Abstract
It has been demonstrated that foot-and-mouth disease virus (FMDV) can utilize at least four members of the αV subgroup of the integrin family of receptors in vitro. The virus interacts with these receptors via a highly conserved arginine-glycine-aspartic acid amino acid sequence motif located within the βG-βH loop of VP1. While there have been extensive studies of virus-receptor interactions at the cell surface, our understanding of the events during viral entry into the infected cell is still not clear. We have utilized confocal microscopy to analyze the entry of two FMDV serotypes (types A and O) after interaction with integrin receptors at the cell surface. In cell cultures expressing both the αVβ3 and αVβ6 integrins, virus adsorbed to the cells at 4°C appears to colocalize almost exclusively with the αVβ6 integrin. Upon shifting the infected cells to 37°C, FMDV capsid proteins were detected within 15 min after the temperature shift, in association with the integrin in vesicular structures that were positive for a marker of clathrin-mediated endocytosis. In contrast, virus did not colocalize with a marker for caveola-mediated endocytosis. Virus remained associated with the integrin until about 1 h after the temperature shift, when viral proteins appeared around the perinuclear region of the cell. By 15 min after the temperature shift, viral proteins were seen colocalizing with a marker for early endosomes, while no colocalization with late endosomal markers was observed. In the presence of monensin, which raises the pH of endocytic vesicles and has been shown to inhibit FMDV replication, viral proteins were not released from the recycling endosome structures. Viral proteins were not observed associated with the endoplasmic reticulum or the Golgi. These data indicate that FMDV utilizes the clathrin-mediated endocytosis pathway to infect the cells and that viral replication begins due to acidification of endocytic vesicles, causing the breakdown of the viral capsid structure and release of the genome by an as-yet-unidentified mechanism.
Foot-and-mouth disease is a highly contagious viral disease of cloven-hoofed livestock caused by Foot-and-mouth disease virus (FMDV), the type species of the genus Aphthovirus of the family Picornaviridae. The virus is a positive-strand RNA virus containing a genome of approximately 8,400 nucleotides (reference 33 and references therein).
FMDV initiates infection in cultured cells by binding to any of four members of the αV subgroup of the integrin family of cellular receptors (αVβ1, αVβ3, αVβ6, and αVβ8) (16, 27, 36, 39, 40, 60) via a highly conserved arginine-glycine-aspartic acid amino acid sequence motif located within the βG-βH loop of VP1 (6, 30, 46, 52). We have recently shown that the αVβ6 integrin acts as a high-affinity receptor for the virus while αVβ3 interacts with virus with a much lower affinity (28). In addition, viruses of the type A serotype utilize both the αVβ3 and αVβ6 integrins as receptors in cultured cells, while serotype O viruses have an affinity for the αVβ6 integrin (27).
While the initial events of FMDV-receptor interactions have been studied in detail, the subsequent events of virion entry and release of the viral genome are still not well defined. Interaction of enteroviruses with their receptors causes a conformational rearrangement of the virion, resulting in the release of VP4 and externalization of the N-terminal extension of VP1. This particle has been called an altered, or A, particle (31, 32, 42, 90). The A particle appears to degrade further to an 80S particle by interaction with membranes and the release of the RNA genome (13). In contrast, FMDV interactions with receptors do not result in structural changes to the virion (5, 28). Rather, this event occurs during viral internalization and results in the breakdown of the virus to 12S pentameric subunits and the release of the RNA (3-5, 21). Compounds which raise intracellular pH inhibit the breakdown, indicating that it occurs within acidic endocytic vesicles (3, 19, 20, 57). Interestingly, the breakdown of the virion within the vesicle, while necessary for productive infection, is not sufficient, suggesting that additional steps are required (45). Direct analysis of FMDV entry and events occurring subsequent to the virion entering the cell, however, has not been reported. Most nonenveloped viruses enter cells via endocytic mechanisms that are either clathrin, caveola, or lipid raft mediated (63, 78). Analysis of the internalization of human parechovirus 1 (HPEV-1) and human adenoviruses, which utilize αV integrins as internalization receptors, have shown that both viruses use the clathrin-mediated endocytosis pathway for entry into the cell (41, 61, 95), while the enterovirus echovirus 1, which utilizes the integrin α2β1 as a receptor, enters via a caveola-mediated, lipid raft-dependent mechanism (49, 64).
In this study, we utilized confocal microscopy to follow viral entry after adsorption and to determine the cell structures which play roles in these events.
MATERIALS AND METHODS
Cell lines, viruses, and plasmids.
Human mammary gland epithelial cells (MCF-10A) were obtained from the American Type Culture Collection (catalogue no. CRL-10317) and were maintained in a mixture of Dulbecco's minimum essential medium and F12-Ham's media (1:1; Life Technologies) containing 5% heat inactivated fetal bovine serum (FBS), 20 ng/ml epidermal growth factor, 100 ng/ml cholera toxin, 10 μg/ml insulin, and 500 ng/ml hydrocortisone. COS-1 cells were maintained in Dulbecco's minimum essential medium containing 10% FBS, an additional 2 mM l-glutamine, and 1 mM sodium pyruvate.
FMDV type A12 strain 119ab (A12) was derived from the infectious cDNA clone pRMC35 (71), and type O1 strain Campos (O1C) was derived from the vesicular fluid of an infected steer. Viral stocks were grown in BHK-21 cells and purified as previously described (51). Titers were determined by plaque assay on BHK-21 cell monolayers using standard techniques (71).
cDNA plasmids encoding the full-length bovine αV, β3, and β6 subunits have been described (27, 59). Transient expression of bovine integrin subunits in COS-1 cells was performed as described previously (59). Briefly, cells were transfected with 2 μg each of cDNA plasmids encoding the bovine αV subunit and the appropriate β subunit, using the transfection reagent FuGene6 (Roche Molecular Biochemicals). After overnight incubation, the transfected cultures were infected with FMDV as described below.
Viral growth curve.
Monolayers of MCF-10A or BHK-21 cells were infected with type A12 or O1C virus at a multiplicity of infection (MOI) of 10 PFU/cell for 1 h at 37°C. At the end of the adsorption period, the inoculum was removed and the cells were rinsed with ice-cold MES (2-morpholinoethanesulfonic acid)-buffered saline (25 mM MES, pH 5.5, 145 mM NaCl) to remove residual virus particles. The monolayers were then rinsed with minimum essential medium containing 1% FBS and 25 mM HEPES, pH 7.4, and incubated at 37°C. At appropriate times postinfection, the cells were frozen at −70°C, and the thawed lysates were used to determine titers by plaque assay on BHK-21 cell monolayers.
Antibodies and reagents.
Monoclonal antibodies (MAbs) 6HC4, directed against FMDV type A12, and 12FB, directed against type O1, have been previously described (8, 80). A rabbit polyclonal antiserum to the C-terminal region of the FMDV 3A protein has been previously described (62). MAb 2D11, directed against the FMDV nonstructural protein 3Dpol, was obtained from E. Brocchi, Instituto Zooprofilattico Sperimentale della Lombardia e dell Emilia-Romagna, Brescia, Italy. MAbs LM609 (catalogue no. MAB1976), which recognizes the αVβ3 heterodimer (23), and CSβ6 (catalogue no. MAB2076), which recognizes the β6 integrin subunit (97) and the αVβ6 heterodimer (28), were purchased from Chemicon. To study virus entry, antibodies against the following human cellular structures were used: a rabbit polyclonal antibody against early endosomal antigen 1 (EEA-1) (Affinity Bioreagents) was used to identify early endosomes, a rabbit polyclonal antibody against the cation-independent mannose-6-phosphate receptor (CI-MPR) (Affinity Bioreagents) was used to identify late endosomes, and a rabbit polyclonal antibody against caveolin-1 and a MAb against clathrin (clone X22) were used as markers for caveolae and clathrin endocytosis pathways, respectively (Affinity Bioreagents). In addition, MAbs directed against the endoplasmic reticulum (ER) marker, protein disulfide isomerase (PDI; clone RL77; Affinity Bioreagents), Golgi zone area (clone 371-4; Sigma), β-COP (clone maD; Sigma), and transferrin receptor (TfnR; clone RVS-10; Chemicon) were used.
