Abstract
Animal pollination is crucial for the reproduction and economic viability of a wide range of crops. Despite the existing data, the extent to which citrus crops depend on pollinators to guarantee fruit production still needs to be determined. Here, we described the composition of potential pollinators in citrus (Citrus spp.) from the main growing areas of Argentina; moreover, we combined Bayesian models and empirical simulations to assess the contribution of animal pollination on fruit set and yield ha−1 in different species and cultivars of lemons, grapefruits, mandarins, and oranges. Honeybee (A. mellifera L.) was the most commonly observed potential pollinator, followed by a diverse group of insects, mainly native bees. Regardless of citrus species and cultivars, the probability of flowers setting fruit in pollinated flowers was 2.4 times higher than unpollinated flowers. Furthermore, our simulations showed that about 60% of the citrus yield ha−1 can be attributable to animal pollination across all species and cultivars. Therefore, it is crucial to maintain environments that support pollinator diversity and increase consumer and to producer awareness and demand in order to ensure the significant benefits of animal pollination in citrus production.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73591-6.
Keywords: Citrus fruit, Fruit set, Pollinator-dependent crops, Pollination service, Crop yield, Food crops
Subject terms: Agroecology, Biodiversity, Ecosystem services
Introduction
The contribution of animal pollination to global agriculture is increasingly acknowledged1. Yet, pollination requirements vary considerably between and even within species and cultivars. Species variability is mainly due to different crop breeding systems and the regional and local communities of pollinators2,3. For instance, crops such as sugarcane, rice, or maize are predominantly wind-pollinated, and thus the presence of insect pollinators does not impact their production. However, for the vast majority of crops, such as pumpkin, almonds, cherries, and tomatoes, insect pollinators are crucial for the fruit or seed quantity and/or quality1,4. Pollination can be a limiting factor for these crops, as yields are expected to increase in the presence of pollinators until the crop is fully pollinated5. Cultivars within crop species may also respond differently to animal pollination6, for example, soybean7, blueberry8, canola9, bean10 and apple11. This variability may be due to cross-cultivar variation in the mating system that affects the interaction with pollinators12 or reduces the need for pollen deposition for ovule fecundation13.
Pollinators contribution to crop yield is evaluated through experiments, contrasting between flowers with open access to pollinators and those with close pollination (pollinator exclusion experiments), or flowers with hand cross-pollination and those with hand self-pollination (pollen supplementation experiments)14,15. The proportion of flowers that set mature fruits or seeds (i.e. fruit/seed set) is then compared across experiments as the difference between open and excluded treatment and a pollinator contribution or pollinator dependence value is reported. The first compilation of studies that valued the contribution of animals to crop production1 had a great impact, as they stated that most of our crops are benefited by animals, to a certain degree. However, nowadays there is a search for more agronomic/economic impacts of pollinators on crop yield that can be communicated more directly to producers16. For example, measures such as fruit quantity, fruit quality, and yield stability per unit area that are lost in the absence of pollinators are more informative. This information will provide growers and stakeholders with an impulse for the conservation of pollinator services and market policy to implement agri-environmental programs with a meaningful focus on pollinators17.
Citrus fruits (Citrus spp. such as oranges, mandarins, lemons, and grapefruits) are one of the main and most widespread crops globally, with a production of over 140 million tons in 202018 and were considered as a group of species with little dependence on pollinators, but with variability between species1. Citrus fruits are a diverse group comprising between 16 and 156 cultivated species with numerous cultivars (cultivated varieties)19, and include all breeding systems ranging from agamospermy and parthenocarpy (asexual reproduction), self-incompatibility, through all forms of self-pollination, to self-incompatibility and cross-pollination. Thus, it is challenging to generalise citrus responses to pollinators due to their numerous cultivars and complex pollination requirements. It is said, that gamospermous or parthenocarpic citrus varieties do not require pollination to bear fruit (e.g., seedless mandarins, Salustiana oranges, some grapefruits, and lemons)20. However, a significant number of citrus varieties, including oranges but especially grapefruits and mandarins, are self-incompatible and require or benefit from cross-pollination facilitated by insects to produce fruit or to enhance the yield and quality21,22. Many studies conclude that cross-pollination by insect pollinators enhances fruit set, with varying degrees of dependency depending on the species and cultivar23–31. In addition to increased fruit set, some studies find that insect pollination results in fruits of higher quality, with larger size, weight, and more juice quantity and sugars25,26,32,33. Thus, the contribution of pollinators to citrus production remains controversial21,34.
Given the wide variability in their breeding systems and the ample differences reported between studies, an actualized assessment of the citrus cultivated species is necessary4,34. In this study we used empirical data from different species and cultivars (1) to describe the main functional groups of pollinators that visit citrus crops in Argentina; also, (2) we evaluated the influence of animal pollination on the probability of the flower setting fruit across cultivars, and (3) its consequences in crop yield ha−1 across citrus cultivars. In light of this, we utilised a dataset containing visits and fruit set percentages of different citrus groups (grapefruit: C. paradisi, mandarins: C. reticulata and C. x clementina, lemon: C. limon and oranges: C. sinensis) belonging to commonly cultivated species and cultivars. Also, using this dataset, we conducted simulations to estimate the contribution of insect pollination to different productivity measures to reach a broader spectrum of stakeholders about these impacts.
Results
Composition of potential citrus pollinators
In total, we recorded 21,553 visits to the flowers of all citrus studied in 323 h. The most common potential pollinator to all citrus flowers was A. mellifera, which accounted for 87% of the total visitors recorded. Bees accounted for half of all observed wild potential pollinators, with 33% being small native bees and 17% being medium/large bees. Among the remaining groups, beetles were the most common (17%), followed by dipterans (14%), and wasps (9%).
In lemons and grapefruits, more than 87% of the insects observed were A. mellifera (Fig. 1a and d), while in mandarins and oranges, observations of wild potential pollinators predominated (61% and 72%, respectively; Fig. 1b and c). In each citrus group, more than half of wild potential pollinators observations were of bees, mainly small bees (32% in lemons, 44% in mandarins, and 37% in grapefruit), except in oranges, where beetles were the most common group (37%) (Fig. 1c).
Fig. 1.
