Abstract
Background
Acne is a common skin disorder among women. Although no uniform approach to the management of acne exists, combination oral contraceptives (COCs), which contain an estrogen and a progestin, often are prescribed for women.
Objectives
To determine the effectiveness of combined oral contraceptives (COCs) for the treatment of facial acne compared to placebo or other active therapies.
Search methods
In January 2012, we searched for randomized controlled trials of COCs and acne in the computerized databases of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, POPLINE, and LILACS. We also searched for clinical trials in ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP) (Aug 2011). For the initial review, we wrote to researchers to seek any unpublished or published trials that we might have missed.
Selection criteria
We considered randomized controlled trials reported in any language that compared the effectiveness of a COC containing an estrogen and a progestin to placebo or another active therapy for acne in women.
Data collection and analysis
We extracted data on facial lesion counts, both total and specific (i.e., open or closed comedones, papules, pustules and nodules); acne severity grades; global assessments by the clinician or the participant, and discontinuation due to adverse events. Data were entered and analyzed in RevMan. For continuous data, we calculated the mean difference (MD) and 95% confidence interval (CI). For dichotomous data, we calculated the Peto odds ratio (OR) and 95% CI.
Main results
The review includes 31 trials with 12,579 participants. Of 24 comparisons made, 6 compared a COC to placebo, 17 different COCs, and 1 compared a COC to an antibiotic. Of nine placebo‐controlled trials with data for analysis, all showed COCs reduced acne lesion counts, severity grades and self‐assessed acne compared to placebo. A levonorgestrel‐COC group had fewer total lesion counts (MD ‐9.98; 95% CI ‐16.51 to ‐3.45), inflammatory and non‐inflammatory lesion counts, and were more likely to have a clinician assessment of clear or almost clear lesions and participant self‐assessment of improved acne lesions. A norethindrone acetate COC had better results for clinician global assessment of no acne to mild acne (OR 1.86; 95% CI 1.32 to 2.62). In two combined trials, a norgestimate COC showed reduced total lesion counts (MD‐9.32; 95% CI ‐14.19 to ‐4.45), reduced inflammatory lesion and comedones counts, and more with clinician assessment of improved acne. For two combined trials of a drospirenone COC, the investigators' assessment of clear or almost clear skin favored the drospirenone group (OR 3.02; 95% CI 1.99 to 4.59). In one trial, the drospirenone‐COC group showed greater (more positive) percent changes for total lesion count (MD 29.08; 95% CI 3.13 to 55.03), inflammatory and non‐inflammatory lesion counts, and papule and closed comedone counts. A dienogest‐COC group had greater percentage decreases in total lesion count (MD ‐15.30; 95% CI ‐19.98 to ‐10.62) and inflammatory lesion count, and more women assessed with overall improvement of facial acne. A CMA‐COC group had more 'responders,' those with 50% or greater decrease in facial papules and pustules (OR 2.31; 95% CI 1.50 to 3.55)
Differences in the comparative effectiveness of COCs containing varying progestin types and dosages were less clear, and data were limited for any particular comparison. COCs that contained chlormadinone acetate or cyproterone acetate improved acne better than levonorgestrel. A COC with cyproterone acetate showed better acne outcomes than one with desogestrel, but the studies produced conflicting results. Likewise, levonorgestrel showed a slight improvement over desogestrel in acne outcomes, but results were not consistent. A drospirenone COC appeared to be more effective than norgestimate or nomegestrol acetate plus 17β‐estradiol but less effective than cyproterone acetate.
Authors' conclusions
This update yielded six new trials but no change in conclusions. The six COCs evaluated in placebo‐controlled trials are effective in reducing inflammatory and non‐inflammatory facial acne lesions. Few important and consistent differences were found between COC types in their effectiveness for treating acne. How COCs compare to alternative acne treatments is unknown since only one trial addressed this issue. The use of standardized methods for assessing acne severity would help in synthesizing results across trials as well as aid in interpretation.
Keywords: Female; Humans; Acne Vulgaris; Acne Vulgaris/drug therapy; Contraceptives, Oral, Combined; Contraceptives, Oral, Combined/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Effect of birth control pills on acne in women
Acne is a common skin problem for women. Several treatments are available. Combined birth control pills, which have the hormones estrogen and progestin, are often prescribed for women with acne. This review looked at how well birth control pills worked to treat facial acne.
In January 2012, we did a computer search for studies of birth control pills and acne treatment. Outcomes could be the amount of acne, how severe the acne was, and how many women dropped out early due to problems. We wrote to researchers to find other trials. We included randomized trials in any language that compared two types of birth control pills, a pill and a placebo or 'dummy,' or a pill and another acne treatment.
The review now includes 31 trials with a total of 12,579 women. Ten studies used dummies. Overall, 24 pairs of treatments or placebos were compared: 6 compared a birth control pill and a placebo, 17 compared different types of birth control pills, and 1 compared a pill and an antibiotic. The six pills studied in trials with placebos worked well to reduce facial acne. When we compared pills with different hormones, we did not see any important and consistent differences.
The conclusions did not change when we added trials in this update. Most trials compared two types of pills for acne treatment. Better quality studies are needed to compare one birth control pill with another. Studies should use standard methods for reporting how severe the acne is. How birth control pills compare to other acne treatments like antibiotics is not clear. Since birth control pills improve acne, they can be used to treat women with acne who also want birth control.
Background
Description of the condition
Acne vulgaris is a skin disorder of the sebaceous follicles that presents as lesions that are either inflamed (i.e., papules, pustules and nodules) or non‐inflamed (i.e., open or closed comedones, papules, pustules and nodules) (Toyoda 2001). It is one of the most common skin conditions requiring medical treatment, yet its pathophysiology is poorly understood. The characteristic localization of acne to the face and upper trunk is a result of the distribution of oil‐secreting structures known as sebaceous glands within the hair follicles. Although the etiology of acne involves a number of interrelated factors (George 2008), a large amount of evidence indicates that one factor may be an increased rate of sebum production, which is predominantly controlled by androgenic sex hormones.
Acne is a common condition. For example, the prevalence of clinical acne among Danish adolescent women aged 15 to 22 years was 24% (Jemec 2002). In a French study of women aged 25 to 40 years, 41% of the respondents reported currently having acne (Poli 2001). A clinical UK study of women aged 25 years or older found that 54% of the participants had facial acne and 12% had clinical facial acne (Goulden 1999). Acne affects an estimated 40 to 50 million people in the US annually (George 2008). More than 25% of acne sufferers in the US consult a physician annually (Bergfeld 1995). In addition to the morbidity associated with lesions and adverse effects of treatments, acne can produce life‐long physical and emotional scars (Baldwin 2002). The cost of acne treatment constitutes an economic burden to both the individual and community.
Description of the intervention
No consensus exists as to the most appropriate approach to the management of acne. Individual acne lesions can remit spontaneously by unknown mechanisms that may be associated with de‐differentiation of follicular cells that produce sebum or by spontaneous resolution of inflammatory changes in the lesions (Downie 2002). Although effective prescribed medications are available, many women rely on over‐the‐counter preparations and herbal remedies, in conjunction with strict skin hygiene routines and dietary modifications (Webster 2002). Treatment options generally target one or more of the factors implicated in acne pathogenesis (i.e., blockage of hair follicle openings, bacteria colonization, abnormal keratinization or excess sebum production). Medications that affect one or more of these mechanisms either alone or in combination are commonly used (Eady 1994; Leyden 1997; Burkhart 2000; Thiboutot 2000; Webster 2002).
A number of COCs, containing different progestins and hormonal dosages, are prescribed for women with acne, often for their dual functions of acne treatment and contraception. COCs traditionally used for acne treatment contain cyproterone acetate (CPA) and ethinyl estradiol (EE). In the UK, for example, the COC containing CPA 2 mg and EE 35 μg is licensed for treatment of women with severe acne that is refractory to prolonged treatment with antibiotics (Seaman 2003). In the US, three COCs have been approved by the Food and Drug Administration for treating moderate acne (O'Connell 2008). The progestins in these COCs are norethindrone, norgestimate, and drospirenone.
How the intervention might work
Combined oral contraceptives (COCs) are thought to alter the prognosis of acne through several mechanisms. First, COCs appear to decrease free testosterone levels by 40% to 50%, on average (Fotherby 1994; Thorneycroft 1999). This is due to a reduced production of the androgen, testosterone, achieved by the suppression of luteinizing hormone, which causes decreased androgen synthesis. Androgen bioavailability also is reduced when COCs increase the level of the protein that binds free androgens (sex hormone‐binding globulin), which in turn, increases binding of testosterone. Secondly, testosterone has to be converted in the hair follicles and skin to dihydrotestosterone by the enzyme 5‐alpha reductase to lead to acne (Cassidenti 1991). COCs prevent this conversion of free testosterone to dihydrotestosterone by blocking androgen receptors and inhibiting 5‐alpha‐reductase activity. Progestin types appear to differ in the degree to which they prevent testosterone production, bioavailability, or conversion (Rabe 2000).
Objectives
The review examined whether any COC is more effective than other COCs, oral or topical anti‐acne medications or placebo in the treatment of facial acne in women. We evaluated the following hypotheses:
COC treatment for facial acne in females is more effective than no treatment or placebo in reducing the severity of facial acne;
COCs do not differ significantly in their efficacy, and are not more efficacious than other oral or topical anti‐acne medications in reducing the severity of facial acne;
COCs do not differ significantly in discontinuation due to side effects.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials reported in any language
Types of participants
Women of any age for whom facial acne vulgaris was assessed
Types of interventions
Any COC compared to a second COC, oral or topical anti‐acne medication, no treatment or placebo. The COC treatment groups also could have included concomitant acne treatment.
Types of outcome measures
Trials must have reported on the effectiveness of drug treatment using at least one of the following outcomes:
Change in specific types of facial lesion (i.e., open or closed comedones, papules, pustules or nodules) counts from baseline to last available evaluation or the specific facial lesion counts at the last available evaluation;
Change in total facial lesion counts from baseline to last available evaluation or the total facial lesion counts at the last available evaluation;
Global assessments made by the clinician or the participant regarding improvement in skin condition (e.g. excellent, good or fair progress versus no change or worse);
Psychosocial function outcomes, such as quality of life and disability indices, and utility outcome (e.g. willingness to pay or accept treatment); and
Early study discontinuation due to adverse events, including worsening of acne.
Search methods for identification of studies
Electronic searches
In January 2012, we searched the computerized databases of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE using PubMed, POPLINE, and LILACS. In addition, we searched for recent clinical trials through ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP) (Aug 2011). The search strategies are given in Appendix 1. Strategies for earlier versions of this review are shown in Appendix 2.
Searching other resources
For the initial review, we also assessed the reference lists of published reports and sought information from colleagues on unpublished trials, technical reports, conference proceedings and dissertations. Other experts and pharmaceutical companies with an interest in this topic were contacted for relevant published or unpublished reports.
Data collection and analysis
Selection of studies
One author assessed all titles and abstracts located in the literature searches to determine their relevance to the objective of the review. Translators were used for potentially eligible studies written in languages other than English.
Data extraction and management
Two authors independently extracted data from the studies identified for inclusion. These data included the method of randomization, allocation concealment, blinding, pre‐treatment washout periods, types of interventions, participant characteristics, premature withdrawals from the trial, and outcome measures. The authors were not blinded to the authors, journals or institutions.
Assessment of risk of bias in included studies
The authors also assessed the quality of each trial and determined its methodological strengths and weaknesses. Guidance for such an assessment can be found in Higgins 2011. The criteria included adequacy of sample size, randomization protocol, allocation concealment, inclusion and exclusion criteria, blinding, the extent of premature withdrawals and loss to follow up and method of analysis. Discrepancies were resolved by discussion. We wrote to the corresponding researchers for clarification or additional data.
Data synthesis
Data were entered and analyzed with RevMan. For trials that included two or more intervention groups, we made all possible comparisons between groups. We calculated the number of events for any outcomes that were published as percentages if the absolute numbers for the numerators or denominators were provided. For continuous data, we extracted means and standard deviation or standard error and calculated the mean difference and 95% confidence interval (CI). For dichotomous data, we calculated the Peto odds ratio (OR) and 95% CI. We combined results of different trials in meta‐analysis to calculate a weighted treatment effect across trials if they had similar types of interventions (i.e., same progestin type and dose, estrogen dose, and number of treatment cycles) and outcome measures.
Results
Description of studies
Results of the search
Previous searches identified 83 trials potentially related to COC for the treatment of acne vulgaris. We located 80 trials through electronic databases and 3 trials by hand searching. Our 2012 search identified eight reports that were possibly eligible for inclusion. One was a pooled analysis of trials already included, and did not provide additional data for this review (Koltun 2011). Another was excluded due to the women not being diagnosed with acne prior to the intervention (Sanam 2011).
Included studies
The 2009 version of this review had 25 primary trials that met the inclusion criteria along with four secondary articles. We obtained additional data from corresponding researchers for three of the included articles.
In 2012, an additional 6 trials met the inclusion criteria for a new total of 31 trials. The new reports included four published trials (Palombo‐Kinne 2009; Plewig 2009; Kelly 2010; Mansour 2011) and two trials from ClinicalTrials.gov that had results available (J&J 2005; Bayer 2011). We also added a 2009 secondary article from Maloney 2008 for a total of five secondary articles.
A total of 12,579 participants were enrolled into the 31 trials. Individual sample sizes varied from 24 to 2152. The trials varied considerably in treatment arms. The doses of EE ranged from 20 μg to 50 μg and one trial used 17β‐estradiol (E2) 1.5 mg. The trials included 11 types of progestin. In this review, 24 comparisons were made: 6 compared a COC to placebo, 17 compared different COC groups, and 1 compared a COC to an antibiotic. The duration of the trials varied from 3 to 13 treatment cycles (mode=6 cycles). Only three studies had fewer than six treatment cycles (Dieben 1994; Thorneycroft 1999; J&J 2005). Most trials (N=27) received support from pharmaceutical companies. The trials used a variety of outcome measures for assessing acne (see Characteristics of included studies).
Risk of bias in included studies
Trial quality was assessed according to criteria related to the potential for selection, performance, attrition and detection biases, which could affect the validity of the results.
Allocation
Eleven studies described their methods of randomization (Palatsi 1984; Koetsawang 1995; Lucky 1997; Redmond 1997; Thiboutot 2001; Leyden 2002; Rosen 2003; Thorneycroft 2004; Koltun 2008; Maloney 2008; Mansour 2011).
Six trials had information on allocation concealment. Five trials attempted to conceal allocation: two used numbered, sealed envelopes (Thiboutot 2001; Leyden 2002), though the researchers did not mention if the envelopes were opaque; Kelly 2010 used 'randomization envelopes'; Redmond 1997 used numbered, sealed boxes; and Mansour 2011 had an interactive voice‐response system. Another researcher corresponded that the randomization list was open (Dieben 1994). The remaining trials did not provide information on allocation concealment.
Blinding
Twenty trials reported some blinding of which 19 described double‐blinding: 11 had double‐blinding involving the investigators and participants (Lachnit‐Fixson 1977; Carlborg 1986; Fugere 1988; Lucky 1997; Redmond 1997; Thiboutot 2001; Leyden 2002; Rosen 2003; Koltun 2008; Maloney 2008; Bayer 2011); 1 blinded the evaluators and participants (Kelly 2010); 7 reported double‐blinding but did not describe who was blinded (Aydinlik 1986; Maloney 2001; Van Vloten 2002; Thorneycroft 2004; J&J 2005; Palombo‐Kinne 2009; Plewig 2009). One trial was single‐blinded (investigator) (Worret 2001), and one did not have information on blinding (Palatsi 1984). Performance bias was likely in 10 trials that were open.
Incomplete outcome data
Exclusions after randomization were reported in 17 studies (Carlborg 1986; Fugere 1988; Dieben 1994; Koetsawang 1995; Charoenvisal 1996; Lucky 1997; Redmond 1997; Halbe 1998; Thiboutot 2001; Vartiainen 2001; Worret 2001; Thorneycroft 2004; J&J 2005; Palombo‐Kinne 2009; Plewig 2009; Bayer 2011; Mansour 2011). Attrition due to loss to follow up, early discontinuation, exclusions, missing data or unexplained reasons ranged from zero to 53% in the trials except for two studies that did not report attrition data (Aydinlik 1986; J&J 2005).
The analytic method varied among the trials. Eleven trials based the analysis on an intent‐to‐treat population or modified intent‐to‐treat population (Lucky 1997; Redmond 1997; Maloney 2001; Thiboutot 2001; Leyden 2002; Van Vloten 2002; Thorneycroft 2004; J&J 2005; Plewig 2009; Kelly 2010; Bayer 2011; Mansour 2011). In Koltun 2008 and Maloney 2008, US Food and Drug Administration changed the indication from "mild to moderate acne" to "moderate acne", thus reducing the primary analysis dataset. The remaining studies used a per‐protocol population, a population based on those completing the study, or an undescribed population.
Effects of interventions
A few studies could be combined in meta‐analysis since they had the same hormone treatments and the same study duration.
Monophasic levonorgestrel plus EE versus a placebo (Thiboutot 2001; Leyden 2002
Triphasic norgestimate plus EE versus a placebo (Lucky 1997; Redmond 1997)
Desogestrel plus EE and gestodene plus EE (Halbe 1998; Koetsawang 1995)
Drospirenone 3 mg plus EE 20 µg versus a placebo (Koltun 2008; Maloney 2008; Bayer 2011) though data for combined analysis were very limited.
COC versus placebo
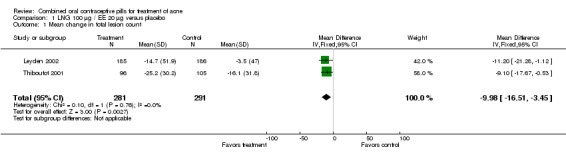
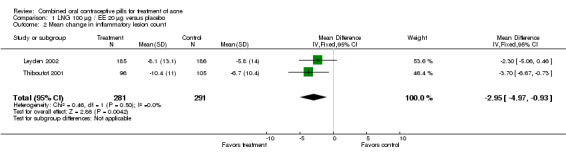
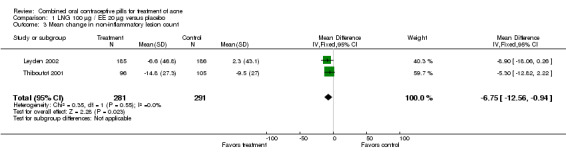
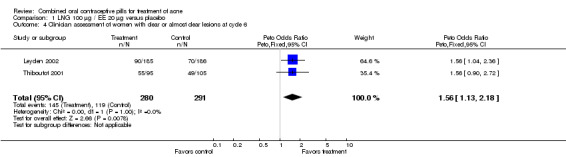
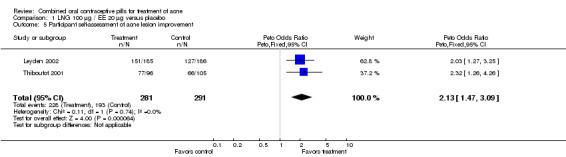
Of 10 trials that included a placebo group, 9 showed improvements in acne associated with the assigned COC, and one did not provide sufficient data for analysis (Koltun 2008). The two trials that compared levonorgestrel (LNG) 100 μg / EE 20 μg with a placebo were combined in a meta‐analysis (Thiboutot 2001; Leyden 2002). The COC group had fewer total lesion counts (mean difference ‐9.98; 95% CI ‐16.51 to ‐3.45), inflammatory lesion counts (mean difference ‐2.95; 95% CI ‐4.97 to ‐0.93), and non‐inflammatory lesion counts (mean difference ‐6.75; 95% CI ‐12.56 to ‐0.94) compared with the placebo group. The LNG/EE group also fared better than the placebo group regarding the clinician assessment of clear or almost clear lesions (OR 1.56; 95% CI 1.13 to 2.18) and the participant self‐assessment of improved acne lesions (OR 2.13; 95% CI 1.47 to 3.09).
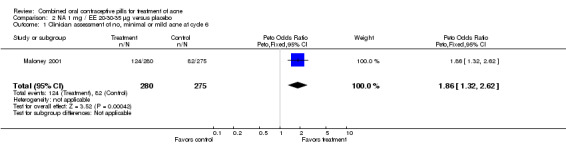
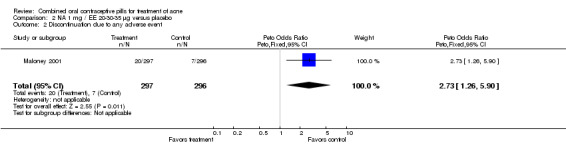
Maloney 2001 compared norethindrone acetate (NA) 1 mg / EE 20‐30‐35 μg with placebo. Women in the NA/EE group fared better for the clinician global assessment of no, minimal, or mild acne than those in the placebo group (OR 1.86; 95% CI 1.32 to 2.62).
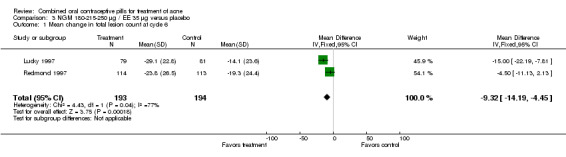
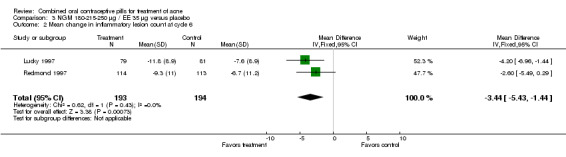
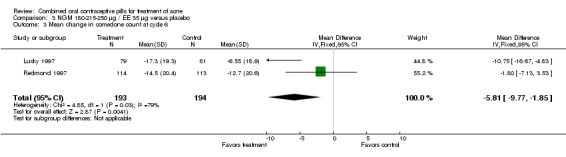
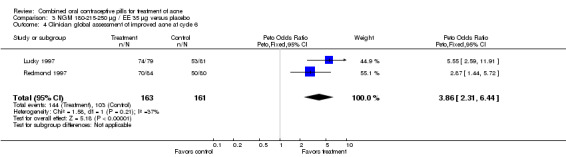
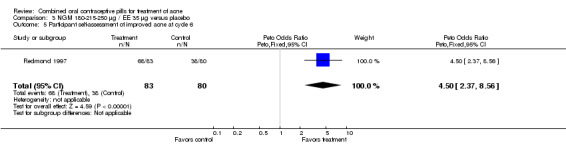
Data were combined from Lucky 1997 and Redmond 1997. Compared to the placebo group, women assigned to norgestimate (NGM) 180‐215‐250 μg / EE 35 μg had reduced total lesion counts (mean difference ‐9.32; 95% CI ‐14.19 to ‐4.45), reduced inflammatory lesion counts (mean difference ‐3.44; 95% CI ‐5.43 to ‐1.44) and reduced comedones counts (mean difference ‐5.81; 95% CI ‐9.77 to ‐1.85). Women in the NGM/EE group also were more likely to have improved acne compared to the placebo group for clinician global assessment (OR 3.86; 95% CI 2.31 to 6.44) for the combined trials (Lucky 1997; Redmond 1997) and for participant self‐assessment (OR 4.50; 95% CI 2.37 to 8.56) in Redmond 1997.
