Abstract
Hepatitis C virus (HCV) circulates in the bloodstream in different forms, including complexes with immunoglobulins and/or lipoproteins. To address the significance of such associations, we produced or treated HCV pseudoparticles (HCVpp), a valid model of HCV cell entry and its inhibition, with naïve or patient-derived sera. We demonstrate that infection of hepatocarcinoma cells by HCVpp is increased more than 10-fold by human serum factors, of which high-density lipoprotein (HDL) is a major component. Infection enhancement requires scavenger receptor BI, a molecule known to mediate HDL uptake into cells as well as HCVpp entry, and involves conserved amino acid positions in hypervariable region 1 (HVR1) of the E2 glycoprotein. Additionally, we show that the interaction with human serum or HDL, but not with low-density lipoprotein, leads to the protection of HCVpp from neutralizing antibodies, including monoclonal antibodies and antibodies present in patient sera. Finally, the deletion or mutation of HVR1 in HCVpp abolishes infection enhancement and leads to increased sensitivity to neutralizing antibodies/sera compared to that of parental HCVpp. Altogether, these results assign to HVR1 new roles which are complementary in helping HCV to survive within its host. Besides immune escape by mutation, HRV1 can mediate the enhancement of cell entry and the protection of virions from neutralizing antibodies. By preserving a balance between these functions, HVR1 may be essential for the viral persistence of HCV.
Hepatitis C poses a major public health problem, with nearly 3% of the world's population infected and approximately 3 to 4 million new infections occurring each year (22). Hepatitis C virus (HCV) infection has become very prevalent, with about 5 million cases in Europe, 4 million in the United States, and 2 million in Japan. In the United States, HCV infection is the most common chronic blood-borne infection, and HCV-associated chronic liver disease is the principal cause of liver transplantation and the 10th leading cause of death among adults (27). At present, there exists no vaccine against HCV infection, and the only authorized treatments, pegylated alpha interferon and ribavirin, have shown limited effects against HCV, with sustained virological response rates of 54% in general and 42% for genotype 1. Furthermore, the treatments cause significant side effects (28).
HCV is transmitted by blood and progresses slowly, causing no symptoms or only mild symptoms in the acute phase of infection. However, only 20% of infected individuals clear the virus spontaneously, while 80% develop chronic disease which leads to various severe hepatic pathologies (cirrhosis and hepatocarcinoma) in the long term in one out of five cases. Spontaneous clearance of HCV has been associated with strong cellular immune responses (reviewed in reference 48), while detailed analysis of the role of the humoral immune response has become possible only recently with the development of HCV pseudoparticles (HCVpp), a recently described model of HCV cell entry and its inhibition (2-4, 19). Understanding the virus-host interactions that enable acute viral clearance or that favor HCV persistence is the key to the development of more-effective therapeutic and prophylactic strategies. Such studies have been difficult because HCV is genetically highly variable, comprising six principal genotypes and numerous subtypes. Furthermore, the small-animal-model systems which are currently emerging for the analysis of HCV pathology and the cell culture systems that support the propagation of HCV in vitro are still technically demanding and restricted.
In human patients, HCV has been described to exist in heterogeneous forms within serum. By density equilibrium centrifugation, HCV genomes are detected in high-density fractions which are thought to represent virions bound to immunoglobulins. In addition, HCV can be detected in fractions of low density, which contain plasma lipoproteins. Indeed, several lines of evidence suggest that HCV associates with lipoprotein particles of very low, low, and high densities (1, 18, 20, 29, 30, 40, 49). Furthermore, several studies have shown a correlation between acute or persistent liver damage and the detection of lipoprotein-associated, rather than immunoglobulin-associated, HCV (18, 20). Yet, it remains unclear whether association with immunoglobulins neutralizes the virus and/or whether lipoproteins influence and/or enhance HCV infection and pathology. To address the significance of lipoprotein particles in HCV biology, we studied the effects of human serum and lipoprotein particles on the infectivity of HCVpp.
(This study was presented at the 11th International Symposium on Hepatitis C Virus and Related Viruses, Heidelberg, Germany, October 2004).
MATERIALS AND METHODS
Expression constructs and production of HCVpp.
Expression vectors for E1 and E2 glycoproteins of genotypes 1a of strain H77 (GenBank accession no. AF009606) and 1b of strain CG1b (accession no. AF333324) and the hypervariable region 1 (HVR1) deletion mutant (with G384 to N411 deleted; strain H77) have been described previously (3, 4) and were used to construct point mutations within HVR1 (G389L, L399R, G406L, and G406R) and E1 (Y276F) (one-letter amino acid code, numbered according to the sequence of the polyprotein precursor [accession no. AF009606]) by site-directed mutagenesis (details available upon request). The murine leukemia virus (MLV) packaging and green fluorescent protein (GFP) transfer vectors and the phCMV-RD114, phCMV-G, phCMV-HA/NA, phCMV-LCMV, and phCMV-HIV expression plasmids encoding glycoproteins of feline endogenous virus RD114, vesicular stomatitis virus, influenza virus, lymphocytic choriomeningitis virus, and human immunodeficiency virus (HIV), respectively, have been described previously (3, 43; unpublished results). 293T cells were transfected with expression vectors encoding viral glycoproteins, retroviral core proteins, and GFP transfer vector by using a CalPhos mammalian transfection kit (Clontech, France). Twenty-four hours after transfection, the medium was replaced with Dulbecco modified Eagle medium (DMEM)-10% fetal calf serum (FCS) for standard particle production or with DMEM containing specified amounts of sera/lipoproteins. Supernatants were harvested 24 h after the medium change, filtered (0.45-μm pore size), and used to infect Huh-7 (32), PLC/PRF/5 (ATCC CRL-8024), SW-13 (CCL-105), SK-Hep1 (HTB-52), CHO-CD81/SRBI (4), HepG2 (HB-8065), and HepG2-CD81 (4) cells. Two hours prior to infection, target cells were preincubated in DMEM containing 0.1% FCS or no serum at all. Then the medium was removed, and dilutions of viral supernatants were added to the cells and incubated for 3 h. As indicated, additional serum or lipoproteins (high-density lipoprotein [HDL], low-density lipoprotein [LDL], or very-low-density lipoprotein [vLDL], containing 2.17 mg/ml, 9.46 mg/ml, and 4.39 mg/ml of cholesterol, respectively; purchased from Calbiochem) were added to the infection reaction mixtures at the indicated concentrations. The HDL preparation (density, 1.063 to 1.2 mg/ml) contained a mixture of HDL2 and HDL3. After 3 h of incubation, the supernatants were removed and the infected cells were kept in regular medium (DMEM, 10% FCS) for 72 h before analysis of the percentage of GFP-positive cells with a fluorescence-activated cell sorter (3). Infections were controlled by using nonenveloped particles, which resulted in background titers below 102 IU/ml. The downregulation of scavenger receptor BI (SR-BI) in Huh-7 cells was achieved via small interfering RNA (siRNA)-expressing lentiviral vectors, as previously described (25).
Reagents and antibodies.
For preparation of sera, blood was incubated on ice for 2 h and centrifuged at 4,000 rpm for 20 min, and the supernatants were harvested and stored in aliquots at −80°C. The human sera used in this study contained, on average, 3.27 ± 1.48 mg/ml LDL (12 samples) and 1.40 ± 0.38 mg/ml HDL. Normal human serum is reported to contain ca. 1.2 to 2.98 mg/ml HDL (i.e., 0.37 to 0.92 mg/ml of cholesterol HDL) (www.doctissimo.fr/html/dossiers/cholesterol.htm). BLTs were obtained from Chembridge and resuspended in dimethyl sulfoxide. 9/27 and AP33 (34) and E2mAb-1 (C. Granier, B. Bartosch, and F.-L. Cosset, unpublished results) are E2-specific monoclonal antibodies. A pool of HCV immunoglobulins G (IgG) (70 mg/ml) was concentrated and purified from a set of 25 different sera from patients with chronic HCV, of genotypes 1a, 1b, and 3, using DEAE Affi-Gel Blue gel (Bio-Rad) according to the manufacturer's instructions. The origins of the sera from a cohort of acutely infected HCV patients have been described previously (24). Anti-RD polyclonal serum was used as previously described (3). Western blot analysis of pseudoparticles purified on 20% sucrose cushions was performed as previously described (3).
RESULTS
Human serum stimulates infectivity of HCVpp.
