Abstract
Background/purpose
Platelet-rich fibrin (PRF) is a promising host-derived scaffold for regenerative endodontic treatment. This study investigated the effects of advanced PRF plus (A-PRF+) and injectable PRF (i-PRF) on the proliferation, migration, and differentiation of stem cells from apical papilla (SCAPs).
Materials and methods
A-PRF+ and i-PRF were prepared using a DUO Quattro centrifuge following a standard protocol. A-PRF+ and i-PRF extract were diluted in Dulbecco's modified Eagle's medium and Ham's F-12 medium (DMEM/F12) to produce the experimental culture medium. DMEM/F12 and DMEM/F12 supplemented with 10% foetal bovine serum (FBS) were used as the negative control (NC) and positive control (PC) media, respectively. The proliferative ability of SCAPs was assessed using a counting method (haemocytometer). The migration ability was examined using a scratch-wound assay. Alkaline phosphatase, bone sialoprotein, dentin matrix protein 1, and dentin sialophosphoprotein expression were measured to determine the differentiation ability.
Results
The proliferation, migration, and differentiation of SCAPs in the A-PRF+ group were similar to those of the PC group. In the i-PRF group, the cell number was significantly (p < 0.01) lower than that of the A-PRF+ group on days 8 and 10; the percentage of the scratched area on days 1 and 2 was significantly higher than in the A-PRF+ group (p < 0.05). The mRNA expression levels of biomarkers in the i-PRF group were similar to those in the A-PRF+ group.
Conclusion
Both A-PRF+ and i-PRF induce SCAPs proliferation, migration, and differentiation. However, A-PRF+ was superior in supporting the proliferation and migration of SCAPs.
Keywords: Platelet-rich fibrin, A-PRF+, i-PRF, Stem cells from apical papilla, Regenerative endodontics
Introduction
Regenerative endodontic treatment (RET) teeth requires three essential components: stem cells, growth factors (GFs), and biomaterial scaffolds.1 Among available scaffolds, host-derived products are of interest because of their biological advantages such as compatibility, degradability, and porosity. These features create a suitable microenvironment for stem cells from apical papilla (SCAPs), which develop and differentiate into a natural root.2
Blood clots (BC), platelet-rich plasma (PRP), and platelet-rich fibrin (PRF) have been used as host-derived scaffolds. Among these, PRF appears to be superior because of its significantly higher amount of GFs, various forms of use, and lack of additives. However, the failure rates of PRF were slightly higher than those of PRP in previous randomised clinical trials.3,4 Some authors have suggested that the high viscosity of PRF could prevent it from approaching the tooth apex and thus reduce the materials efficacy, especially in narrow- or multi-rooted teeth.3 Therefore, injectable PRF (i-PRF) may overcome the drawbacks of solid PRF.5 However, the number of platelets in i-PRF is smaller than in solid PRF; i-PRF could, therefore, be less effective.
To the best of our knowledge, the effect of i-PRF on SCAPs activity has not been investigated or compared with that of solid PRF. This in vitro study investigated the ability of advanced A-PRF plus (A-PRF+) and i-PRF to support the proliferation, migration, and differentiation of SCAPs.
Materials and methods
This study protocol was approved by the Medical Research Ethics Committee of the University of Medicine and Pharmacy at Ho Chi Minh City under No. 22131-ĐHYD (IRB-VN01002/IORG0008603/FWA00023448).
Culture media preparation
PRF preparation was performed at the Department of Oral Surgery. Whole blood was collected from three male donors (24–26 years). From each donor, a nurse withdrew 10 mL of venous blood into an A-PRF+ tube and another 10 mL into an i-PRF tube. These tubes were immediately centrifuged with a DUO Quattro centrifuge (Process for PRF, Nice, France). The machine was set centrifugation speed and time according to the standard protocol; at 1,300 rpm in 8 min for A-PRF+ tube; and at 700 rpm for 3 min for i-PRF.5,6 After centrifugation, the collected samples were stored on ice at 4 °C and delivered to the laboratory for the preparation of PRF media.
