Abstract
Passive antibody prophylaxis against human immunodeficiency virus type 1 (HIV-1) has been accomplished in primates, suggesting that this strategy may prove useful in humans. While antibody specificity is crucial for neutralization, other antibody characteristics, such as subclass, have not been explored. Our objective was to compare the efficiencies of immunoglobulin G (IgG) subclasses from polyclonal human HIV immune globulin (HIVIG) in the neutralization of HIV-1 strains differing in coreceptor tropism. IgG1, IgG2, and IgG3 were enriched from HIVIG by using protein A-Sepharose. All three subclasses bound major HIV-1 proteins, as shown by Western blot assay and enzyme-linked immunosorbent assay. In HIV-1 fusion assays using X4, R5, or X4R5 envelope-expressing effector cells, IgG3 more efficiently blocked fusion. In neutralization assays with cell-free viruses using X4 (LAI, IIIB), R5 (BaL), and X4R5 (DH123), a similar hierarchy of neutralization was found: IgG3 > IgG1 > IgG2. IgG3 has a longer, more flexible hinge region than the other subclasses. To test whether this is important, IgG1 and IgG3 were digested with pepsin to generate F(ab′)2 fragments or with papain to generate Fab fragments. IgG3 F(ab′)2 fragments were still more efficient in neutralization than F(ab′)2 of IgG1. However, Fab fragments of IgG3 and IgG1 demonstrated equivalent neutralization capacities and the IgG3 advantage was lost. These results suggest that the IgG3 hinge region confers enhanced HIV-neutralizing ability. Enrichment and stabilization of IgG3 may therefore lead to improved HIVIG preparations. The results of this study have implications for the improvement of passive immunization with polyclonal or monoclonal antibodies and suggest that HIV-1 vaccines which induce high-titer IgG3 responses could be advantageous.
Passive immune globulin therapy has been successfully used to prevent infection with a variety of viruses, including varicella-zoster virus, measles virus, hepatitis A virus, hepatitis B virus, cytomegalovirus, respiratory syncytial virus, and rabies virus (14, 15, 31, 43). Primate studies in vivo have suggested that human immunodeficiency virus (HIV) infection might be preventable by anti-HIV antibodies (1, 2, 4, 20, 36). A preparation of antibodies against HIV could potentially be useful in the prevention of maternal-fetal transmission or for post-needle stick prophylaxis, such as in cases of accidental inoculation of health care personnel. To this end, several polyclonal preparations of immune globulin manufactured from plasma of HIV-positive persons with high antibody titers have been developed and used in primate and human prophylaxis and treatment studies. Prevention of oral, vaginal, and intravenous HIV type 1 (HIV-1) transmission by passive antibody therapy was demonstrated in primates by using polyclonal and/or neutralizing monoclonal antibodies (MAbs). Polyclonal anti-HIV immunoglobulins protected chimpanzees against low-dose HIV-1 infection and provided additive prophylaxis against vaginal and intravenous infection of primates treated concurrently with anti-HIV MAbs (13, 25–27, 33). Complete abrogation of mucosal infection of neonatal macaques has been achieved by using combinations of MAbs (1). In vivo neutralization mechanisms are not well defined but may include prevention of initial infection and prevention of viral transfer from dendritic cells to activated T cells (16).
In women with HIV-1 infection and in children with advanced infection, anti-HIV antibody preparations decreased p24 antigenemia and resulted in delayed in vitro virus propagation, although CD4 counts and viral loads were minimally affected (23, 41). A preparation of human HIV immune globulin (HIVIG) was tested for the prevention of maternal-fetal transmission in a pivotal study (42). While HIVIG and a placebo showed no statistically significant differences, there was a lower-than-expected transmission rate because zidovudine was given to both treatment groups. HIVIG has not been studied for prophylaxis after accidental exposure to HIV. Thus, the benefit of polyclonal anti-HIV preparations in different prophylactic settings remains an open question.