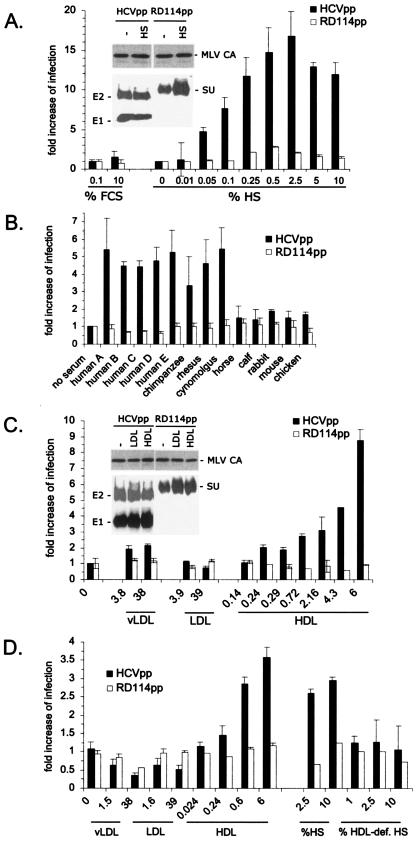
HCVpp have been shown previously to reproduce the cell entry steps of HCV and its neutralization (2-4, 19). We generated HCVpp by transfecting 293T cells with three plasmids encoding the retroviral core proteins, the full-length, unmodified E1 and E2 glycoproteins of HCV of genotype 1a, and a recombinant retroviral genome harboring a GFP marker (3). Infectivity of the HCVpp recovered in the supernatant of transfected 293T cells was determined on Huh-7 hepatocarcinoma cells by measuring the number of GFP-positive cells. Compared to the HCVpp infectivity produced in culture medium devoid of serum, the addition of normal human serum (HS) to the culture medium of transfected 293T cells strongly stimulated infection of Huh-7 cells in a dose-dependent manner, with a maximum increase of 20-fold (Fig. 1A). This increase was not due to increased particle production in the presence of large amounts of human serum, as shown by immunoblotting purified viral particles (Fig. 1A, inset), albeit a maximal twofold increase was seen in some experiments. An enhancement of infection was not observed when FCS, rather than HS, was used to produce HCVpp (Fig. 1A). Infection of control pseudoparticles generated with the RD114 feline endogenous virus glycoprotein (RD114pp) was increased less than threefold (2) (Fig. 1A) and correlated well with a twofold increase in the incorporation of RD114 glycoproteins in the presence of human serum (Fig. 1A, inset). Altogether, these results suggested that a specific component(s) of HS enhances the infectivity of HCVpp. To find out whether this component(s) changed the composition of HCVpp during viral assembly and thus indirectly influenced infection, or whether it acted directly on HCVpp by increasing the infectivity, we tested the effect of HS added in trans to HCVpp produced in serum-free medium. Again, the addition of normal HS, but not FCS, increased infectivity (Fig. 1B), though ca. two- to fourfold less efficiently than production of HCVpp in similar amounts of HS. Enhancement of infection was also detected for the other HCV genotypes/subtypes (genotypes 1 to 6; data not shown) as well as with all types of normal HS we tested (over 12 types of HS; Fig. 1B and data not shown). Facilitation of infection was specifically mediated by primate sera, since incubation of HCVpp with normal sera from other vertebrate species resulted in significantly reduced levels of enhancement (Fig. 1B). Enhancement of infection for other viruses has been described previously to be mediated by antibodies and/or by complement (14, 16, 47). However, IgG-depleted normal HS stimulated infection of HCVpp as efficiently as untreated HS (data not shown), and incubation of HCVpp with immunoglobulins purified from HCV-negative HS was not found to facilitate infection (data not shown). Additionally, serum decomplementation by heat treatment did not abrogate the facilitation of HCVpp infectivity (data not shown). Finally, only HCVpp infectivity, not that of pseudoparticles generated with the glycoproteins from alternative enveloped viruses (including HIV, MLV, lymphocytic choriomeningitis virus, vesicular stomatitis virus, and influenza virus), was stimulated by normal HS (data not shown). These data excluded a role of antibodies or complement in the facilitation of HCVpp infectivity and showed this effect to be highly specific for HCV.
FIG. 1.
Enhancement of HCVpp infectivity by HS. Infection assays were performed on Huh-7 cells using HCVpp of genotype 1a and RD114pp as the controls. The infectious titers of HCVpp and RD114pp produced in 0.1% FCS were ca. 5 × 104 and 1 × 107 IU/ml, respectively. To obtain viral supernatants containing similar amounts of infectious particles, we therefore diluted all preparations of RD114pp 250-fold. Results show the increases in infection determined by calculating the ratios between the average infectious titers determined in the presence or absence of serum, as indicated. (A) Virions were produced in cell culture media containing the indicated quantities of normal HS or FCS. Results are expressed as ratios between the average infectious titers determined in the presence or absence of serum (mean ± SD, 5). The inset shows a Western blot of purified viral particles that were produced in the absence (−) or presence of 1% HS. The glycoproteins of HCVpp and RD114 were revealed using the A4 and H52 monoclonal antibodies against E1 and E2 (9, 13) and an anti-SU antiserum (ViroMed Biosafety Laboratories), respectively. The MLV capsid (MLV CA) proteins of either pseudoparticle were detected with an anticapsid antiserum (ViroMed Biosafety Laboratories). (B) Virions produced in low-serum medium (0.1% FCS) to which sera from the indicated species were added to 1% were used for infections. The results are expressed as ratios between the average infectious titers determined in the presence of the indicated sera and the titers determined in 0.1% FCS (mean ± SD, 4). (C) Virions were produced in cell culture media containing 0.1% FCS and defined quantities of purified vLDL, LDL, and HDL, as indicated in μg/ml of cholesterol lipoprotein. The results are expressed as ratios between the average infectious titers obtained in the presence or absence of the indicated lipoproteins (mean ± SD, 4). The inset shows a Western blot of purified viral particles produced in low-serum medium supplemented with 6 μg/ml LDL and HDL or not supplemented (−), as indicated. (D) Virions produced in low-serum medium (0.1% FCS) were incubated with defined quantities of vLDL, LDL, and HDL, as indicated in μg/ml, or with defined quantities of an HDL-deficient serum from a patient with Tangier disease (HDL-def. HS) and are compared to normal HS. The results are expressed as ratios between the average infectious titers in the presence of lipoproteins or sera and the titers determined in the absence of lipoproteins or sera.
HDL and SR-BI are infection-facilitating components.
Since HCV isolated from the plasma of HCV-infected patients is often associated with lipoproteins (1, 18, 20, 29, 30, 40, 49), we asked whether lipoprotein interactions could enhance infection of HCVpp. As shown in Fig. 1C, HCVpp produced in the presence of purified HDL had an infectivity that was strongly increased compared to that of control RD114pp. Average concentrations of HDL in normal human serum are in the range of 0.37 to 0.92 mg/ml of cholesterol HDL, and maximal infection enhancement by HS is observed at an HS concentration of 0.5 to 2.5% (Fig. 1A), which amounts to 1.85 to 23 μg/ml of cholesterol HDL. This number corresponds well to the amount of purified HDL (4 to 9 μg/ml cholesterol HDL) required for maximal infection enhancement (Fig. 1C). Furthermore, the infectivity of HCVpp produced in optimal concentrations of HDL could not be further enhanced by the addition of HS and vice versa (data not shown), suggesting that HDL is the predominant enhancing factor in HS. Consistently, no or less-than-twofold enhancement of infection was detected when the HCVpp were produced with LDL or vLDL, respectively (Fig. 1C).
To exclude possible indirect effects of lipoproteins on HCVpp infectivity via modification of the membrane lipid composition during viral production, all further experiments were performed in trans by adding HS or lipoproteins to HCVpp produced in low-serum medium. Consistent with previous experiments performed in cis, infection of HCVpp was significantly increased when in trans-purified HDL, but not LDL or vLDL, was added to HCVpp just before infection of Huh-7 cells (Fig. 1D). Also, an HDL-deficient HS from a patient with Tangier disease did not stimulate infection in trans (Fig. 1D), further demonstrating that HDL is an essential infection-enhancing factor in normal HS. Interestingly, the pretreatment of HS with several polyclonal antibodies targeted against Apo-A1, -A2, -C1, -C2, and -C3, which are expressed on HDL, did not abrogate infection enhancement (data not shown). While these results do not exclude the possibility that the active HDL component is a protein, it is possible that lipids or protein/lipid complexes are involved. Finally, the enhancement of infection by normal HS or by HDL was restricted to pseudoparticles harboring glycoproteins derived from HCV and did not include those from alternative enveloped viruses (data not shown), suggesting a specific interplay of the HCV glycoproteins with HDL and their cell surface receptors.