Three trials with similar design examined a COC with drospirenone 3 mg plus EE 20 µg versus a placebo (Koltun 2008; Maloney 2008; Bayer 2011). Most outcomes for Koltun 2008 and Maloney 2008 were shown in figures without specific data to analyze in this review.
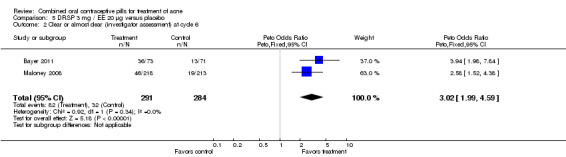
Bayer 2011 and Maloney 2008 were combined in a meta‐analysis for the investigators' assessment of clear or almost clear skin. The result favored the treatment group (OR 3.02; 95% CI 1.99 to 4.59).
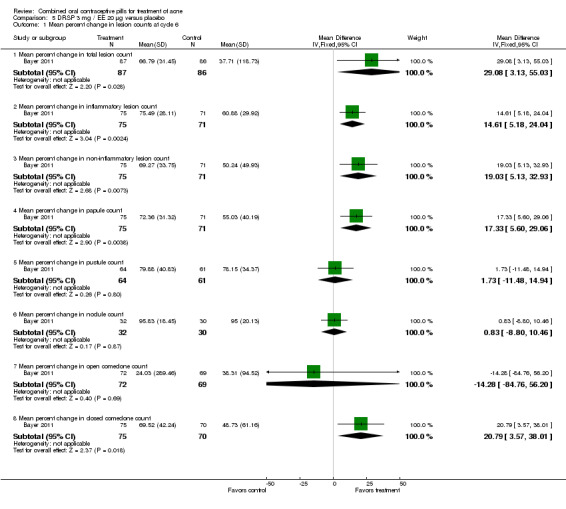
Bayer 2011 reported percent changes in various the lesion counts by cycle six. Improvement in lesion count is indicated by larger percent change: [(count at baseline ‐ count at cycle 6)/count at baseline]*100. Compared to the placebo group, the drospirenone COC group showed greater (more positive) percent changes for total lesion count (MD 29.08; 95% CI 3.13 to 55.03), inflammatory lesion count (MD 14.61; 95% CI 5.18 to 24.04), non‐inflammatory lesion count (MD 19.03; 95% CI 5.13 to 32.93), papule count (MD 17.33; 95% CI 5.60 to 29.06), and closed comedone count (MD 20.79; 95% CI 3.57 to 38.01). The groups were not significantly different for changes in pustule count, nodule count, and open comedone count
Both Koltun 2008 and Maloney 2008 reported the mean percentage reduction in lesion count was significantly greater in the COC group compared to the placebo (reported P < 0.0001 and < 0.001, respectively). The results included inflammatory, non‐inflammatory, and total lesion count. A 2009 secondary paper for Maloney 2008 reported on lesion counts (nodules, papules, pustules, open comedones and closed comedones) but results were provided in figures without absolute numbers. Koltun 2008 reported the odds were greater for the investigator's assessment of clear or almost clear skin among the COC group versus placebo (reported P = 0.0001). Similarly, more women in the COC group reportedly rated their skin improved compared to the placebo group (reported P = 0.0005).
A later publication (Koltun 2011) reported on pooled analysis of data from these two trials. Results for lesions were again presented in figures without numbers; P values were reported. The researchers reported percent of participants rated by investigator as 'clear' or 'almost clear,' as they did in Maloney 2008.
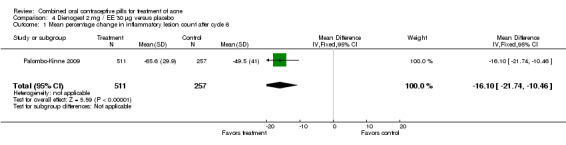
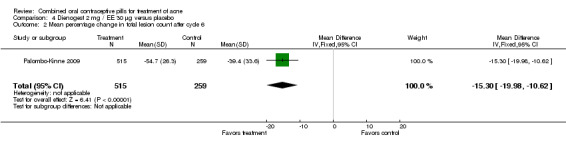
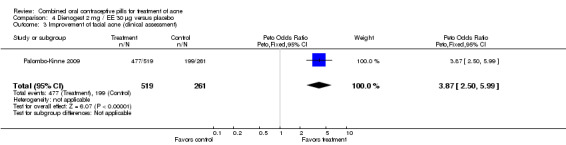
In Palombo‐Kinne 2009, the test product of dienogest 2 mg plus EE 30 µg was compared with a placebo as well as with the control of cyproterone acetate 2 mg plus EE 35 µg. Compared to the placebo group at cycle six, the dienogest COC group had greater percentage decreases in inflammatory lesion count (MD ‐16.10; 95% CI ‐21.74 to ‐10.46) and total lesion count (MD ‐15.30; 95% CI ‐19.98 to ‐10.62) than the placebo group. The treatment group was also more likely to be assessed with overall improvement of facial acne (OR 3.87; 95% CI 2.50 to 5.99).
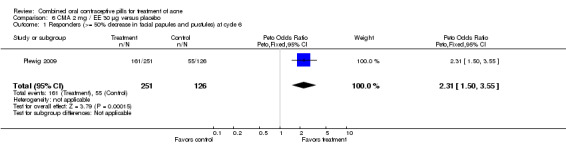
Plewig 2009 compared chlormadinone acetate (CMA) 2 mg plus EE 30 µg versus placebo. The CMA group was more likely to be classed as a 'responder' at cycle six, that is, having at least 50% decrease in facial papules and pustules (OR 2.31; 95% CI 1.50 to 3.55).
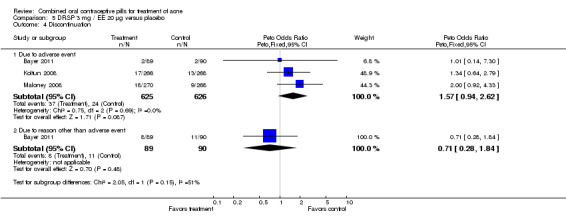
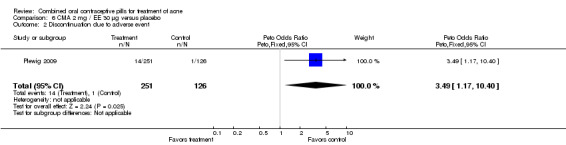
None of these placebo‐controlled trials reported differences in early discontinuation due to worsening of acne. However, women in the treatment group were more likely to discontinue due to adverse events than those assigned to the placebo group in Maloney 2001 (OR 2.73; 95% CI 1.26 to 5.90) and in Plewig 2009 (OR 3.49; 95% CI 1.17 to 10.40). For the other trials, the study arms were not significantly different for discontinuation due to adverse events. The data were inconsistent in Maloney 2008: the 'flow of participants' diagram indicated 18 women in the treatment group discontinued due to adverse events, while the text reported 16.
DRSP 3 mg/EE 30 μg versus other COCs
Four trials compared drospirenone (DRSP) 3 mg / EE 30 μg versus another COC.
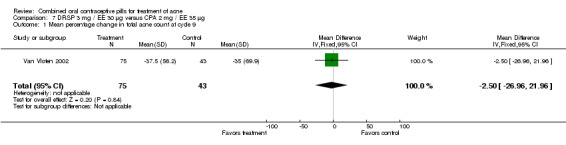
Van Vloten 2002 compared DRSP 3 mg / EE 30 μg versus CPA 2 mg / EE 35 μg. The groups were not significantly different for the percentage change in total acne lesions from baseline to cycle nine.
In Kelly 2010, DRSP 3 mg / EE 30 μg was compared with LNG 150 μg / EE 30 μg. Results were presented in figures (without absolute numbers) for total lesion count and percentage of subjects with acne. The text describes increases and decreases without any actual numbers or any statistical results. Data for discontinuation due to acne were provided; the DRSP group was less likely to discontinue due to acne deterioration (OR 0.16; 95% CI 0.05 to 0.47).
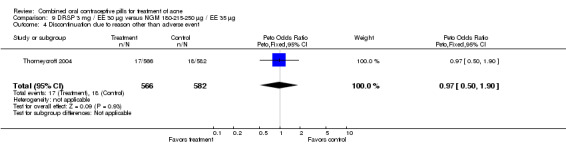
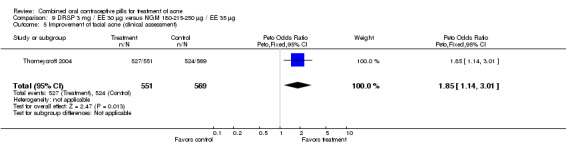
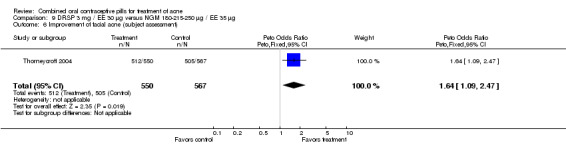
Thorneycroft 2004 compared DRSP 3 mg / EE 30 μg versus the triphasic NGM 180‐215‐250 μg / EE 35 μg. The DRSP group had a greater mean percentage change in total lesion count after cycle 6 (mean difference ‐3.30; 95% CI ‐6.45 to ‐0.15). More women in the DRSP group had improvement in facial acne as assessed by the investigator (OR 1.85; 95% CI 1.14 to 3.01) and by the women themselves (OR 1.64; 95% CI 1.09 to 2.47).
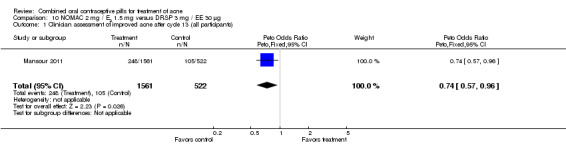
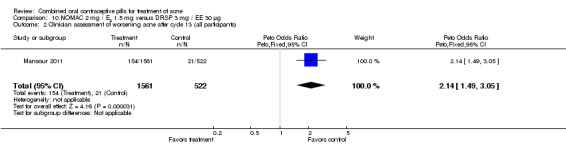
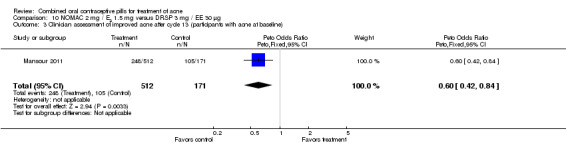
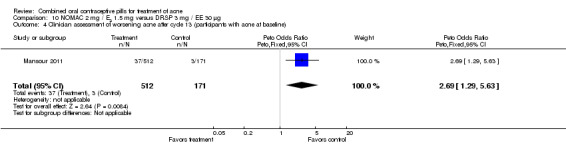
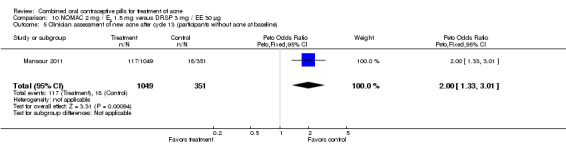
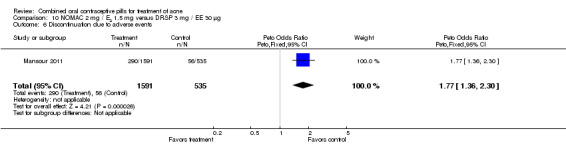
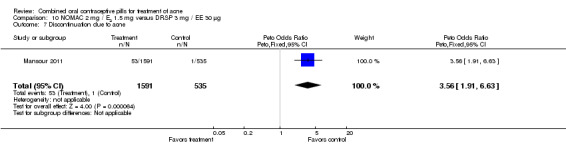
For Mansour 2011, the experimental treatment was nomegestrol acetate (NOMAC) 2.5 mg / E2 1.5 mg and the comparison was DRSP 3 mg / EE 30 μg. After cycle 13, changes in acne severity favored the DRSP‐COC group. For all participants, the investigators were less likely to report improved acne for the NOMAC group than for the DRSP group (OR 0.74; 95% CI 0.57 to 0.96), and more likely to record acne worsening for the NOMAC group (OR 2.14; 95% CI 1.49 to 3.05). For the women with acne at baseline, those in the NOMAC group were less likely to be rated as improved compared to the DRSP group (OR 0.60; 95% CI 0.42 to 0.84), and more likely to be classed as worsening (OR 2.69; 95% CI 1.29 to 5.63). Of those without acne at baseline, women in the NOMAC group were more likely to have new acne after cycle 13 (OR 2.00; 95% CI 1.33 to 3.01). Discontinuation due to adverse events was more likely in the NOMAC group (OR 1.77; 95% CI 1.36 to 2.30) as was discontinuation due to acne (OR 3.56; 95% CI 1.91 to 6.63).
DSG/EE versus CPA/EE or DSG/EE
Three trials compared a desogestrel‐containing COC and CPA/EE.
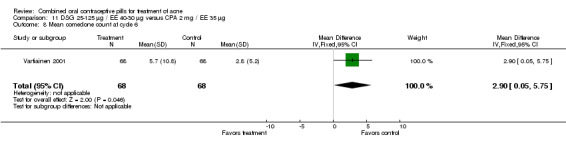
Two compared biphasic desogestrel (DSG) 25‐125 μg / EE 40‐30 μg versus CPA 2 mg / EE 35 μg women (Dieben 1994; Vartiainen 2001). Neither trial found significant differences in acne grades or mean counts of pustules, nodules or papules. One trial (Vartiainen 2001) found a higher mean comedone count (mean difference 2.90; 95% CI 0.05 to 5.75) in the DSG/EE than the CPA/EE group. Dieben 1994 found the two groups were not significantly different in the comedone count, but the trial had only four treatment cycles. In Vartiainen 2001, the groups were not significantly different in discontinuation for adverse events or worsening of acne.
In a third trial (Charoenvisal 1996), women who used DSG 150 μg / EE 30 μg were more likely to have moderate or severe acne (OR 6.35; 95% CI 1.96 to 20.52) compared to those who used CPA 2 mg / EE 50 μg. However, the two groups were not significantly different for the subjective assessment of acne.
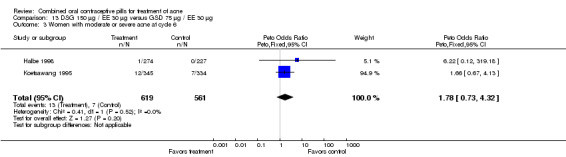
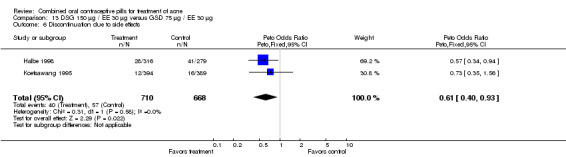
Two multi‐center trials (Koetsawang 1995; Halbe 1998) and a small single‐center trial (Mango 1996) compared DSG 150 μg / EE 30 μg versus gestodene (GSD) 75 μg / EE 30 μg. Mango 1996, which could not be pooled with the larger trials due to different study durations, showed no significant differences in acne outcomes. Likewise, the groups were not significantly different in acne outcomes with the pooled trials (Koetsawang 1995; Halbe 1998). For the combined two trials, women assigned to the DSG/EE group had less discontinuation due to side effects (OR 0.61; 95% CI 0.40 to 0.93) than the GSD/EE users.
LNG/EE versus other COCs
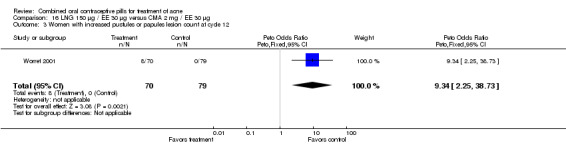
Worret 2001 compared LNG 150 μg / EE 30 μg versus chlormadinone acetate (CMA) 2 mg / EE 30 μg. The odds of having increased pustule or papule lesions at cycle 12 was 9.34 (95% CI 2.25 to 38.73) times greater for women assigned to use LNG/EE than those using CMA/EE. Also, women in the LNG/EE group were less likely to report self‐assessed improvement of acne (OR 0.16; 95% CI 0.04 to 0.57).
Two trials compared a levonorgestrel‐containing COC versus CPA/EE.
Carlborg 1986 compared LNG 150 μg / EE 30 μg with CPA 2 mg / EE 35 μg. The LNG/EE combination resulted in a significantly higher mean pustule count (mean difference 1.80; 95% CI 0.63 to 2.97) and mean papule count (mean difference 2.90; 95% CI 0.20 to 5.60) than the CPA/EE combination. Also, fewer women in the LNG/EE group had a "good" (undefined) acne assessment when measured by either a dermatologist (OR 0.29; 95% CI 0.12 to 0.68) or by self‐assessment (OR 0.23; 95% CI 0.09 to 0.54). Discontinuation due to side effects was not significantly different between the two groups.
Carlborg 1986 also compared the same LNG/EE versus a CPA/EE combination with a higher dose (50 μg) of EE. The LNG/EE group resulted in a higher mean pustule count (mean difference 2.10; 95% CI 0.93 to 3.27) and mean papule count (mean difference 3.60; 95% CI 1.12 to 6.08) than the CPA/EE combination. Women in the LNG/EE group had lower odds of having a "good" acne assessment than women in the CPA/EE group when measured by a dermatologist (OR 0.22; 95% CI 0.09 to 0.52) or by self‐assessment (OR 0.18; 95% CI 0.08 to 0.44). The groups were not significantly different in the proportion of women who discontinued early due to side effects.
A second trial compared LNG 250 μg / EE 50 μg versus CPA 2 mg / EE 50 μg (Lachnit‐Fixson 1977). Women in the LNG/EE group had lower odds of having improved or healed acne than the CPA/EE group (OR 0.24; 95% CI 0.08 to 0.75).
Three trials examined a levonorgestrel‐containing COC versus a desogestrel‐containing COC.
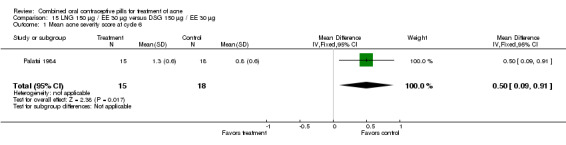
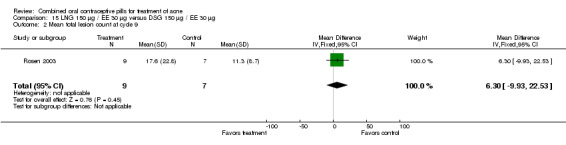
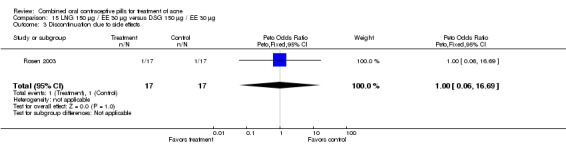
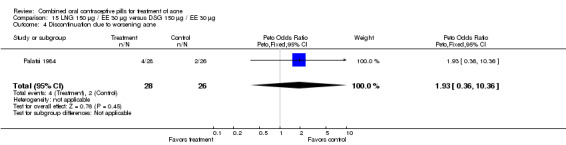
Palatsi 1984 and Rosen 2003 compared LNG 150 μg plus EE 30 μg versus DSG 150 μg plus EE 30 μg. The earlier trial (Palatsi 1984) found a difference in mean acne severity score (mean difference 0.50; 95% CI 0.09 to 0.91) for the LNG/EE compared to the DSG/EE group. However, Rosen 2003 showed the groups were not significantly different for the mean total lesion count. In Palatsi 1984, the groups were not significantly different in early discontinuation due to side effects or worsening acne. These were small trials, however.
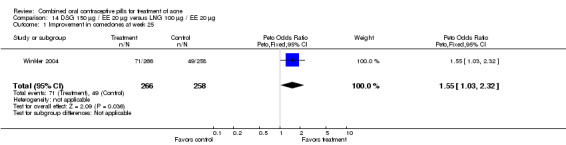
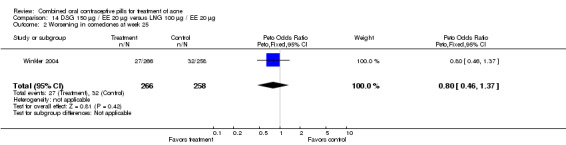
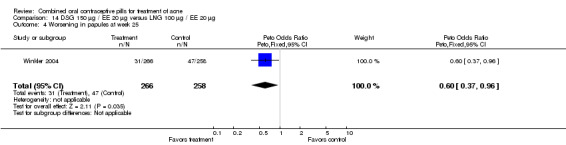
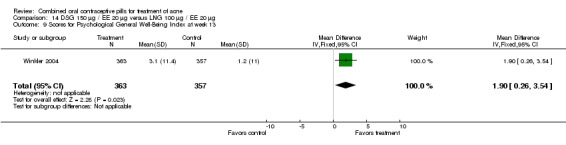
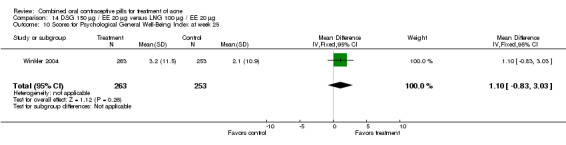
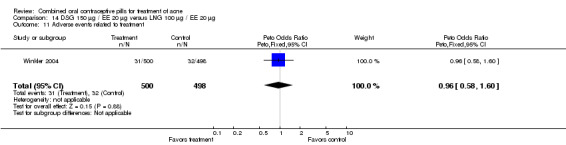
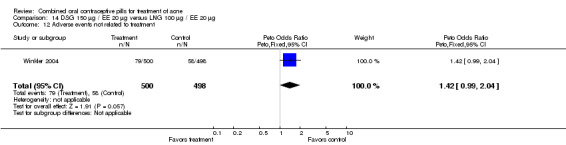
Winkler 2004 compared LNG 100 μg plus EE 20 μg versus DSG 150 μg plus EE 20 μg. At 25 weeks, the DSG group was more likely than the LNG group to show improvement in comedones (OR 1.55; 95% CI 1.03 to 2.32) and less likely to have worsening of papules (OR 0.60; 95% CI 0.37 to 0.96). Criteria for "improvement" or "worsening" were undefined, although the categories were reportedly based on objective counting of lesions. The DSG group also had a higher (better) mean for the Psychological General Well‐Being Index at 13 weeks (mean difference 1.90; 95% CI 0.26 to 3.54) but not at 25 weeks. The groups were not significantly different in the adverse events related to treatment.
The one trial (Thorneycroft 1999) that compared LNG 100 μg plus EE 20 μg to NA 1 mg plus EE 20 μg showed the groups were not significantly different in acne outcomes. The study had only three treatment cycles.
Other COCs versus CPA 2 mg/EE
As noted above, in addition to the placebo, Palombo‐Kinne 2009 compared dienogest 2 mg plus EE 30 µg with the control of cyproterone acetate 2 mg plus EE 35 µg. The groups were not significantly different for changes in inflammatory lesion count, total lesion count and assessment of facial acne improvement.
In J&J 2005, the triphasic norgestimate (NGM) 180‐215‐250 μg / EE 35 μg was compared with CPA 2 mg/ EE 35 μg. In this small trial, the study groups were not significantly different for mean change in total lesion count and discontinuation due to adverse events at cycle three.
Three trials compared CPA 2 mg plus EE 35 μg versus CPA 2 mg plus EE 50 μg (Aydinlik 1986; Carlborg 1986; Fugere 1988). This was the only comparison between two COCs with the same progestin but different doses of estrogen, and the trials found the two groups were not significantly different in acne outcomes and discontinuation rates.
