Abstract
Objectives
The incidence of hemidiaphragmatic paresis (HDP) in superior trunk block (STB) usually depends on the dose of local anesthetic. This study aimed to further evaluate the impact of a lower volume (10 mL) of the same low concentration (0.25%) ropivacaine compared to a conventional volume (15 mL), on diaphragmatic function and analgesic efficacy under a multimodal analgesia regimen for shoulder arthroscopy.
Methods
Patients scheduled to undergo shoulder arthroscopy were randomized allocated to receive either 10 mL or 15 mL of 0.25% ropivacaine in the STB under ultrasound guidance prior to general anesthesia. The primary outcome was the percentage reduction in diaphragm excursion (ΔDE) between baseline and 30 min after block. Secondary outcomes included DE and diaphragm thickening fraction (DTF) before and after block, incidence of HDP, onset of sensory/motor block, duration of analgesia/motor block, dermatomal coverage area of the block, postoperative pain severity, pre- and post-block respiratory function and intraoperative hemodynamic parameters, the use of other anesthetic and analgesic drugs, post-block complications, and adverse events post-surgery.
Results
Compared with 15 mL volume, 10 mL ropivacaine significantly reduced the incidence of post-block HDP (as measured by ΔDE: 39.47% vs. 64.10%; and by post-block DTF: 13.16% vs. 33.33%). There was no significant difference in onset of sensory block, duration of analgesia/motor block, dermatomal coverage area of the block, postoperative pain severity between the two groups, except that the onset of motor block was significantly slower in the 10 mL group than in the 15 mL group. Pre- and post-block respiratory function and intraoperative hemodynamic parameters, the use of other anesthetic and analgesic drugs, post-block complications, or postoperative adverse events were not significantly different between the two groups.
Conclusion
In shoulder arthroscopy, STB with 10 mL of ropivacaine can reduce the incidence of HDP with no significant difference in analgesic effects under a multimodal analgesia regimen compared with 15 mL.
Trial registration: We registered the study at chictr.org ( ChiCTR2200057543 , 14/03/2022. https://www.chictr.ogr.cn
Keywords: Superior trunk block, Hemidiaphragmatic paresis, Diaphragmatic motion, Ropivacaine
Background
Interscalene brachial plexus block (ISB) is an effective analgesic anesthesia method that significantly reduces opioid consumption and shortens hospital stays, playing a crucial role in multimodal analgesia regimens after shoulder surgery [1]. However, conventional ISB can result in almost 100% ipsilateral hemidiaphragmatic paresis (HDP) [2]. Even with the precise injection of small doses (5 mL) of low-concentration local anesthetics (0.125% bupivacaine) into the fascia outside the brachial plexus sheath under ultrasound guidance, the incidence of ipsilateral HDP still exceeds 20% [3–5]. As the diaphragm is the most important respiratory muscle in the human body, 75% of lung volume changes during quiet breathing are due to diaphragm movement, with the remaining 25% resulting from the activity of the intercostal muscles, sternocleidomastoid, and scalene muscles. Unilateral phrenic nerve block or injury leads to dysfunction in the contraction and relaxation of the diaphragm on that side, but it does not directly affect the diaphragmatic function on the contralateral side. When unilateral phrenic nerve block occurs, patients typically do not exhibit symptoms such as dyspnea because the intercostal muscles and other respiratory muscles maintain normal function, and the contralateral lung compensates with normal function [6]. Consequently, this complication is often overlooked in clinical practice. However, for patients with preexisting respiratory diseases or obesity, the risk of developing dyspnea increases [7–9], limiting the clinical application of ISB in patients with concomitant pulmonary disease or obesity.
Kessler et al. [10] demonstrated through cadaveric ultrasonography that the phrenic nerve and brachial plexus are within 2 mm of each other at the cricoid cartilage level, with additional 3 mm separation for every cm more caudal in the neck. Superior trunk block (STB) involves the injection of local anesthetic into the omohyoid muscle plane, very close to the level where the suprascapular nerve branches from the superior trunk. This technique can block the acromioclavicular joint, glenohumeral joint, supraspinatus, and infraspinatus muscles innervated by this nerve. Additionally, the local anesthetic can spread proximally to the superior trunk along other branches of the cervical (C)5 and C6 nerve roots, providing effective analgesia for shoulder surgery. It is important to note that the dose and concentration of the local anesthetic are key risk factors for complications associated with ultrasound-guided STB. Currently, the commonly used clinical doses of ropivacaine under ultrasound guidance vary from 5 to 20 mL [11, 12]. Studies have shown that the use of smaller doses of local anesthetics restricts drug diffusion, while precise positioning and injection under ultrasound guidance ensure adequate concentration around the target nerve for anesthesia and minimize complication occurrence. Therefore, choosing the appropriate dose and concentration is paramount to balancing patient safety with optimal therapeutic outcomes.
Previous research primarily focused on demonstrating the non-inferiority of STB in providing analgesia to ISB, often employing relatively larger concentrations and volumes of local anesthetic agents. Hartrick et al. [13] reported similar analgesic effects with 10 mL and 20 mL of 0.75% ropivacaine. Another study showed a lower incidence of HDP with the use of 10 mL of local anesthetic compared to the conventional volume of 20 mL during ISB, with similar analgesic effects [14]. A cadaveric study also indicated that injecting 5 mL of methylene blue at the level of the superior trunk did not stain the phrenic nerve [15]. These findings suggested that theoretically, smaller volumes of local anesthetics may reduce the risk of HDP. In this study, a lower concentration and dosage of local anesthetic (0.25% ropivacaine 10 mL) compared to previously mentioned studies, was chosen to investigate whether it could reduce the incidence of HDP while ensuring effective local anesthesia and achieving similar analgesic effects compared with 15 mL.
Research has demonstrated a significant correlation between ultrasonographically measured diaphragm thickness and thickness measured directly at autopsy [16]. Furthermore, ultrasonographic assessment of diaphragm excursion (DE) has shown a positive correlation with the level of pulmonary function [17]. These studies suggest the reliability of using ultrasound for assessing diaphragm muscle. DE, diaphragm thickness, and diaphragm thickening fraction (DTF) have been validated in multiple studies as predictive indicators of successful withdrawal in mechanically ventilated patients in the intensive care unit [18–20]. Additionally, diaphragm ultrasound offers advantages such as bedside operation, non-invasiveness, simplicity, and cost-effectiveness. Therefore, this trial assessed diaphragmatic function by measuring DE and DTF before and after the block, as well as HDP incidence.
Methods
Study participants
After obtaining approval from the Ethics Committee of Taizhou Hospital of Zhejiang Province (K20210405), this trial was prospectively registered on March 14, 2022, in the Chinese Clinical Trial Registry (ChiCTR2200057543). Written informed consent was obtained from all participants. We recruited 80 patients scheduled for elective right shoulder arthroscopic surgery at Taizhou Hospital, Zhejiang Province, between April and October 2022. Inclusion criteria were as follows: aged 18 to 75 years old, American Society of Anesthesiologists (ASA) physical status I to II, and body mass index (BMI) between 18 and 24 kg/m2. All surgeries were performed by a single surgeon. Patients meeting the criteria were identified from the surgeon's schedule and contacted a day before surgery to inform them about the research protocol. Patients who refused to participate in the study, had a history of alcohol, analgesic, or sedative abuse, prior allergy to local anesthetics, severe coagulation disorders, infection or ulceration at the puncture site, pre-existing brachial plexus injury, respiratory, hepatic, or renal dysfunction, inability to understand sedation scoring or visual analogue scale (VAS), and those incapable of using patient-controlled analgesia pumps were excluded.
Randomization and blinding
Participants were divided into two groups (n = 40) using a computer-generated randomized sequence (1:1 allocation ratio). The control group (Group C) received 15 mL of 0.25% ropivacaine injection solution (AstraZeneca AB, Sweden), while the experimental group (Group N) received 10 mL of the same solution. To reduce performance bias, the blocks were administered collaboratively by an experienced anesthesiologist and an anesthetic nurse. Upon entering the operating room, the anesthesiologist opened a sequentially numbered, sealed opaque envelope indicating the group assignment of 15 or 10 mL. Both patients and all other study personnel, including outcome assessors and nursing staff, remained blinded to the group assignments.
