Abstract
Coxsackievirus B3 (CVB3) is one of the most common pathogens for viral myocarditis. The lack of effective therapeutics for CVB3-caused viral diseases underscores the importance of searching for antiviral compounds. Pyrrolidine dithiocarbamate (PDTC) is an antioxidant and is recently reported to inhibit ubiquitin-proteasome-mediated proteolysis. Previous studies have shown that PDTC inhibits replication of rhinovirus, influenza virus, and poliovirus. In the present study, we report that PDTC is a potent inhibitor of CVB3. Coxsackievirus-infected HeLa cells treated with PDTC showed a significant reduction of CVB3 viral RNA synthesis, viral protein VP1 expression, and viral progeny release. Similar to previous observation that divalent ions mediate the function of PDTC, we further report that serum-containing copper and zinc are required for its antiviral activity. CVB3 infection resulted in massive generation of reactive oxygen species (ROS). Although PDTC alleviated ROS generation, the antiviral activity was unlikely dependent on its antioxidant effect because the potent antioxidant, N-acetyl-l-cysteine, failed to inhibit CVB3 replication. Consistent with previous reports that PDTC inhibits ubiquitin-proteasome-mediated protein degradation, we found that PDTC treatment led to the accumulation of several short-lived proteins in infected cells. We further provide evidence that the inhibitory effect of PDTC on protein degradation was not due to inhibition of proteasome activity but likely modulation of ubiquitination. Together with our previous findings that proteasome inhibition reduces CVB3 replication (H. Luo, J. Zhang, C. Cheung, A. Suarez, B. M. McManus, and D. Yang, Am. J. Pathol. 163:381-385, 2003), results in this study suggest a strong antiviral effect of PDTC on coxsackievirus, likely through inhibition of the ubiquitin-proteasome pathway.
Coxsackievirus B3 (CVB3), an enterovirus in the family of Picornaviridae, is one of the most common causative pathogens for human viral myocarditis and its sequela, dilated cardiomyopathy (DCM) (3, 41, 42). CVB3-induced viral myocarditis is initially considered an immune-mediated disease (40), but results from our laboratory and others have shown that CVB3 can also directly injure cardiomyocytes in infected hearts (12). In cultured HeLa cells, CVB3 triggers mitochondria-mediated apoptotic pathway through activation of several caspases following cytochrome c release (9, 10). Similar to other viruses, CVB3 can modulate the pre-existing host signaling machinery to facilitate its own replication. Several signaling proteins, such as the extracellular signal-regulated kinase 1 and 2 (ERK1/2) (14, 26, 37, 43) and protein kinase B/Akt (PKB/Akt) (21), are activated following CVB3 infection, and activation of these signaling proteins is important to successfully complete CVB3 life cycle.
Ubiquitin-proteasome pathway, a major intracellular proteolytic system, has been found to be involved in a variety of intracellular functions, including cell cycle regulation, apoptosis, and inflammatory responses (33, 47). In the process of protein degradation, substrates are first tagged by ubiquitin by three enzymes, ubiquitin-activating enzyme (E1 enzyme), ubiquitin-conjugation enzyme (E2 enzyme), and ubiquitin ligase (E3 enzyme). The polyubiquitinated proteins are then rapidly degraded by the proteasome. Recent studies have implicated an important role of the ubiquitin-proteasome pathway in viral life cycle (7, 38, 39, 45, 49, 54). Proteasome-mediated proteolysis of cyclin D1 is associated with CVB3-induced cell growth arrest, and a few other host proteins including p53 are rapidly degraded following CVB3 infection. In addition, proteasome inhibitors reduce the replication or progeny release of several viruses, including CVB3, human cytomegalovirus, and human immunodeficiency virus (HIV) type 1. However, the exact host proteins that are important for viral replication remain undefined. In addition, the role of ubiquitin ligases in CVB3 replication has not been reported.
Pyrrolidine dithiocarbamate (PDTC) is a stable pyrrolidine derivative of dithiocarbamates. It has been commonly used as an inhibitor for oxidative stress-induced NF-κB activation. Recent studies have shown that PDTC may play a role in ubiquitin-proteasome-mediated protein degradation by acting as an inhibitor of E3 ubiquitin ligase (25) or by direct inhibition of proteasome activity (32). Previous studies have shown that PDTC strongly inhibits replication of human rhinoviruses that is independent of its antioxidant activity (24). However, the precise mechanisms are not well elucidated.
In this report, we provide evidence that PDTC effectively reduces CVB3 replication and CVB3 viral progeny release, and such inhibitory effect is independent of its antioxidant activity. Additionally, we find that PDTC apparently inhibits proteasome-mediated degradation of several host proteins, including p53, p21, and MKP-1. Inhibition of the ubiquitin-proteasome pathway by PDTC may contribute to its antiviral effect.
MATERIALS AND METHODS
Cell culture, virus, and materials.
HeLa cells (American Type Culture Collection) were grown and maintained in Dulbecco's modified Eagle's media (DMEM) supplemented with 10% heat-inactivated newborn calf serum (NCS) (Invitrogen). CVB3 (Kandolf strain) was propagated in HeLa cells and stored at −80°C. Virus titer was routinely determined by a plaque assay of HeLa cell monolayer prior to infection as described below.
PDTC, N-acetyl-l-cysteine (NAC), and monoclonal anti-β-actin antibody were purchased from Sigma Chemical Company. The monoclonal anti-VP1 antibody was obtained from DakoCytomation. The monoclonal anti-caspase-3, anti-p21, and anti-p53, polyclonal anti-MKP-1 antibodies and horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology. The polyclonal anti-IκBα antibody was obtained from Cell Signaling, and polyclonal anti-ubiquitin antibody was from Calbiochem.
Virus infection.
HeLa cells were grown in complete medium (DMEM supplemented with 10% NCS) to 70 to 80% confluence prior to infection. HeLa cells were then infected at a multiplicity of infection (MOI) of 10 with CVB3 unless otherwise indicated or sham infected with phosphate-buffered saline (PBS) for 1 h in serum-free DMEM. Cells were then washed with PBS and cultured in serum-free DMEM for the indicated periods of time. For inhibitor experiments, HeLa cells were infected with CVB3 (MOI = 10) for 1 h, washed with PBS, and then incubated with DMEM containing various concentrations of compounds unless otherwise specified.
Western blot analysis.
Cell lysates were prepared as described previously (37). Equal amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. Membranes were blocked for 1 h with nonfat dry milk solution (3% in PBS) containing 0.1% Tween 20. Blots were then incubated for 1 h with the primary antibody followed by incubation for 1 h with the secondary antibody. Immunoreactive bands were visualized by enhanced chemiluminescence (Amersham).
Viral RNA in situ hybridization.
