Abstract
DNA motifs containing unmethylated CpG dinucleotides within the context of certain flanking sequences enhance both innate and antigen-specific immune responses, due in part to the enhanced production of Th1-type cytokines. Here we explored the ability of CpG-containing oligodeoxynucleotides combined with recombinant hepatitis B surface antigen (HBsAg) to induce Th1 responses in mice that are transgenic for this antigen and that represent a model for asymptomatic hepatitis B virus chronic carriers. This was compared to hepatitis B virus-specific DNA-mediated immunization, which we have previously shown to induce the clearance of the transgene expression product and the down-regulation of hepatitis B virus mRNA in this transgenic mouse lineage. In control nontransgenic C57BL/6 mice, three immunizations with HBsAg and CpG triggered the production of anti-HBs antibodies and of HBs-specific T cells that secrete gamma interferon but do not display any HBsAg-specific cytotoxic activity. In the HBsAg-transgenic mice, immunization with HBsAg and CpG oligodeoxynucleotides, but not with CpG alone, induced the clearance of HBsAg circulating in the sera, with a concomitant appearance of specific antibodies, and was able to regulate the hepatitis B virus mRNA constitutively expressed in the liver. Finally, adoptive transfer experiments with CD8+ T cells primed in C57BL/6 mice with HBsAg and CpG oligodeoxynucleotide-based immunization show that these cells were able to partially control transgene expression in the liver and to clear the HBsAg from the sera of recipient transgenic mice without an antibody requirement. CpG oligodeoxynucleotides motifs combined with HBsAg could therefore represent a potential therapeutic approach with which to treat chronically infected patients.
Hepatitis B virus (HBV) causes a common infectious disease, and there are an estimated 350 million chronic HBV carriers worldwide (29). Patients with chronic hepatitis B are at high risk of developing liver cirrhosis, and this is associated with a higher rate of mortality due to the development of hepatocellular carcinoma or noncarcinomatous complications of cirrhosis (20, 21).
Currently, the only therapy for chronic hepatitis that has a lasting beneficial effect is systemic treatment with alpha interferon (IFN-α), but a sustained response is achieved in only one-third of patients with chronic hepatitis B (21). Nucleoside analogues such as lamivudine provide a therapeutic alternative leading to a rapid decrease in serum HBV DNA levels and to histopathological improvement of liver disease. However, cessation of treatment usually leads to a rapid relapse of disease, and long-term treatment often results in the selection of resistant viral variants (27). These outcomes emphasize the need for novel therapeutic approaches. Although the pathogenesis of chronic liver disease is not well understood, there is a consensus that liver damage is immune mediated. Specific immunotherapeutic strategies have been proposed as possible alternatives to the use of IFN or antiviral drugs to enhance or to broaden the defective T-cell responses in chronically infected patients. Among these, specific vaccine therapies with either currently available recombinant anti-hepatitis B vaccines (9, 40), a lipopeptide-based T-cell vaccine (53), or newly developed genetic vaccines (31, 33, 42) have been studied recently with animal models or in human clinical trials (19, 40).
As an animal model for asymptomatic carriers infected at birth, we have used mice that are transgenic (Tg) for hepatitis B surface antigen (HBsAg) (1, 16). In this model, we have previously shown that HBsAg-specific T- and B-cell responses induced after DNA-based immunization are able to mediate antigen clearance in the sera and down-regulation of transgene expression in the liver (33, 34). The Th1 bias of the immune response induced following intramuscular (i.m.) injection of DNA is mostly attributed to immunostimulatory CpG motifs present in the plasmid (44). Thus, we ask whether synthetic CpG-containing oligodeoxynucleotides (ODN) could efficiently replace DNA adjuvanticity for HBsAg immunization in this Tg mouse lineage.
Unmethylated cytosine-guanine dinucleotides within the context of certain flanking sequences (CpG motifs), as originally identified in bacterial DNA, have diverse stimulatory effects on the innate and adaptive immune systems. Several of these effects contribute to the strong Th1-type adjuvant activity for antigen-specific responses. For example, CpG DNA triggers most (>95%) B cells to proliferate, secrete immunoglobulin (Ig) and cytokines, and be protected from apoptosis (24, 26, 57), all of which contribute to a stronger humoral response. CpG DNA also directly activates monocytes, macrophages, and dendritic cells to secrete various Th1 cytokines (18, 24), which in turn induces T and NK cells to secrete additional cytokines (2, 4, 10, 24, 56, 57). Overall, CpG induces a strong Th1-like pattern of cytokine production dominated by interleukin-12 (IL-12) and IFN-γ, with little secretion of Th2 cytokines (24), and these cytokines can provide additional T-cell help for both humoral and cell-mediated immune responses. CpG ODN have been shown to be effective Th1-type vaccine adjuvants in animals with a variety of antigens. For example, mice immunized by i.m. injection of antigen with CpG ODN have strong cytotoxic T lymphocytes (CTL) and predominantly IgG2a antibodies, also indicative of a Th1-type response (8, 12, 30, 43, 55). Since such Th1-type immune responses are thought to be necessary for clearance of HBV infection (3, 17, 23, 39), it is possible that CpG ODN with recombinant HBsAg may be an effective therapeutic vaccine for the treatment of patients chronically infected with HBV. Here, we show that immunization with HBsAg combined with CpG ODN resulted in clearance of the HBsAg from the sera, induction of specific antibodies, and partial down-regulation of HBV mRNA in the livers of HBsAg-Tg mice.
MATERIALS AND METHODS
Antigens used for immunization.
The pCMV-S2.S DNA (37), which served as a positive control, expressed the S and pre-S2 region of the HBV envelope gene (ayw subtype) under the control of the cytomegalovirus (CMV) immediate-early gene promoter. The plasmid DNA used for gene transfer was purified with anion-exchange chromatography columns (Qiagen GmbH, Hilden, Germany), redissolved in endotoxin-free phosphate-buffered saline (PBS) (Sigma, St. Louis, Mo.) at 1 mg/ml, and stored at or below −20°C. The protein vaccine contained recombinant surface proteins of HBV (small and middle proteins encoded by S and pre-S2 genes) of the ayw subtype, which was produced in CHO cells (PMC, Val de Reuil, France) (36) and is referred to hereafter as HBsAg. Unless otherwise indicated, the antigen was used in soluble form, without any adjuvant, at a final concentration of 1 mg/ml. HBsAg was combined with CpG ODN (sequence 1826: 5′ TCCATGACGTTCCTGACGTT 3′ [optimal murine CpG motifs are underlined]) or non-CpG control ODN (sequence 1982: 5′ TCCAGGACTTCTCTCAGGTT 3′) (12). The ODN, which were provided by the Coley Pharmaceutical Group (Wellesley, Mass.), were synthesized with a nuclease-resistant phosphorothioate backbone.
Peptide.
The eight-mer synthetic Kb-binding S 371–378 peptide ILSPFLPL was synthesized by Genosys Biotechnologies (Cambridge, United Kingdom), and the 13-mer 126–138 peptide RGLYFPAGGSSSG was obtained from Neosystem (Strasbourg, France). The numbering of the amino acid sequence of peptides starts from the first methionine of the HBV ayw subtype pre-S domain. These peptides were used for the CTL and enzyme-linked immunospot (ELISPOT) assays.
In vitro splenocyte stimulation assays.
