Abstract
Background
Chitosan oligosaccharides (COS) have great potential for applications in several fields, including agriculture, food industry or medicine. Nevertheless, the large-scale use of COS requires the development of cost-effective technologies for their production. The main objective of our investigation was to develop an effective method of enzymatic degradation of chitosan in a column reactor using Mucor circinelloides IBT-83 cells, immobilized in a polyurethane foam (PUF). These cells serve as a source of chitosanolytic enzymes.
Results
The study revealed that the process of freeze-drying of immobilized mycelium increases the stability of the associated enzymes during chitosan hydrolysis. The use of stabilized preparations as an active reactor bed enables the production of COS at a constant level for 16 reactor cycles (384 h in total), i.e. 216 h longer compared to non-stabilized mycelium. In the hydrolysate, oligomers ranging in structure from dimer to hexamer as well as D-glucosamine were detected. The potential application of the obtained product in agriculture has been verified. The results of phytotests have demonstrated that the introduction of COS into the soil at a concentration of 0.01 or 0.05% w/w resulted in an increase in the growth of Lepidium sativum stem and root, respectively (extensions by 38 and 44% compared to the control sample).
Conclusions
The research has verified that the PUF-immobilized M. circinelloides IBT-83 mycelium, which has been stabilized through freeze-drying, is a promising biocatalyst for the environmentally friendly and efficient generation of COS. This biocatalyst has the potential to be used in fertilizers.
Keywords: Chitooligosaccharides, Whole-cell biocatalyst, Chitosanase, Mucor circinelloides, Promoting plant growth
Introduction
Chitosan is an N-deacetylated derivative of chitin – a copolymer built of β-(1,4)-2-amino-2-deoxy-D-glucopyranose and β-(1,4)-2-acetamido-2-deoxy-D-glucopyranose. Basic properties of this biopolymer that contribute to its wide range of industrial applications are biocompatibility, bioactivity and biodegradability. For many years it has been attracting the attention of agriculture, food, pharmaceutical, medical, cosmetic and textile industries, as well as environmental protection and biotechnology. It should be noted that the number of chitosan applications has been increasing every year, along with the growing number of its modifications and newfound interesting properties of its degradation products [1–5].
Chitosans that have a degree of polymerization (DP) below 20 and an average molecular weight (MW) below 3.9 kDa are called chitooligosaccharides (COS), chitosan oligomers or chitooligomers [6–8]. Both high-molecular chitosan and COS have a number of interesting properties, they are bioactive (they exhibit e.g. antioxidant, anti-inflammatory, antimicrobial, antiviral, antiangiogenic, antitumor and prebiotic properties). Furthermore, they are biocompatible, non-toxic, and non-allergenic. Often, the biological activity of COS obtained from chitosan is higher compared to the starting polysaccharide. In addition, the fractions of degradation products with a low DP value are completely soluble at neutral pH. These solutions possess a low viscosity, thereby enabling the possible application and modification of these compounds [3].
COS can be produced from chitosan by various degradation methods: physical, chemical and enzymatic or a combination of them [8]. The enzymatic hydrolysis of this biopolymer with the use of chitinases (EC 3.2.1.4) and chitosanases (EC 3.2.1.132) seems to be the best method because the reaction is carried out under mild conditions and the distribution of the MW of the product is easier to control [3]. However, the production of well-defined COS in terms of DP, degree of N-deacetylation (DD) and sequence (acetylation pattern, PA) by enzymatic conversion processes is not straightforward. Costly chitinase and chitosanase preparations, also immobilized, limit their use on an industrial scale. COS are produced by several companies, mainly in China and Korea, these products consist of partially characterized COS with clearly defined purities [9].
Currently, a number of studies focus on the modification of COS, including amidation, esterification, epoxy-amine/hydroxyl coupling, Schiff base formation and Michael addition which contributes to their wider application. These modifications enhance the versatility and usefulness of COS [10].
Our previous research demonstrated that the mycelium of Mucor circinelloides IBT-83 is a promising source of chitosan hydrolyzing enzymes. The intracellular chitosanase isolated from this strain was partially purified and subjected to characterization, as described by Struszczyk and coworkers [11]. Moreover, it has been proven that this strain produces intracellular chitin deacetylase (EC 3.5.1.41) [12, 13]. In addition, our research focused on the immobilization of strain cells, and we began the development of a method for obtaining COS using column reactors packed with the obtained preparations [14–16]. We have developed a method of immobilizing whole mold cells, simply through simultaneous culture and immobilization processes. This approach eliminates the need for additional steps like enzyme extraction and purification. This method significantly reduces the costs of biocatalysts production.
The aim of the work was to stabilize proteins associated with the mycelium of M. circinelloides IBT-83 exhibiting chitosanolytic activity and to use it as an active bed for COS production in a column reactor. Additionally, the potential agricultural application of the obtained product was presented.
Materials and methods
Materials
Chitosan from shrimp shells was obtained from Heppe Medical Chitosan GmbH. The MW and DD were 31 × 104 Da and ≥ 92.6%, respectively. All the other chemicals were of analytical or extra pure grade and were obtained from commercial sources. Reticulated open-cell polyurethane foam (PUF, technical foam, purchased from Recticel, Belgium), type Filtren TM30 based on polyether polyol (pore diameter 1.6–2.2 mm, density 19–22 kg/m3, final extension 100%, tensile strength 80 kPa) were used for mold immobilization. Phytotoxkit tests for seed germination and early plant growth were obtained from MicroBioTests Inc., Mariakerke (Gent, Belgium).
Microorganism
The filamentous fungus strain Mucor circinelloides IBT-83 was obtained from the culture collection of the Institute of Molecular and Industrial Biotechnology, Lodz University of Technology. Large subunit ribosomal RNA gene and 18 S ribosomal RNA gene sequencing was performed to identify the genus of the M. circinelloides IBT-83 strain and deposited in GenBank database with accession numbers KR056084 and KR056083, respectively.
