Abstract
Dendritic cells are the most potent antigen-presenting cell for priming naive T cells. Optimal activation of T cells requires that dendritic cells undergo a process of maturation resulting in the increased expression of costimulatory molecules, such as CD40, CD86, and CD80, and the production of cytokines. In this study we analyzed the effect of infection of dendritic cells obtained from two strains of mice, BALB/c and C57BL/6, with the paramyxovirus simian virus 5 (SV5). Our results show that C57BL/6 bone marrow-derived dendritic cells (BMDC) are much more permissive to infection with SV5 at a multiplicity of infection (MOI) of 10 PFU/cell compared to BALB/c BMDC, as determined by the production of viral proteins and progeny. However, infection of BALB/c BMDC with a higher MOI of 50 PFU/cell resulted in a productive infection with the production of significant amounts of viral proteins and progeny. Regardless of the permissivity to infection, both BALB/c and C57BL/6 BMDC efficiently upregulated CD40 and CD86. However, CD80 upregulation correlated with the level of expression of viral proteins and the production of viral progeny. While secreted alpha/beta interferon was required for increased expression of all three molecules, optimal CD80 expression was dependent on an additional signal provided by a productive viral infection. These findings provide evidence that the signals controlling the expression of costimulatory molecules following viral infection are distinct. Further, they suggest that the amount of virus encountered and/or the permissivity of a dendritic cell to infection can alter the resulting maturation phenotype and functional capacity of the infected dendritic cell.
Dendritic cells (DC) are professional antigen-presenting cells that are uniquely capable of priming both naive and memory T cells. They shape the immune response based on their capacity to deliver a complete array of costimulatory and cytokine signals. Immature DC normally reside in peripheral tissues. Upon sensing a microbe, DC are triggered to enter a developmental program where they undergo maturation, making them competent for activation of naïve T cells (5). DC then migrate out of their peripheral site to a secondary lymphoid tissue, where they trigger a potent adaptive immune response. During this transit, they process microbial products to be presented as peptides on major histocompatibility complex (MHC) class I and II molecules, upregulate costimulatory molecules, downregulate their antigen capture receptors, and become competent for cytokine production. A number of studies have shown that both cytokine and costimulatory signals delivered by mature DC are important for the complete activation of T cells (9, 10, 16, 24, 25).
Many viruses, including influenza virus, Sendai virus, murine cytomegalovirus, dengue virus, hantavirus, and measles virus, have been shown to induce the maturation of DC (8, 11, 26, 29, 31, 37). In these studies, infection of DC resulted in the upregulation of costimulatory molecules and the secretion of cytokines. Interestingly, in some cases virus infection resulted in the synthesis of viral proteins without the production of progeny virus, suggesting that the infections were abortive at a stage subsequent to the production of viral proteins.
The goal of the studies presented here was to define the signals elicited by paramyxovirus infection of DC that may promote maturation. Our laboratory has used simian virus 5 (SV5) as a model to study the effects of paramyxovirus infection on DC maturation. SV5 is a prototype member of the Paramyxoviridae family, which contains a number of clinically relevant viruses, including measles virus, respiratory syncytial virus, parainfluenza viruses, and mumps virus. SV5 is an enveloped virus with a single-stranded, negative-sense, nonsegmented RNA genome. Its 15-kb genome consists of seven tandemly linked genes that encode eight proteins. The proteins responsible for virus entry and replication include surface glycoproteins (HN and F), subunits of the RNA dependent-RNA polymerase (P and L), and internal virion structural proteins (NP and M). In addition, SV5 encodes proteins that are involved in regulating the host response following virus infection (V and SH) (27). The V protein has been shown to block alpha/beta interferon (IFN-α/β) signaling in human but not murine cells through the degradation of STAT1 (14, 41), while the small hydrophobic (SH) protein has been shown to prevent tumor necrosis factor alpha (TNF-α) production in murine cells, thereby preventing apoptosis (30). Thus, we hypothesized that SV5 may have the ability to alter DC maturation through the action of immunomodulatory viral proteins.
Previous studies have shown that in BALB/c fibroblast (BF) cells, infection with recombinant SV5 (rSV5) results in production of IFN-α/β, resulting in a low-level, protracted infection where the virus fluxes between active and repressed states. While SV5 infection in BF cells results in an initial wave of SV5 gene expression, virus protein synthesis and growth are attenuated due to the production of IFN-α/β (13, 41). Given these previous findings, we tested the hypothesis that infection of bone marrow-derived DC (BMDC) with rSV5 would result in an initial wave of viral transcription and/or translation which could induce maturation.
In these studies we determined the ability of rSV5 infection to induce maturation of BMDC from two strains of mice, BALB/c and C57BL/6, and the ability of these cells to activate naive CD8+ T cells. Our initial studies indicated that SV5-matured BALB/c BMDC were reduced in their capacity to activate naive T cells compared to SV5-matured C57BL/6 BMDC. Analysis of the maturation state of these BMDC revealed that BMDC from both strains of mice produced a similar array of cytokines but differed in the upregulation of costimulatory molecules. In the studies reported here, the upregulation of three commonly used molecules, CD40, CD86, and CD80, was used as an indicator of DC maturation. BMDC from both BALB/c and C57BL/6 upregulated the expression of CD40 and CD86 following infection with rSV5 with a similar multiplicity of infection (MOI) of 10 PFU/cell. However, CD80 was upregulated significantly only on C57BL/6 BMDC. Increasing the MOI from 10 PFU/cell to 50 PFU/cell caused the upregulation of CD80 on BALB/c BMDC. Intriguingly, our results showed that the differential maturation induced in the BMDC by rSV5 correlated with the permissivity to rSV5 infection. Furthermore, our experiments suggest that, although secreted IFN-α/β is required for the upregulation of CD40, CD80, and CD86, optimal CD80 upregulation requires an additional signal present during a productive viral infection. These observations provide new insights into the interaction of paramyxoviruses with DC and the differential requirement for the upregulation of distinct costimulatory molecules by DC following viral infection.
MATERIALS AND METHODS
Mice and cell lines.
BALB/c and C57BL/6 mice were purchased from the Frederick Cancer Research and Development Center (Frederick, MD). 129S1/SvImJ control mice were purchased from Jackson Laboratories (Bar Harbor, ME). OT-1 Ova257-264-specific T-cell receptor (TCR) transgenic mice, on a C57BL/6 background, were also purchased from Jackson Laboratories. L9.6 listeria p60217-225-specific TCR transgenic (tg) mice on a Rag-1 KO/BALB/cJ background were a generous gift from Eric Pamer (Memorial Sloan Kettering Cancer Center, New York, N.Y.). IFN-α/β receptor-deficient mice (IFN-α/βR−/−), on a 129S1/SvImJ background, were a generous gift from Christian Schindler (Columbia University, New York, N.Y.). P815 is a DBA/2-derived (H-2d) mastocytoma grown in RPMI 1640 medium (Invitrogen, Grand Island, N.Y.) supplemented with 10% fetal calf serum (HyClone, Logan, UT), l-glutamine, sodium pyruvate, nonessential amino acids, HEPES, penicillin, streptomycin (BioWhittaker, Walkersville, MD), and 5 × 10−5 M 2-mercaptoethanol. NTCC L929 is a fibroblast cell line derived from C3H/An (H-2k) mice grown in Dulbecco's modified Eagle's medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal calf serum (HyClone, Logan, UT), l-glutamine, sodium pyruvate, nonessential amino acids, HEPES, penicillin, streptomycin (BioWhittaker, Walkersville, MD), and 5 × 10−5 M 2-mercaptoethanol. All research performed on mice in this study has complied with federal and institutional guidelines set forth by the Wake Forest University Animal Care and Usage Committee.
Generation of bone marrow-derived dendritic cells.
The protocol used to generate BMDC was similar to the protocol used by Inaba et al. (23). Bone marrow was harvested from the femurs and tibias of mice and red blood corpuscles (RBC) were lysed with ammonium chloride buffer (BioWhittaker, Walkersville, MD). Bone marrow cells were then plated at 106 cells/well in a 24-well plate in RPMI 1640 medium (Invitrogen, Grand Island, N.Y.) supplemented with 10% fetal calf serum (HyClone, Logan, UT), HEPES, gentamicin sulfate (BioWhittaker, Walkersville, MD) and 5 × 10−5 M 2-mercaptoethanol and cultured for 7 days in the presence of 20 ng/ml recombinant granulocyte-macrophage colony-stimulating factor (Biosource, Camarillo, CA). Every 2 days, the cell culture medium of the BMDC cultures was removed followed by the addition of fresh medium with 20 ng/ml recombinant granulocyte-macrophage colony-stimulating factor.
Recombinant viruses.
