Abstract
Xeroderris stuhlmannii (Fabaceae) is a medicinal reported in Cameroonian herbal medicine to treat hypertension. The aim of the study was to assess the antihypertensive and antioxidant activities of X. stuhlmannii aqueous leaf extract (AEXS) on cadmium chloride-induced hypertensive rats. The in vitro antioxidant activities of AEXS were investigated for their radical scavenging potency using 2,2-diphenyl-1-picrylhydrazyl (DPPH), Ferric reducing antioxidant power (FRAP), 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid (ABTS), Nitric oxide (NO) and OH- assays completed with oxidative stress markers analyses. Antihypertensive activity of AEXS (35, 100, and 300 mg/kg) was assessed in CdCl2 induced-hypertensive rats. Antihypertensive activities performed include systolic (SBP), diastolic blood pressure (DBP) and heart rate (HR) variation, followed by evaluation of selected biochemical parameters in urine, blood (Alanine aminotransferase (ALT), aspartate aminotransferase (AST)), creatinine, urea and total protein) and histological examination of tissue samples (aorta, heart, kidneys and liver). The amount of the phenols of the leaf extract was estimated in mg gallic acid equivalent and identification of some compounds was done by UPLC-UV-ESI-TOF-MS. Accordingly, the identified phenols were stuhlmannione A (1), formononetin (2), stuhlmarotenoid A (3), 9-methoxymaackiain (4), 4-hydroxymaackiain (5) and 7-hydroxy-3′,4′-methylenedioxy-isoflavone (6). The extract exhibited a significant (P < 0.05–0.001) decrease of SBP, DBP and HR when compare to control. AEXS also reduced (P < 0.05) serum rates of ALT, AST, and urea. The extract showed beneficial effects on alterations observed in the histological structures of the aorta, heart, kidneys and liver. AEXS highlighted high level of phenols (26.48 ± 2.89 mg GAE/g) and a strong antiradical activity on DPPH, ABTS+, OH− and NO with IC50 of 148.8 μg/mL, 27.83 μg/mL, 22.29 μg/mL, 29.84 μg/mL respectively. An optical density of 1.79 nm was obtained with FRAP test. Thus, X. stuhlmannii leaf extract has in vitro antioxidant and antihypertensive effects that may support its use against hypertension.
Keywords: X. stuhlmannii, Hypertension, Cadmium chloride, Oxidative stress, Phenols
1. Introduction
Hypertension occurs when the blood pressure in vessels becomes too high (140 mmHg SBP and 90 mmHg DBP or higher). It represents the main cause of premature death worldwide. Reduce the prevalence of hypertension by 33 % within 2010 and 2030 has become one of the global challenges for non-communicable diseases [1]. An estimation reveals that 1.28 billion of adults aged between 30 and 79 years worldwide are suffering from hypertension, most of them reside in low- and intermediate-income countries [1]. Hypertension has many origins, including exposure to an environmental pollutant such as cadmium (Cd), which is a toxic heavy metal that could be present in food and tobacco [2]. Its industrial exploitation grew at the beginning of the 20th century. Once absorbed, Cd could be accumulated in different tissues, especially in the liver and kidneys [2], and therefore cause harmful effects such as kidney dysfunction [3], pulmonary edema [4], cancer [5] and cardiovascular diseases including hypertension and atherosclerosis [6]. In addition, numbers of clinical and experimental carried out have correlated a hypertension and Cd presence in organism, although the biological mechanisms which explain the link between Cd exposure and high blood pressure are unknown. However, it has been brought evidence that the hypertensive effect of Cd exposure results from complex mechanisms that operate on both vascular smooth muscle cells and on vascular endothelium. The main factors playing a key role in the cardiovascular complications in living organisms and which have been the subject of intensive research are reactive nitrogen species (RNS), reactive oxygen species (ROS), and depletion of antioxidant levels. These factors always lead to a state of oxidant/antioxidant balance [7]. In recent years, numerous studies have reported the role of oxidative stress as a key mechanism which can explain cadmium chloride toxicity [8]. Oxidative stress can cause blood pressure increase by several pathways such as causing a decrease in the bioavailability of NO, which promotes increased peripheral vascular resistance, endothelial dysfunction, and vascular remodeling, platelet and leukocyte adhesion [9]. However, oxidative damage could be suppressed by natural or synthetic antioxidants used for medicinal purposes [10]. Synthetic antioxidants like butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) are actually not indicated due to their carcinogenic effects in vivo [11]. They are now being replaced by natural plant-derived antioxidants due to their excellent non-toxic, free radical detoxifying properties, affordable prices joint to fewer adverse effects [12]. The treatment of hypertension aims to gradually drop blood pressure and also prevent possible damage of organs. It mainly relies on hygieno-dietetic measures and the use of antihypertensive drugs. Due to the substantial cost of health services and medicines on the one hand, and unfavourable socio-economic factors on the other, a large proportion of the population relies on medicinal plants for their health care [13]. Therefore, an interest was given to the study of many plant properties include the antihypertensive and antioxidant properties of the aqueous extract of the leaves of X. stuhlmannii (Fabaceae). It is a species found in open forests or Sudano-Guinean wooded savannahs [14]. The plant is particularly found on well-drained soils, on sandy soils and it is resistant to drought. The species is largely spread in tropical Africa, from eastern Senegal to Kenya, south of Zimbabwe, Mozambique, Cameroon and northern South Africa. The leaves are used to treat colds, coughs, wounds, stomach ache, fever, malaria, and hypertension [14]. Phytochemical screening of the ethanolic leaf extract of X. stuhlmannii indicated the presence of isoflavones (flavonoids) and rotenoids [15]. Although the virtues of this plant are known empirically to treat various pathologies, its effect on high blood pressure has not yet been demonstrated. Thus, this study was undertaken to assess the antioxidant and antihypertensive effects of the aqueous extract from the leaves of X. stuhlmannii against CdCl2-induced hypertension in rats.
2. Materials and methods
2.1. Plant material and extraction procedure
Fresh leaves (Fig. 1A) of X. stuhlmannii, were collected from Bitchoua-Nord, West region of Cameroon in June 2022. The plant was identified at the National Herbarium of Yaounde-Cameroon, in comparison of the harvested leaves and fruits (Fig. 1B) with the Voucher's specimen, deposited under Number 6011/SRFCAM. The leaves were shade-dried and ground into fine powder. The aqueous extract was obtained by infusing 4.84 g of X. stuhlmannii leaves powder into 200 mL of boiling distilled water (100 °C) until complete cooling. Therefore, extract filtration was performed using Whatman paper n°3. The filtrate was dried in an oven at 40°Ϲ and 0.973 g of dried brown powder was obtained, let be a percentage yield of 20.10 %.
Fig. 1.
Photographs of the leaves (A) and fruits (B) of Xeroderris stuhlmannii.
2.2. Experimental animals
Wistar Albino rats, aged 10–14 weeks old and weighing between 182 and 225 g were used for antihypertensive effect. They were housed in a colony inside plexiglass cages, at the animal house of the University of Douala, Cameroon, at room temperature (23–25 °C) with a 12 h dark-light natural cycle. They were fed the standard commercial diet and tap water ad libitum. The institutional ethics committee of the University of Douala approved the research protocol under the reference N° 3082 CEI-UDo/05/2022/T.
2.3. Drugs and chemicals
Analytical-grade reagents were used in this study. Iron-chloride, sodium chloride, Potassium peroxodisulfate and potassium hydroxide were bought from Acros organics (Germany). Amlodipine was purchased from Denk (Germany). Sodium carbonate, Tris, Folin-Ciolcalteu were from Carl Roth (Germany). 2-deoxy-D-ribose, sodium hydroxide and sodium nitrite were obtained from Alfa Aesar (Germany). Phosphate buffered saline, Sodium nitrite, and NED were purchased from VWR life science (Belgium). Hydrogen peroxide HR rapid was purchased from Tintometer group GmbH (Germany). Trichloroacetic acid and gallic acid were from Cayman chemical company (Germany). Alanine aminotransferase, Aspartate aminotransferase, urea, total protein, and creatinine kits were purchased from SGM Italia (Roma). Trolox, potassium ferricyanide, 2,2-diphenyl-1-picrylhydrazyl, thiobarbituric acid, cadmium chloride hydrate, 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid), disodium hydrogen phosphate, ferric chloride, phosphoric acid and ascorbic acid were obtained from Sigma-Aldrich (Germany).
2.4. Quantification of total phenolic content
The total phenolic content was quantified in extract by using the modified method of Singleton and Rossi [16]. Gallic acid was used as a standard and the reaction mixture was prepared with 40 μL of extract (1 mg/mL), mixed with 3.16 mL of distilled water and 200 μL of 10 % Folin-Ciocalteu reagent was added. The mixture was incubated for 8 min, then 600 μL of 20 % of Na2CO3 was added, and incubated for 30 min at 40°Ϲ. Experiment was carried out in triplicate and absorbance was measured at 760 nm. The results obtained for the different concentrations of the standard gallic acid (0–300 μg/mL; y = 0.0007x + 0.0198; R2 = 0.9707) were expressed as the mg gallic acid equivalent per gram of dry weight [17].
2.5. In vitro assays: assessment of the antioxidant activity of X. stuhlmannii leaf extract
Overall, concentration providing 50 % of radical scavenging activity (IC50s) obtained from the tests on DPPH (2,2-diphenyl-1-picrylhydrazyl radical), ABTS (2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid), OH− and NO were determined from the graph depicting inhibition percentages against extract concentrations. Ascorbic acid or trolox were used as reference compounds.