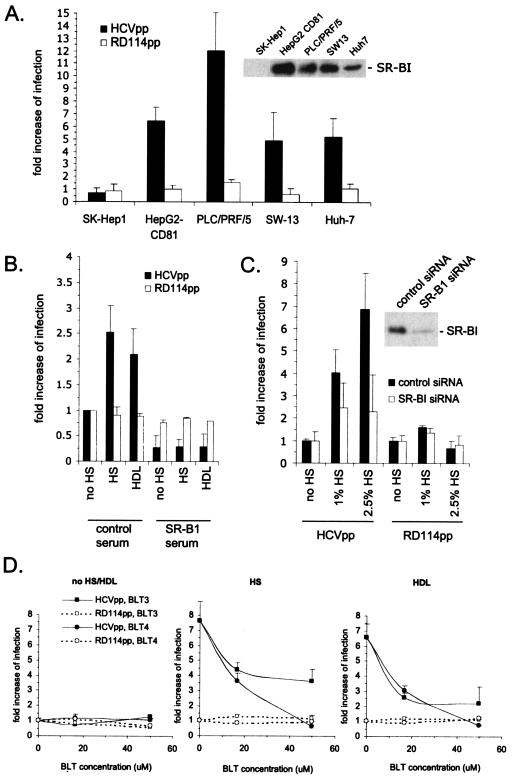
HDL is a ligand of the scavenger receptor SR-BI, a molecule required for HCV entry (4, 44), and its high-affinity binding to SR-BI mediates the selective lipid uptake of cholesteryl esters from lipid-rich HDL to cells (21). We therefore asked whether HDL-mediated facilitation of HCVpp requires SR-BI. While infection enhancement of HCVpp incubated with HS was detected in Huh-7 cells as well as in other SR-BI-expressing cells, such as PLC/PRF/5 hepatocarcinoma cells, or in SW-13 adrenocortical cells, no facilitation of infection could be detected in SK-Hep1 hepatocarcinoma target cells that express undetectable SR-BI levels (Fig. 2A). Additionally, no infection enhancement could be detected when SR-BI was blocked using a polyclonal SR-BI-blocking antibody. While this antibody reduced infection of HCVpp in the absence of HS, as previously reported (4), it completely inhibited the stimulation of infection mediated by both HS and HDL (Fig. 2B). Likewise, the downregulation of SR-BI with lentiviral vectors carrying an siRNA targeted against SR-BI (25) significantly reduced infection enhancement by HS (Fig. 2C). Altogether, these results suggested that enhancement of infection involves an interplay between HCVpp and a serum component(s) such as HDL or SR-BI. Interestingly, infection enhancement by HS was detected in HepG2 hepatoma cells transfected with CD81, another HCV coreceptor (36). However, parental HepG2, as well as CHO cells expressing both CD81 and SR-BI, remained nonpermissive to HCVpp in the presence of HS or HDL (data not shown). Thus, HDL-mediated enhancement of HCVpp cell entry requires SR-BI, CD81, and an as-yet-unidentified coreceptor(s).
FIG. 2.
Role of SR-BI in facilitation of infection. (A) Results of infection assays with Huh-7, PLC/PRF/5, HepG2-CD81, SW-13, and SK-Hep1 cells that express or do not express SR-BI receptors. HCVpp and RD114pp (1/250 dilution) were produced in cell culture medium containing 0.1% FCS. The infectious titers of HCVpp for these cells in the absence of human serum were as follows: for SK-Hep1, 1.2 × 103 IU/ml; HepG2-CD81, 1.3 × 104 IU/ml; PLC/PRF/5, 5.7 × 104 IU/ml; SW-13, 103 IU/ml; and Huh-7, 2.4 × 104 IU/ml. Infection assays were performed in the absence or presence of 1% normal HS, which was added during infection. The results are expressed as ratios between the average infectious titers determined in the presence or absence of serum (mean ± SD, 3). The inset shows the expression levels of SR-BI from immunoblotting equal amounts of cell lysates with an SR-BI rabbit antiserum (ab396; Abcam; 1/1,500), as described previously (25). Note that we could detect very small amountsof SR-BI in SK-Hep1 cells on overexposed autoradiographs. (B) HCVpp or RD114pp (1/250 dilution) were produced in cell culture medium containing 0.1% FCS and used in infection assays with Huh-7 cells in the absence of HS (no HS) or in the presence of 1% HS or 6 μg/ml HDL. The same set of infections was performed in the presence of a 1/50-diluted polyclonal anti-SR-BI mouse serum (4). Results are shown as ratios between the average infectious titers determined in the presence or absence of human serum or HDL (mean ± SD, 2). (C) Huh-7 cells expressing a control or anti-SR-BI siRNA (25) were used as target cells for HCVpp or RD114pp (1/250 dilution) produced in 0.1% FCS in the absence (no HS) or presence of HS, as indicated. Downregulation of SR-BI reduced the HCVpp titer fivefold from that of control siRNA-treated Huh-7 cells, as reported previously (25). The inset shows the expression levels of SR-BI from immunoblotting of all lysates, as described previously (25). Results are shown as ratios between the average infectious titers determined in the presence or absence of serum. (D) Huh-7 target cells were treated with 16.7 μM or 50 μM of BLT compounds before and during infection with HCVpp or RD114pp (1/250 dilution) that were produced in 0.1% FCS. As indicated, no HS/HDL, normal HS (2.5%), or HDL (6 μg/ml) was added to the infection reaction mixture. Results are shown as ratios between the average infectious titers determined in the presence or absence of HS or HDL (mean ± SD, 3).
To investigate which stages of cell entry HS or HDL enhances, we performed experiments where HS/HDL and virus were added to target cells in various orders. Exposure of HCVpp to HS prior to infection did not increase infection enhancement compared to the concomitant addition of HS and HCVpp to Huh-7 cells (Table 1), nor did preincubation of target cells with HS for various amounts of time have an effect on the level of HCVpp infection enhancement. However, if HS was removed from target cells by washing them before HCVpp addition, no infection enhancement was observed (Table 1). When HCVpp prebound to Huh-7 target cells at 4°C were subsequently exposed to HS, infectivity was enhanced (Table 1). Similar results were obtained with HDL, while LDL showed no enhancing effect under any of the conditions described (data not shown). This indicated that serum factor or HDL either interacts with the virus particles or needs to be present at the same time as HCVpp to exert a stimulatory effect. This stimulatory effect may occur after virus attachment to cells, as prebound HCVpp remain sensitive to HS/HDL. Consistently, we were not able to detect an increased binding of HCVpp to SR-BI-positive target cells in the presence of HS or HDL by fluorescence-activated cell sorter analysis (23) (data not shown), confirming that the enhancement of infection may occur at a postbinding stage.
TABLE 1.
Enhancement of HCVpp infection by human serum
| Sequential treatment of Huh-7 target cells
|
Increase in infectiona (fold) | ||
|---|---|---|---|
| Step A | Step B | Step C | |
| Preincubation of HS with HCVpp for 1 minb | NAe | NA | 4.1 ± 0.02 |
| Preincubation of HS with HCVpp for 60 minb | NA | NA | 4.2 ± 0.05 |
| Preincubation of cells with HS for 1 minc | No wash | HCVpp | 4.6 ± 0.04 |
| Preincubation of cells with HS for 60 minc | No wash | HCVpp | 5.2 ± 0.02 |
| Preincubation of cells with HS for 1 minc | Wash | HCVpp | 0.81 ± 0.04 |
| Preincubation of cells with HS for 60 minc | Wash | HCVpp | 0.7 ± 0.02 |
| Prebinding of HCVpp to target cellsd | No wash | HS | 3.8 ± 0.05 |
| Prebinding of HCVpp to target cellsd | Wash | HS | 3.5 ± 0.02 |
Results of infections are expressed as ratios (±standard deviations) of the average infectious titers (n = 3) determined in the presence and absence of HS (2.5%).
Virus and HS had been preincubated for the indicated times at room temperature, before addition of the mixtures to Huh-7 cells.
Huh-7 target cells were preincubated with HS for the indicated times at room temperature and then directly infected with HCVpp or washed twice to remove unbound HS before infection with HCVpp.
HCVpp were prebound to Huh-7 cells for 1 h at 4°C. Then, HS was directly added to the infection, or unbound virus was removed by washing the Huh-7 cells twice before the addition of HS. Removing unbound virus from the infection reaction mixture resulted in a 15-fold reduction in the titer.
