Abstract
Background
The stromal cell derived factor 2 (SDF2) relates closely to the occurrence and development of several kind of cancers. There are few studies to investigate the clinicopathological and prognostic significance of SDF2 in gastric cancer (GC) patients.
Methods
We detected SDF2 expression in GC and normal gastric tissues using bioinformatics, western blot and immunohistochemistry. Furthermore, we tested the relationship between SDF2 expression and clinicopathological characteristics and prognosis of GC patients.
Results
Bioinformatics, western blot and immunohistochemistry results showed that SDF2 expression in GC tissue was higher than that in normal gastric tissue (P < 0.01). SDF2 expression was associated with Borrmann classification III-IV (χ2 = 6.484, P = 0.011), depth of infiltration T3-T4 (χ2 = 9.140, P = 0.003), positive lymph node metastasis (χ2 = 24.945, P = 0.000) and TNM III-IV stage (χ2 = 9.945, P = 0.002) of GC patients. The Cox regression analysis indicated that distant metastasis M1 stage (HR = 6.026, 95% CI: 1.880-19.318, P = 0.003), TNM III-IV (HR = 1.833, 95% CI: 1.023–3.287, P = 0.042) and SDF2 high expression (HR = 2.091, 95% CI: 1.064–4.108, P = 0.032) were independent risk factors for OS of GC patients. Kaplan-Meier test showed that the OS of GC patients with SDF2 high expression was much poorer than that of GC patients with SDF2 low-expression (χ2 = 22.925, P = 0.000).
Conclusion
SDF2 expression is high in GC tissue and is correlated with Borrmann classification III-IV, tumor infiltration depth, positive lymph node metastasis and TNM III-IV stage of GC patients. GC patients with SDF2 high-expression have significantly poor OS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-024-03430-5.
Keywords: SDF2, Gastric cancer, Clinicopathological significance, Prognosis, Bioinformatics
Background
Gastric cancer (GC) is the fifth most common cancers in the world with high prevalence and poor survival and a global health challenge [1, 2]. In recent years, significant progression has been made in the diagnosis and treatment of GC, but many patients are first diagnosed at the advanced stage. When metastasis of GC occurs, the median survival is poor [3]. Early diagnosis is vital for comprehensive treatment of GC patients, which can improve the survival of GC patients [4]. Previous studies showed that the differential expressions of multiple genes are involved in the occurrence and development of GC [5]. So far, there is still lack of indicators for early diagnosis and treatment of GC in clinical practice. Therefore, it is necessary to find a diagnostic and prognostic indicator for GC.
Stromal cell derived factor 2 (SDF2) belongs to stromal cell-derived factors family, and is a small protein of 211 amino acids [6]. Previous studies have shown that SDF2 is a protein involved in the process of endoplasmic reticulum (ER) stress [7]. The abnormal activation of ER stress sensors and their downstream signal pathway have become key regulators of tumor growth, metastasis, chemotherapy, targeted therapy and immunotherapy [8]. SDF2 is an important regulator for trophoblast cells to control cell survival under ER stress [9]. Moreover, in some cancer cells, SDF2 is also associated with cell survival and apoptosis [10]. Recently, studies showed that SDF2 expression is involved in the occurrence and development of several kinds of cancers [11, 12]. Giulianelli et al. indicated that SDF2 was high expression and related to hormone-independent tumor growth in breast cancer [12]. Expression of SDF2 were significantly reduced in patients with metastasis of breast cancer and the patients with poor survival [13]. However, the clinicopathological and prognostic significance of SDF2 in GC has not been reported.
In our study, we predicted the differential level of SDF2 expression in GC and normal gastric tissues and its impact on the clinicopathological characteristics and prognosis of GC patients. Furthermore, we further detected the clinicopathological and prognostic significance of SDF2 in the patients with GC.
