Abstract
Psychiatric symptoms are common in neurodevelopmental movement disorders, including some types of dystonia. However, research has mainly focused on motor manifestations and underlying circuits. Myoclonus-dystonia is a rare and homogeneous neurodevelopmental condition serving as an illustrative paradigm of childhood-onset dystonias, associated with psychiatric symptoms. Here, we assessed the prevalence of psychiatric disorders and the severity of depressive symptoms in patients with myoclonus-dystonia and healthy volunteers (HV). Using resting-state functional neuroimaging, we compared the effective connectivity within and among non-motor and motor brain networks between patients and HV. We further explored the hierarchical organization of these networks and examined the relationship between their connectivity and the depressive symptoms. Comparing 19 patients to 25 HV, we found a higher prevalence of anxiety disorders and more depressive symptoms in the patient group. Patients exhibited abnormal modulation of the cerebellum on the cerebral cortex in the sensorimotor, dorsal attention, salience, and default mode networks. Moreover, the salience network activity was directed by the cerebellum in patients and was related to depressive symptoms. Altogether, our findings highlight the role of the cerebellar drive on both motor and non-motor cortical areas in this disorder, suggesting cerebellar involvement in the complex phenotype of such neurodevelopmental movement disorders.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73386-9.
Keywords: Myoclonus, Dystonia, Cerebellum, MRI, Network
Subject terms: Anxiety, Depression, Developmental disorders, Obsessive compulsive disorder, Limbic system, Prefrontal cortex, Neural circuits, Neurological disorders, Neurological disorders, Psychiatric disorders, Imaging
Introduction
Psychiatric symptoms are a significant component of various neurodevelopmental movement disorders including dystonias1,2, which is abnormal muscles twitches and postures3. Yet, the underlying pathogenesis of these non-motor manifestations remains poorly understood even though they are among the most important predictors of patients’ quality of life2. Among these conditions, myoclonus-dystonia is a rare disorder typically caused by a pathogenic variant in the SGCE gene4. It is characterized by a childhood-onset dystonia associated with jerky movements related to subcortical myoclonus4. Beside these motor symptoms, psychiatric symptoms related to depression, anxiety, and obsessive-compulsive disorders are likely component of its phenotype4,5. Therefore, myoclonus-dystonia constitutes a paradigm of a homogenous group of patients with a neurodevelopmental condition associating dystonia and psychiatric disorders.
Investigations of the myoclonus-dystonia pathophysiology have been primarily focused on the motor network and suggest a cerebello-thalamo-pallido-cortical network dysfunction4,6. However, the SGCE protein is broadly expressed within the brain7 suggesting that, in addition to the motor networks8, networks related to various non-motor functions may be affected in myoclonus-dystonia. Moreover, within the motor system, the SGCE protein is highly expressed within the cerebellum7, which plays a crucial role in the pathogenesis of motor symptoms in myoclonus-dystonia9–12. Yet, through its connections with non-motor networks, the cerebellum is also involved in cognition and emotion regulation13–15. In the healthy brain, the posterior cerebellum contributes to functional networks associated with these non-motor functions, such as the central executive, dorsal attention, salience/ventral attention, limbic, and the default mode networks. On the other hand, the anterior cerebellum is related to the sensorimotor network15,16.
Functionally, these networks can be categorized into different units: (i) the sensorimotor and the dorsal attention networks form an external unit that connects with the external environment, receiving sensory inputs and generating motor outputs (referred to as “psychomotricity”); (ii) the limbic and the salience networks constitute an internal unit, connecting interoceptive inputs with visceromotor outputs (referred to as “affectivity”); (iii) the default mode network is involved in imagination, memory processes, and spontaneous thoughts (referred to as “internal thought”); (iv) the central executive network is responsible for executive functions and behavioral planning (referred to as “executive thought”)17,18. Dysfunction within these networks have been observed in various psychiatric conditions, including affective and anxiety disorders and psychosis17,19–21. The cerebellum serves as a convergence hub for these networks and influences the excitatory-inhibitory balance within the cortex, which is crucial for optimal processing of the neuronal information15,22. Disruption of the posterior cerebellum can lead to cognitive and emotional impairment, together forming the “cognitive and affective cerebellar syndrome”23,24.
Here, we aimed to examine resting state connectivity of non-motor and motor networks in patients with myoclonus-dystonia and evaluate whether the cerebellum, which is a crucial structure in the motor phenotype of the disease, also play a role in the reorganization of non-motor networks.
We hypothesized that (i) connectivity within and among non-motor networks would be impaired in myoclonus-dystonia, potentially contributing to its non-motor phenotype, and (ii) the cerebellum is involved in the reorganization of non-motor networks in myoclonus-dystonia.
Results
Participants
24 patients with myoclonus-dystonia and 25 healthy volunteers (HV) were initially included in the study. Three patients did not complete the neuroimaging procedure and Framewise displacement (FD) values of two patients were identified as outliers, leading to their removal from the analysis. The remaining 19 patients were included in final analysis. As presented in Table 1, groups were similar for age, sex, laterality, and level of education (all p > 0.05). Patients had more severe or more frequent psychiatric manifestations assessed with the Beck Depression Inventory (BDI) (t42 = 2.69, p = 0.01) and the Mini-International Neuropsychiatric Interview (MINI) (social phobia: p = 0.001; generalized anxiety disorder: p < 0.001). Two volunteers exhibited minimal psychiatric symptoms. To ensure these symptoms did not impact the results, all neuroimaging analyses involving group comparisons were also conducted without these subjects (see supplementary results).
Table 1.
