Abstract
Despite being a major component of the pseudorabies virus tegument, VP22 is not required for PRV replication, virulence, or neuroinvasion (T. del Rio, H. C. Werner, and L. W. Enquist, J. Virol. 76:774-782, 2002). In the absence of VP22, tegument assembly compensates in a limited fashion with increased incorporation of cellular actin. Infection of epithelial cell lines expressing fluorescent actin fusion proteins resulted in the incorporation of filamentous and nonfilamentous actin into individual virions that were predominately light, noninfectious particles. We conclude that cellular actin is incorporated in the tegument of wild-type virions and is part of a compensation mechanism for VP22-null virions.
The herpes simplex virus type 1 and bovine herpesvirus homologues of VP22, encoded by the UL49 gene, are major constituents of the tegument layer (13, 27, 31). However, deletion of UL49 from pseudorabies virus (PRV) exhibited no phenotype in numerous in vivo or in vitro assays. For example, the 50% lethal dose and spread were indistinguishable from the parental virus following infection of mouse flank, rat eye, and chicken allantoic membrane (2, 6, 9; unpublished results). Additionally, surface presentation of major histocompatibility complex class I antigen was not affected by loss of VP22 (reference 30 and unpublished results). Virions lacking VP22 are able to infect cells and animals as efficiently as wild-type virus, which suggests that VP22 has no defined or fixed structural interactions during tegument assembly. Consistent with this idea, incorporation of a green fluorescent protein (GFP)-VP22 fusion protein in individual heavy or light particles was heterogeneous, ranging from undetectable to substantial (10). We therefore determined whether PRV VP22 is a major component of a population of virions and whether any proteins may compensate for its loss.
Evidence for tegument compensation in VP22-null virions.
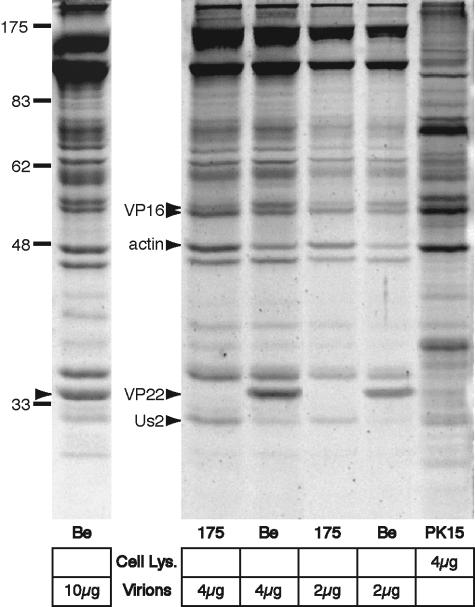
For comparison of virion protein profiles, extracellular virions were purified by centrifugation on a linear tartrate gradient (5 to 20%) (10), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 12.5%), and stained with Sypro Ruby Red (Molecular Probes). Sypro stain is fluorescent and reacts with primary amines at an acidic pH similar to a Coomassie blue binding action (3). Proteins were detected using UV illumination, and VP22 appeared as a single 34-kDa band in wild-type PRV Becker (Be) virions but not in VP22-null virions (PRV 175) (Fig. 1, left) (9). Clearly, VP22 is a major component of wild-type virions (Fig. 1, arrowhead in left lane [10 μg Be]) and a significant portion of the tegument mass (Fig. 2). We therefore investigated the protein profile of VP22-null (PRV 175) virions for differences relative to wild-type virions that may compensate for the loss of VP22. In VP22-null virions, three proteins of apparent molecular masses of 28, 46, and 53 kDa increased relative to other virion proteins (Fig. 1, right arrowheads). The 28-kDa and 46-kDa compensating proteins were sequenced by tandem mass spectrometry at the Harvard Microchemistry Facility and identified as PRV Us2 (a protein with a molecular weight of 28,000 [28K] from suid herpesvirus 1) and cellular actin (actin gamma; cytoskeletal type 5 from African clawed frog), respectively. The cellular actin found in lysates of PK15 cells migrated identically to actin identified in purified virions (Fig. 1). PRV Us2 protein is prenylated in infected cells, but the nonprenylated form is packaged in the tegument of virions (8). The 53-kDa protein was identified as PRV tegument protein VP16 (14) and reacts with a chicken polyclonal antibody against His-tagged PRV VP16 (PAS1200; Pro Science, Inc.) (data not shown). While virion incorporation of the 53-kDa form of VP16 increased following the loss of VP22, the 55-kDa form of VP16 was unchanged (Fig. 1). Western blot analysis confirmed the identity and relative increase of actin and Us2 in VP22-null virions, as well as the decrease of Us3 protein (data not shown).
