Abstract
According to early research, the incidence of claustrum lesions in patients with neurological Wilson’s disease (WD) was inconsistent, ranging from 1.8 to 75% on magnetic resonance imaging (MRI). Our study aims to explore the incidence, clinical presentation features, iconography features, and possible pathological mechanisms in WD patients with claustrum lesions on magnetic resonance imaging (MRI), to characterize the clinical, and brain imaging findings and possible pathological mechanisms in the patients with WD. Retrospective cases meeting the inclusion criteria were studied for analyzing MRI characteristics and associated physicochemical examination data in neurological WD patients with claustrum lesions. 443 (66.3%) with brain MRI abnormalities were screened from 668 WD patients. The three (0.7%) patients with the claustrum lesions characteristics on MRI images were: (a) “bright claustrum” in T2-weighted and FLAIR sequences, (b) bilateral symmetrical, (c) non-isolated lesions, (d) occurred only in severe neurological manifestations. The claustrum lesions are not common in neurological WD and mainly appear in cases with severe neurological symptoms. On MRI, the “bright claustrum” signs may be a radiographic marker of neuroinflammation, the features of the lesions showed bilateral symmetry, and hyperintensity signals on T2-weighted, FLAIR, and DWI.
Keywords: Wilson’s disease, Claustrum, Brain MRI, Copper, Neuroinflammation
Subject terms: Cell death in the nervous system, Diseases of the nervous system, Neurogenesis, Neuroimmunology, Neurological disorders, Neuroscience
Introduction
Wilson’s disease (WD) is a rare, autosomal recessive disorder of copper metabolism caused by an ATP7B gene mutation, resulting in the unregulated accumulation of copper in various tissues such as the liver and the brain1. Most particularly in the brain, and its clinical manifestations are progressive neuropsychiatric symptoms, the most common neurological presentations being extrapyramidal manifestations, including chorea, tremor, and dystonia2, which significantly affect the quality of life and prognosis. Neurological WD occurs in 22–55%, with the first neuropsychiatric symptoms most often occurring between the ages of 20–30 years, and increasing as age advances, early diagnosis and correct treatment are the key to successful rehabilitation of WD3. Magnetic resonance imaging (MRI) is the most frequently used imaging method to evaluate the degree and extent of brain lesions in neurological WD. Typical imaging manifestations include cerebral atrophy, cerebellum, the white matter of the cerebral hemispheres, and symmetrical hyperintensity or mixed intensity on T2-weighted image changes in the basal ganglia, thalamus, and brainstem4,5.
In recent years, potential brain MRI pathognomonic signs, such as “face of the giant panda”, “miniature panda”, “split thalamus”, and “whorl”, have been found and proposed to be pathognomonic for WD6,7. However, these signs have also been described in Leigh syndrome, glutaric aciduria type 1, isoniazid-included ataxia, as well as in other disorders, including methanol poisoning or Japanese B encephalitis8–10, indicating that the frequency and pathological significance of these signs still needs to be further established. The “bright claustrum” sign was described as the claustrum hyper signal in T2 weighted. Proton density sequences in 3/4 (75%) of neurological WD patients, appear to be a new pathology sign and are subsequently considered a potential MRI finding11,12. Recently, a new study came to a new and different conclusion, finding the “bright claustrum” sign occurred in only 1.8% (1/55) of neurological cases13. Therefore, the MRI imaging features and pathological mechanisms of the “bright claustrum” sign need to be further elucidated.
The claustrum was thought to play a key role in sensory integration and consciousness; it may very well be a regional “skeleton key” that is at the root of, and also links and contributes to, a variety of brain disorders14. The “bright claustrum” sign on brain MRI was also found in other neurological diseases, such as Parkinson’s disease (PD), Alzheimer’s disease (AD), Lewy body dementia (LBD), schizophrenia, autism, and especially in neuroinflammatory diseases such as rapidly increasing reported COVID-19 infection and autoimmune epilepsy15–18. Significant changes in the structure and volume of the claustrum were seen in several neurological conditions; similar lesions are described as affecting either the claustrum, the external capsule, or both, so it may not be truly pathognomonic of WD. The latest research methods, such as neuroimaging, proteomics, and tractography, have helped to obtain new information about claustrum lesions. It is required to further explore and study whether the role of neuroinflammation is involved in its pathological mechanism and represents an imaging marker of neuroinflammation in the brain.
Neurological manifestations in neurological WD patients have been frequently described in recent years. There is still debate about the significance of these proposed pathognomonic brain MRI signs, particularly concerning the “bright claustrum” sign, and few large studies have explored the clinical features and possible lesion mechanisms. The claustrum is a brain region that has been investigated for over 200 years, yet its precise function remains unknown. We must maintain curiosity by recognizing the key symptoms and characteristics, manifested with the claustrum lesions signs onset, progression, and pathomechanism, to make a prompt diagnosis and initiate early treatment modalities, drastically improving the disease course, prognosis, and decreasing the risk of complications of neurological WD patients. Our study aimed to evaluate the frequency and significance of claustrum lesions signs on MRI, consolidate the pathognomonic neuroradiological knowledge about the neurological manifestations, and present the pathophysiology of neurological manifestation of the claustrum lesions in WD.
Patients and methods
Participants retrospection
A retrospective study of WD patients (2020.3-2023.12), hospitalized in the department of neurology from our hospital, were undertaken with the following inclusion criteria: (a) patients meeting WD diagnostic criteria, with the WD diagnosis based on the Leipzig score and genetics analysis as described previously19; (b) abnormal brain MRI scan that accords with the main imaging characteristics of WD patients; (c) patients who underwent brain MRI scans at 1.5T or 3.0T before the chelation therapy or follow-up in the treated phase of the illness; (d) no history of previous or concomitant neurologic disorders.