Since partial protection was conferred by HIVIG in many primate studies, a more detailed examination of the immunoglobulin properties associated with neutralization is warranted. While antibody specificity for HIV-1 is essential, one in vitro study using a cloned human variable region of an anti-HIV antibody linked to either the γ1 or the γ3 constant region has suggested that the neutralizing activity against a laboratory-adapted strain of HIV-1 is enhanced by the IgG3 constant region (5). In addition, one of the most broadly cross neutralizing human MAbs (MAb 477-53-D) specific for V3 is of the IgG3 isotype (17, 18). If, indeed, a particular IgG subclass can improve the neutralizing activity of anti-HIV-1 antibodies, it may be useful to develop polyclonal or monoclonal preparations with those characteristics. This goal could be achieved by vaccination with appropriate adjuvants or by molecular engineering of MAbs. To further examine this question, we separated IgG subclasses from a commercially manufactured HIVIG. We compared the abilities of human antibody subclasses IgG1, IgG2, and IgG3 to bind to HIV-1 antigens and to mediate viral neutralization. We found that all three subclasses exhibited binding to solid-phase HIV-1 antigens, with HIVIG and IgG1 showing the greatest binding by enzyme-linked immunosorbent assay (ELISA). However, IgG3 was superior to IgG2 and IgG1 in blocking HIV-1 envelope-mediated cell fusion and in neutralization of cell-free virus. F(ab) fragments of IgG3 generated by papain digestion lost their increased efficacy compared to F(ab) of IgG1, while IgG3 F(ab′)2 fragments generated by pepsin digestion retained their enhanced fusion-inhibiting activity compared to that of IgG1 F(ab′)2. These results suggest that the unique structure of the IgG3 hinge region is a major contributor to its enhanced HIV-1-neutralizing capacity.
MATERIALS AND METHODS
HIVIG preparations.
HIVIG was obtained through the National Heart, Lung, and Blood Institute repository with the kind assistance of Luiz Barbosa of the National Heart, Lung, and Blood Institute and Mark Cosentino. HIVIG was manufactured by North American Biologicals, Inc. (Boca Raton, Fla.), from the plasma of >100 HIV-positive donors with high anti-p24 antibody levels and CD4 counts of > 400/μl who were clinically healthy. Two separate lots (HIVIG-113 and HIVIG-114) were used for these studies. Manufacturing was performed in accordance with good manufacturing practices. Before fractionation, plasma was treated with tri-n-butyl phosphate and 1% Tween 80 to inactivate HIV-1. Immunoglobulin G (IgG) was purified by using a Cohn-Oncley fractionation procedure, followed by QAE-50 Sephadex. The final preparation was formulated as a 5% solution in normal saline that was sterile and nonpyrogenic (9). High-pressure liquid chromatography analysis showed <3% fragmentation and <1% aggregates in the two lots used for these studies.
Intravenous human respiratory syncytial virus immune globulin (RSVIG) was used as a control immunoglobulin in all experiments. RSVIG is a commercial preparation manufactured by Massachusetts Public Health Biological Laboratories (Boston, Mass.). Like HIVIG, RSVIG is prepared by using a modified Cohn-Oncley fractionation procedure, which also includes a solvent-detergent step to inactivate viruses. RSVIG is obtained from plasma of healthy donors with high titers of antibodies against respiratory syncytial virus. The final preparation is a sterile 5% solution.
IgG subclass separations.
IgG subclass separation was performed by pH gradient elution from a protein A affinity column as adapted from the method of Duhamel et al. (11). Recombinant protein A-conjugated Sepharose beads (rProtein A-Sepharose Fast Flow; Amersham Pharmacia Biotech AB, Uppsala, Sweden) were packed into a 1.5-cm-diameter column to a bed height of 14 cm and equilibrated with McIlvaine's citrate-phosphate buffer, pH 6.5 (0.2 M Na2HPO4 titrated to the desired pH with 0.1 M citric acid and preserved with 0.1% sodium azide). HIVIG or RSVIG (0.25 g/run at a concentration of 50 mg/ml) was applied to the column after dialysis into the equilibration buffer. Column flow was controlled by a Bio-Rad Biologic HR fast protein liquid chromatography (FPLC) system, which permits a programmable admixture of buffers to flow through the column at low pressures and a constant rate. Fractions of 8 ml were collected when the A280 (0.5-cm path length) was greater than 0.1. Typically, a two-step gradient was run by programming the admixture of two buffers at pHs 6.5 and 3.5 (buffers A and B, respectively). The first step was 55 to 70% buffer B in a 200-ml volume, and the second step was 70 to 85% buffer B in a 50-ml volume. The flow rate was held at 0.33 ml/min throughout the run. IgG3 does not bind to protein A and was therefore present in the flowthrough; IgG2 was eluted at pHs 4.70 to 4.55, and the majority of IgG1 eluted at pHs 4.50 to 3.70, although there was some tailing at lower pHs. Small amounts of IgG4 were present in both the IgG1 and IgG2 fractions. Because no further separation was attainable, the gradient was steepened after the main IgG1 peak began to elute. The column was regenerated with 1 column volume of 0.1 M citric acid and re-equilibrated with buffer A between separations.