Wild-type recombinant SV5 (rSV5-WT) and rSV5 expressing green fluorescent protein (GFP) (rSV5-GFP) were generated from cDNA clones as described previously (21, 36). RSV5-I10-HA was engineered to express a polypeptide segment containing the I10 epitope from the BH8 clone of human immunodeficiency virus gp160 (residues 878 to 2223) linked at the C terminus to an 11-residue hemagglutinin (HA) tag. RSV5-GFP-ova was engineered to express GFP fused to a C-terminal extension consisting of the ovalbumin immunodominant amino acids 257 to 264 flanked by four additional naturally occurring N- and C-terminal residues. Viruses expressing either the I10-HA polypeptide or GFP-ova as an additional transcriptional unit between the SV5 HN and L genes were recovered as described previously (36). Viral protein expression and growth properties of viruses containing additional genes were indistinguishable from rSV5-WT (data not shown). Wild-type vesicular stomatitis virus (VSV) was a gift from Douglas Lyles and was grown as previously described (33).
DC infection and treatments.
Virus infections were performed directly in the wells where the BMDC were generated to avoid maturation of cells as a result of manipulation. rSV5 was diluted in RPMI containing 0.75% bovine serum albumin (Invitrogen, Grand Island, NY) and added to the BMDC cultures at a multiplicity of infection (MOI) of 2, 10, or 50 PFU/cell. BMDC treated with 200 ng/ml lipopolysaccharide (LPS) (Sigma, St. Louis, MO) were used as a positive control for fully matured cells. UV inactivation of virus was carried out using the standard 115-V 1A lamp (Ultra Lum, CA) for 15 min at a distance of 6.5 cm. These conditions destroyed all detectable infectious units. This length of UV inactivation did not have a detectable effect on the attachment of virus particles as determined by agglutination assays (data not shown). To assess the role of secreted IFN-α/β in the maturation of BMDC, neutralizing antibodies to IFN-α and IFN-β (40 μg/ml) (PBL Biomedical Laboratories, New Brunswick, NJ) were added to BMDC cultures during rSV5 infection. Poly(I:C) (Pharmacia, New York, NY) was added as a positive control for IFN-α/β production by BMDC.
Western blot analysis.
Western blot analysis was used to assess viral protein production. Two and twenty-four hours following infection with rSV5 (MOI, 10 PFU/cell and 50 PFU/cell), BMDC were solubilized in RIPA buffer containing 1 mM phenylmethylsulfonyl fluoride, 1 mM aprotinin, and 1 mM pepstatin. Protein concentrations were calculated using the Bradford assay (Bio-Rad, Hercules, CA). The normalized proteins were then resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) on 12% polyacrylamide gels. Following electrophoresis, gels were immunoblotted onto polyvinylidene difluoride and blocked in Tris-buffered saline (pH 7.5) containing 5% dry milk. Immunoblots were then probed with polyclonal antibodies specific to cellular protein actin or the SV5 phosphoprotein (P). Protein bands were quantified by scanning and analyzed with Quantity One software (densitometry) (Bio-Rad).
Plaque assay.
Supernatants from BMDC cultures that were mock-infected or infected with live or UV-inactivated rSV5 were analyzed for the presence of progeny virions. Serial dilution of the supernatants were performed and plated on CV-1 cells in duplicates in 6 well plates. Following 2 h of virus attachment, the supernatants were removed, cells were washed and overlaid with 1% agar noble. The cells were incubated for 5 days at 37°C and then enumerated for PFU. Note: 50 PFU/ml was regarded as the limit of detection for the assay.
Cell staining and flow cytometry.
Twenty-four and forty-eight hours following treatment, rSV5-infected, mock-infected, and LPS (200 ng/ml)-treated BMDC were stained with the following antibodies: allophycocyanine-conjugated anti-CD11c together with phycoerythrin-conjugated anti-CD40, anti-CD80, or anti-CD86 antibodies (PharMingen, San Diego, CA). The cells were blocked with Fc block prior to staining to prevent nonspecific binding of antibodies. Flow cytometry was performed using a FACSCalibur and the data analyzed with CellQuest Pro software (both from BD, San Diego, CA). The percent of BMDC expressing high levels of costimulatory molecules was determined by gating on the cells that were positive in mock-treated cells.
Immunofluorescence microscopy.
Virus-infected and mock-infected BMDC were harvested 24 h following treatment. The cells were spun onto coverslips and fixed with paraformaldehyde. Following fixation, cells were stained with a primary hamster anti-CD11c antibody (N418), followed by a secondary goat anti-hamster antibody conjugated with Alexa-568 (Molecular Probes, Eugene, OR). Cells were then washed and mounted onto glass slides. The samples were analyzed with the Nikon Eclipse TE300 microscope.
Cytokine detection.
Supernatants from BMDC cultures that were rSV5-infected, mock-infected, or LPS-treated were analyzed 24 h following treatment for the presence of cytokines. Interleukin- (IL-)12p40 was detected by capture enzyme-linked immunosorbent assay (ELISA). IL-12p70, tumor necrosis factor alpha (TNF-α), IL-10, IL-6, and IFN-γ were detected using the cytometric bead assay kit as per the manufacturer's instructions (BD, San Diego, CA).
IFN-α/β was measured using the VSV-based IFN-α/β bioassay. This assay measures the amount of IFN-α/β present based on the decreased susceptibility of L929 cells to VSV-mediated cytolysis. Briefly, infectious virus in the supernatants from BMDC cultures was inactivated at 4°C overnight by acid treatment, the acid was neutralized, and serial dilutions were incubated with L929 cells in 96-well plates overnight at 37°C. As a standard, cells were incubated with serial fivefold dilutions of IFN (Universal IFN-α/β; PBL Biomedical Laboratories, New Brunswick, NJ). The samples were aspirated, and cells were challenged with wild-type VSV at an MOI of 2 in 100 μl of medium. Controls included cells infected with wild-type VSV alone and cells that were not challenged with wild-type VSV. Cells were incubated overnight at 37°C, medium was aspirated, and cells were fixed with 95% ethanol. Cells were then stained with a 0.1% crystal violet solution in methanol. Absorbance was read at 540 nm on an ELISA reader (1). The levels of IFN-α/β were determined by comparison to standard curves.
Antigen presentation assays.
BMDC that were either mock-infected, infected with live or UV-inactivated rSV5, or peptide-pulsed for 24 h were cocultured with naive OT-1 TCR transgenic mice expressing TCR specific for the Ova257-264 epitope (C57BL/6 antigen presentation assay); or I10-specific high avidity CTL (BALB/c antigen presentation assay) at a ratio of DC:T cells of 1:10. Twenty-four hours following initiation of culture, supernatants were harvested and analyzed for IFN-γ by ELISA. The I10 high-avidity cytotoxic T lymphocyte (CTL) line used was an in vitro established CTL line generated using splenocytes from BALB/c mice immunized with 107 PFU of vPE-16, a recombinant vaccinia virus construct that expresses the gp160 envelope protein from human immunodeficiency virus IIIB (15). This line was restimulated on a weekly basis using 10−9 M I10 peptide-pulsed naive BALB/c splenocytes (3). Briefly, cells were cultured together in a 24-well plate containing 2 ml of RPMI 1640 medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal calf serum (HyClone, Logan, UT), l-glutamine, sodium pyruvate, nonessential amino acids, HEPES, penicillin, streptomycin (BioWhittaker, Walkersville, MD), 5 × 10−5 M 2-mercaptoethanol, and 10% concanavalin A supernatant.
T-cell proliferation assay.
Day 7 BMDC from BALB/c or C57BL/6 mice were mock-treated, LPS-treated, or infected with rSV5 for 24 h. Twenty-two hours following treatment the cells were pulsed with Ova peptide or listeria p60 peptide, respectively (10−10 M Ova257-264 [SIINFEKL]-pulsed C57BL/6 BMDC and 10−9 M listeria p60217-225 pulsed-BALB/c BMDC). These peptide concentrations were chosen because they were the minimal concentration of peptide that gave a detectable proliferation of antigen-specific T cells with SV5-matured BMDC. These cells were then washed to remove soluble peptide. Splenocytes from antigen specific transgenic mice were subjected to negative selection to isolate CD8+ T cells (MiniMACS separation columns and CD8+ isolation kit, Miltenyi Biotec, Auburn, CA). CFSE (5 μM) (5- and 6-carboxyfluorescein diacetate, succinimidyl ester, Molecular Probes, Eugene, OR)-labeled, antigen-specific L9.6 listeria p60217-225-specific TCR transgenic CD8+ T cells or OT-1 Ova257-264-specific TCR transgenic CD8+ T cells were then cocultured with DC pulsed with peptides for 3 days at a ratio of DC:T cells of 1:10 at 37°C in 96-well plates. Proliferation was then measured by the decrease in CFSE intensity by flow cytometry. Analysis and quantitation of data was performed using FlowJo software (FlowJo, Ashland, OR). Proliferation index is the average number of divisions in cells that divided and Division index is the average number of divisions the cell population has undergone.