Information including demographic data, clinical features, diagnostic findings, therapeutic interventions, and clinical outcomes of patients were acquired. The pathological brain regions on MRI were recorded. The scanning sequences included T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted imaging (DWI). The severity of brain imaging injury was assessed by the semiquantitative brain MRI score of WD (bMRIsc-WD)20. The typological severity and profile were quantified using the Global Assessment Scale for WD (GAS for WD), composed of two parts: global disability (GAS-Tier 1) and neurological impairment (GAS-Tier 2)21. All the retrospections were completed by a separate team, guided by the assigned researcher.
Literature review of the claustrum lesions
Searches for identification of studies were run from 1990 to the present day in MEDLINE and PubMed. The search keywords were as follows: “claustrum”; “claustrum lesions”; “claustrum sign” “bright claustrum”; “claustrum damage” and “claustrum hyperintensity”. The bibliography of each of the retrieved articles was examined to identify relevant references that may have been missed by the electronic search. For each available citation considered, the abstract was read and then screened for cases in which reported lesions included the claustrum according to neuroimaging.
Results
Clinical and neuroimaging features in patients with neurological WD
443 (66%) patients, 378 underwent MRI scans at 1.5 T, and 65 at 3.0 T, showed brain MRI abnormalities from 668 WD patients. Among them, 89% (395/443) were neurological WD, showing neuropsychiatric symptoms, and 11% (48/443) were hepatic WD, showing hepatic symptoms. The lenticular nucleus was the most commonly affected site, being involved in 80.9% (356/443 ) of patients, the frequency followed by brain atrophy (234/443, 52.8%), thalamus (148/443, 33.4%), brain stem (115/443, 30%), and caudate nucleus (126/443, 28.4%). Unfrequent signal abnormalities were noted: encephalomalacia (12/443, 2.7%), cerebellum (12/443,2.7%), white matter lesion (8/443, 1.8%), corpus callosum (8/443, 1.8%), and claustrum (3/443, 0.7%), (Fig. 1). In the three patients, showing “bright claustrum” signs, the most common signs and symptoms were motor disorders (3/3), speech problems (3/3), nonspecific cognitive impairment (3/3), sleep disturbances (3/3), delusions (1/3), hallucinations (1/3), non-seizure electroencephalography (EEG) abnormalities (1/3), GAS-WD Tier1(12–18), GAS-WD Tier2 (25–35), bMRIsc-WD (11–16) (Table 1).
Fig. 1.
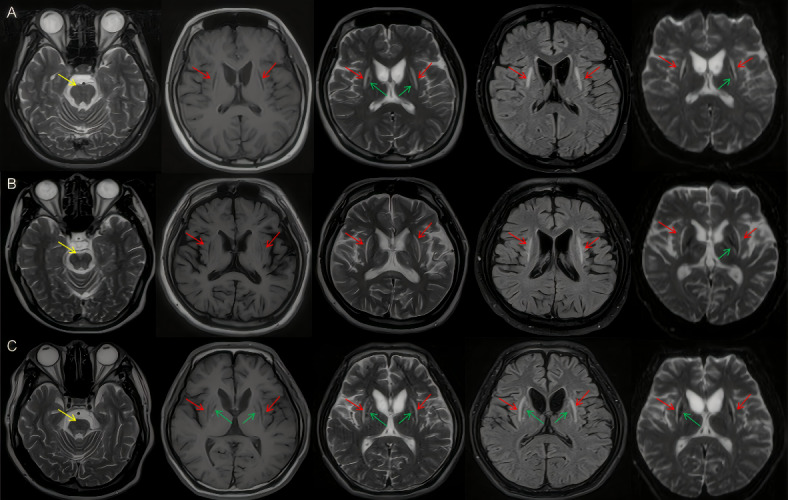
The brain lesions on MRI axial scanning T1-weighted, T2-weighted, FLAIR, DWI sequence in three WD patients. In claustrum, symmetrical bilateral hypointensity on T1-weighted and hyperintensity on T2-weighted, FLAIR, and DWI images (red arrow). In putamen, incomplete symmetrical bilateral mix intensity on T1-weighted, T2-weighted, FLAIR, and DWI images (green arrow). In brainstem, incomplete symmetrical hyperintensity on T2-weighted images (yellow arrow).
Table 1.
Clinical features of the three WD patients with claustrum lesions.
