closed, allowing asanguinous isolated liver perfusion with virus-containing saline. Thirty minutes after virus application, hepatocyes were isolated by collagenase perfusion. In both settings, reporter gene expression in plated hepatocytes was analyzed 48 h postinfection. Four mice per group were analyzed for Ad5L1, Ad5*F, Ad5L2 and Ad5/35L variants with blood and for Ad5L1 without blood. Five mice per group were analyzed for Ad5L2 variant without blood, and seven and eight mice per group were analyzed for Ad5*F and Ad5/35L variants without blood, respectively. **, P < 0.001. There were no statistically significant differences between other paired variants. (C) Hepatocyte transduction with GFP-expressing Ad viruses (UV fluorescence). (D) Hepatocyte transduction with β-galactosidase-expressing Ad viruses after histochemical staining with X-galactosidase.
Ad-blood factor complexes enter liver cells via heparan sulfate proteoglycans and LDL receptor-related protein (LRP).
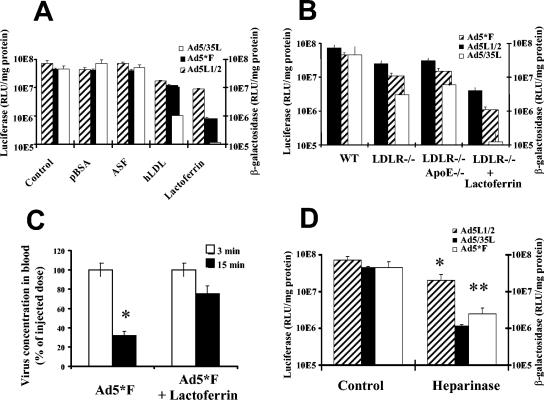
To identify the cellular receptor(s) involved in binding and uptake of the complex(es) formed between Ad and the putative blood factor(s), we injected a number of ligands for known hepatocellular receptors intravenously into mice to test whether they could compete with Ad infection in vivo. These ligands included polymerized BSA to saturate the scavenger receptor SR-BI (54), asialofetuin to saturate the asialoglycoprotein receptor (57), human LDL to saturate the LDL receptor (LDLR) (6), and lactoferrin to saturate the LRP as well as HSPG (19, 24) (Fig. 3A). Among the ligands analyzed, only lactoferrin strongly inhibited infection with Ad5*F (>50-fold reduction in reporter gene activity) and Ad5/35L (>600-fold reduction), whereas the efficiency of Ad5L infection was reduced by only 8 fold (P < 0.001). To test whether the LDLR could serve as a receptor for the Ad blood factor complexes, the in vivo infectivity of the non-CAR-binding Ad5*F and Ad5/35L vectors in wild-type and ldlr−/− mice (6) was compared (Fig. 3B). The absence of LDLR expression in hepatocytes did not affect transduction with these vectors to the extent of the competition with lactoferrin in wild-type mice. Injection of ldlr−/− mice with lactoferrin reduced the efficiency of gene transfer to a degree similar to that seen with wild-type mice. This result suggests that a lactoferrin-interacting receptor(s) provides a major contribution to Ad liver transduction. The observed reduction of Ad infectivity in ldlr−/− mice could be due to elevated blood LDL levels, which interfere with function of the lactoferrin receptors, LRP and HSPG (19, 20, 24). The involvement of ApoE-containing lipoproteins or chylomicrons in Ad infection in vivo was excluded by administering viruses into ldlr−/−/apoE−/− double-knockout mice (23) (Fig. 3B), where reporter gene activities were not further reduced compared to Ldlr −/− mice.
FIG. 3.
Analysis of hepatocellular receptors mediating the uptake of complexes between Ad and blood factors. (A) Competition of Ad infection with different ligands to hepatocellular receptors. The indicated competitors or saline (as a control) were injected into the portal vein through a catheter 5 min before virus administration. Hepatocytes were purified and reporter gene activities were analyzed as described in Materials and Methods. Three mice per group were analyzed for control, pBSA, asialofetuin (ASF), and hLDL groups, and five mice were analyzed for the lactoferrin group. *, P = 0.0056 for the hLDL Ad5L1/2 group, compared to control. For the other viruses in the hLDL group and all viruses in the lactoferrin group, there was a significant difference in hepatocyte transduction compared to the control group (P < 0.001). (B) Analysis of hepatocyte transduction in wild-type, ldlr−/−, and ldlr/ApoE−/− knockout mice. A total of 1011 viral particles were injected into the portal vein. After 15 min, hepatocytes were harvested, and reporter gene activities were analyzed 48 h later. (C) Blood persistence of Ad5*F in blood with or without lactoferrin administration. Lactoferrin was injected into the portal vein 5 min prior virus administration. * P < 0.01 for four samples. (D) Heparinase I was administrated into the tail vein of C57BL/6 mice 30 min before virus injection. Fifteen minutes after virus application, hepatocytes were harvested, plated, and analyzed for reporter gene activities 2 days postinfection. Three mice per group were analyzed for all control groups, four mice per group were analyzed for Ad5L1/2, and five mice per group were analyzed for Ad5F* and Ad5/35L (heparinase treated) groups. *, P < 0.01; **, P < 0.001.
Lactoferrin is a natural antimicrobial and antiviral agent (60). To exclude that lactoferrin inactivates Ad or redirects it to nonhepatic tissues in vivo, we measured the amount of infectious Ad5*F virus present in the blood with and without preinjection of lactoferrin. Fifteen minutes after Ad5*F administration without lactoferrin, >75% of vector was removed from the circulation, whereas <30% of virus was removed upon preinjection of lactoferrin. Since the majority of administered virus remained in the circulation in lactoferrin-preinjected animals, these data indicate that lactoferrin did not mediate significant sequestration to nonhepatic tissues (Fig. 3C). The infectivity of Ad5*F (as determined with 293-DH26 cells) was comparable in blood samples from mice with and without lactoferrin pretreatment (data not shown).