Demographic and clinical data.
| Variable | MD (n = 19) | HV (n = 25) | p (t for t-test) |
|---|---|---|---|
| Agea (range)d | 31.00 ± 2.67 (17–60) | 30.00 ± 2.08 (18–54) | 0.77 (t = 0.3) |
| Years of educationa, d | 13.79 ± 0.39 | 13.12 ± 0.36 | 0.22 (t = 1.24) |
| Handedness (R : L)b, e | 18 : 1 | 20 : 5 | 0.21 |
| Sex (F : M)b, e | 10 : 9 | 15 : 10 | 0.76 |
| BDIa, d | 4.42 ± 0.78 | 1.96 ± 0.53 | 0.01 (t = 2.69) |
| MDDb, e | 1 | 0 | 0.43 |
| Panic disorderb, e | 3 | 0 | 0.07 |
| Agoraphobiab, e | 3 | 1 | 0.30 |
| Social phobiab, e | 7 | 0 | 0.001 |
| GADb, e | 11 | 1 | < 0.001 |
| OCDb, e | 4 | 1 | 0.15 |
| BFMa | 12.11 ± 2.39 | ||
| UMRSa | 35.00 ± 3.97 | ||
| Medicationb, c,d | 9 | 0 | < 0.001 |
aReported as mean ± SEM.
bReported in effective.
cZonisamide (n = 4); Trihexyphenidyl (n = 3); Tetrabenazine (n = 1); Benzodiazepine (n = 4); Propranolol (n = 1); Selective serotonin reuptake inhibitor (n = 2).
dt-test.
eFischer’s exact test.
Abbreviations: MD = Myoclonus-dystonia due to SGCE pathogenic variants; HV = Healthy volunteers; R = righty; L = lefty; F = Female; M = Male; BDI = Beck’s Depression Inventory; GAD = generalized anxiety disorder; OCD = obsessive compulsive disorder; MDD = Major depressive disorder; BFM = Burke–Fahn–Marsden Dystonia Rating Scale; UMRS = unified myoclonus rating scale.
Neuroimaging results
Independent component analysis-based identification of networks of interest
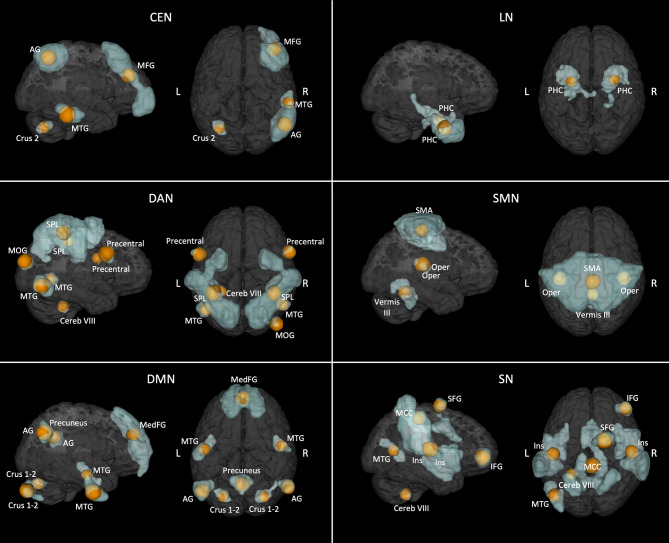
The empirical method we used indicated that a decomposition of the blood-oxygen-level dependent (BOLD) signal by the Independent Component Analysis (ICA) in 22 components gave the best results in term of networks identification (see supplementary results). Six networks of interest (the sensorimotor, dorsal attention, salience, limbic, central executive, and the default mode networks) were identified and parcellated in distinct regions (Fig. 1). The cerebellum was part of all these networks, with exception for the limbic network.
Fig. 1.
Results of the Independent Component analysis. In blue, the networks of interest identified by the Independent Component analysis. In orange, the 8 mm spherical nodes (i.e., those used for the effective connectivity analysis) derived from the parcellation of each network. DAN = Dorsal attention network; SMN = Sensorimotor network; LN = Limbic network; SN = Salience network; DMN = Default mode network; CEN = Central executive network; SMA = Supplementary motor area; Oper = Rolandic operculum; SPL = Superior parietal lobule; MTG = Middle temporal gyrus; MOG = Middle occipital gyrus; IFG = Inferior frontal gyrus; SFG = Superior frontal gyrus; MCC = Middle cingulate cortex; PHC = Parahippoccampal cortex; Cereb = Cerebellum; Ins = Insula; MFG = Middle frontal gyrus; AG = Angular gyrus; MedFG = extended Median frontal region; L = Left; R = Right.
Effective connectivity analysis within networks
We conducted an effective connectivity analysis considering commonalities across patients and HV and group difference (between patients and HV) in different networks of interest (the nodes included in these networks are presented in Fig. 1; see Supplementary Table 2 for the Montreal Neurological Institute – MNI – nodes center coordinates). FD differed between groups (mean ± standard error of the mean – SEM; myoclonus-dystonia = 0.16 ± 0.02, HV = 0.09 ± 0.01; t42 = 2.47, p = 0.02) and was added as covariate of non-interest. Additionally, we examined the effect of FD on each connection (see supplementary results).
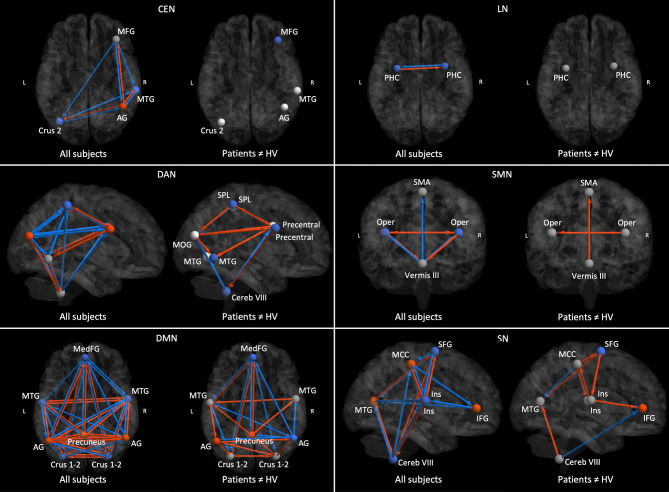
Regarding the commonalities (Fig. 2 and Supplementary Fig. 1), the cerebellum exerted an overall inhibitory effect on the cortical nodes, and received excitatory influences from the cortical nodes, within the central executive, dorsal attention, default mode, sensorimotor and the salience networks. In addition, the cerebellum had an inhibitory intrinsic connectivity (i.e., effect on itself) within the central executive, default mode and the salience networks. Last, we identified several inhibitory and excitatory connections among the cortical areas of each network of interest.