The PRF media were prepared using the spontaneous release method. The PRF kit was used to press A-PRF+ to remove exudate and collect the membrane. Each A-PRF+ membrane was, then, placed in falcon tubes containing 5 mL of Dulbecco's modified Eagle's medium and Ham's F-12 medium (DMEM/F12; Sigma–Aldrich, St. Louis, MO, USA). The tubes were incubated at 5% CO2, 37 °C for 72 h before collecting the A-PRF+ extract. Next, it was diluted in 35 mL DMEM/F12 to obtain the final A-PRF+ culture medium. To prepare i-PRF medium, sterile syringes were used to draw the top 1 mL of the yellowish layer of the i-PRF tubes after centrifugation. The collected i-PRF was diluted in 4 mL DMEM/F12 to obtain the final i-PRF culture medium. The final PRF culture media were filtered using a 0.2 μm filter to remove red blood cells. Finally, the culture media were prepared with the following concentrations: one A-PRF+ membrane in 40 mL of medium and 20% i-PRF. These concentrations were similar to those reported previously.7,8
All of following experiments were conducted with 4 media: A-PRF+, i-PRF, positive control (PC) medium: DMEM/F12 supplemented with 10% foetal bovine serum (DMEM/F12 + 10% FBS; Sigma–Aldrich, St. Louis, MO, USA), and negative control (NC) medium: DMEM/F12. SCAPs stem cells used in this study were provided by Laboratory of Tissue Engineering and Biomedical Materials, University of Science, Vietnam National University, Ho Chi Minh City.
Proliferation assay
A proliferation assay was performed by counting SCAPs using a haemocytometer. SCAPs were seeded into 96-well trays with 2 × 103 cells/well and cultured in different media. The culture medium was replaced every two days. Cell counts were determined on days 2 (D2), D4, D6, D8, and D10 using an optical microscope (Olympus, Tokyo, Japan).
30 μL of trypsin/EDTA (Sigma–Aldrich, St. Louis, MO, USA) was added to each culture well within 1 min and gently shaken to detach SCAPs. DMEM/F12 (30 μL) was added to neutralise trypsin/EDTA. Trypan blue (60 μL, 0.4%) was used to detect dead cells. The number of cells was determined using a haemocytometer under a microscope (100X magnification).
Migration assay
A cell migration assay was performed using the scratch-wound method. SCAPs were seeded into 6-well trays at the concentration 2 × 104 cells/well and cultured in DMEM/F12 + 10% FBS medium. When cell confluency reached 80–90%, the culture medium was replaced with DMEM/F12 and incubated for 24 h to starve the SCAPs. A 100 μL pipet was used to remove the monolayer cells. The debris was removed with 1 mL of phosphate-buffered solution (PBS; Thermo Fisher Scientific, Waltham, MA, USA) and the reference points were marked. The trays were photographed on D, D2, and D3. The images were assessed using ImageJ 1.53 software (University of Wisconsin, Madison, WI, USA) and the “wound_healing_size_tool” add-on.9
Differentiation assay
SCAPs were cultured in the osteo-/odontogenic induction medium for 14 days. Briefly, the culture medium was supplemented with 0.1 μM dexamethasone, 10 mM β-glycerophosphate, and 50 μg/mL ascorbic acid (Thermo Fisher Scientific, Waltham, MA, USA). The RNA of SCAPs was obtained using the TRIzol isolation method. The obtained RNA was then reverse-transcribed using a SensiFAST™ cDNA synthesis kit (Meridian Bioscience, Cincinnati, OH, USA). The relative mRNA expression levels of alkaline phosphatase (ALP), bone sialoprotein (BSP), dentin matrix protein 1 (DMP-1), and dentin sialophosphoprotein (DSPP) were analysed using the SensiFAST™ HRM kit (Meridian Bioscience, Cincinnati, OH, USA) with the primer sequences listed in Table 1. Agarose gel electrophoresis (AGE) and real-time quantitative polymerase chain reaction (RT-qPCR) for ALP, BSP, DMP-1, and DSPP expression were performed on D7 and D14 to assess the osteo-/odontogenic differentiation potential of SCAPs.
Table 1.