All individuals involved in data collection and processing underwent comprehensive training, focusing on data protection and confidentiality. Data security was ensured through encryption for both storage and transmission, with access to protected areas strictly limited to authorized personnel, monitored, and controlled by access permissions. A data and safety monitoring committee oversaw participant safety throughout the trial, and assessments were conducted by two independent assessors who were not involved in the trial’s implementation, management, or data generation of this study.
Ultrasound-guided STB
The patients were routinely instructed to fast before surgery. In the preoperative preparation room, intravenous access was established, and standard monitoring was conducted, including electrocardiography (ECG), heart rate (HR), pulse oximetry (SpO2), respiratory rate, left radial artery cannulation for mean arterial pressure (MAP) measurement, and blood gas analysis. No preoperative medications or oxygen supplementation were administered to any patients before or after STB.
Patients were positioned supine with their heads turned to the contralateral side, a small pillow placed under their shoulders. A high-frequency linear array probe (Edge II ultrasound device, Sonosite, USA) was positioned to scan at the level of the cricoid cartilage, revealing a series of continuous images in the interscalene groove between the anterior and middle scalene muscles. The C5 and C6 nerve roots were located at the most proximal end of the interscalene groove, just beneath the lateral edge of the sternocleidomastoid muscle, with the phrenic nerve lying on the surface of the anterior scalene muscle. Scanning distally with the ultrasound probe revealed the C5 and C6 nerve roots forming the superior trunk, which exhibited a clear hyperechoic boundary beneath the deep fascia. At this point, the suprascapular nerve was seen branching off from the superior trunk (Fig. 1).
Fig. 1.
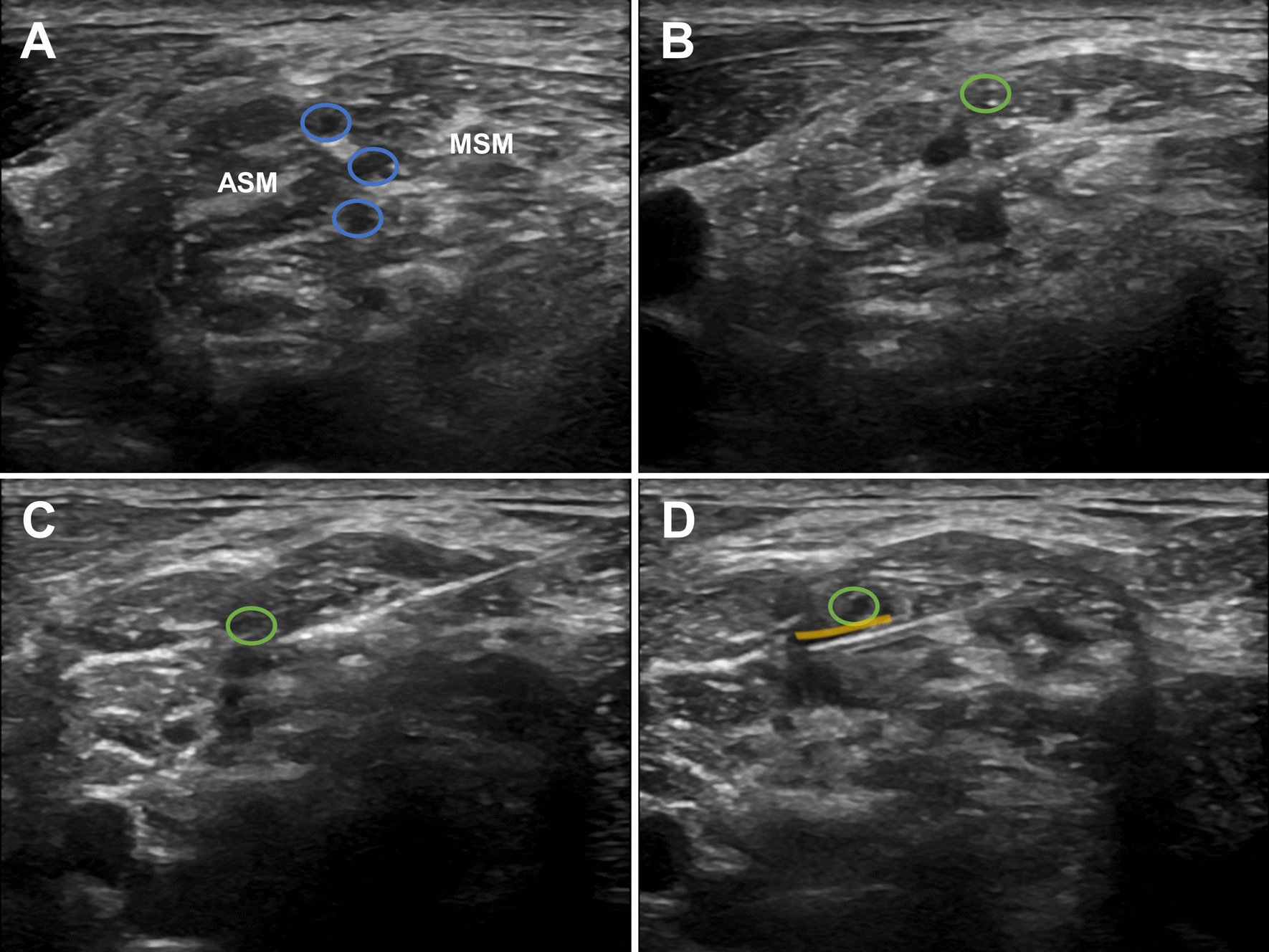
Ultrasound images of the superior trunk block. The green oval indicates the suprascapular nerve. A The blue oval marks the superior, middle, and inferior trunks. B The suprascapular nerve branches off from the superior trunk. C The needle tip is positioned posterior to the superior trunk. D The yellow highlight shows the diffusion of the injected local anesthetic. ASM, anterior scalene muscle; MSM, middle scalene muscle
After sterile skin preparation, the STB was performed under ultrasound guidance according to group allocation. A nerve block needle was advanced from posteriolateral to anteriomedial through the middle scalene muscle towards the lateral aspect of the superior trunk. Confirmation of proper positioning was performed by observing the spread of ropivacaine around the superior trunk. During the injection process, the needle tip was consistently directed towards the superior trunk of the brachial plexus, ensuring targeted spread of the local anesthetic around the superior trunk.
Assessment of diaphragmatic movement
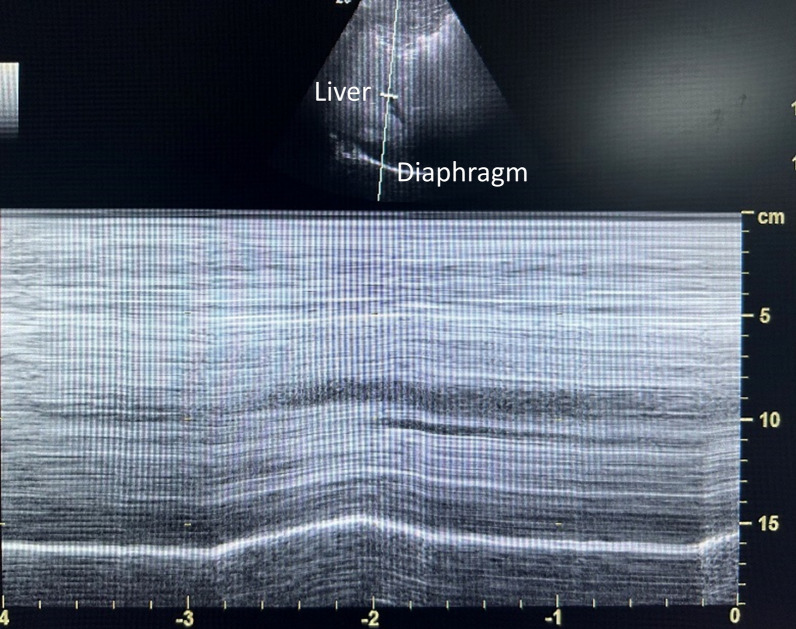
Before the block was initiated (T0) and 30 min after the block (T1), another proficient anesthesiologist, blinded to group allocation, used ultrasound imaging (Edge II, Sonosite, USA) to evaluate the right diaphragmatic excursion. DE was assessed using M-mode ultrasonography with the patient in a supine position. The liver was used as an observation window to record the motion of the right diaphragm. A low-frequency convex array probe (2 to 5 MHz) was placed along the right anterior axillary line and the midclavicular line, just below the right costal margin. The ultrasound probe was moved medially, cephalically, and posteriorly to visualize the posterior elevated part of the right diaphragm. The high-echo line attached to the liver is caused by the attachment of the peritoneum and pleura to the diaphragm. The inspiratory and expiratory movements of the diaphragm shorten and lengthen the distance between the probe and the diaphragm, respectively. After switching to M-mode, the selected line was placed vertically at the top of the elevated part, and the cranial-caudal displacement during inspiration and expiration appeared as a bright line (high echo) in the form of a “sine wave.” DE is the vertical distance from the baseline to the peak of the sine wave. DE between inspiration and expiration was measured in centimeters, and each test was conducted thrice, with the average was recorded. The severity of HDP was measured as the reduction in DE between baseline and 30 min after block (calculated as a percentage difference) (Fig. 2). No HDP indicated a reduction in DE of less than 25%; partial HDP indicated a reduction of 25% to 75%; complete HDP referred to a reduction of 75% to 100% in DE [14].