IgG3 fractions from the early flowthrough from multiple FPLC runs were pooled. Since IgG1- and IgG2-containing fractions overlapped, tubes containing substantial proportions of both subclasses were discarded. To determine the enrichment of the individual fractions, as well as the final pooled fractions, an ELISA for each subclass was performed by using a human IgG subclass detection kit in accordance with the manufacturer's instructions (Central Laboratory of The Netherlands Red Cross Blood Transfusion Service; obtained through Accurate Chemical, Westbury, N.Y.). Pooled fractions were concentrated by ultrafiltration (Millipore, Bedford, Mass.) of stirred cells. The subclass-enriched preparations were dialyzed against phosphate-buffered saline (PBS), and protein concentrations were determined by measuring the A280.
HIV-1 ELISA.
HIVIG subclasses were tested for binding to HIV antigens by using a commercial kit which included a combination of HIV-1 viral lysate (SF2) and recombinant HIV-1 envelope antigen produced in Escherichia coli (rLAV EIA; Genetic Systems, Redmond, Wash.). The HIVIG subclasses were diluted to a concentration of 40 μg/ml and serially diluted fivefold. The protocol was followed in accordance with the manufacturer's instructions, and the A450 was read. Additionally, ELISA plates coated with purified gp120 protein (SF2; Chiron Corp.) or V3 peptide were used for binding assays.
HIV-1 immunoblotting.
A commercial kit, the Novapath HIV-1 Immunoblot kit (Bio-Rad, Hercules, Calif.) was used in accordance with the manufacturer's instructions. Briefly, total HIVIG and HIVIG subclasses were incubated with membrane strips on which HIV-1 proteins were blotted after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. All IgG subclasses were added at a concentration of 0.40 mg/ml and incubated for 30 min at room temperature. After washing of the strips, anti-human IgG conjugated to alkaline phosphatase was added, the mixture was incubated for 10 min, and the reaction was developed with a 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium substrate.
HIV-1 envelope-expressing recombinant vaccinia viruses for fusion inhibition studies.
A recombinant vaccinia virus expressing the HIV-1 envelope derived from R5 strain JR-FL (vCB28) (3) was obtained from Christopher Broder (Uniformed Services University of the Health Sciences, Bethesda, Md.). Recombinant vaccinia viruses expressing the X4 envelope (IIIB), vPE16 (12), and X4R5 (dual tropic envelope), v89.6 (6), were obtained from the laboratory of Bernard Moss (National Institute of Allergy and Infectious Diseases, National Institutes of Health [NIH]). A recombinant vaccinia virus expressing the bacterial β-galactosidase gene, vSC8 (from the laboratory of Bernard Moss, NIH), was used as a negative control in all of the experiments. In addition, the human lymphoid cell line TF228.1.16, which stably expresses the HIV IIIB/BH10 (X4) envelope (22), was used in the fusion assays.
Fusion inhibition assays.
CD4− cell line 12E1 was infected with the HIV-1 envelope-expressing recombinant vaccinia viruses at 10 PFU/cell overnight. Envelope-expressing 12E1 cells or TF228.1.16 (IIIB/BH10 envelope) cells were mixed (1:1) with the PM1 cell line (a CD4+ CXCR4+ CCR5+ derivative of the Hut 78 cell line that is susceptible to infection by both X4 and R5 strains) (24). HIVIG subclasses were added at different concentrations to the envelope-expressing effector cells and incubated for 1 h at 37°C, and then the PM1 target cells were added. The numbers of multinucleated syncytia were scored at various times after initiation of cocultures (peak syncytium numbers were usually observed between 3 and 5 h). All groups were plated at two or three replicates, and all experiments were repeated at least three times. Fifty percent infective doses (ID50) were calculated by using linear regression with GraphPad Prism software.
HIV-1 strains and virus neutralization assays.
HIV-1 viral stocks IIIB (X4 strain) and BaL (R5 strain) were obtained from the AIDS Research and Reference Reagent Program (McKesson HBOC BioServices, Rockville, Md.), the dual-tropic primary isolate DH123 was obtained from Malcolm Martin (National Institute of Allergy and Infectious Diseases, NIH) (35), and the primary isolate LAI strain was obtained from Keith Peden (DVP, OVRR, Food and Drug Administration). The BaL and DH123 viral stocks were propagated and their titers were determined in phytohemagglutinin-activated peripheral blood lymphocytes, and the titer of the IIIB strain was determined on H9 cells by the method of Reed and Muench (34). Viral neutralization by different HIVIG subclasses was conducted as described by Shibata et al. (37). The virus strains were preincubated with the control (RSVIG) or with HIVIG subclasses (threefold dilutions, starting at 4 mg/ml) for 30 min at 37°C. Ten-microliter volumes of virus-antibody mixtures (containing 100 50% tissue culture-infective doses [TCID50]) were added to a 96-well-plate containing PM1 cells (5 × 104 cells/well, five replicates per group). The plates were washed extensively after 3 days to remove residual virions and HIVIG (including anti-p24 antibodies). Every second day thereafter, the supernatants were removed and the cultures were supplemented with fresh medium. Virus production was determined by measuring p24 in the supernatants with ELISA kits (NEN Life Sciences Products Inc., Boston, Mass.). Virus neutralization by the different HIVIG subclasses is expressed either as percent inhibition of p24 production at a given concentration of antibodies (usually based on day 9 to 14 p24 assays) or as the 50% neutralization titer against 100 TCID50 of DH123 as described by Shibata et al. (37).