HeLa cells were grown and maintained on two-chamber culture slides (Becton Dickinson Labware). Subconfluent cells were infected with either PBS or CVB3 (MOI of 10). Following 1 h of incubation at 37°C, cells were washed with PBS and replenished with complete medium in the absence and presence of 100 μM PDTC. HeLa cells were incubated for an additional 5 h before fixation. The culture slides were then washed gently with PBS, fixed with formalin buffer for 15 min, and then air dried at room temperature. Culture slides were then subjected to in situ hybridization assays to detect the sense strand of CVB3 genomic RNA as previously described (2).
Cell viability assay.
A modified 3,4-(5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt (MTS) assay (Promega), which measures mitochondrial function, was used to determine cell viability. HeLa cells were grown in 96-well plates and serum starved for 24 h. Following CVB3 infection (MOI = 1), culture medium was replaced with serum-free DMEM with or without inhibitors. Twenty hours postinfection, cells were incubated in MTS solution for 2 h, and absorbance was measured with an enzyme-linked immunosorbent assay plate reader (490 nm). MTS assays were performed in triplicate.
Plaque assay.
CVB3 titer in cell supernatant was determined on monolayers of HeLa cells by an agar overlay plaque assay in triplicate as described previously (2). Briefly, samples were serially diluted 10-fold and overlaid on 90 to 95% confluent monolayers of HeLa cells in 6-well plates and incubated for 1 h. Medium was aspirated and HeLa cells was washed with PBS twice, and 2 ml of complete DMEM containing 0.75% agar was overlaid in each well. Cells were incubated at 37°C for 72 h, fixed with Carnoy's fixative (75% ethanol-25% acetic acid) for 30 min, and stained with 1% crystal violet. Plaques were counted, and viral concentration was calculated as PFU per milliliter.
Reactive oxygen species (ROS) detection.
HeLa cells were sham infected with PBS or infected with CVB3 (MOI = 10) for the indicated periods of time, then a redox-sensitive fluorescence probe, CM-H2DCFDA (15 μM), was added to the medium. Cells were labeled with CM-H2DCFDA for an hour and visualized under a Nikon fluorescence microscope. Images of cells were captured by a CCD digital camera and then processed using Photoshop.
26S proteasome activity assay.
Fresh cytoplasmic extracts were used to measure 26S proteasome activity as described previously (38). Twenty micrograms of cytoplasmic protein was added to an assay buffer (20 mM Tris-HCl [pH 8.0], 1 mM ATP, and 2 mM MgCl2) in the presence of 75 μM synthetic fluorogenic substrate SLLVY-AMC (Calbiochem) to a final volume of 100 μl. The tubes were incubated at 30°C for 1 h, and the fluorescence product AMC in the supernatant was measured at a 465-nm emission wavelength using a fluorometer.
RESULTS
PDTC inhibits CVB3 viral RNA synthesis, viral protein expression, and viral progeny release.
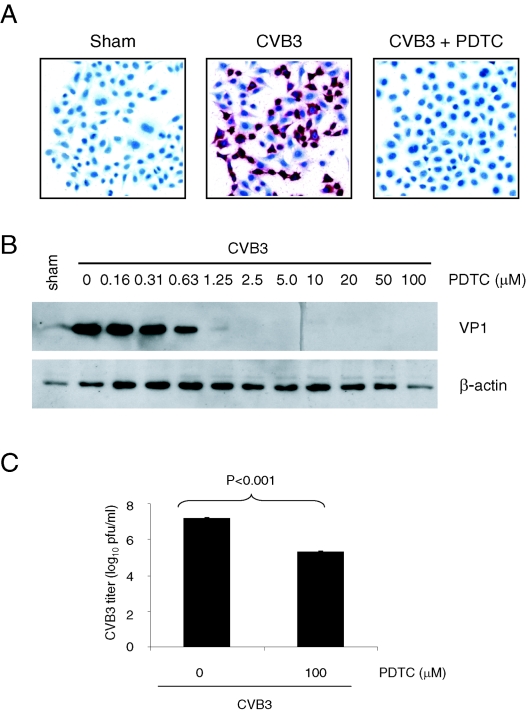
PDTC has been shown to be a potent inhibitor of replication of rhinovirus and influenza virus (24, 58). The life cycle of CVB3 consists of different stages, including viral RNA synthesis, viral proteins expression, and viral progeny release (9). To investigate whether PDTC also has an antiviral effect on replication of CVB3, we first examined the effect of PDTC on CVB3 viral RNA synthesis by in situ hybridization. We found that CVB3 infection resulted in a massive production of viral RNA in the cytoplasm (red staining), and treatment with 100 μM PDTC significantly reduced CVB3 viral RNA expression in these cells (Fig. 1A). We next investigated the effect of PDTC on viral capsid protein VP1 expression by Western blotting. As shown in Fig. 1B, PDTC inhibited VP1 expression in a dose-dependent manner and a complete inhibition of VP1 expression by PDTC was observed at concentrations as low as 2.5 μM. The viral progeny release was also investigated by a plaque formation assay. As shown in Fig. 1C, PDTC treatment resulted in a more than 170-fold reduction of viral progeny release (log10 is 7.325 ± 0.041 versus 5.075 ± 0.073). These results suggest that PDTC potently inhibits CVB3 replication.
FIG. 1.
PDTC decreases CVB3 viral RNA expression, viral protein synthesis, and viral progeny release of infected cells. (A) HeLa cells were sham-infected with PBS or infected with CVB3 (MOI = 10) in the presence or absence of 100 μM PDTC in the complete medium (with 10% NCF). Six hours postinfection, positive-stranded viral RNA was determined by in situ hybridization using antisense riboprobes for CVB3 (red). Cell nuclei were counterstained with hematoxylin (blue). (B) HeLa cells were infected with CVB3 in the presence or absence of various concentrations of PDTC in an identical manner as described above. Seven hours postinfection, cell lysates were collected and immunoblotted with anti-VP1 and anti-β-actin (loading control) antibodies. (C) HeLa Cells were infected with CVB3 (MOI = 1) in the presence of 100 μM PDTC. Twenty-four hours postinfection, medium was collected from CVB3-infected cells and virus titer was determined by plaque assays. The data shown are means ± standard deviations from three independent experiments.
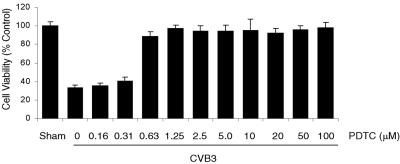
Apoptosis or programmed cell death during the late phase of viral infection has been suggested to play an important role in CVB3 life cycle by facilitating viral progeny release and propagation (9, 10). However, early cell death of infected cells may limit virus replication (51). Other studies have shown that PDTC induces apoptosis by activating caspase-mediated proapoptotic pathways in many cell types (11, 31, 57, 60). We therefore decided to determine whether PDTC can inhibit CVB3 replication via induction of apoptosis during the early stage of viral infection. We investigated the effect of PDTC on cell viability by MTS assay and found that the dosage and the incubation periods of PDTC applied in this study did not cause caspase-3 activation and loss of cell viability in sham-infected cells (data not shown). In contrast, PDTC treatment greatly promoted cell viability in infected cells (Fig. 2). Cells treated with PDTC were almost 100% viable at a concentration of 1.25 μM or higher, which was consistent with previous observed inhibition of VP1 expression (Fig. 1B). These results suggest that PDTC reduces CVB3 replication not through inducing apoptosis of infected cells.