Primer sequences used in quantitative real-time polymerase chain reactions.
| Gene | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| Alkaline phosphatase | 5′-GTGAACCGCAACTGGTACTC-3′ | 3′-GAGCTGCGTAGCGATGTCC-5′ |
| Bone sialoprotein | 5′-CACTGGAGCCAATGCAGAAGA-3′ | 3′-TGGTGGGGTTGTAGGTTCAAA-5′ |
| Dentin matrix protein 1 | 5′-CTCCGAGTTGGACGATGAGG-3′ | 3′-TCATGCCTGCACTGTTCATTC-5′ |
| Dentin sialophosphoprotein | 5′-TTTGGGCAGTAGCATGGGC-3′ | 3′-CCATCTTGGGTATTCTCTTGCCT-5′ |
Statistical analysis
Each experiment was performed in triplicate. Data were analysed using SPSS 26.0 (IBM, Armonk, NY, USA). Data are presented as mean values ± standard deviation. The Kruskal–Wallis H-test was used to compare differences between groups. If the difference was significant, Dunn's test was used to determine which group differed. Statistical significance was set at P < 0.05.
Results
Proliferation assay
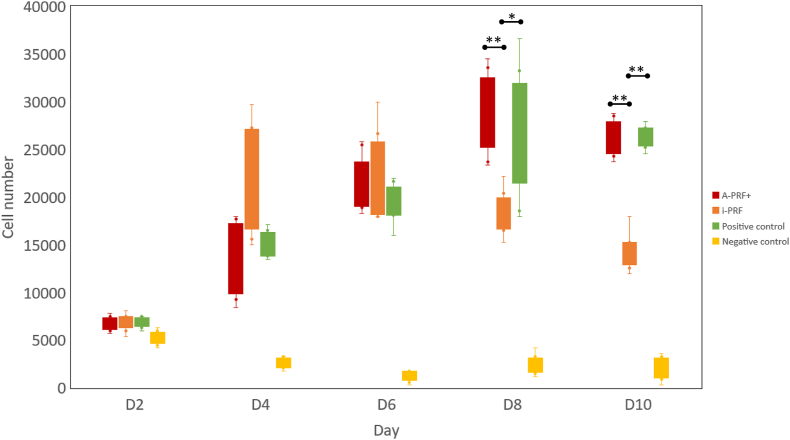
Fig. 1 shows the number of cells in the four groups on D2 to D10. The number of cells in the i-PRF group increased from D2 to D4, no change from D4 to D6, and decreased from D6 to D10. In the A-PRF+ group, the cell number steadily increased during D2 to D8 and decreased slightly on D10. The cell proliferation of the PC group was comparable to that of the A-PRF+ group. The number of SCAPs in the i-PRF group was significantly lower than that of the A-PRF+ group on D8 and D10. The number of cells in the NC group was significantly lower than in all remaining groups from D4 to D10 (P < 0.001).
Figure 1.
Proliferation assay of stem cells from apical papilla cultured in four media from days 2 to 10. The results are presented as mean ± standard deviation. (A-PRF+, advanced platelet-rich fibrin plus; i-PRF, injectable platelet-rich fibrin; D, day; ∗P < 0.05, ∗∗P < 0.01).
Migration assay
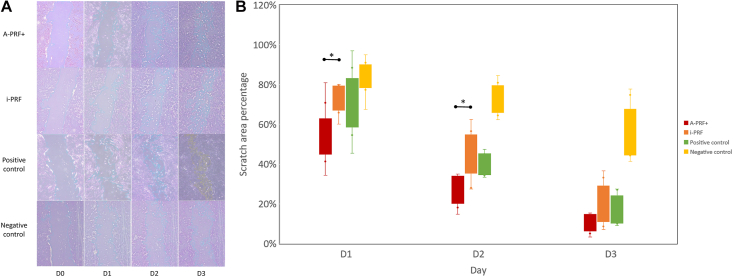
Fig. 2 shows the change in the scratch area as a percentage of the four media. The scratch area significantly reduced during D1 to D3 in all media. However, the scratch area percentage of A-PRF+ was significantly lower than that of i-PRF on D1 and D2 (P < 0.05). The scratch area percentage of NC group was significantly higher than that of A-PRF+ during D1 to D3, and other two groups on D2 and D3 (P < 0.05).
Figure 2.
A. The images of scratch-wound method for measuring the scratch area percentage. B. The scratch area percentage of stem cells from apical papilla cultured in four media from day 1 to 3. Note. A-PRF+, advanced platelet-rich fibrin plus; i-PRF, injectable platelet-rich fibrin; D, day; ∗P < 0.05.