FIG. 1.
The compensating pool of tegument proteins in PRV VP22-null virions is specific and includes cellular actin. Wild-type PRV Becker (Be) and VP22-null (PRV 175) virions were purified by centrifugation on a linear tartrate gradient (5 to 20%), and the indicated amounts were separated by SDS-PAGE. Total protein was stained using Sypro Ruby Red and detected by UV illumination. The PRV VP22 homologue was identified as a 34-kDa virion protein and a major component of Be virions (10 μg, left arrowhead). The levels of three proteins increased in VP22-null (PRV 175) virions in comparison to wild type (Be). Two of these proteins, 28 and 46 kDa in size, were excised and identified using mass spectrometry as the PRV Us2 tegument protein and cellular actin, respectively (right arrowheads). Cellular actin was confirmed to be a major component of extracellular virions. A 53-kDa protein also increased in VP22-null virions and was identified as the tegument protein VP16 (arrowhead). The migration positions of molecular mass markers are shown in kilodaltons.
FIG. 2.
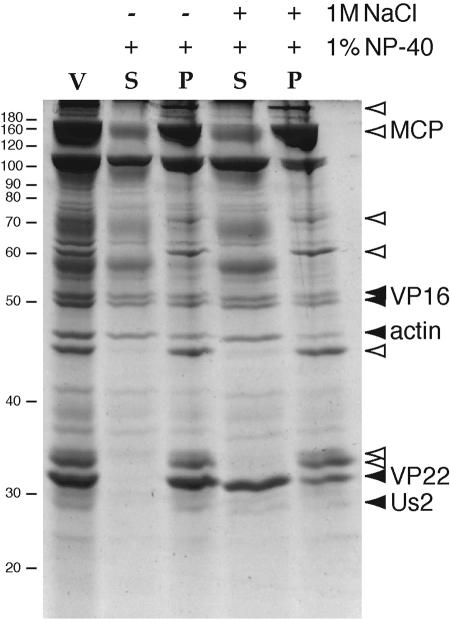
Cellular actin in virions fractionates as a tegument protein. Purified extracellular Becker virions (lane V; 10 μg) were treated with either 1% NP-40 or 1% NP-40 plus 1 M NaCl, from which soluble (S) and pellet (P) fractions were isolated by high-speed centrifugation. Proteins were separated by SDS-PAGE and Sypro staining was used to visualize total protein. Protein bands associated with the capsid structure (open arrowheads) pellet with or without 1 M NaCl and include the MCP. The migration positions of molecular mass markers are shown on the left in kilodaltons.
Actin fractionates with components of the tegument layer in a virion sedimentation assay.
Cellular actin has been previously shown to be a major tegument protein of Epstein-Barr virus (18). To verify the tegument location of actin in PRV, we sedimented 10 μg of purified Becker virions (100-μl volume; 100,000 × g for 30 min at 4°C) (Beckman TLA-100 rotor) de-enveloped with 1% NP-40 detergent and analyzed the protein distribution between supernatant and pellet fractions by SDS-PAGE and Sypro Ruby Red staining. VP22 was not detected in the detergent-solubilized fraction but was abundantly released by treatment with detergent plus 1 M NaCl (9). Unlike VP22, the tegument proteins VP16 and Us2, as well as cellular actin, were partially solubilized by 1% NP-40 treatment (Fig. 2, filled arrowheads). The major capsid protein (MCP), identified by Western blot analysis (data not shown), associated with the pellet in the presence or absence of 1 M NaCl (Fig. 2). At least six other proteins behaved similarly to MCP, suggesting that the intact capsid was pelleted (Fig. 2, open arrowheads). The largest protein is likely to be the VP1/2 tegument protein homologue (>300 kDa [21]), which remains tightly associated with the capsid (Fig. 2). Despite treatment with 1 M NaCl, a fraction of the tegument proteins VP16, Us2, and actin remains associated with the capsid pellet. It is notable that the fractionation properties of virion-associated actin in this assay are similar to those of Us2 and VP16, the other tegument proteins whose incorporation is also detectably elevated in VP22-null virions. One idea is that actin, Us2, and VP16 may form patches or domains of tegument beneath the viral envelope. Another virion component of approximately 105 kDa was significantly solubilized by 1% NP-40 treatment (Fig. 2) and may also be a component of the same outer domain of the tegument layer. We predicted that VP22 would be a component of these patches, but the sedimentation assay was not consistent with this prediction. One explanation for this discrepancy is that the sedimentation properties of VP22 extracted from virions do not reflect these proposed outer tegument patches but, rather, other properties of the protein. For example, VP22 has been reported to interact with microtubules, cell membranes, nonmuscle myosin IIA, and not only DNA but also condensed chromosomes and the cellular DNA chaperone TAF-1 (5, 11, 12, 34, 35), and these varied interaction properties may affect the outcome of the biochemical assay presented here. We conclude that cellular actin in virions fractionates with the tegument proteins Us2 and VP16. Collectively, these proteins may comprise a distinctive outer tegument shell solubilized by detergent.