Preparation of the immobilized whole cell biocatalysts
The M. cicinelloides IBT-83 strain was grown on a medium containing corn steep liquor (3.7% w/v) and rapeseed oil (2.7% v/v). PUF cubes (sized 10 × 10 × 10 mm) were added to the medium in an amount of 1.25% (w/v). The culture medium was inoculated with 6 × 107 sporangiospores per 1 ml of culture medium (suspension prepared by washing 3-day-old agar slants containing brewing broth at a concentration of 8.5°Blg, pH 5.8). The strain was cultivated for 72 h at 30 °C with agitation at 180 rpm. The mycelium immobilized in PUF obtained after cultivation was thoroughly washed with water (three times), and the water was squeezed thoroughly onto a ceramic partition. Throughout these processes, the mycelium remains attached to the PUF due to its strong association with the carrier. The resulting PUFs with immobilized mycelium were divided into two parts. The first half, immediately after the end of the culture, was subjected to various types of stabilizing factors (stabilization methods are described in Chap. 2.4). After completing of the stabilization process, the preparation was transferred to a Reactor-Ready reactor (5 L, Radleys, United Kingdom). The lipids present in the cells were then extracted using acetone (100 mL of acetone for every 10 g of the preparation). This extraction process was repeated three times, with each extraction taking place every 15 min at room temperature. The extracted lipids were subsequently dried at room temperature for approximately 24 h. Regarding the second part of the preparation, the technique was reversed. The initial step involved defatting the preparation using acetone, as previously mentioned. Subsequently, it was left to dry at room temperature for approximately 24 h before being stabilized. The acetone used in the process was completely recycled.
Methods of biocatalysts stabilization
The following methods were used to stabilize the M. circinellopides PUF-immobilized preparation: (1) treatment with 1% glutaraldehyde (GA) solution (pH 3.0, temp. 4 °C for 30 min) and then with 1% glycine solution for 24 h (saturation of free aldehyde groups); (2) treatment with 1% sodium alginate solution (24 °C, 15 min) followed by 1% CaCl2 solution (4 °C, 24 h); (3) freeze drying (-30 °C, 0.37 mbar, Alpha 1–4 freeze dryer, Christ, Germany); (4) treatment with 0.5% polyvinyl alcohol (PVA) solution (4 °C, 24 h), followed by 1% GA solution (pH 3.0, temp. 4 °C for 30 min) and 1% glycine solution for 24 h. All these stabilized PUF-immobilized mycelia were applied in batch chitosan hydrolysis process.
Batch hydrolysis of chitosan
Batch hydrolysis of chitosan was carried out lasting a total of 10 cycles of 24 h each. A PUF-immobilized mycelium preparation was added to a 0.5% w/v chitosan acetate solution (pH 5.5). For this purpose, 50 mg of mold preparation (corresponding to approximately 25 mg DW. of mycelium) was introduced into each 1 cm3 of chitosan solution. Samples were incubated on a shaker (120 rpm) at 37oC for 24 h. Subsequently, the immobilized biocatalyst was separated from the chitosan hydrolysate, washed three times with 0.05 M acetate buffer with pH 5.5 and suspended in a new portion of the substrate (0.5% w/v chitosan acetate solution, pH 5.5.). For the control sample, an enzyme preparation previously subjected to thermal inactivation (temp. 100oC, 10 min) was used. The concentration of reducing sugars in the chitosan hydrolysates and control samples, was determined using the Somogyi-Nelson method. To estimate the amount of protein contained in chitosan hydrolysates (washed out from the mycelium), the Lowry method was applied. The chitosan hydrolysis efficiency measured as the ratio of reducing sugar concentration to the total amount of mycelium utilized, was evaluated in the eluates following each 24-hour cycle.
Hydrolysis of chitosan in the packed bed reactor
The PUF-immobilized mycelium (pre-cut into cubes approximately 1 × 1 × 1 cm) in the amount of 35.63 ± 0.69 g was placed into a glass column (ECO series glass column, SR-version, with heating/cooling jacket, 2.5 cm (ID) × 45 cm, Kronlab, Germany) and thoroughly washed with 0.05 M acetate buffer with pH 5.5 using a peristaltic pump. Washing of the column packed with mycelium was carried out for approx. 24 h. At this point, the buffer was drained from the column. The temperature of the reactor was kept at 37 °C by circulation of water in the jacket surrounding the reactor. The continuous hydrolysis of chitosan was achieved by supplying the substrate solution (0.5 L, in acetic acid) to the reactor continuously. The flow rate of the substrate solution was 0.25 mL/min. The substrate concentration was 0.5% (w/v). After every 24 h a new substrate was connected, and the obtained product was analyzed. According to Santos-Moriano et al. [17] aliquots were mixed with 0.25 M NaOH in a 1:1 (v/v) ratio to stop the reaction and to precipitate the remaining long-chain polysaccharide, which was removed by centrifugation at 10,000 × g for 5 min. The obtained supernatant was lyophilized. COS analysis was performed using high-performance chromatography (HPLC). The efficiency of the reactor was determined by measuring the amount of reducing sugars in the collected samples without prior addition of NaOH and was expressed as the concentration of reducing sugars divided by the total mass of mycelium used in the reactor.
Analytical methods
Determination of the content of reducing substances
The content of reducing amino-chitooligomers was determined by the Somogyi–Nelson method [18] using glucosamine as standards.
Protein assay
Protein concentration was determined by the Lowry method [19] using bovine serum albumin as the standard.
Analysis of COS by high performance chromatography (HPLC)
The HPLC analysis of the chitosan degradation products was carried out using a Gold Beckman chromatography system equipped with a TSKgel Amide-80 column (4.6 mm × 25 cm), a pre-column of the same type (3.2 mm × 1.5 cm) and a refractometric detector. The mobile phase was a mixture of acetonitrile-water-methanol-tetrahydrofuran (6:2.4: 1.5: 1.0, v / v / v / v). The separation was carried out at 24oC.
Plant growth promotion tests
Phytotoxkit tests for seed germination and early plant growth (MicroBioTests Inc., Mariakerke, Gent, Belgium) were used in the research. COS was introduced into the soil in the amount of 0.01–1% w/w. The soil moisture was 30%. The research plates were filled with 100 g of soil, then covered with filter paper upon which cress seeds (Lepidium sativum) were placed. COS was not included in the control sample. The test plates were incubated vertically at 25 °C for 4 days. Subsequently, the length of the root and the stem of the plant were measured.
Statistical analysis
The experiments were performed in triplicate. Statistical analysis was performed with Microsoft Excel 2013. Data were presented as mean ± SD.
Results
PUF-immobilized mycelium stabilization
Polyurethane foam (PUF) is an excellent carrier for the immobilization of microorganisms. It is characterized by high porosity, which provides a large adsorption surface. It is also an inert material with good mechanical properties, i.e. high strength and flexibility. This carrier makes immobilization possible by using the fact that some microorganisms can naturally stick to solid surfaces through physicochemical bonds (adsorption on the surface) [20]. For the M. circinelloides IBT-83 strain, this method proves to be highly effective, yielding mechanically stable preparations where the mycelium remains firmly attached to the carrier, thereby serving as an excellent source of enzymes bound to cell membranes. Only processing the mycelium, such as drying it, may cause it to crumble from the carrier. These characteristics make the preparations suitable for periodic and continuous reactions.
