Abstract
Background/purpose
Erythema multiforme (EM) is considered a hypersensitivity reaction associated with drugs and infections, and remains underestimated due to the lack of precise classification and diagnostic criteria. The aim of this study was to evaluate the triggering factors and clinical manifestations of EM and to present our experience in the diagnosis and management of this disorder.
Materials and methods
All patient records were reviewed, and records of patients admitted, diagnosed, and treated with EM were retrieved. Data on age, gender, medical history, triggering factor(s), clinical form, mucosal/cutaneous involvement, affected oral site(s), recurrence, and treatment were collected. The data were analyzed statistically at a significance level set at P < 0.05.
Results
A total of 36 EM patients were studied. The triggering factor was identified as infection in 25 %, drugs in 16.7 %, infections and drugs in 41.7 %, and none in 16.7 % of the 36 EM patients. EM minor was diagnosed in 77.8 % of the patients. Labial mucosa (86.1 %) was the most commonly affected oral site. Most patients were treated with topical steroids (25 %). No significant differences were detected between demographic and clinical characteristics with regards to gender, triggering factor, and the number of affected oral sites (P > 0.05).
Conclusion
The results of this study, based on the data from 36 EM patients with oral involvement treated at our clinic, can guide dentists in this regard and may be considered as an epidemiological source for the region.
Keywords: Erythema multiforme, Triggering factors, Clinical manifestation, Management
Introduction
Erythema multiforme (EM) is an acute, self-limiting and sometimes recurrent mucocutaneous inflammatory disorder recognized as a hypersensitivity reaction primarily triggered by certain infections and drugs, including vaccinations.1, 2, 3, 4, 5, 6, 7, 8
EM is regarded by numerous authors to be a component of a continuous spectrum of increasingly severe manifestations of a single disease entity, including EM minor, EM major, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN, also known as Lyell disease).2,7,9,10 However, others considered EM to be a distinct disorder due to its strong correlation with infections, especially herpes simplex virus (HSV) infections, which is in contrast to the prevailing drug-induced etiology of SJS and TEN.2,9,11, 12, 13, 14, 15 Some authors suggested that the diagnosis of EM would be appropriate when involvement is limited to less than 10 % of the body surface area, while reserving SJS or TEN for more extensive cases.6,7,9,11,13
First described by Bateman in 1817, Ferdinand von Hebra provided the comprehensive description of the characteristic morphological features and etiologies of this specific eruption, coining the term “erythema exudativum multiforme” in 1866.1,16 Subsequently, in 1950, Thomas proposed a classification for EM, dividing it into “minor” and “major” subtypes.17 EM minor was assigned to cases presenting typical clinical presentation with mild cutaneous involvement as originally described by von Hebra, while EM major was attributed to variants exhibiting more severe mucocutaneous lesions. Additionally, some authors have advocated a further classification, distinguishing cases with a single mucosal site involvement as EM minor and those with two or more mucosal sites involvement as EM major.2,6,15,18 In 1968, Kennett described an oral inflammatory disorder that mimics the typical oral lesions of EM but without any cutaneous involvement.19 Oral EM is considered controversial, not well-recognized, and rare within the spectrum of EM, as some dermatologists continue to strongly argue that the exclusive criteria for EM diagnosis consist of the distinct appearance and distribution of target cutaneous lesions.20,21 Various forms of EM exist in addition to acute cases, including recurrent and chronic persistent forms, which indicate the evolution of the disease over time.4,10,22, 23, 24
Given its acute nature and manifestation in the dental setting, EM proves to be an important condition that necessitates prompt diagnosis and care. This highlights the significance of elucidating its epidemiology and clinical characteristics through a dentist's viewpoint. The lack of established objective markers and distinct diagnostic criteria makes the interpretation of the available information on EM a challenging task. The literature review reveals a scarcity of large-scale studies on EM. Both prospective and retrospective studies are comparatively few in number, while the available case series in the literature are outdated. Additionally, there is a notable lack of case series depicting the diversity of oral presentations of the disease. Therefore, this retrospective study aimed to investigate the epidemiology, triggering factors, clinical characteristics, and treatment modalities in 36 EM patients with oral involvement treated at our clinic. Our aim was to provide insights into the diagnosis and management of this disorder based on our observations and experiences.
Materials and methods
The design of this retrospective study was reviewed and approved by the Clinical Research Ethics Committee of the Faculty of Medicine at Marmara University (Protocol no: 09.2023.752) and was designed in accordance with the Declaration of Helsinki. Written consents of all patients had been obtained and strict measures were implemented throughout the study to protect patient anonymity.
Study design
The study was designed as a retrospective analysis covering all cases diagnosed with EM admitted to the Oral Medicine and Maxillofacial Radiology Clinic at the Faculty of Dentistry, Marmara University, Istanbul, Turkey, from 1999 to 2023. Within the scope of this retrospective study, all patient records were reviewed, and the records of patients admitted, diagnosed, and treated with EM during the aforementioned period were retrieved and reviewed.
Data collection was performed by two researchers (TG and BG) using a digital template. Patient records including complete details of demographic information (age and sex), past and recent medical history, factor(s) that triggered the EM episode (such as previous/concomitant infection(s), antecedent use of drug(s), or other causes), presence and nature of prodromal symptom(s), clinical form, mucosal and/or cutaneous involvement, specific oral site(s) affected, recurrence, treatment(s) administered, and duration of the EM episode were included in the study.
The diagnosis of EM at our clinic is primarily based on clinical diagnostic criteria. Given the lack of specific diagnostic tests for EM, cases where alternative diagnoses were clearly ruled out through clinical evaluation were included in this study. The majority of diagnoses were solely based on clinical symptoms, with a limited number of patients undergoing additional investigations such as histopathology and/or direct immunofluorescence (DIF) for confirmation.
In this study, cases of EM were clinically categorized using the classification system proposed by Al-Johani et al. EM was classified into two types according to the extent of mucous membrane involvement.9 Cases presenting solely with cutaneous lesions or those with cutaneous lesions alongside the involvement of a single mucosal surface were assigned as “EM minor”. The diagnosis of “EM major” was attributed to cases involving skin and at least two mucosal surfaces. Patients diagnosed with SJS and TEN were excluded from the study group.