Fig. 2.
M-mode ultrasound image of the right diaphragm with the liver as an acoustic window
The diaphragmatic thickness was measured using the ABCD [21, 22] method. A high-frequency linear array probe (6-13 MHz) was placed in the 7th to 9th intercostal space along the anterior axillary line, perpendicular to the long axis of the rib. Patients were instructed to breathe while the ultrasound probe was moved downward to obtain corresponding sagittal images of the diaphragm. The diaphragm was shown on ultrasound images as a hypoechoic image between two hyperechoic lines (peritoneum and pleura), and the diaphragmatic thickness was measured as the distance between the two hyperechoic lines. To ensure consistency and accuracy, the angle between the ultrasound beam and the diaphragm was 90 degrees, with measurements taken at the same intercostal position by counting the ribs from top to bottom. Each test was performed three times and averaged. DTF was calculated as [End-inspiratory diaphragmatic thickness—End-expiratory diaphragmatic thickness] / End-expiratory diaphragmatic thickness × 100%. Studies by Sferrazza et al. [23] confirmed that a DTF < 20% indicates diaphragmatic functional paralysis in healthy adults.
Induction of anesthesia
All patients received total intravenous anesthesia while using a supraglottic airway device (i-gel) for airway management. Following positioning the patients in the lateral decubitus position, induction was performed using 1.5–2 mg/kg of propofol emulsion (Fresenius Kabi, Beijing, China), 0.3 μg/kg of sufentanil citrate (Eurocept BV, Norway), and 0.6 mg/kg of rocuronium bromide (Hameln Pharmaceuticals GmbH, Germany). Following induction, an i-gel supraglottic airway device was inserted, with size selection based on the patient's body weight (i-gel size 3 for 30–60 kg and size 4 for 50–90 kg). A suction catheter was routinely placed to aspirate gastric fluids and drain them. The anesthesia machine (GE Aespire, Oramed, USA) parameters were set as follows: Intermittent Positive Pressure Ventilation (IPPV) mode, tidal volume of 6–8 mL/kg, respiratory rate of 10–12 breaths per minute, and maintenance of end-tidal carbon dioxide partial pressure (PETCO2) at 30–40 mmHg.
Maintenance of anesthesia
Throughout the procedure, patients were maintained with an infusion of propofol emulsion at 3–6 mg/kg/h and remifentanil infusion (Yichang Humanwell Pharmaceutical, Hubei, China) at 0.2–0.5 μg/kg/min, with the state entropy (SE) maintained between 40 and 60. Rocuronium bromide was administered as needed based on the duration of the surgery. Twenty minutes before the end of the surgery, 50 mg of flurbiprofen ester was administered intravenously for analgesia, along with 5 mg of dexamethasone injection and 3 mg of granisetron (Fuan Pharmaceutical Group Ningbo Tianheng Pharmaceutical Co., Ltd, Zhejiang, China) to prevent postoperative nausea and vomiting.
Postoperative management
After the surgery, all intravenous anesthetics were discontinued, and patients were monitored to ensure they were fully awake, had no risk of vomiting, demonstrated a tidal volume ≥ 5 ml/kg, a respiratory rate ≤ 30 breaths/min, and could hold their head up for at least 5 s, indicating good ventilation. Once these criteria were met, the supraglottic airway device was removed in the operating room. After device removal, patients were transferred to the Post-Anesthesia Care Unit (PACU) for routine monitoring of SpO2, blood pressure, and HR. Pain control was targeted at a VAS score of ≤ 3; otherwise, PACU nurses administered rescue analgesia with intravenous sufentanil (5 μg) based on the anesthesiologist’s orders. Patients in both groups received a patient-controlled intravenous analgesia (PCIA) regimen postoperatively, using 0.2 mg/mL oxycodone hydrochloride injection (Beijing Mundipharma Pharmaceutical Co., Ltd, Beijing, China) diluted in normal saline, with parameters set for no background dose, a self-controlled dose of 1.6 mg (8 mL) per bolus, a lockout interval of 15 min, and a maximum dose of 6.4 mg per hour.
Outcome measurement
The primary outcome was the percentage reduction in diaphragm excursion (ΔDE) between baseline and 30 min after block, calculated as ΔDE (%) = (DET0-DET1)/DET0 × 100. Secondary outcomes included DE and DTF before and after block, incidence of HDP, onset of sensory/motor block, duration of analgesia/motor block, dermatomal coverage area of the block, postoperative pain severity, respiratory function and intraoperative hemodynamic parameters, the use of other anesthetic and analgesic drugs, post-block complications, and occurrence of adverse events post-operation.
Within 30 min after the block, patients underwent sensory and motor examinations to ensure the onset of the block. Sensory block onset was assessed based on the disappearance of pinprick sensation in the intended surgical area, with the following scoring criteria: 0 points for unchanged sensation with no difference compared to the contralateral limb; 1 point for decreased sensation with a noticeable difference compared to the contralateral limb; and 2 points for absence of pain. Motor block onset was assessed based on the inability to perform shoulder abduction and external rotation, with the following scoring criteria: 0 points for the ability to perform abduction and external rotation of both shoulders without significant difference; 1 point for limited abduction and external rotation on the blocked side, with inability to resist force; and 2 points for complete inability to perform voluntary abduction and external rotation of the blocked shoulder. In this study, a score of 2 was used as the criterion for determining the onset of sensory and motor block. The onset of the sensory/motor block referred to the time interval from completion of the block to the onset of sensory/motor block. The duration of the analgesic block was the time from the onset of sensory block to the reoccurrence of pain at the surgical site. The duration of the motor block was defined as the time from the onset of motor block to the restoration of shoulder abduction and external rotation ability.
Thirty minutes after STB completion, using the healthy contralateral side as a control, the dermatomal coverage area of the STB was assessed by testing for cutaneous loss of sensation using ice cubes from C3 to C8 regions. The ice cubes used were standardized to have a temperature below 0 °C and a volume of at least 8 cm3 to ensure consistent cold stimulation. C3 represented the peri-auricular area, C4 over the acromioclavicular joint, C5 the lateral (radial) side of the antecubital fossa, C6 the distal radial forearm, C7 the dorsum of hand between 2 and 3 fingers, and C8 the dorsum of hand between 4 and 5 fingers. The phrenic nerve originates from C3, C4, and C5, which are important for assessing potential effects on diaphragmatic movement. To assess analgesic effects between the two groups, VAS scores were recorded at extubation, 30 min, 6, 12, 24, 36, and 48 h post-operation, both at rest and with movement. The scale ranged from 0 to 10, with 0 considered 'no pain', 1–3 'mild pain', 4–6 'moderate pain', and 7–10 'severe pain' [24]. Measurements of SpO2, partial pressure of arterial oxygen (PaO2), and partial pressure of arterial carbon (PaCO2) were collected and recorded 30 min pre-block and post-block. HR and MAP were recorded before skin incision, at incision and at the end of skin suturing in both groups.