M. circinelloides IBT-83 mycelium that exhibits chitosanolytic activity was immobilized into PUF. The developed mold cultivation conditions allowed for immobilization of approximately 1 g of defatted and dried mycelium in 1 g of carrier [16]. Our previous attempts to use PUF-immobilized mycelium of M. circinelloides IBT-83 in the chitosan hydrolysis process proved that the post-reaction mixture contained proteins leached from the mycelium during both batch and continuous hydrolysis [15]. It was found that this may impact the limited operational stability of the biocatalyst. Therefore, it was necessary to carry out tests to stabilize proteins associated with mycelium. The following protein stabilization methods were tested as part of the research: cross-linking of mycelium-associated proteins with GA; trapping mycelium in calcium alginate; trapping mycelium in PVA combined with cross-linking of proteins with GA or freeze-drying of biomass. Two types of PUF-immobilized preparations were tested, i.e. defatted before and after the protein stabilization process.
The prepared biocatalysts were used in the batch hydrolysis of chitosan (10 cycles, each lasting 24 h). After each cycle, the concentration of reducing sugars and proteins present in the hydrolysates was determined. The obtained results are presented in Figs. 1–2.
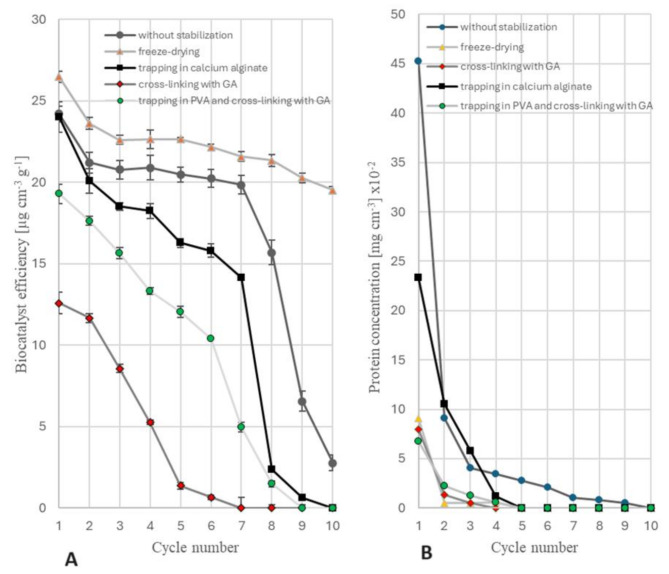
Fig. 1.
Batch hydrolysis of chitosan catalyzed by PUF-immobilized mycelium of M. circinelloides IBT-83: (A) biocatalyst efficiency; (B) concentration of protein eluted from the mycelium. Two-stage preparation of the biocatalyst: 1) lipid extraction with acetone; 2) protein stabilization
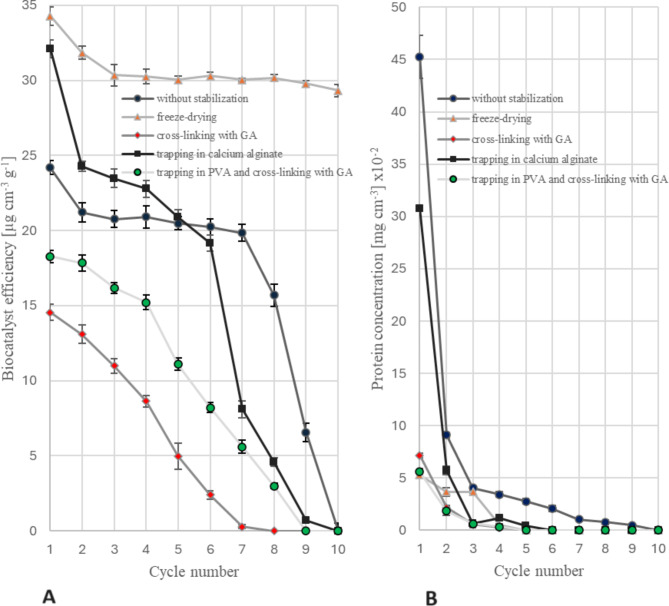
Fig. 2.
Batch hydrolysis of chitosan catalyzed by PUF-immobilized mycelium of M. circinelloides IBT-83: (A) biocatalyst efficiency; (B) concentration of protein eluted from the mycelium. Two-stage preparation of the biocatalyst: 1) protein stabilization, 2) lipid extraction with acetone
The majority of protein was released from the mycelium already during the initial cycle of using enzyme preparations, as illustrated in Figs. 1B and 2B. Hence, it is possible that the proteins liberated during the initial operational phase of the batch reactor played a role in the hydrolysis of chitosan and influenced the level of this hydrolysis, as evidenced by the predominant decline in the efficacy of the biocatalysts during the first cycle. The chitosan hydrolysates collected after subsequent cycles consistently exhibited the presence of proteins, suggesting that their slow release could potentially contribute to the decreased activity observed in the tested biocatalysts.
Freeze-drying of the mycelium proved as the most effective method of stabilizing the proteins responsible for chitosan hydrolysis. The preparations obtained using this method exhibit the highest level of effectiveness for all substrate hydrolysis cycles conducted (Figs. 1A and 2A). The freeze-drying of the preparation is a crucial step followed by the extraction of lipids from the biomass. This method facilitated the utilization of the catalytic properties of the mycelium for a total of 10 cycles of chitosan hydrolysis. In contrast, the non-stabilized, defatted preparations exhibited a consistent level of activity for 7 days (Fig. 2A). The activity of the preparation, which was initially defatted and subsequently lyophilized, showed a gradual decline starting from the 5th hydrolysis cycle (Fig. 1A). Additionally, it was observed that the enzymatic preparation subjected to lyophilization as soon as the cultivation of mold was finished and then defatted exhibited higher activity compared to the preparation first defatted with acetone, with an average increase of 25%.
The study revealed that the use of calcium alginate did not enhance the stability of the biocatalyst. The utilization of GA, in conjunction with PVA trapping, enables the cross-linking of proteins associated with mycelium. This process effectively decreases the quantity of protein that is released from the mycelium during successive cycles of chitosan hydrolysis. Unfortunately, the use of this cross-linking compound reduces the effectiveness of the enzymes responsible for chitosan hydrolysis (the deactivation of chitosanases), regardless its concentration (GA concentrations ranging from 0.1 to 1.0% were investigated, but the results have been omitted and are not shown here). With the exception of freeze-drying, all stabilization methods reduce the mechanical strength of preparations.