Preparation of HIVIG subclass Fab and F(ab′)2 fragments.
IgG1 and IgG3 from HIVIG subclasses (2 to 7 mg/ml) in PBS containing 20 mM EDTA and 20 mM l-cysteine were incubated with papain (Sigma, St. Louis, Mo.). Preliminary studies determined the optimal conditions for complete subclass digestion into Fab fragments. As has been previously reported, IgG3 is much more sensitive to proteinases and complete digestion could be observed after brief exposure to this enzyme, whereas higher concentrations of papain and longer digestion periods are required for the other subclasses (45). IgG3 was digested with papain at 20 μg/ml for 1 h at 37°C. IgG1 was digested with papain (50 μg/ml) for 24 h at 37°C. The reactions were terminated with 50 mM iodoacetamide for 30 min at room temperature, and the products were dialyzed overnight into PBS. To remove residual intact molecules and Fc fragments, the reaction mixtures were passed through a protein G-Sepharose column (Amersham Pharmacia Biotech), which was prewashed with 0.1 M citric acid, pH 3, and equilibrated in PBS. Samples were loaded onto the column, and the flowthrough was collected. The column was washed with 2 ml of PBS, and the wash fraction was combined with the flowthrough. Samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to determine their purity and then concentrated with an Amicon concentrator (Millipore). By using goat anti-human IgG Fc-specific antibodies in binding assays, it was determined that the IgG-Fab preparation contained no contaminating Fc fragments or intact IgG molecules.
For pepsin digestion, IgG1 and IgG3 preparations (7 mg/ml) were dialyzed into acetate buffer (pH 4.0) for 4 h. Protein concentration was determined after dialysis, and pepsin (Sigma) was added at an enzyme-antibody concentration ratio of 1:20. The reaction mixture was incubated for 1 h at 37°C, and the digestion was terminated by adding 2 M Tris base until a pH of 8.0 was attained (approximately 0.2 ml/reaction mixture). The reaction mixtures were dialyzed overnight into PBS. For removal of residual intact molecules and Fc fragments, each reaction mixture was passed through a protein G-Sepharose column (Amersham Pharmacia Biotech) as described for Fab fragments. The purity of the final preparations was assessed by high-pressure liquid chromatography.
RESULTS
Enriched IgG subclasses from HIVIG display similar HIV antigen-binding profiles.
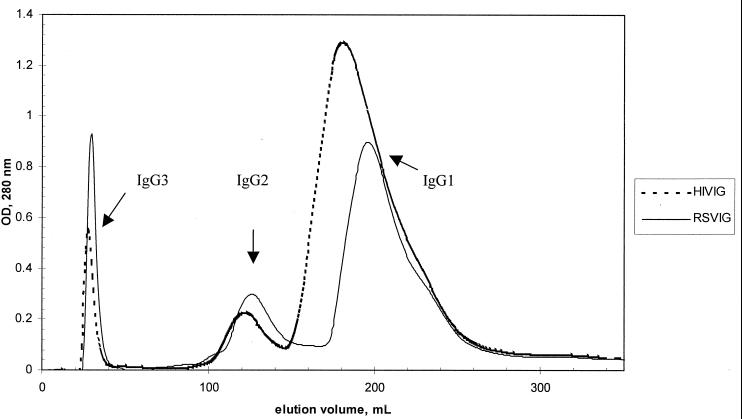
HIVIG subclasses were enriched by using a protein A-Sepharose column (Fig. 1). IgG3, which does not bind to protein A, was recovered in the early flowthrough. IgG2 and then IgG1 were eluted by using a diminishing pH gradient. The amount of IgG3 was lower and that of IgG1 was higher than would be expected from HIVIG manufactured from normal donors. These results confirm subclass distributions reported for previous lots and reflect skewing of the IgG subclass distribution seen in HIV patients (9). The elution fractions were assayed individually and pooled to obtain optimal enrichment of each subclass (Table 1). The small amounts of IgG4 can be attributed to the use during manufacturing of a QAE Sephadex column, which is known to remove IgG4 due to the unique charge properties of this subclass (38).
FIG. 1.