NA, not applicable.
To further examine the role of SR-BI in HDL-mediated infection enhancement of HCVpp, we preincubated target cells with BLT-3 and -4, small-molecule inhibitors that block SR-BI-mediated selective cholesteryl ester uptake from HDL (33) prior to infection. While the BLT-3 and -4 compounds did not significantly reduce the infectivity of HCVpp in the absence of HS or HDL, they abrogated the enhancement of infection by HS or HDL in a dose-dependent manner (Fig. 2D). Infectivity of control RD114pp was not affected by the BLT compounds (Fig. 2D). In conclusion, inhibition of the cholesteryl ester uptake function of SR-BI by two structurally unrelated inhibitors concomitantly blocks HS- or HDL-mediated infection enhancement of HCVpp, suggesting that these two processes may be linked.
HVR1 is a viral component involved in HDL-mediated enhancement of infection.
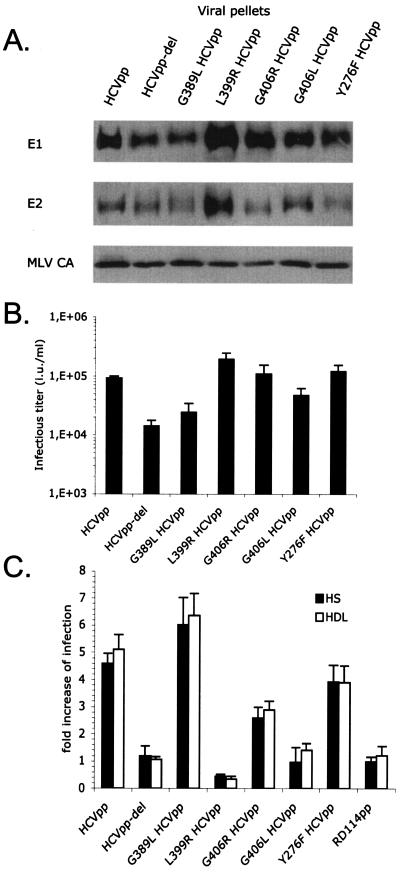
HVR1, the 27 N-terminal amino acids of the E2 HCV glycoprotein, has been implicated previously in mediating interactions of HCVpp with SR-BI during cell entry (4, 44). The removal of HVR1 still allows efficient incorporation of E1 and E2 glycoproteins (Fig. 3A) but results in ca. 10- fold-reduced cell entry (4). Interestingly, we found that the infectivity of HCVpp harboring HVR1-deleted glycoproteins was not stimulated by HS or HDL (Fig. 3C), indicating that HVR1 is required for enhancement of infectivity, perhaps by interacting with the facilitating serum component(s) or HDL. We confirmed this result with HVR1 deletion mutants generated from HCVpp harboring E1 and E2 glycoproteins from alternative HCV genotypes (data not shown).
FIG. 3.
Role of HVR1 in facilitation of infection. (A) Immunoblots of purified HCVpp generated with E1 and E2 wild-type or mutant glycoproteins (genotype 1a) are shown. E2 point mutations G389L, L399R, G406R, and G406L are located in the HVR1 region. Y276F HCVpp, an E1 point mutant, served as the control. The HCV glycoproteins and the MLV capsid (MLV CA) proteins were revealed with A4 and H52 monoclonal antibodies against E1 and E2 (9, 13) or with an anti-MLV capsid antiserum (ViroMed Biosafety Laboratories). (B) Titers of HCVpp harboring point mutations. Results are expressed as average infectious titers determined on Huh-7 cells in the absence of serum or lipoproteins (mean ± SD, 3). (C) Results of infection of Huh-7 cells with HCVpp or RD114pp produced in 0.1% FCS, with the addition of 2.5% normal HS or 6 μg/ml HDL to the infection reaction mixture. The concentrations of viral supernatants were adjusted to obtain infection of ca. 5 to 10% of target cells. The results are expressed as ratios between average infectious titers determined in the presence or absence of serum or lipoproteins (mean ± SD, 3).
To further analyze the role of HVR1 in infection, we introduced changes in conserved amino acid positions of HVR1 (G389L, L399R, G406L, and G406R) that are thought to be essential for its conformation and perhaps for cell interaction (35). All mutants incorporated similar ratios of E1 and E2 glycoproteins (Fig. 3A). The virions exhibited infectious titers between 2.4 × 104 and 1.4 × 105 IU/ml (Fig. 3B), consistent with the <2- to 3-fold differences in envelope incorporation levels. To take into account these slight variations in virion production levels, the inputs of wild-type and mutant HCVpp were adjusted to investigate the effects of HS and HDL on the infectivity of the pseudoparticles. Interestingly, some mutations, i.e., L399R and G406L, abrogated the enhancement of HCVpp infection mediated by HS and HDL (Fig. 3C). The G406R mutation resulted in an intermediate phenotype. In contrast, the G389L mutation increased the infection enhancement in the presence of HS or HDL, underlining the importance of HVR1 conformation in virus entry. Altogether, these results confirmed the important role played by HVR1 in cell entry and its enhancement by HS or HDL and pointed out key amino acid residues mediating these functions.
HVR1 suppresses a neutralizing immune response in HCV-infected patients.
HVR1 is an important target of neutralizing antibodies in vivo, and its variability is thought to allow HCV to persist in vivo (10). Since HVR1 also appeared to be the target of the enhancing serum factor (Fig. 3), we asked to what extent its interplay with HS or HDL could modulate HCVpp inhibition by neutralizing antibodies.
First, we investigated the effects of facilitating serum components on the neutralization of HCVpp in the sera of a cohort of acute-phase patients infected with a single HCV virus of subtype 1b during a nosocomial outbreak in a hemodialysis center (24). One group of patients (7/13 patients) developed neutralizing antibodies of seemingly narrow specificity and showed a >3- to 4-log decrease in HCV RNA titer (24) (Table 2 and Fig. 4A, panels Pt-3 and Pt-4). Indeed, as previously reported (24), the emergence of neutralizing antibodies in these patients could be readily detected using HCVpp displaying the autologous E1 and E2 glycoproteins derived from these patients or displaying highly homologous E1 and E2 sequences of a 1b subtype (strains CG1b and BK). No or poor neutralization was detected when HCVpp of more-divergent strains, including strain 1a or strains of different subtypes/genotypes, were used. Strikingly, upon the deletion of HVR1, we found that HCVpp of genotypes 1b and 1a were both efficiently cross-neutralized (Fig. 4A and Table 2). Conversely, a second group of acute-phase patients (6/13 patients) was characterized by sustained high viral loads (<1-log decrease in HCV RNA titers) and did not develop a neutralizing response detectable with HCVpp harboring autologous E1 and E2 glycoproteins (24) (Table 2 and Fig. 4A, panels Pt-8 and Pt-9). Again, we found that sera from patients 8 and 9 could efficiently cross-neutralize HVR1-deleted HCVpp of both subtypes 1a and 1b (Fig. 4A and Table 2). Similar results were obtained from the analysis of sera from chronic-phase patients. Indeed, the neutralization titers of such sera were at least 10-fold lower when using unmodified HCVpp compared to those with HVR1-deleted HCVpp in infection assays (data not shown). Altogether, these results suggested that the neutralizing activities of antibodies from sera of HCV-infected patients are inhibited or not detectable in the presence of HVR1 on HCVpp.
TABLE 2.
Neutralizing responses in sera from acute-phase HCV-infected patients
| Patienta | Neutralizing responseb in sera from patients infected with:
|
|||
|---|---|---|---|---|
| HCVpp-1b | HCVpp-del1b | HCVpp-1a | HCVpp-del1a | |
| 1 | ++ | +++ | − | ++ |
| 2 | + | ++ | − | +++ |
| 3 | ++ | +++ | +/− | +++ |
| 4 | + | +++ | − | + |
| 5 | + | +++ | − | +++ |
| 6 | ++ | +++ | + | +++ |
| 7 | + | +++ | − | ++ |
| 8 | − | + | − | ++ |
| 9 | − | + | − | + |
| 10 | − | + | − | + |
| 11 | − | + | − | + |
| 12 | − | + | − | + |
| 13 | +/− | + | − | ++ |
The cohort consisted of hemodialysis patients (mean age, 63.7 years; range, 37 to 77 years) with HCV RNA-positive acute HCV infection who had all been infected by a single HCV source, genotype 1b, during a nosocomial outbreak that occurred in a hemodialysis ward in mid-2002, as described in reference 26. They were all followed for 6 months without treatment before a therapeutic decision was made. Acute HCV infection was confirmed in patients 1 to 13 by characterizing seroconversion profiles with a line immunoassay (INNO-LIA HCV IV; Innogenetics, Ghent, Belgium). One group of patients (patients 1 to 7) was characterized by a strong decrease in HCV replication over time, correlated with the emergence of neutralizing responses, whereas a second group (patients 8 to 13) did not clear or control the virus (26).