Lactoferrin binds to both LRP and HSPG (57). Because LRP knockout status in mice is lethal (22), we tested the role of HSPG as a potential receptor for Ad infection in vivo. It has been shown that injection of heparinase in vivo dramatically reduces clearance of proteins from blood whose catabolism depends on HSPG (22). According to the protocols used in these studies, wild-type mice were injected with heparinase, followed by virus administration (Fig. 3D). The reporter gene activities in hepatocytes harvested and cultured after Ad5*F and Ad5/35L infection were almost 2 orders of magnitude lower in mice pretreated with heparinase than in untreated controls. At the same time, transduction with CAR-interacting Ad5L was not dramatically affected by heparinase treatment. These data indicate that HSPG represents a major cellular receptor, enabling Ad5*F and Ad5/35L to transduce hepatocytes in the presence of blood.
Multiple blood factors bind Ad fiber knob domain and can mediate liver cell infection in vivo.
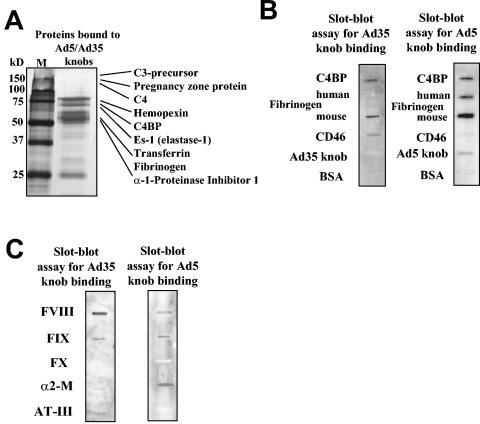
HSPG and LRP interact in vivo with a large variety of structurally unrelated ligands. To identify blood factors interacting with Ad, mouse plasma proteins were precipitated with both Ad5 and Ad35 fiber knob domains and analyzed by tandem mass spectrometry. We identified several knob-interacting proteins that are present in plasma at high concentrations (Table 1 and Fig. 4A and B). Additionally, we screened a number of purified recombinant HSPG-LPR ligands (coagulation factors VIII, IX, and X; tissue factor pathway inhibitor; and antithrombin III) for their ability to bind to Ad knobs by slot blot assays (Fig. 4C). These ligands are less abundant in plasma and, therefore, difficult to detect by the coprecipitation approach. From all identified knob-binding plasma proteins that were available as recombinant proteins, only human coagulation FIX (identified by a slot blot assay) and complement component C4BP (identified by tandem mass spectrometry) conferred CAR-independent Ad infection of HSPG-LRP-expressing immortalized and primary human hepatocytes in vitro (Fig. 5). Importantly, both FIX and C4BP were used at or less than physiological concentrations. We further demonstrated that the infectivity of non-CAR-interacting Ads correlated with the dose of FIX or C4BP and could be efficiently inhibited by lactoferrin and heparin, which bind HSPGs and block the receptor recognition epitopes on activated FIX (38) and C4BP, respectively. With HSPG-LRP-expressing murine cells, however, only human FIX but not human recombinant C4BP (data not shown) allowed for efficient cell transduction with Ad5*F and Ad5/35L (Fig. 6A and B), which might be due to species specificity in human recombinant C4BP-mouse HSPG-LRP interactions. To demonstrate involvement of blood factors in liver cell transduction in mice, we therefore focused on FIX. We perfused mouse livers with Ad5L1/2, Ad5*F, or Ad5/35L virus containing saline with and without supplementation with FIX or human factor X (FX) (as a negative control) (Fig. 6C). Supplementation of saline with FIX allowed for the transduction of hepatocytes with the non-CAR-interacting Ad5*F and Ad5/35L viruses to levels comparable with Ad5L1/2 (P < 0.001).
TABLE 1.
Plasma proteins precipitated with Ad fiber knob domain, identified by tandem mass spectrometry
| Identified protein | gi no.a | No. of peptides matched | Mowse scorea | LRP/HSPG binding |
|---|---|---|---|---|
| Fibrinogen, β polypeptide | 33859809 | 7 | 524 | No |
| Pzp | 34785996 | 9 | 466 | Yes |
| Fibrinogen, γ polypeptide | 19527078 | 4 | 240 | No |
| Proteinase inhibitor, clade A | 6678079 | 3 | 215 | Yes |
| Complement C3 | 1352102 | 5 | 208 | Yes |
| Ceruloplasmin | 6680997 | 4 | 180 | No |
| Hemopexin | 1881768 | 3 | 129 | No |
| Fibrinogen, α polypeptide | 33563252 | 2 | 115 | No |
| C4-binding protein | 309119 | 3 | 107 | Yes |
| Complement C4 | 50242 | 2 | 98 | No |
| α-1-Proteinase inhibitor 2 | 2118392 | 3 | 92 | Yes |
| Es-1 plasma protein | 22135640 | 1 | 34 | ? |
gi, protein sequence identification number (GenBank); Mowse score, individual ion scores of >32 indicate identity or extensive homology to a particular protein in the database with a probability of P < 0.05. Protein scores are derived from ions scores as a nonprobabilistic basis for ranking proteins hits. Only proteins with a Mowse score of >32 are shown. ?, data not available.
FIG. 4.
Analysis of plasma proteins interacting with Ad fiber knob domains by mass spectrometry and protein slot blot assay. (A) Silver-stained polyacrylamide gel of proteins precipitated from mouse plasma with Ad5 and Ad35 fiber knob domains. (B and C) Slot blot analysis of direct binding of Ad fiber knob domains to purified plasma proteins (10 μg/lane) immobilized on nitrocellulose membrane. Human CD46 protein was used as a positive control to detect specific Ad35 fiber knob binding. Ad5, Ad35, and BSA were used to determine the levels of nonspecific binding. FVIII, FIX, and FX, coagulation factors VIII, IX and X, respectively; α2-M, α2-macroglobulin; AT-III, antithrombin III.
