Introduction
Acute generalized exanthematous pustulosis (AGEP) is a potentially severe cutaneous adverse reaction primarily linked to drug exposure. It is characterized by the rapid onset of edema and erythema followed by the development of numerous nonfollicular, sterile pustules, and desquamation upon resolution. AGEP sine pustules is an uncommon variant of AGEP in which the characteristic pustules are conspicuously absent.1
Semaglutide, a glucagon-like peptide-1 (GLP-1) receptor analog, is Food and Drug Administration (FDA) approved for the treatment of type II diabetes, chronic weight management, and cardiovascular risk reduction in certain at-risk patients.2 The most commonly reported adverse events are gastrointestinal, with less than 1% of patients reporting skin-related adverse events in clinical trials.3 To date, dermal hypersensitivity reactions and bullous pemphigoid induced by semaglutide have been reported.3,4 As semaglutide is a relatively novel medication, the full extent of its adverse effects is not yet fully appreciated. Here, we report a unique case of AGEP sine pustules attributed to semaglutide injections.
Case report
A 36-year-old woman presented in January 2024 with approximately a 2-month history of waxing and waning rash affecting her axillae and inframammary area. The rash was associated with burning pain but was minimally pruritic. She denied any systemic symptoms or oral lesions. Her medical history was significant for obesity (BMI 36.5 kg/m2) and nephrolithiasis but was otherwise unremarkable. Her medications included multivitamins, fish oil, and levonorgestrel IUD, none of which were new in the past year. Her only recent medication change was the initiation of weekly injections of compounded semaglutide (0.25-0.75 mg; Olympia Pharmaceuticals) in late October 2023. She otherwise denied recent changes in skincare products or detergents. She had visited urgent care on several occasions for the rash, where she was prescribed clotrimazole-betamethasone 1%/0.05% cream, ketoconazole 2% cream, hydrocortisone 2.5% cream, and fluconazole 150 mg with minimal benefit.
At the initial evaluation in our clinic, she exhibited ill-defined erythematous patches with minimal fine scale lacking vesicles, pustules, or satellite lesions in the axillary and inframammary folds (Fig 1). No lymphadenopathy or mucosal lesions were present. A potassium hydroxide examination was deferred due to recent treatment with antifungals. The differential diagnosis included contact dermatitis, symmetrical drug-related intertriginous and flexural exanthema, and erythema annulare centrifugum. She was started on hydrocortisone 2.5% ointment twice daily and a 21-day taper of prednisone 40 mg. Despite 10 days of treatment, the rash continued to spread down the abdomen, and the patient experienced ongoing burning pain. She was instructed to stop treatment in anticipation of a skin biopsy. Three weeks after her initial evaluation, she returned for a biopsy. During her visit, she reported missing 2 consecutive doses of semaglutide in the past 3 weeks. The rash began resolving after skipping these doses but reappeared rapidly 3 days after her most recent injection. Examination revealed annular erythematous patches with prominent trailing scale in the axillary and inframammary folds (Fig 2, A and B).
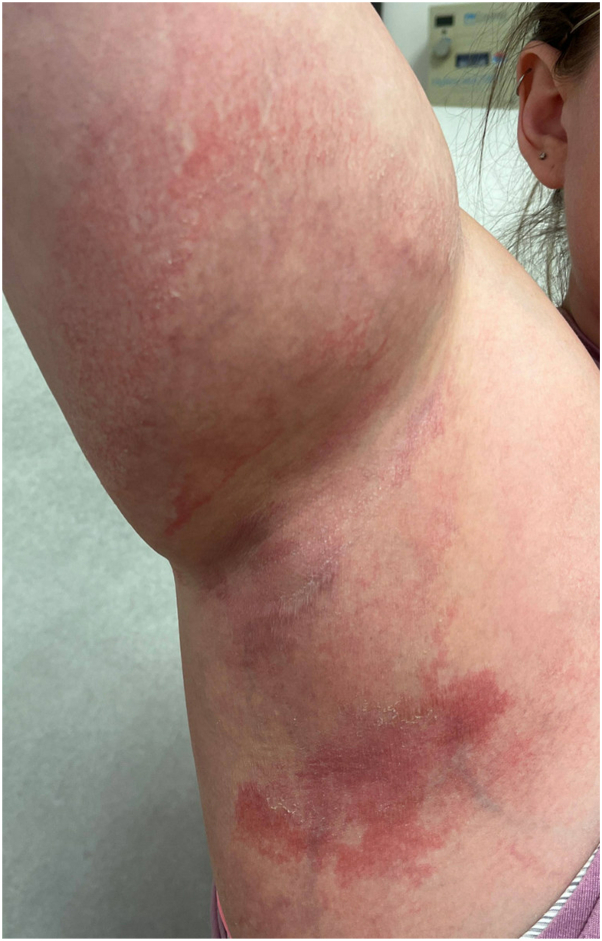
Fig 1.