The following chemicals and inhibitors were used: monensin ionophore (Sigma), a lysosomotropic agent which raises the pH in endocytic vesicles (3), was prepared as a 10 mM stock solution in 95% ethanol and used at a 50 μM concentration diluted in culture medium; nystatin (Gibco), which sequesters cholesterol and disrupts lipid rafts (1, 77, 99), was used at a 25 μM concentration diluted in culture medium; chlorpromazine (Sigma), which causes the loss of coated pits from the surface of the cell and the appearance of clathrin coats composed of the same subunits on endosomal membranes (41, 96), was used at a 12.5 μM concentration diluted in culture medium.
Infection of cells for confocal microscopy.
Subconfluent monolayers of MCF-10A cells or transfected COS-1 cells expressing either bovine αVβ3 or αVβ6, grown on 12-mm glass coverslips in 24-well tissue culture dishes, were infected with FMDV (MOI, 100 PFU/cell) for 1 h at 4°C in minimum essential medium containing 0.5% FBS and 25 mM HEPES, pH 7.4. After the adsorption period, the inoculum was removed, the monolayers were washed with medium, fresh medium was added, and the cells were incubated at 37°C. At the appropriate times after the temperature shift, cells were fixed with 4% paraformaldehyde and processed for immunofluorescence and confocal microscopy as described below. In all of the figures, the times are listed as either minutes postadsorption (p.a.) or hours p.a. These times refer to the amount of time elapsed after the temperature was shifted from 4° to 37°C.
To investigate the effects of the inhibitory compounds (monensin, nystatin, and chlorpromazine), cells were incubated with the compounds for 30 min at 37°C prior to infection, and the compounds were present during the entire experimental period. Virus infection of the cells was monitored by counting the number of immunofluorescence (IF)-positive cells stained with MAbs reactive with viral structural proteins.
IF and confocal microscopy.
After fixation, the paraformaldehyde was removed, and the cells were permeabilized with 0.5% Triton X-100 for 5 min at room temperature (RT) and incubated in blocking buffer (phosphate-buffered saline [PBS], 5% normal goat serum, 2% bovine serum albumin, 10 mM glycine, 0.01% thimerosa) for 1 h at RT. The fixed cells were then incubated with the primary antibodies overnight at 4°C. When double labeling was performed, cells were incubated with both antibodies together. The dilutions of the primary antibodies were as follows: anti-αvβ3 (1/100), anti-β6 (1/100), anti-FMDV (1/5), anti-caveolin-1 (1/200), anti-clathrin (1/100), anti-β-COP (1/200), anti-TfnR (1/50), anti-EEA-1 (1/200), anti-CI-MPR (1/100), anti-PDI (1/200), and anti-Golgi (1/100). After being washed three times with PBS, the cells were incubated with the appropriate secondary antibody, goat anti-rabbit immunoglobulin G (IgG) (1/400; Alexa Fluor 594; Molecular Probes) or goat anti-mouse isotype-specific IgG (1/400; Alexa Fluor 488 or Alexa Fluor 594; Molecular Probes), for 1 h at RT. Following this incubation, the coverslips were washed three times with PBS, counterstained with the nuclear stain TOPRO-iodide 642/661 (Molecular Probes) for 5 min at RT, washed as before, mounted, and examined in a Leica scanning confocal microscope. Data were collected utilizing appropriate prepared controls lacking the primary antibodies, as well as using anti-FMDV antibodies in uninfected cells to give the negative background levels and to determine channel crossover settings. The captured images were adjusted for contrast and brightness using Adobe Photoshop software.
RESULTS
FMDV replication in MCF-10A cells.
Since foot-and-mouth disease affects livestock species such as cattle and pigs and can replicate to high titers in the hamster cell line BHK-21, it would have been advantageous to utilize either bovine cells, porcine cells, or BHK-21 cells for these experiments. However, since almost all of the available antibodies against intracellular organelles react only with human cells, we decided to perform most of these studies with the human breast tissue epithelial cell line MCF-10A. Since it has been shown that FMDV can utilize the human αVβ3 and αVβ6 integrins as viral receptors in vitro (40, 60), we analyzed the expression of these integrins in MCF-10A cells by IF-confocal microscopy. The αVβ3 integrin was distributed on the cell surface as small discrete structures (Fig. 1a). Examination of the distribution of αVβ6, which was done by using a MAb that recognizes only the β6 subunit, showed a cytoplasmic localization for the subunit (Fig. 1b). Both integrins were present in only a subset of cells. The distribution of the integrins in MCF-10A cells was similar to the distribution observed when primary cultures of fetal bovine kidney cells were examined (not shown). In addition, MCF-10A cells also express the β1 integrin subunit and the αVβ5 and α5β1 integrins (not shown). The last two integrins are not FMDV receptors (27, 40, 60). We did not analyze these cells for the expression of the other two FMDV integrin receptors, αVβ1 (39) and αVβ8 (36).
FIG. 1.
Distribution of αVβ3 and β6 integrins in MCF-10A cells. Monolayers of uninfected MCF-10A cells were processed for IF staining as described in Materials and Methods. MAbs LM609, which recognizes the αVβ3 heterodimer (a), and CSβ6, which recognizes the β6 integrin subunit (b), were used as primary antibodies. Alexa Fluor 488-conjugated antibodies were used as secondary antibodies. The bars represent 16 μm in panel a and 20 μm in panel b.
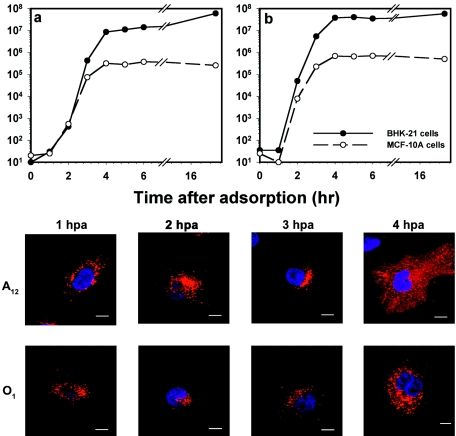
The replication cycle of FMDV in MCF-10A cells was analyzed by growth curve and immunofluorescence. The growth kinetics of types A12 (Fig. 2a) and O1C (Fig. 2b) in MCF-10A cells were similar to their kinetics in BHK-21 cells; however, the human cell line produced 2 to 3 log units lower viral titers than did BHK-21 cells (Fig. 2a and b). Analysis of the localization of the viral capsid protein VP1 during the replication cycle showed that the protein was distributed near the perinuclear area by 1 h p.a., and newly synthesized viral protein was distributed throughout the cytoplasm by 4 h p.a. (Fig. 2 bottom). In addition, the nonstructural proteins 3A and 3Dpol were also detected in the cytoplasm by 4 h p.a. (data not shown). These results indicate that the MCF-10A cells are suitable for the study of the early events in the viral replication cycle in vitro.
FIG. 2.
FMDV replication in MCF-10A cells. Monolayers of MCF-10A and BHK-21 cells were infected with type A12 (a) or O1C (b) at an MOI of 10 PFU/cell for 1 h at 37°C. After the adsorption period, the cells were washed with MES-buffered saline (see Materials and Methods) and incubated at 37°C. At the times indicated, the plates were removed to −70°C. Samples were thawed, and titers were determined by plaque assay on BHK-21 cells. Parallel cultures of MCF-10A cells were processed for IF-confocal microscopy as described in Materials and Methods at the times indicated after the adsorption period (bottom). Viral protein synthesis was visualized with a specific MAb against type A12 or type O1C VP1. Anti-mouse isotype-specific IgG Alexa Fluor 594-labeled conjugate was used as a secondary antibody. The bars represent 8 μm.
FMDV colocalizes with integrins at the cell surface during adsorption.
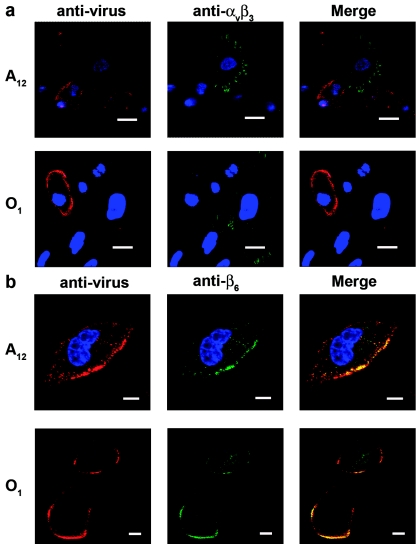
Both types A12 and O1C were adsorbed to cells at 4°C, and localization of virus on the membrane in relation to the integrins αVβ3 and αVβ6 was determined by confocal microscopy. To locate the αVβ6 integrin on the cell surface, we utilized a MAb (CSβ6) which recognizes the β6 subunit (97). However, we have recently shown that this antibody also reacts with the intact heterodimer (28). In nonpermeabilized cells, we can detect the β6 subunit on the cell surface (not shown), and since the ligand binding domains of active surface integrins consist of regions of both the α and β subunits (100), in this report we will consider that staining of this subunit on the cell membrane will identify the intact αVβ6 heterodimer.