FIG. 5.
Interaction of Ad with C4BP and coagulation FIX mediates CAR-independent infection of human cells in vitro. (A) Dose-dependent transduction of human hepatoma HepG2 cells by Ad vectors with or without FIX (in units per milliliter, with 1 U corresponding to the physiological concentration of FIX in plasma). For infection inhibition experiments, 0.5 mg/ml of lactoferrin and 10 U/ml of heparin were used, with four mice per group analyzed. (B) Dose-dependent transduction of human hepatoma HepG2 cells by Ad5*F with or without C4BP (in micrograms per milliliter, where 150 μg/ml corresponds to the physiological concentration of C4BP in plasma), with four mice per group analyzed. (C) Expression of the GFP gene in human hepatoma HepG2 cells infected with Ad5*F in the presence of C4BP and different competitors 24 h post virus infection. Infections were done as described for panel B and in Materials and Methods. Cell nuclei were counterstained with DAPI (4-,6-diamidino-2-phenylindole) (blue). Representative fields with similar cell densities are shown. (D) Expression of GFP in primary human hepatocytes infected with Ad5*F in the presence of FIX and different competitors 24 h post virus infection. Infections were done as described for panel A and in Materials and Methods. Representative fields are shown. Upper panels demonstrate cells illuminated by UV light; lower panels demonstrate the same areas in visible light.
FIG. 6.
Interaction of Ad with coagulation FIX mediates CAR-independent infection of mouse cells in vitro and ex vivo. (A) Transduction of HSPG-expressing (CHO-K1) and HSPG-negative (CHO-pgsA745) cells by Ad5F* or Ad5/35L with or without FIX. (Averages and standard deviations for four independent experiments are shown.) **, P < 0.01. (B) Infection of mouse embryonic fibroblast MEF-1 (lrp+/+) or MEF-2 (lrp−/−) cells by Ad5F* or Ad5/35L with or without FIX. (C) Isolated, asanguinous liver perfusion of C57BL/6 mice with virus or saline plus human FX or FIX (1 U/ml). After virus perfusion, hepatocytes were isolated, and transgene expression was analyzed, with four mice analyzed for each virus group. **, P < 0.001. (D) Accumulation of Cy3-labeled Ad5L and Ad5/35L viruses (red) in F4/80-positive Kupffer cells (green) on liver sections 30 min after isolated liver perfusion with virus-containing saline with and without the addition of FIX. Note the absence of both Ad5L and Ad5/35L virus association with Kupffer cells when the livers were perfused in the absence of FIX. Representative fields of livers are shown, with three samples analyzed. (E) Quantitative representation of Ad accumulation in Kuppfer cells in mouse livers perfused with or without addition of FIX. Quantifications of virus-containing Kupffer cells on liver sections were conducted in a blinded fashion by independent experienced pathologists. At least 10 liver sections per experimental group were utilized to collect the data for statistical analysis. **, P < 0.01.
Our recent data indicate that Ad accumulation in Kupffer cells is mediated by the fiber knob domain but does not depend on virus interaction with CAR (45). To test whether Kupffer cells trap Ad via a blood factor-dependent pathway, we perfused mouse livers with saline containing Cy3-fluorochrome-labeled Ad5L or Ad5/35L vectors. While perfusion of mouse livers with virus-containing saline did not demonstrate considerable accumulation of virus in Kupffer cells, addition of FIX resulted in significantly increased virus accumulation in Kupffer cells (Fig. 6D and E).
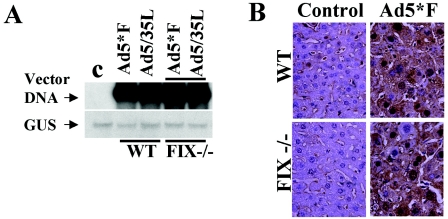
The use of FIX or C4BP knockout mice should enable assessment of the relative contribution of FIX or C4BP in CAR-independent Ad liver cell transduction in vivo. Since C4BP knockout animals were not available, we focused on FIX knockout mice (30). In these mice, the translation initiation site and the first three exons of the FIX gene were deleted, resulting in a lack of functionally active FIX in the blood. We injected Ad5*F and Ad5/35L vectors into FIX knockout and strain-matched wild-type mice and analyzed the amount of vector DNA present in the liver at 72 h postinfusion by quantitative Southern blotting. This analysis revealed that there was no significant difference between the amounts of Ad5*F or Ad5/35L vector DNA in the liver of wild-type or FIX knockout mice (Fig. 7A). Histological evaluation of GFP reporter gene expression corroborated the Southern blot analysis data and showed that CAR-binding ablated Ad5*F (Fig. 7B) and Ad5/35L (data not shown) vectors expressed GFP with equal efficiency in hepatocytes of both wild-type and FIX knockout animals. While our data undoubtedly demonstrate that FIX allows for CAR-independent Ad infection of mouse hepatocytes and Kupffer cells in vitro and in vivo and human hepatocytes in vitro, the data obtained with FIX knockout mice suggest that other blood factors, like C4BP, contribute to in vivo transduction of liver cells.
FIG. 7.
Accumulation of Ad genomic DNA in liver tissue and transduction of hepatocytes after vector administration into wild-type and FIX knockout mice. (A) Analysis of Ad5*F genomic DNA accumulation in liver tissue of wild-type or FIX knockout mice 72 h post vector administration, as determined by quantitative Southern blotting as shown in Fig. 1C. (B) Histological analysis of hepatocyte transduction after intravenous administration of Ad5*F vector to wild-type or FIX knockout mice. All procedures were done as described in Materials and Methods and in the legend to Fig. 1B.
