Abstract
Glucagon-like peptide-1 (GLP-1) analogs are important therapeutics for type 2 diabetes and obesity. Ecnoglutide (XW003) is a novel, long-acting GLP-1 analog. We conducted a Phase 2, randomized, double-blind, placebo-controlled study enrolling 145 adults with T2DM. Participants were randomized to 0.4, 0.8, or 1.2 mg ecnoglutide or placebo as once-weekly injections for 20 weeks. The primary objective was to evaluate the efficacy of ecnoglutide, as measured by HbA1c change from baseline at Week 20. Secondary endpoints included body weight, glucose and lipid parameters, as well as safety. We show that, at end of treatment, the 0.4, 0.8, and 1.2 mg groups had statistically significant HbA1c reductions from baseline of −1.81%, −1.90%, and −2.39%, respectively, compared to −0.55% for placebo (P < 0.0001). At end of treatment, 71.9% of the 1.2 mg group had HbA1c ≤ 6.5% versus 9.1% on placebo, and 33.3% had body weight reductions ≥5% versus 3.0% for placebo. Ecnoglutide was generally safe and well tolerated. China Drug Trials Registry CTR20211014.
Subject terms: Type 2 diabetes, Obesity, Drug development
A Phase 2, randomized, placebo-controlled study evaluated novel GLP-1 analog ecnoglutide in adults with type 2 diabetes. Here we show that ecnoglutide significantly reduced HbA1c from baseline up to -2.39% compared to -0.55% for placebo (P<0.0001), with a safety profile similar to approved GLP-1 analogs.
Introduction
Type 2 diabetes mellitus (T2DM) is an important global health problem, with approximately 483 million adults currently living with the disease. The prevalence of diabetes is increasing, with an estimated 783 million cases expected worldwide in 2045. In China, over 140 million people were living with diabetes in 2021. Diabetes can lead to serious health complications, including retinopathy, nephropathy, neuropathy, and cardiovascular disease, with approximately 6.7 million deaths attributable to the condition annually1.
Over the past two decades, glucagon-like peptide-1 (GLP-1) analogs have been developed as a key therapeutic for T2DM and obesity. GLP-1 analogs approved by the US Food and Drug Administration for the treatment of T2DM include exenatide (Byetta®), liraglutide (Victoza®), lixisenatide (Adlyxin®), dulaglutide (Trulicity®) and semaglutide (Ozempic® and Rybelsus®). Semaglutide (Wegovy®) and liraglutide (Saxenda®) are also approved for the management of obesity. Most GLP-1 analogs are administered by injection, whereas Rybelsus® is a once-daily oral tablet.
GLP-1 analogs mimic the activity of the natural peptide incretin hormone, which is produced by intestinal L-cells in response to a meal. Incretins act to enhance glucose-stimulated insulin secretion and lower blood glucose levels, as well as to slow gastric emptying and promote a sense of fullness that reduces appetite2. While the native GLP-1 peptide is rapidly degraded by dipeptidyl peptidase-IV (DPP-4), with a half-life of approximately 2 min, recombinant analogs have modifications to improve plasma stability, resulting in a half-life as long as 1 week3.
Next generation GLP-1 therapeutics aim to improve efficacy and tolerability, as well as maximize convenience for patients by focusing on the frequency of dosing and route of administration. In addition, peptide sequence optimization seeks to overcome the high cost and complexity of manufacturing for some recombinant peptides that contain unnatural amino acid substitutions, such as semaglutide.
Ecnoglutide (XW003) is a modified GLP-1 (7–37) peptide containing an alanine to valine substitution at position 8, as well as an 18-C fatty acid conjugation at the lysine 30 side chain4. These modifications promote the stability and activity of the peptide. Ecnoglutide is composed entirely of natural amino acids, which facilitates manufacturing compared to semaglutide. Full-length ecnoglutide peptide can be synthesized recombinantly, requiring fewer steps and likely lower costs than semaglutide. The ecnoglutide valine substitution also biases GLP-1 receptor signaling towards cyclic adenosine monophosphate (cAMP) induction over β-arrestin recruitment and receptor internalization4. Ecnoglutide therefore differs from approved single GLP-1 peptide analogs, which are full agonizts of both pathways5. Dual incretin receptor agonists (such as GLP-1/glucose-dependent insulinotropic polypeptide [GIP] analog tirzepatide) also show cAMP bias6. In preclinical models, ecnoglutide showed significant improvements in glucose control and body weight reduction compared to the unbiased analog semaglutide4. Biased agonism is hypothesized to also promote clinical efficacy.
In a Phase 1 clinical trial in healthy participants, once-weekly injection of ecnoglutide demonstrated a favorable safety, tolerability, and pharmacokinetics profile4.
Here we report a randomized, double-blind, placebo-controlled, Phase 2 clinical trial of ecnoglutide as a once-weekly injection designed to explore the efficacy and safety in patients with T2DM inadequately controlled with diet and exercise alone or with a single oral hypoglycemic agent. We show that ecnoglutide was generally safe and well tolerated in this population and resulted in significant improvements in blood glucose, HbA1c levels, and body weight reductions compared to placebo.
Results
Study participants
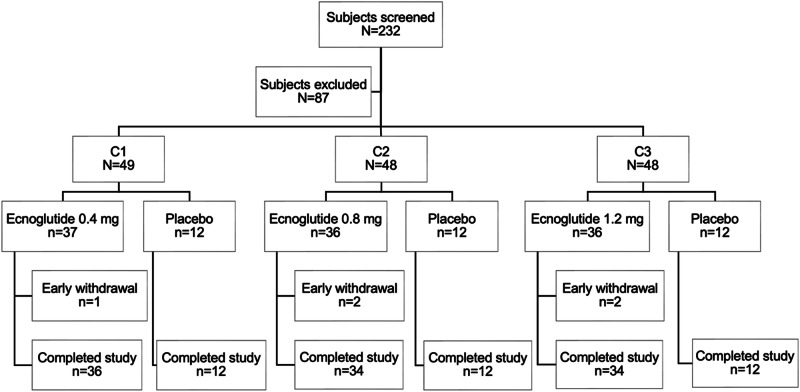
This completed Phase 2, multicenter, randomized, double-blind, placebo-controlled clinical trial was conducted at 21 hospital-based study centers in China. Overall, 232 subjects were screened, 145 underwent randomization, and 109 received at least one dose of ecnoglutide. Of all participants randomized, 140 (96.6%) completed the 20-week (134 day) treatment period (Fig. 1). The baseline characteristics of the randomized participants were generally well-balanced across the treatment groups (Table 1). The mean age was 50.2 and 49.7 years for ecnoglutide and placebo groups, respectively. Most participants were male (67.9 and 52.8% for ecnoglutide and placebo, respectively). The mean duration of diabetes was 49.72 months for ecnoglutide groups and 40.36 months for placebo. Baseline values were comparable across treatment groups for HbA1c (mean of all participants 8.55%), body weight (mean 73.41 kg), and BMI (mean 26.27 kg/m2). Five (3.4%) participants who received ecnoglutide and none receiving placebo discontinued the study prematurely. Reasons for discontinuation were withdrawal by participant, AE, or physician decision.
Fig. 1. Participant disposition.
Screening cases are defined as participants who signed the informed consent. Early withdrawal includes participants who withdrew prior to the end of study visit on Day 169.
Table 1.