Hydrolysis of chitosan in the packed bed column reactors
The biocatalysts were evaluated for their operational stability through the continuous hydrolysis of chitosan. For this purpose, two packed bed reactors (PBRs) were designed, with the following active beds: PBR1 containing PUF-immobilized mycelium not stabilized (only defatted), PBR2 containing PUF-immobilized mycelium, freeze-dried and defatted. Figure 3 illustrates the packed bed reactor used for continuous production of chitosan oligosaccharides.
Fig. 3.
COS production system: (A) PBR with equipment (peristaltic pump, magnetic stirrer). The column is connected to a thermostat (not visible in the photo), (B) PUF-immobilized mycelium in the column reactor
The process of biopolymer hydrolysis was carried out for 20 cycles, each lasting 24 h. The efficiency of chitosan hydrolysis was estimated in eluates from the column collected after each cycle. Each day of the column operation, the protein concentration in the eluent was also tested. The results obtained are given in Figs. 4 and 5.
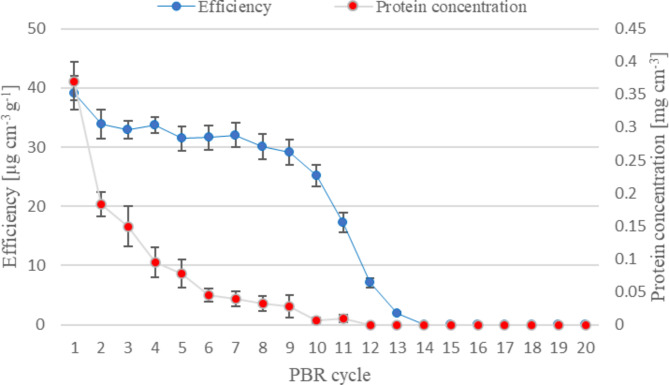
Fig. 4.
Dynamics of continuous process of chitosan hydrolysis catalyzed by PUF-immobilized mycelium of M. circinelloides IBT-83 (PBR1, mycelium was defatted with acetone). Explanation: flow rate of 0.5% w/v chitosan acetate solution − 0.25 cm3 min− 1, mass of biocatalyst − 35.2 ± 0.58 g (PUF containing approximately 17.2 ± 0.28 g of dried mycelium)
A two-phase operation of both PBRs was observed. The initial case (PBR1, Fig. 4) included an unstabilized biocatalyst, in the first phase, lasting for 7 cycles, the efficiency of the reactor bed is gradually, very slowly reduced (only on the first day, it decreases rapidly by 13%). In the second phase, the phenomenon is rapid. The profile of the column operation can be associated with both the specific stability of chitosanolytic enzymes and the phenomenon of their elution from the column bed. The PBR2 operates at a constant level for 16 cycles, after which a decrease in its efficiency can be observed (Fig. 5). It works at a constant level for 384 h in total, which is 216 h longer compared to PBR1 with unstabilized mycelium.
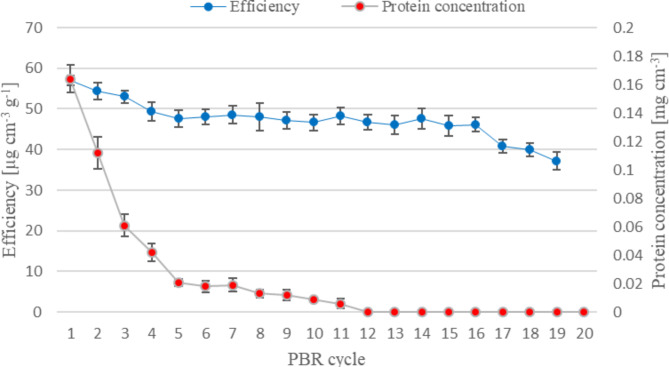
Fig. 5.
Dynamics of continuous process of chitosan hydrolysis catalyzed by PUF-immobilized mycelium of M. circinelloides IBT-83 (PBR 2, two-stage preparation of the biocatalyst: 1) freeze-drying, 2) lipid extraction with acetone.). Explanation: flow rate of 0.5% w/v chitosan acetate solution − 0.25 cm3 min− 1, mass of biocatalyst − 36.1 ± 0.46 g (PUF containing approximately 18.02 ± 0.23 g of dried mycelium)
Chitosan degradation products were analyzed using high performance chromatography (HPLC). Combined samples of hydrolysates taken from PBR2, obtained between cycle 5 and 17, were analyzed. The content of individual products in the sample is presented in Table 1. HPLC analysis confirmed that the analyzed samples contained COS ranging from the di- to the hexamer of D-glucosamine as well as its monomer. The dominant oligomers in the sample were chitobiose, chitotriose and chitotetrose.
Table 1.
Composition of the COS mixture obtained during the hydrolysis of chitosan in PBR
| COS | [%, w/w] |
|---|---|
| GlcN | 12.59 ± 3.14 |
| (GlcN)2 | 23.79 ± 4.31 |
| (GlcN)3 | 32.20 ± 3.73 |
| (GlcN)4 | 22.89 ± 2.98 |
| (GlcN)5 | 5.71 ± 1.48 |
| (GlcN)6 | 2.82 ± 0.60 |
The effect of COS on plant growth
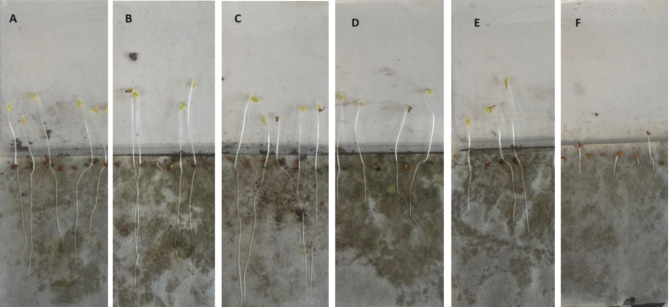
The obtained results from phytotests demonstrate that the introduction of COS into the soil at a concentration of 0.05% w/w promotes the growth of cress roots. The roots were 44% longer compared to the control sample. The longest cress stems were observed in the case of COS at a concentration of 0.01% w/w, i.e. 38% longer than those without the addition of chitosan hydrolysate. However, at a COS concentration of 0.05% w/w, the stems were 11% longer. Higher concentrations of COS (above 0.05% w/w) inhibited the growth of both the root and the above-ground part of the plant. The obtained results are presented in Table 2; Fig. 6.