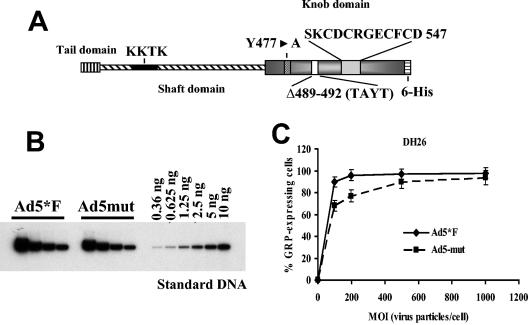
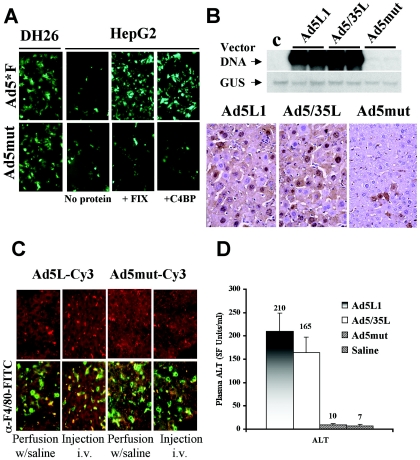
A mutant Ad ablated for binding to CAR and blood factors does not efficiently transduce liver cells and demonstrates reduced hepatotoxicity in vivo. To ultimately prove the involvement of blood factors in Ad liver cell transduction in vivo, we generated an Ad vector possessing mutations within its knob domain that interfere with binding to both C4BP and FIX. Based on the three-dimensional structure of Ad5 fiber knob domain (59), we modified exposed areas within the knob and generated a set of mutant fiber knob domains. In a first screening step, mutant knobs were tested for their inability to bind to FIX and C4BP by slot blot assays (data not shown). For selected mutants, recombinant Ad vectors were generated. The following modifications (Fig. 8A) were found to significantly reduce binding to CAR, C4BP, and FIX and at the same time to allow for virus production. (i) The Y477A single point mutation ablated Ad binding to CAR. (ii) The FG loop deletion (ΔΤAYT) was predicted to change the overall conformation of the knob domain without disturbing its ability to trimerize. (iii) The HI loop was extended by inserting a 12-amino-acid-long heterologous peptide (position 547) to create additional sterical hindrances, preventing interaction with natural ligands. The C terminus of the mutant fiber knob also contained a six-histidine tag that allowed for the purification of recombinant knob and for propagation of a corresponding virus. An Ad vector containing the mutated knob, Ad5mut, was rescued and amplified to high titers on 293-DH26 cells, expressing anti-six-His tag antibody as novel Ad receptor (12). The fact that Ad5mut efficiently infects 293-DH26 cells (Fig. 8B and C and Fig. 9A) demonstrates that this virus is viable and can use the artificial receptor to gain cell entry. At the same time, Ad5mut was unable to efficiently infect cells in vitro that did not express the artificial receptor (HepG2 cells), and addition of FIX or C4BP did not restore its infectivity, in contrast to Ad5*F (Fig. 9A).
FIG. 8.
Fiber structure of Ad mutated for binding to CAR and blood factors and its biological activity. (A) Schematic representations of mutations introduced into the Ad5L fiber knob domain ablating its binding to both CAR and blood factors. (B) Quantitative Southern blot analysis of purified Ad5*F and Ad5mut vector stocks. Virus DNA purified from 10 μl of vector stock was applied (in twofold serial dilutions) on an agarose gel together with serial dilutions of standard DNA (linearized adenovirus genome-containing plasmid). After hybridization with a 32P-labeled Ad-specific DNA probe and quantitation of signals with a phosphorimager, the concentrations of the indicated viruses were calculated. The concentration for Ad5*F was 8.6 × 1012 virus particles per ml and the concentration for Ad5mut was 6.8 × 1012 virus particles per ml. (C) Transduction of 293-DH26 cells with Ad5*F and Ad5mut vectors. (Averages and standard deviations for three independent experiments are shown.) For all further studies, an MOI of 1,000 virus particles per cell was used.
FIG. 9.
FIX-mediated infection of primary human hepatocytes and reduced infection of mouse liver with mutated Ad vector in vivo. (A) Visualization of GFP expression in 293-DH26 and HepG2 cells infected with Ad5*F and Ad5mut in the presence of absence of C4BP (150 μg/ml) and FIX (1 U/ml), with four mice analyzed per group. Representative fields with comparable cell density are shown. (B) Transduction of liver cells with Ad5L1, Ad5/35L-GFP, and Ad5mut vectors. Southern blot analysis (top) of vector DNA in mouse livers 72 h after intravenous administration of the indicated vectors. Control, liver DNA of a mouse injected with saline; GUS, single-copy mouse β-glucuronidase gene. GFP immunohistochemistry (bottom) of formalin-fixed and paraffin-embedded sections of livers harvested from mice injected with indicated viruses. (C) Detection of Cy3-labeled Ad5L1 and Ad5mut viruses (red) and F4/80-positive Kupffer cells (green) on liver sections 1 h after isolated liver perfusion with virus-containing saline with and without addition of FIX or after injection or viruses intravenously. Representative fields of livers are shown, with three mice analyzed per group. (D) Plasma ALT levels 72 h after tail vein injection of 1011 virus particles of the indicated Ad vectors or saline, with five mice analyzed per group.
To evaluate the ability of Ad5mut virus to infect liver cells in vivo, we injected 1011 particles of Ad5L1, Ad5/35L-GFP, and Ad5mut vectors intravenously into mice. Southern blot analysis for vector genomes performed 72 h p.i. demonstrated that, compared to Ad5L1 and Ad5/35L, about 50-fold less Ad5mut vector was present in the liver (Fig. 9B, top). Analysis of GFP expression on liver section corroborated the Southern blot data and showed transduction of only sparse hepatocytes with Ad5mut (Fig. 9B, bottom).
