Abstract
Background/purpose
The incidence of human papillomavirus (HPV)-related oropharyngeal cancer (OPC) is increasing worldwide. HPV vaccines have shown efficacy in preventing diseases in both males and females. Therefore, there is a need to develop cost-effective strategies for HPV vaccines to prevent HPV-related OPC. This meta-analysis aimed to evaluate cost-effectiveness using the global mean of incremental cost-effectiveness ratios compared to the willingness-to-pay threshold and incremental net benefits (INBs) of HPV vaccination strategies between boys’ extension vaccine and girls only. These recommendations will be useful for countries that have not implemented universal HPV vaccines in national programs, such as Taiwan.
Materials and methods
Studies evaluating the cost-effectiveness of HPV vaccination strategies in the prevention of OPC that included both sexes versus girls only were identified through the Cochrane Library, EMBASE, PubMed, ScienceDirect, and Web of Science databases on February 05, 2024, and a meta-analysis of pooled INBs was performed using a random-effects model. The outcome was an effective measurement of the OPC burden. The results are represented in USD (2024).
Results
Fifteen model analyses were included. All the studies were conducted in high-income countries. The global mean of incremental cost-effectiveness ratio was $39,553 (95% CI, $27,008–66,641) per quality-adjusted life years gained, which was below the global mean of the willingness-to-pay threshold of $65,473 (95% CI, $52,138–83,755). Pooled INBs of $9370 (95% CI, $5046–13,695; P < 0.001) favored the extended HPV in boys.
Conclusion
HPV vaccination strategies that include boys are cost-effective compared to those with girls only in preventing OPC burden. By implementing a universal HPV vaccination program, countries can receive $9370 in additional monetary benefits per patient. Given its relevance to high-income countries, this study offers key insights that can aid policymakers in Taiwan.
Keywords: Meta-analysis, Oropharyngeal cancer, Incremental cost-effectiveness ratio, Incremental net benefit
Introduction
Oropharyngeal cancer (OPC) incidence has been increasing over the past few decades globally, including in Taiwan.1,2 The incidence of OPC is expected to increase in the upcoming decades due to changes in its risk factors, notably human papillomavirus (HPV) infection.3 Globally, 33% of OPC is attributable to HPV infection in 2021. The HPV-attributed OPC varies between countries; it is 13–17% in Canada, Brazil, the United Kingdom, the Netherlands, Estonia, Slovakia, and Poland; 29% in Taiwan; 38–56% in Australia, Japan, France, and Switzerland; and 73% in the United States.2,4, 5, 6, 7 Without any screening methods for early OPC detection,8 its prevention solely relies on large-scale vaccination programs.
Over the last two decades, HPV vaccine programs for girls have demonstrated efficacy in reducing the incidence of cervical cancer.9,10 Although Taiwan has made strides in implementing HPV vaccination programs for cervical cancer prevention,11 similar benefits against OPC remain underexplored despite the high treatment costs of OPC. In Taiwan during 2007–2017, OPC cost USD 56,501 in direct and indirect medical costs annually.12 The study analyzed 50,784 patients, including 91.4% males; furthermore, the mean (standard deviation, SD) age at diagnosis was 55.0 (11.9) years with death at 58.5 (12.8) years.12 Understanding the potential benefits of HPV vaccination against OPC in Taiwan is crucial for optimizing public health strategies and resource allocation, especially medical costs.13
Studies have demonstrated the potential utility of HPV vaccines in preventing OPC in both males and females as they target the commonest oral high-risk HPV serotypes—HPV16 and 18.14,15 Although more than 90% of oral HPV infections are sexually acquired, HPV infections are commonly asymptomatic, thus resulting in persistent oral infection acting as the primary cause of OPC.16 Furthermore, despite the differences in the hormonal and immunological systems and sexual behaviors between the sexes, the incidence of HPV-related OPC is disproportionately higher in males.17 To address this issue and based on the herd immunity achieved through universal HPV vaccination of girls, certain countries have extended the HPV vaccine to boys.18,19 However, certain countries may be awaiting cost-effectiveness analyses before extending the HPV vaccine to boys.20
Most economic evaluations are presented as incremental cost-effectiveness ratios (ICERs), which are related to the effective monetary measures gained due to health strategies. ICERs below the willingness-to-pay (WTP) threshold indicate that the health strategy is cost-effective. However, the interpretation of negative ICER values may be ambiguous; they may indicate lower costs relative to higher effectiveness or higher costs compared with lower effectiveness of interventions. To overcome this limitation, incremental net benefits (INBs) are calculated by including WTP in the analysis along with effectiveness and cost differences; positive INBs indicate cost-effectiveness.21
The major strength of this study was the evaluation of a broader societal perspective regarding the cost-effectiveness of HPV vaccines. This meta-analysis aimed to evaluate the cost-effectiveness of the global mean ICERs versus the WTP threshold and pooled INBs of HPV vaccination strategies that include boys versus those that include girls only. Studies have not investigated the global estimations; therefore, a systematic review of published economic evaluation studies is warranted. Considering the lack of published economic evaluations of this issue in Taiwan and restrictions on data utility, this study further emphasized the role of universal HPV vaccination in mitigating the OPC burden in Taiwan.
Materials and methods
Search strategy
The research questions were designed according to the population, intervention, comparator, and outcome (PICO) framework. The population was defined as a health-economic analytical model covering all age groups. Interventions were defined as either boys or girls or sex-neutral HPV vaccination programs. The comparator was defined as girls only HPV vaccination programs. Outcomes were defined as the incremental cost-effectiveness ratio per health outcome metric for OPC. The Boolean operators ″AND″ and ″OR″ were utilized for search terms: cost-effectiveness analysis, HPV vaccine, and OPC in various combinations. We searched for relevant papers published until February 05, 2024, in the Cochrane Library, EMBASE, PubMed, Science Direct, and Web of Science databases. The included studies were limited to original research and review articles. Other types of publications, such as conference papers or letters to the editor, were excluded. This study was reported according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines.22
Study selection
Two researchers (APP and SFC) independently identified papers through databases using designated search terms, screened the retrieved studies, and assessed the full-text eligibility based on the inclusion and exclusion criteria for the meta-analysis. In the initial stage, reference manager software Endnote v.X9 (The EndNote Team, Clarivate, PA, USA) was used to delete duplicate studies before screening. Eligible studies were selected if they met the PICO inclusion criterion. The exclusion criteria were as follows: (1) studies lacking ICERs, (2) studies lacking designated cancer outcomes, and (3) studies that did not compare the universal HPV vaccine or sex-neutral HPV vaccination with girls-only vaccination. The agreement rate in screening based on title and abstract was 98%, and that based on full-text review was 92%. Disagreements between the researchers were resolved by consensus.