Fig. 2.
Results of the within-network effective connectivity analysis. For each network, in the left image red and blue represent respectively excitatory and inhibitory connections across all participants (i.e. patients and controls), whereas in the right image red and blue represent respectively increased excitation (or decreased inhibition) and increased inhibition (or decreased excitation) in patients compared to healthy volunteers (HV). Only the connections with a posterior probability > 0.99 are displayed. DAN = Dorsal attention network; SMN = Sensorimotor network; LN = Limbic network; SN = Salience network; DMN = Default mode network; CEN = Central executive network; SMA = Supplementary motor area; Oper = Rolandic operculum; SPL = Superior parietal lobule; MTG = Middle temporal gyrus; MOG = Middle occipital gyrus; IFG = Inferior frontal gyrus; SFG = Superior frontal gyrus; MCC = Middle cingulate cortex; PHC = Parahippoccampal cortex; Cereb = Cerebellum; Ins = Insula; MFG = Middle frontal gyrus; AG = Angular gyrus; MedFG = extended Median frontal region; L = Left; R = Right.
Regarding the group difference, we found that connectivity was altered in the central executive, dorsal attention, default mode, sensorimotor and the salience networks and preserved in the limbic network in myoclonus-dystonia. In patients, the connections arising from the cerebellum and directed to the cortex were altered in the default mode, sensorimotor, salience and the dorsal attention networks. Their inhibitory effect was decreased in the default mode and the sensorimotor networks while increased in the dorsal attention network. In the salience network we found a loss of cerebellar inhibition on the left middle temporal gyrus and an increase cerebellar inhibition on the right inferior frontal gyrus. There was no difference between groups in cerebellar connectivity within the central executive network. At the cortical level, we also observed changes in excitatory and inhibitory connections depending on the network, namely intracortical connectivity was severely altered within the dorsal attention, default mode and the salience networks while it was overall preserved within the central executive and the limbic networks.
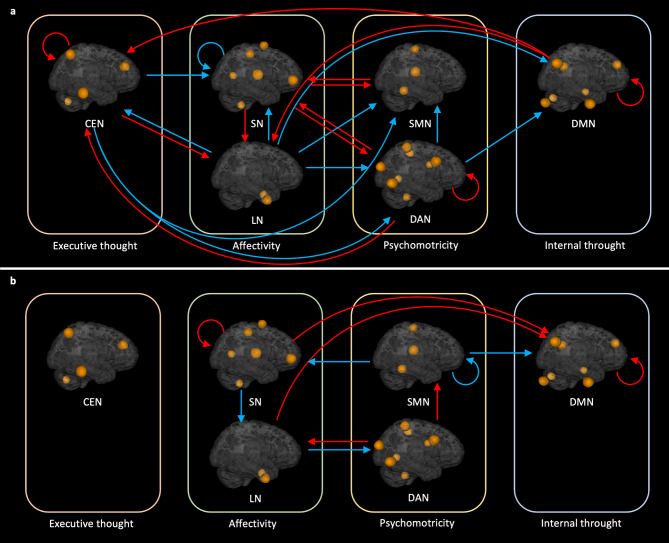
Effective connectivity analysis between networks
Regarding the commonalities (Fig. 3.a and Supplementary Fig. 1), there were several inhibitory influences between the networks, namely (i) central executive on the dorsal attention, (ii) dorsal attention on the default mode and the sensorimotor networks and (iii) the limbic network on the dorsal attention, default mode, central executive, and the sensorimotor networks. We also observed inhibitory self-connectivity within the salience and the dorsal attention networks (Fig. 3.a). Excitatory influences between networks were as follows: (i) the central executive network on the limbic network, (ii) the dorsal attention network on the central executive and the salience network, (iii) the default mode network on the central executive and the limbic networks, (iv) the sensorimotor network on the salience network, and (v) the salience network on the dorsal attention, limbic and the sensorimotor networks. We found excitatory self-connectivity within the central executive, dorsal attention and the default mode networks, while identifying inhibitory self-connectivity within the salience network.
Fig. 3.
Results of the between-network effective connectivity analysis. a Red and blue arrows represent, respectively, excitatory and inhibitory connections between networks across all participants. b Red and blue arrows represent, respectively, increased excitation (or decreased inhibition) and increased inhibition (or decreased excitation) in patients with myoclonus-dystonia. Only the connections with a posterior probability > 0.99 are displayed. DAN = Dorsal attention network ; SMN = Sensorimotor network ; LN = Limbic network ; SN = Salience network ; DMN = Default mode network ; CEN = Central executive network.
Regarding the group differences (Fig. 3.b and Supplementary Fig. 1), patients with myoclonus-dystonia showed (i) a higher disinhibition within the salience network; (ii) increased excitatory influences from the salience network to the default mode network; (iii) decreased inhibitory influences from the limbic network to the default mode network; (iv) increased disinhibition within the default mode network; (v) diminished excitation from the salience network to the limbic network; (vi) increased inhibition from the limbic network to the dorsal attention network. The dorsal attention network exerted a higher excitatory influence on the limbic network and lower inhibitory influences on the sensorimotor network. The sensorimotor network exerted a higher inhibitory effect on the default mode network, a lower excitatory effect on the salience network and, increased self-inhibition.
