Abstract
Antigenic variation is a viral strategy exploited to promote survival in the face of the host immune response and represents a major challenge for efficient vaccine development. Influenza viruses are pathogens with high transmissibility and mutation rates, enabling viral escape from immunity induced by prior infection or vaccination. Intense selection from neutralizing antibody drives antigenic changes in the surface glycoproteins, resulting in emergence of new strains able to reinfect hosts immune to previously circulating viruses. CD8+ cytotoxic T cells (CTLs) also provide protective immunity from influenza virus infection and may contribute to the antigenic evolution of influenza viruses. Utilizing mice transgenic for an influenza virus NP366-374 peptide-specific T-cell receptor, we demonstrated that the respiratory tract is a suitable site for generation of escape variants of influenza virus selected by CTL in vivo. In this report the contributions of the perforin and Fas pathways utilized by influenza virus-specific CTLs in viral clearance and selection of CTL escape variants have been evaluated. While transgenic CTLs deficient in either perforin- or Fas-mediated pathways are efficient in initial pulmonary viral control, variant virus emergence was observed in all the mice studied, although the spectrum of viral CTL escape variants selected varied profoundly. Thus, a less-restricted repertoire of escape variants was observed in mice with an intact perforin cytotoxic pathway compared with a limited variant diversity in perforin pathway-deficient mice, although maximal variant diversity was observed in mice having both Fas and perforin pathways intact. We conclude that selection of viral CTL escape variants reflects coordinate action between the tightly controlled perforin/granzyme pathway and the more promiscuous Fas/FasL pathway.
A central paradigm in viral immunology is that the immune system adapts and responds to a multitude of pathogens in a variety of qualitatively and quantitatively different ways. Innate immune mechanisms lay the groundwork for a more specific, focused attack on the pathogen by the adaptive immune response, consisting of antibody production by B cells to neutralize free virus and the CD8+ cytotoxic T-lymphocyte (CTL) response to directly and rapidly eliminate infected cells, which is promoted and regulated by a virus-specific CD4+ T-cell response (10, 11, 14, 27, 35). As key mediators of the antiviral response, CTLs exert their effects via both secretory and cell contact-dependent pathways following T-cell receptor (TCR) engagement. The secretory pathway involves release of inflammatory cytokines, including gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), to exert paracrine effects on cells in the vicinity, promoting up-regulation of major histocompatibility complex (MHC) antigens and entry into an antiviral state. Cell contact-dependent effector mechanisms include the membrane lytic pathway, operating via perforin and granzyme release from CTL granules, and the receptor-mediated cytotoxic pathway, which triggers apoptotic death of target cells via Fas/Fas ligand (FasL) or TNF receptor 1 interactions. It is generally considered that cell contact-dependent pathways represent the major CTL mechanism for elimination of infected cells (14, 40). Interplay between these effector arms is, for the most part, highly effective at eliminating viruses from the host and occurs within a tightly regulated framework to ensure protection from the pathogen with minimal associated pathology (44). A corollary of this is that viruses utilize a variety of mechanisms to evade the host immune response and promote their survival. This is especially true for the CTL response, where failure to contain replication early in the infection can lead to emergence of T-cell escape variants of the virus bearing mutations which affect the presentation or T-cell recognition of peptide epitopes, a deleterious consequence of immunological selective pressure exerted on the virus by the host (4, 9, 23, 26).
Influenza viruses are classic example of antigenically variable pathogens. A wealth of epidemiologic evidence has demonstrated that these pathogens are, perhaps uniquely, able to escape from immunity induced by prior infection or vaccination to cause acute respiratory infections with significant morbidity and mortality in humans on an epidemic and pandemic scale (16, 17). The basis of this remarkable variability is their unusual, segmented RNA genome, which facilitates two forms of antigenic variability. Antigenic “shift” is brought about by reassortment of viral genes leading to emergence of antigenically novel viruses that have pandemic potential. In contrast, antigenic “drift” occurs within virus subtypes and involves accumulation of minor genetic changes. Such progressive antigenic changes are most obvious in the viral surface glycoproteins hemagglutinin (HA) and neuraminidase (NA), which are the primary targets of antiviral antibody (reviewed in references 8, 17, and 37). Host immune pressure drives the continual and comparatively rapid evolution of HA and NA, effectively preventing cross-protective humoral immunity against multiple virus strains (41, 43) and ultimately allowing sequential reinfection of individuals over successive years by influenza viruses bearing antigenically distinct surface glycoproteins. In addition to the antibody response, which is considered to act primarily by preventing reinfection by antigenically identical viral strains, ample evidence shows that CTL responses protect from the potentially lethal consequences of influenza virus infection. Induction of a robust CTL response is an especially attractive goal for successful vaccine design as, in contrast to the strain specificity of the antibody response, CTLs are cross-reactive between influenza virus subtypes (1, 6, 22) due to the relative conservation of the internal viral proteins, which are inaccessible to antibody selection pressure but are potential CTL targets.
While antibody-mediated selection of variants is the predominant force for evolution of viral strains within a host population, strong evidence is accumulating for the concept that CTLs exert sufficient immunological pressure to drive antigenic variation in MHC class I-restricted CTL epitopes of influenza virus (15, 28, 31, 32). In agreement with this concept, in a previous report utilizing an influenza virus nucleoprotein-specific TCR-transgenic mouse model, we demonstrated selection of CTL escape variant viruses within the lung, indicating that a strong and highly focused CTL response, in combination with the intrinsic genetic variability of influenza virus, is sufficient for CTL-mediated antigenic drift within infected hosts (31). However, the mechanism by which such antiviral CTLs exert this selective pressure upon a viral population in vivo remained unexplored. In this context, it is possible that such selective pressure was preferentially mediated via the secretory or one of the cell contact-dependent CTL effector mechanisms. Influenza virus infection is an especially good model to investigate this question, as it is known that infected cells upregulate Fas expression, and previous studies have demonstrated at least a partial functional overlap between the perforin/granzyme- and Fas/FasL-dependent cytotoxic pathways in virus clearance (5, 7, 20, 38, 39). In this report, we demonstrate that both pathways contribute to virus clearance and selection of CTL escape viral variants.
MATERIALS AND METHODS
Mice and virus infection.
F5 TCR-transgenic mice were kindly provided by D. Kioussis (National Institute for Medical Research, London, United Kingdom) (21). C57BL/6 (B6), B6-gld (FasL−/−), B6 immunoglobulin M (IgM)-deficient (IgM−/−), B6 recombination activating gene 1 (RAG-1−/−)-deficient, and B6 perforin-deficient (P−/−) (13) mice were obtained from Jackson Laboratories, Bar Harbor, ME. F5 mice were crossed with RAG-1−/−, P−/−, or FasL−/− mice or combinations of these backgrounds to obtain mice of F5-RAG-1−/−, F5-RAG-1−/−-P−/−, F5-RAG-1−/−-FasL−/−, or F5-RAG-1−/−-P−/− FasL−/− genotypes. IgM−/−-P−/− or IgM−/−-FasL−/− mice were generated by intercrossing IgM−/− with P−/− or FasL−/− mice. Animals were bred and maintained in a specific-pathogen-free environment, and all procedures involving animals were conducted in accordance with institutional guidelines for animal use and care. For infection, animals (8 to 12 weeks old) were anesthetized with methoxyflurane (Metofane; Pitman-Moore, Mundelein, IL) and inoculated intranasally with 50 μl of virus diluted in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin.
Viruses and cells.
Stocks of influenza virus strains A/Memphis/102/72 (H3N2) (A/Mem) (31) and mouse-adapted A/PR/8/34 (H1N1), a kind gift of Peter Doherty (St. Jude Children's Research Hospital, Memphis TN) were propagated in 10-day-old embryonated hen's eggs. Madin-Darby canine kidney (MDCK) cells and 293T cells were propagated in Eagle's minimal essential medium supplemented with 5% fetal calf serum and l-glutamine. Viruses were titrated by 50% tissue culture infective dose (TCID50) assay on MDCK cells, as described previously (31). The threshold of virus detection in this assay is ∼102 TCID50/g lung tissue.
