Abstract
Prion diseases are characterized by the deposition of PrPSc, an abnormal form of the cellular prion protein PrPC. A growing body of evidence suggests that antibodies to PrPC can antagonize deposition of PrPSc. However, host tolerance hampers the induction of immune responses to PrPC, and cross-linking of PrPC by bivalent anti-PrP antibodies is neurotoxic. In order to obviate these problems, we explored the antiprion potential of recombinant single-chain antibody (scFv) fragments. scFv fragments derived from monoclonal anti-PrP antibody 6H4, flagged with c-myc and His6 tags, were correctly processed and secreted by mammalian RD-4 rhabdomyosarcoma cells. When cocultured with cells secreting anti-PrP scFv, chronically prion-infected neuroblastoma cells ceased to produce PrPSc, even if antibody-producing cells were physically separated from target cells in transwell cultures. Expression of scFv with irrelevant specificity, or of similarly tagged molecules, was not curative. Therefore, eukaryotically expressed scFv exerts a paracrine antiprion activity. The effector functions encoded by immunoglobulin constant domains are unnecessary for this effect. Because of their small size and their monovalent binding, scFv fragments may represent candidates for gene transfer-based immunotherapy of prion diseases.
Prion diseases, or transmissible spongiform encephalopathies, are invariably lethal neurodegenerative illnesses that affect humans and many animal species. They include bovine spongiform encephalopathy of cattle, chronic wasting disease of mule deer and elk, and Creutzfeldt-Jakob disease (CJD) in humans (3). The causative agent is termed a prion (36) and was proposed to be identical to PrPSc, a pathological conformer of the cellular protein PrPc encoded by the Prnp gene (31). PrPC is expressed on the surfaces of almost all cells in the body but at particularly high levels on neurons in the peripheral and central nervous systems. PrPC is essential for the development of prion disease (7), and Prnpo/o mice, which lack PrPC, are resistant to scrapie (8).
The bovine spongiform encephalopathy epidemic (42) can be arguably deemed the direct consequence of flawed technology assessment and is caused by prions that were amplified through the bovine food chain (41). Transmission of bovine prions to humans has then given rise to variant CJD (44). At the time of this writing, Switzerland is experiencing an alarming increase in the incidence of a form of CJD which is clinically and biochemically indistinguishable from sporadic CJD and whose cause has not yet been conclusively determined (11, 12). Also, the recent increase in chronic wasting disease in North America (38) underlines the fact that prion diseases of farm and wildlife animals still represent a threat to public health. For all the reasons enumerated above, there is an urgent and growing need for efficient preventive and/or therapeutic measures against prion diseases (2).
Prions use immune cells to gain access to the brain (14, 20, 35). On the other hand, several reports indicate that humoral immune responses against the prion protein can antagonize prion infections. This is true even when such responses are directed primarily against PrPC and do not selectively target the disease-associated prion protein, PrPSc. For example, monoclonal antibodies directed against PrPC have been shown to prevent de novo scrapie infection and to abrogate prion infectivity and PrPSc from chronically scrapie-infected neuroblastoma cells (10, 33). Transgenic expression (16) or peripheral administration of anti-PrP antibodies in mice (43) can arrest peripheral scrapie pathogenesis, and PrP-Fcγ fusion molecules interfere with prion replication (30).
One major obstacle to devising efficacious regimens of active immunization is host tolerance to endogenous PrPC that inhibits a host-derived anti-PrP antibody immune response. Active immunization attempts have thus far resulted in the induction of meager anti-PrP titers at best (13, 22, 34, 39). Accordingly, the biological efficacy of these immunization series was limited (34, 39), emphasizing the need for alternative strategies.
Conversely, administration of antibodies generated in Prnp-ablated animals (“passive immunization”), while feasible and effective (43), suffers from the intrinsic problem of poor diffusion from vessels into tissues, particularly into the central nervous tissue. This may explain why administration of monoclonal antibodies has been shown to prevent prion pathogenesis only when applied simultaneously or shortly after peripheral prion infection (43). In addition, production of the large amounts of monoclonal antibodies necessary for the treatment of human patients is technically challenging and expensive (18, 25, 28).
Delivery of antibodies by gene therapy may circumvent the limitations described above by providing a steady, localized supply of antibodies which obviates the need for repetitive injections. However, whole antibodies are unwieldy and undergo a complex biogenesis, and their large genes are poorly suitable for efficient genetic transfer with vectors. Worryingly, intracerebral injection of anti-PrP immunoglobulin G (IgG) antibodies was found to provoke neurotoxicity by cross-linking PrPC (40).
One possible solution to the latter problems is offered by the use of phage display-based single-chain miniantibodies (scFvs), which are small and consist of a single fusion polypeptide. ScFvs are often derived from natural or synthetic cDNA libraries, but they can also be engineered from the sequences encoding the variable regions of individual hybridomas. In the latter case, scFvs retain the antigen binding properties of the parent monoclonal antibody (19, 24, 26, 45). Here we tested a novel strategy of paracrine immunization that employs soluble scFvs with anti-PrP specificity. We find that secretion of anti-PrP scFvs by mammalian cells cures chronically prion-infected N2a neuroblastoma cells (ScN2a). These results suggest that open reading frames (ORFs) encoding anti-PrP scFvs may be used in gene therapy against prion diseases.
MATERIALS AND METHODS
Cell culture.
The human rhabdomyosarcoma cell line RD-4 was obtained from David Derse (National Cancer Institute, Frederick, Maryland) and cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal calf serum (Gibco). The chronically prion-infected mouse neuroblastoma cell line N2a/Bos2 (10) was kindly provided by Charles Weissmann (London, United Kingdom) and cultured in OPTI-MEM supplemented with 10% fetal calf serum and penicillin G-streptomycin (Gibco) at 37°C in 5% CO2. Cells were grown in 10-cm dishes and routinely split 1:5 every 3 to 4 days.
Construction and selection of anti-PrP scFv fragment.
