Abstract
We measured the longitudinal responses to 95 HLA class I-restricted human immunodeficiency virus (HIV) epitopes and an immunodominant HLA A2-restricted cytomegalovirus (CMV) epitope in eight treatment-naive HIV-infected individuals, using intracellular cytokine staining. Patients were treated with highly active antiretroviral therapy (HAART) for a median of 78 weeks (range, 34 to 121 weeks). Seven of eight patients maintained an undetectable viral load for the duration of therapy. A rapid decline in HIV-specific CD8+ T-cell response was observed at initiation of therapy. After an undetectable viral load was achieved, a slower decrease in HIV-specific CD8+ T-cell response was observed that was well described by first-order kinetics. The median half-life for the rate of decay was 38.8 (20.3 to 68.0) weeks when data were expressed as percentage of peripheral CD8+ T cells. In most cases, data were similar when expressed as the number of responding CD8+ T cells per microliter of blood. In subjects who responded to more than one HIV epitope, rates of decline in response to the different epitopes were similar and varied by a factor of 2.2 or less. Discontinuation of treatment resulted in a rapid increase in HIV-specific CD8+ T cells. Responses to CMV increased 1.6- and 2.8-fold within 16 weeks of initiation of HAART in two of three patients with a measurable CMV response. These data suggest that HAART quickly starts to restore CD8+ T-cell responses to other chronic viral infections and leads to a slow decrease in HIV-specific CD8+ T-cell response in HIV-infected patients. The slow decrease in the rate of CD8+ T-cell response and rapid increase in response to recurrent viral replication suggest that the decrease in CD8+ T-cell response observed represents a normal memory response to withdrawal of antigen.
The CD8+ T-cell response to human immunodeficiency virus (HIV) infection plays a key role in the control of HIV. Despite the undeniable benefits of highly active antiretroviral therapy (HAART), treatment with HAART results in both diminished cellular and humoral HIV-specific responses. HIV-specific antibodies (11), HIV-specific CD4+ T-cell responses (16), and HIV-specific CD8+ T-cell responses (2, 4, 5, 11, 14, 15, 17) are all reported to decrease with treatment. Recent reports have suggested that the decline in CD8+ T-cell response is particularly rapid after the initiation of therapy. Ogg et al. (14) have reported a half-life (t1/2) of 45 days after initiation of HAART. Ortiz et al. (15) reported a t1/2 of 3 to 8 days, as determined by enzyme-linked immunospot analysis. In response to these findings and reports that treatment interruption increases HIV-responsive peripheral blood CD8+ T cells (19), several strategies such as therapeutic vaccination and structured treatment interruption have been proposed to enhance the patient's immune response while on HAART. Early reports suggest that structured treatment interruption in patients recently infected with HIV may be beneficial (18). In chronic infection, where a mature immune response has already developed, no clear clinical consensus concerning the benefits of structure treatment interruption has developed.
A wide range of values for the rates of decay of CD8+ T-cell responses have been reported in studies using a variety of different patients, epitopes, and methods (3, 5, 6, 13, 15, 18, 19). We report here the CD8+ T-cell responses of eight treatment naive patients to 95 optimized cytotoxic T-lymphocyte (CTL) peptide epitopes and to a putative immunodominant HLA A2 cytomegalovirus (CMV) peptide epitope after the initiation of HAART, using a functional assay for T-cell response to antigen. Seven of these eight patients maintained maximal viral suppression for a median period of 78 weeks of therapy. Our results show an initial rapid decrease in response, followed by a lower rate of decay after viral load becomes undetectable. This lower rate of decay suggests that in chronically infected individuals, interventions to boost the CD8+ T-cell response may not be necessary until later in therapy than previously thought.
MATERIALS AND METHODS
Subjects.
Eight HIV-infected, antiretrovirus-naive patients with an initial CD4+ T-cell count of greater than 200/μl were recruited for this study. Six of these subjects were involved in other clinical trials testing the efficacy of different drug regimens at the time of this study. All eight subjects achieved undetectable viral loads (<400 copies/ml) on HAART. One subject had an episode of detectable viremia due to medical noncompliance during this study; viremia remained undetectable in seven subjects for the duration of the study. These seven patients consisted of six men and one woman with a median age of 36 (26 to 52) years.
Only two of the eight patients studied had documented evidence of recent infection (<1 year after a negative serological test for HIV). Patient 7 presented with a bilateral facial palsy, photophobia, a sterile lumbar puncture with a lymphocytic pleocytosis, and an indeterminate Western blot, consistent with primary HIV infection (Table 1). Patient 6, who presented with a right optic neuritis, reported that he was seronegative 6 months prior to presentation. Medical regimens, clinical data, and HLA typing are reported for all eight subjects in Table 1. HLA typing was performed on all eight subjects, using PCR sequence-specific primer methodology. All patients signed informed consent as approved by the Institutional Review Board of the University of Texas Southwestern Medical Center.