CPA 2 mg/EE 50 μg versus antibiotic
The one trial (Monk 1987) that compared CPA 2 μg plus EE 50 μg to minocycline hydrochloride 50 mg showed the groups were not significantly different in self‐assessed acne improvement or discontinuation rates. However, this was a relatively small trial with imprecise effect estimates.
Discussion
Summary of main results
COC use reduced inflammatory and non‐inflammatory facial lesion counts, severity grades and self‐assessed acne in the nine trials that compared a COC to a placebo group and had data for analysis. Progestins examined included levonorgestrel, norethindrone acetate, norgestimate, drospirenone, dienogest, and chlormadinone acetate.
Differences were less clear in the comparative effectiveness of COCs containing varying progestin types and dosages. Although COCs containing CPA have been traditionally used for acne treatment (Seaman 2003), little evidence shows its superiority over other progestins. The CPA/EE combination appeared to improve acne (i.e., inflammatory lesions and global assessments) better than LNG/EE in the two trials that made this comparison (Lachnit‐Fixson 1977; Carlborg 1986), but the findings had wide confidence intervals, which indicate limited precision. Also, the three trials that compared CPA/EE to DSG/EE produced conflicting results (Dieben 1994; Charoenvisal 1996; Vartiainen 2001). The COCs with CPA/EE were not significantly different from LNG/EE or DSG/EE in discontinuation rates due to adverse events.
Few other differences between COCs regarding acne efficacy were found. DRSP/EE was more effective compared to NGM/EE (Thorneycroft 2004) and to NOMAC/E2 (Mansour 2011), but not more effective than CPA/EE (Van Vloten 2002). Van Vloten 2002 was a much smaller study than Thorneycroft 2004 and Mansour 2011, which had greater power for detecting an important effect. However, Mansour 2011 did not involve lesion counts but relied on the investigator's assessment of acne severity. The COC with CMA seemed to improve acne better than LNG, but this is based on one trial (Worret 2001). Finally, LNG/EE showed a slight improvement over DSG/EE in Palatsi 1984, while Rosen 2003 found the two COC groups were not significantly different. Winkler 2004 had more favorable results with DSG/EE than with LNG/EE, but Winkler 2004 used a lower EE dose than the other two trials and had a larger sample size. Additional potential differences in acne effectiveness among COC types might not have been detected due to the limited number of eligible trials, comparisons made and data reported.
No conclusions can be reached regarding the effect of a COC compared to an antibiotic since only one underpowered trial made this comparison (Monk 1987).
Overall completeness and applicability of evidence
Most studies assessed women over six treatment cycles, a length of time that might not be adequate for a chronic condition like acne. One trial that compared NOMAC/E2 versus DRSP/EE showed a difference in early discontinuation due to total adverse events as well as those due to acne (Mansour 2011). In addition, Kelly 2010 indicated the DRSP/EE group was less likely than the LNG/EE group to discontinue due to acne deterioration. Two placebo‐controlled trials (Maloney 2001; Plewig 2009) showed that the COC group was more likely to discontinue due to adverse events than the placebo group. The COCs were CMA/EE in Plewig 2009 and NA/EE in Maloney 2001. Thus, even if COCs improve acne, women might not be willing to accept their long‐term use as acne treatment due to other side effects. Other acne treatments could also have side effects that limit their acceptability. However, we found only one eligible trial with limited data that compared a COC to an antibiotic (Monk 1987).
Quality of the evidence
Twenty trials reported some blinding of which 19 described double‐blinding. The group assignment was apparent to both participants and investigators in 10 trials. Furthermore, only 11 studies described their methods of randomization, and only 5 trials provided information on their attempts to conceal allocation. The potential for bias is increased with an open study design as well as inadequate method of allocation concealment before group assignment (Schulz 2002a; Schulz 2002b). In addition, most eligible trials were funded by pharmaceutical companies, which could present potential conflicts of interest in terms of the reporting of unfavorable results (Lexchin 2003). Another limitation present in several studies was the high attrition rate due to exclusions after randomization, loss to follow up, early discontinuation, missing data or unexplained reasons. For example, nine trials reported acne outcomes that were measured using less than 70% of the randomized women (Palatsi 1984; Dieben 1994; Lucky 1997; Redmond 1997; Thiboutot 2001; Winkler 2004; Worret 2001; Rosen 2003; Kelly 2010).
Differences in treatment regimens, assessment methods and outcome measures in the studies make synthesis of the evidence difficult. In particular, the lack of use of standardized methods for assessing acne prevents the synthesis of results across trials and complicates their interpretation.
Potential biases in the review process
Several studies had insufficient for analysis in this review due to presenting outcome data in figures without absolute numbers or simply describing selected results in the text (Koltun 2008; Maloney 2008; Kelly 2010). The researchers apparently measured outcomes relevant to our review but did not report those results adequately. Such reporting is inconsistent with CONSORT guidelines and prevented our ability to include those results in the review (CONSORT 2009). In addition, two studies evaluated the effect of treatment on psychosocial function outcomes, but also presented results in graphs without absolute numbers.
Agreements and disagreements with other studies or reviews
The development of acne is complex, and may be influenced by hormonal contraceptives (George 2008; Rich 2008). The level of free testosterone affects sebum production (O'Connell 2008). Ethinyl estradiol can increase sex hormone‐binding globulin (SHBG), which lowers free testosterone (O'Connell 2008; Rich 2008). Some of the included trials examined the mechanisms via the levels of SHBG and testosterone. This review focused on the outcome of acne as assessed by lesion counts. Only three trials compared the same progestin (CPA) with EE doses of 35 µg versus 50 µg. Most of the trials that compared COCs examined different progestins as well as EE doses. The varied results suggest the differences may be related to the type of progestin as well as the estrogen dose. In addition, the estrogen dose may have to be higher than that found in most current COCs (Rich 2008).
Authors' conclusions
Implications for practice.
This update yielded six new studies but no change in conclusions. Since COCs reduce acne lesion count, severity grades and self‐assessed acne in placebo‐controlled trials, they should be considered for women with acne who also want an oral contraceptive. COCs containing CMA or CPA seem to improve acne better than LNG; however, this finding is based on limited evidence. A DRSP‐COC may be more effective than NGM or NOMAC/E2 but the trials used different methods to assess acne severity assessments. Comparisons between other COCs were either conflicting or showed no significant difference in their ability to reduce acne. How COCs compare to alternative acne treatments is unknown since only one trial addressed this issue.
Implications for research.
Future studies should use allocation concealment. More recent studies were reportedly double‐blind but should be clear about who was blinded. The use of standardized methods for assessing acne would help in synthesizing results across trials and aid in interpretation. The comparative effectiveness of COCs in treating acne should be evaluated in randomized controlled trials. In addition, research is needed on the acceptability of, and need for, long‐term use of COCs for acne treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 6 February 2012 | New citation required but conclusions have not changed | Incorporated 6 new trials. |
| 30 January 2012 | New search has been performed | New trials found (J&J 2005; Palombo‐Kinne 2009; Plewig 2009; Kelly 2010; Bayer 2011; Mansour 2011). Secondary paper added for Maloney 2008. Ongoing trial added (Kimball 2011). |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 2 February 2009 | New citation required but conclusions have not changed | Two new trials were added (Koltun 2008; Maloney 2008). |
| 30 January 2009 | New search has been performed | Searches were updated. |
| 14 April 2008 | Amended | Converted to new review format. |
| 30 August 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Carol Manion of FHI 360 helped with the literature searches for earlier versions of this review.
Appendices
Appendix 1. Search strategies, 2012 update
PubMed search of MEDLINE (Oct 2008 to 25 Jan 2012)
((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ("latin square" [tw]) OR placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR cross‐over studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animal [mh] NOT human [mh]))
AND (contraceptives, oral OR (cyproterone acetate OR desogestrel OR ethinodiol diacetate OR gestodene OR levonorgestrel OR lynestrenol OR norethindrone OR norethynodrel OR norgestimate OR drospirenone) AND (acne OR acne vulgaris)
Cochrane Central Register of Controlled Trials (CENTRAL) (2008 to 25 Jan 2012)
(acne OR acne vulgaris) in Title, Abstract, or Keywords
AND contracept* OR (cyproterone acetate OR desogestrel OR ethinodiol diacetate OR gestodene OR levonorgestrel OR lynestrenol OR norethindrone OR norethisterone OR norethynodrel OR norgestimate OR drospirenone) in Title, Abstract, or Keywords NOT hirsutism or polycystic or bulimic in Title.
POPLINE (2008 to 25 Jan 2012)
(oral contraceptives / contraceptive agents / female / ((cyproterone acetate / desogestrel / ethinodiol diacetate / gestodene / levonorgestrel / lynestrenol / norethindrone / norethynodrel / norgestimate) & ethinyl estradiol)) & (acne / acne vulgaris)
LILACS (25 Jan 2012)
((cyproterone acetate or desogestrel or ethinylestrenol or gestodene or levanogestrel or levonorgestrel or linestrenol or lynestrenol or norethindrone or norethynodrel or noretinodrel or norgestimate)
and (ethinyl estradiol or etinilestradiol or etinil estradiol)
OR (contraceptives, oral or anticonceptivos orales or anticoncepcionais orais)) and (acne or acne vulgaris or acne vulgar)
ClinicalTrials.gov (01 Jan 2008 to 24 Aug 2011)
Conditions: acne Interventions: contraceptive OR contraception
ICTRP (24 Aug 2011)
acne AND contraceptive
Appendix 2. Search strategies from earlier versions
2009 search
CENTRAL (2006 to 13 Jan 2009)
(acne or acne vulgaris) in Title, Abstract, or Keywords AND contracept* or (cyproterone acetate or desogestrel or ethinodiol diacetate or gestodene or levonorgestrel or lynestrenol or norethindrone or norethisterone or norethynodrel or norgestimate or drospirenone) in Title, Abstract, or Keywords NOT hirsutism or polycystic or bulimic in Title.
PubMed search of MEDLINE (01 Jan 2006 to 13 Jan 2009)
Cochrane search strategy adapted for PubMed (Robinson 2002): ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ("latin square" [tw]) OR placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR cross‐over studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animal [mh] NOT human [mh])) AND (contraceptives, oral OR (cyproterone acetate OR desogestrel OR ethinodiol diacetate OR gestodene OR levonorgestrel OR lynestrenol OR norethindrone OR norethynodrel OR norgestimate OR drospirenone) AND (acne OR acne vulgaris)
EMBASE (2006 to 27 Jan 2009)
((acne OR acne vulgaris) AND (oral contraceptive agent OR ((cyproterone acetate OR desogestrel OR ethinodiol diacetate OR gestodene OR levonorgestrel OR lynestrenol OR norethindrone OR norethynodrel OR norgestimate) AND ethinyl estradiol))) AND (clinical trial OR controlled study OR randomized controlled trial OR controlled (w) clinical (w) trial? OR random (w) allocation OR multi‐center study OR comparative (w) study OR evidence (w) based (w) medicine OR research (w) design OR double (w) blind (w) procedure OR single (w) blind (w) procedure OR random)
POPLINE (2006 to 27 Jan 2009)
(oral contraceptives / contraceptive agents / female / ((cyproterone acetate / desogestrel / ethinodiol diacetate / gestodene / levonorgestrel / lynestrenol / norethindrone / norethynodrel / norgestimate) & ethinyl estradiol)) & (acne / acne vulgaris)
LILACS (27 Jan 2009)
((cyproterone acetate or desogestrel or ethinylestrenol or gestodene or levanogestrel or levonorgestrel or linestrenol or lynestrenol or norethindrone or norethynodrel or noretinodrel or norgestimate) and (ethinyl estradiol or etinilestradiol or etinil estradiol) or (contraceptives, oral or anticonceptivos orales or anticoncepcionais orais)) and (acne or acne vulgaris or acne vulgar)
ClinicalTrials.gov (15 Jan 2009)
Conditions: acne Interventions: contraceptive OR contraception
ICTRP (15 Jan 2009)
acne AND contraceptive
2003 and 2006 searches
The same strategies were used as listed under 2009 for CENTRAL, PubMEd, EMBASE, POPLINE, and LILACS. These earlier versions also included the following:
Cochrane Skin Group's trial register
Acne*
Biological Abstracts
(acne or acne*) and oral contraceptives or ((cyproterone acetate or desogestrel or ethinodiol diacetate or gestodene or levonorgestrel or norethindrone or norethynodrel or norgestimate or lynestrenol) and estradiol)
Data and analyses
Comparison 1. LNG 100 µg / EE 20 µg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in total lesion count | 2 | 572 | Mean Difference (IV, Fixed, 95% CI) | ‐9.98 [‐16.51, ‐3.45] |
| 2 Mean change in inflammatory lesion count | 2 | 572 | Mean Difference (IV, Fixed, 95% CI) | ‐2.95 [‐4.97, ‐0.93] |
| 3 Mean change in non‐inflammatory lesion count | 2 | 572 | Mean Difference (IV, Fixed, 95% CI) | ‐6.75 [‐12.56, ‐0.94] |
| 4 Clinician assessment of women with clear or almost clear lesions at cycle 6 | 2 | 571 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.56 [1.13, 2.18] |
| 5 Participant self‐assessment of acne lesion improvement | 2 | 572 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.13 [1.47, 3.09] |
| 6 Discontinuation due to non‐acne adverse event | 1 | 350 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.54 [0.55, 4.31] |
| 7 Discontinuation due to lack of acne improvement | 1 | 350 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.31, 2.47] |
1.1. Analysis.
Comparison 1 LNG 100 µg / EE 20 µg versus placebo, Outcome 1 Mean change in total lesion count.
1.2. Analysis.
Comparison 1 LNG 100 µg / EE 20 µg versus placebo, Outcome 2 Mean change in inflammatory lesion count.
1.3. Analysis.
Comparison 1 LNG 100 µg / EE 20 µg versus placebo, Outcome 3 Mean change in non‐inflammatory lesion count.
1.4. Analysis.
Comparison 1 LNG 100 µg / EE 20 µg versus placebo, Outcome 4 Clinician assessment of women with clear or almost clear lesions at cycle 6.
1.5. Analysis.
Comparison 1 LNG 100 µg / EE 20 µg versus placebo, Outcome 5 Participant self‐assessment of acne lesion improvement.
1.6. Analysis.
Comparison 1 LNG 100 µg / EE 20 µg versus placebo, Outcome 6 Discontinuation due to non‐acne adverse event.
1.7. Analysis.
Comparison 1 LNG 100 µg / EE 20 µg versus placebo, Outcome 7 Discontinuation due to lack of acne improvement.
Comparison 2. NA 1 mg / EE 20‐30‐35 µg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinician assessment of no, minimal or mild acne at cycle 6 | 1 | 555 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.86 [1.32, 2.62] |
| 2 Discontinuation due to any adverse event | 1 | 593 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.73 [1.26, 5.90] |
2.1. Analysis.
Comparison 2 NA 1 mg / EE 20‐30‐35 µg versus placebo, Outcome 1 Clinician assessment of no, minimal or mild acne at cycle 6.
2.2. Analysis.
Comparison 2 NA 1 mg / EE 20‐30‐35 µg versus placebo, Outcome 2 Discontinuation due to any adverse event.
Comparison 3. NGM 180‐215‐250 µg / EE 35 µg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in total lesion count at cycle 6 | 2 | 387 | Mean Difference (IV, Fixed, 95% CI) | ‐9.32 [‐14.19, ‐4.45] |
| 2 Mean change in inflammatory lesion count at cycle 6 | 2 | 387 | Mean Difference (IV, Fixed, 95% CI) | ‐3.44 [‐5.43, ‐1.44] |
| 3 Mean change in comedone count at cycle 6 | 2 | 387 | Mean Difference (IV, Fixed, 95% CI) | ‐5.81 [‐9.77, ‐1.85] |
| 4 Clinician global assessment of improved acne at cycle 6 | 2 | 324 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.86 [2.31, 6.44] |
| 5 Participant self‐assessment of improved acne at cycle 6 | 1 | 163 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.50 [2.37, 8.56] |
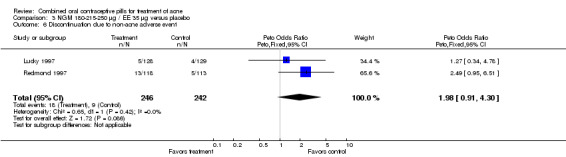
| 6 Discontinuation due to non‐acne adverse event | 2 | 488 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.98 [0.91, 4.30] |
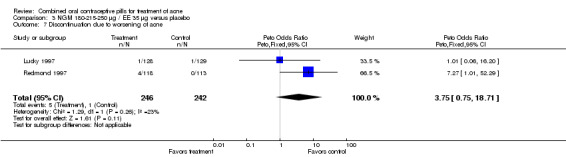
| 7 Discontinuation due to worsening of acne | 2 | 488 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.75 [0.75, 18.71] |
3.1. Analysis.
Comparison 3 NGM 180‐215‐250 µg / EE 35 µg versus placebo, Outcome 1 Mean change in total lesion count at cycle 6.
3.2. Analysis.
Comparison 3 NGM 180‐215‐250 µg / EE 35 µg versus placebo, Outcome 2 Mean change in inflammatory lesion count at cycle 6.
3.3. Analysis.
Comparison 3 NGM 180‐215‐250 µg / EE 35 µg versus placebo, Outcome 3 Mean change in comedone count at cycle 6.
3.4. Analysis.
Comparison 3 NGM 180‐215‐250 µg / EE 35 µg versus placebo, Outcome 4 Clinician global assessment of improved acne at cycle 6.
3.5. Analysis.
Comparison 3 NGM 180‐215‐250 µg / EE 35 µg versus placebo, Outcome 5 Participant self‐assessment of improved acne at cycle 6.
3.6. Analysis.
Comparison 3 NGM 180‐215‐250 µg / EE 35 µg versus placebo, Outcome 6 Discontinuation due to non‐acne adverse event.
3.7. Analysis.
Comparison 3 NGM 180‐215‐250 µg / EE 35 µg versus placebo, Outcome 7 Discontinuation due to worsening of acne.
Comparison 4. Dienogest 2 mg / EE 30 µg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean percentage change in inflammatory lesion count after cycle 6 | 1 | 768 | Mean Difference (IV, Fixed, 95% CI) | ‐16.10 [‐21.74, ‐10.46] |
| 2 Mean percentage change in total lesion count after cycle 6 | 1 | 774 | Mean Difference (IV, Fixed, 95% CI) | ‐15.30 [‐19.98, ‐10.62] |
| 3 Improvement of facial acne (clinical assessment) | 1 | 780 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.87 [2.50, 5.99] |
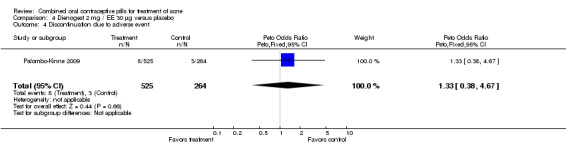
| 4 Discontinuation due to adverse event | 1 | 789 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.33 [0.38, 4.67] |
| 5 Discontinuation due to reason other than adverse event | 1 | 789 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.18, 0.86] |
4.1. Analysis.
Comparison 4 Dienogest 2 mg / EE 30 µg versus placebo, Outcome 1 Mean percentage change in inflammatory lesion count after cycle 6.
4.2. Analysis.
Comparison 4 Dienogest 2 mg / EE 30 µg versus placebo, Outcome 2 Mean percentage change in total lesion count after cycle 6.
4.3. Analysis.
Comparison 4 Dienogest 2 mg / EE 30 µg versus placebo, Outcome 3 Improvement of facial acne (clinical assessment).
4.4. Analysis.
Comparison 4 Dienogest 2 mg / EE 30 µg versus placebo, Outcome 4 Discontinuation due to adverse event.
4.5. Analysis.
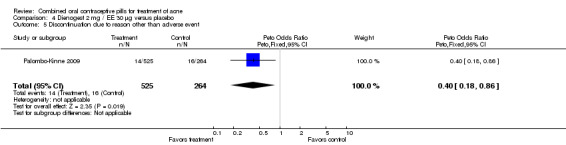
Comparison 4 Dienogest 2 mg / EE 30 µg versus placebo, Outcome 5 Discontinuation due to reason other than adverse event.
Comparison 5. DRSP 3 mg / EE 20 µg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean percent change in lesion counts at cycle 6 | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Mean percent change in total lesion count | 1 | 173 | Mean Difference (IV, Fixed, 95% CI) | 29.08 [3.13, 55.03] |
| 1.2 Mean percent change in inflammatory lesion count | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | 14.61 [5.18, 24.04] |
| 1.3 Mean percent change in non‐inflammatory lesion count | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | 19.03 [5.13, 32.93] |
| 1.4 Mean percent change in papule count | 1 | 146 | Mean Difference (IV, Fixed, 95% CI) | 17.33 [5.60, 29.06] |
| 1.5 Mean percent change in pustule count | 1 | 125 | Mean Difference (IV, Fixed, 95% CI) | 1.73 [‐11.48, 14.94] |
| 1.6 Mean percent change in nodule count | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [‐8.80, 10.46] |
| 1.7 Mean percent change in open comedone count | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐14.28 [‐84.76, 56.20] |
| 1.8 Mean percent change in closed comedone count | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 20.79 [3.57, 38.01] |
| 2 Clear or almost clear (investigator assessment) at cycle 6 | 2 | 575 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.02 [1.99, 4.59] |
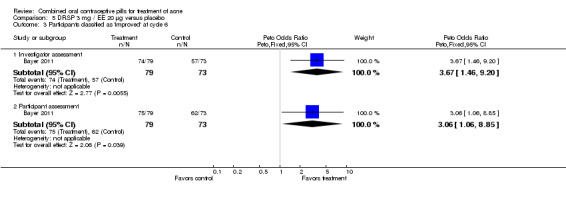
| 3 Participants classified as 'improved' at cycle 6 | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Investigator assessment | 1 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.67 [1.46, 9.20] |
| 3.2 Participant assessment | 1 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.06 [1.06, 8.85] |
| 4 Discontinuation | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Due to adverse event | 3 | 1251 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.94, 2.62] |
| 4.2 Due to reason other than adverse event | 1 | 179 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.28, 1.84] |
5.1. Analysis.
Comparison 5 DRSP 3 mg / EE 20 µg versus placebo, Outcome 1 Mean percent change in lesion counts at cycle 6.
5.2. Analysis.
Comparison 5 DRSP 3 mg / EE 20 µg versus placebo, Outcome 2 Clear or almost clear (investigator assessment) at cycle 6.
5.3. Analysis.
Comparison 5 DRSP 3 mg / EE 20 µg versus placebo, Outcome 3 Participants classified as 'improved' at cycle 6.
5.4. Analysis.
Comparison 5 DRSP 3 mg / EE 20 µg versus placebo, Outcome 4 Discontinuation.
Comparison 6. CMA 2 mg / EE 30 µg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Responders (>= 50% decrease in facial papules and pustules) at cycle 6 | 1 | 377 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.31 [1.50, 3.55] |
| 2 Discontinuation due to adverse event | 1 | 377 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.49 [1.17, 10.40] |
6.1. Analysis.
Comparison 6 CMA 2 mg / EE 30 µg versus placebo, Outcome 1 Responders (>= 50% decrease in facial papules and pustules) at cycle 6.