Data extraction and harmonization
The extracted data included the country, author name, type of vaccine, number of doses, age at vaccination, perspective, type of model, time horizon, discount rate of cost and effectiveness, funding for the study, vaccine efficacy in men and women, vaccine coverage in men and women, duration of protection, and currency year. From individual studies, we extracted ICERs along with their 95% confidence intervals (CIs) or, if unavailable, gathered incremental cost and effectiveness data comparing sex-neutral HPV vaccination with girls-only HPV vaccination from the cost-effectiveness plane.23 We collected the WTP threshold for each study for the corresponding year. Given that the conclusions of the effectiveness do not depend on the outcome chosen in most of the cases, i.e., the cost per life year gained and the cost per quality-adjusted life year gained, we combined them into a single outcome of effectiveness.21
We standardized currencies (€, £, $, and C$) and years by converting to purchasing power parity and consumer price index adjusted to the 2024 USD.21 For instance, Breese et al.24 reported cost, ICERs, and WTP thresholds in 2010 Euros, which were converted to 2010 USD using the historical purchasing power parity of Austria. Subsequently, the 2010 USD was converted to a consumer price index-adjusted 2024 USD.
Statistical analysis
After currency conversions for ICERs, costs, and threshold, the INBs can be further estimated for each study using the formula ΔE × K − ΔC, where ΔE represents the difference in the effectiveness, K indicates the threshold, and ΔC indicates the difference in the costs.21 Where only ΔE is provided and ΔC is not reported, we used the ICERs equation to derive the value of ΔC as ICERs equal ΔC/ΔE.21 Following the availability of 95% CI, we calculated the standard error of INBs using the formula (upper limit – lower limit)/3.92.21
We pooled INBs across studies using a random-effects model chosen to account for heterogeneity among studies and reflect real differences in populations, interventions, or methods.25 This model also considers between-study variability, providing more realistic and generalizable estimates.25 The I-squared (I2) test was used to assess the effect of heterogeneity between studies. We used the I2 >70% defined as high heterogeneity.26 The I2 test quantifies the proportion of total variation in effect estimates that is due to heterogeneity rather than sampling error, offering a clear measure of inconsistency across studies.26
We further performed subgroup analyses according to vaccine type, vaccine doses, age at vaccination, perspective, vaccine coverage, funding source, study year, and geographical region. Subgroup analyses were performed based on the most sensitive parameters from each study. Furthermore, meta-regression was used to individually fit each potential source of heterogeneity.
Sensitivity analysis was performed to determine the consistency of the pooled INBs between the main and leave-one-out models as an alternative model for meta-analysis.27 Statistical analyses were performed using Stata v17.0 (StataCorp, College Station, TX, USA). The results were deemed statistically significant at P < 0.05 (two-tailed).
Risk of bias assessment
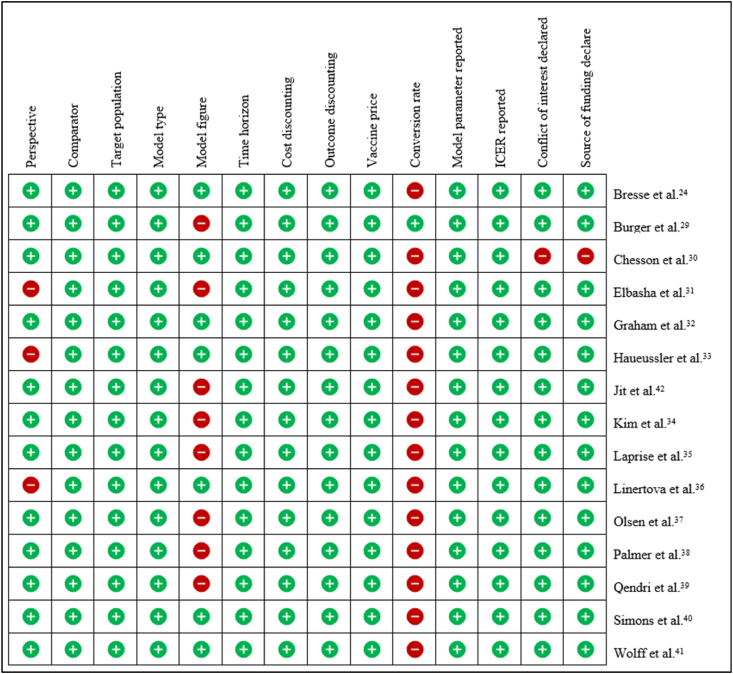
The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist28 was used to evaluate the risk of bias. Our assessment was conducted using the following criteria: study perspective, description of the comparator, time horizon, explanation of discounting of cost and outcome, depiction of the model with accompanying figures, clear delineation of the study population, reporting of ICERs and their units, sensitivity analysis, disclosure of funding sources, and any conflicts of interest. APP and SFC assessed the quality of the studies and discussed discrepancies until a consensus was reached.
Results
Study selection process and characteristics of the study
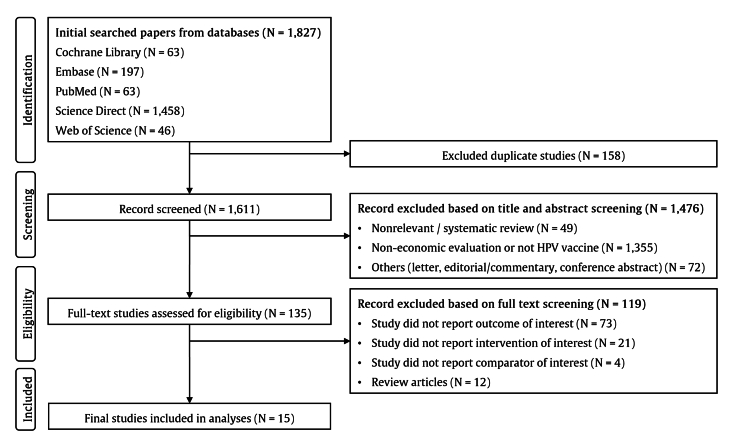
We identified 1827 records from the five databases. After 158 duplicate studies were deleted, 1611 studies were screened for titles and abstracts. The process excluded 1476 studies, thus leaving 135 articles for eligibility assessment. Subsequently, we excluded 119 additional studies, resulting in 15 studies included in the analyses (Fig. 1).
Figure 1.
Flowchart depicting the study selection process. Abbreviations: N = number of studies; HPV = human papillomavirus.
All the studies were conducted in high-income countries. A 2-dose HPV vaccine was used in seven studies, and a 3-dose vaccine was used in eight studies. Majority of studies (73%) used 4-valent HPV vaccine type. Age at vaccine ranged between 9 and 26. The time horizon used in studies is mostly lifetime or 100 years (80%). Across 15 studies, the three perspectives (payer, societal, and healthcare provider) distributed similarly. The median discount rates for costs and outcomes were 3.0% (SD = 0.9%). The vaccine efficacy for preventing persistent HPV infection that leads to OPC in male ranged between 76% and 100%. Vaccine coverage for boys and vaccine price per dose were assumed to be the same as girls-only HPV vaccine program in most studies (Table 1).