Upon adsorption of either type A12 or O1C to MCF-10A cells, virus colocalized with the αVβ6 integrin on the cell membrane (Fig. 3b). However, neither virus appeared to interact with the αVβ3 integrin on these cells (Fig. 3a). We found a few cells that faintly reacted with anti-FMDV antibodies and displayed positive staining for αVβ3 at the cell surface, but we were not able to detect any colocalization. Most of the cells that displayed strong staining for the viral capsid protein did not express αVβ3 at the cell surface (Fig. 3a). Since we have shown that type A viruses can utilize both αVβ3 and αVβ6 integrins as receptors while type O viruses have a preference for the αVβ6 integrin (27), we transiently expressed the bovine αVβ3 or αVβ6 integrin in COS-1 cells and analyzed virus localization by confocal microscopy. Our results showed that type A12 colocalized with both integrins while type O1C colocalized only with αVβ6 (not shown), confirming our previously reported results (27). In addition, these results suggest that when both integrins are expressed on cultured cells, both types A12 and O1C appear to have a higher affinity for the αVβ6 integrin.
FIG. 3.
Distribution of FMDV virions and integrin receptors during the viral adsorption period. Monolayers of MCF-10A cells were infected with FMDV type A12 or O1C at an MOI of 100 PFU/cell for 1 h at 4°C and processed for double IF staining as described in Materials and Methods. FMDV virions were localized with specific MAbs against the capsid protein VP1 and visualized with Alexa Fluor 594 (red). Integrins were stained with anti-αVβ3 (a) or anti-β6 (b) MAbs and visualized with Alexa Fluor 488 (green). The bars represent 20 μm in panel a and 8 μm in panel b.
FMDV internalizes in association with the αVβ6 integrin.
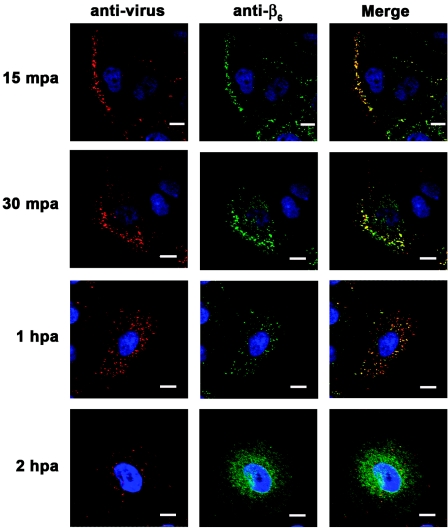
To begin to analyze the internalization process, we followed both the virion and the integrin receptor after binding the virus to MCF-10A cells at 4°C and shifting the temperature to 37°C. Figure 4 shows that viral capsid protein can be seen entering the cell cytoplasm from the membrane as early as 15 min p.a. By 1 h p.a., the virion had translocated to the perinuclear region of the cell (Fig. 4). These micrographs also reveal that αVβ6 is also redistributed and appears to internalize along with the virion. When uninfected cells were examined, we did not detect integrin internalization at 37°C (not shown), suggesting that virus binding to αVβ6 resulted in its internalization. This association can be observed for as long as 1 h p.a. Interestingly, the amount of integrin on the cell surface is decreased drastically by the internalization process (Fig. 4) and does not appear to return to preinfection levels throughout the infectious cycle (not shown). Similar results were obtained when these experiments were performed with either fetal bovine kidney cells or COS-1 cells expressing the bovine αVβ6 integrin (not shown).
FIG. 4.
Internalization of FMDV and αVβ6. FMDV type O1C was adsorbed to MCF-10A monolayers at an MOI of 100 PFU/cell for 1 h at 4°C. The cells were washed with medium and incubated at 37°C. At the times indicated after the temperature shift, the cells were processed for IF-confocal microscopy as described in Materials and Methods. Virus was stained with an anti-VP1 MAb and visualized with Alexa Fluor 594 (red), and the αVβ6 integrin was stained with an anti-β6 MAb and visualized with Alexa Fluor 488 (green). The bars represent 8 μm.
FMDV internalizes via a clathrin-dependent mechanism.
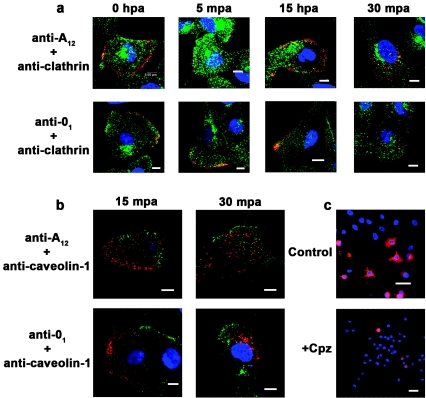
In order to determine the endocytic mechanism utilized by FMDV, we followed virion entry into MCF-10A cells, along with the distribution of either clathrin or caveolin-1. Figure 5a shows colocalization of both type A12 and type O1C with clathrin within 5 min p.a., which has almost completely disappeared by 30 min p.a. In contrast, virus was not observed to colocalize with caveolin-1, a marker for caveolae, even though the surfaces of these cells have a high concentration of the protein (Fig. 5b).
FIG. 5.
Analysis of the FMDV internalization pathway. FMDV types A12 and O1C were adsorbed to monolayers of MCF-10A cells (MOI, 100 PFU/cell) for 1 h at 4°C. The cells were washed, overlaid with warm medium, and transferred to 37°C. At the times indicated after the temperature shift, cells were processed for confocal microscopy as described in Materials and Methods. Virus was stained with anti-VP1 MAbs and visualized with Alexa Fluor 594 (red). Clathrin (a) was stained with an anti-clathrin MAb, and caveolin-1 (b) was stained with a rabbit polyclonal anti-caveolin-1 antibody. Both proteins were visualized with Alexa Fluor 488 (green). Only the merged photographs are shown. In panel c, cells were pretreated with chlorpromazine (Cpz; 12.5 μM) for 30 min at 37°C prior to infection with type O1C (MOI, 100 PFU/cell). The cells were incubated at 37°C in the presence of the drug until 4 h postinfection, when they were processed for confocal microscopy. Virus was stained with an anti-VP1 MAb and visualized with Alexa Fluor 594 (red). The bars represent 8 μm in panels a and b and 40 μm in panel c.
To confirm these results biochemically, we infected cells in the presence of chlorpromazine, which inhibits clathrin-mediated endocytosis (41, 96), and observed the number of virus-positive cells at 4 h p.a. by IF-confocal microscopy. We found that chlorpromazine markedly reduced the number of cells expressing viral antigen (Fig. 5c). By counting infected and noninfected cells in multiple fields, we determined that there were about 50% infected cells per field in the control cultures and only about 4% infected cells per field in the chlorpromazine-treated cultures. In addition, in the presence of the drug, we were able to detect viral capsid protein at the surfaces of a small number of cells for as long as 30 min p.a., but no colocalization with clathrin (not shown). By 1 h p.a., we were unable to detect virus either on the cell surface or in the cytoplasm (not shown). We have previously shown that FMDV, after binding to the surface of the cell at 4°C, begins to elute from the cell surface as early as 5 to 10 min after the temperature is shifted to 37°C (5); thus, we did not expect to see virus on the cell surface at these later time points. These results, therefore, suggest that in the presence of chlorpromazine the virus can bind to the cellular receptor but is unable to internalize.
Although we had already seen that FMDV did not colocalize with the caveolin-1 marker, we investigated if the cholesterol-sequestering and lipid raft-disrupting compound nystatin (1) could inhibit viral infection. By using IF-confocal microscopy, we observed no decrease in the number of virus-infected cells when infection was done in the presence of the drug compared to the control (not shown). Overall, these data indicate that FMDV entry into MCF-10A cells is clathrin dependent.
