Abstract
Respiratory syncytial virus (RSV) is the most important cause of lower respiratory tract disease in infants and children. To study RSV replication, we have developed an in vitro model of human nasopharyngeal mucosa, human airway epithelium (HAE). RSV grows to moderate titers in HAE, though they are significantly lower than those in a continuous epithelial cell line, HEp-2. In HAE, RSV spreads over time to form focal collections of infected cells causing minimal cytopathic effect. Unlike HEp-2 cells, in which wild-type and live-attenuated vaccine candidate viruses grow equally well, the vaccine candidates exhibit growth in HAE that parallels their level of attenuation in children.
Respiratory syncytial virus (RSV) causes severe respiratory illness in infancy (10) and recurrent upper respiratory tract infections in older children and adults (11) and is an important cause of morbidity in the elderly (7). In pathological specimens from human cases, RSV grows exclusively in epithelial cells lining the respiratory tract (17). The clinical impact of RSV must be explained in the context of growth in epithelial cells and in the innate and adaptive immune response that RSV infection initiates in the associated lymphoid tissues lining the respiratory tract.
RSV growth was examined in primary adenoid epithelial cells, human airway epithelium (HAE), derived from tissue removed at adenoidectomy. We have previously described our HAE model and its support of the growth of influenza virus (6, 18). Surgical removal of adenoids is most often performed for hypertrophy and partial airway or eustachian tube obstruction (2). Although chronic inflammatory changes are seen occasionally, the microscopic adenoid anatomy is usually normal. The adenoids have a transitional epithelium with representation of ciliated cells, Clara cells, and mucin-producing goblet cells (6). We hypothesized that examination of RSV replication in HAE would give important clues to key events in the virus replication cycle and host response to infection.
Exploration of the growth of RSV in primary respiratory cells has been largely confined to studies of tracheal organ cultures in animal species. In a bovine model, with a bovine RSV strain, growth was seen without alteration in ciliary function and with virus primarily in the subepithelium (15); nevertheless, RSV is a significant respiratory pathogen in young cattle (1). In a ferret tracheal ring RSV growth was maximal between 5 and 7 days (12). As in the bovine model, no histologic changes or diminution of ciliary activity was seen and RSV was detected on the lamina propria and serosal surface of ferret tracheal ring. In a piglet tracheal ring, destruction of ciliated cells and growth of virus over a 17- to 19-day period were observed. By fluorescence, brightly staining cells scattered in the epithelial layer were seen with no virus in the subepithelium (8). The animal models of RSV in differentiated epithelial cells and in organ cultures emphasize the need to explore the human model, as the studies show sharp dichotomies in the site of replication of RSV.
In a human tracheal ring organ culture, there were multinucleated cells with cytoplasmic inclusions and diminished ciliary activity (12). By fluorescence, RSV was confined to the superficial epithelial layer with selective infection of a single cell surrounded by uninfected cells (12). Some tracheal rings were from a fetus as young as 18 weeks, in which there should be limited development of the immune system, suggesting that the restriction in numbers and types of cells infected is not immunologically mediated. It appeared on electron microscopy that ciliary cells were infected (12). Studies of RSV histopathology in the human lung have shown only isolated cells in the bronchioles and alveoli that are infected (17). In contradistinction, extensive staining of exfoliated cells in intraluminal airway debris is seen, suggesting that infected cells may be rapidly shed into the airway (J. E. Johnson, unpublished data). A recent paper has demonstrated more uniform superficial epithelial cell localization of RSV in ciliated human airway epithelial cells using RSV expressing a green fluorescent protein (GFP) (20).
Our current studies have focused on RSV in adenoid epithelial cells and have involved quantitation of virus growth and extent and pattern of RSV infection by immunofluorescence and immunohistochemistry and correlation of level of growth in HAE of a series of live-attenuated RSV vaccine candidates with their levels of attenuation in animal models (3, 4) and adults and children (13, 19).