6.2. Analysis.
Comparison 6 CMA 2 mg / EE 30 µg versus placebo, Outcome 2 Discontinuation due to adverse event.
Comparison 7. DRSP 3 mg / EE 30 µg versus CPA 2 mg / EE 35 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean percentage change in total acne count at cycle 9 | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐26.96, 21.96] |
7.1. Analysis.
Comparison 7 DRSP 3 mg / EE 30 µg versus CPA 2 mg / EE 35 µg, Outcome 1 Mean percentage change in total acne count at cycle 9.
Comparison 8. DRSP 3 mg / EE 30 µg versus LNG 150 µg / EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation due to acne deterioration | 1 | 424 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.16 [0.05, 0.47] |
8.1. Analysis.
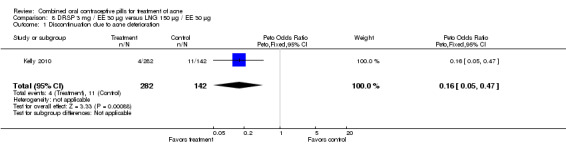
Comparison 8 DRSP 3 mg / EE 30 µg versus LNG 150 µg / EE 30 µg, Outcome 1 Discontinuation due to acne deterioration.
Comparison 9. DRSP 3 mg / EE 30 µg versus NGM 180‐215‐250 µg / EE 35 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean percentage change in inflammatory lesion count after cycle 6 | 1 | 1108 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐5.97, 1.17] |
| 2 Mean percentage change in total lesion count after cycle 6 | 1 | 1108 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐6.45, ‐0.15] |
| 3 Discontinuation due to adverse event | 1 | 1148 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.43, 1.49] |
| 4 Discontinuation due to reason other than adverse event | 1 | 1148 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.50, 1.90] |
| 5 Improvement of facial acne (clinical assessment) | 1 | 1120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.85 [1.14, 3.01] |
| 6 Improvement of facial acne (subject assessment) | 1 | 1117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.64 [1.09, 2.47] |
9.1. Analysis.
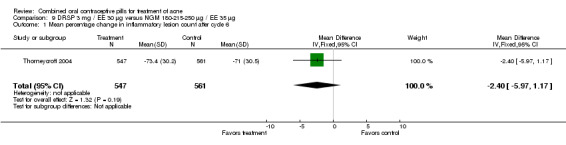
Comparison 9 DRSP 3 mg / EE 30 µg versus NGM 180‐215‐250 µg / EE 35 µg, Outcome 1 Mean percentage change in inflammatory lesion count after cycle 6.
9.2. Analysis.
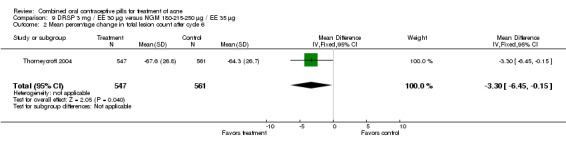
Comparison 9 DRSP 3 mg / EE 30 µg versus NGM 180‐215‐250 µg / EE 35 µg, Outcome 2 Mean percentage change in total lesion count after cycle 6.
9.3. Analysis.
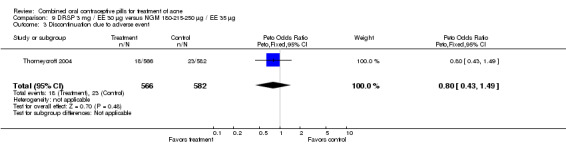
Comparison 9 DRSP 3 mg / EE 30 µg versus NGM 180‐215‐250 µg / EE 35 µg, Outcome 3 Discontinuation due to adverse event.
9.4. Analysis.
Comparison 9 DRSP 3 mg / EE 30 µg versus NGM 180‐215‐250 µg / EE 35 µg, Outcome 4 Discontinuation due to reason other than adverse event.
9.5. Analysis.
Comparison 9 DRSP 3 mg / EE 30 µg versus NGM 180‐215‐250 µg / EE 35 µg, Outcome 5 Improvement of facial acne (clinical assessment).
9.6. Analysis.
Comparison 9 DRSP 3 mg / EE 30 µg versus NGM 180‐215‐250 µg / EE 35 µg, Outcome 6 Improvement of facial acne (subject assessment).
Comparison 10. NOMAC 2 mg / E2 1.5 mg versus DRSP 3 mg / EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinician assessment of improved acne after cycle 13 (all participants) | 1 | 2083 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.57, 0.96] |
| 2 Clinician assessment of worsening acne after cycle 13 (all participants) | 1 | 2083 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.14 [1.49, 3.05] |
| 3 Clinician assessment of improved acne after cycle 13 (participants with acne at baseline) | 1 | 683 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.60 [0.42, 0.84] |
| 4 Clinician assessment of worsening acne after cycle 13 (participants with acne at baseline) | 1 | 683 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.69 [1.29, 5.63] |
| 5 Clinician assessment of new acne after cycle 13 (participants without acne at baseline) | 1 | 1400 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.00 [1.33, 3.01] |
| 6 Discontinuation due to adverse events | 1 | 2126 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.77 [1.36, 2.30] |
| 7 Discontinuation due to acne | 1 | 2126 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.56 [1.91, 6.63] |
10.1. Analysis.
Comparison 10 NOMAC 2 mg / E2 1.5 mg versus DRSP 3 mg / EE 30 µg, Outcome 1 Clinician assessment of improved acne after cycle 13 (all participants).
10.2. Analysis.
Comparison 10 NOMAC 2 mg / E2 1.5 mg versus DRSP 3 mg / EE 30 µg, Outcome 2 Clinician assessment of worsening acne after cycle 13 (all participants).
10.3. Analysis.
Comparison 10 NOMAC 2 mg / E2 1.5 mg versus DRSP 3 mg / EE 30 µg, Outcome 3 Clinician assessment of improved acne after cycle 13 (participants with acne at baseline).
10.4. Analysis.
Comparison 10 NOMAC 2 mg / E2 1.5 mg versus DRSP 3 mg / EE 30 µg, Outcome 4 Clinician assessment of worsening acne after cycle 13 (participants with acne at baseline).
10.5. Analysis.
Comparison 10 NOMAC 2 mg / E2 1.5 mg versus DRSP 3 mg / EE 30 µg, Outcome 5 Clinician assessment of new acne after cycle 13 (participants without acne at baseline).
10.6. Analysis.
Comparison 10 NOMAC 2 mg / E2 1.5 mg versus DRSP 3 mg / EE 30 µg, Outcome 6 Discontinuation due to adverse events.
10.7. Analysis.
Comparison 10 NOMAC 2 mg / E2 1.5 mg versus DRSP 3 mg / EE 30 µg, Outcome 7 Discontinuation due to acne.
Comparison 11. DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
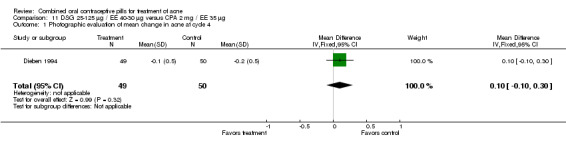
| 1 Photographic evaluation of mean change in acne at cycle 4 | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.1 [‐0.10, 0.30] |
| 2 Women with pustules or nodules at cycle 4 | 1 | 121 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.62, 2.58] |
| 3 Mean change in comedone count at cycle 4 | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐5.39, 12.79] |
| 4 Mean change in papule count at cycle 4 | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐4.16, 2.96] |
| 5 Mean change in pustule count at cycle 4 | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [‐0.15, 4.75] |
| 6 Women with moderate acne at cycle 6 | 1 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.61, 2.34] |
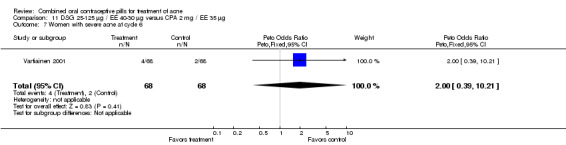
| 7 Women with severe acne at cycle 6 | 1 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.00 [0.39, 10.21] |
| 8 Mean comedone count at cycle 6 | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [0.05, 5.75] |
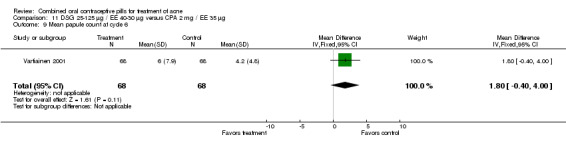
| 9 Mean papule count at cycle 6 | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [‐0.40, 4.00] |
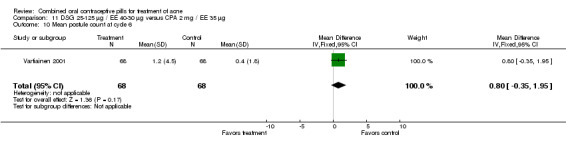
| 10 Mean postule count at cycle 6 | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.35, 1.95] |
| 11 Mean nodule count at cycle 6 | 1 | 136 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.18, 0.18] |
| 12 Discontinuation due to non‐acne adverse event | 1 | 172 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [0.45, 5.73] |
| 13 Discontinuation due to worsening of acne | 1 | 172 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.06, 16.90] |
11.1. Analysis.
Comparison 11 DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg, Outcome 1 Photographic evaluation of mean change in acne at cycle 4.
11.2. Analysis.
Comparison 11 DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg, Outcome 2 Women with pustules or nodules at cycle 4.
11.3. Analysis.
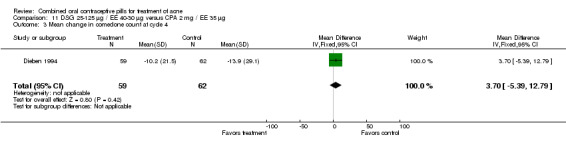
Comparison 11 DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg, Outcome 3 Mean change in comedone count at cycle 4.
11.4. Analysis.
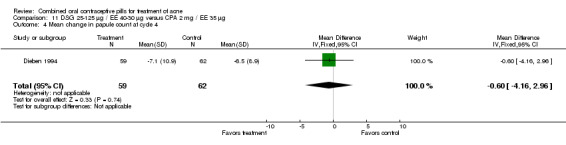
Comparison 11 DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg, Outcome 4 Mean change in papule count at cycle 4.
11.5. Analysis.
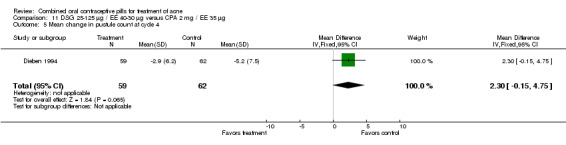
Comparison 11 DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg, Outcome 5 Mean change in pustule count at cycle 4.
11.6. Analysis.
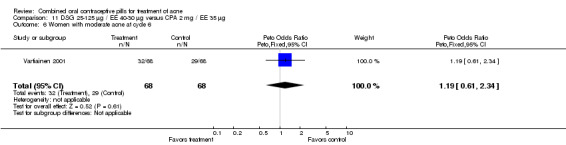
Comparison 11 DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg, Outcome 6 Women with moderate acne at cycle 6.
11.7. Analysis.
Comparison 11 DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg, Outcome 7 Women with severe acne at cycle 6.
11.8. Analysis.
Comparison 11 DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg, Outcome 8 Mean comedone count at cycle 6.
11.9. Analysis.
Comparison 11 DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg, Outcome 9 Mean papule count at cycle 6.
11.10. Analysis.
Comparison 11 DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg, Outcome 10 Mean postule count at cycle 6.
11.11. Analysis.
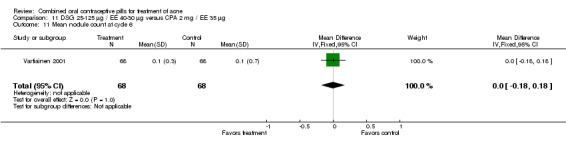
Comparison 11 DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg, Outcome 11 Mean nodule count at cycle 6.
11.12. Analysis.
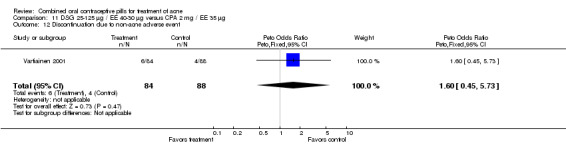
Comparison 11 DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg, Outcome 12 Discontinuation due to non‐acne adverse event.
11.13. Analysis.
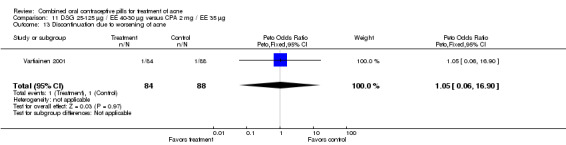
Comparison 11 DSG 25‐125 µg / EE 40‐30 µg versus CPA 2 mg / EE 35 µg, Outcome 13 Discontinuation due to worsening of acne.
Comparison 12. DSG 150 µg / EE 30 µg versus CPA 2 mg / EE 50 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Women with moderate or severe acne at cycle 6 | 1 | 57 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.35 [1.96, 20.52] |
| 2 Women with self‐assessed acne improvement at cycle 6 | 1 | 57 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.41 [0.32, 18.18] |
| 3 Discontinuation due to side effects | 1 | 66 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.12 [0.21, 21.13] |
12.1. Analysis.
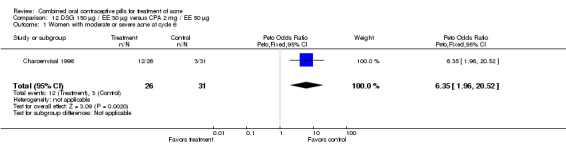
Comparison 12 DSG 150 µg / EE 30 µg versus CPA 2 mg / EE 50 µg, Outcome 1 Women with moderate or severe acne at cycle 6.
12.2. Analysis.
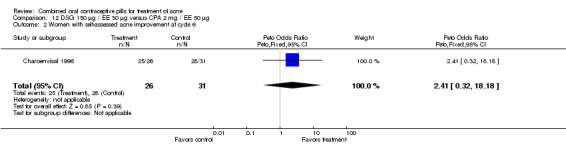
Comparison 12 DSG 150 µg / EE 30 µg versus CPA 2 mg / EE 50 µg, Outcome 2 Women with self‐assessed acne improvement at cycle 6.
12.3. Analysis.
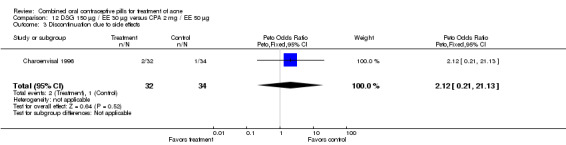
Comparison 12 DSG 150 µg / EE 30 µg versus CPA 2 mg / EE 50 µg, Outcome 3 Discontinuation due to side effects.
Comparison 13. DSG 150 µg / EE 30 µg versus GSD 75 µg / EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Women without acne at cycle 6 | 2 | 1180 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.82, 1.66] |
| 2 Women with mild acne at cycle 6 | 2 | 1180 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.52, 1.10] |
| 3 Women with moderate or severe acne at cycle 6 | 2 | 1180 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.78 [0.73, 4.32] |
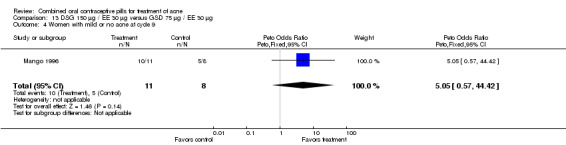
| 4 Women with mild or no acne at cycle 9 | 1 | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.05 [0.57, 44.42] |
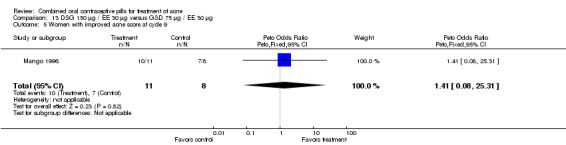
| 5 Women with improved acne score at cycle 9 | 1 | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.41 [0.08, 25.31] |
| 6 Discontinuation due to side effects | 2 | 1378 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.40, 0.93] |
13.1. Analysis.
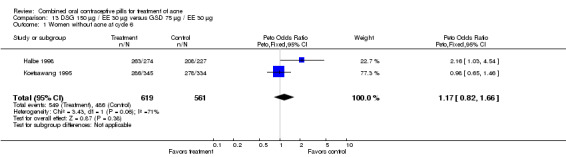
Comparison 13 DSG 150 µg / EE 30 µg versus GSD 75 µg / EE 30 µg, Outcome 1 Women without acne at cycle 6.
13.2. Analysis.
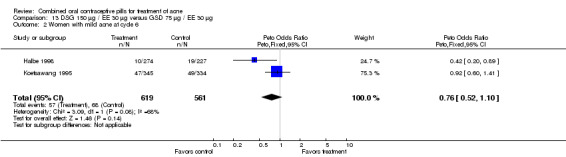
Comparison 13 DSG 150 µg / EE 30 µg versus GSD 75 µg / EE 30 µg, Outcome 2 Women with mild acne at cycle 6.
13.3. Analysis.
Comparison 13 DSG 150 µg / EE 30 µg versus GSD 75 µg / EE 30 µg, Outcome 3 Women with moderate or severe acne at cycle 6.
13.4. Analysis.
Comparison 13 DSG 150 µg / EE 30 µg versus GSD 75 µg / EE 30 µg, Outcome 4 Women with mild or no acne at cycle 9.
13.5. Analysis.
Comparison 13 DSG 150 µg / EE 30 µg versus GSD 75 µg / EE 30 µg, Outcome 5 Women with improved acne score at cycle 9.
13.6. Analysis.
Comparison 13 DSG 150 µg / EE 30 µg versus GSD 75 µg / EE 30 µg, Outcome 6 Discontinuation due to side effects.
Comparison 14. DSG 150 µg / EE 20 µg versus LNG 100 µg / EE 20 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
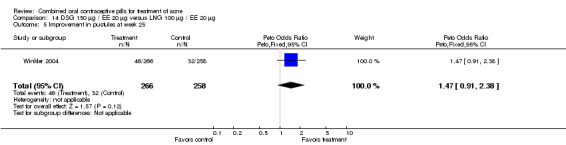
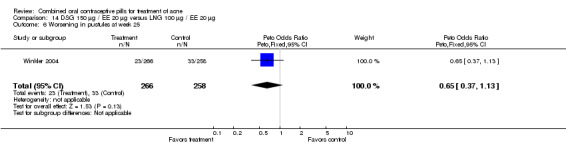
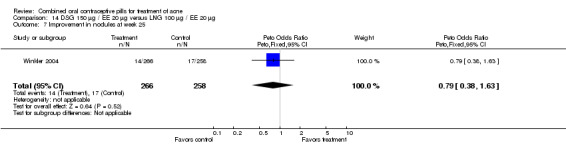
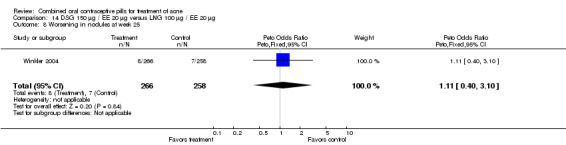
| 1 Improvement in comedones at week 25 | 1 | 524 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.55 [1.03, 2.32] |
| 2 Worsening in comedones at week 25 | 1 | 524 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.46, 1.37] |
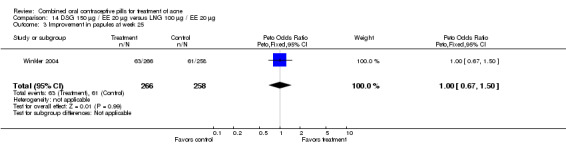
| 3 Improvement in papules at week 25 | 1 | 524 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.67, 1.50] |
| 4 Worsening in papules at week 25 | 1 | 524 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.60 [0.37, 0.96] |
| 5 Improvement in pustules at week 25 | 1 | 524 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.91, 2.38] |
| 6 Worsening in pustules at week 25 | 1 | 524 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.37, 1.13] |
| 7 Improvement in nodules at week 25 | 1 | 524 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.38, 1.63] |
| 8 Worsening in nodules at week 25 | 1 | 524 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.40, 3.10] |
| 9 Scores for Psychological General Well‐Being Index at week 13 | 1 | 720 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [0.26, 3.54] |
| 10 Scores for Psychological General Well‐Being Index at week 25 | 1 | 516 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [‐0.83, 3.03] |
| 11 Adverse events related to treatment | 1 | 998 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.58, 1.60] |
| 12 Adverse events not related to treatment | 1 | 998 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.42 [0.99, 2.04] |
14.1. Analysis.
Comparison 14 DSG 150 µg / EE 20 µg versus LNG 100 µg / EE 20 µg, Outcome 1 Improvement in comedones at week 25.
14.2. Analysis.
Comparison 14 DSG 150 µg / EE 20 µg versus LNG 100 µg / EE 20 µg, Outcome 2 Worsening in comedones at week 25.
14.3. Analysis.
Comparison 14 DSG 150 µg / EE 20 µg versus LNG 100 µg / EE 20 µg, Outcome 3 Improvement in papules at week 25.
14.4. Analysis.
Comparison 14 DSG 150 µg / EE 20 µg versus LNG 100 µg / EE 20 µg, Outcome 4 Worsening in papules at week 25.
14.5. Analysis.
Comparison 14 DSG 150 µg / EE 20 µg versus LNG 100 µg / EE 20 µg, Outcome 5 Improvement in pustules at week 25.
14.6. Analysis.
Comparison 14 DSG 150 µg / EE 20 µg versus LNG 100 µg / EE 20 µg, Outcome 6 Worsening in pustules at week 25.
14.7. Analysis.
Comparison 14 DSG 150 µg / EE 20 µg versus LNG 100 µg / EE 20 µg, Outcome 7 Improvement in nodules at week 25.
14.8. Analysis.
Comparison 14 DSG 150 µg / EE 20 µg versus LNG 100 µg / EE 20 µg, Outcome 8 Worsening in nodules at week 25.
14.9. Analysis.
Comparison 14 DSG 150 µg / EE 20 µg versus LNG 100 µg / EE 20 µg, Outcome 9 Scores for Psychological General Well‐Being Index at week 13.
14.10. Analysis.
Comparison 14 DSG 150 µg / EE 20 µg versus LNG 100 µg / EE 20 µg, Outcome 10 Scores for Psychological General Well‐Being Index at week 25.
14.11. Analysis.
Comparison 14 DSG 150 µg / EE 20 µg versus LNG 100 µg / EE 20 µg, Outcome 11 Adverse events related to treatment.
14.12. Analysis.
Comparison 14 DSG 150 µg / EE 20 µg versus LNG 100 µg / EE 20 µg, Outcome 12 Adverse events not related to treatment.
Comparison 15. LNG 150 µg / EE 30 µg versus DSG 150 µg / EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean acne severity score at cycle 6 | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.09, 0.91] |
| 2 Mean total lesion count at cycle 9 | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 6.30 [‐9.93, 22.53] |
| 3 Discontinuation due to side effects | 1 | 34 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.06, 16.69] |
| 4 Discontinuation due to worsening acne | 1 | 54 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.93 [0.36, 10.36] |
15.1. Analysis.
Comparison 15 LNG 150 µg / EE 30 µg versus DSG 150 µg / EE 30 µg, Outcome 1 Mean acne severity score at cycle 6.