FMDV traffics through early endosomes after entry.
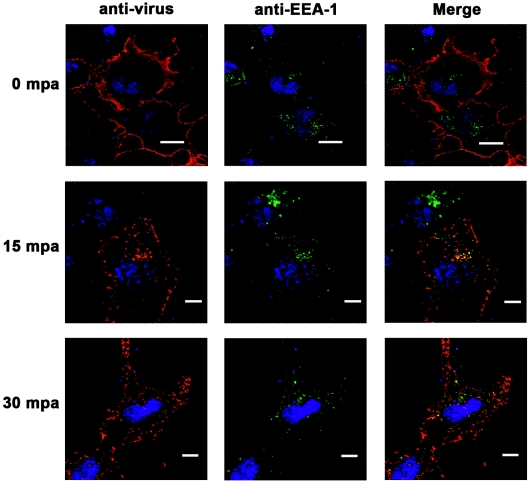
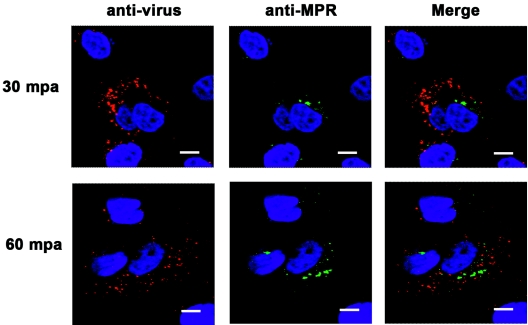
To evaluate the movement of virions through early endosomes, we utilized an antibody against the early endosomal protein EEA-1. Because this antibody did not detect the protein in MCF-10A cells, we used COS-1 cells expressing the bovine integrin αVβ6. Examination of virus-infected cells showed colocalization of the viral capsid protein with the EEA-1 protein at 15 min p.a. which disappeared by 30 min p.a., indicating that the virus had already been translocated from the early endosomes (Fig. 6). Interestingly, we were not able to detect virions in conjunction with CI-MPR, a marker of late endosomes (Fig. 7).
FIG. 6.
Movement of virus into early endosomes. Monolayers of COS-1 cells were cotransfected with cDNA plasmids encoding the bovine αV and β6 subunits as described in Materials and Methods. At 24 h posttransfection, cells were infected with type O1C (MOI, 100 PFU/cell) for 1 h at 4°C. After being washed, the cells were overlaid with warm medium and moved to 37°C. At the times indicated after the temperature shift, cells were processed for confocal microscopy. Virus was stained with an anti-VP1 MAb and visualized with Alexa Fluor 594 (red). Early endosomes were stained with a rabbit polyclonal anti-EEA-1 antiserum and visualized with Alexa Fluor 488 (green). The bars represent 8 μm.
FIG. 7.
Interaction of FMDV with late endosomes. Monolayers of MCF-10A cells were infected with type A12 (MOI, 100 PFU/cell) for 1 h at 4°C. After being washed, the cells were overlaid with warm medium and moved to 37°C. At the times indicated after the temperature shift, cells were processed for confocal microscopy. Virus was stained with an anti-VP1 MAb and visualized with Alexa Fluor 594 (red). Late endosomes were stained with a rabbit polyclonal anti-CI-MPR antiserum and visualized with Alexa Fluor 488 (green). The bars represent 8 μm.
Following internalization, some recycling receptors, such as TfnR, are known to pass through the early endosomes and then accumulate in recycling endosomes before they return to the plasma membrane (69, 92). To further study FMDV entry, we examined the localization of FMDV capsid protein with TfnR. Double labeling with anti-FMDV capsid protein and anti-TfnR antibodies revealed colocalization for up to 1 h p.a. (not shown), resembling the distribution observed for FMDV with αVβ6 (Fig. 4).
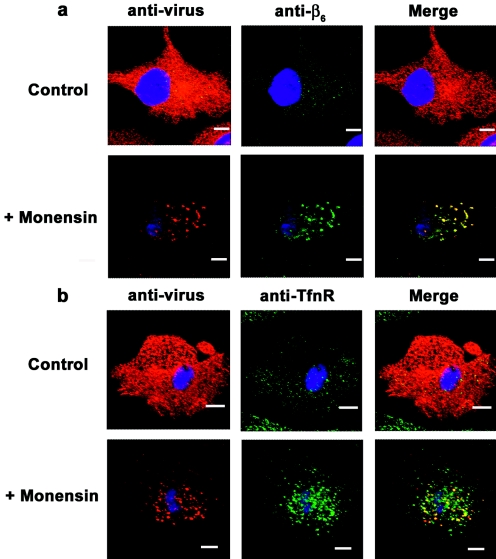
It had been shown that the use of lysosomotropic agents, such as monensin, which raise the pH of endosomal vesicles, inhibited the replication of FMDV (3, 19, 20, 57). To further understand this phenomenon, we examined FMDV-infected MCF-10A cells in the presence of monensin at 4 h p.a. Figure 8 shows that in the presence of the drug, both type A12 (Fig. 8b) and type O1C (Fig. 8a) virions were found in small structures resembling endosomes, quite similar to the viral staining seen at very early times after the temperature shift (Fig. 4 and 5). In contrast, viral proteins were distributed throughout the cytoplasm in untreated infected cells (Fig. 8). In addition, in infected treated cells, the virus was still colocalizing with TfnR and the αVβ6 integrin as late as 4 h p.a. (Fig. 8) and was no longer colocalizing with the clathrin marker, which was not affected by monensin treatment (not shown). Thus, raising the pH of the early endosomes probably prevents the viral genome from being released to the cytoplasm to begin the replication cycle. This result confirms earlier results, which showed that monensin prevented the breakdown of the virus to pentameric subunits and RNA in infected cells (3), and is in agreement with the above-mentioned results on viral trafficking through clathrin-coated vesicles.
FIG. 8.
Effect of monensin on virus internalization. Monolayers of MCF-10A cells were incubated with monensin (50 μM) for 30 min at 37°C prior to infection with type O1C (a) or type A12 (b) (MOI, 100 PFU/cell) for 1 h at 4°C. After being washed, the cells were overlaid with warm medium in the presence of monensin and moved to 37°C. At 4 h p.a., cells were processed for confocal microscopy. Virus was stained with an anti-VP1 MAb and visualized with Alexa Fluor 594 (red). The integrin αVβ6 was stained with an anti-β6 MAb (a), and the TfnR was stained with an anti-TfnR MAb (b). Both proteins were visualized with Alexa Fluor 488 (green). The bars represent 8 μm.
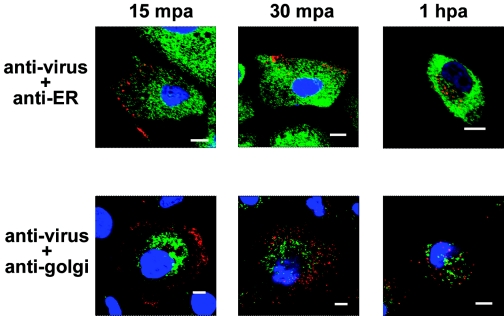
FMDV does not colocalize with the ER or the Golgi.
We also examined the roles of both the ER and the Golgi apparatus during virus internalization. We used anti-PDI antibody as a marker for the ER and compared the distribution with that of the FMDV capsid protein in MCF-10A cells at early times p.a. The infected cells showed no colocalization of the viral capsid protein with the ER (Fig. 9). Similarly, viral proteins were not found colocalized with the Golgi apparatus during early times p.a. (Fig. 9), suggesting that virus is not being delivered to the ER or Golgi during internalization.
FIG. 9.
Interaction of FMDV with ER and Golgi apparatus. Monolayers of MCF-10A cells were infected with type O1C (MOI, 100 PFU/cell) for 1 h at 4°C. After being washed, the cells were overlaid with warm medium and moved to 37°C. At the times indicated after the temperature shift, cells were processed for confocal microscopy. Virus was stained with an anti-VP1 MAb and visualized with Alexa Fluor 594 (red). The ER was stained with an anti-PDI MAb, and the Golgi was stained with an anti-Golgi zone area MAb. Both proteins were visualized with Alexa Fluor 488 (green), and only the merged photographs are shown. The bars represent 8 μm.
