Abstract
We have shown that foot-and-mouth disease virus (FMDV) infection mediated by the integrin αvβ6 takes place through clathrin-dependent endocytosis but not caveolae or other endocytic pathways that depend on lipid rafts. Inhibition of clathrin-dependent endocytosis by sucrose treatment or expression of a dominant-negative version of AP180 inhibited virus entry and infection. Similarly, inhibition of endosomal acidification inhibited an early step in infection. Blocking endosomal acidification did not interfere with surface expression of αvβ6, virus binding to the cells, uptake of the virus into endosomes, or cytoplasmic virus replication, suggesting that the low pH within endosomes is a prerequisite for delivery of viral RNA into the cytosol. Using immunofluorescence confocal microscopy, FMDV colocalized with αvβ6 at the cell surface but not with the B subunit of cholera toxin, a marker for lipid rafts. At 37°C, virus was rapidly taken up into the cells and colocalized with markers for early and recycling endosomes but not with a marker for lysosomes, suggesting that infection occurs from within the early or recycling endosomal compartments. This conclusion was supported by the observation that FMDV infection is not inhibited by nocodazole, a reagent that inhibits vesicular trafficking between early and late endosomes (and hence trafficking to lysosomes). The integrin αvβ6 was also seen to accumulate in early and recycling endosomes on virus entry, suggesting that the integrin serves not only as an attachment receptor but also to deliver the virus to the acidic endosomes. These findings are all consistent with FMDV infection proceeding via clathrin-dependent endocytosis.
For animal viruses, the entry pathway culminates in the uncoating of the viral genome and its transfer across a cellular membrane to the site of virus replication. Genome transfer can occur by direct penetration through the plasma membrane or following virus uptake into the cell via a specific endocytic pathway (26). A number of different endocytic pathways have been recognized, which normally serve to deliver membrane components, receptor-associated ligands, and solute molecules to several distinct intracellular locations. They also regulate membrane composition by the recycling of receptors and other membrane proteins (54). These pathways include clathrin-dependent endocytosis (or receptor-mediated endocytosis), caveola-dependent endocytosis, macropinocytosis, phagocytosis, and as-yet-undefined non-clathrin- and non-caveola-dependent pathways (54).
Receptors and ligands that enter the cell via clathrin-dependent endocytosis are delivered to the early (or sorting) endosomes. The acidic environment within these compartments allows some ligands to dissociate from their receptors. From here, many ligands are transported to the late endosomes and lysosomes and are subsequently degraded, whereas the internalized receptors may enter a number of different trafficking pathways. Along with their ligands, internalized receptors may be transported to the late endosomes and lysosomes, or they may be returned to the plasma membrane either directly from the early endosomes or via the recycling endosomal compartments.
Caveola-dependent endocytosis is mediated by caveolae, which are formed at lipid rafts as flask-shaped invaginations of the plasma membrane (61). Their formation is dependent on the caveolin family of proteins and cholesterol (60). Following internalization, caveolae are delivered to membrane-bound cytoplasmic organelles called caveosomes, which are also enriched for caveolin (61). Lipid rafts are detergent-insoluble membrane microdomains at the cell surface (71). They are composed of saturated glycosphingolipids and sphingomyelin and are also enriched in cholesterol. In addition to caveolae, other endocytic pathways that are dependent on lipid rafts have been described, but unlike caveolae, these pathways are not dependent on caveolin (51). Although all of the endocytic pathways have the potential to be exploited for gaining entry into cells, viruses appear most commonly to utilize the clathrin- and the caveola-dependent (hence lipid raft) pathways (62, 70).
Foot-and-mouth disease virus (FMDV) is the type species of the Aphthovirus genus within the family Picornaviridae and is the etiological agent of foot-and-mouth disease, a severe vesicular disease of cloven-hoofed animals, including domesticated ruminants and pigs (2). In addition to FMDV, the Picornaviridae include several other important pathogens of humans and animals. The virion consists of a positive-sense single-stranded RNA genome enclosed within a nonenveloped icosahedral capsid formed from 60 copies each of four virally encoded proteins, VP1 to -4 (1). VP1 to VP3 form the outer surface, whereas VP4 is located at the inner capsid surface of the virion (1).
Picornaviruses have evolved a variety of strategies for gaining entry into cells. For many picornaviruses infection is accompanied by a major structural transformation of the virion to the “altered” or “A” particle, and this change is thought to be a prerequisite for release of the viral RNA (vRNA). For the enteroviruses and certain members of the major-receptor group of human rhinoviruses (HRV), this structural change is triggered by virus binding to their cognate receptors (5, 14, 18, 35, 39, 41, 45, 65, 69, 83). For other picornaviruses, such as the minor-receptor group of HRV, conversion to A particles is triggered not by virus binding to its receptor but by the acidic pH within endosomes (7, 16, 38, 66, 68, 73). A-particle formation by other members of the major-receptor group of HRV appears to involve aspects of both the enterovirus and minor-receptor group HRV pathways, as A-particle formation is most efficiently achieved by a combination of virus binding to its receptor and exposure to low pH (58).
Although the cellular receptors used to initiate infection by several picornaviruses have been identified (30), and for a number of these viruses the events that lead to uncoating of the viral genome have been studied (see above), the mechanism of vRNA transfer into the cytosol and the identity of the cellular membrane(s) penetrated by the different members of the Picornaviridae are less well understood. Infection by human parechovirus-1 (HPEV-1) (45) and HRV-2 (11, 12, 58, 66, 68, 73) has been shown to follow virus uptake into endosomes. Another important human picornavirus, poliovirus, may also enter the cell via endosomes; however, it is likely that poliovirus uses multiple pathways for infection (6). In HeLa cells, virus particles have been visualized inside endosome-like structures at early times postinfection, suggesting that poliovirus infection requires virus uptake into endosomes (49, 82). This route of infection has also been reported for Hep-2 cells (84) and neural cells (59). However, other studies have concluded that poliovirus infection may not involve virus uptake via clathrin-dependent endocytosis (13, 20, 41, 76), as infection has been reported not to be dependent on the low pH within endosomes (63) or on dynamin, a protein required for the formation of clathrin-coated pits (24). As dynamin is also required for formation of caveolae, these observations have been interpreted as evidence that poliovirus does not enter the cell via caveolae.
Infection by several other picornaviruses has been shown to require caveolae and/or lipid rafts. Echovirus-1 and its receptor, the integrin α2β1, have been localized to caveolae, and infection is inhibited by methyl-β-cyclodextrin (MβCD) and a dominant-negative version of caveolin, both of which inhibit caveola formation and hence caveola-dependent endocytosis (52). Echovirus-11 and its receptor decay-accelerating factor are found in lipid rafts, and infection is inhibited by pretreatment of the cells with the raft-disrupting reagent nystatin (74). Similarly, infection by two other picornaviruses, coxsackievirus B4 and coxsackievirus A9, has been reported to require intact lipid rafts (78, 79).
FMDV has been shown to use a number of αv integrins (αvβ1, αvβ3, αvβ6, and αvβ8) as receptors to initiate infection of cultured cells (15, 42-44). Of these, αvβ6 is of particular interest as it is expressed on the epithelial cells targeted by FMDV in cattle (submitted for publication); however, despite knowing the identity of the receptors used by FMDV, little is known of the events of cell entry that follow receptor binding. For FMDV, there is no evidence for A-particle formation during cell entry. Instead, virus disassembly proceeds directly to its 12S pentameric subunits, RNA and VP4 (9, 10, 17, 28). FMDV is unusual among the picornaviruses, as its capsid is extremely sensitive to acid and dissociates at a pH just below neutrality (19). Within the capsid, the interpentamer contacts are largely ionic, and the trigger for capsid disassembly is believed to be the protonation of specific histidine residues at the pentamer boundary (19, 29). This event is believed to occur following virus exposure to the prevailing low pH within endosomes, as infection by FMDV is inhibited by reagents, such as monensin (8) and concanamycin A (55), which raise the pH within these compartments. However, these experiments were carried out using a high multiplicity of infecting virus per cell, which can cause infection to proceed via endocytic pathways that are not normally used by the virus (74). In addition, these studies did not establish whether the inhibitory effect of these reagents on infection resulted directly from raising the endosomal pH or from nonspecific effects of these reagents. Thus, a role for endosomes in infection by FMDV has not been clearly established. It is against this background that we sought to determine the early events in FMDV infection mediated by αvβ6. For these studies we have used the SW480 cell line stably transfected to express αvβ6 (SW480-αvβ6 cells). SW480 cells are normally nonpermissive for field strains of FMDV but are made susceptible to infection following transfection with β6 cDNA (44). Previously, αvβ6 was shown to be the major receptor for FMDV on these cells, as both virus attachment and infection are inhibited by a function-blocking antibody specific for the αvβ6 heterodimer (44).
We present here analysis of the early events in FMDV infection. Our results show that αvβ6-mediated infection of SW480 cells by FMDV is dependent on clathrin-dependent endocytosis and the low pH within endosomes but not on caveolae or other endocytic pathways that depend on lipid rafts. Similar observations were made using a CHO cell line transfected to express αvβ6 (CHO-αvβ6 cells). These data provide the first evidence for a functional link between the presence of FMDV within endosomes and infection. In addition, we provide evidence that virus binding induces endocytosis of αvβ6 and that the integrin serves to deliver the virus into the early and recycling endosomes.
MATERIALS AND METHODS
Cells and viruses.
SW480-αvβ6 (80) and CHO-αvβ6 (43) cells were cultivated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 20 mM glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and Geneticin (Life Technologies) (1 mg/ml). Preparation of working stocks of FMDV O1K-cad2 by using primary bovine thyroid cells and virus purification on sucrose gradients were as described previously (21, 42). The multiplicity of infection (MOI) was based on the virus titer on BHK cells as described previously (44).