15.2. Analysis.
Comparison 15 LNG 150 µg / EE 30 µg versus DSG 150 µg / EE 30 µg, Outcome 2 Mean total lesion count at cycle 9.
15.3. Analysis.
Comparison 15 LNG 150 µg / EE 30 µg versus DSG 150 µg / EE 30 µg, Outcome 3 Discontinuation due to side effects.
15.4. Analysis.
Comparison 15 LNG 150 µg / EE 30 µg versus DSG 150 µg / EE 30 µg, Outcome 4 Discontinuation due to worsening acne.
Comparison 16. LNG 150 µg / EE 30 µg versus CMA 2 mg / EE 30 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
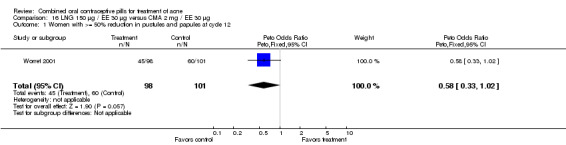
| 1 Women with >= 50% reduction in pustules and papules at cycle 12 | 1 | 199 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.33, 1.02] |
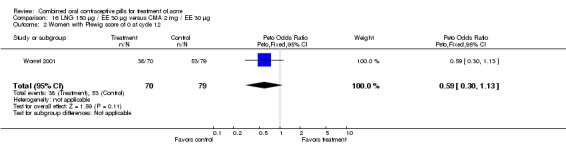
| 2 Women with Plewig score of 0 at cycle 12 | 1 | 149 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.30, 1.13] |
| 3 Women with increased pustules or papules lesion count at cycle 12 | 1 | 149 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.34 [2.25, 38.73] |
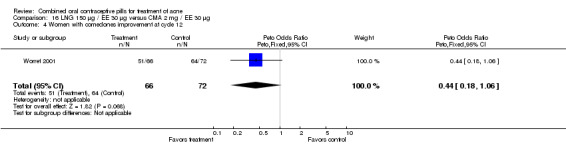
| 4 Women with comedones improvement at cycle 12 | 1 | 138 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.18, 1.06] |
| 5 Women with self‐assessed acne improvement at cycle 12 | 1 | 149 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.16 [0.04, 0.57] |
16.1. Analysis.
Comparison 16 LNG 150 µg / EE 30 µg versus CMA 2 mg / EE 30 µg, Outcome 1 Women with >= 50% reduction in pustules and papules at cycle 12.
16.2. Analysis.
Comparison 16 LNG 150 µg / EE 30 µg versus CMA 2 mg / EE 30 µg, Outcome 2 Women with Plewig score of 0 at cycle 12.
16.3. Analysis.
Comparison 16 LNG 150 µg / EE 30 µg versus CMA 2 mg / EE 30 µg, Outcome 3 Women with increased pustules or papules lesion count at cycle 12.
16.4. Analysis.
Comparison 16 LNG 150 µg / EE 30 µg versus CMA 2 mg / EE 30 µg, Outcome 4 Women with comedones improvement at cycle 12.
16.5. Analysis.
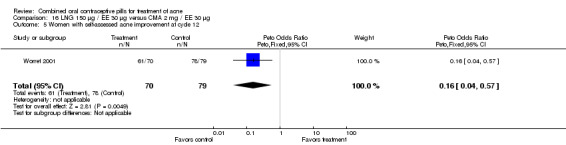
Comparison 16 LNG 150 µg / EE 30 µg versus CMA 2 mg / EE 30 µg, Outcome 5 Women with self‐assessed acne improvement at cycle 12.
Comparison 17. LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 35 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in total acne lesions at cycle 6 | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 2.50 [‐8.81, 13.81] |
| 2 Mean pustule count at cycle 6 | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.63, 2.97] |
| 3 Mean papule count at cycle 6 | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [0.20, 5.60] |
| 4 Mean cyst and nodule count at cycle 6 | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.13, 0.93] |
| 5 Women with dermatologist global "good" acne assessment at cycle 6 | 1 | 81 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.12, 0.68] |
| 6 Women with "good" acne self‐assessment at cycle 6 | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.09, 0.54] |
| 7 Discontinuation due to side effects | 1 | 85 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.40, 4.60] |
17.1. Analysis.
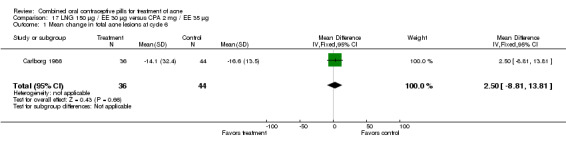
Comparison 17 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 35 µg, Outcome 1 Mean change in total acne lesions at cycle 6.
17.2. Analysis.
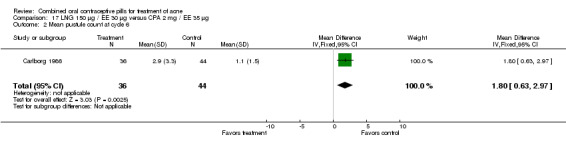
Comparison 17 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 35 µg, Outcome 2 Mean pustule count at cycle 6.
17.3. Analysis.
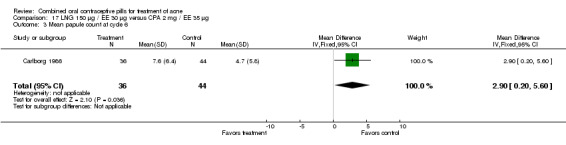
Comparison 17 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 35 µg, Outcome 3 Mean papule count at cycle 6.
17.4. Analysis.
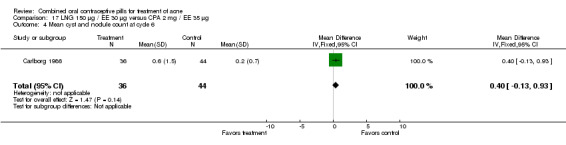
Comparison 17 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 35 µg, Outcome 4 Mean cyst and nodule count at cycle 6.
17.5. Analysis.
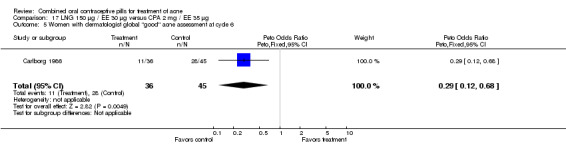
Comparison 17 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 35 µg, Outcome 5 Women with dermatologist global "good" acne assessment at cycle 6.
17.6. Analysis.
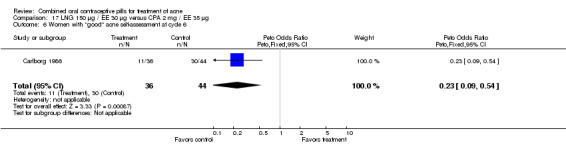
Comparison 17 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 35 µg, Outcome 6 Women with "good" acne self‐assessment at cycle 6.
17.7. Analysis.
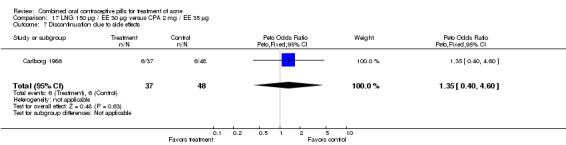
Comparison 17 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 35 µg, Outcome 7 Discontinuation due to side effects.
Comparison 18. LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 50 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in total acne lesions at cycle 6 | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐10.79, 10.99] |
| 2 Mean pustule count at cycle 6 | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [0.93, 3.27] |
| 3 Mean papule count at cycle 6 | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [1.12, 6.08] |
| 4 Mean cyst and nodule count at cycle 6 | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.11, 0.91] |
| 5 Women with dermatologist global "good" acne assessment at cycle 6 | 1 | 81 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.09, 0.52] |
| 6 Women with "good" acne self‐assessment at cycle 6 | 1 | 81 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.08, 0.44] |
| 7 Discontinuation due to side effects | 1 | 85 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.66 [0.47, 5.92] |
18.1. Analysis.
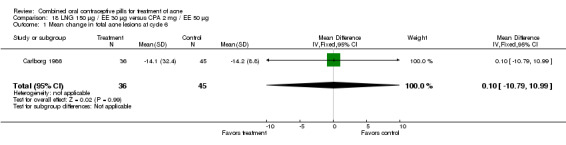
Comparison 18 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 50 µg, Outcome 1 Mean change in total acne lesions at cycle 6.
18.2. Analysis.
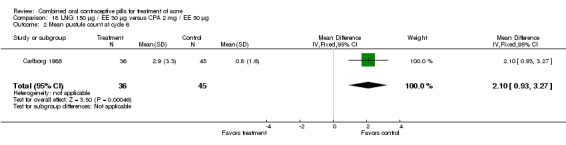
Comparison 18 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 50 µg, Outcome 2 Mean pustule count at cycle 6.
18.3. Analysis.
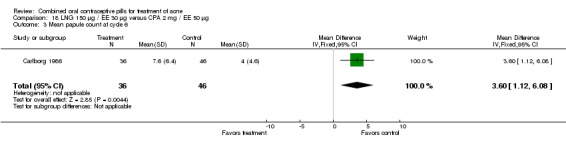
Comparison 18 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 50 µg, Outcome 3 Mean papule count at cycle 6.
18.4. Analysis.
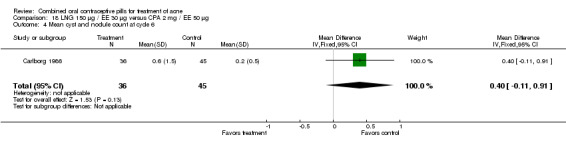
Comparison 18 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 50 µg, Outcome 4 Mean cyst and nodule count at cycle 6.
18.5. Analysis.
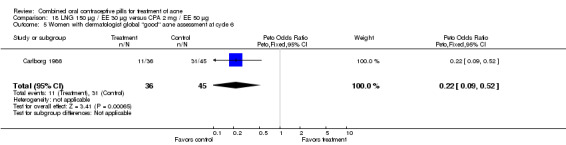
Comparison 18 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 50 µg, Outcome 5 Women with dermatologist global "good" acne assessment at cycle 6.
18.6. Analysis.
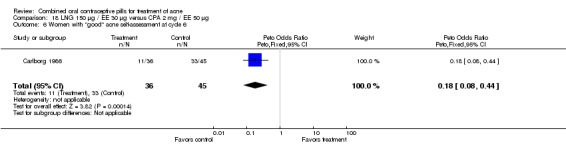
Comparison 18 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 50 µg, Outcome 6 Women with "good" acne self‐assessment at cycle 6.
18.7. Analysis.
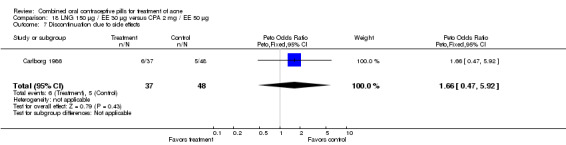
Comparison 18 LNG 150 µg / EE 30 µg versus CPA 2 mg / EE 50 µg, Outcome 7 Discontinuation due to side effects.
Comparison 19. LNG 250 µg / EE 50 µg versus CPA 2 mg / EE 50 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Women with global 'improvement or healing' acne assessment at cycle 6 | 1 | 75 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.24 [0.08, 0.75] |
19.1. Analysis.
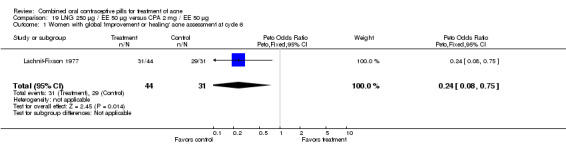
Comparison 19 LNG 250 µg / EE 50 µg versus CPA 2 mg / EE 50 µg, Outcome 1 Women with global 'improvement or healing' acne assessment at cycle 6.
Comparison 20. LNG 100 µg / EE 20 µg versus NA 1 mg / EE 20 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in total lesion count among subset of women with >= 15 lesions at baseline | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [‐12.26, 17.26] |
| 2 Discontinuation due to side effects | 1 | 58 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.37] |
20.1. Analysis.
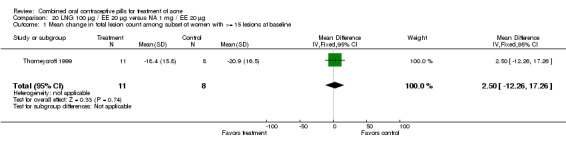
Comparison 20 LNG 100 µg / EE 20 µg versus NA 1 mg / EE 20 µg, Outcome 1 Mean change in total lesion count among subset of women with >= 15 lesions at baseline.
20.2. Analysis.
Comparison 20 LNG 100 µg / EE 20 µg versus NA 1 mg / EE 20 µg, Outcome 2 Discontinuation due to side effects.
Comparison 21. Dienogest 2 mg / EE 30 µg versus CPA 2 mg / EE 35 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean percentage change in inflammatory lesion count after cycle 6 | 1 | 1037 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐4.72, 2.72] |
| 2 Mean percentage change in total lesion count after cycle 6 | 1 | 1043 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐4.37, 2.17] |
| 3 Improvement of facial acne (clinical assessment) | 1 | 1051 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.80, 1.88] |
| 4 Discontinuation due to reason other than adverse event | 1 | 1062 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.37, 1.50] |
| 5 Discontinuation due to adverse event | 1 | 1062 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.56 [0.78, 8.40] |
21.1. Analysis.
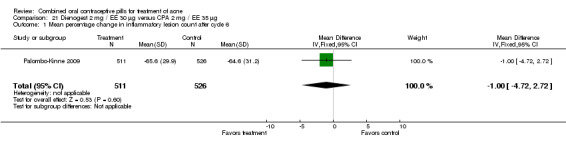
Comparison 21 Dienogest 2 mg / EE 30 µg versus CPA 2 mg / EE 35 µg, Outcome 1 Mean percentage change in inflammatory lesion count after cycle 6.
21.2. Analysis.
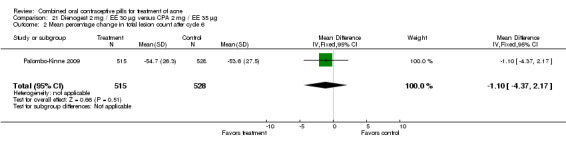
Comparison 21 Dienogest 2 mg / EE 30 µg versus CPA 2 mg / EE 35 µg, Outcome 2 Mean percentage change in total lesion count after cycle 6.
21.3. Analysis.
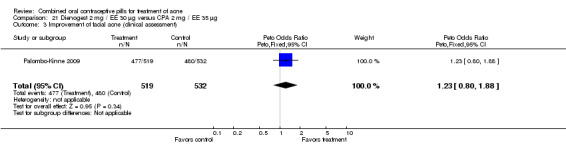
Comparison 21 Dienogest 2 mg / EE 30 µg versus CPA 2 mg / EE 35 µg, Outcome 3 Improvement of facial acne (clinical assessment).
21.4. Analysis.
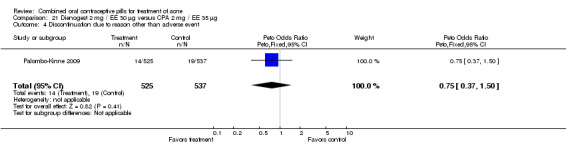
Comparison 21 Dienogest 2 mg / EE 30 µg versus CPA 2 mg / EE 35 µg, Outcome 4 Discontinuation due to reason other than adverse event.
21.5. Analysis.
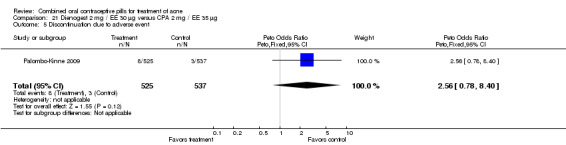
Comparison 21 Dienogest 2 mg / EE 30 µg versus CPA 2 mg / EE 35 µg, Outcome 5 Discontinuation due to adverse event.
Comparison 22. NGM 180‐215‐250 µg / EE 35 µg versus CPA 2 mg / EE 35 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in total lesion count at cycle 3 | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐9.16 [‐24.98, 6.66] |
| 2 Discontinuation due to adverse event | 1 | 45 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.97 [0.47, 18.90] |
22.1. Analysis.
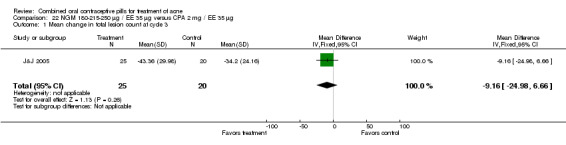
Comparison 22 NGM 180‐215‐250 µg / EE 35 µg versus CPA 2 mg / EE 35 µg, Outcome 1 Mean change in total lesion count at cycle 3.
22.2. Analysis.
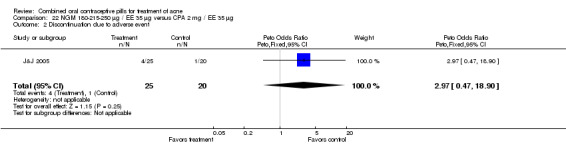
Comparison 22 NGM 180‐215‐250 µg / EE 35 µg versus CPA 2 mg / EE 35 µg, Outcome 2 Discontinuation due to adverse event.
Comparison 23. CPA 2 mg / EE 35 µg versus CPA 2 mg / EE 50 µg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in total acne lesions at cycle 6 | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐7.15, 2.35] |
| 2 Mean pustule count at cycle 6 | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.34, 0.94] |
| 3 Mean papule count at cycle 6 | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐1.47, 2.87] |
| 4 Mean cyst and nodule count at cycle 6 | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.25, 0.25] |
| 5 Women with dermatologist global "good" acne assessment at cycle 6 | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.31, 1.77] |
| 6 Women with "good" acne self‐assessment at cycle 6 | 1 | 89 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.32, 1.94] |
| 7 Women with healed or improved facial acne lesions at cycle 9 | 1 | 425 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.79, 1.70] |
| 8 Women with severe acne score at cycle 12 | 1 | 73 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.94 [0.48, 17.99] |
| 9 Discontinuation due to side effects | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.35, 4.27] |
23.1. Analysis.
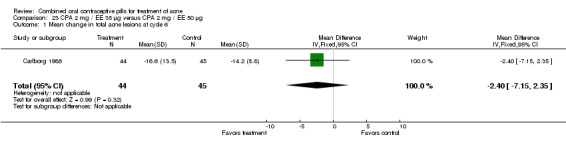
Comparison 23 CPA 2 mg / EE 35 µg versus CPA 2 mg / EE 50 µg, Outcome 1 Mean change in total acne lesions at cycle 6.
23.2. Analysis.
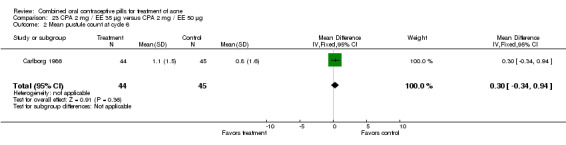
Comparison 23 CPA 2 mg / EE 35 µg versus CPA 2 mg / EE 50 µg, Outcome 2 Mean pustule count at cycle 6.
23.3. Analysis.
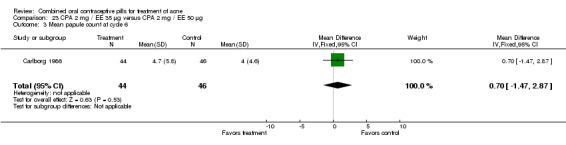
Comparison 23 CPA 2 mg / EE 35 µg versus CPA 2 mg / EE 50 µg, Outcome 3 Mean papule count at cycle 6.
23.4. Analysis.
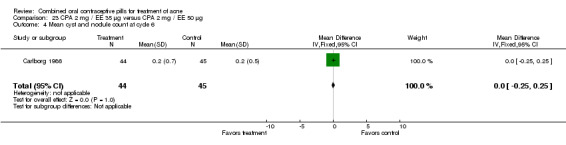
Comparison 23 CPA 2 mg / EE 35 µg versus CPA 2 mg / EE 50 µg, Outcome 4 Mean cyst and nodule count at cycle 6.
23.5. Analysis.
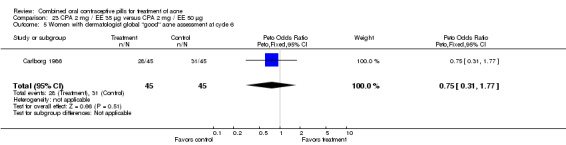
Comparison 23 CPA 2 mg / EE 35 µg versus CPA 2 mg / EE 50 µg, Outcome 5 Women with dermatologist global "good" acne assessment at cycle 6.
23.6. Analysis.
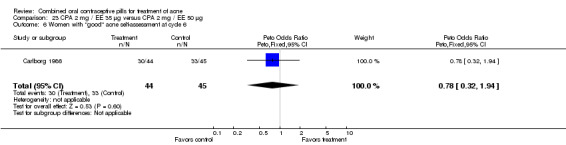
Comparison 23 CPA 2 mg / EE 35 µg versus CPA 2 mg / EE 50 µg, Outcome 6 Women with "good" acne self‐assessment at cycle 6.
23.7. Analysis.
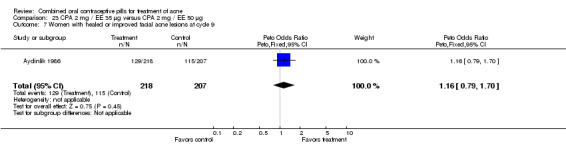
Comparison 23 CPA 2 mg / EE 35 µg versus CPA 2 mg / EE 50 µg, Outcome 7 Women with healed or improved facial acne lesions at cycle 9.
23.8. Analysis.
Comparison 23 CPA 2 mg / EE 35 µg versus CPA 2 mg / EE 50 µg, Outcome 8 Women with severe acne score at cycle 12.
23.9. Analysis.
Comparison 23 CPA 2 mg / EE 35 µg versus CPA 2 mg / EE 50 µg, Outcome 9 Discontinuation due to side effects.
Comparison 24. CPA 2 mg / EE 50 µg versus minocycline hydrochloride 50 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Women with self‐assessed acne improvement at cycle 6 | 1 | 78 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.43, 5.01] |
| 2 Women with self‐assessed lack of acne at cycle 6 | 1 | 78 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.28, 2.60] |
| 3 Discontinuation due to non‐acne adverse event | 1 | 98 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.29, 6.26] |
| 4 Discontinuation due to lack of acne improvement | 1 | 98 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.11, 3.95] |
24.1. Analysis.
Comparison 24 CPA 2 mg / EE 50 µg versus minocycline hydrochloride 50 mg, Outcome 1 Women with self‐assessed acne improvement at cycle 6.
24.2. Analysis.
Comparison 24 CPA 2 mg / EE 50 µg versus minocycline hydrochloride 50 mg, Outcome 2 Women with self‐assessed lack of acne at cycle 6.
24.3. Analysis.
Comparison 24 CPA 2 mg / EE 50 µg versus minocycline hydrochloride 50 mg, Outcome 3 Discontinuation due to non‐acne adverse event.