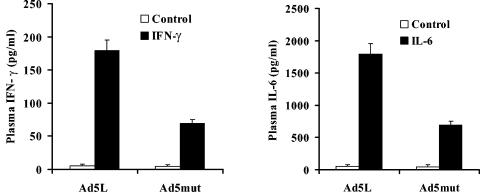
To evaluate whether the fiber knob mutations introduced into Ad5mut also affected uptake into Kupffer cells, the distribution of Cy-3-labeled Ads on liver sections was analyzed 30 min after isolated liver perfusion or tail vein injection (Fig. 9C). As anticipated, in the absence of blood factors, both Ad5L1 and Ad5mut were not taken up by Kupffer cells (Fig. 9C, “perfusion with saline”). In the presence of blood and following tail vein injection, Ad5L1 (Fig. 9C, left) and Ad5/35L (data not shown) efficiently accumulated in Kupffer cells (Fig. 9C, “injection i.v.”). In contrast, Ad5mut, while detectable throughout the liver parenchyma, did not significantly colocalize with Kupffer cell-specific F4/80 antigen staining (Fig. 9C, “injection i.v.” variant). To assess whether reduced accumulation in Kupffer cells and hepatocytes of Ad5mut in vivo resulted in reduced hepatotoxicity, we measured plasma ALT levels. Tail vein injection of 1011 virus particles of Ad5L and Ad5/35L triggered elevation of ALT levels. In contrast, ALT levels after infusion of Ad5mut were comparable to those in mice injected with saline (Fig. 9D). We also analyzed plasma levels of key proinflammatory cytokines 6 h after intravenous administration of Ad5L, Ad5/35L, or Ad5mut vectors. The levels of IFN-γ and IL-6 were significantly elevated, following administration of Ad5L (Fig. 10) and Ad5/35L (data not shown) vectors. In contrast, levels of these cytokines after administration of Ad5mut virus were up-regulated much less (Fig. 10). Taken together, Ad5mut, a vector that is able to utilize an artificial receptor for infection in vitro, is unable to bind blood factors and infect hepatocytes and Kupffer cells after intravenous administration and consequently does not induce liver toxicity.
FIG. 10.
Plasma levels of IFN-γ and IL-6 cytokines after administration of mice with Ad vectors. Mice were administered Ad5L or Ad5mut vectors; 6 h later, plasma samples were collected, and the levels of IFN-γ and IL-6 were analyzed by cytometric bead array as described in Materials and Methods (four mice per group).
DISCUSSION
A new pathway for Ad liver transduction.
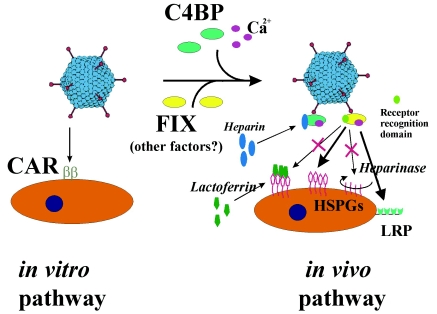
In this study, we describe a new pathway utilized by Ad for infection of liver, which is the organ predominantly transduced after systemic virus application. Our data challenge the currently accepted model of Ad infection that is based mostly on in vitro analyses. According to this model, Ad infects cells in a two-step process (42). The first and limiting step is the binding of Ad fibers to the primary cell surface receptor. Next, RGD motifs within the Ad penton base interact with cellular integrins, allowing for internalization of the attached virus particles into the cell. An important consequence of this model was the generalization that cells that do not efficiently express the primary attachment receptor(s) would be refractory to Ad infection. Here, we demonstrated that Ads, which were unable to infect hepatocytes in vitro, due to an inability to interact with CAR, efficiently infected hepatocytes in vivo. Based on our findings, we propose a new model in which in vivo infection of hepatic cells by Ads occurs through binding of viral particles to blood factors, in particular to coagulation FIX and the complement factor C4BP, and redirecting these complexes to hepatocellular receptors, including HSPGs and LPR (Fig. 11). This model has significant implications for current efforts utilizing systemically applied Ad vectors for in vivo gene therapy. This model is also in conflict with recent papers by Smith et al. reporting that the KKTK motif present within the Ad5 fiber shaft directly interacts with HSPGs, thus enabling Ad to infect liver cells in vivo (52, 53). Our data demonstrate that Ad vectors containing the intact Ad5 fiber shaft (Ad5/35L and especially Ad5F*, possessing only a single point mutation within an exposed loop of Ad knob) are unable to infect hepatocytes in vivo in the absence of blood factors, arguing against a major role of the fiber shaft motif in Ad infection in vivo.
FIG. 11.
Schematic representation of CAR-dependent and blood factor-dependent pathways of Ad infection and action of competitors (lactoferrin, heparin, and heparinase).
Our studies with Ads containing modified fibers possessing Ad5 or Ad35 (subgroup C and B, respectively) knob domains suggest that this new pathway can be utilized by different Ad serotypes. A crucial role of virus interaction with blood factors in viral pathogenesis was suggested for several members of the Flaviviridae family, including hepatitis C and dengue viruses (1, 21). Considering our data, adenoviruses are a new addition to the list of viruses that may utilize an indirect mechanism of target cell infection. It is apparent that Ads use multiple pathways for infection in vivo, which may represent an evolutionary mechanism to extend the spectrum of target cells. However, the importance of this finding for natural Ad pathogenicity remains to be determined.
Ad uptake into Kupffer cells and Ad-associated toxicity.