Peptides, antibodies, and reagents.
The RGD peptide with its sequence derived from the GH loop of VP1 of type O FMDV (VPNLRGDLQVLAQKVAR) was synthesized at the peptide synthesis facility at the Institute for Animal Health, Compton, United Kingdom. The following antibodies were used to identify cellular compartments: early endosomes, EEA-1 (mouse immunoglobulin G1 [IgG1]; BD Transduction Laboratories); transferrin receptor, H68.4 (mouse IgG1; Zymed Laboratories); LAMP-2, H4B4 (mouse IgG1; Developmental Studies Hybridoma Bank); and caveolin-1, N20 (rabbit polyclonal serum; Santa Cruz Biotech). The anti-α-tubulin (DM1A; mouse IgG) and the rabbit polyclonal antiserum to cholera toxin were from Sigma. The antibody to c-myc (9E10; mouse IgG) was from the Developmental Studies Hybridoma Bank. The anti-integrin antibodies used in these studies were 10D5 (mouse IgG2a; anti-αvβ6) and E7P6 (mouse IgG1; anti-αvβ6) from Chemicon and a rabbit monoclonal antibody (MAb), 4B5 (anti-β6) (40), obtained form Dean Sheppard (University of California). The anti-FMDV monoclonal antibodies B2 (mouse IgG1) and D9 (mouse IgG2a), which recognize VP1 of type O FMDV (53), were purified using protein A (Pierce) according to the manufacturers instructions. MAbs B2 and D9 recognize a linear epitope (the GH loop) within VP1 and consequently bind intact virions and their pentameric subunits. Therefore, in the immunofluorescence confocal microscopy studies MAb D9 cannot be used to distinguish between intact virus particles and their uncoating intermediates. MAb 2C2 (23) recognizes the 3A protein of FMDV and was used as a diluted ascites. Acridine orange and Alexa-labeled transferrin and B subunit of cholera toxin (CTB) were from Molecular Probes, as were the Alexa-Fluor-conjugated anti-rabbit and anti-mouse isotype-specific anti-immunoglobulin antibodies. The R-phycoerythrin-conjugated secondary antibodies were from Southern Biotechnology Associates. Concanamycin A was from Fluka, and wortmannin, nystatin, filipin, and MβCD were purchased from Sigma. Stock solutions of concanamycin A and wortmannin were prepared in dimethyl sulfoxide (DMSO). A stock solution of MβCD was prepared in DMEM. Where appropriate, an equivalent dilution of DMSO was included as the mock treatment.
Quantification of infection assays.
For the enzyme-linked immunospot (ELISPOT) assay, cells were prepared in 96-well tissue culture plates and grown overnight at 37°C until approximately 70 to 80% confluent. The cells were incubated (for either 0.5 or 1 h) at 37°C with FMDV at a multiplicity of infection of ∼0.3 PFU/cell. The monolayers were washed and incubated with DMEM at 37°C for a further 4 h. The cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 1 h. The fixed cells were permeabilized with 0.1% Triton in PBS for 15 min. After an incubation for 0.5 h in blocking buffer (10 mM Tris-HCl, pH 7.5; 140 mM NaCl; 1 mM CaCl2; 0.5 mM MgCl2; 10% normal goat serum; 1% fish gelatin), the cells were incubated with MAb 2C2 (mouse IgG2a) for 1 h at room temperature (RT). The cells were then incubated sequentially with a biotinylated goat anti-mouse IgG secondary antibody (Southern Biotechnologies) and streptavidin-conjugated alkaline phosphatase (Caltag Laboratories) prepared in blocking buffer for 1 h each at RT. The alkaline phosphatase substrate (Bio-Rad) was added according to the manufacturer's instructions for 10 min. The cells were then washed with distilled water and allowed to air dry. The infected cells were stained dark blue and counted using an ELISPOT plate reader (Zeiss). Nonspecific labeling was determined by either omitting MAb 2C2 or performing the assay on mock-infected cells. The extents of nonspecific labeling were similar for both conditions (Fig. 1).
FIG. 1.
Quantification of infection by using the ELISPOT assay. SW480-αvβ6 cells were incubated with FMDV (MOI of ∼0.3) for 1 h at 37°C. The cells were washed, incubated in culture medium for a further 4 h, fixed, and permeabilized. The infected cells were detected using MAb 2C2 (which recognizes the viral protein 3A, a marker for virus replication), a biotinylated goat anti-mouse IgG secondary antibody, and streptavidin-conjugated alkaline phosphatase. In the presence of enzyme substrate, the infected cells appear dark blue (shown as black in the figure) and were counted using an ELISPOT plate reader. (A) Mock-infected cell monolayer. (B) Infected cell monolayer.
The ELISPOT assay was used to quantify the effects of various cell treatments on infection. The effect of sucrose on infection was assayed as follows. Cells in triplicate wells were treated with sucrose in DMEM for 0.5 h prior to infection with FMDV for 1 h in the presence of sucrose (note that sucrose was absent throughout the rest of the assay). Similarly, pharmacological reagents were added to cells in triplicate wells for each concentration. Nocodazole, wortmannin, filipin, and nystatin were added 0.5 h before the addition of virus and remained present throughout the assay. MβCD was present for 0.5 h before the addition of and during the 0.5-h incubation with virus but was absent for the rest of the assay. Concanamycin A was present for 0.5 h before and during the 1-h incubation with virus but not after the virus inoculum had been replaced by cell culture medium. In the replication control (Fig. 3), concanamycin A was added for 1.5 h after the 1 h incubation with virus. For the mock treatments, cells were incubated with an equivalent dilution of DMSO or DMEM (see above).
FIG. 3.
Concanamycin A inhibits an early step in cell entry. (A) Concanamycin A inhibits FMDV infection. SW480-αvβ6 cells were treated with concanamycin A (Con-A) for 0.5 h prior to infection (MOI of ∼0.3) with FMDV for 1 h in the presence of the drug. Mock-treated cells were treated with an equivalent dilution of DMSO (see Materials and Methods). In the replication control (RC), the drug was added for 1.5 h after the virus inoculum had been removed. Infection was quantified as described for Fig. 1. The number of infected cells in the drug-treated samples was expressed as a percentage of the number of infected cells in mock-treated samples. The means and standard deviations from three observations are shown. (B to E). Flow cytometric analysis of FMDV binding (B and C) and integrin expression (D and E) on SW480-αvβ6. (B and C). FMDV binding to SW480-αvβ6 is not inhibited by Con-A. Panel B shows the mean fluorescence intensity (MFI) for virus binding to the Con-A- or mock-treated cells. The means and standard deviations from three observations are shown. Panel C shows representative histograms for virus binding to the cells. Mock-treated cells, open histogram; Con-A-treated cells, black histogram; background fluorescence, grey histogram. The background fluorescence was determined in the absence of virus. (D and E). Con-A does not inhibit surface expression of αvβ6. Panel D shows the mean fluorescence intensity for αvβ6 expression on the Con-A- or mock-treated cells. The means and standard deviations from three observations are shown. Panel E shows representative histograms for αvβ6 expression. Mock-treated cells, open histogram; Con-A-treated cells, black histogram; background fluorescence, grey histogram. The background fluorescence was determined in the absence of the primary antibody. The experiments shown in panels A, B, and D were each repeated twice and gave results similar to those shown in the figure.
Transfections with AP180C.
Cells were prepared on glass coverslips as described in “Immunofluorescence confocal microscopy” below and transiently transfected using Lipofectamine 2000 (Invitrogen) with pCMV-MYC containing the coding sequence for the C-terminal fragment (residues 530 to 915) of AP180 (AP180C). The AP180C was fused to the c-myc tag (31), which allowed the transfected cells to be identified using confocal microscopy (see below). At 12 h posttransfection, the cells were infected at an MOI of ∼0.5 with FMDV O1Kcad2 for 5 h at 37°C. The cells were fixed and processed for confocal microscopy as described below. Transfected cells were identified using the anti-c-myc antibody (9E10) and Alexa-488-conjugated goat anti-mouse IgG secondary antibody. Infected cells were identified with a rabbit polyclonal serum to FMDV and an Alexa-568-conjugated goat anti-rabbit IgG secondary antibody. Confocal images were collected sequentially as described below.
Immunofluorescence confocal microscopy. (i) Detection of intracellular antigen.
Cells on 13-mm glass coverslips (BDH) were washed with PBS, fixed with 4% paraformaldehyde (PFM) for 40 min at RT, and permeabilized for 20 min with 0.1% Triton X-100 or 0.5% saponin prepared in blocking buffer (Tris-buffered saline supplemented with 1 mM CaCl2, 0.5 mM MgCl2, 10% normal goat serum, and 1% gelatin). The cells were incubated with primary antibody (5 μg/ml) for 1 h. When saponin was used to permeabilize the cells, 0.1% saponin was included in this and all of the subsequent steps. The cells were washed and incubated with Alexa-Fluor-conjugated secondary antibodies in blocking buffer for 45 min at RT. After washing, the cells were mounted using Vectashield mounting medium with DAPI (4′,6′-diamidino-2-phenylindole) (Vector Labs) and the coverslips sealed with nail varnish. All data were collected sequentially, to eliminate cross talk of the fluorescent dyes, using a Leica SP2 scanning laser confocal microscope. For experiments that involved dual labeling of virus and integrin or of the virus or integrin with a cellular compartment, the specificity of the secondary conjugated antibodies was confirmed by showing a lack of cross-reactivity of the anti-mouse IgG1 for mouse IgG2a and vice versa.
(ii) Detection of surface antigen.
The cells, prepared on coverslips as described above, were cooled on ice and incubated sequentially in the cold with the primary and secondary conjugated antibodies. The cells were then fixed with cold PFM and mounted as described above without permeabilization.