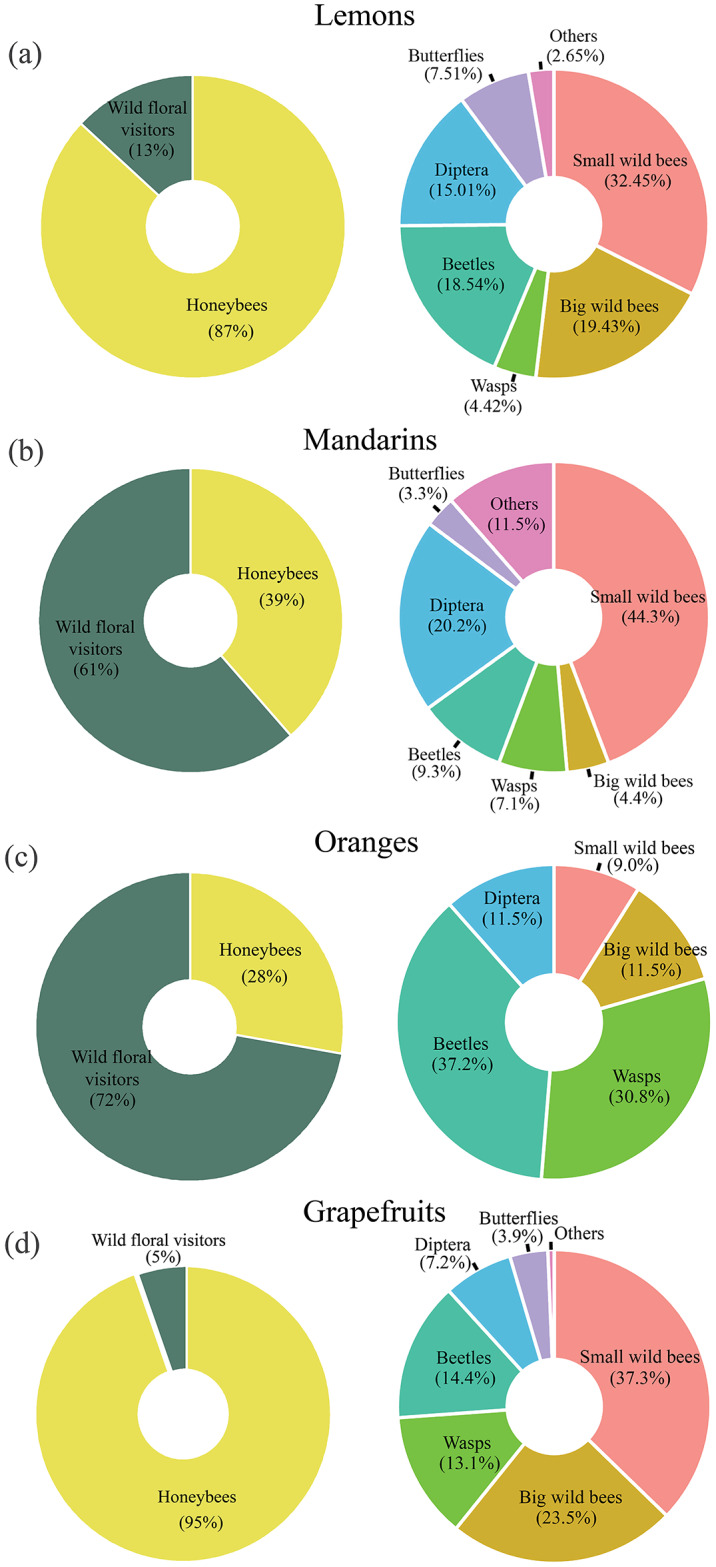
Percentage of observed wild potential pollinators and honeybees visiting lemons (a), mandarins (b), oranges (c), and grapefruits (d) crops in Argentina. The right side panel shows the percentage of functional groups of visitors within the “wild floral visitors” category.
Influence of pollinators in the probability of flowers setting fruit—open versus close pollination
In the close pollination treatment, the probability of flowers setting fruit was low on average for all citrus groups (1.25% CI, 0.78–1.95; Fig. 2), i.e. when flowers were not visited by insects, on average, between one or two fruits are formed per hundred flowers. Meanwhile, in the open pollination treatment, the probability of a flower setting a fruit was 3.07% (CI, 1.94–4.67). This means that flowers from the open treatments had 2.4 times higher probability of setting fruit than those from close pollination (Fig. 2). Indeed, this pattern remained consistent across citrus groups (2.4 ± 0.04 SD) and cultivars (2.4 ± 0.07 SD) (see Supplementary Information 1, Sect. 2.7—Tables S3, S4). Under open pollination conditions, lemon cultivars had the highest probability of flowers setting fruit (11.0% ± 2.01), followed by grapefruits (2.98% ± 0.47), mandarins (2.62% ± 2.03), and oranges (1.98% ± 0.77). The same pattern was observed for the close pollination treatment (lemons = 4.79% ± 1; grapefruits = 1.23% ± 0.2; mandarins = 1.08% ± 0.84; oranges = 0.87% ± 0.31) (Fig. 2, Supplementary Information 1, Sect. 2.7—Table S3). Rhat values and visual diagnostics showed that all chains converged to the same posterior distribution. The effective sampling size (ess) of all parameters was > 1,000 and 96% of pareto k values were below 0.7 (see Supplementary Information 1, Sect. 2.5). The posterior predictive checks indicated that the model was well-fitted (see Supplementary Information 1, Sect. 2.6).
Fig. 2.
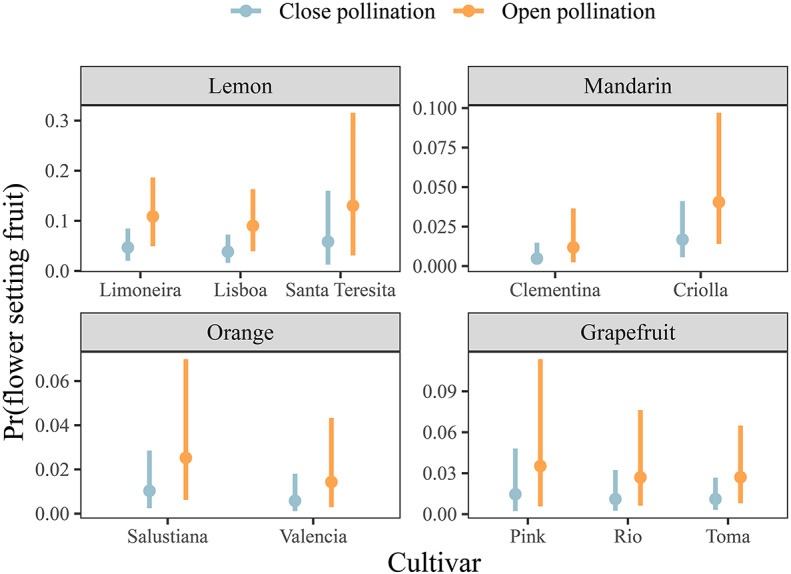
Probability of flowers setting fruit in pollinator exclusion experiments for the main citrus groups grown in Argentina. The x-axis indicates different cultivars. The error bars and the dot show the 95% credibility interval and median of the marginal posterior distribution, respectively.