FIG. 2.
PDTC promotes cell viability of CVB3-infected cells. HeLa cells were infected with CVB3 (MOI = 10) as described in the legend to Fig. 1 in the presence or absence of various concentrations of PDTC. Cell viability was determined at 20 h postinfection by the MTS assay that measures mitochondrial function. One-hundred percent survival was defined as the level of MTS in sham-infected cells. The data shown are means ± standard deviations (n = 6). Similar results were obtained in two independent experiments.
PDTC reduces CVB3 replication in a serum-dependent manner.
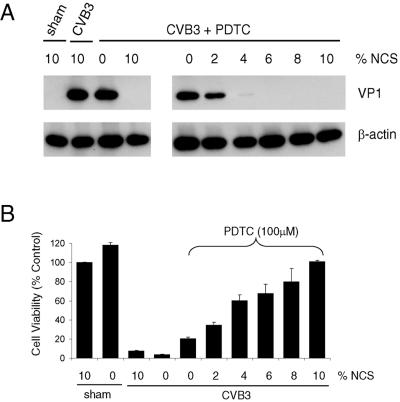
Our preliminary studies showed that withdrawal of serum from the medium abolished the antiviral effects of PDTC, which led us to further investigate whether soluble factors present in the serum were required for the effect of PDTC on viral replication. We first examined the impact of 100 μM PDTC on viral protein expression in the presence of various concentrations of newborn calf serum. We found that 10% NCS or 100 μM PDTC alone did not have any inhibitory effect on CVB3 VP1 expression (left panel in Fig. 3A). However, in the presence of increasing concentrations of serum, PDTC dose dependently inhibited CVB3 VP1 expression (right panel in Fig. 3A) and CVB3-induced cell death (Fig. 3B).
FIG. 3.
Antiviral activity of PDTC depends on serum concentration. (A) HeLa cells were sham infected with PBS or infected with CVB3 (MOI = 10) in serum-free DMEM. One hour postinfection, 100 μM PDTC and various concentrations of NCS were added into the medium. Seven hours postinfection, cell lysates were collected and immunoblotted with anti-VP1 and anti-β-actin (loading control) antibodies. (B) HeLa Cells were sham infected with PBS or infected with CVB3 (MOI = 1) in the presence or absence of 100 μM PDTC and various concentrations of NCS as indicated. Cell viability was determined at 20 h postinfection by the MTS assay. One-hundred percent survival was defined as the level of MTS in sham-infected cells. The data shown are means ± standard deviations (n = 3). Similar results were obtained in two independent experiments.
Copper and zinc ions contribute to the antiviral activity of PDTC.
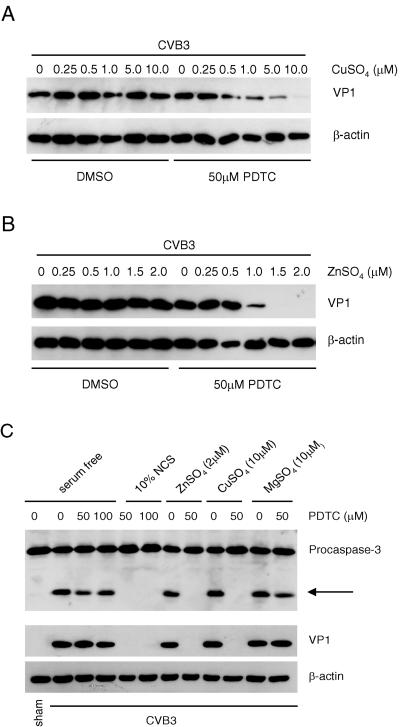
Divalent ions, including copper and zinc, have been reported to mediate PDTC functions, such as blockage of NF-κB activation and induction of cell apoptosis (23, 30). We investigated whether copper and zinc were factors present in the serum that contributed to the antiviral activity of PDTC. HeLa cells were infected with CVB3 and then incubated with increasing concentrations of CuSO4 or ZnSO4 in the presence or absence of 50 μM PDTC. As shown in Fig. 4A and B, the combination of PDTC with copper or zinc, but not copper or zinc alone, potently inhibited CVB3 replication in a dose-dependent manner, suggesting that both copper and zinc ions appeared to be the cofactors present in the serum that were required for PDTC activity.
FIG. 4.
Copper and zinc contribute to the antiviral activity of PDTC. HeLa cells were infected with CVB3 (MOI = 10) in the presence of increased concentrations of CuSO4 (A) or ZnSO4 (B) in the presence or absence of 50 μM PDTC as indicated. Seven hours postinfection, cell lysates were collected and immunoblotted with anti-VP1 and anti-β-actin (loading control) antibodies. (C) HeLa Cells were sham infected with PBS or infected with CVB3 as described in the legend to Fig. 3. PDTC (50 μM or 100 μM), NCS (10%), ZnSO4 (10 μM), CuSO4 (10 μM), and MgSO4 (10 μM) were added to the medium 1 h postinfection as indicated. Nine hours postinfection, cell lysates were collected and immunoblotted with anti-caspase-3, anti-VP1, and anti-β-actin (loading control). Arrow indicates the cleavage of procaspase-3. The data represent one of three independent experiments.
Furthermore, we observed that PDTC alone or in combination with MgSO4, which is abundant in the DMEM, failed to prevent CVB3-induced caspase-3 cleavage, nor did zinc or copper alone. However, PDTC supplemented with serum, zinc, or copper showed potent inhibition of CVB3-induced cleavage of caspase-3 (Fig. 4C).
CVB3 infection leads to ROS generation.
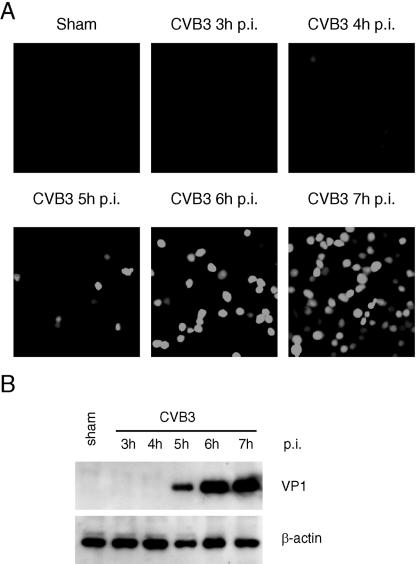
To investigate the possible mechanisms of the observed antiviral effects of PDTC, we first examined whether the antioxidant property of PDTC contributed to its action. To determine the influences of CVB3 replication on intracellular ROS level, HeLa cells were infected with CVB3 for various periods of time, and a redox-sensitive fluorescence probe, CM-H2DCFDA, was added to the medium 1 h prior to taking photographs. As shown in Fig. 5A, exposure to CVB3 resulted in an increased ROS production which began at 5 h postinfection and peaked at 7 h postinfection, corresponding to the increases of viral protein expression (Fig. 5B).
