Abstract
DNA vaccines have been used widely in experimental primate models of human immunodeficiency virus (HIV), but their effectiveness has been limited. In this study, we evaluated three technologies for increasing the potency of DNA vaccines in rhesus macaques. These included DNA encoding Sindbis virus RNA replicons (pSINCP), cationic poly(lactide-co-glycolide) (PLG) microparticles for DNA delivery, and recombinant protein boosting. The DNA-based pSINCP replicon vaccines encoding HIV Gag and Env were approximately equal in potency to human cytomegalovirus (CMV) promoter-driven conventional DNA vaccines (pCMV). The PLG microparticle DNA delivery system was particularly effective at enhancing antibody responses induced by both pCMV and pSINCP vaccines and had less effect on T cells. Recombinant Gag and Env protein boosting elicited rapid and strong recall responses, in some cases to levels exceeding those seen after DNA or DNA/PLG priming. Of note, Env protein boosting induced serum-neutralizing antibodies and increased frequencies of gamma interferon-producing CD4 T cells severalfold. Thus, PLG microparticles are an effective means of delivering DNA vaccines in nonhuman primates, as demonstrated for two different types of DNA vaccines encoding two different antigens, and are compatible for use with DNA prime-protein boost regimens.
Both neutralizing antibodies and cell-mediated immunity (CMI) likely will be required to protect against viruses that can establish chronic infections, such as human immunodeficiency virus (HIV) and hepatitis C virus (HCV). Moreover, in animal models for HIV, both neutralizing antibodies against Env (9, 35-37) and cytotoxic T lymphocytes (CTL) that target multiple viral antigens (3-5, 17, 18, 21, 26, 30, 32, 38, 39, 45, 48, 51-53, 59) can contribute to protection through prevention of infection and clearance of virus-infected cells, respectively. Vaccines consisting of inactivated pathogens or recombinant proteins generally are not effective at inducing CTL and typically are used to induce protective antibodies. In contrast, viruses and intracellular bacteria can induce CTL responses, in part due to neoexpression of the antigens during infection.
Plasmid DNA vaccines were born out of the need for inducing both antibody and CMI responses, including CTL, without the problems associated with live organism-based vaccines, such as potential reversion to virulence and antivector immunity that can limit boosting. Indeed, DNA vaccines that express antigens from strong viral promoters have been used to elicit protective antibodies and CMI in many animal models (14, 23). However, naked DNA vaccines, i.e., plasmid DNA in saline, have proven to be only modestly potent in humans, thereby limiting their utility. Many approaches have been explored to improve DNA vaccine potency, including better expression vectors, DNA formulation and delivery systems, adjuvants, and the use of booster vaccines.
We developed an alternative DNA vector that launches a self-amplifying Sindbis virus (alphavirus) RNA replicon (15, 24). With this vector, once the RNA replicon is transcribed in and exported from the nucleus, the RNA is replicated in the cytoplasm by replicon-encoded enzymes and expresses the gene of interest using a Sindbis subgenomic promoter. Plasmid-based replicons utilizing a Sindbis-derived RNA replicase have been shown to be immunogenic in several murine models at 10- to 1,000-fold lower doses than conventional plasmid vectors (24, 33, 46). Similar DNA vaccines based on Semliki Forest virus (SFV) have also been demonstrated to be effective (1). So far, no data have been reported for alphavirus-based DNA vaccines in primates.
We have addressed the inefficient delivery of DNA vaccines, both within tissues and into cells (16), which has limited their effectiveness. The vast majority of injected DNA is degraded by nucleases in the extracellular spaces and within phagocytic cells. One approach to improved delivery is to facilitate uptake of DNA by antigen-presenting cells (APCs) using microparticles, such as chitosan (34, 49), polyethylenimine (31), and poly(lactide-co-glycolide) (PLG) (25, 27, 28, 41, 54). We have reported that DNA adsorbed to cationic PLG microparticles is a very effective delivery system that markedly enhances immune responses in small animals (6, 42, 54). PLG microparticle adsorption captures DNA with high efficiency but still allows for rapid release of the DNA from particles. The mode of action of the PLG delivery system may involve uptake and expression of DNA by APCs as well as prolonged expression of antigen by protecting plasmid DNA from nuclease digestion (12).
The studies presented here investigated the utility of two DNA vaccine technologies, namely the Sindbis-derived pSINCP DNA vector (versus conventional cytomegalovirus [CMV] promoter-driven) and the PLG microparticle DNA delivery system, in rhesus macaques. Furthermore, we evaluated these technologies both in the context of priming immune responses and following boosting with recombinant proteins to address compatibility and potential synergies.
MATERIALS AND METHODS
Plasmids.
The plasmid pCMVKm2.GagMod.SF2, constructed from the expression vector pCMVKm2 (8) and containing a codon-optimized HIVSF2 gag sequence, has been described previously (61). A codon-optimized env sequence encoding the ectodomain (gp140) of env from the SF162 HIV isolate (2) was cloned into the expression vector pCMVLink (61) to produce the plasmid pCMVLink0.140mod.SF162.
The Sindbis virus-derived plasmid replicon pSINCP was constructed by modifying a previously described version of a Sindbis plasmid replicon, pSIN1.5 (24). The entire nonstructural protein (nsP) gene coding sequences from the human dendritic cell tropic strain of Sindbis virus (SINCR) (22) were substituted for the existing nsP sequences in pSIN1.5. The vector was modified further by substitution of the CMV promoter-containing plasmid backbone with that from pCMVLink (61). To prepare the pSINCP plasmid DNA vaccines, the gag and env inserts were excised from the pCMVKm2 and pCMVLink constructs and were inserted into pSINCP immediately downstream of the Sindbis subgenomic junction region promoter. Plasmid DNA was prepared using endotoxin-free kits (QIAGEN, Valencia, CA).
Production and purification of o-gp140SF162ΔV2 protein.
The sequence encoding a V2-deleted ectodomain of the HIV SF162 env gene protein from HIV-1SF162 (gp140SF162ΔV2) was cloned as a 2.1-kb EcoRI-XbaI DNA fragment containing two mutations in the primary and three mutations in secondary cleavage sites (56). The resulting env sequence was cloned into the EcoRI and XbaI sites of the pCMV3 expression vector, which was transfected into CHO cells, and stable env-expressing cell lines were selected. The highest expressing gp140SF162ΔV2-CHO cell clone was adapted to low serum medium (0.5% fetal bovine serum) and adjusted to a cell density and perfusion rate that facilitated the maximum expression of gp140SF162ΔV2 protein in its oligomeric conformation (o-gp140SF162ΔV2) (55, 56). The bioreactor-adapted gp140ΔV2-CHO cell clone was used to seed a 12.5-liter bioreactor for production runs. At the end of the run, medium was concentrated 20-fold through a 100-kDa membrane and stored at −80°C in the presence of 1 mM EDTA and 1 mM EGTA. The purification of o-gp140SF162ΔV2 was performed as described elsewhere (55, 56).
Preparation of PLG microparticles for DNA adsorption.