Generation of recombinant virus.
A reassortant influenza virus (A/MemH1N1) bearing the HA and NA of A/PR/8/34 but with all other genes derived from A/Mem was generated by plasmid-based reverse genetics methodologies (12). Briefly, cDNA from each viral RNA segment was generated by reverse transcription using a universal primer (5′-AGCAAAAGCAGG-3′) and amplified by PCR using primers complementary to the 3′ and 5′ termini of the viral genome segments (sequences available on request). The resulting PCR products were cloned into pHW2000 vectors (kindly provided by Erich Hoffmann, St. Jude Children's Research Hospital, Memphis, TN) to place each viral cDNA under control of an RNA polymerase I promoter and terminator flanked by the cytomegalovirus immediate-early promoter and BGH poly(A) signal. Recovery of recombinant virus following plasmid transfection into cocultured 293T and MDCK cells was essentially as described elsewhere (12).
Sequence analysis of viral isolates.
Isolates from lungs of infected mice were grown in MDCK cells for 48 h, and virus was precipitated from supernatant with equal volumes of 3 M LiCl and 6 M urea by centrifugation (20,000 × g, 30 min, 4°C). Viral RNA was extracted from this pellet using the RNeasy extraction kit (QIAGEN, Santa Clarita, CA). Reverse transcription and PCR amplification of the viral NP gene fragment was performed as previously described (31).
HI assay.
Serum antibody titers were determined by hemagglutination inhibition (HI) assay, using 8 HI units/50 μl of A/Mem or A/MemH1N1 recombinant viruses, as previously described (30). The limit of detection in this assay is 2 HI units/50 μl of serum.
Quantitation of virus-specific CD8+ T cells in the pneumonic lung and spleen.
Experiments utilized tetramers of H-2Db complexed with NP366-374 (ASNENMDTM) or PA224-233 (SCLENFRAYV) and H-2Kb complexed with NS2114-121 (RTFSFQLI) peptides, prepared as described previously (2). Single-cell suspensions prepared from spleen or lymphocytes isolated from lung were stained with tetramer and antibody for CD8 (clone 53-6.72) in fluorescence-activated cell sorter (FACS) buffer (PBS with 1% bovine serum albumin and 0.2% sodium azide). After staining for 1 h at 4°C, cells were fixed in PBS containing 0.1% paraformaldehyde and acquired on a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA), and data were analyzed using CellQuest software.
Intracellular cytokine secretion assay following peptide stimulation.
Cells recovered by bronchoalveolar lavage (BAL) or from spleens were cultured in 96-well U-bottom plates at 4 × 106 cells/well in 200 μl RPMI 1640 in the presence or absence of NP366-374, PA224-233, or NS2114-121 peptides at a concentration of 1 μg/ml (31). To quantitate total virus-specific CD8+ T-cell responses, virus-infected dendritic cell (DC2.4; kindly provided by Kenneth Rock, Boston, MA) (34) stimulators were used in combination with intracellular cytokine staining. A total of 1 × 106 effector cells were incubated with 4 × 105 DC2.4 cells uninfected or infected 18 h previously with A/Mem at a multiplicity of infection of 1. Stimulations were performed for 6 h at 37°C, in the presence of 10 U/well human interleukin-2 and 1 μg/well Brefeldin A (BD-Pharmingen). After 6 h cells were harvested, washed, and surface stained for CD8α, prior to intracellular cytokine staining (fluorescein isothiocyanate-conjugated antibody to murine IFN-γ; clone XMG1.2; Caltag, Burlingame, CA) using the Cytofix/Cytoperm kit (BD-Pharmingen). Stained cells were washed, fixed, and acquired as above.
Proliferation of T cells in response to peptide stimulation.
Transgenic splenocytes (5 × 104/well) were cultured with irradiated (30 Gy) splenocytes (5 × 105/well) from B6 mice in the given concentrations of peptides in Iscove's modified Dulbecco's medium supplemented with 10% fetal calf serum for 72 h. Proliferation of T cells was determined by incorporation of [3H]thymidine (1 μCi/well) during the last 6 to 8 h of culture.
Histological analysis of lung inflammation.
Lung tissues harvested from infected mice were fixed in 10% neutral buffered formalin, paraffin embedded, and sectioned. Sections were stained with hematoxylin and eosin and subjected to gross and microscopic pathological analysis.
RESULTS
(i) Virus clearance and antibody and CD8+ T-cell responses are not compromised during primary or heterosubtypic challenge infection of perforin- or FasL-deficient mice.
Intranasal infection with a sublethal dose (104 PFU) of A/Mem resulted in a pulmonary infection that was cleared between days 7 and 10, with no significant differences in the kinetics of virus replication and elimination between controls and P−/− or FasL−/− mice. On heterosubtypic challenge with A/MemH1N1 (104 PFU) 1 month after priming with A/Mem (H3N2), virus clearance occurred similarly between controls and P−/− or FasL−/− mice (Fig. 1A). A/MemH1N1 is a recombinant reassortant virus generated by reverse genetics and which expresses the HA and NA of A/PR/8/34 (H1N1) and all other genes from A/Mem. Thus, the neutralizing antibody response to HA and NA is not cross-reactive, but CTL epitopes are conserved between these two viruses. Note that 104 PFU of A/MemH1N1 is lethal for unprimed B6 mice (data not shown), illustrating that the protective effects of a priming heterosubtypic infection are largely CTL mediated. These findings confirm previous observations that mice deficient in perforin- or Fas-mediated cytotoxic pathways are not compromised in their ability to recover from a primary or secondary heterosubtypic influenza virus infection (5).
FIG. 1.
Protection and compensation between influenza virus-specific T-cell-mediated cytotoxic effector pathways in mice with an intact antibody response. Analyses were performed to correlate the kinetics of virus replication (A) with the kinetics of virus-specific CD8+ T-cell responses (B) and kinetics of the HI antibody response (C). Virus titers were measured at the time points indicated in lungs of B6-P−/−, B6-FasL−/−, or control B6 mice following a primary infection with A/Mem (104 PFU) or heterosubtypic challenge with A/MemH1N1 (104 PFU) 1 month after priming with A/Mem (H3N2) (indicated by the arrow in the graph). Data shown are means ± standard errors of the means (SEM) of the log10 TCID50 per gram of lung tissue of 5 to 10 mice. In parallel, total numbers of NP366-374, NS2114-121, or PA224-233 peptide-specific CD8+ T cells in the BAL fluid were measured by staining with MHC tetramers (•) or for intracellular IFN-γ (○) following peptide stimulation of cells. Values were derived by multiplying the percentages of total tetramer-positive cells by the total number of lymphocytes isolated from BAL fluid at a given time point. Data shown are mean (± SEM) log10 virus-specific T cells per lung of 5 to 10 mice. Serum HI antibody titers are shown for both A/Mem (H3N2) (•) and A/MemH1N1 (○) expressed as mean ± SEM log10 HI titers of 5 to 10 mice.