FIG. 5.
Reactive oxygen species generation is increased during CVB3 replication. (A) HeLa cells were sham infected or infected with CVB3 (MOI = 10) for the indicated time. Redox-sensitive fluorescence probe CM-H2DCFDA (15 μM) was added to the medium during the last hour of infection. Representative images of ROS-induced DCF fluorescence were shown at a magnification of 200×. (B) HeLa cells were sham infected with CVB3 in an identical manner as described in the legend to Fig. 3. Seven hours postinfection (p.i.), cell lysates were collected and immunoblotted with anti-VP1 and anti-β-actin (loading control) antibodies. The data represent one of three independent experiments.
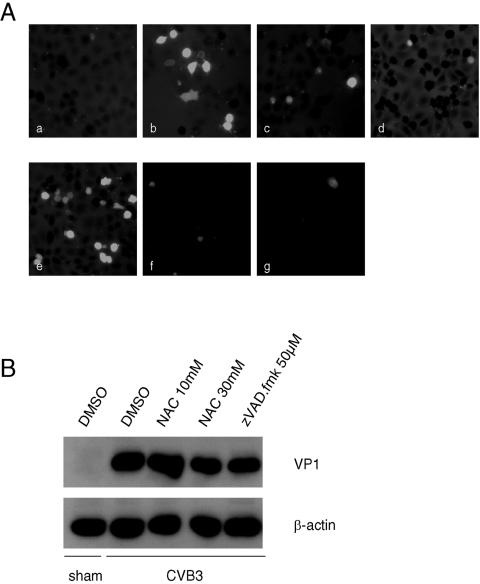
Antioxidants have been shown to have antiviral activities against a variety of unrelated viruses by alleviating the oxidative stress generated by viruses (1, 4, 16, 24, 59). To study the influence of antioxidants on CVB3 replication, another antioxidant, NAC, was used. HeLa cells were either sham infected with PBS or infected with CVB3. One hour postinfection, cells were treated with 30 mM NAC, 50 μM PDTC, or 50 μM zVAD.fmk (a general caspase inhibitor). As shown in Fig. 6A, at 7 h postinfection, CVB3 infection resulted in a drastic increase of intracellular ROS, which was strongly inhibited by NAC and PDTC. Interestingly, caspase inhibitor also greatly suppressed CVB3-induced ROS generation, suggesting oxidative stress was secondary to virus-induced apoptosis. We further showed that, unlike strong inhibitory effects seen using PDTC, either NAC treatment or caspase inhibition by zVADfmk. failed to inhibit VP1 expression (Fig. 6B) and virus-induced cytopathic effects (data not shown). Taken together, our data suggest that the antioxidant property of PDTC is not likely a major contributor to its antiviral activity.
FIG. 6.
Inhibition of CVB3 replication by PDTC is independent of its antioxidant activity. (A) HeLa cells were sham infected with PBS or infected with CVB3 (MOI = 10) in the presence of various inhibitors. Redox-sensitive fluorescence probe CM-H2DCFDA (15 μM) was added to the medium during the last hour of infection. Representative images of ROS-induced DCF fluorescence of infected cells at 7 h postinfection are shown at a magnification of 200×. (a) Sham infected; (b to g) CVB3 infected; (b) no inhibitor added; (c) 30 mM NAC; (d) 50 μM zVAD.FMK; (e) 50 μM PDTC without serum; (f) 50 μM PDTC with 10% NCS; (g) 50 μM PDTC with 10 μM CuSO4. (B) HeLa cells were either sham infected or infected with CVB3 (MOI = 10) in the presence of NAC or zVAD.fmk as indicated. Seven hours postinfection, cell lysates were collected and immunoblotted with anti-VP1 and anti-β-actin (loading control) antibodies. The data represent one of three independent experiments.
PDTC inhibits ubiquitin-proteasome pathway-mediated degradation of host proteins.
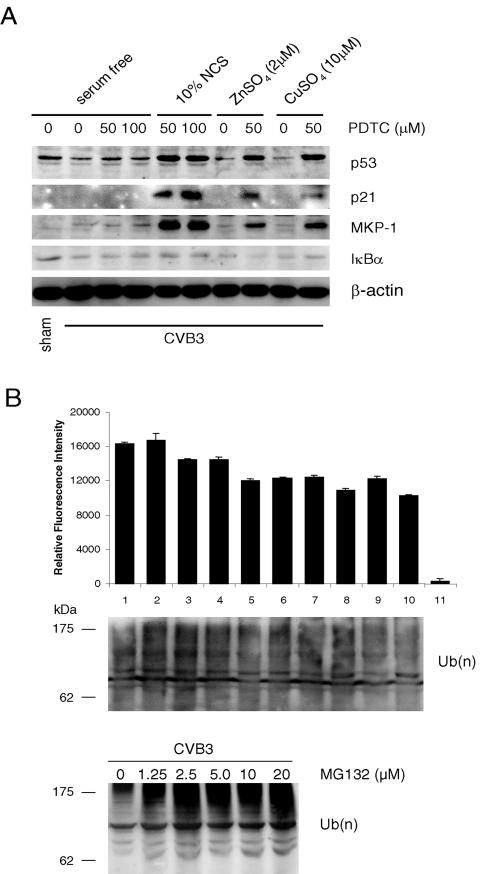
To further explore the possible antiviral mechanisms of PDTC, we examined the effect of PDTC on ubiquitin-proteasome-mediated protein degradation. We first measured the expression of tumor suppressor protein p53, which was previously found to be down-regulated following CVB3 infection (39). As shown in Fig. 7A, treatment with PDTC significantly increased the steady-state levels of p53 and cell cycle inhibitor p21 expression. We also examined the expression of MKP-1, a dual phosphatase capable of dephosphorylating and inactivating mitogen-activated protein kinases (MAPKs), including ERK1/2 and p38 MAPK. It was recently reported that MKP-1 was degraded via the ubiquitin-proteasome pathway and MKP-1 degradation resulted in sustained ERK1/2 activation (36). As shown in Fig. 7A, treatment with PDTC significantly increased MKP-1 expression, suggesting an important role of PDTC in the regulation of protein degradation.
FIG. 7.
PDTC inhibits proteasome-mediated protein degradation. (A) HeLa cells were sham infected with PBS or infected with CVB3 (MOI = 10). PDTC (50 μM or 100 μM), NCS (10%), ZnSO4 (10 μM), or CuSO4 (10 μM) was added to the medium 1 h postinfection as indicated. HeLa cells were maintained in serum-free medium, except for 10% NCS-added groups. Nine hours postinfection, cell lysates were collected and immunoblotted with anti-p53, anti-p21, anti-MKP-1, and anti-IκBα antibodies. β-Actin was probed as a loading control. The data represent one of three independent experiments. (B) Five hours postinfection, proteasome activity was measured as described in Materials and Methods. Lane 1, sham infected; 2 to 11, CVB3 infected; 2, no inhibitor added; 3, PDTC (50 μM); 4, PDTC (100 μM); 5, PDTC (50 μM) with 10% NCS; 6, PDTC (100 μM) with 10% NCS; 7, ZnSO4 (2 μM); 8, ZnSO4 (2 μM) with PDTC (50 μM); 9, CuSO4 (10 μM); 10, CuSO4 (10 μM) with PDTC (50 μM); 11, MG-132 (20 μM). The data shown are means ± standard deviations (n = 5). Similar results were obtained in two independent experiments. Cell lysates were immunoblotted with an anti-ubiquitin antibody to detect polyubiquitinated proteins under the indicated treatment. The masses of protein markers are indicated. Ub(n), polyubiquitin.