(iii)Binding and uptake of virus.
Cells were incubated with FMDV (5 μg/ml) at 4°C in DMEM for 1 h. Excess unbound virus was removed by washing with cold DMEM. For colocalization experiments with surface antigens, the cells were processed as described in “Detection of surface antigen” above. Alternatively, after the cold wash step, virus internalization was initiated by incubation at 37°C. At the times indicated in the figures, the cells were fixed with cold PFM and mounted as described above. Virus was detected using MAb D9 and a goat anti-mouse IgG2a Alexa-conjugated antibody. In the experiment shown in Fig. 2, the prebinding step at 4°C was omitted and the virus was added directly to the cells at 37°C.
FIG. 2.
Clathrin-dependent endocytosis of FMDV. (A) Sucrose inhibits FMDV infection. SW480-αvβ6 cells were treated with sucrose for 0.5 h prior to infection (MOI of ∼0.3) with FMDV for 1 h in the presence of sucrose. Mock-treated cells were treated with DMEM (see Materials and Methods). Infection was quantified as described for Fig. 1. The number of infected cells in the sucrose-treated samples was expressed as a percentage of the number of infected cells in mock-treated samples. The means and standard deviations from three observations are shown. (B to E). Sucrose inhibits uptake of both FMDV and transferrin. Transferrin and FMDV were detected using the confocal microscope as described in Materials and Methods. SW480-αvβ6 cells were treated with 0.4 M sucrose (C and E) for 0.5 h prior to internalization of Alexa-488-conjugated transferrin (green) or FMDV (red) for 20 min in the presence of sucrose. Mock-treated cells were treated as described above in the absence of sucrose (B and D). Sucrose treatment inhibited uptake of both transferrin and FMDV (C and E). (F and G). Expression of AP180C inhibits infection by FMDV. SW480-αvβ6 cells were transfectedto express c-myc-tagged AP180C and subsequently infected with FMDV (see Materials and Methods). Transfected and/or infected cells were detected using the confocal microscope. Transfected cells (green) were detected using the anti-c-myc antibody, and infected cells (red) were detected with a rabbit polyclonal serum to FMDV (see Materials and Methods). The nuclei were stained with DAPI and are shown as blue. Expression of AP180C inhibited infection, as the transfected cells showed a reduced infection compared with the untransfected cells. Bars, •••.
(iv)Binding and uptake of transferrin.
The cells were washed and incubated in serum-free DMEM. Alexa-conjugated transferrin (10 μg/ml) was bound to the cells for 45 min at 4°C. Excess unbound transferrin was removed by washing and transferrin uptake initiated by incubation at 37°C. The cells were fixed and processed for microscopy as described above. In the experiments shown in Fig. 2 and 6, the prebinding step at 4°C was omitted and transferrin was added directly to the cells at 37°C.
FIG. 6.
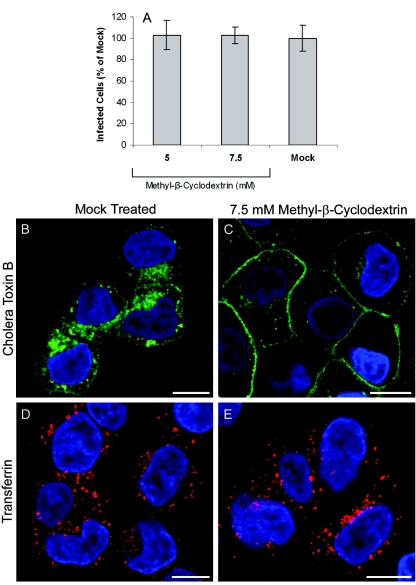
MβCD does not inhibit FMDV infection. (A) SW480-αvβ6 cells were treated with MβCD in DMEM for 0.5 h and infected with FMDV (MOI of ∼0.3) for 0.5 h in the presence of the drug, and infection was quantified as described for Fig. 1. The mock-treated cells were treated with DMEM. The number of infected cells in the drug-treated samples was expressed as a percentage of the number of infected cells in mock-treated samples. The means and standard deviations from three observations are shown. (B to E) Effect on the cellular uptake of CTB or transferrin following cell treatment with 7.5 mM MβCD. SW480-αvβ6 cells were treated with MβCD (C and E) prior to the internalization of Alexa-488-conjugated CTB (green) or Alexa-568-conjugated transferrin (red) for 20 min in the presence of the drug. Mock-treated cells (B and D) were treated with DMEM (see Materials and Methods). Internalization of transferrin was not inhibited by MβCD (E). In contrast, internalization of CTB was inhibited by MβCD (C). The nuclei were stained with DAPI and are shown as blue. Bars, 10 μm.
(v) Uptake of CTB.
Alexa-488-conjugated CTB (0.2 μg/ml) in serum-free DMEM was added to the cells at 37°C. The cells were fixed and processed for microscopy as described above.
(vi) Surface labeling of CTB.
CTB at the cell surfaces was visualized by antibody cross-linking of Alexa-488-conjugated CTB. Cells at 4°C were incubated sequentially with fluorescent CTB (0.2 μg/ml) and a rabbit anti-cholera toxin antibody for 45 min each. The cells were fixed and processed for microscopy as described in “Detection of surface antigen” above.
(vii) Acridine orange staining.
Cells on coverslips were treated with concanamycin A (100 nM) for 0.5 h at 37°C. Mock-treated cells were treated with DMSO (see above). The cells were then incubated with acridine orange (0.5 μg/ml in DMEM) in the presence or absence (mock treatment) of the drug for a further 15 min. The cells were washed in the cold with PBS and mounted and sealed as described above except that PBS was used in place of Vectashield. The cells were visualized immediately on a confocal microscope (λex = 488), collecting fluorescent emission in the green (490 to 550 nm) and red (590 to 640 nm) regions of the spectrum.
Flow cytometry.
Virus binding and αvβ6 expression on SW480-αvβ6 cells were determined by flow cytometry as described previously (42). The cells were pretreated with either DMSO (mock) or concanamycin A (100 nM) for 0.5 h and the cells prepared for flow cytometry. Integrin expression was detected using MAb 10D5, and cell-bound virus was detected with MAb B2 and R-phycoerythrin-conjugated secondary antibodies. Background fluorescence was determined by omitting either the primary antibody or virus from the assay. Fluorescent staining was analyzed by flow cytometry using a FACSCalibur (Becton Dickinson), counting 6,000 cells per sample.
RNA extraction and reverse transcription-PCR.
RNA was extracted from the cells by using Trizol (0.5 ml; Invitrogen) according to the manufacturer's protocol. Random hexamers (Roche) were used to synthesize cDNA from this RNA by using Moloney murine leukemia virus reverse transcriptase (Invitrogen). A PCR was performed using this cDNA as the template, Taq polymerase (Roche), and primers for either actin or human caveolin-1 (actin primers, 5′dGAGAAGCTGTGCTACGTCGC3′ and 5′dCCAGACAGCACTGTGTTGGC3′; human caveolin-1 primers, 5′dATGTCTGGGGGCAAATACGTAGA3′ and 5′dGTAAATGCCCCAGATGAGTGCCA3′).
RESULTS
In this study, we have used inhibitors of various endocytic pathways and immunofluorescence confocal microscopy to map the early events in αvβ6-mediated infection of SW480 cells (SW480-αvβ6) by FMDV.
FMDV infection is dependent on clathrin-dependent endocytosis and active endosomal acidification.
To determine whether αvβ6-mediated infection of SW480-αvβ6 by FMDV requires virus entry into endosomes and the prevailing low pH within these compartments, we investigated the effect of reagents that either inhibit clathrin-dependent endocytosis or raise the intraendosomal pH on infection of cells. These experiments were carried out at a low MOI to favor the most efficient infectious pathway. Infection was quantified by identifying the infected cells by using antibodies to FMDV proteins (see below) and either an ELISPOT plate reader (Fig. 1) or immunofluorescence confocal microscopy (see Materials and Methods).
Treatment of cells with sucrose has been shown to inhibit clathrin-dependent endocytosis (34, 37) and block uptake and infection by viruses that use this pathway for gaining entry into cells (33, 57). Figure 2A shows that when sucrose is present during the incubation with virus, infection of SW480-αvβ6 cells by FMDV is inhibited in a dose-dependent manner. Figure 2D and E show that treatment of SW480-αvβ6 cells with 0.4 M sucrose inhibited the uptake of FMDV. To confirm that this treatment inhibited clathrin-dependent endocytosis, we also investigated the effect of sucrose on uptake of Alexa-488-labeled transferrin (a marker for clathrin-dependent endocytosis). Figure 2B and C show that under these conditions, sucrose inhibited uptake of transferrin, confirming that clathrin-dependent endocytosis was inhibited.
To confirm that FMDV infection is clathrin dependent, we determined the effect of expression of a dominant-negative version of AP180 on infection (31). AP180 binds clathrin and is required for clathrin cage assembly. The C-terminal domain contains the putative clathrin-binding site, and when expressed in mammalian cells this domain (AP180C) acts as a dominant-negative inhibitor of clathrin-dependent endocytosis (31). Cells were transfected with a c-myc-tagged AP180C, which allowed the transfected cells to be identified in the confocal microscope by using the anti-c-myc antibody (see Materials and Methods). In these experiments, the infected cells were similarly identified using a rabbit polyclonal serum which recognizes the FMDV capsid proteins. Figure 2F and G show representative cells that had been transfected with AP180C and subsequently infected with FMDV. Approximately 19% of nontransfected cells were infected with FMDV (n = 901, where n is the number of cells counted), whereas only <2% of the transfected cells were infected (n = 244), indicating that expression of AP180C had inhibited infection by ∼90%. Taken together, these data show that virus uptake and infection of SW480-αvβ6 cells by FMDV are dependent on clathrin-dependent endocytosis.