Spleens from 10- to 12-week-old naïve C57BL/6 mice (Charles River, Wilmington, Mass.) were recovered under sterile conditions, and single-cell suspensions were prepared in RPMI 1640 (Life Technologies, Gaithersburg, Md.) supplemented with l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), and 2% (vol/vol) heat-inactivated normal mouse serum (NMS) (Cedarlane, Hornby, Canada). The splenocytes were plated at 5 × 106/ml for the cytokine assays (100 μl/well) in triplicate in 96-well round-bottom polystyrene plates (Becton Dickinson Labware, Franklin Lakes, N.J.). CpG ODN 1826 and non-CpG control ODN 1982 were suspended in complete RPMI 1640 and plated at 100 μl/well to final concentrations of 10, 3, 1, and 0.3 μg/ml for the cytokine evaluation. For comparison, genomic Escherichia coli DNA, as a source of bacterial DNA, was suspended in RPMI 1640 without sera and plated at 100 μl/well to final concentrations of 10 and 30 μg/ml. The splenocytes were incubated for 96 h at 37°C and 5% CO2. For cytokine evaluation, four identical assays were set up, and culture supernatants were harvested and stored at −80°C following 6, 24, 48, and 72 h of incubation at 37°C and 5% CO2. The levels of tumor necrosis factor alpha (TNF-α), IL-12, IL-6, and IFN-γ were determine by using murine-specific OPTEIA enzyme-linked immunosorbent assay (ELISA) sets (Pharmingen, Mississauga, Ontario, Canada).
Immunization of mice.
Normal C57BL/6 and HBsAg-Tg mice (1) were kept under standard pathogen-free conditions in the animal facility of the Pasteur Institute. The HBV envelope Tg mouse lineage E36 was initially produced on a C57BL/6 × SJL/J background and was then backcrossed against C57BL/6 (H-2b) at least 24 times before use. The transgene in these mice consists of a copy of the HBV genome with the core gene deleted. The transgene is expressed exclusively in the liver, and large amounts of HBsAg particles are secreted in mouse serum (16, 33). C57BL/6 Tg or non-Tg female mice, 5 to 7 weeks old, were immunized two or three times at monthly intervals by i.m. injection bilaterally into the tibialis anterior (TA) muscle with recombinant HBsAg (2 μg) alone or combined with CpG or non-CpG ODN (120 μg) in a total volume of 50 μl per leg. As a positive control, other Tg and non-Tg mice were injected on a single occasion with 100 μg of recombinant plasmid DNA directly into regenerating TA muscles as previously described (32). We have shown this to induce strong HBsAg-specific immunity and to bring about control of transgene expression in the Tg mice (33).
Serologic test.
At various times before and after DNA injection, blood was collected from mice by retrobulbar puncture with heparinized glass pipettes, and sera recovered by centrifugation were assayed for anti-HBs and anti-preS2 antibodies by specific ELISA. Purified recombinant particles containing HBV small S protein (1 μg/ml) or pre-S2 (120–145) synthetic peptide (1 μg/ml) were used as the solid phase. After blocking with PBST (PBS containing 0.1% Tween 20) supplemented with 10% fetal calf serum, serial dilutions of sera were added. After extensive washing, the bound antibodies were detected with antimouse Ig (total IgG) labeled with horseradish peroxidase (Amersham, Little Chalfont, United Kingdom). Antibody titers were determined by the serial end-point dilution method. Mouse sera were tested individually, and titers were the mean of at least three determinations. Serum dilutions below 1/100 were considered negative. The sera from DNA-immunized HBsAg-Tg mice were also used for detection of HBsAg with a commercial ELISA kit (Monolisa AgHBs; Bio-Rad, Marnes la Coquette, France). Anti-HBs and HBsAg titers were expressed as group geometric means ± standard errors of the mean of individual animal values, which represent the average of duplicate or triplicate assays. The significance of differences between values was assessed with a Mann-Whitney test; P values lower than 0.05 were considered significant.
CTL activity assay.
Groups of four C57BL/6 mice were immunized with HBsAg combined with CpG or non-CpG ODN or with pCMV-S2.S DNA vector. Spleens were removed from immunized mice 2 weeks after the last injection of HBsAg and ODN or 4 weeks after the DNA injection. Single-cell suspensions were prepared. Cells (107 cells/well) were suspended in 2 ml of α minimum essential medium (α-MEM) supplemented with 10 mM HEPES buffer, 1 mM sodium pyruvate, nonessential amino acids, 0.05 mM β-mercaptoethanol, antibiotics, glutamine, and 10% fetal calf serum (Myoclone, Gibco BRL; Cergy Pontoise, France) in 24-well plates. Responder spleen cells were cocultured with 106 irradiated autologous transfected cells expressing the small envelope protein (RBL5/S) or with RBL5 cells pretreated with HBsAg particles. After 5 days in culture, half of the medium was replaced with fresh medium, and the cells were used as effectors in a standard chromium release assay performed 2 or 3 days later. For pulsing, 106 RBL5 cells were incubated with 10 μg of HBsAg in 500 μl of complete α-MEM for 2 h at 37°C with 5% CO2. After two washes in α-MEM medium, cells were irradiated at 200 Gy and then used as stimulator or target cells in the CTL activity assay (45, 46). Targets were autologous transfectant cells (RBL5/S), HBsAg-pulsed or peptide (HBs 371–378)-pulsed RBL5 cells, or unpulsed RBL5 cells. Targets were labeled with 51Cr (3.7 MBq/106 cells; Amersham). After a 4-h incubation at 37°C, 50 μl of supernatant was removed from each well and counted on a beta counter as described elsewhere (34). The percentage of specific release was calculated as [(experimental release − spontaneous release)/(total release − spontaneous release)] × 100. Total release was measured by resuspending target cells in lysis buffer. Spontaneous release was obtained from targets incubated with medium alone and is usually less than 15% of the total release.
ELISPOT assay.
The number of splenic IFN-γ-secreting cells was determined by using a modification of the Czerkinsky ELISPOT technique (11). In brief, flat-bottom nitrocellulose ELISA plates (Multiscreen; Millipore, Molsheim, France) were coated overnight with rat anti-mouse IFN-γ (Pharmingen, San Diego, Calif.) and thereafter saturated for 2 h at 37°C with RPMI 1640 containing 10% fetal calf serum (complete RPMI medium). Cells were suspended in complete RPMI medium, transferred onto coated plates (106 cells/well), and incubated for 42 h at 37°C in 5% CO2 with stimulator peptides or antigen. Two different peptides were used at the concentration of 3 μg/ml: the 13-mer major histocompatibility complex (MHC) class II binding 126–138 peptide (34) and the 8-mer H-2Kb-binding HBs 371–378 peptide (47). The cells were removed by flicking the plates and then lysed with water. After being washed with PBS–0.05% Tween 20, biotinylated rat anti-mouse IFN-γ antibody (Pharmingen) was added to each well. Following incubation and subsequent washing, the plates were incubated with streptavidin-alkaline phosphatase conjugate (Boehringer-Mannheim, Germany). Next, a 2.3 mM solution of 5-bromo-4-chloro-3-indolyl phosphate (Promega, Madison, Wis.) diluted in alkaline buffer solution was added. When spots were visible, the color reaction was stopped by rinsing the plates with distilled water. Then, after drying, the number of IFN-γ-secreting blue spots was counted. Each cell population was titrated in triplicate, but data were derived only from wells with more than 10 spots.
CD8+ T-lymphocyte subset fractionation and adoptive transfer.