Differentiation assay
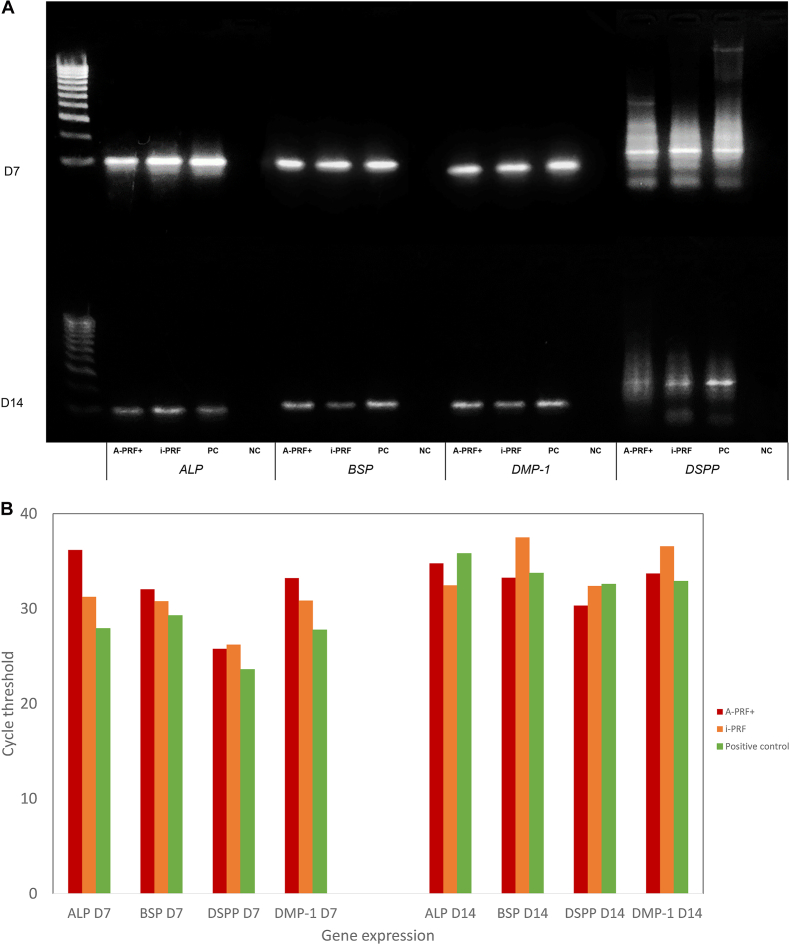
Fig. 3 shows the results of AGE and RT-qPCR for ALP, BSP, DMP-1, and DSPP on D7 and D14 after exposure to osteo-/odontogenic induction media. SCAPs that were previously cultured in A-PRF+, i-PRF, and PC media expressed a similar differentiation potential to the osteo-/odontogenic media.
Figure 3.
A. The agarose-electrophoresis results of stem cells from apical papilla cultured in four media. B. mRNA expression of osteo-/odontogenic biomarkers of stem cells from apical papilla after differentiation. Note. A-PRF+, advanced platelet-rich fibrin plus; i-PRF, injectable platelet-rich fibrin; D, day; PC, positive control; NC, negative control; ALP, alkaline phosphatase; BSP, bone sialoprotein; DMP-1, dentin matrix protein 1; DSPP, dentin sialophosphoprotein.
Discussion
PRF is of interest in RET due to their outstanding biocompatibility and cellular components.1,2 Previous studies have reported the positive effect of PRF on SCAPs.10,11 However, none of them compared the effects of the most advanced products of PRF, including A-PRF+ and i-PRF. This study showed that these PRFs could induce SCAPs proliferation and migration as compared to DMEM/F12 + 10% FBS, which is considered the gold standard for culturing MSCs. Both PRFs are able to induce SCAPs differentiation to osteo-/odontogenic media. Additionally, A-PRF+ was superior to i-PRF in inducing the proliferation and migration of SCAPs.