Cationic PLG-cetyltrimethylammonium bromide (CTAB) microparticles were prepared using a modified solvent evaporation process as described previously (6, 54). The microparticles were prepared using an homogenizer (IKA, Wilmington, N.C.) at high speed to emulsify 10 ml of a 5% (wt/vol) PLG polymer solution in methylene chloride with 1 ml of phosphate-buffered saline (PBS). The primary emulsion was then added to 50 ml of distilled water containing CTAB (0.5% [wt/vol]). This resulted in the formation of a water-in-oil-in-water emulsion that was stirred at 6,000 rpm for 12 h at room temperature, allowing the methylene chloride to evaporate. The resulting microparticles were washed four times in distilled water by centrifugation at 10,000 × g and lyophilized. Plasmid DNA was adsorbed to PLG-CTAB microparticles by incubating 1 mg of DNA in 1 ml of 1× Tris-EDTA (TE) buffer with 100 mg of microparticles overnight at 4°C with gentle rocking. The microparticles were then pelleted by centrifugation at 11,424 × g for 10 min, washed with 1× TE buffer, recentrifuged, and suspended in 5 ml of deionized water and lyophilized. The size distribution of the microparticles was determined using a particle size analyzer (Mastersizer; Malvern Instruments).
Preparation of HIV p55 gag protein adsorbed onto anionic PLG microparticles.
As described previously (29), microparticles were prepared by homogenizing 10 ml of 6% (wt/vol) PLG polymer solution in methylene chloride with 40 ml of distilled water containing SDS (1% [wt/vol]) at high speed using a 10-mm probe. This resulted in an oil-in-water emulsion, which was stirred at 1,000 rpm for 12 h at room temperature, and the methylene chloride was allowed to evaporate. The resulting microparticles were filtered through 38-μm mesh, washed three times in distilled water, and lyophilized. The size distribution of the microparticles was determined using a Mastersizer (Malvern Instruments) particle size analyzer.
Fifty micrograms of lyophilized PLG-sodium dodecyl sulfate (SDS) particles were incubated with 0.5 mg of p55Gag protein in 10 ml of 25 mM Borate buffer, pH 9, with 6 M urea. Particles were left on a lab rocker at room temperature for 5 h. The microparticles were separated from the incubation medium by centrifugation, washed once with borate buffer with 6 M urea, washed three times with distilled water, and lyophilized.
The amount of protein adsorbed to microparticles was determined by dissolving 10 mg of the microparticles in 2 ml of 5% SDS-0.2 M NaOH solution at room temperature. Protein concentration was measured by bicinchoninic acid protein assay (Pierce, Rockford, IL). The Zeta potential for both blank and adsorbed microparticles was measured using a Zeta analyzer (Malvern Instruments).
Vaccination.
Rhesus macaques were housed at Southern Research Institute (Frederick, MD) in accordance with Institutional Animal Care and Use Committee guidelines. Animals were immunized by intramuscular injection on weeks 0, 4, and 14 with DNA vaccines encoding HIVSF2 p55Gag (0.5 mg) and HIVSF162 gp140Env (1.0 mg), with or without adsorption to PLG microparticles. Rhesus were boosted with yeast-derived p55Gag protein (0.2 mg) adsorbed to anionic PLG microparticles (Gag/PLG) (44) at week 29. Finally, the animals were boosted with 0.1 mg CHO cell-derived o-gp140SF162ΔV2 (oligomeric, V2 loop-deleted gp140Env protein) administered with the oil-in-water MF59 adjuvant (Env/MF59) (47) at weeks 38 and 75.
Antibody assays.
Antibodies against Env and Gag proteins were measured by an enzyme-linked immunosorbent assay (ELISA). MaxiSorp plates (Nalge Nunc, Rochester, NY) were coated overnight at 4°C with 50 μl of 5 μg/ml of Env protein or Gag protein in PBS, pH 7.0. The coated wells were blocked for 1 h at 37°C with 150 μl of 5% goat serum (Gibco BRL, Grand Island, NY) in phosphate-buffered saline (PBS). Serum samples were diluted initially 1:25 or 1:100 in the blocking buffer followed by threefold serial dilutions. The bound antibodies were detected with horseradish peroxidase-conjugated goat anti-monkey immunoglobulin G (IgG) (Southern Biotechnology Associates, Birmingham, AL) diluted 1:5,000 with the blocking buffer and incubated for 1 h at 37°C. For development, 3,3′,5,5′-tetramethylbenzidine was incubated for 15 min, and the reaction was stopped by adding 2 N HCl. The assay plates were then read on an ELISA plate reader at an absorbance wavelength of 450 nm. A standard was included on each plate, and a reference value of the standard was used for the normalization of the sample ELISA titers. The titers represent the dilution factors producing an optical density of 0.5.
Virus-neutralizing antibodies were assessed against human peripheral blood mononuclear cells (PBMC)-grown, homologous HIV-1SF162 virus, as previously described (7). Titers were calculated as the greatest serum dilution that reduced virus growth in humans by 80%, as measured by p24Gag antigen production. Preimmune sera were used as negative controls.
Purification of Rhesus peripheral blood mononuclear cells (PBMC) and derivation of B-lymphoblastoid cell lines (B-LCL).
Rhesus PBMC were separated from heparinized whole blood on Ficoll-Hypaque gradients. To derive rhesus B-lymphoblastoid cell lines, PBMC were exposed to herpesvirus papio-containing culture supernatant from the 594S cell line in the presence of 0.5 μg/ml Cyclosporine A (Sigma). Rhesus PBMC were cultured at 2 × 106 to 3 × 106 per well in 1.5 ml in 24-well plates for 8 days in AIM-V:RPMI 1640 (50:50) culture medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (AR10). Antigen-specific cells were stimulated by the addition of a pool of either Gag or Env peptides (20-mers, overlapping by 10; 10.7 μg/ml total peptide). Recombinant human IL-7 (15 ng/ml; R&D Systems, Minneapolis, MN) was added at the initiation of culture. Recombinant human IL-2 (Proleukin; 20 IU/ml; Chiron) was added on days 1, 3, and 6.
51Cr-release assay for CTL activity.
Autologous B-LCL were infected with recombinant vaccinia viruses (rVV) expressing gag (rVVgag-polSF2) or env (rVVgp160envSF162) and then labeled overnight with Na2[51Cr]O4 (NEN, Boston, MA; 10 μCi per 2.5 × 105 B-LCL) and washed. rVV-infected, 51Cr-labeled B-LCL were added (2,500 per round-bottom well) to duplicate wells containing threefold serial dilutions of cultured PBMC. Unlabeled B-LCL (1 × 105 per well) were added to inhibit nonspecific cytolysis. After 4 h, 50 μl of supernatant from each culture was harvested, added to Lumaplates (Packard, Meriden, CT), and counted with a Wallac Microbeta TriLux liquid scintillation counter (Perkin Elmer Life Sciences, Boston, MA). 51Cr released from lysed targets was normalized by the formula percent specific 51Cr release = 100 × (mean experimental release − spontaneous release)/(maximum release − spontaneous release), where spontaneous release is mean counts per minute (cpm) released from target cells in the absence of PBMC and maximum release is mean cpm released from target cells in the presence of 0.1% Triton X-100. For each dilution of PBMC culture, a response toward a given antigen (Gag or Env) was scored as positive if, for PBMC stimulated in culture with that particular antigen, lysis of antigen-bearing targets exceeded lysis of irrelevant targets by at least 10% and if, for targets bearing that antigen, lysis by PBMC stimulated with that antigen exceeded lysis by PBMC stimulated with the irrelevant antigen by at least 10%. Cultures were scored as positive if the above criteria were satisfied for at least two consecutive dilutions of culture.
Lymphoproliferation assay (LPA).