TABLE 1.
Patient summarya
| Patient | Age (yr) at initiation of therapy | Class 1 HLA type | CD4+ T cells/μl at initiation of therapy | Antiviral | Clinical comment |
|---|---|---|---|---|---|
| 1 | 29 | A3,A31 B18,B39 | 653 | AZT 3TC IDV | Chronic HIV; CDC classification, A1 |
| 2 | 52 | A3,A33 B50,B35 | 268 | AZT 3TC IDV | Chronic HIV; neurosyphilis at presentation; CDC classification, A2 |
| 3 | 38 | A23,A33 B53,Bw4 | 415 | AZT 3TC ABC | Chronic HIV; CDC classification, A2 |
| 4 | 52 | A1,A3 B8,B35 | 274 | AZT 3TC IDV | Chronic HIV; CDC classification, A2 |
| 5 | 36 | A3,A24 B60,B65 | 565 | AZT 3TC IDV | Chronic HIV; CDC classification, A1 |
| 6 | 44 | A2,A3 B7,B44 | 519 | ABC 3TC NVP | Seronegative 6 mo before presentation; at presentation, seropositive; right optic neuritis at presentation; CDC classification, B1 |
| 7 | 26 | A2,A31 B51,B58w4 | 704 | AZT 3TC EVZ | Acute HIV syndrome (bilateral facial nerve palsy); CDC classification, A1 |
| 8 | 32 | A2,A2 B44,B70 | 298 | AZT 3TC IDV | Chronic HIV; CDC classification, A2 |
AZT, zidovudine; 3TC, lamivudine; ABC, abacavir; NVP, nevirapine; EVZ, efavirenz; IDV, indinavir; CDC, Centers for Disease Control and Prevention.
Peptide screening.
Ninety-five optimally defined HIV epitopes described in the HIV Molecular Immunology Database (10) were used to screen for CD8+ T-cell responses and included peptides from the HIV gag, pol, env, and nef gene products. Lyophilized peptides were resuspended in dimethyl sulfoxide at stock concentrations of 100 mg/ml for peptide mixes or 10 mg/ml for single-peptide experiments. For epitope screening, peptides were used in a matrix format as described by Kern et al. (9) and Betts et al. (1). The final concentration of each peptide was 2 μg/106 cells in all experiments described. After reactive peptide epitopes were identified, only those epitopes were followed longitudinally. Two individuals, patients 3 and 5, were screened using peptide epitopes restricted to their specific HLA class I genotypes because of a limited amount of frozen peripheral blood mononuclear cells (PBMC). Twelve peptides were screened for patient 3; 14 were screened for patient 5.
Antibodies.
Unconjugated mouse anti-human CD28, unconjugated mouse anti-human CD49d, fluorescein isothiocyanate (FITC)-conjugated mouse anti-human gamma interferon (IFN-γ), phycoerythrin (PE)-conjugated mouse anti-human CD69, peridinin chlorophyll protein-conjugated mouse anti-human CD3, and allophycocyanin-conjugated mouse anti-human CD8 monoclonal antibodies were obtained from Becton Dickinson Immunocytometry Systems, San Jose, Calif.
Cell preparation.
PBMC were obtained by standard Ficoll-Hypaque density centrifugation (Pharmacia, Uppsala, Sweden). Both fresh and frozen PBMC were used for intracellular IFN-γ staining. PBMC were frozen in complete RPMI medium containing 10% heat-inactivated fetal calf serum (R-10 medium)–dimethyl sulfoxide (90:10) in a Forma CryoMed cell freezer. Cells were stored at −140°C. Cells were thawed at 37°C, immediately diluted at least 1:15 fold-with ice-cold R-10 medium, centrifuged at 300 × g for 8 min at 4°C, and then washed once again before being counted and diluted to 106 cells/ml. Cells were allowed to come to room temperature over at least a 2-h period before cell stimulation.
Cell stimulation.
Stimulation was performed as described previously (8). One million PBMC in 1 ml of R-10 medium were incubated with 1 μg each of costimulatory CD28 and CD49d monoclonal antibodies and 2 μg of each peptide to be tested. To control for spontaneous production of cytokine and activation of cells prior to addition of peptides, cells incubated with only costimulatory antibodies were included at every time point. Cultures were incubated at 37°C in a 5% CO2 incubator for 1 h, followed by an additional 5-h incubation after addition of the secretory inhibitor brefeldin A (10 μg/ml; Sigma, St. Louis, Mo.).