In cases where a preceding illness was noted without any history of drug intake, an infectious etiology was suspected for EM. Any infections noted in the clinical records within 3 weeks prior to EM diagnosis were referred to as “concomitant” infections. In cases where drug intake was reported during an infection, both factors were considered in relation to the onset of EM.25 HSV was attributed as the cause of EM when patients presented either a positive HSV culture result or acute HSV serological evidence in association with their mucocutaneous eruptions. Similar to a previous study, HSV infection was considered to be the cause of EM if the patient presented with consistent clinical features (e.g., a history of HSV lesions before each EM episode and clinically evident subacute to resolving HSV lesions), even in the absence of a positive culture result in the medical records.10 The study also investigated drug use for symptoms or signs that were unrelated to EM. If multiple drugs came under suspicion in a patient, each drug was thoroughly examined. One of the researchers (SG) conducted a specific evaluation of the antecedent use of drug(s).
To investigate the seasonal effect on the onset of EM, the month of presentation for each EM case between 1999 and 2023 was also recorded.
Data analysis
The statistical analysis was performed using IBM SPSS Statistics Version 22.0 software (SPSS Inc., Chicago, IL, USA). Kolmogorov–Smirnov and Shapiro Wilks tests were used to assess data normality. For the comparison of quantitative data, Kruskal Wallis and Mann Whitney U tests were employed in addition to providing descriptive statistics such as mean, standard deviation, and frequency. The comparison of qualitative data was conducted using Fisher's exact chi-square test, Fisher Freeman Halton exact chi-square test, and Continuity (Yates) correction. Statistical significance was evaluated at a P value of <0.05.
Results
Demographic characteristics
A total of 36 patient charts, including 22 female (61.1 %) and 14 male (38.9 %) patients with the diagnosis of EM, were retrospectively analyzed. Table 1 provides a summary of the demographic, etiological, and clinical data for these patients.
Table 1.
Data regarding demographic, etiological, and clinical features of the 36 patients with erythema multiforme.
| No | Age | Sex | Preceding infection(s) | Drug antecedent(s) | Cutaneous lesions | Affected mucosa(e) | Clinical form | Involved oral site(s) |
Recurrence | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lips | Labial mucosa | Buccal mucosa | Hard palate | Soft palate | Tongue | Floor of the mouth | Uvula | Gingiva | |||||||||
| 1 | 32 | M | Influenza | Paracetamol | – | Oral and nasal | Major | X | X | X | – | X | – | – | X | – | X |
| 2 | 43 | F | – | – | X | Oral and genital | Major | X | X | X | – | – | X | – | – | – | – |
| 3 | 18 | M | Urinary tract infection | Cephalosporin | X | Oral | Minor | X | X | X | – | – | – | – | – | X | – |
| 4 | 23 | F | Genital infection | Cephalosporin | – | Oral | Minor | X | X | – | – | – | – | – | – | – | – |
| 5 | 19 | M | – | Mesalazine | – | Oral | Minor | X | X | X | – | – | X | – | – | X | – |
| 6 | 20 | M | HSV infection | – | – | Oral | Minor | X | – | X | – | – | X | – | – | – | – |
| 7 | 25 | F | HSV infection | – | – | Oral | Minor | X | X | – | X | – | X | – | – | – | X |
| 8 | 19 | M | Odontogenic infection | Penicillin | X | Oral | Minor | X | X | – | – | – | – | – | – | – | – |
| 9 | 24 | F | Odontogenic infection | Penicillin | – | Oral | Minor | X | X | – | – | – | – | – | – | – | – |
| 10 | 38 | M | HSV infection | – | – | Oral and nasal | Major | – | X | X | – | – | – | – | – | – | – |
| 11 | 18 | F | Odontogenic infection | Ciprofloxacin | – | Oral | Minor | X | X | – | X | – | – | – | – | – | – |
| 12 | 58 | F | – | Naproxen | – | Oral | Minor | – | – | – | X | – | X | – | – | – | – |
| 13 | 24 | M | Influenza HSV infection |
Cephalosporin | – | Oral | Minor | X | X | X | – | – | – | – | – | – | – |
| 14 | 45 | F | – | – | – | Oral and ocular | Major | – | X | X | – | X | – | – | – | – | X |
| 15 | 23 | M | HSV infection | – | X | Oral | Minor | – | X | X | X | – | – | – | – | – | X |
| 16 | 34 | M | – | – | – | Oral | Minor | X | X | – | – | – | – | – | – | – | X |
| 17 | 24 | F | HSV infection | – | – | Oral | Minor | X | X | X | – | – | X | – | – | – | X |
| 18 | 21 | M | – | Naproxen | – | Oral and genital | Major | X | – | – | X | – | – | – | – | – | X |
| 19 | 36 | F | Odontogenic infection | Penicillin | X | Oral and genital | Major | – | X | X | – | – | X | – | – | – | – |
| 20 | 30 | M | – | Naproxen Metronidazole |
– | Oral | Minor | X | – | – | X | – | X | – | – | – | X |
| 21 | 22 | F | Influenza | Penicillin | – | Oral | Minor | X | – | X | – | – | – | X | – | – | |
| 22 | 24 | M | Odontogenic infection | Penicillin Naproxen |
– | Oral and genital | Major | X | X | X | X | – | X | X | – | – | X |
| 23 | 6 | F | Influenza | Paracetamol | – | Oral | Minor | X | X | – | – | – | – | – | – | – | – |
| 24 | 15 | F | Influenza | Paracetamol | – | Oral | Minor | X | X | – | – | – | – | – | – | X | – |
| 25 | 62 | F | – | – | X | Oral and nasal | Major | X | X | X | – | – | – | – | – | – | – |
| 26 | 14 | F | – | – | – | Oral | Minor | – | X | X | – | – | – | – | – | – | – |
| 27 | 28 | F | HSV infection | – | – | Oral | Minor | X | X | – | X | – | – | – | – | – | X |
| 28 | 66 | F | Cryptic tonsillitis | Paracetamol Penicillin |
– | Oral | Minor | X | X | – | – | – | – | – | – | – | – |
| 29 | 28 | M | Cryptic tonsillitis | Paracetamol Cephalosporin Naproxen |
– | Oral | Minor | X | X | X | X | X | – | – | – | X | – |
| 30 | 53 | F | Odontogenic infection | Macrolide Etodolac |
– | Oral | Minor | X | X | – | – | – | – | – | – | – | – |
| 31 | 53 | F | HSV infection | – | – | Oral | Minor | X | X | – | – | – | – | – | – | – | – |
| 32 | 24 | F | Influenza | – | – | Oral | Minor | X | X | X | X | X | – | – | – | – | X |
| 33 | 22 | F | – | Depakin Levetiracetam |
– | Oral | Minor | – | X | – | X | – | – | – | – | – | – |
| 34 | 66 | F | – | – | – | Oral | Minor | X | X | X | – | – | – | – | – | – | – |
| 35 | 51 | M | Influenza | – | – | Oral | Minor | X | X | – | – | – | – | – | – | – | X |
| 36 | 48 | F | – | Levodopa Amantadine sulfate |
– | Oral | Minor | X | X | – | – | – | – | – | – | – | – |
The mean age at diagnosis was 32.1 ± 15.8 years, ranging from 6 to 66 years. Among the 36 patients, females outnumbered males, and young adults aged 21–30 years (38.9 %) were predominant. The demographic characteristics of patients are presented in Table 2.