Contribution of pollinators to citrus production per tree and ha—simulation results
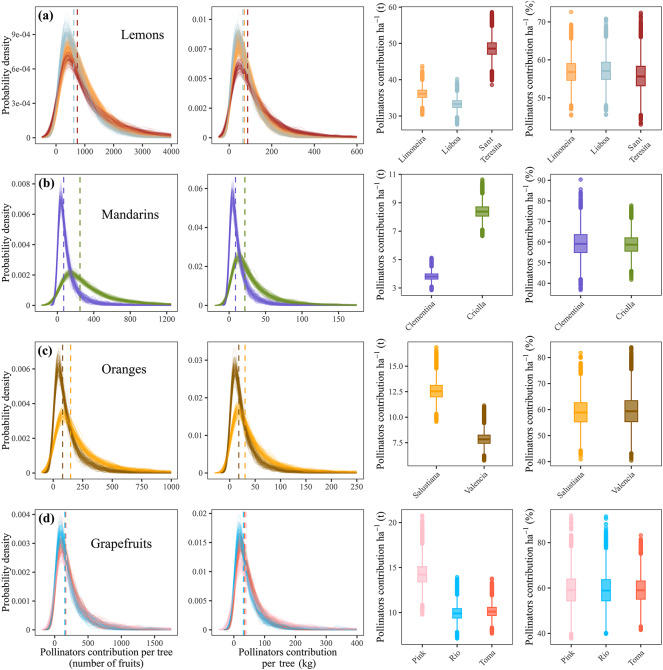
When comparing open vs. close pollination treatments, our simulations showed that, regardless of the citrus groups or cultivars, there is an ~ 80% probability that trees exposed to animal pollination produce more fruits (see Supplementary Information 1, Sect. 3.7.2). Considering the contribution of animal pollination to citrus production, i.e. the posterior distribution of the contrast between open and close pollination, the simulations showed that animal pollination contributes to the production of 739 fruits tree−1 (SD ± 70.4) of lemons (accounting for 71.5 kg tree−1 ± 11.2), 159.25 fruits tree−1 (SD ± 126.2) of mandarins (accounting for 15.2 kg tree−1± 9.2), 114 fruits tree−1 (SD ± 48.1) of oranges (accounting for 24.03 kg ± 8.7), and 168 fruits tree−1 (SD ± 11.3) of grapefruits (accounting for 35.4 kg ± 4.2) (Fig. 3). Considering the density of trees planted per ha, we found that animal pollination contributed to 36.1 t ha−1 (SD ± 6.8) of lemons (56.5% ± 3.5 of total production), 5.9 t ha−1 (SD ± 2.3) of mandarins (58.9% ± 5.9 of total production), 10.1 t ha−1 (SD ± 2.4) of oranges (59.1% ± 5.7 of total production), and 10.5 t ha−1 (SD ± 2.2) of grapefruits (58.9% ± 6.6 of total production) (Fig. 3).
Fig. 3.
Estimated crop production attributed to animal pollination in four citrus groups and their cultivars: (a) lemons, (b) mandarins, (c) oranges and (d) grapefruits. Each density line and colour denotes different simulations (only 100 are shown) and cultivars, respectively. The vertical discontinuous lines show the median value for each cultivar, and the box plots show the values from 10,000 simulations per cultivar.
Discussion
The influence of animal pollination on crop yield can vary due to several factors. In citrus crops, the contribution of pollinators is still a matter of debate, mainly because of the wide variety of reproductive strategies within citrus species and cultivars, but also due to spatial, temporal and biological factors4,35. Using data from main citrus species and cultivars (i.e., cultivated varieties) in large regions of Argentina, we found that animal pollination increases about two times the probability of flowers setting fruit in the citrus crop, resulting in a contribution of about 60% of the total yield at the hectare level. Therefore, despite the sometimes neglected role of animal pollination for this crop, our study provides evidence that justifies safeguarding pollinators in citrus agricultural landscapes.
The exotic honeybee A. mellifera was the main potential pollinator to the citrus flowers. A diverse group of native insects, mainly bees, participated in the visits to a lesser extent. The widespread occurrence of honeybees is common in citrus pollination studies21, but it should be considered that honeybees in these agricultural landscapes can be either managed or feral. In agroecosystems of the region, the abundance of honeybees is linked to the proximity of natural habitats34 and the abundance of locally managed honeybee hives for the production of honey. Studies have shown that the introduction of managed honeybees can increase flower visitation rates, which can result in a reduction in fruit set and lower fruit quality in various crops36,37, including citrus38. It is crucial to acknowledge that an increase in pollinator visitation does not necessarily lead to enhanced fruit production, and the native pollinators may be as or more efficient in fruit production than managed bees39. In our study, the most abundant wild potential pollinators were small bees, mainly stingless bees, and the eusocial bees Lasioglossum spp. Curtis. These bees could prove to be valuable for citrus even in the presence of managed hives, as highlighted by previous studies40–42. Consequently, the adoption of effective local and landscape management strategies to enhance flowering and nesting resources and to reduce current environmental pressures on wild pollinators may be sufficient to achieve optimal benefits.
Our results have shown that in all the citrus groups and cultivars studied, insect pollination has a positive effect on the proportion of flowers setting fruit, increasing more than double the number of fruits compared with non-pollinated flowers. Therefore, according to our data, insects are certainly important for citrus fruit production. In the lemon cultivars of our study, the percentage of fruit set in open pollinated flowers was lower than that reported in the literature for C. limon (29.7% Layek et al.43). However, the increase in fruit set in open versus closed treatments was very similar in both studies (44% in our study and 45% in Layek et al.43). The fruit set of mandarins and oranges varies greatly due to the wide variety of cultivars and breeding. The Clementine mandarin is facultative parthenocarpic, i.e. it requires pollen stimulation by pollinators to set fruit44. However, the probability of fruit set was doubled when the flowers were pollinated by insects compared to excluded flowers25. Studies on mandarin pollination have found either a smaller increase41 or a similar number of mature fruits32 in insect pollinated flowers compared to our study. Previous studies have found that the increase in cross-pollination treatments is dependent on the cultivars used25,28–30. In studies on oranges, the values in flowers with open pollination for the Pera Rio variety were 30–78% higher than in excluded flowers26,45,46. For grapefruit, the only published results available in the literature correspond to the data used in this work22. In comparison to previous studies, we did not observe a significant difference in fruit set between open and close treatments for citrus cultivars of the same species. Therefore, the variations in fruit set reported in previous studies may be attributed to differences in estimation methods, limited replication, and a small number of studies with other species and cultivars that are less dependent on pollinators.