Direct visualization of GFP-actin in individual wild-type virions.
We investigated whether ectopically expressed GFP-actin could be incorporated into wild-type virions and whether there is a preference to incorporate actin monomers or filaments. A commercially available pEGFP-actin (with enhanced GFP; BD-Clontech) construct was used to produce PK15 cell lines directing incorporation of GFP fused to human β-actin into microfilaments. We also introduced a Gly13Arg (G13R) point mutation previously shown to prevent incorporation into filaments (26). During site-directed mutagenesis (Transformer Site-Directed Mutagenesis Kit; BD-Clontech), a second site mutation, Asp11Glu (D11E), which does not affect actin incorporation into filaments or interaction with myosin-S1 (29), arose spontaneously.
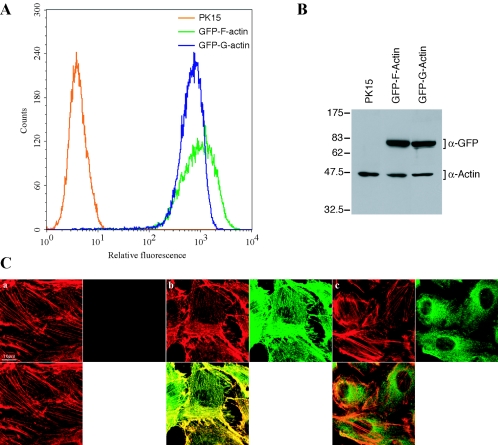
PK15 cells stably expressing the fusion proteins GFP-F-actin (GFP-filamentous-actin from pEGFP-actin) or GFP-G-actin (GFP-globular-actin from pTD58) were made by the calcium phosphate transfection method (15) and subsequent expansion under G418 selection (800 μg/ml) (geneticin; Gibco-BRL). Two homogenous cell lines expressing comparable levels of fusion proteins, GFP-F-actin line 74 and GFP-G-actin line 52, were selected by several rounds of fluorescence-activated cell sorting and expansion (Fig. 3A). Despite easy visualization by fluorescence microscopy, ectopic GFP-actin was not readily detected by Western blotting using the actin AC15 antibody (Sigma) (data not shown). However, the fusion proteins were detected using a GFP monoclonal antibody (Chemicon) (Fig. 3B). Phalloidin-rhodamine staining (Molecular Probes) detected filamentous actin and revealed the incorporation of GFP-F-actin into microfilaments (Fig. 3C, frames a and b). In contrast, GFP-G-actin is not incorporated into filamentous actin (Fig. 3C, frame c), is largely nonnuclear, and likely remains associated with the cytoplasmic pool of monomeric or globular actin. GFP-F-actin must also still exist in the globular state, but as evident by the localization of GFP-F-actin in PK15 cells (Fig. 3C), the steady-state concentration of the globular form is reduced.
FIG. 3.