Table 1.
General characteristics of studies.
| Author | Country | Geographical region | Income level | Vaccine type; dose | Age at vaccine | Perspective | Type of model | Time horizon | Discount rate, % | Study funder | Vaccine efficacy, % | Vaccine coverage, % | Duration of protection, year | Sensitivity analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bresse et al.24 | Austria | Western Europe | High-income | 4v; 3 | 9 | Payer | DTM | 100 | 3.0 | Pharmacy | 78–96 | 65 | Lifelong | One-way |
| Burger et al.29 | Norway | Northern Europe | High-income | 4v; 3 | 12 | Societal | DTM | Lifetime | 3.0 | Non-pharmacy | 90 | 71 | 20 | One- and multi-way |
| Chesson et al.30 | US | Northern America | High-income | 4v; 3 | 12 | Societal | DTM | 100 | 3.0 | Non-pharmacy | 95 | 20–75 | Lifelong | One way, PSA |
| Elbasha et al.31 | US | Northern America | High-income | 4v; 3 | 9–26 | NS | DTM | 100 | 3.0 | Pharmacy | 76–96 | 50–90 | Lifelong | Multiway |
| Graham et al.32 | Canada | Northern America | High-income | 4v; 3 | 12 | HCP | DTM | Lifetime | 5.0 | Non-pharmacy | 99 | 70 | NS | One-way |
| Haeussler et al.33 | Italy | Southern Europe | High-income | 4v; 3 | 12 | NS | DTM | 55 | 3.0 | Pharmacy | 50 | 90 | Lifelong | PSA |
| Jit et al.42 | UK | Northern Europe | High-income | 4v; 2 | 12 | HCP | DTM | 100 | 3.5 | Pharmacy | 100 | 80 | 20 | One-way |
| Kim et al.34 | US | Northern America | High-income | 4v; 3 | 12 | Societal | DTM | 100 | 3.0 | Pharmacy | 90 | 75 | Lifelong | One-way |
| Laprise et al.35 | Canada | Northern America | High-income | 4v; 2 | 12 | Payer | DTM | 70 | 3.0 | Pharmacy | 95 | 80 | 20 | One- and multi-way |
| Linertova et al.36 | Spain | Southern Europe | High-income | 9v; 2 | 12 | NS | DTM | Lifetime | 3.0 | Non-pharmacy | 78.5 | 87.7 | Lifelong | PSA |
| Olsen et al.37 | Denmark | Northern Europe | High-income | 4v; 3 | 12 | HCP | DTM | 62 | 3.0 | Pharmacy | 100 | 85 | Lifelong | One-way, PSA |
| Palmer et al.38 | Japan | Asia | High-income | 4v; 2 | 12–16 | Payer | DTM | 100 | 2.5 | Pharmacy | 90.2 | 15 | 20 | One-way |
| Qendri et al.39 | Austria | Western Europe | High-income | 9v; 2 | 9–12 | Payer | DTM | 100 | 3.0 | Non-pharmacy | 98.0 | 60 | Lifelong | PSA |
| Belgium | Western Europe | High-income | 9v; 2 | 12–14 | Payer | DTM | 100 | 2.3 | Non-pharmacy | 98.0 | 70 | Lifelong | PSA | |
| Croatia | Central and Eastern Europe | High-income | 9v; 2 | 14 | Payer | DTM | 100 | 5.0 | Non-pharmacy | 98.0 | 20 | Lifelong | PSA | |
| Estonia | Northern Europe | High-income | 9v; 2 | 15–18 | Payer | DTM | 100 | 5.0 | Non-pharmacy | 98.0 | 70 | Lifelong | PSA | |
| Italy | Southern Europe | High-income | all; 2 | 12 | Payer | DTM | 100 | 3.0 | Non-pharmacy | 98.0 | 70 | Lifelong | PSA | |
| Latvia | Central and Eastern Europe | High-income | 2v; 2 | 12–17 | Payer | DTM | 100 | 5.0 | Non-pharmacy | 98.0 | 40 | Lifelong | PSA | |
| Netherlands | Western Europe | High-income | 2v; 2 | 10 | Payer | DTM | 100 | 2.8 | Non-pharmacy | 98.0 | 50 | Lifelong | PSA | |
| Poland | Central and Eastern Europe | High-income | 2v,4v; 2 | 12–13 | Payer | DTM | 100 | 4.3 | Non-pharmacy | 98.0 | 20 | Lifelong | PSA | |
| Slovenia | Central and Eastern Europe | High-income | 9v; 2 | 11–12 | Payer | DTM | 100 | 5.0 | Non-pharmacy | 98.0 | 50 | Lifelong | PSA | |
| Spain | Southern Europe | High-income | all; 2 | 12 | Payer | DTM | 100 | 3.0 | Non-pharmacy | 98.0 | 80 | Lifelong | PSA | |
| Sweden | Northern Europe | High-income | 4v; 2 | 10–12 | Payer | DTM | 100 | 3.0 | Non-pharmacy | 98.0 | 80 | Lifelong | PSA | |
| Simons et al.40 | Netherlands | Western Europe | High-income | 2v; 2 | 12 | HCP | DTM | Lifetime | 2.3 | Non-pharmacy | 93.2 | 30 | Lifelong | One-way, PSA |
| Wolff et al.41 | Sweden | Northern Europe | High-income | 2v,4v; 2 | 10–12 | Societal | DTM | 100 | 3.0 | Non-pharmacy | 84.0 | 80 | Lifelong | DSA |
Abbreviation: DSA = deterministic sensitivity analysis; DTM = dynamic transmission model; HCP = healthcare payers; NS = not stated; PSA = probability sensitivity analysis; UK = United Kingdom; US = United States; v = valent.