More than 95% of the blood cells in PRF are platelets, which are critical for wound healing. The secretion of alpha granules by platelets provides numerous GFs that induce regeneration.12 Among several PRF generations, A-PRF+ and i-PRF are the most advanced solid and liquid one, respectively.6 To extract GFs from the PRF, we used a spontaneous release method with minor modifications.13,14 The authors increased the immersion time to 72 h to collect GFs that are released slowly after the first 24 h.6
As a member of MSC family, SCAPs activity is also regulated by numerous GFs which are released by platelets.15 The activated platelets release hundreds of GFs, chemokines, and other products.16 Only a few GFs have been investigated for their effects on MSC activity, and the role of other secreted particles remains elusive.15,17 The effect of a single GF on cell activity is complicated and may have biphasic dose-dependent characteristics, such as insulin-like growth factor 2.18 Therefore, the impact of PRF media on SCAPs activity is a comprehensive result of platelet quantity, PRF nature, and dilution ratio. These discrepancies may be the main reason for the different results of SCAPs proliferation, migration, and differentiation between the two PRF groups in this study.
Compared with the i-PRF medium, the proliferation of SCAPs cultured in A-PRF+ medium reached its peak later, but the maximum number of SCAPs was significantly higher and comparable to the PC group. Conversely, previous studies have reported a superior effect of PRF media on SCAPs differentiation in comparison with PC media.7,10 However, it should be noted that the PRF medium was prepared by adding the PRF extract to the culture medium which was already supplemented with FBS. This preparation method makes it difficult to determine the efficacy of PRF. Using PRF medium without FBS, a previous study on PDLSCs showed a similar proliferation curve between the PC and PRF medium groups.13 That is, A-PRF+ medium induced SCAPs proliferation at a rate comparable to the PC medium. The effect of i-PRF was less pronounced than that of A-PRF+.
The results of this study demonstrated the positive effect of A-PRF+ and i-PRF media on SCAPs migration. From D1 to D3, the scratch percentage of the A-PRF+ medium group was consistently lower than that of the i-PRF and control medium groups. As SCAPs were starved before the migration experiment and the proliferation in A-PRF+ and i-PRF media was comparable in the first two days, the reduction in the scratch area mostly occurred due to cell migration activity. Further, SCAPs cultured in the A-PRF+ medium migrated more prolifically than the control groups and those cultured in the i-PRF medium. Previous studies have reported that SCAPs cultured in PRF medium can migrate significantly better than control cells.7,10 As mentioned above, different procedures for medium preparation may lead to this discrepancy. When using PRF to replace FBS in the culture medium, it has been reported that the effects on PDLSC migration are comparable.13 Within the first 72 h, SCAPs cultured in A-PRF+ media showed improved migration capacity compared to those cultured in i-PRF media. The rate of SCAPS migration in i-PRF media was comparable to the PC media.
ALP, BSP, DSPP, and DMP-1 are biomarkers of osteo-and odontogenic differentiation. ALP and BSP are crucial enzymes that participate in the initial formation of mineralised tissues, such as enamel, cementum, dentin, and alveolar bone.19,20 Subsequently, DSPP and BMP-1 complement these tissues to adapt to the physiological functions of human dentition.21,22 AGE images showed that all differentiation biomarkers were expressed in both A-PRF+ and i-PRF media on D7 and D14. In addition, RT-qPCR revealed that the expression levels of all biomarkers in both PRF media were comparable to those of the PC group. Although previous studies showed that PRF improved osteo-odontogenic differentiation in SCAPs compared to a control medium, these results should be interpreted carefully.7,10 The PRF medium used in these studies was also supplemented with FBS; therefore, the higher biomarker expression could be the result of the cumulative impact of FBS and PRF. In another study on PDLSCs that used PRF as the sole factor, ALP and BSP expression was significantly lower than the control group at D7. However, the difference became insignificant on D14.23 Notably, as dental stem cells derived from different niches express various proliferation and differentiation levels during the first week and became more homogenous in the second week, comparisons at D14 are more reliable.24,25 Thus, both PRF media induced osteo-/odontogenic differentiation in SCAPs.