Results of analysis of the hierarchical networks organization
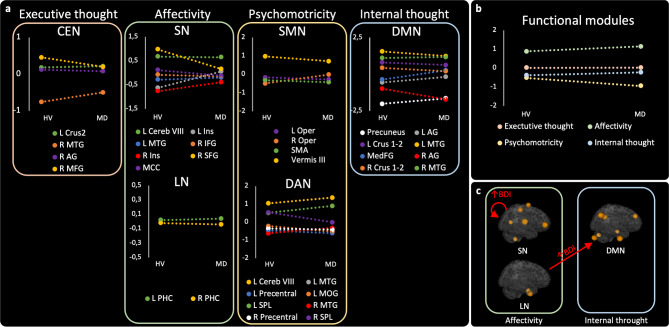
Here, we focused on the brain areas at the top of the hierarchy, i.e. acting as drivers.
In the “within network” analysis (Fig. 4.a), the hierarchy was notably changed in the salience network where the superior frontal gyrus was at the top in HV, whereas it was the cerebellum in patients with myoclonus-dystonia. In the other networks, the top of the hierarchy was held by similar structures in both groups. In addition to the salience network, the cerebellum was at the top of the hierarchy in the sensorimotor and the dorsal attention networks and shared this position with the right middle frontal gyrus in the central executive network in patients. The left middle temporal gyrus and the left parahippocampal gyrus respectively dominated the hierarchy of the default mode and the limbic networks.
Fig. 4.
Hierarchical organization of networks and relationships between change of connectivity and BDI scores in patients. a Comparison of the hierarchical organization within each network between the groups of healthy volunteers (HV) and patients (MD). Nodes at the top act as drivers, while nodes at the bottom serve as sinks. b Comparison of the hierarchical organization of functional units between the groups of healthy volunteers and patients. c, results of the analysis of the DCM PEB model with the Beck depression inventory as covariate of interest in the group of patients. Only connections that differed between groups were considered. DAN = Dorsal attention network; SMN = Sensorimotor network; LN = Limbic network; SN = Salience network; DMN = Default mode network; CEN = Central executive network; SMA = Supplementary motor area; Oper = Rolandic operculum; SPL = Superior parietal lobule; MTG = Middle temporal gyrus; MOG = Middle occipital gyrus; IFG = Inferior frontal gyrus; SFG = Superior frontal gyrus; MCC = Middle cingulate cortex; PHC = Parahippoccampal cortex; Cereb = Cerebellum; Ins = Insula; MFG = Middle frontal gyrus; AG = Angular gyrus; MedFG = extended Median frontal region; L = Left; R = Right.
In the “between networks” analysis (Fig. 4.b), we did not find any differences between groups. The unit related to affectivity (i.e. the salience and the limbic networks) was ranked at the first place.
Results of relationships analysis between clinical and imaging data
We found that among the connections among networks of interest that differed between groups, the BDI score was associated with the connectivity parameters related to the affective unit. Indeed, a higher BDI score was associated with increased abnormal disinhibition of the salience network and decreased inhibitory influences from the limbic network to the default mode network. The connectivity parameters with the largest effect size regarding the BDI score was the abnormal disinhibition of the salience network.
Discussion
Patients with myoclonus-dystonia showed increased non-motor symptoms, including general anxiety, social phobia, and a higher and pathological score on BDI scale. Neuroimaging approaches demonstrated abnormal connectivity within and between both motor and non-motor functional networks. Specifically, impairments were observed in the cerebello-cerebral modulation of the salience, sensorimotor, dorsal attention, and default mode networks. The cerebellum dominated the hierarchy of sensorimotor as well as several non-motor networks in patients. Notably, the cerebellum emerged as a key driver of abnormal functioning within the salience network, and higher levels of disinhibition in this network were associated with higher scores on BDI in patients. Interactions between the limbic, salience, dorsal attention, sensorimotor, and default mode networks also exhibited excitatory-inhibitory imbalances. Within this disorganized system, the affective unit, which includes the salience network, drove the other units activity, exerting abnormal influence over the psychomotor and internal thoughts units. These findings suggest that dysfunction across widespread networks may contribute to the development of non-motor symptoms in myoclonus-dystonia, with the salience network playing a significant role. Furthermore, the results support the notion that the cerebellum is involved in the reorganization of both motor and non-motor networks in this neuropsychiatric developmental disorder.
Our study has several limitations. First, compared to task-based functional MRI, resting-state functional MRI is less specific for assessing the link between brain network and brain function. However, in this study we aimed to clarify whether the cerebellum, which is a key structure in the motor phenotype, is also involved in the reorganization of non-motor networks in myoclonus-dystonia and thus we chose the most appropriate method to address this question.
A second limitation of our study is the lack of specific measurements for the severity of anxiety. However, anxiety has several dimensions leading to various disorders with different connectivity patterns of large-scale neuronal networks25 and thus, larger-scale multicentre studies are warranted to address this question due to rarity of myoclonus-dystonia.
Third, the dimension of the models (i.e. the number of nodes within each network) used in the analysis was heterogeneous, which could influence our findings. This is a results of our data-driven approach, which ensures that our conclusions are supported by empirical evidence rather than assumptions. This approach leads to include the cerebellum in most of the networks, which aligns with the existing literature15,16.
Another limitation is that patients had more frequent head movements during image acquisition compared to the HV, likely due to their motor symptoms. To limit the impact of these involuntary movements on our analysis, we incorporated framewise displacement as a covariate of non-interest in the effective connectivity analysis and ensured that FD had no effect on the main results.
Finally, we acknowledge that the number of participants remains limited. However, it represents one of the largest cohorts for neuroimaging studies in myoclonus dystonia, considering the rarity of this disorder. Additionally, two volunteers exhibited minimal psychiatric symptoms, however they did not affect the results of the study.
