Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD) brings heavy clinical and economic burdens to patients worldwide. High fasting plasma glucose (HFPG) was proven to be an important modifiable risk factor. However, the global burden distribution of HFPG-attributable MASLD has not been fully studied. This study aimed to describe the epidemiological distribution and trends of the burden of HFPG-attributable MASLD worldwide. The data source was the 2021 Global Burden of Disease Study. Descriptive statistics were mainly conducted using disability-adjusted life years (DALYs) and deaths of HFPG-attributable MASLD from 1990 to 2021, as well as their age-standardized rates (ASRs) and population-attributable fractions. Subgroup analyses were conducted by region, age group, and sex. We found that 213.48 thousand DALYs and 10.02 thousand deaths in MASLD were attributable to HFPG worldwide in 2021, with an increase of 2.96 and 3.32 times compared with 1990, respectively. Over the past 32 years, age-standardized DALY rates (ASDRs) have fluctuated upward, reaching 2.45 per 100,000 people in 2021, with an increase of 81.21%. The ASDRs continued to rise in low, low-middle, and high social demographic index (SDI) regions, fluctuated upward at high levels in middle SDI regions, and were relatively low in high-middle SDI regions. People aged 50–69 accounted for the largest proportion of DALYs, while people over 70 had the largest increase of 3.73 times. Men had higher ASDRs, and the sex difference has been gradually expanding over the past 32 years, peaking at the age of 45–49. In conclusion, the burden of HFPG-attributable MASLD has continued to increase globally, with differences in geographical area, age, and sex distribution. HFPG, as a modifiable risk factor, should be given more importance. The implementation of targeted health intervention strategies is recommended for each country based on trends in the burden of HFPG-attributable MASLD.
Keywords: Global burden of disease, Metabolic dysfunction-associated steatotic liver disease, Non-alcoholic fatty liver disease, High fasting plasma glucose, Disability-adjusted life years, Ecological study
Subject terms: Non-alcoholic fatty liver disease, Pre-diabetes
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD) until June 20231, is the world’s most common chronic liver disease with a heavy clinical burden2. The global prevalence of MASLD has been increasing in recent years2, and a meta-analysis conducted in 2021 estimated that the global prevalence of MASLD has reached 32.4% (95% CI 29.9–34.9)3. The global deaths due to MASLD almost doubled from 93.7 thousand in 1990 to 169.0 thousand in 2019, and global DALYs regarding MASLD were elevated from 2.7 million in 1990 to 4.4 million in 20194. Long-term MASLD may lead to cirrhosis, end-stage liver disease, hepatocellular carcinoma, and other severe adverse outcomes5, and MASLD has gradually become an important cause of liver transplantation6. The large number of MASLD patients brings great pressure to clinical diagnosis, treatment, and health management in our health system. In the United States, the average annual healthcare cost for each MASLD patient was $19,908, with patients progressing to compensated or decompensated cirrhosis reaching $26,538 and $74,454 respectively, and patients receiving liver transplantation spending up to $129,2767. Even in low-middle-income countries such as Thailand, the total lifetime cost of non-alcoholic steatohepatitis patients with significant fibrosis in 2019 was as high as $5,174 per case, totaling $15.2 billion, accounting for approximately 3% of Thailand’s GDP8.
Notably, the global increase in the prevalence of MASLD was accompanied by a parallel increase in the prevalence of type 2 diabetes (T2D)9. These two diseases often coexist10 and can synergistically increase the risk of adverse clinical outcomes11. Large-sample cohort studies have shown that high fasting plasma glucose (HFPG) has been confirmed to be an important risk factor for the development of MASLD12,13. Basic medicine research has shown that hyperglycemia promotes an increase in fat synthesis in the liver, inhibits fatty acid oxidation, leads to fat accumulation in the liver, and ultimately promotes the development of MASLD14. Meanwhile, hyperglycemia can also trigger inflammation, activate inflammatory cells and cytokines in the liver, further damage liver tissue, exacerbate the progression of MASLD, and even lead to liver fibrosis and cirrhosis15. In addition, some antihyperglycemic drugs have shown certain effectiveness in treating MASLD11. Therefore, HFPG-attributable MASLD may be regarded as a complication of T2D. Moreover, owing to population aging, changes in dietary patterns, reduction of physical activity, and increased prevalence of obesity, the global fasting plasma glucose level continues to rise16. A study estimates that the global prevalence of impaired fasting glucose was 5.8% (298 million) in 2021 and is predicted to rise to 6.5% (414 million) by 204517. HFPG, as a modifiable risk factor for MASLD, needs urgent attention. However, there are currently few international studies on the disease burden of HFPG-attributable MASLD. If this risk factor is not fully studied and controlled, the number of patients with HFPG-attributable MASLD will continue to increase, aggravating the global burden of MASLD.
The Global Burden of Disease (GBD) Study systematically assessed the burden of multiple diseases from a global perspective, and its methodological framework and analytical strategy have been widely accepted by the academic community18,19. Therefore, this study aimed to describe the epidemiological distribution and trends in the burden of HFPG-attributable MASLD globally and in different regions, age groups, and sexes by using GBD 2021 data, and to provide a reference for the formulation of prevention strategies.
Methods
Data sources
The GBD 2021 Study (https://ghdx.healthdata.org/gbd-2021) systematically updated and evaluated age- and sex-specific epidemiological data on 371 diseases and injuries and 88 risk factors in 204 countries and territories worldwide. This study obtained the global burden data on HFPG-attributable MASLD from 1990 to 2021 from the GBD 2021 Study. These data included disability-adjusted life years (DALYs), deaths, years lived with disability (YLDs), years of life lost (YLLs), and their age-standardized rates (ASRs), where the ASRs of DALYs are abbreviated as ASDRs (age-standardized DALY rates). Since the disease burden of a country or territory is often closely related to its socio-economic development, the GBD collaborators developed the socio-demographic index (SDI)18. The SDI is a summary indicator used to quantify the level of socioeconomic development by comprehensively considering per capita income, average education level, and total fertility rate. The GBD study divided 204 countries and territories into 5 levels according to the SDI quintiles: high (> 0.81), high-middle (0.71–0.81), middle (0.62–0.71), low-middle (0.47–0.62) and low (< 0.47). In addition, these countries and territories were divided into 21 GBD regions based on their geographic contiguity (Table S1). Ethical approval and informed consent were waived since the GBD is a publicly available summary-level database, and no identifiable information was involved.