Statistical analysis.
A paired Student's t test was used to compare significance of individual time points and BMDC treatments. P ≤ 0.05 was considered statistically significant.
RESULTS
SV5-matured C57BL/6 BMDC are more efficient inducers of T-cell proliferation compared to SV5-matured BALB/c BMDC.
Numerous studies have indicated that virus infection can induce maturation of DC to become efficient stimulators of naive T cells (8, 11, 12, 26, 29, 31, 37). Thus, we tested whether infection with rSV5 resulted in BMDC that are capable of inducing proliferation in naive T cells. rSV5-infected BMDC were compared to LPS-matured BMDC. LPS is known to efficiently mature BMDC (12) making them competent for T-cell activation. BMDC from two strains of mice, C57BL/6 and BALB/c, were studied. BMDC were generated from the bone marrow of 6- to 8-week-old mice by culture in the presence of recombinant granulocyte-macrophage colony-stimulating factor. A highly enriched DC population (>90%) was generated in these cultures after 7 days as determined by staining of cells with CD11c (data not shown). BMDC were mock-infected, exposed to LPS or infected with rSV5 (MOI = 10 PFU/cell) for 24 h. Twenty-two hours after treatment, cells were pulsed with Ova257-264 peptide (C57BL/6 BMDC) or Listeria p60217-225 peptide (BALB/c BMDC) for two hours, followed by washing to remove soluble peptide. The choice of peptides was made based on the availability of TCR transgenic mice. Peptide pulsing of DC with similar amounts of peptide ensured that all T cells receive a constant signal through the T-cell receptor (TCR), thus allowing assessment of the selective contribution of costimulatory molecules. C57BL/6 and BALB/c BMDC were then cocultured with CFSE-labeled antigen specific CD8+ T cells (OT-1 TCR transgenic [tg] CD8+ T cells or L9.6 Listeria p60 TCR transgenic CD8+ T cells, respectively) for 3 days and proliferation of CD8+ T cells was determined by flow cytometry.
Figure 1A and 1B show representative proliferation profiles of antigen-specific CD8+ T cells exposed to 10−10M Ova257-264-pulsed C57BL/6 BMDC (Fig. 1A) and 10−9M p60217-225-pulsed BALB/c BMDC (Fig. 1B). The concentrations of peptide used in this assay were the lowest that resulted in significant proliferation of antigen-specific T cells, thereby allowing the contribution of costimulatory or cytokine signals to be revealed. Figure 1C shows the percentage of cells that have undergone division as well as the proliferation and division indices obtained from analyzing the proliferation of antigen-specific CD8+ T cells. Ova257-264-specific OT-1 TCR transgenic CD8+ T cells cultured with peptide-pulsed C57BL/6 BMDC exposed to SV5 were slightly better at activating T cells compared to peptide-pulsed, LPS-treated BMDC as demonstrated by the increased division and proliferation indices (Fig. 1A and 1C). In contrast, the percentage of listeria p60217-225 specific L9.6 TCR transgenic CD8+ T cells cultured with peptide-pulsed BALB/c BMDC exposed to rSV5 that underwent division was significantly lower compared to peptide-pulsed, LPS-treated BMDC (11.6 versus 53.9%). Further, the average number of divisions in the T cells stimulated with DC exposed to SV5 was highly reduced (0.2 versus 1.1) compared to peptide-pulsed, LPS-treated DC (Fig. 1B and 1C). These data show that C57BL/6 BMDC infected with rSV5 were much more effective inducers of T-cell proliferation compared to BALB/c BMDC infected with rSV5. Of note, the data suggest the decrease in proliferation is not due to an inherently reduced ability of the p60-specific T cells to respond to antigen compared to Ova-specific T cells, as the p60 T cells proliferated similar to Ova-specific T cells when stimulated with LPS-matured cells.
FIG. 1.
SV5-matured C57BL/6 BMDC are much more efficient inducers of T-cell proliferation than rSV5-matured BALB/c BMDC. (A) C57BL/6 BMDC infected with rSV5 or treated with LPS for 24 h were pulsed with 10−10 M Ova peptide and then added to CFSE-labeled, Ova257-264-specific TCR transgenic T cells for 3 days. (B) BALB/c BMDC infected with rSV5 or treated with LPS for 24 h were pulsed with 10−9 M listeria p60217-225 peptide and then added to CFSE-labeled, listeria p60-specific TCR transgenic T cells for 3 days. (C) Table showing the analysis from the proliferation assay of T cells. The proliferation index is the average number of divisions in cells that divided and the division index is the average number of divisions the cell population has undergone. The proliferation profiles shown are representative of three independent experiments. Note: the peptide concentration used was the minimal concentration of peptide that gave detectable proliferation of antigen-specific T cells with SV5-matured DC.
C57BL/6 and BALB/c BMDC produce a similar subset of pro-inflammatory cytokines following infection with SV5.
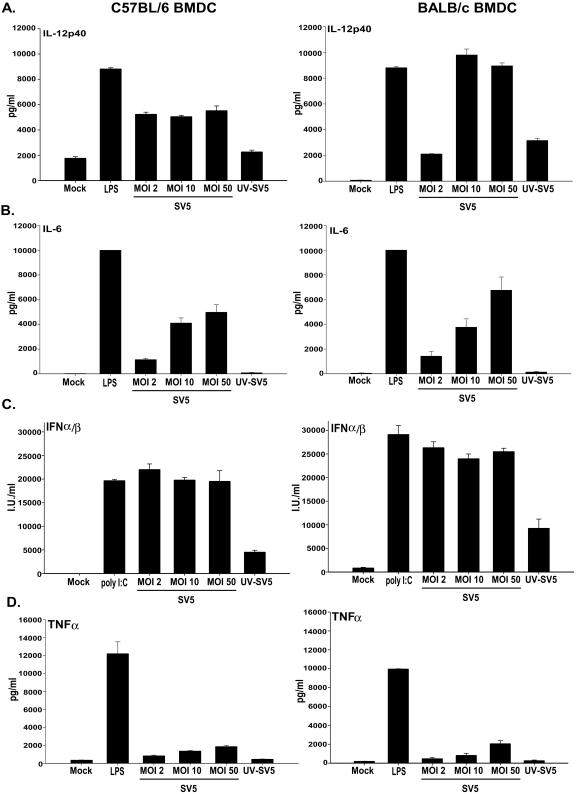
To determine if the difference observed in the proliferation of CD8+ T cells was the result of differences in the cytokines secreted by C57BL/6 versus BALB/c BMDC following infection with rSV5, in vitro-cultured BMDC were exposed to rSV5 at an MOI of 2, 10, or 50 PFU/cell as well as to UV-inactivated rSV5 (MOI of 10 PFU/cell). Twenty-four hours after infection, supernatants from these cell cultures were harvested and IL-12, TNF-α, IL-6, IL-10, and IFN-γ were measured using ELISA or cytometric bead analysis. IFN-α/β was measured using a biological assay based on the inhibition of vesicular stomatitis virus (VSV)-mediated cytolysis of NTCC L929 cells.
The data shown in Fig. 2 show that the pattern of cytokines produced in response to rSV5 infection was similar in BMDC from both strains of mice, with large amounts of IL-12-p40 (Fig. 2A), IL-6 (Fig. 2B), and IFN-α/β (Fig. 2C) produced at all the MOIs tested. The data show that rSV5-infected BALB/c BMDC secreted higher amounts of IL-12p40 (1.9-fold) compared to C57BL/6 BMDC; whereas rSV5-infected BMDC from both strains of mice secreted similar levels of IL-6 and IFN-α/β. TNF-α was also detected, but the level produced was very low compared to LPS-treated BMDC (Fig. 2D). UV inactivation of rSV5 caused a significant decrease in the amount of all cytokines secreted compared to live virus, suggesting that the induction of cytokine production was dependent on live virus (Fig. 2). Neither BALB/c nor C57BL/6 BMDC secreted detectable levels of IL-12p70, IFN-γ, or IL-10 following rSV5 infection compared to mock-infected cells (data not shown). The production of IL-12p40 in the absence of p35 is not unexpected given a previous report from Schultz et al. showing that the optimal production of IL-12p70 involves synergy between microbial stimulation (e.g., virus) and CD40 engagement (39). Together our data show that while there are some differences in the absolute amount (IL-12p40), a similar pattern of cytokines is produced following SV5-infection of BMDC from C57BL/6 or BALB/c mice.