2.5.1. Scavenging test on 2,2-diphenyl-1-picrylhydrazyl radical
The ability of AEXS to scavenge DPPH radical was determined as described by Brand Williams et al. [18] with modifications. For each test tube containing 1000 μL of various concentrations (1-3-10-30-100-300-700 and 1000 μg/mL) of the plant extract or ascorbic acid, 500 μL of DPPH solution (0.063 mg/mL) were added and incubated for 20 min at room temperature in the absence of light. The absorbance was measured against blank (methanol) at 517 nm.
Experiments were run in triplicate and the inhibition percentage was calculated as follows:
Where Ac and At are the absorbances of the blank and test substances (extract/ascorbic acid), respectively.
2.5.2. Scavenging test on 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid radical
ABTS radical scavenging assay was determined as described by Re et al. [19] with modifications. The solutions of potassium persulfate (2.45 mM) and ABTS (7 mM) previously dissolved in distilled water, were stirred at equal volume. The mixture was kept to react in the dark at room temperature within 12–16 h. Distilled water was supplemented to that working solution until an absorbance of 0.70 ± 0.02 (734 nm) was obtained. Thereafter, 1 mL of ABTS solution was stirred with 0.1 mL of extract or trolox at different concentrations (1–1000 μg/mL), and was analyzed after 7 min against the blank (working solution and distilled water). All the concentrations were tested in triplicate. The results were presented as percentage of inhibition of ABTS:
where Ac and At are the absorbances of control and test substances (extract/trolox) respectively.
2.5.3. OH radical Scavenging assay
The scavenging of the OH radical produced by Fenton reaction was determined according to the modified method of Kunchandy and Rao [20]. Five hundred microliter (500 μL) of different concentrations (1–1000 μg/mL) of extract or trolox were added to 100 μL of 2-deoxy-D-ribose (28 mM), 200 μL mixture of EDTA (1.04 mM) and FeCl3 (0.2 mM) [1:1 v/v], 100 μL H2O2 (1 mM) and 100 μL of ascorbic acid (1 mM). All the reagents and extract contained in the M1 mixture were diluted in 20 mM of KH2PO4-KOH buffer, pH 7.4. This mixture (M1) was incubated at 37°Ϲ for 1 h. Thiobarbituric acid (1 %, 500 μL) and trichloroacetic acid (2.8 %, 500 μL) was added successively to M1 (500 μL) and incubated at 100 °C for 30 min. After cooling, the absorbance was measured at 532 nm against a control sample. The assays were performed in triplicate. The results were expressed as a percentage of 2-deoxyribose degradation.
Where Ac and At are the absorbances of the control and test substances (extract/trolox), respectively.
2.5.4. Ferric reducing antioxidant power
The reducing capacity of the extract was evaluated according to a previously described method with modifications [21]. Half milliliter (0.5 mL) of various concentrations of extract/ascorbic acid (1–300 μg/mL), 1.25 mL of phosphate buffer (200 mM, pH = 6.6), and 1.25 mL of potassium ferricyanide (1 %) were introduced in the test tubes. The mixture was incubated at 50°Ϲ for 20 min, then 1.25 mL trichloroacetic acid (10 %) was added. After centrifugation at 3000 rpm for 10 min, 1.25 mL of supernatant was taken and 1.25 mL of distilled water and 0.25 mL of FeCl3 (0.1 %) were added. The mixture was incubated for 10 min at 37°Ϲ and the absorbance was recorded at 700 nm in triplicate against the blank solution. The antioxidant power was estimated by plotting of the variation of the optical density as a function of the concentration of the assay tubes compared to the standard tubes (ascorbic acid).
2.5.5. Nitric oxide Scavenging test
The nitric oxide (NO) released by sodium nitroprusside was determined using the Griess reaction [22]. A volume of 50 μL of sodium nitroprusside (10 mM in phosphate buffered saline, pH 7.4) was transferred to other tubes containing 950 μL of extract or ascorbic acid at different concentrations (1–1000 μg/mL). The mixture reaction was left to stand at room temperature (25°Ϲ) for 3 h under light. Thereafter, 500 μL of the mixture was removed and then stirred with 500 μL of Griess reagent (1 % sulfanilamide prepared in distilled water and 0.1 % napthylethylenediamine prepared in 2 % phosphoric acid) in dark. The absorbance of the chromatophore was recorded immediately at 546 nm against the blank (distilled water, sodium nitroprussiate, and Griess reagent) and referred to the standard solution (ascorbic acid prepared in the same way) absorbance. The experiment was carried out in triplicate [23]. The results were expressed as percentage of inhibition of nitrite ions by the following formula:
Where Ac and At were the absorbance of the control and test substances (extract/ascorbic acid), respectively.
2.6. Antihypertensive activity of Xeroderris stuhlmannii against cadmium chloride-induced hypertension
2.6.1. Animal preselection, distribution and drug administration
After 5 days acclimatization period, animals were screened to exclude anyone with blood pressure higher than 140/90 mmHg. Therefore, 48 animals divided into 2 separate groups were fasted 12 h prior to the experiment, which was curative and ongoing during 3 weeks for the preliminary test. Accordingly, the first group of 06 rats, named normotensive rats (control), daily received NaCl (0.9 %, i.p) as vehicle. The second group (42 rats) were administrated cadmium chloride (1 mg/kg, i.p daily) to induce hypertension. At the end of 3 weeks, the pressure of the animals was recorded and those in the second group with blood pressure values higher than or equal to 140/90 mmHg were considered as hypertensive animals and selected for the following experience. Accordingly, 36 rats which were hypertensive were randomized into 5 groups of 6 rats each, which received different solutions as follows:
-
-
Hypertensive group (control) continuous to receive cadmium chloride (CdCl2)
-
-
Standard group which received amlodipine (1 mg/kg body weight) concomitantly with CdCl2
-
-
Three assay groups which received the extract at different doses of 35, 100 and 300 mg/kg of body weight concomitantly with CdCl2 (1 mg/kg, i.p daily).
Throughout the experiment, the rats of group 1 were receiving tap water while the other groups were receiving NaCl (1 %) as drinking water.
Following animal redistribution, the experiment was extended for more two weeks, blood pressure and heart rate were recorded twice a week following the non-invasive method with the use of CODA system (Kent Scientific Co, USA) [24].
2.6.2. Sample collection and analysis
At the end of experiment, the urine sample of each animal was collected using metabolic cages and transferred to laboratory tubes. Animals were anesthetized with dual intraperitoneal injection of diazepam and of ketamine (10 mg/kg; 50 mg/kg) and euthanized by cervical decapitation. Blood specimen was also collected in heparin-tubes. Blood and urine samples were centrifuged at 3000 rpm for 15 min to obtain plasma and supernatant respectively. Aliquot solutions collected were used to perform various biochemical measurements including alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, urea, and total protein. Aorta, heart, kidneys, and liver tissues were excised quickly, freed of fat and connective tissues, rinsed in physiological saline (0.9 % NaCl) and weighed. Known weights representative fragments of tissues (aorta and heart) were homogenized in Mac Even or in Tris-HCl 50 mM buffer solution pH = 7.4 for liver and kidney (20 %, w/v). After centrifugation at 3000 rpm for 30 min (aorta and heart) and for 15 min (liver and kidney), the supernatant was collected to assess oxidative stress parameters (catalase, malondialdehyde, superoxide dismutase and nitric oxide).
2.6.3. Histopathological examination
The remaining portions of tissue specimens (aorta, heart, kidneys, and liver) were kept in 10 % buffered formalin (pH = 7.4) solution, dehydrated in graded alcohol and embedded in paraffin for further histopathological examination. 5 μm sections obtained were mounted on glass slides and stained with hematoxylin-eosin for observation under an optical microscope ( × 400-magnification). Parameters such as media diameter in the aorta, aspect of muscle fibers in heart, aspect of hepatocytes in liver, and aspect of glomerulus in the kidney were observed and photographed.
2.7. UPLC-UV-ESI-TOF-MS analysis of X. stuhlmannii leaf extract
Aliquots 2 μL of the leaf extract (1 mg/10 mL, 50 % MeCN) were analyzed by means of UPLC-ESI-TOF MS on a Waters Synapt G2-S HDMS mass spectrometer (Waters, Manchester, UK) coupled to an Acquity UPLC core system (Waters, Milford, MA, USA) equipped with a 2 × 150 mm, 1.7 μm, BEH C18 column (Waters, Manchester) consisting of a binary solvent manager, sample manager and column oven. Operated with a flow rate of 0.4 mL/min at 50 °C, the following gradient was used for chromatography: starting with a mixture (1/99, v/v) of aqueous HCO2H (0.1 % in H2O) and MeCN (0.1 % HCO2H) for 0.3 min, the MeCN content was increased to 100 % within 8 min, kept constant for 2 min, decreased to 1 % within 1 min and finally kept constant for 1 min at 1 %. Scan time for the MSe method (centroid) was set to 0.1 s. Analyses were performed with negative and positive ESI in high resolution mode using the following ion source parameters: capillary voltage −2.0 or +2.5 kV, sampling cone 50 V, source offset 30 V, source temperature 120 °C, desolvation temperature 450 °C, cone gas flow 2 L/h, nebulizer 6.5 bar and desolvation gas 800 L/h. Data processing was performed by using Mass Lynx 4.1 SCN 9.16 (Waters, Manchester) and the elemental composition tool for determining the accurate mass. All data were locked mass corrected on the pentapeptide leucine enkephaline (Tyr-Gly-Gly-Phe-Leu, m/z 554.2615, [M − H]-) in a solution (1 ng/μL) of MeCN/0.1 % HCO2H (1/1, v/v). Scan time for the lock mass was set to 0.3 s, an interval of 15 s and 3 scans to average with a mass window of ±0.3 Da. Calibration of the Synapt G2-S in the range from m/z 50 to 1200 was performed using a solution of HCO2Na (5 mmol/L) in 2-propanol/H2O (9/1, v/v). The UPLC and Synapt G2-S systems were operated with MassLynx™ software (Waters). Collision energy ramp for MSe was set from 20 to 40 eV.