We have previously demonstrated that BHK-21 cells infected with FMDV or transfected with a cDNA plasmid encoding the nonstructural protein 3A disrupted the Golgi apparatus (62), resembling the disruption reported in studies examining the mechanism of action of brefeldin A (47, 48). We have shown that FMDV replicates in the presence of brefeldin A (62), which affects the budding of coatomer protein I (COPI)-coated membranes (44). To further study if FMDV uses the retrograde transport system, we examined by IF-confocal microscopy whether a component of COPI, β-COP, colocalizes with FMDV capsid proteins during the internalization process. No colocalization was observed in MCF-10A cells infected with FMDV (not shown), supporting the data shown above.
DISCUSSION
In general, picornaviruses utilize endocytic pathways, which are either clathrin mediated, caveola mediated, or lipid raft dependent, to enter cells after binding to their receptors (11, 24, 25, 35, 49, 63, 76, 78, 81, 84). The current data suggest that the nature of the viral receptor determines the pathway of entry. At least four different picornaviruses utilize integrin receptors to infect cells. Echovirus 1, which utilizes the integrin α2β1 as a receptor (14, 15, 89), has been shown to utilize a caveola-mediated entry pathway (49, 64), while HPEV-1, which utilizes the αVβ3 or αVβ1 integrin as a receptor (67, 83, 86), uses the clathrin-mediated endocytosis pathway to enter the cell (41). Coxsackievirus A9 can utilize the integrin αVβ3 as a receptor (74, 87, 88) and glucose-regulated protein 78 as a coreceptor (82). The latter molecule appears to associate with a major histocompatibility complex class I molecule that is responsible for virus internalization via a lipid raft-dependent mechanism (85). The results presented in this study indicate that FMDV, which utilizes the αV integrins as receptors (references 9, 10, and 38 and references therein), enters the cell via a clathrin-mediated endocytic pathway.
Most of these studies were performed with the human breast epithelial cell line MCF-10A, since almost all the reagents available to detect cellular structures react only with human cells. Staining MCF-10A cells for the expression of the αVβ3 and αVβ6 integrins showed a heterogeneous cell population for integrin expression (Fig. 1). While the two integrins appear to be expressed in different subpopulations of cells, we were not able to determine if there was coexpression in individual cells. MCF-10A cells were shown to be susceptible to infection with both serotypes, A12 and O1C, with growth kinetics similar to those of BHK-21 cells (Fig. 2). In addition, IF studies showed strong staining for newly synthesized capsid proteins after 4 h p.a. (Fig. 2), as well as for nonstructural proteins 3A and 3Dpol (not shown), indicating that this cell line is suitable for study of the early events during the viral replication cycle in vitro.
We have previously shown that αVβ6 acts as a high-affinity receptor for the virus, while αVβ3 interacts with the virus with a much lower affinity (28). We have also shown that type A viruses can utilize both the αVβ3 and the αVβ6 integrins as receptors, while the type O viruses appear to preferentially utilize the αVβ6 integrin (27). The experiments with MCF-10A cells support these data, showing that in cultures of cells which express either αVβ3 or αVβ6, both types A12 and O1C preferentially bind to the αVβ6 integrin (Fig. 3). However, when COS-1 cells were transfected with cDNAs encoding either the bovine αVβ3 or αVβ6 integrin, we were able to detect colocalization of type A12 with both integrins, while type O1C colocalized only with αVβ6 (not shown).
Following the binding of FMDV to the cell surface, we analyzed the entry route of the virus into the cell. FMDV appears to be internalized, in association with the αVβ6 integrin, into a structure resembling an endocytic vesicle and is translocated from the plasma membrane to the perinuclear region within 1 h p.a. (Fig. 4). Interestingly, in contrast to the results presented here, the αVβ3 integrin does not appear to internalize or colocalize with HPEV-1 during the early phases of internalization (41). Under normal conditions, αVβ3, under the control of cellular growth factors, intracellular kinases, and GTPases, is internalized, traffics through endosomes, and then recycles to the plasma membrane for reutilization (72, 73, 98). Similar studies have not been done with the αVβ6 integrin, but it appears that the integrin receptor does not recycle back to the surface, since we cannot detect surface integrin at 2 h p.a. (Fig. 4) and even at later times in the infectious cycle (not shown). It is not clear whether the surface integrin utilized to internalize the virus is degraded; however, since the virus inhibits cap-dependent cellular protein synthesis (26, 43), it is unlikely that any newly synthesized integrin would be generated.
In order to determine the endocytic pathway used by FMDV to enter the cell, the distribution of virus with markers for the clathrin or caveola pathway was examined. After adsorption at 4°C, virus appears to colocalize with clathrin as early as 5 min after the temperature is shifted to 37°C (Fig. 5). By 30 min after the temperature shift, little or no colocalization of virus and clathrin can be observed (Fig. 5), which probably is the result of the uncoating of clathrin from the clathrin-coated pit after separation from the plasma membrane (55). In addition, virions did not colocalize with caveolin-1, a marker for the caveola-mediated endocytosis pathway, during the internalization process. To verify these results biochemically, we utilized chlorpromazine, a member of a class of compounds that inhibits the formation of clathrin-coated pits and causes pits to disappear from the cell surface (41, 96). In the presence of this drug, viral infection was markedly inhibited. Finally, viral infection was not inhibited in the presence of nystatin, a cholesterol-sequestering and lipid-raft disrupting compound (1, 77, 99). Clathrin-mediated endocytosis is generally not dependent on lipid rafts (63).
It has also been shown that the virus can utilize at least two surrogate receptors to infect cells, Fc receptors (7, 51, 52) and a chimeric receptor consisting of a single-chain anti-FMDV MAb fused to ICAM-1 (70). While Fc receptors have been shown to internalize via a clathrin-mediated route (50, 56), ICAM-1, which is a receptor for the major group of human rhinoviruses (HRV) (12), does not contain endocytosis signals, and neither the transmembrane nor the cytoplasmic domain is essential for HRV infection (29, 79). It has been shown, however, that major-group HRV is internalized into intracellular endosomes (76) via a dynamin-dependent mechanism (25), but it is not clear whether the clathrin or caveola pathway is used. Cell culture-adapted FMDV has also been shown to utilize cell surface heparan sulfate (HS) as a receptor in cultured cells (37, 60), which results in a loss of virulence for cattle (75). Although the pathway of HS internalization of ligands is not clear, some recent results suggest that HS-mediated internalization of growth factors occurs via a caveola-dependent pathway (68). Thus, the pathway utilized by FMDV for internalization appears to be a function of the receptor and not the virus.
We have not examined the role of dynamins during FMDV internalization. Dynamins are a family of GTPases that facilitate the budding of clathrin-coated pits, leading to the formation of coated vesicles (91), and also mediate the caveola-dependent pathway of internalization (34). Although the role of dynamin in the internalization of HPEV-1 or CAV9, both of which utilize αV integrins as receptors, has not been determined, the internalization of human adenovirus, which is mediated by the αV integrins, is dynamin dependent (53, 54, 95).
We detected virus in early endosomes within 15 min after shifting the temperature to 37°C (Fig. 6). By 30 min, however, there was no staining of viral proteins in early endosomes, indicating that the virus was already translocated from the vesicle. We were not able to detect virus within late endosomes at times after 30 min, suggesting that virus was not being translocated to this cellular structure. To further examine this, we studied the distribution of FMDV with the TfnR, which passes sequentially through clathrin-coated early endosomes and recycling endosomes before returning to the plasma membrane in a dynamin-dependent manner (69, 92). We found that FMDV and TfnR colocalized in the presence of monensin, which inhibits receptor recycling (2), indicating that FMDV is moving from the early endosomes to recycling endosomes rather than to the late endosomes, as was described for HPEV-1 (41).
We were not able to detect any colocalization of FMDV capsid proteins with either ER or Golgi markers (Fig. 9). Again, these results are in contrast to those observed with HPEV-1 (41). However, these data confirm our previous results, demonstrating that FMDV replicates in the presence of brefeldin A, a compound that disrupts the Golgi apparatus and affects the budding of COPI-coated membranes (62). We also found that virus did not colocalize with the COPI complex or with tubulin, a microtubule marker, during the internalization process (not shown). In addition, nocodazole, which disrupts microtubules and inhibits endosomal transport from early to late endosomes, did not inhibit FMDV replication (S. J. Berryman, S. Clark, A. Burman, P. Monaghan, and T. Jackson, Abstr. Seventh Int. Symp. Pos. Strand RNA Viruses, abstr. P1-B4, 2004), indicating that virus is not transported to the ER and Golgi apparatus. Thus, the data presented here indicate that neither the ER nor the Golgi is used by the virus during the internalization event.