Right axilla with ill-defined erythematous patches with minimal fine scale.
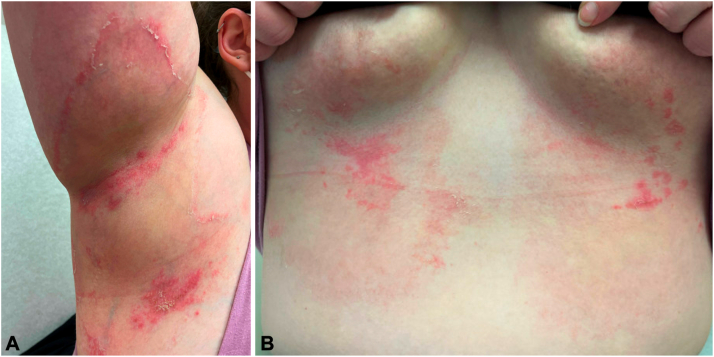
Fig 2.
Annular erythematous patches with prominent trailing scale in the (A) axillary and (B) inframammary folds.
A punch biopsy from the left axilla revealed a subcorneal pustular dermatosis with parakeratosis, mild acanthosis, mild spongiosis, and a lymphocytic and neutrophilic infiltrate in the superficial dermis (Fig 3). Periodic acid-Schiff stain was negative. Based on these findings, the differential diagnosis included AGEP, Sneddon–Wilkinson disease, and early evolving pustular psoriasis. Given the constellation of clinicopathologic findings and the rapid onset and resolution of the eruption following drug initiation and discontinuation, a diagnosis of AGEP sine pustules was favored. The Naranjo adverse drug reaction probability score was 9 and indicative of a definite adverse drug reaction.5 The patient had an AGEP validation score of 6, indicating probable AGEP, based on the EuroSCAR (European Severe Cutaneous Adverse Drug Reactions) diagnostic criteria.6 The patient’s semaglutide was discontinued, and the eruption cleared within 2 weeks. Patch testing to further verify the diagnosis of AGEP was considered but deferred due to the durable clearance of the eruption and patient preference.
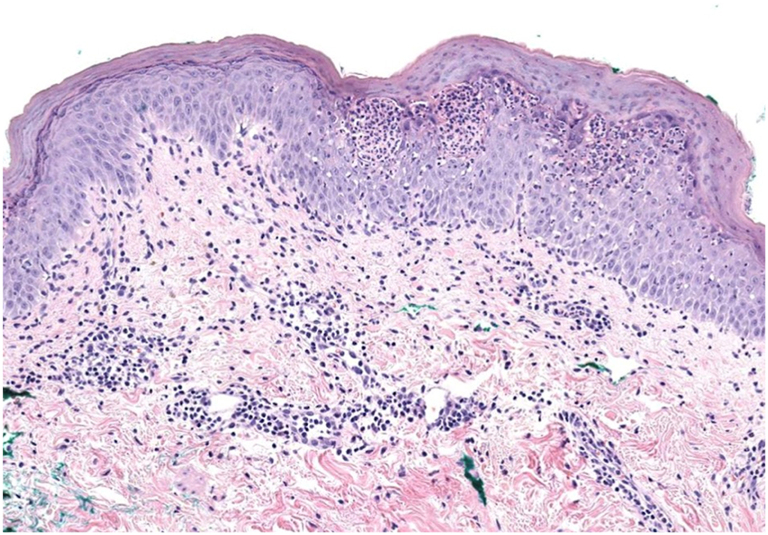
Fig 3.
Subcorneal pustular dermatosis with parakeratosis, mild acanthosis, mild spongiosis, and a lymphocytic and neutrophilic infiltrate in the superficial dermis.