Demographic and baseline characteristics
| Characteristic* | Ecnoglutide | Placebo (N = 36) | |||
|---|---|---|---|---|---|
| 0.4 mg (N = 37) | 0.8 mg (N = 36) | 1.2 mg (N = 36) | Total (N = 109) | ||
| Age (years) | 49.1 (8.87) | 51.8 (10.34) | 49.6 (9.65) | 50.2 (9.62) | 49.7 (10.54) |
| Gender, n (%) | |||||
| Male | 25 (67.6) | 28 (77.8) | 21 (58.3) | 74 (67.9) | 19 (52.8) |
| Female | 12 (32.4) | 8 (22.2) | 15 (41.7) | 35 (32.1) | 17 (47.2) |
| Body weight (kg) | 72.8 (14.6) | 76.6 (13.4) | 72.5 (12.1) | 74.0 (13.4) | 71.8 (12.2) |
| BMI (kg/m2) | 25.8 (3.7) | 26.6 (3.4) | 26.2 (3.0) | 26.2 (3.4) | 26.4 (3.2) |
| HbA1c (%) | 8.45 (0.64) | 8.65 (0.76) | 8.67 (0.75) | 8.59 (0.72) | 8.44 (0.67) |
| FPG (mmol/L) | 9.70 (1.605) | 11.08 (1.678) | 10.00 (1.889) | 10.26 (1.811) | 10.50 (1.800) |
| Diabetes duration (months) | 46.23 (51.93) | 57.64 (44.18) | 45.39 (49.55) | 49.72 (48.56) | 40.36 (35.58) |
| Previous antihyperglycemic treatment, n (%) | 15 (40.5) | 23 (63.9) | 22 (61.1) | 60 (55.0) | 19 (52.8) |
*Mean (SD) unless otherwise noted.
SD standard deviation, kg, kilogram, BMI body mass index, HbA1c Hemoglobin A1c, FPG fasting plasma glucose.
Primary outcome
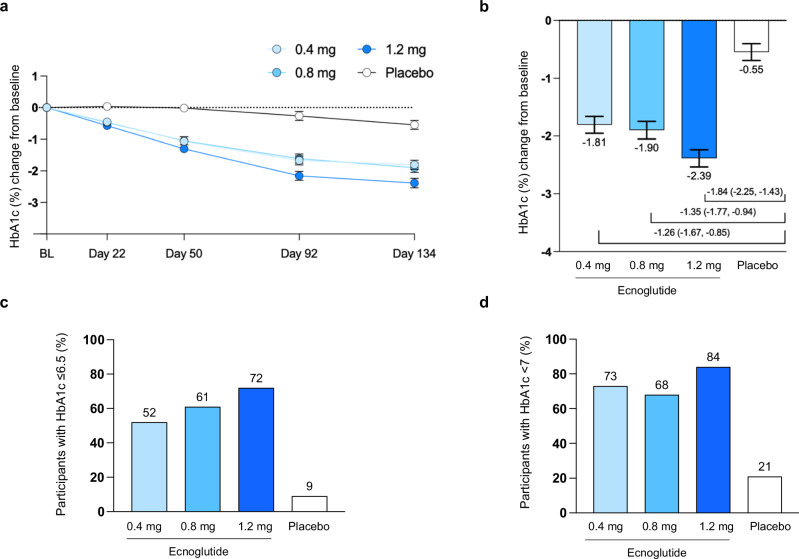
HbA1c levels declined for all ecnoglutide dose groups over the course of treatment (Fig. 2). At the end of treatment (Day 134), participants receiving target doses of 0.4, 0.8, and 1.2 mg ecnoglutide had mean HbA1c reductions from baseline of −1.81% (standard error [SE] 0.15), −1.90% (0.15), and −2.39% (0.15), respectively, compared to −0.55% (0.15) in the placebo group. The mean treatment differences in HbA1c between ecnoglutide 0.4, 0.8, 1.2 mg, and placebo were −1.26% (95% CI −1.67, −0.85), −1.35% (−1.77, −0.94), and −1.84% (−2.25, −1.43), respectively. Based on a −0.3% superiority margin, all doses of ecnoglutide were significantly (P < 0.0001) superior to placebo at reducing HbA1c. The decrease in HbA1c was dose dependent, linear, and had not plateaued by the end of treatment. Sensitivity analysis, including evaluation of the per protocol population, indicated that the analysis results were robust (Supplemental Table S1). Individual participant data distribution is shown in Supplemental Fig S2.
Fig. 2. HbA1c changes from baseline in participants treated with ecnoglutide or placebo.
a HbA1c change from baseline to end of treatment (Day 134), derived from mixed model for repeated measures (MMRM) analysis. Least squares mean and SE are shown. Numbers of participants (n) for the 0.4, 0.8, 1.2 mg ecnoglutide and placebo groups, respectively, are as follows: BL, 37, 36, 36, 36; Day 22, 36, 35, 35, 36; Day 50, 35, 34, 35, 36; Day 92, 35, 34, 33, 34; and Day 134, 33, 31, 32, 33. b HbA1c change from baseline at Day 134 and difference from placebo, derived from MMRM analysis, with n = 33, 31, 32, 33 participants in the 0.4, 0.8, 1.2 mg ecnoglutide and placebo groups, respectively. Least squares mean and SE are shown, as well as mean difference from placebo and 95% confidence interval. All ecnoglutide treatment groups were significantly different from placebo (P < 0.0001) using σ = 0.3 superiority margin. c, d The proportion of participants with HbA1c values of ≤6.5% and <7%, respectively, at Day 134. Intent to treat (ITT) population is shown for all analyses. BL baseline. Source data are provided as a Source Data file.
A significantly higher proportion of participants given ecnoglutide reached an HbA1c target concentration of <7.0% and ≤6.5% at the end of treatment versus placebo (P < 0.0001). An HbA1c value of <7.0% was achieved in 68 to 84% of participants receiving ecnoglutide versus 21% for placebo. A HbA1c value of ≤6.5% was achieved in 52–72% of participants in the ecnoglutide groups versus 9% with placebo.
Secondary outcomes
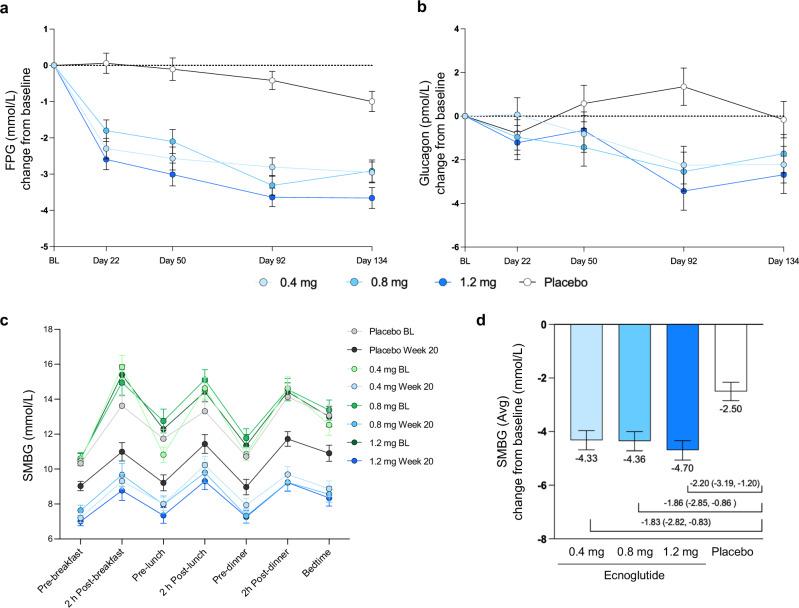
Fasting plasma glucose (FPG) significantly decreased over the treatment period in all ecnoglutide treatment groups compared to placebo (P < 0.0001 for all dose groups from Day 22 to Day 134). Reductions in FPG levels were apparent as early as the first assessment on Day 22 and remained stable in all ecnoglutide cohorts throughout the treatment period. At end of treatment (Day 134), the estimated mean (SE) treatment differences versus placebo were −2.0 (0.4), −1.9 (0.4), and −2.7 (0.4) mmol/L for 0.4, 0.8, and 1.2 mg ecnoglutide cohorts, respectively (P < 0.0001 for all ecnoglutide groups compared to placebo) (Fig. 3a).