Efficient viral control following primary or secondary viral challenge was reflected by strong polyclonal CTL responses measured by MHC-tetramer (Db-NP366-374, Kb-NS2114-121, or Db-PA224-233) staining and IFN-γ production after peptide stimulation. Overall, similar kinetic patterns, magnitude of expansion, and recruitment of virus-specific CTLs to the lung were observed among the different mice (Fig. 1B). Functionally, there was no deficit in activation of CTLs in any of the mouse strains tested, as assessed by intracellular staining for IFN-γ, and no significant alteration in the immunodominance hierarchy, as revealed by a more prevalent CD8+ T-cell response to the NP366-374 epitope than to the NS2114-121 or PA224-233 epitopes during primary infection or secondary challenge of mice. To determine whether the overall response to the virus was altered in different mice, the virus-specific CTL response in the lung and spleen was examined by in vitro restimulation using virus-infected dendritic cells (DC2.4) in combination with IFN-γ staining. As expected, the sum of NP366-374-, NS2114-121-, and PA224-233-specific CTL populations essentially comprised the overall virus-specific CTL response as measured by intracellular staining for IFN-γ (Fig. 2A), confirming the results observed above for individual epitope-specific CTL populations. As a percentage of CD8+ T cells, the number of tetramer-positive cells (the sum of NP366-374-, NS2114-121-, and PA224-233-specific CD8+ T cells) was higher in the lung than the spleen, with no significant differences observed among the mouse strains studied (Fig. 2B). Representative flow cytometry profiles to illustrate tetramer staining patterns and dendritic cell restimulation of the total virus-specific CD8+ CTL population are presented in Fig. 3. These findings are in agreement with previous reports (5) and suggest that perforin or Fas/FasL cytotoxic pathways play no major role in shaping the hierarchy of antigen-specific CD8+ T-cell responses in the influenza virus system. Finally, P−/− or FasL−/− mice or their controls developed comparable A/Mem- or A/MemH1N1-specific HI antibody responses (Fig. 1C). Though other studies have reported a role for the perforin-dependent CTL effector pathway during primary infection of mice with highly virulent mouse-adapted virus strains (20), under the experimental conditions described here (sublethal primary infection with a strain of comparatively low virulence followed by challenge with a more virulent strain) multiple immunological pathways compensate for deficiencies in CTL effector mechanisms and efficiently mediate virus clearance from the host. In summary, in antibody-sufficient mice, T-cell-mediated perforin or Fas/FasL cytotoxicity does not play a critical role in either viral clearance or in dictating the immunological hierarchy of epitope-specific CTL responses and has little or no impact on the neutralizing antibody response.
FIG. 2.
Kinetics of total virus-specific CD8+ T-cell responses in lung and spleen based on tetramer versus intracellular IFN-γ secretion by stimulation of lymphocytes with DC2.4 virus-infected cells. (A) B6-P−/−, B6-FasL−/−, or control B6 mice were infected with 104 PFU A/Mem or challenged with 104 PFU of A/MemH1N1 1 month after priming with A/Mem (indicated by arrow), and total numbers of virus-specific CD8+ T cells (sum of Db/NP366-374, Kb/NS2114-121, and Db/PA224-233 tetramer-positive T cells) were determined at the indicated time points (•). Virus-specific CD8+ T cells were tested for their ability to produce IFN-γ following short-term culture with virus-infected DC2.4 cells on the indicated days after infection (○). Data shown are means ± standard errors of the means (SEM) of log10 virus-specific T cells per tissue of three to six mice. (B) Percentages of total virus-specific CD8+ T cells (sum of NP366-374, NS2114-121, and PA224-233 peptide-specific T cells) in BAL fluid (▪) or spleen (•) were calculated based on data presented in panel A. Data shown are mean ± SEM log10 virus-specific T cells per tissue of three to six mice.
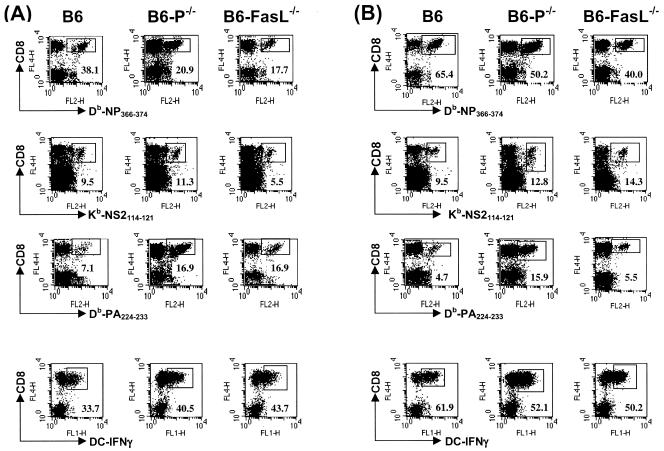
FIG. 3.
Epitope-specific CD8+ T-cell populations in BAL fluid in relation to the total of virus-specific IFN-γ-producing CD8+ T cells during a primary and secondary influenza virus infection. B6-P−/−, B6-FasL−/−, or control B6 mice were infected as described in the legend for Fig. 1, and BAL cells were isolated 10 days after primary (A) or secondary (B) infection. The percentage of antigen-specific CD8+ T cells was assessed by staining with MHC tetramers (indicated) and antibody against CD8, and total numbers of virus-specific CD8+ T cells producing IFN-γ after stimulation with virus-infected DC2.4 cells were determined by concurrent analyses (bottom panels). The percentage of CD8+ T cells staining positive by MHC tetramer or producing IFN-γ are indicated in the lower corners of the corresponding panels. Plots shown are gated for live cells.
(ii) Significant role for perforin in T-cell-mediated virus clearance but not on homeostatic regulation of epitope-specific CD8+ T-cell responses in the absence of antibody.
To observe the action of T cells in clearance of an acute influenza virus infection and the regulation of the CTL response in the absence of antibody, B6 mice deficient in IgM (and hence lacking mature B cells and antibody secretion) in combination with perforin or FasL were infected with 104 PFU of A/Mem. As expected, B6-IgM−/− mice and B6-IgM−/−-FasL−/− mice were able to efficiently clear the virus between days 7 and 10. However, while a fraction (30 to 50%) of IgM−/−-P−/− mice were able to control the infection, a large portion of these mice maintained virus within the lung at variable levels for 20 to 30 days after infection (Fig. 4A), although this residual virus was eventually controlled by day 30 to 40 after infection. Examination of the overall CTL response in BAL fluid from these mice revealed no major differences in either the magnitude or functional capacity of these cells (as determined by tetramer staining and IFN-γ production on restimulation with infected dendritic cells, as described above). Similarly, there was no detectable alteration to the hierarchy of the CTL response against individual epitopes (Fig. 4B). This illustrates that while deficiency in individual CTL effector pathways may be compensated for in the presence of an intact immune system, overall virus control may be seriously compromised in situations where multiple immunological pathways are affected. In this case, it is also clear that antiviral protection is more efficiently mediated via perforin than by FasL-dependent pathways.
FIG. 4.
Contribution for perforin-mediated cytotoxicity in clearance of influenza virus but not in homeostatic regulation of the CD8+ T-cell responses in the absence of antibody. (A) IgM−/−-P−/−, IgM−/−-FasL−/−, or control IgM−/− mice were infected with 104 PFU A/Mem, and virus titers were measured at the time points indicated. Data shown are means ± standard errors of the means (SEM) of the log10 TCID50 per gram of lung of 5 to 10 mice. In the case of IgM−/−-P−/− mice, variation in viral titers was observed. Symbols in the middle panel represent individual mice with detectable viral titers (○) or mice with viral titers below the detection limits (•), with the number of mice falling into each group indicated for each time point. (B) Parallel total numbers of NP366-374, NS2114-121, or PA224-233 peptide-specific CD8+ T cells in the BAL fluid were measured by staining with MHC tetramers (•) or for intracellular IFN-γ production (○) following peptide stimulation. Total numbers of virus-specific CD8+ T cells (sum of NP366-374, NS2114-121, and PA224-233 peptide-specific T cells) were determined by staining with tetramers (•) or by intracellular IFN-γ staining following short-term culture with virus-infected DC2.4 cells (○) as described above (Fig. 2). Data shown are mean ± SEM log10 virus-specific T cells per lung of 5 to 10 mice.