As expected, and in accordance with motor phenotype of this disorder, the sensorimotor network was impacted in patients. We also observed that the cerebellum dominated the sensorimotor network hierarchy in both groups (i.e. acted as the main driver) suggesting its major contribution to the sensorimotor network activity. The cerebellum via the cerebello-thalamo-cortical tract exerts an extensive but dual excitatory-inhibitory effect on the motor cortex with a dominant inhibitory component according to our findings (Fig. 2)22. Such balance is involved in the construction of a transient and synchronized activation of cortical neurons, and further shapes a directional cortical flow of information involved in motor timing22. Patients with myoclonus-dystonia showed alteration of this excitatory-inhibitory balance characterized by diminished cerebellar inhibition over the supplementary motor area. According to the sensorimotor network hierarchy, this abnormal cerebellar modulation of the motor cortex may play a major role in the disorganization of the motor system functioning in myoclonus-dystonia and has been suggested to be involved in several dystonic syndromes26–28.
Crucially, our study revealed changes of connectivity in all non-motor networks, particularly from the cerebellum to the cortex in the salience, dorsal attention and default mode networks. Among these networks, the cerebellum acted as a driver in the dorsal attention network in both groups and took on this role in the salience network in patient group. These networks are involved in emotion regulation29 and cognition30. The disrupted cerebellar modulation of cortical regions shared by motor and non-motor networks aligns with the “dysmetria of thought” hypothesis, which suggests that cerebellar dysfunction can result in computational impairments across various domains, including motor, emotional, and cognitive processing31. Interestingly, alongside motor and psychiatric disorders, patients with myoclonus-dystonia may also exhibit a mild impairment of executive functions32,33 supporting the potential alteration of networks associated with these various domains.
Importantly, the hierarchical organization analysis helped us identify regions driving network activity among those involved in connectivity changes. While the networks hierarchy suggests a significant involvement of the cerebellum, cortical regions may also exhibit abnormal activity within these networks. This statement is in line with recent findings showing an hyperexcitable phenotype in cortical neurons with SGCE mutations34 and suggesting an additional cortical vulnerability involved in its pathogenesis. Moreover, we acknowledge that structures other than the one occupying the top position may also play a pathological role.
Beside these modifications in functional connectivity within networks, we also found changes between networks in patients with myoclonus-dystonia. Across all participants, the “affective” and “psychomotricity” units exerted an inhibitory effect on the “internal thought” unit (default mode network), consistent with previous research in healthy participants35. Disruptions in the connectivity patterns of the salience, default mode, and the sensorimotor networks are associated with various psychiatric disorders, including depression17. Depression, characterized by inhibited psychomotor behavior and excessive self-focused thinking, is associated with specific alterations in the amplitude of low-frequency fluctuations at rest in the sensorimotor, default mode, and the salience networks36,37. Similarly, patients with myoclonus-dystonia exhibiting higher scores on BDI had decreased self-inhibition in the salience network, increased disinhibition in the default mode network, and increased self-inhibition in the sensorimotor network. This pattern in patients may arise from an excitatory-inhibitory dysfunction cascade driven by the affective unit, at the top of the hierarchical position (Fig. 4.b). Within this unit, both the salience and the limbic networks enhanced their excitatory effects on the default mode network, resulting in increased disinhibition within the default mode network. This is consistent with evidence supporting the role of the salience network in regulating other networks, such as switching between the default mode and the central executive networks during tasks requiring cognitive control38,39. We propose that the affective unit, and particularly the salience network, plays a critical role in the reorganization of connectivity patterns and the emergence of non-motor manifestations, including depressive mood in patients with myoclonus-dystonia. The association between the BDI score and the burden of the disinhibition of the salience network and the excitatory influence of the limbic network on the default mode network supports this hypothesis, acknowledging that our study design is limited to infer any causal links.
Complex developmental disorders with dystonic and psychiatric symptoms involve widespread dysfunction across numerous brain networks, as suggested by our results. We acknowledge that myoclonus-dystonia may not reflect the pathophysiology of all these syndromes. However, our results suggested that specific regions within networks may act as hubs, supporting their dysfunctions. We demonstrated that the cerebellum, extensively connected with both motor and non-motor cortices, might play a critical role. Indeed, this structure is implicated not only in dystonic syndromes3 but also cognitive and emotional developmental pathologies such as autism40,41, schizophrenia42 or attention-deficit hyperactivity disorder41. Moreover, a current hypothesis supports the idea that the cerebellum may guide the maturation of the neocortex43. Therefore, dysfunction in this developmental process may have a broad impact44 and could lead to varied symptoms depending on the pathology. It is also important to note that the connectivity anomalies related to non-motor symptoms observed here are not necessarily specific to myoclonus-dystonia. Whether they could be shared with other conditions characterized by depression and anxiety was beyond the scope of this study, but it is warranted in future research to examine if the mechanisms identified in the present study are specific to myoclonus-dystonia.
These findings have potential therapeutic implications. Here, our results suggest that interventions modulating the cerebello-cortical pathways, as transcranial magnetic stimulation45, may have effects on both motor and non-motor networks in myoclonus-dystonia.
In summary, using the paradigm of the myoclonus-dystonia syndrome, our study reveals alterations in effective functional connectivity within both motor and non-motor networks and suggests a framework to understand the complex presentation of patients with mixed phenotype of dystonia and psychiatric symptoms. Further investigations are needed to validate a direct causality of such abnormal connectivity.
Materials and methods
Participants
All participants signed informed consent according to the Declaration of Helsinki, and the study was approved by the ethics committee (CPP/AU-1360). Patients’ inclusion criteria were: (i) age between 15 and 60 years-old (ii) clinical diagnosis of myoclonus-dystonia with SGCE mutation, (iii) at least three months OFF of botulinum toxin injection (iv) absence of pharmacological treatment modification for at least one month prior to the study. HV were matched to patients for age, sex, educational level, handedness and had no history of chronic illness or habitual use of medication (except for oral contraception).
Common psychiatric disorders were screened using the MINI46 and depressive symptoms were assessed using the short form of the BDI (BDI-13), which defines depressive disorders for scores above 4 (mild)47. In patients, motor symptoms were assessed using the Burke–Fahn–Marsden Dystonia Rating Scale (BFM)48 and the unified myoclonus rating scale (UMRS)49.