2.8. Statistical analysis
Results in both studies (in vitro and in vivo) were computed as mean ± standard error of the mean. For the in vitro antioxidant assays, IC50 values were determined using a nonlinear regression curve followed by normalized and logarithmic transformation. Data from in vivo experiments were submitted to one-way ANOVA followed by Tukey's post test for organ weight variation and biochemical parameters and two-way ANOVA with repeated measures followed by Bonferroni post test for blood pressure and body weight. A result with P < 0.05 was considered statistically significant.
3. Results
3.1. Total phenols content
Total phenols content of X. stuhlmannii leaf extract expressed as gallic acid equivalent (GAE) was found to be 26.48 ± 2.89 mg GAE/g.
3.2. Antioxidant activities of X. stuhlmannii leaf extract
3.2.1. Antiradical activity of X. stuhlmannii leaf extract against DPPH
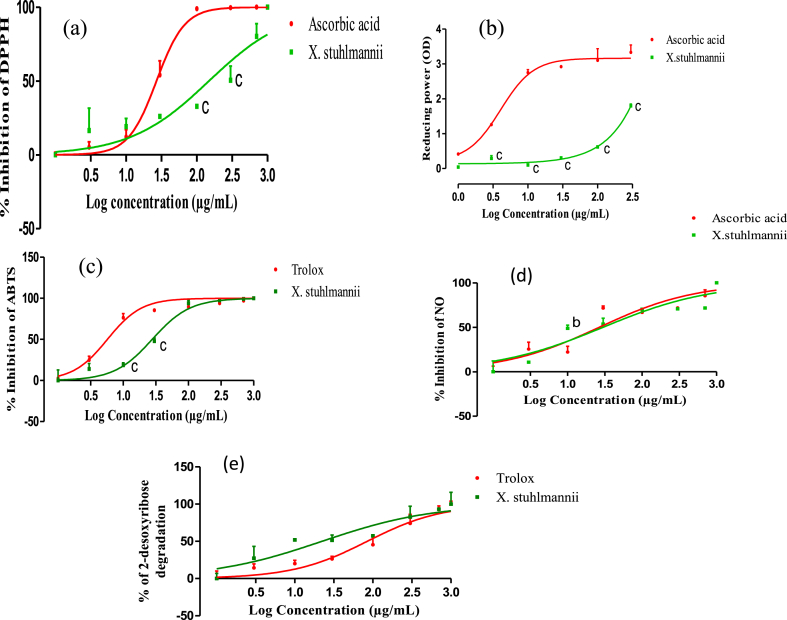
The leaf extract of X. stuhlmannii developed a concentration-dependent scavenging activity on DPPH radical with an inhibitory concentration 50 (IC50) of 148.8 μg/mL, while the standard ascorbic acid exhibited an IC50 of 26.95 μg/mL (Fig. 2a).
Fig. 2.
DPPH radical scavenging (a), ferric reducing antioxidant power (b), ABTS radical scavenging (c), NO radical scavenging (d) and OH radical scavenging (e) capacity of the aqueous leaf extract of X. stuhlmannii and ascorbic acid or Trolox. Data are presented as mean ± SEM, n = 3; bP < 0.01; cP < 0.001 vs, ascorbic acid or Trolox.
3.2.2. Antioxidant effect of X. stuhlmannii through ferric reducing power/reducing potential assay
The ferric reducing antioxidant power of the aqueous leaf extract and the standard increased proportionally with the concentration (Fig. 2b). The optical density of the extract was 1.79 nm, while that of ascorbic acid was 3.33 nm.
3.2.3. Scavenging activity of X. stuhlmannii leaf extract against ABTS radical
The inhibition of the absorbance of ABTS radical by X. stuhlmannii extract and Trolox are represented in Fig. 2c. The extract and Trolox exhibited significant scavenging activity on ABTS radical cation at various tested concentrations. The concentration of X. stuhlmannii extract necessary for 50 % inhibition was found to be 27.83 μg/mL while 5.72 μg/mL was required for the standard Trolox.
3.2.4. Scavenging activity of X. stuhlmannii against NO radical
The result of the NO radical scavenging (Fig. 2d) showed that the inhibition of NO release in presence of X. stuhlmannii leaf extract and ascorbic acid is concentration dependent manner. The IC50 value obtained for the extract was 29.84 μg/mL compared to that of ascorbic acid of 26.55 μg/mL.
3.2.5. Scavenging activity of X. stuhlmannii against hydroxyl radical
Scavenging ability of hydroxyl radical was concentration dependent for the aqueous extract and Trolox (Fig. 2e). The IC50 value obtained with the extract was 22.29 μg/mL compared to that of Trolox which was 84.71 μg/mL.
The concentration providing 50 % of radical scavenging activity (IC50) was obtained using graphpad prism software version 5.03.
3.3. Effect of cadmium chloride and aqueous leaf extract of X. stuhlmannii on organs relative weight, blood pressure and heart rate in rats
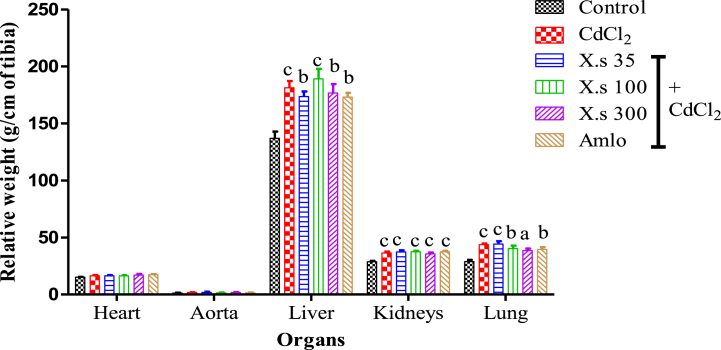
Compared to the control the administration of CdCl2 induced significant variation of relative weight of the liver (32.36 %; P < 0.001), kidneys (27.40 %; P < 0.001) and lungs (51.45 %; P < 0.001). The concomitant administration of the extract or amlodipine with CdCl2 did not induce any significant variation in the heart, aorta the liver, kidneys, and lungs relative weight compared to the hypertensive group (CdCl2 group) (Fig. 3).
Fig. 3.
Relative weight of some organs Data are presented as mean ± SEM, n = 6; aP < 0.05, bP < 0.01, and cP < 0.001: compared to the control; CdCl2 = cadmium chloride; X.s = X.s stuhlmannii; Amlo = Amlodipine.
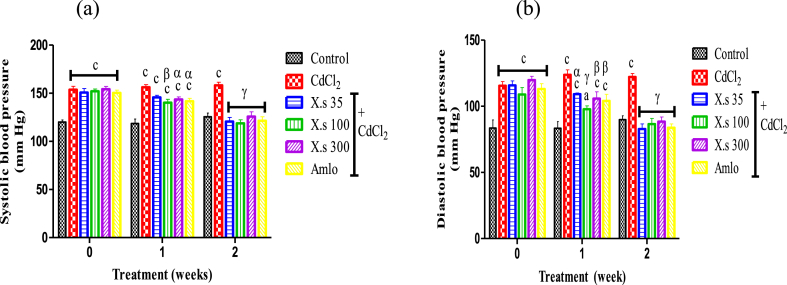
Two weeks of treatment with CdCl2 induced a significant increase (P < 0.001) of SBP and DBP in rats compared to control. Concomitant administration of CdCl2 and aqueous extract of X. stuhlmannii or amlodipine resulted in a progressive decrease (P < 0.001) in SBP and DBP in all groups compared to the hypertensive group. At the end of the first week of treatment the administration of the extract (300 mg/kg) induced a significant fall of the SBP from 158.30 mmHg to 115.10 mmHg (Fig. 4a). At the end of second week of treatment, the maximum decrease in DBP by 32.19 % as compared to hypertensive group was observed (Fig. 4b).
Fig. 4.
Effects of different treatments on systolic (a) and diastolic (b) blood pressures Data are presented as mean ± SEM, n = 6; aP < 0.05, bP < 0.01 and cP < 0.001: compared to the control; αP < 0.05; βP < 0.01 and γP < 0.001: compared to CdCl2; CdCl2 = Cadmium chloride; X.s = X. stuhlmannii; Amlo = Amlodipine.
The heart rate did not change significantly during experimental induction of hypertension as well as treatment with plant extract. Only the dose of 100 mg/kg of extract significantly reduced (P < 0.01) the HR compared to hypertensive group.