(iii) A differential contribution of perforin and Fas/FasL pathways in CD8+ T-cell-mediated selection of CTL escape variants in an influenza virus-specific TCR-transgenic mouse model.
Limited TCR diversity of responding T cells has been associated with viral escape from antigen-specific CTLs and is a factor influencing the outcome of viral infection in humans (24). This phenomenon originally described for lymphocytic choriomeningitis virus (29), a pathogen causing systemic persistent infections, has been more recently confirmed for influenza virus, a pathogen causing acute infections largely confined to the respiratory tract (31). Here, transgenic CTLs specific for influenza virus NP reduce pulmonary virus below the threshold of detection by days 8 to 10. Virus then remains undetectable for a period of 2 to 3 weeks, but after this period virus resistant to CTL recognition reemerges and persists at high titer in the lungs for several weeks. Experimentally, this has been demonstrated by infecting F5-RAG-1−/− mice with 102 or 104 PFU of A/Mem (Fig. 5).
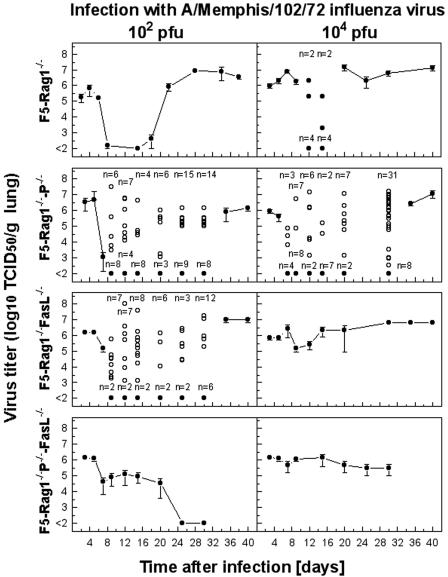
FIG. 5.
Selection and propagation of CTL escape variants in F5-RAG-1−/− mice deficient in perforin, FasL, or both cytolytic pathways following infection with A/Mem influenza virus. Mice were infected with 102 or 104 PFU A/Mem, and virus titers were measured at the time points indicated. Data shown are means ± standard errors of the means of the log10 TCID50 per gram of lung of 5 to 10 mice. Variation in viral titers was observed, and symbols in the relevant panels represent individual mice with detectable viral titers (○) or mice with viral titers below the detection limits (•), with the number of mice falling into each group indicated for each time point.
As CTL-mediated selection of influenza virus variants in F5-RAG-1−/− mice involves several effector functions, including cytotoxicity via perforin/granzyme or Fas/FasL pathways and cytokine secretion, we sought to determine the contributions of these effector mechanisms to the variant selection process by studying viral kinetics in the lungs of F5-RAG-1−/− mice deficient in perforin, FasL, or both perforin and FasL. Specifically, we wished to test the hypothesis that tightly regulated and highly directional release of perforin following TCR engagement would provide a strong selective pressure on the virus and hence promote escape from the CTL response. Conversely, we hypothesized that FasL expression on activated T cells combined with upregulated Fas expression by infected cells may result in T-cell-mediated apoptotic killing of infected cells in a less-cognate TCR-ligand-restricted manner. In the later case, we wished to determine whether Fas/FasL-mediated CTL killing was sufficiently effective to counteract or delay the virus-CTL escape variant selection process. As shown in Fig. 5, F5-RAG-1−/−-P−/− mice infected with either 102 or 104 PFU of A/Mem initially reduced the pulmonary virus load but were unable to fully eliminate the infection. This was evident by an initial large drop in lung virus titer followed by an increasing proportion of mice retaining moderate to high levels of virus, until eventually (after approximately 4 weeks) all mice had moderately high (∼106 TCID50/g) virus levels. The observed pattern of virus retention increasing over time most likely reflects a transitional period during which CTL escape variant viruses are selected and become established in the lung, a process that seems to occur with variable efficiency in individual animals. While F5-RAG-1−/−-FasL−/− mice infected with a comparatively low dose (102 PFU) of A/Mem showed a similar pattern of initial virus reduction and a transitional phase before complete emergence of virus to high levels, F5-RAG-1−/−-FasL−/− mice infected with a higher dose (104 PFU) uniformly showed only a minor drop in virus levels around day 7 to 10, followed by an immediate rebound and persistence of high virus titers for the duration of the experiment. Surprisingly, double-deficient F5-RAG-1−/−-P−/−-FasL−/− mice infected with 102 PFU A/Mem were able to initially reduce the pulmonary virus load to a moderate level by day 10 and eventually eliminate detectable virus from the lung by day 25 but were wholly unable to control infection with a high dose (104 PFU). However, it should be noted that F5-RAG-1−/−-P−/−-FasL−/− mice infected with either dose exhibited progressively more severe symptoms of infection (lethargy, ruffled fur, and hunched posture) and on sacrifice were found to possess enlarged edematous lungs.
To investigate the process of CTL escape variant virus selection, sequence analysis of the NP gene was performed on lung virus isolates obtained during the onset and later stages of infection (Table 1). In the initial stages of infection (days 3, 5, and 7), no amino acid variations in the NP366-374 epitope were observed in virus isolated from any of the transgenic or effector pathway-deficient transgenic mice. As expected, isolates derived from F5-RAG-1−/−-P−/− or F5-RAG-1−/−-FasL−/− mice at later time points in the infection revealed the appearance of amino acid substitutions within the NP366-374 epitope at TCR contact residues (positions 371 and 372) or MHC anchor residues (positions 370 and 374). However, as demonstrated in Table 2, isolates derived from F5-RAG-1−/− - P−/− mice showed a more limited spectrum of variation than those derived from F5-RAG-1−/− or F5-RAG-1−/−-FasL−/− mice. While in all three mouse strains the most commonly observed variation was the 371 M→I mutant, which effectively abrogates recognition of the NP366-374 peptide by the F5-TCR, a wide diversity of mutations predicted to affect either TCR recognition or peptide binding to MHC was clearly observed in perforin-sufficient mice. However, the maximal diversity of variant virus was observed where both perforin and Fas/FasL pathways were intact, suggesting interplay between these two mechanisms. It is worthy to note that once established in individual animals the variant pool appears to be determined by a single variant mutant type, implying that a single mutation event is required for a variant virus to emerge and clonally predominate in the lung. This propensity for mutation was specific for the epitope recognized by transgenic CTLs, as no variations were observed in residues flanking the NP366-374 epitope. Curiously, isolates obtained from F5-RAG-1−/−-P−/−-FasL−/− mice showed no amino acid changes within the NP gene fragment analyzed. This strongly implies that cytokine-mediated selection pressure is insufficient to induce escape mutations in MHC class I-restricted epitopes. Limited analyses of the NS1 gene of viruses isolated from F5-RAG-1−/−-P−/−-FasL−/− mice revealed no evidence of mutations that may contribute to cytokine resistance (33).
TABLE 1.
CTL escape variants with mutations in the NP366-374 epitope of influenza virusa
| Mouse strain | Time after infection (days) | Coding alterations in the NP366-374 CTL epitope | No. of lung isolate variants/total |
|---|---|---|---|
| F5-Rag-1−/− | 3 | WT | 0/4 |
| 5 | WT | 0/6 | |
| 7 | WT | 0/6 | |
| 9 | ND | ||
| 12 | ND | ||
| 15 | ND | ||
| 20-40 | Variants | 70/70 | |
| F5-Rag-1−/−-P−/− | 3 | WT | 0/3 |
| 5 | WT | 0/6 | |
| 7 | WT | 0/3 | |
| 9 | ND | ||
| 12 | ND | ||
| 15 | ND | ||
| 20-40 | Variants | 25/25 | |
| F5-Rag-1−/−-FasL−/− | 3 | WT | 0/2 |
| 5 | WT | 0/7 | |
| 7 | WT | 0/7 | |
| 9 | ND | ||
| 12 | ND | ||
| 15 | ND | ||
| 20-40 | Variants | 22/22 | |
| F5-Rag-1−/−-P−/−-FasL−/− | 3 | ND | |
| 5 | WT | 0/4 | |
| 7 | WT | 0/3 | |
| 9 | WT | 0/3 | |
| 12 | WT | 0/4 | |
| 15 | ND | ||
| 20-40 | WT | 0/8 |
The fraction of virus isolates (bulk virus) recovered from individual mice infected with A/Mem over time displaying amino acid changes within the NP366-374 CTL epitope was compared with the number of analyzed isolates. WT, no amino acid changes; ND, not determined.