To confirm that FMDV infection requires virus entry into the acidic endosomes, we investigated the inhibitory effect of concanamycin A on FMDV infection. Concanamycin A is a potent and specific inhibitor of the vacuolar proton ATPase, and consequently treatment of cells with this reagent raises the pH within endosomes. SW480-αvβ6 cells were treated with concanamycin A for 0.5 h prior to FMDV infection (MOI of ∼0.3) for 1 h in the presence of the drug (see Materials and Methods). Under these conditions, infection was inhibited by 95% compared with the mock-treated cells, in a dose-dependent manner, reaching a maximum inhibitory effect at 100 nM (Fig. 3A). In contrast, when concanamycin A was added for 1.5 h after the virus inoculums had been removed (Fig. 3A, RC) infection proceeded normally, indicating that the drug was inhibiting an event in cell entry and not a subsequent step of intracellular virus replication.
A possible consequence of raising the pH within endosomes is to inhibit the recycling of internalized surface receptors back to the plasma membrane. Therefore, the inhibitory effect of concanamycin A on FMDV infection could have resulted from the depletion of αvβ6 from the surfaces of the cells. To rule out this possibility, we determined the effect of concanamycin A on the surface expression of αvβ6 and on virus binding to the cells. Figure 3 shows that neither the amount of αvβ6 at the cell surface (Fig. 3D and E) nor the amount of virus binding (Fig. 3B and C) was affected by concanamycin A treatment, showing that the inhibitory effect of concanamycin A on infection does not result from a lack of surface αvβ6 or virus binding to the cell.
To ensure that the concentration of drug used in the experiments described above inhibited acidification of the intracellular acidic vesicles, the cells were stained with acridine orange following treatment with or without (mock treatment) concanamycin A and visualized under a fluorescence microscope. The emission spectrum of acridine orange is dependent on its local concentration. At low concentrations it emits green fluorescence, whereas at higher concentrations the emission is red. Acridine orange is a weak base, and it is trapped and concentrated within acidic endosomes due to protonation and thereby emits red fluorescence, which is used frequently as a marker for these compartments (3, 4, 85). Figure 4A shows SW480-αvβ6 cells that had been incubated with acridine orange. The figure shows an overlay of the red and green fluorescences, and the acidic vesicles are seen as a granular orange-yellow fluorescence. These structures were not seen in cells treated with 100 nM concanamycin A (Fig. 4B), confirming that at this concentration, concanamycin A inhibited endosomal acidification.
FIG. 4.
Concanamycin A raises the intraendosomal pH. SW480-αvβ6 cells, prepared on coverslips, were treated with either concanamycin A (100 nM) or DMSO (mock treated) for 0.5 h at 37°C. The cells were then incubated with acridine orange and visualized with the confocal microscope (see Materials and Methods). Fluorescence emission was collected for green and red regions of the spectrum. The figure shows an overlay of the green and red fluorescence. Panel A shows the intracellular acidic vesicles (orange labeling) in the mock-treated cells. This staining was completely abolished in the concanamycin A treated cells (B), showing that the intraendosomal pH had been raised. Bars, 10 μm.
The studies described above show that FMDV infection is dependent on clathrin-dependent endocytosis and active endosomal acidification and strongly suggest that during cell entry, FMDV is taken up into the acidic endosomes. To further understand the route of infection followed by FMDV, we investigated the effect of nocodazole and wortmannin, which interfere with vesicular trafficking through the endosomal pathway, on infection. Nocodazole treatment causes the depolymerization of the microtubules and inhibits trafficking between early and late endosomes. At low concentrations, wortmannin is a specific inhibitor of phosphatidylinositol 3-kinase. In many animal cell types, although it is not required for receptor uptake into early endosomes, phosphatidylinositol 3-kinase activity is required for both endosomal fusion and membrane trafficking, and as with nocodazole, wortmannin has been shown to arrest vesicular transport between the early and late endosomes (25, 46-48, 72). Pretreatment of SW480-αvβ6 cells with either 40 μM nocodazole or 200 to 25 nM wortmannin (Fig. 5) did not inhibit infection suggesting that delivery of FMDV to the late endosomal compartments (the late endosomes and lysosomes) is not required for a productive infection. At the concentration used, nocodazole was shown to disrupt the microtubules as revealed by indirect immunofluorescence confocal microscopy (data not shown).
FIG. 5.
Nocodazole and wortmannin do not inhibit FMDV infection. SW480-αvβ6 cells were pretreated with nocodazole or wortmannin (see Materials and Methods) and infected with FMDV (MOI of ∼0.3). Infection was quantified as described for Fig. 1. The number of infected cells in the drug-treated samples was expressed as a percentage of the number of infected cells in mock-treated samples. The means and standard deviations from three observations are shown.
FMDV infection is not inhibited by methyl-β-cyclodextrin.
A number of different endocytic pathways, including uptake mediated by lipid rafts and caveolae, are dependent on cholesterol. MβCD binds cholesterol and depletes it from the plasma membrane, thereby disrupting lipid rafts and impairing the endocytic pathways (including caveola-dependent endocytosis) that are dependent on their integrity. Therefore, to investigate the role of cholesterol in αvβ6-mediated infection, we determined the effect of MβCD on infection of SW480-αvβ6 cells by FMDV. Figure 6A shows that when MβCD is present before and during the incubation with virus, FMDV infection is not inhibited. Similar results were obtained using other cholesterol-depleting reagents (nystatin and filipin), as these reagents also did not inhibit infection (data not shown).
To confirm that at the concentrations used in the study described above MβCD resulted in disruption of raft-dependent endocytosis, we investigated the effect of MβCD on the uptake of Alexa-Fluor-488-labeled CTB by immunofluorescence confocal microscopy. CTB binds to GM-1 gangliosides which are enriched in lipid rafts, and consequently, for a number of different cell types CTB enters cells predominantly via lipid raft-dependent endocytosis (36, 56, 67, 75, 77). Lipid rafts are also enriched for cholesterol, and depletion of cholesterol from rafts by use of MβCD disrupts raft integrity and inhibits raft-mediated endocytic pathways. For some cell types, MβCD has been reported to inhibit clathrin-dependent endocytosis (73) in a dose-dependent manner. Therefore, we also investigated the effect of MβCD on uptake of Alexa-Fluor-568-labeled transferrin, a marker for clathrin-dependent endocytosis.
Figure 6B and D show that both Alexa-labeled CTB (green) and Alexa-labeled transferrin (red) are taken up by SW480-αvβ6 cells. Figure 6C shows that uptake of CTB is inhibited by treatment with MβCD, indicating that raft-dependent endocytosis had been inhibited. In contrast, Fig. 6E shows that clathrin-dependent endocytosis is not affected by this treatment, as uptake of transferrin was not inhibited. These data indicate that FMDV infection of SW480-αvβ6 cells is not dependent on endocytic pathways that require intact lipid rafts.
Immunofluorescence confocal microscopy studies of FMDV endocytosis.
The studies described above showed that αvβ6-mediated infection of SW80-αvβ6 cells is dependent on clathrin-dependent endocytosis and active endosomal acidification, but not on intact lipid rafts, and suggest that FMDV most likely infects its host cell following virus uptake into the early or recycling endosomes. These experiments were carried out using a low multiplicity of infecting virus per cell to limit the possibility of virus uptake (and hence infection) proceeding via endocytic pathways that are not normally used by FMDV. To verify that on entry, FMDV is taken up in to endosomes, we investigated the intracellular trafficking of virus at early times postentry by using indirect immunofluorescence confocal microscopy and MAb D9 (which recognizes VP1 and hence both intact virions and viral pentamers). For these experiments we used virus that had been purified by sucrose gradient sedimentation, and to visualize the virus during entry, the amount of virus used was necessarily large (see Materials and Methods).
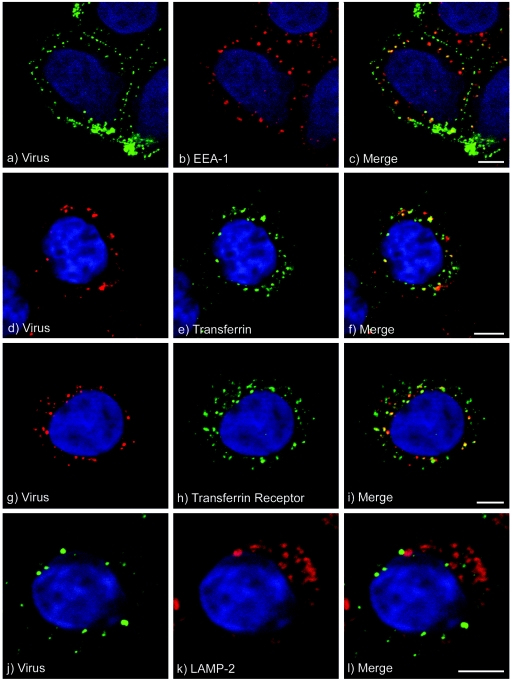
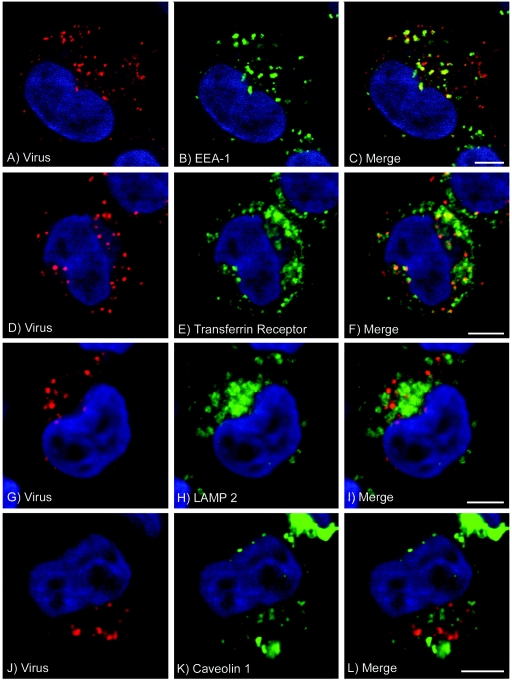
Figure 7 shows that at 37°C, FMDV is rapidly internalized into the cell. During the first 15 min of entry, virus was seen in vesicular structures, the majority of which appeared to be located at the periphery of the cell with a distribution that resembles that of early endosomes (Fig. 7a to c). At 0.5 h postentry, virus was seen to accumulate in larger vesicles (Fig. 7d). To identify these structures, we colocalized virus with markers for specific endosomal compartments. Figure 8a to c show that at 5 min postentry, virus was colocalized with labeling for EEA-1, a marker for early endosomes. Similarly, at this time point virus was colocalized with fluorescence-labeled transferrin, a marker for clathrin-dependent endocytosis (Fig. 8d to f). At 10 min postentry, the extent of virus colocalization with EEA-1 was similar to that at 5 min, whereas the extent of virus colocalization with labeled transferrin was increased (data not shown). At 15 min postentry, virus was seen to be colocalized with labeling for transferrin (data not shown) and the transferrin receptor (Fig. 8g to i), a marker for early and recycling endosomes. At 30 min postentry, virus was no longer colocalized with early endosomes (data not shown). We did not detect virus in vesicles that were labeled for LAMP-2, a marker for lysosomes, at 5 min and 10 min (data not shown) and 30 min (Fig. 8j to l) postentry. These data are consistent with the transit of FMDV through the early and recycling endosomes and support our conclusion that for infection, virus is taken up into endosomes but does not require transport to the late endosomes and lysosomes. However, although we did not detect virus in the lysosomes, we cannot rule out that virus is also transported to these compartments and is rapidly degraded, thereby preventing its detection by microscopy.