RNA was prepared from 6H4 hybridoma cell pellets (23), and cDNA was obtained by subsequent reverse transcription-PCR. VH and VL fragments were amplified, joined by PCR assembly, and cloned into the phage display vector pAK100 (19, 24) as described previously (16). Functionality of phages displaying 6H4 single-chain antibodies was analyzed in a standard enzyme-linked immunosorbent assay. The resulting scFvs were amplified by PCR with the primers 5′-GCC GTA CGA AGC TTG ATG GCG GAC TAC AAA GAC ATT GTT-3′ and 5′-GGG CCC TCC TCG AGC GAT CAG CTT CTG CTC GAA TTC GGC-3′, introducing unique HindIII and XhoI restriction sites (underlined) at the 5′ and 3′ ends, respectively. The modified scFvs ORFs were cloned in frame into the plasmid vector pSecTag2/HygroA (Invitrogen), resulting in the generation of pSecTag2/Hygro4.1 and pSecTag2/Hygro4.5. RD-4 cells were transfected with 30 μl of GenePorter transfection reagent and 6 μg of plasmid DNA in a six-well plate according to the manufacturer's guidelines (Gene Therapy Systems). Cells were then treated with 300 μg/ml of hygromycin B (Roche), and resulting single-cell clones were selected to be expanded in presence of hygromycin B for 2 weeks. HEK-293 EBNA cells were maintained as serum-free suspension cultures in Excell-293 medium (JRH Biosciences, Lenexa, KS) in glass spinner flasks (Bellco Glass Inc., Vineyard, NY) and transiently transfected with polyethyleneimine as described previously (5).
Northern blotting.
Total RNA was isolated using the TRIzol reagent (BRL) according to the manufacturer's instructions. RNA was denatured, electrophoresed on an agarose gel containing formaldehyde, blotted onto nitrocellulose, and hybridized with 32P-labeled probes according to standard methods.
Western blotting.
Twenty microliters of medium, corresponding to 70 μg of total protein, or 10 μl of purified scFVs was electrophoresed through 12% sodium dodecyl sulfate-polyacrylamide gels and transferred to nitrocellulose membranes by electroblotting. ScFvs were detected by horseradish peroxidase-conjugated monoclonal anti-c-myc antibody (Invitrogen) and visualized by enhanced chemiluminescence (ECL kit; Pierce, Rockford, IL). Alternatively, membranes were incubated with monoclonal anti-c-myc antibody 4A6 (Upstate) and probed with horseradish peroxidase-labeled anti-mouse IgG1 antibody (Zymed).
Dot and slot blotting.
Recombinant PrP at a concentration of 200 ng was diluted in 100 μl of phosphate-buffered saline (PBS) and dotted or slotted onto a nitrocellulose membrane. The membrane was air dried, blocked with 5% skim milk in TBST (10 mM Tris-HCl, pH 7.8, 10 mM NaCl, and 0.05% Tween 20), and incubated with a 1:2 dilution of conditioned medium with TBST for 2 h in a final volume of 3 ml. After three washing steps (5 min) with TBST, scFv binding was detected with horseradish peroxidase-conjugated monoclonal anti-His6 antibody (Invitrogen) and thereafter visualized by enhanced chemiluminescence (ECL kit; Pierce, Rockford, IL).
Cell blot assay.
The cell blot assay was performed as described by Bosque and Prusiner (6). Cells were transferred to nitrocellulose membrane, treated with proteinase K, denatured, immunostained with monoclonal antibody 6H4 followed by a horseradish peroxidase-conjugated goat anti-mouse IgG1 antibody, and visualized by enhanced chemiluminescence (ECL kit; Pierce, Rockford, IL). To assess the extent of transfer of cells to the nitrocellulose membrane, membranes were stained with 0.5 μg/ml ethidium bromide for 15 min and photographed in UV light as described previously (10).
Enrichment and purification of scFvs.
Supernatants derived from transiently transfected HEK-293 cells were enriched for scFvs of clone 4.1 or 4.5 by use of centrifugal filter devices with an ability to retain molecules above 10 kDa (Amicon Ultra; Millipore) or purified by His6 affinity and fast protein liquid chromatography using a nickel-nitrilotriacetic acid (Ni-NTA) kit (QIAGEN) according to the manufacturer's description.
Anti-PrP ELISA.
Ninety-six-well plates were coated with 5 μg/ml mouse recombinant PrP23-231 (PrPREC) overnight at 4°C. Plates were washed with PBS containing 0.1% (vol/vol) Tween 20 (PBST) and blocked with PBST containing 5% bovine serum albumin for 2 h at room temperature (RT). After washing, plates were incubated with 50 μl of serially twofold diluted cell culture supernatant purified for scFvs or, as technical control, monoclonal antibody POM1 to mouse PrP. After 2 h at RT, plates were thoroughly washed and probed with a horseradish peroxidase-conjugated monoclonal anti-c-myc or anti-His antibody (both from Invitrogen; 1:5000 dilution) for 1 h at RT, except for wells incubated with POM1, which were probed with horseradish peroxidase-conjugated rabbit anti-mouse IgG (Zymed; 1:000 dilution). Plates were developed with tetramethyl benzidine, and optical density was measured at 450 nm. For competition experiments, PrPREC-coated plates were pretreated with serial 10-fold dilutions of monoclonal antibody POM1 or POM2 (from 2 μg to 2 ng/well) for 2 h at RT before addition of purified scFvs of clone 4.1 or 4.5.
Antibodies.
Monoclonal mouse anti-PrP antibodies POM1 and POM2 (both IgG1) were obtained by immunization of Prnpo/o mice with PrPREC. Splenocytes were isolated and fused with immortalized myeloma cells according to standard protocols. PrP-specific clones were selected by ELISA, Western blotting, and fluorescence-activated cell sorter analysis. POM1 recognizes a mouse PrP epitope which competes with the 6H4 epitope (amino acids 144 to 152) (23), while POM2 binds to a linear contiguous N-terminal epitope consisting of residues 57 to 88 of mouse PrP (M. Polymenidou and A. Aguzzi, unpublished results).
RESULTS
Expression and secretion of anti-PrP scFv in mammalian cells.