The neutralizing responses were analyzed by incubating patient sera with viral particles harboring the E1 and E2 glycoproteins of genotype 1b (HCVpp-1b), genotype 1a (HCVpp-1a), and the HVR1-deleted versions of these glycoproteins (HCVpp-del1b and HCVpp-del1a, respectively). The results display the maximal neutralizing activity detected during the 6-month followup and are expressed as the mean percentages of inhibition of the average infectious titers relative to that from incubation with medium devoid of HS. −, no detectable neutralization; +/−, <25% neutralization; +, 25 to 80% neutralization; ++, 80 to 90% neutralization; +++, >90% neutralization. The results were derived from at least three independent experiments, and the standard deviations (Fig. 4A) did not exceed 15% of the mean values.
FIG. 4.
Human serum or HDL protects HCVpp from neutralization. (A) Effects of sera from a cohort of acutely infected HCV patients (24) on infectivity of the indicated HCVpp. Data were obtained over a time interval of 16 weeks and are shown for four patients who are representative of the cohort. The HCV RNA kinetics for each patient were measured weekly after inclusion in the cohort (HCV RNAs [given in IU/ml], analyzed by means of a third-generation, branched-DNA-based assay [Versant HCV RNA 3.0 assay; Bayer Diagnostics, Tarrytown, N.J.]). The 13-patient cohort consisted of two groups. The first group (7/13 patients), represented by patients 3 and 4 (Pt-3 and Pt-4), showed a >3- to 4-log reduction in HCV RNA titers in the second half of the study period (24). Patients 8 and 9 (Pt-8 and Pt-9) represent the second group (6/13 patients), in whom replication levels remained high (<1-log decrease in HCV RNA titers) throughout the entire study period (24). Serum samples chosen from the beginning, middle, and end of the study period from these four patients were investigated in neutralization assays at 1/50 dilutions with HCVpp (104 IU) of genotype 1b, strain CG1b (HCVpp-1b); HCVpp of genotype 1a, strain H77 (HCVpp-1a); or their HVR1-deleted counterparts (HCVpp-del1b and HCVpp-del1a, respectively) by incubation for 30 min at room temperature before infection of Huh-7 target cells. The results are expressed as mean percentages (±standard deviations [SD]; n = 3) of inhibition of the infectious titers relative to inhibition from incubation with medium devoid of patient sera. Note that no or poor neutralization was detected in Pt-3 and Pt-4 (group 1) with HCVpp-1a, and no neutralization at all was detected in Pt-8 and Pt-9 (group 2) with both HCVpp-1a and HCVpp-1b (Table 2). The specificity of neutralization was controlled with RD114pp, against which no antibodies were detected in HS (3). Nonspecific inhibition of RD114pp (data not shown) over a value of ±20% was never detected. (B) Titration of neutralizing antibodies in total IgG purified from chronically infected HCV patients. Neutralization assays were performed in the absence of HS (no HS) or in the presence of 2.5% HS, 39 μg/ml LDL, or 6 μg/ml HDL. The results are expressed as mean percentages (±SD; n = 4) of inhibition of the infectious titers relative to inhibition from incubation with medium devoid of antibodies. (C) Neutralization curves of the AP33 monoclonal HCV E2 antibody in the absence (no HS) or presence of 2.5% HS using HCVpp harboring the indicated point mutations in HVR1. The results are expressed as the mean percentages (±SD; n = 3) of inhibition of the infectious titers relative to incubation with medium devoid of antibodies. ID50 values are indicated by dotted lines.
The presence of HVR1 and HDL protects HCV from neutralizing antibodies.
To investigate this issue further, we performed neutralization assays of HCVpp with purified antibodies in the presence or absence of HS or lipoproteins. In the absence of HS or in the presence of LDL, the infectivity of HCVpp could be neutralized by over 95% by antibodies purified from patients chronically infected with HCV (Fig. 4B and Table 3) or by some E2 monoclonal antibodies (Table 3). In contrast, in the presence of HS or HDL, the neutralization of HCVpp was much less efficient. Indeed, the polyclonal and some monoclonal HCV antibodies, even at high concentrations, could not neutralize the HCVpp by more than 80% and 60%, respectively (Fig. 4B and Table 3). Interestingly, the 9/27 monoclonal antibody, raised against HVR1, formed an exception since no difference of neutralization titers could be detected in the absence of HS or in the presence of HS or HDL (Table 3).
TABLE 3.
Modulation of HCVpp antibody-mediated neutralization by HS or HDL
| Antibody | Neutralization in presence ofa: | Antibody concn (μg/ml) atd:
|
|||
|---|---|---|---|---|---|
| ID60 | ID80 | ID90 | ID95 | ||
| E2mAb-1b | No serum | 0.4 | 4 | 10 | 13.89 |
| HDL | 8.4 | — | — | — | |
| HS | 6.2 | — | — | — | |
| 9/27b | No serum | 1.95 | 2.62 | 3.06 | 3.38 |
| HDL | 1.83 | 2.85 | 3.98 | 7 | |
| HS | 1.54 | 2.33 | 3.24 | 4.48 | |
| AP33b | No serum | 4.53 | 13.83 | 19.02 | 21.86 |
| HDL | 18.86 | — | — | — | |
| HS | 21.46 | — | — | — | |
| HCV Igb | No serum | 14 | 70 | 350 | 700 |
| LDL | 11 | 70 | 400 | 700 | |
| HDL | 70 | 350 | — | — | |
| HS | 90 | 450 | — | — | |
| Anti-RD114c | No serum | 0.431 | 0.559 | 2.33 | 4 |
| HS | 0.459 | 0.559 | 2.33 | 5 | |
HS (2.5%), HDL (6 μg/ml), or LDL (39 μg/ml) was added to target cells concomitantly with the indicated antibodies, before infection with HCVpp or RD114pp.
Neutralization of HCVpp was achieved with the indicated antibodies. The antibodies recognize a conformational epitope (E2mAb-1), the HVR1 (9/27), and the amino acid 412 to 423 region (AP33) in E2.
Neutralization of RD114pp was achieved with a polyclonal antibody against RD114 glycoproteins.
Concentration of antibodies in infection medium required to neutralize the HCVpp or RD114pp at the indicated ID values. —, not applicable, as neutralization could not reach the indicated ID value at saturating antibody concentrations.
By performing serial dilutions of neutralizing antibodies, we calculated that, depending on the antibody, 4- to 20-fold more antibody was required to neutralize HCVpp in the presence of HS or HDL than was required to neutralize HCVpp in the presence of LDL or in the absence of HS (Fig. 4B and Table 3). This was most likely an underestimation, since for most antibodies, these ratios could be calculated only for the 60% infective dose (ID60) (and some ID80) values (Table 3). These findings were specific to HCVpp, as RD114pp were neutralized with the same efficiency by an anti-RD114 polyclonal antibody in the absence or in the presence of HS (Table 3). These results suggested that the interaction of HVR1 with a component(s) of normal HS provides a mechanism to protect HCV from neutralization by antibodies targeted outside the HVR1. These findings are consistent with the increased capacity of acute-phase patient sera to neutralize HVR1-deleted HCVpp (Fig. 4A). Furthermore, the capacity of HVR1 to mediate both infection enhancement and protection from neutralization was confirmed using HCVpp harboring mutated HVR1 sequences. Indeed, the mutations that inactivated HVR1-mediated infection enhancement (e.g., L399R and G406L [Fig. 3C]) also abolished the protection by HS from inhibition by neutralizing antibodies (Fig. 4C).
DISCUSSION
Enhancement of infection in vivo has been reported previously for several viruses: virus-specific antibodies can enhance viral infectivity, both in vitro and in vivo, through the binding of virus-antibody complexes to cellular Fc receptors (expressed in, e.g., monocytes/macrophages) via the Fc portion of the antibodies (38). Fixation of the C3 or C1q complement proteins, activated by virus-antibody complexes, can also facilitate virus entry, as has been shown for HIV (14), dengue virus (17), and Ebola virus (47). Finally, antibody-independent enhancement of HIV infection in vitro, via a mechanism that involves receptors of the classical and alternative complement pathways, has also been reported (16). However, none of these previously described mechanisms appear to be involved in the facilitation of HCVpp infection by HS observed in this study. Indeed, sera from healthy donors did not lose their ability to facilitate HCVpp infection after decomplementation by heat treatment, and incubation with normal purified human immunoglobulin or monoclonal antibodies did not augment infectivity. Our results demonstrate that HCVpp infection is significantly increased by factors within HS, a major component of this enhancement being HDL, which is in agreement with a recent study (51). Furthermore, HDL-mediated infection enhancement requires the HCV receptor candidate, scavenger receptor BI (SR-BI), involves the HVR1 of the E2 glycoprotein, and leads to protection from neutralizing antibodies.