This study was conducted using a standard protocol used to assess MSCs proliferation, migration, and differentiation. Among the numerous methods used to count cell numbers, using a haemocytometer is presently considered the gold standard with affordability and versatility.26 For migration evaluation, a scratch assay was conducted because of its convenience, simplicity and suitability.27 The differentiation was assayed using AGE and RT-qPCR was used to quantify the expression of osteo-/odontogenic biomarkers which has been used in previous studies.28 However, this study has a few limitations that should be considered. First, both PRFs should be examined prior to determine the best dilution ratio for supporting SCAPs activity. Although the authors reviewed the medical literature for culture medium preparation, different types of PRF and targeted stem cells might result in up- or downregulation of selective genes. Second, the differentiation experiment did not examine the expression of glyceraldehyde 3-phosphate dehydrogenase. This deficiency can weaken the differentiation result although this was conducted by Laboratory of Tissue Engineering and Biomedical Materials, University of Science, Vietnam National University, Ho Chi Minh City. Finally, to continue closure of the tooth apex, stem cells must differentiate into other tissues such as vascular or neurogenic cells. Therefore, future studies should examine the induction of PRF differentiation under other conditions using appropriate biomarkers to provide a more comprehensive understanding.
Within the limitations of this study, both A-PRF+ and i-PRF induced SCAPs proliferation, migration, and osteo-/odontogenic differentiation. A-PRF+ was found to be superior in supporting SCAPs proliferation and migration. These results showed that both PRFs are potential biomaterials for further research in RET.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
The authors sincerely thank the participants who volunteered to participate in this study.
References
- 1.Liu H., Lu J., Jiang Q., et al. Biomaterial scaffolds for clinical procedures in endodontic regeneration. Bioact Mater. 2022;12:257–277. doi: 10.1016/j.bioactmat.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raddall G., Mello I., Leung B.M. Biomaterials and scaffold design strategies for regenerative endodontic therapy. Front Bioeng Biotechnol. 2019;7:317. doi: 10.3389/fbioe.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shivashankar V.Y., Johns D.A., Maroli R.K., et al. Comparison of the effect of PRP, PRF and induced bleeding in the revascularization of teeth with necrotic pulp and open apex: a triple blind randomized clinical trial. J Clin Diagn Res. 2017;11:Zc34–Zc39. doi: 10.7860/JCDR/2017/22352.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulusoy A.T., Turedi I., Cimen M., Cehreli Z.C. Evaluation of blood clot, platelet-rich plasma, platelet-rich fibrin, and platelet pellet as scaffolds in regenerative endodontic treatment: a prospective randomized trial. J Endod. 2019;45:560–566. doi: 10.1016/j.joen.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Liang Y., Ma R., Chen L., et al. Efficacy of i-PRF in regenerative endodontics therapy for mature permanent teeth with pulp necrosis: study protocol for a multicentre randomised controlled trial. Trials. 2021;22:436. doi: 10.1186/s13063-021-05401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujioka-Kobayashi M., Miron R.J., Hernandez M., Kandalam U., Zhang Y., Choukroun J. Optimized platelet-rich fibrin with the low-speed concept: growth factor release, biocompatibility, and cellular response. J Periodontol. 2017;88:112–121. doi: 10.1902/jop.2016.160443. [DOI] [PubMed] [Google Scholar]
- 7.Bi J., Liu Y., Liu X.M., Lei S., Chen X. Platelet-rich fibrin improves the osteo-/odontogenic differentiation of stem cells from apical papilla via the extracellular signal-regulated protein kinase signaling pathway. J Endod. 2020;46:648–654. doi: 10.1016/j.joen.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Thanasrisuebwong P., Kiattavorncharoen S., Surarit R., Phruksaniyom C., Ruangsawasdi N. Red and yellow injectable platelet-rich fibrin demonstrated differential effects on periodontal ligament stem cell proliferation, migration, and osteogenic differentiation. Int J Mol Sci. 2020;21:5153. doi: 10.3390/ijms21145153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suarez-Arnedo A., Torres Figueroa F., Clavijo C., Arbeláez P., Cruz J.C., Muñoz-Camargo C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS One. 2020;15 doi: 10.1371/journal.pone.0232565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong S., Chen W., Jiang B. A comparative evaluation of concentrated growth factor and platelet-rich fibrin on the proliferation, migration, and differentiation of human stem cells of the apical papilla. J Endod. 