FMDV is structurally unaffected by interaction with its receptor (5, 28). The initial alteration of the 140S virion to 12S pentameric subunits probably occurs within acidified endocytic vesicles once the virus is internalized, resulting in the release of the RNA genome (4, 5, 21). Further evidence of this is the finding that agents that raise the pH of endocytic vesicles inhibit FMDV replication and prevent this initial alteration (3, 19, 20, 57). In the present work, we found that in the presence of monensin FMDV is adsorbed to the cell surface and internalized normally but remains in structures that resemble endocytic vesicles for as long as 4 h after infection (Fig. 8). These data further confirm previous experiments and indicate that preventing the acid-induced virion breakdown inhibits the release of the RNA genome to the cytosol. Thus, FMDV resembles the minor-group HRV, which are also dependent on low-pH-induced virion degradation for release of the viral genome through virus-induced pores in the endosomal membrane (65, 66).
The role, if any, of integrin cytoplasmic domain internalization signals in FMDV internalization has not been determined. Integrin β subunit cytoplasmic domains contain a tetrameric NPXY sequence, which has been shown to be an internalization signal for the human low-density lipoprotein receptor (22). It has been suggested, however, that while this sequence is not required for integrin internalization (93), it is required for integrin signaling functions and adhesion (17, 18). We have previously reported that deletion of almost all of the cytoplasmic domains of the αV and β3 subunits does not prevent the integrin from mediating FMDV infection in cultured cells (58). In addition, we also observed that mutation of the tyrosine within the NPXY sequence of the β3 subunit, which abolishes phosphorylation of the subunit and affects integrin avidity (17), did not affect the ability of the αVβ3 integrin to function as a receptor for type A virus (not shown). In contrast, Miller and coworkers demonstrated that removal of at least 80% of the C-terminal region of the β6 cytoplasmic domain, or deletion of the central region containing the NPXY motif, resulted in loss of the ability of the αVβ6 integrin to mediate viral infection subsequent to adsorption (57). In addition, exchange of the β6 cytoplasmic domain with the β8 cytoplasmic domain abolished the ability of the αVβ6 integrin to both bind virus and mediate infection (36). Taken together, these results indicate that the αVβ3 integrin is active in virus receptor activity even when its activity for cell attachment to vitronectin is abolished by either removing or mutating the cytoplasmic domain. There is a possibility that viral entry mediated by αVβ3 might not use the clathrin-dependent pathway. In contrast, the αVβ6 integrin requires the cytoplasmic domain for binding, internalization, or both. Removal of the three C-terminal residues of the β5 subunit prevented the release of the human adenovirus genome mediated by the αVβ5 integrin to the cytosol but did not prevent virus internalization (94).
The data presented here indicate that FMDV interacts at the cell surface with integrins, and a mechanism that regulates receptor recycling is used by the virus during the internalization process. The virus enters the cell using the clathrin-mediated endocytosis pathway, trafficking throughout the acidified endocytic vesicles, where its capsid rapidly dissociates into pentamers, resulting in the release of the RNA genome.
Acknowledgments
We thank Terry Jackson for sharing his data with us prior to publication.
This work was supported by the U.S. Department of Agriculture, Agricultural Research Service, through CRIS project no. 1940-32000-035-00D.
REFERENCES
- 1.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu, S. K., J. L. Goldstein, R. G. Anderson, and M. S. Brown. 1981. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell 24:493-502. [DOI] [PubMed] [Google Scholar]
- 3.Baxt, B. 1987. Effect of lysosomotropic compounds on early events in foot-and-mouth disease virus replication. Virus Res. 7:257-271. [DOI] [PubMed] [Google Scholar]
- 4.Baxt, B., and H. L. Bachrach. 1982. The adsorption and degradation of foot-and-mouth disease virus by isolated BHK-21 cell plasma membranes. Virology 116:391-405. [DOI] [PubMed] [Google Scholar]
- 5.Baxt, B., and H. L. Bachrach. 1980. Early interactions of foot-and-mouth disease virus with cultured cells. Virology 104:42-55. [DOI] [PubMed] [Google Scholar]
- 6.Baxt, B., and Y. Becker. 1990. The effect of peptides containing the arginine-glycine-aspartic acid sequence on the adsorption of foot-and-mouth disease virus to tissue culture cells. Virus Genes 4:73-83. [DOI] [PubMed] [Google Scholar]
- 7.Baxt, B., and P. W. Mason. 1995. Foot-and-mouth disease virus undergoes restricted replication in macrophage cell cultures following Fc receptor-mediated adsorption. Virology 207:503-509. [DOI] [PubMed] [Google Scholar]
- 8.Baxt, B., D. O. Morgan, B. H. Robertson, and C. A. Timpone. 1984. Epitopes on foot-and-mouth disease virus outer capsid protein VP1 involved in neutralization and cell attachment. J. Virol. 51:298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baxt, B., S. Neff, E. Rieder, and P. Mason. 2002. Foot-and-mouth disease virus-receptor interactions: role in pathogenesis and tissue culture adaptation, p. 115-123. In B. L. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. ASM Press, Washington, D.C.
- 10.Baxt, B., and E. Rieder. 2004. Molecular aspects of foot-and-mouth disease virus virulence and host range: role of host cell receptors and viral factors, p. 145-172. In F. Sobrino and E. Domingo (ed.), Foot and mouth disease: current perspectives. Horizon Bioscience, Norfolk, England.
- 11.Bayer, N., D. Schober, M. Huttinger, D. Blaas, and R. Fuchs. 2001. Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry. J. Biol. Chem. 276:3952-3962. [DOI] [PubMed] [Google Scholar]
- 12.Bella, J., and M. G. Rossmann. 1999. Rhinoviruses and their ICAM receptors. J. Struct. Biol. 128:69-74. [DOI] [PubMed] [Google Scholar]
- 13.Belnap, D. M., D. J. Filman, B. L. Trus, N. Cheng, F. P. Booy, J. F. Conway, S. Curry, C. N. Hiremath, S. K. Tsang, A. C. Steven, and J. M. Hogle. 2000. Molecular tectonic model of virus structural transitions: the putative cell entry states of poliovirus. J. Virol. 74:1342-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergelson, J. M., M. P. Shepley, B. M. Chan, M. E. Hemler, and R. W. Finberg. 1992. Identification of the integrin VLA-2 as a receptor for echovirus 1. Science 255:1718-1720. [DOI] [PubMed] [Google Scholar]
- 15.Bergelson, J. M., N. F. St. John, S. Kawaguchi, R. Pasqualini, F. Berdichevsky, M. E. Hemler, and R. W. Finberg. 1994. The I domain is essential for echovirus 1 interaction with VLA-2. Cell Adhes. Commun. 2:455-464. [DOI] [PubMed] [Google Scholar]
- 16.Berinstein, A., M. Roivainen, T. Hovi, P. W. Mason, and B. Baxt. 1995. Antibodies to the vitronectin receptor (integrin αVβ3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J. Virol. 69:2664-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blystone, S. D., M. P. Williams, S. E. Slater, and E. J. Brown. 1997. Requirement of integrin β3 tyrosine 747 for β3 tyrosine phosphorylation and regulation of αVβ3 avidity. J. Biol. Chem. 272:28757-28761. [DOI] [PubMed] [Google Scholar]
- 18.Calderwood, D. A., Y. Fujioka, J. M. de Pereda, B. Garcia-Alvarez, T. Nakamoto, B. Margolis, C. J. McGlade, R. C. Liddington, and M. H. Ginsberg. 2003. Integrin β cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. USA 100:2272-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrillo, E. C., C. Giachetti, and R. Campos. 1985. Early steps in FMDV replication: further analysis on the effects of chloroquine. Virology 147:118-125. [DOI] [PubMed] [Google Scholar]
- 20.Carrillo, E. C., C. Giachetti, and R. H. Campos. 1984. Effect of lysosomotropic agents on the foot-and-mouth disease virus replication. Virology 135:542-545. [DOI] [PubMed] [Google Scholar]
- 21.Cavanagh, D., D. J. Rowlands, and F. Brown. 1978. Early events in the interaction between foot-and mouth disease virus and primary pig kidney cells. J. Gen. Virol. 41:255-264. [DOI] [PubMed] [Google Scholar]