Our study reveals the contribution of pollinators to citrus yield, but we did not explore the contribution of pollinators to seed setting in these fruits, which is an important marketable trait in citrus, i.e. seedlessness. Seedlessness is important for fresh citrus use, whereas this trait is less important for industrial citrus26,47. Seedless varieties are a desirable productive trait in most citrus, and for most species, pollination and fertilisation result in seed formation47. Therefore, there is a potential trade-off between the need of animal pollination to increase the probability of flowers setting fruit (i.e. production quantity), and the market demand of seedless production (i.e. quality production). Yet, for certain cultivars, such as some mandarins, lemons, and grapefruits, seedlessness is not required by the market or even possible22–24,38,48. In addition to production quantity, pollination can also improve the sugar content26,38 and weight of the fruits42 due to pollen deposition and fecundation of the ovules which activate hormonal processes related to fruit growth, seed formation, pulp firmness and thus increase the post-harvest quality of fruits49. Despite the numerous studies that provide evidence of the benefits of pollination in this production system, when the number of seeds is relevant, growers often have to choose based on market demand. They must choose between increasing the yield and quality of the fruit or reducing a trait that affects the destination market of the production, which alters the value of the product49.
Our results indicate that without animal pollination, the amount of fruit produced per hectare (equivalent in tonnes/ha) would be reduced by an average of 58.35%. All the species and cultivars studied showed similar levels of pollinator dependence, despite differences in environmental conditions, spatial arrangements, farm management practices, and composition of potential pollinators. The degree of pollinator dependence on citrus fruits has been previously reported in the literature in compilations, with widely varying results1,4,15. Klein et al.1 reported that pollinators make a small contribution to citrus fruit production, with less than 10% dependence. Mallinger et al.4 report values of pollinator contribution in Florida (USA), of 73% for grapefruits, 31% for lemons and oranges, and over 80% for tangelo and mandarins. Siopa et al.15 reported high levels of pollination dependence in citrus of around 65–80%, except for oranges which had a lower dependence of around 20%. This latter study used hand pollen supplementation to assess the yield associated with animal pollination, in contrast to our study, which used the open pollination treatment. It is important to note that the use of hand pollen supplementation tends to show higher values of pollinator contribution15, which indicates that there can be some level of pollen deficit. Therefore, although the contribution of animal pollination in most citrus studies indicates a relative/high dependence of pollinators on citrus yields, values of pollinator dependence and reporting of these values may vary according to the estimation methods used.
In this study, we considered the contribution of pollinators to citrus production in terms of yield ha−1, including aspects such as fruit set, fruit weight, and literature value of floral display, in contrast to previous studies that use fruit set to calculate this contribution. Previous studies classified crop pollination dependence (for example, little, modest, high, and essential in Klein et al.1), where the difference between close pollination treatments and pollinator-associated production treatments (open pollination or hand pollen supplementation) was calculated as 1-(close/pollinator-associated production). However, the fruit set al.one does not fully reflect the true dependence on pollinators in commercial crop production17. These studies do not take into account quantitative differences in fruit, such as the weight of fruit per tree or per hectare lost in the absence of pollinators, as calculated here. Also, in contrast to the above-mentioned approaches, our results reflect the potential implications of pollinator absences on farms and allow us to assess the precise value of pollinators on agricultural production using commercially relevant yield metrics. This approach is advantageous as it is more effective and allows for more concrete communication for the decision-making of growers, industry, and stakeholders.
To estimate the contribution of animal pollination in citrus, we fitted Bayesian models that necessarily neglect the role of plant physiological state and other agronomic inputs (e.g. pruning practices, irrigation, fertilisation, pest control), which should be taken into account in future studies. We also recommend that future research should assess whether the contribution of pollinators to the production quantity is reflected in certain aspects of citrus quality that also have a significant impact on market value, such as weight, seed quantity, size, sugar concentration, and others. Furthermore, although not documented in our study, the presence of managed beehives at sites close to the crops certainly influenced their high abundance. To achieve this, the number of managed hives in the vicinity and their distance from citrus plantations should be recorded, to assess the pollen limitation (in addition to open and hand-pollen supplementation treatment) and the ideal number of visits to achieve high fruit yields in citrus production.
This study showed that animal pollination more than doubled the fruit set and contributed around 60% of citrus yield/ha−1, regardless of species and cultivar. In light of these results and the future of the national citrus market, which is increasingly focused on management practices that emphasise the absence of animal pollination to develop ‘seedless citrus’, and in the face of an imminent advance of diseases that threaten the quality of fresh fruit for export (e.g. citrus Huanglongbing or HLB), it will be necessary to evaluate a change in future market strategy or a re-evaluation of the quality standard to be marketed. In this context, animal pollination ensures a higher quantity and quality of fruit, both in terms of the weight of individual fruits and the sugar content of the juice produced26,34,38,42. Therefore, changing market demands, based on pollinator conservation may also be a possibility, especially when the number of seeds is not so relevant. Empirical studies, market analysis, and policy interventions aimed at promoting pollinator conservation and sustainable agriculture can assist stakeholders in better understanding and mitigating the risks associated with pollinator declines while harnessing the economic benefits of animal pollination for food systems globally.
Methods
Citrus crops
Citrus (Citrus spp., such as oranges, mandarins, lemons, and grapefruits) represent a significant portion of Argentina’s fruit production, covering 23.9% of the total production area50. The mean production area of all cultivated citrus species in Argentina is approximately 132,669 hectares per year, generating nearly USD 378 million in export earnings from the marketing of over 3.3 million tons of fruit (period 2018-202251). The citrus flowers are perfect, containing both pistils and stamens with the potential for self-pollination, which may obviate the need for pollinators21. The fruits display a variety of shapes, sizes, and other quality parameters according to species and cultivars52. A vigorous citrus tree can produce between 20,000 and 250,000 floral units during the flowering period53,54. However, typically, a very low percentage (between 0.1 and 3%) of these flowers develop fruits53,55. In general, citrus flowers are highly attractive to pollinators. Citrus trees experience mass flowering, typically in early spring when there are still few wildflowers, thus providing valuable nectar and pollen resources for pollinators. The diversity of pollinators in citrus varies according to geographical area and the flowering season. The use of managed pollinators for pollination services is not a conventional practice in these crops, because of the common belief that citrus does not require insect pollination to set fruits and even in some citrus, pollination is intentionally avoided, which is a relatively common practice in some mandarins for a desirable ‘seedless citrus’.