24.4. Analysis.
Comparison 24 CPA 2 mg / EE 50 µg versus minocycline hydrochloride 50 mg, Outcome 4 Discontinuation due to lack of acne improvement.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aydinlik 1986.
| Methods | Multicenter trial in 8 European countries. Double blind but no mention of who was blinded. Pretreatment wash‐out period not stated. 9 treatment cycles. | |
| Participants | 425 women of reproductive age with mild to moderate acne and seborrhea. Exclusion criteria not stated. | |
| Interventions | Group 1: Cyproterone acetate (CPA) 2 mg plus ethinyl estradiol (EE) 35 µg Group 2: CPA 2 mg plus EE 50 µg | |
| Outcomes | Healing or improvement of acne cases from baseline. | |
| Notes | Methods of randomization and allocation concealment were not described. No mention of early discontinuation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Placebo group received identical‐appearing tablets. |
Bayer 2011.
| Methods | Multicenter trial conducted in China from 2008 to 2010 Double‐blind (subject and investigator) No information on how the randomization schedule was generated. |
|
| Participants | 179 women, 14 to 45 years old. Inclusion criteria: >1 year post‐menarche with moderate acne vulgaris with no contraindication to COCs; healthy except for moderate acne; smokers up to age 30 (inclusive). Exclusion criteria: pregnancy, lactation (< 3 menstrual cycles since delivery, abortion, or lactation); obesity (Body Mass Index > 30 kg/m2); hypersensitivity to ingredient of study drug; any disease or condition that may worsen under hormonal treatment |
|
| Interventions | Group 1: Drospirenone (DRSP) 3 mg plus EE 20 µg Group 2: Placebo Duration: 6 cycles |
|
| Outcomes | Primary: percent change in total lesion count in full analysis and per‐protocol analysis. Secondary: percentage of participants classified as "0" (clear) or "1" (almost clear) on 6‐point ISGA (Investigator Static Global Assessment); percent change in inflammatory and non‐inflammatory lesion counts; percent change in lesion counts of papules, pustules, nodules, open comedones, and closed comedones; percentage of participants classified as 'improved' according to investigator's and participant's overall ratings. Improvement in lesion count is indicated by larger percent change: ((count at baseline ‐ count at cycle 6)/ count at baseline)*100 |
|
| Notes | Results were posted on ClinicalTrials.gov Study was sponsored by Bayer Schering Pharma Excluded after randomization 6 women who did not take study drug (2 DRSP and 4 placebo) Lost to follow up: DRSP 4/89 (4.5%); placebo 6/90 (6.7%). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Carlborg 1986.
| Methods | Multicenter trial in Sweden. Clinicians and participants were blinded. 4‐week washout period for acne treatments. 6 treatment cycles. | |
| Participants | 160 healthy women over 15 years of age with at least 8 lesions on the face. Excluded women with contraindications to COCs. | |
| Interventions | Group 1: CPA 2 mg plus EE 35 µg Group 2: CPA 2 mg plus EE 50 µg Group 3: Levonorgestrel (LNG) 150 µg plus EE 30 µg | |
| Outcomes | Acne lesion counts. Gynecologist, dermatologist and participant assessment of acne ("good," "moderate" or "poor"). Early discontinuation due to side effects. | |
| Notes | Randomization by manufacturer. No information on allocation concealment. Blinding with numbered packages. 35 women were excluded, discontinued early, were lost to follow up or were missing data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Charoenvisal 1996.
| Methods | Two‐center, open trial in Thailand and Bangkok. 3‐month washout for OCs or injectables use. 6 treatment cycles. | |
| Participants | 66 women aged 16 to 30 years. Excluded women with contraindications to COC use, current systemic anti‐acne medication, delivery or abortion within the last 6 months or breastfeeding. | |
| Interventions | Group 1: Desogestrel (DSG) 150 µg plus EE 30 µg Group 2: CPA 2 mg plus EE 50 µg | |
| Outcomes | Acne severity score ("absent" no acne; "mild" <= 15 comedones, <= 10 papules and <= 5 pustules; "moderate" > 15 comedones or > 10 papules or > 5 pustules and < 5 nodulocystic lesions; "severe" >= 5 nodulocystic lesions). Participant self‐assessment of acne severity. Early discontinuation due to side effects. | |
| Notes | Methods of randomization and allocation concealment were not described. Separate statistical analysis was performed for each center due to fundamental differences in scale rating. 9 women discontinued early or were lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Dieben 1994.
| Methods | Multicenter trial in 4 European countries. Open trial except that photograph assessor was blinded. Washout period of 2 months for hormonal contraceptives and 4 weeks for anti‐acne medication. 4 treatment cycles. | |
| Participants | 183 women aged 18 to 35 years with at least 5 facial acne lesions. Excluded women with contraindications to COC use. | |
| Interventions | Group 1: Biphasic DSG 25‐125 µg plus EE 40‐30 µg Group 2: CPA 2 mg plus EE 35 µg | |
| Outcomes | Acne lesion counts. Photograph assessment using modified Burke and Cunliffe grades ("Grade 0" no comedos, papules, pustules or nodules; "Grade 1" comedos only; "Grade 2" papules, no nodules, no pustules; "Grade 3" pustules, no nodules; "Grade 4" nodules). | |
| Notes | Randomization list was generated by sponsors. According to correspondence from the author, the randomization list was open so no allocation concealment was done. 47 women were excluded, discontinued early or were lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Not used |
Fugere 1988.
| Methods | Two‐center trial in Canada. Participants and investigators were blinded. Six‐week washout period. 12 treatment cycles. | |
| Participants | 93 healthy women, aged 17 to 35 years, with moderate to severe acne. Number randomized to each group not provided. Excluded women with thromboembolic disorders, cerebral vascular disease, liver disease or dysfunction, malignancy of the breast, Cushing syndrome, congenital adrenal hyperplasia, ovarian or adrenal tumors, estrogen dependent neoplasia, undiagnosed abnormal vaginal bleeding, ophthalmic vascular disease, myocardial infarction or classical migraine, or women receiving hormonal therapy or medications that might interfere with study drug. | |
| Interventions | Group 1: CPA 2 mg plus EE 35 µg Group 2: CPA 2 mg plus EE 50 µg | |
| Outcomes | Acne lesion counts. Acne severity score using an 8‐point scale. Overall acne "severity" outcome not defined. | |
| Notes | Randomization was by an independent pharmaceutical company. No information on allocation concealment. Blinding done by using similarly colored medications in boxes. 20 women were excluded, discontinued early or were lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Halbe 1998.
| Methods | Multicenter open trial in Brazil. Pretreatment wash‐out was not described. 6 treatment cycles. | |
| Participants | 595 healthy women of fertile age with regular ovulatory cycles. Excluded women with contraindications to oral contraceptives, breast feeding or regular use of drugs that impair the efficacy of oral contraceptives. | |
| Interventions | Group 1: DSG 150 µg plus EE 30 µg Group 2: Gestodene (GSD) 75 µg plus EE 30 µg | |
| Outcomes | Acne severity score ("absent" no acne lesions; "mild" < 6 comedones or papules; "moderate" 6‐15 comedones or papules and < 4 inflamed lesions; "severe" > 15 comedones or papules and > 3 inflamed lesions). Early discontinuation due to side effects. | |
| Notes | Methods of randomization and allocation concealment were not described. 94 women were excluded, discontinued early or were lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
J&J 2005.
| Methods | Randomized controlled trial conducted in Taiwan from Sep 2004 to Sep 2005 No information on randomization method. Double‐blind (did not specify who was blinded) |
|
| Participants | 48 women, 15 to 49 years. Inclusion Criteria: moderate acne vulgaris (grade II or III), 6 to 100 comedones (non‐inflammatory lesions), 10 to 50 inflammatory lesions (papules or pustules), fewer than 5 nodules; agree to condoms or diaphragm, and spermicide or other medically approved effective barrier contraceptive or non‐hormonal IUD; agree to take as treatment for acne only supplied study drug during 3‐month treatment phase.
Exclusion Criteria: in investigator's opinion, cannot understand or follow study instructions, pregnant or nursing, clinical depression and suicidal or require immediate treatment for depression, known hypersensitivity to any ingredient, significant adverse experiences from ethinyl estradiol or norgestimate, coexisting medical condition or taking concomitant medication likely to interfere with safe administration of TriCilest or Diane‐35; took systemic retinoids, systemic antimicrobials, and topical acne treatments in past 6 months, 1 month, and 2 weeks, respectively; any contraindication to oral contraceptives: current or past thrombophlebitis or thromboembolic disorder, cerebral vascular or coronary artery disease or known severe hypertension, diabetes with vascular involvement, known or suspected carcinoma of the breast, known or suspected estrogen‐dependent neoplasia; undiagnosed, abnormal genital bleeding; liver tumor, jaundice or severe liver disease, neurovascular lesion of the eye or serious visual disturbances, known allergic reaction or sensitivities to TriCilest or Diane 35; took an investigational medication in past 30 days (or in period of five times its half‐life or the half‐life of its metabolites) |
|
| Interventions | Group 1: norgestimate 180‐215‐250 µg plus ethinyl estradiol 35 µg Group 2: cyproterone acetate 2 mg plus EE 35 µg Duration: 3 cycles |
|
| Outcomes | Primary: change in total lesion count from baseline to latest available evaluation Secondary: change in inflammatory lesion count, change in individual lesion count, percentage of participants showing improvement on investigator's global assessment, participant's end‐of‐therapy self‐assessment. |
|
| Notes | Unpublished report was obtained from a link on ClinicalTrials.gov. For secondary outcomes, no data were provided. Report noted the study groups were not significantly different regarding treatment effect for these counts. Study was sponsored by Johnson & Johnson Taiwan Ltd. Excluded from analysis 3 participants who did not take any study drug (comparison). Loss to follow up: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Kelly 2010.
| Methods | Randomized multicenter trial. Allocated in 2:1 ratio (DRSP to LNG). Participants and evaluators were blinded to treatment assignment. Principal investigator was not blinded (required to open envelopes and dispense drugs). Sample size of 420 planned for 80% power to detect difference in Menstrual Distress Questionnaire between groups. |
|
| Participants | 424 healthy women, aged 16 to 40 years (up to age 35 if smoker), with established menstrual cycle and requesting contraception. Inclusion criteria: healthy gynecological status by exam and cervical smear, willing to not use other hormonal treatment (except thyroxine and insulin). Exclusion criteria: contraindication to combined OC, history of herpes, obesity, concurrent treatment with preparation that induces hepatic enzymes | |
| Interventions | Group 1: drospirenone 3 mg plus ethinyl estradiol (EE) 30 µg (N=282) (21 + 7 regimen) Group 2: levonorgestrel (LNG) 150 µg plus EE 30 µg (N=142) 7 treatment cycles |
|
| Outcomes | Acne lesions counted at randomization and each visit: inflammatory (papules, pustules, nodules) or non‐inflammatory (open and closed comedones). Results presented in figures (without absolute numbers) for total lesion count and percentage of subjects with acne. Text describes increases and decreases without any actual numbers or any statistical results. Discontinuation due to acne was given. An earlier attempt to obtain additional data from the sponsor was unsuccessful. The sponsor communicated that data (from daily diary cards) were available for the luteal phase but not for the premenstrual phase. |
|
| Notes | Sites not specified; authorship suggests 5 sites in UK and 1 in Australia Study was funded by Bayer Schering Pharma. Loss to follow up: 6% overall; DRSP group 5%; LNG group 9%. Discontinuation and losses: 34% overall; DRSP group 32%; LNG group 39%. Analysis reportedly included all non‐missing data for all randomized participants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomization envelopes |
Koetsawang 1995.
| Methods | Multicenter, open trial in Thailand. Pretreatment washout period was not described. 6 treatment cycles. | |
| Participants | 783 healthy women of fertile age with regular cycles. No specific age range was reported. Excluded women with contraindications to oral contraceptives, complete breast feeding or regular use of drugs that impair oral contraceptives. | |
| Interventions | Group 1: DSG 150 µg plus EE 30 µg Group 2: GSD 75 µg plus EE 30 µg | |
| Outcomes | Acne lesion counts. Acne severity grades ("absent" no acne lesions; "mild" < 6 comedones or papules; "moderate" 6‐15 comedones or papules and/or < 4 inflamed lesions; "severe" > 15 comedones or papules and/or > 3 inflamed lesions). Early discontinuation due to side effects. | |
| Notes | Randomization by random number tables. No information on allocation concealment. 104 women were excluded, discontinued early or were lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Koltun 2008.
| Methods | Double‐blind RCT (participants and investigators were blinded); 32 centers in the US. Same design as Maloney 2008. Randomization 1:1 based on "computer‐generated randomization code" A priori sample size calculation indicated 250 women were needed per group for 90% power to detect differences in lesion counts. Differences were based on previous studies (treatment versus placebo: 48% versus 30% for inflammatory lesions and 42% versus 28% for total lesions). For non‐inflammatory lesions, the estimate was based on detecting a difference of 18 lesions. | |
| Participants | 458 women, aged 14 to 45 years, with minimum of 20 inflammatory (papules or pustules) and 20 non‐inflammatory (comedones) facial lesions. Inclusion criteria: negative pregnancy test and normal Pap smear and at least 1 menstruation within last 3 months; agreed not to use topical or systemic acne treatment. Exclusion criteria: contraindications for COC use; use of additional steroid hormones, heparin, warfarin, hydantoins, barbiturates, phenytoin, primidone, carbamazepine, rifampicin, griseofulvin, topiramate, felbamate, ritonavir and products containing St John's wort, spironolactone, and continuous use of antibiotics; having acne and atopy, comedonal acne or acne conglobata, sandpaper acne or acne with multiple large nodes; cysts, fistular comedones, or abscessing fistular ducts; taking medication with "acne‐inducing effect." | |
| Interventions | Group 1: DRSP 3 mg plus EE 20 µg (24 days + 4 days inactive tablets) Group 2: placebo for 28 days 6 treatment cycles | |
| Outcomes | Baseline to endpoint (after cycle 6): percent reduction in inflammatory, non‐inflammatory, and total lesion counts; percent of participants rated by investigator as "clear" or "almost clear"
Results were presented in figures without actual numbers to use in analysis, except for discontinuation. Koltun 2011 reported on pooled analysis of data from this trial and Maloney 2008. Results presented for lesions were limited to figures and P values. Again, the authors reported percent of participants rated by investigator as "clear" or "almost clear". |
|
| Notes | US Food and Drug Administration changed indication from "mild to moderate acne" to "moderate acne", thus reducing the primary analysis data set from 534 to 458. Losses from original 534: 6% DRSP and 8% placebo lost to follow up. Loss data were not presented separately for the amended sample of 458. Attempted to contact author regarding outcome data for use in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No mention of concealment before assignment |
Lachnit‐Fixson 1977.
| Methods | Multicenter trial in Germany and Austria. Participant and investigators were blinded. 6 to 12 treatment cycles. | |
| Participants | 88 women aged 16 to 45 years with acne, seborrhea or hirsutism graded from none to severe. Excluded women with contraindications to oral contraceptives or abnormal gynecological evaluation. | |
| Interventions | Group 1: CPA 2 mg plus EE 50 µg Group 2: LNG 250 µg plus EE 50 µg | |
| Outcomes | Clinician global assessment. | |
| Notes | Randomization method not stated. No information on allocation concealment before assignment. Pills distributed in numbered kits with 21‐day pill packs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Leyden 2002.
| Methods | Multicenter trial in U.S. Participants and investigators were blinded. 6‐week washout period for retinoid use, 1 month for systemic acne, 2 weeks for topical acne and 3 months for oral or injectable contraception; 6 treatment cycles | |
| Participants | 371 healthy women at least 14 years of age with regular menstrual cycles and moderate facial acne. Other inclusion criteria were normal or low grade abnormal Papanicolaou smear within the past 6 months, negative pregnancy test and agreement to use a non‐hormonal contraceptive if at risk of pregnancy. Excluded women with contraindications to OCs, smoking in women over 35 years old or use of sex hormones within 6 months of enrollment. | |
| Interventions | Group 1: LNG 100 µg plus EE 20 µg Group 2: Placebo | |
| Outcomes | Acne lesion counts. Clinician global assessment using 4‐point scale ("clear," "almost clear/mild," "moderate" or "severe") adapted from an American Academy of Dermatology consensus statement. Participant self‐assessment. | |
| Notes | Randomization in blocks of 4 using a computer‐generated schedule. Medication code was provided in individual sealed envelopes labeled according to the randomization schedule. Did not specify if envelopes were opaque or not. 125 women discontinued early or were lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Medication code was provided in individual sealed envelopes labeled according to the randomization schedule. Did not specify if envelopes were opaque or not. |
Lucky 1997.
| Methods | Multicenter, placebo‐controlled trial in U.S. Investigators, study staff, data analyst and participants were blinded. Washout periods were 3 months for OCs and levonorgestrel implant, 6 months for depomedroxyprogesterone acetate and systemic retinoids, 1 month for systemic antibiotics and 2 weeks for topical medications. 6 treatment cycles. | |
| Participants | 257 healthy women aged 15 to 49 years with moderate acne vulgaris. Women at risk of pregnancy had to be willing to use a non‐hormonal contraceptive method. Excluded women with hirsutism or contraindications to COCs. | |
| Interventions | Group 1: Triphasic norgestimate (NGM) 180‐215‐250 µg plus EE 35 µg Group 2: Placebo | |
| Outcomes | Acne lesion counts. Clinician global assessment of acne. Participant self‐assessment of acne. Early discontinuation due to side effects and due to worsening of acne. | |
| Notes | Computer‐generated randomization schedule. No information on allocation concealment. 85 women were excluded, discontinued early or were lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Maloney 2001.
| Methods | Multicenter, placebo‐controlled trial in U.S. Double blind but no mention of who was blinded. No washout period described. 6 treatment cycles. | |
| Participants | 593 women aged 14 to 49 years with at least moderate acne. Excluded women with significant endocrinopathy, testosterone level > 150 ng/dL, DHEA sulfate > 600 µg/dL, concomitant systemic disease, pregnancy or breast feeding. | |
| Interventions | Group 1: Norethindrone acetate (NA) 1 mg plus EE 20‐30‐35 µg Group 2: Placebo | |
| Outcomes | Clinician assessment of "no, minimal or mild" acne. Early discontinuation due to side effects. | |
| Notes | Methods of randomization and allocation concealment were not described. 190 women were excluded, discontinued early or were lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Maloney 2008.
| Methods | Double‐blind RCT (participants and investigators were blinded); 28 centers in the US. Same design as Koltun 2008. Randomization 1:1 based on "computer‐generated randomization code" A priori sample size calculation indicated 250 women were needed per group for 90% power to detect differences in lesion counts. Differences were based on previous studies (treatment versus placebo: 48% versus 30% for inflammatory lesions and 42% versus 28% for total lesions). For non‐inflammatory lesions, the estimate was based on detecting a difference of 18 lesions. | |
| Participants | 431 women, aged 14 to 45 years, with minimum of 20 inflammatory (papules or pustules) and 20 non‐inflammatory (comedones) facial lesions classified as grade 3, 4, or 5. Inclusion criteria: age 14 to 30 years if smoked >10 cigarettes/day, 14 to 35 years if smoked <10 cigarettes/day, and 14 to 45 years for nonsmokers; normal Pap smear in last 6 months and at least 1 menstruation within last 3 months; agreed not to use topical or systemic acne treatment. Exclusion criteria: contraindications for COC use; use of additional steroid hormones, heparin, warfarin, hydantoins, barbiturates, phenytoin, primidone, carbamazepine, rifampicin, griseofulvin, topiramate, felbamate, ritonavir and products containing St John's wort, spironolactone, and continuous use of antibiotics; having acne and atopy, comedonal acne or acne conglobata, sandpaper acne or acne with multiple large nodes; cysts, fistular comedones, or abscessing fistular ducts. | |
| Interventions | Group 1: DRSP 3 mg plus EE 20 µg (24 days + 4 days inactive tablets) Group 2: placebo for 28 days 6 treatment cycles | |
| Outcomes | Baseline to endpoint (after cycle 6): percent reduction in inflammatory, non‐inflammatory, and total lesion counts; percent of participants rated by investigator as "clear" or "almost clear" Except for investigator ratings, results were presented in figures without actual numbers to use in analysis. Secondary paper (Maloney 2009) reported on secondary outcomes but still presented results in figures without absolute numbers (lesion counts by nodules, papules, pustules, open comedones and closed comedones). Koltun 2011 reported on pooled analysis of data from this trial and Koltun 2008. Results for lesions were presented in figures. The researchers again reported percent of participants rated by investigator as "clear" or "almost clear". |
|
| Notes | US Food and Drug Administration changed indication from "mild to moderate acne" to "moderate acne", thus reducing the primary analysis dataset from 538 to 431. Losses from original 538: 7% DRSP and 9% placebo. Loss data were not presented separately for the amended sample of 431. Attempted to contact author regarding outcome data for use in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No mention of concealment before assignment |
Mango 1996.
| Methods | Single‐center, open trial in Italy. 6 month pre‐treatment wash‐out period. 9 treatment cycles. | |
| Participants | 24 nulligravid women aged 18 to 35 years with either late onset or persistent acne vulgaris and normal cycles or oligomenorrhea. Excluded women who heavy smoking and overweight contraindications to COCs, abnormalities in blood coagulation, lipid profile, liver and thyroid function, androgen secreting tumors, congenital adrenal hyperplasia, hyperprolactinemia. | |
| Interventions | Group 1: DSG 150 µg plus EE 30 µg Group 2: GSD 75 µg plus EE 30 µg | |
| Outcomes | Acne severity score using modified Pillsbury method ("grade 0" no acne; "grade I" isolated comedones, no papules or pustules; "grade II" 1‐9 comedones and/or papules or pustules; "grade III" 10‐19 comedones and/or papules or pustules; "grade IV" 20 lesions and inflammation extending to total face". | |
| Notes | Methods for randomization and allocation concealment were not described. 9 women did not complete the treatment cycles or clinical examinations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Mansour 2011.
| Methods | Open‐label, multicenter trial conducted in Europe, Asia, and Australia. Computer‐generated randomization schedule with blocks of four; allocated 3:1 (NOMAC/E2 or DRSP/EE); stratified by age (18 to 35 years and 36 to 50 years). Sample size based on precision of Pearl Index in women <=35 years old, 80% power; used guidance of Committee for Medicinal Products for Human Use. |
|
| Participants | 2152 healthy, sexually active women (18 to 50 years) with body mass index between 17 and 35 kg/m2 who needed contraception and did not plan to use condoms. Exclusion criteria: contraindications for contraceptive steroids; abnormal cervical smear at screening; clinically relevant abnormal laboratory result at screening; use of injectable hormonal contraception within 6 months of injection with 3‐month duration, within 4 months of injection with 2‐month duration, within 2 months of injection with 1‐month duration; present use or use in past 2 months: phenytoin, barbiturates, primidone, carbamazepine, oxcarbazepine, topiramate, felbamate, rifampicin, nelfinavir, ritonavir, griseofulvin, ketoconazole, sex steroids (except allowed contraceptive methods used before and after treatment period), and herbal remedies containing Hypericum perforatum (St John’s Wort). |
|
| Interventions | Group 1: Nomegestrol acetate (NOMAC) 2.5 mg + 17β‐estradiol (E2) 1.5 mg (24/4) (N=1613)
Group 2: Drospirenone (DRSP) 3 mg + EE 30 μg (21/7) (N=539) 13 treatment cycles |
|
| Outcomes | Acne was secondary outcome. Study staff assessed acne at screening and all visits; scored acne as ‘none’, ‘mild ’, ‘moderate’ or ‘severe’ according to own judgment. Staff recorded any increase in severity of acne from baseline on the adverse events form. Outcomes presented as 'improved', 'no change', 'worsening' for all participants and for participants with acne at baseline. For those without acne at baseline, results included 'free from acne' or 'new acne'. By correspondence, researcher provided absolute counts for results that had been presented in figures. |
|
| Notes | Study funded by Merck Sharp & Dohme (MSD). Three authors employed by MSD (Oss, the Netherlands) Analysis population excluded those who did not take any study drug: NOMAC n=22 (1.4%); DRSP n=4 (0.7%). Loss to follow up: NOMAC 2.5%; DRSP 3.2% Loss overall (discontinuations, exclusions): NOMAC 471/1613 (29%); DRSP 129/539 (24%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system; central allocation in order of randomization call |
Monk 1987.