Cost-effectiveness based on global mean incremental cost-effectiveness ratios per unit gained and pooled incremental net benefits of universal vaccination vs. girls-only vaccination
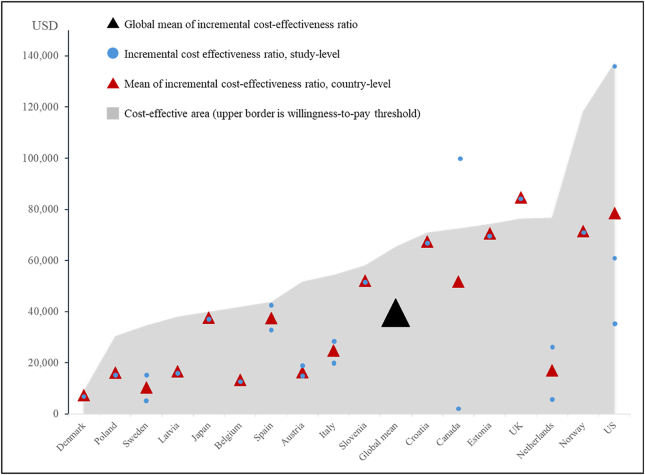
Most studies24,29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 (93%) reported that the universal HPV vaccine program was cost-effective. Only one study42 concluded that it was not cost-effective compared with the girls-only HPV vaccine program. The studies performed cost-utility analyses using quality-adjusted life years (93%) and life years gained (7%) as effective outcome. Across 16 countries across among 15 studies, the ICERs ranged from $2681 to $137,640 per unit gained, and the WTP threshold ranged from $6096 to $148,568. Vaccine price ranged from $13.31 in Sweden and $290.42 in Austria. Summary of ICERs, WTP-thresholds and INBs for each study were available in Table 2. The global mean of ICERs were $39,553 (95% CI, $27,008–66,641) per unit gained, which was below the global mean WTP threshold of $65,473 (95% CI, $52,138–83,755). Mean of ICERs in country-level and interpretation based on respective WTP-thresholds were calculated and depicted in Fig. 2.
Table 2.
Characteristics of economic evaluation.
| Author | Country | Currency, year | Change incidence in male | Purchasing power parity | Vaccine price per dosea | WTP thresholda | ICERs per unit gaineda | Unit | INB |
|---|---|---|---|---|---|---|---|---|---|
| Bresse et al.24 | Austria | € 2010 | 0.73 | 0.84 | 290.42 | 59,235 | 16,980 | €/QALY | 30,761 |
| Burger et al.29 | Norway | $ 2010 | 0.22 | 1.00 | 106.88 | 118,276 | 71,535 | $/QALY | 10,283 |
| Chesson et al.30 | US | $ 2008 | 0.48 | 1.00 | 178.28 | 148,568 | 61,507 | $/QALY | 41,789 |
| Elbasha et al.31 | US | $ 2008 | 0.56 | 1.00 | 198.09 | 109,000 | 36,643 | $/QALY | 40,198 |
| Graham et al.32 | Canada | C$ 2012 | 0.60 | 1.25 | 145.69 | 28,077 | 2681 | C$/QALY | 15,161 |
| Haeussler et al.33 | Italy | € 2015 | 0.51 | 0.74 | 100.11 | 57,995 | 20,550 | €/QALY | 19,097 |
| Jit et al.42 | UK | ₤ 2001 | 0.30 | 0.69 | 205.02 | 76,409 | 84,764 | ₤/QALY | −2535 |
| Kim et al.34 | US | $ 2006 | 0.31 | 1.00 | 142.80 | 154,773 | 137,640 | $/QALY | 5311 |
| Laprise et al.35 | Canada | C$ 2010 | 0.25 | 1.22 | 88.40 | 116,804 | 100,685 | C$/QALY | 4,030 |
| Linertova et al.36 | Spain | € 2020 | 0.87 | 0.61 | 73.37 | 49,467 | 42,857 | €/QALY | 5729 |
| Olsen et al.37 | Denmark | € 2012 | 0.13 | 7.56 | 14.23 | 8997 | 7377 | €/QALY | 211 |
| Palmer et al.38 | Japan | $ 2022 | 0.45 | 1.00 | 46.30 | 40,013 | 37,612 | $/QALY | 1092 |
| Qendri et al.39 | Austria | $ 2017 | 0.33 | 1.00 | 105.79 | 44,343 | 15,815 | $/LYG | 9414 |
| Belgium | $ 2017 | 0.33 | 1.00 | 66.51 | 41,809 | 13,384 | $/LYG | 9380 | |
| Croatia | $ 2017 | 0.21 | 1.00 | 120.99 | 70,949 | 67,341 | $/LYG | 758 | |
| Estonia | $ 2017 | 0.21 | 1.00 | 99.96 | 74,207 | 70,532 | $/LYG | 772 | |
| Italy | $ 2017 | 0.21 | 1.00 | 61.57 | 50,678 | 28,895 | $/LYG | 4574 | |
| Latvia | $ 2017 | 0.21 | 1.00 | 44.82 | 38,008 | 16,664 | $/LYG | 4482 | |
| Netherlands | $ 2017 | 0.33 | 1.00 | 26.61 | 25,339 | 5526 | $/LYG | 6538 | |
| Poland | $ 2017 | 0.37 | 1.00 | 69.18 | 30,407 | 16,207 | $/LYG | 5254 | |
| Slovenia | $ 2017 | 0.37 | 1.00 | 103.51 | 58,008 | 52,023 | $/LYG | 2214 | |
| Spain | $ 2017 | 0.21 | 1.00 | 56.00 | 38,008 | 32,212 | $/LYG | 1217 | |
| Sweden | $ 2017 | 0.49 | 1.00 | 20.90 | 63,347 | 14,960 | $/LYG | 23,710 | |
| Simons et al.40 | Netherlands | € 2018 | 0.28 | 0.78 | 102.09 | 128,000 | 28,651 | €/QALY | 28,099 |
| Wolff et al.41 | Sweden | € 2014 | 0.44 | 8.73 | 13.36 | 6096 | 5775 | €/QALY | 142 |
Abbreviation: C$ = Canada dollar; ICERs = incremental cost-effectiveness ratios; LYG = life year gained; QALY = quality-adjusted life year; WTP = willingness-to-pay.
Value converted to USD using Power Purchasing Parity and inflated to 2024 USD currency.
Figure 2.
Global mean of incremental cost-effectiveness ratios of universal HPV vaccination by respective country's mean willingness-to-pay threshold. Abbreviations: ICER = incremental cost-effectiveness ratio; UK = United Kingdom; US = United States; USD = United States Dollar; WTP = willingness-to-pay. ICER within area of cost-effectiveness (area under WTP threshold) were considered as cost-effective strategy. For countries with more than one data point (more than one study), mean ICER and mean WTP threshold were calculated. Minimum ICER in Sweden, Spain, Austria, Italy, Canada, and Netherlands represent ICER from study Wolff et al.,41 Qendri et al.,39 Qendri et al.,39 Haeussler et al.,33 Graham et al.,32 and Qendri et al.,39 respectively. Maximum ICER in Sweden, Spain, Austria, Italy, Canada, and Netherlands represent ICER from study Qendri et al.,39 Linertova et al.,36 Bressee et al.,24 Qendri et al.,39 Laprise et al.,35 and Simons et al.,40 respectively. In the US, data points from lowest to highest represent ICER from study Elbasha et al.,31 Chesson et al.,30 and Kim et al.,34 respectively). Minimum and maximum WTP threshold were not shown.