PBMC (2 × 105) were incubated in flat-bottom microtiter wells in a volume of 0.2 ml AR10 in the absence or presence of p55 Gag protein (3 μg/ml) or a pool of Env peptides (16 μg/ml). Six replicate cultures were established. After 4 days of incubation, [3H]thymidine ([3H]TdR; Amersham, Piscataway, NJ) was added (1 μCi/well). Following overnight incubation, cultures were harvested onto glass microfiber filters. Cellular uptake of [3H]TdR was measured with a Microbeta TriLux liquid scintillation counter (Perkin Elmer). Stimulation indices (SI) were calculated using the formula SI = (mean cpm in the presence of antigen)/(mean cpm in the absence of antigen). SI values of ≥5.0 were considered positive.
Intracellular cytokine staining (ICS) and flow cytometry.
Rhesus PBMC were incubated overnight at 37°C in the absence or presence of antigen (Gag peptide pool, 30 μg/ml, or Env peptide pool, 30 μg/ml). Anti-CD28 (1 μg/ml; Pharmingen, San Diego, CA) was added as a source of costimulation, and Brefeldin A (1:1,000; Pharmingen) was added to prevent cytokine secretion. Duplicate cultures were prepared for each stimulation condition. After overnight incubation, PBMC were stained for cell surface CD4 (anti-CD4 allophycocyanin conjugate, clone SK3; Becton Dickinson, San Jose, CA) and CD8 (anti-CD8α peridinin chlorophyll protein conjugate, clone SK1; Becton Dickinson), permeabilized with Cytofix/Cytoperm (Pharmingen), and then stained for intracellular gamma interferon (IFN-γ) (monoclonal antibody 4S.B3, phycoerythrin conjugate; Pharmingen) and tumor necrosis factor alpha (TNF-α) (MAb11, fluorescein isothiocyanate [FITC] conjugate; Pharmingen). Stained cells were analyzed with a FACSCalibur flow cytometer (Becton Dickinson). For each culture condition, the mean frequency of cytokine-positive cells was calculated for the duplicate cultures. To estimate the antigen-specific frequency, the unstimulated mean frequency was subtracted from the stimulated mean frequency.
RESULTS
Antibody responses to pCMV and pSINCP DNA vaccines adsorbed to cationic PLG microparticles.
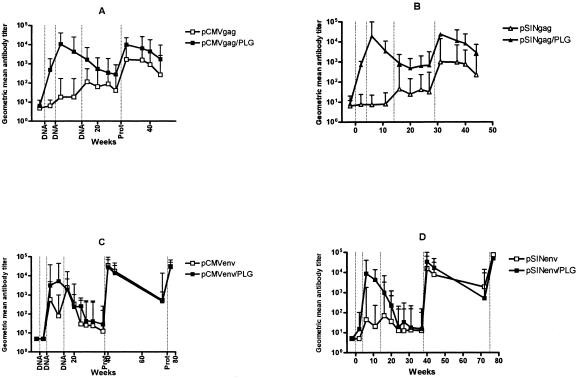
When administered in saline, both pCMV and pSINCP vectors induced low to intermediate titers of gag- and env-specific antibodies (Fig. 1). At no time was there a significant difference in the gag antibody titers induced by the two vectors; however, pCMVenv-vaccinated animals had higher titers of env-specific antibodies (peak geometric mean titer, 2,460) than those vaccinated with pSINCPenv (peak geometric mean titer, 70). When pCMV and pSINCP vectors were adsorbed to PLG microparticles, high titers of gag- and env-specific antibodies (peak geometric mean titers, 5,000 to 20,000) were induced with just two doses. The enhancing effects of PLG formulation were statistically significant (P < 0.05, analysis of variance [ANOVA] on log-transformed titers), with the exception of pCMVenv (Fig. 1C), where env titers 2 weeks after vaccination with pCMVenv/PLG were nonetheless fivefold greater than pCMVenv-induced titers. Env-specific antibody titers induced by PLG-adsorbed pSINCP and pCMV were indistinguishable. Thus, PLG-adsorption of pCMV and pSINCP vectors expressing either env or gag led to substantial increases in antibody titers. Despite high env-specific antibody titers in rhesus vaccinated with PLG-adsorbed pCMVenv or pSINCPenv, no HIV neutralizing activity was detected (Fig. 2).
FIG. 1.
Gag and Env-specific antibodies induced by DNA or DNA/PLG priming and protein boosting. Groups of 5 rhesus macaques were vaccinated at weeks 0, 4, and 14 with pCMVgag and pCMVenv DNA/saline or DNA/PLG (A and C) or pSINCPgag and pSINCPenv DNA or DNA/PLG (B and D). Rhesus were boosted at week 29 with recombinant Gag protein adsorbed onto anionic PLG microparticles and at weeks 38 and 75 with oligomeric gp140env protein formulated with MF59 adjuvant. Anti-Gag or -Env antibodies are plotted as group geometric mean ELISA titers for DNA/saline (open symbols) and DNA/PLG (closed symbols), and error bars extend to 95% confidence upper limits.
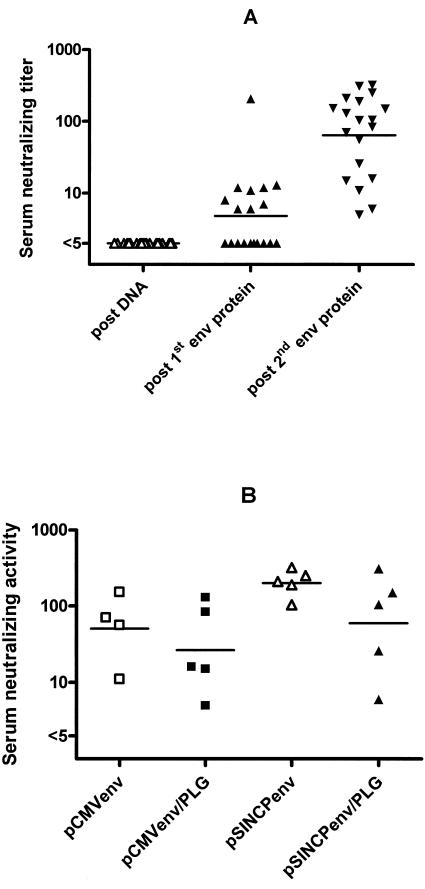
FIG. 2.
Serum-neutralizing antibody titers in vaccinated rhesus. HIV-1SF162 neutralizing titers of sera obtained 2 weeks after the third DNA vaccination and after each Env protein boost are shown for all vaccines (A). Neutralizing titers of sera obtained 2 weeks after the second Env protein boost are grouped by env DNA and formulation used in the vaccine priming phase (B). Horizontal bars indicate group geometric mean titers.
CD4 T-cell responses to DNA vaccines adsorbed to cationic PLG microparticles.