Table 2.
Demographic characteristics of the patients with erythema multiforme.
| Age | Female |
Male |
Total |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| 1–10 | 1 (4.6%) | 0 (0%) | 1 (2.8%) |
| 11–20 | 3 (13.6%) | 4 (28.6%) | 7 (19.4%) |
| 21–30 | 8 (36.4%) | 6 (42.9%) | 14 (38.9%) |
| 31–40 | 1 (4.5%) | 3 (21.4%) | 4 (11.1%) |
| 41–50 | 3 (13.6%) | 0 (0%) | 3 (8.3%) |
| 51–60 | 3 (13.6%) | 1 (7.1%) | 4 (11.1%) |
| 61–70 | 3 (13.6%) | 0 (0%) | 3 (8.3%) |
25 out of 36 patients (69.4 %) had no known co-morbid conditions whilst 11 (30.6 %) presented with systemic diseases, the most frequent of which was epilepsy (18.2 %).
Etiological associations
The cases attributed to infectious agents, drugs, both, or none were analyzed. Infections stood as the sole trigger in 9 of the cases (25 %), while drugs were the single cause in 6 cases (16.7 %). Both infectious agents and drugs acted as triggers in 15 cases (41.7 %). However, neither an infectious agent nor any drug was identified as the cause (no triggering factor) in 6 patients (16.7 %). Overall, over 80 % of the cases involved either infectious and/or pharmacological triggers.
The most commonly identified triggering factor for EM onset was preceding infection with HSV (19.4 %), typically herpes labialis. Drugs were suspected as triggers in 21 patients (58.3 %), with penicillin (19 %), paracetamol (14.3 %), cephalosporin (14.3 %), and naproxen (9.5 %) being the most frequently implicated. The complete list of implicated infections and drugs is provided in Table 3.
Table 3.
Implicated etiology of erythema multiforme.
| n | % | ||
|---|---|---|---|
| Infection (n = 24) | Influenza | 6 | 16.7 |
| Influenza and herpes∗ | 1 | 2.8 | |
| Urinary | 1 | 2.8 | |
| Genitalia | 1 | 2.8 | |
| Herpes | 7 | 19.4 | |
| Odontogenic | 6 | 16.7 | |
| Cryptic tonsillitis | 2 | 5.6 | |
| Drug (n = 21) | Paracetamol | 3 | 14.3 |
| Paracetamol, cephalosporin, and naproxen∗∗ | 1 | 4.8 | |
| Paracetamol and penicillin∗∗ | 1 | 4.8 | |
| Depakin and levetiracetam∗∗ | 1 | 4.8 | |
| Levodopa and amantadine sulfate∗∗ | 1 | 4.8 | |
| Cephalosporin | 3 | 14.3 | |
| Mesalazine | 1 | 4.8 | |
| Penicillin | 4 | 19 | |
| Penicillin and naproxen∗∗ | 1 | 4.8 | |
| Ciprofloxacin | 1 | 4.8 | |
| Naproxen | 2 | 9.5 | |
| Naproxen and metronidazole∗∗ | 1 | 4.8 | |
| Macrolide and etodolac∗∗ | 1 | 4.8 |
Some patients reported more than one infection∗ or drug∗∗.
The incidence of EM was comparatively higher during spring and autumn, whereas it was lower during summer and winter.
Clinical manifestations
EM minor was diagnosed in 28 (77.8 %), while EM major was diagnosed in 8 patients (22.2 %). 12 patients (33.3 %) reported recurrence. Prodromal symptoms, including fever, malaise, or loss of appetite, preceded the onset of EM in 8 patients (22.2 %). Regional lymphadenopathy was detected in 13 patients (36.1 %).
All patients exhibited oral mucosal involvement. Moreover, 11.1 % (n = 4) presented also with genital, 8.3 % (n = 3) with nasal and 2.8 % (n = 1) with ocular involvement. Cutaneous involvement was observed in 16.7 % (n = 6) of patients and target or iris lesions located on the feet/legs, hands/arms, abdominal region and pectoral region were identified. Details are provided in Table 1.
Oral manifestations presented as erythema, erosions, bullae, and ulcerations on both non-keratinized and keratinized mucosal surfaces and bloodstained crusts on the lips (Figure 1, Figure 2). The labial mucosa was the most commonly affected oral site (86.1 %), followed by the upper and lower lips (80.6 %), and the buccal mucosa (50 %). The site least affected was the floor of the mouth (2.8 %). The number of affected oral sites ranged between 2 and 6 (mean: 3.03 ± 1.18, median: 3). Details of affected sites for each case can be found in Table 1.