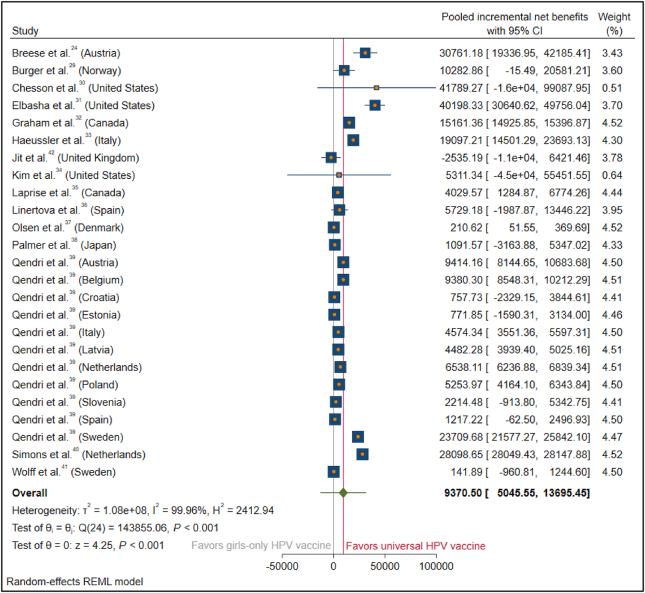
The INBs were calculated for each country and pooled across countries using a random-effects model, which yielded a pooled INB of $9370 (95% CI, $5046–13,695) across 25 study group (Cochran's Q = 143,855.06; df = 24; P < 0.001). All the study group has similar weighting factor, however two studies (Chesson et al.30 and Kim et al.,34 that were conducted in the United States) have the widest 95% CI and thus the lowest weighting factor to the effect estimate of pooled INB. Overall, the INBs of introducing a universal HPV vaccination program were equal to $9370 per individual in universal vaccination programs compared with the girls-only HPV vaccination programs in preventing OPC. The overall I2 was 99.96%, indicating high heterogeneity among these studies (Fig. 3).
Figure 3.
Forest plot of studies on the incremental net benefit of universal HPV vaccine vs. girls-only HPV vaccine. Abbreviations: CI = confidence interval; INB = incremental net benefit; HPV = human papillomavirus. Two studies (Chesson et al.30 and Kim et al.,34 that were conducted in the United States) have the widest 95% CI, therefore the lowest weighting factor to the effect estimate of pooled INB.
Subgroup analysis, risk of bias assessment, and sensitivity analysis
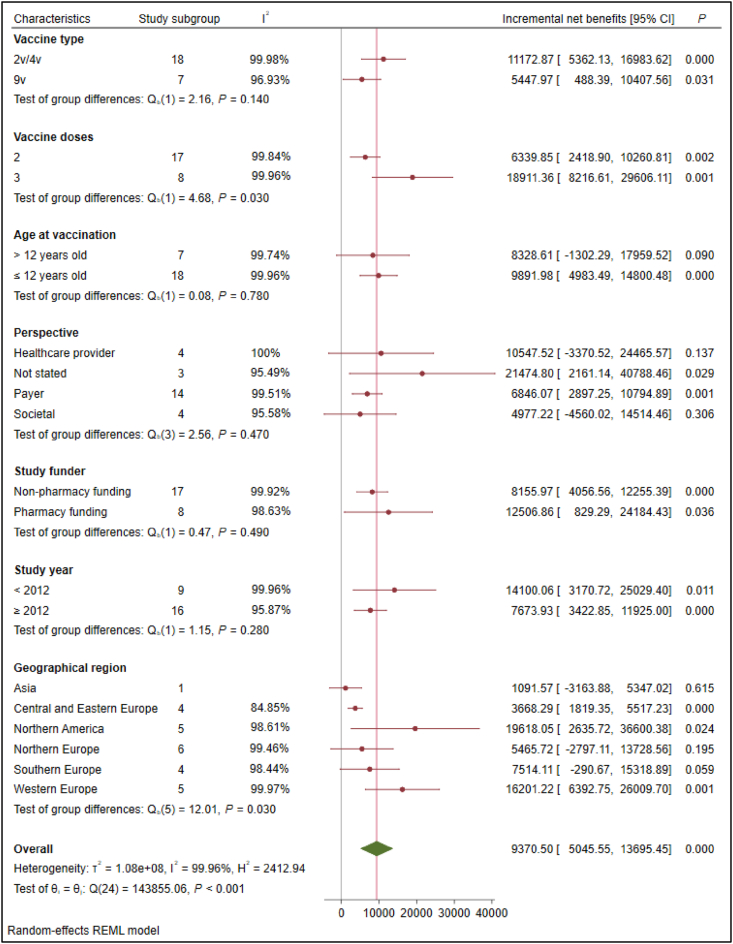
Across all categorical characteristics in subgroup analysis, INBs were consistent and positive. Further, I2 that was computed for subgroup differences showed the high percentage of heterogeneity in effect estimates from the different subgroups (Fig. 4). Based on the meta-regression analysis, the sources of heterogeneity included the threshold (R2 = 19.51%) and vaccine price per dose (R2 = 17.95%) (Table 3).
Figure 4.
Subgroup analysis according to various characteristics. Abbreviations: CI, confidence interval; v = valent.
Table 3.
Summary of R2 from various continuous variables across studies.
| Variable | Na | INBs (95% CI) | R2 |
|---|---|---|---|
| Discount rate | 25 | $18,737.46 ($1834.66–35,640.27) | 0.41% |
| Time horizon | 25 | $7856.507 ($1782.71–13,930.30) | 0.52% |
| Vaccine efficacy | 25 | $38,145.23 ($1471.56–74,818.90) | 6.09% |
| Vaccine coverage | 25 | $11,759.76 (−$731.59–24,251.10) | 0.16% |
| Vaccine price per dose | 25 | $1846.32 (−$5159.03–8851.67) | 17.95% |
| Administration cost | 16 | $7752.16 ($2267.71–13,236.60) | 0.61% |
| Threshold | 26 | $1040.97 (−$6653.66–8735.60) | 19.51% |
CI = confidence interval, INBs = incremental net benefits, N = number of observations.
Number of observations in every variable is different due to missing value.
The risk of bias resulted in the most unlikely risk of bias (Fig. 5). We performed a sensitivity analysis by excluding studies individually as an alternative meta-analysis model. The pooled INBs of the universal HPV vaccination differed from those of the main model by less than 10% (Table 4).
Figure 5.
Summary of risk of bias assessment. Green plus indicates that the article declared such information (low risk of bias) and red minus indicates that the article did not declare such information (high risk of bias). Abbreviations: ICER = incremental cost-effectiveness ratio.
Table 4.