FIG. 2.
rSV5-infected C57BL/6 and BALB/c BMDC produce a similar subset of proinflammatory cytokines. In vitro-cultured BMDC were mock-infected, LPS-treated, and infected with rSV5 at an MOI of 2, 10, or 50 or with UV-inactivated rSV5 (MOI = 10). Twenty-four hours after infection, supernatants from the cultures were harvested and cytokines were measured using ELISA (IL-12p40) or cytometric bead analysis (TNF-α and IL-6). IFN-α/β was measured using a biological assay based on vesicular stomatitis virus (VSV)-mediated cytolysis of NTCC L929 cells. Poly(I:C) was used as a positive control for IFN-α/β production by BMDC. (A) IL-12p40, (B) IL-6, (C) IFN-α/β, (D) TNF-α. The graphs show the average ± standard error of the mean of four independent experiments.
C57BL/6 and BALB/c BMDC differ in the costimulatory molecules expressed following infection with rSV5.
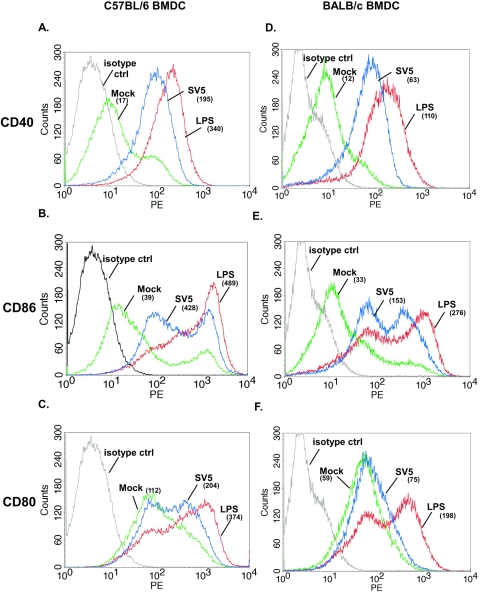
Since the cytokines secreted by BMDC from both strains of mice were similar, we determined whether there was a difference in the costimulatory molecules expressed following infection with rSV5. The expression of three commonly used markers of DC maturation, CD40, CD86, and CD80, was assessed. LPS was used as a positive control for maturation. BMDC were infected for 24 h with rSV5 (MOI 10 PFU/cell). The cells were then stained with a monoclonal antibody to CD11c, together with antibodies to CD40, CD86 or CD80 and analyzed by flow cytometry. Figure 3 shows the level of expression of costimulatory molecules expressed on CD11c+ DC. LPS strongly upregulated all three molecules on both C57BL/6 and BALB/c BMDC. Similarly, rSV5 induced significant upregulation of CD40 (Fig. 3A and 3D) and CD86 (Fig. 3B and 3E) on both C57BL/6 and BALB/c BMDC compared to mock-infected cells (albeit to a slightly lesser extent on a per cell basis, compared to LPS-treated DC). However, although CD80 expression was increased on SV5-infected C57BL/6 BMDC compared to mock-treated cells, no increased expression over mock-infected cells was detected on BALB/c BMDC following infection with SV5 (Fig. 3C and 3F). The upregulation of these costimulatory molecules (CD40, CD86, and CD80) on C57BL/6 and BALB/c BMDC was the result of rSV5 infection as maturation was prevented by neutralization of rSV5 using an antibody that binds the surface glycoprotein HN (data not shown).
FIG. 3.
C57BL/6 and BALB/c BMDC infected with rSV5 efficiently upregulated the expression of CD40 and CD86, while CD80 is efficiently upregulated only on C57BL/6 BMDC. Day 7 in vitro-cultured C57BL/6 BMDC (A-C) or BALB/c (D-F) were infected for 24 h with rSV5 (MOI = 10), stained with antibodies to CD11c and CD40, CD86, or CD80, and analyzed by flow cytometry. LPS (200 ng/ml)-treated BMDC served as a positive control. Colors: isotype control (black histogram), mock-infected BMDC (green histogram), LPS-treated BMDC (red histogram), SV5-infected BMDC (blue histogram). The numbers shown in parentheses are the geometric mean fluorescence intensity (MFI). The histograms shown are representative of four independent experiments. Upregulation of costimulatory molecules following infection with rSV5 compared to mock-infected BMDC was statistically significant (average of four experiments). C57BL/6 BMDC: CD40 (P = 0.045), CD86 (P = 0.011), CD80 (P = 0.024); BALB/c BMDC: CD40 (P = 0.040), CD86 (P = 0.015).
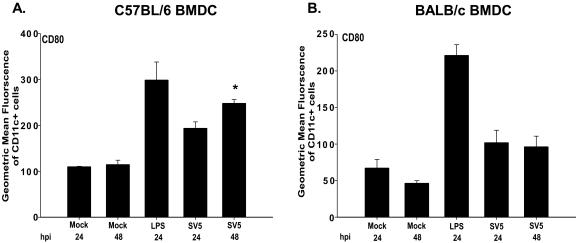
Analysis of the time course of CD80 expression on C57BL/6 and BALB/c BMDC with rSV5 (MOI = 10 PFU/cell) showed that CD80 expression continued to increase between 24 h and 48 h on CD11c+ C57BL/6 BMDC. However, CD80 expression on BALB/c BMDC remained similar to mock-infected cells even at 48 h postinfection (Fig. 4). These studies indicated that infection of BALB/c BMDC with rSV5 results in the selective upregulation of a subset of costimulatory molecules, while CD40, CD80 and CD86 are all upregulated on C57BL/6 BMDC following infection with rSV5.
FIG. 4.
CD80 expression continues to increase only on C57BL/6 BMDC between 24 and 48 h postinfection with rSV5. Day 7 BMDC from both strains of mice were mock infected, treated with LPS, or infected with rSV5 (MOI = 10) for 24 h or 48 h. Cells were then stained with antibodies to CD11c and CD80 and analyzed by flow cytometry. The graphs in the figure show the geometric mean fluorescent intensity of CD80 for CD11c+ cells for both C57BL/6 (A) and BALB/c (B) BMDC. The graphs show the average ± standard error of the mean of four independent experiments. P values were calculated using the paired t test. *, upregulation of CD80 on C57BL/6 BMDC following infection with rSV5 compared to mock-infected BMDC was statistically significant (24 h: P = 0.024; 48 h: P = 0.040).
Upregulation of costimulatory molecules on BALB/c and C57BL/6 BMDC is dependent on live virus.
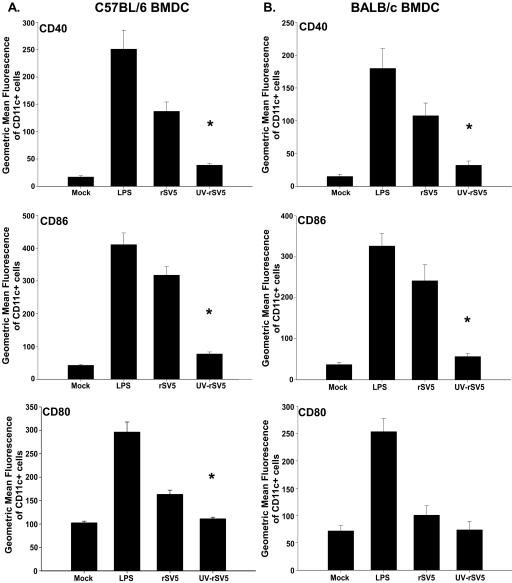
We next determined whether the maturation of DC by rSV5 was dependent on live virus or whether UV-inactivated virus could induce upregulation of costimulatory molecule expression. Figure 5 shows the average expression of CD40, CD86 and CD80 following infection with live or UV-inactivated rSV5 (UV-rSV5) at an MOI of 10 PFU/cell for both C57BL/6 and BALB/c BMDC. In C57BL/6 mice, there was approximately an 82%, 87%, and 86% decrease in the expression of CD40, CD86 and CD80, respectively, following infection with UV-rSV5 compared with live rSV5 (Fig. 5A). Similarly, compared to BALB/c BMDC exposed to live virus, there was an approximately 82% and 90% reduction in the expression of CD40 and CD86 following exposure to UV-rSV5 (Fig. 5B). This indicated that the optimal maturation of BMDC was highly dependent on live virus. The inability to completely abolish upregulation of costimulatory molecules following exposure to UV-rSV5 suggested that some cell surface interaction might contribute to the upregulation of costimulatory molecules or, alternatively, that UV inactivation of rSV5 was incomplete.
FIG. 5.
Upregulation of costimulatory molecules on C57BL/6 and BALB/c BMDC is dependent on live virus. Day 7 C57BL/6 (A) and BALB/c (B) BMDC were infected with live or UV-inactivated rSV5 (UV-rSV5, MOI = 10). LPS-treated and mock-infected BMDC were used as positive controls. Twenty-four hours after infection, cells were stained with antibodies to CD11c and CD40, CD86 or CD80 and analyzed using flow cytometry. The level of expression of these molecules on CD11c+ cells is shown. The graphs show the average ± standard error of the mean of four independent experiments. P values were calculated using the paired t test. *, C57BL/6 BMDC: CD40 (P = 0.030), CD86 (P = 0.012), CD80 (P = 0.025); BALB/c BMDC: CD40 (P = 0.045), CD86 (P = 0.040).
