Abstract
Infection of the oral mucosa of human immunodeficiency virus type 1 (HIV-1)-infected individuals remains an under-evaluated and somewhat enigmatic process. Nonetheless, it is of profound importance in the ongoing AIDS pandemic, based on its potential as a site of person-to-person transmission of the virus as well as a location of HIV-1 pathogenesis and potential reservoir of disease in the setting of virally suppressive highly active antiretroviral therapy. We utilized molecular and virological techniques to analyze HIV-1 infection of primary human mucosal cells and also evaluated the proapoptotic potential of selected HIV-1 proteins in primary isolated human oral keratinocytes. Primary isolated human oral keratinocytes were plated on 0.4 μM polyethylenetetraphthalate cell culture inserts to form an in vitro oral mucosal layer. The strength of this layer in forming a barrier was determined by measuring trans-epithelial electrical current passage across the monolayer. The oral keratinocyte monolayers had trans-epithelial electrical resistance of approximately 176 to 208 Ω. For viral infectivity assays, the macrophage-tropic (R5) HIV-1 strains, YU-2 and ADA, and T-cell-line-tropic (X4), NL4-3 virions, incubated with or without deoxynucleoside triphosphates (dNTPs) and/or the polyamines spermine and spermidine, were used to infect oral keratinocytes. Of importance, polyamines and dNTPs have been shown to enhance natural endogenous reverse transcription (NERT), a step essential for early lentiviral infection, and are abundantly present in human semen. The infectivities of HIV-1 strains YU-2, ADA, and NL4-3 for these primary keratinocytes were dramatically increased by the addition of physiological concentrations of dNTPs, spermine, and spermidine. Binding and viral internalization assay studies showed no differences in these oral mucosal cells, with or without NERT-altering agents. It was also observed that the recombinant, cell-free HIV-1 proteins Nef, Tat, and gp120 (R5) induced apoptosis in primary oral keratinocytes compared with the results seen with nontreated cells or cells treated with glutathione S-transferase protein as a control under similar conditions. Microarray analyses suggested that HIV-1 gp120 and Tat induce apoptosis in primary human oral keratinocytes via the Fas/FasL apoptotic pathway, whereas induction of apoptosis by Nef occurs through both Fas/FasL and mitochondrial apoptotic pathways. Thus, these findings suggest molecular mechanisms by which semen in particular, as well as other bodily fluids such as cervicovaginal secretions, could increase oral transmission of HIV-1 via increasing infectivity in confluent and low-replicating oral keratinocytes. As well, the induction of apoptosis in human oral keratinocytes with relevant HIV-1-specific proteins suggests another potential complementary mechanism by which the oral mucosa barrier may be disrupted during HIV-1 infection in vivo.
Infection of the oral mucosa by human immunodeficiency virus type I (HIV-1) in vivo is a critical area of HIV-1 pathogenesis which has been investigated previously, but an extensive number of important questions remain unanswered (5, 7, 20, 31, 57). This field of research is key, as the oral mucosa is increasingly found to be associated with horizontal (and vertical) transmission of this human lentivirus, as well as being potentially involved with a variety of pathogenic processes in the oral cavities of HIV-1-infected individuals (10, 26, 84, 106).
HIV-1 had been thought previously to rarely be transmitted between individuals via oral-genital and oral-oral contact of mucosa or through infected saliva (56, 60, 109). Nevertheless, several recent studies have suggested that a receptive oral sexual contact may lead to significant HIV-1 transmission in certain cohorts, especially in male homosexuals and crack cocaine and intravenous drug users (34, 97, 99, 102). A number of studies have demonstrated that HIV-1 can be detected in the saliva of infected individuals by culture, reverse transcriptase PCR, and DNA-PCR. Although the levels are usually lower than in peripheral blood or semen and cervical/vaginal secretions of infected-individuals, certain patients have been shown to be “hyper-secretors” with respect to saliva (62, 92, 107). A recent study has demonstrated by heteroduplex mapping that the viral quasispecies in peripheral blood is quite similar to viral strains in the saliva of HIV-1-infected individuals (40). As well, both in vitro and in vivo studies have suggested that epithelial cells may be infected with HIV-1 (23, 61, 71). Our laboratories have previously shown, utilizing in situ PCR, that HIV-1 could be detected in a significant majority of infected individuals within their oral mucosal epithelial cells (92). As well, detection of HIV-1 in oral mucosal cells was demonstrated by electron microscopy (91). Recent studies have demonstrated that oral keratinocytes can be infected by HIV-1 using galactosylceramide and CXCR4 as receptors (61), and ethanol may increase infectivity (23). As well, other studies have suggested that HIV-1 infection, both cell free and cell associated, of salivary gland cell lines via CXCR4 and CCR5 with a CD4-independent mechanism can be demonstrated in vitro (70). In addition, secretory immunoglobulin A from parotid glands may inhibit CCR5- but not CXCR4-tropic viruses. Data also suggest that oral shedding of HIV-1 RNA correlates with antiretroviral therapy, CD4+ T-cell counts, and tonsillectomy status (134).
It has been well described for the past decade or more that a variety of salivary inhibitors decrease HIV-1 infectivity in the oral cavity (108, 109). Nonetheless, ongoing studies have suggested that oral transmission of the virus remains quite common in a number of important settings in vivo (6, 7, 51). These enigmatic processes require significant evaluations to dissect, in a prioritized manner, the full virus:host cell interactions which occur in vivo and lead to oral HIV-1 transmission and pathogenesis. As such, in this study, we have utilized selected virological and molecular techniques to analyze HIV-1 infection and interactions with primary human oral keratinocytes. As well, we evaluated the potential for selected recombinant HIV-1 proteins to induce apoptosis in these primary human cells.
MATERIALS AND METHODS
Isolation of primary human oral mucosal cells.
Primary oral mucosal cells were obtained from the gingiva of HIV-1-seronegative dental patients and processed as described, with some modifications (71). Briefly, the epithelial layer from the oral tissue was digested for 60 min at 37°C with collagenase type II (Millipore Corp., New Bedford, MA) (1.0 mg/ml) and dispase grade II (Boehringer Mannheim, Indianapolis, IN) (2.4 mg/ml) to separate the epithelium from the underlying submucosa. Epithelial sheets were then dispersed into individual cells by using trypsin. The cells were washed twice with Dulbecco's phosphate-buffered saline (PBS) containing amphotericin B (fungizone) plus penicillin/streptomycin and resuspended in keratinocyte growth medium (Clonetics Corp., San Diego, CA). Aliquots of 104 cells were plated in fibronectin-coated six-well plastic tissue culture dishes (BD Biosciences Discovery Labware). Purity of keratinocytes was assessed by immunocytochemical analyses with the epithelial cell markers keratin and CD99. Cell isolates showing ∼98% purity were used in subsequent studies. All studies were approved by our institutional review board.
Measurements of TEER.
To measure the trans-epithelial electrical resistance (TEER) of confluent oral mucosal cells, the cells were cultured on 0.40 μM polyethylenetetraphthalate track-etched membranes (BD Biosciences, Bedford, MA) to form an in vitro oral mucosa barrier layer. The integrity was determined by measuring trans-epithelial electrical resistance across the monolayer with the aid of a Millicell-ERS system (Millipore Corp., Bedford, MA), as described previously (72).
HIV-1 infection of primary oral mucosal cells.
Viral infectivity assays were performed to determine the effects of natural endogenous reverse transcription (NERT) on HIV-1 infection of primary human oral mucosal cells. Aliquots (100 ng of p24 antigen equivalents) of the CXCR4-tropic HIV-1 strain, NL4-3, and the CCR5-tropic HIV-1 strains, YU-2 and ADA, were treated with either medium or NERT-stimulating agents, the polyamines spermine (3.0 mM) and spermidine (0.1 mM), with or without deoxynucleoside triphosphates (dNTPs) (100 nM), which are the physiological concentrations of these molecules in human semen (129). After incubation for 20 h at 37°C, aliquots were used to infect primary oral mucosal cells grown to confluence in fibronectin-coated, six-well tissue culture plates. After incubation for 4 h, the cells were washed 3 times with medium and viral growth was monitored for 1 week. Aliquots of the supernatants were collected at specified time points for quantitation of the HIV-1 p24 antigen levels by enzyme-linked immunosorbent assays (ELISA) (Dupont).
Virion binding, uptake, and internalization studies.
Aliquots of the CXCR4-tropic HIV-1 strain, NL4-3, and the CCR5-tropic HIV-1 strain, YU-2 (100 ng of HIV-1 p24 antigen), were incubated with NERT-stimulating agents, the polyamines spermine (3 mM) and spermidine (0.1 mM), with or without 100 nM dNTPs at 37°C for 4 h, according to a previously described methodology (29, 129-131). The ensuing cocktail was used to infect primary human oral mucosal cells. As described previously (29), controls included incubated but untreated virions. The unbound virions are then washed off with phosphate-buffered saline (PBS) (twice), and subsequently the uninternalized virions were removed by incubation with 0.25% trypsin (Sigma) for 5 min at 37°C. Three further washings, including one wash with a serum-containing medium, were then performed. The levels of HIV-1 p24 antigen in the cell lysates were quantitated by ELISA.
HIV-1 protein induction of programmed cell death in primary oral keratinocytes.
To investigate the potential ability of selected HIV-1 proteins to induce apoptotic cell death in primary oral keratinocytes, three specific HIV-1 proteins, gp120, Tat, and Nef, which we and other groups have shown to induce apoptosis in various human cells (1, 82, 83), were evaluated. Nef and gp120 were expressed in our laboratory in baculovirus and vaccinia virus systems, respectively, whereas HIV-1 Tat was obtained from the National Institutes of Health AIDS Reagent and Repository Program. Low-passage, primary human oral keratinocytes were seeded onto fibronectin-coated, four-well chamber slides (BD Biosciences Discovery Labware). The cells were allowed to attach for 48 h before being exposed to 10 ng/ml of recombinant HIV-1 Nef, gp120 BaL (R5), Tat, and glutathione S-transferase (GST) (recombinant control protein). Selection of this particular initial concentration was based on our previous studies of primary human brain microvascular endothelial cells (1). Following treatment with viral proteins, cells were incubated for 48 h at 37°C and 5% CO2 in a humidified environment. As additional negative controls, cells that had not been treated with any protein were used. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays were performed on treated and untreated cells, using an in situ cell death detection system, TMR Red (Roche Diagnostics, Mannheim, Germany), following the manufacturer's instructions. Briefly, cells were washed twice with 1× PBS and fixed for 10 min with acetone at room temperature. The fixed cells were washed three times with 1× PBS, and 50 μl of TUNEL reaction mixture was added to each well. Cells treated with the TUNEL mixture were incubated at 37°C in a humidified environment for 1 h. On completion of incubation, the treated cells were washed four times with PBS and evaluated by fluorescence microscopy. As positive controls, primary oral keratinocytes were treated with DNase (Life Technologies, Gaithersburg, MD) at a concentration of 1.0 μg/ml and incubated for 10 min at room temperature to induce DNA strand breaks. Untreated cells were also used as negative apoptosis controls, in addition to the GST-treated cells.
Semiquantitative apoptosis determinations.
For semiquantitative measurements of apoptosis, images generated with a charge-coupled device array camera (RT Color; Diagnostic Instruments, Inc., Sterling Heights, MI) were subjected to fluorescence brightness value determinations on a monochromatic scale in red, blue, and green (RGB) values (0 to 255 ASCII numbers). Apoptosis was defined based on a red monochromatic scale in the range of 0 to 255, as described previously (1). Blank values were subtracted from the average of seven random values from different cells. Each image was further analyzed on a visual scale ranging from 1 to 10. This number was multiplied with ASCII numbers to generate final semiquantitative values for each image.