2018;44:977–983. doi: 10.1016/j.joen.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Sequeira D.B., Oliveira A.R., Seabra C.M., et al. Regeneration of pulp-dentin complex using human stem cells of the apical papilla: in vivo interaction with two bioactive materials. Clin Oral Invest. 2021;25:5317–5329. doi: 10.1007/s00784-021-03840-9. [DOI] [PubMed] [Google Scholar]
- 12.Fujioka-Kobayashi M., Miron R.J., editors. Platelet rich fibrin in regenerative dentistry: biological background and clinical indications. John Wiley & Sons; New Jersey: 2017. [Google Scholar]
- 13.Ji B., Sheng L., Chen G., et al. The combination use of platelet-rich fibrin and treated dentin matrix for tooth root regeneration by cell homing. Tissue Eng Part A. 2015;21:26–34. doi: 10.1089/ten.tea.2014.0043. [DOI] [PubMed] [Google Scholar]
- 14.Clipet F., Tricot S., Alno N., et al. In vitro effects of Choukroun's platelet-rich fibrin conditioned medium on 3 different cell lines implicated in dental implantology. Implant Dent. 2012;21:51–56. doi: 10.1097/ID.0b013e31822b9cb4. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues M., Griffith L.G., Wells A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 2010;1:32. doi: 10.1186/scrt32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaumenhaft R., Sharda A. In: Platelets. 4th ed. Michelson A.D., editor. Academic Press; Massachusetts: 2019. Platelet secretion; pp. 349–370. [Google Scholar]
- 17.Fu X., Liu G., Halim A., Ju Y., Luo Q., Song A.G. Mesenchymal stem cell migration and tissue repair. Cells. 2019;8:784. doi: 10.3390/cells8080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diao S., Yang H., Cao Y., Yang D., Fan Z. IGF2 enhanced the osteo-/dentinogenic and neurogenic differentiation potentials of stem cells from apical papilla. J Oral Rehabil. 2020;47(Suppl 1):S55–S65. doi: 10.1111/joor.12859. [DOI] [PubMed] [Google Scholar]
- 19.Chen J., Sasaguri K., Sodek J., Aufdemorte T.B., Jiang H., Thomas H.F. Enamel epithelium expresses bone sialoprotein (BSP) Eur J Oral Sci. 1998;106(Suppl 1):S331–S336. doi: 10.1111/j.1600-0722.1998.tb02194.x. [DOI] [PubMed] [Google Scholar]
- 20.Haniastuti T., Susilowati H., Rinastiti M. Viability and alkaline phosphatase activity of human dental pulp cells after exposure to yellowfin tuna bone-derived hydroxyapatite in vitro. Int J Dent. 2020;2020 doi: 10.1155/2020/8857534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye L., MacDougall M., Zhang S., et al. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004;279:19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- 22.Lim D., Wu K.C., Lee A., Saunders T.L., Ritchie H.H. DSPP dosage affects tooth development and dentin mineralization. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y.H., Zhang M., Liu N.X., et al. The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. Biomaterials. 2013;34:5506–5520. doi: 10.1016/j.biomaterials.2013.03.079. [DOI] [PubMed] [Google Scholar]
- 24.Kotova A.V., Lobov A.A., Dombrovskaya J.A., et al. Comparative analysis of dental pulp and periodontal stem cells: differences in morphology, functionality, osteogenic differentiation and proteome. Biomedicines. 2021;9:1606. doi: 10.3390/biomedicines9111606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakopoulou A., Leyhausen G., Volk J., et al. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP) Arch Oral Biol. 2011;56:709–721. doi: 10.1016/j.archoralbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Cadena-Herrera D., Esparza-De Lara J.E., Ramírez-Ibañez N.D., et al. Validation of three viable-cell counting methods: manual, semi-automated, and automated. Biotechnol Rep (Amst) 2015;7:9–16. doi: 10.1016/j.btre.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moutasim K.A., Nystrom M.L., Thomas G.J. In: Cancer cell culture: methods and protocols. 2nd ed. Cree I.A., editor. Humana Press; New Jersey: 2011. Cell migration and invasion assays; pp. 333–343. [Google Scholar]
- 28.Nada O.A., El Backly R.M. Stem cells from the apical papilla (SCAP) as a tool for endogenous tissue regeneration. Front Bioeng Biotechnol. 2018;6:103. doi: 10.3389/fbioe.2018.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]