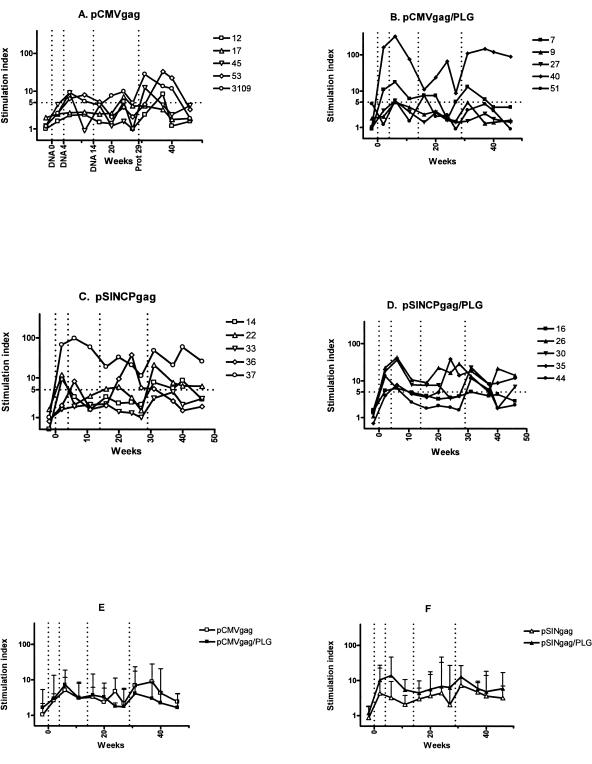
CD4 T-cell responses to DNA vaccination were measured both by LPA and by cytokine flow cytometry. Gag-specific proliferation was detected 2 weeks after the first DNA vaccination and reached peak levels 2 weeks after the second DNA vaccination (Fig. 3). A third DNA vaccination did not increase stimulation indices. Considering stimulation indices (SI) of ≥5 as indicating significant proliferation, we noted that the numbers of vaccinees with SI ≥ 5 2 weeks after the first vaccination were 0 (pCMVgag), 2 (pCMVgag/PLG), 3 (pSINCPgag), and 4 (pSINCPgag/PLG), suggesting that PLG-formulated DNA vaccines were better than unformulated DNA and that the pSINCP vector was more effective than pCMV. However, these differences between the vaccination groups did not achieve statistical significance (P = 0.08, Kruskal-Wallis test). Rhesus #40 (pCMVgag/PLG) and rhesus #37 (pSINCP) had much higher gag-specific proliferative responses than the others in their respective groups. There were no obvious factors that contributed to the high responses of these two individuals. When these outliers were excluded from the analysis, no statistically significant differences in the magnitudes of the stimulation indices between four groups were observed (Fig. 3E and F), although the pSINCPgag/PLG-vaccinated rhesus had higher responses on average than the pSINCPgag-vaccinated rhesus.
FIG. 3.
Gag-specific lymphoproliferation of rhesus PBMC. Stimulation indices for individual animals vaccinated with pCMVgag/saline (A), pCMVgag/PLG (B), pSINCPgag/saline (C), or pSINCPgag/PLG (D). Figure legends show animal numbers. Vertical dotted lines denote DNA vaccinations at weeks 0, 4, and 14 and Gag protein boost at week 29. Group geometric mean stimulation indices and 95% confidence upper limits are shown for pCMVgag (E)- and for pSINCPgag (F)-vaccinated animals. Outliers (#40 and #37) were excluded.
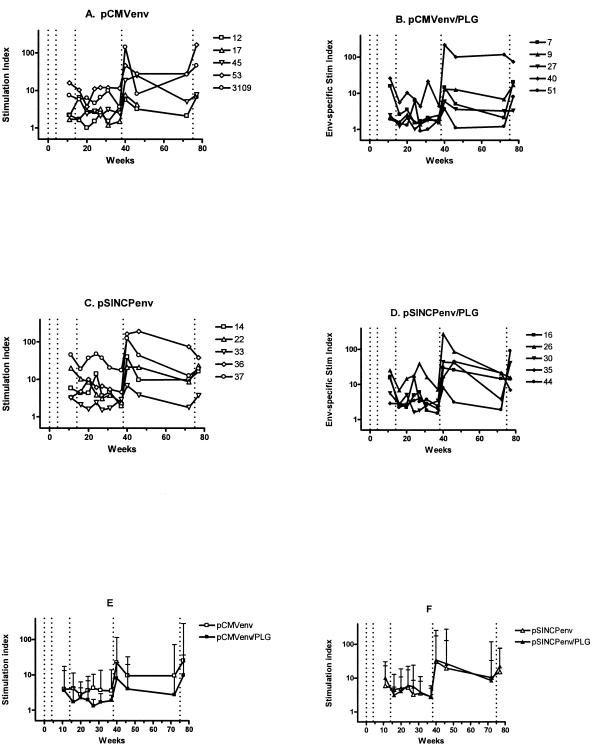
For env-specific proliferation assays, we used a pool of overlapping env peptides that stimulated higher responses than recombinant env protein, which may modulate T-cell proliferation through a direct interaction with CD4. We were unable to perform env LPA until 7 weeks after the second DNA vaccination and therefore may not have observed maximal responses, which were likely to have occurred 2 weeks after the second DNA vaccination, as observed for Gag (Fig. 3). The pCMVenv and pSINCPenv DNA vaccines and their PLG-formulated versions induced env-specific T cells capable of proliferating in LPA assays (Fig. 4). As observed for Gag, rhesus #37 and #40 had much higher stimulation indices than the others in their respective groups, indicating that these two animals were unusually responsive to vaccination. Overall, stimulation indices of the rhesus vaccinated with PLG-formulated env DNA were not greater than those of the rhesus vaccinated with unformulated env DNA (Fig. 4E and F). Somewhat higher stimulation indices were seen in the pSINCP-vaccinated animals compared to the pCMV-vaccinated animals, but the differences were not statistically significant.
FIG. 4.
Env-specific lymphoproliferation of rhesus PBMC. Stimulation indices for individual animals vaccinated with pCMVenv/saline (A), pCMVenv/PLG (B), pSINCPenv/saline (C), or pSINCPenv/PLG (D). Figure legends show animal numbers. Vertical dotted lines denote DNA vaccinations at weeks 0, 4, and 14 and Env protein boosts at weeks 38 and 75. Group geometric mean stimulation indices and 95% confidence upper limits are shown for pCMV (E)- and for pSINCP (F)-vaccinated animals. Outliers (#40 and #37) were excluded.
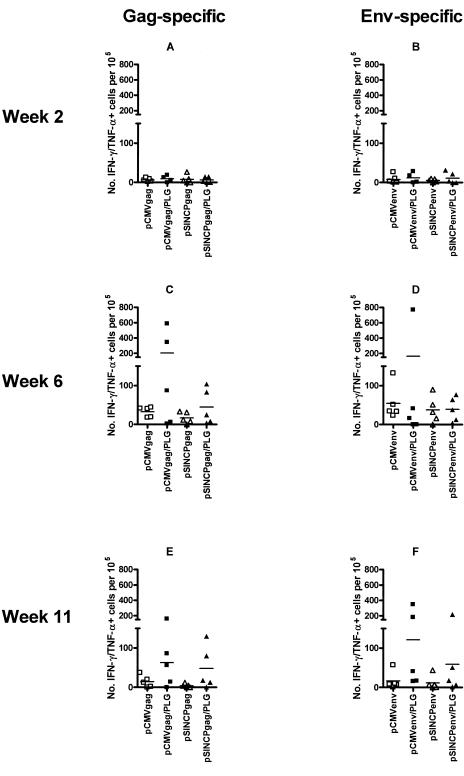
To assess antigen-specific T-cell function and differentiation, PBMC were stimulated overnight with pools of synthetic peptides, stained for cell surface CD4 and CD8 and for intracellular IFN-γ and TNF-α, and analyzed by flow cytometry for cytokine-positive cells. We focused our analysis on IFN-γ/TNF-α-double positive cells, which were the most prevalent antigen-specific cells. Antigen-specific IFN-γ/TNF-α-double positive CD4 T-cell frequencies were near background 2 weeks after the first vaccination (Fig. 5A and B). This was in contrast to proliferative responses that were detectable at that time (Fig. 3). Peak levels of antigen-specific IFN-γ/TNF-α-double positive CD4 T cells were observed 2 weeks after the second DNA vaccination (Fig. 5C and D) and decreased thereafter (Fig. 5E and F), with no apparent increase after the third DNA vaccination (data not shown). Frequencies of antigen-specific CD4 T cells were higher to some extent for the groups vaccinated with PLG-formulated DNA, but the differences were not statistically significant, in part due to animal-to-animal variations.