Separation of antibody subclasses from polyclonal immune globulin preparations by FPLC. RSVIG and HIVIG were run under identical conditions, and the graphs indicating the protein concentrations of the elution-fractions are superimposed. IgG3 protein was present in the initial flowthrough, as indicated. Upon application of a gradually diminishing pH gradient, IgG2 was eluted, followed and partially overlapped by IgG1. OD, optical density.
TABLE 1.
Purity of enriched pooled subclass fractionsa
| Fraction | % IgG1 | % IgG2 | % IgG3 | % IgG4 |
|---|---|---|---|---|
| IgG1 | 94.7 | 4.7 | 0 | 0.6 |
| IgG2 | 10 | 89.4 | 0 | 0.6 |
| IgG3 | 0 | 0 | 100 | 0 |
Collection tubes from FPLC were tested by ELISA for the presence of all four IgG subclasses. HIVIG lot 113 is shown here; results for lot 114 were similar.
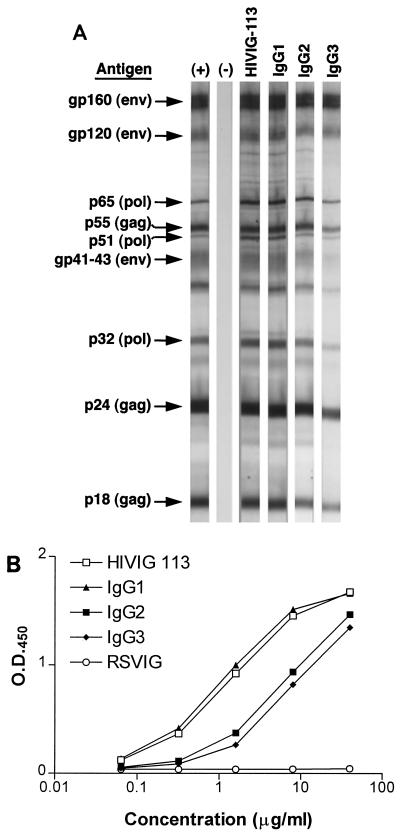
To determine whether all of the subclasses bound multiple HIV antigens, enriched subclass fractions were compared with unfractionated HIVIG for binding in a commercial HIV-1 Western blot assay. Identical viral bands were recognized by all three subclasses and by the original, unfractionated HIVIG from which the subclasses were derived (Fig. 2A). To compare subclass binding to solid-phase antigens more quantitatively, IgG subclasses and HIVIG were also tested in ELISAs using HIV-1 lysate (SF2)–LAV envelope antigen. Binding of unseparated HIVIG was used as an internal reference in order to demonstrate that the subclass separation process did not result in a significant reduction in the binding capacity of the immunoglobulins. While all of the IgG subclasses displayed binding to HIV-1 antigens in an ELISA, the rank order of binding was IgG1 ≥ HIVIG > IgG3-IgG2 (Fig. 2B). No significant binding to HIV-1 proteins was observed with RSVIG. In addition, binding to plates coated with either purified gp120 or V3 peptide was determined by ELISA. Again, a similar pattern of binding was observed, as depicted in Fig. 2 (data not shown).
FIG. 2.
HIVIG subclass binding to viral antigens. (A) Binding of unfractionated HIVIG and HIVIG subclasses (or the RSVIG control) to an immunoblot of HIV antigens. The same amount of protein was incubated with each strip. Intact HIVIG and all three subclasses bound to the same bands similarly. (B) Unfractionated HIVIG and HIVIG subclasses demonstrate quantitative differences in binding to HIV lysate by ELISA. Serial dilutions of antibody preparations were applied to HIV lysate-coated ELISA plates and developed in accordance with the manufacturer's instructions. The control immune globulin, RSVIG, did not exhibit binding. Similar results were obtained by using ELISA plates coated with viral envelope (SF2) and V3 peptide (not shown). O.D.450, optical density at 450 nm.
HIV fusion inhibition and neutralization by HIVIG subclasses.