Definitions
In the GBD 2021 Study, MASLD (NAFLD) was defined as a spectrum of diseases including hepatic fat deposition without cirrhosis, cirrhosis due to fat deposition and inflammation, and liver cancer due to non-alcoholic steatohepatitis. (When the GBD 2021 Study was conducted, “MASLD” had not yet been updated as the new name of the disease, but the old name “NAFLD” was used.) Although biopsy provides the gold-standard clinical case definition, this invasive procedure is not typically employed in population-based surveys or screening programs. Therefore, the GBD 2021 Study used ultrasound or other imaging imaging modalities as our reference case diagnostics. Since the majority of MASLD cases are asymptomatic, the GBD 2021 Study used active case-finding methods (e.g., screening data in representative populations in specific locations) as the primary data source and used administrative data from hospitals or claims as secondary data sources based on the International Classification of Diseases (ICD)-10 code K76.0 and ICD-9 code 571.8. Detailed disease diagnosis and assessment methods have been published previously18. High fasting plasma glucose (HFPG) was defined as 4.88–5.30 mmol/L in the GBD 2021 Study19.
Burden indicators estimation
Age-standardized rates of DALY, YLD, YLL, death, and their population-attributable fractions (PAFs) were used as indicators to describe the burden of HFPG-attributable MASLD. DALYs were calculated by summing YLDs and YLLs across different locations, ages, sexes, and years. YLDs were derived through a microsimulation process, which involved using estimated prevalent counts of non-fatal disease sequelae specific to age, sex, location, and year, alongside disability weights for each sequela. To address the co-occurrence of multiple non-fatal causes within the population, YLDs were adjusted for comorbidity, ensuring that they were additive within the GBD 2021 Study cause hierarchy. This simulation was conducted with a population of 20,000 individuals for each combination of age, sex, location, and year18. YLLs were calculated by multiplying the estimated number of deaths, categorized by age, sex, location, and year, with the standard life expectancy at the age of death for each cause20.
The Cause of Death Ensemble model (CODEm) was employed to estimate cause-specific deaths. CODEm integrates an ensemble of statistical models and systematically evaluates various covariate combinations based on their out-of-sample predictive validity. The model then synthesizes these results to estimate the number of deaths by location, age, sex, and year for each cause20.
The ASR (per 100,000 people) of those indicators was calculated according to the following formula:
where represents the disease burden indicator for the ith age group. represents the number of individuals (or weights) in the same ith age group in the standard population. In addition, 95% uncertainty intervals (UIs) of the estimates were calculated to reflect random and systematic errors in the statistical modeling.
The relative risk (RR) between the risk factor and outcome was estimated based on published systematic reviews and meta-analyses. Based on published exposure data for each risk factor in large population surveys or reports, the Bayesian network meta-regression model (DisMod-MR 2.1) and spatiotemporal Gaussian process regression model were used to summarize the data and determine the theoretical minimum-risk exposure levels (TMREL). Attributable proportions of age-standardized DALY or mortality rates by sex, age, location, and year were assessed using population-attributable fractions (PAFs), which represent the ideal reduction in DALY or mortality rates if the risk factor was eliminated. The equation for the PAF for HFPG is defined as follows19:
where is the function of the RR to the exposure level (x) under a specific risk factor (j, i.e., HFPG in this study), outcome (o, i.e., MASLD), age group (a), sex (s), and location (g) with the lowest level of observed exposure as l and the highest as u. is the distribution of exposure at x under a specific risk factor (j), age group (a), sex (s), location (g), and year (t). is the TMREL under a specific risk factor (j), age group (a), and sex (s).
Statistical analyses
Frequencies and percentages were used to describe the distribution of categorical variables. The changing patterns of HFPG-attributable MASLD burden from 1990 to 2021 were described globally and by SDI region, age, and sex subgroup. Pearson correlation analysis was used to evaluate the correlation between the HFPG-attributable MASLD burden indicators and the SDI. Statistical analyses and visualization were performed in R 4.1.2 (R Core Team). A P value < 0.05 was considered to indicate statistical significance.
Results
Regional differences in the disease burden of HFPG-attributable MASLD
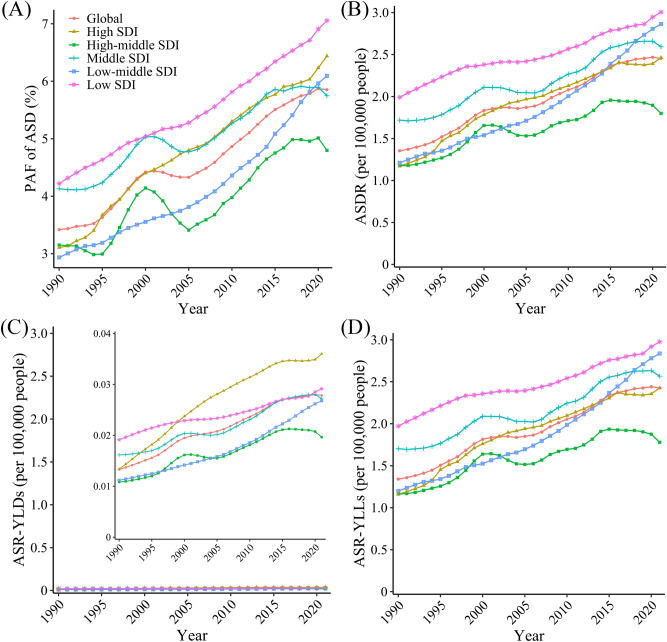
In 2021, 213.48 (95% UI 23.80–432.14) thousand DALYs and 10.02 (95% UI 1.10–19.98) thousand deaths from MASLD globally were attributable to HFPG, with increases of 2.96 (95% UI 2.48–3.51) times and 3.32 (95% UI 2.76–3.93) times, respectively, compared with 1990 (Table 1). Over the past 32 years, the ASDRs of HFPG-attributable MASLD have shown a fluctuating upward trend globally, reaching 2.45 (95% UI 0.27–4.95) per 100,000 people in 2021, an increase of 81.21% (Table 1; Fig. 1B). The increase in the ASMR was also as high as 90.86%, with a trend similar to that of the ASDR (Fig. S1B). The PAF for HFPG-attributable MASLD ASDs increased from 3.42% in 1990 to 5.85% in 2021 (Fig. 1A), and the PAF of age-standardized death increased from 4.07% to 7.32% with a similar trend to the PAF of ASD (Fig. S1A). In addition, both the YLLs and YLDs of HFPG-attributable MASLD patients showed a fluctuating upward trend (Fig. 1C, D).
Table 1.