Immunofluorescent staining.
Peptide-stimulated and control cultures were washed and spun down at 300 × g for 8 min in cold Dulbecco's phosphate-buffered saline containing 1% bovine serum albumin and 0.1% sodium azide (FACS [fluorescence-activated cell sorting] buffer), and transferred into 5-ml Falcon polystyrene tubes for staining. After an additional wash, the cells were stained with directly conjugated CD3+ and CD8+ antibodies for 30 min on ice. The cells were washed once with cold FACS buffer and resuspended in 750 μl of a solution containing 50 μl of enzyme-grade Tween (Sigma) per 100 ml of a 2× concentration FACS-lyse solution (Becton Dickinson Immunocytometry Systems) for 10 min in the dark at room temperature. Permeabilized cells were immediately washed twice with cold FACS buffer and spun down at 600 × g for 8 min. The cell pellet was resuspended in minimal volume and stained with directly conjugated anti-IFN-γ and anti-CD69 antibodies for 30 min on ice. After a final wash, the cells were resuspended in Dulbecco's phosphate-buffered saline containing 1% paraformaldehyde (Electron Microscopy Systems, Fort Washington, Pa.) and stored at 4°C until analysis.
Flow cytometric analysis.
Six-parameter flow cytometric analysis was performed on a FACScalibur flow cytometer (Becton Dickinson Immunocytometry Systems), using FITC, PE, peridinin chlorophyll protein, and allophycocyanin as the fluorescent parameters. Between 50,000, and 130,000 live events were acquired, gated on small viable lymphocytes and CD3+ and CD8+ expression. Results were analyzed using PAINT-A-GATEPlus software (Becton Dickinson Immunocytometry Systems). In all experiments, responses were rated positive when a population of IFN-γ+ and CD69+ events representing ≥0.05% (above background) of CD3+ CD8+ lymphocytes was observed.
Reproducibility of intracellular cytokine assay in fresh and frozen cells.
Replicate analysis of the CD8+ T-cell response to the HLA A2 CMV peptide epitope pp65 NLVPMVATV was performed on both freshly isolated (n = 5) and cryopreserved (n = 5) PBMC from a subject with a known response to this epitope. Incubation of this peptide with freshly prepared PBMC resulted in IFN-γ production in CD69+ cells in 2.12 ± 0.16% of total CD8+ T cells. In frozen cells, 2.03 ± 0.14% of the CD8+ cells responded. This difference was not significant, as judged by Student's t test (P = 0.42). Responses to HIV epitopes in cells frozen for as long as 6 months were similar to data obtained from freshly prepared PBMC 6 months earlier.
Statistics.
Student's t tests, paired t tests, and linear regression analysis were done using a SPSS version 9.0 statistics package.
RESULTS
Suppression of HIV load and changes in T cells with HAART.
Eight patients were recruited for this study. All but one patient, patient 8, maintained an undetectable viral load for the duration of this study. Median viral load prior to initiation of therapy was 23,620 (range, 10,782 to 245,000) copies/ml in the seven patients who maintained a viral load of <400 copies/ml. Median CD4+ T-cell count was 519 (268 to 705) cells/μl at initiation of therapy. Median duration of study was 78 (34 to 121) weeks. In all but one patient, viral load was <400 copies/ml within 16 weeks of therapy. In the patient with the highest viral load, an undetectable viral load was not achieved until week 24 (Fig. 1).
FIG. 1.
Plot of HIV load versus time of therapy. Viral loads at week 0 are from samples drawn at initiation of therapy.
CD4+ T cells significantly increased from 519 (268 to 705) to 1,001 (531 to 1,287) cells/μl during treatment (Fig. 2). Absolute CD8+ T-cell counts were unavailable for two patients. In the other five patients, CD8+ T cells showed a rapid decrease in number early in therapy and then stabilized or increased.
FIG. 2.
Plots of CD4+ and CD8+ T cells per microliter of blood versus time of therapy.
Peptide screening.
Of the 95 optimized HLA class I-restricted HIV-derived CTL epitopes, 15 different epitopes were recognized. Of these 15 epitopes, 12 were recognized in only a single patient (Table 2). The most commonly recognized region was amino acids 18 to 29 of p17 (IRLRPGGKKKY), which was recognized by four of the eight subjects studied. In three of these subjects, the initial response to the peptide epitope from this region was the strongest of the responses noted. Between one and three different epitopes were recognized by each subject (Table 2). At the initial time point, the median percentage of CD8+ T cells responding to each epitope was 0.52% (0.11 to 3.81%).
TABLE 2.