Our data suggest that while infection of hepatocytes involves both the CAR- and blood factor-mediated pathway, uptake of Ad vectors into Kupffer cells is CAR independent and mediated by blood factors. The identification of a mechanism for Ad uptake by Kupffer cells has important practical implications. Uptake of Ad particles into Kupffer cells is associated with release and/or production of proinflammatory cytokines, which directly or indirectly (via activation of other cell types of the reticuloendothelial system) are responsible in turn for toxic side effects observed after the intravenous injection of Ad vectors in animals and humans. Consequently, preventing Kupffer cell activation by blocking the pathways involved in Ad binding and uptake will improve the safety profile of Ad vectors. We showed in this study that an Ad vector ablated for binding to FIX and C4BP (Ad5mut) demonstrated reduced hepatic and innate toxicity after systemic application. Importantly, Ad5mut is a retargeted vector; it possesses a six-His ligand linked on the fiber C terminus, which allowed efficient infection of cells expressing membrane-anchored single-chain anti-His antibody (serving as an artificial virus attachment receptor). Notably, it is unclear whether the FIX/C4BP-mediated pathway is also dominant in the presence of anti-Ad antibodies, which could mediate Ad uptake via Fc receptors present on Kupffer cells.
Because our study was limited to factors that were either commercially available or provided by other investigators as purified recombinant proteins, the role of other blood factors or their complexes in liver uptake cannot be excluded. In this context, however, it is worth noting that the specific mutations introduced into the fiber knob domain were sufficient to prevent both Ad accumulation in Kupffer cells and transduction of hepatocytes, resulting in reduced overall Ad toxicity in vivo. Administration of CAR-binding-ablated vectors to FIX knockout mice resulted in no significant reduction of liver cell transduction, compared to wild-type animals. One of the potential explanations of this data is based on the dramatic difference in the levels of FIX and C4BP in peripheral blood. While the physiological concentration of FIX in plasma is only 5 μg/ml, C4BP (one of the most abundant plasma proteins) is present in plasma at levels of 250 to 300 μg/ml. Since both FIX and C4BP allow CAR-independent Ad cell infection, deletion of only one low abundance protein (such as FIX) was apparently not sufficient to prevent CAR-independent liver cell infection. Clearly, until C4BP knockout or C4BP-FIX double-knockout mice are available, the relative contribution of each of these proteins to Ad liver cell infection remains unclear.
Our study contributes to a better understanding of adenovirus vector-host interactions, when virus is applied intravenously. It also has immediate practical implications aiding the development of safe and effective adenovirus vectors with modified tropism that might prove useful for treatment of numerous human inborn and acquired human diseases, including cancer.
Acknowledgments
We thank Arthur Thompson (Puget Sound Blood Center, Seattle, Wash.), Michael Gotthardt (VCAPP, Pullman, and MDC-Berlin), Ramon Alemany (Institut Catala d'Oncologia, Barcelona, Spain), Katherine High (Children's Hospital of Philadelphia), Anna Blöm and Björn Dahlback (Lund University, Sweden), and Joanne T. Douglas (University of Alabama at Birmingham) for providing valuable materials. We thank Jeffrey Chamberlain, David Dichek, Renee Le Boeuf, Matt Mealiffe (University of Washington), and our colleagues for critical discussions of the manuscript.
This work was supported by NIH grants HL53750, CA 80192, and DK 47754 (to A.L.), and AI060807 (to D.M.S).
REFERENCES
- 1.Agnello, V., G. Abel, M. Elfahal, G. B. Knight, and Q. X. Zhang. 1999. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA 96:12766-12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alemany, R., and D. T. Curiel. 2001. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 8:1347-1353. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Gary, H., R. L. McKinney, T. Rosengart, M. L. Lesser, and R. G. Crystal. 2002. Systemic interleukin-6 responses following administration of adenovirus gene transfer vectors to humans by different routes. Mol. Ther. 6:287-297. [DOI] [PubMed] [Google Scholar]
- 4.Bernt, K. M., D. S. Steinwaerder, S. Ni, Z. Y. Li, S. R. Roffler, and A. Lieber. 2002. Enzyme-activated prodrug therapy enhances tumor-specific replication of adenovirus vectors. Cancer Res. 62:6089-6098. [PubMed] [Google Scholar]
- 5.Branchereau, S., D. Calise, and N. Ferry. 1994. Factors influencing retroviral-mediated gene transfer into hepatocytes in vivo. Hum. Gene Ther. 5:803-808. [DOI] [PubMed] [Google Scholar]
- 6.Brown, M. S., and J. L. Goldstein. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science 232:34-47. [DOI] [PubMed] [Google Scholar]
- 7.Brunetti-Pierri, N., D. J. Palmer, A. L. Beaudet, K. D. Carey, M. Finegold, and P. Ng. 2004. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into non-human primates. Hum. Gene Ther. 15:35-46. [DOI] [PubMed] [Google Scholar]
- 8.Carlson, C. A., D. S. Steinwaerder, H. Stecher, D. M. Shayakhmetov, and A. Lieber. 2002. Rearrangements in adenoviral genomes mediated by inverted repeats. Methods Enzymol. 346:277-292. [DOI] [PubMed] [Google Scholar]
- 9.Chroboczek, J., and B. Jacrot. 1987. The sequence of adenovirus fiber: similarities and differences between serotypes 2 and 5. Virology 161:549-554. [DOI] [PubMed] [Google Scholar]