We next investigated whether PDTC inhibited ubiquitin-proteasome-mediated proteolysis through direct inhibition of proteasome activity. Five hours postinfection, 26S proteasome activities were measured using a synthetic fluorogenic substrate, SLLVY-AMC. As shown in Fig. 7B, MG132, a known proteasome inhibitor, significantly reduced proteasome activities. However, proteasome activity was not significantly altered after addition of PDTC. In addition, we did not observe a significant accumulation of polyubiquitinated proteins after PDTC treatment by Western blot analysis, while MG132 treatment markedly increased the levels of polyubiquitinated proteins (Fig. 7B). These results suggest that PDTC appears to inhibit early components (i.e., E1, E2, or E3 ligase) of the ubiquitin-proteasome pathway rather than directly inhibit 26S proteasome activity, which is consistent with previous studies that PDTC is an inhibitor of SCF ubiquitin E3 ligase (25). Indeed, we found that the expression of some other proteasome substrates, such as IκBα (Fig. 7A) and β-catenin (data not shown), was not altered upon PDTC treatment, implicating that PDTC selectively inhibited proteasome-mediated degradation of proteins following CVB3 infection.
DISCUSSION
PDTC has been reported to possess strong antiviral activity against replication of influenza virus, rhinovirus, and poliovirus. However, the mechanism was not well elucidated (24, 58). In the current study, we have explored the manner in which PDTC inhibits both coxsackievirus B3 (CVB3) viral RNA synthesis and virus structural protein expression. We provide evidence that PDTC exerts its antiviral activity intracellularly, most likely independent of viral entry.
Divalent metal ions including copper and zinc have been reported to play an essential role in mediating various biological functions of PDTC (11, 13, 20, 30). Studies by Furuta et al. and Kim et al. suggest that PDTC may act as an ionophore, recruiting extracellular copper and zinc into the cells (23, 31). Treatment with PDTC resulted in an increase of intracellular zinc and redox reactive Cu2+, and depletion of extracellular copper and zinc prevented PDTC-induced cell death and inhibition of NF-κB activation. In the present study, we found that the antiviral activity of PDTC required the presence of copper and zinc, consistent with previous reports.
One of the plausible mechanisms by which PDTC inhibits CVB3 replication is via its antioxidant activity. The intracellular oxidation status has been implicated in the pathogenesis of several viral infections, including influenza, hepatitis, HIV, and coxsackievirus (8, 27, 35, 44, 46, 52). We showed for the first time that CVB3 replication was associated with a dramatically increased intracellular ROS production (Fig. 5). To determine whether PDTC reduces CVB3 replication through inhibition of ROS production, we employed another potent ROS scavenger, NAC. We found that both inhibitors prevented CVB3-induced intracellular ROS generation. However, NAC failed to inhibit CVB3 replication. This finding, along with our observation that caspase inhibitor prevented CVB3-induced ROS generation but had no effect on CVB3 VP1 expression, suggests that (i) ROS generation is a relatively later event as compared to viral RNA synthesis and viral protein expression, which are potently inhibited by PDTC, and that (ii) the antiviral activity of PDTC is unlikely to be mediated through its antioxidant property. Such a view is consistent with previous studies in other systems (24, 58).
PDTC has been shown to prevent agonist-induced cell death in lymphocytes and tumor cell lines (5, 50); in contrast, it induces apoptosis in endothelial cells, smooth muscle cells, and B cells (31, 57, 60). Apoptosis or cell death at late viral infection can facilitate virus progeny release (9, 10). However, premature cell death will decrease the ability of host cells to foster virus replication (51). In this study, we showed that PDTC alone or in combination with zinc or copper did not cause apoptosis or cell death in sham-infected cells. In contrast, CVB3-induced apoptosis or cell death was dramatically blocked after treatment with PDTC/Cu2+ or PDTC/Zn2+, and such was accompanied by decreased viral protein expression. Our data suggest that PDTC inhibits CVB3 replication via a mechanism independent of apoptosis. Furthermore, the inhibitory effect of PDTC on CVB3-induced cell death appears to be due to reduced viral replication, although we cannot rule out the direct impact of PDTC on virus-induced apoptosis.
We have previously shown that CVB3 infection results in rapid degradation of several host proteins, including cyclin D1 and p53, through the ubiquitin-proteasome pathway (38, 39). Inhibition of 26S proteasome activities by synthetic inhibitors MG-132 and lactacystin greatly reduces viral replication and viral progeny release (38, 39). Given the evidence that PDTC inhibits proteasome-dependent proteolysis (32), it is possible that PDTC exerts its inhibitory effect on viral replication by inhibiting the ubiquitin-proteasome pathway. Kim and colleagues have showed that PDTC and zinc treatment results in an accumulation of polyubiquitinated proteins (32); however, we did not find significant changes of proteasome activity and accumulation of polyubiquitinated proteins under our experimental conditions. Nevertheless, the observation that PDTC inhibited proteasome-mediated degradation of several short-lived proteins, such as p53, p21, and MKP-1 (23, 32), but not other proteins (IκB, β-catenin) suggests that PDTC targets the early stages of ubiquitin-proteasome processes, a view which is supported by recent evidence that PDTC serves as an inhibitor of ubiquitin ligase (25). Ubiquitin ligases (E3 enzyme) recognize specific ubiquitin signals present in substrates and transfer the ubiquitin moiety from ubiquitin-conjugating enzymes (E2 enzyme) to lysine residues of substrates (or ubiquitin) to form polyubiquitinated proteins (48). Two families of E3 enzymes, HECT domain family and RING finger domain family, have been identified to date. RING domain E3 enzymes are either single-subunit enzymes (i.e., c-Cbl and Mdm2) or multisubunit enzymes (i.e., SCF ubiquitin ligase) in which the RING domain binds to zinc ions through four cysteine and histidine residues and plays a scaffold role. Accumulation of various short-lived proteins, such as p53, p21, and MKP-1, shown by us and others (23, 32) suggests that PDTC acts on common components of E3 enzymes, since p53 and p21 were targeted by different E3 ligases (34, 62). It is likely that RING domain of E3 ligase is altered by PDTC, because PDTC treatment increases intracellular zinc and copper levels which, in turn, may cause conformational changes of RING domains and reduce their binding to E2 enzymes.