3.4. Effect of X. stuhlmannii extract on some enzymes and renal markers
3.4.1. Effect of aqueous extract of X. stuhlmannii on serum transaminases activities
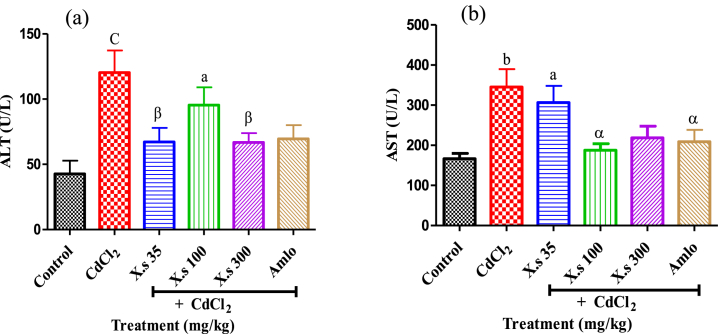
The results revealed that the hypertensive group had significantly (P < 0.001) increased serum transaminases (ALT and AST) activity compared to controls. Concomitant administration of CdCl2 and the aqueous leaf extract of X. stuhlmannii led to a decrease (P < 0.01) in ALT activity as compared to hypertensive group. This decrease reached 67.22 U/I and 66.81 U/I at doses of 35 and 300 mg/kg, respectively (Fig. 5a). The administration of the plant extract or amlodipine and cadmium chloride led to a decrease (P < 0.05) in AST activity compared to the hypertensive group (Fig. 5b).
Fig. 5.
Variation of serum transaminases activities after administration of leaf aqueous extract of X. stuhlmannii in rats Data are presented as mean ± SEM, n = 6; aP < 0.05, bP < 0.01 and cP < 0.001: compared to the control; αP < 0.05 and βP < 0.01: compared to CdCl2; CdCl2 = Cadmium chloride; X.s = X. stuhlmannii; Amlo = Amlodipine.
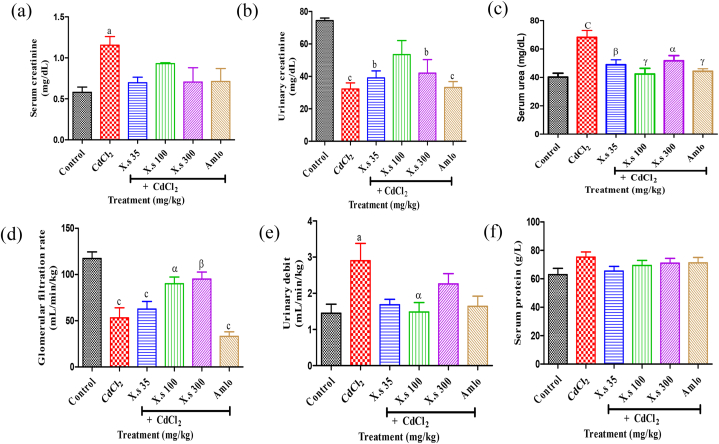
3.4.2. Effect of extract on some kidneys functions parameters
The repeated administration of cadmium chloride to rats provoked an increase (P < 0.05) of the serum creatinin from 0.579 ± 0.064 mg/dL for the control to 1.155 ± 0.105 mg/dL in hypertensive rats (Fig. 6a). However, a decrease of urinary creatinin concentration was observed in the hypertensive group (32.29 ± 3.739 mg/dL) compared to the control (74.41 ± 1.568 mg/dL). Compared to the hypertensive group oral administration of EAXS caused an increase (P > 0.05) of urinary creatinin concentration in all the assays groups (Fig. 6b). The serum urea level in rats was significantly higher (P < 0.001) in hypertensive group than in control group, with a percentage of depletion of 40.99 % (Fig. 6c). The treatment of CdCl2 induced-hypertensive rats with EAXS significantly prevented (P < 0.05–0.001) the elevation of urea level by 28.32 %, 37.88 %, and 24.20 %, respectively for the doses 35, 100 and 300 mg/kg compared to the hypertensive group. The reference drug, amlodipine also caused a decrease (P < 0.001) in the urea level.
Fig. 6.
Effects of different treatments on serum (a) and urinary (b) creatinine, serum urea (c), glomerular filtration rate (d), urinary debit (e) and serum protein (f) Data are presented as mean ± SEM, n = 6; aP < 0.05, bP < 0.01 and cP < 0.001: compared to the control; αP < 0.05, βP < 0.01 and γP < 0.001: compared to CdCl2; CdCl2 = Cadmium chloride; X.s = X. stuhlmannii; Amlo = Amlodipine.
The glomerular filtration rate (GFR) was low (P < 0.001) in the hypertensive group compared to the control. During the treatment, the concomitant administration of the extract and CdCl2 led to an increase (P < 0.05) in this flow rate of 90.07 ± 7.22 and 95.05 ± 7.62 mL/min/kg respectively for 100 and 300 mg/kg against 53.23 ± 10.85 mL/min/kg for the hypertensive group (Fig. 6d).
Urinary debit was significantly higher in the hypertensive group (2.903 ± 0.474 mL/min/kg) compared to the control (1.449 ± 0.249 mL/min/kg) (Fig. 6e). The plant extract in concomitant administration with CdCl2 caused a significant decrease in the urinary debit in all the assay groups, in the order of 1.483 ± 0.262 mL/min/kg for the extract (100 mg/kg) vs. The hypertensive group.
The effects of AEXS on serum proteins are presented in Fig. 6f. No significant variation between the different batches treated was observed.
3.5. Effect of the aqueous extract of X. stuhlmannii on oxidative stress markers
The effects of AEXS on catalase and superoxide dismutase (SOD) activities, as well as malondialdehyde (MDA), reduced glutathione (GSH) and nitrite oxide (NO) concentrations are summarized in Table 1.
Table 1.
Effect of X. stuhlmannii and amlodipine on oxidative stress markers in cadmium chloride-induced hypertension.
| Parameters |
Organs |
Control |
CdCl2 |
CdCl2 + |
|||
|---|---|---|---|---|---|---|---|
| X.s 35 | X.s 100 | X.s 300 | Amlo | ||||
| Catalase (μmol of H2O2/min/g organ) | Aorta | 689.1 ± 60.66 | 163.8 ± 12.69c | 445.7 ± 76.26 | 363.6 ± 88.06a | 279.5 ± 69b | 287.7 ± 64.94b |
| Heart | 88.80 ± 19.04 | 43.52 ± 8.24 | 93.62 ± 13.51α | 109.7 ± 5.62β | 104.2 ± 2.97β | 74.92 ± 7.20 | |
| Liver | 133.1 ± 16.24 | 52.24 ± 10.03c | 106.7 ± 7.27β | 53.58 ± 11.45c | 35.70 ± 7.05c | 56.15 ± 4.95c | |
| Kidneys | 91.51 ± 5.67 | 35.92 ± 5.05c | 79.30 ± 6.44γ | 73.43 ± 5.46β | 61.03 ± 5.75a | 61.40 ± 6.58aα | |
| SOD (unity of SOD/g Organ) | Heart | 327.5 ± 29.81 | 318.2 ± 9.66 | 421.2 ± 38.86 | 449.4 ± 56.67 | 428.4 ± 62.15 | 300.8 ± 23.51 |
| Liver | 288.9 ± 18.27 | 222.8 ± 32.39 | 178.8 ± 11.23 | 249.8 ± 60.16 | 259.6 ± 49.12 | 207.8 ± 39.04 | |
| Kidneys | 177.3 ± 21.18 | 117.8 ± 6.41 | 122.3 ± 7.28 | 149.2 ± 12.13 | 158.1 ± 21.64 | 146.3 ± 9.77 | |
| Gluthatione (mmol/g Organ) | Aorta | 0.47 ± 0.06 | 0.38 ± 0.03 | 0.31 ± 0.06 | 0.58 ± 0.08 | 0.28 ± 0.05 | 0.43 ± 0.04 |
| Heart | 0.06 ± 0.01 | 0.05 ± 0.006 | 0.04 ± 0.005 | 0.03 ± 0.005 | 0.04 ± 0.008 | 0.04 ± 0.004 | |
| Liver | 0.03 ± 0.008 | 0.03 ± 0.003 | 0.02 ± 0.002 | 0.05 ± 0.003 | 0.02 ± 0.002 | 0.06 ± 0.007bγ | |
| Kidneys | 0.022 ± 0.002 | 0.003 ± 0.001c | 0.006 ± 0.001c | 0.009 ± 0.0009c | 0.017 ± 0.002γ | 0.016 ± 0.001aγ | |
| MDA (mmol/g Organ) | Liver | 0.003 ± 0.001 | 0.011 ± 0.001b | 0.01 ± 0.001a | 0.002 ± 0.001β | 0.002 ± 0.001β | 0.005 ± 0.001 |
| Kidneys | 0.008 ± 0.0003 | 0.007 ± 0.0005 | 0.006 ± 0.0006 | 0.002 ± 0.001 | 0.005 ± 0.0001cγ | 0.006 ± 0.0005 | |
| NO (μmol/g organ) | Aorta | 2.33 ± 0.40 | 1.44 ± 0.25 | 1.32 ± 0.56 | 1.43 ± 0.64 | 2.23 ± 0.19 | 2.05 ± 0.12 |
| Heart | 0.16 ± 0.004 | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.18 ± 0.01 | 0.12 ± 0.01 | 0.14 ± 0.01 | |
| Liver | 0.065 ± 0.006 | 0.046 ± 0.005 | 0.062 ± 0.004 | 0.059 ± 0.007 | 0.043 ± 0.005 | 0.044 ± 0.0008 | |
| Kidneys | 0.084 ± 0.005 | 0.054 ± 0.002b | 0.058 ± 0.005a | 0.052 ± 0.006b | 0.056 ± 0.004b | 0.054 ± 0.003b | |
Data are presented as mean ± SEM, n = 6;aP < 0.05, bP < 0.01 and cP < 0.001: compared to the control; αP < 0.05, βP < 0.01 and γP < 0.001: compared to CdCl2; SOD = Super oxide dismutase, MDA = Malondialdehyde, NO = nitric oxide, CdCl2 = Cadmium chloride; X.s = X. stuhlmannii; Amlo = Amlodipine.