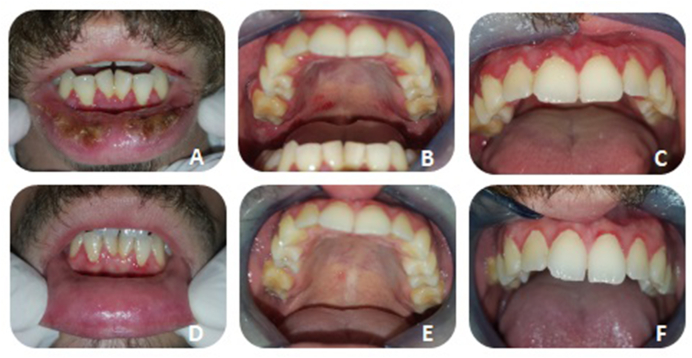
Figure 1.
Clinical photographs of the patient #20. (A, B, and C) The initial clinical photographs showing crusted erosions of the lips and irregular ulcerations on the hard palate and tongue. (D, E, and F) The clinical photographs after 3 weeks of treatment exhibiting complete healing of the oral mucosal lesions.
Figure 2.
Clinical photographs of the patient #29. (A, B, and C) The initial clinical photographs showing crusted erosions of the lips and irregular erosive and ulcerative lesions on the hard palate and gingivae. (D, E, and F) The clinical photographs after 2 weeks of treatment exhibiting complete healing of the oral mucosal lesions.
No significant differences were identified in demographic and clinical characteristics relating to triggering factor(s), clinical form, cutaneous/mucosal involvement, affected oral site(s), and recurrence (P > 0.05). On the other hand, a significantly higher incidence of EM major (40 %) was detected in patients above the age of 30 when compared to those under 30 (9.5 %) (P: 0.046; P < 0.05).
No significant differences were found among the triggering factor(s) in terms of clinical form, cutaneous/mucosal involvement, affected oral sites, and recurrence (P > 0.05). While there was a tendency for infection to be associated with recurrence (66.7 %), this trend was not statistically significant.
Treatment approaches
Management was related to the severity of the condition. Eleven (30.6 %) patients were referred to relevant medical specialties due to cutaneous or other mucosal involvement besides the oral mucosa. The most common form of treatment was solely topical steroid therapy (25 %), followed by topical antiseptic (8.3 %), topical steroid with topical antiseptic (8.3 %), and topical antiseptic with topical anesthetic therapy (8.3 %). The mean duration of the disease was 15.9 ± 4.1 days (range: 10–28, median: 14).
Discussion
EM presents a challenge for study because of its certain characteristics, such as the absence of a “gold standard” for clinical assessment, difficulty in estimating the onset, lack of reliable in vitro or in vivo tests for assessment of risk factors, and the high number of drugs, infections, or other potential risk factors that may predispose to the reaction. These features lead to difficulties in comparing EM data from different researchers (Table 4).13,14,26,27
Table 4.
Similar previous studies listed chronologically with reference number, author(s), country(s), institution(s), study design/time period, and study population.
| Year | Reference number | Author(s) | Country(s) | Institution(s) | Study design and time period | Study population |
|---|---|---|---|---|---|---|
| 1965 | 31 | Hellgren and Hersle | Sweden | Dermatology department | Retrospective 1953–1963 (no oral findings mentioned) |
224 patients range: 0–86 years 116 (51.8%) females, 108 (48.2%) males |
| 1976 | 32 | Al-Ubaidy and Nally | England | Oral medicine department | Retrospective – |
26 patients range: 18–49 years, mean: 32 years 16 (61.5%) females, 10 (38.5%) males |
| 1978 | 38 | Lozada and Silverman | USA | Oral diagnosis and oral medicine department | Prospective – |
50 patients range: 11–75 years 30 (60%) females, 20 (40%) males |
| 1984 | 33 | Howland et al. | USA | Dermatology department | Prospective – |
42 patients range: 2–79 years 19 (45.2%) females, 23 (54.8%) males |
| 1984 | 18 | Ting and Adam | Malaysia | Medical wards of a university hospital | Retrospective 1967–1982 |
59 patients range: 13–72 years 30 (50.%) females, 29 (49.1%) males |
| 1985 | 34 | Nethercott and Choi | Canada | Four different hospitals | Retrospective 1974–1980 |
123 patients 62 (50.4%) females, 61 (49.6%) males |
| 1989 | 35 | Lozada-Nur et al. | USA | Oral medicine clinic of a dentistry faculty | Retrospective 1976–1986 |
95 patients range: 17–77 years, mean: 43 years 57 (60%) females, 38 (40%) males |
| 1999 | 39 | Villiger et al. | Switzerland | Department of pediatrics | Retrospective 1978–1997 |
42 patients range: 0.1–15.8 years, mean: 6.1 years 15 (35.7%) females, 27 (64.3%) males |
| 2010 | 40 | Sanchis et al. | Spain | Service of stomatology | Retrospective 2000–2009 |
22 patients range: 18–85 years, mean: 47 years 8 (36.4%) females, 14 (63.6%) males |
| 2010 | 36 | Shabahang | Iran | Dermatology clinic | Prospective 2009–2010 (only 1 patient with oral mucosal involvement) |
61 patients range: 18–57 years, mean: 26.8 years 27 (44.3%) females, 34 (55.7%) males |
| 2015 | 29 | Celentano et al. | Italy and Romania | Oral medicine unit and oral medicine-oral pathology department | Retrospective 1982–2014 |
60 patients range: 7–78 years, mean: 37.9 years 29 (48.3%) females, 31 (51.7%) males |
| 2015 | 30 | Creţu et al. | Romania | Dermatology clinic | Retrospective 2006–2009 |
40 patients range: 12–78 years 23 (57.50%) females, 17 (42.50%) males |
| 2018 | 37 | Yavuz et al. | Turkey | Dermatology department | Retrospective 2005–2017 |
66 patients mean: 36.7 ± 13.9 years 44 (63.7%) females, 22 (33.3%) males |
| 2020 | 21 | Oluwadaisi et al. | Nigeria | Oral medicine clinic | Retrospective 2009–2019 |
19 patients range: 9–73 years, mean: 35.53 years 10 (52.6%) females, 9 (47.4%) males |
| 2023 | – | Current study Gungor et al. |
Turkey | Oral medicine clinic of a dentistry faculty | Retrospective 1999–2023 |
36 patients range: 6–66, mean: 32 years 22 (61.1%) females, 14 (38.9%) males |
Although epidemiological data on EM are limited and controversial predominantly because of the acute nature of the disease, the absence of a universally accepted classification, and the under-reporting of cases, particularly those of shorter duration that do not require hospitalization, accumulating evidence indicates that the reported worldwide prevalence of EM is lower than 1 %.2,14,18,21,28, 29, 30 This study further verified that EM constitutes a relatively rare disorder. Only 36 cases of EM were identified in an approximately 25-year period in our institution, a large tertiary care dental hospital serving as a referral center for a major metropolitan area.