Total doses of sufentanil injection, propofol emulsion, and remifentanil injection during surgery and cases requiring sufentanil injection in the PACU post-operation were tracked. Additionally, doses of oxycodone hydrochloride injection and flurbiprofen ester injection were recorded upon PACU entry and at 0–6, 6–12, 12–18, 18–24, 24–36, and 36–48 h post-operation. For data collection occurring during late night hours, ward nurses completed it, and if the patient was in a good sleep state, indicating a VAS score ≤ 3. Both groups also recorded post-operative adverse events such as respiratory depression, sedation scores, post-operative nausea and vomiting, dizziness, and muscle weakness, with respiratory rates < 8 breaths per minute and SpO2 < 90% considered respiratory depression.
Sample size calculation
This study was a randomized controlled trial evaluating the impact of different local anesthetic volumes during STB on diaphragmatic motion, with ΔDE as the primary outcome. The sample size was determined using an a priori power analysis conducted with Microsoft Office Excel. Based on a pilot with a delta DE between groups of 6.21 and a standard deviation (SD) of 2.78, and under the conditions of α = 0.05 and 1-β = 0.9, the calculated minimum sample size per group is 33 subjects. Considering potential dropouts during the study, an additional 20% sample size is added, resulting in 40 subjects per group, totaling 80 recruited patients.
Statistical analysis
Data analysis was conducted using SPSS version 20 software (IBM Corp., Armonk, NY, USA). Following confirmation of normality using the Shapiro–Wilk test, normally distributed continuous variables were presented as mean ± SD, while non-normally distributed continuous variables were represented by median and interquartile range (IQR). Categorical variables were expressed as numbers and percentages. Continuous variables were assessed using t-tests for normally distributed data or Mann–Whitney tests for skewed distributions, while categorical variables were analyzed using chi-square or Fisher's exact test. Kaplan–Meier survival analysis and log-rank tests were employed to compare the onset time (sensory and motor) and duration of blockage (sensory vs. motor) between the two groups. All analyses were considered statistically significant at P < 0.05.
Results
Baseline measurements
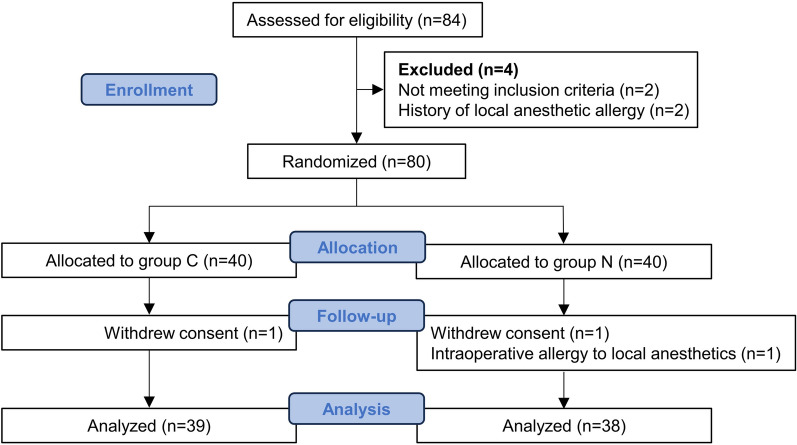
Figure 3 depicted the Consolidated Standards of Reporting Trials (CONSORT) flowchart illustrating patient enrollment and attrition. A total of 80 patients were enrolled, with 40 individuals in each group. Within the C and N groups, one participant each declined participation in the trial after providing informed consent. Additionally, one participant in the N group, without any prior history of local anesthetic allergies, experienced an allergic reaction (rash on chest and abdomen) to the local anesthetic used during the study. These three participants, who did not receive the intended intervention, were therefore excluded from further analysis. All other patients completed the primary outcome assessment. As shown in Table 1, there were no statistically significant differences between the two groups in terms of demographic characteristics (gender, age, BMI), ASA classification, surgical time (min), or surgical approach (P > 0.05).
Fig. 3.
Consolidated Standards of Reporting Trials (CONSORT) diagram of patient flow through the study
Table 1.
Patient characteristics and demographics
| Group C (n = 39) | Group N (n = 38) | P value | |
|---|---|---|---|
| Age (years); median (IQR) | 54 (49–61) | 53 (47–55) | 0.293 |
| Sex; n (%) | 0.707 | ||
| Male | 16 (41.03) | 14 (36.84) | |
| Female | 23 (58.97) | 24 (63.16) | |
| BMI (kg/m2); mean ± SD | 20.65 ± 3.18 | 20.42 ± 3.76 | 0.771 |
| ASA grade; n (%) | 0.430 | ||
| I | 15 (38.46) | 18 (47.37) | |
| II | 24 (61.54) | 20 (52.63) | |
| Surgery time (min); median (IQR) | 78 (69–90) | 78 (66–90) | 0.457 |
| Surgical approach; n (%) | 0.746 | ||
| Supraspinatus tendon repair | 25 (64.10) | 23 (60.53) | |
| Infraspinatus tendon repair | 14 (35.90) | 15 (39.47) |
ASA, American Society of Anesthesiologists; BMI, body mass index; IQR, interquartile range; SD, standard deviation
Diaphragmatic motor function
As shown in Table 2, there were no statistically significant differences in DE (during eupnea and forced respiration) between the two groups before the block (P > 0.05). After 30 min of block, DE was reduced in both groups during eupnea and forceful breathing, and DE T1 was higher in group N than in group C and was statistically different in the forceful breathing state (P < 0.05). Regardless of eupnea or forced respiration, the ΔDE before and after the block was significantly lower in group N than in group C. After 30 min of block, 15 cases (39.47%) in the N group experienced HDP, whereas 25 cases (64.10%) in the C group experienced HDP, demonstrating a statistically significant difference (P < 0.05).
Table 2.
Comparison of diaphragmatic motor function between the two groups
| Parameters | Group C (n = 39) | Group N (n = 38) | P value |
|---|---|---|---|
| Eupnea | |||
| DE T0 (cm); mean ± SD | 2.50 ± 0.22 | 2.43 ± 0.18 | 0.098 |
| DE T1 (cm); median (IQR) | 1.70 (1.40–2.01) | 1.83 (1.67–2.01) | 0.137 |
| ΔDE (%); median (IQR) | 31.84 (21.45–41.95) | 24.05 (18.09–30.69) | 0.026 |
| DTF T0 (%); mean ± SD | 59.18 ± 4.72 | 57.89 ± 4.26 | 0.214 |
| DTF T1 (%); median (IQR) | 36 (19–42) | 43 (35–46) | 0.015 |
| Forced respiration | |||
| DE T0 (cm); mean ± SD | 4.87 ± 0.41 | 4.81 ± 0.39 | 0.479 |
| DE T1 (cm); median (IQR) | 3.40 (2.69–3.82) | 3.60 (3.17–3.92) | 0.030 |
| ΔDE (%); median (IQR) | 31.07 (22.90–42.99) | 23.02 (16.94–29.78) | 0.003 |
| DTF T0 (%); median (IQR) | 57 (55–63) | 57 (53–61) | 0.363 |
| DTF T1 (%); median (IQR) | 31 (19–39) | 35 (28–44) | 0.041 |
| HDP [ΔDE ≥ 25%]; n (%) | 25 (64.10) | 15 (39.47) | 0.031 |
| HDP degree; n (%) | 0.031 | ||
| Absent | 14 (35.90) | 23 (60.53) | |
| Partial | 22 (56.41) | 15 (39.47) | |
| Complete | 3 (7.69) | 0 (0.00) | |
| HDP [DTF < 20%]; n (%) | 13 (33.33) | 5 (13.16) | 0.036 |
DE, diaphragm excursion; DTF, diaphragm thickening fraction; HDP, hemidiaphragmatic paresis; IQR, interquartile range; SD, standard deviation
There were no statistically significant differences in DTF (during eupnea and forced respiration) between the two groups before the block (P > 0.05). After 30 min of block, DTF decreased during both eupnea and forced respiration in both groups. The DTF in the N group was significantly higher than in the C group (P < 0.05). Using DTF as an indicator for determining the occurrence of HDP, after 30 min of block, 5 cases (13.16%) in the N group and 13 cases (33.33%) in the C group experienced HDP, showing a statistically significant difference (P < 0.05).