Animals only treated with CdCl2 showed a low catalase activity compared to controls. This decrease (P < 0.001) of activities reached 60.75 %, 60.75 %, and 76.23 % for hepatic, renal, and aortic tissue, respectively, compared to control. Administration of the extract (35 and 100 mg/kg) resulted in a significant increase in catalase activity in the liver, heart aorta and kidneys tissues compared to the hypertensive group. Amlodipine (1 mg/kg) used as reference substance also increased the catalase activity with respective percentages of 70.93 % for the extract in the heart compared to hypertensive group.
It appears that the injection of CdCl2 led to a reduction (P > 0.05) in the activity of SOD in all tissue's specimen. Treatment with the extract or amlodipine resulted in an increase (P > 0.05) at all doses tested in all organs assayed.
The administration of CdCl2 dropped GSH levels in the kidneys. This decrease was 49.12 % (P < 0.001) compared to control. However, administration of the plant extract or amlodipine concomitantly with CdCl2 resulted in an increase (P < 0.001) in GSH levels in the liver and kidney.
The treatment with CdCl2 significantly increased the activity of MDA in liver. This increase was 0.01196 ± 0.001 mmol/g organ for the control versus 0.00325 ± 0.001 mmol/g organ for the hypertensive group. Concomitant administration of the extract with CdCl2 resulted in a decrease in MDA activity. As for renal MDA, the concomitant administration of the extract and CdCl2 led to a significant decrease (P < 0.001) in the activity of MDA at a dose of 100 mg/kg compared to the control and hypertensive groups, with respective reduction percentages of 73.21 % and 70.92 %.
The level of NO significantly (P < 0.01) decreased in the kidneys of rats receiving only CdCl2 compared to controls. In addition, the plant extract and amlodipine could not significantly remediate this decrease in NO levels compared to the hypertensive group.
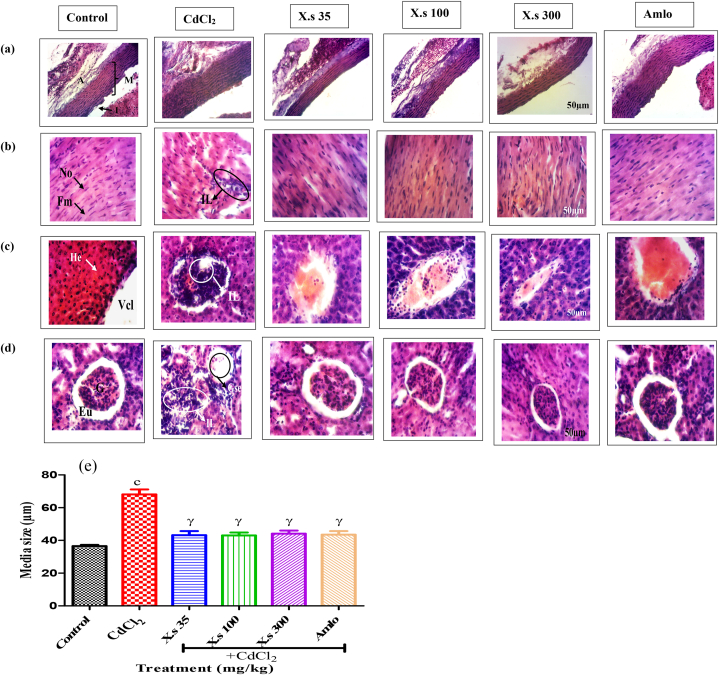
3.6. Effect of X. stuhlmannii on histological structures of aorta, heart, liver, and kidney
The effects of AEXS on the microarchitecture of the heart, aorta, kidney and liver are represented in Fig. 7. The aorta of control rats showed a normal size of the media.
Fig. 7.
Photomicrographs of Aorta (a), heart (b), liver (c) and kidney (d) (HE, 200×) and the effect of different treatments on media length (e). Aorta: I = intima, M = media, A = adventice; Heart: No = nucleus, Fm = muscular fiber, IL = leukocyte infiltration; Liver: Vcl = centrilobular Vein, He = hepatocyte, IL = leukocyte infiltration; kidneys: G = glomerulus, Gse = glomerulosclerosis, EU = urinary space. Data are presented as mean ± SEM, n = 6; cP < 0.001: compared to the control; γP < 0.001: compared to CdCl2; CdCl2 = cadmium chloride; X.s = X. stuhlmannii; Amlo = amlodipine.
CdCl2-induced experimental hypertension is linked to the wall hypertrophy. The media size which was 36.50 ± 1.00 μm for the control and increased to 68.09 ± 3.73 μm for hypertensive group (Fig. 7e). After administration of plant extract or amlodipine resulted in a decrease of the media size of 36.64, 36.77, 35.23 and 36.06 % respectively at the dose of 35, 100, 300 mg/kg and amlodipine (Fig. 7a).
At the level of heart, the chronic administration of CdCl2 revealed leukocyte infiltration for hypertensive group compared to control. The different doses of extract and amlodipine presented a similar architecture to control, with normal nuclei and muscle fibers (Fig. 7b).
The liver section of control indicated normal architecture. However, in negative control, we observed a leukocyte infiltration compared to the control. The architecture of rat's liver that concomitantly received CdCl2 and extract or amlodipine highlighted a structural organization similar to those of the control (Fig. 7c).
The kidneys of control animals showed anormal glomeruli and the urinary space as well as convoluted tubules. Hypertensive animals revealed glomerulosclerosis and leukocyte infiltration. The concomitant administration of CdCl2 and extract or amlodipine highlight showed structural organization similar to those of the control rats (Fig. 7d).
3.7. UPLC-UV-ESI-TOF-MS analysis of X. stuhlmannii leaf extract
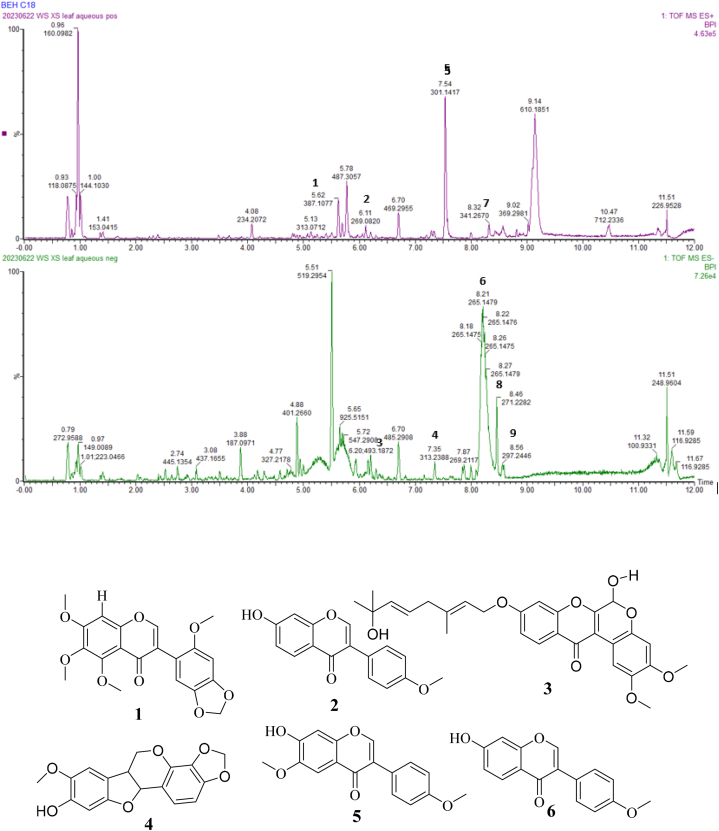
Fig. 8 shows the Base Peak Ion Chromatogram (BPIC) of the aqueous leaf extract of X. stuhlmannii, obtained by UPLC-UV-ESI-TOF-MS in positive and negative ion modes. This chromatogram allowed us to visualize some of the chemical constituents with broad polarity ranging from 5 to 100 % of organic solvent. Compounds 1–6 were identified via reference compounds [15] as: stuhlmannione A (1), formononetin (2), stuhlmarotenoid A (3), 9-methoxymaackiain (4), 4-hydroxymaackiain (5) and 7-hydroxy-3′,4′-methylenedioxy-isoflavone (6). (Table 2 and Fig. 7).
Fig. 8.
Base peak chromatograms of leaf aqueous extract of X. stuhlmannii obtained by UPLC-UV-ESI-TOF-MS. The number on each peak corresponds to the compound numbers presented in Table 2.
Table 2.
Identified phenols compounds in X. stuhlmannii leaf extract using UPLC-UV-ESI-TOF-MS.
| Compound Number | Retention time (min) | MS (m/z) | Ion adducts | Molecular Formular | Proposed identification | References |
|---|---|---|---|---|---|---|
| 1 | 3.62 | 387.1077 | [M+H]+ | C20H18O8 | Stuhlmannione A | Mekuete et al. [15] |
| 2 | 6.11 | 269.0825 | [M+H]+ | C16H12O4 | Formononetin | |
| 3 | 6.20 | 493.1872 | [M − H]- | C28H30O8 | Stuhlmarotenoid A | |
| 4 | 7.35 | 313.2388 | [M − H]- | C17H14O6 | 9-methoxymaackiain | Mekuete et al. [15] |
| 5 | 7.54 | 301.1417 | [M+H]+ | C16H12O6 | 4-hydroxymaackiain | Mekuete et al. [15] |
| 6 | 8.21 | 265.1479 | [M − H]- | C16H10O4 | 7-hydroxy-3′,4′-methylenedioxy-isoflavone | Mekuete et al. [15] |
| 7 | 8.32 | 341.2670 | [M+H]+ | C19H16O6 | Unknown | / |
| 8 | 8.46 | 271.2282 | [M − H]- | C15H14O5 | Unknown | / |
| 9 | 8.56 | 297.2446 | [M − H]- | C17H14O5 | Unknown | / |
4. Discussion
The study purposed to assess the effects of the aqueous leaves extract of X. stuhlmannii against cadmium chloride-induced hypertension in rats and oxidative stress. The aqueous extract was adopted for experiment to follow the traditional use of the plant material by local population and traditional healer as observed during ethnobotanical investigations. Cd is widely used in various industrial applications and is an ubiquitous environmental toxicant, causing oxidative stress and hypertension [25].