| Clinical data | Case #1 | Case #2 | Case #3 |
|---|---|---|---|
| General information | 22-year-old man | 19-year-old man | 21-year-old female |
| Disease duration (years) | 15 | 4 | 3 |
| Age of onset (years) | 7 | 15 | 18 |
| Initial indication | Blood ALT elevated | Cirrhosis | Movement clumsy |
| Motor symptoms | Slurred speech, limb torsion, swallowing difficulty | Slurred speech, clumsy movements, hands trembled, walking difficulty |
Unsteady walking, unclear speech, swallowing difficulty |
| Non-motor symptoms | Cognitive impairment, Insomnia, delusion | Cognitive impairment, depression, insomnia, | Cognitive impairment, Drowsiness, hallucinations |
| Serum ceruloplasmin (mg/L) | 48.7 | 34.0 | 34.5 |
| Copper oxidase (OD) | 0.035 | 0.025 | 0.018 |
| Serum copper (µmol/L) | 1.12 | 2.03 | 1.10 |
| Blood ammonia (µg/L) | 4 | 10 | 15 |
| Serum iron (µmol/L) | 8.70 | 11.26 | 16.39 |
| Serum zinc (µmol/L) | 21.97 | 23.46 | 16.45 |
| Serum uric acid (UA) (mol/L) | 167 | 142 | 251 |
| C-reactive protein (CRP) (mg/L) | 0.70 | 2.95 | 3.51 |
| D-dimer (mg/L) | 0.48 | 0.63 | 0.81 |
| Superoxide dismutase (SOD) (u/ml) | 241.30 | 137 | 225.90 |
| 24-h urinary copper (mg/24 h) | 144.61 | 107.69 | 187.91 |
| Kayser-Fleischer ring (grade) | ++++ | ++++ | +++ |
| Modified Leipzig score | 8 | 7 | 7 |
| GAS-Tier1 (points) | 18 | 16 | 18 |
| GAS-Tier2 (points) | 35 | 32 | 32 |
| BMRIsc-WD (points) | 16 | 14 | 13 |
Neuroimaging features on MRI in WD with claustrum lesions
All three patients with claustrum lesions underwent a brain MRI 1.5T scan before chelation therapy, symmetrical and obscure hypointensity was seen in the claustrum, and the extended lentiform nucleus presented high, low, or mixed signals on axial T1-weighted images. Axial T2-weighted and FLAIR images demonstrated bilateral high signals in the claustrum, with the exact location and alteration extent of the signal across varying the four cases. The claustrum lesions showed a high signal on both DWI images, without restricted diffusion. all bilateral involvement was present in the patients, and the “bright claustrum” sign was easily recognizable in each case, (Fig. 1).
Literature review of the claustrum lesions on MRI
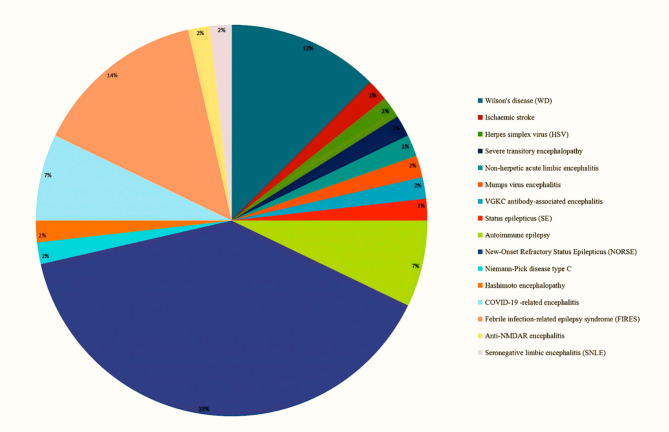
A total of 72 studies were initially identified. After excluding duplicates, 48 studies were screened by title and abstract, and 22 studies were excluded because the cases or MRI images were incomplete and did not meet the eligibility criteria. Finally, 27 papers, including 22 individual cases and 5 cohort studies, with a total of 56 patients and 16 disease species, were included in this literature review (Fig. 2). Among them, the distribution was as follows: 35 (62%) in epilepsy-related diseases; 13 (23%) in encephalitis-related diseases; 7 (13%) in WD; and 1 (1%) in ischaemic stroke. In terms of spatial distribution, 55 (98%) were bilateral, except for 1 (2%) ischaemic stroke which was unilateral. Isolated the claustrum lesions on MRI were present in 20 (35.7%) (Table 2).
Fig. 2.
Disease species distribution of the claustrum lesions on MRI from reported literature cases (%).
Table 2.
The claustrum lesions on MRI reported and clinical data of literature review cases.
| Authors | Year | Cases (n) | Claustrum lesions | Extend lesion sites | Disease species |
|---|---|---|---|---|---|
| Sener RN.11 | 1993 | 3 | Bilaterally thickened and bright claustrum long-TR MR images | The bilateral basal ganglionic and subcortical white matter involvement | WD |
| Kimura S, et al.22 | 1994 | 1 | Bilateral the claustrum high signals on T2-weighted, mild low signal on T1-weighted | Bilateral lesions of the external capsule | HSV |
| Sperner J, et al.23 | 1996 | 1 | Reversible bilateral claustrum hyperintense on T2-weighted, hypointense on Tl-weighted | Bilateral lesions of the external capsule | Severe transitory encephalopathy |
| Ishida H, et al.24 | 2006 | 1 | Bilateral claustrum on T2-weighted | Right hippocampus | NHALE |
| Ishii K, et al.25 | 2011 | 1 | High-intensity lesions of the bilateral claustrum on T2-weighted | No | Mumps virus encephalitis |
| Panda AK.12 | 2013 | 1 | Hyperintensity in the bilateral claustrum on T2-weighted | Putamen, caudate nucleus, and thalamus | WD |
| Hiraga A, et al.26 | 2014 | 1 | Hyperintensity in the bilateral claustrum on T1-weighted | Bilateral medialtemporal lobes | VGKC antibody-associated encephalitis |
| Hwang KJ, et al.27 | 2014 | 1 | Hyperintensities in the right claustrum on T2-weighted/FLAIR | Bilateral external capsule | SE |
| Goyal S, et al.28 | 2015 | 1 | Bilateral hyperintense in claustrum on T2-weighted/FLAIR | Bilateral thalami, basal ganglia, and dorsal mesencephalon, red nucleus. | WD |
| Steriade C, et al.29 | 2017 | 4 | Claustrum hyperintensities on T2-weighted/FLAIR | Hippocampal tail/fornix | Autoimmune epilepsy |
| Meletti S, et al.30 | 2017 | 12 | Hyperintense foci in bilateral claustrum on T2-weighted/FLAIR | No | NORSE |
| Georgi K, et al.31 | 2018 | 1 | Isolated left claustrum lesion | No | Ischaemic stroke |
| Choi JY, et al.32 | 2019 | 10 | Bilateral claustrum on T2-weighted/FLAIR | Neocortex, bilateral mesial tempora and pulvinar | NORSE-LE |
| Kułak-Bejda A, et al.33 | 2020 | 1 | Bilateral symmetrical hyperintensities in claustrum on T2-weighted | The putamen, globus pallidus, heads of caudate nucleus, and thalamus | WD |