Cell entry of HCV is thought to involve several cell surface molecules, including the LDL receptor (1); the type-C lectins (DC-SIGN and L-SIGN), which act as virion capture receptors (37); the tetraspanin CD81 (36); and the SR-BI receptor (44) that acts as a cell entry coreceptor. The involvement of some of these molecules in cell entry has been confirmed by using HCVpp (3, 4, 19, 52). SR-BI is a 509-amino-acid glycoprotein with two C- and N-terminal cytoplasmic domains separated by a large extracellular domain. Although a direct interaction between soluble E2 glycoproteins and SR-BI could be demonstrated (44), how this receptor mediates HCV entry requires further investigation. It has become clear that the HVR1 domain located at the amino-terminal end of the E2 glycoprotein is a critical region required for the functional interaction between E2 and SR-BI. Indeed, deletion of this region decreases E2 binding to SR-BI (44) and SR-BI-mediated cell entry (4) and lowers infectivity of HVR1-deleted HCVpp more than 10-fold.
SR-BI is a lipoprotein receptor responsible for the selective uptake of cholesteryl ester from HDL via a two-step mechanism involving the binding of lipoproteins to its extracellular domain followed by lipid uptake (8). It is possible that HDL interacts with HCVpp, via protein/protein or lipid/protein interactions, and hence stimulates interactions between SR-BI and the HCV glycoproteins, leading to increased HCVpp entry. However, we have not been able to detect an enhancement of HCVpp binding to target cells in the presence of HDL or a stable physical association of HDL with HCVpp using sucrose gradients and coimmunoprecipitation assays performed with apolipoprotein or E1 and E2 antibodies (data not shown). These negative results could mean that interactions between HDL and HCVpp are transient and/or of low affinity, just as interactions between HDL and SR-BI are of a transient nature due to loss of affinity to SR-BI after cholesteryl uptake (26). Alternatively, as HDL enhances infection of HCVpp which are already bound to target cells, infection enhancement may be linked to cholesteryl ester uptake from HDL by SR-BI. Our finding that inhibitors of cholesterol transfer (BLT-3 and -4) (33) abolished HDL-mediated enhancement of HCVpp entry supports this hypothesis. However, even though BLT-3 and BLT-4 are structurally very different molecules (33), we cannot exclude the possibility that BLTs may simply inhibit HCVpp infection enhancement by HDL by modulating the physical interaction(s) of SR-BI and/or HDL with HCVpp.
One consequence of SR-BI-mediated lipid uptake is an increase of the cholesterol contents of target cell membranes, which is known to facilitate the entry of many different viruses, such as influenza virus and HIV (6, 41). Indeed, cholesterol influx is essential for regulating the properties of cell membranes, probably by maintaining sphingolipid rafts, which are platforms for virus entry (6, 41), in a functional state (46). However, under the experimental conditions described here, HCVpp are many times more sensitive to HDL-mediated infection enhancement than are other cholesterol-sensitive viruses. Furthermore, preincubation of target cells with HDL did not facilitate HCVpp entry, suggesting that other mechanisms or mechanisms in addition to simple cholesterol enrichment of cell membranes are involved in HCVpp enhancement of infection. More specifically, HCVpp may interact with SR-BI and HDL to specifically target cholesterol-enriched microdomains and/or to stimulate local cholesterol enrichment and thus enhance its entry. Alternatively, the direct interaction(s) of HDL with HCVpp, bound or not bound to SR-BI, may induce or accelerate conformational changes within the HCV glycoproteins or SR-BI which are required for the fusion process.
We did not identify here the components in HDL which mediate infection enhancement of HCVpp, but we do know that this effect is limited to HDL from primate species, which differ not only in their apolipoprotein sequences but also in their lipid compositions. It remains unclear, therefore, whether proteins and/or lipids contained in HDL are involved in HCV infection enhancement. Using a number of antibodies directed against apolipoproteins contained within HDL (anti-Apo-A1, -A2, -C2, and -C3), we could not inhibit HCVpp infection, although the antibodies we tested here may not have bound the relevant epitopes. Of note, in a separate study, we found that recombinant apolipoprotein C1 protein enhanced HCVpp infectivity ca. twofold (29a).
The involvement of HVR1 in infection enhancement via HDL and SR-BI is highly significant, given that its genetic variability, which is thought to be the result of a continuous selection process by the host humoral immune response, may allow the virus to adapt to its host. Indeed, the early development of HCV quasispecies and particularly the variation of HVR1 have been suggested to correlate with persistent infection (10), whereas reduction of genetic diversity, leading to increasingly homogenous virus populations, has been shown to be a consistent feature associated with viral clearance in sustained responders (12). Consistently, HVR1 has been shown to contain at least one neutralization epitope (2, 4, 11, 19, 45). Furthermore, the emergence of antibodies against HVR1 in inoculated chimpanzees was associated with variations in HVR1, whereas no variation was detected in the absence of detectable HVR1 antibodies (50). These findings and other evidence in support of HVR1 selection by the humoral response (5, 42) have led to the notion that HVR1 may function as an “immunological decoy,” stimulating a strong immune response that causes variant selection but that is ineffective for viral clearance (31).
Our results shed new light on the functions of HVR1 as an essential component of sustained HCV infection. Indeed, we found that via an interplay with HDL, HVR1 not only promotes infection enhancement but also increases protection from neutralization >4- to 20-fold (Fig. 4). The mechanism underlying protection from neutralizing antibodies is currently unclear. A shielding of neutralizing epitopes on the virion by HDL would be the most straightforward explanation. Indeed, HCV purified from the plasma of infected patients is found in a number of forms which vary at different stages of the disease. Fractionation of blood samples by ultracentrifugation techniques reveals three fractions in which HCV RNA is present: a low-density fraction (<1.063 g/ml) containing infectious virions associated with LDL that is detected early in the infection but not in chronically infected patients (18, 39); an intermediate-density fraction (1.063 to 1.21 g/ml) containing HDL that is predominant in chronically infected patients (29) and that accumulates mutations in HVR1 (7); and a high-density, noninfectious fraction (>1.21 g/ml) containing immunoglobulins complexed with virions (7, 18). While HCV isolated from the intermediate-density fraction was not detectably associated with HDL, the available evidence suggests that it can be neither immunoprecipitated by HCV antibodies nor cleared by the humoral immune response, in contrast to HCV detected in the low-density fraction (40). These data are consistent with our findings that HCVpp are protected from neutralizing antibodies in the presence of HDL but not LDL (Fig. 4). The implications of these results are significant for our understanding of viral propagation and persistence in vivo. Indeed, HDL strongly stimulates HCVpp infectivity because it enhances infection and also because neutralization in the presence of HDL requires at least 4- to 20-fold more antibody. Importantly, a polyclonal antibody purified from a pool of chronically infected patients could not neutralize more than 80% of the virions treated with HDL or normal HS, even when it was used at high concentrations. Interestingly, at saturation, a set of E2 monoclonal antibodies targeted to epitopes outside HVR1 neutralized HCVpp significantly less efficiently in the presence of normal HS or HDL, while a monoclonal antibody against HVR1 could inhibit infection almost totally, most likely as a result of inactivation of the protection mechanism itself.
Furthermore, we found that HVR1 impaired the detection of neutralizing and cross-neutralizing antibodies in both acutely and chronically infected patients. A recent study of the humoral response in a cohort of acute-phase patients infected by a single-source HCV revealed that HCV RNA loads decreased in some patients, which correlated with the progressive emergence of neutralizing antibodies of narrow specificity, while in other patients, high and stable HCV RNA levels correlated with a lack of neutralizing antibodies despite seroconversion (24). Strikingly, the use of HVR1-deleted HCVpp in neutralization assays with sera from this latter group of patients revealed the existence of a relatively strong neutralization response against both homologous and heterologous HCV sequences. Moreover, the use of HVR1-deleted HCVpp, in contrast to wild-type HCVpp, led to the detection of a stronger and broader neutralizing response in sera from the first group of patients, indicating that HS/HDL may somehow mask HCVpp from the cross-neutralizing antibodies present within these sera. However, we have not yet been able to demonstrate an interaction of HCVpp with HDL in order to explain such a “masking” effect. Yet, interactions with lipoproteins may be very transient in nature. Furthermore, interactions between, e.g., lipid moieties in HDL and the HCV glycoproteins may be of low affinity and thus hard to demonstrate. Alternatively, the affinity of the glycoproteins to HDL may decrease after conformational changes are induced. Reconstitution of HDL particles with defined populations of lipids and apolipoproteins may help to identify the HDL components required for HCVpp enhancement of infection and interactions and allow us to shed more light on how HDL helps HCVpp to escape from neutralization.