Summary of sensitivity analyses.
| Omitted study | INBs ($) | 95% CI ($) | Difference, % |
|---|---|---|---|
| Netherlands (Simons et al.40) | 8403 | 4318.410–12,486.591 | 0.90 |
| Denmark (Olsen et al.37) | 9809 | 5352.910–14,265.947 | 1.05 |
| Canada (Graham et al.32) | 9112 | 4610.780–13,613.717 | 0.97 |
| Netherlands (Qendri et al.39) | 9526 | 4988.089–14,064.472 | 1.02 |
| Latvia (Qendri et al.39) | 9620 | 5098.182–14,141.849 | 1.03 |
| Belgium (Qendri et al.39) | 9394 | 4848.994–13,938.099 | 1.00 |
| Italy (Qendri et al.39) | 9615 | 5092.953–14,137.894 | 1.03 |
| Poland (Qendri et al.39) | 9585 | 5055.859–14,113.243 | 1.02 |
| Sweden (Wolff et al.41) | 9811 | 5356.136–14,266.213 | 1.05 |
| Austria (Qendri et al.39) | 9392 | 4847.842–13,935.949 | 1.00 |
| Spain (Qendri et al.39) | 9764 | 5288.448–14,239.670 | 1.04 |
| Sweden (Qendri et al.39) | 8670 | 4381.599–12,957.926 | 0.93 |
| Estonia (Qendri et al.39) | 9780 | 5312.677–14,246.592 | 1.04 |
| Canada (Laprise et al.35) | 9636 | 5121.560–14,150.206 | 1.03 |
| Croatia (Qendri et al.39) | 9776 | 5310.265–14,242.714 | 1.04 |
| Slovenia (Qendri et al.39) | 9714 | 5223.506–14,203.980 | 1.04 |
| Japan (Palmer et al.38) | 9755 | 5283.731–14,225.471 | 1.04 |
| Italy (Haueussler et al.33) | 8935 | 4508.094–13,361.895 | 0.95 |
| Spain (Linertova et al.36) | 9540 | 5030.466–14,048.837 | 1.02 |
| United Kingdom (Jit et al.42) | 9838 | 5432.601–14,242.712 | 1.05 |
| United States (Elbasha et al.31) | 8098 | 4358.423–11,837.132 | 0.86 |
| Norway (Burger et al.29) | 9355 | 4855.101–13,854.600 | 1.00 |
| Austria (Bresse et al.24) | 8580 | 4403.210–12,757.123 | 0.92 |
| United States (Kim et al.34) | 9400 | 5044.215–13,755.973 | 1.00 |
| United States (Chesson et al.30) | 9205 | 4872.634–13,537.769 | 0.98 |
Sensitivity analysis was performed using the leave-one-out model relative to the main model, INBs = $9370 (95% CI, $5045–13,695). CI = confidence interval, INBs = incremental net benefits.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to assess the cost-effectiveness of universal HPV vaccination in comparison with girls-only vaccination by pooling INBs data. Based on 15 studies across 16 countries, the pooled INBs was $9370 (95% CI, $5046–13,695).
A few systematic reviews have compared universal HPV vaccination programs with girls-only programs. In 2018, Siok et al. published a systematic review of universal HPV vaccines using various stratifications across 14 studies.43 However, a meta-analysis was not conducted. In 2022, Tejada et al. conducted a meta-analysis across 24 studies from Latin America.44 However, the meta-analysis did not focus on the OPC burden. A study published in 2022 conducted an analysis based on the recalculation of ICERs and compared them with different WTP thresholds but did not pool INBs; INBs include WTP threshold in their estimation, thus making them easier to interpret.21Another advantage of INBs is that they are not ratio-based measures. Consequently, they allow for a method of pooling that facilitates the meta-analysis of cost-effectiveness.45 Our meta-analysis reported that the pooled INBs were positive, thus indicating that extending the HPV vaccine program to boys was cost-effective. The countries included in the analysis gained a net benefit of $9370 per individual (95% CI, $5046–13,695) by introducing a universal HPV vaccine program to prevent OPC in comparison with a girls-only HPV vaccine program.
Our subgroup analyses demonstrated the cost-effectiveness of all variables in the universal HPV vaccination program. We observed that the sources of heterogeneity in our study were the WTP threshold and the vaccine price. The WTP threshold and the vaccine price were two major source of heterogeneity similar to the meta-analysis of economic evaluation studies that remains to be explored.46 The wide range in the WTP threshold across regions ($8997 in Denmark and $118,276 in the USA) is influenced by societal preferences, economic conditions, policy contexts, and methodological differences. In the United States, the healthcare system is characterized by higher healthcare expenditures per capita and a greater emphasis on individualism, which may have resulted in a higher WTP for healthcare interventions.47 Additionally, the United States has a diverse population with varying income levels and healthcare needs.47 In contrast, Denmark has a universal healthcare system with comprehensive coverage and a strong emphasis on egalitarian principles, which may have resulted in a lower WTP threshold.48 Cultural differences regarding healthcare access, expectations, and attitudes towards risk and uncertainties can contribute to variations in WTP between countries.49 The cost of the vaccine varied due to the expected price for the following year of study based on several factors, including the manufacturing location of the vaccine. However, in many countries, the cost tends to decrease due to tendering.50
Based on our analysis, HPV vaccine effectively prevents OPC in male (mean = 92.7%, SD = 10.5%). This study highlights the importance of expanding HPV vaccination programs for boys in Taiwan. Epidemiological data indicates that HPV infections and HPV-related cancers are significant public health issues in Taiwan, due to economic implication.51 This program will protect not only males from HPV infections (gender equality), but also provide broad public health benefits by reducing the transmission of the virus to their sexual partners and the community.52 Furthermore, OPC is one of the top three cancer cost burdens in Taiwan, which is predominantly prevalent in males; therefore, it requires urgent attention from the government in optimizing the allocation of national health resources.
This study has several strengths. First, the new method of pooling INBs was applied after data harmonization. INBs higher than the ICERs as the economic measure upon recommendation from comparative efficiency research (COMER) methods demonstrate generalizability and easier interpretation.21 The random effects model in meta-analysis offers significant advantages by accounting for variability between studies, making it particularly suitable for cases with heterogeneity. This model provides more realistic and generalizable estimates by assuming that the included studies are a random sample from a larger population.25 Consequently, it results in wider confidence intervals, reflecting greater uncertainty and promoting more conservative inference.25 Additionally, the random-effects model is less sensitive to the size of individual studies and facilitates a comprehensive exploration of heterogeneity sources through subgroup analyses and meta-regression, making it a flexible and robust option for synthesizing diverse research findings.25 Second, subgroup analyses consistently showed positive INBs in all grouping, with high heterogeneity among all variables suggesting genuine subgroup differences rather than sampling errors. Third, the sensitivity analyses revealed a small difference between the original and alternative models, thus suggesting the robustness of the results.