Discussion
AGEP, a severe cutaneous adverse drug reaction, is frequently associated with various medications, including antibiotics, antifungals, hydroxychloroquine, and diltiazem.7 Typically, onset occurs within 1-11 days of drug exposure with flexural accentuation and may manifest with systemic symptoms.8 AGEP sine pustules can be a challenging diagnosis due to the absence of typical pustules and is likely under-recognized. However, rapid onset and resolution after drug discontinuation, along with characteristic histopathologic findings, support the diagnosis. Given the rarity of reported cases of AGEP sine pustules, the proportion of this variant among all AGEP cases remains unclear.1 While semaglutide has not previously been implicated in AGEP, 2 cases of dermal hypersensitivity reaction and a case of bullous pemphigoid have been reported to be attributed to semaglutide use.3,4
The pathophysiology of AGEP has long been considered to be a T cell-mediated process (type IV hypersensitivity) driven by IL-8-producing drug-specific T cells that recruit neutrophils. However, more recent evidence suggests that early activation of IL-36-producing monocytes/macrophages by drug exposure stimulates drug-specific T cells and may represent the initiating event.9 Atypical presentations of AGEP, such as AGEP sine pustules, may be influenced by patient-level differences in drug metabolism or immune system regulation, necessitating further research to clarify the underlying mechanisms. Regardless, prompt recognition of this entity is crucial to discontinue the culprit drug, given its potential to produce severe complications.1
Semaglutide, a GLP-1 agonist, is an antihyperglycemic agent that functions via stimulation of insulin secretion and inhibition of glucagon release from pancreatic islet cells in a glucose-dependent manner. Furthermore, GLP-1 agonists have the potential to assist with pronounced weight loss, leading to their increased use and popularity in recent years.3 As use has increased, so has the frequency of reported drug reactions. The FDA has cautioned against compounded semaglutide formulations, highlighting concerns about compounded salt forms such as semaglutide sodium and semaglutide acetate, which do not contain the same active ingredient as FDA approved semaglutide and lack demonstrated safety and efficacy.10 Although the compounded formulation used by our patient did not involve a salt form, it is crucial to maintain vigilance toward these emerging medications, particularly due to their increasing utilization, given that the complete range of potential adverse effects is not yet fully appreciated.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
Patient consent: The authors obtained written consent from patients for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available. Patient consent forms were not provided to the journal but are retained by the authors.
IRB approval status: Not applicable.
References
- 1.Svoboda S.A., Bisbee E.L., Bender N., Motaparthi K. Acute generalized exanthematous pustulosis sine pustules: a case series. JAAD Case Rep. 2022;23:24–26. doi: 10.1016/j.jdcr.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration . The U.S. Food and Drug Administration; 2024. FDA Approves First Treatment to Reduce Risk of Serious Heart Problems Specifically in Adults with Obesity or Overweight. [Google Scholar]
- 3.Ouellette S., Frias G., Shah R., Alamgir M., Wassef C. Dermal hypersensitivity reaction to semaglutide: two case reports. J Drugs Dermatol. 2023;22:413–416. doi: 10.36849/JDD.6550. [DOI] [PubMed] [Google Scholar]
- 4.Burruss C.P., Jones J.M., Burruss J.B. Semaglutide-associated bullous pemphigoid. JAAD Case Rep. 2021;15:107–109. doi: 10.1016/j.jdcr.2021.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naranjo C.A., Busto U., Sellers E.M., et al. Naranjo adverse drug reaction probability scale. Clin Pharm Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 6.De A., Das S., Sarda A., Pal D., Biswas P. Acute generalised exanthematous pustulosis: an update. Indian J Dermatol. 2018;63:22–29. doi: 10.4103/ijd.IJD_581_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szatkowski J., Schwartz R.A. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843–848. doi: 10.1016/j.jaad.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Sidoroff A., Dunant A., Viboud C., et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) Br J Dermatol. 2007;157:989–996. doi: 10.1111/j.1365-2133.2007.08156.x. [DOI] [PubMed] [Google Scholar]
- 9.Stadler P.C., Oschmann A., Kerl-French K., et al. Acute generalized exanthematous pustulosis: clinical characteristics, pathogenesis, and management. Dermatology. 2023;239:328–333. doi: 10.1159/000529218. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration Medications Containing Semaglutide Marketed for Type 2 Diabetes or Weight Loss. 2024. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/medications-containing-semaglutide-marketed-type-2-diabetes-or-weight-loss Accessed March 2024.