| Mukherjee D, et al.34 | 2021 | 1 | Bilateral symmetrical hyperintensities in claustrum on axial T2-weighted/FLAIR | Medial temporal lobe, uncus, basifrontal region and insularregion | Niemann-Pick disease type C |
| Ayatollahi P, et al.35 | 2021 | 1 | Bilateral claustrum hyperintensities on axial T2-weighted/FLAIR | Extreme capsules, insular cortices | Acute SARS-CoV-2 Infection |
| Jegatheeswaran V, et al.36 | 2021 | 1 | Bilateral claustrum hyperintensities on axial T2-weighted/FLAIR | Right mesial temporal lobe | HE |
| Zuhorn F, et al.37 | 2021 | 1 | Bilateral claustrum signal alterations on FLAIR, DWI, and ADC map | External capsule regions | Parainfectious encephalitis in COVID-19 |
| Laura Susani E, et al.38 | 2021 | 1 | Hyperintensities bilaterally but especially on the left side of the claustrum on FLAIR | Bilaterally but especially on the left side of the putamen, the insula cortex, and the frontotemporal cortex | Parainfectious COVID-19 encephalitis |
| Yang F, et al.39 | 2021 | 5 | Symmetrical low signal lesions inclaustrum on T1, high signal lesions on T2-weighted, and FLAIR. | No | FIRES |
| Acampora R, et al.40 | 2022 | 1 | Hyperintense inclaustrum on FLAIR | Insular | FIRES |
| Muccioli L, et al.41 | 2022 | 1 | Right-predominant hyperintensity and Swelling of the Claustrumon FLAIR and T2-weighted | The pulvinar, and hippocampus | FIRES |
| Datta AK, et al.42 | 2022 | 1 | Bilateral claustrum hyperintensities on FLAIR and T2-weighted | Hippocampal tail/fornix | Anti-NMDAR encephalitis |
| Humayun MB, et al.43 | 2023 | 1 | Bilateral hyperintense claustrum on T2-weighted | No | Post-COVID-19 Encephalitis |
| Safan AS, et al.44 | 2023 | 1 | Sparing of the intervening claustrum bilaterally on FLAIR and T2-weighted | Left thalamus, symmetric external and extreme capsules | SNLE |
| Di Dier K, et al.45 | 2023 | 1 | Bilateral claustrumT2-hyperintense | The mesiotemporal structures | FIRES |
| Guo K, et al.46 | 2023 | 1 | Symmetric or asymmetric T2/FLAIR hyperintensity in the bilateral claustrum | FIRES | |
| Rędzia-Ogrodnik B, et al.13 | 2023 | 1 | Bilateral hyperintense in claustrum on T2-weighted/ FLAIR | Bilateral putamen, globus pallidus, heads of caudate nucleus and thalamus | WD |
MRI magnetic resonance imaging, DWI diffusion-weighted imaging, FLAIR fluid-attenuated inversion recovery, WD Wilson’s disease, SNLE seronegative limbic encephalitis, HE Hashimoto encephalopathy, FIRES febrile infection-related epilepsy syndrome, HSV herpes simplex virus, VGKC voltage-gated potassium channel, NORSE new-onset refractory status epilepticus, LE limbic encephalitis, NHALE non-herpetic acute limbic encephalitis, SE status epilepticus.
Discussion
The T2-weighted/FLAIR hyperintensity of the claustrum, the so-called claustrum sign or “bright claustrum” in neurological WD, has been described11,13, however, whether it is a new potential sign of MRI in patients with WD is controversial. In our study, the sign-on MRI was found in 3/443 (0.7%) of neurological WD, and the incidence rate is lower than that reported in the literature. As shown in our study results, MR imaging examinations of the four patients with a variety of claustral disorders, our study show that the claustrum lesions on MRI imaging in WD patients have the following features: (a) “bright claustrum” on T2-weighted and FLAIR sequences, (b) bilateral symmetrical, (c) non-isolated lesions, (d) occurred only in severe neurological manifestations. Thus, the sign can be seen as a potential brain MRI pathognomonic sign that occurs relatively rarely across all patients, most commonly in patients with severe neurological symptoms, and this knowledge may advance the pathophysiology of WD.
Our study in the literature revealed that the claustrum sign is more associated with neuroimmune diseases, it may not be as pathognomonic as the majority of these indications, including potential brain MRI pathognomonic signs “face of the giant panda” and “miniature panda”. Therefore, it did not attract sufficient attention from the clinicians. A minority of WD patients with neurological involvement may not have brain MRI abnormalities or the Kayser-Fleischer ring, while patients with liver illness or presymptomatic subjects may have these abnormalities due to the wide variability of the WD phenotype45. Since the WD phenotype varies widely, it is still unknown what factors contribute to it. Although the absence of these imaging signs does not exclude a WD, clinicians should be aware that their presence may be useful to diagnose it. Patients suffering from WD experience a large build-up of copper in their brains, which leads to long-term damage to the brain parenchyma and morphological abnormalities as well as different degrees of brain shrinkage46. The most prevalent kind of neurologic WD is putaminal involvement; sensitivity of T2-weighted and FLAIR sequences is comparable; the number of MRI lesions is linked with disease severity but not with prognosis47. The basal ganglia exhibit characteristic brain MRI alterations associated with WD that are typically characterized as symmetric, hyperintense, or with mixed intensity in T2-weighted imaging, showing gliosis, edema, and demyelination. When the disease reaches its severe stages, they are located in the same area as hypointense alterations in T1-weighted sequences, which indicate necrosis13,20.