Stable expression of filamentous and nonfilamentous GFP-actin in PK15 epithelial cell lines. Expression of GFP-F-actin (filamentous) or the GFP-G-actin (globular) fusion proteins in PK15 cells. (A) Flow cytometric analysis of 10,000 nonfixed PK15 cells or cells from lines expressing similar levels of either GFP-F-actin (cell line 74) or GFP-G-actin (cell line 52). These lines were isolated following several rounds of fluorescence-activated cell sorting and expansion. (B) Cell lysates of PK15 cells and the cell lines were analyzed by Western blot analysis using monoclonal antibodies to β-actin and GFP. (C) Monolayers of PK15 cells (a) and cell lines expressing similar levels of GFP-F-actin (b) or GFP-G-actin (c) were grown on glass coverslips. Cells were fixed and stained for filamentous actin with rhodamine-conjugated phalloidin (red) and, along with GFP autofluorescence (green), imaged by confocal microscopy.
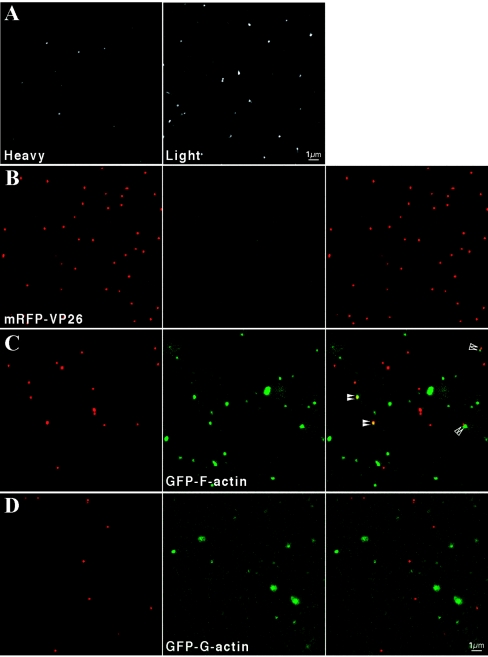
To determine virion incorporation and detection levels, the nonsorted cell line GFP-F-actin 2, which was available before the sorted lines but exhibited heterogeneous amounts of fluorescence (data not shown), was first infected with PRV Becker. Extracellular virions produced from GFP-actin-expressing cells were purified as above and diluted with an equal volume of glycerol. The punctate GFP autofluorescence of single virions was revealed by confocal microscopy and was brighter in particles isolated from the light band relative to those from the heavy band (Fig. 4A). Since light particles contain more tegument mass than heavy particles and incorporate an increased amount of the GFP-VP22 tegument fusion (10), it is likely that light particles contain more actin and incorporate more GFP-actin. We tested this using a recombinant PRV expressing a fluorescent red capsid (PRV 180), which enables the direct identification of individual heavy and light particles (10). When isolated from infected PK15 cells, red capsid virions exhibit no green fluorescence (Fig. 4B). Occasionally, GFP-F-actin colocalized with red capsid puncta (Fig. 4C, filled arrowheads), but the majority of the green signal localized to light particles. Similar to our previous observations for GFP-VP22, we also occasionally observed GFP-F-actin puncta juxtaposed to red capsid puncta (Fig. 4C, open arrowheads), reminiscent of the asymmetrical tegument cap described by Grunewald et al. (17). Incorporated GFP-G-actin was more difficult to detect and, in this assay, was only observed in light particles (Fig. 4D). We have not established the lower limits of detection of GFP-actin; therefore, negative results must be interpreted accordingly. However, similar to results for GFP-VP22, an increased amount of GFP-actin is detected in light particles relative to heavy particles.
FIG. 4.
Detection of filamentous and nonfilamentous GFP-actin in single virions. Becker virions produced from the infection of heterogeneous GFP-F-actin expressing cells (line 2) were purified on a linear tartrate gradient (5 to 20%). (A) Particles isolated from the heavy (left) or light (right) bands were combined with an equal volume of glycerol and imaged by confocal microscopy. Particles from the light band exhibited increased fluorescence relative to those from the heavy band. (B) Recombinant PRV 180 virions, containing an mRFP-VP26 (red-capsid) fusion and isolated from infected PK15 cells, exhibited red fluorescence (left) but no detectable green fluorescence (middle). (C) A similar infection and analysis of homogenous GFP-F-actin-expressing cells (line 74) revealed the green fluorescence overwhelmingly localized to light particles (right panel, merged red and green). Occasionally, GFP-F-actin was observed to colocalize with red capsid puncta (filled arrowheads) or to be juxtaposed to red capsid (open arrowheads) in heavy particles. (D) PRV 180 infection of GFP-G-actin cells (line 52) also revealed that green fluorescence predominantly localized to light particles.