Age-standardized rates and numbers of MASLD burden attributable to HFPG in 1990 and 2021.
| Characteristic | ASR (per 105) (95% UI) | Percentage change (× 100%) (95% UI) 1990–2021 | Numbers × 103 (95% UI) | Percentage change (× 100%) (95% UI) 1990–2021 | ||
|---|---|---|---|---|---|---|
| 1990 | 2021 | 1990 | 2021 | |||
| DALYs attribute to HFPG | ||||||
| Global | 1.35 (0.14, 2.81) | 2.45 (0.27, 4.95) | 0.81 (0.60, 1.05) | 53.93 (5.77, 111.05) | 213.48 (23.80, 432.14) | 2.96 (2.48, 3.51) |
| Men | 1.50 (0.16, 3.12) | 2.80 (0.31, 5.59) | 0.86 (0.61, 1.18) | 27.88 (2.96, 57.83) | 114.07 (12.76, 230.07) | 3.09 (2.51, 3.79) |
| Women | 1.22 (0.13, 2.53) | 2.14 (0.23, 4.42) | 0.76 (0.49, 1.08) | 26.04 (2.79, 53.81) | 99.41 (10.79, 205.00) | 2.82 (2.23, 3.50) |
| High SDI | 1.17 (0.14, 2.40) | 2.46 (0.28, 4.71) | 1.10 (0.91, 1.32) | 13.02 (1.51, 26.52) | 51.60 (5.91, 99.31) | 2.96 (2.58, 3.40) |
| High-middle SDI | 1.17 (0.12, 2.43) | 1.80 (0.20, 3.63) | 0.53 (0.29, 0.86) | 11.88 (1.26, 24.68) | 36.06 (4.12, 72.63) | 2.04 (1.55, 2.70) |
| Middle SDI | 1.72 (0.18, 3.54) | 2.59 (0.28, 5.28) | 0.51 (0.28, 0.81) | 17.55 (1.81, 36.28) | 70.22 (7.51, 144.39) | 3.00 (2.35, 3.77) |
| Low-middle SDI | 1.21 (0.12, 2.58) | 2.86 (0.32, 5.90) | 1.37 (0.87, 1.92) | 7.23 (0.72, 15.47) | 41.12 (4.59, 85.38) | 4.69 (3.52, 6.03) |
| Low SDI | 1.99 (0.19, 4.49) | 3.01 (0.29, 6.53) | 0.51 (0.19, 1.00) | 4.20 (0.41, 9.18) | 14.33 (1.43, 31.73) | 2.41 (1.73, 3.46) |
| Deaths attribute to HFPG | ||||||
| Global | 0.06 (0.01, 0.13) | 0.12 (0.01, 0.23) | 0.91 (0.67, 1.16) | 2.32 (0.25, 4.80) | 10.02 (1.10, 19.98) | 3.32 (2.76, 3.93) |
| Men | 0.07 (0.01, 0.14) | 0.13 (0.01, 0.26) | 0.97 (0.72, 1.30) | 1.13 (0.12, 2.37) | 5.11 (0.57, 10.11) | 3.51 (2.90, 4.29) |
| Women | 0.06 (0.01, 0.12) | 0.11 (0.01, 0.21) | 0.85 (0.58, 1.17) | 1.19 (0.13, 2.41) | 4.91 (0.52, 10.02) | 3.14 (2.51, 3.88) |
| High SDI | 0.05 (0.01, 0.11) | 0.12 (0.01, 0.23) | 1.27 (1.07, 1.50) | 0.61 (0.07, 1.22) | 2.75 (0.31, 5.29) | 3.51 (3.09, 4.05) |
| High-middle SDI | 0.05 (0.01, 0.11) | 0.08 (0.01, 0.17) | 0.64 (0.40, 0.96) | 0.50 (0.05, 1.03) | 1.68 (0.19, 3.35) | 2.39 (1.89, 3.08) |
| Middle SDI | 0.08 (0.01, 0.17) | 0.12 (0.01, 0.25) | 0.56 (0.30, 0.88) | 0.73 (0.07, 1.53) | 3.18 (0.33, 6.35) | 3.37 (2.68, 4.24) |
| Low-middle SDI | 0.06 (0.01, 0.12) | 0.13 (0.01, 0.27) | 1.36 (0.85, 1.94) | 0.30 (0.03, 0.64) | 1.76 (0.19, 3.60) | 4.86 (3.63, 6.26) |
| Low SDI | 0.10 (0.01, 0.22) | 0.15 (0.01, 0.31) | 0.52 (0.18, 1.04) | 0.18 (0.02, 0.40) | 0.63 (0.06, 1.37) | 2.50 (1.78, 3.63) |
ASR age-standardized rate, DALY disability-adjusted life-year, HFPG high fasting plasma glucose, MASLD, metabolic dysfunction-associated steatotic liver disease, SDI sociodemographic index, UI uncertainty interval.
Fig. 1.
Global trends in the PAF of ASD (A), ASDRs (B), ASR-YLDs (C), ASR-YLLs (D) of HFPG-attributable MASLD, 1990–2021. ASD age-standardized disability-adjusted life-years, ASDR age-standardized disability-adjusted life-years rate, ASR age-standardized rate, HFPG high fasting plasma glucose, MASLD metabolic dysfunction-associated steatotic liver disease, PAF population-attributable fraction, SDI sociodemographic index, YLD years lived with disability, YLL years of life lost.
Geographically, the maximum ASDRs and PAFs of ASD of HFPG-attributable MASLD have always occurred in low SDI regions over the past 32 years. Meanwhile, low-middle SDI regions had the highest increase by up to 1.37 and 1.08 times, respectively (Fig. 1A, B). From 1990 to 2021, the ASDRs exhibited various trends in different regions. The ASDRs continued to rise in low, low-middle, and high SDI regions and fluctuated upward at high levels in middle SDI region. However, the ASDRs in high-middle SDI region fluctuated upward at a relatively low level and showed a slight downward trend in 2020–2021 (Fig. 1B).
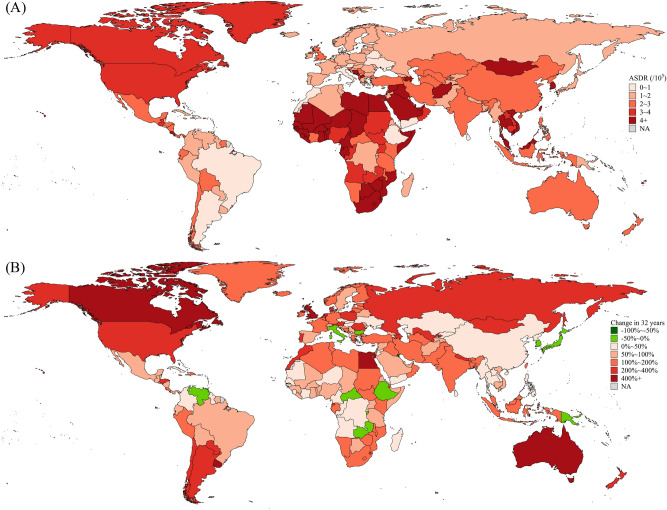
Among 204 countries and territories, the 5 highest ASDRs (per 100,000 people) in 2021 occurred in Qatar (20.95), Tonga (20.05), Gambia (19.07), Egypt (16.26) and Eswatini (15.99), and the 5 lowest ASDRs occurred in Ukraine (0.43), Morocco (0.54), Argentina (0.59), Denmark (0.68) and Mauritius (0.70) (Table S2; Fig. 2A). Over the past 32 years, the 5 largest increases in ASDR occurred in Lesotho (5.04 times), Uruguay (4.88 times), Australia (4.61 times), Canada (4.24 times) and Egypt (4.09 times), and the 5 largest decreases occurred in Mauritius (− 75.83%), Kuwait (− 57.52%), South Korea (− 42.97%), Japan (− 36.72%) and Bulgaria (− 30.46%) (Table S2; Fig. 2B).