Human apoptosis gene microarrays.
The CLONTECH Atlas cDNA expression human apoptosis array was used in this study. Total cellular RNA was extracted from third-passage human oral keratinocytes with an ULTRASPEC RNA isolation kit (Biotecx Laboratories, Inc., Houston, TX), following the manufacturer's instructions. After RNA extraction, cDNA probe mixtures were synthesized by reverse transcribing respective RNA using cDNA synthesis primer mix provided by the manufacturer and [α-32P]dATP (Perkin Elmer Sciences, Inc., Boston, MA). Each radioactively labeled probe mix was then hybridized to separate Atlas arrays overnight at 68°C. After high-stringency washes, as suggested by the manufacturer, the hybridization patterns were analyzed by autoradiography and quantified with a phosphorimager (Molecular Dynamics). The relative expression levels of a given cDNA from RNA obtained from oral keratinocytes treated with recombinant HIV-1 Nef, Tat, and gp120 protein were assessed by comparing the signal obtained with a probe of RNA from treated keratinocytes to that obtained with a probe of control RNA.
RESULTS
Isolation and characterization of primary human oral keratinocytes.
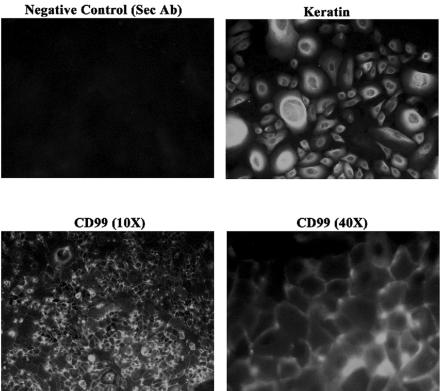
To analyze the ability of HIV-1 to infect primary oral mucosa cells, we first isolated and cultured pure keratin-positive, human oral mucosal epithelial cells. The purity of the cells was determined by immunocytochemical staining with monoclonal anti-pan cytokeratin antibodies from Sigma Aldrich Corp. (St. Louis, MO). The results are illustrated in Fig. 1. Keratin staining was robustly demonstrated using the CY2 chromophore. In these analyses, using immunofluorescence microscopy, few if any cells were shown to be fibroblastic by morphology or negative staining. In addition, we have begun to characterize the intercellular proteins that may be important in further studies of HIV-1-infected cell migration across an oral mucosal barrier. As demonstrated by initial studies illustrated in Fig. 1, CD99 was shown to have reproducible and significant expression in these primary oral mucosal epithelial explants. This is an extremely important molecule that has been demonstrated recently to play a major role in the migration of monocytes across endothelial junctions (101). As such, this may be a key molecule in understanding transmigration of HIV-1-infected monocytes and potentially CD4+ T lymphocytes across an oral mucosal barrier.
FIG. 1.
Illustration of immunofluorescence staining for keratin, a specific characterization marker for keratinocytes, and CD99. Aliquots of 104 cells were plated in fibronectin-coated six-well plastic tissue culture dishes (BD Biosciences Discovery Labware) and incubated for 72 h. Purity of keratinocytes was then assessed by immunocytochemical analyses with the epithelial cell markers keratin and CD99. Sec Ab, secondary antibody only.
In addition, we sought to ascertain whether or not cultured human oral keratinocytes were able to generate an oral mucosal barrier system in vitro, analogous to our studies of in vitro blood-brain barrier models (72). We utilized near-100%-pure primary oral mucosal cells cultured on 0.4 μM polyethylenetetraphthalate membranes. These cultures, in multiple separate and independent samples, demonstrated a transepithelial electrical resistance (TEER) of approximately 176 to 208 Ω (Table 1). This is somewhat lower than that obtained for human brain microvascular endothelial cells but is still significantly elevated compared to baseline (e.g., brain microvascular endothelial cells frequently lead to approximately 250 Ω in models of this type in our hands). Whether these TEER values obtained for oral mucosal keratinocytes barriers reveal properties similar to other epithelial barriers requires further elucidation. Of note, we were unable to detect the expression of zonula occludens proteins, mainly involved in the creation of barrier properties of certain epithelial layers (not illustrated). These initial studies have suggested, however, not only that human oral keratinocytes can be grown in an in vitro explant system but also that they do have barrier functions, as described previously (25, 94).
TABLE 1.
TEER measurements of human oral keratinocytes
| Sample | TEER (ohms) |
|---|---|
| Control | 131, 130 |
| 1 | 177 |
| 2 | 181 |
| 3 | 187 |
| 4 | 176 |
| 5 | 178 |
| 6 | 208 |
| 7 | 181 |
| 8 | 186 |
Virological and microenvironmental factors which alter HIV-1 infection of oral keratinocytes.
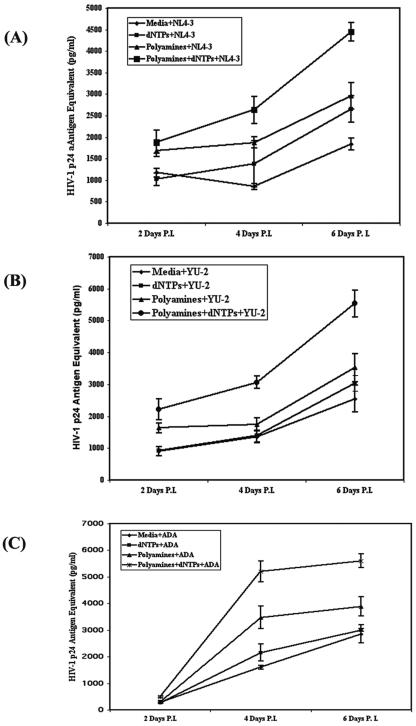
To evaluate critical virological and environmental parameters which alter HIV-1 infection of the oral keratinocytes, we first infected the cells in the presence of the NERT-stimulating agents: deoxynucleoside triphosphates (dNTPs) and polyamines. Of note, NERT represents stimulated reverse transcription within intact virions, which augments viral infectivity in many human cell types (29, 129). Following infection of oral keratinocytes with HIV-1 NL4-3, YU-2, and ADA in the presence or absence of these agents, we assayed HIV-1 gag p24 production in cell culture supernatants by ELISA. As shown in Fig. 2, in all three strains of HIV-1, the CXCR4-tropic NL4-3 virus as well as the CCR5-tropic viruses YU2 and ADA, viral infectivity of these primary oral explant mucosal cells could be dramatically increased with the use of agents which stimulate NERT, including polyamines and dNTPs, at physiological concentrations demonstrated in human semen (129). Although the CXCR4-tropic NL4-3 virus as well as the CCR5-tropic YU2 and ADA viruses all had their infectivities increased by these NERT-stimulating agents, it is interesting that YU-2 demonstrated a more dramatic effect from NERT stimulation. Control viral binding and internalization studies showed no differences in these oral cells with or without NERT-altering agents, demonstrating the specificity of these molecular effects (data not illustrated).
FIG. 2.
Natural endogenous reverse transcription (NERT) stimulation of HIV-1. Virions increase infectivity on human oral keratinocytes. Primary human keratinocytes were grown to confluence, and then 100 ng of p24 antigen equivalents of the CXCR4-tropic HIV-1 strain, NL4-3 (A), and the CCR5-tropic HIV-1 strains YU-2 (B) and ADA (C) were treated with either medium or NERT-stimulating agents (the polyamines spermine [3 mM] and spermidine [0.1 mM] and/or dNTPs [100 nM], which are the physiological concentrations in human semen), prior to infection of 1 × 106 primary human oral keratinocytes. Certain viral inputs were treated with dNTPs alone and others with polyamines alone, while still others were treated with both polyamine and dNTPs. After infection for 4 h, the cells were vigorously washed three times with medium and viral growth was monitored for 1 week. Viral outgrowth using ELISA for HIV-1 p24 antigen expression in the culture supernatants was analyzed. These data are representative of three independent experiments performed in triplicate. P.I., postinfection.
Induction of apoptosis in human primary keratinocytes by HIV-1 proteins.
HIV-1 infection of cells can release specific viral products (123), which may induce apoptosis in bystander cells. It has been shown that HIV-1 infection can also lead to the production and release of proinflammatory cytokines (90), which are toxic to cells. Therefore, in addition to determining the effects of NERT on HIV-1 replication in primary human oral keratinocytes, we wished to determine whether HIV-1 viral proteins released during infection induce apoptosis in these cells. The ability of HIV-1 proteins to induce apoptotic cell death in primary keratinocytes, by exposing the cells to three selected HIV-1 specific proteins, gp120, Tat, and Nef, was investigated.
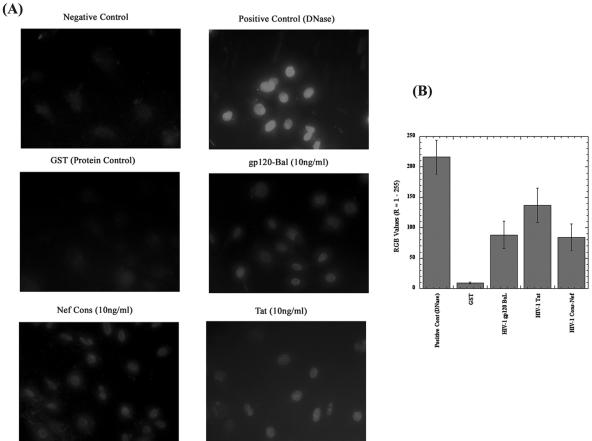
Primary oral keratinocytes cells were exposed to recombinant viral proteins Nef, Tat, gp120 BaL, and GST at a concentration of 10 ng/ml. At 48 h postexposure, apoptosis was evaluated utilizing the TUNEL assay; the results are depicted in Fig. 3A. It was observed that the use of nontreated cells and a control protein, GST, led to little or no programmed cell death in the primary oral keratinocytes. Individually each of the viral proteins showed apoptosis in oral keratinocytes, but with quite different intensities compared with control results. Strict quantitation is difficult in these assay systems, but in evaluations of multiple independent assays, visual examination of the photomicrographs suggested that the effect of Tat was the most pronounced, followed by gp120 BaL and Nef. However, comparison of all treatments using double-tailed Student's t tests indicated that the differences between Tat and gp120 did not rise to significance (P > 0.08). Similarly, there was no significant difference between gp120 and Nef (P > 0.8), but there was a significant difference between Tat and Nef (P < 0.05). Of importance, though, there were various degrees of quite significant differences between all three HIV-1 proteins and the GST-treated, control oral keratinocytes, with all P values less than 0.001.
FIG. 3.
(A) TUNEL assays of human oral keratinocytes treated with selected HIV-1-specific proteins. Representative TUNEL staining of HIV-1 Nef-, gp120-, and Tat-treated primary human oral keratinocytes is shown. Cells were exposed to HIV-1 Nef, gp120, and Tat for 72 h, after which cells were subjected to TUNEL staining. The concentration of protein was 10 ng/ml. As a positive control, keratinocytes were exposed to DNase at a concentration of 10 μg/ml for 10 min at room temperature. TUNEL assays were performed using an in situ cell death detection kit, TMR Red (Roche Diagnostics). The cells were analyzed with fluorescence microscopy (Olympus System microscope, model BX60, with fluorescence attachment BX-FLA). The top panels illustrate a negative control and a positive control with apoptosis-induced nuclei of primary oral keratinocytes after treatment with nuclease (DNase; Life Technologies). These results are representative of experiments performed in triplicate and repeated at least twice. The other photomicrographs of the figure illustrate apoptosis-induced nuclei of cells treated with selected HIV-1-specfic proteins. Nef Cons, Nef consensus sequence protein. (B) Semiquantitative analysis of apoptosis in primary human oral keratinocytes, using red, blue, and green values (R = 1 to 255). The details of this methodology for evaluating TUNEL-positive cell numbers are presented in Materials and Methods.