Nerve block and analgesic effect
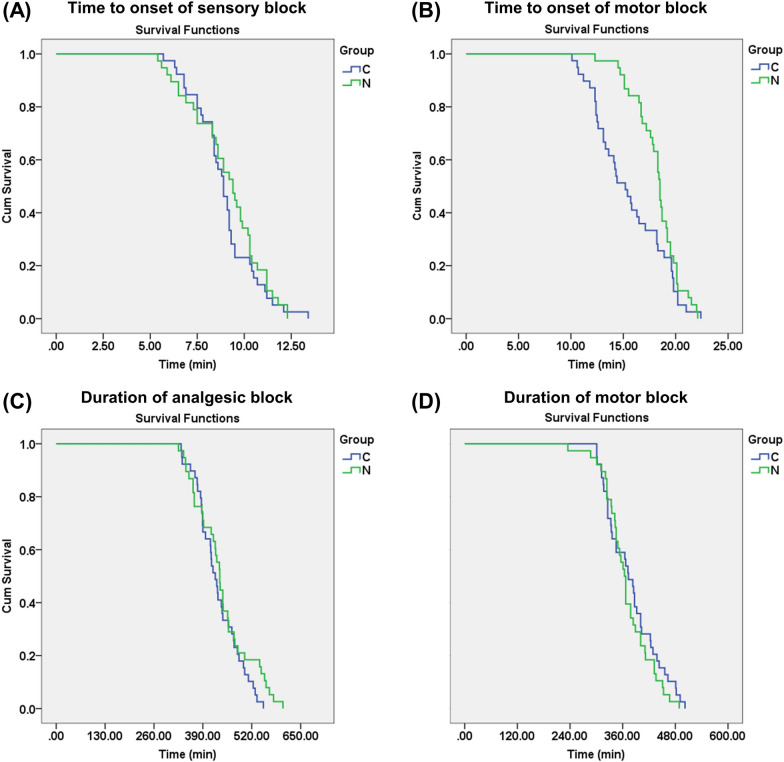
As shown in Fig. 4, there were no statistically significant differences between the two groups in terms of the onset and duration of sensory block, as well as the duration of motor block (P > 0.05). However, the onset time of motor block in the N group was significantly longer than in the C group (P = 0.033). The dermatomal coverage area of the STB in both groups was concentrated within the nerve innervation areas of C5 and C6, extending upwards to C4 and downwards to C7. There was no diffusion observed to C3 and C8 after the block. The dermatomal coverage area of the STB showed no statistically significant differences between the two groups (Table 3). As presented in Table 4, there were no significant differences in the severity of pain between the two groups at various time points such as 6, 12, 24, 36, and 48 h post-operation, whether in a resting or active state (P > 0.05). Although the pain severity in group C was significantly lower than that in group N at rest 30 min after extubation, overall, it remained below a mild level.
Fig. 4.
Comparison of time characteristics of nerve block effects between the two groups
Table 3.
Comparison of dermatomal coverage area of the STB between the two groups, reported as n (%)
| Dermatome | Group C (n = 39) | Group N (n = 38) | P value |
|---|---|---|---|
| C3 | 0 (0.00) | 0 (0.00) | / |
| C4 | 13 (33.33) | 6 (15.79) | 0.074 |
| C5 | 39 (100.00) | 38 (100.00) | > 0.999 |
| C6 | 39 (100.00) | 38 (100.00) | > 0.999 |
| C7 | 7 (17.95) | 4 (10.53) | 0.352 |
| C8 | 0 (0.00) | 0 (0.00) | / |
C, cervical
Table 4.
Comparison of pain severity (no/mild/moderate/severe; n) between the two groups at different time points after extubation in resting and motion state
| Time (h) | Group C (n = 39) | Group N (n = 38) | P value |
|---|---|---|---|
| Resting state | |||
| 30 min | 15/24/0/0 | 5/32/1/0 | 0.018 |
| 6 h | 0/39/0/0 | 0/38/0/0 | / |
| 12 h | 0/30/8/1 | 0/33/5/0 | 0.458 |
| 24 h | 1/38/0/0 | 0/38/0/0 | 0.506 |
| 36 h | 0/39/0/0 | 0/38/0/0 | / |
| 48 h | 6/33/0/0 | 5/33/0/0 | 0.780 |
| Motion state | |||
| 30 min | 25/14/0/0 | 27/11/0/0 | 0.515 |
| 6 h | 8/31/0/0 | 8/30/0/0 | 0.953 |
| 12 h | 0/38/0/1 | 0/37/1/0 | > 0.999 |
| 24 h | 0/39/0/0 | 0/38/0/0 | / |
| 36 h | 3/36/0/0 | 2/36/0/0 | > 0.999 |
| 48 h | 2/37/0/0 | 5/33/0/0 | 0.407 |
Respiratory function, intraoperative hemodynamic parameters, and the use of other anesthetic and analgesic drugs
There were no significant differences between the groups pertaining to respiratory function, intraoperative hemodynamic parameters, intraoperative drug use, and postoperative analgesic use (P > 0.05, Tables 5, 6, 7).
Table 5.
Comparison of respiratory function before and after block in the two groups
| Parameters | Group C (n = 39) | Group N (n = 38) | P value |
|---|---|---|---|
| T0 | |||
| SpO2 (%); median (IQR) | 99 (99–100) | 99 (99–100) | 0.367 |
| PaCO2 (mmHg); median (IQR) | 46 (45–47) | 46 (44–47) | 0.262 |
| PaO2 (mmHg); mean ± SD | 84.49 ± 3.34 | 84.61 ± 2.42 | 0.860 |
| T1 | |||
| SpO2 (%); median (IQR) | 99 (98–99) | 98 (98–99) | 0.465 |
| PaCO2 (mmHg); median (IQR) | 47 (46–48) | 46 (45–47) | 0.079 |
| PaO2 (mmHg); mean ± SD | 83.51 ± 2.53 | 84.24 ± 2.15 | 0.181 |
IQR, interquartile range; PaCO2, partial pressure of arterial carbon; PaO2, partial pressure of arterial oxygen; SD, standard deviation; SpO2, peripheral oxygen saturation
Table 6.
Comparison of intraoperative hemodynamic parameters between the two groups
| Parameters | Group C (n = 39) | Group N (n = 38) | P value |
|---|---|---|---|
| HR (beat/min) | |||
| Before skin incision; mean ± SD | 80.54 ± 7.19 | 76.68 ± 7.11 | 0.620 |
| Immediately after skin incision; mean ± SD | 64.13 ± 5.11 | 64.69 ± 5.24 | 0.557 |
| At the end of skin closure; median (IQR) | 67 (65–70) | 69 (65–72) | 0.410 |
| MAP (mmHg) | |||
| Before skin incision; mean ± SD | 83.54 ± 4.63 | 83.03 ± 5.60 | 0.658 |
| Immediately after skin incision; mean ± SD | 76.05 ± 5.20 | 75.72 ± 4.80 | 0.872 |
| At the end of skin closure; mean ± SD | 76.97 ± 5.51 | 77.81 ± 4.19 | 0.451 |
HR, heart rate; IQR, interquartile range; MAP, mean arterial pressure; SD, standard deviation
Table 7.