- 22.Chen, W. J., J. L. Goldstein, and M. S. Brown. 1990. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J. Biol. Chem. 265:3116-3123. [PubMed] [Google Scholar]
- 23.Cheresh, D. A., and R. C. Spiro. 1987. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J. Biol. Chem. 262:17703-17711. [PubMed] [Google Scholar]
- 24.Danthi, P., and M. Chow. 2004. Cholesterol removal by methyl-β-cyclodextrin inhibits poliovirus entry. J. Virol. 78:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17:4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devaney, M. A., V. N. Vakharia, R. E. Lloyd, E. Ehrenfeld, and M. J. Grubman. 1988. Leader protein of foot-and-mouth disease virus is required for cleavage of the p220 component of the cap-binding protein complex. J. Virol. 62:4407-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duque, H., and B. Baxt. 2003. Foot-and-mouth disease virus receptors: comparison of bovine αV integrin utilization by type A and O viruses. J. Virol. 77:2500-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duque, H., M. LaRocco, W. T. Golde, and B. Baxt. 2004. Interactions of foot-and-mouth disease virus with soluble bovine αVβ3 and αVβ6 integrins. J. Virol. 78:9773-9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dustin, M. L., and T. A. Springer. 1991. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu. Rev. Immunol. 9:27-66. [DOI] [PubMed] [Google Scholar]
- 30.Fox, G., N. R. Parry, P. V. Barnett, B. McGinn, D. J. Rowlands, and F. Brown. 1989. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid). J. Gen. Virol. 70:625-637. [DOI] [PubMed] [Google Scholar]
- 31.Fricks, C. E., and J. M. Hogle. 1990. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J. Virol. 64:1934-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez Yafal, A., G. Kaplan, V. R. Racaniello, and J. M. Hogle. 1993. Characterization of poliovirus conformational alteration mediated by soluble cell receptors. Virology 197:501-505. [DOI] [PubMed] [Google Scholar]
- 33.Grubman, M. J., and B. Baxt. 2004. Foot-and-mouth disease. Clin. Microbiol. Rev. 17:465-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henley, J. R., E. W. Krueger, B. J. Oswald, and M. A. McNiven. 1998. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber, M., M. Brabec, N. Bayer, D. Blaas, and R. Fuchs. 2001. Elevated endosomal pH in HeLa cells overexpressing mutant dynamin can affect infection by pH-sensitive viruses. Traffic 2:727-736. [DOI] [PubMed] [Google Scholar]
- 36.Jackson, T., S. Clark, S. Berryman, A. Burman, S. Cambier, D. Mu, S. Nishimura, and A. M. King. 2004. Integrin αVβ8 functions as a receptor for foot-and-mouth disease virus: role of the β-chain cytodomain in integrin-mediated infection. J. Virol. 78:4533-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. Newman, and A. M. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson, T., A. M. King, D. I. Stuart, and E. Fry. 2003. Structure and receptor binding. Virus Res. 91:33-46. [DOI] [PubMed] [Google Scholar]
- 39.Jackson, T., A. P. Mould, D. Sheppard, and A. M. King. 2002. Integrin αVβ1 is a receptor for foot-and-mouth disease virus. J. Virol. 76:935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson, T., D. Sheppard, M. Denyer, W. Blakemore, and A. M. King. 2000. The epithelial integrin αVβ6 is a receptor for foot-and-mouth disease virus. J. Virol. 74:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joki-Korpela, P., V. Marjomaki, C. Krogerus, J. Heino, and T. Hyypia. 2001. Entry of human parechovirus 1. J. Virol. 75:1958-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaplan, G., M. S. Freistadt, and V. R. Racaniello. 1990. Neutralization of poliovirus by cell receptors expressed in insect cells. J. Virol. 64:4697-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirchweger, R., E. Ziegler, B. J. Lamphear, D. Waters, H. D. Liebig, W. Sommergruber, F. Sobrino, C. Hohenadl, D. Blaas, R. E. Rhoads, and T. Skern. 1994. Foot-and-mouth disease virus leader proteinase: purification of the Lb form and determination of its cleavage site on eIF-4g. J. Virol. 68:5677-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klausner, R. D., J. G. Donaldson, and J. Lippincott-Schwartz. 1992. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116:1071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knipe, T., E. Rieder, B. Baxt, G. Ward, and P. W. Mason. 1997. Characterization of synthetic foot-and-mouth disease virus provirions separates acid-mediated disassembly from infectivity. J. Virol. 71:2851-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leippert, M., E. Beck, F. Weiland, and E. Pfaff. 1997. Point mutations within the βG-βH loop of foot-and-mouth disease virus O1K affect virus attachment to target cells. J. Virol. 71:1046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lippincott-Schwartz, J., L. Yuan, C. Tipper, M. Amherdt, L. Orci, and R. D. Klausner. 1991. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell 67:601-616. [DOI] [PubMed] [Google Scholar]
- 48.Lippincott-Schwartz, J., L. C. Yuan, J. S. Bonifacino, and R. D. Klausner. 1989. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56:801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marjomaki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of echovirus 1 in caveolae. J. Virol. 76:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin-Belmonte, F., J. A. Martinez-Menarguez, J. F. Aranda, J. Ballesta, M. C. de Marco, and M. A. Alonso. 2003. MAL regulates clathrin-mediated endocytosis at the apical surface of Madin-Darby canine kidney cells. J. Cell Biol. 163:155-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mason, P. W., B. Baxt, F. Brown, J. Harber, A. Murdin, and E. Wimmer. 1993. Antibody-complexed foot-and-mouth disease virus, but not poliovirus, can infect normally insusceptible cells via the Fc receptor. Virology 192:568-577. [DOI] [PubMed] [Google Scholar]
- 52.Mason, P. W., E. Rieder, and B. Baxt. 1994. RGD sequence of foot-and-mouth disease virus is essential for infecting cells via the natural receptor but can be bypassed by an antibody-dependent enhancement pathway. Proc. Natl. Acad. Sci. USA 91:1932-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meier, O., K. Boucke, S. V. Hammer, S. Keller, R. P. Stidwill, S. Hemmi, and U. F. Greber. 2002. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 158:1119-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meier, O., and U. F. Greber. 2003. Adenovirus endocytosis. J. Gene Med. 5:451-462. [DOI] [PubMed] [Google Scholar]
- 55.Merrifield, C. J., M. E. Feldman, L. Wan, and W. Almers. 2002. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 4:691-698. [DOI] [PubMed] [Google Scholar]
- 56.Miettinen, H. M., K. Matter, W. Hunziker, J. K. Rose, and I. Mellman. 1992. Fc receptor endocytosis is controlled by a cytoplasmic domain determinant that actively prevents coated pit localization. J. Cell Biol. 116:875-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller, L. C., W. Blakemore, D. Sheppard, A. Atakilit, A. M. King, and T. Jackson. 2001. Role of the cytoplasmic domain of the β-subunit of integrin αVβ6 in infection by foot-and-mouth disease virus. J. Virol. 75:4158-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neff, S., and B. Baxt. 2001. The ability of integrin αVβ3 to function as a receptor for foot-and-mouth disease virus is not dependent on the presence of complete subunit cytoplasmic domains. J. Virol. 75:527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neff, S., P. W. Mason, and B. Baxt. 2000. High-efficiency utilization of the bovine integrin αVβ3 as a receptor for foot-and-mouth disease virus is dependent on the bovine β3 subunit. J. Virol. 74:7298-7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neff, S., D. Sa-Carvalho, E. Rieder, P. W. Mason, S. D. Blystone, E. J. Brown, and B. Baxt. 1998. Foot-and-mouth disease virus virulent for cattle utilizes the integrin αVβ3 as its receptor. J. Virol. 72:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nemerow, G. R., and P. L. Stewart. 1999. Role of αV integrins in adenovirus cell entry and gene delivery. Microbiol. Mol. Biol. Rev. 63:725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Donnell, V. K., J. M. Pacheco, T. M. Henry, and P. W. Mason. 2001. Subcellular distribution of the foot-and-mouth disease virus 3A protein in cells infected with viruses encoding wild-type and bovine-attenuated forms of 3A. Virology 287:151-162. [DOI] [PubMed] [Google Scholar]