The CD8+ T-cell subpopulation was isolated from the total spleen cells by negative selection by magnetic cell sorting (MACS; Miltenyi Biotec, Paris, France). The purity of the CD8+ T cell was confirmed by cytofluorometry analysis with a FACScan flow cytometer (Becton Dickinson, Sunnyvale, Calif.) with Cell Quest software and following staining with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-CD8 and anti-CD4 monoclonal antibodies (Pharmingen, San Diego, Calif.). The percentage of undesirable CD4+ T cells in the population was <0.4%. The remaining leukocytes were pooled according to the type of immunization and then counted and resuspended in 200 μl of endotoxin-free PBS. CD8+ T cells (6 × 106) were injected into the retroorbital cavities of recipient mice that had been sublethally irradiated (5 Gy) before transfer.
Northern blot analysis.
Total RNA of liver was extracted from mechanically pulverized frozen tissue with RNA-PLUS (Bioprobe Systems, Montreuil-sous-Bois, France). The RNA (40 μg) was fractionated on 1% formaldehyde–agarose gels and blotted onto nylon membranes, which were then hybridized with 32P-DNA probes synthesized from a 2.4-kb BglII HBV DNA fragment or from a 0.2-kb PstI cDNA fragment of the murine 18S rRNA gene (Valbiotech, France) by using the Rediprime DNA labeling system (Amersham). Quantification of HBV mRNA was performed with a PhosphorImager.
Statistical test.
The statistical test used to calculate P values for differences in mRNA levels was the nonparametric Mann-Whitney test. It was used with Stat View 4.5 software (Abacus Concepts, Berkeley, Calif.). P values ≤ 0.05 were considered significant.
RESULTS
In vitro effect of ODN on cytokine production by naïve C57BL/6 splenocytes.
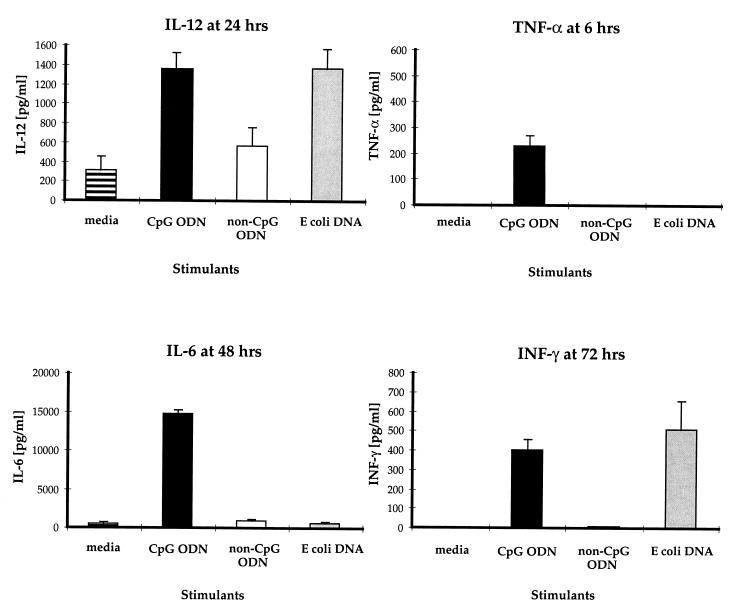
The immunostimulatory effects of CpG ODN were evaluated with spleen cells derived from nonimmune C57BL/6 mice. Pooled splenocytes from five naïve mice were cultured in vitro either alone or with CpG ODN, non-CpG control ODN, or E. coli DNA. The CpG ODN clearly showed a superior ability to induce a non-antigen-specific lymphoproliferative response when compared to medium, the control ODN, or E. coli DNA (data not shown). With respect to cytokine production, the CpG ODN, but not the control ODN, induced significant levels of TNF-α, IL-6, and IFN-γ (Fig. 1). Comparable amounts of IFN-γ and IL-12 were induced from splenocytes after stimulation with CpG ODN and E. coli DNA. Presumably because of the optimized CpG motifs and a nuclease-resistant phosphorothioate backbone, the CpG ODN was superior to both control ODN and E. coli DNA at inducing detectable TNF-α and IL-6 production.
FIG. 1.
In vitro cytokine production by splenocytes from naïve C57BL/6 mice stimulated with CpG (solid bars) or non-CpG ODN (open bars) at 3 μg/ml or E. coli DNA (shaded bars) at 30 μg/ml. Supernatants were collected at different time points after stimulation, and cytokines were quantified by ELISA. The results show the means of quadruplicate experiments.
Effect of ODN on humoral response in C57BL/6 mice immunized with HBsAg.
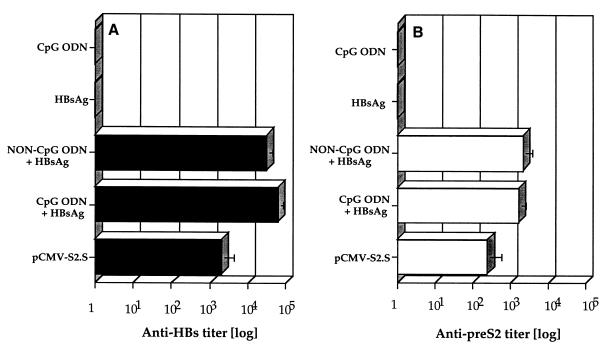
Groups of six mice were immunized i.m. once with pCMV-S2.S DNA or three times with HBsAg alone or combined with CpG or non-CpG control ODN, with other controls receiving CpG ODN alone. Antibodies specific for HBsAg (Fig. 2A) or the pre-S2 domain of HBV middle protein (Fig. 2B) were detected by ELISA 3 weeks after the last injection. Both ODN combined with HBsAg induced a significant production of anti-HBs and anti-pre-S2 antibodies in C57BL/6 mice (Fig. 2). In contrast, HBsAg alone was not able to induce specific antibodies. As expected, no anti-HBs antibodies were detected in mice receiving CpG ODN alone. Interestingly, the antibody titers were comparable (P ≤ 0.165) in the sera of mice receiving HBsAg with either CpG ODN or non-CpG control ODN. As previously shown (37), a single injection of pCMV-S2.S DNA was sufficient to induce anti-HBs and anti-pre-S2 antibodies. Anti-HBs antibody titers induced after injection of HBsAg combined with CpG and non-CpG ODN were 30- and 16-fold higher, respectively, than those obtained after DNA immunization. Similarly, titers of anti-pre-S2 antibodies were six- and eightfold higher in mice receiving HBsAg with CpG and non-CpG ODN, respectively. However, it should be noted that these antibodies were induced after three injections of HBsAg with ODN, whereas the DNA was given only once.
FIG. 2.
Anti-hepatitis B Ig-specific responses. Groups of six C57BL/6 mice were immunized once with pCMV-S2.S (100 μg) or three times at monthly intervals with CpG ODN alone (120 μg), HBsAg alone (2 μg), or HBsAg combined with CpG or non-CpG control ODN. Antibodies specific for HBsAg (A) or the pre-S2 domain of HBV middle protein (B) were investigated by ELISA 3 weeks after the last injection. Antibody titers are expressed as a serial end point dilutions.
The CpG ODN coinjected with the HBsAg in the C57BL/6 mice induced a production of high levels of anti-HBs and anti-pre-S2-specific antibodies. This specific antibody production was concomitant with a significant increase in the IgG2b titer in the C57BL/6 mice (data not shown). In this strain, IgG2a antibodies are not detectable due to a missing gene (22). The adjuvant effect of control ODN was unexpected, since this had not been seen before in BALB/c mice (12). This could be due in part to the ODN dose, which was 10 times higher than that used in previous studies with BALB/c mice. In addition, some adjuvant properties of the nuclease-resistant phosphorothioate backbone had previously been observed with mucosal administration (35).