FIG. 7.
FMDV entry into SW480-αvβ6 cells. SW480-αvβ6 cells, prepared on coverslips, were incubated with FMDV at 4°C for 1 h, washed to remove excess unbound virus, and shifted to 37°C. Virus that had entered the cells was detected using the confocal microscope. Shown is labeling (in white) for FMDV after incubation at 37°C for (a) 5, (b) 10, (c) 15, and (d) 30 min. The nuclei were stained with DAPI and are shown as grey. Bars, 5 μm.
FIG. 8.
Endocytosis of FMDV by SW480-αvβ6 cells. FMDV colocalization with cellular compartments was observed using immunofluorescence confocal microscopy. SW480-αvβ6 cells, prepared on coverslips, were incubated with FMDV at 4°C for 1 h, washed to remove excess unbound virus, and shifted to 37°C. Intracellular virus was detected using MAb D9, and cellular compartments were detected with either compartment-specific antibodies or Alexa-Fluor-488-labeled-transferrin (see Materials and Methods). The cell nuclei are shown in blue. Panels a to f, g to i, and j to l show virus at 5, 15, and 30 min postentry, respectively. Panel a shows virus (green), and panel b shows early endosomes (red) labeled with EEA-1. Panel c shows an overlay of panels a and b. Panel d shows virus (red), and panel e shows transferrin (green). Panel f shows an overlay of panels d and e. Panel g shows virus (red), and panel h shows the transferrin receptor (green). Panel i shows an overlay of panels g and h. Panel j shows virus (green), and panel k shows the lysosomes (red) labeled using the LAMP-2 antibody. Panel l shows an overlay of panels j and k. Virus was colocalized with early endosomes (c), transferrin (f), and transferrin receptor-positive compartments (i) but not with LAMP-2-positive lysosomes (l). Bars, 5 μm.
It is possible that the inhibitory effect of concanamycin A (Fig. 3) on infection may have resulted from inhibition of FMDV endocytosis (see Discussion). Therefore, to understand better the effect of concanamycin A on infection, we investigated the intracellular trafficking of FMDV in concanamycin A-treated cells. This study showed that the inhibitory effect of concanamycin A on infection is primarily due to its effect on endosomal pH, as in cells treated with this reagent, the majority of the internalized virus was still colocalized in EEA-1- and transferrin receptor-positive compartments (data not shown), indicating that entry of FMDV proceeds normally in concanamycin A-treated cells.
SW480-αvβ6 cells do not express caveolin-1.
During the experiments described above, we investigated the colocalization of FMDV with caveolae by using antibodies specific for caveolin-1, a marker for caveolae and caveosomes. However, using a number of different antibodies, we were unable to detect fluorescent labeling for caveolin-1 (data not shown). This observation prompted us to investigate expression of caveolin-1 in SW480-αvβ6 cells by using PCR. For this study, CHO cells were included as a positive control, as they are known to express caveolin-1. Figure 9 shows that a PCR product was obtained for actin when using cDNAs prepared from both SW480-αvβ6 and CHO cells, whereas a product for caveolin-1 was detected only for CHO cells. These data suggest that SW480-αvβ6 cells do not express caveolin-1 and hence would not be expected to have caveolae (32, 50).
FIG. 9.
SW480-αvβ6 cells do not express caveolin-1. Reverse transcription-PCR was carried out for the presence of actin or caveolin-1 mRNA in CHO and SW480-αvβ6 cells. A PCR product (∼275 bp) was detected for actin in both cell lines (lanes 2 and 4). In contrast, a PCR product (∼350 bp) for caveolin-1 was detected for CHO cells (lane 3) but not for SW480-αvβ6 cells (lane 1). Lane M, size markers.
αvβ6-mediated infection of CHO cells is dependent on the acidic endosomes but not caveolae.
Although exposure of FMDV to the neutral pH within caveolae would not be expected to trigger the acid-dependent capsid disassembly that is required for a productive infection, the studies described above do not rule out the possibility that FMDV infection may occur through caveolae on cells other than SW480 cells, which express caveolin-1. Therefore, we investigated the endocytic uptake of FMDV by using a CHO cell line also transfected to express αvβ6. On these cells (CHO-αvβ6), αvβ6 has previously been shown to serve as the major receptor for virus binding and infection (43).
Immunofluorescence studies using CHO-αvβ6 cells showed that on virus uptake, FMDV entered early endosomes (and possibly recycling endosomes), as virus was found in EEA-1- and transferrin receptor-positive vesicles (Fig. 10A to F). In contrast, virus was not detected within LAMP-2- or caveolin-1-positive vesicles, which are markers for lysosomes and caveolae, respectively (Fig. 10G to L, confirming that internalized virus did not enter these vesicles. The pH dependency of infection of these cells was confirmed by showing that infection is inhibited by concanamycin A (100 nM) (data not shown). Taken together, these data suggest that on cells which express caveolin-1, αvβ6-mediated infection by FMDV is also dependent on virus uptake into an acidic vesicle and not on caveolae.
FIG. 10.
Endocytosis of FMDV by CHO-αvβ6 cells. FMDV colocalization with cellular compartments was observed using immunofluorescence confocal microscopy. CHO-αvβ6 cells, prepared on coverslips, were incubated with FMDV at 4°C for 1 h, washed to remove excess unbound virus, and shifted to 37°C. Intracellular virus was detected using MAb D9, and cellular compartments were detected with compartment-specific antibodies. The cell nuclei are shown in blue. Panels A to C and D to L shows virus colocalization at 10 min and 30 min postentry, respectively. FMDV labeling is shown in red, and cellular compartments are shown in green. Panel A shows virus, and panel B shows early endosomes (EEA-1). Panel C shows an overlay of panels A and B. Panel D shows virus, and panel E shows the transferrin receptor. Panel F shows an overlay of panels D and E. Panel G shows virus, and panel H shows LAMP-2-positive lysosomes. Panel I shows an overlay of panels G and H. Panel J shows virus, and panel K shows labeling for caveolin-1 (a marker for caveolae). Panel L shows an overlay of panels J and K. Virus was colocalized with early endosomes (C) and transferrin receptor-positive compartments (F) but not with LAMP-2-positive lysosomes (I) or caveolin-1. Bars, 5 μm.
Role of αvβ6 in infection.
The integrin αvβ6 has been shown to serve as the receptor for virus attachment on SW480-αvβ6 cells, but it is not known whether it plays any further role in the entry process. To gain a better understanding of the role of αvβ6 in infection, we colocalized FMDV with αvβ6 at early times postentry. Figure 11A to C show that FMDV is extensively colocalized with αvβ6 at the cell surface. At 5 min postentry, FMDV and αvβ6 were extensively colocalized, suggesting that the integrin and virus had been taken up into the same intracellular compartment(s) (Fig. 11D to F). Figure 11G to L also show that FMDV was not associated with CTB-labeled rafts, supporting our conclusion that FMDV does not require lipid rafts for infection. Rafts are small, and consequently raft resident molecules often appear to be diffusely distributed over the cell surface rather that present as a punctate pattern (64). Therefore, to aid raft identification, the rafts were visualized as puncta by cross-linking Alexa-conjugated CTB at the cell surface.
FIG. 11.
Colocalization of FMDV with αvβ6. Colocalization of FMDV with αvβ6 was observed using immunofluorescence confocal microscopy. SW480-αvβ6 cells, prepared on coverslips, were incubated with FMDV and, where appropriate, Alexa-488 conjugated CTB at 4°C for 1 h and washed to remove excess unbound ligands. Surface virus, integrin, and Alexa-488-conjugated CTB (a marker for lipid rafts) were detected as described in Materials and Methods (“Detection of surface antigen”). Internalized virus and integrin were detected following incubation of the cells at 37°C for 5 min as described in Materials and Methods (“Detection of intracellular antigen”). FMDV was detected using MAb D9, and αvβ6 was detected with MAb E7P6. The cell nuclei are shown in blue. Panel A shows αvβ6 (red), and panel B shows FMDV (green) at the cell surface. Panel C shows an overlay of panels A and B. Panel D shows αvβ6 (red), and panel E shows FMDV (green) at 5 min post entry. Panel F shows an overlay of panels D and E. FMDV and αvβ6 showed extensive colocalization at the cell surface (C) and inside the cell (F). Panel G shows CTB (green), and panel H shows FMDV (red) at the cell surface. Panel I shows an overlay of panels G and H. Panels J, K, and L show an enlargement of an area of the cell membrane (boxed) taken from panels G, H, and I, respectively. FMDV showed little colocalization with CTB (I and L). Bars, 5 μm.