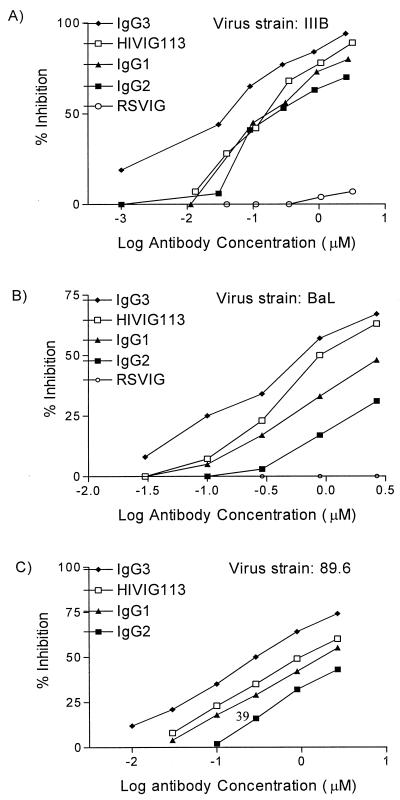
Several important HIV-neutralizing epitopes on the viral envelope are noncontinuous and conformation dependent. Such conformational epitopes are often lost when HIV antigens are processed for solid-phase assays such as ELISA and Western blot assay. Therefore, functional assays, rather than binding assays, are more accurate predictors of anti-HIV antibody activity in vivo (25, 36). Fusion of HIV gp120/41 envelope protein with cells expressing CD4 and the appropriate coreceptors is the essential first step in HIV entry into cells. A syncytium assay, which measures the fusion between HIV-1 envelope-expressing effector cells and target cells expressing CD4-chemokine receptors, is a surrogate assay for cell-to-cell HIV-1 transmission. The abilities of total HIVIG and HIVIG IgG subclasses to inhibit fusion were tested by coincubating cells expressing either R5-dependent, X4-dependent, or dual-tropic envelope protein with PM1 target cells that express CD4 and both CCR5 and CXCR4. The IgG preparations were added to the envelope-expressing cells for 60 min prior to the mixing of effector and target cells. Syncytium formation, an indicator of cell fusion, was assessed 3 to 6 h after effector-target cell mixing at a 1:1 ratio. Interestingly, among the HIVIG subclasses, IgG3 displayed superior inhibition of X4-, R5-, and X4-R5 (dual-tropic)-mediated envelope fusion (Fig. 3A to C). The ID50 of IgG3 were 3.5-, 5.7-, and 3-fold lower than those of IgG1 for inhibition of fusion with X4, R5, and X4R5 envelope-expressing cells, respectively. The overall ability to inhibit R5-mediated syncytium formation was less than for X4 envelope-expressing cells, as previously reported for R5 viruses (12).
FIG. 3.
Inhibition of HIV-1 envelope-mediated fusion by HIVIG and HIVIG subclasses. Serial dilutions of antibody preparations were added to envelope-expressing cells for 1 h prior to addition of PM1 target cells. Fusion was assessed 3 to 6 h later. Fusion inhibition was compared to that of a control culture without IgG additives, which defined 0% inhibition. RSVIG was used as a negative control. Results are expressed as micromolar concentrations to account for the slightly greater molecular weight of IgG3. (A) HIV IIIB (X4) envelope-mediated fusion inhibition using TF228 cells. The ID50 (micromolar) were as follows: HIVIG, 0.18; IgG1, 0.21; IgG2, 0.37; IgG3, 0.06. (B) HIV-1 BaL (R5) envelope-mediated fusion inhibition using vCB28-infected 12E1 cells. The ID50 (micromolar) were as follows: HIVIG, 1.2; IgG1, 4.0; IgG2, 73.2; IgG3, 0.7. (C) HIV-1 89.6 (X4R5)-mediated envelope fusion inhibition using vaccinia virus strain 89.6-infected 121E1 cells. The ID50 (micromolar) were as follows: HIVIG, 1.2; IgG1, 1.75; IgG2, 4.2; IgG3, 0.6. The experimental results shown are representative of at least two fusion inhibition assays for each envelope.
Neutralization of infection with cell-free HIV is also an important measure of antibody potential to prevent infection. The ability of HIVIG subclasses to inhibit viral infection was tested by preincubation of antibodies with HIV strain BaL (R5) or IIIB (X4) or a primary isolate, DH123 (X4R5), followed by addition of the virus-antibody mixtures to PM1 cells. p24 antigen production was measured at various time points as a marker of the extent of productive infection. Again, IgG3 anti-HIV antibodies were found to be more effective than IgG1, IgG2, and HIVIG in neutralizing IIIB and BaL (Tables 2 and 3). Neutralization of primary HIV isolates, as opposed to laboratory strains, is believed to be a more relevant predictor of in vivo virus neutralization. Attenuation of infection with the primary isolate, DH123, by HIVIG subclasses was determined after 13 days of infection of PM1 cells at 100 TCID50 (Table 4). The dose of antibody preparation that inhibited p24 antigen production by 50% was 23- to 26-fold lower for IgG3 than for HIVIG, IgG1, and IgG2. Taken together, these results consistently show that IgG3 antibodies from HIVIG are more efficacious in neutralizing HIV-1 in vitro.
TABLE 2.