Human apoptosis gene expression.
The mechanisms involved with induction of apoptosis in primary human oral keratinocytes are not known. Therefore, to elucidate the molecular mechanisms involved in HIV-1 protein-induced apoptosis, we utilized targeted gene microarrays to investigate the up-regulation of apoptosis-related genes in primary human oral keratinocytes exposed to HIV-1 Nef, gp120, and Tat proteins. Cells exposed to GST protein were used as controls.
Table 2, Table 3, and Table 4 depict apoptosis-related genes up-regulated in response to gp120, Nef, and Tat, respectively. The genes up-regulated when cells were exposed to gp120 included tumor necrosis factor (TNF) receptor super family member 1, and caspases 3, 6, and 8, as well as tumor proteins p53, Bcl-2, NF-κB, cyclin B1, cyclin-dependent kinase 1 (cdk1), and other apoptosis-related genes. As Table 3 indicates, Nef-treated oral keratinocytes showed up-regulation in the expression of p53, DAXX, mitogen-activated protein kinase (MAPK) 7, and caspases 3, 6, 8, 9, and 10, as well as TNF receptor super family members 1 and 2 and Bcl-xL plus other apoptosis-related genes, compared with the GST-treated control. The results from the Tat-treated primary human oral keratinocytes are depicted in Table 4. As indicated in this table, modest up-regulation in the expression of MAPK 7, cyclin B, caspases 9 and 10, TNF receptor super family 12, and Ras homolog gene family member A were observed in primary oral keratinocytes exposed to Tat. PICTAIRE protein kinase 3 and E2F transcription factor 5 were modestly down-modulated. As can be observed in Tables 2, 3, and 4, it is clear that most of the up-regulated genes have been implicated in the Fas/FasL apoptotic pathways. Therefore, increases in their mRNA expression levels suggest possible involvement of the Fas/FasL apoptotic pathway in HIV-1 gp120-, Nef-, and Tat-induced apoptosis in oral keratinocytes. We also observed up-regulation in mRNA expression levels of genes involved in the mitochondrial apoptotic pathway, notably caspase 9, in cells exposed to HIV-1 Nef and Tat. It could therefore be strongly suggested that HIV-1 Nef and Tat may utilize more than one pathway in inducing apoptosis in primary human oral keratinocytes.
TABLE 2.
cDNA microarray analyses of human oral mucosa (keratinocytes) exposed to extracellular HIV-1 gp120 and GST protein as a control
| Gene | Severalfold increase in gene expression |
|---|---|
| TNF receptor superfamily member 1 | 2.00 |
| Retinoblastoma binding protein 4 | 2.30 |
| Tumor protein p53 | 1.80 |
| NF-κB | 2.40 |
| Cyclin dependent kinase 1 (Cdk 1) | 2.50 |
| Proliferating cell nuclear antigen | −2.00a |
| Caspase 3 | 1.7 |
| Caspase 6 | 1.71 |
| Caspase 8 | 1.83 |
| Bcl-2 | −2.50 |
| Growth arrest-specific gene 1 (GAS 1) | 2.15 |
| Cyclin B1 | 1.80 |
| MAPK/ERK | 2.00 |
Negative (−) signs indicate genes that were moderately down-modulated.
TABLE 3.
cDNA microarray analyses of human oral mucosa (keratinocytes) exposed to extracellular HIV-1 Nef and GST protein as a control
| Gene | Severalfold increase in gene expression |
|---|---|
| Tumor protein p53 | 2.00 |
| DAXX | 2.00 |
| Mitogen-activated protein kinase (MAPK) 7 | 2.50 |
| Caspase 3 | 1.5 |
| Caspase 6 | 1.8 |
| Caspase 8 | 1.71 |
| Caspase 9 | 1.83 |
| Caspase 10 | 2.45 |
| Proline dehydrogenase (oxygenase) | 2.15 |
| TNF receptor superfamily member 1 | 1.85 |
| TNF receptor superfamily member 12 | 1.50 |
| Ras homolog gene family member A | 1.75 |
| Metalloproteinase-disintegrin TNF-α convertase (TACE) | 2.00 |
| Bcl-xL | −1.75a |
Negative (−) signs indicate genes that were down-modulated.
TABLE 4.
cDNA microarray analyses of human oral mucosa (keratinocytes) exposed to extracellular HIV-1 Tat and GST protein as a control
| Gene | % Increase in gene expression |
|---|---|
| PCTAIRE protein kinase 3 (apoptosis related) | −1.3a |
| Cyclin C | 18.5 |
| Mitogen-activated protein kinase (MAPK) 7 | 2.4 |
| E2F transcription factor 5 (transcription activators and repressors) | −62.0 |
| Cyclin-dependent kinase inhibitor | 28.0 |
| Transcription factor Dp-2 | 17.8 |
| Caspase 9 (apoptosis related) | 30.0 |
| Caspase 10 (apoptosis related) | 25.0 |
| Proline dehydrogenase (oxygenase) | 31.0 |
| TNF receptor superfamily member 12 | 17.6 |
| Insulin-like growth factor 1 | 27.0 |
| Insulin-like growth factor binding protein 6 | 9.0 |
| Retinoid X receptor beta (nuclear receptor, death receptor) | 34.0 |
| Ras homolog gene family member A | 22.0 |
Negative (−) signs indicate genes that were down-modulated.
DISCUSSION
These studies evaluated virological and microenvironmental factors which potentially may increase HIV-1 infection of oral mucosal epithelial cells. First, natural endogenous reverse transcription (NERT) (i.e., intravirion reverse transcription), which has been described previously (29, 129) as a new phase of the viral life cycle, was analyzed for its effects on augmenting infection of human oral keratinocytes in vitro.
Our laboratories were involved in the initial discovery of NERT in lentivirions. These studies demonstrated that reverse transcription could be driven in replication-competent virions by microenvironments containing physiological concentrations of polyamines and dNTPs. This process increases viral infectivity, being especially prominent in resting cells, and may increase transmissibility of the virus in vivo. In a study by Zhang et al. (129), strong-stop negative-strand DNA was detected in HIV-1 virions obtained from a cell culture system. Most importantly, it was demonstrated that without detergent, addition of dNTPs to isolated HIV-1 virions could stimulate RT activity in these extracellular virions, leading to higher levels of virions carrying strong-stop negative-strand moieties and more-complete negative-strand intermediates. This was demonstrated at nanomolar concentrations of dNTPs. As well, several human physiological fluids, including seminal and blood plasma, could drive intravirion HIV-1 DNA synthesis. This was hypothesized to be due to dNTPs and polyamines within these fluids (129). These data suggested that in physiological fluids, HIV-1 virions might synthesize significant quantities of partial, and possibly full-length, reverse transcripts. This hypothesis was then supported by our initial data in which HIV-1 virions, isolated from the peripheral blood of infected individuals, were demonstrated to harbor HIV-1 DNA (for a review, see references 130 and 131). Based on the studies in the present report, it could be hypothesized that these molecular processes at the oral mucosa may also alter interhost vertical and horizontal transmission of HIV-1.
To test the ability of NERT-stimulating agents in augmenting HIV-1 infection of primary human oral keratinocytes in vitro, we exposed the cells to HIV-1 NL4-3, ADA, and YU2. Our results show that oral keratinocytes are productively infected by both R5- and X4-tropic HIV-1 isolates and that R5 isolates may do so most efficiently. These findings are fully consistent with the results of other studies (70, 71, 132). However, Liu et al. (61) have reported productive infection of oral keratinocytes with X4- and dual-tropic isolates (X4/R5) and minimal infection with R5 isolates. In contrast, Quinones-Mateu et al. (91) could not demonstrate infection of oral keratinocytes with either X4 or R5 isolates. The apparent discrepancy between the results of our study and those of the other studies may be explained by the differing experimental conditions which were used. For example, the amount of virus used by Liu et al. to infect their cells was about 30-fold higher than the level we used in our study. We also did not add Polybrene in our viral infections, as we have found this to be toxic to these cells and also because it is not representative of the physiological environment. In addition, Liu et al. used 2-week-old cultures of oral keratinocytes, whereas ours were approximately 3 days old. Liu et al. (61) also reported minimal expression of CCR5 in comparison with the expression of CXCR4. It is not clear whether the age of their cultures adversely affected the expression of CCR5 in oral keratinocytes, since other studies have reported the expression of this receptor at levels comparable to that of CXCR4 (50, 70, 71).
In addition to our observations of the infection of oral keratinocytes by both HIV-1 R5 and X4 isolates, we also demonstrated that stimulation by NERT increases infectibility of these cell-free virions on oral mucosal epithelium, as well as virus newly produced from HIV-1-infected cell types. Importantly, these factors model oral sexual contact and/or oral inflammation. Higher concentrations of these agents may not only increase HIV-1 replication but also increase HIV-1-specific protein-induced apoptosis (programmed cell death) in target cells (see below).
HIV-1 proteins and apoptosis in oral keratinocytes.
One of the hallmarks of HIV-1 infection is programmed cell death of infected cells (9, 12). Of note, HIV-1 proteins individually have also been shown to induce and/or augment apoptosis in various primary isolated human cells, as well as in established cell lines in vitro (9, 12, 82, 83). Thus, in addition to studying the effects of NERT in augmenting HIV-1 infection of oral keratinocytes, we also sought to discern the role of HIV-1 proteins on oral mucosa cells. To validate the experiments in this study, two complementary controls were utilized, a non-HIV-1 recombinant protein expressed in our laboratories, GST, and nontreated cells. In the present study, we observed that several selected HIV-1 proteins mediated apoptosis of human oral keratinocytes. Previous studies have suggested that apoptosis (or programmed cell death) of CD8+ and/or CD4+ T lymphocytes may play a major role in AIDS pathogenesis (81). Increased apoptosis in neurons, astrocytes, and microvascular endothelial cells in the central nervous system of macaques infected with a neurovirulent strain of simian immunodeficiency virus has been described (2). Recent studies have also shown in vitro apoptosis induced by HIV-1 gp120 and/or SDF-1α/β, binding to the chemokine coreceptor, CXCR4, on certain T-lymphocyte as well as neuronal cell populations (3, 45, 59, 74, 103), and HIV-1 envelope-specific apoptosis was demonstrated in primary brain mixed-cell cultures (78). The present study adds to these data and shows that HIV-1 Tat, gp120, and Nef induced substantial apoptosis in primary human oral keratinocytes.
In addition to HIV-1 gp120, which has been well studied in previous apoptosis experiments, other lentiviral proteins were evaluated for their effects on oral keratinocytes. Tat is an HIV-1 regulatory protein and the major transcriptional transactivator of this lentivirus (58). Tat is expressed in the early stage of infection from multiply spliced HIV-1 mRNA and, importantly, is also excreted into the extracellular milieu from infected cells (33). Tat can be taken up by noninfected cells at levels which, when internalized, can alter expression from the HIV-1 long terminal repeat and the promoters of certain cellular genes, both directly and by increasing nuclear factor-kappa B (NF-κB) levels (33). HIV-1 Tat may also induce T-lymphocyte cell death by apoptosis (98). Thus, HIV-1 Tat has been shown to have deleterious effects on certain cells in vitro. This was clearly demonstrated in the present study for human oral keratinocytes.