CdCl2 intraperitoneal injection in rats for five weeks provoked an increase of SBP and DBP compared to controls. By acting as a partial agonist of calcium channels, cadmium produces a direct contractile response on vascular smooth muscle, or could also act to alter cation transport, leading to an increase in blood pressure [[26], [27], [28], [29], [30]]. Treatment of hypertensive animals for two weeks with AEXS lowered blood pressure at all doses tested. This antihypertensive activity could be related to the extract's interference with calcium channels or to variable protective effects of its metabolites on the cardiovascular system [31].
When penetrate in the body, cadmium specifically accumulates in the heart, aorta, liver, and kidneys where it can induce severe tissue damage [32]. In this study, a significant increase in the weight of lungs, liver and kidneys was observed in all assay groups compared to controls. Thus, once absorbed by the gastrointestinal tract, cadmium is transported in direction to the liver and kidneys. The high weight of hepatic and renal tissues should be an indication of the presence of this heavy metal bound to metallothionein (MT) in the cytoplasm of their cells. When the hepatic concentration of MT becomes insufficient to bind to cadmium, it causes hepatocyte membrane lyses and promotes the release of Cd-MT complexes into the bloodstream in direction to the kidneys [33]. The extract could not reverse the increase in the relative weight of these organs.
AST and ALT constitute the key functional indicators used to detect liver damage or functional integrity [34]. Previous results revealed an increase in serum transaminase levels during chronic administration of CdCl2 in rats [35]. Indeed, the extract counteracted the liver damaging by activating the thiol group thus preventing the cadmium from binding to the thiol group; or else by inactivating the Kupffer cells and some inflammatory mediators. Alternatively, the extract may provide protection by stabilizing the cell membrane in liver damage-associated Cd [36,37]. The histological analysis of the rats’ liver allowed to confirm this result, since it has been observed a reduction of leucocytes infiltration, a well-differentiated architecture of the centrilobular vein and of hepatocytes in AEXS treated rats.
Chronic Cd poisoning mainly target kidneys [2]. The exposition of animals to this metal, could led to the development of renal tubular dysfunction [38]. Our study revealed that the administration of Cd resulted in an increase in serum creatinine, serum urea, and urinary debit but a decrease in urinary creatinine and GFR. These dysfunctions are caused by the cadmium-thionein complex, which is very nephrotoxic [39]. Administration of AEXS for a period of two weeks has been able to decline the rate of urea in the blood, as well as the urinary debit. At the dose of 300 mg/kg, the extract significantly increased the GFR compared to hypertensive group, demonstrating the ability of the aqueous extract to remove the creatinine from the blood in the urine. These results were confirmed by histopathological analysis of the kidneys which showed protective effects in AEXS treated rats compared to the control.
Oxidative stress is another manifestation of CdCl2 damage playing an important role in the toxicity of many xenobiotics [35].
The ability of Cd to induce oxidative stress has been established in vivo, in vitro, and in some epidemiologic studies [40]. AEXS exerted a remarkable antioxidant activity in vitro and in vivo.
The in vitro antioxidant effect of AEXS was evaluated using various known assays [41]. From our results, AEXS developed DPPH radical scavenging activity with an IC50 lower than that of the ascorbic acid. Indeed, in alcoholic solution, DPPH forms a stable free radical which can be stabilize by antioxidant chemicals through hydrogen releasing or electrons transferring [42]. The phytochemical analysis revealed an interesting concentration of phenols in the extract, which are rich in hydroxyl groups [43]. Thus, antioxidant molecules such as phenols scavenge DPPH radicals by donating hydrogen or electrons [44]. FRAP test was performed and the extract reveals an activity. Indeed, reducing agents such as OH groups enriched phenols present in extract reduce ferric iron (Fe3+) into ferrous iron (Fe2+) by donating electrons [45]. Moreover, the ABTS+ radical scavenging capacity observed with the AEXS was significant. DPPH and ABTS are two free radicals used to bring evidence of the antiradical potential of substances. However, the ABTS assay strictly relies on hydrogen atom transfer [46]. AEXS has developed lower antiradical activity than the standard (trolox) by reducing ABTS+ cation radical through donating protons [47]. Excessive generation of NO is related to a number of pathological conditions, including intracellular oxidative damage and cell death [48,49]. The extract inhibited the nitrite formation by direct competition with oxygen in its reaction with NO, hence protecting cells and organelles from damages [50,51]. The hydroxyl radical was formed by the Fe3+-ascorbate-EDTA-H2O2 system. The scavenging activity on OH radical was measured through inhibition of deoxyribose degradation [20]. AEXS at all concentrations tested inhibited the degradation of deoxyribose. Thus, when the extract was added to the reaction mixture, it removed the hydroxyl radicals from the sugar to prevent reaction [50]. Overall, the antioxidant activity of the extract could be partially attributed to its phenols content. Nevertheless, the quantified value (26.48 ± 2.89 mg GAE/g) of phenols was considered not as much, thus explaining the lowest activity of extract compared with the reference compounds (ascorbic acid and trolox) tested. In addition, the antioxidant activity of phenolic compounds varies remarkably, depending on their chemical structure, such as the position occupied by the -OH group in the whole molecule [52]. Furthermore, some authors such as Salim et al. [53] and Lee et al. [54] have shown that the selection of the solvent for extraction is beneficial to have a maximum of secondary metabolites (like phenolic compounds). This could be observed for example by the strong antioxidant power (IC50 = 42,653 μg/mL) of the methanol-chloroform extract of Chenopodium murale as compared to that of ascorbic acid (IC50 = 55,004 μg/mL) during DPPH test [53]. Most of the identified compounds have only have 1 or 2 OH functional groups, which is not too strong for antioxidant. The plant also contains many other metabolites that have not been identified as contributing to the extract's biological activity. Phytochemical studies are underway to provide more information about the plant's metabolite content.
Antioxidant enzymes contribute as essential part of the cellular defense against reactive oxygen species (ROS) [55]. Cd administration was associated with a reduction of aortic, hepatic and renal catalase, renal glutathione, and renal NO compared to control. Cd has been shown to directly inhibit catalase activity via an interaction between cadmium and the enzyme, which leads to a disruption of the enzyme's topography, important for catalytic activity [56]. The treatment with different doses of AEXS had increased the liver and kidneys catalase. The GSH is a non-enzymatic antioxidant. The administration of Cd to rats reduces renal glutathione compared to controls. Cd could increase oxidative stress by damaging the antioxidant defense systems of cells by depleting GSH [57]. But, the administration of the extract at the dose of 300 mg/kg has increased the level of renal GSH. The extract might play an important role in the metabolism of GSH, thus increasing intracellular antioxidant power [58]. Another study demonstrated that cadmium-induced hypertension is linked with the decrease of NO [59]. This potent free radical can switch endothelial nitric oxide synthase (eNOS). The extract was not able to increase the level of renal NO. It may be possible that the extract does not significantly interfere on peroxynitrite formation. A direct link exists between the level of tissue damage and the quantity of MDA produced [60]. Hence, the amount of MDA can be used as an index of peroxidative damage in vivo and the evaluation of the sensibility of tissues to underlying oxidative stress. The elevated level of MDA in the liver of hypertensive group compared to controls may be due to the increase of membrane lipid peroxidation. The reduction in MDA levels by AEXS treatment could be due to its ability to attenuate Cd-induced lipid peroxidation. Histological alterations observed in the aorta section showed the increase of media size of the hypertensive group compared to controls. For all groups tested, AEXS remedied all alterations, since no significant difference was observed between the cardiac architecture of normal control animals and that of all groups tested. The extract could therefore potentially contribute to restoring Cd-induced cardiac damage [61].
The step of identification of some phenols in AEXS was carried out using UPLC-UV-ESI-TOF-MS data compared to those of the isolates obtained during previous investigation of X. stuhlmannii leaf extract [15]. These metabolites mainly belong to the isoflavonoid and pterocarpan classes, which are classes of compounds which characterize Fabaceae family. Isoflavonoid belongs to the flavonoid family, which is a group of bioactive polyphenolic compounds abundant in dietary plants and herbs. The flavonoids proved to have cardiovascular (hypertension) benefits affecting blood pressure [62]. The isoflavonoid formononetin attenuates oxidative stress [63] and hypertension. The antihypertensive effect of this compound may cause vasodilatation, probably due to the inhibition of voltage-dependent Ca2+ channels and intracellular Ca2+ release and by release of NO [64]. Additionally, some studies revealed the strongest antioxidant potential of compounds belonging to the pterocarpan class [65].