Table 2.
Root and stem length of cress cultivated for 96 h with different concentrations of COS
| COS concentration in soil [%, w/w] | |||||
|---|---|---|---|---|---|
| 0 | 0.01 | 0.05 | 0.1 | 0.5 | |
| Root length [cm] | 4.16 ± 0.48 | 5.41 ± 0.52 | 5.97 ± 0.49 | 3.20 ± 0.56 | 3.06 ± 0.54 |
| Stem length [cm] | 3.24 ± 0.49 | 4.47 ± 0.41 | 3.59 ± 0.42 | 3.38 ± 0.12 | 2.50 ± 0.34 |
Fig. 6.
The growth of cress (96 h) on soil supplemented with COS at the concentration % w/w of: (A) 0; (B) 0.01; (C) 0.05; (D) 0.1; (E) 0.5; (F) 1.0
Discussion
Enzymatic production of chitooligosaccharides was first conducted in batch reactors. An example can be found in the experiment proposed by Izume and Ohtakara [21]. The chitosanase from Bacillus sp. No. 7-M mixed with the substrate catalyzed the hydrolysis of β-1,4-glycosidic bonds occurring inside the biopolymer chain. However, that approach did not allow the obtaining of products with a predetermined DP, but only a mixture of mono- and oligomers. Its main disadvantage was the enormous cost resulting from the need to use large amounts of the enzyme without the possibility of its reuse. Subsequently, chitosan hydrolysis methods were developed using immobilized enzymes filling the reactor column, which enabled their re-(repeated) use in subsequent production cycles. The study focused on the efficiency of the process, which was influenced by various parameters including: temperature, column filling (such as the amount and porosity of the biocatalyst bed), initial substrate concentration and the speed of its flow through the reactor column. A much weaker affinity for the immobilized enzyme chitosan compared to its native form was the main problem of such systems due to application reasons [21–23]. The enzymatic method was successively modernized by extending the system with a module containing an ultrafiltration membrane (UF), resulting in increased efficiency of the process and obtain products with the required, assumed molecular weight [24–28]. Using this method, Qin et al. [28] obtained COS with a high degree of polymerization (DP 5–10) accounting for 56.17% (w/w) of the total mixture of COS. However, because of the UF membrane’s clogging due to the substrate’s high viscosity, it was often impossible to carry out the process for a long time. Additionally, a two-reactor system for continuous hydrolysis of chitosan was tested [29]. The system consisted of two interconnected components: a column reactor filled with the immobilized enzyme and a reactor equipped with an ultrafiltration membrane. In the first stage, the substrate underwent partial hydrolysis due to its contact with the immobilized biocatalyst constituting the column bed. Following that, the products which exhibited a lower DP compared to the original chitosan, were transferred into the reactor containing the native enzyme, where they were further decomposed. Subsequently, the emerged product was fractionated based on its mass using an ultrafiltration membrane, and then concentrated and dried. Santos-Morino et al. [17] conducted a study to test the dual-reactor system for continuous production of COS. The researchers used commercial α-amylase from Bacillus amylolyquefaciens (with confirmed chitosanolytic activity) covalently immobilized on glyoxal agarose beads. Chitotriose and chitobiose were found to be the predominant components in the COS mixture formed when chitosans with different PD and DD were used. It should be emphasized that the search for cost-effective technologies for large-scale COS production is still being ongoing. The main drawbacks of chitosanolytic enzymes are undoubtedly their high cost (which also includes the cost of their purification and immobilization) and the requirement to improve their operating stability.
Another example of the COS production method is our proposed approach of using continuous chitosan hydrolysis using whole cells of M. circinelloides IBT-83 immobilized in a PUF carrier, which are the source of chitosanolytic enzymes. The results from our previous studies demonstrated that such a system enables the production of COS [14, 15]. The active bed in which the reactor was packed only required improvement in its operational stability. During the operation of the reactor proteins, including those with chitosanolytic activity, are washed out of the bed. It was discovered that a crucial factor influencing the possibility of using the designed reactor, affecting in COS large-scale production, is its low operational stability. Preliminary research was carried out to stabilize the active reactor bed by cross-linking proteins with GA [15]. Therefore, further research included optimization of cross-linking conditions by selecting the optimal concentration and type of cross-linking agent. Additionally, alternative techniques for stabilizing whole cell chitosanolytic enzymes were employed. The study examined several methods, including protein cross-linking using GA; trapping in calcium alginate; simultaneous trapping in PVA and cross-linking with GA or freeze-drying (lyophilization). These methods have been previously tested for stabilizing mycelium (pellets) of M. circinelloides, as a lipase source, for application in non-aqueous media [30]. Based on the obtained results, it can be concluded that the stability of the enzyme preparation during chitosan hydrolysis increases through the process of freeze-drying. Lyophilization is a widely recognized preservation technique, that involves the removal of frozen water from proteins or cells in suspension through the process of sublimation [31]. This technique has significant importance in the pharmaceutical and biotechnology sectors, and it has become a standard method used for the stabilization, storage, and prolongation of the shelf life of enzyme preparations and other biological products. In the case of M. cicinelloides IBT-83 mycelium, freeze-drying allows the removal of water from the cells, which in turn contributes to the effective extraction of lipids using organic solvents (e.g. acetone).
Our research has demonstrated that the effectiveness of PUF-immobilized mycelium stabilized with GA is lower to that unstabilized, defatted mycelium. The use of the previously mentioned cross-linking agent reduces the amount of protein that is released from the mycelium. However, it is also associated to a significant decrease in the activity of the preparation in the hydrolysis of chitosan. In addition, it was noticed that all tested stabilization procedures, with the exception of freeze-drying, reduced the mechanical properties of the mycelium immobilized in PUF. This was visible in the susceptibility of the mycelium to chipping from the carrier. Bifunctional compounds, such as glutaraldehyde, are commonly used for the purpose of stabilizing proteins through cross-linking. The approach used by Szczęsna-Antczak et al. [30] proved to be effective in stabilizing M. circinelloides pellets containing lipase. Ban and coauthors [32] successfully maintained lipase activity at a constant level for six full cycles of periodic methanolysis of soybean oil using PUF-immobilized mycelium of Rhizopus oryzae, treated with GA solution. The efficiency of this process ranges from 70 to 83%. However, the same preparations, without cross-linking, gradually lost activity during subsequent cycles of this process. By retaining 83% yields of alkene epoxidation even after four cycles of reactions, Xu and coworkers found that the catalytic performance of R. oryzae cells that were cross-linked was significantly enhanced in comparison to the untreated cells [33]. High transfructosylation activity was subsequently achieved by Goncalves et al. [34] through the entrapment of GA-crosslinked cells from Aspergillus oryzae IPT-301 in calcium alginate. Free cells not cross-linked with GA exhibited approximately 70% less activity than cells encapsulated in calcium alginate and cross-linked with GA. Finally, until the third cycle of continuous fructo-oligosaccharide production, the activity of the cross-linked and entrapped cells persisted.