Some limitations of this study should be noted when interpreting the results. First, the results should be generalized only after considering specific country information (e.g., perspective, time horizon, currency, and gross domestic product or WTP threshold). This study demonstrated high heterogeneity in pooled INBs. However, stratified analyses and meta-regression revealed that different WTP thresholds cause high heterogeneity. The second limitation is the method for pooling INBs using ICERs and 95% CI. Owing to the limited data, we used sensitivity analysis and 95% CIs for ICERs to pool INBs. We encourage further meta-analytic studies to compare the pooled INBs of girls-only HPV vaccination programs and universal HPV vaccination programs, irrespective of the ICERs provided. Third, due to the limited number of published economic analysis studies in low- and middle-income countries, this study was restricted to high-income countries, which may affect the generalizability of the results. Conducting similar research in low- and middle-income countries is necessary and would provide a more comprehensive perspective.
In conclusion, all studies included in this meta-analysis suggest that the universal HPV vaccination is cost-effective compared to the girls-only HPV vaccination, and the pooled INBs indicate a high monetary benefit per patient in terms of preventing OPC burden. This study would benefit from including specific recommendations for policymakers, particularly for countries like Taiwan that have not yet implemented a universal HPV vaccination program. Providing detailed steps and considerations for the implementation of such a program would be highly valuable.
Declaration of competing interest
The authors have no conflict of interest relevant to this article.
Acknowledgments
This study did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors. The authors thank Dr. Shin-Hu Chen, Dr. Wei-Chin Hsu, Professor Wen-Chao Ho, Professor Bing-Fang Hwang, and Ms. Pei-Jia Tsai for their contributions to this study.
Contributor Information
Wei-Chia Su, Email: davidsujeff168@gmail.com.
Jian-Hong Yu, Email: kenkoyu@mail.cmu.edu.tw.
References
- 1.Zumsteg Z.S., Luu M., Rosenberg P.S., et al. Global epidemiologic patterns of oropharyngeal cancer incidence trends. J Natl Cancer Inst. 2023;115:1544–1554. doi: 10.1093/jnci/djad169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C.P., Chen T.C., Hsu W.L., et al. Rising incidence of HPV positive oropharyngeal cancer in Taiwan between 1999 and 2014 where betel nut chewing is common. BMC Cancer. 2022;22:296. doi: 10.1186/s12885-022-09407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonsêca T.C., Jural L.A., Marañón-Vásquez G.A., et al. Global prevalence of human papillomavirus-related oral and oropharyngeal squamous cell carcinomas: a systematic review and meta-analysis. Clin Oral Invest. 2023;28:62. doi: 10.1007/s00784-023-05425-0. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi A.K., Anderson W.F., Lortet-Tieulent J., et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lechner M., Liu J., Masterson L., Fenton T.R. HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management. Nat Rev Clin Oncol. 2022;19:306–327. doi: 10.1038/s41571-022-00603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roman B.R., Aragones A. Epidemiology and incidence of HPV-related cancers of the head and neck. J Surg Oncol. 2021;124:920–922. doi: 10.1002/jso.26687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senkomago V., Henley S.J., Thomas C.C., Mix J.M., Markowitz L.E., Saraiya M. Human papillomavirus-attributable cancers - United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2019;68:724–728. doi: 10.15585/mmwr.mm6833a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreimer A.R., Shiels M.S., Fakhry C., et al. Screening for human papillomavirus-driven oropharyngeal cancer: considerations for feasibility and strategies for research. Cancer. 2018;124:1859–1866. doi: 10.1002/cncr.31256. [DOI] [PubMed] [Google Scholar]
- 9.Falcaro M., Castañon A., Ndlela B., et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. 2021;398:2084–2092. doi: 10.1016/S0140-6736(21)02178-4. [DOI] [PubMed] [Google Scholar]
- 10.Lei J., Ploner A., Elfström K.M., et al. HPV vaccination and the risk of Invasive cervical cancer. N Engl J Med. 2020;383:1340–1348. doi: 10.1056/NEJMoa1917338. [DOI] [PubMed] [Google Scholar]
- 11.Tsai S.A., Lu C.Y., Chen T.I., Huang S.P., Chen Y.C. Adverse events from HPV vaccination in Taiwan. Vaccine. 2023;41:7444–7449. doi: 10.1016/j.vaccine.2023.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Huang S.Y., Chen H.M., Liao K.H., Ko B.S., Hsiao F.Y. Economic burden of cancers in Taiwan: a direct and indirect cost estimate for 2007-2017. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-036341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenzatti Hiles G., Chang K.P., Bellile E.L., et al. Understanding the impact of high-risk human papillomavirus on oropharyngeal squamous cell carcinomas in Taiwan: a retrospective cohort study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson S., Isaac A., Jeffery C.C., et al. Practices regarding human papillomavirus counseling and vaccination in head and neck cancer: a Canadian physician questionnaire. J Otolaryngol-Head N. 2017;46:61. doi: 10.1186/s40463-017-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J.Z., Jou J., Cohen E. Vaccine strategies for human papillomavirus-associated head and neck cancers. Cancers (Basel) 2021;14:33. doi: 10.3390/cancers14010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ducatman B.S. The role of human papillomavirus in oropharyngeal squamous cell carcinoma. Arch Pathol Lab Med. 2018;142:715–718. doi: 10.5858/arpa.2018-0083-RA. [DOI] [PubMed] [Google Scholar]
- 17.El Hussein M.T., Dhaliwal S. HPV vaccination for prevention of head and neck cancer among men. Nurse Pract. 2023;48:25–32. doi: 10.1097/01.NPR.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 18.Colzani E., Johansen K., Johnson H., Pastore Celentano L. Human papillomavirus vaccination in the European Union/European Economic Area and globally: a moral dilemma. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.50.2001659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsu V.D., LaMontagne D.S., Atuhebwe P., Bloem P.N., Ndiaye C. National implementation of HPV vaccination programs in low-resource countries: lessons, challenges, and future prospects. Prev Med. 2021;144 doi: 10.1016/j.ypmed.2020.106335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linertová R., Guirado-Fuentes C., Mar Medina J., Imaz-Iglesia I., Rodríguez-Rodríguez L., Carmona-Rodríguez M. Cost-effectiveness of extending the HPV vaccination to boys: a systematic review. J Epidemiol Community Health. 2021;75:910–916. doi: 10.1136/jech-2020-216305. [DOI] [PubMed] [Google Scholar]