With the approval of the Vanderbilt Institutional Review Board, adenoids were obtained from surgeries performed for independently defined clinical indications (2). The isolation and growth of primary epithelial cells from adenoidal tissue were previously described by our group (6, 18). Cells were grown on the collagen matrix of Vitrogen 100 (Cohesion, Palo Alto, CA) at 37°C under 5% CO2 as a “submerged culture” or in Transwell collagen-coated inserts (Corning Inc., Corning, NY). The experiments were performed as soon as the cells were confluent, 8 to 14 days, at which time the ciliary cell population was approximately 10% and the cells were one to three layers thick. These cells are different in site of origin and length of time in culture (2 weeks versus 5 to 6 weeks) from the uniformly ciliated, multilayered population of cells described by Zhang et al. (20). They were used at a point when resistance was greater than 300 Ω · cm2 as measured by an epithelial volt ohmmeter (World Precision Instruments, Sarasota, FL).
Respiratory syncytial virus in primary epithelial cells.
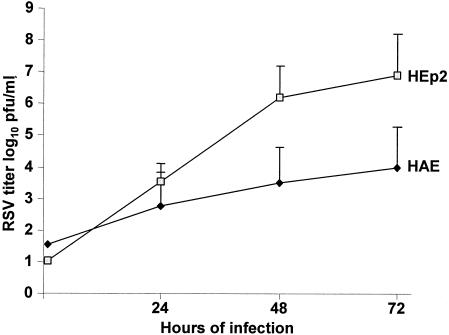
RSV grew to a significantly lower titer in HAE than in HEp-2 cells in parallel experiments done with the same low multiplicity of input (MOI) of 0.01 PFU per cell (Fig. 1). Pairwise comparisons of growth in the two cell lines were done by both t test and Mann-Whitney test. A low MOI limited residual virus from the inoculum and thus increased the capacity to demonstrate replication. The lower initial growth in HAE, the target cells for RSV infection, was somewhat surprising; nevertheless, the increasing titer of virus in HAE indicated that RSV underwent productive infection (one-way analysis of variance was used to test trend over time). The titer of virus recovered from epithelial cells reached a plateau between 48 and 72 h. However, virus could be recovered at sustained high titers for at least 30 days with no visible alteration in microscopic appearance of the cells and with continued ciliary activity.
FIG. 1.
Growth of RSV in respiratory epithelial tissue culture systems. Titers of RSV strains were determined by plaque assay on HEp-2 cell monolayer cultures maintained under semisolid overlay at 37°C for wild-type RSV and at 32°C for live-attenuated mutants as previously described (13). Virus recovery was significantly greater from HEp-2 cells than from primary human epithelial cells (HAE) (P < 0.01 at 48 and 72 h).
Several aspects of RSV growth in HAE were explored to help identify causes of limited initial viral replication. Recovery of virus from HAE at 24 and 72 h was directly influenced by the initial MOI of virus. The titer at 72 h was 103.65 with an MOI of 0.01 and 105.5 with an MOI of 1. This indicates limitation in the spread of newly formed virus in HAE. The growth of RSV A2, an extensively laboratory-adapted strain, and that of a recently isolated RSV A strain were comparable. Fetal bovine serum concentrations between 0 and 10% had no effect on virus growth in both HEp-2 cells and HAE. Titers of RSV in supernatants and cell lysates were the same in both cell systems. Using a Transwell insert, RSV growth was seen only with apical application of virus, and release of newly produced virus was also from the apical surface.
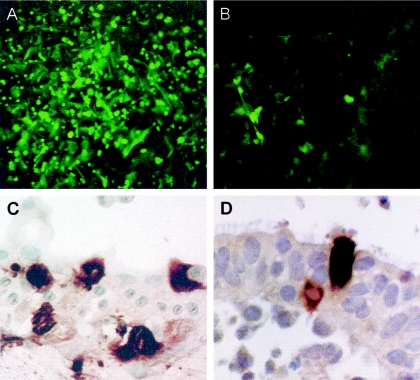
In HAE RSV infection remains focal whereas all cells in HEp-2 monolayers become infected. The pattern of fluorescence with the rgRSV A2 strain with the GFP insert demonstrates this, as does immunohistochemistry of both HAE and an infected adenoid organ culture (Fig. 2). This pattern is also seen in the human lung of infants dying of RSV infection (17). Possible explanations for the more limited growth in HAE appear to be that, even with a high multiplicity, RSV is selective in the cells in which it will replicate, is influenced in replication by innate immunity, or has a limitation in cell-to-cell spread.