Studies from other laboratories have demonstrated the important roles of the proteasome pathway in the regulation of the life cycle of certain viruses. Monoubiquitination of HIV proteins enhances its transactivation and budding from cells (6, 29, 54). In addition, HIV vif protein interacts with host SCF ubiquitin ligase and promotes the degradation of innate antiviral enzyme APOBEC3G via 26S proteasome in order to support its replication (53, 61). Eom et al. have also shown that neuronal F-Box 42-kDa protein (NFB42), a subunit of an SCF ubiquitin ligase, binds to the herpes simplex virus 1 (HSV-1) UL9 protein and regulates its nuclear export, ubiquitination, and degradation via the 26S proteasome (18, 19). Such interaction may prevent the active replication and promote the latency of HSV-1 in neuronal cells. These examples illustrate the elegant interplay between viruses and cellular ubiquitination machineries. At present, selective inhibition of specific ubiquitin ligases appears to be an attractive strategy to limit replication or latency of many life-threatening viruses.
In addition to the best known function in the regulation of cell growth and apoptosis (22, 28), the tumor suppressor protein p53 has been reported to interfere directly with the replication of several viruses, such as human immunodeficiency virus type 1, simian virus, and herpesvirus, by various mechanisms (15, 17, 56). Wild-type p53 binds to simian virus 40 large T antigen and blocks the function of large T antigen in mediating viral replication. p53 regulates human immunodeficiency virus type 1 gene expression by suppressing transcriptional activation of the long terminal repeat. Previous studies by our laboratory have shown that CVB3 infection resulted in a down-regulation of steady-state levels of p53 (39). Reduction of p53 by CVB3 infection may represent a strategy that the virus utilizes to maximize its own infectivity by attenuating the inhibitory effect of p53 on virus replication. In the current study we found that PDTC treatment increased the steady-state level of p53, leading us to speculate that increased p53 may contribute, at least in part, to the antiviral activities of PDTC.
MKP-1 is a dual phosphatase that specifically dephosphorylates and inactivates MAPKs, including ERK1/2 and p38 MAPK (55). It was recently reported that MKP-1 is degraded via the ubiquitin-proteasome pathway, and MKP-1 degradation results in sustained ERK1/2 activation (36). We have previously reported that inhibition of the ERK1/2 signaling pathway reduces CVB3 replication, suggesting a role of ERK1/2 activation in CVB3 infectivity. In the current study, we found that PDTC treatment increased expression of MKP-1 in infected cells, accompanied by decreased phosphorylation of ERK1/2 (data not shown). Thus, prevention of ERK1/2 phosphorylation appears to contribute to the inhibition of CVB3 replication by PDTC.
In summary, through this series of experiments we demonstrate for the first time that PDTC is a potent inhibitor of CVB3 and that blockage of CVB3 by PDTC is most likely through selective inhibition of host protein degradation. Together with our previous reports, the present study supports an important role for the ubiquitin-proteasome pathway in the regulation of coxsackievirus life cycle.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) (to H.L.) and the Heart and Stroke Foundation of New Brunswick (to B.M.M.). X.S. is a recipient of the CIHR/HSFC IMPACT Post-Doctoral Fellowship, Michael Smith Fellowship, and the Heart and Stroke Foundation of Canada (HSFC) Research Fellowship. H.L. is a New Investigator of the CIHR/St. Paul's Hospital Foundation Award and a Scholar of the Michael Smith Foundation for Health Research (MSFHR). J.Y. is a recipient of a Doctoral Traineeship from the HSFC. C.C. is supported by a Doctoral Traineeship from the CIHR, and M.E. is supported by the Heart and Stroke Foundation of British Columbia and Yukon and the MSFHR.
We gratefully thank B. Wong for critical reading of the manuscript and S. Greene and D. English for graphical support.
REFERENCES
- 1.Allard, J. P., E. Aghdassi, J. Chau, C. Tam, C. M. Kovacs, I. E. Salit, and S. L. Walmsley. 1998. Effects of vitamin E and C supplementation on oxidative stress and viral load in HIV-infected subjects. AIDS 12:1653-1659. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D. R., J. E. Wilson, C. M. Carthy, D. Yang, R. Kandolf, and B. M. McManus. 1996. Direct interactions of coxsackievirus B3 with immune cells in the splenic compartment of mice susceptible or resistant to myocarditis. J. Virol. 70:4632-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck, M. A., N. M. Chapman, B. M. McManus, J. C. Mullican, and S. Tracy. 1990. Secondary enterovirus infection in the murine model of myocarditis. Pathologic and immunologic aspects. Am. J. Pathol. 136:669-681. [PMC free article] [PubMed] [Google Scholar]
- 4.Beloqui, O., J. Prieto, M. Suarez, B. Gil, C. H. Qian, N. Garcia, and M. P. Civeira. 1993. N-acetyl cysteine enhances the response to interferon-alpha in chronic hepatitis C: a pilot study. J. Interferon Res. 13:279-282. [DOI] [PubMed] [Google Scholar]
- 5.Bessho, R., K. Matsubara, M. Kubota, K. Kuwakado, H. Hirota, Y. Wakazono, Y. W. Lin, A. Okuda, M. Kawai, R. Nishikomori, et al. 1994. Pyrrolidine dithiocarbamate, a potent inhibitor of nuclear factor kappa B (NF-kappa B) activation, prevents apoptosis in human promyelocytic leukemia HL-60 cells and thymocytes. Biochem. Pharmacol. 48:1883-1889. [DOI] [PubMed] [Google Scholar]
- 6.Bres, V., R. E. Kiernan, L. K. Linares, C. Chable-Bessia, O. Plechakova, C. Treand, S. Emiliani, J. M. Peloponese, K. T. Jeang, O. Coux, M. Scheffner, and M. Benkirane. 2003. A non-proteolytic role for ubiquitin in Tat-mediated transactivation of the HIV-1 promoter. Nat. Cell Biol. 5:754-761. [DOI] [PubMed] [Google Scholar]
- 7.Bultmann, A., J. Eberle, and J. Haas. 2000. Ubiquitination of the human immunodeficiency virus type 1 env glycoprotein. J. Virol. 74:5373-5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bureau, C., J. Bernad, N. Chaouche, C. Orfila, M. Beraud, C. Gonindard, L. Alric, J. P. Vinel, and B. Pipy. 2001. Nonstructural 3 protein of hepatitis C virus triggers an oxidative burst in human monocytes via activation of NADPH oxidase. J. Biol. Chem. 276:23077-23083. [DOI] [PubMed] [Google Scholar]