It is claimed that EM mostly affects young adults aged 20 to 40 years.4,6,9,10,18,21,28,30, 31, 32, 33, 34, 35, 36, 37 Some studies suggest that females may be slightly more susceptible than males, although conclusive statistics on this topic are lacking.30, 31, 32,35,37,38 Nonetheless, there are also studies which report a slight male predominance in EM,33,36,39,40 or no sex differences at all (Table 4).18,21,29,34 In the present study, the highest incidence was observed in the age group between 20 and 29 years, with a greater representation of female patients. It is noteworthy that only one patient under the age of 10 with EM was included in the study group, possibly due to a preference for primary care by pediatricians. As our patients may not be a random sample of the population, this observation may only reflect our clinical referral population.
Although the etiopathogenesis of EM remains unclear, it has been associated with a type IV cytotoxic immune reaction, mediated by T-lymphocytes due to various factors including a number of infectious agents (viral or bacterial) and certain drugs (as well as several vaccines).6,9,25,32 Furthermore, this study comprising 36 EM patients has validated the highly heterogeneous etiological profile of this condition.
Many studies have indicated that HSV infection is the most frequently identified triggering factor in the onset of EM, particularly in the recurrent cases.2, 3, 4, 5, 6, 7, 8, 9,12,13,18,24,25,28, 29, 30,33,37,38 It has been reported that the most common site of HSV infection was the lips, and many patients observed a correlation between the size and severity of the HSV lesion and the occurrence and severity of the EM.4,30 In this study, the patient history and clinical examination indicated a potential association between HSV infection in 7 (19.4 %) EM patients as well as 5 (41.7 %) recurrent EM patients. Unfortunately, there is a paucity of studies using confirmatory PCR to ascertain the existence of HSV-DNA, and it is noteworthy that the identification of HSV infection relied mainly on clinical history, as was the case in our study. Apart from HSV infections, acute upper respiratory tract infections, such as influenza, were found to be a significant trigger for EM in a considerable number of cases, consistent with the findings of previous studies.18,39
The literature consistently indicates that adverse drug reactions typically account for less than 10 % of EM cases and are commonly related to non-steroidal anti-inflammatory drugs (NSAIDs), antipyretics, antiepileptics, and antibiotics.2,7,26,28,29,32 However, recent studies indicated that this rate may rise to 50 %, as the list of drugs associated with EM expands with the emergence of new drug categories.7,9,10,29,30,36,37,40 Identifying the suspected drug presents challenges as patients are often on multiple medications. A causal relationship can only be established when the outbreak occurs following the introduction of a new drug.30,40 Additionally, EM has been linked to the administration of influenza, measles, rubella, varicella, smallpox, mumps, diphtheria, tetanus, Hepatitis B, pneumococcal, and meningococcal vaccines.41, 42, 43 Recently, an association has also been reported between EM and the novel coronavirus [the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)], which was accused for the Coronavirus pandemic in 2019 (COVID-19), as well as the COVID-19 vaccine.44,45 Our research indicated that, in 6 patients (16.7 %), drug therapy alone - either prescribed by a physician or self-administered – might be associated with subsequent EM (implying a highly suggestive causative role). The most frequently implicated drugs in our series as triggers of EM were penicillin, paracetamol, cephalosporin, and naproxen, listed in order of decreasing prevalence. It is worth noting that this list shows considerable variation in different studies. Interestingly, no cases of EM due to SARS-CoV-2 infection or COVID-19 vaccine were detected in our group. Ulcerative colitis, a condition reported by Cameron et al. to cause EM due to sulfonamides which are frequently used in its treatment, was seen in only one patient in our study.46
In addition, it should be noted that in the 15 patients (41.7 %) with a notable history of drug use, both drugs and infectious agents were identified as possible triggers of the disease simultaneously. It is important to mention that, in general, these patients had taken drugs either for clinical complaints similar to the prodromal symptoms of EM or for causes unrelated to prodromal EM (possible causative role). As drugs were frequently prescribed to treat suspected or confirmed infections, there was an overlap between drugs and infections as underlying causes.18,29,30 The difficulty in attributing the illness to a single causal factor made data analysis more challenging. Frequent prescription of antibiotics and NSAIDs poses a challenge for causal analysis. It may be biologically possible that an infectious agent interacting with drug or its metabolite may provoke EM.14,47,48
The seasonal distribution in this study showed a relatively higher incidence in spring and autumn and a lower incidence in summer and winter. Occurrences observed mainly in spring and autumn have also been reported by others in the literature.32 However, Hellgren and Hersle reported a higher incidence in spring and early summer and a lower incidence in late summer, autumn, and winter.31
EM is classified into EM minor and EM major based on the presence or absence of mucous membrane involvement or the extent or number of mucosal sites involved.2,8,9 In this study, we adopted the classification system proposed by Al-Johani et al., which defines EM minor and EM major as the involvement of one or more mucosal sites, respectively.9 A diagnosis of EM minor was established in 28 patients (77.8 %) while EM major in 8 patients (22.2 %). The low number of EM major cases may be attributed to the fact that most patients with considerable cutaneous lesions seek care at dermatology clinics, with only those exhibiting oral mucosal involvement are referred to oral medicine clinics, as reported in previous studies.21,29 Due to considerable differences in clinical features and natural history between the two groups, the characteristics of a particular patient population with EM will vary depending on the proportion of patients with minor or major EM.18
Interestingly, Celentano et al. reported a substantial sex preference for the clinical forms, with EM major more common in females and EM minor more common in males, and also significantly correlated the clinical forms with age.29 In our study, no significant differences were identified between the clinical forms in relation to sex. However, our analysis highlighted a significantly higher occurrence of EM major in individuals over the age of 30 (40 %) in comparison to those under 30 years of age (9.5 %) (P: 0.046; P < 0.05).