FIG. 2.
Demonstration of the limited growth and focal nature of the infection of RSV in HAE. Shown are expression of GFP by rgRSV in (A) HEp-2 cells and (B) HAE at 72 h after initiation of infection and results of immunoperoxidase staining with a goat biotin-conjugated antibody to RSV (Biodesign International, Saco, ME) in (C) HAE and (D) an adenoid organ culture.
Attenuated respiratory syncytial virus strains in HAE.
The isolation and characterization of wild-type RSV strain A2, cold-passaged RSV A2 strain 3131, strain cp, further-attenuated cpA2 derivatives (rA2cp530/1009, rA2cp248/955, rA2cp248/404, and rA2cp248/404/1030ΔSH), and vaccines with deletions of the NS2 gene have been described previously (5). All have been evaluated in an ongoing clinical vaccine development program (13, 19) in a slow, iterative process of evaluation of strains in adults and progressively younger children. Although animal models have given important clues to the level of attenuation of strains (5), a tissue culture system in which attenuation could be predicted would be helpful. Towards that end RSV vaccines that had been evaluated in humans and/or chimpanzees were compared to wild-type virus in HAE cells at three temperatures, 32°C, 35°C, and 37°C. Each vaccine was from an experimental vaccine lot with titers of between 105 and 106 PFU/ml, and each experiment was repeated two to four times. In HAE the progressively more attenuated derivatives of cpA2 at permissive temperatures mirrored their growth in young children and infants (Table 1). Two candidates, rA2cp248/404 and rA2cp248/404/1030ΔSH, were considered sufficiently attenuated to give to infants, although the former caused mild congestion in infants (19). rA2cp248/404/1030ΔSH has an excellent safety profile with limited but consistent replication even in the fully susceptible child (R. Karron, unpublished data). The introduction of the NS2 deletion (14) into three prototype vaccine candidates caused a 3- to 40-fold decrease in growth in HAE (Table 1) but not in HEp-2 cells. These findings parallel the growth of these strains in children, results in which rA2cpNS2 grew to a limited extent in seropositive children, indicating partial attenuation, and the rA2cp248/404ΔNS2 and rA2cp530/1009NS2 strains were highly attenuated with limited growth in seronegative children. Evaluation in chimpanzees also suggests attenuation associated with deletion of NS2 (Table 1) (16). Growth in HEp-2 cells of the attenuated mutants was generally equivalent to that of wild type at 32°C, though influenced by temperature sensitivity, so that most of the attenuated growth was limited at 37°C or even 35°C. There was a significant correlation between growth in the chimpanzee and that in HAE in ranking of titer (Spearman's rho correlation using rank P = 0.03) and in absolute titer (Pearson correlation [two-tailed], P = 0.03). The growth in HAE approached significance in comparison with growth in seronegative children, 0.07, by rank and by titer. The chimpanzee data were not a significant predictor of growth of RSV attenuated strains in seronegative children. Limited growth in HAE, below 3.0 logs, was predictive of attenuation in the most susceptible seronegative children. This was true for the vaccines derived by sequential mutation from the original cold-adapted vaccine (9) and those with attenuation conveyed by deletion of the NS2 gene (16). Although clinical trials in humans are essential, it appears that HAE represents a very effective screening step for comparing attenuation of vaccine candidates. This concept is strengthened by the demonstration that effective attenuating mutations in three other viruses, influenza A, influenza B, and parainfluenza type 3 virus, all limit growth in HAE compared to that of their parent strain (18).
TABLE 1.