- 9.Carthy, C. M., D. J. Granville, K. A. Watson, D. R. Anderson, J. E. Wilson, D. Yang, D. W. Hunt, and B. M. McManus. 1998. Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. J. Virol. 72:7669-7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carthy, C. M., B. Yanagawa, H. Luo, D. J. Granville, D. Yang, P. Cheung, C. Cheung, M. Esfandiarei, C. M. Rudin, C. B. Thompson, D. W. Hunt, and B. M. McManus. 2003. Bcl-2 and Bcl-xL overexpression inhibits cytochrome c release, activation of multiple caspases, and virus release following coxsackievirus B3 infection. Virology 313:147-157. [DOI] [PubMed] [Google Scholar]
- 11.Chen, S. H., S. H. Liu, Y. C. Liang, J. K. Lin, and S. Y. Lin-Shiau. 2000. Death signaling pathway induced by pyrrolidine dithiocarbamate-Cu(2+) complex in the cultured rat cortical astrocytes. Glia 31:249-261. [DOI] [PubMed] [Google Scholar]
- 12.Chow, L. H., K. W. Beisel, and B. M. McManus. 1992. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Lab. Investig. 66:24-31. [PubMed] [Google Scholar]
- 13.Chung, K. C., J. H. Park, C. H. Kim, H. W. Lee, N. Sato, Y. Uchiyama, and Y. S. Ahn. 2000. Novel biphasic effect of pyrrolidine dithiocarbamate on neuronal cell viability is mediated by the differential regulation of intracellular zinc and copper ion levels, NF-kappaB, and MAP kinases. J. Neurosci. Res. 59:117-125. [PubMed] [Google Scholar]
- 14.Cunningham, K. A., N. M. Chapman, and S. D. Carson. 2003. Caspase-3 activation and ERK phosphorylation during CVB3 infection of cells: influence of the coxsackievirus and adenovirus receptor and engineered variants. Virus Res. 92:179-186. [DOI] [PubMed] [Google Scholar]
- 15.Devireddy, L. R., and C. J. Jones. 1999. Activation of caspases and p53 by bovine herpesvirus 1 infection results in programmed cell death and efficient virus release. J. Virol. 73:3778-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Docherty, J. J., M. M. Fu, B. S. Stiffler, R. J. Limperos, C. M. Pokabla, and A. L. DeLucia. 1999. Resveratrol inhibition of herpes simplex virus replication. Antiviral Res. 43:145-155. [DOI] [PubMed] [Google Scholar]
- 17.Duan, L., I. Ozaki, J. W. Oakes, J. P. Taylor, K. Khalili, and R. J. Pomerantz. 1994. The tumor suppressor protein p53 strongly alters human immunodeficiency virus type 1 replication. J. Virol. 68:4302-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eom, C. Y., W. D. Heo, M. L. Craske, T. Meyer, and I. R. Lehman. 2004. The neural F-box protein NFB42 mediates the nuclear export of the herpes simplex virus type 1 replication initiator protein (UL9 protein) after viral infection. Proc. Natl. Acad. Sci. USA 101:4036-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eom, C. Y., and I. R. Lehman. 2003. Replication-initiator protein (UL9) of the herpes simplex virus 1 binds NFB42 and is degraded via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA 100:9803-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erl, W., C. Weber, and G. K. Hansson. 2000. Pyrrolidine dithiocarbamate-induced apoptosis depends on cell type, density, and the presence of Cu(2+) and Zn(2+). Am. J. Physiol. Cell Physiol. 278:C1116-C11125. [DOI] [PubMed] [Google Scholar]
- 21.Esfandiarei, M., H. Luo, B. Yanagawa, A. Suarez, D. Dabiri, J. Zhang, and B. M. McManus. 2004. Protein kinase B/Akt regulates coxsackievirus B3 replication through a mechanism which is not caspase dependent. J. Virol. 78:4289-4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fridman, J. S., and S. W. Lowe. 2003. Control of apoptosis by p53. Oncogene 22:9030-9040. [DOI] [PubMed] [Google Scholar]
- 23.Furuta, S., F. Ortiz, X. Zhu Sun, H. H. Wu, A. Mason, and J. Momand. 2002. Copper uptake is required for pyrrolidine dithiocarbamate-mediated oxidation and protein level increase of p53 in cells. Biochem. J. 365:639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudernak, E., J. Seipelt, A. Triendl, A. Grassauer, and E. Kuechler. 2002. Antiviral effects of pyrrolidine dithiocarbamate on human rhinoviruses. J. Virol. 76:6004-6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayakawa, M., H. Miyashita, I. Sakamoto, M. Kitagawa, H. Tanaka, H. Yasuda, M. Karin, and K. Kikugawa. 2003. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J. 22:3356-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber, M., K. A. Watson, H. C. Selinka, C. M. Carthy, K. Klingel, B. M. McManus, and R. Kandolf. 1999. Cleavage of RasGAP and phosphorylation of mitogen-activated protein kinase in the course of coxsackievirus B3 replication. J. Virol. 73:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Israel, N., and M. A. Gougerot-Pocidalo. 1997. Oxidative stress in human immunodeficiency virus infection. Cell Mol. Life Sci. 53:864-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jimenez, G. S., S. H. Khan, J. M. Stommel, and G. M. Wahl. 1999. p53 regulation by post-translational modification and nuclear retention in response to diverse stresses. Oncogene 18:7656-7665. [DOI] [PubMed] [Google Scholar]
- 29.Kikonyogo, A., F. Bouamr, M. L. Vana, Y. Xiang, A. Aiyar, C. Carter, and J. Leis. 2001. Proteins related to the Nedd4 family of ubiquitin protein ligases interact with the L domain of Rous sarcoma virus and are required for gag budding from cells. Proc. Natl. Acad. Sci. USA 98:11199-11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, C. H., J. H. Kim, C. Y. Hsu, and Y. S. Ahn. 1999. Zinc is required in pyrrolidine dithiocarbamate inhibition of NF-kappaB activation. FEBS Lett. 449:28-32. [DOI] [PubMed] [Google Scholar]
- 31.Kim, C. H., J. H. Kim, J. Xu, C. Y. Hsu, and Y. S. Ahn. 1999. Pyrrolidine dithiocarbamate induces bovine cerebral endothelial cell death by increasing the intracellular zinc level. J. Neurochem. 72:1586-1592. [DOI] [PubMed] [Google Scholar]
- 32.Kim, I., C. H. Kim, J. H. Kim, J. Lee, J. J. Choi, Z. A. Chen, M. G. Lee, K. C. Chung, C. Y. Hsu, and Y. S. Ahn. 2004. Pyrrolidine dithiocarbamate and zinc inhibit proteasome-dependent proteolysis. Exp. Cell Res. 298:229-238. [DOI] [PubMed] [Google Scholar]
- 33.Kisselev, A. F., and A. L. Goldberg. 2001. Proteasome inhibitors: from research tools to drug candidates. Chem. Biol. 8:739-758. [DOI] [PubMed] [Google Scholar]