The role of Nef in inducing apoptosis in T lymphocytes is quite controversial (28, 79, 93, 110). In a recent study, we observed that baculovirally expressed consensus HIV-1 recombinant Nef induced apoptosis in primary human brain microvascular endothelial cells at concentrations of 10 to 100 ng/ml. These apoptotic effects of Nef were augmented with the addition of 0.3% ethanol (1). In the present study, we observed Nef-mediated apoptosis in primary human oral keratinocytes. It therefore appears that contrary to the results of certain studies of other cell types, HIV-1 Nef induces apoptosis, in vitro, in human oral mucosal cells.
Mechanisms of apoptosis in oral mucosal cells induced by HIV-1 proteins.
The molecular mechanisms involved in apoptosis of primary human oral keratinocytes are mostly unknown. Therefore, to begin to elucidate the molecular mechanisms involved, we employed targeted gene microarrays to analyze the regulation of apoptotic genes in primary human oral keratinocytes exposed to soluble HIV-1 gp120, Nef, and Tat proteins. The gene microarray analyses demonstrated that Tat-treated primary human oral keratinocytes induced increased expression of TNFR12, whereas both gp120- and Nef-treated oral keratinocytes induced up-regulation in the expression of TNFR 1 and caspases 6 and 8. In addition, Nef up-regulated the expression of DAXX, a protein that has also been implicated in apoptosis.
Apoptosis is comprised of two major pathways, the mitochondrial apoptotic pathway and the Fas/Fas ligand plus the TNF/TNFR-1 apoptotic pathways (14, 53, 105). In all cases, the downstream target of these apoptotic pathways is the activation of caspase 3. Caspase 8 is the downstream indicator of activation of the Fas-Fas ligand pathway, whereas caspase 9 is associated with the mitochondrial apoptotic pathway (53, 105). Receptors in the TNF receptor super family are involved with the induction of apoptosis as well as inflammatory signaling. In response to Fas ligation, Fas-associated death domain protein binds and recruits caspase 8 to form the death-induced signaling complex, thereby activating Fas apoptotic signaling (13, 66, 76). It has also been shown that upon Fas engagement, molecules such as DAXX may also bind to Fas and mediate Fas-associated death domain protein-independent apoptotic signaling (54). Thus, the fact that in the present studies, genes involved in the TNF/TNF-R and Fas/Fas ligand pathways were found up-regulated suggests the involvement of the TNF/TNF-R and Fas/Fas ligand pathways in HIV-1 gp120-, Nef-, and Tat-induced apoptosis in primary human oral keratinocytes. These results are consistent with previous studies which suggest that Nef (41-44), gp120 (125), and Tat (126, 127) up-regulate the expression of genes in the TNF/TNF-R and Fas/Fas ligand pathways, resulting in apoptosis. For instance, the HIV-1 proteins gp120 (96, 115), Tat (32, 120), and Nef (128) have been reported to increase CD95 and FasL levels, which enhance cellular susceptibility to Fas-mediated apoptosis. Nef, on the other hand, is thought to induce FasL expression by interacting specifically with the zeta chain of the TCR complex (124), whereas Tat has been shown to up-regulate the expression of the initiator, caspase 8 (11).
In addition to the induction of the TNF/TNF-R and Fas/Fas ligand pathways, oral keratinocytes exposed to Nef showed up-regulation in the expression of caspases 3, 6, 8, 9, and 10, whereas Tat-induced expression of caspase 9 and 10 and gp120 induced the expression of caspases 3, 6, and 8. The caspase 9 activation pathway involves the release of cytochrome c from mitochondria, which associates with pro-caspase 9 and Apaf-1 in the presence of dATP to form a proapoptotic complex (53, 105). The pro-caspase 9-APf-1-cytochrome c complex so formedinduces autoactivation of caspase 9 by aggregation (133). Once activated, caspase 9 initiates a cascade involving caspase 3, 6, and 7, which act by themselves to cleave cellular targets such as poly(ADP-ribose) polymerase, leading to cell death (133). In this study, we also observed increases in the expression of various caspases when mucosal cells were exposed to HIV-1 gp120, Nef, and Tat. This increase in caspase 9 expression in the cells exposed to HIV-1 Nef and Tat suggests that induction of apoptosis by these specific proteins may also involve the mitochondrial apoptotic pathway. Furthermore, this observation supports the hypothesis that synergy between the up-stream caspase pathways may have an important role in HIV-1 proteins-induced oral keratinocyte apoptosis. Together, the data presented herein support an important function for caspases in HIV-1-induced injury and apoptosis in this cell type.
In addition to the TNF/TNF-R and Fas/Fas ligand pathways and the caspase cascades initiated by exposure of oral keratinocytes to gp120, Nef, and Tat, cells exposed to Nef exhibited down-regulation in the expression of Bcl-xL. Similarly, gp120 induced down-modulation in the expression of Bcl-2. Bcl-2 is a human proto-oncogene located on chromosome 18. Its product is an integral membrane protein (entitled Bcl-2) located in the membranes of the endoplasmic reticulum and nuclear envelope and in the outer membranes of the mitochondria. At least 16 members of the Bcl-2 family of proteins have been sequenced in recent years, both in mammals and in viruses (19, 95, 112). The Bcl-2 family of proteins regulates the mitochondrial potential and early apoptotic changes in cells (75). Some of these, such as Bcl-2, Bcl-xL, Bcl-w, or Mcl-1, protect cells against Fas-induced programmed cell death by decreasing the permeability of the mitochondrial membrane, while others, such as Bad, Bak, Bax, Bid, Bim, or Hrk, induce apoptosis (96). The antiapoptotic activity of these proteins is related to their ability to form heterodimers (96). For instance, in murine FL5.12 cells, Oltvai et al. (80) showed that overexpression of Bax after withdrawal of interleukin 3 led to the formation of homodimers, resulting in programmed cell death. Overexpression of antiapoptotic proteins such as Bcl-2 and Bcl-xL resulted in the formation of Bcl-2/Bax or Bcl-xL/Bax heterodimers, thereby inhibiting the lethal functions of Bax.
Although the mechanism of apoptosis regulation by Bcl-2 is not yet fully understood, whether or not a cell becomes susceptible to programmed cell death may depend on the balance between proapoptotic proteins, such as Bax, and antiapoptotic proteins, such as Bcl-2 or Bcl-xL. It has been reported that the HIV-1-encoded proteins gp120, Tat, and Nef inhibit expression of Bcl-2, and Nef alone inhibits the expression of Bcl-xL (4, 100). In the present study, moderate down-modulation in the expression of Bcl-2 was observed in keratinocytes exposed to gp120. Similarly, oral keratinocytes exposed to Nef exhibited moderate decreases in the expression of Bcl-xL. The decrease in the relative amounts of these proteins in this study is likely important, as increased quantities of antiapoptotic proteins may be able to overwhelm even larger numbers of proapoptotic signals. The results also suggest that the reduced expression of antiapoptotic Bcl-2 family of proteins in gp120- and Nef-treated cells might have disturbed the balance between the pro- and antiapoptotic regulators, thereby promoting proapoptotic signaling in response to exposure to these HIV-1 proteins. Thus, these results further confirm the involvement of the Fas/FasL apoptotic pathway in both gp120- and Nef-induced apoptosis in oral keratinocytes.
Besides the expression of Bcl-2 proteins, our targeted microarray analyses also revealed the up-regulation in the expression of p53, NF-κB, MAPK/extracellular signal-regulated kinase (ERK), growth arrest-specific gene 1 (GAS) 1, retinoblastoma binding protein 4, cyclin B1, and cyclin-dependent kinase 1 (Cdk 1) in primary human oral keratinocytes exposed to gp120. There was down-modulation in the expression of proliferating cell nuclear antigen (PCNA) in these same cells. Oral keratinocytes exposed to Nef, on the other hand, demonstrated up-regulation in the expression of the tumor protein p53, mitogen activated protein kinase (MAPK) 7, and Ras homolog gene family member A. Similarly, Tat induced increased expression of Ras homolog gene family member A, MAPK 7, and cyclin C plus other genes and down-regulated the expression of the apoptotic-related PICTAIRE and E2F transcription factor 5.
Ras is an essential component of signal transduction pathways that control cell proliferation, differentiation, and survival (73, 104). The gene and its expression products have been established as modulators of apoptosis and, depending on the cell type and the presence of other proapoptotic or antiapoptotic stimuli (21, 30), can either inhibit or promote apoptosis. Numerous studies have shown that Ras activity is manifested through ceramide signaling in the Fas/Fas ligand apoptotic pathway and that overexpression of ras accelerates the Fas-induced death process (21, 47). For example, in human or mouse lymphocytes, it has been shown that expression of Ras resulted in Fas-induced apoptosis (48), and this apoptotic process was blocked by the overexpression of bcl-2 (21, 22). Similarly, it has been demonstrated in Jurkat T-cells that overexpression of ras resulted in sensitization of the cells to Fas engagement and low levels of expression of Bcl-2 (22, 35). In a study to examine the cellular responses to high-intensity Ras signaling, Joneson and Bar-Sagi (52) reported that the expression of increasing amounts of the oncogenic form of human HRas, HRasV12, resulted in a dose-dependent induction of apoptosis in both primary and immortalized cells (52). At low levels of Ras expression, this group reported that the antiapoptotic pathway is activated to an extent that is sufficient to counteract the apoptotic signals. At high levels of Ras signaling, this balance was tilted in favor of the proapoptotic signals presumably because the component(s) of the antiapoptotic pathway are limiting. The apoptotic effect of HRasV12 requires the activation of both the ERK and Jun N-terminal protein kinase (JNK) mitogen-activated protein kinase cascade and is independent of the tumor protein, p53 (52). It is important to note that the p53-independent apoptotic effects may be exclusive to the HRasV12, since the expression of the molecule has been shown to play important roles in apoptosis (67, 68). For instance, wild-type p53 activates BH-3-containing proteins and mediates the down-modulation of Bcl-2 expression and up-regulation of Bax, thereby predisposing the cells to apoptosis (67, 68).
Our targeted microarray analyses clearly demonstrated that HIV-1 Nef and gp120 down-modulated the expression Bcl-xL and Bcl-2, respectively. Also, Nef and Tat induced increased expression of Ras mRNAs in primary oral keratinocytes. Taken together, these data suggest that HIV-1 Nef, gp120, and Tat-induced apoptosis in oral keratinocytes occurs potentially through the down-modulation of Bcl-2 and Bcl-xL and the up-regulation of Ras expression. It is possible that, in the present studies, the reduced expression of Bcl-2, in the case of gp120, and Bcl-xL, in the case of Nef, may have prevented the binding of these proteins to Ras, thereby enhancing the apoptotic signaling mediated by Ras, during the Fas-mediated apoptotic process. Furthermore, p53-dependent apoptotic pathways may have contributed to gp120- and Nef-elicited Bcl-2 expression.
The tumor protein, p53, is constitutively expressed in cells, and when bound to the ubiquitinase-like enzyme HDM2, is transported to the nucleus from the cytoplasm. The binding of p53 to the ubiquitinase-like enzyme HDM2 results in the degradation of p53 (39), and phosphorylation of p53 reduces its affinity for HDM2, thereby causing an increase in the levels of p53. Phosphorylated p53 induction mediates the down-modulation of Bcl-2 expression and up-regulation of Bax, thereby predisposing the cells to cells-to-cell cycle arrest and apoptosis (67, 68). Furthermore, Cande et al. (15) recently reported that activation of cyclin-dependent kinase-1 (Cdk1) resulted in the activation of mammalian target of rapamycin (mTOR), phosphorylation of p53 at S15, and apoptosis. They also showed that neutralization of p53 expression in peripheral blood mononuclear cells from HIV-1-infected patients suppressed apoptosis. These cells also exhibited an increase in cyclin B and mTOR expression, correlating with p53S15 phosphorylation and viral load. Thus, it appears that the sequential activation of cyclin B-Cdk1, mTOR, and p53 leads to induction of apoptosis. Unlike p53, MAPK and MAPK/ERK have not been directly linked to the mitochondrial apoptotic pathway. Also, the relationship between the activation of MAPK cascades and induction of apoptosis is still not clear and may differ among different cell types (118, 122).