In vivo adjuvant effect of CpG ODN on the cellular immune response in C57BL/6 mice immunized with HBsAg.
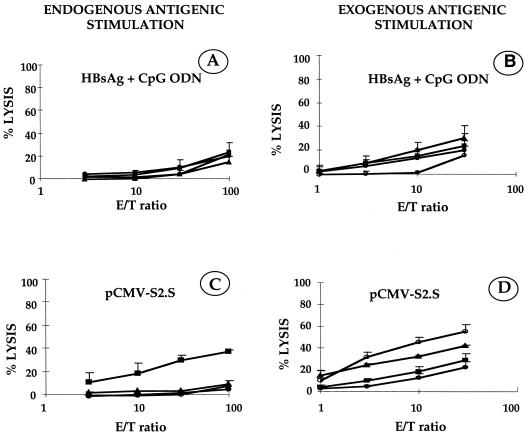
C57BL/6 mice were immunized twice (1 month apart) with HBsAg (2 μg) together with the CpG ODN (120 μg). Spleen cells were taken 2 weeks after the second injection. As a positive control, C57BL/6 mice were immunized with pCMV-S2.S (100 μg) as already described (32) and sacrificed 4 weeks after the single injection. Primed T splenocytes recovered from C57BL/6 mice were restimulated in vitro with either irradiated HBsAg-pulsed RBL5 cells (Fig. 3 B and D, exogenous antigenic stimulation) or irradiated HBs-expressing RBL5/S transfected cells (Fig. 3A and C, endogenous antigenic stimulation). The targets were autologous transfectant cells expressing or not expressing the HBV small envelope protein or cells pulsed with HBV peptide (HBs 371–378) or with HBsAg. The results show that immunization with HBsAg with CpG ODN (Fig. 3A and B) did not induce HBsAg-specific CTL irrespective of whether in vitro endogenous or exogenous restimulation was used. In contrast, DNA-based immunization with HBsAg-encoding plasmid pCMV-S2.S (Fig. 3C and D) triggered the emergence of HBsAg-specific CTL after in vitro restimulation with endogenously processed antigen (Fig. 3C). Likewise, after stimulation with exogenously processed antigen, the DNA vaccination generated CTL specific for target cells pulsed with HBsAg or with the peptide HBs 371–378 (Fig. 3D), but not for peptides presented by RBL5/S transfected cells. HBsAg-specific CTL were not observed in C57BL/6 mice immunized with HBsAg alone or in control mice administered CpG ODN without antigen (data not shown).
FIG. 3.
CTL responses of C57BL/6 mice immunized once with pCMV-S2.S (100 μg) or twice, 1 month apart, with HBsAg (2 μg) combined with CpG ODN (120 μg). Splenocytes harvested, respectively, 4 and 2 weeks postimmunization were stimulated in vitro for 5 days with irradiated HBsAg-pulsed RBL5 cells (exogenous stimulation) or with irradiated HBs-expressing RBL5/S transfectant cells (endogenous stimulation). The target cells used were RBL5 cells (●), RBL5/S transfectant cells (▪), HBsAg-pulsed RBL5 cells (▴), or peptide 371–378-pulsed RBL5 cells (○). The percentage of specific lysis was calculated as [(experimental 51Cr release − spontaneous 51Cr release)/(total 51Cr release − spontaneous 51Cr release)] × 100. Results represent the mean for four mice.
Effect of CpG ODN on T-cell cytokine secretion in C57BL/6 mice immunized with HBsAg.
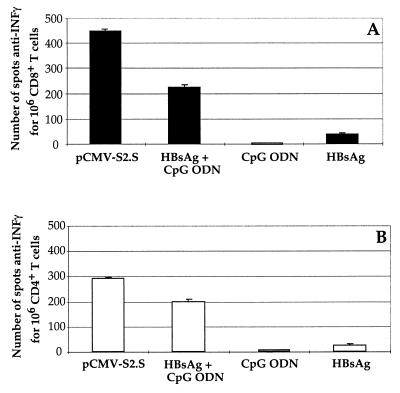
We performed an anti-IFN-γ ELISPOT assay to evaluate the frequency of HBsAg-specific T cells in the spleens of C57BL/6 mice after immunization with HBsAg plus CpG ODN, with an HBsAg-expressing DNA vector, or with HBsAg or CpG ODN alone. The number of epitope-specific T cells producing IFN-γ was measured with the HBs 371–378 Kb-binding peptide (Fig. 4A) or the HBs 126–138 T helper peptide (Fig. 4B) as stimulatory peptides. The results show that, compared to the immunization with HBsAg alone, immunization with HBsAg plus CpG ODN or with pCMV-S2.S DNA was able to activate, respectively, 6 or 12 times more HBs-specific IFN-γ-secreting CD8+ T cells (Fig. 4A). In addition, stimulation with the T helper peptide HBs 126–138 induced IFN-γ production by spleen cells from mice immunized with pCMV-S2.S DNA, HBsAg plus CpG ODN, or HBsAg (Fig. 4B), but the frequency of such IFN-γ-secreting CD4+ T cells was significantly higher in mice immunized with HBsAg plus CpG ODN than in mice receiving HBsAg alone. In DNA-immunized control mice, the number of INF-γ-secreting T cells was 10 times higher than that in mice immunized with HBsAg alone.
FIG. 4.
INF-γ production by ELISPOT. Groups of four C57BL/6 mice were immunized (i.m.) twice at monthly intervals with recombinant HBsAg (2 μg) alone or combined with CpG ODN (120 μg), with CpG ODN alone (120 μg), or once with pCMV-S2.S (100 μg). Each bar represents the mean of triplicate values for spot-forming cells following stimulation of splenocytes with peptide 371–378 for 106 CD8+ T cells (A) or with peptide 126–138 for 106 CD4+ T cells (B).
DNA or CpG ODN immunization of HBsAg-Tg mice induced the clearance of circulating HBsAg and the concomitant appearance of anti-HBs and anti-pre-S2 antibodies.
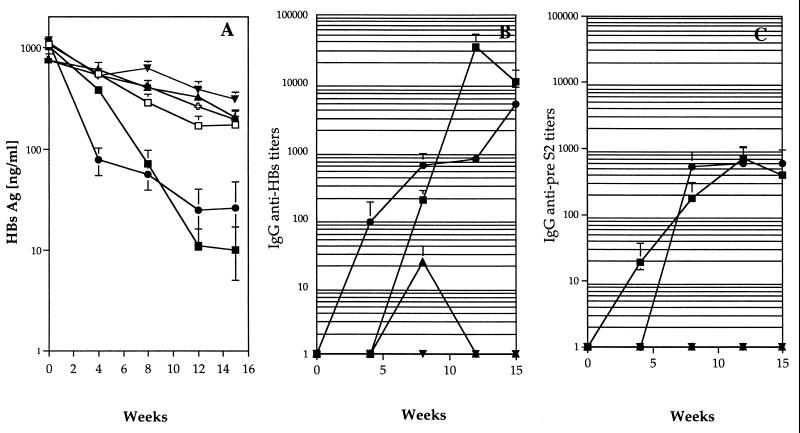
We have previously described how a single injection of pCMV-S2.S DNA that encodes HBsAg, but not an irrelevant DNA, into HBsAg-Tg mice induced a persistent decrease in circulating HBsAg particles and a concomitant appearance of serum anti-HBs antibodies, which were maintained over time (33). Figure 5A shows that three injections of HBsAg combined with immunostimulatory CpG ODN were sufficient to clear HBsAg from the sera of HBsAg-Tg mice to a similar degree to clearance with the DNA vaccine. In contrast, three injections of CpG ODN alone, HBsAg alone, or HBsAg combined with non-CpG control ODN did not result in major changes in HBsAg levels (Fig. 5A).