| Methods | Multicenter, open trial in the UK. 1 month pre‐treatment wash‐out period. 6 treatment cycles. | |
| Participants | 98 women with acne "of sufficient severity to merit systemic antibiotic therapy". Excluded pregnancy, contraindications to OCs, severe nodular, cystic or nodulo‐cystic acne. | |
| Interventions | Group 1: CPA 2 mg plus EE 50 µg Group 2: minocycline hydrochloride 50 mg | |
| Outcomes | Participant self‐assessment. Early discontinuation due to side effects and due to lack of acne improvement. | |
| Notes | Methods for randomization and allocation concealment were not described. 20 women discontinued early or were lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Palatsi 1984.
| Methods | Single‐center trial in Finland. Blinding was not described. 3‐month washout period for hormonal medication use. 6 treatment cycles. | |
| Participants | 54 women aged 18 to 35 years with persistent acne. Excluded women with contraindications to COCs or thyroid, liver or cardiovascular diseases. | |
| Interventions | Group 1: DSG 150 µg plus EE 30 µg Group 2: LNG 150 µg plus EE 30 µg | |
| Outcomes | Acne severity grade using scale similar to Allen and Smith scale ("grade 0" perfectly clear or only few small lesions; "grade 1" few pustules and about 10 papules; "grade 2" about half of the face is affected and numerous lesions; "grade 3" numerous active lesions and general inflammation of the facial skin). Early discontinuation due to worsening acne. | |
| Notes | According to correspondence from the author, a randomization table, produced by a statistician, provided the numbers for balanced blocks with size of four. No information on allocation concealment. 21 women discontinued early, were lost to follow up or had missing samples. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Palombo‐Kinne 2009.
| Methods | Multicenter trial conducted Mar 2004 to May 2005 (65 centers in Czech Republic, Poland, Russian Federation, Slovakia, and Ukraine) Double‐blind, three‐arm trial; no information on who was blinded Random assignment 2:2:1 (DNG/EE: CPA/EE: placebo); no information on how randomization schedule was generated. |
|
| Participants | 1338 women, 16 to 45 years old, with mild to moderate facial papulopustular acne and no contraindication to COC use. Inclusion criteria: normal Papanicolaou test result in past 6 months; non‐hormonal contraception use, given the placebo arm, for sexually active patients; and avoidance of topical or systemic acne treatments (e.g., covering creams or sunscreens, light therapy, chemical peelings). Exclusion criteria: presence of known contraindication to OCs; smoking, if age > 30 years; pregnancy and lactation (at least 3 regular cycles to elapse before treatment); and a body mass index < 30 kg/m2. Dermatological exclusion criteria: other forms of acne and atopy and intake of preparations with known or suspected acne‐inducing effects (e.g., vitamin B, anabolics, corticoids). |
|
| Interventions | Group 1: dienogest 2 mg plus EE 30 µg (test product); received active and placebo tablets Group 2: cyproterone acetate 2 mg plus EE 35 µg (control product); received active and placebo tablets Group 3: placebo; received 2 placebo tablets, identical in appearance to active tablets Treatment duration: 6 cycles |
|
| Outcomes | Primary: percentage change in inflammatory lesion count (sum of papules, pustules and nodules); percentage change in total lesion count (sum of open and closed comedones, papules, pustules and nodules); and percentage of patients with improvement of facial acne (6‐point scale). Secondary: Investigator Static Global Assessment at each visit [six‐point scale from normal to highly inflammatory]; absolute changes in facial lesion count over time; patient's self‐assessment at end of therapy. |
|
| Notes | Study was funded by Schering (now Bayer Schering Pharma) Analysis excluded those who did not receive intervention (n=10) or did not return (n=2): 5 DNG/EE, 4 CPA/EE, and 3 placebo. Loss to follow up: 11/1338 (0.8%) Discontinuations: 63/1338 (4.7%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information. |
Plewig 2009.
| Methods | Multicenter randomized (2:1) trial from 2000 to 2001 (28 centers in Bulgaria, Estonia, France, Germany, Lithuania, Sweden and the UK) Double blind; no information on who was blinded No information on how randomization schedule was generated. |
|
| Participants | 387 women, 18 to 40 years old (smokers up to age 30), with moderate papulopustular acne of face. Participants were instructed to use condoms and were not allowed to take hormonal contraception or topical or systemic moderate acne therapy during the trial. Exclusion criteria: systemic moderate acne therapy (e.g., with 'antiandrogens' or retinoids) in past 6 months; hormonal combinations containing 'antiandrogens,' norgestimate or desogestrel in past 3 months; oral antibiotic or topical moderate acne treatment in past 4 weeks. |
|
| Interventions | Group 1: chlormadinone acetate 2 mg plus EE 30 µg Group 2: placebo Duration: 6 treatment cycles |
|
| Outcomes | Primary: change in number of papules and pustules on face from baseline to final efficacy examination. Satisfactory 'response' defined as >=50% decrease in facial papules and pustules. Secondary: number of comedones on face and moderate papulopustular acne lesions of 'décolleté' (lower neck line) and back regions. During final efficacy examination, subjects' did a global self‐assessment of therapeutic influence on their moderate acne. |
|
| Notes | Study was sponsored by Grünenthal GmbH Excluded from analysis 10 who did not take any study drug (groups not specified). Losses due to drop outs: CMA/EE 15% (37/251); placebo 18% (23/126). No information on losses to follow up. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Redmond 1997.
| Methods | Multicenter, placebo‐controlled trial in U.S. Investigator, study staff, participants and data analysts were blinded. Washout period for systemic retinoids, antimicrobials and topical anti‐acne agents of 6 months, 1 month and 2 weeks, respectively. 6 treatment cycles. | |
| Participants | 250 healthy women aged 15 to 49 years with moderate acne vulgaris. Excluded women with contraindications to COCs. Participants with risk of pregnancy were required to use a barrier method of contraception. | |
| Interventions | Group 1: norgestimate (NGM) 180‐215‐250 µg plus EE 35 µg Group 2: Placebo | |
| Outcomes | Acne lesion counts. Clinician global assessment of acne. Early discontinuation due to side effects and due to worsening of acne. | |
| Notes | Randomization by central computer‐generated codes. The study drug was dispensed in individual, sealed, numbered identical boxes according to the randomization schedule. After assignment, the drug was dispensed. 86 women were excluded, discontinued early or were lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | The study drug was dispensed in individual, sealed, numbered identical boxes according to the randomization schedule. After assignment, the drug was dispensed. |
Rosen 2003.
| Methods | Single‐center trial in U.S. Investigators and participants were blinded. Washout period of 2 months for OCs and 6 months for long‐acting progestins. 9 treatment cycles. | |
| Participants | 34 women aged between 18 to 46 years with moderate facial acne. Excluded women with androgen secreting ovarian tumor, congenital adrenal hyperplasia or Cushing syndrome. | |
| Interventions | Group 1: LNG 150 µg plus EE 30 µg Group 2: DSG 150 µg plus EE 30 µg | |
| Outcomes | Acne scores. Early discontinuation due to side effects. | |
| Notes | Block randomization by pharmacy. No information on allocation concealment. 18 women discontinued early or were lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Thiboutot 2001.
| Methods | Multicenter, placebo‐controlled trial in Australia, Canada and U.S. Investigators and participants were blinded. Washout period of 6 months for injectable hormones, 3 months for oral or implantable hormones and 2 to 6 weeks for systemic or topical acne treatment. 6 treatment cycles. | |
| Participants | 350 healthy women aged 14 or more years with normal menstrual cycle and moderate facial acne vulgaris. Excluded women with contraindications to COCs. | |
| Interventions | Group 1: LNG 100 µg plus EE 20 µg Group 2: Placebo | |
| Outcomes | Acne lesion counts. Clinician global assessment using 4‐point scale ("clear," "almost clear/mild," "moderate" or "severe") adapted from an American Academy of Dermatology consensus statement. Participant self‐assessment. Early discontinuation due to side effects and due to lack of acne improvement. | |
| Notes | Randomization by computer‐generated schedule using blocks of 4. Allocation was concealed in sealed envelopes labeled according to the randomization code. Did not specify whether envelopes were opaque. 124 women were excluded, discontinued early or were lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed envelopes labeled according to the randomization code. Did not specify whether envelopes were opaque. |
Thorneycroft 1999.
| Methods | Multicenter, open trial in U.S. 3‐month washout period for sex hormone use. 3 treatment cycles. | |
| Participants | 58 healthy women aged 18 to 28 years. Excluded women with menstrual irregularity within 3 months of study, clinical evidence of androgen abnormality or current treatment for acne. | |
| Interventions | Group 1: LNG 100 µg plus EE 20 µg Group 2: NA 1 mg plus EE 20 µg | |
| Outcomes | Acne lesions count among subset of women with >15 lesions at baseline. Acne lesions located in the hair line, eye brows and nose were not counted. Discontinuation due to side effects. | |
| Notes | Randomization by computer‐generated code. No information on allocation concealment. 6 women discontinued early or were lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Thorneycroft 2004.
| Methods | Double‐blind randomized trial in 56 centers in Russia (17), Germany (13), Ukraine (12), Czech Republic (9), and Netherlands (5); 6 treatment cycles. No mention of who was blinded. Randomization done via a computer‐generated randomization list. A priori sample size calculations provided. Analysis conducted by intent to treat and per protocol methods. | |
| Participants | 1154 healthy women, age 15 to 40 years. Inclusion criteria: 6 to 100 comedones, 10 to 50 papules or pustules, not more than 5 nodules on face; washout for OCs (3 cycles), systemic retinoids or DMPA (6 months), systemic anti‐acne agents (4 weeks), topical retinoids (4 weeks), and other topical treatments (2 weeks); normal gynecologic exam and cervical smear within 6 months; negative pregnancy test; 3 spontaneous withdrawal bleeds after delivery, abortion, or lactation; no comedogenic cosmetics or sunscreens, sex hormone preparation, or anti‐acne therapy. Exclusion criteria: > 30 years and smoking; pregnant or lactating; acne comedonica or nodulocystic/conglobate acne; acne with multiple large nodes, cysts, fistular comedones, or abscessing fistular ducts; previous acne treatment failure with sex hormones for >= 3 months; or need for other medication with acne‐inducing effects. | |
| Interventions | Group 1: DRSP 3 mg plus EE 30 µg Group 2: NGM 180‐215‐250 µg plus EE 35 µg | |
| Outcomes | Primary: from baseline to cycle 6 ‐ percent change in inflammatory lesion count (papules, pustules, nodules) and percent change in total lesion count (papules, pustules, nodules, and open and closed comedones); proportion of subjects with facial acne improvement by investigators' assessment. Secondary: subject's and investigator's assessment of treatment effect; changes in sebum production and hormone levels; and bleeding record. Discontinuation. | |
| Notes | No information on allocation concealment. Study conducted from May 2000 to September 2001. Exclusions after randomization, 2 from DRSP group and 4 from NGM group. Early discontinuation: 6% DRSP group and 7% NGM group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Van Vloten 2002.
| Methods | Multicenter trial in The Netherlands and Germany. Double blind but no mention of who was blinded. 2‐cycle washout period. Randomization in 2:1 ratio. 9 treatment cycles. | |
| Participants | 128 healthy women, aged 16 to 35 years, with mild‐to‐moderate facial acne (at least 8 papulopustular lesions) and minor seborrhea or hair growth on upper lip, chin and chest. Excluded certain medical conditions, lack of least one normal menstrual cycle following recent birth, abortion or lactation, obesity, use of injectable depot contraceptives in prior 6 months, severe acne (multiple large nodes, cysts, fistular comedos or abscessing fistular ducts), anti‐androgenic hormone treatment in prior 3 months or isotretinoin treatment in prior 12 months. | |
| Interventions | Group 1: drospirenone (DRSP) 2 mg plus EE 30 µg Group 2: CPA 2 mg plus EE 35 µg | |
| Outcomes | Percentage change in acne lesion counts. Participant self‐assessment and dermatologist and gynecologist global assessments of acne. | |
| Notes | Methods of randomization and allocation concealment were not described. 30 women excluded from intent‐to‐treat analysis due to failure to start treatment, lack of at least one observation after dosing or an undescribed reason. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Vartiainen 2001.
| Methods | Multicenter, open trial in Belgium, Finland and the Netherlands. 6‐month washout period for injectable contraceptives, 2 months for other hormonal contraceptives and 2 months for prescription acne medication. 6 treatment cycles. | |
| Participants | 172 women aged 16 to 35 years and weighing 45 to 85 kg with acne. Excluded women with very severe acne needing oral antimicrobial or retinoid acid, use of either trial medications prior to the study, concomitant use of barbiturates, anticonvulsants, griseofulvin, phenylbutazone, rifampicin, penicillin, tetracycline or anti‐acne medication. | |
| Interventions | Group 1: DSG 25‐125 µg plus EE 40‐30 µg Group 2: CPA 2 mg plus EE 35 µg | |
| Outcomes | Acne lesion count. Acne severity score ("no acne" no visible lesions; "mild acne" < 6 comedones or papules and no pustules; "moderate acne" 6‐15 comedones or papules and/or < 4 pustules; "severe acne" > 15 comedones or papules and/or > 3 pustules and/or any nodules). Early discontinuation due to adverse events or due to worsening of acne. | |
| Notes | Methods of randomization and allocation concealment were not described. 36 women were excluded, discontinued early or were lost to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
Winkler 2004.
| Methods | Open‐label, randomized controlled trial conducted in 50 practices in Germany and the Netherlands; 6 treatment cycles. According to correspondence from the author, the block size was 2 for randomization. | |
| Participants | 1027 women. Inclusion criteria 18 to 45 years of age, good physical and mental condition, sexually active, with body mass index from 18 to 29 kg/m2. Exclusion criteria: menstrual cycle < 24 days or > 35 days, being older than 35 years and smoking, taking concomitant medications or addictive drugs, or having a mental or psychiatric disorder or depression that might interfere with the trial, using OCs, IUD. Also excluded were those who had contraceptive implant within past month or injectable contraceptive within past 6 months. | |
| Interventions | Group 1: DSG 150 µg plus 20 µg EE Group 2: LNG 100 µg plus EE 20 µg | |
| Outcomes | Acne lesion count (comedones, papules, pustules, nodules) by investigators; subject judgment (no, mild, moderate, severe); Psychological General Well‐Being Index; Profile of Mood States. Report had limited data for analysis. Author provided some data via correspondence for outcomes that had been presented in figures: numbers and percents with worsening or improvement of comedones, papules, pustules, and nodules (not clear how these were calculated for an individual) and mean scores for Psychological General Well‐Being Index. | |
| Notes | Losses were 22% in DSG Group and 25% for LNG group according to the report. However, change data for the main outcomes indicated losses of 47% and 48%, respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment. |
Worret 2001.
| Methods | Multicenter trial in Germany. Single‐blind (investigator) trial. 6‐month washout period for systemic acne therapy. 12 treatment cycles. | |
| Participants | 199 women aged 18 to 40 years (smokers up to 30 years) with mild to moderate acne on the face. | |
| Interventions | Group 1: Chlormadinone acetate (CMA) 2 mg plus EE 30 µg Group 2: LNG 150 µg plus EE 30 µg | |
| Outcomes | Acne lesion count. Acne severity score using a modified Plewig score based on number of papules or pustules per half of the face ("grade 0" < 3 papules or pustules; "grade 1" 4‐10; "grade 2" 11‐20; "grade 3" 21‐30; "grade 4" > 30). Participant self‐assessment of acne severity. | |
| Notes | Methods of randomization and allocation concealment were not described. 49 women were excluded or discontinued early. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information |
OC = oral contraceptive COC = combined oral contraceptive DMPA = depot medroxyprogesterone acetate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barranco 1974 | Not a randomized controlled trial. |
| Carmina 2002 | Used subset of women from two trials: Lucky 1997 and Redmond 1997. |
| Chapdelaine 1989 | Insufficient acne data reported. |
| Coney 2001 | Report focused on weight as outcome, although data were from a trial that looked at acne originally. |
| Cremoncini 1976 | Not a randomized controlled trial. |
| Erdmann 1994 | Not a randomized controlled trial. |
| Erkkola 1990 | Insufficient acne data reported. |
| Greenwood 1985 | Insufficient acne data reported. |
| Gruber 1998 | Insufficient acne data reported. |
| Grund 1975 | Not a randomized controlled trial. |
| Huber 2000 | Insufficient acne data reported. |
| Katz 2000 | Insufficient acne data reported. |
| Lachnit‐Fixson 1979 | Insufficient acne data reported. |
| Mehrl 1988 | News article reporting on two trials. Insufficient acne data reported. |
| Miller 1986 | Insufficient acne data reported. |
| Nilsson 1967 | Insufficient acne data reported. |
| Perrone 1987 | Not a randomized controlled trial. |
| Sanam 2011 | Sample sizes for analysis not provided for acne data. Unable to obtain further information from researcher. |
| Sanhueza 1979 | Insufficient acne data reported. |
| Spona 1996 | Insufficient acne data reported. |
| Vegetti 1996 | Insufficient acne data reported. |
| Vermeulen 1988 | Acne was not outcome. |
| Vexiau 2002 | Insufficient acne data reported (acne was grouped with hirsutism as an assessment of 'hyperandrogenism'). Treatments were a COC plus topical minoxidil versus a COC containing cyproterone acetate/ethinyl estradiol plus cyproterone acetate supplement. |
| Volpe 1994 | Not a randomized controlled trial. |
| Weber‐Diehl 1993 | Insufficient acne data reported. |
| Wendler 1995 | Randomization not implemented; group assignment affected by acne. |
| Wishart 1991 | Not a randomized controlled trial. |
Characteristics of ongoing studies [ordered by study ID]
Kimball 2011.
| Trial name or title | A study to examine the safety and efficacy of drospirenone and ethinyl estradiol (YAZ) compared with placebo In the treatment of moderate truncal acne vulgaris |
| Methods | Randomized, double‐blind trial |
| Participants | 100 females, 18 to 45 years old. Inclusion criteria: achieved spontaneous menarche, clinical diagnosis of truncal acne vulgaris and desire for oral contraceptive for birth control, minimum of 10 but not more than 50 inflammatory lesions on back and chest combined, maximum of 5 nodules. Exclusion criteria:
|
| Interventions | Drospirenone and ethinyl estradiol (YAZ) daily versus placebo (daily) |
| Outcomes | Primary: percent change in truncal lesion counts (24 weeks) Secondary: percentage of subjects rated clear or almost clear on Investigator's Global Assessment (IGA) of truncal acne and all acne at week 24 and change in truncal lesion counts, subject's assessment of acne at week 24/early termination |
| Starting date | Jul 2008; estimated completion Jul 2012 |
| Contact information | Lynne M Hermosilla, 617‐726‐5066, harvardskinstudies@partners.org |
| Notes |
Contributions of authors
For the initial review, Ayodele Arowojolu performed the literature search, selected the eligible articles and drafted the review. Maria Gallo assisted with the initial literature search and selection of eligible articles and revised the draft. Sarah Garner helped with the initial literature search, reviewed the manuscript and provided clinical expertise. David Grimes developed the topic idea, edited and advised on the manuscript, and provided clinical expertise, and did the secondary data abstractions for the updates. For the updates (2006, 2009, 2012), Laureen Lopez conducted the searches, did the primary data abstraction, incorporated the new trials, and revised the text. All authors reviewed the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute of Child Health and Human Development, USA.
US Agency for International Development, USA.
Declarations of interest
Dr Grimes has consulted with the pharmaceutical companies Bayer Healthcare Pharmaceuticals and Merck & Co, Inc.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aydinlik 1986 {published data only}
- Aydinlik S, Lachnit‐Fixson U, Lehnert J. Reduced estrogen ovulation inhibitor in acne therapy. Double‐blind study comparing Diane‐35 to Diane [Ostrogenreduzierter ovulationshemmer zur aknetherapie.]. Fortschritte der Medizin 1986;104:547‐50. [PubMed] [Google Scholar]
Bayer 2011 {published data only}
- Bayer Schering Pharma AG. GA YAZ ACNE in China Phase III. http://clinicaltrials.gov/ct2/show/NCT00818519 (accessed 25 Aug 2011).
Carlborg 1986 {published and unpublished data}
- Carlborg L. Cyproterone acetate versus levonorgestrel combined with ethinyl estradiol in the treatment of acne. Results of a multicenter study. Acta Obstetricia et Gynecologica Scandinavica 1986;134:29‐32. [DOI] [PubMed] [Google Scholar]
Charoenvisal 1996 {published data only}
- Charoenvisal C, Thaipisuttikul Y, Pinjaroen S, Krisanapan O, Benjawang W, Koster A, et al. Effects on acne of two oral contraceptives containing desogestrel and cyproterone acetate. International Journal of Fertility and Menopausal Studies 1996;41:423‐9. [PubMed] [Google Scholar]
Dieben 1994 {published and unpublished data}
- Dieben TO, Vromans L, Theeuwes A, Bennink HJ. The effects of CTR‐24, a biphasic oral contraceptive combination, compared to Diane‐35 in women with acne. Contraception 1994;50:373‐82. [DOI] [PubMed] [Google Scholar]
Fugere 1988 {published data only}
- Fugere P, Percival‐Smith R, Lussier‐Cacan S, Tetrault C, Farquhar DJ. The comparative efficacy and safety of Diane‐35 versus Diane‐50 in the treatment of moderate to severe acne and seborrhea: 12‐month results. Recent Research on Gynecological Endocrinology 1988;1:590‐608. [Google Scholar]
- Fugere P, Percival‐Smith RK, Lussier‐Cacan S, Davignon J, Farquhar D. Cyproterone acetate/ethinyl estradiol in the treatment of acne. A comparative dose‐response study of the estrogen component. Contraception 1990;42:225‐34. [DOI] [PubMed] [Google Scholar]
Halbe 1998 {published data only}
- Halbe HW, Melo NR, Bahamondes L, Petracco A, Lemgruber M, Andrade RP, et al. Efficacy and acceptability of two monophasic oral contraceptives containing ethinylestradiol and either desogestrel or gestodene. European Journal of Contraception and Reproductive Health Care 1998;3:113‐20. [DOI] [PubMed] [Google Scholar]
J&J 2005 {published data only}
- Johnson, Johnson Taiwan Ltd. Comparison of efficacy and safety of norgestimate‐ethinyl estradiol and cyproterone acetate‐ethinyl estradiol in the treatment of acne vulgaris. http://clinicaltrials.gov/ct2/show/NCT00752635 (accessed 25 Aug 2011).