We report the construction of two clones of phages (termed sc4.1 and sc4.5) displaying the Fv portions of the heavy and light chains of monoclonal antibody 6H4 (23) as a single-chain fusion protein (scFv). Antibody 6H4 displays a high affinity for PrPC and was previously shown to interfere with the buildup of PrPSc and of prions in vitro (10) and in transgenic mice (16). The clones were selected by a panning assay against recombinant full-length PrP (PrPREC) and sequenced. The ORFs of clones sc4.1 and sc4.5 were inserted into the plasmid vector pSecTag2/Hygro (Invitrogen) in order to allow for eukaryotic expression. pSecTag2/Hygro contains the CMV promoter, an Igκ leader sequence specifying secretion of heterologous proteins, a c-myc tag at the C terminus, and a hexahistidine tag allowing for detection and purification through immobilized metal affinity chromatography with nickel-chelating resin (Fig. 1a). As a positive control for expression and secretion in mammalian cells, a clone of pSecTag2/Hygro containing the open reading frame for human prostate-specific antigen (PSA) (pSecTag2/Hygro-PSA; Invitrogen) was constructed (Fig. 1a).
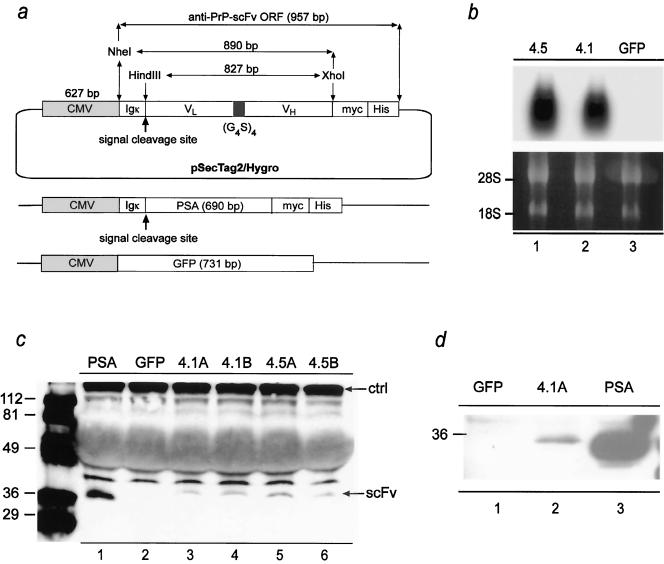
FIG. 1.
Constructs and cells expressing anti-PrP scFv. a: Constructs containing the ORF of anti-PrP scFv (clones 4.1 and 4.5; 957 bp) with VL- and VH-coding sequences joined by a flexible linker composed of four repeats consisting of four glycine residues and one serine residue [(G4S)4]. Constructs were placed under the control of the CMV enhancer and promoter (627 bp) and fused to an amino-terminal Igκ leader sequence and a carboxy-terminal c-myc/His6 tag. The restriction sites utilized for the cloning and the signal peptide cleavage site are indicated. For a control, the ORF of PSA (690 bp) was fused to the amino-terminal Igκ leader sequence and a carboxy-terminal c-myc/His6 tag. In order to be able to monitor the efficiency of transfection, the ORF of GFP (731 bp) was put under the control of the CMV enhancer and promoter. b: Transcription of anti-PrP scFvs (clones sc4.5 and sc4.1 in lanes 1 and 2; GFP as negative control in lane 3) was analyzed by Northern blotting of transiently transfected RD-4 cells. Lower panel: rRNA bands as loading control. c: Secretion of anti-PrP scFv in supernatants of transiently transfected RD-4 cells, analyzed by Western blotting using an anti-c-myc antibody. Duplicates of each transfection were analyzed in lanes A and B, respectively. Supernatants derived from cells transfected with PSA (lane 1) or GFP (lane 2) served as positive and negative controls, respectively. A cross-reacting protein was used as a loading control (ctrl). d: Western blot analysis of anti-PrP scFv protein (clone 4.1A, lane 2) purified by Ni2+ affinity chromatography. Supernatants derived from cells transfected with PSA (lane 3) or GFP (lane 1) served as positive and negative controls, respectively. Markers (left) indicate molecular masses in kDa.
To assess transcription of pSecTag2/Hygro-sc4.1 and pSecTag2/Hygro-sc4.5, the human rhabdomyosarcoma cell line RD-4 (9) was transiently transfected, total RNA was isolated 48 h after transfection, and Northern blotting was performed using a probe spanning the full scFv ORF (Fig. 1b). A plasmid expressing green fluorescent protein (GFP) under the control of the CMV promoter (pEGFP-C1) was cotransfected to monitor the efficiency of transfection.
Next, we assessed translation and secretion of pSecTag2/Hygro-sc4.1 and pSecTag2/Hygro-sc4.5 in the supernatant of transiently transfected RD-4 cells by means of Western blotting. Seventy-two hours after transfection, 20 μl of conditioned medium was loaded on a 12% sodium dodecyl sulfate-polyacrylamide gel. Correct protein expression of sc4.1 and sc4.5 was indicated by the presence of a protein with a molecular mass of ∼36 kDa visualized by antibodies to the fused c-myc tag (Fig. 1c). Further, Ni2+ chelation chromatography resulted in an enrichment of anti-PrP scFvs (Fig. 1d). These findings indicate that anti-PrP scFvs were translated and correctly processed by the cellular export apparatus, resulting in their secretion into the medium of cultured cells.
Secreted anti-PrP scFvs recognize PrPREC.
In order to test whether secreted anti-PrP scFvs retain their anti-PrP binding activity, supernatants from transiently transfected RD-4 cells were analyzed by dot blotting and by slot blotting. Binding of scFvs was visualized with anti-His antibodies. We found that conditioned medium derived from sc4.1, but not that derived from sc4.5 or from any of the negative controls, recognized PrPREC (Fig. 2a).
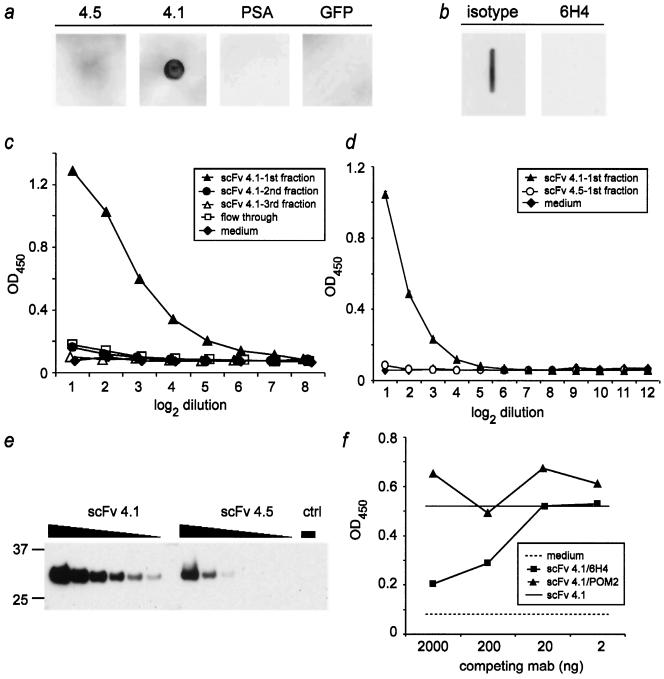
FIG. 2.