To verify that on FMDV uptake, αvβ6 also enters endosomes, we performed experiments involving colocalization of αvβ6 with either EEA-1 or the transferrin receptor (Fig. 12). In the absence of FMDV, the labeling for αvβ6 was relatively weak, and the integrin was present at the cell surface and distributed in small vesicles throughout the cytosol which showed little or no colocalization with either EEA-1 (Fig. 12A to C) or the transferrin receptor (Fig. 12G to I). However, in the presence of virus, the integrin labeling was mostly contained within discrete cellular vesicles that colocalized with both EEA-1 (Fig. 12D to F) and the transferrin receptor (Fig. 12J to L). These data suggest that FMDV binding to αvβ6 stimulates endocytosis of the integrin and that the integrin serves to deliver the virus into the early and recycling endosomes. This virus-induced uptake of αvβ6 may have resulted from ligation of the RGD-binding site, from cross-linking of the integrin by a multivalent ligand such as FMDV, or from a combination of both ligation and integrin cross-linking. In order to better understand the mechanism of virus-induced integrin uptake, we repeated these experiments using an RGD-containing peptide (with its sequence derived from the RGD-containing loop of FMDV) in place of whole virus. Previously, this peptide has been shown to bind αvβ6, as it inhibits virus binding to SW480-αvβ6 cells (44). This experiment showed that cross-linking of the integrin is most likely required to stimulate its uptake, as in the presence of the peptide, αvβ6 did not colocalize with EEA-1 (data not shown), suggesting that ligation of the RGD-binding site alone is insufficient to stimulate endocytosis of αvβ6.
FIG. 12.
FMDV induces endocytosis of αvβ6. Colocalization was observed using immunofluorescence confocal microscopy. SW480-αvβ6 cells, prepared on coverslips, were incubated with or without FMDV at 4°C for 1 h, washed to remove excess unbound virus, and shifted to 37°C for 10 min to allow for virus internalization. The integrin αvβ6 (red) was detected using the rabbit MAb 4B5, and cellular compartments (green) were detected with compartment-specific antibodies. The cell nuclei are shown in blue. Panels A to C show labeling for EEA-1 and αvβ6, and panels G to I show labeling for αvβ6 and the transferrin receptor in the absence of virus. Panels D to F show labeling for EEA-1 and αvβ6, and panels J to L show labeling for αvβ6 and the transferrin receptor following 10 min of incubation in the presence of virus. Panels A, D, G, and J show αvβ6 (red). Panels B and E shows early endosomes (EEA-1) (green). Panels H and K shows transferrin receptor-positive compartments (green). Panel C shows an overlay of panels A and B. Panel F shows an overlay of panels D and E. Panel I shows an overlay of panels G and H. Panel L shows an overlay of panels J and K. Little or no colocalization is seen for αvβ6 with early endosomes or the transferrin receptor in the absence of virus (C and I). In the presence of virus, αvβ6 is colocalized with early endosomes or the transferrin receptor (F and L). Bars, 5 μm.
DISCUSSION
The events that lead to uncoating of the vRNA genome have been studied for a number of picornaviruses (see the introduction); however, the identities of the cellular membranes penetrated by the different picornaviruses are less well understood.
FMDV has been shown to use a number of αv integrins, including αvβ6, as receptors to initiate infection of cultured cells. In this study we have used a β6-transfected cell line (SW480-αvβ6) to investigate the early events in αvβ6-mediated infection by FMDV. We have shown that infection of SW480-αvβ6 cells is dependent on clathrin-dependent endocytosis, as virus uptake and infection are blocked by reagents (sucrose and a dominant-negative AP180C) that inhibit this endocytic pathway. In contrast, infection was shown not to be dependent on caveolae or other endocytic pathways that depend on lipid rafts. These observations were made using a low multiplicity of infecting virus per cell to encourage the use of the most efficient uptake pathway and suggest that for a productive infection to occur, FMDV must be delivered to endosomes by clathrin-dependent endocytosis.
We have also shown that blocking endosomal acidification with concanamycin A inhibited an early step in infection. Blocking endosomal acidification with concanamycin A inhibited infection but did not interfere with surface expression of αvβ6 (the receptor used by FMDV to infect these cells), virus binding to the cells, uptake of the virus into endosomes, or the cytoplasmic replication of the virus. Taken together, these experiments suggest that for a productive infection to occur, FMDV must not only be taken up into the endosomes but also exposed to the prevailing low pH within these compartments.
The endosomal route of infection was supported by our immunofluorescence confocal microscopy studies. FMDV was shown to colocalize with αvβ6 at the cell surface but not with the B subunit of cholera toxin, a marker for lipid rafts. At 37°C, virus was rapidly taken up into the cells and colocalized with markers for early and recycling endosomes but not with a marker for the lysosomes. Although we cannot rule out that the virus is transported to the lysosomes and rapidly degraded, our data suggest that the majority of the internalized virus is not transported to these compartments. This conclusion was supported by showing that FMDV infection was not inhibited by treatment of the cells with nocodazole or wortmannin, drugs that arrest vesicular transport between early and late endosomes. Taken together, these observations suggest that the low pH within endosomes is a prerequisite for delivery of the vRNA into the cytosol and that this step occurs at an early stage of the entry process. Although we have not yet identified the specific compartment in which virus uncoating and vRNA translocation occur, the extreme sensitivity of the FMDV capsid to low pH implies that virus uncoating would most likely occur in the early endosomes, i.e., before the virus has reached the recycling compartments.
Infection by two other picornaviruses, HPEV-1 (45) and HRV-2 (66, 68, 73), has also been shown to require the endocytic uptake of the virus. Similar to that of FMDV, the vRNA of HPEV-1 is thought to be released and translocated into the cytosol at an early stage of the entry process, as infection is not inhibited by nocodazole, which trapped the virus within the early endosomes. By contrast, infection by HRV-2 requires virus transport to the late endosomes, as conversion to A particles (and hence infection; see the introduction) requires virus exposure to a pH lower (∼5.6) than that within the early endosomes. These studies reported that infection by HRV-2 is sensitive to bafilomycin. Like concanamycin A, bafilomycin is a potent and specific inhibitor of the vacuolar proton ATPase, and consequently treatment of cells with bafilomycin raises the pH within endosomes (27). Although infection by HRV-2 is dependent on the acidic pH within endosomes, the inhibitory effect of bafilomycin on infection could not be attributed solely to its effect on the endosomal pH, as bafilomycin was found to arrest transport of the virus in early endosomes, thereby preventing the virus from being exposed to a pH sufficiently low for infection. Our results with FMDV have shown that the inhibitory effect of concanamycin A on infection is primarily due to its effect of raising the endosomal pH, as this reagent did not interfere with FMDV endocytosis or virus trafficking to the early and recycling endosomes.
We have shown that αvβ6-mediated infection of SW480 cells by FMDV is not dependent on cellular cholesterol-rich lipid rafts, as the cholesterol-depleting agent MβCD did not inhibit infection. Similarly, our studies have shown that αvβ6-mediated infection does not require caveola-dependent endocytosis, as SW480 cells were found not to express caveolin-1, which is an essential component for the formation of caveolae. Although exposure of FMDV to the neutral pH within caveolae (and hence caveosomes) would not be expected to trigger the acid-dependent capsid disassembly that is required for a productive infection, these studies do not rule out the possibility that FMDV infection may occur through caveolae on other cell types which posses these structures. To confirm that FMDV infection does not require virus to be taken up into caveolae, we used immunofluorescence confocal microscopy to investigate the endocytic uptake of FMDV by CHO cells expressing αvβ6 as the major receptor for infection. These studies showed that as for SW480-αvβ6 cells, internalized virus entered early endosomes and compartments that are positive for the transferrin receptor (early and recycling endosomes) but did not enter the lysosomes. Importantly, these cells express caveolin-1 (Fig. 9) and therefore have caveolae, and upon entry virus was not seen to enter these compartments. The role for acidic endosomes in infection of CHO-αvβ6 cells by FMDV was confirmed by showing that infection of these cells is blocked by inhibiting the vacuolar ATPase with concanamycin A. Taken together, these studies suggest that in cells which express caveolin-1, infection by FMDV is also dependent on virus uptake into an acidic vesicle and not on caveolae.
Although poliovirus infection is thought not to be dependent on caveolae (see the introduction), recent studies by Danthi and Chow (22) have shown that poliovirus infection is dependent on cholesterol. However, as neither the poliovirus receptor nor viral capsid proteins were shown to localize in lipid rafts, those authors concluded that poliovirus infection was not dependent on the integrity of the rafts but rather that cholesterol may be required for transfer of the vRNA into the cytosol. Our studies have shown that infection by FMDV is not dependent on cholesterol, suggesting that the molecular mechanism of vRNA translocation into the cytosol may differ for FMDV and poliovirus or that the genomes of these viruses may enter the cytosol by penetration through different cellular membranes.