FIG. 5.
Cytokine-producing antigen-specific CD4 T cells induced by DNA or DNA/PLG vaccination. Antigen-specific CD4 T cells that produced both IFN-γ and TNF-α were enumerated by flow cytometry. Frequencies of cytokine-positive CD4 T cells are plotted for individual animals. Horizontal bars indicate group mean frequencies. Gag-specific (A, C, E) and Env-specific (B, D, F) responses are shown for 2 weeks after the first vaccination (week 2) (A and B) and for 2 weeks (C and D) and 7 weeks (E and F) after the second DNA vaccination (weeks 6 and 11, respectively).
CD8 T-cell responses to DNA vaccines adsorbed to cationic PLG microparticles.
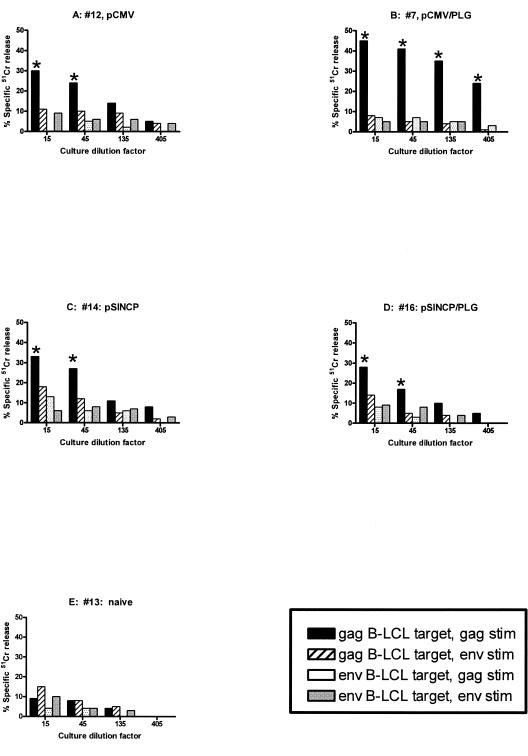
Because the PBMC were stained for both CD4 and CD8, we could also study the CD8+ CD4− T-cell population; however, antigen-specific, IFN-γ/TNF-α-double positive CD8 T cells were generally at background levels. Therefore, PBMC were cultured with antigens and growth factors under conditions that stimulated the production of CTL. Assays for cytolytic activity were performed using as targets 51Cr-labeled autologous B-LCL that had been infected with recombinant vaccinia virus vectors for gag (rVVgagpolSF2) or env (rVVgp160envSF162). Figure 6 illustrates antigen-specific cytolytic activity from individual animals that were observed 2 weeks after the second vaccination. Levels of cytolysis were heterogeneous and ranged from undetectable to high. Because of this heterogeneity, we defined criteria for positivity (Materials and Methods) and scored each culture against those criteria (Table 1). CTL response rates for pCMVgag/saline and pCMVgag/PLG were similar, although CTL appeared earlier with pCMVgag/PLG vaccination. The pCMVgag vaccines (saline or PLG) appeared more effective at inducing Gag CTL responses than pSINCPgag (saline or PLG), with a higher frequency of responders (Table 1). However, neither pCMV nor pSINCP was effective at inducing Env CTL, with or without PLG. The differences between Gag and Env CTL response rates could reflect vector-related differences, i.e., the gag vaccines were superior to the env vaccines, or differences in the assays, e.g., inhibitory peptides in the env pool but not in the gag pool, inefficient processing and presentation of env by the rVVenv-infected B-LCL targets, or more epitopes for CTL recognition in gag than in env.
FIG. 6.
CTL activity from cultured PBMC obtained 2 weeks after the second DNA or DNA/PLG vaccination. Shown are CTL from individual animals vaccinated with pCMV/saline (A), pCMV/PLG (B), pSIN/saline (C), pSIN/PLG (D), or not vaccinated (E). Gag- and Env-stimulated (stim) cultures were tested against both rVVgag-infected targets and rVVenv-infected targets. Culture dilutions meeting the criteria for positivity (see Materials and Methods) are indicated (*).
TABLE 1.
Gag and Env CTL responses of monkeys vaccinated with DNA and boosted with proteina
| Antigen | Vaccine | No. of Gag- or Env-CTL-positive rhesus at wk:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 6 | 11 | 16 | 20 | 24 | 31 | 40 | 77 | ||
| Gag | pCMV | 0 | 4 | 1 | 4 | 3 | 1 | 0 | 2 | 0 |
| pCMV/PLG | 3 | 3 | 2 | 3 | 1 | 1 | 1 | 2 | 1 | |
| pSINCP | 1 | 0 | 0 | 0 | 0 | NDb | 0 | 2 | 0 | |
| pSINCP/PLG | 0 | 3 | 1 | 1 | 0 | ND | 0 | 2 | 0 | |
| Env | pCMV | 0 | 0 | 0 | 0 | 1 | 1 | 0 | ND | 1 |
| pCMV/PLG | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ND | 0 | |
| pSINCP | 0 | 0 | 0 | 0 | 0 | ND | 1 | ND | 2 | |
| pSINCP/PLG | 0 | 1 | 0 | 0 | 0 | ND | 1 | ND | 2 | |
Anti-Gag and anti-Env CTL responses are shown as the number of responding animals per group of 5, based on criteria described in Materials and Methods.
ND, not determined.
p55Gag protein boosting.
As noted above, the third DNA vaccination did not lead to increases in either antibodies or T cells beyond what was observed after the second vaccination. Because recombinant proteins have been shown in other systems to boost immune responses in DNA-primed rhesus all animals were given intramuscular recombinant gag protein that was adsorbed to anionic PLG microparticles. This formulation has been reported to be immunogenic in rhesus (44). As shown in Fig. 1A and B, after boosting with gag protein, the anti-gag antibody titers were approximately 10-fold higher in the animals primed with PLG/CTAB-DNA than those primed with naked DNA. Thus, more effective priming by PLG-formulated DNA was associated with higher antibody titers after gag protein boosting.
Most animals showed increased gag-specific lymphoproliferation after boosting with Gag protein/PLG (Fig. 3A to D); however, postboosting stimulation indices mostly did not exceed those induced by DNA priming. When we compared rhesus primed with pCMVgag/PLG to those primed with pCMVgag/saline (Fig. 3E) or pSINCPgag/PLG-primed to pSINCPgag/saline rhesus (Fig. 3F), there were no differences in their postboosting proliferative responses. Consistent with the proliferation data, no increases in IFN-γ/TNF-α double-positive gag-specific CD4 T cells were seen in the cytokine flow cytometry assays performed after the Gag protein boosting (data not shown). Finally, recombinant Gag protein did not restore Gag-specific CD8 CTL to the levels seen 2 weeks after the second DNA priming vaccination. Thus, in this DNA prime-protein boost regimen, PLG-adsorbed Gag protein was more effective at boosting antibodies than T cells.
Env protein boosting.