We demonstrate that actin incorporation increases in the absence of the major tegument protein VP22 and fractionates with other tegument proteins. In addition, GFP-actin is more readily detectable in light particles, which contain an increased tegument mass relative to infectious heavy particles. At present, given the current technology and lack of understanding of light-particle formation, we cannot easily quantitate and compare differences in single components of the tegument mass of light and heavy particles. The fact that we were able to detect GFP-actin in light particles and only occasionally in heavy particles is consistent with the idea that light particles contain more tegument mass and, in addition, that we are at or near the detection limit of GFP-actin in the tegument. Taken together, the data demonstrate that the majority of actin in PRV virions resides within the tegument layer. These data confirm and extend previous results suggesting that actin is internally localized in the pseudorabies virion (32). Virion-associated actin has been reported in other herpesviruses (1, 17, 19, 32, 36, 38) and other enveloped viruses including paramyxovirus, retrovirus, and rhabdovirus (4, 23, 24, 25, 28, 33). One hypothesis to explain this general finding is that actin plays an active role in virus budding. For example, it has been proposed that filamentous actin depolymerization occurs coincident with human immunodeficiency virus virion budding (7). Similarly, microfilaments of a size consistent with polymerized actin have been visualized within the reticular structure of the herpes simplex virion tegument (17). It may be that actin microfilament depolymerization occurs only at the forming herpesvirus envelope-tegument interface, while polymerized actin remains embedded in the outer tegument layer following budding. However, this model does not provide a rationale for the incorporation of globular actin. Therefore, we cannot rule out the possibility that some tegument-associated actin is acquired passively during secondary envelopment.
Actin is a dynamic component of pseudorabies virus assembly whose levels of incorporation can be altered by the deletion of a major tegument protein (Fig. 1). One key question concerns the mechanism that can provide such flexibility in outer tegument formation. While little is understood about tegument assembly and incorporation in light particles, it is likely that the innermost tegument layer consisting in part of UL36 and UL37 proteins interacts with the capsid in heavy particles (20, 37). This conclusion is supported by entry assays demonstrating that UL36 (VP1/2), UL37, and Us3 remain associated with cytoplasmic capsids following entry and penetration (16, 22). In addition, these same studies found that UL11, UL47 (VP13/14), UL48 (VP16), and UL49 (VP22) tegument proteins are lost following virion entry and uncoating. These proteins are likely to be components of the outer tegument layer. One interpretation of these data and our findings is that cellular actin, Us2, and the 53-kDa form of VP16 are components of the outer tegument layer that contains VP22. We have reported that the incorporation of a GFP-VP22 fusion protein in single virions ranged from undetectable to highly abundant (10). Therefore, the composition of the outer tegument shell, which may be equivalent to the tegument cap (17), appears to be flexible and heterogeneous. We postulate that the 55-kDa form of VP16, whose incorporation does not increase in VP22-null virions, is not a component of the flexible network of protein interactions in this outer tegument layer. At present, it is unclear whether the 55-kDa form of VP16 is associated with the inner tegument layer as predicted by this model of tegument assembly. It is also unknown whether the flexible outer tegument network is present only at the tegument-envelope interface or at other sites within the tegument. It is possible that actin-binding sites in the outer tegument layer increase in the absence of VP22, but we have been unable to localize or resolve such putative binding sites. Viral proteins involved in the assembly process may interact with actin and lead to specific incorporation of a fraction of the abundant cellular protein in the tegument. Nevertheless, our data are consistent with the formation of a dynamic, flexible outer tegument shell in herpesvirus particles. One prediction, therefore, is that PRV virions lacking other specific tegument components may contain variable amounts of actin. Further study of the origin and function of actin in PRV virions is likely to provide insight into herpes virion assembly and envelopment.
Acknowledgments
We acknowledge Joe Goodhouse for expert help with confocal microscopy. We thank Alex Flood for purification of the His-tagged VP16 used for antibody production and for the gift of a monoclonal antibody specific for PRV major capsid protein.
This work was supported by National Institutes of Health (NIH) grant 2ROI-NS33506 to L.W. Enquist and an NIH Research Supplement for Underrepresented Minority to T. del Rio.