The HIV-1 envelope protein gp120 elicits the transcriptional activation of p53 (18), resulting in the activation of the bax gene (69) and subsequent apoptosis. In a recent publication, Perfettini et al. (86) reported that p53 and NF-κB are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. Utilizing microarray technology, they identified several HIV-1 envelope-elicited, p53-dependent proapoptotic transcripts that include Puma, a proapoptotic “BH-3-only” protein from the Bcl-2 family, which is known to activate Bax/Bik. These authors further reported that p53 inhibition prevented the expression of several proapoptotic genes and disabled the apoptotic machinery. Furthermore, Castedo et al. (18) have suggested that in gp120-elicited apoptosis, gp120 interacts with CD4 and CXCR4 to form a gp120/CD4/CXCR4 complex. The formation of this complex transiently results in the up-regulation in the expression of cyclin B at the protein level, which in turn induces cyclin B-dependent kinase 1-dependent (cyclin B/Cdk 1) activation of the mTOR, a serine/threonine kinase of the phosphatidyl inositol kinase family. Accumulation of the mTOR in the nucleus results in the direct phosphorylation of p53 at the Ser15 residue and the subsequent transcriptional activation of proapoptotic Bcl-2 protein family member Bax. This ultimately causes a reduction in the mitochondrial membrane potential and the release from the mitochondria of cytochrome c and other proapoptotic proteins, particularly apoptosis-inducing factor 1 (AIF), resulting in programmed cell death (17, 36, 37). Note also that retinoblastoma binding protein 4, which was demonstrated to be up-regulated in oral keratinocytes exposed to gp120, is a substrate for Cdk1 and has been found to be induced in apoptotic cells (46).
Cyclin B1 is a key regulator of apoptosis in some cell types (88). Recently, it was reported that the cyclin B1 protein is important for the induction of radiation-induced apoptosis in hematopoietic cells (89). It has also been shown that accumulation of the protein is necessary for the induction of apoptosis caused by T-cell-receptor activation (38). Recently, it was demonstrated that induced cell death required the expression of cyclin B1 in T cells undergoing activation and that overexpression of the protein enhances the expression of FasL, which may ultimately lead to cell death (116). This effect of cyclin B1 is mediated by the binding of the protein to Cdk1 to form the cyclin B1/Cdk1 complex. The regulation of the transcription of FasL by the cyclin B1/Cdk1 complex is achieved through the control of NF-κB activation. Overexpression of the NF-κB subunit, Rel A, enhanced the activation and induction of FasL promoter activity and subsequent cell death (116).
Consistent with the HIV-1 gp-120-induced apoptotic pathway described above, our microarray analyses demonstrated concomitant up-regulation in the expression of p53, NF-κB, MAPK/ERK, growth-arrest-specific gene 1 (GAS 1), retinoblastoma binding protein 4, cyclin B1, and cyclin-dependent kinase 1 (Cdk 1) in primary human oral keratinocytes exposed to gp120. The formation of cyclin B1/Cdk1 complex might have contributed to gp120-induced FasL expression and subsequent programmed cell death of primary oral keratinocytes. The data also strongly suggest that in addition to the Fas/FasL and TNF/TNFR apoptotic pathways, gp120 might induce apoptosis in primary human oral keratinocytes via the mitochondrial apoptotic pathway.
The proliferating cell nuclear antigen (PCNA), whose expression was up-regulated by gp120, is a protein involved with the major DNA replication and repair machinery of the cell (55, 64). Depending on the physiological conditions of the cell, the protein may perform several functions in mammalian cells. For instance, in the absence of p53, the presence of large quantities of PCNA results in DNA replication in cells. The presence of p53 leads to DNA repair. However, low quantities or a complete absence of p53 may drive the cells into apoptosis (85). Consistent with this idea, there was a decrease in the expression of PCDNA in keratinocytes exposed to gp120, and this may have led to apoptosis. The increase in the expression of growth-arrest-specific gene 1 (GAS 1) may have also contributed to overall apoptotic mechanism, since the gene is known to inhibit DNA synthesis in vivo (24) and also to act as a mediator of apoptosis (49).
Nef possesses the capacity to regulate numerous transcriptional activities by targeting and controlling various src family kinases and MAPK and p53 activities. However, most reports regarding Nef-mediated transcriptional activation of p53 and MAPK pathways have been less than conclusive. Some of these studies suggest that Nef inhibits while others suggest that Nef enhances p53 and MAPK activities (63, 111). Nevertheless, it appears that the regulation of the transcriptional activities of various kinases and p53 by Nef is very complicated, and whether or not Nef inhibits or enhances these activities may depend on the cell type and possibly on the nature of the Nef protein involved. In the present study, Nef was found to up-regulate the expression of p53, and oral keratinocytes exposed to Nef and Tat were found to undergo MAPK cascade activation. In view of the fact that these proteins have been shown in some studies to contribute to the induction of apoptosis and that Nef and Tat were also found in the present study to up-regulate the expression of other proapoptotic genes, it could be argued these HIV-1 proteins might modulate the sensitivity of oral keratinocytes to proapoptotic molecules, thus contributing to the induction of programmed cell death. This is consistent with other studies that have shown that Tat and Nef induce certain cells to undergo MAPK cascade activation and apoptosis (27, 117). Thus, the complexity of the differential gene expression regulated by p53, as identified by our targeted DNA microarray in primary human oral keratinocytes exposed to all three HIV-1 proteins, suggests that a diverse array of genes may potentially play synergistic or additive roles in p53-mediated mitochondrial apoptosis in these cells in vitro.
In addition to the increased expression of p53-regulated genes by keratinocytes exposed to gp120, Nef, and Tat, it was also observed that both Nef and Tat induced increased expression ofproline dehydrogenase. Nef alone induced the expression of metalloproteinase-disintegrin TNF-α convertase (TACE or ADAM17), and Tat induced the expression of PCTAIRE protein kinase, insulin-like growth factor 1 (IGF-1), insulin-like growth factor binding protein 6 (IGFBP-6), and retinoid X receptor beta.
Proline dehydrogenase, a mitochondrial enzyme, is involved in the transfer of redox potential across the mitochondrial membrane through the proline/delta-P5C (proline-P5C) pathway (87). In vitro, it has been shown that the proline dehydrogenase gene is induced early by the tumor suppressor protein p53 and that it inhibits cell proliferation plus localizes to the mitochondria and triggers programmed cell death. Similarly, TACE and the insulin-like growth factor binding protein 6 (IGFBP-6) are inducers of apoptosis whereas IGF-1 inhibits apoptosis. TACE, a key enzyme (sheddase) that releases TNF-α from its inactive cell-bound precursor (119), has been shown to induce apoptosis in U937 cells via the release of TNF-α and soluble FasL (114).
IGFBP-6 is a family of six high-affinity secreted proteins that modulate the availability and hence the antiapoptotic actions of the IGFs. Thus, IGF-1 and IGFBP-6 exert opposing effects on cell growth and apoptosis (77, 113). Unlike proline dehydrogenase, TACE, and IGFBP-6, IGF-1 is the most prevalent and potent survival factor for many cells. Studies have shown that IGF-1 inhibits apoptosis, promotes matrix formation, and can decrease TNF-α-induced proteoglycan degradation and apoptosis in numerous cells (8).
Given their proapoptotic role, the production of proline dehydrogenase, TACE, and IGFBP-6 in keratinocytes exposed to gp120, Nef, or Tat might have impacted the induction of apoptosis favorably. The increase in the expression of IGF-1 by keratinocytes exposed to Tat may have been an attempt by these cells to counteract the effects of IGFBP-6 and apoptosis. Indeed, some studies suggest that Tat triggers IGF-I gene expression, resulting in IGF-1-induced resistance to apoptosis in several cell types (27, 121).
The role of HIV-1 Tat in inducing apoptosis in cells is not quite clear. Tat has been described as pro- and antiapoptotic depending on the cell type, microenvironment, and culture conditions. For example, it has been shown in vitro that Tat induces apoptosis of uninfected T cells (65) through a Fas/FasL apoptotic pathway (120), whereas it exerts the opposite effect in HIV-1-infected T cells (65). Similarly, this protein has been shown to be antiapoptotic in different tumor cell lines of neuronal, epithelial, and lymphoid origin (16). Tat is able to trigger the expression of several genes responsible for cytokines and growth factors, and the differential expression of these may determine the sensitivity of cells to apoptosis (27). In the present study, Tat elicited the expression of numerous proapoptotic and growth factors. Collectively, the increase in the expression these genes may have resulted in the release of proapoptotic proteins, cytokines, and growth factors released in the cell microenvironment, leading to the proapoptotic effects of Tat.
In summary, the present studies strongly suggest, for the first time, that infection of human oral keratinocytes can be augmented by microenvironments containing physiological concentrations of polyamines and dNTPs and that cell-free lentiviral proteins may lead to programmed cell death in these cells. The data regarding NERT augmentation of HIV-1 infection of oral mucosa cells suggest that seminal fluids, and possibly cervical-vaginal fluids, in which NERT-stimulating molecules are at relatively high concentrations may antagonize the HIV-1 salivary inhibitors. In addition, cell-free HIV-1-specific proteins (i.e., Tat, Nef, and gp120) may lead to the disruption of the oral mucosal barrier in vivo. As such, these represent two potential molecular mechanisms by which HIV-1 infects humans after orogenital contact with infected individuals and by which infants of HIV-1-infected women may acquire HIV-1 infection perinatally, during vaginal delivery.
Acknowledgments
We thank Rita M. Victor and Brenda O. Gordon for excellent secretarial assistance. HIV-1 Tat was obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
This work was supported in part by U.S. Public Health Service grants AA13849, AI52732, and NS41864 to R.J.P.