Recurrent EM cases constituted 33.3 % of the patients (12 cases) in our study, of which 5 were females (41.7 %) and 7 were males (58.3 %). Two cases were idiopathic (16.7 %) and 4 cases were caused by HSV infection (33.3 %). Wetter and Davis conducted a retrospective analysis of recurrent EM patients and reported that 58 % of 48 patients were female, and HSV caused recurrent EM in 11 (23 %) patients, while the cause was unknown in 28 (58 %).10 In contrast to this study, in the study of Shabahang, no cases of recurrent EM were attributed to HSV infection.36 Wetter and Davis discovered genital lesions in 10 (21 %) patients and ocular disease in 1 patient, implying that recurrent EM may involve mucosal surfaces other than the oral cavity less commonly.10 Schofield et al. reported that out of 65 patients, 25 % had genital involvement and 17 % had ocular involvement.4 Pope and Krafchik reported a case of recurrent EM induced by HSV, affecting 3 mucous membranes including oral, genital, and ocular mucosa.49 In our study, of the 12 patients with recurrent EM, 2 had genital, 1 had nasal, and 1 had ocular lesions along with the oral mucosal lesions.
EM may display a wide spectrum of clinical disease. Cutaneous involvement is usually in the form of typical “target” or “iris” lesions symmetrically distributed over the extremities and trunk, with or without oral or other mucosal (nasal, ocular, pharyngeal, tracheal, and genital) involvement.2, 3, 4,7,8,32,38 According to the findings of this study and those reported in the literature, the most frequent locations for cutaneous involvement were found to be the feet and legs (42.8 %) and hands and arms (28.5 %).4,30, 31, 32, 33,36,40
The reported incidence of oral lesions varies notably (16–70 %), depending partly on whether the study is conducted in an oral medicine or a dermatology/internal medicine clinic population.4,7,9,10,19,29,30,35,37,38 In our series, all patients presented with oral lesions, whilst 28 (77.8 %) had exclusive oral involvement. In EM, oral lesions manifest as painful, erythematous, erosive or ulcerative patches on the non-keratinized mucosa and crusted erosions on the lips.4,19,20,32,38 Consistent with prior research, the labial mucosa and upper and lower lips were detected to be the most frequent sites of mucosal involvement in this study.9,10,21,29,35
The diagnosis of EM is a challenging task, particularly when the disease is confined to the oral mucosa.32 The primary basis for EM diagnosis is the patient history and clinical presentation, since pathognomonic histopathological features are unavailable and laboratory investigations, except for an increased erythrocyte sedimentation rate in severe cases, lack specificity.7,28,32,38 In this study, an intraoral biopsy was carried out on only 3 patients to distinguish between various diseases affecting the oral cavity, including pemphigus, pemphigoid, and oral lichen planus.
The management of EM varies depending on the severity of the lesions, and there is no single specific treatment that has been universally recognized as optimal.6,9,32 Identification and elimination of the suspected triggering agent and adequate symptomatic supportive treatment suffice for most patients.8,14 Nonetheless, patients with severe EM may necessitate hospitalization for systemic therapy.4,8 Interdisciplinary collaboration is vital in cases of EM major, given the potential need for consultations with ophthalmology, ear-nose-throat, gynecology, urology, and gastroenterology.30 Of the patients in this study, 11 (30.6 %) were referred to relevant medical specialties due to cutaneous or mucosal involvement other beyond the oral mucosa (nasal, genital, or ocular). The symptoms of the remaining patients were relieved using various topical agents and systemic drugs. Therapeutic management for alleviating oral symptoms incorporated interventions such as antiseptic and analgesic mouthwashes, anesthetic ointments, oral corticosteroid hydrogel, and in some cases systemic treatment with corticosteroids.
The limitations of this study primarily stem from its retrospective design. The authors are aware of the limitations of making decisions based on reported clinical features rather than by direct examination. A prospective study with standardized data collection along with clinical photographs of patients could completely eliminate this problem. A multicenter design would be beneficial due to the low incidence of the disease. Etiological factors may have been inaccurately identified in some patients within the study group, as they were self-reported. Therefore, we suggest treating this aspect of our findings as descriptive. Recurrence rates were established through a review of patient histories and/or readmissions pertaining to this condition. It is possible that some patients experiencing recurrence may not have been readmitted, potentially leading to an underestimation of recurrence rates. As previously stated, our clinic acts as a tertiary referral center. Since these patients were selectively referred for treatment, our patient cohort may not accurately represent the general population.