TABLE 2.
Spectrum of CTL escape variants with mutations in the NP366-374 epitope of influenza virusa
| Mouse strain | Virus isolate | Amino acid sequence | No. (%) of variant isolates/total tested |
|---|---|---|---|
| ASNENMDTM | |||
| F5-Rag-1−/− | 370N→T | ----T---- | 15/70 (21) |
| 370N→S | ----S---- | 5/70 (7) | |
| 370N→D | ----D---- | 2/70 (3) | |
| 371M→I | -----I--- | 37/70 (53) | |
| 371M→T | -----T--- | 4/70 (6) | |
| 371M→V | -----V--- | 2/70 (3) | |
| 372D→E | ------E-- | 3/70 (4) | |
| 374M→T | --------T | 2/70 (3) | |
| F5-Rag-1−/−-P−/− | 370N→S | ----S---- | 2/25 (8) |
| 371M→I | -----I--- | 23/25 (92) | |
| F5-Rag-1−/−-FasL−/− | 370N→H | ----H---- | 1/22 (4.5) |
| 370N→K | ----K---- | 1/22 (4.5) | |
| 371M→I | -----I--- | 18/22 (82) | |
| 371M→T | -----T--- | 1/22 (4.5) | |
| 372D→N | ------N-- | 1/22 (4.5) | |
| F5-Rag-1−/−-P−/−-FasL−/− | WT | --------- | 0/8 (0) |
The deduced amino acid sequences of the NP366-374 epitope of wild-type and reemergent escape variants recovered from the lungs of individual mice 20 to 40 days after infection with A/Mem are indicated. Changes in amino acid sequences are indicated by arrows. The fraction of virus isolates displaying such changes within the NP366-374 CTL epitope was compared with the number of analyzed isolates. WT, no amino acid changes; ND, not determined.
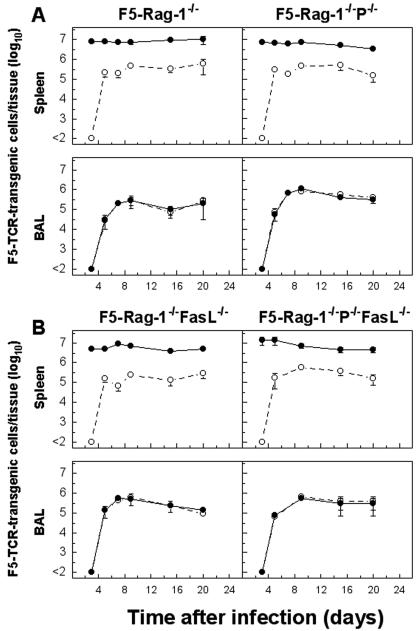
To examine whether activation and recruitment of transgenic CTL to the lung was affected by deficiency in either perforin, FasL, or both, cells were recovered from the spleen and BAL fluid at various times after infection with 104 PFU of A/Mem (Fig. 6). As expected, over the course of infection we could observe no differences in the cellularity of the spleen, the number of transgenic cells, or the ability of a fraction of splenocytes to produce IFN-γ on response to peptide stimulation (Fig. 6), the latter parameter being an indication of T-cell activation. NP tetramer and IFN-γ staining on peptide restimulation revealed recruitment into the lung of near-identical numbers of transgenic CTLs for all four mouse strains. In contrast to splenocytes, transgenic CTL from BAL were uniformly able to produce IFN-γ on peptide restimulation. Splenocytes from uninfected transgenic mice deficient in effector pathways compared to F5-RAG-1−/− controls showed no differences in overall number or proliferative capacity following in vitro stimulation with NP366-374 peptide (Fig. 7 and data not shown). This indicates that there are no inherent defects in the ability of CTL from these mice to recognize and respond to their cognate MHC-peptide ligand.
FIG. 6.
Activation and recruitment of transgenic CTL to the lung were not affected by deficiency in either perforin, FasL, or both effector pathways. Mice were infected with 104 PFU A/Mem, and the total number of transgenic cells in the BAL fluid and spleen was determined by staining with Db/NP366-374 tetramer (•) or for intracellular IFN-γ (○) production following peptide stimulation. Note that transgenic cells from uninfected mice stimulated with cognate peptide under similar conditions did not show any intracellular IFN-γ staining. Data shown are mean ± the standard error of the mean log10 transgenic cells per lung or spleen of three mice.
FIG. 7.
T-cell proliferative responses of transgenic cells from uninfected transgenic mice deficient in perforin, FasL, or both cytolytic pathways to cognate NP366-374 peptide. Spleen cells (105/well) from uninfected F5-RAG-1−/− (A), F5-RAG-1−/−-P−/− (B), F5-RAG-1−/− - FasL−/− (C), or F5-RAG-1−/−-P−/−-FasL−/− (D) mice were stimulated with the given concentration of NP366-374 peptide of A/Mem for 3 days. Proliferation was determined by incorporation of [3H]thymidine pulsed during the last 6 h of culture. Stimulation indices were calculated in relation to proliferation in the medium control.
(iv) Contribution of perforin and Fas/FasL cytolytic pathways by CD8+ CTLs in pulmonary influenza virus pathology.
Lung inflammation and injury following influenza virus infection may result from direct destruction of infected cells or indirectly through secretion of proinflammatory cytokines by activated CD8+ CTLs. In addition, direct viral cytopathic effects and production of cytokines and other factors by infected cells can promote the inflammatory process in the respiratory tract. Although the role of the T-cell response in development of tissue damage for both systemic and anatomically restricted viral infections has been well documented, the relative contributions of adaptive immunity and direct effects of the virus itself in influenza pneumonia are not well understood. In this context, the F5-TCR transgenic model is unique in that participation of both the virus and the CTL response to it in development of lung pathology can be dissected under conditions where virus-specific CD8+ T-cell interaction with infected cells in the lung is restricted to a few days as a result of virus CTL escape variation.