Two weeks after boosting with oligomeric Env protein (at week 38), antibody titers increased to high levels (geometric mean ≈ 27,000) in all animals (Fig. 1). Unlike Gag, there were no significant differences in Env antibody titers between the DNA/PLG-primed and DNA/saline-primed animals. Over the next 34 weeks, Env antibody titers declined but remained above preboost levels. The second Env protein boost at week 75 restored antibody titers to levels seen after the first Env protein boost. Env protein boosting was sufficient to induce neutralizing antibodies: 50% of the animals had measurable neutralizing antibodies after the first protein boost and 100% had neutralizing antibodies after the second protein boost (Fig. 2A). Furthermore, neutralizing titers increased from a geometric mean of 5 after the first Env boost to a geometric mean of 64 after the second boost (P < 0.001, t test on log-transformed titers). The neutralizing titers were not statistically different between the different DNA-primed groups (Fig. 2B, ANOVA on log-transformed titers).
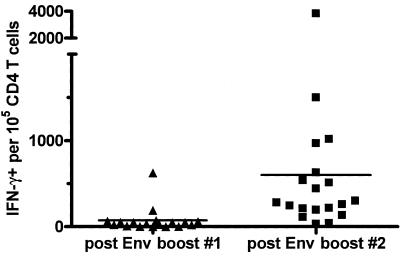
Two weeks after Env protein boosting, Env-specific lymphoproliferation SIs had increased 4- to 12-fold over preboost levels (Fig. 4) and were 2- to 5-fold higher than after env DNA priming (7 weeks after second DNA prime). Despite strongly increased env-specific proliferation after the first env protein boost, frequencies of IFN-γ-positive CD4 T cells were relatively low (<0.1%; Fig. 7). However, after the second Env protein boost, these frequencies increased substantially and significantly (P < 0.001, Mann-Whitney) to a mean of 0.6%. Four animals had env-specific frequencies of ≥1%, and the highest was 3.8%. As expected, CTL responses were not boosted by Env protein boosts (Table 1).
FIG. 7.
IFN-γ-producing antigen-specific CD4 T cells induced by Env protein boosting of DNA (saline or PLG)-primed rhesus. PBMC were assayed by CFC 2 weeks after Env protein boosts. Frequencies of env-specific IFN-γ-positive CD4 T cells are plotted for individual animals. Horizontal bars indicate group mean frequencies: 73 × 10−5 after first Env protein boost, 601 × 10−5 after second Env protein boost.
In summary, boosting DNA-primed macaques with Env protein resulted in neutralizing antibodies and IFN-γ-producing, proliferation-competent CD4 T cells that were markedly higher than achieved by DNA priming.
DISCUSSION
This study evaluated DNA vaccine expression and formulation technologies for plasmids encoding HIVSF2 p55Gag and HIVSF162 gp140 Env that were sequence modified for high levels of expression. First, two fundamentally different types of DNA vectors were compared: a conventional plasmid vector, pCMV, containing genes driven by a highly active CMV promoter, and a plasmid-based Sindbis replicon vector, pSINCP. The present study demonstrates that in rhesus macaques the pSINCP Sindbis-based DNA replicon vaccines were similar in potency to pCMV, at least at the dose used. Thus, pSINCP may provide an alternative to pCMV for clinical testing. It remains to be determined whether pSINCP is more potent than pCMV at limiting doses, as shown previously for rodents (24, 33, 46, 50).
We have developed DNA-adsorbed PLG microparticles as a means of increasing the delivery of DNA into cells, particularly APCs (16). DNA/PLG vaccines are very potent in small animals (42, 54), and the results presented here demonstrate that DNA/PLG led, in particular, to a marked enhancement of antibody responses, with a lesser effect on CD4 T cells and CTL. Why antibody titers were more greatly increased than were T-cell responses is not known. Our T-cell assays measured proliferative capacity, cytokines (IFN-γ, TNF-α), and CTL, and it is possible that we may have seen a greater PLG effect had we directly measured T-cell help for B cells. On the other hand, relatively greater effect of PLG delivery on antibody response may have implications for understanding the mode of action of DNA vaccines in primates. Substantially increased antibody titers may be a result of prolonged expression of antigen in situ, as was seen in mice (54). In vitro studies have shown that PLG microparticles protect plasmid DNA from nuclease digestion (12), thereby potentially prolonging the availability of the plasmid in vivo. This may be important, since the amount of antigen produced in situ by a DNA vaccine is very small (60). For T-cell responses, the relatively lower increase in responses suggests that expression of antigen in APCs is not a limiting step in primates, assuming PLG functioned similarly here as it may in mice. It has been shown that expression of DNA vaccine antigens in APCs is not required for induction of T-cell responses, including CTL (57). In fact, in mice it appears that cross-priming of antigens produced in non-APCs such as muscle cells is the predominant means of eliciting CTL responses after needle injection (10, 11, 13, 19). These results suggest that means to targeting DNA vaccines for expression in non-APCs may also be useful, particularly for T-cell responses. Indeed, electroporation, which is a physical delivery system that results in increased uptake of DNA by myocytes (40, 58), was relatively more effective for enhancement of T-cell responses in rhesus macaques than it was for antibodies (43).
To determine the optimal number of DNA or DNA/PLG vaccinations, we monitored immune responses after each vaccination. Two vaccinations were required to obtain maximal antibody (Fig. 1) and T-cell responses (Fig. 2, 3, 4, Table 1). Interestingly, while Gag-specific lymphoproliferative responses were detectable after the first vaccination, IFN-γ- and TNF-α-producing CD4 T cells were detected only after the second DNA vaccination, suggesting that two vaccinations were required for effector cell differentiation. We noted that a third DNA vaccination at week 14 did not result in further increases in antibody titers or antigen-specific T cells, particularly for the DNA/PLG groups. The vaccination regimen may not have allowed sufficient time between immunizations, which has been suggested previously to be important in primates (20). Another contributing factor may have been the antibody levels at the time of the third immunization. In the DNA/PLG groups, the anti-Gag and anti-Env antibody titers were still high (Fig. 1), which may have prevented effective boosting by neutralizing the small amount of antigen produced in situ.
Despite the apparent ineffectiveness of the third DNA vaccination, the effectiveness of boosting DNA-primed macaques with recombinant protein was demonstrated here, as has been reported previously (9). In this case, we sought to determine if a strong DNA prime could affect the quantity and quality of responses after the protein boost. Interestingly, in only some cases did a stronger DNA prime result in higher levels of immune responses after the protein boost, such as for anti-Gag antibodies in the DNA/PLG groups versus naked DNA groups. In contrast, anti-Env antibodies (both ELISA and neutralizing) were not different after the protein boost, despite significantly higher responses in the DNA/PLG groups after the DNA prime. Reasons for these differences are not known but may be related to the nature of the recombinant antigens (Gag versus Env) or the adjuvant/delivery systems used (anionic PLG microparticles versus MF59 oil-in-water emulsion). Importantly though, the quality of the anti-Env T-cell responses was influenced by DNA vaccine priming. Strong Th1-type cytokine (IFN-γ) production from CD4 T cells was observed after Env protein boosting, despite the use of MF59, a Th2-promoting adjuvant. In fact, in all animals the CD4 IFN-γ response was higher after the second Env protein boost compared to the DNA prime. In contrast, priming of macaques with Env protein vaccines in other studies did not induce this type of Th1 response (unpublished observations). Therefore, strong DNA vaccine priming induces a Th1 type of T-cell response that can be substantially boosted with recombinant Env protein.