REFERENCES
- 1.Acheampong, E., M. Mukhtar, Z. Parveen, N. Ngoubilly, N. Ahmad, C. Patel, and R. J. Pomerantz. 2002. Ethanol strongly potentiates apoptosis induced by HIV-1 proteins in primary human brain microvascular endothelial cells. Virology 304:222-234. [DOI] [PubMed] [Google Scholar]
- 2.Adamson, D. C., T. M. Dawson, M. C. Zink, J. E. Clements, and V. L. Dawson. 1996. Neurovirulent simian immunodeficiency virus infection induces neuronal, endothelial, and glial apoptosis. Mol. Med. 2:417-428. [PMC free article] [PubMed] [Google Scholar]
- 3.Alimonti, J. B., T. B. Ball, and K. R. Fowke. 2003. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J. Gen. Virol. 84:1649-1661. [DOI] [PubMed] [Google Scholar]
- 4.Ameisen, J. C. 2001. Apoptosis subversion: HIV-Nef provides both armor and sword. Nat. Med. 7:1181-1182. [DOI] [PubMed] [Google Scholar]
- 5.Anonymous. 1993. HIV antibody detection in oral fluids. Can. Commun. Dis. Rep. 19:94-95. [PubMed] [Google Scholar]
- 6.Anonymous. 2003. Oral sex and HIV investigated. AIDS Patient Care STDS 17:316. [PubMed] [Google Scholar]
- 7.Anonymous. 2003. Study looks at how HIV may spread through oral sex. AIDS Read 13:207-208. [PubMed] [Google Scholar]
- 8.Anwar, A., A. A. Zahid, K. J. Scheidegger, M. Brink, and P. Delafontaine. 2002. Tumor necrosis factor-alpha regulates insulin-like growth factor-1 and insulin-like growth factor binding protein-3 expression in vascular smooth muscle. Circulation 105:1220-1225. [DOI] [PubMed] [Google Scholar]
- 9.Arnoult, D., F. Petit, J. D. Lelievre, and J. Estaquier. 2003. Mitochondria in HIV-1-induced apoptosis. Biochem. Biophys. Res. Commun. 304:561-574. [DOI] [PubMed] [Google Scholar]
- 10.Baba, T. W., A. M. Trichel, L. An, V. Liska, L. N. Martin, M. Murphey-Corb, and R. M. Ruprecht. 1996. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science 272:1486-1489. [DOI] [PubMed] [Google Scholar]
- 11.Bartz, S. R., and M. Emerman. 1999. Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J. Virol. 73:1956-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell, D. J., and D. H. Dockrell. 2003. Apoptosis in HIV-1 infection. J. Eur. Acad. Dermatol. Venereol. 17:178-183. [DOI] [PubMed] [Google Scholar]
- 13.Boldin, M. P., T. M. Goncharov, Y. V. Goltsev, and D. Wallach. 1996. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell 85:803-815. [DOI] [PubMed] [Google Scholar]
- 14.Brunner, T., R. J. Mogil, D. LaFace, N. J. Yoo, A. Mahboubi, F. Echeverri, S. J. Martin, W. R. Force, D. H. Lynch, and C. F. Ware. 1995. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 373:441-444. [DOI] [PubMed] [Google Scholar]
- 15.Cande, C., I. Cohen, E. Daugas, L. Ravagnan, N. Larochette, N. Zamzami, and G. Kroemer. 2002. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie 84:215-222. [DOI] [PubMed] [Google Scholar]
- 16.Cantaluppi, V., L. Biancone, M. Boccellino, S. Doublier, R. Benelli, S. Carlone, A. Albini, and G. Camussi. 2001. HIV type 1 Tat protein is a survival factor for Kaposi's sarcoma and endothelial cells. AIDS Res. Hum. Retrovir. 17:965-976. [DOI] [PubMed] [Google Scholar]
- 17.Castedo, M., K. F. Ferri, J. Blanco, T. Roumier, N. Larochette, J. Barretina, A. Amendola, R. Nardacci, D. Metivier, J. A. Este, M. Piacentini, and G. Kroemer. 2001. Human immunodeficiency virus 1 envelope glycoprotein complex-induced apoptosis involves mammalian target of rapamycin/FKBP12-rapamycin-associated protein-mediated p53 phosphorylation. J. Exp. Med. 194:1097-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castedo, M., J. L. Perfettini, K. Andreau, T. Roumier, M. Piacentini, and G. Kroemer. 2003. Mitochondrial apoptosis induced by the HIV-1 envelope. Ann. N. Y. Acad. Sci. 1010:19-28. [DOI] [PubMed] [Google Scholar]
- 19.Chao, D. T., and S. J. Korsmeyer. 1998. BCL-2 family: regulators of cell death. Annu. Rev. Immunol. 16:395-419. [DOI] [PubMed] [Google Scholar]
- 20.Cheingsong-Popov, R., D. Callow, J. Weber, C. Holm-Hansen, and N. T. Constantine. 1993. Antibody to specific HIV-1 proteins in oral mucosal transudates. Lancet 341:1659-1660. [DOI] [PubMed] [Google Scholar]
- 21.Chen, C. Y., and D. V. Faller. 1995. Direction of p21ras-generated signals towards cell growth or apoptosis is determined by protein kinase C and Bcl-2. Oncogene 11:1487-1498. [PubMed] [Google Scholar]
- 22.Chen, C. Y., and D. V. Faller. 1996. Phosphorylation of Bcl-2 protein and association with p21Ras in Ras-induced apoptosis. J. Biol. Chem. 271:2376-2379. [DOI] [PubMed] [Google Scholar]
- 23.Chen, H., J. Zha, R. E. Gowans, P. Camargo, J. Nishitani, J. L. McQuirter, S. W. Cole, J. A. Zack, and X. Liu. 2004. Alcohol enhances HIV type 1 infection in normal human oral keratinocytes by up-regulating cell-surface CXCR4 coreceptor. AIDS Res. Hum. Retrovir. 20:513-519. [DOI] [PubMed] [Google Scholar]
- 24.Del Sal, G., M. E. Ruaro, L. Philipson, and C. Schneider. 1992. The growth arrest-specific gene, gas1, is involved in growth suppression. Cell 70:595-607. [DOI] [PubMed] [Google Scholar]
- 25.Delvenne, P., P. Hubert, N. Jacobs, S. L. Giannini, L. Havard, I. Renard, D. Saboulard, and J. Boniver. 2001. The organotypic culture of HPV-transformed keratinocytes: an effective in vitro model for the development of new immunotherapeutic approaches for mucosal (pre)neoplastic lesions. Vaccine 19:2557-2564. [DOI] [PubMed] [Google Scholar]
- 26.DePond, W., J. W. Said, T. Tasaka, S. de Vos, D. Kahn, E. Cesarman, D. M. Knowles, and H. P. Koeffler. 1997. Kaposi's sarcoma-associated herpesvirus and human herpesvirus 8 (KSHV/HHV8)-associated lymphoma of the bowel. Report of two cases in HIV-positive men with secondary effusion lymphomas. Am. J. Surg. Pathol. 21:719-724. [DOI] [PubMed] [Google Scholar]
- 27.Deregibus, M. C., V. Cantaluppi, S. Doublier, M. F. Brizzi, I. Deambrosis, A. Albini, and G. Camussi. 2002. HIV-1-Tat protein activates phosphatidylinositol 3-kinase/AKT-dependent survival pathways in Kaposi's sarcoma cells. J. Biol. Chem. 277:25195-25202. [DOI] [PubMed] [Google Scholar]
- 28.Dickie, P. 1996. HIV type 1 Nef perturbs eye lens development in transgenic mice. AIDS Res. Hum. Retrovir. 12:177-189. [DOI] [PubMed] [Google Scholar]
- 29.Dornadula, G., H. Zhang, O. Bagasra, and R. J. Pomerantz. 1997. Natural endogenous reverse transcription of simian immunodeficiency virus. Virology 227:260-267. [DOI] [PubMed] [Google Scholar]
- 30.Downward, J. 1998. Ras signalling and apoptosis. Curr. Opin. Genet. Dev. 8:49-54. [DOI] [PubMed] [Google Scholar]
- 31.Emmons, W. W., S. F. Paparello, C. F. Decker, J. M. Sheffield, and F. H. Lowe-Bey. 1995. A modified ELISA and western blot accurately determine anti-human immunodeficiency virus type 1 antibodies in oral fluids obtained with a special collecting device. J Infect. Dis. 171:1406-1410. [DOI] [PubMed] [Google Scholar]
- 32.Ensoli, B., G. Barillari, S. Z. Salahuddin, R. C. Gallo, and F. Wong-Staal. 1990. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature 345:84-86. [DOI] [PubMed] [Google Scholar]
- 33.Ensoli, B., L. Buonaguro, G. Barillari, V. Fiorelli, R. Gendelman, R. A. Morgan, P. Wingfield, and R. C. Gallo. 1993. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faruque, S., B. R. Edlin, C. B. McCoy, C. O. Word, S. A. Larsen, D. S. Schmid, J. C. Von Bargen, and Y. Serrano. 1996. Crack cocaine smoking and oral sores in three inner-city neighborhoods. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13:87-92. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Sarabia, M. J., and J. R. Bischoff. 1993. Bcl-2 associates with the ras-related protein R-ras p23. Nature 366:274-275. [DOI] [PubMed] [Google Scholar]
- 36.Ferri, K. F., and G. Kroemer. 2001. Mitochondria—the suicide organelles. Bioessays 23:111-115. [DOI] [PubMed] [Google Scholar]
- 37.Ferri, K. F., and G. Kroemer. 2001. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 3:E255-E263. [DOI] [PubMed] [Google Scholar]
- 38.Fotedar, R., J. Flatt, S. Gupta, R. L. Margolis, P. Fitzgerald, H. Messier, and A. Fotedar. 1995. Activation-induced T-cell death is cell cycle dependent and regulated by cyclin B. Mol. Cell. Biol. 15:932-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freedman, D. A., and A. J. Levine. 1998. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol. Cell. Biol. 18:7288-7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freel, S. A., J. M. Williams, J. A. Nelson, L. L. Patton, S. A. Fiscus, R. Swanstrom, and D. C. Shugars. 2001. Characterization of human immunodeficiency virus type 1 in saliva and blood plasma by V3-specific heteroduplex tracking assay and genotype analyses. J. Virol. 75:4936-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujii, Y., K. Otake, Y. Fujita, N. Yamamoto, Y. Nagai, M. Tashiro, and A. Adachi. 1996. Clustered localization of oligomeric Nef protein of human immunodeficiency virus type 1 on the cell surface. FEBS Lett. 395:257-261. [DOI] [PubMed] [Google Scholar]
- 42.Fujii, Y., K. Otake, M. Tashiro, and A. Adachi. 1996. Human immunodeficiency virus type 1 Nef protein on the cell surface is cytocidal for human CD4+ T cells. FEBS Lett. 393:105-108. [DOI] [PubMed] [Google Scholar]
- 43.Fujii, Y., K. Otake, M. Tashiro, and A. Adachi. 1996. In vitro cytocidal effects of human immunodeficiency virus type 1 Nef on unprimed human CD4+ T cells without MHC restriction. J. Gen. Virol. 77(Pt. 12):2943-2951. [DOI] [PubMed] [Google Scholar]
- 44.Fujii, Y., K. Otake, M. Tashiro, and A. Adachi. 1996. Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells. FEBS Lett. 393:93-96. [DOI] [PubMed] [Google Scholar]
- 45.Garden, G. A., S. L. Budd, E. Tsai, L. Hanson, M. Kaul, D. M. D'Emilia, R. M. Friedlander, J. Yuan, E. Masliah, and S. A. Lipton. 2002. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J. Neurosci. 22:4015-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glozak, M. A., and M. B. Rogers. 2001. Retinoic acid- and bone morphogenetic protein 4-induced apoptosis in P19 embryonal carcinoma cells requires p27. Exp. Cell Res. 268:128-138. [DOI] [PubMed] [Google Scholar]