- 21.World Crespo C., Monleon A., Díaz W., Ríos M. Comparative efficiency research (COMER): meta-analysis of cost-effectiveness studies. BMC Med Res Methodol. 2014;14:139. doi: 10.1186/1471-2288-14-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagepally B.S., Chaikledkaew U., Chaiyakunapruk N., Attia J., Thakkinstian A. Meta-analysis of economic evaluation studies: data harmonisation and methodological issues. BMC Health Serv Res. 2022;22:202. doi: 10.1186/s12913-022-07595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bresse X., Goergen C., Prager B., Joura E. Universal vaccination with the quadrivalent HPV vaccine in Austria: impact on virus circulation, public health and cost-effectiveness analysis. Expert Rev Pharm Out. 2014;14:269–281. doi: 10.1586/14737167.2014.881253. [DOI] [PubMed] [Google Scholar]
- 25.Riley R.D., Higgins J.P.T., Deeks J.J. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J.P. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37:1158–1160. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
- 27.Willis B.H., Riley R.D. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat Med. 2017;36:3283–3301. doi: 10.1002/sim.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husereau D., Drummond M., Augustovski F., et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMC Med. 2022;20:23. doi: 10.1186/s12916-021-02204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burger E.A., Sy S., Nygar̊d M., Kristiansen I.S., Kim J.J. Prevention of HPV-related cancers in Norway: cost-effectiveness of expanding the HPV vaccination program to include pre-adolescent boys. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chesson H.W., Ekwueme D.U., Saraiya M., Dunne E.F., Markowitz L.E. The cost-effectiveness of male HPV vaccination in the United States. Vaccine. 2011;29:8443–8450. doi: 10.1016/j.vaccine.2011.07.096. [DOI] [PubMed] [Google Scholar]
- 31.Elbasha E.H., Dasbach E.J. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28:6858–6867. doi: 10.1016/j.vaccine.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Graham D.M., Isaranuwatchai W., Habbous S., et al. A cost-effectiveness analysis of human papillomavirus vaccination of boys for the prevention of oropharyngeal cancer. Cancer. 2015;121:1785–1792. doi: 10.1002/cncr.29111. [DOI] [PubMed] [Google Scholar]
- 33.Haeussler K., Marcellusi A., Mennini F.S., et al. Cost-effectiveness analysis of universal human papillomavirus vaccination using a dynamic bayesian methodology: the BEST II study. Value Health. 2015;18:956–968. doi: 10.1016/j.jval.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Kim J.J., Simms K.T., Killen J., et al. Human papillomavirus vaccination for adults aged 30 to 45 years in the United States: a cost-effectiveness analysis. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laprise J.F., Drolet M., Boily M.C., et al. Comparing the cost-effectiveness of two- and three-dose schedules of human papillomavirus vaccination: a transmission-dynamic modelling study. Vaccine. 2014;32:5845–5853. doi: 10.1016/j.vaccine.2014.07.099. [DOI] [PubMed] [Google Scholar]
- 36.Linertová R., Guirado-Fuentes C., Mar-Medina J., Teljeur C. Cost-effectiveness and epidemiological impact of gender-neutral HPV vaccination in Spain. Hum Vaccines Immunother. 2022;18 doi: 10.1080/21645515.2022.2127983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsen J., Jorgensen T.R. Revisiting the cost-effectiveness of universal HPV-vaccination in Denmark accounting for all potentially vaccine preventable HPV-related diseases in males and females. Cost Eff Resour Allocation. 2015;13:4. doi: 10.1186/s12962-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer C., Tobe K., Negishi Y., You X., Chen Y.T., Abe M. Health impact and cost effectiveness of implementing gender-neutral HPV vaccination in Japan. J Med Econ. 2023;26:1546–1554. doi: 10.1080/13696998.2023.2282912. [DOI] [PubMed] [Google Scholar]
- 39.Qendri V., Bogaards J.A., Baussano I., Lazzarato F., Vänskä S., Berkhof J. The cost-effectiveness profile of sex-neutral HPV immunization in European tender-based settings: a model-based assessment. Lancet Public Health. 2020;5:e592–e603. doi: 10.1016/S2468-2667(20)30209-7. [DOI] [PubMed] [Google Scholar]
- 40.Simons J.J.M., Vida N., Westra T.A., Postma M.J. Cost-effectiveness analysis of a gender-neutral human papillomavirus vaccination program in The Netherlands. Vaccine. 2020;38:4687–4694. doi: 10.1016/j.vaccine.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 41.Wolff E., Elfström K.M., Haugen Cange H., et al. Cost-effectiveness of sex-neutral HPV-vaccination in Sweden, accounting for herd-immunity and sexual behaviour. Vaccine. 2018;36:5160–5165. doi: 10.1016/j.vaccine.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 42.Jit M., Choi Y.H., Edmunds W.J. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ. 2008;337 doi: 10.1136/bmj.a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng S.S., Hutubessy R., Chaiyakunapruk N. Systematic review of cost-effectiveness studies of human papillomavirus (HPV) vaccination: 9-Valent vaccine, gender-neutral and multiple age cohort vaccination. Vaccine. 2018;36:2529–2544. doi: 10.1016/j.vaccine.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 44.Tejada R.A., Malagón T., Franco E.L. Cost-effectiveness of human papillomavirus vaccination in girls living in Latin American countries: a systematic review and meta-analysis. Vaccine. 2022;40:2667–2678. doi: 10.1016/j.vaccine.2022.03.046. [DOI] [PubMed] [Google Scholar]
- 45.Haider S., Chaikledkaew U., Thavorncharoensap M., Youngkong S., Islam M.A., Thakkinstian A. Systematic review and meta-analysis of cost-effectiveness of rotavirus vaccine in low-income and lower-middle-income countries. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dilokthornsakul P., Veettil S.K., Lan L.M., et al. Combining cost-effectiveness results into a single measurement: what is the value?: authors response. EClinicalMedicine. 2022;51 doi: 10.1016/j.eclinm.2022.101565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papanicolas I., Woskie L.R., Jha A.K. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024–1039. doi: 10.1001/jama.2018.1150. [DOI] [PubMed] [Google Scholar]
- 48.Eriksen J., Ebbesen M., Eriksen K.T., et al. Equity in digital healthcare – the case of Denmark. Front Public Health. 2023;11 doi: 10.3389/fpubh.2023.1225222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hussain B., Latif A., Timmons S., Nkhoma K., Nellums L.B. Overcoming COVID-19 vaccine hesitancy among ethnic minorities: a systematic review of UK studies. Vaccine. 2022;40:3413–3432. doi: 10.1016/j.vaccine.2022.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garattini L., Padula A. Pricing of HPV vaccines in Europe: back to the future? Appl Health Econ Health Pol. 2018;16:275–277. doi: 10.1007/s40258-018-0375-9. [DOI] [PubMed] [Google Scholar]
- 51.Wu Y.H., Lai C.H., Chien L., et al. Economic burden of cervical and head and neck cancer in Taiwan from a societal perspective. Int J Environ Res Publ Health. 2023;20:3717. doi: 10.3390/ijerph20043717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grandahl M., Nevéus T., Dalianis T., Larsson M., Tydén T., Stenhammar C. 'I also want to be vaccinated!' - adolescent boys' awareness and thoughts, perceived benefits, information sources, and intention to be vaccinated against Human papillomavirus (HPV) Hum Vaccines Immunother. 2019;15:1794–1802. doi: 10.1080/21645515.2018.1551670. [DOI] [PMC free article] [PubMed] [Google Scholar]