Materials and methods
Bioinformatics prediction
To detect the level of SDF2 expression in GC and gastric tissues, TIMER (http://timer.cistrome.org/) and UALCAN databases (https://ualcan.path.uab.edu/) were used to analyze the clinicopathological significance of SDF2 expression in GC patients. Furthermore, we utilize the GEPIA database (http://gepia.cancer-pku.org/) online to analysis the impact of SDF2 expression on prognosis of GC patients.
Patients and methods
Our study included 94 GC patients who underwent surgical resection in the Central Hospital of Dalian University of Technology (Dalian Municipal Central Hospital) from October 2014 to June 2016. The period of follow-up was more than 5 years. The recruited criteria were as follows: (1) GC patients undergoing surgical resection. (2) histological diagnosis of GC. (3) the availability of complete clinicopathological parameters and follow-up data. (4) the patients had signed the informed consent. The exclusion criteria were as follows: (1) patients died before discharge. (2) patients with preoperative radiotherapy and chemotherapy. (3) patients with multiple cancers. The general information of 94 GC patients was listed in Table 1. The study was approved by the Ethics Committee of the Central Hospital of Dalian University of Technology (Dalian Municipal Central Hospital) (approval number: YN2023-100-01), and all the participants signed the written informed consent.
Table 1.
The general information of included GC patients
| Clinicopathological parameters | Cases(N = 94) |
|---|---|
| Gender | |
| Male | 67(71.28%) |
| Female | 27(28.72%) |
|
Age(years) <65 ≥ 65 |
53(56.38%) 41(43.62%) |
| T stage | |
| T1 | 22 (23.41%) |
| T2 | 9 (9.57%) |
| T3 | 60(63.83%) |
| T4 | 3 (3.19%) |
| N stage | |
| No | 35 (37.23%) |
| Yes | 59 (62.77%) |
| M stage | |
| M0 | 90 (95.74%) |
| M1 | 4 (4.26%) |
| TNM stage | |
| I | 29 (30.85%) |
| II | 32 (34.04%) |
| III | 29 (30.85%) |
| IV | 4 (4.26%) |
| Differentiation | |
| Well/ moderate | 32 (34.04%) |
| Poor | 62 (65.96%) |
| Borrmann classification | |
| I- II | 23(24.47%) |
| III- IV | 71(75.53%) |
| Tumor size (cm) | |
| ≤ 5 | 61 (64.89%) |
| >5 | 33 (35.11%) |
Western blot analysis
GC tissue and normal gastric tissue specimens (2 cm from the edge of the GC) were collected from 15 GC patients who underwent surgical treatment at Central Hospital of Dalian University of Technology (Dalian Municipal Central Hospital) in March 2023. All patients were diagnosed with GC through pathological examination and did not receive radiotherapy or chemotherapy before surgery. The patients included 9 males and 6 females, and aged 52–77 years old (average age was 64.20 ± 7.66 years old). GC and normal gastric tissue specimens are fresh surgical specimens that have been removed. They are rinsed with normal saline and stored in liquid nitrogen.
GC and normal gastric tissues samples were lysed in RIPA buffer (Beyotime, Shanghai, China). Protein quantified and equal amounts of protein were separated and electrophoretically transferred to polyvinylidene difluoride membranes. The membrane was blocked and incubated with SDF2 primary antibody (1:1000, ab82804, Abcam, UK). Then the membrane was incubated with anti-β-actin antibody (1:1,000, TransGen Biotech, Beijing, China). The proteins were visualized using ECL reagents (Beyotime, Jiangsu, China).
Immunohistochemical staining
Paraffin specimens from 94 GC and 47 normal gastric tissues (2 cm from the edge of the GC) who met the inclusion criteria were collected at Dalian Municipal Central Hospital from October 2014 to June 2016. The GC tissues and normal gastric tissues were dehydrated, embedded in paraffin. Paraffin tissue section was 4 μm and deparaffinized in 90%-70% alcohol for 3 min. Then sections were incubated in citrate buffer and hydrogen peroxide. After blocking nonspecific binding sites with 5% goat serum albumin for 30 min, sections were incubated with SDF2-specifc antibody (1:200, ab82804, Abcam, UK). After washed, samples were counterstained with hematoxylin.