Kelly 2010 {published data only (unpublished sought but not used)}
- Kelly S, Davies E, Fearns S, McKinnon C, Carter R, Gerlinger C, et al. Effects of oral contraceptives containing ethinylestradiol with either drospirenone or levonorgestrel on various parameters associated with well‐being in healthy women: a randomized, single‐blind, parallel‐group, multicentre study. Clinical Drug Investigation 2010; Vol. 30, issue 5:325‐36. [DOI] [PubMed]
Koetsawang 1995 {published data only}
- Koetsawang S, Charoenvisal C, Banharnsupawat L, Singhakovin S, Kaewsuk O, Punnahitanont S. Multicenter trial of two monophasic oral contraceptives containing 30 mcg ethinylestradiol and either desogestrel or gestodene in Thai women. Contraception 1995;51:225‐9. [DOI] [PubMed] [Google Scholar]
Koltun 2008 {published data only}
- Koltun W, Lucky AW, Thiboutot D, Niknian M, Sampson‐Landers C, Korner P, et al. Efficacy and safety of 3 mg drospirenone/ 20 mcg ethinylestradiol oral contraceptive administered in 24/4 regimen in the treatment of acne vulgaris: a randomized, double‐blind, placebo‐controlled trial. Contraception 2008;77:249‐56. [DOI] [PubMed] [Google Scholar]
- Lucky AW, Koltun W, Thiboutot D, Niknian M, Sampson‐Landers C, Korner P, et al. A combined oral contraceptive containing 3‐mg drospirenone/ 20‐µg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double‐blind, placebo‐controlled study evaluating lesion counts and participant self‐assessment. Cutis 2008;82:143‐50. [PubMed] [Google Scholar]
Lachnit‐Fixson 1977 {published data only}
- Lachnit‐Fixson U, Kaufmann J. [Therapy of androgenization symptoms: double blind study of an antiandrogen preparation (SH B 209 AB) against neogynon]. Medizinische Klinik 1977;72:1922‐6. [PubMed] [Google Scholar]
Leyden 2002 {published data only}
- Leyden J, Shalita A, Hordinsky M, Swinyer L, Stanczyk FZ, Weber ME. Efficacy of a low‐dose oral contraceptive containing 20 ug of ethinyl estradiol and 100 ug of levonorgestrel for the treatment of moderate acne: a randomized, placebo‐controlled trial. Journal of the American Academy of Dermatology 2002;47:399‐409. [DOI] [PubMed] [Google Scholar]
Lucky 1997 {published data only}
- Lucky AW, Henderson TA, Olson WH, Robisch DM, Lebwohl M, Swinyer LJ. Effectiveness of norgestimate and ethinyl estradiol in treating moderate acne vulgaris. Journal of the American Academy of Dermatology 1997;37:746‐54. [DOI] [PubMed] [Google Scholar]
Maloney 2001 {published data only}
- Maloney JM, Arbit DI, Flack M, McLaughlin‐Miley C, Sevilla C, Derman R. Use of low‐dose oral contraceptive containing norethindrone acetate and ethinyl estradiol in the treatment of moderate acne vulgaris. Clinical Journal of Women's Health 2001;1:123‐31. [Google Scholar]
Maloney 2008 {published data only}
- Maloney JM, Dietze P, Watson D, Niknian M, Lee‐Rugh S, Sampson‐Landers C, et al. A randomized controlled trial of a low‐dose combined oral contraceptive containing 3 mg drospirenone plus 20 microg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self‐assessment. Journal of drugs in dermatology 2009, issue 9:837‐44. [PubMed]
- Maloney JM, Dietze P, Watson D, Niknian M, Lee‐Rugh S, Sampson‐Landers C, et al. Treatment of acne using a 3‐milligram drospirenone/ 20‐microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstetrics and Gynecology 2008;112:773‐81. [DOI] [PubMed] [Google Scholar]
Mango 1996 {published data only}
- Mango D, Ricci S, Manna P, Miggiano GA, Serra GB. Clinical and hormonal effects of ethinylestradiol combined with gestodene and desogestrel in young women with acne vulgaris. Contraception 1996;53:163‐70. [DOI] [PubMed] [Google Scholar]
Mansour 2011 {published data only}
- Mansour D, Verhoeven C, Sommer W, Weisberg E, Taneepanichskul S, Melis GB, et al. Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β‐oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen. European Journal of Contraception and Reproductive Health Care. 2011/10/15 2011; Vol. 16, issue 6:430‐43. [DOI] [PMC free article] [PubMed]
Monk 1987 {published data only}
- Monk BE, Almeyda JA, Caldwell IW, Green B, Pelta D, Leonard J, et al. Efficacy of low‐dose cyproterone acetate compared with minocycline in the treatment of acne vulgaris. Clinical and Experimental Dermatology 1987;12:319‐22. [DOI] [PubMed] [Google Scholar]
Palatsi 1984 {published and unpublished data}
- Palatsi R, Hirvensalo E, Liukko P, Malmiharju T, Mattila L, Riihiluoma P, et al. Serum total and unbound testosterone and sex hormone binding globulin (SHBG) in female acne patients treated with two different oral contraceptives. Acta Dermato‐Venereologica 1984;64:517‐23. [PubMed] [Google Scholar]
- Palatsi R, Reinila M, Kivinen S. Pituitary function and DHEA‐S in male acne and DHEA‐S, prolactin and cortisol before and after oral contraceptive treatment in female acne. Acta Dermato‐Venereologica 1986;66:225‐30. [PubMed] [Google Scholar]
Palombo‐Kinne 2009 {published data only}
- Palombo‐Kinne E, Schellschmidt I, Schumacher U, Gräser T. Efficacy of a combined oral contraceptive containing 0.030 mg ethinylestradiol/2 mg dienogest for the treatment of papulopustular acne in comparison with placebo and 0.035 mg ethinylestradiol/2 mg cyproterone acetate. Contraception 2009; Vol. 79, issue 4:282‐9. [DOI] [PubMed]
Plewig 2009 {published data only}
Redmond 1997 {published data only}
- Olson WH, Lippman JS, Robisch DM. The duration of response to norgestimate and ethinyl estradiol in the treatment of acne vulgaris. International Journal of Fertility and Women's Medicine 1998;43:286‐90. [PubMed] [Google Scholar]
- Redmond GP, Olson WH, Lippman JS, Kafrissen ME, Jones TM, Jorizzo JL. Norgestimate and ethinyl estradiol in the treatment of acne vulgaris: a randomized, placebo‐controlled trial. Obstetrics and Gynecology 1997;89:615‐22. [DOI] [PubMed] [Google Scholar]
Rosen 2003 {published data only}
- Rosen MP, Breitkopf DM, Nagamani M. A randomized controlled trial of second‐ versus third‐generation oral contraceptives in the treatment of acne vulgaris. American Journal of Obstetrics and Gynecology 2003;188:1158‐60. [DOI] [PubMed] [Google Scholar]
Thiboutot 2001 {published data only}
- Thiboutot D, Archer DF, Lemay A, Washenik K, Roberts J, Harrison DD. A randomized, controlled trial of a low‐dose contraceptive containing 20 µg of ethinyl estradiol and 100 µg of levonorgestrel for acne treatment. Fertility and Sterility 2001;76:461‐8. [DOI] [PubMed] [Google Scholar]
Thorneycroft 1999 {published data only}
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, Ballagh SA, Nichols M, Weber ME. Effect of low‐dose oral contraceptives on androgenic markers and acne. Contraception 1999;60:255‐62. [DOI] [PubMed] [Google Scholar]
Thorneycroft 2004 {published data only}
- Thorneycroft H, Gollnick H, Schellschmidt I. Superiority of a combined contraceptive containing drospirenone to a triphasic preparation containing norgestimate in acne treatment. Cutis 2004;74:123‐30. [PubMed] [Google Scholar]
Van Vloten 2002 {published data only}
- Vloten WA, Haselen CW, Zuuren EJ, Gerlinger C, Heithecker R. The effect of 2 combined oral contraceptives containing either drospirenone or cyproterone acetate on acne and seborrhea. Cutis 2002;69 Suppl (4):2‐15. [PubMed] [Google Scholar]
Vartiainen 2001 {published data only}
- Vartiainen M, Gezelle H, Broekmeulen CJ. Comparison of the effect on acne with a combiphasic desogestrel‐containing oral contraceptive and a preparation containing cyproterone acetate. European Journal of Contraception and Reproductive Health Care 2001;6:46‐53. [PubMed] [Google Scholar]
Winkler 2004 {published and unpublished data}
- Winkler UH, Ferguson H, Mulders JAPA. Cycle control, quality of life and acne with two low‐dose oral contraceptives containing 20 µg ethinylestradiol. Contraception 2004;69:469‐76. [DOI] [PubMed] [Google Scholar]
Worret 2001 {published data only}
- Worret I, Arp W, Zahradnik HP, Andreas JO, Binder N. Acne resolution rates: results of a single‐blind, randomized, controlled, parallel phase III trial with EE/CMA (Belara) and EE/LNG (Microgynon). Dermatology 2001;203:38‐44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barranco 1974 {published data only}
- Barranco VP. Effect of androgen‐dominant and estrogen‐dominant oral contraceptives on acne. Cutis 1974;4:384‐6. [Google Scholar]
Carmina 2002 {published data only}
- Carmina E, Godwin AJ, Stanczyk FZ, Lippman JS, Lobo RA. The association of serum androsterone glucuronide with inflammatory lesions in women with adult acne. Journal of Endocrinological Investigation 2002;25:765‐8. [DOI] [PubMed] [Google Scholar]
Chapdelaine 1989 {published data only}
- Chapdelaine A, Desmarais JL, Derman RJ. Clinical evidence of minimal androgenic activity of norgestimate. International Journal of Fertility 1989;34:347‐52. [PubMed] [Google Scholar]
Coney 2001 {published data only}
- Coney P, Washenik K, Langley RGB, DiGiovanna JJ, Harrison DD. Weight change and adverse event incidence with a low dose contraceptive: two randomized, placebo‐controlled trials. Contraception 2001;63:297‐302. [DOI] [PubMed] [Google Scholar]
Cremoncini 1976 {published data only}
- Cremoncini C, Vignati E, Libroia A. Treatment of hirsutism and acne in women with two combinations of cyproterone acetate and ethinylestradiol [Trattamento dell'irsutismo e dell'acne nella donna con due associazioni di ciproterone acetato ed etinil‐estradiolo]. Acta Europaea Fertilitatis 1976;7:299‐314. [PubMed] [Google Scholar]
Erdmann 1994 {published data only}
- Erdmann D, Schindler EM, Schindler AE. Ovarian suppression with Diane 35/50 [Die ovarielle Suppression unter Diane 35/50]. Geburtshilfe und Frauenheilkunde 1994;54:627‐33. [DOI] [PubMed] [Google Scholar]
Erkkola 1990 {published data only}
- Erkkola R, Hirvonen E, Luikku J, Lumme R, Mannikko H, Aydinlik S. Ovulation inhibitors containing cyproterone acetate or desogestrel in the treatment of hyperandrogenic symptoms. Acta Obstetricia et Gynecologica Scandinavica 1990;69:61‐5. [DOI] [PubMed] [Google Scholar]
Greenwood 1985 {published data only}
- Greenwood R, Brummitt L, Burke B, Cunliffe WJ. Acne: double blind clinical and laboratory trial of tetracycline, oestrogen‐cyproterone acetate, and combined treatment. British Medical Journal 1985;291:1231‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gruber 1998 {published data only}
- Gruber DM, Sator MO, Joura EA, Kokoschka EM, Heinze G, Huber JC. Topical cyproterone acetate treatment in women with acne: a placebo‐controlled trial. Archives of Dermatology 1998;134:459‐63. [DOI] [PubMed] [Google Scholar]
Grund 1975 {published data only}
- Grund E, Schmidt‐Elmendorff H. The treatment of virilizing syndromes. Comparative clinical studies of 2 antiandrogen‐active gestagens (cyproterone acetate, megestrol acetate) [Behandlung von Virilisierungs‐erscheinungen. Vergleichende klinische Untersuchung zweier antiandrogenwirksamer Gestagene (Cyproteronazetat, Megestrolazetat)]. Die Medizinische Welt 1975;26:2180‐7. [PubMed] [Google Scholar]
Huber 2000 {published data only}
- Huber J, Foidart JM, Wuttke W, Merki‐Feld GS, The HS, Gerlinger C, et al. Efficacy and tolerability of a monophasic oral contraceptive containing ethinylestradiol and drospirenone. European Journal of Contraception and Reproductive Health Care 2000;5:25‐34. [DOI] [PubMed] [Google Scholar]
Katz 2000 {published data only}
- Katz HI, Kempers S, Akin MD, Dunlap F, Whiting D, Norbart TC. Effect of a desogestrel‐containing oral contraceptive on the skin. European Journal of Contraception and Reproductive Health Care 2000;5:248‐55. [DOI] [PubMed] [Google Scholar]
Lachnit‐Fixson 1979 {published data only}
- Lachnit‐Fixson U. The development and evaluation of an ovulation inhibitor (DIAne) containing an antiandrogen. Acta Obstetricia et Gynecologica Scandinavica. Supplement 1979;88:33‐42. [DOI] [PubMed] [Google Scholar]
Mehrl 1988 {published data only}
- Mehrl J. Acne: long‐term therapy with side effect. Sebaceous gland activity is androgen dependent‐‐acne therapeutic drug is effective as a contraceptive [Akne: Langzeittherapie mit Nebeneffekt. Talgdrüsenaktivität androgenabhängig ‐ Aknetherapeutikum auch kontrazeptiv wirksam]. Fortschritte der Medizin 1988;106:50‐1. [PubMed] [Google Scholar]
Miller 1986 {published data only}
- Miller JA, Wojnarowska FT, Dowd PM, Ashton RE, O'Brien TJ, Griffiths WA, et al. Anti‐androgen treatment in women with acne: a controlled trial. British Journal of Dermatology 1986;114:705‐16. [DOI] [PubMed] [Google Scholar]
Nilsson 1967 {published data only}
- Nilsson L, Solvell L. Clinical studies on oral contraceptives‐‐a randomized, doubleblind, crossover study of 4 different preparations (Anovlar(R) mite, Lyndiol(R) mite, Ovulen(R), and Volidan). Acta Obstetricia et Gynecologica Scandinavica 1967;46: Suppl (8):1‐31. [DOI] [PubMed] [Google Scholar]
Perrone 1987 {published data only}
- Perrone G, Calzolari E, Mancone M, Masci A, Steffe M, Tesseri E. Oral contraceptives and their minor side effects: comparison between three low‐dose estroprogestinic associations [Contraccettivi orali ed effeti collatereali minori: Confronto fra tre estropogestinici a basso dosaggio]. Patologia e Clinica Ostetrica e Ginecologica 1987;15:6‐11. [PubMed] [Google Scholar]
Sanam 2011 {published data only}
- Sanam M, Ziba O. Desogestrel+ethinylestradiol versus levonorgestrel+ethinylestradiol. Which one has better affect on acne, hirsutism, and weight change. Saudi Medical Journal. 2011/01/08 2011; Vol. 32, issue 1:23‐6. [PubMed]
Sanhueza 1979 {published data only}
- Sanhueza H, Sivin I, Kumar S, Kessler M, Carrasco A, Yee J. A randomized double blind study of two oral contraceptives. Contraception 1979;20:29‐48. [DOI] [PubMed] [Google Scholar]
Spona 1996 {published data only}
- Spona J, Elstein M, Feichtinger W, Sullivan H, Ludicke F, Muller U, et al. Shorter pill‐free interval in combined oral contraceptives decreases follicular development. Contraception 1996;54:71‐7. [DOI] [PubMed] [Google Scholar]
Vegetti 1996 {published data only}
- Vegetti W, Testa G, Maggioni P, Motta T, Falsetti L, Crosignani PG. An open randomized comparative study of an oral contraceptive containing ethinyl estradiol and cyproterone acetate with and without the GnRH analogue goserelin in the long‐term treatment of hirsutism. Gynecologic and Obstetric Investigation 1966;41:260‐8. [DOI] [PubMed] [Google Scholar]
Vermeulen 1988 {published data only}
- Vermeulen A, Rubens R. Effects of cyproterone acetate plus ethinylestradiol low dose on plasma androgens and lipids in mildly hirsute or acneic young women. Contraception 1988;38:419‐28. [DOI] [PubMed] [Google Scholar]
Vexiau 2002 {published data only}
- Vexiau P, Chaspoux C, Boubou P, Fiet J, Jouanique C, Hardy N, et al. Effect of minoxidil 2% vs. cyproterone acetate treatment on female androgenetic alopecia: a controlled, 12‐month randomized trial. British Journal of Dermatology 2002;146:992‐9. [DOI] [PubMed] [Google Scholar]
Volpe 1994 {published data only}
- Volpe A, Silferi M, Mauri A, Deiana P, Angioni S, Grasso A, et al. Efficacy on hyperandrogenism and safety of a new oral contraceptive biphasic formulation containing desogestrel. European Journal of Obstetrics and Gynecology and Reproductive Biology 1994;53:205‐9. [DOI] [PubMed] [Google Scholar]
Weber‐Diehl 1993 {published data only}
- Weber‐Diehl F, Lehnert J, Lachnit U. Comparison of two triphasic oral contraceptives containing either gestodene or norethindrone: a randomized, controlled trial. Contraception 1993;48:291‐301. [DOI] [PubMed] [Google Scholar]
Wendler 1995 {published data only}
- Wendler J, Siegert C, Schelhorn P, Klinger G, Gurr S, Kaufmann J, et al. The influence of Microgynon and Diane‐35, two sub‐fifty ovulation inhibitors, on voice function in women. Contraception 1995;52:343‐8. [DOI] [PubMed] [Google Scholar]
Wishart 1991 {published data only}
- Wishart JM. An open study of Triphasil and Diane 50 in the treatment of acne. Australasian Journal of Dermatology 1991;32:51‐4. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Kimball 2011 {published data only}
- Kimball AB, Alora‐Palli MB. A study to examine the safety and efficacy of drospirenone and ethinyl estradiol (YAZ) compared with placebo In the treatment of moderate truncal acne vulgaris. http://clinicaltrials.gov/ct2/show/NCT00722761 (accessed 25 Aug 2011). [PubMed]
Additional references
Baldwin 2002
- Baldwin HE. The interaction between acne vulgaris and the psyche. Cutis 2002;70:133‐9. [PubMed] [Google Scholar]
Bergfeld 1995
- Bergfeld WF. The evaluation and management of acne: economic considerations. Journal of American Academy of Dermatology. 1995;32 Suppl (5 Pt 3):52‐6. [DOI] [PubMed] [Google Scholar]
Burkhart 2000
- Burkhart CG, Butcher C, Burkhart CN, Lehmann P. Effects of benzoyl peroxide on lipogenesis in sebaceous glands using animal model. Journal of Cutaneous Medicine and Surgery 2000;4:138‐41. [DOI] [PubMed] [Google Scholar]
Cassidenti 1991
- Cassidenti DL, Paulson RJ, Serafini P, Stanczyk FZ, Lobo RA. Effects of sex steroids on skin 5 alpha‐reductase activity in vitro. Obstetrics and Gynecology 1991;78:103‐7. [PubMed] [Google Scholar]
CONSORT 2009
- CONSORT group. CONSORT: Transparent reporting of trials. http://www.consort‐statement.org/ (accessed 15 Jul 2009).
Downie 2002
- Downie MM, Sanders DA, Kealey T. Modelling the remission of individual acne lesion in vitro. British Journal of Dermatology 2002;147:869‐78. [DOI] [PubMed] [Google Scholar]
Eady 1994
- Eady EA, Farmery MR, Ross JI, Cove JH, Cunliffe WJ. Effects of benzoyl peroxide and erythromycin alone and in combination against antibiotic‐sensitive and ‐resistant skin bacteria from acne patients. British Journal of Dermatology 1994;131:331‐6. [DOI] [PubMed] [Google Scholar]
Fotherby 1994
- Fotherby K, Caldwell AD. New progestogens in oral contraception. Contraception 1994;49:1‐32. [DOI] [PubMed] [Google Scholar]
George 2008
- George R, Clarke S, Thiboutot D. Hormonal therapy for acne. Seminars in Cutaneous Medicine and Surgery 2008;27:188‐96. [DOI] [PubMed] [Google Scholar]
Goulden 1999
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. Journal of the American Academy of Dermatology 1999;41:577‐80. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1[updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, (accessed 04 Oct 2011).
Jemec 2002
- Jemec GB, Linneberg A, Nielsen NH, Frolund L, Madsen F, Jorgensen T. Have oral contraceptives reduced the prevalence of acne? A population‐based study of acne vulgaris, tobacco smoking and oral contraceptives. Dermatology 2002;204:179‐84. [DOI] [PubMed] [Google Scholar]
Koltun 2011
- Koltun W, Maloney JM, Marr J, Kunz M. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 mug plus drospirenone 3mg administered in a 24/4 regimen: a pooled analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology 2011;155(2):171‐5. [DOI] [PubMed] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. British Medical Journal 2003;326:1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leyden 1997
- Leyden JJ. Therapy for acne vulgaris. New England Journal of Medicine 1997;336:1156‐62. [DOI] [PubMed] [Google Scholar]
O'Connell 2008
- O'Connell K, Westhoff C. Pharmacology of hormonal contraceptives and acne. Cutis 2008;81(1 Suppl):8‐12. [PubMed] [Google Scholar]
Poli 2001
- Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. Journal of the European Academy of Dermatology and Venereology 2001;15:541‐5. [DOI] [PubMed] [Google Scholar]
Rabe 2000
- Rabe T, Kowald A, Ortmann J, Rehberger‐Schneider S. Inhibition of skin 5 alpha‐reductase by oral contraceptive progestins in vitro. Gynecological Endocrinology 2000;14:223‐30. [DOI] [PubMed] [Google Scholar]
Rich 2008
- Rich p. Hormonal contraceptives for acne management. Cutis 2008;81:13‐8. [PubMed] [Google Scholar]
Schulz 2002a
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002;359:614‐8. [DOI] [PubMed] [Google Scholar]
Schulz 2002b
- Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet 2002;359:696‐700. [DOI] [PubMed] [Google Scholar]
Seaman 2003
- Seaman HE, Vries CS, Farmer RD. Differences in the use of combined contraceptives amongst women with and without acne. Human Reproduction 2003;18:515‐21. [DOI] [PubMed] [Google Scholar]
Thiboutot 2000
- Thiboutot D. New treatments and therapeutic strategies for acne. Archives of Family Medicine 2000;9:179‐87. [DOI] [PubMed] [Google Scholar]
Toyoda 2001
- Toyoda M, Morohashi M. Pathogenesis of acne. Medical Electron Microscopy 2001;34:29‐40. [DOI] [PubMed] [Google Scholar]
Webster 2002
- Webster GF. Acne vulgaris. British Medical Journal 2002;325:475‐9. [PMC free article] [PubMed] [Google Scholar]