Specificity and PrP binding properties of scFv fragments. a: Recombinant PrP (PrPREC; 200 ng) was spotted onto nitrocellulose and incubated with medium derived from RD-4 cells stably expressing anti-PrP scFv (clone 4.5 and clone 4.1), GFP, and PSA. Only supernatant derived from RD-4 cells transfected with anti-PrP scFv clone 4.1 recognized PrPREC. b: monoclonal antibody 6H4, but not the IgG isotype control, antagonized binding to PrPREC when administered prior to incubation with sc4.1. c, d: Affinity-purified sc4.1 (filled triangles) resulted in concentration-dependent binding to PrPREC as assessed by ELISA. In contrast, none of the negative controls or sc4.5 (d) bound PrPC. e: The relative concentrations of sc4.1 and sc4.5 in the preparations used in panels c and d were estimated by probing serially twofold diluted scFvs with an antibody to the myc tag upon Western blotting. The negative control (ctrl) was Ni-NTA-purified medium of nontransfected HEK-293 cells. Numbers on the left indicate molecular masses in kDa. f: Pretreatment of PrPREC-coated ELISA plates with increasing amounts of monoclonal antibody 6H4 (recognizing mouse PrP144-152) inhibited binding of 4 μg purified scFvs (clone 4.1) to PrPREC (squares), while anti-PrP antibody POM2 (which recognizes an amino-terminal epitope of PrPC) did not alter PrP reactivity of sc 4.1 (triangles). Solid line, binding of scFvs 4.1 to PrPREC in the absence of competing antibodies; dashed line, background as determined by the unspecific binding of medium to PrPREC. In panels c, d, and f, each symbol represents the mean of duplicate measurements, except in panel d, where the mean and standard deviation of triplicates are depicted. Abscissa, twofold serial dilutions of scFvs (c and d) or amount of competing monoclonal antibody (mab) per well (in ng) (f). Ordinate, optical density at 450 nm.
If the binding of clone sc4.1 to PrPREC represents a specific scFv-antigen interaction, it should compete with the binding of its parental monoclonal antibody 6H4, from which both sc4.1 and sc4.5 were derived. To investigate this question, nitrocellulose membranes were blotted with PrPREC and preincubated with monoclonal antibody 6H4 (100 ng/ml), which recognizes residues 144 to 152 of mouse PrPC (23). After such pretreatment of the membrane, sc4.1 no longer reacted to PrPREC. In contrast, preincubation with an IgG isotype control antibody (100 ng/ml; Zymed) did not prevent binding (Fig. 2b).
To investigate the specificity and sensitivity of binding to PrPREC, Ni-NTA-purified scFvs of transiently transfected HEK-293 cells were analyzed by ELISA on plates coated with PrPREC and visualized with anti-His antibodies. We detected strong specific binding of sc4.1, whereas sc4.5 showed no reactivity (Fig. 2c and d), even when taking into account the fact that the concentration of sc4.5 was ∼4-fold lower than that of sc4.1 (Fig. 2e).
Competition assays further demonstrated that sc4.1 binds a PrP epitope overlapping that of monoclonal antibody 6H4 (Fig. 2f). Remarkably, sc4.1 was displaced from PrPREC by 6H4 only when the latter holoantibody was added at concentrations of 2 μg/ml (corresponding to 200 ng/well) or higher (Fig. 2f). Such concentrations of 6H4 were previously shown to be sufficient to clear prion infection from cultured chronically prion-infected N2a cells (10).
To explore their differences in binding to PrPREC, the primary amino acid sequences of sc4.1 and sc4.5 were analyzed and found to differ in three amino acids at positions 58, 61, and 293 (Fig. 3). Dissimilarities at residues 58 and 61 were detected within framework region 1 of the light chain, whereas the mutation at amino acid 293 was positioned in framework region 4 of the heavy chain right after the complementarity-determining region 3. The complementarity-determining regions form loops which contribute to the critical interaction between the antibody and ligand. Differences in the framework regions, particularly in close vicinity to complementarity-determining region 3, which is the main determinant for antigen binding (4), may destabilize folding (32, 47) and therefore may explain the lack of PrPC affinity in sc4.5. Therefore, in all subsequent experiments, clone sc4.5 was operationally regarded as of irrelevant specificity and served as a negative control.
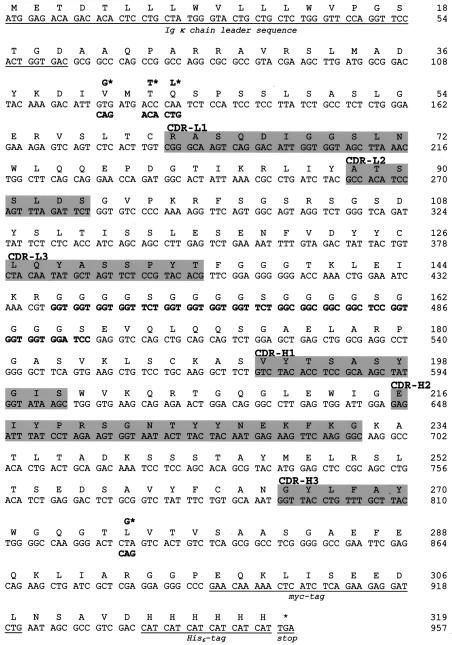
FIG. 3.