Accurate diagnosis and management of EM, which presents with variable signs and symptoms, is of utmost importance. To the best of our knowledge, this study is one of the few studies in the literature that assesses the characteristics of EM outpatients and the results may be considered as an epidemiological source for the region. Large-scale, multicenter, controlled prospective clinical studies are necessary to gain a more profound understanding of the underlying mechanisms involved in the development of EM, and to further validate our research findings. It is essential to inform patients about potential exposure to drugs and infectious agents, and to encourage them to exercise caution.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
References
- 1.von Hebra F. vol. 1. The New Sydenham Society; London: 1866. (On diseases of the skin including the exanthemata). [Google Scholar]
- 2.Huff J.C., Weston W.L., Tonnesen M.G. Erythema multiforme: a critical review of characteristics, diagnostic criteria, and causes. J Am Acad Dermatol. 1983;8:763–775. doi: 10.1016/s0190-9622(83)80003-6. [DOI] [PubMed] [Google Scholar]
- 3.Brice S.L., Huff J.C., Weston W.L. Erythema multiforme. Curr Probl Dermatol. 1990;2:5–25. [Google Scholar]
- 4.Schofield S.L., Tatnall F.M., Leigh I.M. Recurrent erythema multiforme: clinical features and treatment in a large series of patients. Br J Dermatol. 1993;128:542–545. doi: 10.1111/j.1365-2133.1993.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 5.Weston W.L. What is erythema multiforme? Pediatr Ann. 1996;25:106–109. doi: 10.3928/0090-4481-19960201-11. [DOI] [PubMed] [Google Scholar]
- 6.Farthing P., Bagan J.V., Scully C. Mucosal disease series. Number IV. Erythema multiforme. Oral Dis. 2005;11:261–267. doi: 10.1111/j.1601-0825.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 7.Scully C., Bagan J. Oral mucosal diseases: erythema multiforme. Br J Oral Maxillofac Surg. 2008;46:90–95. doi: 10.1016/j.bjoms.2007.07.202. [DOI] [PubMed] [Google Scholar]
- 8.Sokumbi O., Wetter D.A. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889–902. doi: 10.1111/j.1365-4632.2011.05348.x. [DOI] [PubMed] [Google Scholar]
- 9.Al-Johani K.A., Fedele S., Porter S.R. Erythema multiforme and related disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:642–654. doi: 10.1016/j.tripleo.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Wetter D.A., Davis M.D.P. Recurrent erythema multiforme: clinical characteristics, etiologic associations, and treatment in a series of 48 patients at Mayo Clinic, 2000 to 2007. J Am Acad Dermatol. 2010;62:45–53. doi: 10.1016/j.jaad.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 11.Bastuji-Garin S., Rzany B., Stern R.S., Shear N.H., Naldi L., Roujeau J.C. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92–96. [PubMed] [Google Scholar]
- 12.Assier H., Bastuji-Garin S., Revuz J., Roujeau J.C. Erythema multiforme with mucous membrane involvement and Stevens-Johnson syndrome are clinically different disorders with distinct causes. Arch Dermatol. 1995;131:539–543. [PubMed] [Google Scholar]
- 13.Auquier-Dunant A., Mockenhaupt M., Naldi L., Correia O., Schröder W., Roujeau J.C. SCAR (Severe Cutaneous Adverse Reactions) Study Group. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch Dermatol. 2002;138:1019–1024. doi: 10.1001/archderm.138.8.1019. [DOI] [PubMed] [Google Scholar]
- 14.Forman R., Koren G., Shear N.H. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a review of 10 years' experience. Drug Saf. 2002;25:965–972. doi: 10.2165/00002018-200225130-00006. [DOI] [PubMed] [Google Scholar]
- 15.Lerch M., Mainetti C., Terziroli Beretta-Piccoli B., Harr T. Current perspectives on erythema multiforme. Clin Rev Allergy Immunol. 2018;54:177–184. doi: 10.1007/s12016-017-8667-7. [DOI] [PubMed] [Google Scholar]
- 16.Bateman T. Longman; London: 1817. Delineations of cutaneous diseases: exhibiting the characteristic appearances of the principal genera and species comprised in the classification of the late Dr. Willan and completing the series of engravings begun by that author. Hurst, Rees, Orme, and Brown. [Google Scholar]
- 17.Thomas B.A. The so-called Stevens-Johnson syndrome. Br Med J. 1950;1:1393–1397. doi: 10.1136/bmj.1.4667.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ting H.C., Adam B.A. Erythema multiforme: epidemiology, clinical characteristics and natural history in fifty-nine patients. Australas J Dermatol. 1984;25:83–88. doi: 10.1111/j.1440-0960.1984.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 19.Kennett S. Erythema multiforme affecting the oral cavity. Oral Surg Oral Med Oral Pathol. 1968;25:366–373. doi: 10.1016/0030-4220(68)90010-8. [DOI] [PubMed] [Google Scholar]
- 20.Woo S.B., Setterfield J.F., Greenberg M.S. In: Burket's oral medicine. 13th ed. Glick M., Greenberg M.S., Lockhart P.B., Challacombe S.J., editors. John Wiley & Sons, Inc.; USA: 2021. Ulcerative, Vesicular, and Bullous Lesions; pp. 35–85. [Google Scholar]
- 21.Oluwadaisi A.M., Adewale A.A., Mogaji I.K., Oyetola E.O., Owotade F.J. Erythema multiforme: a retrospective study of the clinical manifestations of patients attending an oral medicine clinic in Nigeria. Int J Res Rep Dent. 2020;3:6–14. [Google Scholar]
- 22.Chen C.W., Tsai T.F., Chen Y.F., Hung C.M. Persistent erythema multiforme treated with thalidomide. Am J Clin Dermatol. 2008;9:123–127. doi: 10.2165/00128071-200809020-00006. [DOI] [PubMed] [Google Scholar]
- 23.Levin J., Hofstra T. Recurrent erythema multiforme. JAMA. 2014;312:426–427. doi: 10.1001/jama.2014.3611. [DOI] [PubMed] [Google Scholar]
- 24.Turnbull N., Hawkins D., Atkins M., Francis N., Roberts N. Persistent erythema multiforme associated with Epstein-Barr virus infection. Clin Exp Dermatol. 2014;39:154–157. doi: 10.1111/ced.12243. [DOI] [PubMed] [Google Scholar]