Fig. 2.
ASDRs in 2021 (A) and percentage of changes in ASDRs in 32 years (B) of HFPG-attributable MASLD in 204 countries and territories. ASDR age-standardized disability-adjusted life-year rate, HFPG high fasting plasma glucose, MASLD metabolic dysfunction-associated steatotic liver disease.
Age differences in the disease burden of HFPG-attributable MASLD
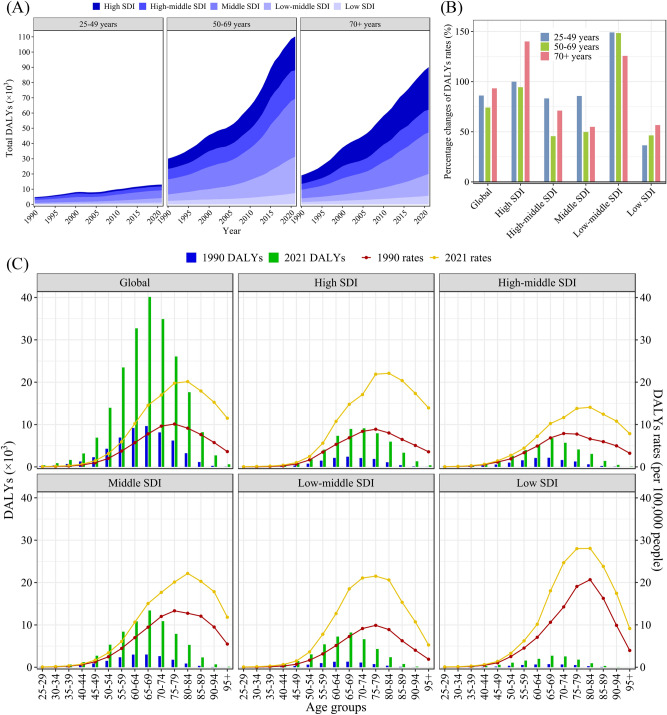
From 1990 to 2021, the 50–69 age group contributed the largest proportion to the total DALYs of HFPG-attributable MASLD, however, people over 70 years had the highest DALY increase of 3.73 times (Fig. 3A). The over 70 years age group contributed the largest proportion to the total deaths and had the highest increase of 3.98 times (Fig. S2A). Over the past 32 years, the DALY rate among people over 70 years of age has increased the most globally. In different regions, the changes in the DALY rate and SDI exhibited U-shaped trends. The DALY rates in high SDI and low-middle SDI regions increased significantly among all age groups, while the increase in high-middle SDI, middle SDI, and low SDI regions was relatively small. (Fig. 3B). Changes in the mortality rate among age groups in different regions were similar to those in DALY rates (Fig. S2B). The peak of HFPG-attributable MASLD DALYs occurred in the 65–69 years age group globally, while the peak of DALY rates occurred in the 75–84 age group, and this phenomenon was basically similar in different SDI regions (Fig. 3C). The peak number of HFPG-attributable MASLD deaths occurred in the 70–74 years old age group globally, while the peak number of death rates occurred in the 85–89 age group (Fig. S2C).
Fig. 3.
Trends of DALYs of HFPG-attributable MASLD in different regions by age, 1990–2021. (A) Total DALYs; (B) Percentage of changes in DALY rates in 32 years; (C) DALY rates and DALYs in 15 age groups. DALY disability-adjusted life-year, HFPG high fasting plasma glucose, MASLD metabolic dysfunction-associated steatotic liver disease, SDI sociodemographic index.
Sex differences in disease burden of HFPG-attributable MASLD
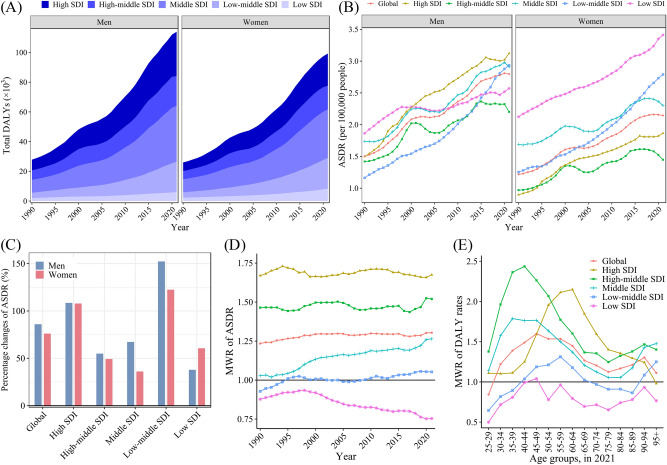
From 1990 to 2021, the ASDR of HFPG-attributable MASLD was always greater in men than in women globally, and the increase in men was as high as 86.02% in 32 years, while that in women increased by only 76.10% (Table 1; Fig. 4B). The DALYs for men changed from 7.07% higher than those for women in 1990 to 14.75% higher in 2021 (Table 1; Fig. 4A). In all regions except the low SDI region, the ASDRs of men increased more than those of women, and the difference in the change of ASDR between men and women was greatest in the middle SDI region (Fig. 4C). In the past 32 years, the sex difference in the ASDR of HFPG-attributable MASLD gradually increased worldwide, with the men-to-women ratio (MWR) increasing from 1.23 to 1.30. Among them, the MWRs in low-middle SDI and middle SDI regions increased significantly, while the MWRs in high SDI and high-middle SDI regions remained relatively stable, at approximately 1.69 and 1.47, respectively, while the MWR in low SDI regions showed a fluctuating downward trend (Fig. 4D). In 2021, the MWR of the global DALY rates showed a trend of first increasing and then decreasing as age increased, with the sex difference peaking at the age of 45–49 (Fig. 4E). From 1990 to 2021, the sex differences in death-related indicators of HFPG-attributable MASLD showed a trend similar to that of DALY-related indicators (Fig. S3).
Fig. 4.
Trends of DALYs of HFPG-attributable MASLD in different regions by sex, 1990–2021. (A) Total DALY; (B) ASDRs; (C) Percentage of changes in 32 years; (D) MWRs of ASDRs; (E) MWRs of DALY rates. ASDR age-standardized disability-adjusted life-year rate, DALY disability-adjusted life-year, HFPG high fasting plasma glucose, MWR men-to-women ratio, MASLD metabolic dysfunction-associated steatotic liver disease, SDI sociodemographic index.