Nucleotide and corresponding amino acid sequences of the anti-PrP scFv clones 4.1 and 4.5, displaying distinct codon substitutions (asterisks). The underlined sequence shows the Igκ chain leader sequence of clones 4.1 and 4.5 and ends with a signal cleavage site. VL and VH sequences are joined by a (G4S)4 linker (boldface) and followed by the carboxy terminus with the c-myc as well as the His6 tag (both underlined). The complementarity-determining regions of the light (CDR-L1-3) and heavy (CDR-H1-3) chains are highlighted in grey (29, 46). The differences between the VL and VH chain sequence of clones 4.5 and 4.1 are highlighted by asterisks and are likely to be responsible for the difference in binding to PrP.
Secreted anti-PrP scFvs abolish PrPSc in cocultured prion-infected N2a/Bos2 cells.
In order to test for the prionostatic effects of anti-PrP scFvs, we cocultured RD-4 cells stably transfected with pSecTag2/Hygro4.1 in the presence of chronically scrapie-infected N2a/Bos2 neuroblastoma cells (6). Stably transfected RD-4 cells producing anti-PrP scFvs (Fig. 4a) were separated by transwell membranes from chronically prion-infected N2a/Bos2 cells (Fig. 4b). Although chronically prion-infected N2a/Bos2 cells shared their medium with anti-PrP-scFv-producing cells, no direct cell-cell contact between stably transfected RD-4 and N2a/Bos2 cells was possible. This experimental design was chosen in order to simulate a paracrine situation, in which cells expressing scFv may coexist in the same organ with cells that may be infected with prions but do not express the antibody.
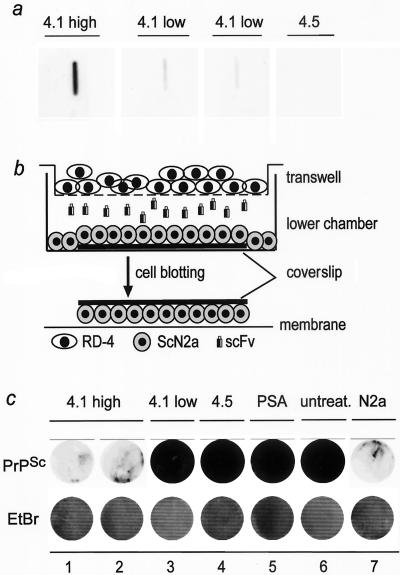
FIG. 4.
Anti-PrP scFvs rescue chronically scrapie-infected ScN2a cells. a: Supernatants of different clones of RD-4 cells stably transfected with respective anti-PrP scFv constructs (either clone 4.1 or 4.5) were tested by slot immunoblotting for their binding to PrPREC. One clone with high expression was termed 4.1 high, whereas two clones with low expression were termed 4.1 low. Clone 4.5 again served as a negative control. b: Schematic illustration of the transwell model used for coculturing stably transfected anti-PrP-scFv-producing RD-4 cells with chronically scrapie prion-infected N2a/Bos2 cells (ScN2a, lower chamber). The transwell consists of a permeable membrane and allows diffusion of soluble factors, e.g., anti-PrP scFvs, between RD-4 cells (cultured on the transwell membrane) and ScN2a cells (cultured on a glass coverslip in the lower chamber). Thereafter, coverslips with ScN2a cells were removed and a cell-blot assay was performed. c: Decrease in PrPSc content in ScN2a cells cocultured with stably transfected RD-4 cells producing large amounts of anti-PrP scFvs (4.1 high, in duplicate, lanes 1 and 2, upper row). Small amounts of anti-PrP scFv (4.1 low, lane 3, upper row) or the negative controls (4.5 and PSA; lanes 4 and 5, upper row) as well as untransfected RD-4 cells (untreat., lane 6, upper row) did not alter the PrPSc content in ScN2a cells. The displayed PrP levels represent PrPSc, since uninfected N2a cells upon proteinase K digestion displayed only some background signal (N2a, lane 7, upper row). Ethidium bromide staining demonstrates the presence of equal amounts of cells per well upon transfer onto nitrocellulose membrane (lower row).
After 3 weeks of coculturing, including subpassaging of N2a/Bos2 cells, and change of the transwell membranes with stably transfected RD-4 cells, the presence of PrPSc in N2a/Bos2 cells was analyzed by cell blotting (6, 10). We found a drastic reduction of PrPSc in prion-infected N2a/Bos2 cells cocultured with RD-4 cells producing anti-PrP scFvs (sc4.1), whereas no reduction in PrPSc load was observed in N2a/Bos2 cells cocultured with any of the controls (Fig. 4c). Interestingly, the decrease in PrPSc was dependent on the amount of anti-PrP scFv expression, since clones of RD-4 cells stably transfected with the same anti-PrP scFvs construct (clone sc4.1) with low expression were less efficient in clearing PrPSc than clones with high expression (Fig. 4a to c). N2a/Bos2 cells treated with anti-PrP scFvs (sc4.1) maintained reduced levels of PrPSc for at least three further passages (corresponding to 12 days) in the absence of additional anti-PrP scFv treatment (Fig. 5).
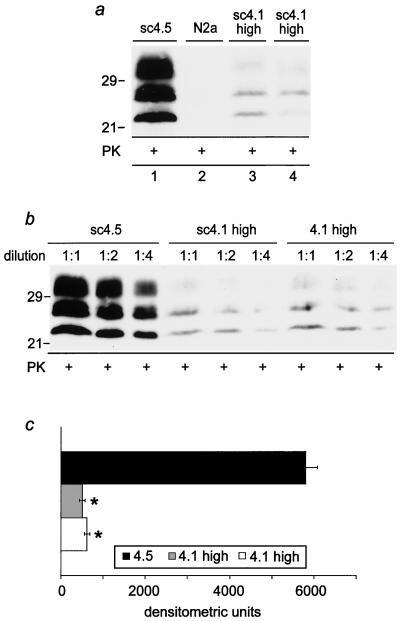
FIG. 5.