Imaging acquisition
All scans were acquired on the 3T MRI scanner (Siemens Prisma, Germany) with a 64-channel head coil at the Paris Brain Institute.
Resting state functional (rs-fMRI) images were acquired using a multi-echo echo-planar imaging sequence (EPI), with a multi-slice, multi-echo acquisition scheme (TR = 1.9s, TE = 17/36/56ms, iPAT acceleration factor 2, multi-band 2, isotropic voxel size 3 mm) with eyes open and fixating a cross (details are available in supplementary materials). The participants were monitored with an eye tracker system to assure that they were not falling asleep.
3D T1-weighted images were acquired for rs-fMRI preprocessing (parameters are provided in supplementary materials).
Imaging preprocessing
Rs-fMRI data were preprocessed using combined AFNI-TEDANA (see supplementary materials for details), an advanced method to efficiently perform movement corrections50. Despiking, slice timing correction and motion correction (with extraction of the framewise displacement - FD) were achieved using AFNI (v19.3.0). Then, TEDANA (v0.0.0a1) was used to remove both thermal noise and physiological noise. Using SPM12, denoised rs-fMRI images were co-registered to structural images. The normalization to the MNI space was applied to the structural image and then to the rs-fMRI image. Finally, data were smoothed (FWHM = 4 mm) and filtered (0.01–0.1 Hz) using the CONN toolbox51.
Statistical analysis
Overview of the analysis plan
We first identified motor and non-motor networks using a data-driven approach based on an (independent component analysis) ICA. Next, the effective connectivity within and between networks was compared between patients and HV using dynamic causal modeling. Then, we analyzed the hierarchical organization of networks to identify regions driving network activity, comparing groups. Finally, using the example of depressive symptoms, we attempted to determine whether certain connectivity changes between networks were associated with these manifestations in patients.
ICA-based identification of networks of interest
Using an ICA on individual data, we separated patterns of BOLD signal covariation into a set of independent sources. Using the CONN toolbox, we performed a Group-ICA (with group ICA - GICA3 - back-projection model using a FastICA) to extract the networks of interest that best explained the percentage of variance and were commonly identified over all participants (i.e., the sensorimotor, dorsal attention, salience, limbic, central executive, and the default mode networks). We tested the effectiveness of ICA decomposition in 20 to 30 components52,53 on our dataset to fit with the same networks from the Yeo 7-Networks atlas (see supplementary materials)54. The resulting networks were parcellated using the CONN toolbox into distinct regions of contiguous voxels. We considered regions with a minimum size of 150 voxels55,56. For nodes definition for the effective connectivity analysis, we considered spheres57 of 8 mm diameter centered on the global maxima with the highest factor loading within regions of the different networks of interest from the ICA analysis (Fig. 1).
Effective connectivity analysis within and between networks of interest
We investigated the effective connectivity within and between ICA-based networks of interest using dynamic causal modeling (DCM) analysis performed with SPM12. The “within network” analysis aimed to isolate focal nodes with altered connectivity among nodes belonging to the same network. The “between network” analysis aimed to evaluate global impairments at a macro-scale, including alteration of connectivity among motor and non-motor networks.
We considered the first eigenvariate of BOLD signals time series of regions of interest (ROIs). For the “within network” analysis, we extracted the signal of the voxels within each spherical ROI of a given network of interest. For the “between networks” analysis, we extracted the signal of each network of interest mask58 defined as the union of the spherical ROIs belonging a particular network.
We used the Parametric Empirical Bayes (PEB) methodology to determine the direction and strength of excitatory and inhibitory connections following the classical SPM approach (see https://en.wikibooks.org/wiki/SPM/Parametric_Empirical_Bayes_(PEB) for more details)57,59. The same steps were performed for the within network and the between networks analyses.
Statistical analysis of clinical and neuroimaging data
Statistical analysis was performed using SPSS v21 (SPSS Inc. Chicago, IL, USA). Clinical and demographic data were compared between groups using t-tests and Fisher’s exact tests when appropriate.
For neuroimaging data analysis, the PEB employs Bayesian statistics. Consequently, the outcomes do not rely on p-values but are presented in terms of posterior probabilities (pp) and this approach eschews the multiple-comparisons problem60. For each analysis (i.e. the within networks and the between networks analyses), the PEB model run on all participants tested for two aspects: the commonalities across all participants and between-group differences. FD was added as covariate of non-interest. Then, we used a data-driven approach in the form of an automatic search over connection parameters to prune away any parameters from the PEB which did not contribute to the model evidence for each contrast. The parameter values from the 256 models from the final iteration of the search were averaged and weighted by their model evidence (Bayesian model averaging) considering all participants59. We only reported estimated parameters with a high level of evidence (i.e. pp > 0.99) based on free energy59.
Hierarchical network organization
Hierarchical network organization refers to the relative degree of influence that each node exerts over the other nodes belonging to the same network, i.e. identifying the nodes that act as drivers or sinks. Following similar approaches from previous works35,58, we established this hierarchy by considering the balance between the strength of outputs and inputs for each node. For the “within network” analysis, we estimated a PEB model in each group separately (patients, HV) to appreciate network specificity of each group, considering their own priors. The model parameter values were averaged and weighted by their model evidence, this time separately in each group. Then, in each PEB model, we computed the discrepancy between the absolute efferent and afferent connections (with a pp > 0.99) of each node.
We employed a similar methodology to explore the hierarchical organization of functional units for the “between networks” analysis.
Relationships between clinical and imaging data
Given the well-established abnormal interactions among the networks of interest in depression20, we explored the relationships between altered connectivity in the between networks analysis and the depressive symptoms in the group of patients. To do so, we estimated a PEB model in the group of patients with the BDI as covariate of interest (after verifying normal distribution and absence of outliers). To focus our analysis on connectivity parameters that differed between groups in the between networks analysis, only these relevant connections were switch on. The model parameter values were averaged and weighted by their model evidence. Among the connectivity parameters with a high level of evidence (pp > 0.99), we identified the one with the largest effect size, indicating the strongest association with the BDI.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Drs Lucie Guyant-Marechal, Elisabeth Sadot, Philippe Damier, Marion Simonetta-Moreau, Damien Ricard for their help with participants’ inclusions.