Study area and potential pollinator sampling
This study is based on empirical data from various sources which cover the main citrus-growing areas of Argentina50. Argentinian citrus-growing differs from each other (i.e. Mediterranean) in terms of the phytogeography influence they receive. For instance, the citrus areas in the northwest are influenced by the Yungas forests or by the Dry Chaco region. The citrus area in northeast Argentina is influenced by the Paranaense region56. Citrus growing in the NW is commonly associated with large areas of well-protected forest of Yungas or Chaco, while those in the NE are surrounded by a mixed area of crops with a relatively low proportion of natural habitats.
Grapefruits
Samplings in grapefruit (C. paradisi Macf) were carried in three different cultivars (‘Pink’, ‘Río Red’, and ‘Rouge La Toma’) in NW Argentina (see Supplementary Information 2, Fig. S1). Four grapefruit plantations were selected and sampled over three consecutive years (2000 to 2002). The activity of potential pollinators was recorded during 15-minute observation sessions on randomly selected branches. All selected plantations were conventionally managed with fields predominantly dedicated to citrus (see Chacoff and Aizen22,57 for more detail).
Mandarins
We studied the Criolla mandarin (C. reticulata var. Criolla Blanco) in NW Argentina, and Clementine mandarin (Citrus x clementina Hort.) in NE Argentina. Studies on Criolla mandarin crops were conducted in 2019 over ten family citrus farms (see Supplementary Information 2, Fig S1). These citrus crops were situated in rural areas surrounded by secondary semi-deciduous forests and shrublands, patches of old-growth forests, fruit trees (primarily citrus and olives), and fodder crops. Potential pollinators activity was recorded during 15-minute observation sessions on randomly selected branches (see Monasterolo et al.38 for more details). The Clementine mandarin was studied in 2021, over an experimental area property of INTA (National Institute of Agricultural Technology). The potential pollinators sampling was conducted along linear transects, where observations were made on one side of the planting line, across the width of the plot (100 m), for a period of 10 min.
Lemons
Lemon plantations (C. limon L. Burm. F.) were sampled in NW Argentina, in 2015, 2020, and 2021 (see Supplementary Information 2, Fig. S1). Lemons were sourced from three different cultivars: ‘Lisboa’, ‘Limoneira’, and ‘Santa Teresita’. Two plantations were sampled in 2015 and 2021, while another one was sampled in 2020. The potential pollinators samplings were conducted in 5-minute intervals on randomly selected branches. The selected plantations had conventional management.
Oranges
Sampling was carried out in 2021, focusing on Salustiana (C. sinensis L. Osbeck cv. Salustiana) and Valencia oranges (C. sinensis L. Osbeck cv. Valencia), located in NE Argentina (see Supplementary Information 2, Fig. S1). We selected four productive plots, within the experimental area, each approximately one hectare in size, one for each cultivar examined. The potential pollinators sampling was conducted along linear transects, where observations were made on one side of the planting line, across the width of the plot (100 m), for a period of 10 min. The fields where we studied oranges and Clementine mandarin were situated in an area with high environmental variability, including cultivated and natural environments. These include blueberry plantations, palm groves, riverine vegetation, and fields of pecan trees and small blocks of Eucalyptus spp. forest plantations.
Composition of potential citrus pollinators
In all citrus species, the potential pollinators were identified in flight or collected and taken to the laboratory. A visit was considered when a visitor contacted reproductive parts of any of the citrus flowers observed, thus they can be reasonably assumed as pollinators. We acknowledge that to state that a flower visitor is a pollinator, more detailed studies are needed, as pollen deposition and pollen tube elongation, however, from the perspective of the ecosystem service, they are indicative of pollination. Accordingly, the term “potential pollinators’’ was used consistently throughout the manuscript. Due to the varying sampling methodologies and identification limitations in some studies (lack sufficient data at the species or morphospecies level), the potential pollinators were categorised into eight functional groups, based on their morphological features, taxonomic classification, and relative abundance of observations. The studies included various insect groups such as flies, beetles, wasps, butterflies/moths, and others (such as ants, hemipterans, and homopterans). Furthermore, bees were categorised into three groups: one consisting solely of the exotic bee A. mellifera due to its widespread abundance, while the remaining two groups were composed of native bees, classified based on size, determined by inter-tegular distance58. The small bees were grouped together (mainly stingless bees and small halictids). While medium to large bees were also grouped together (which mainly included the medium to large Apidae bees like some members of the tribe Eucerini, Bombus spp., and Xylocopa spp., as well as medium-sized megachilids and halictids such as Augochlora spp. and Augochloropsis spp.), due to the low representation of medium bees in the citrus flowers (2% of the total observations).
Effect of pollinators on the probability of flower setting fruits
To assess the influence of pollinators on the fruit set of grapefruit, lemons, oranges and mandarins, experiments were conducted comprising two treatments: open pollination and close pollination through bagging branches with flower buds (for more details on these methodologies see Chacoff and Aizen22; Monasterolo et al.38). In each tree, we selected at least two branches with flowering buds and applied one of two pollination treatments: open pollination and close pollination treatment. In the open pollination treatment, we recorded the number of mature flowering buds when flowering season was starting and we counted the number of initial fruits when fruits were formed (one month after flowering). In this treatment, flowers were exposed to natural levels of pollination, including both animal pollination and self-pollination due to wind pollination. For close pollination treatment, a branch with flowering buds was excluded from pollinators by using a voile that permitted the action of the wind but not the visits from insects, here only self-pollination due to wind pollination can occur. The bags were removed after flowering and the number of fruits formed in each treatment was counted to calculate the fruit set (fruits/flowers). The number of sampled plants and branches is provided in Table 1 (for more details see Supplementary Information 1, Sect. 2.2—Table S1).
Table 1.
Number of branches sampled per treatment, species, and cultivar.