In our study, T1-weighted sequences on MRI located in the claustrum in cases showed varying degrees of hypointense changes, which may be reflecting necrosis. In the T2-weighted, FLAIR, DWI sequence located in the claustrum, all patients showed varying degrees of hyperintensity changes, reflecting existing necrosis and edema. Despite the presence of excess copper within the brain in WD, pathological findings are limited primarily to the basal ganglia, thalamus, midbrain, and pons. The excess copper accumulation and deposition are toxic to the central nervous tissue and can further lead to the generation of oxygen-free radicals, cell membrane damage, and reactive astrocytes48. Histopathology studies showed atrophy, spongy softening, cavitations, neuronal loss, increased cellularity, and the presence of opalski cells49. Currently, the pathophysiological mechanisms of patients can be partially revealed by MRI imaging techniques, such as cytotoxic edema-like, and vasogenic edema-like two patterns on diffusion MRI, which were observed by quantitative evaluations of the ADC maps in WD50. Our study found that the claustrum lesions showed high signals on both DWI, indicating that there may be cytotoxic edema in the claustrum lesions local tissue.
The claustrum is a thin sheet of grey matter lying underneath the surface of the insular cortex having widespread connections with neocortical and subcortical structures, and it forms part of the brain circuitry that plans and executes motor behaviors51,52. While the claustrum’s anatomy renders lesion studies challenging, isolated claustrum lesions are rare and predominantly occur in the spectrum of autoimmune encephalitis. The most common clinical manifestations of claustrum lesions are that patients experience different levels of cognitive, perceptual, and motor dysfunction, as well as seizures, mental states, and sleep disorders53. Literature studies of claustrum lesions reveal that the disease species distribution was as follows: 35 (62%) in epilepsy-related diseases; 13 (23%) in encephalitis-related diseases; 7 in WD (13%); and 1 (2%) in ischaemic stroke. In space distribution, 55 (98%) were bilateral, except for 1 (2%) ischaemic stroke unilateral. Limitation to the claustrum lesions site as the sole region of involvement on MRI was seen in 20 (35.7%) of our literature review studies. Our studies showed that only a few clinical studies have described neurological lesions or conditions where the claustrum was the sole region of involvement. This may support the idea that dysfunction in the claustrum results primarily in disruption of higher behavioral and cognitive functions associated with the functional networks, rather than a specific, unitary deficit54. In our study, 3 patients with claustrum lesions were unable to stand and walk, 3 patients were accompanied by sleep disorder, and 2 patients were accompanied by psychiatric symptoms; these may be related to the participation of claustrum lesions.
Understanding how the claustrum operates is difficult because it is located deep within the brain and injury to it is extremely rare. Earlier reported findings of lesions involving the claustrum comprised cases with asphyxia, WD, ischemic white matter disease, thalamic arteriovenous malformation, mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome, viral encephalitis, and PD55. Current evidence shows that the bilateral claustrum hyperintensity as a unique radiological marker on T2-weighted/FLAIR sequences of MRI brain, described typically in association with immune-inflammatory-mediated encephalopathy, the sign has been specifically identified in the background of autoimmune epilepsy, anti-VGKC, anti-glutamic acid decarboxylase (GAD), anti-Ma-2 antibodies and post-COVID26,56–58. In WD, mixed intensity in the basal ganglia and hyperintense linear rim at the peripheral putamen were similar to those seen in Japanese encephalitis59. These immune effector cell-associated neurotoxicity syndromes may represent a specific marker of cytokine-mediated neuroinflammation60. Therefore, the claustrum may have a potential role and constitute a therapeutic target in neurological WD patients associated with disturbances of cognition, behavior, and cortical excitability. Accumulating evidence reveals that inflammation is not confined to archetypal inflammatory diseases like multiple sclerosis, but rather is an intrinsic characteristic of many psychiatric and neurological conditions that are not traditionally classed as neuroinflammatory61.
Excessive copper deposition can lead to neuroinflammation in the brain of WD patients, excessive free copper leads to its participation in the redox reactions that produce reactive oxygen species (ROS), leading to catastrophic damage to lipids, proteins, and DNA62,63. Growing evidence suggests that extracellular copper accumulation triggers pathological microglial activation and subsequent neurotoxicity, copper overload-mediated activation of NLRP3 inflammasome leads to progressive neuropathology, and levels of inflammatory cytokines are elevated in the brain of murine WD models64,65. Besides WD, there was robust evidence that copper has been linked to AD, PD, amyotrophic lateral sclerosis, Huntington’s disease, and prion-mediated encephalopathies66. From here, we see that neuroinflammation occurs as one of the most common pathological outcomes in various neurological disorders, making it a promising therapeutic target67. These studies can be used to evaluate therapeutic strategies to alleviate behavioral disturbances and cerebral pathology observed in WD when the claustrum is damaged. The effects are severe and better therapies are urgently needed. Therefore, starting from neuroinflammation, it may be a potential therapeutic strategy for neurological WD. At present, the pathological mechanism of neuroinflammation caused by copper deposition in WD has not been fully clarified. Specific markers of neuroinflammation are lacking clinically, so serum levels of uric acid (UA), c-reactive protein (CRP), superoxide dismutase (SOD), and d-dimers have been used as biomarkers of inflammatory or oxidative stress, but they are nonspecific to neuroinflammation68,69. Due to the little-understood area of the claustrum, it may be the next frontier in improving outcomes for brain-damaged patients. Based on recommendations for neurological follow-up in these patients, brain MRI should also be considered as part of long-term treatment monitoring during neurological examinations70. In conclusion, so-called “bright claustrum” imaging signs on MRI may be an imaging marker of neuroinflammation.