Altogether, our results and those of others assign to HVR1 three different roles which are complementary in their aim to help the virus to survive within its host: enhancement of cell entry, masking of virions from (cross-)neutralizing antibodies by HDL, and escape from a selective humoral immune response by mutation. Preserving the three functions of HVR1 may be essential for viral persistence and is consistent with the notions that, despite its high degree of genetic variability, highly conserved amino acid positions are found throughout HVR1 and that, even at various positions, the physicochemical properties of amino acids are maintained (35). Indeed, we found that the nonconservative substitution of conserved amino acids had a dramatic effect on infection enhancement, suggesting that the genetic diversification of HVR1 may compromise immune escape and enhancement of cell entry. While the conserved amino acids may be responsible for maintaining HVR1 in a conformation that allows interaction with HDL and/or SR-BI, the truly variable positions are likely involved in HVR1 antigenicity, a type of organization similar to those of immunoglobulin and T-cell-receptor variable domains that exhibit variable sequences but conserved conformations (15). Deciphering the molecular aspects of HVR1 in immune escape and infection enhancement will provide valuable information for HCV biology and the development of antiviral therapies.
Acknowledgments
We thank J. Dubuisson, C. Granier, J. McKeating, and A. H. Patel for providing the A4 anti-E1, the H52 and E2mAb-1 anti-E2, the 9/27 anti-HVR1, and the AP33 anti-E2 monoclonal antibodies. We thank J. C. Piette and C. d’Alba for providing the serum from a patient with Tangier disease and G. Germanidis for providing the sera from the acute-phase patients.
This work was supported by La Ligue Nationale Contre le Cancer, Agence Nationale pour la Recherche sur le SIDA et les Hépatites Virales (ANRS), the European Community (LSHB-CT-2004-005246), Région Rhône-Alpes, and the Institut National de la Santé et de la Recherche Médicale, Action Thématique Concertée “Hépatite C.” B.B. was supported by a Marie Curie fellowship from the European Community.
REFERENCES
- 1.Agnello, V., G. Ábel, M. Elfahal, G. B. Knight, and Q.-X. Zhang. 1999. Hepatitis C virus and other Flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA 96:12766-12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartosch, B., J. Bukh, J. C. Meunier, C. Granier, R. E. Engle, W. C. Blackwelder, S. U. Emerson, F. L. Cosset, and R. H. Purcell. 2003. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc. Natl. Acad. Sci. USA 100:14199-14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartosch, B., J. Dubuisson, and F. L. Cosset. 2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J. Exp. Med. 197:633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartosch, B., A. Vitelli, C. Granier, C. Goujon, J. Dubuisson, S. Pascale, E. Scarselli, R. Cortese, A. Nicosia, and F.-L. Cosset. 2003. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J. Biol. Chem. 278:41624-41630. [DOI] [PubMed] [Google Scholar]
- 5.Booth, J. C., U. Kumar, D. Webster, J. Monjardino, and H. C. Thomas. 1998. Comparison of the rate of sequence variation in the hypervariable region of E2/NS1 region of hepatitis C virus in normal and hypogammaglobulinemic patients. Hepatology 27:223-227. [DOI] [PubMed] [Google Scholar]
- 6.Chazal, N., and D. Gerlier. 2003. Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 67:226-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choo, S. H., H. S. So, J. M. Cho, and W. S. Ryu. 1995. Association of hepatitis C virus particles with immunoglobulin: a mechanism for persistent infection. J. Gen. Virol. 76:2337-2341. [DOI] [PubMed] [Google Scholar]
- 8.Connelly, M. A., and D. L. Williams. 2003. SR-BI and cholesterol uptake into steroidogenic cells. Trends Endocrinol. Metab. 14:467-472. [DOI] [PubMed] [Google Scholar]
- 9.Dubuisson, J., H. H. Hsu, R. C. Cheung, H. B. Greenberg, D. G. Russell, and C. M. Rice. 1994. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J. Virol. 68:6147-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farci, P., A. Shimoda, A. Coiana, G. Diaz, G. Peddis, J. C. Melpolder, A. Strazzera, D. Y. Chien, S. J. Munoz, A. Balestrieri, R. H. Purcell, and H. J. Alter. 2000. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288:339-344. [DOI] [PubMed] [Google Scholar]
- 11.Farci, P., A. Shimoda, D. Wong, T. Cabezon, D. De Gioannis, A. Strazzera, Y. Shimizu, M. Shapiro, H. J. Alter, and R. H. Purcell. 1996. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc. Natl. Acad. Sci. USA 93:15394-15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farci, P., R. Strazzera, H. J. Alter, S. Farci, D. Degioannis, A. Coiana, G. Peddis, F. Usai, G. Serra, L. Chessa, G. Diaz, A. Balestrieri, and R. H. Purcell. 2002. Early changes in hepatitis C viral quasispecies during interferon therapy predict the therapeutic outcome. Proc. Natl. Acad. Sci. USA 99:3081-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flint, M., C. Maidens, L. D. Loomis-Price, C. Shotton, J. Dubuisson, P. Monk, A. Higginbottom, S. Levy, and J. A. McKeating. 1999. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J. Virol. 73:6235-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fust, G. 1997. Enhancing antibodies in HIV infection. Parasitology 115:S127-S140. [DOI] [PubMed] [Google Scholar]
- 15.Garcia, K. C., L. Teyton, and I. A. Wilson. 1999. Structural basis of T cell recognition. Annu. Rev. Immunol. 17:369-397. [DOI] [PubMed] [Google Scholar]
- 16.Gras, G. S., and D. Dormont. 1991. Antibody-dependent and antibody-independent complement-mediated enhancement of human immunodeficiency virus type 1 infection in a human, Epstein-Barr virus-transformed B-lymphocytic cell line. J. Virol. 65:541-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halstead, S. B. 2003. Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 60:421-467. [DOI] [PubMed] [Google Scholar]
- 18.Hijikata, M., Y. K. Shimizu, H. Kato, A. Iwamoto, J. W. Shih, H. J. Alter, R. H. Purcell, and H. Yoshikura. 1993. Equilibrium centrifugation studies of hepatitis C virus: evidence for circulating immune complexes. J. Virol. 67:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu, M., J. Zhang, M. Flint, C. Logvinoff, C. Cheng-Mayer, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. USA 100:7271-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanto, T., N. Hayashi, T. Takehara, H. Hagiwara, E. Mita, M. Naito, A. Kasahara, H. Fusamoto, and T. Kamada. 1994. Buoyant density of hepatitis C virus recovered from infected hosts: two different features in sucrose equilibrium density-gradient centrifugation related to degree of liver inflammation. Hepatology 19:296-302. [PubMed] [Google Scholar]
- 21.Krieger, M. 2001. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J. Clin. Investig. 108:793-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavanchy, D., R. Purcell, F. B. Hollinger, C. Howard, A. Alberti, M. Kew, G. Dusheiko, M. Alter, E. Ayoola, P. Beutels, R. Bloomer, B. Ferret, R. Decker, R. Esteban, O. Fay, H. Fields, E. C. Fuller, P. Grob, M. Houghton, N. Leung, S. A. Locarnini, H. Margolis, A. Meheus, T. Miyamura, M. K. Mohamed, B. Tandon, D. Thomas, H. T. Head, A. U. Toukan, P. Van Damme, A. Zanetti, R. Arthur, M. Couper, R. D'Amelio, J. C. Emmanuel, K. Esteves, P. Gavinio, E. Griffiths, Z. Hallaj, C. C. Heuck, D. L. Heymann, S. E. Holck, M. Kane, L. J. Martinez, F. Meslin, I. S. Mochny, A. Ndikuyeze, A. M. Padilla, G. M. Rodier, C. Roure, F. Savage, and G. Vercauteren. 1999. Global surveillance and control of hepatitis C. J. Viral Hepat. 6:35-47.10847128 [Google Scholar]