The strain of M. circinelloides IBT-83 produces and accumulates a large amount of oil in the cells. In optimal conditions (culture medium composed of corn steep solids, plant oil, glucose and NO3−) the mold produces over 50 g DW/dm3 of biomass containing over 60% of lipids [35]. Also, in the case of the medium used in these studies, the amount of lipids was high. Therefore, it was necessary to employ acetone for defatting the mycelium immobilized in PUF in order to inhibit the infiltration of lipids into the chitosan hydrolysates. It was found that the sequence of lipid removal and mycelium stabilization is very important. Initially, it is important to stabilize the immobilized mycelium, followed by the extraction of lipids using acetone. This extraction process aids to maintain the activity of the preparation. The subsequent approach facilitated the utilization of the catalytic characteristics of the mycelium in ten cycles of batch chitosan hydrolysis. The activity of mycelium is also influenced by the way of its preparation. The utilization of a freeze-dried and subsequently defatted formulation for the batch hydrolysis of chitosan resulted in a 25% increase in efficiency when compared to a preparation where the method was reversed. The preparations dehydrated using these two procedures exhibited varying water content and likely possessed distinct internal compositions, which likely impacted their efficacy in chitosan hydrolysis.
The tested mycelium preparations, stabilized by freeze-drying, can be successfully used in the process of continuous hydrolysis of chitosan, thus enabling the production of COS. Chitosan hydrolysis was carried out under optimal conditions for chitosanase from M. circinelloides IBT-83 [11, 16]. The stability of the column filled with immobilized mycelium is mostly determined by the rate of washing out of the associated protein. In our process, high protein concentrations were observed in hydrolysates, especially in the initial reactor cycles. The amount of protein determined in hydrolysates after cycles no. 1, 2, and 3 was 0.37, 0.18, and 0.15 mg cm− 3, respectively, in the case of using an unstabilized enzyme preparation. Then its concentration in hydrolysates decreased significantly. The eluted proteins probably also include chitosanolytic enzymes. Their slow release may be one of the reasons for the loss of PBR effectiveness. A drastic drop in PBR efficiency occurs from cycle no. 10, when enzyme activity/stability is likely lost. The freeze-drying stabilization of enzyme preparations allowed for a reduction in the amount of eluted protein. The concentration of protein determined in hydrolysates taken after the completion of subsequent PBR cycles was approximately 55% lower compared to unstabilized preparations (in cycles no. 1, 2 and 3, the protein concentration was 0.16, 0.11 and 0.06 mg cm− 3, respectively). We also noted a gradual elution of protein from the mycelium, which was most drastic in the first PBR cycles. However, since PBR operates at a constant level, we can hypothesize that the eluted proteins do not participate in the hydrolysis of chitosan. Reduction the phenomenon of protein elution, while maintaining chitosanase activity, it become enabled the production of COS at a constant level for 16 reactor cycles (384 h in total), 216 h longer compared to non-stabilized mycelium. HPLC analysis confirmed that the obtained chitosan hydrolysate contained: oligomers of D-glucosamine (from dimer to hexamer) and its monomer. The presence of the GlcN monomer is probably related to the activity of exochitosanolytic enzymes produced by M. circinelloides IBT-83. As a result of applying whole cells of filamentous fungi, which serve as a source of chitin- and chitosanolytic enzymes, the elimination of their activity and, consequently, removal of GlcN in the hydrolyzate remains unachievable. Further research is needed to examine the impact of chitosan hydrolysis time on the hydrolyzate composition and the feasibility of using chitosans with different parameters, including DD and MW.
The use of chitosan in various industries is limited mainly due to its high viscosity and insolubility at neutral pH. This problem can be solved by applying chitosan hydrolysis products, i.e. oligomers with various degrees of polymerization. COS are now being tested in various industries, including agriculture. The research focuses on issues related to sustainable agriculture, such as the development and application of organic fertilizers. COS promote growth and induces reactions linked with both primary and secondary metabolism in plants [36]. The research findings demonstrate the ability of COS to combat fungi and viral plant diseases, support plant growth and yield, as well as extend the shelf life of flowers and fruits [37]. It has been confirmed that the germination of wheat (Triticum aestivum) [39, 40] or barley (Hordeum vulgare L.) [40] seeds can be improved if the seeds are pre-treated by soaking in a COS solution. In the study by Ma et al. [38], the effect of soaking wheat seeds in various concentrations of chitosan oligomers (0.03–1.00%) was tested, including: on seed germination, root activity and the activity of wheat antioxidant enzymes. The effect of low concentration COS was greater in comparison to that of high concentration. In order to improve the growth and development of wheat, it was proposed to soak the seeds in a 0.0625% COS solution. The research conducted by Chatelain et al. [41] involved the cultivation of common beans (Phaseolus vulgaris) in a hydroponic environment. The objective of the study was to evaluate the impact of three different concentrations of COS (0.01, 0.05, and 0.1 g/L) on both plant development and mineral accumulation. Research has revealed that the application of COS alters the length of plant roots, the accumulation of minerals, and the biomass of plant shoots. The study investigated the potential impact of the acquired COS on the root and stem length of cress (Lepidium sativum). The research was carried out using Phytotoxkit plate tests. The findings of the study indicate that the introduction of COS into the soil at concentrations of 0.01 or 0.05% w/w resulted in an increase of stem and root growth, respectively (extensions by 38% and 44% compared to the control sample). The use of higher concentrations of COS, i.e. above 0.05% w/w, inhibits the growth of the plant. Plants grown at high COS levels had shorter shoots and roots, suggesting that COS can be phytotoxic to the plant [41]. The results presented indicate the possibility of using the produced COS mixture in agriculture to promote plant growth. It should be emphasized, however, that these are preliminary studies that require extension and thorough analysis.