Fig. 3. Changes in glucose and glucagon from baseline.
a Fasting blood glucose (FBG) change from baseline to end of treatment (Day 134), using mixed model for repeated measures (MMRM) analysis. Least squares mean and SE are shown. Numbers of participants (n) for the 0.4, 0.8, 1.2 mg ecnoglutide and placebo groups, respectively, are as follows: BL, 37, 34, 34, 36; Day 22, 36, 35, 35, 36; Day 50, 35, 34, 35, 36; Day 92, 36, 34, 34, 36; and Day 134, 33, 31, 32, 34. P < 0.0001 for all ecnoglutide cohorts compared to placebo at Days 22, 50, 92, and 134. b Glucagon change from baseline to end of treatment (Day 134), using MMRM analysis. Least squares mean and SE are shown. Numbers of participants (n) for the 0.4, 0.8, 1.2 mg ecnoglutide and placebo groups, respectively, are as follows: BL, 37, 36, 36, 36; Day 22, 36, 35, 35, 36; Day 50, 36, 35, 35, 36; Day 92, 36, 34, 34, 36; and Day 134, 34, 31, 32, 34. P < 0.0001 for ecnoglutide 1.2 mg compared to placebo at Day 92. P < 0.002 for ecnoglutide 0.4 and 0.8 mg on Day 92. P < 0.02 for ecnoglutide 1.2 mg on Day 134. c Self-monitored blood glucose pre- and post-meals at baseline and end of treatment (sampled between Days 135–140). Means and SE are shown. d SMBG average change from baseline to Week 20, derived from analysis of variance (one-way ANOVA). Least squares mean and SE are shown, as well as mean difference from placebo and 95% confidence interval. P values were 0.0002, 0.0002, and <0.0001, for 0.4, 0.8, 1.2 mg ecnoglutide compared to placebo, respectively. Numbers of participants (n) for SMBG for the 0.4, 0.8, 1.2 mg ecnoglutide and placebo groups, respectively, are BL, 34, 35, 35, 34; Week 20, 29, 30, 28, 30. BL baseline. Source data are provided as a Source Data file.
Seven-point self-monitored blood glucose (SMBG) readings at end of treatment (Day 134) were reduced from baseline for all dose groups for both pre- and post-prandial measurements (Fig. 3c). Average SMBG measurements decreased significantly by a mean (SE) of −4.33 (0.36), −4.36 (0.36), and −4.70 (0.36) mmol/L for 0.4, 0.8, and 1.2 mg ecnoglutide cohorts, respectively, versus −2.50 (0.35) mmol/L with placebo (P < 0.001 for all dose groups). (Fig. 3d and Supplemental Fig. S2). Decreases in the largest amplitude of glucose excursion (LAGE) ranged from −2.87 to −3.20 mmol/L with ecnoglutide versus −1.79 mmol/L with placebo. Decreases in the standard deviation on mean blood glucose (SDBG) ranged from −1.09 to −1.19 mmol/L with ecnoglutide versus −0.64 mmol/L with placebo. Decreases in postprandial glucose excursion (PPGE) ranged from −1.30 to −1.52 mmol/L with ecnoglutide versus −1.07 mmol/L with placebo. Ecnoglutide at 1.2 mg showed significantly greater reductions in LAGE and SDBG than placebo (P = 0.008 for LAGE and P = 0.009 for SDBG, respectively). These results indicate a dose dependent change across all glucose measures, which is important in diabetes management.
Glucagon levels also decreased with ecnoglutide treatment. The maximum change from baseline was seen at Day 92, with mean (SE) difference in glucagon levels compared to placebo of −3.6 (1.2), −3.9 (1.2), and −4.8 (1.2) pmol/L for 0.4, 0.8, and 1.2 mg ecnoglutide groups, respectively. At end of treatment (Day 134), the mean (SE) difference in glucagon levels compared to placebo were −2.1 (1.2), −1.6 (1.2), and −2.5 (1.2) pmol/L for 0.4, 0.8, and 1.2 mg ecnoglutide cohorts, respectively. The change from baseline was significantly different from placebo for all ecnoglutide dose groups at Day 92 (P < 0.002 for 0.4 and 0.8 mg groups; P < 0.0001 for 1.2 mg group) and for 1.2 mg ecnoglutide at Day 134 (P < 0.02) (Fig. 3b).
No significant changes from baseline or compared to placebo were observed for blood insulin levels for any of the ecnoglutide dose groups.
LDL decreases from baseline to end of treatment (Day 134) ranged from −0.14 to −0.42 mmol/L for the ecnoglutide cohorts versus −0.09 mmol/L with placebo; these differences were not statistically significant. No significant changes were observed in mean changes from baseline in triglycerides and HDL.
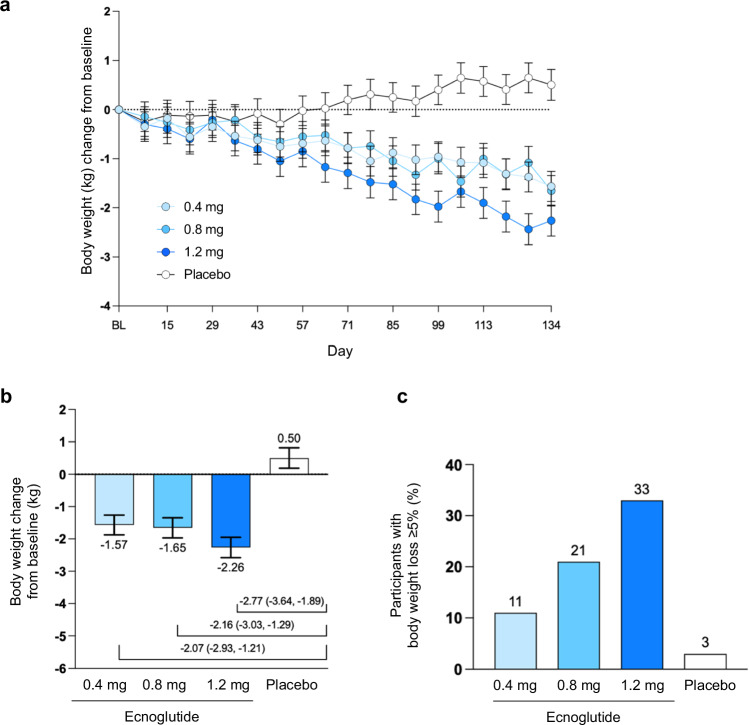
Body weight decreased dose-dependently from baseline to end of treatment in all ecnoglutide dose groups. The reduction in body weight at end of treatment (Day 134) was significantly more pronounced for the ecnoglutide cohorts, which ranged from −1.57 to −2.26 kg of weight loss, compared to the placebo group, which showed a mean gain of 0.50 kg (P < 0.0001). Significant weight loss was observed with 1.2 mg ecnoglutide beginning from Day 64. The proportion of participants achieving a ≥5% weight reduction at end of treatment increased dose-dependently, up to 33.3% with 1.2 mg ecnoglutide compared to 3.0% for placebo (Fig. 4 and Supplemental Fig. S2).