5. Conclusion
We can conclude that the aqueous leaf extract of AEXS exhibited scavenging activities on DPPH, ABTS+, OH− and NO radicals, and expressed interesting ferric reducing power. The extract also enhanced significant decreased of systolic and diastolic blood pressures as well as heart rate, which is correlated with a reduction in serum transaminases activities, urea concentrations, and urinary debit while increasing the glomerular filtration rate at all the doses tested. Therefore, AEXS leaf extract has in vitro antioxidant and antihypertensive effects that may support its use against hypertension. Secondary metabolites such as stuhlmannione A (1), formononetin (2), stuhlmarotenoid A (3), 9-methoxymaackiain (4), 4-hydroxymaackiain (5) and 7-hydroxy-3′,4′-methylenedioxy-isoflavone (6), were identified in AEXS. They belong to isoflavonoid and pterocarpan classes, which are known to possess antioxidant and antihypertensive effects. We intend in further studied to isolate the identified active compounds and elucidate the mechanism of activities underlining the observed antioxidant and antihypertensive effects of AEXS which were reported in the current study.
Ethics statement
The Institutional Ethics Committee for Research on Human Health (CEI-UD) of the University of Douala, Cameroon approved (Ref No 3082 CEI-UDo/05/2022/T) all experimental protocols. Procedures and animals handling were conducted according to animal welfare guidelines of the NIH publications No 8083, revised 1978.
Data availability
Data will be made available on request.
CRediT authorship contribution statement
Augustine Nkojap Kuinze: Methodology, Investigation, Conceptualization. Edwige Laure Nguemfo: Conceptualization. William Nana Yousseu: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Jacquy Joyce Wanche Kojom: Methodology, Conceptualization. Calvin Zangueu Bogning: Methodology, Conceptualization. Christelle Stéphanie Sonfack: Methodology. Willifred Tsopgni Dongmo Tekapi: Writing – original draft, Formal analysis. Timo D. Stark: Writing – review & editing, Writing – original draft, Funding acquisition. Guy Blaise Anatole Azebaze: Methodology, Formal analysis. Alain Bertrand Dongmo: Writing – review & editing, Writing – original draft, Supervision, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:DONGMO Alain Bertrand reports equipment, drugs, or supplies was provided by Alexander von Humboldt Foundation. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Alexander von Humboldt Foundation Germany, through a donation of research equipment
Abbreviations
- AEXS
Aqueous extract of Xeroderris stuhlmannii
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- FRAP
Ferric reducing antioxidant power
- ABTS
2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid
- NO
Nitric oxide
- IC50
Median inhibitory concentration
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- HR
Heart rate
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- GFR
Glomerular filtration rate
- SOD
Superoxide dismutase
- GSH
Reduced glutathione
- MDA
Malondialdehyde
- MT
Metallothionein
References
- 1.WHO Hypertension. 2023 https://www.who.int/news-room/fact-sheets/detail/hypertension [Google Scholar]
- 2.Andujar P., Bensefa-colas L., Descatha A. Acute and chronic cadmium poisoning, La Revue de Médecine Interne. Elsevier. 2010;31(2):107–115. doi: 10.1016/j.revmed.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Satarug S., Nishijo M., Ujjin P., Vanavanitkun Y., Moore M.R. Cadmium-induced nephopathy in the development of high blood pressure. Toxicol. Lett. 2005;157(1):57–68. doi: 10.1016/j.toxlet.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W., Fievez L., Cheu E., Bureau F., Rong W., Zhang F., Zhang Y., Advenier C., Gustin P. Anti-inflammatory effects of formoterol and ipratropium bromide against acute cadmium-induced pulmonary inflammation in rats. Eur. J. Pharmacol. 2010;628(1–3):171–178. doi: 10.1016/j.ejphar.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Menke A., Muntner P., Silbergeld E.K., Platz E.A., Gualler E. Cadmium levels in urine and mortality among U.S. adults. Environ. Health Perspect. 2009;117(2):190–196. doi: 10.1289/ehp.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afridi H.I., Kazi T.G., Kazi N.G., Jamali M.K., Arain M.B., Sirajuddin, Baig J.A., Kandhro G.A., Wadhwa S.K., Shah A.Q. Evaluation of cadmium, lead, nickel and zinc status in biological samples of smokers and nonsmokers' hypertensive patients. J. Hum. Hypertens. 2010;24(1):34–43. doi: 10.1038/jhh.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Fang J., Leonard S.S., Rao K.M. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 2004;36(11):1434–1443. doi: 10.1016/j.freeradbiomed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Branca J.J.V., Fiorillo C., Carrino D., Paternostro F., Taddei N., Gulisano M., Pacini A., Becatti M. Cadmium-induced oxidative stress: focus on the central nervous system. Antioxidants. 2020;9(6):492. doi: 10.3390/antiox9060492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaziri N.D. Causal link between oxidative stress, inflammation, and hypertension, Iran J. Kidney Dis. 2008;2(1):1–10. [PubMed] [Google Scholar]
- 10.Lee H.G., Kim H.S., Oh J.Y., Lee D.S., Yang H.W., Kang M.C., Kim E.A., Kang N., Kim J., Heo S.J., Jeon Y.J. Potential antioxidant properties of enzymatic hydrolysates from Stichopus japonicas against hydrogen peroxide–induced oxidative stress. Antioxidants. 2021;10(1):110. doi: 10.3390/antiox10010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeed A., Rehman S., Akram M., Bhatti M.Z., Naz R., Latif A., Ali A., Ahmad A., Saeed A. Evaluation of antioxidant effects and inhibitory activity of medicinal plants against lipid peroxidation induced by iron and sodium nitroprusside in the mouse brain. J. Chem. Soc. Pakistan. 2016;38(2):333–340. [Google Scholar]
- 12.De Britto A.J., Gracelin D.H.S., Sebastian S.R., SR. Antibacterial activity of a few medicinal plants against Xanthomonas campestris and Aeromonas hydrophila. J. Biopestic. 2011;4(1):57. [Google Scholar]
- 13.Agban A., Gbogbo K.A., Hoekou Y., Atchou K., Tchacondo T., Komlan B., Costa de Souza C., Gbeassor M. Evaluation de l’activité antifongique des extraits de Cassia alata L. et de Piliostigma thonningii (Schumach.) MilneRedh. (Fabaceae) sur Candida albicans. Int. J. Biol. chem.Sci. 2013;7(3):1041–1104. doi: 10.4314/ijbcs.v7i3.12. [DOI] [Google Scholar]
- 14.Arbonnier Arbres M. UICN; Montpellier: 2000. arbustes et lianes des zones sèches d'Afrique de l'Ouest, CIRAD, MNHN; p. 541p. [Google Scholar]
- 15.Mekuete L.B.K., Tsopgni W.D.T., Nkojap A.K., Kojom J.J.W., Stark T.D., Fouokeng Y., Dongmo A.B., Azeufack L.T., Azebaze A.G.B. Rotenoids and isoflavones from Xeroderris stuhlmannii (taub.) mendonça & E.P. Souza and their biological activities. Molecules. 2023;28(2846):1–15. doi: 10.3390/molecules28062846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16(3):144–158. [Google Scholar]
- 17.Ahmed D., Zara S., Baig H. In vitro analysis of antioxidant activities of Oxalis corniculata Linn. fractions in various solvents, Afr. J. Tradit. Complement. Altern. Med. 2012;10(1):158–165. [PMC free article] [PubMed] [Google Scholar]
- 18.Brand-Williams W., Cuvelier M.E., Berset C. Use of free radical method to evaluate antioxidant activity. Food Science and Technology. 1995;28(1):25–30. [Google Scholar]
- 19.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 20.Kunchandy E., Rao M.N.A. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990;58:37–240. [Google Scholar]
- 21.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma as a measure of “antioxidant power” the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 22.Marcocci L., Maguire J.J., Droy-Lefaix M.T., Packer L. The nitric oxide scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994;201(2):748–755. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- 23.Ashokkumar D., Thamilselvan V., Senthikumar G.P., Mazumder U.K., Gupta M. Antioxidant and free radical scavenging effects of Lippia nodiflora. Pharm. Biol. 2008;46:762–771. [Google Scholar]
- 24.Kojom J.J.W., Nguemfo E.L., Djouatsa N.Y.L., Bogning Z.C., Azebaze A.G.B., J Llorent-Martínez E., Fernández-de Córdova M.L., Dongmo A.B. Phytochemical, antihypertensive and nephroprotective study of aqueous extract of the stems and roots of Selaginella vogelii Spring (Selaginellaceae) in rat. S. Afr. J. Bot. 2019;127:256–264. [Google Scholar]
- 25.Veljkovic A.R., Nikolic R.S., Kocic G.M., Pavlovic D.D., Cvetkovic T.P., Sokolovic D.T., Jevtovic T.M., Basic J.T., Laketic D.M., Marinkovic M.R., Stojanovic S.R., Djordjevic B.S., Krsmanovic M.M. Protective effects of glutathione and lipoic acid against cadmium induced oxidative stress in rat's kidney. Ren. Fail. 2012;34(10):1281–1287. doi: 10.3109/0886022X.2012.723661. [DOI] [PubMed] [Google Scholar]
- 26.Mollaoglu H., Gokcimen A., Ozguner F. Caffeic acid phenethyl ester prevents cadmium-induced cardiac impairment in rat. Toxicology. 2006;227(1–2):15–20. doi: 10.1016/j.tox.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed M.A. Effect of angiotensin II type 1 receptor blocker on renal function, arterial blood pressure and parathyroid hormone related protein over expression in cadmium induced nephrotoxicity in adult male rats. Int. J. Physiol. Pathophysiol. Pharmacol. 2013;5(2):109–119. [PMC free article] [PubMed] [Google Scholar]