We used the immunoreactive score (IRS) method [14] to evaluate the degree of staining: no was 0, light yellow was 1, brown was 2 and dark was brown. Then the percentage of positively stained cells in each region was calculated, and the score was evaluated as follow: the score for 0% of positive cells was 0, that for < 10% was 1, that for 10–50% was 2, that for 51–80% was 3, and that for > 80% was 4. Calculate the IRS (range from 0 to 12) for each slice, which is generated by multiplying the staining intensity by the positively stained cells. ISR 0–6 is considered as the SDF2 low expression group, and IRS 7–12 is considered as the SDF2 high expression group.
Statistical analysis
SPSS 26.0 software (SPSS, Inc., Chicago, IL, USA) was used for statistically analysis. Chi-squared test (χ2) was used to estimate the SDF2 expression and clinicopathologic characteristics of GC patients. The Cox regression analysis was used for assessing prognostic factors of OS of GC patients. Kaplan-Meier method and log-rank test were used to analysis the relationship between SDF2 expression and OS of GC patients. P ≤ 0.05 was considered as statistically significant.
Results
SDF2 is high-expression in GC tissues
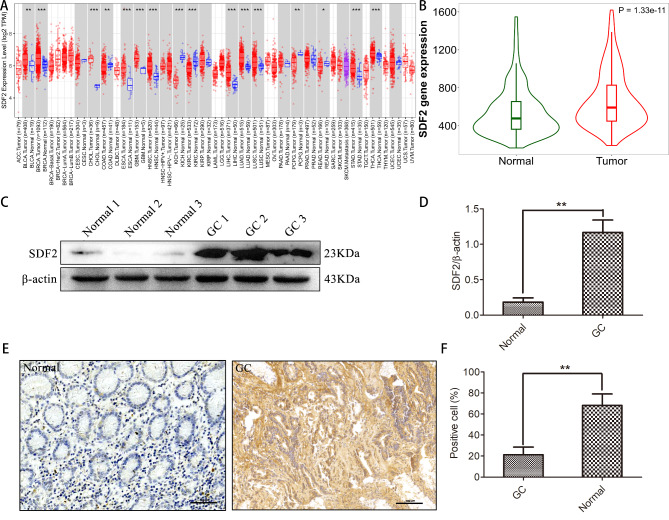
To explore the differential expressions of SDF2 between GC and normal gastric tissues, we initially used TIMER and TNM plot databases. Our results showed that the SDF2 expression was higher in GC compared to normal gastric tissues (P < 0.05, Fig. 1A-B). Then western blot was used to detect SDF2 expression in GC and normal gastric tissues. We observed that SDF2 expression was significantly higher in GC tissues (Fig. 1C-D). Moreover, we chose 94 GC and 47 normal gastric tissues to verify the expression of SDF2 by immunohistochemistry. (Fig. 1E). Our results indicated that SDF2 high expression was detected in 44.68% (42/94) of GC tissues, while 23.40% (11/47) was observed in normal gastric tissues, the SDF2 expression was obviously higher in GC tissues and normal gastric tissues (χ2 = 6.046, P = 0.014) (Table 2). Therefore, these results showed that the SDF2 was high expression in GC tissues.
Fig. 1.