Through this research, our findings regarding the incidence, imaging characteristics, distribution population, and possible pathomechanism of claustrum lesions in WD patients were preliminarily discussed. However, this study has some limitations. This study was a retrospective analysis of the data, and MRI neuroimaging changes in WD only provide us with clues to thinking. It is already known that there are also links between the claustrum and perception, salience, and the sleep-wake cycle, in addition to movement disorders. We did not observe a significant relationship between relevant clinical-specific signs and the claustrum lesions of WD, which could be due to a small sample size and insufficient recognition of the lesion. It follows that the lack of clinical focus on the claustrum lesions may mean there are many more cases yet to be uncovered. Moreover, as a neurology department, we did not receive and treat patients with extremely severe hepatic type WD, such as acute and chronic liver failure. Therefore, our sample may not be fully representative of all WD patients.
Conclusions
Here, our study has summarized the suggestive, but not conclusive, body of evidence that implicates the claustrum in the underlying pathology of neuroinflammation. The claustrum lesions are not common in neurological WD and mainly appear in cases with severe neurological symptoms. Clinically, it can manifest as severe motor function limitations and a specific sleep disorder, depression, or mental excitement. On MRI, the “bright claustrum” signs may be an imaging marker of neuroinflammation, the features of the lesions showed bilateral symmetry, and hyperintensity signals on T2-weighted, FLAIR, and DWI. Cytotoxicity and neuroinflammation induced by copper deposition may be involved in the pathological mechanism of the claustrum lesions, but it needs to be further clarified.
Acknowledgements
We gratefully acknowledge the patients for their previous cooperation and patience in leaving us with valuable case data that made this work possible.
Author contributions
CRediT authorship contribution statement M.X.F.,and F.L.Y. evaluated the specimens. L.K. collected the data; J.P.and T.G.A. analyzed the data;W.G.Q. conceived the study, drafted and wrote the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Funding
This study was supported by the Scientific Research Fund Project of Colleges and Universities in Anhui Province (2023AH050793, 2024AH050969). The funding body had no role or interference in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study was performed in line with the principles of the Declaration of Helsinki, approval was granted by the ethical committee of the Affiliated Hospital, Anhui University of Chinese Traditional Medicine (NO: AHSYS-2023LC-015). The data were anonymous, no patient privacy information was exposed, and The ethics exemption certificate was issued by the ethics committee.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nagral, A. et al. Wilson’s disease: clinical practice guidelines of the Indian National Association for Study of the liver, the Indian Society of Pediatric Gastroenterology, Hepatology and Nutrition, and the Movement Disorders Society of India. J. Clin. Exp. Hepatol. 9, 74–98. 10.1016/j.jceh.2018.08.009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srinivas, K. et al. Dominant psychiatric manifestations in Wilson’s disease: a diagnostic and therapeutic challenge! J. Neurol. Sci. 266, 104–108. 10.1016/j.jns.2007.09.009 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Jopowicz, A. & Tarnacka, B. Neurological Wilson’s disease signs-hepatic encephalopathy or copper toxicosis? Diagnostics (Basel) 13, 893. 10.3390/diagnostics13050893 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs, D. A. et al. The double panda sign in Wilson’s disease. Neurology 61, 969. 10.1212/01.wnl.0000085871.98174.4e (2003). [DOI] [PubMed] [Google Scholar]
- 5.Prashanth, L. K. et al. Do MRI features distinguish Wilson’s disease from other early onset extrapyramidal disorders? An analysis of 100 cases. Mov. Disord. 25, 672–678. 10.1002/mds.22689 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Gupta, A. et al. Face of giant panda’: a rare imaging sign in Wilson’s disease. QJM 107, 579. 10.1093/qjmed/hct217 (2014). [DOI] [PubMed] [Google Scholar]
- 7.George, U. et al. Split thalamus: internal medullary involvement in Wilson’s disease. Neurol. India 58, 680. 10.4103/0028-3886.68701 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Sonam, K. et al. The double panda sign in Leigh disease. J. Child. Neurol. 29, 980–982. 10.1177/0883073813484968 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Vella, S. & Grech, R. Highlighting an atypical cause of the face of the giant panda sign. BJR Case Rep. 4, 20170046. 10.1259/bjrcr.20170046 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holla, V. V. et al. Face of the giant panda sign and bilateral thalamic hyperintensity in isoniazid-included ataxia. J. Mov. Disord. 17, 99–101. 10.14802/jmd.23112 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sener, R. N. The claustrum on MRI: normal anatomy, and the bright claustrum as a new sign in Wilson’s disease. Pediatr. Radiol. 23, 594–596. 10.1007/BF02014975 (1993). [DOI] [PubMed] [Google Scholar]