- 47.Gulbins, E., R. Bissonnette, A. Mahboubi, S. Martin, W. Nishioka, T. Brunner, G. Baier, G. Baier-Bitterlich, C. Byrd, and F. Lang. 1995. FAS-induced apoptosis is mediated via a ceramide-initiated RAS signaling pathway. Immunity 2:341-351. [DOI] [PubMed] [Google Scholar]
- 48.Gulbins, E., B. Brenner, U. Koppenhoefer, O. Linderkamp, and F. Lang. 1998. Fas or ceramide induce apoptosis by Ras-regulated phosphoinositide-3-kinase activation. J. Leukoc. Biol. 63:253-263. [DOI] [PubMed] [Google Scholar]
- 49.Guo, K., V. Wolf, A. M. Dharmarajan, Z. Feng, W. Bielke, S. Saurer, and R. Friis. 1998. Apoptosis-associated gene expression in the corpus luteum of the rat. Biol. Reprod. 58:739-746. [DOI] [PubMed] [Google Scholar]
- 50.Han, Y., C. L. Ventura, K. P. Black, J. E. Cummins, Jr., S. D. Hall, and S. Jackson. 2000. Productive human immunodeficiency virus-1 infection of epithelial cell lines of salivary gland origin. Oral Microbiol. Immunol. 15:82-88. [DOI] [PubMed] [Google Scholar]
- 51.Herz, A. M., M. N. Robertson, J. B. Lynch, A. Schmidt, M. Rabin, C. Sherbert, M. B. Agy, D. Anderson, S. L. Hu, P. D. Greenberg, and W. R. Morton. 2002. Viral dynamics of early HIV infection in neonatal macaques after oral exposure to HIV-2287: an animal model with implications for maternal-neonatal HIV transmission. J. Med. Primatol. 31:29-39. [DOI] [PubMed] [Google Scholar]
- 52.Joneson, T., and D. Bar-Sagi. 1999. Suppression of Ras-induced apoptosis by the Rac GTPase. Mol. Cell. Biol. 19:5892-5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joza, N., S. A. Susin, E. Daugas, W. L. Stanford, S. K. Cho, C. Y. Li, T. Sasaki, A. J. Elia, H. Y. Cheng, L. Ravagnan, K. F. Ferri, N. Zamzami, A. Wakeham, R. Hakem, H. Yoshida, Y. Y. Kong, T. W. Mak, J. C. Zuniga-Pflucker, G. Kroemer, and J. M. Penninger. 2001. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410:549-554. [DOI] [PubMed] [Google Scholar]
- 54.Juo, P., M. S. Woo, C. J. Kuo, P. Signorelli, H. P. Biemann, Y. A. Hannun, and J. Blenis. 1999. FADD is required for multiple signaling events downstream of the receptor Fas. Cell Growth Differ. 10:797-804. [PubMed] [Google Scholar]
- 55.Krishna, T. S., D. Fenyo, X. P. Kong, S. Gary, B. T. Chait, P. Burgers, and J. Kuriyan. 1994. Crystallization of proliferating cell nuclear antigen (PCNA) from Saccharomyces cerevisiae. J. Mol. Biol. 241:265-268. [DOI] [PubMed] [Google Scholar]
- 56.Lafferty, W. E., J. P. Hughes, and H. H. Handsfield. 1997. Sexually transmitted diseases in men who have sex with men. Acquisition of gonorrhea and nongonococcal urethritis by fellatio and implications for STD/HIV prevention. Sex. Transm. Dis. 24:272-278. [DOI] [PubMed] [Google Scholar]
- 57.Langford, A., R. Kunze, S. Schmelzer, H. Wolf, H. D. Pohle, and P. Reichart. 1992. Immunocytochemical detection of herpes viruses in oral smears of HIV-infected patients. J. Oral Pathol. Med. 21:49-57. [DOI] [PubMed] [Google Scholar]
- 58.LeGuern, M., T. Shioda, J. A. Levy, and C. Cheng-Mayer. 1993. Single amino acid change in Tat determines the different rates of replication of two sequential HIV-1 isolates. Virology 195:441-447. [DOI] [PubMed] [Google Scholar]
- 59.Lelievre, J. D., D. Arnoult, F. Petit, and J. Estaquier. 2003. [HIV1-associated CD4 T lymphocyte apoptosis]. Rev. Med. Interne 24:522-529. [DOI] [PubMed] [Google Scholar]
- 60.Lifson, A. R., P. M. O'Malley, N. A. Hessol, S. P. Buchbinder, L. Cannon, and G. W. Rutherford. 1990. HIV seroconversion in two homosexual men after receptive oral intercourse with ejaculation: implications for counseling concerning safe sexual practices. Am. J. Public Health 80:1509-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu, X., J. Zha, H. Chen, J. Nishitani, P. Camargo, S. W. Cole, and J. A. Zack. 2003. Human immunodeficiency virus type 1 infection and replication in normal human oral keratinocytes. J. Virol. 77:3470-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liuzzi, G., A. Chirianni, M. Clementi, P. Bagnarelli, A. Valenza, P. T. Cataldo, and M. Piazza. 1996. Analysis of HIV-1 load in blood, semen and saliva: evidence for different viral compartments in a cross-sectional and longitudinal study. AIDS 10:F51-F56. [DOI] [PubMed] [Google Scholar]
- 63.Marsh, J. W. 1999. The numerous effector functions of Nef. Arch. Biochem. Biophys. 365:192-198. [DOI] [PubMed] [Google Scholar]
- 64.Matsumiya, S., Y. Ishino, and K. Morikawa. 2001. Crystal structure of an archaeal DNA sliding clamp: proliferating cell nuclear antigen from Pyrococcus furiosus. Protein Sci. 10:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCloskey, T. W., M. Ott, E. Tribble, S. A. Khan, S. Teichberg, M. O. Paul, S. Pahwa, E. Verdin, and N. Chirmule. 1997. Dual role of HIV Tat in regulation of apoptosis in T cells. J. Immunol. 158:1014-1019. [PubMed] [Google Scholar]
- 66.Medema, J. P., C. Scaffidi, F. C. Kischkel, A. Shevchenko, M. Mann, P. H. Krammer, and M. E. Peter. 1997. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16:2794-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyashita, T., S. Kitada, S. Krajewski, W. A. Horne, D. Delia, and J. C. Reed. 1995. Overexpression of the Bcl-2 protein increases the half-life of p21Bax. J. Biol. Chem. 270:26049-26052. [DOI] [PubMed] [Google Scholar]
- 68.Miyashita, T., S. Krajewski, M. Krajewska, H. G. Wang, H. K. Lin, D. A. Liebermann, B. Hoffman, and J. C. Reed. 1994. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 9:1799-1805. [PubMed] [Google Scholar]
- 69.Miyashita, T., and J. C. Reed. 1995. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293-299. [DOI] [PubMed] [Google Scholar]
- 70.Moore, J. S., S. D. Hall, and S. Jackson. 2002. Cell-associated HIV-1 infection of salivary gland epithelial cell lines. Virology 297:89-97. [DOI] [PubMed] [Google Scholar]
- 71.Moore, J. S., F. Rahemtulla, L. W. Kent, S. D. Hall, M. R. Ikizler, P. F. Wright, H. H. Nguyen, and S. Jackson. 2003. Oral epithelial cells are susceptible to cell-free and cell-associated HIV-1 infection in vitro. Virology 313:343-353. [DOI] [PubMed] [Google Scholar]
- 72.Mukhtar, M., and R. J. Pomerantz. 2000. Development of an in vitro blood-brain barrier model to study molecular neuropathogenesis and neurovirologic disorders induced by human immunodeficiency virus type 1 infection. J. Hum. Virol. 3:324-334. [PubMed] [Google Scholar]
- 73.Mulcahy, L. S., M. R. Smith, and D. W. Stacey. 1985. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature 313:241-243. [DOI] [PubMed] [Google Scholar]
- 74.Muthumani, K., A. Y. Choo, D. S. Hwang, M. A. Chattergoon, N. N. Dayes, D. Zhang, M. D. Lee, U. Duvvuri, and D. B. Weiner. 2003. Mechanism of HIV-1 viral protein R-induced apoptosis. Biochem. Biophys. Res. Commun. 304:583-592. [DOI] [PubMed] [Google Scholar]
- 75.Muthumani, K., D. S. Hwang, B. M. Desai, D. Zhang, N. Dayes, D. R. Green, and D. B. Weiner. 2002. HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J. Biol. Chem. 277:37820-37831. [DOI] [PubMed] [Google Scholar]
- 76.Muzio, M., A. M. Chinnaiyan, F. C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J. D. Bretz, M. Zhang, R. Gentz, M. Mann, P. H. Krammer, M. E. Peter, and V. M. Dixit. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85:817-827. [DOI] [PubMed] [Google Scholar]
- 77.Ngo, T. H., R. J. Barnard, P. S. Leung, P. Cohen, and W. J. Aronson. 2003. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell growth and apoptosis: possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology 144:2319-2324. [DOI] [PubMed] [Google Scholar]
- 78.Ohagen, A., S. Ghosh, J. He, K. Huang, Y. Chen, M. Yuan, R. Osathanondh, S. Gartner, B. Shi, G. Shaw, and D. Gabuzda. 1999. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J. Virol. 73:897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okada, H., R. Takei, and M. Tashiro. 1997. HIV-1 Nef protein-induced apoptotic cytolysis of a broad spectrum of uninfected human blood cells independently of CD95(Fas). FEBS Lett. 414:603-606. [DOI] [PubMed] [Google Scholar]
- 80.Oltvai, Z. N., C. L. Milliman, and S. J. Korsmeyer. 1993. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609-619. [DOI] [PubMed] [Google Scholar]
- 81.Oyaizu, N., and S. Pahwa. 1995. Role of apoptosis in HIV disease pathogenesis. J. Clin. Immunol. 15:217-231. [DOI] [PubMed] [Google Scholar]
- 82.Patel, C. A., M. Mukhtar, S. Harley, J. Kulkosky, and R. J. Pomerantz. 2002. Lentiviral expression of HIV-1 Vpr induces apoptosis in human neurons. J. Neurovirol. 8:86-99. [DOI] [PubMed] [Google Scholar]
- 83.Patel, C. A., M. Mukhtar, and R. J. Pomerantz. 2000. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J. Virol. 74:9717-9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pauk, J., M. L. Huang, S. J. Brodie, A. Wald, D. M. Koelle, T. Schacker, C. Celum, S. Selke, and L. Corey. 2000. Mucosal shedding of human herpesvirus 8 in men. N. Engl. J. Med. 343:1369-1377. [DOI] [PubMed] [Google Scholar]
- 85.Paunesku, T., S. Mittal, M. Protic, J. Oryhon, S. V. Korolev, A. Joachimiak, and G. E. Woloschak. 2001. Proliferating cell nuclear antigen (PCNA): ringmaster of the genome. Int. J. Radiat. Biol. 77:1007-1021. [DOI] [PubMed] [Google Scholar]
- 86.Perfettini, J. L., T. Roumier, M. Castedo, N. Larochette, P. Boya, B. Raynal, V. Lazar, F. Ciccosanti, R. Nardacci, J. Penninger, M. Piacentini, and G. Kroemer. 2004. NF-kappaB and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. J. Exp. Med. 199:629-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phang, J. M. 1985. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr. Top. Cell Regul. 25:91-132. [DOI] [PubMed] [Google Scholar]
- 88.Porter, L. A., and D. J. Donoghue. 2003. Cyclin B1 and CDK1: nuclear localization and upstream regulators. Prog. Cell Cycle Res. 5:335-347. [PubMed] [Google Scholar]
- 89.Porter, L. A., G. Singh, and J. M. Lee. 2000. Abundance of cyclin B1 regulates gamma-radiation-induced apoptosis. Blood 95:2645-2650. [PubMed] [Google Scholar]
- 90.Puelacher, W., R. Zangerle, S. Kulmer, E. Waldhart, P. Fritsch, and H. Hintner. 1989. [Oral manifestations of HIV infection]. Z. Stomatol. 86:539-548. [PubMed] [Google Scholar]
- 91.Quinones-Mateu, M. E., M. M. Lederman, Z. Feng, B. Chakraborty, J. Weber, H. R. Rangel, M. L. Marotta, M. Mirza, B. Jiang, P. Kiser, K. Medvik, S. F. Sieg, and A. Weinberg. 2003. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS 17:F39-F48. [DOI] [PubMed] [Google Scholar]