In previous studies, αvβ6 had been shown to serve as an attachment receptor for FMDV, but it was not known whether it has a role in the subsequent steps in cell entry. In the present study, we have shown that virus labeling was colocalized with αvβ6 inside the cell after 5 min of incubation at 37°C. This observation shows that αvβ6 and the virus were present in the same endocytic vesicle and suggests that the integrin may serve to deliver the virus to the endosomes. This conclusion was supported by showing that on virus binding and entry, the distribution of αvβ6 changed and the integrin accumulated in the early and recycling endosomes, which is again suggestive of virus binding leading to the endocytosis of αvβ6. This redistribution of αvβ6 was not observed on binding of an RGD-containing peptide, suggesting that ligation of the RGD-binding site alone is insufficient to induce integrin internalization and that cross-linking of the integrin is also required. These observations are consistent with those of Weinreb et al. (81), who have shown that αvβ6 is internalized following binding of a number of different ligand-mimetic, anti-αvβ6 antibodies. Taken together, these observations suggest that the integrin serves not only for virus attachment but also to deliver the virus to the early endosomes and that virus binding induces the endocytosis of αvβ6.
In summary, our data are consistent with a model for FMDV cell entry involving virus-induced, clathrin-dependent endocytosis of an FMDV-αvβ6 complex into the early and recycling endosomes, where the prevailing low pH triggers capsid disassembly and the translocation of the vRNA into the cytosol. These results will lead to future studies to identify the host proteins that are required for virus endocytosis and delivery of the vRNA into the cytosol.
Acknowledgments
Stephen Berryman was supported by an IAH studentship.
We thank Harvey McMahon (Laboratory of Molecular Biology, Neurobiology Division, Cambridge, United Kingdom) for providing AP180C.
REFERENCES
- 1.Acharya, R., E. Fry, D. Stuart, G. Fox, D. Rowlands, and F. Brown. 1989. The three-dimensional structure of foot-and-mouth disease virus at 2.9 A resolution. Nature 337:709-716. [DOI] [PubMed] [Google Scholar]
- 2.Alexandersen, S., Z. Zhang, A. I. Donaldson, and A. J. Garland. 2003. The pathogenesis and diagnosis of foot-and-mouth disease. J. Comp. Pathol. 129:1-36. [DOI] [PubMed] [Google Scholar]
- 3.Altan, N., Y. Chen, M. Schindler, and S. M. Simon. 1998. Defective acidification in human breast tumor cells and implications for chemotherapy. J. Exp. Med. 187:1583-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altan, N., Y. Chen, M. Schindler, and S. M. Simon. 1999. Tamoxifen inhibits acidification in cells independent of the estrogen receptor. Proc. Natl. Acad. Sci. USA 96:4432-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arita, M., S. Koike, J. Aoki, H. Horie, and A. Nomoto. 1998. Interaction of poliovirus with its purified receptor and conformational alteration in the virion. J. Virol. 72:3578-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arita, M., S. Ohka, Y. Sasaki, and A. Nomoto. 1999. Multiple pathways for establishment of poliovirus infection. Virus Res. 62:97-105. [DOI] [PubMed] [Google Scholar]
- 7.Baravalle, G., M. Brabec, L. Snyers, D. Blaas, and R. Fuchs. 2004. Human rhinovirus type 2-antibody complexes enter and infect cells via Fc-gamma receptor IIB1. J. Virol. 78:2729-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxt, B. 1987. Effect of lysosomotropic compounds on early events in foot-and-mouth disease virus replication. Virus Res. 7:257-271. [DOI] [PubMed] [Google Scholar]
- 9.Baxt, B., and H. L. Bachrach. 1982. The adsorption and degradation of foot-and-mouth disease virus by isolated BHK-21 cell plasma membranes. Virology 116:391-405. [DOI] [PubMed] [Google Scholar]
- 10.Baxt, B., and H. L. Bachrach. 1980. Early interactions of foot-and-mouth disease virus with cultured cells. Virology 104:42-55. [DOI] [PubMed] [Google Scholar]
- 11.Bayer, N., D. Schober, M. Huttinger, D. Blaas, and R. Fuchs. 2001. Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry. J. Biol. Chem. 276:3952-3962. [DOI] [PubMed] [Google Scholar]
- 12.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blaas, and R. Fuchs. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72:9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belnap, D. M., D. J. Filman, B. L. Trus, N. Cheng, F. P. Booy, J. F. Conway, S. Curry, C. N. Hiremath, S. K. Tsang, A. C. Steven, and J. M. Hogle. 2000. Molecular tectonic model of virus structural transitions: the putative cell entry states of poliovirus. J. Virol. 74:1342-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belnap, D. M., B. M. McDermott, Jr., D. J. Filman, N. Cheng, B. L. Trus, H. J. Zuccola, V. R. Racaniello, J. M. Hogle, and A. C. Steven. 2000. Three-dimensional structure of poliovirus receptor bound to poliovirus. Proc. Natl. Acad. Sci. USA 97:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berinstein, A., M. Roivainen, T. Hovi, P. W. Mason, and B. Baxt. 1995. Antibodies to the vitronectin receptor (integrin alpha V beta 3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J. Virol. 69:2664-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brabec, M., G. Baravalle, D. Blaas, and R. Fuchs. 2003. Conformational changes, plasma membrane penetration, and infection by human rhinovirus type 2: role of receptors and low pH. J. Virol. 77:5370-5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown, F., and B. Cartwright. 1961. Dissociation of foot-and-mouth disease virus into its nucleic acid and protein components. Nauchni Tr. Vissh. Med. Inst. Sofiia 192:1163-1164. [DOI] [PubMed] [Google Scholar]
- 18.Crowell, R. L., and L. Philipson. 1971. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J. Virol. 8:509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curry, S., C. C. Abrams, E. Fry, J. C. Crowther, G. J. Belsham, D. I. Stuart, and A. M. King. 1995. Viral RNA modulates the acid sensitivity of foot-and-mouth disease virus capsids. J. Virol. 69:430-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curry, S., M. Chow, and J. M. Hogle. 1996. The poliovirus 135S particle is infectious. J. Virol. 70:7125-7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curry, S., E. Fry, W. Blakemore, R. Abu-Ghazaleh, T. Jackson, A. King, S. Lea, J. Newman, D. Rowlands, and D. Stuart. 1996. Perturbations in the surface structure of A22 Iraq foot-and-mouth disease virus accompanying coupled changes in host cell specificity and antigenicity. Structure 4:135-145. [DOI] [PubMed] [Google Scholar]
- 22.Danthi, P., and M. Chow. 2004. Cholesterol removal by methyl-beta-cyclodextrin inhibits poliovirus entry. J. Virol. 78:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Diego, M., E. Brocchi, D. Mackay, and F. De Simone. 1997. The non-structural polyprotein 3ABC of foot-and-mouth disease virus as a diagnostic antigen in ELISA to differentiate infected from vaccinated cattle. Arch. Virol. 142:2021-2033. [DOI] [PubMed] [Google Scholar]
- 24.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17:4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Hondt, K., A. Heese-Peck, and H. Riezman. 2000. Protein and lipid requirements for endocytosis. Annu. Rev. Genet. 34:255-295. [DOI] [PubMed] [Google Scholar]
- 26.Dimitrov, D. S. 2004. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2:109-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drose, S., K. U. Bindseil, E. J. Bowman, A. Siebers, A. Zeeck, and K. Altendorf. 1993. Inhibitory effect of modified bafilomycins and concanamycins on P- and V-type adenosinetriphosphatases. Biochemistry 32:3902-3906. [DOI] [PubMed] [Google Scholar]
- 28.Duque, H., M. LaRocco, W. T. Golde, and B. Baxt. 2004. Interactions of foot-and-mouth disease virus with soluble bovine alphaVbeta3 and alphaVbeta6 integrins. J. Virol. 78:9773-9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellard, F. M., J. Drew, W. E. Blakemore, D. I. Stuart, and A. M. King. 1999. Evidence for the role of His-142 of protein 1C in the acid-induced disassembly of foot-and-mouth disease virus capsids. J. Gen. Virol. 80:1911-1918. [DOI] [PubMed] [Google Scholar]
- 30.Evans, D. J., and J. W. Almond. 1998. Cell receptors for picornaviruses as determinants of cell tropism and pathogenesis. Trends Microbiol. 6:198-202. [DOI] [PubMed] [Google Scholar]
- 31.Ford, M. G. J., B. M. F. Pearse, M. K. Higgins, Y. Vallis, D. J. Owen, A. Gibson, C. R. Hopkins, P. R. Evans, and H. T. McMahon. 2001. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291:1051-1055. [DOI] [PubMed] [Google Scholar]
- 32.Galbiati, F., B. Razani, and M. P. Lisanti. 2001. Emerging themes in lipid rafts and caveolae. Cell 106:403-411. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert, J. M., and T. L. Benjamin. 2000. Early steps of polyomavirus entry into cells. J. Virol. 74:8582-8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen, S. H., K. Sandvig, and B. van Deurs. 1993. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J. Cell Biol. 121:61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He, Y., V. D. Bowman, S. Mueller, C. M. Bator, J. Bella, X. Peng, T. S. Baker, E. Wimmer, R. J. Kuhn, and M. G. Rossmann. 2000. Interaction of the poliovirus receptor with poliovirus. Proc. Natl. Acad. Sci. USA 97:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henley, J. R., E. W. A. Krueger, B. J. Oswald, and M. A. McNiven. 1998. Dynamin-mediated Internalization of Caveolae. J. Cell Biol. 141:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heuser, J. E., and R. G. Anderson. 1989. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hewat, E. A., and D. Blaas. 2004. Cryoelectron microscopy analysis of the structural changes associated with human rhinovirus type 14 uncoating. J. Virol. 78:2935-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoover-Litty, H., and J. M. Greve. 1993. Formation of rhinovirus-soluble ICAM-1 complexes and conformational changes in the virion. J. Virol. 67:390-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang, X., J. Wu, W. Zhu, R. Pytela, and D. Sheppard. 1998. Expression of the human integrin beta6 subunit in alveolar type II cells and bronchiolar epithelial cells reverses lung inflammation in beta6 knockout mice. Am. J. Respir. Cell. Mol. Biol. 19:636-642. [DOI] [PubMed]