Comparison of the use of other anesthetic and analgesic drugs between the two groups
| Drugs | Group C (n = 39) | Group N (n = 38) | P value |
|---|---|---|---|
| Sufentanil (μg); median (IQR) | 15 (15–20) | 15 (15–20) | 0.081 |
| Propofol (mg); mean ± SD | 490.2 ± 140.79 | 466.34 ± 113.85 | 0.416 |
| Remifentanil (mg); mean ± SD | 0.94 ± 0.24 | 0.93 ± 0.23 | 0.895 |
| Sufentanil in the PACU; n (%) | 0 (0) | 1 (2.63) | 0.494 |
| Oxycodone hydrochloride (mg); median (IQR) | |||
| In the PACU | 1.6 (1.6–1.6) | 1.6 (1.6–1.6) | > 0.999 |
| 0 ~ 6 h after extubation | 1.6 (1.6–1.6) | 1.6 (1.6–1.6) | 0.855 |
| 6 ~ 12 h after extubation | 8.0 (6.4–9.6) | 6.4 (4.8–8) | 0.087 |
| 12 ~ 18 h after extubation | 4.8 (3.2–7.2) | 4.8 (1.6–6.4) | 0.330 |
| 18 ~ 24 h after extubation | 1.6 (1.6–1.6) | 1.6 (1.6–1.6) | 0.816 |
| 24 ~ 36 h after extubation | 1.6 (0–3.2) | 1.6 (0–1.6) | 0.073 |
| 36 ~ 48 h after extubation | 0 (0–0) | 0 (0–0) | > 0.999 |
| Flurbiprofenate ester; n (%) | |||
| 0 ~ 6 h after extubation | 3 (7.69) | 2 (5.26) | > 0.999 |
| 6 ~ 12 h after extubation | 6 (15.38) | 4 (10.53) | 0.768 |
| 12 ~ 18 h after extubation | 4 (10.26) | 3 (7.89) | > 0.999 |
| 18 ~ 24 h after extubation | 5 (12.82) | 7 (18.42) | 0.498 |
| 24 ~ 36 h after extubation | 4 (10.26) | 9 (23.68) | 0.116 |
| 36 ~ 48 h after extubation | 3 (7.69) | 5 (13.16) | 0.680 |
IQR, interquartile range; PACU, Post-Anesthesia Care Unit; SD, standard deviation
Post-block complications and postoperative adverse events
After 30 min of block, neither group exhibited complications such as Horner's syndrome, local anesthetic toxicity, or pneumothorax. Each group had one patient experiencing hoarseness, with no statistically significant difference observed between them (P > 0.05). As presented in Table 8, there were no statistically significant differences between the two groups in the occurrence of adverse events such as respiratory depression, sedation scores, nausea/vomiting, dizziness, or muscle weakness (P > 0.05).
Table 8.
Comparison of postoperative adverse events between the two groups, reported as n (%)
| Adverse events | Group C (n = 39) | Group N (n = 38) | P value |
|---|---|---|---|
| Respiratory depression | 0 (0.0) | 0 (0.0) | / |
| Sedation score | > 0.999 | ||
| 0 | 35 (89.74) | 35 (92.11) | |
| 1 | 3 (7.69) | 2 (5.26) | |
| 2 | 1 (2.56) | 1 (2.63) | |
| 3 | 0 (0.0) | 0 (0.0) | |
| Nausea/vomiting | 6 (15.38) | 4 (10.5) | 0.768 |
| Dizziness | 4 (10.26) | 0 (0.0) | 0.130 |
| Muscle weakness | 0 (0.0) | 0 (0.0) | / |
Discussion
Since the 1970s, the conventional ultrasound-guided ISB targeting the C5 and C6 nerve roots has been efficient in providing analgesia for shoulder arthroscopy [25]. However, it often poses the risk of HDP [26, 27]. In recent years, an improved technique, STB, has garnered attention as an alternative to traditional ISB. This technique, first reported by Burckett-st et al. [28], has shown the capability to reduce phrenic nerve block. Campoy et al. [15] preliminarily validated the anatomical feasibility of the technique of applying less local anesthetic with more limited diffusion, combined with ultrasound-guided precise localization and injection to ensure drug infiltration around the target nerve. However, there exist differences between cadaveric and clinical studies, such as variations in local anesthetic and dye volumes and differing diffusion rates. Dye may not entirely reflect the infiltration and diffusion of local anesthetics [29]. To address this, this randomized controlled trial evaluated the effect of 10 mL versus 15 mL of 0.25% ropivacaine in ultrasound-guided STB. Our research findings indicated that, under a multimodal analgesia regimen with no significant differences in other anesthetic and analgesic drugs, administering 10 mL of 0.25% ropivacaine solution provides comparable anesthesia and analgesia effects to 15 mL while having less impact on diaphragmatic function and significantly reducing the incidence of HDP.
In this work, compared to Group C, Group N exhibited higher DE during forced respiration after 30 min of block, lower ΔDE during eupnea and forced respiration, and a significantly reduced incidence of HDP (64.10% vs. 39.47%). Furthermore, both calm and forced respiration DTF significantly decreased after 30 min of block. These research findings align with those of Kim et al. [30]. However, some studies indicated that even with lower doses of local anesthetics in ISB, the incidence of HDP remains relatively high [11, 31, 32]. The differing impact of local anesthetic doses between ISB and STB on HDP occurrence may relate to injection techniques, speed, and site of administration. Kessler et al. [10] demonstrated that as the level moves downward from the cricoid cartilage, the distance between the phrenic nerve and the brachial plexus increases. Therefore, administering medication posterior-inferior to the superior trunk of brachial plexus nerves increases the distance from the phrenic nerve, thus reducing the incidence of HDP. Similarly, the incidence of HDP when injecting the same volume of local anesthetic was 8% for supraclavicular brachial plexus block injected from the backward outside, whereas the incidence of HDP when injected at the level of the ISB was 66.7% [33]. Further research is required to understand the impact of different injection positions in STB on the incidence of HDP. Despite some studies showing that ultrasound-assessed DE, diaphragm thickness, and DTF are predictive indicators of successful withdrawal in mechanically ventilated patients [18, 34], there remains ongoing debate about the pros and cons of these metrics. A meta-analysis demonstrated that while DTF and DE have similar sensitivities in assessing diaphragm function, DTF exhibits greater specificity [35]. DE primarily correlates with inspiratory capacity and is susceptible to the influence of forceful inhalation, while DTF primarily reflects the contractile capacity of the diaphragm itself, potentially explaining its lower misdiagnosis rate in assessing diaphragm function [35]. Interesting, the incidence of HDP determined by DE was higher than that determined by DTF in this study. However, after 30 min of block, there were 13 cases in Group C where the block had spread to C4, while in Group N, there were 6 cases. These observations align more closely with the incidence of HDP determined by DTF. Further analysis of the ROC curve assessing HDP occurrence based on DE and DTF might provide additional clinical significance.
This study demonstrated that low-volume local anesthetics, compared with 15 mL, exhibit similar efficacy in terms of sensory block onset time, duration of sensory and motor block, intraoperative hemodynamic parameters, opioid consumption, analgesic effects, and postoperative adverse events, except for a delayed onset of motor block. It is worth noting that with multimodal analgesia, the vast majority of patients in both groups had good analgesia up to 6 h postoperatively, but by 12 h several patients were in moderate pain at rest, suggesting the need for more opioids. There is enough evidence to suggest that lowering the anesthetic concentration and maintaining volume reduces HDP in nerve blocks, but unfortunately also reduces analgesia efficacy [36]. This is due to the fact that high concentrations of local anesthetics take longer to wear off, prolonging the blocking effect, providing a longer analgesic effect, and improving patient comfort. Therefore, for procedures that require prolonged analgesic efficacy, such as more painful shoulder surgery, maintaining a higher concentration of anesthetic may be more appropriate for effective postoperative pain relief. Besides, one patient in Group C experienced severe, indescribable pain at 12 h postoperatively, with increased use of oxycodone, possibly attributed to rebound pain upon the disappearance of the peripheral nerve block effect. Rebound pain often occurs after the resolution of an ISB and can persist severely for 2 to 6 h [37–39]. The causes and mechanisms behind rebound pain are multifaceted. Barry et al. [40] have demonstrated that being young, female, and undergoing orthopedic surgery are three risk factors for "rebound pain." [41][42, 43] Further investigation into the optimal use of dexamethasone in the STB could be valuable. In this study, all patients received PCIA regimen using oxycodone hydrochloride injection postoperatively. Adverse events occurred in four cases in Group C, experiencing dizziness, while none were reported in Group N. This could potentially be linked to the extensive use of oxycodone hydrochloride injection within a short postoperative period.