PrPSc load in anti-PrP-scFv-treated ScN2a cells 12 days after treatment. a: After 3 weeks of coculturing ScN2a cells with anti-PrP-scFv-producing RD-4 cells (clone 4.1), ScN2a cells were left in culture for three further passages (corresponding to 12 days) without further anti-PrP scFvs treatment. Thereafter, cells were harvested, total proteins were extracted and proteinase K (PK) digested, and Western blotting for PrPSc with the 6H4 monoclonal antibody was performed (20 μg protein per sample). A strong reduction of PrPSc was observable in ScN2a cells that had previously been exposed to RD-4 cells producing high levels of anti-PrP scFv (clone 4.1 high, in duplicate; lanes 3 and 4), in contrast to ScN2a cells exposed to RD-4 cells producing the 4.5 control clone (lane 1). Lane 2 (N2a) represents the control for PK digestion on non-prion-infected N2a cells. b: Semiquantitative analysis of the residual PrPSc amount in ScN2a cells 12 days after anti-PrP scFv treatment. Limiting dilutions of the samples (1:1, 1:2, and 1: 4), as indicated, were analyzed by Western blotting, and the respective bands were assessed densitometrically (Molecular Dynamics system); values are calculated as the mean ± standard error of the mean of the average band density for each dilution. c: Bar graph displaying the densitometric differences in the PrPSc load in anti-PrP-scFv-treated ScN2a cells (as shown in panels a and b). ScN2a cells treated with anti-PrP scFvs (clone 4.1) maintained a 90% reduction in the PrPSc load in ScN2a cells 12 days after anti-PrP-scFv treatment compared to ScN2a cells cocultured with RD-4 cells stably transfected with the control clone 4.5. Statistical significance (P < 0.005) was assessed by Student's t test. +, proteinase K digestion.
DISCUSSION
The prospect of immunotherapy against prion diseases has received considerable attention in recent years. Encouraging results with cell culture systems and with mouse models have raised hopes that antibodies may prove useful at retarding or even at blocking prion replication. However, classical vaccination in wild-type animals appears to be extremely difficult because of host tolerance to endogenous PrPC (15, 34). We therefore reasoned that delivery of antibody-like molecules by gene transfer may be worth investigating, as it might bypass the tolerance problem described above. We have therefore set out to employ protein engineering to determine whether it is possible to express and secrete monovalent single-chain miniantibodies (scFvs, which are most frequently produced in prokaryotic systems) specific to PrP in mammalian cells.
Genetic engineering of antibodies has been utilized in a variety of ways to address the limitations encountered with monoclonal antibodies (17). In particular, generation of scFv moieties with specificities identical to those of their parental monoclonal antibodies has allowed reduction of neutralizing and potentially adverse immune responses against holoantibodies and improvement of biodistribution (37). In this respect, the results presented here suggest that secreted forms of anti-PrP scFv may represent a useful approach to the delivery of antiprion immunotherapeutic agents.
Administration of anti-PrP scFv presents several distinct advantages over the use of monoclonal holoantibodies. In particular, the delivery of scFvs by genetic transduction in vivo, e.g., by means of viral vectors, may allow for sustained production of scFvs at predefined sites for prolonged periods of time. This is difficult to obtain by direct systemic administration of monoclonal antibodies, which are very large molecules and therefore may present limited bioavailability, and by injection of recombinant scFvs, which have extremely short half-lives in blood.
On the other hand, scFv fragments lack all effector domains of holoantibodies, including Fcγ and complement binding domains. Since components of the complement system are involved in pathogenesis of scrapie (21, 27), we deemed it particularly important to assess whether the antiprion biological activity of scFv fragments is similar to that of full-fledged holoantibodies. The coculture studies presented here indicate that this may be the case: effector functions encoded by the constant domains of antibodies are unnecessary for their antiprion effect on cultured cells.
Intracerebellar or intrahippocampal injection of monoclonal anti-PrP holoantibodies induces neurotoxicity, whereas injection of monovalent F(ab)1 fragments prepared from the same monoclonal antibodies was innocuous, suggesting that antibody-induced cross-linking of PrPC may be toxic (40). However, if toxicity is inherent to the bivalent character of monoclonal IgG holoantibodies, as the results mentioned above appear to suggest, one would expect that anti-PrP scFvs, which are monovalent and therefore cannot cross-link PrPC, should be safer than bivalent full-fledged antibodies.
Curing scrapie-infected neuroblastoma cells is likely to be easier than eradicating prions from infected organisms. The bewildering variety of chemicals that appear to suppress prion replication in N2a cells bears witness to the suspicion that activation of a variety of pathways may impair the ability of N2a cells to support prion replication (1). For this reason, it will be important to confirm the efficacy of any proposed scFv-based immunotherapy in animal models. The comparatively small size of the cDNA that encodes for the scFv described here (957 base pairs) makes it easy to efficiently package it into a variety of viral vectors. Further studies will need to address the feasibility and scalability of this approach. For basic studies of prion replication, transduction of eukaryotic cells with anti-PrP scFv fragments may represent a useful tool to suppress prion replication in localized tissue compartments or in specific cell types without interfering with Prnp transcription. With respect to translational research, mammalian cells expressing anti-PrP scFvs may present a therapeutic tool to interfere with prion replication while avoiding problems encountered with classical active or passive PrP immunization.
Acknowledgments
We thank L. Baldi and F. M. Wurm (Lausanne, Switzerland) for providing supernatant of transiently transfected HEK-293 cells.
This work is supported by grants from the Swiss National Research Fund, the Swiss Federal Office of Animal Health, the NCCR on Neural Plasticity and Repair, and the European Union (APOPIS and Priovax) to A.A. and from the Stammbach foundation to F.L.H. M.P. is supported by a Ph.D. fellowship of the Zentrum für Neurowissenschaften Zürich and by UBS grant BA29 AKRB-DZZ (675/B).