Conclusion
This study focused on developing a method for the manufacturing of COS that is both effective and ecologically sustainable, while also being straightforward. The experimental setup involved the utilization of a column reactor containing an active bed consisting of Mucor circinelloides IBT-83 cells immobilized in PUF. The process of freeze-drying the immobilized mycelium has been found to be a highly successful technique for stabilizing proteins that are linked to mycelium. This method effectively prevents the loss of these proteins during the operation of the reactor. This allowed for the uninterrupted generation of COS at a consistent rate during 16 cycles of the reactor, resulting in a total of 384 h. The COS that have been acquired exhibit complete solubility in water, and their solutions possess a low viscosity. Consequently, they can be effectively employed in many industries, including the manufacturing of environmentally friendly fertilizers that aid plant growth.
Acknowledgements
Not applicable.
Abbreviations
- COS
Chitosan oligosaccharides
- DD
Degree of N-deacetylation
- DP
Degree of polymerization
- GA
Glutaraldehyde
- GlcN
D-glucosamine
- MW
Average molecular weight
- PA
Acetylation pattern
- PBR
Packed bed reactor
- PUF
Polyurethane foam
- PVA
Polyvinyl alcohol
Author contributions
Conceptualization, KS-Ś; Data curation, KS-Ś; Formal analysis, KS-Ś, MBK; Investigation, KS-Ś, MBK ; Methodology KS-Ś; Project administration, KS-Ś, OM-M; Resources, KS-Ś; Supervision, KS-Ś, OM-M, TA; Validation, KS-Ś; Visualization, KS-Ś; Writing—original draft KS-Ś. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gierszewska M, Struszczyk-Świta K, Hudson S. Chitin and chitosan. In: Seidel A, editor. Encyclopedia of Polymer Science and Technology. Wiley; 2021. pp. 1–16. 10.1002/0471440264.pst052.pub2
- 2.Hudson S. The outlook for chitosan in textiles: a review of the literature since the start of the 2020’s. PCACD. 2023;28:7. 10.15259/PCACD.28.001 [Google Scholar]
- 3.Kaczmarek MB, Struszczyk-Świta K, Li X, Szczęsna-Antczak M, Daroch M. Enzymatic modifications of chitin, Chitosan, and Chitooligosaccharides. Front Bioeng Biotechnol. 2019;7:243. 10.3389/fbioe.2019.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piekarska K, Sikora M, Owczarek M, Jóźwik-Pruska J, Wiśniewska-Wrona M. Chitin and Chitosan as polymers of the Future-Obtaining, Modification, Life Cycle Assessment and Main directions of application. Polymers. 2023;15:793. 10.3390/polym15040793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yadav M, Goswami P, Paritosh K, Kumar M, Pareek N, Vivekanand V. Seafood waste: a source for preparation of commercially employable chitin/chitosan materials. Bioresour Bioprocess. 2019;6:8. 10.1186/s40643-019-0243-y [Google Scholar]
- 6.Hao W, Li K, Li P. Review: advances in preparation of chitooligosaccharides with heterogeneous sequences and their bioactivity. Carbohydr Polym. 2021;252:117206. 10.1016/j.carbpol.2020.117206 [DOI] [PubMed] [Google Scholar]
- 7.Lodhi G, Kim J-S, Hwang J-W, Kim S-K, Jeon Y-J, Je J-Y, Ahn C-B, Moon SH, Jeon B-T, Park P-J. Chitooligosaccharide and Its Derivatives: Preparation and Biological Applications. Biomaterials: Chitosan and Collagen for Regenerative Medicine. Hindawi Publishing Corporation, BioMed Research International. 2014; Article ID 654913, 10.1155/2014/654913 [DOI] [PMC free article] [PubMed]
- 8.Miguez N, Kidibule P, Santos-Moriano P, Ballesteros AO, Fernandez-Lobato M, Plou FJ. Enzymatic synthesis and characterization of different families of Chitooligosaccharides and their Bioactive properties. Appl Sci. 2021;11(7):3212. 10.3390/app11073212 [Google Scholar]
- 9.Liaqat F, Eltem R. Chitooligosaccharides and their biological activities: a comprehensive review. Carbohydr Polym. 2018;184:243–59. 10.1016/j.carbpol.2017.12.067 [DOI] [PubMed] [Google Scholar]
- 10.Chapelle C, David G, Caillol S, Negrell C. Desroches Le Foll M. Advances in chitooligosaccharides chemical modifications. Biopolymers. 2021;112(9). 10.1002/bip.23461 [DOI] [PubMed]
- 11.Struszczyk K, Szczęsna-Antczak M, Walczak M, Pomianowska E, Antczak T. Isolation and purification of Mucor circinelloides intracellular chitosanolytic enzymes. Carbohydr Polym. 2009;78(1):16–24. [Google Scholar]
- 12.Kaczmarek MB, Struszczyk-Świta K, Florczak T, Szczęsna-Antczak M, Antczak T. Isolation, molecular cloning and characterisation of two genes coding chitin deacetylase from Mucor circinelloides IBT-83. PCACD. 2016;21:93–103. 10.15259.PCACD.21.09. [Google Scholar]
- 13.Kaczmarek MB, Struszczyk-Świta K, Szczęsna-Antczak MH, Antczak T, Gierszewska M, Steinbuchel A, Daroch M. Polycistronic expression system for Pichia pastoris composed of chitin deacetylase, chitinase, and chitosanase enabling one-pot enzymatic modification of chitin substrates. Front Bioeng Biotechnol. 2021;9:710922. 10.3389/fbioe.2021.710922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Struszczyk K, Szczęsna-Antczak M, Walczak M, Pomianowska E, Wojciechowska J, Antczak T. Enzymatic preparations from Mucor moulds and their application in oligoaminosaccharides production. PCACD. 2009;14:89–100. [Google Scholar]
- 15.Struszczyk K, Szczęsna-Antczak M, Pomianowska E, Stańczyk Ł, Wojciechowska J, Antczak T. Process of continuous production of oligoaminosaccharides in a column reactor. PCACD. 2010;15:177–88. [Google Scholar]
- 16.Struszczyk-Świta K, Stańczyk Ł, Szczęsna-Antczak M, Antczak T. Scale-up of PUF-immobilized fungal chitosanase–lipase preparation production. Prep Biochem Biotechnol. 2017;47(9):909–17. 10.1080/10826068.2017.1365240 [DOI] [PubMed] [Google Scholar]