This research had several limitations. Firstly, diaphragmatic movement can be categorized into active and passive motions. The diaphragm on the side where HDP occurs might still experience passive movement due to the influence of the healthy side, making the ultrasound assessment of DE and diaphragm thickness not entirely indicative of the diaphragmatic movement changes. Bao et al. [44] assessed diaphragmatic motor function by examining diaphragmatic electrophysiology after brachial plexus block, using a nerve monitor to measure the diaphragmatic compound action potentials and diaphragmatic nerve conduction time, which might offer a more direct and accurate evaluation of diaphragmatic function compared to ultrasound assessment. Skaarup et al. [45] proposed assessing diaphragmatic movement through ultrasound-measured area, showing improved accuracy and correlation. Thus, future investigations should explore diaphragmatic function through methods like phrenic nerve stimulation, nerve conduction, area measurement, and pulmonary function tests. Secondly, this study presented the occurrence rate of HDP during STB in healthy adult patients without specifically incorporating changes in diaphragmatic function in obese individuals or those with respiratory system disorders. Thirdly, the timing of dexamethasone administration in this study was not optimally considered. Guidelines and clinical findings suggest that administering dexamethasone before or immediately after induction is more effective, which was not adequately considered in our protocol. Additionally, the dose of rocuronium bromide was not standardized and its postoperative effects were not tracked. Lastly, this study utilized ice cube testing for assessing the dermatomal coverage area of the STB, which introduces subjectivity. Research indicates that laser Doppler technology measuring changes in skin blood flow might be more sensitive and reliable in assessing neurological block [46].
In conclusion, this randomized controlled trial demonstrated that in patients undergoing arthroscopic shoulder surgery, 10 mL of 0.25% ropivacaine injection had a lesser effect on diaphragmatic motor function, had a lower incidence of HDP, and provided similar postoperative analgesia compared with 15 mL. Based on these findings, 10 mL may be considered a viable alternative to 15 mL, especially in obese, respiratory patients.
Acknowledgements
Not applicable.
Author contributions
Study design/planning: H.J.W., Q.Q.B., D.H.C., L.J.Z., L.Y.C., Y.L.Y. Acquisition of data: H.J.W., Q.Q.B. Data analysis: Q.Q.B., D.H.C., L.J.Z. Writing the article: H.J.W., L.Y.C., Y.L.Y. All authors reviewed the manuscript.
Funding
This work was funded by grants from Public Welfare Technology Application Research Program Project of Zhejiang Province (Huijun Wang, LGF21H150003) and Medical Science and Technology Project of Zhejiang Province (Yulong Yu, 2020KY1039).
Data availability
The datasets analyzed during the current study are available from the corresponding authors on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Taizhou Hospital of Zhejiang Province (K20210405) and written informed consent was obtained from all enrolled patients. All methods were performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huijun Wang and Qianqian Bao contributed equally to this work.
Contributor Information
Lingyang Chen, Email: chenly@enzemed.com.
Yulong Yu, Email: yyl@enzemed.com.
References:
- 1.Kang R, Jeong JS, Yoo JC, Lee JH, Choi SJ, Gwak MS, et al. Effective dose of intravenous dexmedetomidine to prolong the analgesic duration of interscalene brachial plexus block: a single-center, prospective, double-blind, randomized controlled trial. Reg Anesth Pain Med. 2018;43(5):488–95. [DOI] [PubMed] [Google Scholar]
- 2.Urmey WF, McDonald M. Hemidiaphragmatic paresis during interscalene brachial plexus block: effects on pulmonary function and chest wall mechanics. Anesth Analg. 1992;74(3):352–7. [DOI] [PubMed] [Google Scholar]
- 3.Palhais N, Brull R, Kern C, Jacot-Guillarmod A, Charmoy A, Farron A, et al. Extrafascial injection for interscalene brachial plexus block reduces respiratory complications compared with a conventional intrafascial injection: a randomized, controlled, double-blind trial. Br J Anaesth. 2016;116(4):531–7. [DOI] [PubMed] [Google Scholar]
- 4.Riazi S, Carmichael N, Awad I, Holtby RM, McCartney CJ. Effect of local anaesthetic volume (20 vs 5 ml) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth. 2008;101(4):549–56. [DOI] [PubMed] [Google Scholar]
- 5.Thackeray EM, Swenson JD, Gertsch MC, Phillips KM, Steele JW, Burks RT, et al. Diaphragm function after interscalene brachial plexus block: a double-blind, randomized comparison of 0. 25% and 0.125% bupivacaine. J Shoulder Elbow Surg. 2013;22(3):381–6. [DOI] [PubMed] [Google Scholar]
- 6.Wiesmann T, Feldmann C, Muller HH, Nentwig L, Beermann A, El-Zayat BF, et al. Phrenic palsy and analgesic quality of continuous supraclavicular vs. interscalene plexus blocks after shoulder surgery. Acta Anaesthesiol Scand. 2016;60(8):1142–51. [DOI] [PubMed] [Google Scholar]
- 7.Grassi L, Kacmarek R, Berra L. Ventilatory mechanics in the patient with obesity. Anesthesiology. 2020;132(5):1246–56. [DOI] [PubMed] [Google Scholar]
- 8.De Jong A, Wrigge H, Hedenstierna G, Gattinoni L, Chiumello D, Frat JP, et al. How to ventilate obese patients in the ICU. Intensive Care Med. 2020;46(12):2423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang J, Cui X, Zhang J, Huang Y. Dyspnea induced by hemidiaphragmatic paralysis after ultrasound-guided supraclavicular brachial plexus block in a morbidly obese patient. Medicine (Baltimore). 2022;101(2):e28525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler J, Schafhalter-Zoppoth I, Gray AT. An ultrasound study of the phrenic nerve in the posterior cervical triangle: implications for the interscalene brachial plexus block. Reg Anesth Pain Med. 2008;33(6):545–50. [PubMed] [Google Scholar]
- 11.Gunjiyal MS, Mohammed S, Bhatia P, Chhabra S, Kumar M, Sharma A. Effect of combined versus sequential injection of 2% lidocaine and 0.5% bupivacaine on the onset and duration of supraclavicular brachial plexus block: a double blinded randomised controlled trial. J Clin Anesth. 2021;72:110313. [DOI] [PubMed] [Google Scholar]
- 12.Aliste J, Bravo D, Fernandez D, Layera S, Finlayson RJ, Tran DQ. A randomized comparison between interscalene and small-volume supraclavicular blocks for arthroscopic shoulder surgery. Reg Anesth Pain Med. 2018;43(6):590–5. [DOI] [PubMed] [Google Scholar]
- 13.Hartrick CT, Tang YS, Siwek D, Murray R, Hunstad D, Smith G. The effect of initial local anesthetic dose with continuous interscalene analgesia on postoperative pain and diaphragmatic function in patients undergoing arthroscopic shoulder surgery: a double-blind, randomized controlled trial. BMC Anesthesiol. 2012;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang R, Jeong JS, Chin KJ, Yoo JC, Lee JH, Choi SJ, et al. Superior trunk block provides noninferior analgesia compared with interscalene brachial plexus block in arthroscopic shoulder surgery. Anesthesiology. 2019;131(6):1316–26. [DOI] [PubMed] [Google Scholar]
- 15.Cros Campoy J, Domingo Bosch O, Pomes J, Lee J, Fox B, Sala-Blanch X. Upper trunk block for shoulder analgesia with potential phrenic nerve sparing: a preliminary anatomical report. Reg Anesth Pain Med. 2019;5:96. [DOI] [PubMed] [Google Scholar]
- 16.Wait JL, Nahormek PA, Yost WT, Rochester DP. Diaphragmatic thickness-lung volume relationship in vivo. J Appl Physiol. 1989;67(4):1560–8. [DOI] [PubMed] [Google Scholar]