Initial frequency of HIV-specific CD8+ T-cell response and t1/2s based on the percentage of CD8+ T cells responding
| Patient | Peptide (amino acid sequence) | Initial % CD8+ T-cell responsea |
|---|---|---|
| 1 | Nef 135–143 (YPLTFGWCY) | 1.59b |
| 2 | RT 323–332 (SPAIFQSSMT) | 0.35 |
| gp 41 843–851 (IPRRIRQGL) | 0.52 | |
| 3 | p24 311–319 (QASQEVKNW) | 2.63c |
| 4 | p24 259–267 (EIYKRWII) | 3.01 |
| Nef 135–143 (YPLTFGWCY) | 2.85 | |
| p17 19–27 (IRLRPGGKK) | 1.77 | |
| 5 | p17 20–28 (RLRPGGKKK) | 2.94 |
| RT 325–333 (AIFQSSMTK) | 0.64 | |
| Nef 73–82 (QVPLRPMTYK) | 0.54 | |
| 6 | p17 20–29 (RLRPGGKKKY) | 0.62d |
| Nef 73–82 (QVPLRPMTYK) | 0.37d | |
| 7 | p17 77–85 (SLYNTVATL) | 0.15 |
| p17 20–28 (RLRPGGKKK) | 0.31 | |
| RT 295–302 (TAFTIPSI) | 1.59 | |
| 8 | p17 77–85 (SLYNTVATL) | 0.62 |
Pretreatment value unless otherwise indicated.
Initial response determined at week 12 of treatment.
Initial response determined at week 8 of treatment.
Initial response determined at week 14 of treatment.
Longitudinal analysis of CD8+ T-cell responses to peptide epitopes during HAART.
When expressed as a percentage of peripheral CD8+ lymphocytes, the frequency of all HIV-specific CD8+ T cells decreased during treatment with effective antiretroviral therapy. Serial FACS plots for the response to CTL epitope p24 259–267 for patient 4 are shown in Fig. 3. When the percentage of responding CD8+ T cells is plotted versus time, a nonlinear decay is apparent in most subjects. We also analyzed the data after expressing the results as the total number of epitope-specific CD8+ T cells per microliter of blood peripheral (when CD8+ T-cell counts were available), since reporting the results as a percentage would not reflect changes in the size of the peripheral CD8+ T-cell pool. These data are shown in log-linear representation in Fig. 4. Linear regression analyses assuming first-order kinetics were performed using results obtained from the time patients achieved an undetectable viral load onward or until the CD8+ T-cell response to the peptide was less than 0.05% for two consecutive samples. Evidence of a more rapid decline in CD8+ T-cell response before an undetectable viral load is achieved can be seen in patients 1 and 2 when the data are presented as a function of percent CD8+ T-cell response. This more rapid early decline is suggested in patients 1 to 4 when the data are presented as total CD8+ T-cell response per microliter of blood.
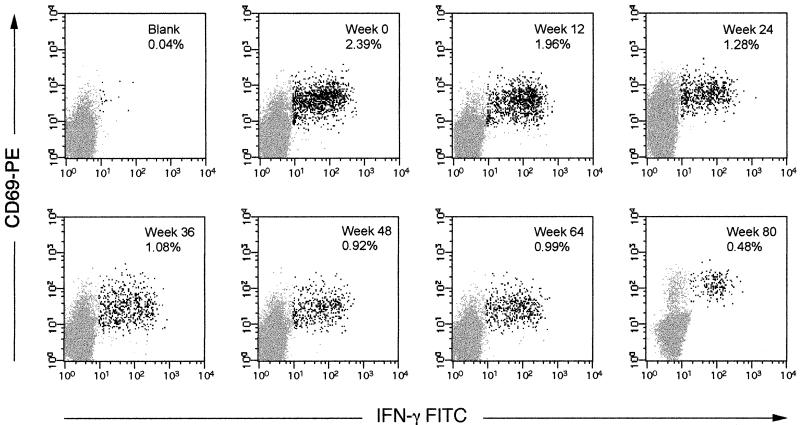
FIG. 3.
FACS plots of longitudinal CD8+ T-cell response to peptide p24 259–267 from patient 4. Darkened dots represent responsive CD8+ T cells as judged by staining with anti-CD69-PE and anti-IFN-γ-FITC. Weeks after initiation of therapy and percent response are indicated in the upper right corner of each plot. The blank shown represents PBMC incubated with anti-CD28 and anti-CD49d in the absence of peptide for cells from day 0. Separate blanks were run for each time point and ranged between 0.03 and 0.07% for this patient.
FIG. 4.