Author contributions
Design: YW, ER, CG; execution: All authors; analysis: CT, EM, BB; writing: CT, YW, ER, CG; editing of final version of the manuscript: All authors.
Funding
The study received financial support from the Dystonia Medical Research Foundation (Chicago, USA), Association AMADYS under the Fonds de Dotation Brou de Laurière, and the European Union’s Horizon 2020 research and innovation programme under the EJP RD COFUND-EJP N° 825575 – EurDyscover.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Declarations
Competing interests
C. Galléa, C. Tranchant, B. Beranger, V. Brochard, C. Atkinson-Clement, L. Defebvre, P. Krystkowiak, J-L. Houeto, J.C. Corvol, E. Apartis, D. Martino, (A) Degardin, J-M Pedespan, M. Vidailhet, Y. Worbe have no disclosures in relation to this work.C. Tarrano received a PhD grant from the “Fondation pour la Recherche Médicale”. C. Delorme has received travel funding from Merz Pharma, Abbvie, and received honoraria from Merz Pharma. (B) Degos received honoraria for acting as a member of Scientific Advisory Boards/Consultancy for Merz Pharma, speaker honoraria from Merz Pharma, Abbvie and Ipsen, and travel support for congresses from Merz Pharma. S. Thobois received honorarium from Abbvie and Merz. E. McGovern has received speaking honoraria from AbbVie, Dutch MDS symposium, served on an advisory board for AbbVie and received research grants from the STAR MD and the RCSI Richard Steeven’s Scholarship. D Grabli served on scientific advisory boards for AbbVie, received speech honoraria from Abbvie, Merz and received travel funding from Abbvie and Merz. E.Roze. received honorarium for participating in an advisory board from Merz-Pharma, received research support from Merz-Pharma, Ipsen, AMADYS, Agence Nationale de la Recherche, Societé Française de Médecine Esthétique, Dystonia Medical Reasearch Foundation.
.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Emmanuel Roze and Yulia Worbe.
References
- 1.Ben-Pazi, H., Jaworowski, S. & Shalev, R. S. Cognitive and psychiatric phenotypes of movement disorders in children: a systematic review: review. Dev. Med. Child. Neurol.53, 1077–1084 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Kuyper, D. J., Parra, V., Aerts, S., Okun, M. S. & Kluger, B. M. Nonmotor manifestations of dystonia: a systematic review. Mov. Disord. 26, 1206–1217 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balint, B. et al. Dystonia. Nat. Rev. Dis. Primer. 4, 25 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Roze, E., Lang, A. E. & Vidailhet, M. Myoclonus-dystonia: classification, phenomenology, pathogenesis, and treatment. Curr. Opin. Neurol.31, 484–490 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Peall, K. J. et al. Psychiatric disorders, myoclonus dystonia and SGCE: an international study. Ann. Clin. Transl Neurol. 3, 4–11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menozzi, E. et al. Twenty years on: myoclonus-dystonia and ε-sarcoglycan - neurodevelopment, channel, and signaling dysfunction. Mov. Disord. 34, 1588–1601 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Ritz, K. et al. SGCE isoform characterization and expression in human brain: implications for myoclonus-dystonia pathogenesis? Eur. J. Hum. Genet. 19, 438–444 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beukers, R. J. et al. Disorganized sensorimotor integration in mutation-positive myoclonus-dystonia: a functional magnetic resonance imaging study. Arch. Neurol. 67, 469–474 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Weissbach, A. et al. Alcohol improves cerebellar learning deficit in myoclonus–dystonia: a clinical and electrophysiological investigation. Ann. Neurol. 82, 543–553 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Hubsch, C. et al. Impaired saccadic adaptation in DYT11 dystonia. J. Neurol. Neurosurg. Psychiatry. 82, 1103–1106 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Popa, T. et al. The neurophysiological features of myoclonus-dystonia and differentiation from other dystonias. JAMA Neurol. 71, 612–619 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Meer, J. N. et al. White matter abnormalities in gene-positive myoclonus-dystonia. Mov. Disord. 27, 1666–1672 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Stoodley, C. J., Valera, E. M. & Schmahmann, J. D. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 59, 1560–1570 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmahmann, J. D. The cerebellum and cognition. Neurosci. Lett. 688, 62–75 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Guell, X. & Schmahmann, J. Cerebellar functional anatomy: a didactic Summary based on human fMRI evidence. Cerebellum. 19, 1–5 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Brissenden, J. A., Levin, E. J., Osher, D. E., Halko, M. A. & Somers, D. C. Functional evidence for a cerebellar node of the dorsal attention network. J. Neurosci. 36, 6083–6096 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martino, M. & Magioncalda, P. Tracing the psychopathology of bipolar disorder to the functional architecture of intrinsic brain activity and its neurotransmitter modulation: a three-dimensional model. Mol. Psychiatry. 27, 793–802 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Li, B. J. et al. A brain network model for depression: from symptom understanding to disease intervention. CNS Neurosci. Ther. 24, 1004–1019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greicius, M. D., Srivastava, G., Reiss, A. L. & Menon, V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc. Natl. Acad. Sci.101, 4637–4642 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulders, P. C., Van Eijndhoven, P. F., Schene, A. H., Beckmann, C. F. & Tendolkar, I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci. Biobehav Rev. 56, 330–344 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Imperatori, C. et al. Default mode network alterations in individuals with high-trait-anxiety: an EEG functional connectivity study. J. Affect. Disord. 246, 611–618 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Nashef, A., Cohen, O., Israel, Z., Harel, R. & Prut, Y. Cerebellar shaping of motor cortical firing is correlated with timing of motor actions. Cell. Rep. 23, 1275–1285 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Schmahmann, J. The cerebellar cognitive affective syndrome. Brain. 121, 561–579 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Hoche, F., Guell, X., Vangel, M. G., Sherman, J. C. & Schmahmann, J. D. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain. 141, 248–270 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tozzi, L. et al. Personalized brain circuit scores identify clinically distinct biotypes in depression and anxiety. Nat. Med.30, 2076–2087 (2024). [DOI] [PMC free article] [PubMed]