- 41.Huang, Y., J. M. Hogle, and M. Chow. 2000. Is the 135S poliovirus particle an intermediate during cell entry? J. Virol. 74:8757-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson, T., S. Clark, S. Berryman, A. Burman, S. Cambier, D. Mu, S. Nishimura, and A. M. King. 2004. Integrin alphavbeta8 functions as a receptor for foot-and-mouth disease virus: role of the beta-chain cytodomain in integrin-mediated infection. J. Virol. 78:4533-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson, T., A. P. Mould, D. Sheppard, and A. M. King. 2002. Integrin alphavbeta1 is a receptor for foot-and-mouth disease virus. J. Virol. 76:935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson, T., D. Sheppard, M. Denyer, W. Blakemore, and A. M. King. 2000. The epithelial integrin alphavbeta6 is a receptor for foot-and-mouth disease virus. J. Virol. 74:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joki-Korpela, P., V. Marjomaki, C. Krogerus, J. Heino, and T. Hyypia. 2001. Entry of human parechovirus 1. J. Virol. 75:1958-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joly, M., A. Kazlauskas, and S. Corvera. 1995. Phosphatidylinositol 3-kinase activity is required at a postendocytic step in platelet-derived growth factor receptor trafficking. J. Biol. Chem. 270:13225-13230. [DOI] [PubMed] [Google Scholar]
- 47.Jones, A. T., and M. J. Clague. 1995. Phosphatidylinositol 3-kinase activity is required for early endosome fusion. Biochem. J. 311:31-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones, A. T., I. G. Mills, A. J. Scheidig, K. Alexandrov, and M. J. Clague. 1998. Inhibition of endosome fusion by wortmannin persists in the presence of activated Rab5. Mol. Biol. Cell 9:323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kronenberger, P., D. Schober, E. Prchla, O. Ofori-Anyinam, R. Vrijsen, B. Rombaut, D. Blaas, R. Fuchs, and A. Boeye. 1998. Uptake of poliovirus into the endosomal system of HeLa cells. Arch. Virol. 143:1417-1424. [DOI] [PubMed] [Google Scholar]
- 50.Kurzchalia, T. V., and R. G. Parton. 1999. Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 11:424-431. [DOI] [PubMed] [Google Scholar]
- 51.Lamaze, C., A. Dujeancourt, T. Baba, C. G. Lo, A. Benmerah, and A. Dautry-Varsat. 2001. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol. Cell 7:661-671. [DOI] [PubMed] [Google Scholar]
- 52.Marjomaki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of echovirus 1 in caveolae. J. Virol. 76:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCahon, D., J. R. Crowther, G. J. Belsham, J. D. Kitson, M. Duchesne, P. Have, R. H. Meloen, D. O. Morgan, and F. De Simone. 1989. Evidence for at least four antigenic sites on type O foot-and-mouth disease virus involved in neutralization; identification by single and multiple site monoclonal antibody-resistant mutants. J. Gen. Virol. 70:639-645. [DOI] [PubMed] [Google Scholar]
- 54.Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12:575-625. [DOI] [PubMed] [Google Scholar]
- 55.Miller, L. C., W. Blakemore, D. Sheppard, A. Atakilit, A. M. King, and T. Jackson. 2001. Role of the cytoplasmic domain of the beta-subunit of integrin alpha(v)beta6 in infection by foot-and-mouth disease virus. J. Virol. 75:4158-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montesano, R., J. Roth, A. Robert, and L. Orci. 1982. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature 296:651-653. [DOI] [PubMed] [Google Scholar]
- 57.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nurani, G., B. Lindqvist, and J. M. Casasnovas. 2003. Receptor priming of major group human rhinoviruses for uncoating and entry at mild low-pH environments. J. Virol. 77:11985-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohka, S., N. Matsuda, K. Tohyama, T. Oda, M. Morikawa, S. Kuge, and A. Nomoto. 2004. Receptor (CD155)-dependent endocytosis of poliovirus and retrograde axonal transport of the endosome. J. Virol. 78:7186-7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parton, R. G. 1996. Caveolae and caveolins. Curr. Opin. Cell Biol. 8:542-548. [DOI] [PubMed] [Google Scholar]
- 61.Pelkmans, L., and A. Helenius. 2002. Endocytosis via caveolae. Traffic 3:311-320. [DOI] [PubMed] [Google Scholar]
- 62.Pelkmans, L., and A. Helenius. 2003. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 15:414-422. [DOI] [PubMed] [Google Scholar]
- 63.Perez, L., and L. Carrasco. 1993. Entry of poliovirus into cells does not require a low-pH step. J. Virol. 67:4543-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pike, L. J. 2003. Lipid rafts: bringing order to chaos. J. Lipid Res. 44:655-667. [DOI] [PubMed] [Google Scholar]
- 65.Powell, R. M., T. Ward, D. J. Evans, and J. W. Almond. 1997. Interaction between echovirus 7 and its receptor, decay-accelerating factor (CD55): evidence for a secondary cellular factor in A-particle formation. J. Virol. 71:9306-9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prchla, E., E. Kuechler, D. Blaas, and R. Fuchs. 1994. Uncoating of human rhinovirus serotype 2 from late endosomes. J. Virol. 68:3713-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schnitzer, J. E., P. Oh, and D. P. McIntosh. 1996. Role of GTP hydrolysis in fission of caveolae directly from plasma membranes. Science 274:239-242. [DOI] [PubMed] [Google Scholar]
- 68.Schober, D., P. Kronenberger, E. Prchla, D. Blaas, and R. Fuchs. 1998. Major and minor receptor group human rhinoviruses penetrate from endosomes by different mechanisms. J. Virol. 72:1354-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shafren, D. R., D. J. Dorahy, S. J. Greive, G. F. Burns, and R. D. Barry. 1997. Mouse cells expressing human intercellular adhesion molecule-1 are susceptible to infection by coxsackievirus A21. J. Virol. 71:785-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. [DOI] [PubMed] [Google Scholar]
- 71.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 72.Simonsen, A., R. Lippe, S. Christoforidis, J. M. Gaullier, A. Brech, J. Callaghan, B. H. Toh, C. Murphy, M. Zerial, and H. Stenmark. 1998. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394:494-498. [DOI] [PubMed] [Google Scholar]
- 73.Snyers, L., H. Zwickl, and D. Blaas. 2003. Human rhinovirus type 2 is internalized by clathrin-mediated endocytosis. J. Virol. 77:5360-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stuart, A. D., H. E. Eustace, T. A. McKee, and T. D. Brown. 2002. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J. Virol. 76:9307-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Torgersen, M. L., G. Skretting, B. van Deurs, and K. Sandvig. 2001. Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 114:3737-3747. [DOI] [PubMed] [Google Scholar]
- 76.Tosteson, M. T., H. Wang, A. Naumov, and M. Chow. 2004. Poliovirus binding to its receptor in lipid bilayers results in particle-specific, temperature-sensitive channels. J. Gen. Virol. 85:1581-1589. [DOI] [PubMed] [Google Scholar]
- 77.Tran, D., J.-L. Carpentier, F. Sawano, P. Gorden, and L. Orci. 1987. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc. Natl. Acad. Sci. USA 84:7957-7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Triantafilou, K., and M. Triantafilou. 2003. Lipid raft microdomains: key sites for coxsackievirus A9 infectious cycle. Virology 317:128-135. [DOI] [PubMed] [Google Scholar]
- 79.Triantafilou, K., and M. Triantafilou. 2004. Lipid-raft-dependent coxsackievirus B4 internalization and rapid targeting to the Golgi. Virology 326:6-19. [DOI] [PubMed] [Google Scholar]
- 80.Weinacker, A., A. Chen, M. Agrez, R. I. Cone, S. Nishimura, E. Wayner, R. Pytela, and D. Sheppard. 1994. Role of the integrin alpha v beta 6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J. Biol. Chem. 269:6940-6948. [PubMed] [Google Scholar]
- 81.Weinreb, P. H., K. J. Simon, P. Rayhorn, W. J. Yang, D. R. Leone, B. M. Dolinski, B. R. Pearse, Y. Yokota, H. Kawakatsu, A. Atakilit, D. Sheppard, and S. M. Violette. 2004. Function-blocking integrin alphavbeta6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J. Biol. Chem. 279:17875-17887. [DOI] [PubMed] [Google Scholar]
- 82.Willingmann, P., H. Barnert, H. Zeichhardt, and K. O. Habermehl. 1989. Recovery of structurally intact and infectious poliovirus type 1 from HeLa cells during receptor-mediated endocytosis. Virology 168:417-420. [DOI] [PubMed] [Google Scholar]
- 83.Xing, L., K. Tjarnlund, B. Lindqvist, G. G. Kaplan, D. Feigelstock, R. H. Cheng, and J. M. Casasnovas. 2000. Distinct cellular receptor interactions in poliovirus and rhinoviruses. EMBO J. 19:1207-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zeichhardt, H., K. Wetz, P. Willingmann, and K. O. Habermehl. 1985. Entry of poliovirus type 1 and Mouse Elberfeld (ME) virus into HEp-2 cells: receptor-mediated endocytosis and endosomal or lysosomal uncoating. J. Gen. Virol. 66:483-492. [DOI] [PubMed] [Google Scholar]
- 85.Zelenin, A. V. 1993. Acridine orange as a probe for molecular and cell biology, p. 83-99. In W. T. Mason (ed.), Fluorescent and luminescent probes for biological activity. Academic Press Inc., San Diego, Calif.