REFERENCES
- 1.Baldick, C. J., Jr., and T. Shenk. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 70:6097-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banfield, B. W., G. S. Yap, A. C. Knapp, and L. W. Enquist. 1998. A chicken embryo eye model for the analysis of alphaherpesvirus neuronal spread and virulence. J. Virol. 72:4580-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berggren, K., E Chernokalskaya, T. H. Steinberg, C. Kemper, M. F. Lopez, Z. Diwu, R. P. Haugland, and W. F. Patton. 2000. Background-free, high sensitivity staining of proteins in one- and two-dimensional sodium dodecyl sulfate-polyacrylamide gels using a luminescent ruthenium complex. Electrophoresis 21:2509-2521. [DOI] [PubMed] [Google Scholar]
- 4.Bohn, W., K. Mannweiler, H. Hohenberg, and G. Rutter. 1987. Replica-immunogold technique applied to studies on measles virus morphogenesis. Scanning Microsc. 1:319-330. [PubMed] [Google Scholar]
- 5.Brignati, M. J., J. S. Loomis, J. W. Wills, J., and R. J. Courtney. 2003. Membrane association of VP22, a herpes simplex virus type 1 tegument protein. J. Virol. 77:4888-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brittle, E. E., A. E. Reynolds, and L. W. Enquist. 2004. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J. Virol. 78:12951-12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, C., O. A. Weisz, D. B. Stolz, S. C. Watkins, and R. C. Montelaro. 2004. Differential effects of actin cytoskeleton dynamics on equine infectious anemia virus particle production. J. Virol. 78:882-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clase, A. C., M. G. Lyman, T. del Rio, J. A. Randall, C. M. Calton, L. W. Enquist, and B. W. Banfield. 2003. The pseudorabies virus Us2 protein, a virion tegument component, is prenylated in infected cells. J. Virol. 77:12285-12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Rio, T., H. C. Werner, and L. W. Enquist. 2002. The pseudorabies virus VP22 homologue (UL49) is dispensable for virus growth in vitro and has no effect on virulence and neuronal spread in rodents. J. Virol. 76:774-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Rio, T., T. H. Ch'ng, A. E. Flood, S. P. Gross, and L. W. Enquist. 2005. Heterogeneity of a fluorescent tegument component in single pseudorabies virions and enveloped axonal assemblies. J. Virol. 79:3903-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott, G., and P. O'Hare. 1998. Herpes simplex virus type 1 tegument protein VP22 induces the stabilization and hyperacetylation of microtubules. J. Virol. 72:6448-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott, G., and P. O'Hare. 1999. Live-cell analysis of a green fluorescent protein-tagged herpes simplex virus infection. J. Virol. 73:4110-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott, G. D., and D. M. Meredith. 1992. The herpes simplex virus type 1 tegument protein VP22 is encoded by gene UL49. J. Gen. Virol. 73:723-726. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs, W., H. Granzow, B. G. Klupp, M. Kopp, and T. C. Mettenleiter. 2002. The UL48 tegument protein of pseudorabies virus is critical for intracytoplasmic assembly of infectious virions. J. Virol. 76:6729-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham, R. L., and A. S. Van der Eb. 1973. A new technique for the assay of infection of human adenovirus 5 DNA. Virology 52:456-467. [DOI] [PubMed] [Google Scholar]
- 16.Granzow, H., B. G. Klupp, and T. C. Mettenleiter. 2005. Entry of pseudorabies virus: an immunogold-labeling study. J. Virol. 79:3200-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grunewald, K., P. Desai, D. C. Winkler, J. B. Heymann, D. M. Belnap, W. Baumeister, and A. C. Steven. 2003. Three-dimensional structure of herpes simplex virus from cryo-electron tomography. Science 302:1396-1398. [DOI] [PubMed] [Google Scholar]