(a) Plots of the natural log of percent responding CD8+ T cells versus time of therapy. Responses for specific HIV epitopes for both plots are as indicated in the graphs. Straight lines represent linear regressions based on data obtained from blood samples taken after viral load became undetectable. (b) Plots of the natural log of number of responding CD8+ T cells per microliter of blood versus time of therapy. Responses for specific HIV epitopes for both plots are as indicated in the graphs. Straight lines represent linear regressions based on data obtained from blood samples taken after viral load became undetectable.
The median t1/2 for the decrease in CD8+ T-cell response to the different peptide epitopes was 38.8 (20.3 to 68.0) weeks when values were calculated from data expressed as percentage of total CD8+ T cells (Table 3). The median r2 value was 0.80 (0.21 to 0.91). Where more than one CD8+ T-cell response was followed in the same individual, t1/2s were remarkably similar. The greatest variation in t1/2 for different epitopes in the same individual was seen in patient 7, 20.7 ± 25.9 and 46.5 ± 25.0, a factor of 2.2, which did not represent a significant difference. No correlation was apparent between the frequency of the initial response and the rate of decay within individuals (Table 2). In the five patients for whom absolute CD8+ T-cell values were available, similar t1/2s were obtained when data were presented either as percent IFN-γ-producing cells or as the number of CD8+ T cells per microliter responding to peptide stimulation. The median t1/2 for the decay in the CD8+ T-cell response was 49.8 weeks when the results were calculated as a function of the number of HIV-responsive CD8+ T cells per microliter of blood. In these same five patients, when the results were presented as percent response, the median t1/2 was 41.2 weeks. This difference was not significant by paired t-test analysis (P = 0.34). The one marked difference occurred in patient 3 because of a sustained increase in CD8+ T cells, which started at week 16 and continued through week 48 of therapy, increasing from 359 to 650 CD8+ T cells/μl during this period of time. This led to an essentially constant number of responsive HIV-specific CD8+ T cells despite a percent fall in frequency within the CD8+ T-cell fraction in the blood (Fig. 4).
TABLE 3.
t1/2s of decay based on the percentage and total number of CD8+ T cells per microliter responding to class I-restricted optimized HIV peptides as a function of the time of treatment with HAART after achieving a sustained undetectable viral load
| Patient | Peptide (amino acid sequence) | Result for CD8+ T-cell response expressed asa:
|
|||
|---|---|---|---|---|---|
| %
|
Total/μl
|
||||
| t1/2 (wk) ± SEM | r2 | t1/2 (wk) ± SEM | r2 | ||
| 1 | Nef 135–143 (YPLTFGWCY) | 68.0 ± 13.3 | 0.86 | 64.7 ± 18.2 | 0.84 |
| 2 | RT 323–332 (SPAIFQSSMT) | 20.3 ± 3.0 | 0.89 | 18.9 ± 3.6 | 0.88 |
| gp 41 843–851 (IPRRIRQGL) | 28.6 ± 14.2 | 0.44 | 28.9 ± 13.2 | 0.54 | |
| 3 | p24 311–319 (QASQEVKNW) | 23.7 ± 4.8 | 0.84 | — | |
| 4 | p24 259–267 (EIYKRWII) | 45.0 ± 11.6 | 0.80 | 52.5 ± 39.8 | 0.47 |
| Nef 135–143 (YPLTFGWCY) | 37.4 ± 8.1 | 0.85 | 47.1 ± 22.4 | 0.71 | |
| p17 19–27 (IRLRPGGKK) | 49.0 ± 9.1 | 0.89 | 80.5 ± 28.0 | 0.77 | |
| 5 | p17 20–28 (RLRPGGKKK) | 48.8 ± 6.9 | 0.79 | 37.9 ± 4.1 | 0.72 |
| RT 325–333 (AIFQSSMTK) | 53.3 ± 20.5 | 0.43 | 57.8 ± 33.7 | 0.28 | |
| Nef 73–82 (QVPLRPMTYK) | 26.7 ± 4.1 | 0.85 | 26.7 ± 9.2 | 0.34 | |
| 6 | p17 20–29 (RLRPGGKKKY) | 38.8 ± 28.3 | 0.49 | NA | |
| Nef 73–82 (QVPLRPMTYK) | 48.1 ± 10.0 | 0.91 | |||
| 7 | p17 77–85 (SLYNTVATL) | 20.7 ± 25.9 | 0.24 | NA | |
| p17 20–28 (RLRPGGKKK) | 22.6 ± 31.0 | 0.21 | |||
| RT 295–302 (TAFTIPSI) | 46.5 ± 25.0 | 0.63 | |||
—, t1/2 was in excess of 10 years with an r2 = 0; NA, CD8+ T-lymphocyte counts were not available.