- 23.Lavillette, D., B. Boson, S. Russell, and F.-L. Cosset. 2001. Activation of membrane fusion by murine leukemia viruses is controlled in cis or in trans by interactions between the receptor-binding domain and a conserved disulfide loop of the carboxy terminus of the surface glycoprotein. J. Virol. 75:3685-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavillette, D., Y. Morice, G. Germanidis, P. Donot, A. Soulier, E. Pagkalos, G. Sakellariou, L. Intrator, B. Bartosch, J.-M. Pawlotsky, and F.-L. Cosset. 2005. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J. Virol. 79:6023-6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavillette, D., A. W. Tarr, C. Voisset, P. Donot, B. Bartosch, C. Bain, A. H. Patel, J. Dubuisson, J. K. Ball, and F.-L. Cosset. 2005. Characterization of host-range and cell entry properties of hepatitis C virus of major genotypes and subtypes. Hepatology 41:265-274. [DOI] [PubMed] [Google Scholar]
- 26.Liu, B., and M. Krieger. 2002. Highly purified scavenger receptor class B, type I reconstituted into phosphatidylcholine/cholesterol liposomes mediates high affinity high density lipoprotein binding and selective lipid uptake. J. Biol. Chem. 277:34125-34135. [DOI] [PubMed] [Google Scholar]
- 27.Major, M. E., B. Rehermann, and S. M. Feinstone. 2001. Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 28.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 29.Manzini, P., G. M. Dusheiko, D. J. Brown, S. I. Khakoo, and J. S. Owen. 1998. Hepatitis C virus (HCV) tends to associate preferentially with high-density lipoproteins by standard ultracentrifugal fractionation of plasma from patients with chronic HCV infection. Hepatol. Res. 11:158-165. [Google Scholar]
- 29a.Meunier, J.-C., R. E. Engle, K. Faulk, M. Zhao, B. Bartosch, H. Alter, S. U. Emerson, F.-L. Cosset, R. H. Purcell, and J. Bukh. 2005. Evidence for cross-genotype neutralization of hepatitis C virus pseudoparticles and enhancement of infectivity by apolipoprotein C1. Proc. Natl. Acad. Sci. USA 102:4560-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monazahian, M., S. Kippenberger, A. Muller, H. Seitz, I. Bohme, S. Grethe, and R. Thomssen. 2000. Binding of human lipoproteins (low, very low, high density lipoproteins) to recombinant envelope proteins of hepatitis C virus. Med. Microbiol. Immunol. 188:177-184. [DOI] [PubMed] [Google Scholar]
- 31.Mondelli, M. U., A. Cerino, L. Segagni, A. Meola, A. Cividini, E. Silini, and A. Nicosia. 2001. Hypervariable region 1 of hepatitis C virus: immunological decoy or biologically relevant domain? Antivir. Res. 52:153-159. [DOI] [PubMed] [Google Scholar]
- 32.Nakabayashi, H., K. Taketa, K. Miyano, T. Yamane, and J. Sato. 1982. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 42:3858-3863. [PubMed] [Google Scholar]
- 33.Nieland, T. J., A. Chroni, M. L. Fitzgerald, Z. Maliga, V. I. Zannis, T. Kirchhausen, and M. Krieger. 2004. Cross-inhibition of SR-BI- and ABCA1-mediated cholesterol transport by the small molecules BLT-4 and glyburide. J. Lipid Res. 45:1256-1265. [DOI] [PubMed] [Google Scholar]
- 34.Owsianka, A., R. F. Clayton, L. D. Loomis-Price, J. A. McKeating, and A. H. Patel. 2001. Functional analysis of hepatitis C virus E2 glycoproteins and virus-like particles reveals structural dissimilarities between different forms of E2. J. Gen. Virol. 82:1877-1883. [DOI] [PubMed] [Google Scholar]
- 35.Penin, F., C. Combet, G. Germanidis, P.-O. Frainais, G. Deléage, and J.-M. Pawlotsky. 2001. Conservation of the conformation and positive charges of hepatitis C virus E2 envelope glycoprotein hypervariable region 1 points to a role in cell attachment. J. Virol. 75:5703-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pileri, P., Y. Uematsu, S. Campagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282:938-941. [DOI] [PubMed] [Google Scholar]
- 37.Pöhlmann, S., J. Zhang, F. Baribaud, Z. Chen, G. J. Leslie, G. Lin, A. Granelli-Piperno, R. W. Doms, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J. Virol. 77:4070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porterfield, J. S. 1986. Antibody-dependent enhancement of viral infectivity. Adv. Virus Res. 31:335-355. [DOI] [PubMed] [Google Scholar]
- 39.Prince, A. M., T. Huima-Byron, T. S. Parker, and D. M. Levine. 1996. Visualization of hepatitis C virions and putative defective interfering particles isolated from low-density lipoproteins. J. Viral Hepat. 3:11-17. [DOI] [PubMed] [Google Scholar]
- 40.Pumeechockchai, W., D. Bevitt, K. Agarwal, T. Petropoulou, B. C. Langer, B. Belohradsky, M. F. Bassendine, and G. L. Toms. 2002. Hepatitis C virus particles of different density in the blood of chronically infected immunocompetent and immunodeficient patients: implications for virus clearance by antibody. J. Med. Virol. 68:335-342. [DOI] [PubMed] [Google Scholar]
- 41.Rawat, S. S., M. Viard, S. A. Gallo, A. Rein, R. Blumenthal, and A. Puri. 2003. Modulation of entry of enveloped viruses by cholesterol and sphingolipids. Mol. Membr. Biol. 20:243-254. [DOI] [PubMed] [Google Scholar]
- 42.Ray, S. C., Y.-M. Wang, O. Laeyendecker, J. R. Ticehurst, S. A. Villano, and D. L. Thomas. 1999. Acute hepatitis C virus structural gene sequences as predictors of persistent viremia: hypervariable region 1 as a decoy. J. Virol. 73:2938-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandrin, V., B. Boson, P. Salmon, W. Gay, D. Nègre, R. LeGrand, D. Trono, and F.-L. Cosset. 2002. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and non-human primates. Blood 100:823-832. [DOI] [PubMed] [Google Scholar]
- 44.Scarselli, E., H. Ansuini, R. Cerino, R. Roccasecca, S. Acali, G. Filocamo, C. Traboni, A. Nicosia, R. Cortese, and A. Vitelli. 2002. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 21:5017-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimizu, Y. K., H. Igarashi, T. Kiyohara, T. Cabezon, P. Farci, R. H. Purcell, and H. Yoshikura. 1996. A hyperimmune serum against a synthetic peptide corresponding to the hypervariable region 1 of hepatitis C virus can prevent viral infection in cell cultures. Virology 223:409-412. [DOI] [PubMed] [Google Scholar]
- 46.Simons, K., and E. Ikonen. 2000. How cells handle cholesterol. Science 290:1721-1726. [DOI] [PubMed] [Google Scholar]
- 47.Takada, A., H. Feldmann, T. G. Ksiazek, and Y. Kawaoka. 2003. Antibody-dependent enhancement of Ebola virus infection. J. Virol. 77:7539-7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thimme, R., J. Bukh, H. C. Spangenberg, S. Wieland, J. Pemberton, C. Steiger, S. Govindarajan, R. H. Purcell, and F. V. Chisari. 2002. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc. Natl. Acad. Sci. USA 99:15661-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomssen, R., S. Bonk, C. Propfe, K. H. Heermann, H. G. Kochel, and A. Uy. 1992. Association of hepatitis C virus in human sera with beta-lipoprotein. Med. Microbiol. Immunol. 181:293-300. [DOI] [PubMed] [Google Scholar]
- 50.van Doorn, L.-J., I. Capriles, G. Maertens, R. DeLeys, K. Murray, T. Kos, H. Schellekens, and W. Quint. 1995. Sequence evolution of the hypervariable region in the putative envelope region E2/NS1 of hepatitis C virus is correlated with specific humoral immune responses. J. Virol. 69:773-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voisset, C., N. Callens, E. Blanchard, A. Op De Beeck, J. Dubuisson, and N. Vu-Dac. 2005. High density lipoproteins facilitate hepatitis C virus entry through the scavenger receptor class B type I. J. Biol. Chem. [DOI] [PubMed]
- 52.Zhang, J., G. Randall, A. Higginbottom, P. Monk, C. M. Rice, and J. A. McKeating. 2004. CD81 is required for hepatitis C virus glycoprotein-mediated viral infection. J. Virol. 78:1448-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]