In summary, we have demonstrated that two fundamentally different DNA vectors (pCMV, pSINCP) are effective in macaques. The potencies of both DNA vaccines were enhanced by delivery via PLG microparticles, and boosting with recombinant proteins further increased antibodies and T cells. The combined use of alternative DNA vectors, improved formulation and delivery systems, and adjunct technologies such as booster vaccines will likely be vital to effective use of DNA vaccines in humans.
Acknowledgments
We gratefully acknowledge contract support from NIH-NIAID-N01-AI-15429.
We thank Nancy Miller (NIAID) for helpful suggestions.
REFERENCES
- 1.Andersson, C., P. Liljestrom, S. Stahl, and U. F. Power. 2000. Protection against respiratory syncytial virus (RSV) elicited in mice by plasmid DNA immunisation encoding a secreted RSV G protein-derived antigen. FEMS Immunol. Med. Microbiol. 29:247-253. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briones, M., M. Singh, M. Ugozzoli, J. Kazzaz, S. Klakamp, G. Ott, and D. O'Hagan. 2001. The preparation, characterization, and evaluation of cationic microparticles for DNA vaccine delivery. Pharm. Res. 18:709-712. [DOI] [PubMed] [Google Scholar]
- 7.Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 16:2019-2035. [DOI] [PubMed] [Google Scholar]
- 8.Chapman, B. S., R. M. Thayer, K. A. Vincent, and N. L. Haigwood. 1991. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 19:3979-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherpelis, S., I. Shrivastava, A. Gettie, X. Jin, D. D. Ho, S. W. Barnett, and L. Stamatatos. 2001. DNA vaccination with the human immunodeficiency virus type 1 SF162DeltaV2 envelope elicits immune responses that offer partial protection from simian/human immunodeficiency virus infection to CD8(+) T-cell-depleted rhesus macaques. J. Virol. 75:1547-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corr, M., D. J. Lee, D. A. Carson, and H. Tighe. 1996. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J. Exp. Med. 184:1555-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corr, M., A. von Damm, D. J. Lee, and H. Tighe. 1999. In vivo priming by DNA injection occurs predominantly by antigen transfer. J. Immunol. 163:4721-4727. [PubMed] [Google Scholar]
- 12.Denis-Mize, K. S., M. Dupuis, M. L. MacKichan, M. Singh, B. Doe, D. O'Hagan, J. B. Ulmer, J. J. Donnelly, D. M. McDonald, and G. Ott. 2000. Plasmid DNA adsorbed onto cationic microparticles mediates target gene expression and antigen presentation by dendritic cells. Gene Ther. 7:2105-2112. [DOI] [PubMed] [Google Scholar]
- 13.Doe, B., M. Selby, S. Barnett, J. Baenziger, and C. M. Walker. 1996. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow-derived cells. Proc. Natl. Acad. Sci. USA 93:8578-8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 15.Dubensky, T. W., Jr., D. A. Driver, J. M. Polo, B. A. Belli, E. M. Latham, C. E. Ibanez, S. Chada, D. Brumm, T. A. Banks, S. J. Mento, D. J. Jolly, and S. M. Chang. 1996. Sindbis virus DNA-based expression vectors: utility for in vitro and in vivo gene transfer. J. Virol. 70:508-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupuis, M., K. Denis-Mize, C. Woo, C. Goldbeck, M. J. Selby, M. Chen, G. R. Otten, J. B. Ulmer, J. J. Donnelly, G. Ott, and D. M. McDonald. 2000. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J. Immunol. 165:2850-2858. [DOI] [PubMed] [Google Scholar]
- 17.Egan, M. A., W. A. Charini, M. J. Kuroda, J. E. Schmitz, P. Racz, K. Tenner-Racz, K. Manson, M. Wyand, M. A. Lifton, C. E. Nickerson, T. Fu, J. W. Shiver, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J. Virol. 74:7485-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowke, K. R., R. Kaul, K. L. Rosenthal, J. Oyugi, J. Kimani, W. J. Rutherford, N. J. Nagelkerke, T. B. Ball, J. J. Bwayo, J. N. Simonsen, G. M. Shearer, and F. A. Plummer. 2000. HIV-1-specific cellular immune responses among HIV-1-resistant sex workers. Immunol. Cell Biol. 78:586-595. [DOI] [PubMed] [Google Scholar]
- 19.Fu, T. M., J. B. Ulmer, M. J. Caulfield, R. R. Deck, A. Friedman, S. Wang, X. Liu, J. J. Donnelly, and M. A. Liu. 1997. Priming of cytotoxic T lymphocytes by DNA vaccines: requirement for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol. Med. 3:362-371. [PMC free article] [PubMed] [Google Scholar]
- 20.Fuller, D. H., M. Murphey-Corb, J. Clements, S. Barnett, and J. R. Haynes. 1996. Induction of immunodeficiency virus-specific immune responses in rhesus monkeys following gene gun-mediated DNA vaccination. J. Med. Primatol. 25:236-241. [DOI] [PubMed] [Google Scholar]
- 21.Fuller, D. H., P. A. Rajakumar, L. A. Wilson, A. M. Trichel, J. T. Fuller, T. Shipley, M. S. Wu, K. Weis, C. R. Rinaldo, J. R. Haynes, and M. Murphey-Corb. 2002. Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J. Virol. 76:3309-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner, J. P., I. Frolov, S. Perri, Y. Ji, M. L. MacKichan, J. zur Megede, M. Chen, B. A. Belli, D. A. Driver, S. Sherrill, C. E. Greer, G. R. Otten, S. W. Barnett, M. A. Liu, T. W. Dubensky, and J. M. Polo. 2000. Infection of human dendritic cells by a sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J. Virol. 74:11849-11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 24.Hariharan, M. J., D. A. Driver, K. Townsend, D. Brumm, J. M. Polo, B. A. Belli, D. J. Catton, D. Hsu, D. Mittelstaedt, J. E. McCormack, L. Karavodin, T. W. Dubensky, Jr., S. M. Chang, and T. A. Banks. 1998. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J. Virol. 72:950-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedley, M. L., J. Curley, and R. Urban. 1998. Microspheres containing plasmid-encoded antigens elicit cytotoxic T-cell responses. Nat. Med. 4:365-368. [DOI] [PubMed] [Google Scholar]
- 26.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones, D. H., S. Corris, S. McDonald, J. C. Clegg, and G. H. Farrar. 1997. Poly(DL-lactide-co-glycolide)-encapsulated plasmid DNA elicits systemic and mucosal antibody responses to encoded protein after oral administration. Vaccine 15:814-817. [DOI] [PubMed] [Google Scholar]
- 28.Kaur, R., M. Rauthan, and S. Vrati. 2004. Immunogenicity in mice of a cationic microparticle-adsorbed plasmid DNA encoding Japanese encephalitis virus envelope protein. Vaccine 22:2776-2782. [DOI] [PubMed] [Google Scholar]
- 29.Kazzaz, J., J. Neidleman, M. Singh, G. Ott, and D. T. O'Hagan. 2000. Novel anionic microparticles are a potent adjuvant for the induction of cytotoxic T lymphocytes against recombinant p55 gag from HIV-1. J. Control Release 67:347-356. [DOI] [PubMed] [Google Scholar]
- 30.Kim, J. J., J. S. Yang, L. K. Nottingham, D. J. Lee, M. Lee, K. H. Manson, M. S. Wyand, J. D. Boyer, K. E. Ugen, and D. B. Weiner. 2001. Protection from immunodeficiency virus challenges in rhesus macaques by multicomponent DNA immunization. Virology 285:204-217. [DOI] [PubMed] [Google Scholar]