- 17.Santos-Moriano P, Woodley JM, Plou FJ. Continuous production of chitooligosaccharides by an immobilized enzyme in a dual-reactor system. J Mol Catal B Enzym. 2016;133:211–7. 10.1016/j.molcatb.2016.09.001 [Google Scholar]
- 18.Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153(2):375–80. [Google Scholar]
- 19.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 20.de Ory I, Cabrera G, Ramirez M, Blandino A. Immobilization of Cell on Polyurethane Foam. In: Guisan JM, editor. Immobilization of Enzymes and Cells. Methods. Mol. Biol. Humana Press Inc. New Jersey; 2008. p. 357-365. 10.1007/978-1-59745-053-9_31
- 21.Izume M, Ohtakara A. Preparation of D-glucosamine oligosaccharides by the enzymatic hydrolysis of chitosan. Agric. Biol. Chem. 1987;51:1189–1191. 10.1080/00021369.1987.10868158
- 22.Jeon YJ, Park PJ, Byun HG, Song BK, Kim SK. Production of chitosan oligosaccharides using chitin-immobilized enzyme. KSBB J. 1998;13:147–54. [Google Scholar]
- 23.Kuroiwa T, Ichikawa S, Sato S, Hiruta O, Sato S, Mukataka S. Factors affecting the composition of oligosaccharides produced in chitosan hydrolysis using immobilized chitosanases. Biotechnol Prog. 2002;18:969–74. 10.1021/bp020011r [DOI] [PubMed] [Google Scholar]
- 24.Kuroiwa T, Ichikawa S, Sato S, Mukataka S. Improvement of the yield of physiologically active oligosaccharides in continuous hydrolysis of chitosan using immobilized chitosanases. Biotechnol Bioeng. 2003;84:121–7. 10.1002/bit.10758 [DOI] [PubMed] [Google Scholar]
- 25.Jeon YJ, Kim SK. Production of chitooligosaccharides using an ultrafiltration membrane reactor and their antibacterial activity. Carbohydr Polym. 2000;41:133–41. [Google Scholar]
- 26.Jeon YJ, Kim SK. Antitumor activity of chitosan oligosaccharides produced in an ultra filtration membrane reactor system. J Microbiol Biotechnol. 2002;12:503–7. [Google Scholar]
- 27.Kuroiwa T, Izuta M, Nabetani M, Sato S, Mukataka S, Ichikawa S. Selective and stable production of physiologically active chitosan oligosaccharides using an enzymatic membrane reactor. Process Biochem. 2009;44:283–7. 10.1016/J.PROCBIO.2008.10.020 [Google Scholar]
- 28.Lin Y-W, Hsiao Y-C, Chiang B-H. Production of high degree polymerized chitooligosaccharides in a membrane reactor using purified chitosanase from Bacillus cereus. Food Res Int. 2009;42(9):1355–61. 10.1016/j.foodres.2009.06.008 [Google Scholar]
- 29.Qin Z, Luo S, Li Y, Chen Q, Qiu Y, Zhao L, Jiang L, Zhou J. Biochemical properties of a novel chitosanase from Bacillus amyloliquefaciens and its use in membrane reactor. LWT. 2018;97:9–16. 10.1016/j.lwt.2018.06.027 [Google Scholar]
- 30.Jeon YJ, Kim SK. Continuous production of chitooligosaccharides using a dual reactor system. Process Biochem. 2000;35:623–32. [Google Scholar]
- 31.Szczesna-Antczaka M, Antczak T, Rzyska M, Modrzejewska Z, Patura J, Kalinowska H, Bielecki S. Stabilization of an intracellular Mucor circinelloideslipase forapplication in non-aqueous media. J Mol Catal B Enzym. 2004;29:163–71. 10.1016/j.molcatb.2004.02.010 [Google Scholar]
- 32.Blanch HW, Clark DS. Biochemical Engineering. NewYork: Marcel Dekker, Inc.; 1997. [Google Scholar]
- 33.Ban K, Hama S, Nishizka K, Kaieda M, Matsumato T, Kondo A, Noda A, Fukuda M. Repeated use of whole-cell biocatalysts immobilized within biomass support particles for biodiesel fuel production. J Mol Catal B Enzym. 2002;17:157–65. 10.1016/S1381-1177(02)00023-1 [Google Scholar]
- 34.Xu L, Qin Y, Song Y, Tang A, Liu Y. Glutaraldehyde-crosslinked Rhizopus oryzae whole cells show improved catalytic performance in alkene epoxidation. Microb Cell Factories. 2023;22:33. 10.1186/s12934-023-02026-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonçalves MCP, Morales SAV, Silva ES, Maiorano AE, Perna RF, Kieckbusch TG. Entrapment of glutaraldehyde-crosslinked cells from aspergillus oryzae IPT-301 in calcium alginate for high transfructosylation activity. JCTB. 2020;95(9). 10.1002/jctb.6429
- 36.Szczęsna-Antczak M, Struszczyk-Świta K, Rzyska M, Szeląg J, Stańczyk Ł, Antczak T. Oil accumulation and in situ trans/esterification by lipolytic fungal biomass. Bioresour Technol. 2018;265:110–8. 10.1016/j.biortech.2018.05.094 [DOI] [PubMed] [Google Scholar]
- 37.Mukhtar Ahmed KB, Khan MMA, Siddiqui H, Jahan A. Chitosan and its oligosaccharides, a promising option for sustainable crop production- a review. Carbohydr Polym. 2020;227:115331. 10.1016/j.carbpol.2019.115331 [DOI] [PubMed] [Google Scholar]
- 38.Zhao X, Wang M, Wang W, Liu Q, Li J, Yin H. The application of Chito/Chitin oligosaccharides as Plant vaccines. In: Zhao L, editor. Oligosaccharides of chitin and Chitosan. Springer; 2019. pp. 289–323. 10.1007/978-981-13-9402-7_10
- 39.Ma LJ, Li YY, Wang LL, Li XM, Liu T, Bu N. Germination and physiological response of wheat (Triticum aestivum) to presoaking with oligochitosan. Int J Agric Biol. 2014;16:766–70. [Google Scholar]
- 40.Fu X, Zhu L, Li L, Zhang T, Li M, Mou H. Ecofriendly preparation of chitooligosaccharides with diferent degrees of deacetylation from shrimp shell waste and their efects on the germination of wheat seeds. Mar Life Sci Technol. 2019;1:95–103. 10.1007/s42995-019-00012-3 [Google Scholar]
- 41.Lan W, Wang W, Yu Z, Qin Y, Luan J, Li X. Enhanced germination of barley (Hordeum vulgare L.) using chitooligosaccharide as an elicitor in seed priming to improve malt quality. Biotechnol Lett. 2016;38:1935–40. [DOI] [PubMed] [Google Scholar]
- 42.Chatelain PG, Pintado ME, Vasconcelos MW. Evaluation of chitooligosaccharide application on mineral accumulation and plant growth in Phaseolus vulgaris. Plant Sci. 2014;215–216:134–40. 10.1016/j.plantsci.2013.11.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.