The relationship between SDI and the burden of HFPG-attributable MASLD
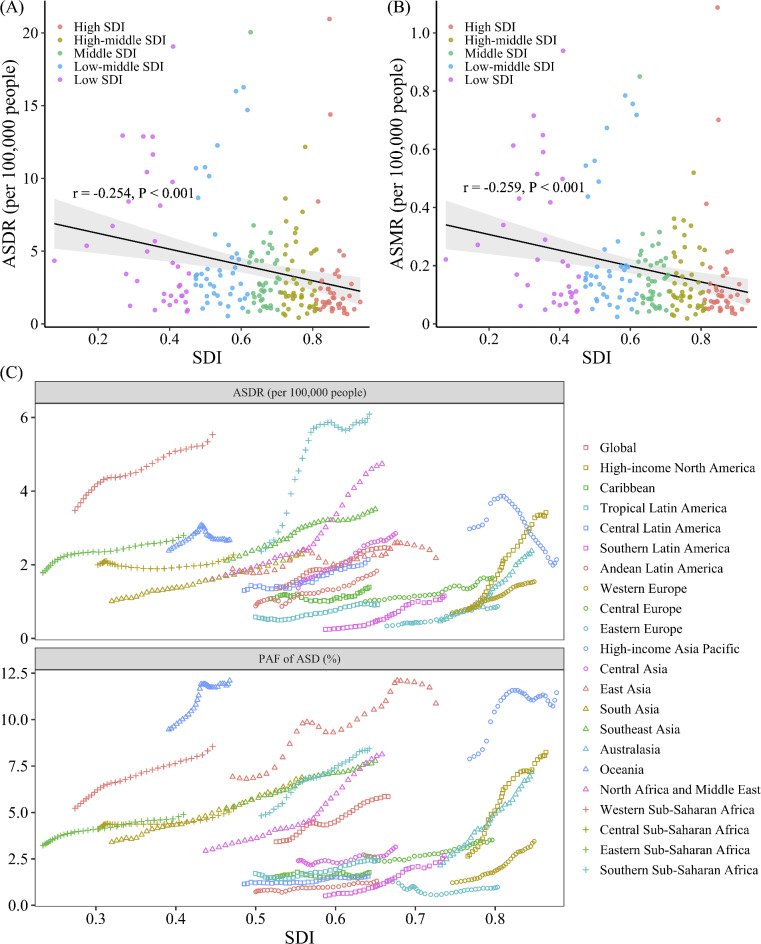
In 2021, the SDI in 204 countries and territories worldwide was weakly negatively correlated with the ASDR of HFPG-attributable MASLD patients (r = − 0.25, P < 0.001) and with the ASMR (r = − 0.26, P < 0.001) (Fig. 5A, 5B). However, no significant correlation was found between the SDI and the PAF of age-standardized DALYs or deaths (Fig. S4A, S4B).
Fig. 5.
The trends between SDI and the burden of HFPG-attributable MASLD across 204 countries and territories and 21 GBD regions, 1990–2021. (A) ASDRs in 2021; (B) ASMRs in 2021; (C) ASDRs and PAFs of ASD across 21 GBD regions, 1990–2021. ASD age-standardized disability-adjusted life-years, ASDR age-standardized disability-adjusted life-years rate, ASMR age-standardized mortality rate, GBD global burden of disease, HFPG high fasting plasma glucose, MASLD metabolic dysfunction-associated steatotic liver disease, PAF population-attributable fraction; SDI, sociodemographic index.
Among the 21 GBD regions, the PAF and ASDR of HFPG-attributable MASLD in most regions showed a fluctuating upward trend over the past 32 years (Fig. 5C). The PAF of ASD in High-income North America (high SDI region) increased rapidly and reached a high level (Fig. 5C). Meanwhile, the ASDR in middle SDI regions such as Southern Sub-Saharan Africa, North Africa and Middle East increased significantly and remained high (Fig. 5C). Among the low-SDI regions, Western Sub-Saharan Africa had the highest ASDR, while Oceania had the highest PAF of ASD (Fig. 5C). On the other hand, Eastern Europe and Southern Latin America, as the high-middle and middle SDI regions, respectively, had maintained low ASDRs and PAFs of ASDs over the past 32 years (Fig. 5C). Notably, the ASDR in High-income Asia Pacific showed a significant downward trend over the past 20 years. The death-related indicators in various regions exhibited similar trends (Fig. S4C).
Discussion
This study analyzed the global burden of HFPG-attributable MASLD from 1999 to 2021, excluding the effect of other risk factors, and conducted subgroup analysis by geographic region, SDI level, age group, and sex by using GBD data. We found an increasing trend in the global burden of HFPG-attributable MASLD over the past 32 years. In addition to socioeconomic development and changes in people’s lifestyles, the improvement in awareness and diagnostic capabilities of MASLD is also an important reason why its burden has maintained a long-term upward trend1. Historically, MASLD was believed to be a disease of rich people and the developed world21. However, in this study, the SDI showed a weakly negative correlation to the ASDR of HFPG-attributable MASLD. Obesity is a well-known risk factor for prediabetes and MASLD, and its prevalence typically increases first in economically developed regions and then shifts towards less developed areas as the global economy improves22. Previous studies indicated that the prevalence of T2D showed a similar trend23. Besides, our study showed that the burden of HFPG-attributable MASLD was heavy among people over 50 years, with the burden increasing the fastest among those over 70 years in particular. One prior study noted that the burden of HFPG-attributable chronic non-communicable diseases increased with age24. Meanwhile, our results also found that the burden was greater in men than in women, and the sex difference was more obvious in socioeconomically developed regions, which was consistent with the findings of a meta-analysis of the global MASLD prevalence and incidence rate3.
The burden of HFPG-attributable MASLD varied significantly among regions with different SDI levels. Over the past 32 years, the burden in low, low-middle, and high SDI regions continued to rise. The burden in middle SDI region fluctuated upward at a high level. High-middle SDI regions exhibited a fluctuated upward at a relatively low level of burden and showed a slight downward trend in recent years. Tinajero’s research showed that North America and Australasia region had the largest total number of patients with T2D, and the growth rate of prevalence was accelerating in the Middle East and North Africa25, which was similar to the burden trend in HFPG-attributable MASLD burden in our study. The reasons for the regional differences in the burden of HFPG-attributable MASLD may be related to factors such as dietary patterns, exercise habits, residents’ education level, disease awareness, health service quality, health policy, and diagnostic capability.