- 28.Bernhoft R.A. Cadmium toxicity and treatment. Sci. World J. 2013 doi: 10.1155/2013/394652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan S.A., Choudhary R., Singh A., Bodakhe S.H. Hypertension potentiates cataractogenesis in rat eye through modulation of oxidative stress and electrolyte homeostasis. J. curr. Ophthalmol. 2016;28(3):123–130. doi: 10.1016/j.joco.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al Hashem F., Dallak M., Bashir N., Abbas M., Khalil M., Alkhateeb M.A. Camel's milk protects against cadmium chloride induced toxicity in white albino rats. Am. J. Pharmacol. Toxicol. 2009;4(3):107–117. doi: 10.3844/ajptsp.2009.107.117. [DOI] [Google Scholar]
- 31.Kalyani B.H., Ginpreet K., Aadesh K., Saloni D. The effect of Murraya koenigii extract on therapeutic efficacy of Amlodipine in rats: possible drug-herb interaction, Gen. Med. 2015;3(1) [Google Scholar]
- 32.Yeh T.K., Wu J.P., Chang L.W., Tsai M.H., Chang W.H., Tsai H.T., Yang C.S., Lin P. Comparative tissue distributions of cadmium chloride and cadmium-based quantum dot 705 in mice: safety implications and applications. Nanotoxicology. 2011;5(1):91–97. doi: 10.3109/17435390.2010.502260. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y., Liu J., Habeebu S.M., Waalkes M.P., Klaassen C.D. Metallothionein-I/II null mice are sensitive to chronic oral cadmium-induced nephrotoxicity. Toxicol. Sci. 2000;57(1):167–176. doi: 10.1093/toxsci/57.1.167. [DOI] [PubMed] [Google Scholar]
- 34.Renugadevi D.J., Milton Pradu S. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010;62:171–181. doi: 10.1016/j.etp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Amamou F., Nemmiche S., Meziane R.K., Didi A., Yazit S.M., Chabane-Sari D. Protective effect of olive oil and colocynth oil against cadmium-induced oxidative stress in the liver of wistar rats. Food Chem. Toxicol. 2015;78:177–184. doi: 10.1016/j.fct.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Rikans L.E., Yamano T. Mechanisms of cadmium mediated-acute hepatotoxicity. J. Biochem. Mol. Toxicol. 2000;14:110–117. doi: 10.1002/(sici)1099-0461(2000)14:2<110::aid-jbt7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 37.Oyinloye B.E., Adenowo A.F., Osunsanmi F.O., Ogunyinka B.I., Nwozo S.O., Kappo A.P. vol. 5. Springer Plus; 2016. p. 641. (Aqueous Extract of Monodora Myristica Ameliorates Cadmium-Induced Hepatotoxicity in Male Rats). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friberg L., Piscator M., Nordberg G., Kjellstrom T. second ed. CRC Press; Cleveland, OH: 1974. Cadmium in the Environment. [Google Scholar]
- 39.El Heni J., Messaoudi I., Hamouda F., Kerkeni A. Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver and kidney of the rat: histology and Cd accumulation. Food Chem. Toxicol. 2008;46:3522–3527. doi: 10.1016/j.fct.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 40.Liu J., Qu W., Kadiiska M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009;238(3):209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evenamede K.S., Kpegba K., Simalou O., Boyode P., Agbonon A., Gbeassor M. Etude comparative des activités antioxydantes d’extraits éthanoliques de feuilles, d’écorces et de racines de Cassia sieberiana. Int. J. Biol. Chem. 2017;11(6):2924–2935. [Google Scholar]
- 42.Zhang J., Ding Y., Dong H., Hou H., Zhang X. Distribution of phenolic acids and antioxidant activities of different bran fractions from three pigmented wheat varieties. J. Chem. 2018;9 Article ID 6459243. [Google Scholar]
- 43.Shipeng Y., Woo H.C., Choi J.H., Park Y.B., Chun B.S. Measurement of antioxidant activities and phenolic and flavonoid contents of brown seaweed Sargassum horneri: comparison of supercritical CO2 and various solvent extractions. J. Fish. Aqut. Sci. 2015;18:123–130. [Google Scholar]
- 44.Chanda S., Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: an overview. Afr. J. Microbiol. Res. 2018;3(13):981–996. [Google Scholar]
- 45.Al-Farsi M., Alasalvar C., Morris A., Barron M., Shahidi F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005;53:7592–7599. doi: 10.1021/jf050579q. [DOI] [PubMed] [Google Scholar]
- 46.Zhao H., Dong J., Lu J., Chen J., Li Y., Shan L., Lin Y., Fan W., Gu G. Effects of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in Barley (Hoedeum vulgare L.) J. Agric. Food Chem. 2006;54(19):7277–7286. doi: 10.1021/jf061087w. [DOI] [PubMed] [Google Scholar]
- 47.Kabra A., Sharma R., Hano C., Kabra R., Martins N., Baghel U.S. Phytochemical composition, antioxidant, and antimicrobial attributes of different solvent extracts from Myrica esculenta Buch. Ham. ex. D. Don Leaves. Biomolecules. 2019;9:357. doi: 10.3390/biom9080357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wojtunik-kulesza K.A., Oniszczuk A., Oniszczuk T., Waksmundzka-hajnos M. The infuence of common free radicals and antioxidants on development of Alzheimer's disease. Biomed. Pharmacother. 2016;78:39–49. doi: 10.1016/j.biopha.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 49.Yermilov V., Rubio J., Becchi M., Friesen M.D., Pignatelli B., Ohshima H. Formation of 8-nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis. 1995;16:2045–2050. doi: 10.1093/carcin/16.9.2045. [DOI] [PubMed] [Google Scholar]
- 50.Hazra B., Biswas S., Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement. Altern. Med. 2008;8:63. doi: 10.1186/1472-6882-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrade A.W.L., Machado K.D.C., C Machado K.D., Figueiredo D.D.R., David J.M., Islam M.T., Uddin S.J., Shilpi J.A., Costa J.P. In vitro antioxidant properties of the biflavonoid agathisflavone. Chem. Central J. 2018;12(1):75. doi: 10.1186/s13065-018-0443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satue-Gracia M.T., Heinonen M., Frankel E.N. Antioxidant activity of anthocyanin in LDL and lecithin liposome systems. J. Agric. Food Chem. 1997;(45):3362–3367. [Google Scholar]
- 53.Salim M., Saeed A., iqbal M., Khan B.A., Khan N., Rabbani I., Alsenani F., Rasul A. Phytochemical screening and evaluation of antioxidant, total phenolic and flavonoid contents in various weed plants associated with wheat crops. Braz. J. Biol. 2022;82 doi: 10.1590/1519-6984.256486. [DOI] [PubMed] [Google Scholar]
- 54.Lee J.H., Kim H.J., Jee Y., Jeon Y.-J., Kim H.J. Antioxidant potential of Sargassum horneri extract against urban particulate matter-induced oxidation. Food Sci. Biotechnol. 2020;29(6):855–865. doi: 10.1007/s10068-019-00729-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ighodaro O.M., Akinloye O.A. First line defense antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defense grid, Alex. J. Med. 2018;54(4):287–293. [Google Scholar]
- 56.Obioha E.U., Suru M.S., Ola-Muclathir F.K., Faremi Y.T. Hepatoprotective potentials of onion and garlic extracts on cadmium-induced oxidative damage in rats. Biol. Trace Elem. Res. 2009;129:143–156. doi: 10.1007/s12011-008-8276-7. [DOI] [PubMed] [Google Scholar]
- 57.Gaubin Y., Vaissade F., Croute F., Beau B., Soleilhavoup J., Murat J. Implication of free radicals and glutathione in the mechanism of cadmium-induced expression of stress proteins in the A549 human lungcell-line. Biochim. Biophys. Acta. 2000;10(1495):4–13. doi: 10.1016/s0167-4889(99)00149-4. [DOI] [PubMed] [Google Scholar]
- 58.Morales A.I., Vicente-Sanchez C., Sandoval J.M., Egido J., Mayoral P., Arévalo M.A., Fernandez-Tagarro M., Lopez-Novoa J.M., Pérez-Barriocanal F. Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem. Toxicol. 2006;44(12):2092–2100. doi: 10.1016/j.fct.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 59.Beckman Js J.S., Koppenol W.H. Nitric oxide, superoxide and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271:1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 60.Ayala A., Munoz M.F., Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014;31 doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milton P.S., Muthumani M. Shagirtha, Quercetin potentially attenuates cadmium induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. Eur. Rev. Med. Pharmacol. 2013;17(5):582–595. [PubMed] [Google Scholar]
- 62.Cao Y., Xie L., Liu K., Liang Y., Dai X., Wang X., Lu J., Zhang X., Li X. The antihypertensive potential of flavonoids from Chinese herbal medicine: a review. Pharmacol. Res. 2021;174 doi: 10.1016/j.phrs.2021.105919. [DOI] [PubMed] [Google Scholar]
- 63.Yi L., Cui J., Wang W., Tang W., Teng F., Zhu X., Qin J., Wuniqiemu T., Sun J., Wei Y., Dong J. Formononetin attenuates airway inflammation and oxidative stress in Murine Allergic Asthma. Front. Pharmacol. 2020;4(11) doi: 10.3389/fphar.2020.533841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun T., Liu R., Cao Y.X. Vasorelaxant and antihypertensive effect of formononetin through endothelium dependent and independent mechanisms. Acta Pharmacol. Sin. 2011;32(8):1009–1018. doi: 10.1038/aps.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.E Ajaegbu E., Eboka C.J., Okoye F.B.C., Proksch P. Cytotoxic effect and antioxidant activity of Pterocarpans from Millettia aboensis root. Nat. Prod. Res. 2023;37(5):829–834. doi: 10.1080/14786419.2022.2089984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.