SDF2 is high expression in GC tissues. (A) The expression level of SDF2 in different kinds cancers and normal tissue samples in TIMER database (STAD: stomach adenocarcinoma, CHOL: cholangio carcinoma, LIHC: liver hepatocellular carcinoma, COAD: colon adenocarcinoma, KIRC: Kidney renal clear cell carcinoma, READ: rectum adenocarcinoma, ACC: adrenocortical carcinoma, BLCA: bladder urothelial carcinoma, BRCA: breast cancer, CESC: cervical squamous cell carcinoma, ESCA: esophageal carcinoma, GBM: glioblastoma multiforme, HNSC: head and neck squamous cell carcinoma, KICH: kidney chromophobe, KIRP: kidney renal papillary cell carcinoma, KIRC: kidney renal clear cell carcinoma, LAML: acute myeloid leukemia, LUAD: lung adenocarcinoma, LUSC: lung squamous cell carcinoma, PAAD: pancreatic adenocarcinoma, PCPG: pheochromocytoma and paraganglioma, PRAD: prostate adenocarcinoma, SARC: sarcoma, SKCM: skin cutaneous melanoma, TGCT: testicular germ cell tumors, THCA: thyroid carcinoma, THYM: thymoma, UCEC: uterine corpus endometrial carcinoma, UVM: uveal melanoma). (B) The expression level of SDF2 in GC tissue samples is significantly higher compared with normal gastric tissue samples in the database (t test was used to compare the two groups). (C) The expression of SDF2 was determined by western blotting assay. (D) SDF2 protein expression levels in GC tissues were higher compared with normal tissues by western blotting (n = 15). **P˂0.01 compared with the normal group. (E) SDF2 immunohistochemical staining in clinical normal gastric tissue and GC tissues samples (bar = 100 μm). (F) Statistical analysis showed that SDF2 expression in GC tissues were higher than that in normal gastric tissues by immunohistochemical staining (n = 10). **P˂0.01 compared with the normal group
Table 2.
SDF2 expression in GC and normal gastric tissues
| n | SDF2 | χ2 | P value | ||
|---|---|---|---|---|---|
| positive | negative | ||||
| GC | 94 | 42 | 52 | 6.046 | 0.014 |
| Normal | 47 | 11 | 36 | ||
Correlation between SDF2 and clinicopathological parameters in GC patients
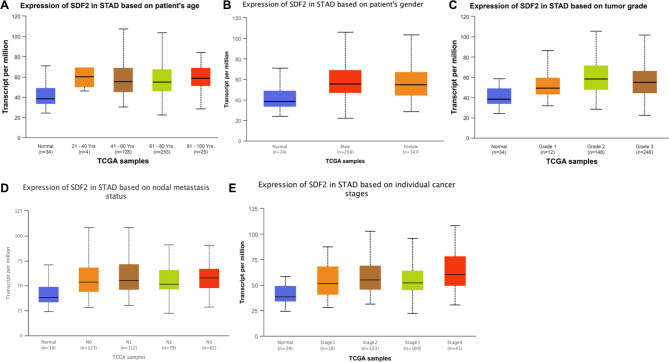
To detect the clinicopathological significance of SDF2 high expression in GC tissues, we tested the relationship between SDF2 expression and clinicopathological parameters of GC patients. First, the UALCAN database showed that SDF2 expression was associated with lymph node metastasis and TNM stage of GC patients (P < 0.05) (Fig. 2). We further detected the relationship between the level of SDF2 expression and clinicopathological parameters of 94 GC patients. We found that SDF2 expression was correlated with the Borrmann classification (χ2 = 6.484, P = 0.011), depth of tumor invasion (χ2 = 9.140, P = 0.003), lymph node metastasis (χ2 = 24.945, P = 0.000) and TNM stage (χ2 = 9.945, P = 0.002). However, there were no significant differences between SDF2 expression and age, gender, tumor size and differentiation (Table 3).
Fig. 2.
The relationship between SDF2 expression and clinicopathological parameters of GC patients in UALCAN database. (A) age. (B) gender. (C) differentiation. (D) lymph node metastasis. (E) TNM stage
Table 3.