Fig. 4. Mean change in body weight from baseline.
a Body weight change (kg) from baseline to end of treatment (Day 134), derived from mixed model for repeated measures (MMRM) analysis. Least squares mean and SE are shown. Numbers of participants (n) for the 0.4, 0.8, 1.2 mg ecnoglutide and placebo groups, respectively, are as follows: BL, 37, 36, 36, 36; Day 8, 36, 36, 35, 36; Day 15, 36, 34, 35, 35; Day 22, 36, 35, 35, 36; Day 29, 36, 35, 33, 36; Day 36, 36, 33, 34, 36; Day 43, 35, 35, 35, 36; Day 50, 36, 34, 33, 36; Day 57, 36, 34, 33, 36; Day 64, 35, 34, 34, 32; Day 71, 35, 33, 32, 36; Day 78, 35, 33, 32, 34; Day 85, 35, 34, 33, 36; Day 92, 36, 34, 34, 34; Day 99, 35, 34, 33, 36; Day 106, 35, 33, 32, 34; Day 113, 36, 33, 33, 35; Day 120, 35, 33, 33, 35; Day 127, 35, 29, 33, 35; Day 134, 35, 34, 33, 33. P < 0.0001 for all ecnoglutide cohorts compared to placebo at Days 106, 120, 127, and 134. b Change in body weight compared to baseline (kg) and difference from placebo, derived from MMRM analysis. Least squares mean and SE are shown, with n = 35, 34, 33, 33 participants for the 0.4, 0.8, 1.2 mg ecnoglutide and placebo groups, respectively. Mean difference from placebo and 95% confidence interval for mean difference are indicated. P < 0.0001 for all ecnoglutide cohorts compared to placebo. c Proportion of participants with body weight loss ≥5% from baseline at Day 134. BL baseline. Source data are provided as a Source Data file.
Waist circumference at end of treatment showed a mean (SE) difference from placebo of −1.3 (0.8), −1.16 (0.8), −3.9 (0.8) cm for 0.4, 0.8, and 1.2 mg ecnoglutide dose groups, respectively; the 1.2 mg group was significantly different from placebo at this timepoint (P < 0.0001). Similarly, hip circumference mean (SE) difference from placebo at Day 134 was −0.7 (0.8), −0.7 (0.8), and −1.4 (0.8) cm for 0.4, 0.8, and 1.2 mg ecnoglutide groups, respectively (P < 0.05 for the 1.2 mg ecnoglutide group).
None of the participants developed treatment-emergent anti-ecnoglutide antibodies in any dose group.
Safety outcomes
Ecnoglutide was generally safe and well tolerated in the T2DM population studied. There were no treatment-related ≥Grade 3 adverse events (AEs), no treatment-related serious adverse events (SAEs), and no deaths occurred during the study (Table 2).
Table 2.
Summary of adverse events
| AEs, n (%) | Ecnoglutide | Placebo N = 36 | ||
|---|---|---|---|---|
| 0.4 mg N = 37 | 0.8 mg N = 36 | 1.2 mg N = 36 | ||
| All AE | 29 (78.4) | 28 (77.8) | 26 (72.2) | 22 (61.1) |
| All TRAE | 18 (48.6) | 16 (44.4) | 16 (44.4) | 6 (16.7) |
| TEAE ≥Grade 3 | 2 (5.4) | 0 | 2 (5.6) | 3 (8.3) |
| TRAE ≥Grade 3 | 0 | 0 | 0 | 0 |
| All AESI | 14 (37.8) | 13 (36.1) | 17 (47.2) | 6 (16.7) |
| Treatment-related AESI | 14 (37.8) | 12 (33.3) | 15 (41.7) | 2 (5.6) |
| TEAE leading to drug discontinuation | 0 | 0 | 1 (2.8) | 0 |
| TRAE leading to drug discontinuation | 0 | 0 | 1 (2.8) | 0 |
| All SAE | 0 | 0 | 1 (2.8) | 0 |
| Treatment-related SAE | 0 | 0 | 0 | 0 |
| TEAE leading to study withdrawal | 0 | 0 | 2 (5.6) | 0 |
| TRAE leading to study withdrawal | 0 | 0 | 1 (2.8) | 0 |
| TEAE leading to death | 0 | 0 | 0 | 0 |
| TRAE leading to death | 0 | 0 | 0 | 0 |
AE adverse event, TRAE treatment-related AE, TEAE treatment-emergent AE, AESI adverse events of special interest, SAE serious adverse event.
The percentages of participants who reported any AE were similar in the three ecnoglutide groups, ranging from 72.2 to 78.4%, compared to 61.1% in the placebo group. Treatment-related adverse events (TRAE) were reported in 48.6, 44.4, 44.4, and 16.7%, of participants receiving 0.4, 0.8, or 1.2 mg ecnoglutide, and placebo, respectively. There were four ≥Grade 3 treatment emergent AEs (TEAEs) in the ecnoglutide cohorts (hypertension and hypercholesterolemia in the ecnoglutide 0.4 mg group; upper respiratory tract infection and ectopic pregnancy in ecnoglutide 1.2 mg group) and 3 (two cases of hypercholesterolemia and one of hypertension) in the placebo group. None of these events were considered by the investigators to be related to study treatment. One SAE (ectopic pregnancy) occurred in the ecnoglutide 1.2 mg treatment group; the participant withdrew from the study. One additional participant in the 1.2 mg ecnoglutide group withdrew from the study due to an AE of nausea; this AE was considered treatment related.
The most frequently reported AEs by SOC were gastrointestinal, metabolic, and nutritional disorders (Table 3). TEAEs with >5% incidence in ecnoglutide-treated participants included diarrhea (14.7%), nausea (11.9%), constipation (7.3%), hyperlipidemia (8.3%), loss of appetite (6.4%), hypoglycemia (5.5%), elevated lipase (5.5%), upper respiratory tract infection (6.4%), and proteinuria (7.3%). Rates of nausea appeared to be dose-dependent, while the incidence of diarrhea was lower in the 0.8 mg ecnoglutide cohort than in the other two dose groups.
Table 3.