Secreted IFN-α/β is required for the upregulation of CD40, CD86, and CD80.
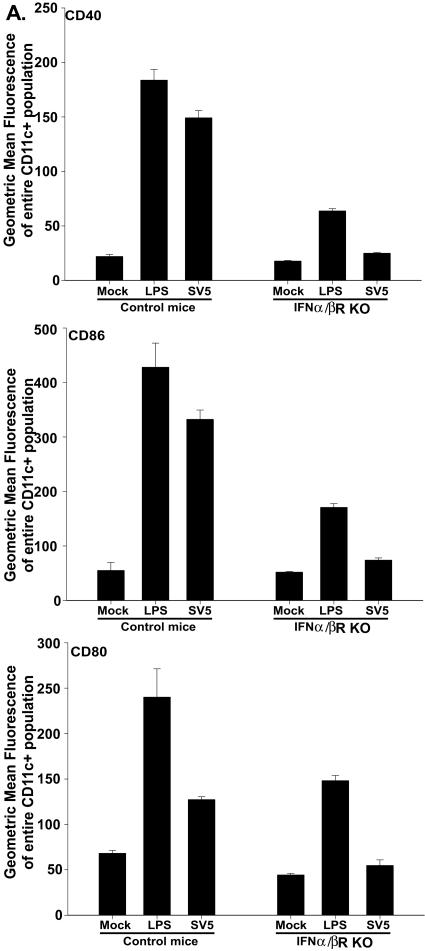
We next wanted to determine the signals responsible for the increased expression of CD40, CD86 and CD80 on BMDC following infection with rSV5. IFN-α/β has been shown to promote the maturation of BMDC (22, 31, 32, 35, 38). Our previous studies had demonstrated that both BALB/c and C57BL/6 BMDC secreted significant amounts of IFN-α/β following infection with rSV5 (Fig. 2C). Thus, it was possible that IFN-α/β produced as a result of rSV5 infection was responsible for the maturation of BMDC in our studies. To address this question, day 7 in vitro-cultured BMDC from IFN-α/β receptor-deficient mice (IFN-α/βR−/−) and control mice (129S1/SvImJ) were infected with rSV5 (MOI of 10 PFU/cell). Upregulation of CD40, CD80, and CD86 was significantly decreased (to near background levels) on BMDC derived from mice that lacked the IFN-α/β receptor compared to control mice (Fig. 6). In agreement with these results, the addition of neutralizing antibodies to IFN-α and IFN-β prevented the upregulation of CD40 and CD86 on BALB/c BMDC and all three molecules on C57BL/6 BMDC (data not shown). Together these results indicated that secreted IFN-α/β is required for the rSV5-induced upregulation of CD40, CD86 and CD80.
FIG. 6.
Binding of secreted IFN-α/β to its receptor is required for the upregulation of CD40, CD80, and CD86. BMDC from IFN-α/βR−/− and control mice (129S1/SvImJ) were mock infected, treated with LPS, or infected with rSV5. Twenty-four hours later, cells were stained for CD11c and CD40, CD80, or CD86 and analyzed using flow cytometry. The graphs in the figure show the geometric mean fluorescence intensity of CD40, CD86, and CD80 for CD11c+ cells. The graphs show the average ± standard error of the mean of three independent experiments.
SV5 infection of BALB/c BMDC does not prevent LPS-induced maturation of BMDC.
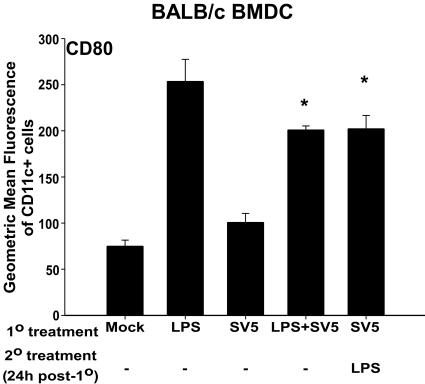
Our previous results showed that CD80 expression was not upregulated on BALB/c BMDC at 24 and 48 h posttreatment with rSV5 compared to LPS-treated BMDC (Fig. 3F and 4B). We hypothesized that the failure to upregulate CD80 on BALB/c BMDC, even at 48 h following infection with rSV5, could be the result of the active inhibition of CD80 expression. Alternatively, it was possible that infection with rSV5 did not provide the positive signal required for the upregulation of this molecule.
To distinguish between these two possibilities, BALB/c BMDC were treated with LPS and rSV5 at an MOI of 10 PFU/cell, either in combination or consecutively, as shown in Fig. 7. The levels of CD80 resulting from exposure to LPS alone or the combination of LPS and rSV5 were not statistically different. This finding suggested that, if the failure of SV5 to induce CD80 upregulation in BALB/c mice was the result of an inhibitory signal, it was not present immediately following infection. However, it was possible that gene products of rSV5 that might inhibit the expression of CD80 were present in the infected cell only at later times postinfection. To determine if this were the case, LPS was added 24 h after infection with rSV5. However, even 24 h of prior infection with rSV5 did not alter LPS-induced upregulation of CD80, i.e., CD80 expression induced by LPS alone was not statistically different compared to infection with rSV5 for 24 h followed by treatment with LPS (Fig. 7). Similar results were obtained using poly I:C as a maturation stimulus (data not shown). These data suggest that rSV5 infection does not negatively regulate CD80 expression and instead suggests that a positive signal required for its upregulation may be lacking following infection of BALB/c BMDC with rSV5.
FIG. 7.
rSV5 infection of BALB/c BMDC does not prevent LPS-induced maturation of BMDC. BALB/c BMDC were LPS treated, mock infected, rSV5 infected, or treated with combinations of LPS (200 ng/ml) and rSV5 (MOI = 10). LPS and rSV5 were added concurrently to BMDC for 24 h, or LPS was added 24 h following infection with rSV5. The graph shows the geometric mean fluorescence intensity of CD80 for CD11c+ cells for BALB/c BMDC. The graphs show the average ± standard error of the mean of three independent experiments. P values were calculated using the paired t test. *, upregulation of CD80 on BALB/c BMDC treated with LPS and rSV5 (P = 0.009) and rSV5 for 24 h followed by LPS (P = 0.003) was statistically significant relative to that of rSV5-infected BMDC.
Newly synthesized SV5 proteins are detected in significant quantities in C57BL/6 BMDC, but not in BALB/c BMDC following infection with SV5.
Our data have shown that CD80 was upregulated on C57BL/6 BMDC but not BALB/c BMDC (Fig. 3 and 4). In addition, although secreted IFN-α/β was required for the upregulation of all three costimulatory molecules, CD80 was not upregulated significantly on BALB/c BMDC, even though these cells secreted significant amounts of IFN-α/β (Fig. 4 and 2C). Furthermore, our data have shown that rSV5 infection in BALB/c BMDC may not be providing the positive signal required for the upregulation of CD80 (Fig. 7). These data suggested that the infection of C57BL/6 BMDC may differ from that of BALB/c BMDC.
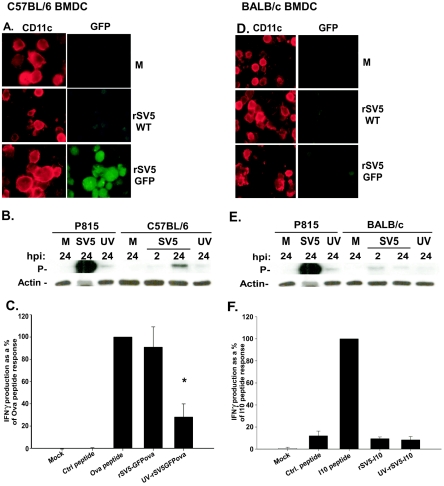
To test this possibility, day 7 in vitro-cultured BMDC from C57BL/6 and BALB/c mice were mock-infected (M) or infected at an MOI of 10 PFU/cell with rSV5-wt or rSV5 expressing GFP (rSV5-GFP). GFP is not packaged into the SV5 virion, therefore the presence of GFP in an infected cell is indicative of new viral gene expression. P815, a cell line that is highly permissive to SV5 infection (19, 20), was used as a positive control and mock-infected cells were used as negative controls. Twenty-four hours after infection, fluorescence microscopy was used to assess GFP expression as a measure of viral protein synthesis. Figures 8A and 8D are representative of three independent experiments and show images of mock, rSV5-wt and rSV5-GFP infected C57BL/6 or BALB/c BMDC. As seen in the images, high levels of GFP were detected in the CD11c+ BMDC from C57BL/6 mice, but no increase in the level of fluorescence was detected in the CD11c+ BMDC from BALB/c mice infected with rSV5-GFP versus rSV5-wt.