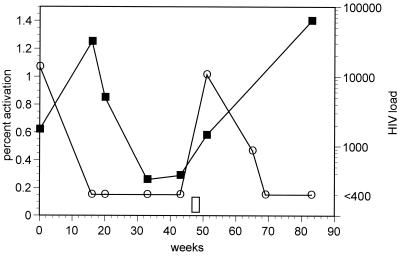
Patient 8 temporarily discontinued his antiretroviral drug therapy after achieving an undetectable viral load for 26 weeks (46 weeks after starting therapy). As shown in Fig. 5, discontinuation of therapy resulted in a rapid rise in viral load and HIV-responsive CD8+ T cells. The frequencies of these CD8+ T cells were at pre-HAART levels at the time of the next blood draw, 31 weeks later.
FIG. 5.
Plot of percent CD8+ T cells responding to peptide p17 77-85 (■) and viral load (○) in a patient who discontinued all antiretroviral drug therapy for a 1.5-week period prior to his 51-week clinic visit, as indicated by the open bar above the x axis. The limit of detection for HIV load was 400 copies/ml.
Longitudinal response to CMV pp65 NLVPMVATV with HAART therapy in HLA A2+ individuals.
Of the eight patients studied, three were HLA class I A2 positive. In these patients, responses to the A2-restricted CMV CTL epitope pp65 NLVPMVATV were followed (Fig. 6). Two of these three subjects showed a marked increase in response to this epitope, increasing from 0.17 and 0.7% prior to therapy to peak levels of 2.9 and 2.15%. Both of these subjects had significant increases in response to this epitope within 16 weeks of starting antiretroviral therapy, 1.6-fold in 9 weeks (patient 8) and 2.8-fold in 16 weeks (patient 7). Patient 6 had a relatively low frequency response, 0.1% at initiation of therapy, and remained essentially unchanged during the 78-week follow-up. None of these patients received treatment for CMV or had any evidence of CMV-specific end organ disease.
FIG. 6.
HIV load (○) and CD8+ T-cell response to CMV epitope pp65 NLMVPMVATV (■) in three HLA A2+ individuals. Day 0 is the day of HAART initiation. The lower limit of detection for HIV viral load was 400 copies/ml.
DISCUSSION
We used intracellular cytokine staining to measure the response to optimized CTL epitopes in eight treatment-naive HIV-infected individuals. The ability to measure the response to multiple peptides at the same time allowed the use of a simplified screening protocol (1). This allowed us to accurately screen CD8+ T-cell responses to 95 different epitopes with limited amounts of patient blood. This assay is similar in sensitivity to tetramer assays in both our hands and others' (3). Mollet et al. (12) and Murali-Krishna et al. (13) have found similar results when comparing tetramer staining and intracellular cytokine staining using a slightly different intracellular staining method. In addition, intracellular IFN-γ staining measures a functional response to antigen that has an antiviral effect and is known to occur in activated CTL.
After complete viral suppression by HAART, we found that the median t1/2 of decay for HIV-specific CD8+ T-cell responses was 49.8 weeks in the five patients for whom results could be reported as number of responding cells per microliter. In the seven patients for whom t1/2s were calculated using data expressed as percent responding cells, the median t1/2 was 38.8 weeks (Table 3). Most patients responded to more than one HIV-specific epitope, and the decay rates for different epitope responses in the same individual varied by a factor of 2.2 or less for each patient. The decay rate for the frequency of response to HIV-specific CD8+ T-cell epitopes was not related to the initial strength of the response and appeared to be more a function of the patient than of the antigen. Reexposure to measurable levels of virus resulted in a rapid increase in the frequency of HIV-specific CD8+ T cells in the one patient who discontinued therapy. The relatively slow decrease in the frequency of HIV-specific CD8+ T-cell response during therapy and the rapid increase during viral breakthrough suggest that the decrease in CD8+ T-cell response observed while patients are on HAART represents a normal response to withdrawal of antigen.
The difference between the median t1/2 that we report for percent responding cells, 38.8 weeks, and that reported by Ogg et al. (14), 45 days, is most likely due to the length of time the patients were studied. Ogg et al. (14) used data from the time of initiation of therapy to a median period of 100 days to calculate his t1/2s. We used data from the time the patients achieved an undetectable viral load, usually within 112 days of starting therapy, to a median period of 78 weeks to calculate our t1/2s. This suggests a rapid early decay of HIV-specific CD8+ T-cell response with initiation of HAART. Our data agree with this hypothesis. A rapid early decay is most apparent when our results are expressed as the absolute number of HIV-specific CD8+ T cells per microliter of blood (Fig. 4b). Using data from the first 8 weeks of therapy for patient 2, the calculated t1/2 for the response to peptide gp41 843–851 was 3.8 weeks, compared to 28.6 weeks for data obtained after an undetectable viral load was achieved. This is much closer to the t1/2 reported by Ogg et al. (14). Unfortunately, our data do not include the number of early time points required to accurately measure an initial decay rate.