The association between SDF2 expression and clinicopathological parameters of GC
| Clinical factors | Total (n = 94) | SDF2 expression | χ2 | P-value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Age | 0.305 | 0.581 | |||
| < 65 | 53 | 28 | 25 | ||
| ≥ 65 | 41 | 24 | 17 | ||
| Gender | 0.895 | 0.344 | |||
| Male | 67 | 35 | 32 | ||
| Female | 27 | 17 | 10 | ||
| Tumor size | 3.421 | 0.064 | |||
| ≤ 5 cm | 61 | 38 | 23 | ||
| ˃ 5 cm | 33 | 14 | 19 | ||
| Histology | 0.017 | 0.896 | |||
| Differentiated | 32 | 18 | 14 | ||
| Undifferentiated | 62 | 34 | 28 | ||
| Borrmann | 6.484 | 0.011 | |||
| I-II | 23 | 18 | 5 | ||
| III-IV | 71 | 34 | 37 | ||
| Depth of tumor invasion | 9.140 | 0.003 | |||
| T1-T2 | 31 | 24 | 7 | ||
| T3-T4 | 63 | 28 | 35 | ||
| Lymph node metastasis | 24.945 | 0.000 | |||
| N | 35 | 31 | 4 | ||
| Y | 59 | 21 | 38 | ||
| Distant metastasis | 1.554 | 0.321 | |||
| M0 | 90 | 51 | 39 | ||
| M1 | 4 | 1 | 3 | ||
| TNM stage | 9.945 | 0.002 | |||
| I-II | 61 | 41 | 20 | ||
| III-IV | 33 | 11 | 22 | ||
| CEA | 0.018 | 0.894 | |||
| High | 41 | 23 | 18 | ||
| Low | 53 | 29 | 24 | ||
| CA199 | 0.024 | 0.877 | |||
| High | 35 | 19 | 16 | ||
| Low | 59 | 33 | 26 | ||
Association between SDF2 expression and OS of GC patients
We further explored the prognostic factors of GC patients by Cox regression analysis. Univariate Cox regression analysis showed that the OS of GC patients was related with depth of tumor invasion (HR = 2.839, P = 0.001), lymph node metastasis (HR = 2.101, P = 0.008), distant metastasis (HR = 8.334, P = 0.000), TNM stage (HR = 2.915, P = 0.000) and SDF2 expression (HR = 3.252, P = 0.000). Then we used multivariate Cox regression analysis, and we found that distant metastasis (HR = 6.026, P = 0.003), TNM stage (HR = 1.833, P = 0.042) and SDF2 expression (HR = 2.091, P = 0.032) were independent factors of OS of GC patients (Table 4).
Table 4.
Results of the univariate and multivariate analyses of the OS
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | ||
| Gender | 1.152 (0.658,2.017) | 0.621 | |||
| Age(years) | 0.809 (0.489,1.338) | 0.408 | |||
| Tumor size(cm) | 1.365 (0.812,2.293) | 0.240 | |||
| Histology | 1.167 (0.678,2.007) | 0.577 | |||
| Borrmann | 1.684 (0.875,3.241) | 0.118 | |||
| Depth of tumor invasion | 2.839 (1.505,5.355) | 0.001 | 1.537 (0.735,3.217) | 0.254 | |
| Lymph node metastasis | 2.101 (1.197,3.686) | 0.010 | 1.269 (0.635,2.538) | 0.500 | |
| Distant metastasis | 8.334 (2.753,25.232) | 0.000 | 6.026 (1.880,19.318) | 0.003 | |
| TNM stage | 2.915 (1.749,4.857) | 0.000 | 1.833 (1.023,3.287) | 0.042 | |
| SDF2 expression | 3.252 (1.943,5.443) | 0.000 | 2.091 (1.064,4.108) | 0.032 | |
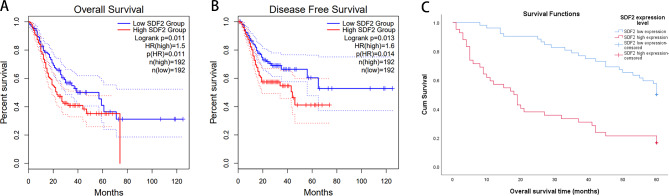
Next, the prognostic significance of SDF2 expression in GC patients was explored, we investigated the relationship between SDF2 expression and prognosis (including OS and disease-free survival) of GC patients through the GEPIA database, and we found that SDF2 high expression in GC patients resulted in a remarkably poorer OS (P = 0.011) (Fig. 3A) and disease-free survival (P = 0.013) (Fig. 3B). In addition, the relationship between SDF2 expression and OS of 94 GC patients was evaluated by Kaplan-Meier method. Our results showed that the OS of GC patients with SDF2 high expression was significantly poorer than that of GC patients with SDF2 low expression (χ2 = 22.925, P = 0.000) (Fig. 3C).