- 18.Johannsen, E., M. Luftig, M. R. Chase, S. Weicksel, E. Cahir-McFarland, D. Illanes, D. Sarracino, and E. Kieff. 2004. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 101:16286-16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kattenhorn, L. M., R. Mills, M. Wagner, A. Lomsadze, V. Makeev, M. Borodovsky, H. L. Ploegh, and B. M. Kessler. 2004. Identification of proteins associated with murine cytomegalovirus virions. J. Virol. 78:11187-11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klupp, B. G., W. Fuchs, H. Granzow, R. Nixdorf, and T. C. Mettenleiter. 2002. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J. Virol. 76:3065-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klupp, B. G., C. J. Hengartner, T. C. Mettenleiter, and L. W. Enquist. 2004. Complete, annotated sequence of the pseudorabies virus genome. J. Virol. 78:424-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luxton, G. W. G., S. Haverlock, K. E. Coller, S. E. Antinone, A. Pincetic, and G. A. Smith. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. USA 102:5832-5837. [DOI] [PMC free article] [PubMed]
- 23.Naito, S., and S. Matsumoto. 1978. Identification of cellular actin within the rabies virus. Virology 91:151-163. [DOI] [PubMed] [Google Scholar]
- 24.Orvell, C. 1980. Structural polypeptides of canine distemper virus. Arch. Virol. 66:193-206. [DOI] [PubMed] [Google Scholar]
- 25.Ott, D. E., L. W. Coren, B. P. Kane, L. K. Busch, D. G. Johnson, R. C. Sowder II, E. N. Chertova, L. O. Arthur, and L. E. Henderson. 1996. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J. Virol. 70:7734-7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posern, G., A. Sotiropoulos, and R. Treisman. 2002. Mutant actins demonstrate a role for unpolymerized actin in control of transcription by serum response factor. Mol. Biol. Cell 13:4167-4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren, X., J. S. Harms, and G. A. Splitter. 2001. Tyrosine phosphorylation of bovine herpesvirus 1 tegument protein VP22 correlates with the incorporation of VP22 into virions. J. Virol. 75:9010-9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagara, J., S. Tsukita, S. Yonemura, and A. Kawai. 1995. Cellular actin-binding ezrin-radixin-moesin (ERM) family proteins are incorporated into the rabies virion and closely associated with viral envelope proteins in the cell. Virology 206:485-494. [DOI] [PubMed] [Google Scholar]
- 29.Solomon, T. L., L. R. Solomon, L. S. Gay, and P. A. Rubenstein. 1988. Studies on the role of actin's aspartic acid 3 and aspartic acid 11 using oligodeoxynucleotide-directed site-specific mutagenesis. J. Biol. Chem. 263:19662-19669. [PubMed] [Google Scholar]
- 30.Sparks-Thissen, R. L., and L. W. Enquist. 1999. Differential regulation of Dk and Kk major histocompatibility complex class I proteins on the cell surface after infection of murine cells by pseudorabies virus. J. Virol. 73:5748-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spear, P. G., and B. Roizman. 1972. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpes virion. J. Virol. 9:143-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong, M. L., and C. H. Chen. 1998. Evidence for the internal location of actin in the pseudorabies virion. Virus Res. 56:191-197. [DOI] [PubMed] [Google Scholar]
- 33.Vainiopaa, R., B. Ziola, and A. Salmi. 1978. Measles virus polypeptides in purified virions and in infected cells. Acta Pathol. Microbiol. Scand. Sect. B 86B:379-385. [DOI] [PubMed] [Google Scholar]
- 34.van Leeuwen, H., G. Elliott, and P. O'Hare. 2002. Evidence of a role for nonmuscle myosin II in herpes simplex virus type 1 egress. J. Virol. 76:3471-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Leeuwen, H., M. Okuwaki, R. Hong, D. Chakravarti, K. Nagata, and P. O'Hare. 2003. Herpes simplex virus type 1 tegument protein VP22 interacts with TAF-I proteins and inhibits nucleosome assembly but not regulation of histone acetylation by INHAT. J. Gen. Virol. 84:2501-2510. [DOI] [PubMed] [Google Scholar]
- 36.Varnum, S. M., D. N. Streblow, M. E. Monroe, P. Smith, K. J. Auberry, L. Pasa-Tolic, D. Wang, D. G. Camp II, K. Rodland, S. Wiley, W. Britt, T. Shenk, R. D. Smith, and J. A. Nelson. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 78:10960-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou, Z. H., D. H. Chen, J. Jakana, F. J. Rixon, and W. Chiu. 1999. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J. Virol. 73:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu, F. X., J. M. Chong, L. Wu, and Y. Yuan. 2005. Virion proteins of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:800-811. [DOI] [PMC free article] [PubMed] [Google Scholar]