In high SDI regions, Western dietary patterns may be associated with higher disease burden. Among G7 countries, Canada had a higher meat consumption, with a higher proportion of red meat and a lower proportion of fish. Meanwhile, the consumption of sugar, sweeteners, and fruits in Canada was also significantly higher than that of other G7 countries26. In addition, a review showed that the top two countries in the world for ultra-processed food consumption (mainly sweets and pastries) were the United States and Canada27, which may be one of the reasons for the large increase in disease burden. At the same time, sedentary behavior and low exercise frequency are becoming increasingly common and are widely known as risk factors for obesity, T2D, and MASLD28. A cross-sectional survey in the United States showed that the incidence of adult obesity had increased from 30.5% in 1999 to 42.4% in 201729. In addition, research has shown that even in socioeconomically developed regions, the level of awareness of MASLD was very low, as was the case in the United States where awareness of only 2.4–3.1%30. Unbalanced dietary patterns, unhealthy behavioral habits, and low levels of disease awareness may be the reasons for the high and increasing burden of HFPG-attributable MASLD in high SDI regions. In addition, high SDI countries have developed medical diagnostic technology, and their early diagnosis ability to diagnose diseases early may also contribute to higher DALYs. However, Japan, a high SDI country, has reduced its disease burden by one-third over the past 32 years. In the Japanese diet, the intake of meat, dairy products, sugar, and oil is relatively low, while the intake of fish and seafood, soybeans, and green tea is relatively high. Studies have shown that dietary fiber and isoflavones in soybeans can reduce the risk of MASLD31,32. Meanwhile, Japan introduced a regulation named Specific Health Checkups (SHC) in 2008, which stipulates that local governments and companies should include waist circumference, blood lipids, and blood glucose indicators of employees aged 40–75 in annual physical examinations33,34. Employees who exceed the standards would be fined and receive at least 3 months of health education and intervention under the supervision of doctors, community nurses, and nutritionists. This is likely an important reason for the decline in disease burden in Japan.
The burden of HFPG-attributable MASLD in high-middle SDI regions was relatively low and has even shown a slight downward trend in 2015–2021, represented by Italy and Chinese mainland. The characteristics of Italy’s Mediterranean dietary pattern include having a rich variety of plant-based foods supplemented by fish and poultry, using olive oil in cooking, and emphasizing the principles of a moderate and balanced diet and an optimistic attitude toward life. The Mediterranean dietary pattern has been proven to have a protective effect on T2D35 and could improve indicators such as serum cholesterol and liver fat scores in MASLD patients36, which was probably one of the reasons for the reduction in HFPG-attributable MASLD burden in Italy. The Chinese government proposed the “Healthy China 2020/2030 Initiative” in 2012 and 2016, respectively, aiming to increase government health investment, promote health equity, and attach importance to the prevention of risk factors37,38. In 2017, the project “China Healthy Lifestyle for All (2017–2025)” was issued, emphasizing “three reductions and three healths” (reducing salt, oil, and sugar; promoting oral health, bone health, and healthy weight), and widely carrying out health education to guide the public to understand the amount of added sugar on food nutrition labels39. The timing of the introduction of this series of health policies coincides with the reduction of China’s HFPG-attributable MASLD burden from 2015 to 2021.
With the process of globalization, middle SDI, low-middle SDI, and low-SDI regions have been increasingly affected by Western dietary patterns and low physical activity lifestyles40. For example, in India, from 1983 to 2004, the average dietary quality index of urban populations decreased significantly, and fat increasingly contributed to energy sources41. Moreover, factors such as insufficient coverage of primary health services, backward disease diagnosis and treatment technology, low education levels, and insufficient awareness of sugar intake control of the dangers of HFPG may all explain the continuously increasing burden of HFPG-attributable MASLD.
It is recommended that countries around the world pay more attention to HFPG-attributable MASLD. For countries with high or rapidly increasing disease burdens, successful experiences in controlling HFPG-attributable MASLD burdens can be learned from countries such as Japan, Italy, and China. Measures such as promoting healthy dietary patterns and lifestyles and conducting health education to enhance public awareness of sugar intake control and the harm of MASLD can be implemented. Targeted interventions can be carried out based on the country’s disease burden trends and the context of its health system.
It is well-known that aging people are more susceptible to T2D, and our study also found that the global burden of HFPG-attributable MASLD was heaviest in people over 50 years old. Previous studies showed that the prevalence of MASLD increased with age42, which was likely to be related to the pathogenesis of MASLD. Metabolic syndrome (MetS) is one of the main risk factors for MASLD43. The presence of each component of MetS and the number of comorbidities increased the risk and severity of MASLD44, and the prevalence of MetS increased with age45,46. A survey in the United States showed that the prevalence of MetS in people over 70 years was as high as 42%47. Besides, the serum total cholesterol and low-density lipoprotein cholesterol levels increased with age48, and dyslipidemia was also very common in patients with MASLD49. Patients with MASLD complicated with T2D and atherogenic dyslipidemia are at risk of increased liver stiffness, which is detrimental to prognosis50. Additionally, although the HFPG-attributable MASLD burden in people aged 20–49 is relatively low in our study, it has nearly doubled in the past 32 years, which also deserves attention. Therefore, we not only recommend attaching importance to the control of HFPG in people over 50 years but also establishing a health concept of sugar control in early adulthood. This will help prevent the occurrence of hyperlipidemia, metabolic syndrome, and atherosclerosis in the causation chains of MASLD to reduce the proportion of them developing MASLD and delay the progression of MASLD to cirrhosis.
The burden of HFPG-attributable MASLD in men was significantly greater than that in women, and the sex difference has gradually expanded over the past 32 years, which was consistent with previous research conclusions on the sex differences in the prevalence of MASLD in patients with T2D51. A cross-sectional study in China also showed that among MASLD patients with T2D, men had higher triglyceride/high-density lipoprotein cholesterol ratios than women did49. We also found that the sex difference peaked at 45–49 years of age and gradually decreased with age thereafter. This age window coincided with the age of menopause, and the underlying mechanism may be related to hormones. A previous study showed that postmenopausal women who received hormone replacement therapy had a significantly lower incidence of MASLD than women who did not receive this treatment52, supporting this interpretation. Besides, we found that the MWRs of disease burden were greater in high, high-middle, and middle SDI regions. This may be because there were differences in dietary habits between men and women in these regions, with women having relatively healthy dietary habits. Previous studies in Poland53 (high SDI), China54(high-middle SDI region), and Brazil55 (middle SDI) all showed that women were more inclined than men to have a high-quality healthy diet. In addition, although the GBD Study excluded the impact of smoking and alcohol consumption on MASLD, men may have a greater burden of MASLD due to a greater opportunity of exposure to other unhealthy lifestyles (such as high work stress) than women. Notably, the burden of HFPG-attributable NAFLD was higher in women than in men in low SDI regions, similar to the pattern of T2D burden56. This may be because women in low SDI regions tend to have limited access to healthcare and poorer metabolic health than men, and national cultural norms always limit their dietary choices57,58. The social restrictions on outdoor physical activity for women in several countries may also contribute to the situation59. Therefore, it is recommended that men (especially those in economically developed regions), postmenopausal women, and women in underdeveloped regions be given more weight as a high-risk group for MASLD and that targeted health education be provided to prevent MASLD and promote health equity.