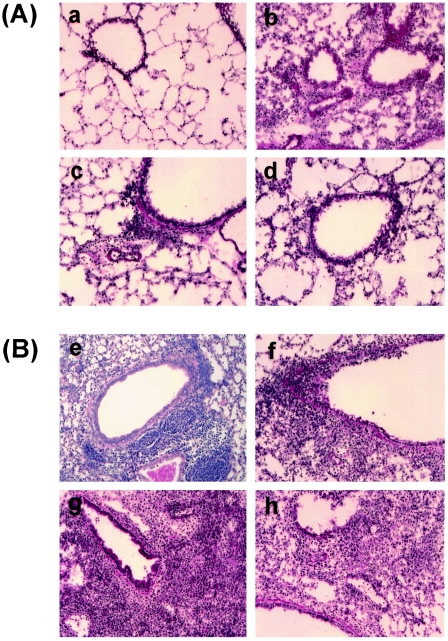
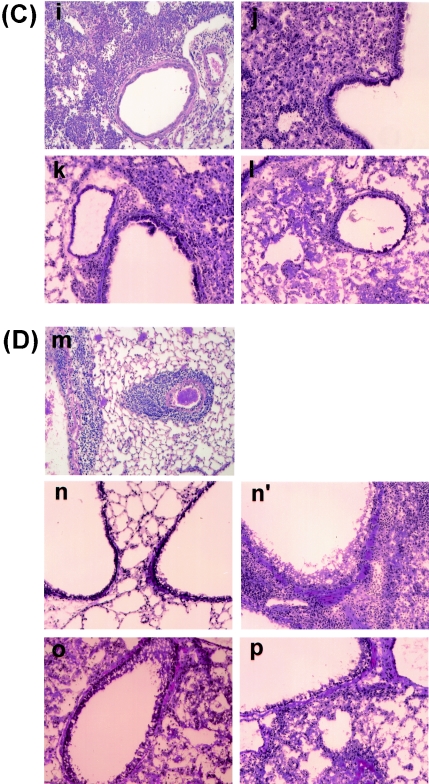
We previously reported using the F5-TCR-transgenic model that CD8+ T cells conferred protection against low doses of infection but exacerbated influenza pneumonia and mortality at high viral doses (>106 PFU) (25). This enhanced pathology was associated with extensive inflammation, primarily consisting of transgenic CTLs and macrophages/monocytes, compromising pulmonary function and resulting in morbidity and death a few days after infection. Therefore, we decided to study the pulmonary pathology following a moderate dose of infection (104 PFU) where no early lethality was expected. As anticipated, histological examination of lung tissues recovered from F5-RAG-1−/− mice over a period of a month revealed moderate lung pathology associated with inflammatory reactions containing transgenic cells and, later, macrophages/monocytes. This increased over the first few days of infection to a maximal level by day 9 before gradually declining in magnitude to persist as a focal perivascular and peribronchial inflammation throughout the duration of the experiment (Fig. 8a, e, i, and m). Pulmonary pathology started earlier in infected F5-RAG-1−/−-P −/− mice, in that inflammatory reactions became detectable on day 2 after infection and rapidly increased in magnitude until day 9 (Fig. 8b, f, and j). At this stage, a divergent pattern was observed, with one fraction of the mice retaining a high degree of pulmonary inflammation associated with detectable virus within the lung and a second fraction essentially exhibiting a resolution of the inflammatory reaction, having reduced pulmonary virus to below the limit of detection (Fig. 8, panels n versus n′). In the case of F5-RAG-1−/−-FasL−/− mice, lung pathology consisted of early peribronchial infiltrates before ultimate development of a multilobar pneumonitis with small, restricted patches of hemorrhagic pathology most evident at day 15. This pronounced accumulation of inflammatory cells was associated with retention of virus in the lung throughout the duration of the study (Fig. 8c, g, k, and o). A similar pulmonary inflammatory process was observed in F5-RAG-1−/−-P−/−-FasL−/− mice, although a progressively more severe hemorrhagic pathology was observed in the interstitial spaces of these animals (Fig. 8d, h, l, and p). A summary of the pulmonary inflammatory process in these animals, together with that from infected B6 or IgM−/− mice deficient in perforin or FasL cytotoxic effector pathways, is presented in Table 3. Note that the inflammatory process begins earlier and lasts longer in either B6 or IgM−/− mice deficient in perforin and appears to correlate with pulmonary virus load.
FIG. 8.
Histological examination of lung tissues from transgenic mice deficient in perforin, FasL, or both cytolytic pathways and infected with influenza virus. F5-RAG-1−/− (a, e, i, and m), F5-RAG-1−/−-P−/− (b, f, j, n, and n′), F5-RAG-1−/−-FasL−/− (c, g, k, and o), or F5-RAG-1−/− -P−/−-FasL−/− (d, h, l, and p) mice were infected with 104 PFU of A/Mem. Lungs taken on day 2 (A), day 5 (B), day 9 (C), or day 15 (D) were stained with hematoxylin and eosin and subjected to gross and microscopic pathological analysis. Original magnification, ×200.
TABLE 3.
Extent of pulmonary inflammatory process of perforin-, Fas/FasL-, or both perforin- and Fas/FasL-deficient mice infected with influenza A virusa
| Mice | Extent of inflammation at time (days) after infection
|
|||||
|---|---|---|---|---|---|---|
| 2 | 5 | 9 | 11 | 16 | 31 | |
| B6 | ND | − | + | ± | − | − |
| B6-P−/− | ND | ± | + | ± | ± | − |
| B6-FasL−/− | ND | − | + | ± | − | − |
| IgM−/− | ND | − | + | ± | − | − |
| IgM-P−/− | ND | ± | + | ++ | + | − |
| IgM-FasL−/− | ND | − | + | ± | − | − |
| F5-Rag-1−/− | − | + | ++ | ND | + | ± |
| F5-Rag-1−/−-P−/− | + | ++ | ++ | ++/− (a) | ++/− (a) | ++/− (a) |
| F5-Rag-1−/−-FasL−/− | + | ++ | ++ | ++ | ++ | ND |
| F5-Rag-1−/−-P−/−-FasL−/− | + | ++ | ++ | ++ | ++ | ++ |
Lung tissues were analyzed histologically at the indicated times following intranasal infection of mice with 104 PFU of A/Mem influenza virus. ++, intensive pathology with cell inflammation encompassing several pulmonary segments; development of extensive lung edema and congestion with thickened of intralaveolar septa and loss of alveoli. +, inflammatory reaction of a few foci of peribronchiolar and perivascular infiltrates on medium and small airways with modest evidence of epithelial necrosis. ±, lung tissues with a few infiltrates; no epithelial necrosis or desquamation. −, lung tissues well aerated; no infiltrates or pathological alteration of lung tissue. a, inflammatory reactions varying between mice; at this time point lungs of mice with undetectable viral titers were free of inflammatory reaction, whereas the rest of animals with high levels of viral titers exhibited intense inflammatory reactions. ND, not determined.
DISCUSSION
A complex and tightly regulated immune system utilizing multiple effector arms is necessary for an effective response to antigenically variable pathogens. On one hand the immune system must be efficient at targeting the pathogen while minimizing unnecessary tissue damage, while a virus with an optimal balance between adaptability and genomic integrity may be able to exploit any weakness in the response to it and persist within the host. This results in an intricate coevolutionary balance between host and pathogen (44). In this context, this study was undertaken to dissect the contribution of CTL effector pathways in viral clearance, immune pathogenesis, and CTL escape variant virus selection during influenza virus pneumonia of mice, a model for a disease of great medical importance to humans. The results indicate that, in agreement with previous reports (5, 39), virus elimination in the absence of perforin- or Fas-dependent CTL effector pathways was readily compensated for in mice with normal CD4+ T-cell and antibody responses. However, in B-cell-deficient mice the perforin pathway is more efficient than FasL-dependent mechanisms in CTL-mediated virus clearance, but either pathway is ultimately able to mediate control of influenza virus infection.
The degree to which host immunity induced against a particular virus strain enables reliable control of antigenic variants arising from that strain during primary or secondary infection and, more importantly, the contribution of various immune elements are as yet far from understood. Our findings suggest that the CTL response against influenza virus employs a sophisticated strategy by exploiting the diverging modes of action of perforin- and Fas/FasL-mediated cytolysis to cope with this highly variable virus. Previous studies showed that effector CTLs rapidly kill influenza virus-infected target cells via a perforin-dependent mechanism (39) and that this effector pathway is highly directional in that release of perforin and granzymes from CTL granules only occurs shortly after TCR activation at or near the contact point between CTL and the infected target cell. This tight regulation of perforin-mediated cytotoxicity is likely critical to avoid unnecessary damage to noninfected tissue. However, Fas expression is specifically up-regulated in influenza virus-infected cells (7), and a recent report has suggested that expression of Fas and related molecules may be essential for efficient influenza virus replication (42). Clearly, such induction of Fas renders influenza virus-infected cells sensitive to CTL attack via the Fas/FasL cytotoxic pathway. Importantly, in contrast to perforin, which is only released from CTL granules for a short period following TCR stimulation, FasL remains expressed on the surface of activated CTL for prolonged periods (18), raising the possibility that FasL-mediated CTL functions may be less dependent on TCR engagement and hence more promiscuous against Fas-expressing target cells than the tightly regulated perforin lytic pathway.