REFERENCES
- 1.Aguzzi, A., M. Glatzel, F. Montrasio, M. Prinz, and F. L. Heppner. 2001. Interventional strategies against prion diseases. Nat. Rev. Neurosci. 2:745-749. [DOI] [PubMed] [Google Scholar]
- 2.Aguzzi, A., M. Heikenwalder, and G. Miele. 2004. Progress and problems in the biology, diagnostics, and therapeutics of prion diseases. J. Clin. Investig. 114:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguzzi, A., and M. Polymenidou. 2004. Mammalian prion biology. One century of evolving concepts. Cell 116:313-327. [DOI] [PubMed] [Google Scholar]
- 4.Amit, A. G., R. A. Mariuzza, S. E. Phillips, and R. J. Poljak. 1986. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science 233:747-753. [DOI] [PubMed] [Google Scholar]
- 5.Baldi, L., N. Muller, S. Picasso, R. Jacquet, P. Girard, H. P. Thanh, E. Derow, and F. M. Wurm. Transient gene expression in suspension HEK-293 cells: application to large-scale protein production. Biotechnol. Prog., in press. [DOI] [PubMed]
- 6.Bosque, P. J., and S. B. Prusiner. 2000. Cultured cell sublines highly susceptible to prion infection. J. Virol. 74:4377-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandner, S., S. Isenmann, A. Raeber, M. Fischer, A. Sailer, Y. Kobayashi, S. Marino, C. Weissmann, and A. Aguzzi. 1996. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature 379:339-343. [DOI] [PubMed] [Google Scholar]
- 8.Büeler, H. R., A. Aguzzi, A. Sailer, R. A. Greiner, P. Autenried, M. Aguet, and C. Weissmann. 1993. Mice devoid of PrP are resistant to scrapie. Cell 73:1339-1347. [DOI] [PubMed] [Google Scholar]
- 9.Donofrio, G., S. Cavirani, and V. L. van Santen. 2000. Establishment of a cell line persistently infected with bovine herpesvirus-4 by use of a recombinant virus. J. Gen. Virol. 81:1807-1814. [DOI] [PubMed] [Google Scholar]
- 10.Enari, M., E. Flechsig, and C. Weissmann. 2001. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc. Natl. Acad. Sci. USA 98:9295-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glatzel, M., P. M. Ott, T. Lindner, J. O. Gebbers, A. Gmur, W. Wuest, G. Huber, H. Moch, M. Podvinec, B. Stamm, and A. Aguzzi. 2003. Human prion diseases: epidemiology and integrated risk assessment. Lancet Neurol. 2:757-763. [DOI] [PubMed] [Google Scholar]
- 12.Glatzel, M., C. Rogivue, A. Ghani, J. R. Streffer, L. Amsler, and A. Aguzzi. 2002. Incidence of Creutzfeldt-Jakob disease in Switzerland. Lancet 360:139-141. [DOI] [PubMed] [Google Scholar]
- 13.Gregoire, S., C. Logre, P. Metharom, E. Loing, J. Chomilier, M. B. Rosset, P. Aucouturier, and C. Carnaud. 2004. Identification of two immunogenic domains of the prion protein-PrP-which activate class II-restricted T cells and elicit antibody responses against the native molecule. J. Leukoc. Biol. 76:125-134. [DOI] [PubMed] [Google Scholar]
- 14.Heikenwalder, M., N. Zeller, H. Seeger, M. Prinz, P. C. Kloehn, P. Schwarz, N. H. Ruddle, C. Weissmann, and A. Aguzzi. 2005. Chronic lymphocytic inflammation specifies the organ tropism of prions. Science 307:1107-1110. [DOI] [PubMed] [Google Scholar]
- 15.Heppner, F. L., and A. Aguzzi. 2004. Recent developments in prion immunotherapy. Curr. Opin. Immunol. 16:594-598. [DOI] [PubMed] [Google Scholar]
- 16.Heppner, F. L., C. Musahl, I. Arrighi, M. A. Klein, T. Rulicke, B. Oesch, R. M. Zinkernagel, U. Kalinke, and A. Aguzzi. 2001. Prevention of scrapie pathogenesis by transgenic expression of anti-prion protein antibodies. Science 294:178-182. [DOI] [PubMed] [Google Scholar]
- 17.Hudson, P. J., and C. Souriau. 2001. Recombinant antibodies for cancer diagnosis and therapy. Expert Opin. Biol. Ther. 1:845-855. [DOI] [PubMed] [Google Scholar]
- 18.Isaacs, J. D. 1990. The antiglobulin response to therapeutic antibodies. Semin. Immunol. 2:449-456. [PubMed] [Google Scholar]
- 19.Kalinke, U., A. Krebber, C. Krebber, E. Bucher, A. Pluckthun, R. M. Zinkernagel, and H. Hengartner. 1996. Monovalent single-chain Fv fragments and bivalent miniantibodies bound to vesicular stomatitis virus protect against lethal infection. Eur. J. Immunol. 26:2801-2806. [DOI] [PubMed] [Google Scholar]
- 20.Klein, M. A., R. Frigg, E. Flechsig, A. J. Raeber, U. Kalinke, H. Bluethmann, F. Bootz, M. Suter, R. M. Zinkernagel, and A. Aguzzi. 1997. A crucial role for B cells in neuroinvasive scrapie. Nature 390:687-690. [DOI] [PubMed] [Google Scholar]
- 21.Klein, M. A., P. S. Kaeser, P. Schwarz, H. Weyd, I. Xenarios, R. M. Zinkernagel, M. C. Carroll, J. S. Verbeek, M. Botto, M. J. Walport, H. Molina, U. Kalinke, H. Acha-Orbea, and A. Aguzzi. 2001. Complement facilitates early prion pathogenesis. Nat. Med. 7:488-492. [DOI] [PubMed] [Google Scholar]
- 22.Koller, M. F., T. Grau, and P. Christen. 2002. Induction of antibodies against murine full-length prion protein in wild-type mice. J. Neuroimmunol. 132:113-116. [DOI] [PubMed] [Google Scholar]
- 23.Korth, C., B. Stierli, P. Streit, M. Moser, O. Schaller, R. Fischer, W. Schulz-Schaeffer, H. Kretzschmar, A. Raeber, U. Braun, F. Ehrensperger, S. Hornemann, R. Glockshuber, R. Riek, M. Billeter, K. Wuthrich, and B. Oesch. 1997. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 390:74-77. [DOI] [PubMed] [Google Scholar]
- 24.Krebber, A., S. Bornhauser, J. Burmester, A. Honegger, J. Willuda, H. R. Bosshard, and A. Pluckthun. 1997. Reliable cloning of functional antibody variable domains from hybridomas and spleen cell repertoires employing a reengineered phage display system. J. Immunol. Methods 201:35-55. [DOI] [PubMed] [Google Scholar]
- 25.Kuus-Reichel, K., L. S. Grauer, L. M. Karavodin, C. Knott, M. Krusemeier, and N. E. Kay. 1994. Will immunogenicity limit the use, efficacy, and future development of therapeutic monoclonal antibodies? Clin. Diagn. Lab. Immunol. 1:365-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lecerf, J. M., T. L. Shirley, Q. Zhu, A. Kazantsev, P. Amersdorfer, D. E. Housman, A. Messer, and J. S. Huston. 2001. Human single-chain Fv intrabodies counteract in situ huntingtin aggregation in cellular models of Huntington's disease. Proc. Natl. Acad. Sci. USA 98:4764-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mabbott, N. A., M. E. Bruce, M. Botto, M. J. Walport, and M. B. Pepys. 2001. Temporary depletion of complement component C3 or genetic deficiency of C1q significantly delays onset of scrapie. Nat. Med. 7:485-487. [DOI] [PubMed] [Google Scholar]
- 28.Maloney, D. G., A. J. Grillo-Lopez, C. A. White, D. Bodkin, R. J. Schilder, J. A. Neidhart, N. Janakiraman, K. A. Foon, T. M. Liles, B. K. Dallaire, K. Wey, I. Royston, T. Davis, and R. Levy. 1997. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 90:2188-2195. [PubMed] [Google Scholar]
- 29.Martin, A. C. R. 2000. Antibodies—structure and sequence. http://www.bioinf.org.uk/abs.