Correspondence of RSV growth in HAE cells and chimpanzee models with that in seronegative children
| Clinical assessment | Vaccine strain | Recovery of RSV in indicated system (PFU/ml)
|
|||||
|---|---|---|---|---|---|---|---|
| HEp-2a | HAECa
|
Chimpanzeesb | Seronegative childrenb (% of children infected) | ||||
| 32°C | 35°C | 37°C | |||||
| Virulent virus | A2wt | 6.5 | 3.7 | 4.3 | 4.8 | 5.4 | Not tested |
| Insufficiently attenuated for | Cp | 4.5 | 3.1 | 3.7 | 3.9 | 5.1 | Not specified (100) |
| seronegative children | 530/1009 | 4.8 | 4.0 | 3.4 | 3.6 | 3.6 | 4.5 (100) |
| ΔNS2 | 5.3 | 2.5 | 3.2 | 2.1 | 3.8 | Not specified (100) | |
| 248/955 | 5.2 | 4.0 | 4.2 | 3.0 | 4.6 | 4.4 (88) | |
| ts1 | 5.0 | 3.2 | 3.2 | 2.2 | 4.2 | 3.7 (96) | |
| Appropriately attenuated for seronegative children; insufficiently attenuated | 248/404 | 5.2 | 4.0 | 3.8 | 2.6 | 2.5 | 4.2 (79) |
| for infants | 248/404ΔSH | 5.9 | 3.7 | 2.6 | Not tested | 2.1 | 4.3 (100) |
| Appropriately attenuated for seronegative children and infants | 248/404/1030ΔSH | 5.9 | 2.6 | 2.4 | 1.7 | Not tested | 2.5 (100) |
| Overly attenuated for seronegative children; | ts2 | 6.0 | 1.3 | <1 | <1.0 | 2.2 | Not specified (14) |
| not tested in infants | 248/404ΔNS2 | Not tested | 3.0 | 2.2 | 2.2 | Not tested | 2.8 (40) |
Mean titer of two to four experiments at 72 h with the input virus titer between 105 and 106 PFU/ml for each vaccine candidate.
Mean peak titer over course of infection.
Acknowledgments
This work was supported by NO1-AI-65298, Respiratory Mucosal Pathogens Unit.
The contributions of Sandra Yoder and Sarah E. Winbourn to the work in this paper are gratefully acknowledged. Live-attenuated RSV vaccines were provided by Brian Murphy, Laboratory of Infectious Disease, NIAID, NIH, Bethesda, MD, or by Wyeth Lederle Vaccines, Pearl River, NY (Valerie Randolph). The wild-type RSV A2 recombinant green fluorescent protein-expressing construct, rgRSV, was used in several experiments (kindly supplied by Mark Peeples, Rush-Presbyterian-St. Luke' s Medical Center, Chicago, IL).
REFERENCES
- 1.Bryson, D. G., M. S. McNulty, E. F. Logan, and P. F. Cush. 1983. Respiratory syncytial virus pneumonia in young calves: clinical and pathologic findings. Am. J. Vet. Res. 44:1648-1655. [PubMed] [Google Scholar]
- 2.Coyte, P. C., R. Croxford, W. McIsaac, W. Feldman, and J. Friedberg. 2001. The role of adjuvant adenoidectomy and tonsillectomy in the outcome of the insertion of tympanostomy tubes. N. Engl. J. Med. 344:1188-1195. [DOI] [PubMed] [Google Scholar]
- 3.Crowe, J. E., Jr., P. T. Bui, A. R. Davis, R. M. Chanock, and B. R. Murphy. 1994. A further attenuated derivative of a cold-passaged temperature-sensitive mutant of human respiratory syncytial virus retains immunogenicity and protective efficacy against wild-type challenge in seronegative chimpanzees. Vaccine 12:783-790. [DOI] [PubMed] [Google Scholar]
- 4.Crowe, J. E., Jr., P. T. Bui, G. R. Siber, W. R. Elkins, R. M. Chanock, and B. R. Murphy. 1995. Cold-passaged, temperature-sensitive mutants of human respiratory syncytial virus (RSV) are highly attenuated, immunogenic, and protective in seronegative chimpanzees, even when RSV antibodies are infused shortly before immunization. Vaccine 13:847-855. [DOI] [PubMed] [Google Scholar]
- 5.Crowe, J. E., Jr. 2001. Respiratory syncytial virus vaccine development. Vaccine 20:S32-S37. [DOI] [PubMed] [Google Scholar]