Several aspects make the F5-RAG-based experimental model uniquely suited for studies on the CTL effector mechanisms involved in selection of variant viruses. First, the lung represents an ideal site for selection of CTL escape variants, because the virus has several days to become established in the respiratory tract before CTL appear at sites of virus replication, producing a pool of virus that is a potential source of variants. As a consequence of virus restriction to the respiratory tract, the virus-specific CTL response is targeted to the lung and effector CTLs can reach extremely high frequencies in the BAL (25, 31). These factors combine to exaggerate CTL-mediated pressure on the virus, leading to selection of escape variants. Our findings show that cytokines (e.g., IFN-γ and TNF-α) secreted by activated T cells or by other cellular components of the inflammatory reaction play an ancillary role for the control of this infection in the lung, as illustrated by late control of infection in low-dose-infected TCR-transgenic mice lacking both perforin and Fas cytotoxic effector functions. Despite the prolonged persistence of virus in transgenic mice lacking both perforin and FasL, we could find no evidence for variant virus selection in these mice, suggesting that the overall immunological pressure exerted by T-cell-mediated cytokine production is comparatively weak and insufficient to drive variant virus selection. However, it is possible that cytokine secretion could partially control mutant virus replication, especially after Fas or perforin contact-dependent cytotoxicity is diminished following variant outgrowth. This provides a plausible explanation for the similar kinetics of escape mutant selection and propagation in TCR-transgenic mice deficient in perforin or FasL and infected with a low virus dose. Infection with a higher viral dose, where a large and more diverse repertoire of mutant viruses in the onset of infection can more easily overcome cytokine-mediated virus control, revealed a role for Fas-mediated cytotoxicity in suppression of mutant virus. However, limitations in this effector pathway may merit further comment. The FasL-mediated pathway requires T cells to become activated to trigger surface FasL expression and also requires Fas expression of target cells, raising the possibility that FasL may initially be efficient in suppressing variant emergence while wild-type virus is still present but, once wild-type virus is eliminated or reduced below a threshold level, CTL activation via cognate TCR and MHC-peptide interaction is abolished and consequently FasL expression is down-regulated, eventually making this effector mechanism ineffective against variant virus. Confinement of virus to one anatomic location, such as the lung, may exaggerate the variant suppression effects of the FasL effector pathway, because in the early stages of the selection process the pool of wild-type viruses and variant viruses derived from it must coexist at or near the same site, ensuring that cognate antigen (derived from wild-type virus) is available to maintain CTL activation and drive FasL expression. Such time and activation status limitations to the FasL cytolytic pathway may explain why mutant virus eventually emerges and replicates at high levels in FasL-sufficient mice. Clearly, Fas-directed cytotoxic activity is beneficial, but of limited effectiveness, in suppression of mutant virus and is perhaps regulated in this way to prevent collateral damage to uninfected tissue.
In our model, development of the initial inflammatory response and recruitment of transgenic CTLs to the lung was unaffected by the absence of either or both CTL cytolytic pathways. This initial recruitment of CTL was clearly associated with a decline in viral titers in mice retaining either FasL or perforin effector pathways, indicating that these cells retained significant antiviral function. Subsequent inflammatory events in these mice were influenced by persistence of variant virus within the lung, with animals able to reduce the pulmonary virus load also exhibiting minimal pulmonary pathology. This latter aspect was especially apparent in the fraction of F5-RAG-1−/−-P−/− mice that were essentially free of lung inflammation in the absence of detectable virus, suggesting that direct cytolysis via perforin has little overall pathological consequence. It must be noted, however, that a qualitatively different, more macrophage-dependent inflammatory pattern was reestablished once variant virus had reemerged to high titer in the lung. As such a variant virus can no longer be recognized by transgenic CTL, this clearly demonstrates that the inflammatory process is driven by the presence of virus rather than by the T-cell infiltrate. This correlates well with the situation observed in RAG-1−/− mice, which possess no T or B cells, where the inflammation is wholly virus driven (25). However, in F5-RAG-1−/−-P−/−-FasL−/− mice the inflammatory process is sustained by nonvariant virus driving cytokine secretion by CTL. This more severe T-cell-mediated inflammatory response manifests in extensive hemorrhagic damage, presumably as a result of excessive cytokine secretion by continual activation of transgenic CTL, leading to the increased vascular leakage and edema characteristic of viral pneumonia. Our results confirm and extend the concept that perforin and FasL CTL cytolytic pathways have limited contributions in the development and overall course of pulmonary inflammation during viral pneumonia, but rather a more significant role is played by CTL secretion of excessive amounts of proinflammatory cytokines (19, 36). While evidence that a robust CD4+ T-cell response may be essential for effective antiviral CD8+ T-cell immunity has been obtained in several experimental settings (3), the F5-RAG model does not seem to require CD4+ T-cell help to mount an antiviral response, due to the extremely high precursor frequency of the monoclonal transgenic CTLs. It is possible that, were CD4+ T cells available in this system, the antiviral effectiveness of transgenic CTLs might be significantly enhanced, which may conceivably delay the selection of escape variants.
In summary, our study demonstrates that where adaptive immune control of influenza virus infection is restricted to a highly focused CTL response, virus mutants escaping CTL control readily emerge and dominate the pool of persisting virus. Both perforin- and Fas-mediated cytolytic pathways are actively engaged in this process but appear to have differential contributions in determining the spectrum of emergent variant clones. Finally, our results demonstrate that CTLs contribute to pulmonary pathology primarily via inflammatory cytokine secretion and to a much lesser degree by lysis of their cellular targets.
Acknowledgments
This work was supported by NIH grant AI42114 to D.M.
REFERENCES
- 1.Ada, G. L., and P. D. Jones. 1986. The immune response to influenza infection. Curr. Top. Microbiol. Immunol. 128:1-54. [DOI] [PubMed] [Google Scholar]
- 2.Altman, J. D., P. A. H. Moss, P. J. R. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 3.Battegay, M., D. Moskophidis, A. Rahemtulla, H. Hengartner, T. W. Mak, and R. M. Zinkernagel. 1994. Enhanced establishment of a virus carrier state in adult CD4+ T-cell deficient mice. J. Virol. 68:4700-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow, P., and G. M. Shaw. 1998. Cytotoxic T-lymphocyte escape viral variants: how important are they in viral evasion of immune clearance in vivo? Immunol. Rev. 164:37-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, W., J. R. Bennink, P. A. Morton, and J. W. Yewdell. 2002. Mice deficient in perforin, CD4+ T cells, or CD28-mediated signaling maintain the typical immunodominance hierarchies of CD8+ T-cell responses to influenza virus. J. Virol. 76:10332-10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doherty, P. C., W. Allan, M. Eichelberger, and S. R. Carding. 1992. Roles of αβ and γδ T cell subsets in viral immunity. Annu. Rev. Immunol. 10:123-151. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto, I., T. Takizawa, Y. Ohba, and Y. Nakanishi. 1998. Co-expression of Fas and Fas-ligand on the surface of influenza virus-infected cells. Cell Death Differ. 5:426-431. [DOI] [PubMed] [Google Scholar]
- 8.Gorman, O. T., W. J. Bean, and R. G. Webster. 1992. Evolutionary processes in influenza viruses—divergence, rapid evolution, and stasis. Curr. Top. Microbiol. Immunol. 176:75-97. [DOI] [PubMed] [Google Scholar]
- 9.Goulder, P. J., and D. I. Watkins. 2004. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 8:630-640. [DOI] [PubMed] [Google Scholar]
- 10.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 11.Harty, J. T., A. R. Tvinnereim, and D. W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275-308. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann, E., G. Neumann, Y. Kawaoka, G. Hobom, and R. G. Webster. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. USA 97:6108-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kagi, D., B. Ledermann, K. Burki, B. Odermatt, K. J. Olsen, E. Podack, R. M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369:31-37. [DOI] [PubMed] [Google Scholar]
- 14.Kagi, D., B. Ledermann, K. Burki, R. M. Zinkernagel, and H. Hengartner. 1996. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu. Rev. Immunol. 14:207-232. [DOI] [PubMed] [Google Scholar]
- 15.Kashiwagi, T., N. Hamada, J. Iwahashi, K. Hara, T. Ueda, H. Noguchi, and T. Toyoda. 2000. Emergence of new influenza A viruses which carry an escape mutation of the HLA-B27-restricted CTL epitope of NP in Japan. Microbiol. Immunol. 44:867-870. [DOI] [PubMed] [Google Scholar]
- 16.Kilbourne, E. D. 1987. Influenza. Plenum Medical Book Company, New York, N.Y.