FIG. 5.
Immunization of HBsAg-Tg mice. Groups of Tg mice were injected with PBS instead of DNA (nonimmunized, 13 mice [ ]) or immunized once with pCMV-S2.S (12 mice; ●) or three times at monthly intervals with CpG ODN alone (5 mice; □) or with either HBsAg alone (6 mice; ▾) or combined with CpG (12 mice; ▪) or the non-CpG control (9 mice; ▴) ODN as in Fig. 2. Mice were bled at weekly intervals, and the sera were analyzed for HBsAg expressed as nanograms per milliliter (A) and for anti-HBs-specific (B) and anti-pre-S2-specific (C) antibodies (expressed as serial end point dilutions). Each point represents the mean titer for the group, and error bars represent the standard error.
Specific antibodies were detectable at the time of HBsAg decrease in the sera of Tg mice. Anti-HBs (Fig. 5B) and anti-pre-S2 (Fig. 5C) antibodies appeared in the sera of pCMV-S2.S-immunized Tg mice by 4 and 8 weeks, respectively, after a single injection of DNA, and these continued to increase over time. Using HBsAg combined with CpG ODN, three injections were required to obtain comparable anti-preS2 antibody titers (Fig. 5C) and anti-HBs titers that were 50-fold greater at the peak of the response than those induced by the DNA vaccine (Fig. 5B). As with the DNA vaccine, injections of HBsAg plus CpG ODN in the HBsAg-Tg mice resulted in the clearance of serum HBsAg and the appearance of detectable specific antibodies.
In contrast, compared to the C57BL/6 non-Tg mice (Fig. 2), injections of HBsAg combined with non-CpG control ODN did not induce any detectable anti-pre-S2 antibodies and only very low levels of anti-HBs antibodies (in two out of nine mice), which failed to trigger clearance of HBsAg.
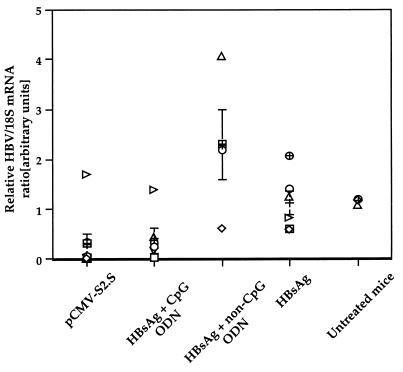
Combination of CPG ODN with HBsAg was able to regulate the HBV mRNA expression in the livers of HBsAg Tg mice.
Northern blot analysis of mRNA from livers of Tg mice sacrificed at the time of HBsAg clearance (15 weeks after immunization) was performed, and HBV mRNA was quantified with a PhosphorImager after correction for mRNA loading and variations in transfer efficiency as assessed by 18S rRNA expression. We have previously shown that the immune response induced after a single injection of pCMV-S2.S DNA into HBsAg-Tg mice reduced the level of HBVmRNA in the liver, in some cases to undetectable levels (33). In the present study, both the DNA vaccine and HBsAg plus CpG ODN reduced the mean HBV mRNA level in the livers of mice to a similar degree (P ≤ 0.30) (Fig. 6). The mean HBV mRNA levels in the livers of mice receiving HBsAg plus non-CpG ODN or HBsAg alone were not significantly different (P ≤ 0.09). Untreated age- and sex-matched Tg mice displayed a range of HBV mRNA levels identical to that of mice receiving HBsAg alone (P ≤ 0.90). In contrast, the mean HBV mRNA levels in the livers of mice receiving HBsAg plus non-CpG ODN or HBsAg alone were significantly higher than in mice treated with HBsAg plus CpG ODN (P ≤ 0.02 and P ≤ 0.025, respectively) and appeared not to be reduced. These data showed that three injections of HBsAg combined with CpG ODN were as efficient as a single injection of pCMV-S2.S DNA at reducing HBV mRNA in the livers of HBsAg-Tg mice and that the effect was CpG dependent.
FIG. 6.
Analysis of HBV mRNA content in the livers of Tg mice was performed by Northern blotting and quantification with PhosphorImager. Livers from Tg mice were taken 15 weeks after immunizations with pCMV-S2.S or with HBsAg alone or combined with CpG or non-CpG-control ODN as in Fig. 5. The HBV/18S mRNA ratios from untreated mice are shown as a control. DNA probes specific for HBV and 18S RNA were used, and the HBV/18S mRNA ratio is expressed in arbitrary units. Values representing individual data, the mean, and the standard error are shown.
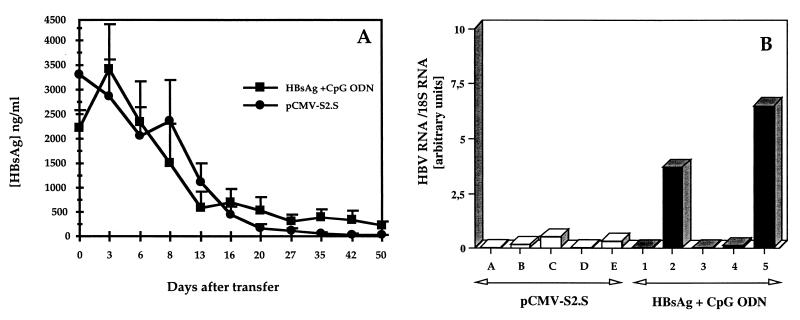
The CD8+ T cells primed by HBsAg plus CpG ODN immunization induced the clearance of HBsAg in the sera and the control of HBV mRNA in the livers of HBsAg-Tg mice.
Because CD8+ T cells detected after immunization with HBsAg plus CpG ODN did not display any detectable cytolytic activity in vitro, we sought to determine whether they might nevertheless be responsible for the control of HBV mRNA expression observed in vaccinated Tg mice. We thus performed adoptive transfer experiments. As a positive control, we used CD4+-depleted spleen cells from DNA-immunized mice, which have been shown previously to down-regulate transgene expression in the livers of HBsAg-Tg mice. Indeed, HBsAg was completely cleared from the sera of all five recipient mice by 50 days following transfer of pCMV-S2.S-primed CD4+-depleted T cells, whereas it was cleared in only three mice and reduced in the other two after transfer of HBsAg plus CpG ODN-primed cells (Fig. 7A). No anti-HBs antibodies were detected in the sera of the recipient mice 50 days after adoptive transfer. Northern blot analysis of RNA prepared from the livers of the recipient Tg mice shows that HBV mRNA was still clearly detectable in the livers of the two of five mice receiving HBsAg plus CpG ODN-primed T cells that failed to clear HBsAg and was almost undetectable in the other three mice, as well as the five of five mice receiving pCMV-S2.S-primed T cells (Fig. 7B).
FIG. 7.
Adoptive transfer of primed CD4+-depleted splenocytes into HBsAg Tg-mice. CD4+-depleted splenocytes, isolated from the spleens of non-Tg mice immunized with either pCMVS2.S (●) or HBsAg combined with CpG ODN (▪), as in Fig. 4, were adoptively transferred into sublethally irradiated Tg mice. The Tg mice were bled, and the level of HBsAg (nanograms per milliliter) in the sera was evaluated on a weekly basis (A). HBV mRNA content (B) in the livers of HBsAg-Tg recipient mice was quantified by Northern blot analysis and PhosphorImaging 50 days after transfer of primed CD8+ T cells from mice immunized with pCMV-S2.S (open bars) or with HBsAg plus CpG ODN (black bar). Each bar represents an individual value expressed as an HBV RNA/18S RNA ratio in arbitrary units.