- 6.Endo, Y., K. N. Carroll, M. R. Ikizler, and P. F. Wright. 1996. Growth of influenza A virus in primary, differentiated epithelial cells derived from adenoids. J. Virol. 70:2055-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falsey, A. R. 1998. Respiratory syncytial virus infection in older patients. Vaccine 16:1775-1778. [DOI] [PubMed] [Google Scholar]
- 8.Fishaut, M., J. D. Schwartzman, K. McIntosh, and S. R. Mostow. 1978. Behavior of respiratory syncytial virus in piglet tracheal organ culture. J. Infect. Dis. 138:644-649. [DOI] [PubMed] [Google Scholar]
- 9.Friedewald, W. T., B. R. Forsyth, C. B. Smith, M. A. Gharpure, and R. M. Chanock. 1968. Low-temperature-grown RS virus in adult volunteers. JAMA 203:690-694. [PubMed] [Google Scholar]
- 10.Glezen, W. P., F. A. Loda, W. A. Clyde, R. J. Senior, C. I. Sheaffer, W. G. Conley, and F. W. Denny. 1971. Epidemiologic patterns of acute lower respiratory disease of children in a pediatric group practice. J. Pediatr. 78:397-406. [DOI] [PubMed] [Google Scholar]
- 11.Hall, C. B. 2001. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 344:1917-1928. [DOI] [PubMed] [Google Scholar]
- 12.Henderson, F. W., S. C. Hu, and A. M. Collier. 1978. Pathogenesis of respiratory syncytial virus infection in ferret and fetal human tracheas in organ culture. Am. Rev. Respir. Dis. 118:29-37. [DOI] [PubMed] [Google Scholar]
- 13.Karron, R. A., P. F. Wright, J. E. Crowe, Jr., M. L. Clements-Mann, J. Thompson, M. Makhene, R. Casey, and B. R. Murphy. 1997. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine in chimpanzees and in human adults, infants and children. J. Infect. Dis. 176:1428-1436. [DOI] [PubMed] [Google Scholar]
- 14.Schlender, J., B. Bossert, U. Bucholz, and K. K. Konzelmann. 2000. Bovine respiratory syncytial virus nonstructural proteins NS1 and NS2 cooperatively antagonize alpha/beta interferon-induced antiviral response. J. Virol. 74:8234-8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas, L. H., E. J. Stott, J. Jebbett, and S. Hamilton. 1976. The growth of respiratory syncytial virus in organ cultures of bovine foetal trachea. Arch. Virol. 52:251-258. [DOI] [PubMed] [Google Scholar]
- 16.Whitehead, S. S., A. Bukreyev, M. N. Teng, C.-Y. Firestone, M. St. Claire, W. R. Elkins, P. L. Collins, and B. R. Murphy. 1999. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J. Virol. 73:3438-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright, C., K. C. Oliver, F. I. Fenwick, N. M. Smith, and G. L. Toms. 1997. A monoclonal antibody pool for routine immunohistochemical detection of human respiratory syncytial virus antigens in formalin-fixed, paraffin-embedded tissue. J. Pathol. 182:238-244. [DOI] [PubMed] [Google Scholar]
- 18.Wright, P. F., M. Ikizler, K. N. Carroll, and Y. Endo. 1996. Interactions of viruses with respiratory epithelial cells. Semin. Virol. 7:227-235. [Google Scholar]
- 19.Wright, P. F., R. A. Karron, R. B. Belshe, J. Thompson, J. E. Crowe, Jr., T. G. Boyce, L. L. Halburnt, G. W. Reed, S. S. Whitehead, R. Casey, M. Eichelberger, B. Thumar, V. B. Randolph, and B. R. Murphy. 2000. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine in infancy. J. Infect. Dis. 182:1331-1342. [DOI] [PubMed] [Google Scholar]
- 20.Zhang, L., M. E. Peeples, R. C. Boucher, P. L. Collins, and R. J. Pickles. 2002. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 76:5654-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]