This study has several limitations. First, since the data in this study were all from the GBD 2021 Study, the reliability of the study depended on the quality and representativeness of its original epidemiological study. Second, GBD collaborators divided the global population by country and territory, and no more detailed divisions (such as urban or rural, race, and so forth) were made. It is difficult to further study the differences in disease burden among urban/rural or racial subgroups within the country.
Conclusions
The burden of HFPG-attributable MASLD has increased significantly worldwide over the past 32 years. The disease burden continued to rise in low, low-middle, and high SDI regions, fluctuated upward at high levels in middle SDI regions, and was relatively low in high-middle SDI regions. The disease burden among people of all ages has increased significantly, with people over 50 years having the heaviest burden. There was a greater disease burden in men (especially in developed regions), and the sex difference peaked at the age of 45–49 years. Since MASLD and T2D often complicate and interact with each other and often synergistically cause adverse clinical outcomes, HFPG should receive increased attention as a modifiable risk factor for MASLD. Each country is recommended to implement targeted health intervention strategies based on its own trends in the burden of HFPG-attributable MASLD and its context of the health system.
Supplementary Information
Acknowledgements
We thank the Global Burden of Disease Study 2021 collaborators and Na Li for her valuable advice on the methodology.
Abbreviations
- ASD
Age-standardized disability-adjusted life-years
- ASDR
Age-standardized disability-adjusted life-years rate
- ASR
Age-standardized rate
- CI
Confidence interval
- CODEm
Cause of Death Ensemble model
- DALY
Disability-adjusted life-year
- GBD
Global burden of disease
- HFPG
High fasting plasma glucose
- ICD
International classification of diseases
- MASLD
Metabolic dysfunction-associated steatotic liver disease
- MetS
Metabolic syndrome
- MWR
Men-to-women ratio
- NAFLD
Non-alcoholic fatty liver disease
- PAF
Population-attributable fraction
- RR
Relative risk
- SDI
Sociodemographic index
- TMREL
Theoretical minimum-risk exposure levels
- UI
Uncertainty interval
- YLD
Years lived with disability
- YLL
Years of life lost
Author contributions
Z.Y. contributed to the study concept and design, data acquisition, analysis, interpretation, supervision, and drafting of the manuscript. Y.Jiang contributed to the data acquisition, analysis, interpretation, and drafting of the manuscript. A.L., X.M., and Y.W. contributed to the data interpretation and drafting of the manuscript. Y.Jin contributed to the study concept and design, obtaining funding, administration, supervision, and critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Gates Foundation (INV-0045085). The funding organization was not involved in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Data availability
The datasets supporting the conclusions of this article are available from the Global Health Data Exchange GBD Results Tool repository (https://ghdx.healthdata.org/gbd-2021).
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: In the original version of this Article, Figure 5 was incorrectly displayed. Full information regarding the corrections made can be found in the correction for this Article.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/25/2024
A Correction to this paper has been published: 10.1038/s41598-024-80624-7
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-72795-0.
References
- 1.Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol.79, 1542–1556. 10.1016/j.jhep.2023.06.003 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology64, 73–84. 10.1002/hep.28431 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Riazi, K. et al. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol.7, 851–861. 10.1016/S2468-1253(22)00165-0 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Chen, H. et al. The global, regional, and national burden and trends of NAFLD in 204 countries and territories: An analysis from global burden of disease 2019. JMIR Public Health Surveill.8, e34809. 10.2196/34809 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazarus, J. V. et al. Advancing the global public health agenda for NAFLD: A consensus statement. Nat. Rev. Gastroenterol. Hepatol.19, 60–78. 10.1038/s41575-021-00523-4 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Terrault, N. A. & Pageaux, G.-P. A changing landscape of liver transplantation: King HCV is dethroned, ALD and NAFLD take over!. J. Hepatol.69, 767–768. 10.1016/j.jhep.2018.07.020 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Gordon, S. C. et al. Disease severity is associated with higher healthcare utilization in nonalcoholic steatohepatitis medicare patients. Am. J. Gastroentero.l115, 562–574. 10.14309/ajg.0000000000000484 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Phisalprapa, P. et al. Economic burden of non-alcoholic steatohepatitis with significant fibrosis in Thailand. BMC Gastroenterol.21, 135. 10.1186/s12876-021-01720-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med.24, 908–922. 10.1038/s41591-018-0104-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantovani, A. et al. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism111, 154170. 10.1016/j.metabol.2020.154170 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Targher, G., Corey, K. E., Byrne, C. D. & Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol.18, 599–612. 10.1038/s41575-021-00448-y (2021). [DOI] [PubMed] [Google Scholar]
- 12.Bedogni, G. et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: The dionysos nutrition and liver study. Hepatology42, 44–52. 10.1002/hep.20734 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Golabi, P. et al. Nonalcoholic fatty liver disease (NAFLD) and associated mortality in individuals with type 2 diabetes, pre-diabetes, metabolically unhealthy, and metabolically healthy individuals in the United States. Metab. Clin. Exp.146, 155642. 10.1016/j.metabol.2023.155642 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Tanase, D. M. et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J. Diabetes Res.2020, 3920196. 10.1155/2020/3920196 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saponaro, C., Gaggini, M. & Gastaldelli, A. Nonalcoholic fatty liver disease and type 2 diabetes: Common pathophysiologic mechanisms. Curr. Diab. Rep.15, 607. 10.1007/s11892-015-0607-4 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Liang, R. et al. The global burden of disease attributable to high fasting plasma glucose in 204 countries and territories, 1990–2019: An updated analysis for the Global Burden of Disease Study 2019. Diabetes Metab. Res. Rev.38, e3572. 10.1002/dmrr.3572 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Rooney, M. R. et al. Global prevalence of prediabetes. Diabetes Care46, 1388–1394. 10.2337/dc22-2376 (2023). [DOI] [PubMed] [Google Scholar]
- 18.GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet403, 2133–2161. 10.1016/S0140-6736(24)00757-8 (2024). [DOI] [PMC free article] [PubMed]
- 19.GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet403, 2162–2203. 10.1016/S0140-6736(24)00933-4 (2024). [DOI] [PMC free article] [PubMed]
- 20.GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet403, 2100–2132. 10.1016/S0140-6736(24)00367-2 (2024). [DOI] [PMC free article] [PubMed]
- 21.Golabi, P., Owrangi, S. & Younossi, Z. M. Global perspective on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis—prevalence, clinical impact, economic implications and management strategies. Aliment. Pharmacol. Ther.59(Suppl 1), S1–S9. 10.1111/apt.17833 (2024). [DOI] [PubMed] [Google Scholar]