- 30.Meier, P., N. Genoud, M. Prinz, M. Maissen, T. Rulicke, A. Zurbriggen, A. J. Raeber, and A. Aguzzi. 2003. Soluble dimeric prion protein binds PrP(Sc) in vivo and antagonizes prion disease. Cell 113:49-60. [DOI] [PubMed] [Google Scholar]
- 31.Oesch, B., D. Westaway, M. Walchli, M. P. McKinley, S. B. Kent, R. Aebersold, R. A. Barry, P. Tempst, D. B. Teplow, L. E. Hood, and C. Weissmann. 1985. A cellular gene encodes scrapie PrP 27-30 protein. Cell 40:735-746. [DOI] [PubMed] [Google Scholar]
- 32.Panka, D. J., M. Mudgett-Hunter, D. R. Parks, L. L. Peterson, L. A. Herzenberg, E. Haber, and M. N. Margolies. 1988. Variable region framework differences result in decreased or increased affinity of variant anti-digoxin antibodies. Proc. Natl. Acad. Sci. USA 85:3080-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peretz, D., R. A. Williamson, K. Kaneko, J. Vergara, E. Leclerc, G. Schmitt-Ulms, I. R. Mehlhorn, G. Legname, M. R. Wormald, P. M. Rudd, R. A. Dwek, D. R. Burton, and S. B. Prusiner. 2001. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 412:739-743. [DOI] [PubMed] [Google Scholar]
- 34.Polymenidou, M., F. L. Heppner, E. C. Pellicioli, E. Urich, G. Miele, N. Braun, F. Wopfner, H. Schaetzl, B. Becher, and A. Aguzzi. 2004. Humoral immune response to native eukaryotic prion protein correlates with anti-prion protection. Proc. Natl. Acad. Sci. USA 101:14670-14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prinz, M., M. Heikenwalder, T. Junt, P. Schwarz, M. Glatzel, F. L. Heppner, Y. X. Fu, M. Lipp, and A. Aguzzi. 2003. Positioning of follicular dendritic cells within the spleen controls prion neuroinvasion. Nature 425:957-962. [DOI] [PubMed] [Google Scholar]
- 36.Prusiner, S. B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136-144. [DOI] [PubMed] [Google Scholar]
- 37.Raag, R., and M. Whitlow. 1995. Single-chain Fvs. FASEB J. 9:73-80. [DOI] [PubMed] [Google Scholar]
- 38.Race, R. E., A. Raines, T. G. Baron, M. W. Miller, A. Jenny, and E. S. Williams. 2002. Comparison of abnormal prion protein glycoform patterns from transmissible spongiform encephalopathy agent-infected deer, elk, sheep, and cattle. J. Virol. 76:12365-12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sigurdsson, E. M., D. R. Brown, M. Daniels, R. J. Kascsak, R. Kascsak, R. Carp, H. C. Meeker, B. Frangione, and T. Wisniewski. 2002. Immunization delays the onset of prion disease in mice. Am. J. Pathol. 161:13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solforosi, L., J. R. Criado, D. B. McGavern, S. Wirz, M. Sanchez-Alavez, S. Sugama, L. A. DeGiorgio, B. T. Volpe, E. Wiseman, G. Abalos, E. Masliah, D. Gilden, M. B. Oldstone, B. Conti, and R. A. Williamson. 2004. Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science 303:1514-1516. [DOI] [PubMed] [Google Scholar]
- 41.Weissmann, C., and A. Aguzzi. 1997. Bovine spongiform encephalopathy and early onset variant Creutzfeldt-Jakob disease. Curr. Opin. Neurobiol. 7:695-700. [DOI] [PubMed] [Google Scholar]
- 42.Wells, G. A., A. C. Scott, C. T. Johnson, R. F. Gunning, R. D. Hancock, M. Jeffrey, M. Dawson, and R. Bradley. 1987. A novel progressive spongiform encephalopathy in cattle. Vet. Rec. 121:419-420. [DOI] [PubMed] [Google Scholar]
- 43.White, A. R., P. Enever, M. Tayebi, R. Mushens, J. Linehan, S. Brandner, D. Anstee, J. Collinge, and S. Hawke. 2003. Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature 422:80-83. [DOI] [PubMed] [Google Scholar]
- 44.Will, R. G., J. W. Ironside, Zeidler, M., Cousens, S. N., Estibeiro, K., Alperovitch, A., Poser, S., Pocchiari, M., Hofman, A., and P. G. Smith. 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347:921-925. [DOI] [PubMed] [Google Scholar]
- 45.Worn, A., and A. Pluckthun. 2001. Stability engineering of antibody single-chain Fv fragments. J. Mol. Biol. 305:989-1010. [DOI] [PubMed] [Google Scholar]
- 46.Wu, T. T., and E. A. Kabat. 1970. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J. Exp. Med. 132:211-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiang, J. H., J. Roder, Z. G. Pan, C. Roifman, and N. Hozumi. 1991. Modification in framework region I results in a decreased affinity of chimeric anti-TAG72 antibody. Mol. Immunol. 28:141-148. [DOI] [PubMed] [Google Scholar]