Neutralization of HIV IIIB (X4, T-tropic virus)a
| IgG added | p24 concn (ng/ml) | % Inhibition |
|---|---|---|
| Virus alone | 339 ± 87 | |
| Total RSVIG | 361 ± 103 | 0 |
| Total HIVIG | 258 ± 23 | 24 |
| HIVIG IgG1 | 206 ± 143 | 39 |
| HIVIG IgG2 | 404 ± 32 | 0 |
| HIVIG IgG3 | 33 ± 22 | 90 |
RSVIG or HIVIG subclasses (lot 113) were incubated with virus stocks (100 TCID50) for 30 min at 37°C prior to addition to PM1 cells. The final concentration of each antibody was 80 μg/ml. p24 antigen was measured on day 9. The data shown are the mean ± the standard deviation of five replicate wells. The results shown are representative of two experiments. Similar results were observed on day 11 of culture.
TABLE 3.
Neutralization of BaL (R5, M-tropic virus)a
| IgG added | p24 concn (ng/ml) | % Inhibition |
|---|---|---|
| Virus alone | 402 ± 16 | 0 |
| Total HIVIG | 209 ± 13 | 48 |
| HIVIG IgG1 | 156 ± 14 | 61 |
| HIVIG IgG2 | 211 ± 20 | 48 |
| HIVIG IgG3 | 74 ± 8 | 82 |
p24 antigen was measured on day 11 after infection. Virus was added at 100 TCID50/well, and the final concentration of each antibody was 80 μg/ml. The data shown are the mean ± the standard deviation of five replicate wells.
TABLE 4.
Neutralization of primary isolate DH123
| Ig | ID50a (μg/ml) |
|---|---|
| Total HIVIG | 51.9 |
| IgG1 | 55.5 |
| IgG2 | 60.8 |
| IgG3 | 2.3 |
The ID50 of each subclass was determined after 13 days of infection of PM1 cells with DH123 at 100 TCID50. The ID50 was determined by the method of Reed and Muench (34).
The enhanced syncytium inhibition by IgG3 depends upon the presence of an intact hinge region but not Fc.
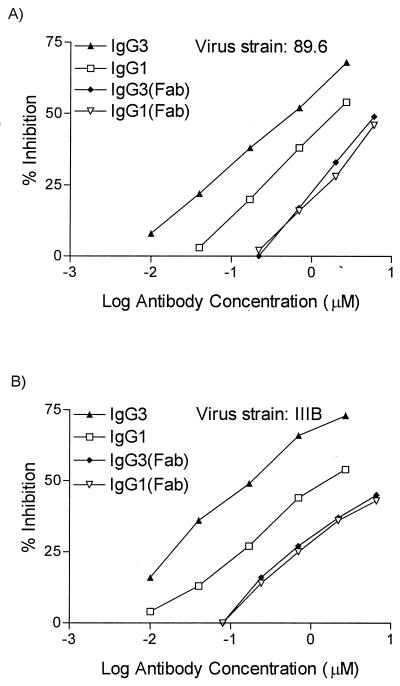
A possible explanation for the superior neutralization activity of IgG3 is that it has a greater content of neutralizing paratopes than do the other subclasses. Alternatively, the unique structural properties of the IgG3 protein may contribute to its greater effectiveness. The structure of IgG3 differs from that of the other subclasses in that human IgG3 has a long hinge region, consisting of 11 disulfide bonds, compared to only 2 disulfide bonds in IgG1 and 4 in IgG2 and IgG4. The distance between the Fab and Fc domains is therefore greater in IgG3 than in the other subclasses. This long hinge region permits the Fab arms to move more freely away from each other, since steric hindrance from the more distant Fc portion is diminished (7, 10, 21, 28). It has been theorized that enhanced hinge region flexibility allows more opportunity for antibodies to bind divalently to virions and cells expressing multivalent epitopes at certain densities (7, 21). It has also been suggested that certain murine subclasses exhibit noncovalent cooperative binding to each other via Fc, thus increasing their functional avidity for antigens, although this has not been demonstrated for human antibody subclasses (19). To test whether the Fab, hinge, or Fc region is responsible for the increased IgG3 neutralizing ability, IgG3 and IgG1 preparations were digested with either papain or pepsin. Fab monomers were tested for syncytium-inhibiting activity and compared with the uncleaved antibodies. Binding studies by flow cytometry indicated an about fivefold reduction in the binding of Fab fragments to 89.6-expressing 12E1 cells, compared to intact antibodies (data not shown). As expected, the fusion inhibition capacities of both IgG1 and IgG3 Fab were reduced compared to that of the intact divalent antibodies, as evidenced by the marked shifts in the fusion inhibition curves and the ID50 (Fig. 4). Importantly, the IgG3 Fab did not have a superior fusion inhibition capacity compared to IgG1 Fab fragments. Similar results were obtained with Fab fragments from two different HIVIG lots, with cells expressing either the IIIB (X4) or the 89.6 (R5) envelope. These results argue against the possibility that IgG3 from HIVIG has more or better paratopes against HIV-1 than does IgG1.