- 10.Connelly, S. 1999. Adenoviral vectors for liver-directed gene therapy. Curr. Opin. Mol. Ther. 1:565-572. [PubMed] [Google Scholar]
- 11.Crystal, R. G., B. G. Harvey, J. P. Wisnivesky, K. A. O'Donoghue, K. W. Chu, J. Maroni, J. C. Muscat, A. L. Pippo, C. E. Wright, R. J. Kaner, P. L. Leopold, P. D. Kessler, H. S. Rasmussen, T. K. Rosengart, and C. Hollmann. 2002. Analysis of risk factors for local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of comorbid conditions. Hum. Gene Ther. 13:65-100. [DOI] [PubMed] [Google Scholar]
- 12.Douglas, J. T., C. R. Miller, M. Kim, I. Dmitriev, G. Mikheeva, V. Krasnykh, and D. T. Curiel. 1999. A system for the propagation of adenoviral vectors with genetically modified receptor specificities. Nat. Biotechnol. 17:470-475. [DOI] [PubMed] [Google Scholar]
- 13.Einfeld, D. A., R. Schroeder, P. W. Roelvink, A. Lizonova, C. R. King, I. Kovesdi, and T. J. Wickham. 2001. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J. Virol. 75:11284-11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fechner, H., A. Haack, H. Wang, X. Wang, K. Eizema, M. Pauschinger, R. Schoemaker, R. Veghel, A. Houtsmuller, H. P. Schultheiss, J. Lamers, and W. Poller. 1999. Expression of coxsackie adenovirus receptor and αv-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 6:1520-1535. [DOI] [PubMed] [Google Scholar]
- 15.Gaggar, A., Z-Y. Li, V. Ternovoi, D. M. Shayakhmetov, N. Kiviat,and A. Lieber. 2002. Using adenoviral fiber knob domains to determine tumor susceptibility to viral cancer therapy. Mol. Ther. 5:S206. [Google Scholar]
- 16.Gaggar, A., D. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 17.Graham, F. L., and A. J. van der Eb. 1973. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52:456-467. [DOI] [PubMed] [Google Scholar]
- 18.Hardonk, M. J., F. W. Dijkhuis, C. E. Hulstaert, and J. Koudstaal. 1992. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J. Leukoc. Biol. 52:296-302. [DOI] [PubMed] [Google Scholar]
- 19.Herz, J., D. E. Clouthier, and R. E. Hammer. 1992. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 71:411-421. [DOI] [PubMed] [Google Scholar]
- 20.Herz, J., and D. K. Strickland. 2001. LRP: a multifunctional scavenger and signaling receptor. J. Clin. Investig. 108:779-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilgard, P., and R. Stockert. 2000. Heparan sulfate proteoglycans initiate dengue virus infection of hepatocytes. Hepatology 32:1069-1077. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi, S., J. Herz, N. Maeda, J. L. Goldstein, and M. S. Brown. 1994. The two-receptor model of lipoprotein clearance: tests of the hypothesis in “knockout” mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc. Natl. Acad. Sci. USA 91:4431-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji, Z. S., W. J. Brecht, R. D. Miranda, M. M. Hussain, T. L. Innerarity, and R. W. Mahley. 1993. Role of heparan sulfate proteoglycans in the binding and uptake of apolipoprotein E-enriched remnant lipoproteins by cultured cells. J. Biol. Chem. 268:10160-10167. [PubMed] [Google Scholar]
- 24.Ji, Z. S., and R. W. Mahley. 1994. Lactoferrin binding to heparan sulfate proteoglycans and the LDL receptor-related protein. Further evidence supporting the importance of direct binding of remnant lipoproteins to HSPG. Arterioscler. Thromb. 14:2025-2031. [DOI] [PubMed] [Google Scholar]
- 25.Kochanek, S., G. Schiedner, and C. Volpers. 2001. High-capacity ‘gutless’ adenoviral vectors. Curr. Opin. Mol. Ther. 3:454-463. [PubMed] [Google Scholar]
- 26.Leissner, P., V. Legrand, Y. Schlesinger, D. A. Hadji, M. van Raaij, S. Cusack, A. Pavirani, and M. Mehtali. 2001. Influence of adenoviral fiber mutations on viral encapsidation, infectivity and in vivo tropism. Gene Ther. 8:49-57. [DOI] [PubMed] [Google Scholar]
- 27.Lieber, A., C. Y. He, L. Meuse, C. Himeda, C. Wilson, and M. A. Kay. 1998. Inhibition of NF-κB activation in combination with Bcl-2 expression allows for persistence of first-generation adenovirus vectors in the mouse liver. J. Virol. 72:9267-9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieber, A., C. Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71:8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieber, A., M. J. Vrancken Peeters, and M. A. Kay. 1995. Adenovirus-mediated transfer of the amphotropic retrovirus receptor cDNA increases retroviral transduction in cultured cells. Hum. Gene Ther. 6:5-11. [DOI] [PubMed] [Google Scholar]
- 30.Lin, H. F., N. Maeda, O. Smithies, D. L. Straight, and D. W. Stafford. 1997. A coagulation factor IX-deficient mouse model for human hemophilia B. Blood 90:3962-3966. [PubMed] [Google Scholar]
- 31.Liu, Q., and D. A. Muruve. 2003. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 10:935-940. [DOI] [PubMed] [Google Scholar]
- 32.Lozier, J. N., G. Csako, T. H. Mondoro, D. M. Krizek, M. E. Metzger, R. Costello, J. G. Vostal, M. E. Rick, R. E. Donahue, and R. A. Morgan. 2002. Toxicity of a first-generation adenoviral vector in rhesus macaques. Hum. Gene Ther. 13:113-124. [DOI] [PubMed] [Google Scholar]
- 33.Lundstrom, K. 2003. Latest development in viral vectors for gene therapy. Trends Biotechnol. 21:117-122. [DOI] [PubMed] [Google Scholar]
- 34.Martin, K., A. Brie, P. Saulnier, M. Perricaudet, P. Yeh, and E. Vigne. 2003. Simultaneous CAR- and αv integrin-binding ablation fails to reduce Ad5 liver tropism. Mol. Ther. 8:485-494.. [DOI] [PubMed] [Google Scholar]
- 35.Mickelson, C. A. 2000. Department of Health and Human Services National Institutes of Health Recombinant DNA Advisory Committee. Minutes of meeting March 8-10, 2000. Hum. Gene Ther. 11:2159-2192. [DOI] [PubMed] [Google Scholar]