- 25.Léauté-Labrèze C., Lamireau T., Chawki D., Maleville J., Taïeb A. Diagnosis, classification, and management of erythema multiforme and Stevens-Johnson syndrome. Arch Dis Child. 2000;83:347–352. doi: 10.1136/adc.83.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan H.L., Stern R.S., Arndt K.A., et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990;126:43–47. [PubMed] [Google Scholar]
- 27.Rzany B., Mockenhaupt M., Baur S., et al. Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990-1992): structure and results of a population-based registry. J Clin Epidemiol. 1996;49:769–773. doi: 10.1016/0895-4356(96)00035-2. [DOI] [PubMed] [Google Scholar]
- 28.Samim F., Auluck A., Zed C., Williams P.M. Erythema multiforme: a review of epidemiology, pathogenesis, clinical features, and treatment. Dent Clin. 2013;57:583–596. doi: 10.1016/j.cden.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Celentano A., Tovaru S., Yap T., Adamo D., Aria M., Mignogna M.D. Oral erythema multiforme: trends and clinical findings of a large retrospective European case series. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:707–716. doi: 10.1016/j.oooo.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Creţu A., Dimitriu A., Brănişteanu D., Brinişteanu D.E. Erythema multiforme - etiopathogenic, clinical and therapeutic aspects. Rev Med-Chir Soc Med Nat Iasi. 2015;119:55–61. [PubMed] [Google Scholar]
- 31.Hellgren L., Hersle K. Erythema multiforme. Statistical evaluation of clinical and laboratory data in 224 patients and matched healthy controls. Acta Allergol. 1966;21:45–51. [PubMed] [Google Scholar]
- 32.Al-Ubaidy S.S., Nally F.F. Erythema multiforme. Review of twenty-six cases. Oral Surg Oral Med Oral Pathol. 1976;41:601–606. doi: 10.1016/0030-4220(76)90312-1. [DOI] [PubMed] [Google Scholar]
- 33.Howland W.W., Golitz L.E., Weston W.L., Huff J.C. Erythema multiforme: clinical, histopathologic, and immunologic study. J Am Acad Dermatol. 1984;10:438–446. doi: 10.1016/s0190-9622(84)80090-0. [DOI] [PubMed] [Google Scholar]
- 34.Nethercott J.R., Choi B.C. Erythema multiforme (Stevens-Johnson syndrome) - chart review of 123 hospitalized patients. Dermatol. 1985;171:383–396. doi: 10.1159/000249463. [DOI] [PubMed] [Google Scholar]
- 35.Lozada-Nur F., Gorsky M., Silverman S., Jr. Oral erythema multiforme: clinical observations and treatment of 95 patients. Oral Surg Oral Med Oral Pathol. 1989;67:36–40. doi: 10.1016/0030-4220(89)90299-5. [DOI] [PubMed] [Google Scholar]
- 36.Shabahang L. Characteristics of adult outpatients with erythema multiforme. Pakistan J Biol Sci. 2010;13:1106–1109. doi: 10.3923/pjbs.2010.1106.1109. [DOI] [PubMed] [Google Scholar]
- 37.Yavuz I.H., Yavuz G.Ö., Bilgili S.G., Bayram I., Savaş H. Erythema multiforme; sixty six case series with review of literature. E J Med. 2018;23:308–312. [Google Scholar]
- 38.Lozada F., Silverman S., Jr. Erythema multiforme. Clinical characteristics and natural history in fifty patients. Oral Surg Oral Med Oral Pathol. 1978;46:628–636. doi: 10.1016/0030-4220(78)90458-9. [DOI] [PubMed] [Google Scholar]
- 39.Villiger R.M., von Vigier R.O., Ramelli G.P., Hassink R.I., Bianchetti M.G. Precipitants in 42 cases of erythema multiforme. Eur J Pediatr. 1999;158:929–932. doi: 10.1007/s004310051244. [DOI] [PubMed] [Google Scholar]
- 40.Sanchis J.M., Bagán J.V., Gavaldá C., Murillo J., Diaz J.M. Erythema multiforme: diagnosis, clinical manifestations and treatment in a retrospective study of 22 patients. J Oral Pathol Med. 2010;39:747–752. doi: 10.1111/j.1600-0714.2010.00912.x. [DOI] [PubMed] [Google Scholar]
- 41.Griffith R.D., Miller O.F., 3rd Erythema multiforme following diphtheria and tetanus toxoid vaccination. J Am Acad Dermatol. 1988;19:758–759. doi: 10.1016/s0190-9622(88)80355-4. [DOI] [PubMed] [Google Scholar]
- 42.Di Lernia V., Lo Scocco G., Bisighini G. Erythema multiforme following hepatitis B vaccine. Pediatr Dermatol. 1994;11:363–364. doi: 10.1111/j.1525-1470.1994.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 43.Patja A., Davidkin I., Kurki T., Kallio M.J., Valle M., Peltola H. Serious adverse events after measles-mumps-rubella vaccination during a fourteen-year prospective follow-up. Pediatr Infect Dis J. 2000;19:1127–1134. doi: 10.1097/00006454-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Jimenez-Cauhe J., Ortega-Quijano D., Carretero-Barrio I., et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol. 2020;45:892–895. doi: 10.1111/ced.14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMahon D.E., Amerson E., Rosenbach M., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cameron A.J., Baron J.H., Priestley B.L. Erythema multiforme, drugs, and ulcerative colitis. Br Med J. 1966;2:1174–1178. doi: 10.1136/bmj.2.5523.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carducci M., Latini A., Acierno F., Amantea A., Capitanio B., Santucci B. Erythema multiforme during cytomegalovirus infection and oral therapy with terbinafine: a virus-drug interaction. J Eur Acad Dermatol Venereol. 2004;18:201–203. doi: 10.1111/j.1468-3083.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- 48.González-Delgado P., Blanes M., Soriano V., Montoro D., Loeda C., Niveiro E. Erythema multiforme to amoxicillin with concurrent infection by Epstein-Barr virus. Allergol Immunopathol. 2006;34:76–78. doi: 10.1157/13086752. [DOI] [PubMed] [Google Scholar]
- 49.Pope E., Krafchik B.R. Involvement of three mucous membranes in herpes-induced recurrent erythema multiforme. J Am Acad Dermatol. 2005;52:171–172. doi: 10.1016/j.jaad.2004.06.048. [DOI] [PubMed] [Google Scholar]