Fig. 3.
Association between SDF2 expression and OS of GC patients. (A) OS curve based on TCGA data. (B) Disease-free survival curve based on TCGA data. (C) Kaplan-Meier method evaluated SDF2 expression and OS of GC patients (χ2 = 22.925, P = 0.000)
Discussion
GC is one of tumors of digestive tract with high morbidity and mortality, which is characterized by strong invasiveness, high recurrence and metastasis rate and poor survival [15–17]. Although the progression of surgical technology, radiotherapy, chemotherapy and other diagnostic and therapeutic technologies have significantly improved the prognosis of GC patients, the mortality rate is still high [18, 19]. At present, great progression has been made in understanding the pathogenesis of GC from the perspective of biology and genomics, which makes the targeted therapy of advanced GC enter clinical research and practice. Previously, researchers identified the SDF2 gene on human chromosome 17 qll.2 through in situ hybridization [20]. In addition, there is some evidence that SDF2 is involved in occurrence and development of malignant tumors. However, the expression and role of SDF2 in GC has not been reported. In our results, for the first time, we found: (1) SDF2 is high expression in GC tissues. (2) High expression of SDF2 is associated with Borrmann classification, depth of tumor invasion, positive lymph node metastasis and TNM stage of GC patients. (3) GC patients with high expression of SDF2 have significantly poor OS.
Previous studies have revealed that different expressions of SDF2 in several malignant tumors and is related to the prognosis of different tumors. For example, Vendrell et al. analyzed genomics and transcriptomics data in a series of Dukes B and C colorectal carcinomas, and 68 genes were identified, including low expression of SDF2 [11]. Takahashi et al. revealed that the SDF2 expression was up-regulated in oxaliplatin-resistant GC cells [21]. In our study, we found the different levels of SDF2 expression in GC tissue and normal gastric tissue through bioinformatics analysis. Furthermore, our results confirmed that the expression of SDF2 in GC tissue was higher than that in normal gastric tissue by western blot and immunohistochemistry.
SDF2 is one of the resident proteins of endoplasmic reticulum. It is reported that chronic ER stress is involved in cancer, diabetes and degenerative disease [22]. Moreover, Schott et al. demonstrated that SDF2 protein can be up-regulated by ER stress [13]. Further understand the mechanism of SDF2 in ER stress may help to identify its possible downstream targets in cancer [23]. Although the level of SDF2 expression has been detected in several types of tumors, there is no data to detect its clinicopathological significance of GC patients. Based on the data of 94 patients with GC, we found that the SDF2 expression was related with Borrmann classification, tumor invasion, positive lymph node metastasis and TNM stage. However, the expression of SDF2 was not related with gender, age, tumor size, histological differentiation. Furthermore, In the TCGA samples, the relationship between SDF2 expression and age, gender, tumor grade, lymph node metastasis, cancer stage of GC patients was analyzed, and the age was analyzed by age stratification. The results were consistent with the conclusions of our clinical data. These results indicated that SDF2 may be involved in the tumor malignant biological behavior, especially metastasis. These differences may be partly due to limitations of sample size. Therefore, this needs to be further studied in involving more patients with GC.