- 26.Argyelan, M. et al. Cerebellothalamocortical connectivity regulates Penetrance in Dystonia. J. Neurosci. 29, 9740–9747 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fremont, R., Tewari, A., Angueyra, C. & Khodakhah, K. A role for cerebellum in the hereditary dystonia DYT1. eLife. 6, e22775 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sondergaard, R. E. et al. Cerebellar Brain Inhibition Is Associated With the Severity of Cervical Dystonia. J. Clin. Neurophysiol. Publish Ahead of Print, (2021). [DOI] [PubMed]
- 29.Yankouskaya, A. et al. Neural connectivity underlying reward and emotion-related Processing: evidence from a large-Scale Network Analysis. Front. Syst. Neurosci. 16, 833625 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maillet, D., Beaty, R. E., Kucyi, A. & Schacter, D. L. Large-scale network interactions involved in dividing attention between the external environment and internal thoughts to pursue two distinct goals. NeuroImage. 197, 49–59 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmahmann, J. D. An Emerging Concept: the cerebellar contribution to higher function. Arch. Neurol. 48, 1178 (1991). [DOI] [PubMed] [Google Scholar]
- 32.Coenen, M. A., Eggink, H., Spikman, J. M. & Tijssen, M. A. Cognition in children and young adults with myoclonus dystonia – a case control study. Parkinsonism Relat. Disord. 89, 162–166 (2021). [DOI] [PubMed] [Google Scholar]
- 33.van Tricht, M. J. et al. Cognition and psychopathology in myoclonus-dystonia. J. Neurol. Neurosurg. Psychiatry. 83, 814–820 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Sperandeo, A. et al. Cortical neuronal hyperexcitability and synaptic changes in SGCE mutation-positive myoclonus dystonia. Brain10.1093/brain/awac365 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou, Y. et al. The Hierarchical Organization of the default, dorsal attention and salience networks in adolescents and young adults. Cereb. Cortex. 28, 726–737 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, C. H. et al. Abnormal baseline brain activity in bipolar depression: a resting state functional magnetic resonance imaging study. Psychiatry Res. Neuroimaging. 203, 175–179 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Martino, M. et al. Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc. Natl. Acad. Sci. 113, 4824–4829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sridharan, D., Levitin, D. J. & Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci.105, 12569–12574 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goulden, N. et al. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. NeuroImage. 99, 180–190 (2014). [DOI] [PubMed] [Google Scholar]
- 40.D’Mello, A. M. & Stoodley, C. J. Cerebro-cerebellar circuits in autism spectrum disorder. Front. Neurosci.9, 408 (2015). [DOI] [PMC free article] [PubMed]
- 41.Cundari, M., Vestberg, S., Gustafsson, P., Gorcenco, S. & Rasmussen, A. Neurocognitive and cerebellar function in ADHD, autism and spinocerebellar ataxia. Front. Syst. Neurosci.17, 1168666 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andreasen, N. C. & Pierson, R. The role of the Cerebellum in Schizophrenia. Biol. Psychiatry. 64, 81–88 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, S. S. H., Kloth, A. D. & Badura, A. The Cerebellum, sensitive periods, and Autism. Neuron. 83, 518–532 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sathyanesan, A. et al. Emerging connections between cerebellar development, behaviour and complex brain disorders. Nat. Rev. Neurosci.20, 298–313 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pauly, M. G. et al. Cerebellar rTMS and PAS effectively induce cerebellar plasticity. Sci. Rep.11, 3070 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lecrubier, Y. et al. The Mini International Neuropsychiatric interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur. Psychiatry. 12, 224–231 (1997). [Google Scholar]
- 47.Beck, A. T., Rial, W. Y. & Rickels, K. Short form of depression inventory: cross-validation. Psychol. Rep.34, 1184–1186 (1974). [PubMed] [Google Scholar]
- 48.Burke, R. E. et al. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 35, 73–77 (1985). [DOI] [PubMed] [Google Scholar]
- 49.Frucht, S. J., Leurgans, S. E., Hallett, M. & Fahn, S. The Unified Myoclonus Rating Scale. Adv. Neurol.89, 361–376 (2002). [PubMed] [Google Scholar]
- 50.Kundu, P. et al. Multi-echo fMRI: a review of applications in fMRI denoising and analysis of BOLD signals. NeuroImage. 154, 59–80 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Whitfield-Gabrieli, S., Nieto-Castanon, A. & Conn A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect.2, 125–141 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Smith, S. M. et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci.106, 13040–13045 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu, X. & Duyn, J. H. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc. Natl. Acad. Sci.110, 4392–4397 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas Yeo, B. T. et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol.106, 1125–1165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thapaliya, K., Marshall-Gradisnik, S., Staines, D. & Barnden, L. Mapping of pathological change in chronic fatigue syndrome using the ratio of T1- and T2-weighted MRI scans. NeuroImage Clin. 28, 102366 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yadav, S. K. et al. Brain microstructural changes support cognitive deficits in HIV uninfected children born to HIV infected mothers. Brain Behav. Immun. - Health. 2, 100039 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeidman, P. et al. A guide to group effective connectivity analysis, part 1: first level analysis with DCM for fMRI. NeuroImage. 200, 174–190 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frässle, S. et al. Regression dynamic causal modeling for resting-state fMRI. Hum. Brain Mapp. 42, 2159–2180 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeidman, P. et al. A guide to group effective connectivity analysis, part 2: second level analysis with PEB. NeuroImage. 200, 12–25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friston, K. J., Harrison, L. & Penny, W. Dynamic causal modelling. NeuroImage. 19, 1273–1302 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.