These results show that CD8+ T cells primed by HBsAg adjuvanted with CpG ODN were able to down-regulate HBV mRNA in the livers of HBsAg-Tg mice in the absence of detectable in vitro cytolytic activity, but to a somewhat lesser extent than those primed by the DNA vaccine.
DISCUSSION
In this study, we have evaluated the adjuvant effect of CpG ODN on the immune response to HBsAg in either naive or HBsAg-Tg mice. With this mouse model of HBV chronic carriers, we have previously shown that vaccination with DNA plasmid encoding HBsAg can break nonresponsiveness to this antigen and resulted in the clearance of HBsAg from the sera and the down-regulation of HBV mRNA in the liver. However, the efficiency of the immune response obtained after DNA-based immunization has been described as being due in part to the presence of immunostimulatory CpG motifs contained in the plasmid backbone (44). These sequences are thought to be responsible for the Th1 response observed after genetic immunization, whereas protein vaccines induce mostly Th2 responses. Thus, CpG ODN could represent an alternative way of modulating the immune response to classical recombinant antigens towards a Th1 profile.
The CpG and the control ODN coinjected with the HBsAg in C57BL/6 mice induced a strong humoral response with high production of anti-HBs and anti-pre-S2-specific antibodies. However, in HBsAg-Tg mice, only the ODN containing consensus CpG motifs were able to induce antibodies, which efficiently clear the transgene-encoded HBsAg from mice sera. Antibody response in non-Tg C57BL/6 mice is dependent on T helper cells specific for epitopes present in both S and pre-S2 domains (38). In HBeAg-Tg mice, it has been shown that T helper cells survive deletion or anergy in the presence of circulating antigen by virtue of low avidity, but they are nevertheless capable of being activated (5). Thus, in our HBsAg-Tg mice, activation of such T helper cells may require a strong activation through CpG ODN to provide help for antibody production. Alternatively, CpG DNA has been shown to directly activate B cells to proliferate in a T-cell-independent manner, and this effect is synergistic with B-cell activation through the antigen receptor (25).
The CpG ODN but not the control ODN has a Th1-type effect and allows IFN-γ secretion from splenocytes stimulated either in vitro or in vivo after immunization. In vitro, after stimulation with the CpG ODN, the C57BL/6 splenocytes produced Th1 cytokines such as IL-12, INF-γ, and TNF-α (Fig. 1). This could favor a Th1 environment for the development of antigen-specific T cells after immunization. In addition, IL-6, a cytokine known to activate B cells, is produced by CpG ODN-activated splenocytes. In vivo, coinjection of CpG ODN with HBsAg has a clear adjuvant effect in inducing HBsAg-specific CD4+ and CD8+ T cells that secrete IFN-γ (Fig. 4) as well as B cells, which produce anti-HBs antibodies (Fig. 2).
Thus, in C57BL/6 mice, the HBsAg plus CpG ODN immunization mimicked the DNA vaccine with respect to humoral response and to IFN-γ production by T cells. However, these results are different from those reported for BALB/c mice, in which HBsAg plus CpG ODN induced a potent CTL response as well (12). After a single pCMV-S2.S DNA injection, the immunized C57BL/6 mice produced a strong HBV-specific CTL response. As shown by our model of i.m. immunization with pCMV-S2.S, we have some experimental evidence that DNA vaccination results in the in vivo production of HBsAg particles (13). It has been demonstrated that HBsAg can be processed by an alternative pathway for peptide presentation by MHC class I molecules (46). This results in peptides different from those derived from the classical endogenous pathway (47). Therefore, induction of CTL specific for the peptide HBs 371–378 that result only from the exogenous pathway of degradation indicates that the antigen produced after DNA-based immunization has been endocytosed or captured, processed in the alternative pathway, and presented as peptides in association with MHC class I molecules. In contrast after immunization of C57BL/6 mice with HBsAg and CpG ODN, we did not detect a cytotoxic response. To be sure that the lack of CTL response in the C57BL/6 mice was not due to technical difficulties, we stimulated splenocytes in vitro under the following different experimental conditions: (i) with autologous RBL5/S transfectant cells (46) expressing the small envelope protein (endogenous condition) (45), (ii) with HBsAg-pulsed RBL5 cells (exogenous condition), and (iii) with unpulsed RBL5 cells as a control. None of these experimental strategies has been able to elicit in vitro a cytotoxic activity of lymphocytes primed in vivo with HBsAg plus CpG ODN. Thus the CD8+ T cells induced by HBsAg plus CpG ODN immunization secreted IFN-γ, but did not display cytotoxic activity in vitro (52). Nevertheless, both DNA vaccine and HBsAg plus CpG ODN broke the nonresponsiveness to HBsAg in Tg mice by inducing clearance of the transgene expression product and control of HBV mRNA in the liver. After direct immunization of HBsAg-Tg mice, the clearance of HBsAg is mediated in part by the high-titer anti-HBs antibody production triggered by immunization, but also by the regulation of transgene expression at the mRNA level. In this Tg mouse model, we have previously shown that antibodies alone are not sufficient to achieve a persistent clearance of the antigen and that IFN-γ-secreting T cells are required (33, 34). In addition, overcoming nonresponsiveness to HBsAg in Tg mice immunized with HBsAg plus CpG ODN seems to be mediated by CD8+ T cells, as demonstrated by experiments involving adoptive transfer of CD4+-depleted splenocytes into Tg mice. In these adoptive transfer experiments, although B cells were transferred with CD8+ T cells, no specific antibodies were detected at the time of HBsAg elimination, ruling out a role for an antibody-mediated clearance. Recent experiments with adoptive transfer of CD8+-depleted splenocytes into Tg mice resulted in the clearance of HBsAg from the sera as well. Moreover, detection of CTL specific for the HBs 371–378 Kb-binding peptide after peptide stimulation of splenocytes from the recipient Tg mice confirms the role of CD8+ T cells in the control of transgene expression (E. Malanchère-Bres, unpublished results).
It should be noted that CpG ODN required more doses than the DNA vaccine and that the response was less complete, likely due to a weaker T-cell response, as shown by a lower frequency of IFN-γ- producing T cells. Even so, CpG ODN are powerful adjuvants for the induction of T cells regulating transgene expression in HBsAg-Tg mice.
Recently conflicting results have been reported (48) for another model of HBsAg-Tg mice (7) in which different vaccination techniques achieved neither antigen clearance nor suppression of transgene expression in the liver. This may reflect a difference in the tolerance to HBsAg in these two lineages (i.e., peripheral versus neonatal tolerance). The possibility that the control of HBV mRNA expression could be related to DNA methylation patterns is unlikely, since modifications of methylation have only been reported very early in life (41) and during tumor development (15). However, E36 HBsAg-Tg mice never developed tumors, even in advanced age. Interestingly, in the C57BL/6 genetic background, mice with high (16 μg/ml) or low (<100 ng/ml) levels of serum HBsAg have similar levels of DNA methylation, and methylation is also independent of age (49; C. Pourcel, personal communication).
In conclusion, this study has shown the efficiency of CpG ODN as an adjuvant to HBsAg vaccination to trigger specific antibodies and Th1-biased immune responses.
Vaccination of chronically HBV-infected patients has already been performed with classical recombinant vaccine adjuvanted with alum. Despite induction of efficient HBs-specific B and T helper responses in some patients, the long-term clearance of HBV DNA was not achieved in all patients (9).