- 12.Panda, A. K. Classic neuroimaging, the bird’s eye view in Wilson’s disease. BMJ Case Rep. 2013, bcr2013200701. 10.1136/bcr-2013-200701 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rędzia-Ogrodnik, B. et al. Pathognomonic neuroradiological signs in Wilson’s disease - truth or myth? Parkinsonism Relat. Disord. 107, 105247. 10.1016/j.parkreldis.2022.105247 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Nikolenko, V. N. et al. The mystery of claustral neural circuits and recent updates on its role in neurodegenerative pathology. Behav. Brain Funct. 17, 8. 10.1186/s12993-021-00181-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torgerson, C. M. et al. The DTI connectivity of the human claustrum. Hum. Brain Mapp. 36, 827–838. 10.1002/hbm.22667 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuhorn, F. et al. Parainfectious encephalitis in COVID-19: the claustrum sign. J. Neurol. 268, 2031–2034. 10.1007/s00415-020-10185-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steriade, C. et al. Claustrum hyperintensities: a potential clue to autoimmune epilepsy. Epilepsia Open 2, 476–480. 10.1002/epi4.12077 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Horn, J. D. What is old is New Again: investigating and analyzing the mysteries of the claustrum. Neuroinformatics 17, 1–3. 10.1007/s12021-018-9411-z (2019). [DOI] [PubMed] [Google Scholar]
- 19.Ferenci, P. et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 23, 139–142. 10.1034/j.1600-0676.2003.00824.x (2003). [DOI] [PubMed] [Google Scholar]
- 20.Dusek, P. et al. Semiquantitative scale for assessing brain MRI abnormalities in Wilson disease: a validation study. Mov. Disord. 35, 994–1001. 10.1002/mds.28018 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal, A. et al. A novel global assessment scale for Wilson’s disease (GAS for WD). Mov. Disord. 24, 509–518. 10.1002/mds.22231 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Kimura, S. et al. Symmetrical external capsule lesions in a patient with herpes simplex encephalitis. Neuropediatrics 25, 162–164. 10.1055/s-2008-1073016 (1994). [DOI] [PubMed] [Google Scholar]
- 23.Sperner, J. et al. Severe transitory encephalopathy with reversible lesions of the claustrum. Pediatr. Radiol. 26, 769–771. 10.1007/BF01396197 (1996). [DOI] [PubMed] [Google Scholar]
- 24.Ishida, H. et al. A child with non-herpetic acute limbic encephalitis affecting the claustrum and hippocampus. No Hattatsu 38, 443–447 (2006). [PubMed] [Google Scholar]
- 25.Ishii, K. et al. Mumps virus encephalitis with symmetric claustrum lesions. AJNR Am. J. Neuroradiol. 32, E139. 10.3174/ajnr.A2603 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiraga, A. et al. Voltage-gated potassium channel antibody- associated encephalitis with claustrum lesions. Intern. Med. 53, 2263–2264. 10.2169/internalmedicine.53.2701 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Hwang, K. J. et al. Unusual lesion in the bilateral external capsule following status epilepticus: a case report. J. Epilepsy Res. 4, 88–90. 10.14581/jer.14019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goyal, S. et al. Wilson’s disease presenting with unusual radiological features. Iran. J. Neurol. 14, 177–179 (2015). [PMC free article] [PubMed] [Google Scholar]
- 29.Meletti, S. et al. Claustrum damage and refractory status epilepticus following febrile illness. Neurology 85, 1224–1232. 10.1212/WNL.0000000000001996 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georgi, K. et al. Ischemic stroke of the left claustrum in a 55-year-old female: a case report. Claustrum 3(1), 1528135. 10.1080/20023294.2018.1528135 (2018). [Google Scholar]
- 31.Choi, J. Y. et al. Prognostic significance of subsequent extra-temporal involvement in cryptogenic new onset refractory status epilepticus (NORSE) initially diagnosed with limbic encephalitis. Epilepsy Res. 158, 106215. 10.1016/j.eplepsyres.2019.106215 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Kułak-Bejda, A. et al. Primarily depression manifestation of Wilson’s disease-case report. Clin. Neurol. Neurosurg. 190, 105651. 10.1016/j.clineuro.2019.105651 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee, D. et al. Claustrum hyperintensity: a rare radiological correlate in Niemann-pick disease. BMJ Case Rep. 14, e239630. 10.1136/bcr-2020-239630 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayatollahi, P. et al. Possible autoimmune encephalitis with claustrum sign in case of acute SARS-CoV-2 infection. Can. J. Neurol. Sci. 48, 430–432. 10.1017/cjn.2020.209 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jegatheeswaran, V. et al. MRI findings of two patients with hashimoto encephalopathy. Cureus 13, e15697. 10.7759/cureus.15697 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laura Susani, E. et al. The claustrum sign in a parainfectious COVID-19 encephalitis. J. Neurol. Sci. 429, 119874. 10.1016/j.jns.2021.119874 (2021). [Google Scholar]
- 37.Yang, F. et al. Repetitive seizures after febrile period exclusively involving bilateral claustrum. Medicine (Baltimore) 100, e27129. 10.1097/MD.0000000000027129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acampora, R. et al. A case of febrile infection-related epilepsy syndrome (FIRES) in young adult: still a diagnostic and therapeutic challenge. Neurol. Sci. 43, 4555–4558. 10.1007/s10072-022-06106-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muccioli, L. et al. Teaching neuroImage: Claustrum sign in febrile infection-related epilepsy syndrome. Neurology 98, e1090–e1091. 10.1212/WNL.0000000000013261 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Datta, A. K. et al. Claustrum hyperintensity as a marker of anti-NMDAR encephalitis. Postgrad. Med. J. 98, e24. 10.1136/postgradmedj-2021-140396 (2022). [DOI] [PubMed] [Google Scholar]