- 17.Lamb, R. A., and R. M. Krug. 1996. Orthomyxoviridae: the viruses and their replication, p. 1353-1395. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa.
- 18.Li, J. H., D. Rosen, D. Ronen, C. K. Behrens, P. H. Krammer, W. R. Clark, and G. Berke. 1998. The regulation of CD95 ligand expression and function in CTL. J. Immunol. 161:3943-3949. [PubMed] [Google Scholar]
- 19.Liu, A. N., A. Z. Mohammed, W. R. Rice, D. T. Fiedeldey, J. S. Liebermann, J. A. Whitsett, T. J. Braciale, and R. I. Enelow. 1999. Perforin-independent CD8+ T-cell-mediated cytotoxicity of alveolar epithelial cells is preferentially mediated by tumor necrosis factor-alpha: relative insensitivity to Fas ligand. Am. J. Respir. Cell Mol. Biol. 20:849-858. [DOI] [PubMed] [Google Scholar]
- 20.Liu, B., I. Mori, M. J. Hossain, L. Dong, Z. Chen, and Y. Kimura. 2003. Local immune responses to influenza virus infection in mice with a targeted disruption of perforin gene. Microb. Pathog. 34:161-167. [DOI] [PubMed] [Google Scholar]
- 21.Mamalaki, C., T. Norton, Y. Tanaka, A. R. Townsend, P. Chandler, E. Simpson, and D. Kioussis. 1992. Thymic depletion and peripheral activation of class I major histocompatibility complex-restricted T cells by soluble peptide in T-cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA 89:11342-11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMichael, A. 1994. Cytotoxic T lymphocytes specific for influenza virus. Curr. Top. Microbiol. Immunol. 189:75-91. [DOI] [PubMed] [Google Scholar]
- 23.McMichael, A. J., and R. E. Phillips. 1997. Escape of human immunodeficiency virus from immune control. Annu. Rev. Immunol. 15:271-296. [DOI] [PubMed] [Google Scholar]
- 24.Meyer-Olson, D., N. H. Shoukry, K. W. Brady, H. Kim, D. P. Olson, K. Hartman, A. K. Shintani, C. M. Walker, and S. A. Kalams. 2004. Limited T cell receptor diversity of HCV-specific T cell responses is associated with CTL escape. J. Exp. Med. 200:307-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moskophidis, D., and D. Kioussis. 1998. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J. Exp. Med. 188:223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskophidis, D., and R. M. Zinkernagel. 1996. Immunobiology of cytotoxic T-cell resistant virus variants: studies on lymphocytic choriomeningitis virus (LCMV). Semin. Virol. 7:3-11. [Google Scholar]
- 27.Oxenius, A., R. M. Zinkernagel, and H. Hengartner. 1998. CD4+ T-cell induction and effector functions: a comparison of immunity against soluble antigens and viral infections. Adv. Immunol. 70:313-367. [DOI] [PubMed] [Google Scholar]
- 28.Parker, C. E., and K. G. Gould. 1996. Influenza A virus—a model for viral antigen presentation to cytotoxic T-lymphocytes. Semin. Virol. 7:61-73. [Google Scholar]
- 29.Pircher, H.-P., D. Moskophidis, U. Rohrer, K. Burki, H. Hengartner, and R. M. Zinkernagel. 1990. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature 346:629-633. [DOI] [PubMed] [Google Scholar]
- 30.Price, G. E., A. Gaszewska-Mastarlarz, and D. Moskophidis. 2000. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J. Virol. 74:3996-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price, G. E., R. Ou, H. Jiang, L. Huang, and D. Moskophidis. 2000. Viral escape by selection of cytotoxic T cell-resistant variants in influenza A virus pneumonia. J. Exp. Med. 191:1853-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rimmelzwaan, G. F., E. G. Berkhoff, N. J. Nieuwkoop, R. A. Fouchier, and A. D. Osterhaus. 2004. Functional compensation of a detrimental amino acid substitution in a cytotoxic-T-lymphocyte epitope of influenza a viruses by comutations. J. Virol. 78:8946-8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo, S. H., E. Hoffmann, and R. G. Webster. 2002. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 8:950-954. [DOI] [PubMed] [Google Scholar]
- 34.Shen, Z., G. Reznikoff, G. Dranoff, and K. L. Rock. 1997. Cloned dendritic cells can present antigen on both MHC class I and class II molecules. J. Immunol. 158:2723-2730. [PubMed] [Google Scholar]
- 35.Slifka, M. K., and J. L. Whitton. 2000. Antigen-specific regulation of T cell-mediated cytokine production. Immunity 12:451-457. [DOI] [PubMed] [Google Scholar]
- 36.Small, B. A., S. A. Dressel, C. W. Lawrence, D. R. R. Drake, M. H. Stoler, R. I. Enelow, and T. J. Braciale. 2001. CD8+ T cell-mediated injury in vivo progresses in the absence of effector T cells. J. Exp. Med. 194:1835-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stohr, K. 2002. Influenza—W.H.O. cares. Lancet Infect. Dis. 2:517. [DOI] [PubMed] [Google Scholar]
- 38.Takizawa, T., R. Fukuda, T. Miyawaki, K. Ohashi, and Y. Nakanishi. 1995. Activation of the apoptotic Fas antigen-encoding gene upon influenza virus infection involving spontaneously produced beta-interferon. Virology 209:288-296. [DOI] [PubMed] [Google Scholar]
- 39.Topham, D. J., R. A. Tripp, and P. C. Doherty. 1997. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 159:5197-5200. [PubMed] [Google Scholar]
- 40.Trapani, J. A., and M. J. Smyth. 2002. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 10:735-747. [DOI] [PubMed] [Google Scholar]
- 41.Webster, R. G., W. G. Laver, G. M. Air, and G. C. Schild. 1982. Molecular mechanisms of variation in influenza viruses. Nature 296:115-121. [DOI] [PubMed] [Google Scholar]
- 42.Wurzer, W. J., C. Ehrhardt, S. Pleschka, F. Berberich-Siebelt, T. Wolff, H. Walczak, O. Planz, and S. Ludwig. 2004. NF-κB-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas/FasL is crucial for efficient influenza virus propagation. J. Biol. Chem. 279:30931-30937. [DOI] [PubMed] [Google Scholar]
- 43.Yewdell, J. W., R. G. Webster, and W. U. Gerhard. 1979. Antigenic variation in three distinct determinants of an influenza type A haemagglutinin molecule. Nature 279:246-248. [DOI] [PubMed] [Google Scholar]
- 44.Zinkernagel, R. M. 1996. Immunology taught by viruses. Science 271:173-178. [DOI] [PubMed] [Google Scholar]