In recent years, many scholars began to explore the expression and role of SDF2 in tumors. Kang et al. demonstrated that different levels of SDF2 expression in breast cancer patients, and its upregulation was associated with better clinical outcomes [24]. In colorectal cancer, it has been reported that SDF2 expression is down-regulated, and the low expression of SDF2 may be associated with low survival rate [11]. Recent study has shown that the inhibition of SDF2 leads to the enhancement of Oxaliplatin-induced anti-proliferation and apoptosis, and SDF2 may be a new therapeutic target of Oxaliplatin-resistant GC cells [21]. At present, there is no study to detect the relationship between SDF2 expression and prognosis of GC patients. Consistent with the prognosis predicted by GEPIA database, our study showed that the OS of GC patients with SDF2 high expression was significantly poorer than that of GC patients with SDF2 low expression, and SDF2 expression may be an independent predictor of poor OS of GC patients.
In our research, several shortcomings cannot be ignored. First, it was a single center retrospective study, and the sample of included GC patients was relatively small. A multicenter study including large sample of GC patients is needed to detect the significance of SDF2 expression in GC patients. Second, we used immunohistochemistry to explore the SDF2 expression in GC tissue, and IRS method is a semi-quantitative score in each region which could not be representative of the whole region of GC tissue. Therefore, the heterogeneity in our study could not be ignored.
Conclusion
In this study, we found that SDF2 was high expression in GC tissues. High expression of SDF2 was related with Borrmann classification, tumor invasion, positive lymph node metastasis, TNM stage and poor prognosis of GC. These data suggest that SDF2 may be a potential target for therapeutic intervention of GC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not application.
Abbreviations
- GC
gastric cancer
- SDF2
stromal cell derived factor 2
- IRS
immunoreactive score
Author contributions
Guo Zu conceived and designed the experiments, and revised the manuscript; Yuhang Wang, Mingcan Zheng and Shaohua Du collected the data and wrote the manuscript; Yuhang Wang, Mingcan Zheng, Shaohua Du, Puxu Wang and Taotao Zhang provided the study materials; Yuhang Wang, Mingcan Zheng, Shaohua Du, Puxu Wang, Taotao Zhang and Xiangwen Zhang analyzed the data. All authors approved the final version for publication.
Funding
This work was supported by Fund of Ph.D. research initiation program of Liaoning province [2022-BS-358].
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics declarations statements
The experiment protocol has been reviewed and approved by the ethics committee of The Central Hospital of Dalian University of Technology (Dalian Municipal Central Hospital) (approval number: YN2023-100-01). All the participants signed the written informed consent.
Consent for publication
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuhang Wang, Mingcan Zheng and Shaohua Du are co-first authors.
References
- 1.Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer Lancet. 2020;396(10251):635–48. [DOI] [PubMed] [Google Scholar]
- 2.Chia NY, Tan P. Molecular classification of gastric cancer. Ann Oncol. 2016;27(5):763–9. [DOI] [PubMed] [Google Scholar]
- 3.Patel TH, Cecchini M. Targeted therapies in Advanced Gastric Cancer. Curr Treat Options Oncol. 2020;21(9):70. [DOI] [PubMed] [Google Scholar]
- 4.Rosa F, Tortorelli AP, Quero G, et al. The impact of preoperative ASA-physical status on postoperative complications and long-term survival outcomes in gastric cancer patients. Eur Rev Med Pharmacol Sci. 2019;23(17):7383–90. [DOI] [PubMed] [Google Scholar]
- 5.Boonyanugomol W, Rukseree K, Kongkasame W, et al. Genetic polymorphisms of CXCL8 (-251) are Associated with the susceptibility of Helicobacter pylori infection increased the risk of inflammation and gastric Cancer in Thai Gastroduodenal patients. Iran J Allergy Asthma Immunol. 2019;18(4):393–401. [DOI] [PubMed] [Google Scholar]