| Species | Cultivars | Pollination treatment | ||
|---|---|---|---|---|
| Close pollination | Open pollination | Total | ||
| Lemon | Limoneira | 137 | 133 | 270 |
| Lisboa | 55 | 51 | 106 | |
| Santa Teresita | 12 | 14 | 26 | |
| Total lemon | 204 | 198 | 402 | |
| Mandarin | Clementine | 11 | 11 | 22 |
| Criolla | 690 | 206 | 896 | |
| Total mandarin | 701 | 217 | 918 | |
| Orange | Salustiana | 12 | 11 | 23 |
| Valencia | 11 | 9 | 20 | |
| Total orange | 23 | 20 | 43 | |
| Grapefruit | Pink | 11 | 10 | 21 |
| Rio Red | 33 | 34 | 67 | |
| Rouge La Toma | 37 | 37 | 74 | |
| Total grapefruit | 81 | 81 | 162 | |
| Total | 1009 | 516 | 1525 | |
Data analysis
Effect of pollinators on the probability of flower setting fruits
We used a Bayesian hierarchical model to assess the effect of animal pollination exclusion on the probability of the flowers setting fruit:
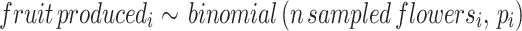 |
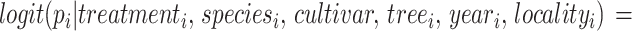 |
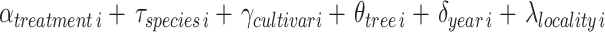 |
We considered the pollination treatment as the population effect, and citrus species, cultivar, sampled tree, year of sampling, and locality as group-level effects. To account for variation in sampling size among citrus species and cultivar and reduce overfitting, we used partial pooling for parameter estimation. The probability of flowers setting fruit tends to be low for Citrus spp55. , hence we defined αtreatment i ~ Normal(5, 2.5) as prior (i.e. number of fruits developed from n observed flowers), and a Normal(0, 1) for all group-level parameters. We estimated the joint posterior distribution of pi (i.e. probability of flowers setting fruit) using the Hamiltonian Monte Carlo algorithm through Stan 2.32.2 and the R package cmdstanr. We fitted the model by setting three chains, 4000 sampling and 500 warming iterations, and a thinning rate of 3. We conducted sampling diagnostics of all parameters through visual assessment of chains convergence, Rhat < 1.1, ess > 1000, and pareto k values < 0.7. We also assessed the quality of the model fit through posterior predictive checks. See Supplementary Information 1, Sect. 2.3 for the mathematical notation of the model and Sect. 2.4 for the Stan code to fit the model.
Contribution of animal pollination to citrus production
Assuming that the differences in the probability of flowers setting fruit between experimental treatments can be attributed to animal pollination, we build simulations to estimate the contribution of pollinators to fruit production and yield of lemon, mandarin, orange, and grapefruit. Annotated R code and main functions conducting the simulations can be consulted in Supplementary Information 1, Sect. 3.1. The simulations followed this reasoning.
Step 1 Considering the floral display size of Citrus spp. reported in the literature (25000 to 200000 flowers in oranges54, or 20000 to 50000 flowers in grapefruits55), and information from citric producers in the localities studied, we hypothesise that the number of flowers produced by Citrus spp. trees can be recreated through a negative binomial probability distribution:
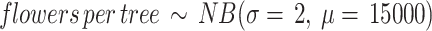 |
σ denotes the dispersion parameter of the distribution, and we defined µ = 15,000 to remain conservative on the average number of flowers produced per tree (Supplementary Information 1, Sect. 3.1).
Step 2 We used step 1 to simulate as many random trees with n flowers as trees expected per ha for each citrus species. For lemon cultivars, we assumed 400 trees ha−1 (5 × 5 m); orange and mandarin cultivars 278 trees ha−1 (6 × 6 m); and grapefruit cultivars 278 trees ha−1 (8 × 6 m). We replicated this process 10,000 times per citrus species and cultivars (i.e., 10000 simulated hectares per citrus species and cultivars). We defined the plantation distance according to information provided by growers (Supplementary Information 1, Sect. 3.2).
Step 3 Then, we used the marginal posterior distribution of the probability of flowers setting fruit per pollination treatment to predict the number of fruits produced per simulated tree in step 2: See Supplementary Material 1, Sect. 3.3.2.1 for this simulation in lemon (Limoneira cultivar).
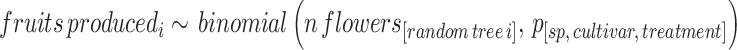 |
Step 4 We used the simulated fruits in step 3 for each pollination treatment per citrus species and cultivar to estimate the probability that trees exposed to animal pollination (i.e. open pollination treatment) produce more fruits than those from close pollination treatment (Supplementary Information 1, Sect. 3.3.2.1).
Step 5 We calculated the contrast of the marginalised posterior distribution of the probability of flowers setting fruit between pollination treatments. Then, we used the contrast and the simulated trees in step 2, to simulate the number of fruits attributed to animal pollination (Supplementary Information 1, Sect. 3.3.2.2).
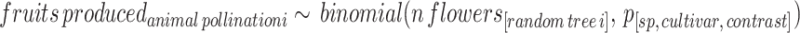 |
Step 6 We fitted Bayesian models to estimate the posterior distribution of fruit weight of lemon, grapefruit, and Criolla mandarin (Supplementary Information 1, Sect. 3.3.1, 3.4.1 and 3.6.1, respectively). The fitting and models diagnostics procedure follows the above-mentioned protocol. Since raw data on fruit weight for orange (Valencia and Salustiana) and Clementine mandarin was not available, we used mean and SD values reported in the literature59 to parameterize normal distributions (Supplementary Information 1, Sect. 3.5.1). Then, we used these distributions to simulate fruit weights for each citrus species and cultivar (Supplementary Information 1, Sect. 3.3.4.2).
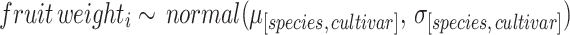 |
Step 7 Finally, we used the distributions of step 6 to simulate as many fruit weights as fruits per tree were generated in step 5. The sum of weights per tree and the sum of trees per ha denoted the production attributed to animal pollination in kg and Tn, respectively. We conducted 10,000 simulations per citrus species and cultivar. We repeat steps 5 to 7 with the posterior distribution of open pollination treatment and use the simulations from the contrast to estimate the percentage of crop production per ha that can be attributed to animal pollination.
We performed all simulations and statistical procedures in R 4.3.160, we did data wrangling operations using base and dplyr packages, and plotted the models and simulations outputs with ggplot2 and cowplot packages. We provide the R script with annotated code to reproduce the simulations and models fit in Supplementary Information 1, Sect. 3.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Field work in Criolla mandarin was supported by CONICET (PIO/2015-1520150100023CO) and PICT-2018-02508. Field work in lemon and grapefruit was supported by Fundación ProYungas, PICT-2021-01152, PICT-2021-00092, and PRÉSTAMO BCIE. We thank the staff of the growers for logistic support during fieldwork and many field assistants as Candela Russo, Lorena Escobar, Beatriz Velazquez, Martín Lepiscopo, Carla J. Cardenas, Sebastian Albanesi, and Lorena Luna. We emphasize that our study does no cause environmental problems and complies with local regulations, as the species used (Citrus paradisi, C. reticulata, C. x clementina, C. limon and C. sinensis) are exotic to our country and are propagated for productive use in commercial nurseries. Furthermore, we had the relevant authorizations from the family farmers, plantation owners (R. Manero, J. Campos, R. Burgos and D. Lorenzo), and institutions/companies (INTA, University of Catamarca, San Miguel S.A., Citrusvil S.A. and Citrus Salta S.A. authorizations) where the citrus data were collected.