- 41.Humayun, M. B. et al. Post-COVID-19 Encephalitis with claustrum sign responsive to immunomodulation. Cureus 15, e35363. 10.7759/cureus.35363 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Safan, A. S. et al. Claustrum sparing sign in seronegative limbic encephalitis. eNeurologicalSci 31, 100465. 10.1016/j.ensci.2023.100465 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Dier, K. et al. The Claustrum sign in febrile infection-related epilepsy syndrome (FIRES). J. Belg. Soc. Radiol. 107, 45. 10.5334/jbsr.3142 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo, K. & Hong, Z. Claustrum sign in febrile infection-related epilepsy syndrome (FIRES). Neurol. Sci. 44, 3357–3359. 10.1007/s10072-023-06887-6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Dato, F. & Iorio, R. Wilson’s disease: is it time to leave behind old clichés? Parkinsonism Relat. Disord. 107, 105284. 10.1016/j.parkreldis.2023.105284 (2023). [DOI] [PubMed] [Google Scholar]
- 46.Fritzsch, D. et al. Seven-tesla magnetic resonance imaging in Wilson disease using quantitative susceptibility mapping for measurement of copper accumulation. Invest. Radiol. 49, 299–306. 10.1097/RLI.0000000000000010 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Ranjan, A. et al. A study of MRI changes in Wilson disease and its correlation with clinical features and outcome. Clin. Neurol. Neurosurg. 138, 31–36. 10.1016/j.clineuro.2015.07.013 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Kipker, N. et al. Neurological-type Wilson disease: Epidemiology, clinical manifestations, diagnosis, and management. Cureus 15, e38170. 10.7759/cureus.38170 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim, T. J. et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. AJNR Am. J. Neuroradiol. 27, 1373–1378 (2006). [PMC free article] [PubMed] [Google Scholar]
- 50.Sener, R. N. Diffusion MRI findings in Wilson’s disease. Comput. Med. Imaging Graph. 27, 17–21. 10.1016/s0895-6111(02)00047-2 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Chevée, M. et al. Neural activity in the mouse claustrum in a cross-modal sensory selection task. Neuron 110, 486–501e7. 10.1016/j.neuron.2021.11.013 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whalley, K. A new direction for the claustrum? Nat. Rev. Neurosci. 23, 67. 10.1038/s41583-021-00554-5 (2022). [DOI] [PubMed] [Google Scholar]
- 53.Atilgan, H. et al. Human lesions and animal studies link the claustrum to perception, salience, sleep and pain. Brain 145, 1610–1623. 10.1093/brain/awac114 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torgerson, C. M. & Van Horn, J. D. A case study in connectomics: the history, mapping, and connectivity of the claustrum. Front. Neuroinform. 8, 83. 10.3389/fninf.2014.00083 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sener, R. N. Lesions affecting the claustrum. Comput. Med. Imaging Graph. 22, 57–61. 10.1016/s0895-6111(97)00043-8 (1998). [DOI] [PubMed] [Google Scholar]
- 56.Specchio, N. & Pietrafusa, N. New-onset refractory status epilepticus and febrile infection-related epilepsy syndrome. Dev. Med. Child. Neurol. 62, 897–905. 10.1111/dmcn.14553 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Trandafir, C. et al. New-onset refractory status epilepticus-related claustral hyperintensities. Eur. Neurol. 83, 327–329. 10.1159/000508268 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Vanderheiden, A. & Klein, R. S. Neuroinflammation and COVID-19. Curr. Opin. Neurobiol. 76, 102608. 10.1016/j.conb.2022.102608 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saha, M. et al. Similarities and differences of MR findings between Japanese encephalitis and Wilson’s disease. Eur. Radiol. 12, 872–876. 10.1007/s003300101058 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Pensato, U. et al. Brain dysfunction in COVID-19 and CAR-T therapy: cytokine storm-associated encephalopathy. Ann. Clin. Transl. Neurol. 8, 968–979. 10.1002/acn3.51348 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oestreich, L. K. L. & O’Sullivan, M. J. Transdiagnostic in vivo magnetic resonance imaging markers of neuroinflammation. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 7, 638–658. 10.1016/j.bpsc.2022.01.003 (2022). [DOI] [PubMed] [Google Scholar]
- 62.Gaier, E. D. et al. Copper signaling in the mammalian nervous system: synaptic effects. J. Neurosci. Res. 91, 2–19. 10.1002/jnr.23143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, H. et al. Serum pentraxin 3 is elevated in patients with neurological Wilson’s disease. Clin. Chim. Acta 462, 178–182. 10.1016/j.cca.2016.08.010 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Terwel, D. et al. Neuroinflammatory and behavioural changes in the Atp7B mutant mouse model of Wilson’s disease. J. Neurochem. 118, 105–112. 10.1111/j.1471-4159.2011.07278.x (2011). [DOI] [PubMed] [Google Scholar]
- 65.Dong, J. et al. Inhibiting NLRP3 inflammasome activation prevents copper-induced neuropathology in a murine model of Wilson’s disease. Cell. Death Dis. 12, 87. 10.1038/s41419-021-03397-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huat, T. J. et al. Metal toxicity links to Alzheimer’s disease and neuroinflammation. J. Mol. Biol. 431, 1843–1868. 10.1016/j.jmb.2019.01.018 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mishra, A. et al. Neuroinflammation in neurological disorders: pharmacotherapeutic targets from bench to bedside. Metab. Brain Dis. 36, 1591–1626. 10.1007/s11011-021-00806-4 (2021). [DOI] [PubMed] [Google Scholar]
- 68.Liu, Y. et al. Simple and effective serum biomarkers potential for predicting status epilepticus in anti-N-methyl-D-aspartate receptor encephalitis. BMC Neurol. 22, 27. 10.1186/s12883-021-02545-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katarina, V. et al. Oxidative stress and neuroinflammation should be both considered in the occurrence of fatigue and depression in multiple sclerosis. Acta Neurol. Belg. 120, 853–861. 10.1007/s13760-018-1015-8 (2020). [DOI] [PubMed] [Google Scholar]
- 70.Litwin, T. et al. Brain Magnetic resonance imaging in Wilson’s disease-significance and practical aspects-a narrative review. Brain Sci. 14, 727. 10.3390/brainsci14070727 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]