Treatment-emergent adverse events with total incidence ≥2% by preferred term (safety analysis set)
| TEAEs, n (%) | Ecnoglutide | Placebo N = 36 | Total N = 145 | |||
|---|---|---|---|---|---|---|
| 0.4 mg N = 37 | 0.8 mg N = 36 | 1.2 mg N = 36 | Total N = 109 | |||
| Participants with at least one TEAE | 29 (78.4) | 28 (77.8) | 26 (72.2) | 83 (76.1) | 21 (58.3) | 104 (71.7) |
| Diarrhea | 6 (16.2) | 3 (8.3) | 7 (19.4) | 16 (14.7) | 1 (2.8) | 17 (11.7) |
| Nausea | 2 (5.4) | 4 (11.1) | 7 (19.4) | 13 (11.9) | 1 (2.8) | 14 (9.7) |
| Upper respiratory tract infection | 1 (2.7) | 4 (11.1) | 2 (5.6) | 7 (6.4) | 4 (11.1) | 11 (7.6) |
| Hyperlipidemia | 5 (13.5) | 0 | 4 (11.1) | 9 (8.3) | 2 (5.6) | 11 (7.6) |
| Constipation | 2 (5.4) | 3 (8.3) | 3 (8.3) | 8 (7.3) | 2 (5.6) | 10 (6.9) |
| Proteinuria | 2 (5.4) | 3 (8.3) | 3 (8.3) | 8 (7.3) | 0 | 8 (5.5) |
| Decreased appetite | 2 (5.4) | 2 (5.6) | 3 (8.3) | 7 (6.4) | 0 | 7 (4.8) |
| Hypoglycemia | 1 (2.7) | 4 (11.1) | 1 (2.8) | 6 (5.5) | 1 (2.8) | 7 (4.8) |
| Elevated amylase | 3 (8.1) | 1 (2.8) | 1 (2.8) | 5 (4.6) | 1 (2.8) | 6 (4.1) |
| Elevated lipase | 2 (5.4) | 3 (8.3) | 1 (2.8) | 6 (5.5) | 0 | 6 (4.1) |
| Urinary tract infection | 2 (5.4) | 1 (2.8) | 1 (2.8) | 4 (3.7) | 2 (5.6) | 6 (4.1) |
| Dyslipidemia | 2 (5.4) | 2 (5.6) | 0 | 4 (3.7) | 1 (2.8) | 5 (3.4) |
| GERD | 2 (5.4) | 1 (2.8) | 1 (2.8) | 4 (3.7) | 0 | 4 (2.8) |
| Hyperuricemia | 2 (5.4) | 0 | 0 | 2 (1.8) | 2 (5.6) | 4 (2.8) |
| WBC in urine | 2 (5.4) | 0 | 2 (5.6) | 4 (3.7) | 0 | 4 (2.8) |
| Palpitations | 2 (5.4) | 0 | 1 (2.8) | 3 (2.8) | 1 (2.8) | 4 (2.8) |
| Headache | 0 | 1 (2.8) | 2 (5.6) | 3 (2.8) | 1 (2.8) | 4 (2.8) |
| Dizziness | 0 | 0 | 2 (5.6) | 2 (1.8) | 2 (5.6) | 4 (2.8) |
| Toothache | 2 (5.4) | 0 | 1 (2.8) | 3 (2.8) | 0 | 3 (2.1) |
| Flatulence | 1 (2.7) | 2 (5.6) | 0 | 3 (2.8) | 0 | 3 (2.1) |
| Vomiting | 1 (2.7) | 0 | 2 (5.6) | 3 (2.8) | 0 | 3 (2.1) |
| Hyperhomocysteinemia | 1 (2.7) | 1 (2.8) | 1 (2.8) | 3 (2.8) | 0 | 3 (2.1) |
| Myocardial ischemia | 1 (2.7) | 1 (2.8) | 0 | 2 (1.8) | 1 (2.8) | 3 (2.1) |
| Diabetic nephropathy | 2 (5.4) | 0 | 1 (2.8) | 3 (2.8) | 0 | 3 (2.1) |
| Hypertension | 2 (5.4) | 0 | 0 | 2 (1.8) | 1 (2.8) | 3 (2.1) |
| Insomnia | 0 | 0 | 1 (2.8) | 1 (0.9) | 2 (5.6) | 3 (2.1) |
GERD gastroesophageal reflux disease, TEAE treatment-emergent adverse events, WBC white blood cells.
Mild hypoglycemia was reported in 1 (2.7%) participant who received ecnoglutide 0.4 mg, 4 (11.1%) who received 0.8 mg, 1 (2.8%) who received 1.2 mg, and 1 (2.8%) with placebo. No clinically significant or severe hypoglycemia was reported in participants given ecnoglutide. Hypoglycemia events were primarily reported as due to skipped meals and/or increased intensity of physical activity.
Rescue therapy for persistent hyperglycemia was administered to 23 (15.9%) of the participants, including 1 (2.7%) participant who received ecnoglutide 0.4 mg, 6 (16.7%) who received 0.8 mg, 1 (2.8%) who received 1.2 mg, and 15 (41.7%) with placebo. Metformin was the most common antihyperglycemic medication used as rescue therapy during this study.
Injection site conditions were noted in 2 participants receiving ecnoglutide (bleeding at injection site in one participant in the 0.4 mg group, itching at the administration site in one participant the 0.8 mg group); no injection site reactions were observed in the placebo group.
No cases of pancreatitis, medullary thyroid cancer, or treatment-emergent diabetic retinopathy were reported during this study. No clinically relevant changes in ECG were observed. At end of treatment, decreases in mean systolic blood pressure ranged from −3.4 to −6.6 mmHg for the ecnoglutide cohorts versus 1.6 mmHg with placebo. Changes in diastolic blood pressure were similar between the ecnoglutide and placebo groups. At end of treatment, increases in mean pulse rate ranged from 3.8–5.5 beats per minute with ecnoglutide versus 0.9 beats per minute with placebo. No treatment-related changes in hematology, blood biochemistry, coagulation, and calcitonin were observed.
These results indicate that the safety profile of ecnoglutide is consistent with other approved GLP-1 analogs.
Pharmacokinetics
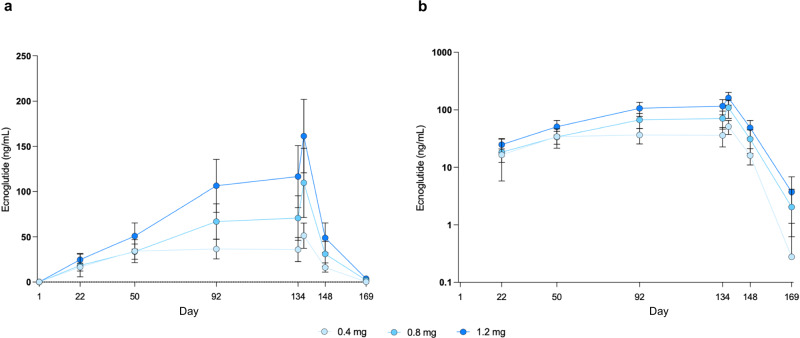
Blood concentrations of ecnoglutide over time are shown in Fig. 5. Ecnoglutide was titrated to a target dose of 0.4 mg (Cohort C1) on Day 29 and steady state was reached at a trough concentration (Ctrough, mean [SD]) of 34.2 (12.7) ng/mL on Day 50. Titration to a target dose of 0.8 mg (Cohort C2) was reached on Day 57, with a steady state Ctrough of 66.9 (19.6) ng/mL on Day 92. Titration to a target dose of 1.2 mg (Cohort C3) was also reached on Day 57, with a steady state Ctrough of 106.4 (29.1) ng/mL on Day 92. The delay between starting the top dose and reaching steady state is due to the long half-life of ecnoglutide. At steady state, ecnoglutide Ctrough concentrations showed approximate dose proportionality over the range of 0.4–1.2 mg. The last day of dosing was Day 134.
Fig. 5. Pharmacokinetics of ecnoglutide.
a Linear and b semilog plots of ecnoglutide concentrations (ng/mL) over time. Mean and standard deviation (SD) are shown. Numbers of participants (n) for the 0.4, 0.8, 1.2 mg ecnoglutide groups, respectively, are as follows: Day 1, 37, 36, 36; Day 22, 36, 35, 35; Day 50, 36, 34, 35; Day 92, 36, 34, 34; Day 134, 33, 31, 32; Day 137, 31, 28, 29; Day 148, 33, 28, 29; Day 169, 35, 34, 34. Source data are provided as a Source Data file.