Author contributions
M.M. supervised. M.M., A.F.R.M., N.P.C., P.C. and P.S. wrote, reviewed and edited. A.F.R.M. analyzed the data. M.M., N.P.C., P.C, V.C., and C.M.C. collected the samples. All authors contributed critically to drafting and gave final approval to publish.
Data availability
Data are available from the Zenodo repository: 10.5281/zenodo.13315510.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Klein, A. M. et al. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 274, 303–313 (2007). [DOI] [PMC free article] [PubMed]
- 2.Potts, S. G. et al. Safeguarding pollinators and their values to human well-being. Nature540, 220–229 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Tamburini, G., Bommarco, R., Kleijn, D., van der Putten, W. H. & Marini, L. Pollination contribution to crop yield is often context- dependent: a review of experimental evidence. Agric. Ecosyst. Environ.280, 16–23 (2019). [Google Scholar]
- 4.Mallinger, R. E., Ternest, J. J., Weaver, S. A., Weaver, J. & Pryer, S. Importance of insect pollinators for Florida agriculture: a systematic review of the literature. Fla. Entomol.104, 222–229 (2021). [Google Scholar]
- 5.Reilly, J. R. et al. Crop production in the USA is frequently limited by a lack of pollinators. Proc. Biol. Sci. 287, 20200922 (2020). [DOI] [PMC free article] [PubMed]
- 6.Marini, L. et al. Crop management modifies the benefits of insect pollination in oilseed rape. Agric. Ecosyst. Environ.207, 61–66 (2015). [Google Scholar]
- 7.da Cunha, N. L. et al. Soybean dependence on biotic pollination decreases with latitude. Agric. Ecosyst. Environ.347, 108376 (2023). [Google Scholar]
- 8.Ramirez-Mejia, A. F., Lomáscolo, S. & Blendinger, P. G. Hummingbirds, honeybees, and wild insect pollinators affect yield and berry quality of blueberries depending on cultivar and farm’s spatial context. Agric. Ecosyst. Environ.342, 108229 (2023). [Google Scholar]
- 9.Ouvrard, P. & Jacquemart, A. L. Review of methods to investigate pollinator dependency in oilseed rape (Brassica napus). Field Crops Res.231, 18–29 (2019). [Google Scholar]
- 10.Bishop, J., Garratt, M. P. D. & Breeze, T. D. Yield benefits of additional pollination to faba bean vary with cultivar, scale, yield parameter and experimental method. Sci. Rep.10, 2102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garratt, M. P. D. et al. Apple pollination: demand depends on variety and supply depends on pollinator identity. PLoS ONE11, e0153889 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courcelles, D. M. M., Button, L. & Elle, E. Bee visit rates vary with floral morphology among highbush blueberry cultivars (Vaccinium corymbosum L). J. Appl. Entomol.137, 693–701 (2013). [Google Scholar]
- 13.Kendall, L. K. et al. Self-compatible blueberry cultivars require fewer floral visits to maximize fruit production than a partially self-incompatible cultivar. J. Appl. Ecol.57, 2454–2462 (2020). [Google Scholar]
- 14.Bartomeus, I. et al. Contribution of insect pollinators to crop yield and quality varies with agricultural intensification. PeerJ2, e328 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siopa, C., Carvalheiro, L., Castro, H., Loureiro, J. & Castro, S. Animal-pollinated crops and cultivars—A quantitative assessment of pollinator dependence values and evaluation of methodological approaches. J. Appl. Ecol.61 (6), 1279–1288 (2024). [Google Scholar]
- 16.Velado-Alonso, E., Kleijn, D. & Bartomeus, I. Reassessing science communication for effective farmland biodiversity conservation. Trends Ecol. Evol. 10.1016/j.tree.2024.01.007 (2024). [DOI] [PubMed] [Google Scholar]
- 17.Melathopoulos, A. P., Cutler, G. C. & Tyedmers, P. Where is the value in valuing pollination ecosystem services to agriculture? Ecol. Econ.109, 59–70 (2015). [Google Scholar]
- 18.FAO. Citrus Fruit Statistical Compendium 2020 (FAO, 2021).
- 19.Ollitrault, P. & Navarro, L. Citrus. Fruit Breed. 623–662 (2012).
- 20.Zhou, X. et al. Parthenocarpy in citrus accessions with special focus on relatives of Kunenbo (C. nobilis Lour. Var. Kunep Tanaka). Sci. Hortic.232, 29–39 (2018). [Google Scholar]
- 21.Sanford, M. T. Pollination of Citrus by Honey Bees (University of Florida Cooperative Extension Service, Institute of Food and Agriculture Sciences, EDIS, 1992).
- 22.Chacoff, N. P. & Aizen, M. A. Pollination requirements of pigmented grapefruit (Citrus paradisi Macf.) from Northwestern Argentina. Crop Sci.47, 1143–1150 (2007). [Google Scholar]
- 23.Hearn, C. J., Reece, P. C. & Fenton, R. Effects of pollen source on fruit characteristics and set of four citrus hybrids. Proc. Florida State Hortic. Soc.81, 94–98 (1968).
- 24.Vithanage, V. Effect of different pollen parents on seediness and quality of ‘Ellendale’ tangor. Sci. Hortic.48, 253–260 (1991). [Google Scholar]
- 25.Wallace, H. M. & Lee, L. S. Pollen source, fruit set and xenia in mandarins. J. Hortic. Sci. Biotechnol.74, 82–86 (1999). [Google Scholar]
- 26.Malerbo-Souza, D. T., Nogueira-Couto, R. H. & Couto, L. A. Honey bee attractants and pollination in sweet orange, Citrus sinensis (L.) Osbeck, var. Pera-Rio. J. Venom. Anim. Toxins Incl. Trop. Dis.10, 144–153 (2004). [Google Scholar]
- 27.Wallace, H. M. Pollination effects on quality in ‘oroval clementine’ mandarin in Australia. Acta Hortic.632, 99–103 (2004). [Google Scholar]
- 28.Chao, C. C. T. Pollinations study of mandarins and the effect on seediness and fruit set: implications for seedless mandarins production. HortScience40, 362–365 (2005). [Google Scholar]
- 29.Papadakis, I. E., Protopapadakis, E. E. & Therios, I. N. Yield and fruit quality of ‘Nova’ hybrid [Citrus clementina hort. Ex Tanaka (C. reticulata Blanco x C. paradisi Macfad)] and two Clementine varieties (C. clementina Hort. Ex Tana Ka) as affected by self- and cross-pollination. Sci. Hortic.121, 38–41 (2009). [Google Scholar]
- 30.Yildiz, E. & Kaplankiran, M. The effect of cross-pollination on fruit set and quality in ‘Robinson’ and ‘Fremont’ mandarins. Ege Üniv Ziraat Fak Derg54, 107–112 (2017). [Google Scholar]
- 31.da Santos, S. R. et al. Bee pollination services and the enhancement of fruit yield associated with seed number in self-incompatible tangelos. Sci. Hortic.276, 109743 (2021). [Google Scholar]
- 32.Manzoor-ul-Haq, M. U. H., Rafie-ul-Din, M. & Ghaffar, A. Effect of insect pollination on fruit bearing in Kinnow Mandarin (Citrus reticulata), and physical and chemical properties of the fruit. J. Apic. Res.17, 47–49 (1978). [Google Scholar]
- 33.Vanlalhmangaiha, R., Singh, H. K., Boopathi, T., Lalhruaitluangi, S. & Sangma, T. T. Impact of insect pollination on the quantitative and qualitative characteristics of sweet orange, Citrus sinensis (L.) Osbeck. J. Apic. Res.62, 767–776 (2023). [Google Scholar]
- 34.Gurung, S. & Chettri, A. Threat to citrus in a global pollinator decline scenario: current understanding of its pollination requirements and future directions. Plant Reprod. Ecol. Recent. Adv.134 (2021).