FIG. 8.
Newly synthesized SV5 proteins are readily detected in significant quantities in C57BL/6 BMDC but not in BALB/c BMDC following infection with rSV5. BMDC from C57BL/6 (A) or BALB/c (D) mice were cultured in vitro for 7 days as described in Materials and Methods. On day 7, cells were mock infected (M) or infected with rSV5-wt or rSV5-GFP (MOI = 10). At 24 h postinfection, cells were stained with a primary hamster anti-mouse antibody to CD11c, followed by the secondary antibody (goat anti-hamster Alexa-568) and immunofluorescence was analyzed. The images shown are representative of three independent experiments. P815 cells, C57BL/6 BMDC (B), or BALB/c BMDC (E) were either mock infected (M) or infected with live or UV-inactivated rSV5 (MOI = 10). Two and 24 hours after infection, cells were lysed and analyzed by Western blotting for SV5 P protein and cellular actin. The blot shown is representative of three independent experiments. Note: The amount of SV5-infected P815 lysate used was 15 μg, whereas 30 μg of the other samples were loaded. (C) C57BL/6 BMDC were infected on day 7 with live or UV-inactivated rSV5-GFP-Ova (MOI = 10). Twenty-four hours after infection, C57BL/6 BMDC were cocultured with naive OT-1 TCR transgenic CD8+ T cells (specific for Ova257-264 peptide) at a ratio of DC:T cells of 1:10. Twenty-four hours following coculture, IFN-γ present in the supernatant was measured by ELISA. (F) BALB/c BMDC were infected on day 7 with live or UV-inactivated rSV5-I10 (MOI = 10). At 24 h postinfection, BMDC were cocultured with an I10-specific high avidity CTL line at a ratio of DC:T cells of 1:10. Twenty-four hours following coculture, IFN-γ present in the supernatant was measured by ELISA. The graphs show the average ± standard error of the mean of three independent experiments. P values were calculated using the paired t test. *, a significant decrease (P = 0.029) in the amount of IFN-γ produced by CD8+ cocultured with C57BL/6 BMDC infected with UV-inactivated rSV5-GFPOva relative to IFN-γ produced by CD8+ T cells cultured with BMDC infected with live rSV5-GFPOva.
We next determined whether a 3′ SV5 gene product (the P protein) could be detected in C57BL/6 and BALB/c BMDC following infection. Day 7 in vitro-cultured BMDC were infected with rSV5 or UV-inactivated rSV5 at an MOI of 10 PFU/cell. P protein was assessed by Western blot analysis at 2 and 24 h after infection. Again P815 cells were used as a positive control for SV5 infection. As shown in Fig. 8B, a time-dependent increase in the expression of P protein was detected in C57BL/6 BMDC infected with SV5, which was prevented when virus was UV inactivated prior to infection. However, no time dependent increase in P protein expression was seen in BALB/c BMDC (Fig. 8E). To determine if virus infection of BMDC resulted in the production of progeny virions, we performed a plaque assay using supernatants from C57BL/6 and BALB/c BMDC following infection with rSV5 for 24 h. A titer of 1.2 × 103 PFU/ml was detected in supernatants from rSV5-infected C57BL/6 BMDC, while virus in the BALB/c BMDC supernatants was below the limit of detection.
Peptide recognition by CTL is a highly sensitive measure of protein production. Thus, day 7 C57BL/6 BMDC were infected with a recombinant SV5 that was engineered to express the H-2b-restricted Ova257-264 peptide fused to GFP (rSV5-GFPOva) as an additional gene. GFPOva is not packaged in the SV5 virion and thus must be synthesized in the cell upon infection in order to be presented to T cells. Twenty-four hours after infection, BMDC were cocultured with CD8+ T lymphocytes from a naive OT-1 TCR transgenic mouse, which expresses a TCR specific for the Ova257-264 epitope. Ova257-264-pulsed BMDC were used as a positive control. Following culture, supernatants were harvested and IFN-γ production by Ova257-264 specific CD8+ T cells measured by ELISA. As expected, IFN-γ was produced by CD8+ T cells that were cultured with rSV5-GFPOva-infected BMDC at levels similar to Ova257-264 peptide-pulsed BMDC (Fig. 8C). UV inactivation of virus prior to infection resulted in a significant decrease in the amount of IFN-γ produced following stimulation, confirming significant dependency on newly synthesized viral proteins.
To determine whether we could detect evidence of newly synthesized viral proteins in BALB/c BMDC using this highly sensitive readout, we performed a similar antigen presentation assay. In this case a high avidity CTL line previously shown to be highly efficient at recognizing very low amounts of peptide on the surface of antigen-presenting cells (15), was used as a probe for newly synthesized viral proteins. BALB/c BMDC were infected on day 7 with live or UV-inactivated rSV5-I10, a recombinant SV5 containing an epitope from the HIVgp160 protein recognized in the context of H-2d. As before, because this protein is not packaged in the rSV5 virion, processing and presentation to CTL require synthesis of the gp160 protein fragment. Twenty-four hours after infection the DC were cocultured with the high-avidity I10-specific CTL line. I10 peptide-pulsed BMDC served as a positive control, while mock-infected BMDC pulsed with an irrelevant peptide served as a negative control. Following 24 h of coculture, supernatants were harvested and IFN-γ production by the I10-specific CTL quantified by ELISA. As shown in Fig. 8F, IFN-γ was not detected above background in samples where CTL were cultured with BMDC infected with rSV5-I10, compared to those exposed to I10 peptide-pulsed BMDC. BMDC infected with rSV5-I10 or UV-inactivated rSV5-I10 were, however, capable of stimulating IFN-γ secretion in SV5-specific CTL (data not shown), suggesting that BALB/c BMDC were capable of presenting peptides from proteins that were present in the incoming virion. The results from the functional assay strongly support the immunofluorescence and Western blot data. Together these data establish that following infection at a similar MOI, newly synthesized SV5 proteins are highly expressed in C57BL/6, but not in BALB/c BMDC.
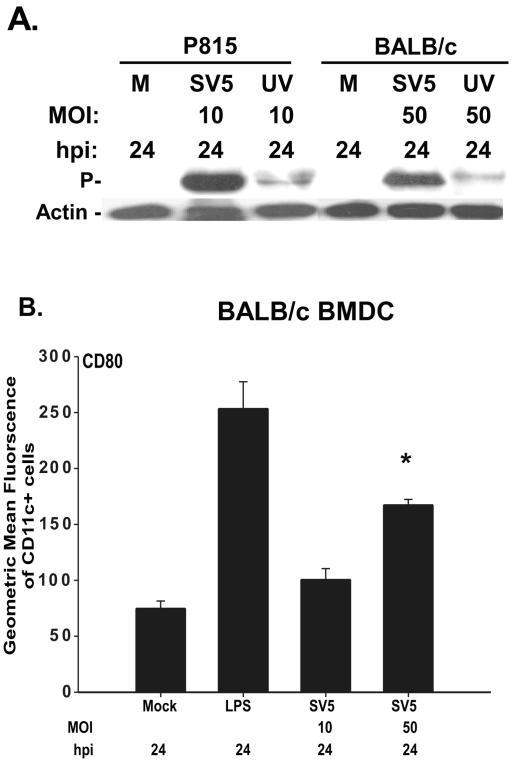
CD80 upregulation on BALB/c BMDC correlates with the expression of viral protein following infection with high MOI.
The data presented thus far have demonstrated a difference in the permissivity of BALB/c versus C57BL/6-derived BMDC to infection with rSV5 at a similar MOI of 10 PFU/cell, in that viral protein was undetectable in BALB/c BMDC following infection. These data suggested that rSV5 infection was highly abortive in BALB/c but not C57BL/6 BMDC at the MOI tested. Therefore, we determined if increasing the MOI might overcome the abortive nature of rSV5 infection of BALB/c BMDC. To address this question, BMDC from BALB/c were infected with rSV5 at a higher MOI (50 PFU/cell) and P protein expression was assessed by Western blot analysis at 24 h postinfection. Although there was no time-dependent increase in the detection of P protein in samples infected with rSV5 at an MOI of 10 PFU/cell between 2 and 24 h, a significant amount of P protein was detected in BALB/c BMDC infected with rSV5 at an MOI of 50 PFU/cell, and this elevated expression was reduced by UV inactivation of the virus (Fig. 8E and 9A). Further, infection of BALB/c BMDC with rSV5 at an MOI of 50 PFU/cell also resulted in the production of progeny virus (data not shown).
FIG. 9.