- 36.Morral, N., W. K. O'Neal, K. Rice, M. M. Leland, P. A. Piedra, E. Aguilar-Cordova, K. D. Carey, A. L. Beaudet, and C. Langston. 2002. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum. Gene Ther. 13:143-154. [DOI] [PubMed] [Google Scholar]
- 37.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10:965-976. [DOI] [PubMed] [Google Scholar]
- 38.Neels, J. G., B. M. van Den Berg, K. Mertens, H. ter Maat, H. Pannekoek, A. J. van Zonneveld, and P. J. Lenting. 2000. Activation of factor IX zymogen results in exposure of a binding site for low-density lipoprotein receptor-related protein. Blood 96:3459-3465. [PubMed] [Google Scholar]
- 39.Nunes, F. A., E. E. Furth, J. M. Wilson, and S. E. Raper. 1999. Gene transfer into the liver of nonhuman primates with E1-deleted recombinant adenoviral vectors: safety of readministration. Hum. Gene Ther. 10:2515-2526. [DOI] [PubMed] [Google Scholar]
- 40.Raper, S. E., M. Yudkoff, N. Chirmule, G. P. Gao, F. Nunes, Z. J. Haskal, E. E. Furth, K. J. Propert, M. B. Robinson, S. Magosin, H. Simoes, L. Speicher, J. Hughes, J. Tazelaar, N. A. Wivel, J. M. Wilson, and M. L. Batshaw. 2002. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum. Gene Ther. 13:163-175. [DOI] [PubMed] [Google Scholar]
- 41.Reid, T., E. Galanis, J. Abbruzzese, D. Sze, J. Andrews, L. Romel, M. Hatfield, J. Rubin, and D. Kirn. 2001. Intra-arterial administration of a replication-selective adenovirus (dl1520) in patients with colorectal carcinoma metastatic to the liver: a phase I trial. Gene Ther. 8:1618-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schnell, M. A., Y. Zhang, J. Tazelaar, G. P. Gao, Q. C. Yu, R. Qian, S. J. Chen, A. N. Varnavski, C. LeClair, S. E. Raper, and J. M. Wilson. 2001. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 3:708-722. [DOI] [PubMed] [Google Scholar]
- 44.Seglen, P. O. 1979. Hepatocyte suspensions and cultures as tools in experimental carcinogenesis. J. Toxicol. Environ. Health 5:551-560. [DOI] [PubMed] [Google Scholar]
- 45.Shayakhmetov, D. M., Z.-Y. Li, S. Ni, and A. Lieber. 2004. Analysis of adenovirus liver sequestration, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J. Virol. 78:5368-5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shayakhmetov, D. M., Z. Y. Li, S. Ni, and A. Lieber. 2002. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res. 62:1063-1068. [PubMed] [Google Scholar]
- 47.Shayakhmetov, D. M., Z. Y. Li, V. Ternovoi, A. Gaggar, H. Gharwan, and A. Lieber. 2003. The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses. J. Virol. 77:3712-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shayakhmetov, D. M., and A. Lieber. 2000. Dependence of adenovirus infectivity on length of the fiber shaft domain. J. Virol. 74:10274-10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shayakhmetov, D. M., T. Papayannopoulou, G. Stamatoyannopoulos, and A. Lieber. 2000. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J. Virol. 74:2567-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shenk, T. 1996. Adenoviridea, p. 2111-2148. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 51.Smith, T., N. Idamakanti, H. Kylefjord, M. Rollence, L. King, M. Kaloss, M. Kaleko, and S. C. Stevenson. 2002. In vivo hepatic adenoviral gene delivery occurs independently of the coxsackievirus-adenovirus receptor. Mol. Ther. 5:770-779. [DOI] [PubMed] [Google Scholar]
- 52.Smith, T. A., N. Idamakanti, J. Marshall-Neff, M. L. Rollence, P. Wright, M. Kaloss, L. King, C. Mech, L. Dinges, W. O. Iverson, A. D. Sherer, J. E. Markovits, R. M. Lyons, M. Kaleko, and S. C. Stevenson. 2003. Receptor interactions involved in adenoviral-mediated gene delivery after systemic administration in non-human primates. Hum. Gene Ther. 14:1595-1604. [DOI] [PubMed] [Google Scholar]
- 53.Smith, T. A., N. Idamakanti, M. L. Rollence, J. Marshall-Neff, J. Kim, K. Mulgrew, G. R. Nemerow, M. Kaleko, and S. C. Stevenson. 2003. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum. Gene Ther. 14:777-787. [DOI] [PubMed] [Google Scholar]
- 54.Takami, M., I. Kasuya, and H. Tsunoo. 1992. Polymerized albumin receptor on rat liver cells. J. Biochem. (Tokyo) 111:714-721. [DOI] [PubMed] [Google Scholar]
- 55.Thomas, C. E., A. Ehrhardt, and M. A. Kay. 2003. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 4:346-358. [DOI] [PubMed] [Google Scholar]
- 56.Vrancken Peeters, M. J., A. L. Perkins, and M. A. Kay. 1996. Method for multiple portal vein infusions in mice: quantitation of adenovirus-mediated hepatic gene transfer. BioTechniques 20:278-285. [DOI] [PubMed] [Google Scholar]
- 57.Windler, E., J. Greeve, B. Levkau, V. Kolb-Bachofen, W. Daerr, and H. Greten. 1991. The human asialoglycoprotein receptor is a possible binding site for low-density lipoproteins and chylomicron remnants. Biochem. J. 276:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Worgall, S., G. Wolff, E. Falck-Pedersen, and R. G. Crystal. 1997. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum. Gene Ther. 8:37-44. [DOI] [PubMed] [Google Scholar]
- 59.Xia, D., L. J. Henry, R. D. Gerard, and J. Deisenhofer. 1994. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 A resolution. Structure 2:1259-1270. [DOI] [PubMed] [Google Scholar]
- 60.Yu, K. C., W. Chen, and A. D. Cooper. 2001. LDL receptor-related protein mediates cell-surface clustering and hepatic sequestration of chylomicron remnants in LDLR-deficient mice. J. Clin. Investig. 107:1387-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]