- 34.Kubbutat, M. H., S. N. Jones, and K. H. Vousden. 1997. Regulation of p53 stability by Mdm2. Nature 387:299-303. [DOI] [PubMed] [Google Scholar]
- 35.Levander, O. A. 2000. Coxsackievirus as a model of viral evolution driven by dietary oxidative stress. Nutr. Rev. 58:S17-S24. [DOI] [PubMed] [Google Scholar]
- 36.Lin, Y. W., S. M. Chuang, and J. L. Yang. 2003. ERK1/2 achieves sustained activation by stimulating MAPK phosphatase-1 degradation via the ubiquitin-proteasome pathway. J. Biol. Chem. 278:21534-21541. [DOI] [PubMed] [Google Scholar]
- 37.Luo, H., B. Yanagawa, J. Zhang, Z. Luo, M. Zhang, M. Esfandiarei, C. Carthy, J. E. Wilson, D. Yang, and B. M. McManus. 2002. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 76:3365-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo, H., J. Zhang, C. Cheung, A. Suarez, B. M. McManus, and D. Yang. 2003. Proteasome inhibition reduces coxsackievirus B3 replication in murine cardiomyocytes. Am. J. Pathol. 163:381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo, H., J. Zhang, F. Dastvan, B. Yanagawa, M. A. Reidy, H. M. Zhang, D. Yang, J. E. Wilson, and B. M. McManus. 2003. Ubiquitin-dependent proteolysis of cyclin D1 is associated with coxsackievirus-induced cell growth arrest. J. Virol. 77:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason, J. W., J. B. O'Connell, A. Herskowitz, N. R. Rose, B. M. McManus, M. E. Billingham, and T. E. Moon. 1995. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N. Engl. J. Med. 333:269-275. [DOI] [PubMed] [Google Scholar]
- 41.McManus, B. M., L. H. Chow, S. J. Radio, S. M. Tracy, M. A. Beck, N. M. Chapman, K. Klingel, and R. Kandolf. 1991. Progress and challenges in the pathological diagnosis of myocarditis. Eur. Heart J. 12(Suppl. D):18-21. [DOI] [PubMed] [Google Scholar]
- 42.McManus, B. M., B. Yanagawa, N. Rezai, H. Luo, L. Taylor, M. Zhang, J. Yuan, J. Buckley, T. Triche, G. Schreiner, and D. Yang. 2002. Genetic determinants of coxsackievirus B3 pathogenesis. Ann. N. Y. Acad. Sci. 975:169-179. [DOI] [PubMed] [Google Scholar]
- 43.Opavsky, M. A., T. Martino, M. Rabinovitch, J. Penninger, C. Richardson, M. Petric, C. Trinidad, L. Butcher, J. Chan, and P. P. Liu. 2002. Enhanced ERK-1/2 activation in mice susceptible to coxsackievirus-induced myocarditis. J. Clin. Investig. 109:1561-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pace, G. W., and C. D. Leaf. 1995. The role of oxidative stress in HIV disease. Free Radic. Biol. Med. 19:523-528. [DOI] [PubMed] [Google Scholar]
- 45.Patnaik, A., V. Chau, and J. W. Wills. 2000. Ubiquitin is part of the retrovirus budding machinery. Proc. Natl. Acad. Sci. USA 97:13069-13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterhans, E. 1997. Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J. Nutr. 127:962S-965S. [DOI] [PubMed] [Google Scholar]
- 47.Pickart, C. M. 2004. Back to the future with ubiquitin. Cell 116:181-190. [DOI] [PubMed] [Google Scholar]
- 48.Pickart, C. M. 2001. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70:503-533. [DOI] [PubMed] [Google Scholar]
- 49.Prosch, S., C. Priemer, C. Hoflich, C. Liebenthaf, N. Babel, D. H. Kruger, and H. D. Volk. 2003. Proteasome inhibitors: a novel tool to suppress human cytomegalovirus replication and virus-induced immune modulation. Antivir. Ther. 8:555-567. [PubMed] [Google Scholar]
- 50.Sandstrom, P. A., M. D. Mannie, and T. M. Buttke. 1994. Inhibition of activation-induced death in T cell hybridomas by thiol antioxidants: oxidative stress as a mediator of apoptosis. J. Leukoc. Biol. 55:221-226. [DOI] [PubMed] [Google Scholar]
- 51.Schwarz, E. M., C. Badorff, T. S. Hiura, R. Wessely, A. Badorff, I. M. Verma, and K. U. Knowlton. 1998. NF-κB-mediated inhibition of apoptosis is required for encephalomyocarditis virus virulence: a mechanism of resistance in p50 knockout mice. J. Virol. 72:5654-5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwarz, K. B. 1996. Oxidative stress during viral infection: a review. Free Radic. Biol. Med. 21:641-649. [DOI] [PubMed] [Google Scholar]
- 53.Sheehy, A. M., N. C. Gaddis, and M. H. Malim. 2003. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat. Med. 9:1404-1407. [DOI] [PubMed] [Google Scholar]
- 54.Strack, B., A. Calistri, M. A. Accola, G. Palu, and H. G. Gottlinger. 2000. A role for ubiquitin ligase recruitment in retrovirus release. Proc. Natl. Acad. Sci. USA 97:13063-13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun, H., C. H. Charles, L. F. Lau, and N. K. Tonks. 1993. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75:487-493. [DOI] [PubMed] [Google Scholar]
- 56.Tiemann, F., and W. Deppert. 1994. Stabilization of the tumor suppressor p53 during cellular transformation by simian virus 40: influence of viral and cellular factors and biological consequences. J. Virol. 68:2869-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai, J. C., M. Jain, C. M. Hsieh, W. S. Lee, M. Yoshizumi, C. Patterson, M. A. Perrella, C. Cooke, H. Wang, E. Haber, R. Schlegel, and M. E. Lee. 1996. Induction of apoptosis by pyrrolidinedithiocarbamate and N-acetylcysteine in vascular smooth muscle cells. J. Biol. Chem. 271:3667-3670. [PubMed] [Google Scholar]
- 58.Uchide, N., K. Ohyama, T. Bessho, B. Yuan, and T. Yamakawa. 2002. Effect of antioxidants on apoptosis induced by influenza virus infection: inhibition of viral gene replication and transcription with pyrrolidine dithiocarbamate. Antiviral Res. 56:207-217. [DOI] [PubMed] [Google Scholar]
- 59.Weiss, L., E. Hildt, and P. H. Hofschneider. 1996. Anti-hepatitis B virus activity of N-acetyl-L-cysteine (NAC): new aspects of a well-established drug. Antiviral Res. 32:43-53. [DOI] [PubMed] [Google Scholar]
- 60.Wu, M., H. Lee, R. E. Bellas, S. L. Schauer, M. Arsura, D. Katz, M. J. FitzGerald, T. L. Rothstein, D. H. Sherr, and G. E. Sonenshein. 1996. Inhibition of NF-kappaB/Rel induces apoptosis of murine B cells. EMBO J. 15:4682-4690. [PMC free article] [PubMed] [Google Scholar]
- 61.Yu, X., Y. Yu, B. Liu, K. Luo, W. Kong, P. Mao, and X. F. Yu. 2003. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science 302:1056-1060. [DOI] [PubMed] [Google Scholar]
- 62.Yu, Z. K., J. L. Gervais, and H. Zhang. 1998. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc. Natl. Acad. Sci. USA 95:11324-11329. [DOI] [PMC free article] [PubMed] [Google Scholar]