- 35.Bishop, J. & Nakagawa, S. Quantifying crop pollinator dependence and its heterogeneity using multi-level meta‐analysis. J. Appl. Ecol.58, 1030–1042 (2021). [Google Scholar]
- 36.Rollin, O. & Garibaldi, L. A. Impacts of honeybee density on crop yield: a meta-analysis. J. Appl. Ecol.56 (5), 1152–1163 (2019). [Google Scholar]
- 37.Aizen, M. A. et al. Invasive bees and their impact on agriculture. Adv. Ecol. Res.63, 49–92 (2020). [Google Scholar]
- 38.Monasterolo, M., Chacoff, N. P., Segura, A. D., Benavidez, A. & Schliserman, P. Native pollinators increase fruit set while honeybees decrease the quality of mandarins in family farms. Basic Appl. Ecol.64, 79–68 (2022). [Google Scholar]
- 39.Garibaldi, L. A. et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science339, 1608–1611 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Grajales-Conesa, J., Meléndez Ramírez, V., Cruz-López, L. & Sánchez, D. Native bees in blooming orange (Citrus sinensis) an lemon (C. limon) orchards in Yucatán, México. Acta Zool. Mex.29, 437–440 (2013). [Google Scholar]
- 41.Pradhan, U. & Devy, S. Pollinators of Sikkim mandarin orange Citrus reticulata (Sapindales: Rutaceae). J. Threat. Taxa11, 13625–13628 (2019). [Google Scholar]
- 42.Nurdiansyah, M. A., Abduh, M. Y. & Permana, A. D. Effects of meliponiculture Tetragonula laeviceps on pollinator diversity and visitation rate and citrus productivity in West Java, Indonesia. Biodivers. J. Biol. Divers.24 (2023).
- 43.Layek, U., Kundu, A. & Karmakar, P. Floral ecology, floral visitors and breeding system of Gandharaj lemon (Citrus × limon L. Osbeck). Bot. Pac. J. Plant. Sci. Conserv.9, 113–119 (2020). [Google Scholar]
- 44.Talon, M., Zacarias, L. & Primo-Millo, E. Hormonal changes associated with fruit set and development in mandarins differing in their parthenocarpic ability. Physiol. Plant79, 400–406 (1990). [Google Scholar]
- 45.Gamito, L. M. & Malerbo-Souza, D. T. Visitantes florais e produção de frutos em cultura de laranja (Citrus sinensis L. Osbeck). Acta Sci. Anim. Sci.28, 483–488 (2006). [Google Scholar]
- 46.Ribeiro, G. S., Alves, E. & Carvalho, C. A. L. Biology of pollination of Citrus sinensis variety ‘pera rio’. Rev. Bras. Fruticult.39, e–033 (2016). [Google Scholar]
- 47.Abouzari, A. & Nezhad, N. M. The investigation of citrus fruit quality. Popular characteristic and breeding. Acta Univ. Agric. Silvicult. Mendelianae Brunensis64, 725–740 (2016). [Google Scholar]
- 48.Sykes, S. R. The effect on citrus fruit of excluding pollinating insects at flowering and implications for breeding new seedless cultivars. J. Hortic. Sci. Biotechnol.83, 713–718 (2008). [Google Scholar]
- 49.Gazzea, E., Batáry, P. & Marini, L. Global meta-analysis shows reduced quality of food crops under inadequate animal pollination. Nat. Commun.14, 4463 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez, E. E. Programa Nacional Frutales. Superficie Ocupada Por Plantaciones Frutales En El País Y Cambios En Su Estructura Productiva (Ediciones INTA, 2020).
- 51.FAOSTAT. Database Collection of the Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/#data (FAO).
- 52.Raveh, E. et al. Conventional breeding of cultivated citrus varieties. Citrus Genome33, 48 (2020). [Google Scholar]
- 53.Palacios, J. Citricultura (2005).
- 54.Agustí, M., García-Marí, F. & Guardiola, J. L. The influence of flowering intensity on the shedding of reproductive structures in sweet orange. Sci. Hortic.12, 343–352 (1982). [Google Scholar]
- 55.Bustan, A. & Goldschmidt, E. E. Estimating the cost of flowering in a grapefruit tree. Plant. Cell. Environ.21, 217–224 (1998). [Google Scholar]
- 56.Morrone, J. J. Biogeographical Regionalisation of the Neotropical Region (2014). [DOI] [PubMed]
- 57.Chacoff, N. P. & Aizen, M. A. Edge effects on flower-visiting insects in grapefruit plantations bordering premontane subtropical forest. J. Appl. Ecol.43, 18–27 (2006). [Google Scholar]
- 58.Greenleaf, S. S., Williams, N. M., Winfree, R. & Kremen, C. Bee foraging ranges and their relationship to body size. Oecologia153, 589–596 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Micheloud, N. G. Comportamiento fenológico—reproductivo de variedades de cítricos en la zona centro de la provincia de Santa Fé 141 (Facultad De Ciencias Agrarias, Universidad Nacional del Litoral, 2013).
- 60.R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (2023).
- 61.Goldenberg, L., Yaniv, Y., Porat, R. & Carmi, N. Mandarin fruit quality: a review. J. Sci. Food. Agric.98, 18–26 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Zenodo repository: 10.5281/zenodo.13315510.