Discussion
We conducted a randomized, double-blind, Phase 2 clinical trial of once-weekly injection ecnoglutide compared to placebo in participants with T2DM inadequately controlled with diet, exercise, or single oral hypoglycemic agent.
In this study, ecnoglutide showed significant improvement in glycemic control at once-weekly doses of 0.4, 0.8, and 1.2 mg compared with placebo. Reductions in HbA1c were observed by the first assessment at Day 22 and continued to progress until the end of treatment. After 20 weeks of treatment, 68 to 84% of participants given ecnoglutide reached the American Diabetes Association (ADA) recommended HbA1c target of <7.0% and 52 to 72% of participants reached an HbA1c target of ≤6.5% as recommend by the American Association of Clinical Endocrinologists (AACE), without increasing hypoglycemic risk. Achievement of these targets is a key metric used in the clinical standard of care. The beneficial effect on glycemic control was further supported by the significant improvements in FPG, as well as in glycemic variability indices calculated from SMBG, including the average SMBG, LAGE, SDBG, and PPGE, reflecting reduced glucose excursion throughout the day.
Compared with other GLP-1 based therapies, ecnoglutide showed comparable or greater HbA1c reductions at all three doses tested. Change from baseline in HbA1c was −1.81, −1.90, and −2.39% for ecnoglutide (0.4, 0.8 and 1.2 mg at 20 weeks). This compared to −0.84 to −1.14% reported for liraglutide (1.2 and 1.8 mg at 52 weeks)7, −1.45 to −1.55% for semaglutide (0.5 and 1.0 mg at 30 weeks)8, −1.86% for semaglutide (1.0 mg at 40 weeks)9, and −1.25 to −1.46% for dulaglutide (0.75 and 1.5 mg at 26 weeks)10. In these studies, the proportion of participants achieving HbA1c concentration <7.0% was 43–51% for liraglutide, 72–74% for semaglutide, and 63–72% for dulaglutide, compared to 68–84% seen here for ecnoglutide. Ecnoglutide (1.2 mg) also showed comparable HbA1c reductions to the dual GLP-1/GIP receptor agonist, tirzepatide. Tirzepatide at 5, 10, and 15 mg weekly resulted in −2.01, −2.24, and −2.30% HbA1c reductions, respectively, from baseline to 40 weeks9.
In addition to glycemic effects, we observed dose-dependent body weight reductions over 20 weeks of treatment with ecnoglutide. Between 11 and 33% of participants who received ecnoglutide reached the ADA recommended weight loss target of ≥5%, versus 3% with placebo. Body weight changes from baseline of −1.57, −1.65, and −2.26 kg for ecnoglutide (0.4, 0.8 and 1.2 mg at 20 weeks) were greater than reported for dulaglutide (−1.0 to −1.5 kg for 0.75 and 1.5 mg doses at 26 weeks10. Longer studies in populations with T2DM and obesity are needed to compare ecnoglutide-induced weight loss to other GLP-1 analogs, which included −2.05 to −2.45 kg for liraglutide (1.2 and 1.8 mg) at 52 weeks7, −3.73 to −4.53 kg for semaglutide (0.5 to 1.0 mg) at 30 weeks8, and −5.7 kg for semaglutide (1.0 mg) at 40 weeks9. Ecnoglutide weight reductions were progressive during the study and did not reach a plateau in any of the three dose groups at the end of 20 weeks of treatment. Continued weight loss is therefore expected with longer treatment periods. In addition, potential cardiovascular benefits may result from the favorable changes in LDL and systolic blood pressure observed with ecnoglutide in this study.
The strong HbA1c lowering activity seen for ecnoglutide supports the hypothesis that cAMP signaling bias enhances the efficacy of GLP-1 analogs. While approved long-acting GLP-1-based peptides, such as semaglutide, dulaglutide, and liraglutide, are full agonists of both cAMP and β-arrestin pathways5, ecnoglutide is designed to promote cAMP signaling bias. Dual GLP-1/GIP peptide analogs, such as tirzepatide, also show signaling bias for cAMP induction6. The ecnoglutide sequence is similar to semaglutide but with signaling bias achieved through the introduction of valine at position 84,11. In preclinical studies, ecnoglutide showed similar in vitro potency to semaglutide, as measured by cAMP induction, but reduced β-arrestin signaling and lower β-arrestin-mediated receptor internalization4. The value of cAMP bias was supported in rodent models, where ecnoglutide showed significantly improved glucose control and body weight reduction compared to semaglutide4. The current study suggests that the observed preclinical potency translates into improved clinical efficacy in T2DM patients. The HbA1c lowering effect of 1.2 mg weekly ecnoglutide at 20 weeks (−2.39%) surpassed that of the similar peptide 1.0 mg semaglutide (−1.86%) at 40 weeks9, and was comparable to 15 mg weekly tirzepatide (−2.30%) at 40 weeks9. The contribution of cAMP bias versus dual GLP-1/GIP targeting to the efficacy of tirzepatide has been a matter of debate5,12. These results suggest that cAMP bias may contribute to efficacy for GLP-1 receptor agonists, although interpretation is limited by the study design, which did not directly assess the mechanism of action or compare agents head-to-head in the same population.
The safety profile of ecnoglutide in this T2DM population was similar to that of other GLP-1 based therapies. The most commonly reported TEAEs were gastrointestinal disorders, including diarrhea, nausea, and constipation. A slightly higher incidence of hypoglycemic episodes was noted in the ecnoglutide cohorts (6 participants, 5.5%) compared to placebo (1 participant, 2.8%). These events were reported as mild, did not show dose dependance, and were mainly due to skipped meals and/or increased physical activity. The difference in incidence of hypoglycemia between placebo and ecnoglutide groups was not statistically significant.
A limitation of this study was its relatively short duration of 20 weeks. A longer study duration could provide additional insights into the therapeutic benefits of ecnoglutide for glycemic control, as well as weight management. In addition, eligible study participants were currently treating their diabetes with lifestyle modifications and/or a single oral hypoglycemic agent. Participants receiving these first-line interventions may be more responsive to therapy. Two Phase 3 studies are currently ongoing to assess the long-term efficacy and safety of ecnoglutide during one year of treatment. These studies are evaluating ecnoglutide as a monotherapy and as an add-on to metformin in patients with T2DM.
In conclusion, ecnoglutide, a biased long-acting GLP-1 agonist, when given once a week at doses 0.4, 0.8, and 1.2 mg for 20 weeks as monotherapy for T2DM, showed beneficial glycemic control and body weight reduction, and a safety profile consistent with GLP-1 based therapies. These results support the development of ecnoglutide as a potential initial treatment option for patients with T2DM early in the course of the disease.
Methods
Study design
This study was a Phase 2, multicenter, randomized, double-blind, placebo-controlled clinical trial conducted in China with the participation of 21 hospital-based certified study centers between June 30, 2021 and June 20, 2022. The primary objective of the study was to evaluate the efficacy of ecnoglutide, as measured by HbA1c change from baseline at Week 20.