- 6.Ponting CP. Novel repeats in ryanodine and IP3 receptors and protein O-mannosyltransferases. Trends Biochem Sci. 2000;25(2):48–50. [DOI] [PubMed] [Google Scholar]
- 7.Schott A, Ravaud S, Keller S, et al. Arabidopsis stromal-derived Factor2 (SDF2) is a crucial target of the unfolded protein response in the endoplasmic reticulum. J Biol Chem. 2010;285(23):18113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Cubillos-Ruiz JR. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat Rev Cancer. 2021;21(2):71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorenzon-Ojea AR, Guzzo CR, Kapidzic M, et al. Stromal cell-derived factor 2: a Novel protein that interferes in endoplasmic reticulum stress pathway in human placental cells. Biol Reprod. 2016;95(2):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black S, Kadyrov M, Kaufmann P, et al. Syncytial fusion of human trophoblast depends on caspase 8. Cell Death Differ. 2004;11(1):90–8. [DOI] [PubMed] [Google Scholar]
- 11.Vendrell E, Ribas M, Valls J, et al. Genomic and transcriptomic prognostic factors in R0 dukes B and C colorectal cancer patients. Int J Oncol. 2007;30(5):1099–107. [PubMed] [Google Scholar]
- 12.Giulianelli S, Herschkowitz JI, Patel V, et al. MPA-induced gene expression and stromal and parenchymal gene expression profiles in luminal murine mammary carcinomas with different hormonal requirements. Breast Cancer Res Treat. 2011;129(1):49–67. [DOI] [PubMed] [Google Scholar]
- 13.Kang H, Escudero-Esparza A, Douglas-Jones A, et al. Transcript analyses of stromal cell derived factors (SDFs): SDF-2, SDF-4 and SDF-5 reveal a different pattern of expression and prognostic association in human breast cancer. Int J Oncol. 2009;35(1):205–11. [DOI] [PubMed] [Google Scholar]
- 14.Segami K, Aoyama T, Hiroshima Y, et al. Clinical significance of TAP1 and DLL4 expression in patients with locally advanced gastric Cancer. Vivo. 2021;35(5):2771–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melloni M, Bernardi D, Asti E, et al. Perforated gastric Cancer: a systematic review. J Laparoendosc Adv Surg Tech A. 2020;30(2):156–62. [DOI] [PubMed] [Google Scholar]
- 16.Rawicz-Pruszyński K, Mielko J, Pudło K, et al. Yield of staging laparoscopy in gastric cancer is influenced by Laurén histologic subtype. J Surg Oncol. 2019;120(7):1148–53. [DOI] [PubMed] [Google Scholar]
- 17.Lee K, Kim KW, Lee JB, et al. Impact of remnant stomach volume and anastomosis on nutrition and body composition in gastric cancer patients. Surg Oncol. 2019;31:75–82. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira C, Pinheiro H, Figueiredo J, et al. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015;16(2):e60–70. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Q, Cao L, Guan L, et al. Immunotherapy for gastric cancer: dilemmas and prospect. Brief Funct Genomics. 2019;18(2):107–12. [DOI] [PubMed] [Google Scholar]
- 20.Hamada T, Tashiro K, Tada H, et al. Isolation and characterization of a novel secretory protein, stromal cell-derived factor-2 (SDF-2) using the signal sequence trap method. Gene. 1996;176(1–2):211–4. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Tanaka M, Yashiro M, et al. Protection of stromal cell-derived factor 2 by heat shock protein 72 prevents oxaliplatin-induced cell death in oxaliplatin-resistant human gastric cancer cells. Cancer Lett. 2016;378(1):8–15. [DOI] [PubMed] [Google Scholar]
- 22.Lorenzon-Ojea AR, Yung HW, Burton GJ, et al. The potential contribution of stromal cell-derived factor 2 (SDF2) in endoplasmic reticulum stress response in severe preeclampsia and labor-onset. Biochim Biophys Acta Mol Basis Dis. 2020;1866(2):165386. [DOI] [PubMed] [Google Scholar]
- 23.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–6. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda S, Sumii M, Masuda Y, et al. Murine and human SDF2L1 is an endoplasmic reticulum stress-inducible gene and encodes a new member of the Pmt/rt protein family. Biochem Biophys Res Commun. 2001;280(1):407–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.