Upregulated CD80 expression on BALB/c BMDC following infection with a high MOI of 50 PFU/cell correlates with significant expression of viral proteins. (A) BMDC from BALB/c mice were mock infected (M) or infected with live or UV-inactivated rSV5 (MOI = 50). Twenty-four hours after infection, cells were lysed and analyzed by Western blotting for SV5 P protein and cellular actin. The blot shown is representative of three independent experiments. (B) Day 7 in vitro-cultured BALB/c BMDC were infected for 24 h with rSV5 (MOI 10 and 50 PFU/cell), stained with antibodies to CD11c and CD80 and analyzed by flow cytometry. Mock- and LPS (200 ng/ml)-treated BMDC served as negative and positive controls, respectively. The graphs show a representative of three independent experiments. P values were determined using the paired t test. *, upregulation of CD80 on BALB/c BMDC infected with rSV5 at an MOI of 50 PFU/cell (P = 0.001) relative to mock-infected cells.
Given the increased expression of viral proteins following infection at an MOI of 50 PFU/cell, we determined whether CD80 expression was also upregulated on these cells. The results in Fig. 9B show that indeed a significant increase in the expression of CD80 was observed on BALB/c BMDC following infection with rSV5 at an MOI of 50 PFU/cell, although to a lesser extent on a per cell basis compared to LPS-treated BMDC. Thus, using BMDC from the same strain of mice, we found that the maturation state of the DC was altered depending on the productivity of the infection, with increased CD80 expression correlating with new viral protein synthesis.
DISCUSSION
Our results have shown that BMDC obtained from two different stains of mice, BALB/c and C57BL/6, exhibit differential susceptibility to infection by rSV5 as measured by new viral protein synthesis, with BALB/c BMDC exhibiting reduced permissivity to infection (Fig. 8). However, increasing the MOI used for infection of BALB/c BMDC from 10 to 50 PFU/cell resulted in the production of significant amounts of viral proteins and viral progeny (Fig. 9A and data not shown). The requirement for a higher MOI in BALB/c versus C57BL/6 BMDC could be the result of a reduced efficiency in any of the steps involved in determining viral protein steady-state levels (attachment, penetration, uncoating, or viral replication). Increasing the MOI may simply increase the probability for these steps to occur. Alternatively, it is possible that infecting BALB/c BMDC with a higher MOI of 50 PFU/cell resulted in the ability to overwhelm an intrinsically superior antiviral response in BALB/c BMDC, leading to the production of significant amounts of viral proteins.
DC can upregulate an array of costimulatory molecules following microbial stimulation which have been shown to contribute to the potent ability of these cells to activate naïve T lymphocytes. The best characterized are CD40, CD80, and CD86. Both CD80 and CD86 bind CD28 on T cells and provide the second signal required for the activation and expansion in response to antigen (16, 24, 25). CD40, on the other hand, provides an activating signal to the DC by virtue of its binding to CD40L on T cells. CD40 engagement leads to the increased upregulation of MHC, adhesion, and costimulatory molecules (7). Our experiments have demonstrated that, regardless of permissivity to infection with rSV5, BMDC obtained from both strains of mice upregulated CD40 and CD86 (Fig. 3). Interestingly, however, the upregulation of CD80 in the context of an rSV5 infection appeared to be dependent on some signal that was present during productive infection, i.e., C57BL6 BMDC infected at an MOI of 10 PFU/cell and BALB/c BMDC infected at an MOI of 50 PFU/cell (Fig. 3, 4, and 9). The failure to upregulate CD80 on BALB/c BMDC following infection with rSV5 at an MOI of 10 PFU/cell suggested that maturation of these cells was incomplete. The observed correlation between viral protein expression and CD80 upregulation suggested that qualitatively and/or quantitatively distinct signals present following infection with rSV5 were probably required for the expression of distinct costimulatory molecules, as exemplified by CD80, CD86, and CD40. To our knowledge, this is the first study to report a correlation between the efficiency of viral infection (as measured by the level of viral protein expressed and production of viral progeny) and the upregulation of select costimulatory molecules during DC maturation.
The production of IFN-α/β, which is induced by rSV5 infection in murine BMDC (Fig. 2C), has been shown to be associated with the maturation of BMDC in a number of previous studies (2, 18, 22, 28, 32, 35, 38). However, maturation is not always due to secreted IFN-α/β itself, as shown in a study by Lopez et al., where activation of the IFN-α/β pathway, as opposed to secreted IFN-α/β, was responsible for BMDC maturation following infection with influenza or Sendai viruses (31). Using both IFN-α/β receptor-deficient mice and neutralizing antibodies, we have found that upregulation of CD40, CD86, and CD80 following SV5 infection is highly dependent on IFN-α/β binding to its receptor (Fig. 6 and data not shown). Further, our preliminary studies suggest that the addition of exogenous IFN-α/β to noninfected BMDC can induce upregulation of CD40, CD86 and CD80 in a dose-dependent manner (data not shown). This is intriguing given that CD80 was upregulated following rSV5 infection at an MOI of 50, but not at an MOI of 10 (Fig. 3 and 9), even though similar amounts of IFN-α/β were produced in both cases (Fig. 2C). This finding suggests that rSV5 infection may modify the response of BMDC to IFN-α/β such that an additional trigger is now required to induce significant increases in CD80 expression which is present only during productive infection. Studies are under way to test this possibility.
We propose a model where infection with SV5 induces the production of IFN-α/β, which then, in an autocrine or paracrine fashion, induces the upregulation of CD40 and CD86. However, while CD80 upregulation is also dependent on IFN-α/β, an additional signal is necessary that is lacking in BALB/c BMDC infected with rSV5 at a lower MOI (10 PFU/cell). In C57BL/6 BMDC (MOI of ≥10 PFU/cell) or BALB/c BMDC (MOI of 50 PFU/cell), where infection results in the production of significant amounts of viral protein and progeny, this signal is present, which together with IFN-α/β triggers the upregulation of CD80. These studies demonstrate that the expression of costimulatory molecules, as exemplified by CD40, CD80, and CD86, can be controlled by distinct signals that may vary in nature and/or level.
What is the functional outcome of the failure of DC to undergo complete maturation? Complete activation of T lymphocytes requires optimal signals 1 (TCR-MHC-peptide complex), 2 (costimulatory molecules), and 3 (cytokines) to be provided by the antigen-presenting cell (9, 10). Incomplete DC maturation has been shown to result in the failure to provide adequate signal 2 (costimulatory molecules) and/or signal 3 (cytokines), which are necessary for the optimal activation and differentiation of T cells (4, 6, 9, 10). In our studies we found that BALB/c BMDC matured by infection with rSV5 at an MOI of 10 PFU/cell were weak stimulators of CD8+ T-cell proliferation compared to LPS-matured BALB/c BMDC (Fig. 1). This corresponded to the failure of these cells to upregulate CD80. While CD80 has been shown in numerous studies to be important for T-cell activation (16, 17, 34, 40), it is important to note that other costimulatory molecules, not assessed here, may also fail to be upregulated under conditions where CD80 is not induced. Therefore while the failure of CD80 may contribute to the observed proliferative differences, it may not be directly responsible for the differences and may instead serve as a marker for incompletely matured DC.
To begin to address this possibility, we have performed preliminary analyses to determine whether BALB/c DC infected at an MOI of 50, which have increased expression of CD80, exhibit an increased capacity to activate naïve T cells. In these studies we have observed only a slightly increased ability to induce CD8+ T-cell proliferation (data not shown). However, importantly, although our data showed that there was an increase in CD80 upregulation on BALB/c BMDC infected with rSV5 at an MOI of 50 compared to those infected at an MOI of 10, the levels are still lower than those seen on LPS-treated DC (Fig. 9B). Thus, it is possible that CD80 expression, while increased in cells infected at an MOI of 50 versus 10, is still below the level required for optimal activation of T cells. Alternatively, as noted above, another molecule that fails to be upregulated under these conditions may be critical for optimal proliferation of naïve cells.
In summary, we have found that a difference exists in BMDC from two strains of mice in permissivity to SV5 infection. Intriguingly, regardless of the susceptibility to infection, SV5 induced upregulation of the costimulatory molecules CD40 and CD86 on BMDC. In contrast, the increased expression of CD80 was correlated with the permissivity of the DC to infection with rSV5. The correlation between increased expression of CD80 and the detection of new viral protein suggested that CD80 upregulation in the context of viral infection required a signal present only when significant amounts of viral RNA and/or protein were produced in these cells. These results suggest that there is a difference in the quantity and/or quality of signal required for the upregulation of distinct costimulatory molecules, e.g., CD80, CD86, and CD40. Further, they suggest that the potency of the DC for naive T-cell activation may be regulated by the level of virus encountered. In total, this work provides new insights into the requirements for maturation of DC as a result of virus infection. A further understanding of the signals that control the expression of these costimulatory molecules will likely provide important insights into the generation of an antiviral immune response.