- 31.Kircheis, R., S. Schuller, S. Brunner, M. Ogris, K. H. Heider, W. Zauner, and E. Wagner. 1999. Polycation-based DNA complexes for tumor-targeted gene delivery in vivo. J. Gene Med. 1:111-120. [DOI] [PubMed] [Google Scholar]
- 32.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leitner, W. W., H. Ying, D. A. Driver, T. W. Dubensky, and N. P. Restifo. 2000. Enhancement of tumor-specific immune response with plasmid DNA replicon vectors. Cancer Res. 60:51-55. [PMC free article] [PubMed] [Google Scholar]
- 34.MacLaughlin, F. C., R. J. Mumper, J. Wang, J. M. Tagliaferri, I. Gill, M. Hinchcliffe, and A. P. Rolland. 1998. Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery. J. Control Release 56:259-272. [DOI] [PubMed] [Google Scholar]
- 35.Mascola, J. R. 2002. Passive transfer studies to elucidate the role of antibody-mediated protection against HIV-1. Vaccine 20:1922-1925. [DOI] [PubMed] [Google Scholar]
- 36.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 38.Matano, T., M. Kano, H. Nakamura, A. Takeda, and Y. Nagai. 2001. Rapid appearance of secondary immune responses and protection from acute CD4 depletion after a highly pathogenic immunodeficiency virus challenge in macaques vaccinated with a DNA prime/Sendai virus vector boost regimen. J. Virol. 75:11891-11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathiesen, I. 1999. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther. 6:508-514. [DOI] [PubMed] [Google Scholar]
- 41.McKeever, U., S. Barman, T. Hao, P. Chambers, S. Song, L. Lunsford, Y. Y. Hsu, K. Roy, and M. L. Hedley. 2002. Protective immune responses elicited in mice by immunization with formulations of poly(lactide-co-glycolide) microparticles. Vaccine 20:1524-1531. [DOI] [PubMed] [Google Scholar]
- 42.O'Hagan, D., M. Singh, M. Ugozzoli, C. Wild, S. Barnett, M. Chen, M. Schaefer, B. Doe, G. R. Otten, and J. B. Ulmer. 2001. Induction of potent immune responses by cationic microparticles with adsorbed human immunodeficiency virus DNA vaccines. J. Virol. 75:9037-9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otten, G., M. Schaefer, B. Doe, H. Liu, I. Srivastava, J. zur Megede, D. O'Hagan, J. Donnelly, G. Widera, D. Rabussay, M. G. Lewis, S. Barnett, and J. B. Ulmer. 2004. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine 22:2489-2493. [DOI] [PubMed] [Google Scholar]
- 44.Otten, G., M. Schaefer, C. Greer, M. Calderon-Cacia, D. Coit, J. Kazzaz, A. Medina-Selby, M. Selby, M. Singh, M. Ugozzoli, J. zur Megede, S. W. Barnett, D. O'Hagan, J. Donnelly, and J. Ulmer. 2003. Induction of broad and potent anti-human immunodeficiency virus immune responses in rhesus macaques by priming with a DNA vaccine and boosting with protein-adsorbed polylactide coglycolide microparticles. J. Virol. 77:6087-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. VanCott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nacsa, M. Klein, J. Tartaglia, and G. Franchini. 2002. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasetti, M. F., E. M. Barry, G. Losonsky, M. Singh, S. M. Medina-Moreno, J. M. Polo, J. Ulmer, H. Robinson, M. B. Sztein, and M. M. Levine. 2003. Attenuated Salmonella enterica serovar Typhi and Shigella flexneri 2a strains mucosally deliver DNA vaccines encoding measles virus hemagglutinin, inducing specific immune responses and protection in cotton rats. J. Virol. 77:5209-5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Podda, A., and G. Del Giudice. 2003. MF59-adjuvanted vaccines: increased immunogenicity with an optimal safety profile. Expert Rev. Vaccines 2:197-203. [DOI] [PubMed] [Google Scholar]
- 48.Robinson, H. L., D. C. Montefiori, R. P. Johnson, K. H. Manson, M. L. Kalish, J. D. Lifson, T. A. Rizvi, S. Lu, S. L. Hu, G. P. Mazzara, D. L. Panicali, J. G. Herndon, R. Glickman, M. A. Candido, S. L. Lydy, M. S. Wyand, and H. M. McClure. 1999. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat. Med. 5:526-534. [DOI] [PubMed] [Google Scholar]
- 49.Roy, K., H. Q. Mao, S. K. Huang, and K. W. Leong. 1999. Oral gene delivery with chitosan-DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat. Med. 5:387-391. [DOI] [PubMed] [Google Scholar]
- 50.Schlesinger, S. 2001. Alphavirus vectors: development and potential therapeutic applications. Expert. Opin. Biol. Ther. 1:177-191. [DOI] [PubMed] [Google Scholar]
- 51.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 52.Seth, A., I. Ourmanov, J. E. Schmitz, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, L. Wyatt, M. Carroll, B. Moss, D. Venzon, N. L. Letvin, and V. M. Hirsch. 2000. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J. Virol. 74:2502-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 54.Singh, M., M. Briones, G. Ott, and D. O'Hagan. 2000. Cationic microparticles: a potent delivery system for DNA vaccines. Proc. Natl. Acad. Sci. USA 97:811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srivastava, I. K., L. Stamatatos, H. Legg, E. Kan, A. Fong, S. R. Coates, L. Leung, M. Wininger, J. J. Donnelly, J. B. Ulmer, and S. W. Barnett. 2002. Purification and characterization of oligomeric envelope glycoprotein from a primary R5 subtype B human immunodeficiency virus. J. Virol. 76:2835-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Srivastava, I. K., K. VanDorsten, L. Vojtech, S. W. Barnett, and L. Stamatatos. 2003. Changes in the immunogenic properties of soluble gp140 human immunodeficiency virus envelope constructs upon partial deletion of the second hypervariable region. J. Virol. 77:2310-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulmer, J. B., R. R. Deck, C. M. Dewitt, J. J. Donnelly, and M. A. Liu. 1996. Generation of MHC class I-restricted cytotoxic T lymphocytes by expression of a viral protein in muscle cells: antigen presentation by non-muscle cells. Immunology 89:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Widera, G., M. Austin, D. Rabussay, C. Goldbeck, S. W. Barnett, M. Chen, L. Leung, G. R. Otten, K. Thudium, M. J. Selby, and J. B. Ulmer. 2000. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 164:4635-4640. [DOI] [PubMed] [Google Scholar]
- 59.Wilson, J. D., G. S. Ogg, R. L. Allen, C. Davis, S. Shaunak, J. Downie, W. Dyer, C. Workman, S. Sullivan, A. J. McMichael, and S. L. Rowland-Jones. 2000. Direct visualization of HIV-1-specific cytotoxic T lymphocytes during primary infection. AIDS 14:225-233. [DOI] [PubMed] [Google Scholar]
- 60.Wolff, J. A., R. W. Malone, P. Williams, W. Chong, G. Acsadi, A. Jani, and P. L. Felgner. 1990. Direct gene transfer into mouse muscle in vivo. Science 247:1465-1468. [DOI] [PubMed] [Google Scholar]
- 61.zur Megede, J., M. C. Chen, B. Doe, M. Schaefer, C. E. Greer, M. Selby, G. R. Otten, and S. W. Barnett. 2000. Increased expression and immunogenicity of sequence-modified human immunodeficiency virus type 1 gag gene. J. Virol. 74:2628-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]