- 92.Qureshi, M. N., C. E. Barr, T. Seshamma, J. Reidy, R. J. Pomerantz, and O. Bagasra. 1995. Infection of oral mucosal cells by human immunodeficiency virus type 1 in seropositive persons. J. Infect. Dis. 171:190-193. [DOI] [PubMed] [Google Scholar]
- 93.Rasola, A., D. Gramaglia, C. Boccaccio, and P. M. Comoglio. 2001. Apoptosis enhancement by the HIV-1 Nef protein. J. Immunol. 166:81-88. [DOI] [PubMed] [Google Scholar]
- 94.Read, J., and F. M. Watt. 1988. A model for in vitro studies of epidermal homeostasis: proliferation and involucrin synthesis by cultured human keratinocytes during recovery after stripping off the suprabasal layers. J. Investig. Dermatol. 90:739-743. [DOI] [PubMed] [Google Scholar]
- 95.Reed, J. C. 1994. Bcl-2 and the regulation of programmed cell death. J. Cell Biol. 124:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reed, J. C. 1997. Double identity for proteins of the Bcl-2 family. Nature 387:773-776. [DOI] [PubMed] [Google Scholar]
- 97.Rothenberg, R. B., M. Scarlett, C. del Rio, D. Reznik, and C. O'Daniels. 1998. Oral transmission of HIV. AIDS 12:2095-2105. [DOI] [PubMed] [Google Scholar]
- 98.Salzman, S. A., and J. K. Burmester. 2000. HIV-1 tat induced apoptosis of T-cells is not mediated by TGF-beta. Med. Oncol. 17:211-217. [DOI] [PubMed] [Google Scholar]
- 99.Samuel, M. C., N. Hessol, S. Shiboski, R. R. Engel, T. P. Speed, and W. Winkelstein, Jr. 1993. Factors associated with human immunodeficiency virus seroconversion in homosexual men in three San Francisco cohort studies, 1984-1989. J Acquir. Immune Defic. Syndr. 6:303-312. [PubMed] [Google Scholar]
- 100.Sastry, K. J., M. C. Marin, P. N. Nehete, K. McConnell, A. K. el-Naggar, and T. J. McDonnell. 1996. Expression of human immunodeficiency virus type I tat results in down-regulation of bcl-2 and induction of apoptosis in hematopoietic cells. Oncogene 13:487-493. [PubMed] [Google Scholar]
- 101.Schenkel, A. R., Z. Mamdouh, X. Chen, R. M. Liebman, and W. A. Muller. 2002. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat. Immunol. 3:143-150. [DOI] [PubMed] [Google Scholar]
- 102.Schwarcz, S. K., T. A. Kellogg, R. P. Kohn, M. H. Katz, G. F. Lemp, and G. A. Bolan. 1995. Temporal trends in human immunodeficiency virus seroprevalence and sexual behavior at the San Francisco municipal sexually transmitted disease clinic, 1989-1992. Am. J. Epidemiol. 142:314-322. [DOI] [PubMed] [Google Scholar]
- 103.Selliah, N., J. Shackelford, J. F. Wang, F. Traynor, J. Yin, and T. H. Finkel. 2003. T cell signaling and apoptosis in HIV disease. Immunol. Res. 27:247-260. [DOI] [PubMed] [Google Scholar]
- 104.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 105.Shi, Y. 2001. A structural view of mitochondria-mediated apoptosis. Nat. Struct. Biol. 8:394-401. [DOI] [PubMed] [Google Scholar]
- 106.Shine, N., K. Konopka, and N. Duzgunes. 1997. The anti-HIV-1 activity associated with saliva. J. Dent. Res. 76:634-640. [DOI] [PubMed] [Google Scholar]
- 107.Shugars, D. C., L. L. Patton, S. A. Freel, L. R. Gray, R. T. Vollmer, J. J. Eron, Jr., and S. A. Fiscus. 2001. Hyper-excretion of human immunodeficiency virus type 1 RNA in saliva. J. Dent. Res. 80:414-420. [DOI] [PubMed] [Google Scholar]
- 108.Shugars, D. C., S. P. Sweet, D. Malamud, S. H. Kazmi, K. Page-Shafer, and S. J. Challacombe. 2002. Saliva and inhibition of HIV-1 infection: molecular mechanisms. Oral. Dis. 8(Suppl.)2:169-175. [DOI] [PubMed] [Google Scholar]
- 109.Shugars, D. C., and S. M. Wahl. 1998. The role of the oral environment in HIV-1 transmission. J. Am. Dent. Assoc. 129:851-858. [DOI] [PubMed] [Google Scholar]
- 110.Silvestris, F., G. Camarda, A. Del Prete, M. Tucci, and F. Dammacco. 1999. Nef protein induces differential effects in CD8+ cells from HIV-1-infected patients. Eur. J. Clin. Investig. 29:980-991. [DOI] [PubMed] [Google Scholar]
- 111.Skowronski, J., M. E. Greenberg, M. Lock, R. Mariani, S. Salghetti, T. Swigut, and A. J. Iafrate. 1999. HIV and SIV Nef modulate signal transduction and protein sorting in T cells. Cold Spring Harbor Symp. Quant. Biol. 64:453-463. [DOI] [PubMed] [Google Scholar]
- 112.Strasser, A., D. C. Huang, and D. L. Vaux. 1997. The role of the bcl-2/ced-9 gene family in cancer and general implications of defects in cell death control for tumourigenesis and resistance to chemotherapy. Biochim. Biophys. Acta 1333:F151-F178. [DOI] [PubMed] [Google Scholar]
- 113.Sueoka, N., H. Y. Lee, G. L. Walsh, B. Fang, L. Ji, J. A. Roth, R. LaPushin, W. K. Hong, P. Cohen, and J. M. Kurie. 2000. Insulin-like growth factor binding protein-6 inhibits the growth of human bronchial epithelial cells and increases in abundance with all-trans-retinoic acid treatment. Am. J. Respir. Cell. Mol. Biol. 23:297-303. [DOI] [PubMed] [Google Scholar]
- 114.Tamura, F., R. Nakagawa, T. Akuta, S. Okamoto, S. Hamada, H. Maeda, S. Kawabata, and T. Akaike. 2004. Proapoptotic effect of proteolytic activation of matrix metalloproteinases by Streptococcus pyogenes thiol proteinase (Streptococcus pyrogenic exotoxin B). Infect. Immun. 72:4836-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tateyama, M., N. Oyaizu, T. W. McCloskey, S. Than, and S. Pahwa. 2000. CD4 T lymphocytes are primed to express Fas ligand by CD4 cross-linking and to contribute to CD8 T-cell apoptosis via Fas/FasL death signaling pathway. Blood 96:195-202. [PubMed] [Google Scholar]
- 116.Torgler, R., S. Jakob, E. Ontsouka, U. Nachbur, C. Mueller, D. R. Green, and T. Brunner. 2004. Regulation of activation-induced Fas (CD95/Apo-1) ligand expression in T cells by the cyclin B1/Cdk1 complex. J. Biol. Chem. 279:37334-37342. [DOI] [PubMed] [Google Scholar]
- 117.Varin, A., S. K. Manna, V. Quivy, A. Z. Decrion, C. Van Lint, G. Herbein, and B. B. Aggarwal. 2003. Exogenous Nef protein activates NF-kappa B, AP-1, and c-Jun N-terminal kinase and stimulates HIV transcription in promonocytic cells. Role in AIDS pathogenesis. J. Biol. Chem. 278:2219-2227. [DOI] [PubMed] [Google Scholar]
- 118.Verheij, M., R. Bose, X. H. Lin, B. Yao, W. D. Jarvis, S. Grant, M. J. Birrer, E. Szabo, L. I. Zon, J. M. Kyriakis, A. Haimovitz-Friedman, Z. Fuks, and R. N. Kolesnick. 1996. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature 380:75-79. [DOI] [PubMed] [Google Scholar]
- 119.Wang, X., G. Z. Feuerstein, L. Xu, H. Wang, W. A. Schumacher, M. L. Ogletree, R. Taub, J. J. Duan, C. P. Decicco, and R. Q. Liu. 2004. Inhibition of tumor necrosis factor-alpha-converting enzyme by a selective antagonist protects brain from focal ischemic injury in rats. Mol. Pharmacol. 65:890-896. [DOI] [PubMed] [Google Scholar]
- 120.Westendorp, M. O., R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H. Walczak, K. M. Debatin, and P. H. Krammer. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497-500. [DOI] [PubMed] [Google Scholar]
- 121.Wu, W., W. L. Lee, Y. Y. Wu, D. Chen, T. J. Liu, A. Jang, P. M. Sharma, and P. H. Wang. 2000. Expression of constitutively active phosphatidylinositol 3-kinase inhibits activation of caspase 3 and apoptosis of cardiac muscle cells. J. Biol. Chem. 275:40113-40119. [DOI] [PubMed] [Google Scholar]
- 122.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]
- 123.Xiong, H., J. Zheng, M. Thylin, and H. E. Gendelman. 1999. Unraveling the mechanisms of neurotoxicity in HIV type 1-associated dementia: inhibition of neuronal synaptic transmission by macrophage secretory products. AIDS Res. Hum. Retrovir. 15:57-63. [DOI] [PubMed] [Google Scholar]
- 124.Xu, X. N., B. Laffert, G. R. Screaton, M. Kraft, D. Wolf, W. Kolanus, J. Mongkolsapay, A. J. McMichael, and A. S. Baur. 1999. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor zeta chain. J. Exp. Med. 189:1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu, Y., J. Kulkosky, E. Acheampong, G. Nunnari, J. Sullivan, and R. J. Pomerantz. 2004. HIV-1-mediated apoptosis of neuronal cells: proximal molecular mechanisms of HIV-1-induced encephalopathy. Proc. Natl. Acad. Sci. USA 101:7070-7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang, Y., B. Dong, P. R. Mittelstadt, H. Xiao, and J. D. Ashwell. 2002. HIV Tat binds Egr proteins and enhances Egr-dependent transactivation of the Fas ligand promoter. J. Biol. Chem. 277:19482-19487. [DOI] [PubMed] [Google Scholar]
- 127.Yang, Y., J. Ma, Z. Song, and M. Wu. 2002. HIV-1 TAT-mediated protein transduction and subcellular localization using novel expression vectors. FEBS Lett. 532:36-44. [DOI] [PubMed] [Google Scholar]
- 128.Zauli, G., D. Gibellini, P. Secchiero, H. Dutartre, D. Olive, S. Capitani, and Y. Collette. 1999. Human immunodeficiency virus type 1 Nef protein sensitizes CD4+ T lymphoid cells to apoptosis via functional upregulation of the CD95/CD95 ligand pathway. Blood 93:1000-1010. [PubMed] [Google Scholar]
- 129.Zhang, H., G. Dornadula, and R. J. Pomerantz. 1996. Endogenous reverse transcription of human immunodeficiency virus type 1 in physiological microenvironments: an important stage for viral infection of nondividing cells. J. Virol. 70:2809-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang, H., G. Dornadula, and R. J. Pomerantz. 1998. Natural endogenous reverse transcription of HIV-1. J. Reprod. Immunol. 41:255-260. [DOI] [PubMed] [Google Scholar]
- 131.Zhang, H., G. Dornadula, and R. J. Pomerantz. 1998. Natural endogenous reverse transcription of HIV type 1. AIDS Res. Hum. Retrovir. 14(Suppl. 1):S93-S95. [PubMed] [Google Scholar]
- 132.Zheng, J., O. O. Yang, Y. Xie, R. Campbell, I. S. Chen, and S. Pang. 2004. Ethanol stimulation of HIV infection of oral epithelial cells. J. Acquir. Immune Defic. Syndr. 37:1445-1453. [DOI] [PubMed] [Google Scholar]
- 133.Zou, H., Y. Li, X. Liu, and X. Wang. 1999. An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 274:11549-11556. [DOI] [PubMed] [Google Scholar]
- 134.Zuckerman, R. A., W. L. Whittington, C. L. Celum, T. Collis, A. Lucchetti, J. L. Sanchez, J. P. Hughes, and R. W. Coombs. 2003. Factors associated with oropharyngeal human immunodeficiency virus shedding. J. Infect. Dis. 188:142-145. [DOI] [PubMed] [Google Scholar]