We have attempted to describe the rate of decline of HIV-specific CD8+ T lymphocytes in peripheral blood. Probably nothing measured in the blood reflects whole-body frequencies or number of responding cells. Data expressed as percentages of the total peripheral CD8+ T-cell count do not reflect changes in the total CD8+ cell count. Absolute numbers are subject to wide changes because of redistribution and may not reflect the true status of the immune system. We have reported our data as both responding cells per microliter of blood and percent responding cells whenever possible. Our data for all patients except one, patient 3, are similar when reported in either manner. In patient 3, an increase in the peripheral blood CD8+ pool during treatment resulted in an increased in the t1/2 for the response to peptide p24 311–319 from 28 weeks to >10 years when data were calculated as total number of responding cells per microliter. This illustrates the possible effect of changes in the peripheral CD8+ pool on kinetic analysis. Similarly, reporting CD8+ T-cell responses as percentage of responding cells may underestimate the rate of decay early in therapy when CD8+ numbers decrease.
Rinaldo et al. (17) and Mollet et al. (12) have also reported an increase in CD8+ T-cell responses to the CMV peptide epitope pp65 NLVPMVATV in patients on effective antiretroviral therapy. Clinical data showing immune reconstitution syndrome associated with CMV vitritis (6, 7) suggest that increased responsiveness to CMV starts soon after initiation of HAART in patients with CD4+ cell counts below 100 cells/μl. In our patients, the frequency of CMV-specific CD8+ T-cell responses increased with initiation of HAART even though there was no sign of CMV-specific end organ disease and CD4+ cell counts were 274 and 704 cells/μl, well above where immune reconstitution syndrome occurs.
These data show that in medically compliant patients who achieve an undetectable viral load, a measurable HIV-specific CD8+ T-cell response can persist for >2 years after the initiation of HAART. These data also indicate that CD8+ T-cell responses to different HIV epitopes all decay at approximately the same rate within an individual. This indicates that HIV-specific CD8+ T cells with different T-cell receptors recognizing different epitopes in different HIV proteins all respond similarly to the loss of viral antigen in vivo. The rate of decay appears to be constant throughout this study, without evidence of establishment of a new immunologic set point. Discontinuation of therapy results in a prompt increase in HIV-specific CD8+ T cells to levels similar to those seen before therapy. Our data do not allow us to address the reports of early fluctuation in CD8+ response seen by Ogg et al. (14) in the first 2 weeks of therapy or the increased responsiveness seen by Mollet et al. (12) in the first month of therapy. However, our data suggest that there is a lower rate of HIV-specific CD8+ T-cell decline after an undetectable viral load is achieved. Any estimate of the longevity of antigen-specific CD8+ T cells that relies heavily on data obtained prior to the clearance of viremia may underestimate the persistence of these cells because of a rapid initial decrease in HIV-specific CD8+ T cells with initiation of HAART. The original justification for structured treatment interruption in patients with chronic infection is based on the assumption that the CTL response to HIV drops rapidly with therapy. Our data demonstrate a slower decrease in HIV-specific CD8+ T-cell responses. This lower decay rate needs to be taken into consideration in the design of structured treatment interruption and therapeutic vaccination protocols.
ACKNOWLEDGMENTS
This work was supported by grants R37 AI35522 (R.A.K.), R01 A147603 (R.A.K.), UOI AI43638 (R.A.K.), and UOI AI43638 (L.J.P.) from the National Institutes of Health. R.A.K. is an Elizabeth Glaser Scientist of the Pediatric AIDS Foundation.
We thank Phil Keiser, Charla Andrews, and Fred Scott for sample procurement and processing.