Successful immunization and protective efficacy in numerous animal models (14, 51) and induction of cellular immune responses in humans have been demonstrated with DNA vaccines (54). However, despite induction of CTL responses, problems regarding induction of antibody responses in humans remain to be resolved (28, 50). This could be particularly important for hepatitis B, for which both antibodies and cellular immune responses have been implicated in disease resolution (6). Thus, CpG ODN could represent an alternative method for modulating the immune response by combining the advantages of both classical and DNA vaccines.
ACKNOWLEDGMENTS
We thank C. Pourcel for critical reading of the manuscript and helpful discussions. We thank R. Vinas (Avantis Pasteur) for generously providing purified recombinant HBsAg.
REFERENCES
- 1.Babinet C, Farza H, Morello D, Hadchouel M, Pourcel C. Specific expression of hepatitis B surface antigen (HBsAg) in transgenic mice. Science. 1985;230:1160–1163. doi: 10.1126/science.3865370. [DOI] [PubMed] [Google Scholar]
- 2.Ballas Z K, Rasmussen W L, Krieg A M. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157:1840–1845. [PubMed] [Google Scholar]
- 3.Bertoletti A, D'Elios M M, Boni C, De Carli M, Zignego A L, Durazzo M, Missale G, Penna A, Fiaccadori F, Del Prete G, Ferrari C. Different cytokine profiles of intraphepatic T cells in chronic hepatitis B and hepatitis C virus infections. Gastroenterology. 1997;112:193–199. doi: 10.1016/s0016-5085(97)70235-x. [DOI] [PubMed] [Google Scholar]
- 4.Chace J H, Hooker N A, Mildenstein K L, Krieg A M, Cowdery J S. Bacterial DNA-induced NK cell IFN-gamma production is dependent on macrophage secretion of IL-12. Clin Immunol Immunopathol. 1997;84:185–193. doi: 10.1006/clin.1997.4380. [DOI] [PubMed] [Google Scholar]
- 5.Chen M, Sallberg M, Thung S N, Hughes J, Jones J, Milich D R. Nondeletional T-cell receptor transgenic mice: model for the CD4+T-cell repertoire in chronic hepatitis B virus infection. J Virol. 2000;74:7587–7599. doi: 10.1128/jvi.74.16.7587-7599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chisari F V. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 7.Chisari F V, Klopchin K, Moriyama T, Pasquinelli C, Dunsford H A, Sell S, Pinkert C A, Brinster R L, Palmiter R D. Molecular pathogenesis of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell. 1989;59:1145–1156. doi: 10.1016/0092-8674(89)90770-8. [DOI] [PubMed] [Google Scholar]
- 8.Chu R S, Targoni O S, Krieg A M, Lehmann P V, Harding C V. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couillin I, Pol S, Mancini M, Driss F, Brechot C, Tiollais P, Michel M L. Specific vaccine therapy in chronic hepatitis B: induction of T cell proliferative responses specific for envelope antigens. J Infect Dis. 1999;180:15–26. doi: 10.1086/314828. . (Erratum, 180:1756.) [DOI] [PubMed] [Google Scholar]
- 10.Cowdery J S, Chace J H, Yi A K, Krieg A M. Bacterial DNA induces NK cells to produce IFN-gamma in vivo and increases the toxicity of lipopolysaccharides. J Immunol. 1996;156:4570–4575. [PubMed] [Google Scholar]
- 11.Czerkinsky C, Andersson G, Ekre H P, Nilsson L A, Klareskog L, Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 12.Davis H, Weeranta R, Waldschmidt T J, Tyett L, Schorr J, Krieg A M. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis surface antigen. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 13.Davis H L, Michel M-L, Whalen R G. DNA based immunization for hepatitis B induces continuous secretion of antigen and high levels of circulating antibody Hum. Mol Genet. 1993;2:1847–1851. doi: 10.1093/hmg/2.11.1847. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly J J, Ulmer J B, Liu M A. DNA vaccines. Dev Biol Stand. 1998;95:43–53. [PubMed] [Google Scholar]
- 15.Farza H, Dragani T A, Metzler T, Manenti G, Tiollais P, Della Porta G, Pourcel C. Inhibition of hepatitis B virus surface antigen gene expression in carcinogen-induced liver tumors from transgenic mice. Mol Carcinog. 1994;9:185–192. doi: 10.1002/mc.2940090402. [DOI] [PubMed] [Google Scholar]
- 16.Farza H, Salmon A-M, Hadchouel M, Moreau J-L, Babinet C, Tiollais P, Pourcel C. Hepatitis B surface antigen gene expression is regulated by sex-steroids and glucocorticoids in transgenic mice. Proc Natl Acad Sci USA. 1987;84:1187–1191. doi: 10.1073/pnas.84.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 18.Halpern M D, Kurlander R J, Pisetsky D S. Bacterial DNA induces murine interferon-gamma production by stimulation of IL-12 and tumor necrosis factor-alpha. Cell Immunol. 1996;167:72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- 19.Heathcote J, McHutchison J, Lee S, Tong M, Benner K, Minuk G, Wright T, Fikes J, Livingston B, Sette A, Chestnut R. A pilot study of the CY-1899 T-cell vaccine in subjects chronically infected with hepatitis B virus. The CY1899 T Cell Vaccine Study Group. Hepatology. 1999;30:531–536. doi: 10.1002/hep.510300208. [DOI] [PubMed] [Google Scholar]
- 20.Hoofnagle J H, di Bisceglie A M. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347–356. doi: 10.1056/NEJM199701303360507. [DOI] [PubMed] [Google Scholar]
- 21.Hoofnagle J H, Lau D. New therapies for chronic hepatitis B. J Viral Hepatitis. 1997;4:41–50. doi: 10.1111/j.1365-2893.1997.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 22.Jouvin-Marche E, Morgado M G, Leguern C, Voegtle D, Bonhomme F, Cazenave P A. The mouse Igh-1a and Igh-1b H chain constant regions are derived from two distinct isotypic genes. Immunogenetics. 1989;29:92–97. doi: 10.1007/BF00395856. [DOI] [PubMed] [Google Scholar]
- 23.Jung M C, Hartmann B, Gerlach J T, Diepolder H, Gruber R, Schraut W, Gruner N, Zachoval R, Hoffmann R, Santantonio T, Wachtler M, Pape G R. Virus-specific lymphokine production differs quantitatively but not qualitatively in acute and chronic hepatitis B infection. Virology. 1999;261:165–172. doi: 10.1006/viro.1999.9833. [DOI] [PubMed] [Google Scholar]
- 24.Klinman D M, Yi A K, Beaucage S L, Conover J, Krieg A M. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krieg A M. Immune effects and mechanisms of action of CpG motifs. Vaccine. 2000;19:618–22. doi: 10.1016/s0264-410x(00)00249-8. [DOI] [PubMed] [Google Scholar]
- 26.Krieg A M, Yi A K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 27.Lai C L, Chien R N, Leung N W, Chang T T, Guan R, Tai D I, Ng K Y, Wu P C, Dent J C, Barber J, Stephenson S L, Gray D F. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 28.Le T P, Coonan K M, Hedstrom R C, Charoenvit Y, Sedegah M, Epstein J E, Kumar S, Wang R, Doolan D L, Maguire J D, Parker S E, Hobart P, Norman J, Hoffman S L. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine. 2000;18:1893–1901. doi: 10.1016/s0264-410x(99)00407-7. [DOI] [PubMed] [Google Scholar]
- 29.Lee W M. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 30.Lipford G B, Sparwasser T, Bauer M, Zimmermann S, Koch E S, Heeg K, Wagner H. Immunostimulatory DNA: sequence-dependent production of potentially harmful or useful cytokines. Eur J Immunol. 1997;27:3420–3426. doi: 10.1002/eji.1830271242. [DOI] [PubMed] [Google Scholar]