Acknowledgments
We thank Douglas Lyles, Elizabeth Hiltbold, and Jason Grayson for critical reading of the manuscript. We thank Daniel Gaddy, Virginia Young, Ellen Palmer, and Gerald Capraro for help with the biochemical techniques and Hiltbold for advice on BMDC preparation. We are also grateful to Christian Schindler and Eric Pamer for providing the IFN-α/βR−/− mice and L9.6 listeria p60 transgenic mice, respectively.
This work was supported by the NIH grants AI060642 and HL71985 (M.A.A.M.) and AI46282 (G.D.P.).
REFERENCES
- 1.Ahmed, M., M. O. McKenzie, S. Puckett, M. Hojnacki, L. Poliquin, and D. S. Lyles. 2003. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 77:4646-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 3.Alexander-Miller, M. A., G. R. Leggatt, and J. A. Berzofsky. 1996. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. USA 93:4102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, D. E., L. J. Ausubel, J. Krieger, P. Hollsberg, G. J. Freeman, and D. A. Hafler. 1997. Weak peptide agonists reveal functional differences in B7-1 and B7-2 costimulation of human T-cell clones. J. Immunol. 159:1669-1675. [PubMed] [Google Scholar]
- 5.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 6.Boise, L. H., A. J. Minn, P. J. Noel, C. H. June, M. A. Accavitti, T. Lindsten, and C. B. Thompson. 1995. CD28 costimulation can promote T-cell survival by enhancing the expression of Bcl-XL. Immunity 3:87-98. [DOI] [PubMed] [Google Scholar]
- 7.Caux, C., C. Massacrier, B. Vanbervliet, B. Dubois, C. Van Kooten, I. Durand, and J. Banchereau. 1994. Activation of human dendritic cells through CD40 cross-linking. J. Exp Med. 180:1263-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cella, M., M. Salio, Y. Sakakibara, H. Langen, I. Julkunen, and A. Lanzavecchia. 1999. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 189:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtsinger, J. M., C. M. Johnson, and M. F. Mescher. 2003. CD8 T-cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 171:5165-5171. [DOI] [PubMed] [Google Scholar]
- 10.Curtsinger, J. M., D. C. Lins, and M. F. Mescher. 2003. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 197:1141-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalod, M., T. Hamilton, R. Salomon, T. P. Salazar-Mather, S. C. Henry, J. D. Hamilton, and C. A. Biron. 2003. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J. Exp. Med. 197:885-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Smedt, T., B. Pajak, E. Muraille, L. Lespagnard, E. Heinen, P. De Baetselier, J. Urbain, O. Leo, and M. Moser. 1996. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 184:1413-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. Sendai virus and simian virus 5 block activation of interferon-responsive genes: importance for virus pathogenesis. J. Virol. 73:3125-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 73:9928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earl, P. L., S. Koenig, and B. Moss. 1991. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J. Virol. 65:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields, P. E., R. J. Finch, G. S. Gray, R. Zollner, J. L. Thomas, K. Sturmhoefel, K. Lee, S. Wolf, T. F. Gajewski, and F. W. Fitch. 1998. B7.1 is a quantitatively stronger costimulus than B7.2 in the activation of naive CD8+ TCR-transgenic T cells. J. Immunol. 161:5268-5275. [PubMed] [Google Scholar]
- 17.Gajewski, T. F. 1996. B7-1 but not B7-2 efficiently costimulates CD8+ T lymphocytes in the P815 tumor system in vitro. J. Immunol. 156:465-472. [PubMed] [Google Scholar]
- 18.Gallucci, S., M. Lolkema, and P. Matzinger. 1999. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5:1249-1255. [DOI] [PubMed] [Google Scholar]
- 19.Gray, P. M., G. D. Parks, and M. A. Alexander-Miller. 2003. High avidity CD8+ T cells are the initial population elicited following viral infection of the respiratory tract. J. Immunol. 170:174-181. [DOI] [PubMed] [Google Scholar]
- 20.Gray, P. M., G. D. Parks, and M. A. Alexander-Miller. 2001. A novel CD8-independent high-avidity cytotoxic T-lymphocyte response directed against an epitope in the phosphoprotein of the paramyxovirus simian virus 5. J. Virol. 75:10065-10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He, B., R. G. Paterson, C. D. Ward, and R. A. Lamb. 1997. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology 237:249-260. [DOI] [PubMed] [Google Scholar]
- 22.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins, M. K., D. M. Pardoll, J. Mizuguchi, H. Quill, and R. H. Schwartz. 1987. T-cell unresponsiveness in vivo and in vitro: fine specificity of induction and molecular characterization of the unresponsive state. Immunol. Rev. 95:113-135. [DOI] [PubMed] [Google Scholar]
- 25.Kaye, P. M. 1995. Costimulation and the regulation of antimicrobial immunity. Immunol. Today. 16:423-427. [DOI] [PubMed] [Google Scholar]
- 26.Klagge, I. M., V. ter Meulen, and S. Schneider-Schaulies. 2000. Measles virus-induced promotion of dendritic cell maturation by soluble mediators does not overcome the immunosuppressive activity of viral glycoproteins on the cell surface. Eur. J. Immunol. 30:2741-2750. [DOI] [PubMed] [Google Scholar]
- 27.Lamb, R. A. a. D. K. 1996. Paramyxoviridae: the viruses and their replication, p. 1174-1204. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 28.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432-436. [DOI] [PubMed] [Google Scholar]
- 29.Libraty, D. H., S. Pichyangkul, C. Ajariyakhajorn, T. P. Endy, and F. A. Ennis. 2001. Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J. Virol. 75:3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, Y., A. C. Bright, T. A. Rothermel, and B. He. 2003. Induction of apoptosis by paramyxovirus simian virus 5 lacking a small hydrophobic gene. J. Virol. 77:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez, C. B., A. Garcia-Sastre, B. R. Williams, and T. M. Moran. 2003. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J. Infect. Dis. 187:1126-1136. [DOI] [PubMed] [Google Scholar]
- 32.Luft, T., K. C. Pang, E. Thomas, P. Hertzog, D. N. Hart, J. Trapani, and J. Cebon. 1998. Type I IFN-s enhance the terminal differentiation of dendritic cells. J. Immunol. 161:1947-1953. [PubMed] [Google Scholar]
- 33.Lyles, D. S., M. O. McKenzie, M. Ahmed, and S. C. Woolwine. 1996. Potency of wild-type and temperature-sensitive vesicular stomatitis virus matrix protein in the inhibition of host-directed gene expression. Virology 225:172-180. [DOI] [PubMed] [Google Scholar]
- 34.Matulonis, U., C. Dosiou, G. Freeman, C. Lamont, P. Mauch, L. M. Nadler, and J. D. Griffin. 1996. B7-1 is superior to B7-2 costimulation in the induction and maintenance of T-cell-mediated antileukemia immunity. Further evidence that B7-1 and B7-2 are functionally distinct. J. Immunol. 156:1126-1131. [PubMed] [Google Scholar]
- 35.Montoya, M., G. Schiavoni, F. Mattei, I. Gresser, F. Belardelli, P. Borrow, and D. F. Tough. 2002. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 99:3263-3271. [DOI] [PubMed] [Google Scholar]
- 36.Parks, G. D., K. R. Ward, and J. C. Rassa. 2001. Increased readthrough transcription across the simian virus 5 M-F gene junction leads to growth defects and a global inhibition of viral mRNA synthesis. J. Virol. 75:2213-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raftery, M. J., A. A. Kraus, R. Ulrich, D. H. Kruger, and G. Schonrich. 2002. Hantavirus infection of dendritic cells. J. Virol. 76:10724-10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santini, S. M., C. Lapenta, M. Logozzi, S. Parlato, M. Spada, T. Di Pucchio, and F. Belardelli. 2000. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 191:1777-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz, O., A. D. Edwards, M. Schito, J. Aliberti, S. Manickasingham, A. Sher, and C. Reis e Sousa. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity 13:453-462. [DOI] [PubMed] [Google Scholar]
- 40.van Dijk, A. M., H. G. Otten, S. M. Vercauteren, F. L. Kessler, M. de Boer, L. F. Verdonck, and G. C. de Gast. 1996. Human B7-1 is more efficient than B7-2 in providing costimulation for alloantigen-specific T cells. Eur. J. Immunol. 26:2275-2278. [DOI] [PubMed] [Google Scholar]
- 41.Young, D. F., N. Chatziandreou, B. He, S. Goodbourn, R. A. Lamb, and R. E. Randall. 2001. Single amino acid substitution in the V protein of simian virus 5 differentiates its ability to block interferon signaling in human and murine cells. J. Virol. 75:3363-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]