REFERENCES
- 1.Betts M R, Casazza J P, Patterson B A, Waldrop S, Trigona W, Fu T-M, Kern F, Picker L J, Koup R A. Immunodominant human immunodeficiency virus-specific CD8+ T-cell response cannot be predicted by major histocompatibility complex class I haplotype. J Virol. 2000;74:9144–9151. doi: 10.1128/jvi.74.19.9144-9151.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalod M, Dupuis M, Deschemin J C, Sicard D, Salmon D, Delfraissy J F, Venet A, Sinet M, Guillet J G. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8+ responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J Virol. 1999;73:7108–7116. doi: 10.1128/jvi.73.9.7108-7116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goulder P J R, Tang Y, Brander C, Betts M R, Trocha A K, He S, Rosenberg E S, Ogg G, Kalams S A, McKinney R E, Mayer K, Koup R A, Pelton S I, Burchett S K, McIntosh K, Walker B D. Functionally inert HIV-specific cytotoxic T lymphocytes do not play a major role in chronically infected adults and children. J Exp Med. 2000;192:1819–1832. doi: 10.1084/jem.192.12.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray C M, Lawrence J, Schapiro J M, Altman J D, Winters M A, Crompton M, Loi M, Kundu S K, Davis M M, Merigan T C. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART) J Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 5.Kalams S A, Goulder P J, Shea A K, Jones N G, Trocha A K, Ogg G S, Walker B D. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karavellas M P, Lowder C Y, Macdonald C, Avila C P, Jr, Freeman W R. Immune recovery vitritis associated with inactive cytomegalovirus retinitis: a new syndrome. Arch Ophthalmol. 1998;116:169–175. doi: 10.1001/archopht.116.2.169. [DOI] [PubMed] [Google Scholar]
- 7.Karavellas M P, Plummer D J, Macdonald J C, Torriani F J, Shufelt C L, Azen S P, Freeman W R. Incidence of immune recovery vitritis in cytomegalovirus retinitis patients following institution of successful highly active antiretroviral therapy. J Infect Dis. 1999;179:697–700. doi: 10.1086/314639. [DOI] [PubMed] [Google Scholar]
- 8.Kern F, Surel I P, Brock C, Freistedt B, Radtke H, Scheffold A, Blasczyk R, Reinke P, Schneider-Mergener J, Radbruch A, Walden P, Volk H D. T-cell epitope mapping by flow cytometry. Nat Med. 1998;4:975–978. doi: 10.1038/nm0898-975. [DOI] [PubMed] [Google Scholar]
- 9.Kern F, Surel I P, Faulhaber N, Frommel C, Schneider-Mergener J, Schonemann C, Reinke P, Volk H D. Target structures of the CD8+ T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J Virol. 1999;73:8179–8184. doi: 10.1128/jvi.73.10.8179-8184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korber B T, Haynes B F, Koup R A, Brander C, Moore J P, Walker B D, Watkins D I. HIV molecular immunology database 1999. Theoretical Biology and Biophysics Group T-10. N.Mex: Los Alamos; 1998. [Google Scholar]
- 11.Markowitz M, Vesanen M, Tenner-Racz K, Cao Y, Binley J M, Talal A, Hurley A, Jin X, Chaudhry M R, Yaman M, Frankel S, Heath-Chiozzi M, Leonard J M, Moore J P, Racz P, Nixon D F, Ho D D. The effect of commencing combination antiretroviral therapy soon after human immunodeficiency virus type 1 infection on viral replication and antiviral immune responses J. Infect Dis. 1999;179:527–537. doi: 10.1086/314628. [DOI] [PubMed] [Google Scholar]
- 12.Mollet L, Li T S, Samri A, Tournay C, Tubiana R, Calvez V, Debre P, Katlama C, Autran B. Dynamics of HIV-specific CD8+ T lymphocytes with changes in viral load. The RESTIM and COMET Study Groups. J Immunol. 2000;165:1692–1704. doi: 10.4049/jimmunol.165.3.1692. [DOI] [PubMed] [Google Scholar]
- 13.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 14.Ogg G S, Jin X, Bonhoeffer S, Moss P, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Hurley A, Markowitz M, Ho D D, McMichael A J, Nixon D F. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortiz G M, Nixon D F, Trkola A, Binley J, Jin X, Bonhoeffer S, Kuebler P J, Donahoe S M, Demoitie M A, Kakimoto W M, Ketas T, Clas B, Heymann J J, Zhang L, Cao Y, Hurley A, Moore J P, Ho D D, Markowitz M. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Investig. 1999;104:R13–R18. doi: 10.1172/JCI7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 17.Rinaldo C R, Jr, Huang X L, Fan Z, Margolick J B, Borowski L, Hoji A, Kalinyak C, McMahon D K, Riddler S A, Hildebrand W H, Day R B, Mellors J W. Anti-human immunodeficiency virus type 1 (HIV-1) CD8+ T-lymphocyte reactivity during combination antiretroviral therapy in HIV-1-infected patients with advanced immunodeficiency. J Virol. 2000;74:4127–4138. doi: 10.1128/jvi.74.9.4127-4138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg E S, Altfeld M, Poon S H, Phillips M N, Wilkes B M, Eldridge R L, Robbins G K, D'Aquila R T, Goulder P J R, Walker B D. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]