- 63.Pelkmans, L., and A. Helenius. 2003. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 15:414-422. [DOI] [PubMed] [Google Scholar]
- 64.Pietiainen, V., V. Marjomaki, P. Upla, L. Pelkmans, A. Helenius, and T. Hyypia. 2004. Echovirus 1 endocytosis into caveosomes requires lipid rafts, dynamin II, and signaling events. Mol. Biol. Cell 15:4911-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prchla, E., E. Kuechler, D. Blaas, and R. Fuchs. 1994. Uncoating of human rhinovirus serotype 2 from late endosomes. J. Virol. 68:3713-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prchla, E., C. Plank, E. Wagner, D. Blaas, and R. Fuchs. 1995. Virus-mediated release of endosomal content in vitro: different behavior of adenovirus and rhinovirus serotype 2. J. Cell Biol. 131:111-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pulli, T., E. Koivunen, and T. Hyypia. 1997. Cell-surface interactions of echovirus 22. J. Biol. Chem. 272:21176-21180. [DOI] [PubMed] [Google Scholar]
- 68.Reilly, J. F., E. Mizukoshi, and P. A. Maher. 2004. Ligand dependent and independent internalization and nuclear translocation of fibroblast growth factor (FGF) receptor 1. DNA Cell Biol. 23:538-548. [DOI] [PubMed] [Google Scholar]
- 69.Richardson, D. R., and P. Ponka. 1997. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim. Biophys. Acta 1331:1-40. [DOI] [PubMed] [Google Scholar]
- 70.Rieder, E., A. Berinstein, B. Baxt, A. Kang, and P. W. Mason. 1996. Propagation of an attenuated virus by design: engineering a novel receptor for a noninfectious foot-and-mouth disease virus. Proc. Natl. Acad. Sci. USA 93:10428-10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rieder, E., T. Bunch, F. Brown, and P. W. Mason. 1993. Genetically engineered foot-and-mouth disease viruses with poly(C) tracts of two nucleotides are virulent in mice. J. Virol. 67:5139-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roberts, M., S. Barry, A. Woods, P. van der Sluijs, and J. Norman. 2001. PDGF-regulated rab4-dependent recycling of αVβ3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr. Biol. 11:1392-1402. [DOI] [PubMed] [Google Scholar]
- 73.Roberts, M. S., A. J. Woods, P. E. Shaw, and J. C. Norman. 2003. ERK1 associates with αVβ3 integrin and regulates cell spreading on vitronectin. J. Biol. Chem. 278:1975-1985. [DOI] [PubMed] [Google Scholar]
- 74.Roivainen, M., L. Piirainen, T. Hovi, I. Virtanen, T. Riikonen, J. Heino, and T. Hyypia. 1994. Entry of coxsackievirus A9 into host cells: specific interactions with αVβ3 integrin, the vitronectin receptor. Virology 203:357-365. [DOI] [PubMed] [Google Scholar]
- 75.Sa-Carvalho, D., E. Rieder, B. Baxt, R. Rodarte, A. Tanuri, and P. W. Mason. 1997. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J. Virol. 71:5115-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schober, D., P. Kronenberger, E. Prchla, D. Blaas, and R. Fuchs. 1998. Major and minor receptor group human rhinoviruses penetrate from endosomes by different mechanisms. J. Virol. 72:1354-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 78.Smith, A. E., and A. Helenius. 2004. How viruses enter animal cells. Science 304:237-242. [DOI] [PubMed] [Google Scholar]
- 79.Staunton, D. E., A. Gaur, P. Y. Chan, and T. A. Springer. 1992. Internalization of a major group human rhinovirus does not require cytoplasmic or transmembrane domains of ICAM-1. J. Immunol. 148:3271-3274. [PubMed] [Google Scholar]
- 80.Stave, J. W., J. L. Card, and D. O. Morgan. 1986. Analysis of foot-and-mouth disease virus type O1 Brugge neutralization epitopes using monoclonal antibodies. J. Gen. Virol. 67:2083-2092. [DOI] [PubMed] [Google Scholar]
- 81.Stuart, A. D., H. E. Eustace, T. A. McKee, and T. D. Brown. 2002. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J. Virol. 76:9307-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Triantafilou, K., D. Fradelizi, K. Wilson, and M. Triantafilou. 2002. GRP78, a coreceptor for coxsackievirus A9, interacts with major histocompatibility complex class I molecules which mediate virus internalization. J. Virol. 76:633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Triantafilou, K., and M. Triantafilou. 2001. A biochemical approach reveals cell-surface molecules utilised by Picornaviridae: Human Parechovirus 1 and Echovirus 1. J. Cell Biochem. 80:373-381. [DOI] [PubMed] [Google Scholar]
- 84.Triantafilou, K., and M. Triantafilou. 2004. Lipid-raft-dependent Coxsackievirus B4 internalization and rapid targeting to the Golgi. Virology 326:6-19. [DOI] [PubMed] [Google Scholar]
- 85.Triantafilou, K., and M. Triantafilou. 2003. Lipid raft microdomains: key sites for Coxsackievirus A9 infectious cycle. Virology 317:128-135. [DOI] [PubMed] [Google Scholar]
- 86.Triantafilou, K., M. Triantafilou, Y. Takada, and N. Fernandez. 2000. Human parechovirus 1 utilizes integrins αVβ3 and αVβ1 as receptors. J. Virol. 74:5856-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Triantafilou, M., K. Triantafilou, K. M. Wilson, Y. Takada, and N. Fernandez. 2000. High affinity interactions of coxsackievirus A9 with integrin αVβ3 (CD51/61) require the CYDMKTTC sequence of β3, but do not require the RGD sequence of the CAV-9 VP1 protein. Hum. Immunol. 61:453-459. [DOI] [PubMed] [Google Scholar]
- 88.Triantafilou, M., K. Triantafilou, K. M. Wilson, Y. Takada, N. Fernandez, and G. Stanway. 1999. Involvement of β2-microglobulin and integrin αVβ3 molecules in the coxsackievirus A9 infectious cycle. J. Gen. Virol. 80:2591-2600. [DOI] [PubMed] [Google Scholar]
- 89.Triantafilou, M., K. M. Wilson, and K. Triantafilou. 2001. Identification of echovirus 1 and coxsackievirus A9 receptor molecules via a novel flow cytometric quantification method. Cytometry 43:279-289. [DOI] [PubMed] [Google Scholar]
- 90.Tsang, S. K., B. M. McDermott, V. R. Racaniello, and J. M. Hogle. 2001. Kinetic analysis of the effect of poliovirus receptor on viral uncoating: the receptor as a catalyst. J. Virol. 75:4984-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Urrutia, R., J. R. Henley, T. Cook, and M. A. McNiven. 1997. The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc. Natl. Acad. Sci. USA 94:377-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Dam, E. M., and W. Stoorvogel. 2002. Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol. Biol. Cell 13:169-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vignoud, L., Y. Usson, F. Balzac, G. Tarone, and M. R. Block. 1994. Internalization of the α5β1 integrin does not depend on “NPXY” signals. Biochem. Biophys. Res. Commun. 199:603-611. [DOI] [PubMed] [Google Scholar]
- 94.Wang, K., T. Guan, D. A. Cheresh, and G. R. Nemerow. 2000. Regulation of adenovirus membrane penetration by the cytoplasmic tail of integrin β5. J. Virol. 74:2731-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang, K., S. Huang, A. Kapoor-Munshi, and G. Nemerow. 1998. Adenovirus internalization and infection require dynamin. J. Virol. 72:3455-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weinacker, A., A. Chen, M. Agrez, R. I. Cone, S. Nishimura, E. Wayner, R. Pytela, and D. Sheppard. 1994. Role of the integrin αVβ6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J. Biol. Chem. 269:6940-6948. [PubMed] [Google Scholar]
- 98.Woods, A. J., D. P. White, P. T. Caswell, and J. C. Norman. 2004. PKD1/PKCmicro promotes αVβ3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 23:2531-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xavier, R., T. Brennan, Q. Li, C. McCormack, and B. Seed. 1998. Membrane compartmentation is required for efficient T cell activation. Immunity 8:723-732. [DOI] [PubMed] [Google Scholar]
- 100.Xiong, J. P., T. Stehle, R. Zhang, A. Joachimiak, M. Frech, S. L. Goodman, and M. A. Arnaout. 2002. Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science 296:151-155. [DOI] [PubMed] [Google Scholar]