The trial was conducted per the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice. Study protocol was approved by ethics committees at the following institutions, Nanjing Drum Tower Hospital, Nanjing First Hospital, Beijing Luhe Hospital Capital Medical University, The First Affiliated Hospital of Henan University of Science and Technology, Central Hospital Affiliated to Shan Dong First Medical University, The Fourth Affiliated Hospital of Harbin Medical University, Emergency General Hospital, The Affiliated Hospital of Xuzhou Medical University, Changde First People’s Hospital, The Second Affiliated Hospital of Heilongjiang University of Chinese Medicine, Nanjing Jiangning Hospital, Luoyang Third People’s Hospital, Zaozhuang Centre Hospital Of Shandong Yiyang Health Group, Binzhou Medical University Hospital, Hebei Petro China Central Hospital, The First Hospital of Handan, Genertec Liaoyou Gem Flower Hospital, Shijiazhuang People’s Hospital, Nanyang First People’s Hospital, and Daqing People’s Hospital. Participants were compensated for travel and lost pay due to time off work for study visits. All the participants provided written informed consent before participating.
The study enrolled 145 adult participants with T2DM, whose disease was inadequately controlled through lifestyle management or single oral antidiabetic therapy. Participants were randomly assigned in a 1:1:1:1 ratio to receive once-weekly subcutaneous (SC) injections of ecnoglutide at target dose of 0.4 mg (cohort [C]1), 0.8 mg (C2), or 1.2 mg (C3), or placebo (ecnoglutide vehicle). The randomization method stratified by HbA1c level (≤8.5%, >8.5%) as the stratification factor. The randomization list was generated by the independent randomization statistician using SAS version 9.4, employing fixed randomization with one-time generation of enough randomization numbers. A centralized interactive web response system was used for treatment randomization and drug dispensing. The random seed, random allocation sequence, and randomization list were concealed remotely. Participants were enrolled by trial staff using the centralized interactive web response system, which allocated a treatment code to each participant. The study drugs and placebo were identically labeled and indistinguishable in appearance. All participants, investigators, study site personnel involved in treating and evaluating participants and the sponsor were blinded to treatment assignment until database lock. Investigational product was dispensed by trial staff at each study site.
Study drug was administered using injector pens with a pre-set volume. Placebo participants were assigned in equal numbers to receive a dosage volume matched to the ecnoglutide dose in cohort C1, C2, or C3. After a 3-week run-in period to acclimatize to injection procedures, all participants received SC injections of ecnoglutide or matched placebo once a week for 20 weeks (134 days), followed by a 5-week safety follow-up period (Fig. S1). Ecnoglutide was administered as a slow dose escalation regimen, starting at 0.2 or 0.3 mg with fixed double-dose increments every 4 weeks until the target dose was reached. Ecnoglutide was supplied in single-use vials containing 1 mL of ecnoglutide at a 2 mg/mL concentration.
Sciwind study number SCW0502-1021. China Drug Trials Registry number CTR20211014.
Participants
Eligible male and female (non-pregnant and non-lactating) participants were 18–65 years of age inclusive and with a diagnosis of T2DM according to WHO criteria13. Participant gender was self-reported. In the three months prior to screening, they were to have been treated with diet and/or exercise alone or with one oral hypoglycemic agent. Oral hypoglycemic agents allowable within 3 months prior to screening included biguanides, glycosidase inhibitors, sulfonylurea, glinides, thiazolidinediones, DPP-4 inhibitors, and sodium-glucose cotransporter 2 (SGLT2) inhibitors. Participants taking an oral hypoglycemic drug underwent a washout period of 2 weeks prior to study entry. Eligible participants had an HbA1c level between ≥7.0% and ≤10.5%, a fasting plasma glucose (FPG) ≥ 7.0 mmol/L and ≤13.9 mmol/L at randomization, and a body mass index (BMI) between ≥20.0 kg/m2 and ≤35.0 kg/m2. Participants must have been willing and able to self-monitor blood glucose (SMBG), as well as to understand and comply with the requirements of the study.
Exclusion criteria included type 1 or other types of diabetes mellitus, history of pancreatitis, and family or personal history of type 2 multiple endocrine neoplasia syndrome or medullary thyroid cancer. Subjects were excluded if they had used any investigational product/device, used any form of insulin (except for ≤7 days use for diabetes-related complications), received medical or non-medical body weight management, or experienced body weight change >5% within three months before screening. Subjects were not eligible if they had received any GLP-1 analog or major gastrointestinal surgery (except cholecystectomy and appendectomy) prior to screening. Subjects were also excluded if they had a history of coronary or cerebrovascular events within 12 months prior to screening, mental or nervous system disease, impaired liver or renal function, malignancy within 5 years of screening (except for skin basal cell carcinoma or carcinoma in situ of cervix), positive screening results for hepatitis B, hepatitis C, or HIV, or other severe disease. Subjects were not eligible if they had a history of an episode or suspected episode of severe hypoglycemia, diabetic ketoacidosis, or hyperglycemic hyperosmolar syndrome within 12 months prior to screening or had been diagnosed with proliferative diabetic retinopathy or stage 3 non-proliferative diabetic retinopathy. Additional exclusion criteria included alcohol or drug abuse, blood transfusion or significant blood loss within 3 months prior to screening, history of severe allergy to drug, food, or other substances. Pregnant or lactating women, male participants who had plans to donate sperm or female partners of male participants who had plans to donate eggs within three months of completing the study, or participants who were unwilling to use effective contraceptives were also not eligible to participate in this study. Subjects were excluded if they were unable to complete the entire course of the study or were deemed by PI unsuitable to participate.
Participants were required to use one or more non-drug contraceptives (e.g., condom, intrauterine device, or contraceptive surgery).
Study endpoints and assessments
The primary efficacy endpoint was mean change from baseline in HbA1c levels at Week 20 (Day 134). Key secondary efficacy endpoints included mean change from baseline in fasting plasma glucose (FPG), self-monitoring blood glucose (SMBG), insulin and glucagon levels, blood lipid profile (triglyceride, low- and high-density lipoprotein), body weight, and waist and hip circumference. The pharmacokinetic (PK) endpoint was plasma trough concentration (Ctrough) of ecnoglutide before dosing on Days 1, 22, 50, 92, 134, and at the end of the study (Day 169). The immunologic endpoint was the formation of anti-drug antibodies (ADA) for ecnoglutide as measured on Day 1, 50, and 169 (end of study).
Safety endpoints included treatment-emergent adverse events (AE), serious adverse events (SAE), and adverse events of special interest (AESI), including gastrointestinal intolerance reactions (vomiting, nausea, diarrhea, abdominal pain, constipation, etc.), and hypoglycemic events. Safety assessments also included pulse rate, systolic and diastolic blood pressure, ECG, and serum calcitonin.
Statistical analyses
Statistical analyses were completed using the statistical software SAS version 9.4 or above. The study was designed to preliminarily evaluate the efficacy and safety of ecnoglutide injection compared with placebo.
The sample size calculation assumed at least a 1.0% difference of mean change from baseline in HbA1c, between ecnoglutide groups and placebo, a common standard deviation of 0.9%, and a dropout rate of 20%. It was estimated that a sample size of 144 participants (36 participants in each group) provided at least 80% power to establish superiority for an ecnoglutide dose compared with placebo (superiority margin of 0.3%) at a one-sided significance level of 0.025, using the formula below.
where nT and nC is the minimum sample size of the ecnoglutide group and the placebo group, respectively, d is the expected difference of mean change from baseline in HbA1c between the ecnoglutide groups and the control group, δ is the superiority margin (0.3%), Sc is the common standard deviation of change from baseline in HbA1c, α is the type I error (one-sided), which is 0.025 in this study, and β is the type II error.