- 31.Lu M, Hilken G, Kruppenbacher J, Kemper T, Schirmbeck R, Reimann J, Roggendorf M. Immunization of woodchucks with plasmids expressing woodchuck hepatitis virus (WHV) core antigen and surface antigen suppresses WHV infection. J Virol. 1999;73:281–289. doi: 10.1128/jvi.73.1.281-289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancini M, Davis H L, Tiollais P, Michel M-L. DNA-based immunization against the envelope proteins of the hepatitis B virus. J Biotechnol. 1996;44:47–57. doi: 10.1016/0168-1656(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 33.Mancini M, Hadchouel M, Davis H L, Whalen R G, Tiollais P, Michel M-L. DNA-mediated immunization in a transgenic mouse model of the hepatitis B surface antigen chronic carrier state. Proc Natl Acad Sci USA. 1996;93:12496–12501. doi: 10.1073/pnas.93.22.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancini M, Hadchouel M, Tiollais P, Michel M L. Regulation of hepatitis B virus mRNA expression in a hepatitis B surface antigen transgenic mouse model by IFN-gamma-secreting T cells after DNA-based immunization. J Immunol. 1998;161:5564–5570. [PubMed] [Google Scholar]
- 35.McCluskie M J, Brazolot Millan C L, Gramzinski R A, Robinson H L, Santoro J C, Fuller J T, Widera G, Haynes J R, Purcell R H, Davis H L. Route and method of delivery of DNA vaccine influence immune responses in mice and non-human primates. Mol Med. 1999;5:287–300. [PMC free article] [PubMed] [Google Scholar]
- 36.Michel M-L, Pontisso P, Sobczak E, Malpiece Y, Streeck R E, Tiollais P. Synthesis in animal cells of hepatitis B surface antigen particles carrying a receptor for polymerized human serum albumin. Proc Natl Acad Sci USA. 1984;81:7708–7712. doi: 10.1073/pnas.81.24.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michel M L, Davis H L, Schleef M, Mancini M, Tiollais P, Whalen R G. DNA-mediated immunization to the hepatitis B surface antigen in mice: aspects of the humoral response mimic hepatitis B viral infection in humans. Proc Natl Acad Sci USA. 1995;92:5307–5311. doi: 10.1073/pnas.92.12.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milich D R. Immune response to hepatitis B virus proteins: relevance of the murine model. Semin Liver Dis. 1991;11:93–112. doi: 10.1055/s-2008-1040428. [DOI] [PubMed] [Google Scholar]
- 39.Penna A, Del Prete G, Cavalli A, Bertoletti A, D'Elios M M, Sorrentino R, D'Amato M, Boni C, Pilli M, Fiaccadori F, Ferrari C. Predominant T-helper 1 cytokine profile of hepatitis B virus nucleocapsid-specific T cells in acute self-limited hepatitis B. Hepatology. 1997;25:1022–1027. doi: 10.1002/hep.510250438. [DOI] [PubMed] [Google Scholar]
- 40.Pol S, Michel M L, Bréchot C. Immune therapy of hepatitis B virus chronic infection. Hepatology. 2000;31:548–549. doi: 10.1002/hep.510310249. [DOI] [PubMed] [Google Scholar]
- 41.Pourcel C, Tiollais P, Farza H. Transcription of the S gene in transgenic mice is associated with hypomethylation at specific sites and with DNase I sensitivity. J Virol. 1990;64:931–935. doi: 10.1128/jvi.64.2.931-935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rollier C, Sunyach C, Barraud L, Madani N, Jamard C, Trepo C, Cova L. Protective and therapeutic effect of DNA-based immunization against hepadnavirus large envelope protein. Gastroenterology. 1999;116:658–665. doi: 10.1016/s0016-5085(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 43.Roman M, Martin-Orozco E, Goodman J S, Nguyen M D, Sato Y, Ronaghy A, Kornbluth R S, Richman D D, Carson D A, Raz E. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 44.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M D, Silverman G J, Lotz M, Carson D A, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 45.Schirmbeck R, Böhm W, Ando K, Chisari F V, Reimann J. Nucleic acid vaccination primes hepatitis B virus surface antigen-specific cytotoxic T lymphocytes in nonresponder mice. J Virol. 1995;69:5929–5934. doi: 10.1128/jvi.69.10.5929-5934.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schirmbeck R, Melber K, Reimann J. Hepatitis B virus small surface antigen particles are processed in a novel endosomal pathway for major histocompatibility complex class I-restricted epitope presentation. Eur J Immunol. 1995;25:1063–1070. doi: 10.1002/eji.1830250431. [DOI] [PubMed] [Google Scholar]
- 47.Schirmbeck R, Wild J, Reimann J. Similar as well as distinct MHC class I-binding peptides are generated by exogenous and endogenous processing of hepatitis B virus surface antigen. Eur J Immunol. 1998;28:4149–4161. doi: 10.1002/(SICI)1521-4141(199812)28:12<4149::AID-IMMU4149>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 48.Schirmbeck R, Wild J, Stober D, Blum H E, Chisari F V, Geissler M, Reimann J. Ongoing murine T1 or T2 immune responses to the hepatitis B surface antigen are excluded from the liver that expresses transgene-encoded hepatitis B surface antigen. J Immunol. 2000;164:4235–4243. doi: 10.4049/jimmunol.164.8.4235. [DOI] [PubMed] [Google Scholar]
- 49.Schweizer J, Valenza-Schaerly P, Goret F, Pourcel C. Control of expression and methylation of a hepatitis B virus transgene by strain-specific modifiers. DNA Cell Biol. 1998;17:427–435. doi: 10.1089/dna.1998.17.427. [DOI] [PubMed] [Google Scholar]
- 50.Tacket C O, Roy M J, Widera G, Swain W F, Broome S, Edelman R. Phase 1 safety and immune response studies of a DNA vaccine encoding hepatitis B surface antigen delivered by a gene delivery device. Vaccine. 1999;17:2826–2829. doi: 10.1016/s0264-410x(99)00094-8. [DOI] [PubMed] [Google Scholar]
- 51.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 52.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 53.Vitiello A, Ishioka G, Grey H M, Rose R, Farness P, LaFond R, Yuan L, Chisari F V, Furze J, Bartholomeuz R, et al. Development of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. I. Induction of a primary cytotoxic T lymphocyte response in humans. J Clin Investig. 1995;95:341–349. doi: 10.1172/JCI117662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang R, Doolan D L, Le T P, Hedstrom R C, Coonan K M, Charoenvit Y, Jones T R, Hobart P, Margalith M, Ng J, Weiss W R, Sedegah M, de Taisne C, Norman J A, Hoffman S L. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 55.Weiner G J, Liu H M, Wooldridge J E, Dahle C E, Krieg A M. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA. 1997;94:10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto S, Yamamoto T, Kataoka T, Kuramoto E, Yano O, Tokunaga T. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J Immunol. 1992;148:4072–4076. [PubMed] [Google Scholar]
- 57.Yi A K, Chace J H, Cowdery J S, Krieg A M. IFN-gamma promotes IL-6 and IgM secretion in response to CpG motifs in bacterial DNA and oligodeoxynucleotides. J Immunol. 1996;156:558–564. [PubMed] [Google Scholar]