- 17.Scheibe N, Sosnowski N, Pinkhasik A, Vonderbank S, Bastian A. Sonographic evaluation of diaphragmatic dysfunction in COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:1925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian Z, Yang M, Li L, Chen Y. Ultrasound assessment of diaphragmatic dysfunction as a predictor of weaning outcome from mechanical ventilation: a systematic review and meta-analysis. BMJ Open. 2018;8(9):e021189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahgat E, El-Halaby H, Abdelrahman A, Nasef N, Abdel-Hady H. Sonographic evaluation of diaphragmatic thickness and excursion as a predictor for successful extubation in mechanically ventilated preterm infants. Eur J Pediatr. 2021;180(3):899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Chen Z, Yan W. Application of bedside ultrasound in predicting the outcome of weaning from mechanical ventilation in elderly patients. BMC Pulm Med. 2021;21(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsui JJ, Tsui BC. A novel systematic ABC approach to Diaphragmatic Evaluation (ABCDE). Can J Anaesth. 2016;63(5):636–7. [DOI] [PubMed] [Google Scholar]
- 22.Khurana J, Gartner SC, Naik L, Tsui BCH. Ultrasound identification of diaphragm by novices using ABCDE technique. Reg Anesth Pain Med. 2018;43(2):161–5. [DOI] [PubMed] [Google Scholar]
- 23.Sferrazza Papa GF, Pellegrino GM, Di Marco F, Imeri G, Brochard L, Goligher E, et al. A review of the ultrasound assessment of diaphragmatic function in clinical practice. Respiration. 2016;91(5):403–11. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Hu J, Hu X, Li R, Li Y, Wong G, et al. Preemptive intravenous nalbuphine for the treatment of post-operative visceral pain: a multicenter, double-blind, placebo-controlled. Randomized Clinical Trial Pain Ther. 2021;10(2):1155–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.la Grange P, Foster PA, Pretorius LK. Application of the Doppler ultrasound bloodflow detector in supraclavicular brachial plexus block. Br J Anaesth. 1978;50(9):965–7. [DOI] [PubMed] [Google Scholar]
- 26.Lemke E, Johnston DF, Behrens MB, Seering MS, McConnell BM, Swaran Singh TS, et al. Neurological injury following peripheral nerve blocks: a narrative review of estimates of risks and the influence of ultrasound guidance. Reg Anesth Pain Med. 2024;49(2):122–32. [DOI] [PubMed] [Google Scholar]
- 27.Kim YD, Yu JY, Shim J, Heo HJ, Kim H. Risk of encountering dorsal scapular and long thoracic nerves during ultrasound-guided interscalene brachial plexus block with nerve stimulator. Korean J Pain. 2016;29(3):179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burckett-St Laurent D, Chan V, Chin KJ. Refining the ultrasound-guided interscalene brachial plexus block: the superior trunk approach. Can J Anaesth. 2014;61(12):1098–102. [DOI] [PubMed] [Google Scholar]
- 29.de Miguel GC, Whyte M, St James M, Ferreira TH. Effect of contrast and local anesthetic on dye spread following transversus abdominis plane injection in dog cadavers. Vet Anaesth Analg. 2020;47(3):391–5. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Han JU, Lee W, Jeon YS, Jeong J, Yang C, et al. Effects of local anesthetic volume (Standard Versus Low) on incidence of hemidiaphragmatic paralysis and analgesic quality for ultrasound-guided superior trunk block after arthroscopic shoulder surgery. Anesth Analg. 2021;133(5):1303–10. [DOI] [PubMed] [Google Scholar]
- 31.Wong AK, Keeney LG, Chen L, Williams R, Liu J, Elkassabany NM. Effect of local anesthetic concentration (0.2% vs. 0.1% Ropivacaine) on pulmonary function, and analgesia after ultrasound-guided interscalene brachial plexus block: a randomized controlled study. Pain Med. 2016;17(12):2397–403. [DOI] [PubMed] [Google Scholar]
- 32.Aliste J, Bravo D, Layera S, Fernandez D, Jara A, Maccioni C, et al. Randomized comparison between interscalene and costoclavicular blocks for arthroscopic shoulder surgery. Reg Anesth Pain Med. 2019;5:96. [DOI] [PubMed] [Google Scholar]
- 33.Santana PV, Cardenas LZ, de Albuquerque ALP, de Carvalho CRR, Caruso P. Diaphragmatic ultrasound findings correlate with dyspnea, exercise tolerance, health-related quality of life and lung function in patients with fibrotic interstitial lung disease. BMC Pulm Med. 2019;19(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eltrabili HH, Hasanin AM, Soliman MS, Lotfy AM, Hamimy WI, Mukhtar AM. Evaluation of diaphragmatic ultrasound indices as predictors of successful liberation from mechanical ventilation in subjects with abdominal sepsis. Respir Care. 2019;64(5):564–9. [DOI] [PubMed] [Google Scholar]
- 35.Llamas-Alvarez AM, Tenza-Lozano EM, Latour-Perez J. Diaphragm and lung ultrasound to predict weaning outcome: systematic review and meta-analysis. Chest. 2017;152(6):1140–50. [DOI] [PubMed] [Google Scholar]
- 36.Fredrickson MJ, Abeysekera A, White R. Randomized study of the effect of local anesthetic volume and concentration on the duration of peripheral nerve blockade. Reg Anesth Pain Med. 2012;37(5):495–501. [DOI] [PubMed] [Google Scholar]
- 37.Munoz-Leyva F, Cubillos J, Chin KJ. Managing rebound pain after regional anesthesia. Korean J Anesthesiol. 2020;73(5):372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park KB, Cho HO, Kim MS, Jeon YD. Rebound pain after arthroscopic cuff repair with interscalene brachial plexus block anesthesia is reduced by surgeon administered multimodal shoulder injections: a prospective randomized controlled trial. Arthroscopy. 2024;6:96. [DOI] [PubMed] [Google Scholar]
- 39.Morita S, Oizumi N, Suenaga N, Yoshioka C, Yamane S, Tanaka Y. Dexamethasone added to levobupivacaine prolongs the duration of interscalene brachial plexus block and decreases rebound pain after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2020;29(9):1751–7. [DOI] [PubMed] [Google Scholar]
- 40.Barry GS, Bailey JG, Sardinha J, Brousseau P, Uppal V. Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br J Anaesth. 2021;126(4):862–71. [DOI] [PubMed] [Google Scholar]
- 41.Woo JH, Lee HJ, Oh HW, Lee JW, Baik HJ, Kim YJ. Perineural dexamethasone reduces rebound pain after ropivacaine single injection interscalene block for arthroscopic shoulder surgery: a randomized controlled trial. Reg Anesth Pain Med. 2021;46(11):965–70. [DOI] [PubMed] [Google Scholar]
- 42.YaDeau JT, Paroli L, Fields KG, Kahn RL, LaSala VR, Jules-Elysee KM, et al. Addition of dexamethasone and buprenorphine to bupivacaine sciatic nerve block: a randomized controlled trial. Reg Anesth Pain Med. 2015;40(4):321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orebaugh SL, Dewasurendra A. Has the future arrived? Liposomal bupivacaine versus perineural catheters and additives for interscalene brachial plexus block. Curr Opin Anaesthesiol. 2020;33(5):704–9. [DOI] [PubMed] [Google Scholar]
- 44.Bao X, Huang J, Feng H, Qian Y, Wang Y, Zhang Q, et al. Effect of local anesthetic volume (20 mL vs 30 mL ropivacaine) on electromyography of the diaphragm and pulmonary function after ultrasound-guided supraclavicular brachial plexus block: a randomized controlled trial. Reg Anesth Pain Med. 2019;44(1):69–75. [DOI] [PubMed] [Google Scholar]
- 45.Skaarup SH, Lokke A, Laursen CB. The Area method: a new method for ultrasound assessment of diaphragmatic movement. Crit Ultrasound J. 2018;10(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hermanns H, Werdehausen R, Hollmann MW, Stevens MF. Assessment of skin temperature during regional anaesthesia-What the anaesthesiologist should know. Acta Anaesthesiol Scand. 2018;62(9):1280–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding authors on reasonable request.