The placebo arm of 36 participants consisted of three groups, with 12 participants in each group receiving a placebo injection that was volume matched to one of the three ecnoglutide regimens (0.4, 0.8, and 1.2 mg). Since during randomization all participants had the same opportunity to be assigned to any dose group and to active or placebo within that group, pooling the placebo participants was warranted and in line with the sample size calculation. Data for all participants randomized to receive a placebo were analyzed and reported as one placebo group.
All efficacy endpoints are presented for the intent to treat (ITT) population, unless otherwise noted. The mixed model for repeated measures (MMRM) was used for primary efficacy endpoint analysis, in which the change in HbA1c from the baseline is used as the dependent variable, and the baseline age, sex, baseline HbA1c level, visit time points, treatment grouping, and treatment by visit interaction are used as the explanatory variables. The model is used to obtain the concomitant variable adjusted mean change in the HbA1c of each group at Week 20 from the baseline and its standard error, estimated 95% confidence interval, inter-group mean difference between different ecnoglutide dose groups and the placebo group and its 95% confidence interval. If the lower limit of the interval is greater than the superiority margin (0.3%), it indicates that the corresponding dose group is superior to the placebo group; otherwise, it cannot indicate that the dose group is superior to the placebo. All MMRM conform to the underlying model assumptions.
Given that this was a Phase 2, exploratory dose finding study, no adjustment was made for type 1 error inflation due to multiple comparisons. Sensitivity analyses were conducted, including comparing MMRM and analysis of variance (ANOVA), with and without imputation, ITT and per protocol population, and evaluating center as a random effect.
Each secondary efficacy indicator was summarized by treatment group and protocol-specified time point. Mean difference from baseline and placebo was determined by MMRM or ANOVA for secondary endpoints, as indicated. For ANOVA, change in the secondary endpoint from baseline is used as the dependent variable; baseline values of age, sex, HbA1c, secondary endpoint, and treatment group are used as explanatory variables.
Subgroup analyses were conducted for effects on change in HbA1c (by baseline factors HbA1c [>8.5% or ≤8.5%], prior treatment [yes/no], and sex [male/female]) and change in body weight (by baseline factors body weight [>75 kg or ≤75 kg] and sex [M/F]). Subgroup analyses showed no significant differences and are therefore not reported.
Sensitivity analysis and solutions to the models for primary and secondary endpoints are provided in Supplemental Tables S1 to S5.
All safety assessments, including concomitant medications, AEs, laboratory assessments, vital signs, ECGs, and other safety assessments, were analyzed using the safety population.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
We thank all the participants and the study investigators who cared for them. We thank Ceara Axelrod and Jennifer Giovanni (Synterex, Inc.) for editorial assistance. We thank Minguang Zhang and Katherine Kacena for statistical consulting. Part of the data from this study were presented at the American Diabetes Association’s 83rd Scientific Sessions, June 2023. The study sponsor was responsible for study design, data collection, analysis, and paper writing.
Author contributions
D.Z., W.W., G.T., G.M., J.M., J.H., X.Z., Y. Liu, S.G., H.Q., Q.Z., J.N., Z.Z., M.G., Y.B., Y. Li, and H.P. designed and performed the study. D.Z., Q.Z., J.N., Z.Z., M.G., Y.B., Y. Li, C.L.J., M.F., S.X., M.K.J., and H.P. analyzed and interpreted the data. D.Z., C.L.J., M.F., S.X., and M.K.J. drafted the paper. All authors approved the final paper.
Peer review
Peer review information
Nature Communications thanks David D'Alessio and Mintu Nath and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data generated in this study are provided in the Article, Supplementary Information, and the Source Data file. Source data are provided with this publication. The full dataset and protocol are not publicly available due to data privacy laws and contractual obligations. Relevant individual de-identified participant data (IPD) are displayed in the Supplementary Information and shared in the Source Data file. Sciwind Biosciences will provide additional individual de-identified participant data underlying the reported results upon request. Data are available after acceptance of this article with no expiration of data requests currently set. Requests should be made by contacting corresponding authors D. Zhu (zhudalong@nju.edu.cn) or H. Pan (hai.pan@sciwindbio.com) and will be evaluated within 6 months of receipt. Access will be provided after the proposed use of the data has been approved by a review committee and receipt of a signed data access agreement with Sciwind Biosciences. Source data are provided with this paper.
Code availability
Code supporting this Article is available within the Supplementary Information.
Competing interests
This study was funded and sponsored by Sciwind Biosciences. D.Z., W.W., G.T., G.M., J.M., J.H., X.Z., Y. Liu, and S.G. received funding from Sciwind to their institutions as trial investigators. H.Q., Q.Z., J.N., Z.Z., M.G., Y.B., Y. Li, C.L.J., M.F., M.K.J., S.X., and H.P. are, or were at the time of the study, employees of Sciwind.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dalong Zhu, Weimin Wang.
Contributor Information
Dalong Zhu, Email: zhudalong@nju.edu.cn.
Hai Pan, Email: hai.pan@sciwindbio.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-52353-y.
References
- 1.International Diabetes Federation. Diabetes prevalence. IDF Diabetes Atlas, 10th edition. 34–38 (2021)
- 2.Heppner, K. M. & Perez-Tilve, D. GLP-1 based therapeutics: simultaneously combating T2DM and obesity. Front. Neurosci.9, 92 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nauck, M. A., Quast, D. R., Wefers, J. & Meier, J. J. GLP-1 receptor agonists in the treatment of type 2 diabetes—state-of-the-art. Mol. Metab.46, 101102 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo, W. et al. Discovery of ecnoglutide—a novel, long-acting, cAMP-biased glucagon-like peptide-1 (GLP-1) analog. Mol. Metab.75, 101762 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones, B. The therapeutic potential of GLP-1 receptor biased agonism. Br. J. Pharmacol.179, 492–510 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willard, F. S. et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight5, e140532 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garber, A. et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. The Lancet373, 473–481 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Sorli, C. et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol.5, 251–260 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Frías, J. P. et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N. Engl. J. Med.385, 503–515 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Shi, L. X. et al. Efficacy and safety of dulaglutide monotherapy compared with glimepiride in Chinese patients with type 2 diabetes: post-hoc analyses of a randomized, double-blind, phase III study. J. Diabetes Investig.11, 142–150 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Der Velden, W. J. C. et al. GLP-1 Val8: a biased GLP-1R agonist with altered binding kinetics and impaired release of pancreatic hormones in rats. ACS Pharmacol. Transl. Sci.4, 296–313 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knerr, P. J. et al. Next generation GLP-1/GIP/glucagon triple agonists normalize body weight in obese mice. Mol. Metab.63, 101533 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization, Department of noncommunicable Disease Surveillance. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO Consultation. 3–7 (1999).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are provided in the Article, Supplementary Information, and the Source Data file. Source data are provided with this publication. The full dataset and protocol are not publicly available due to data privacy laws and contractual obligations. Relevant individual de-identified participant data (IPD) are displayed in the Supplementary Information and shared in the Source Data file. Sciwind Biosciences will provide additional individual de-identified participant data underlying the reported results upon request. Data are available after acceptance of this article with no expiration of data requests currently set. Requests should be made by contacting corresponding authors D. Zhu (zhudalong@nju.edu.cn) or H. Pan (hai.pan@sciwindbio.com) and will be evaluated within 6 months of receipt. Access will be provided after the proposed use of the data has been approved by a review committee and receipt of a signed data access agreement with Sciwind Biosciences. Source data are provided with this paper.
Code supporting this Article is available within the Supplementary Information.