- 22.Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol.15, 288–298. 10.1038/s41574-019-0176-8 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Sun, H. et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract.183, 109119. 10.1016/j.diabres.2021.109119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye, L. et al. Global burden of noncommunicable diseases attributable to high fasting plasma glucose. J. Diabetes12, 807–818. 10.1111/1753-0407.13072 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Tinajero, M. G. & Malik, V. S. An update on the epidemiology of type 2 diabetes: A global perspective. Endocrinol. Metab. Clin.50, 337–355 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Tsugane, S. Why has Japan become the world’s most long-lived country: Insights from a food and nutrition perspective. Eur. J. Clin. Nutr.75, 921–928. 10.1038/s41430-020-0677-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martini, D., Godos, J., Bonaccio, M., Vitaglione, P. & Grosso, G. Ultra-processed foods and nutritional dietary profile: A meta-analysis of nationally representative samples. Nutrients10.3390/nu13103390 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolb, H. & Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med.15, 131. 10.1186/s12916-017-0901-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hales, C. M., Carroll, M. D., Fryar, C. D. & Ogden, C. L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief Natl. Center Health Stat.360, 1 (2020). [PubMed] [Google Scholar]
- 30.Zhou, J. et al. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology71, 1851–1864 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Xia, Y. et al. Insoluble dietary fibre intake is associated with lower prevalence of newly-diagnosed non-alcoholic fatty liver disease in Chinese men: a large population-based cross-sectional study. Nutr. Metab. (Lond)17, 4. 10.1186/s12986-019-0420-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neshatbini Tehrani, A. et al. The effect of soy isoflavones supplementation on metabolic status in patients with non-alcoholic fatty liver disease: A randomized placebo controlled clinical trial. BMC Public Health24, 1362. 10.1186/s12889-024-18812-3 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohro, T. et al. The Japanese national health screening and intervention program aimed at preventing worsening of the metabolic syndrome. Int. Heart J.49, 193–203 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Tsushita, K. et al. Rationale and descriptive analysis of specific health guidance: The nationwide lifestyle intervention program targeting metabolic syndrome in Japan. J. Atheroscler.Thromb.25, 308–322. 10.5551/jat.42010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papamichou, D., Panagiotakos, D. B. & Itsiopoulos, C. Dietary patterns and management of type 2 diabetes: A systematic review of randomised clinical trials. Nutr. Metab. Cardiovasc. Dis.29, 531–543. 10.1016/j.numecd.2019.02.004 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Gelli, C. et al. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J. Gastroenterol.23, 3150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Health Commission of the People’s Republic of China. Interpretation of the “Healthy China 2020 Initiative”, <http://www.nhc.gov.cn/wjw/zcjd/201304/f70f8fc52d6a422494789f65c7ad134d.shtml>
- 38.National Health Commission of the People’s Republic of China. Interpretation of the “Healthy China 2030 Initiative”, <http://www.nhc.gov.cn/guihuaxxs/s3586s/201610/a2325a1198694bd6ba42d6e47567daa8.shtml>
- 39.Chinese National Bureau of Disease Control and Prevention. Notice on Issuing the “China Healthy Lifestyle for All (2017–2025)”, <http://www.nhc.gov.cn/jkj/s5878/201704/e73c1934c7f84c709e445f01bf832b17.shtml>
- 40.Leong, D. P. et al. Reducing the global burden of cardiovascular disease, part 2: Prevention and treatment of cardiovascular disease. Circ. Res.121, 695–710 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Paul, S. & Paul, S. Transition in dietary quality: evidence from India. British Journal of Nutrition, 1–13 (2022). [DOI] [PubMed]
- 42.Paschos, P. & Paletas, K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia13, 9 (2009). [PMC free article] [PubMed] [Google Scholar]
- 43.Ismail, M. H. Nonalcoholic fatty liver disease and type 2 diabetes mellitus: the hidden epidemic. Am. J. Med. Sci.341, 485–492 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Kosmalski, M., Ziółkowska, S., Czarny, P., Szemraj, J. & Pietras, T. The coexistence of nonalcoholic fatty liver disease and type 2 Diabetes mellitus. J. Clin. Med.11, 1375 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae, C.-Y. et al. Biological age and lifestyle in the diagnosis of metabolic syndrome: The NHIS health screening data, 2014–2015. Sci. Rep.11, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meigs, J. B. et al. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and Framingham Offspring Studies. Diabetes52, 2160–2167 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Ford, E. S., Giles, W. H. & Dietz, W. H. Prevalence of the metabolic syndrome among US adults: Findings from the third national health and nutrition examination survey. Jama287, 356–359 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Liu, H.-H. & Li, J.-J. Aging and dyslipidemia: A review of potential mechanisms. Ageing Res. Rev.19, 43–52 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Yi, M., Chen, R. P., Yang, R. & Chen, H. Increased prevalence and risk of non-alcoholic fatty liver disease in overweight and obese patients with Type 2 diabetes in South China. Diabetic Med.34, 505–513 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Julián, M. T. et al. Atherogenic dyslipidemia, but not hyperglycemia, is an independent factor associated with liver fibrosis in subjects with type 2 diabetes and NAFLD: a population-based study. Eur. J. Endocrinol.184, 587–596 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Lonardo, A. et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology70, 1457–1469. 10.1002/hep.30626 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark, J. M., Brancati, F. L. & Diehl, A. M. Nonalcoholic fatty liver disease. Gastroenterology122, 1649–1657 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Krzemińska, S., Lomper, K., Chudiak, A., Ausili, D. & Uchmanowicz, I. The association of the level of self-care on adherence to treatment in patients diagnosed with type 2 diabetes. Acta Diabetol.58, 437–445 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, Y. et al. Geographic, gender, and seasonal variation of diabetes: A nationwide study with 1.4 million participants. J. Clin. Endocrinol. Metab.106, e4981–e4992 (2021). [DOI] [PubMed] [Google Scholar]
- 55.de Oliveira Otto, M. et al. Global Burden of Diseases, Injuries, and Risk Factors Metabolic Risk Factors of Chronic Diseases Expert Group and Nutrition and Chronic Diseases Expert Group (NutriCoDE). The impact of dietary and metabolic risk factors on cardiovascular diseases and type 2 diabetes mortality in Brazil. PLoS One11, 0151503 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie, J. et al. Global burden of type 2 diabetes in adolescents and young adults, 1990–2019: Systematic analysis of the global burden of disease study 2019. BMJ379, e072385. 10.1136/bmj-2022-072385 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hasan, S. U. & Siddiqui, M. A. R. Epidemiology of diabetes mellitus in Pakistan: A systematic review protocol. BMJ Open14, e079513. 10.1136/bmjopen-2023-079513 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kennedy, E. et al. Gender inequalities in health and wellbeing across the first two decades of life: An analysis of 40 low-income and middle-income countries in the Asia-Pacific region. Lancet Glob. Health8, e1473–e1488. 10.1016/S2214-109X(20)30354-5 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Misra, A., Ramchandran, A., Jayawardena, R., Shrivastava, U. & Snehalatha, C. Diabetes in South Asians. Diabet. Med.31, 1153–1162. 10.1111/dme.12540 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are available from the Global Health Data Exchange GBD Results Tool repository (https://ghdx.healthdata.org/gbd-2021).