FIG. 4.
Fab monomers of IgG1 and IgG3 from HIVIG have similar syncytium-inhibiting abilities. Fab monomers generated by papain digestion were tested for the ability to block fusion by viral envelopes. Fab fragments were compared to the intact IgG preparations from which they were derived. (A) X4R5 strain 89.6. The ID50 (micromolar) were as follows: IgG1, 1.9; IgG3, 0.53; IgG1 Fab, 6.6; IgG3 Fab, 8.2. (B) X4 strain IIIB. The ID50 (micromolar) were as follows: IgG1, 1.7; IgG3, 0.18; IgG1 Fab, 9.0; IgG3 Fab, 9.0. The results shown are for lot 113. The same results were obtained in experiments using lot 114 IgG Fab fragments.
To determine whether an intact hinge region structure or the presence of Fc provides the advantage to IgG3, both IgG1 and IgG3 from HIVIG (lot 113) were digested with pepsin, which removes the Fc but not the hinge region. The resulting F(ab′)2 fragments were compared to intact HIVIG antibodies and to each other for the ability to block fusion mediated by the dual-tropic 89.6 envelope (Table 5) and the IIIB envelope (data not shown). In replicate experiments, IgG3 F(ab′)2 was better able to prevent syncytium formation than was IgG1 F(ab′)2, as evidenced by the 3.5-fold lower ID50 of IgG3 F(ab′)2. As before, when intact molecules were compared, the IgG3 ID50 was four- to fivefold lower than that of IgG1 antibodies.
TABLE 5.
Syncytium inhibition (89.6 envelope) by intact IgG versus F(ab′)2 and Faba
| HIVIG | ID50 (nM)
|
IgG1/IgG3 ratio | |
|---|---|---|---|
| IgG1 | IgG3 | ||
| Intact | 1,200 | 267 | 4.5 |
| F(ab′)2 fragment | 920 | 260 | 3.5 |
| Fab fragment | 5,600 | 4,800 | 1.2 |
12E1 cells were infected overnight with recombinant vaccinia virus expressing the envelope of dual-tropic HIV-1 strain 89.6 at 10 PFU/cell. Envelope-expressing cells were preincubated for 60 min with HIVIG subclasses, either intact or after enzymatic digestion into Fab and F(ab′)2 fragments. The cells were then mixed with PM1 target cells at a 1:1 ratio in triplicate, and syncytia were scored after 4 h of incubation at 37°C. The number of syncytia in control RSVIG-treated cultures was 437 ± 49 (mean ± standard deviation). ID50 were derived from linear regression analyses of fusion inhibition values at multiple doses of each Ig preparation.
Similar experiments were conducted with cell-free viruses DH123 and LAI. Again, it was found (Table 6 and data not shown) that both uncleaved and F(ab′)2 fragments of IgG3 had a lower ID50 than IgG1 (G1-to-G3 ID50 ratios of 5.0 and 5.2, respectively). The ID50 of the Fab fragments was higher for both subclasses, and no IgG3 advantage was observed for Fab fragments (Table 6).
TABLE 6.
Neutralization of virus strain DH123 with intact HIV IgG versus F(ab′)2 and Faba
| HIVIG | ID50 (μM)
|
IgG1/IgG3 ID50 ratio | |
|---|---|---|---|
| IgG1 | IgG3 | ||
| Intact | 254 | 51 | 5.0 |
| F(ab′)2 fragment | 187 | 36 | 5.2 |
| Fab fragment | 1,520 | 1,520 | 1.0 |
HIV-1 strain DH123 (100 TCID50) was preincubated with HIVIG subclasses, either intact or after enzymatic digestion into F(ab′)2 and Fab fragments. ID50 were determined 11 days after infection of PM1 cells. ID50 were determined by the method of Reed and Muench (34) and converted to micromolar concentrations based on the sizes of the fragments [150 kDa for intact molecules, 100 kDa for F(ab′)2, and 50 kDa for Fab fragments].
Overall, these results strongly suggest that the enhanced ability of IgG3 in HIVIG to neutralize HIV can be attributed to the longer heavy-chain hinge region and not the Fab or Fc portion of this subclass.
DISCUSSION
Passively transferred antibodies against HIV-1 can prevent infection in primates. However, the qualitative nature of optimal HIV-1-neutralizing antibodies has yet to be determined. Clearly, the specificity of the binding sites is critical and effective antibody prophylaxis in primate models has been achieved with mixtures of several broadly reactive MAbs targeting known neutralizing epitopes in gp120 and gp41 (1, 25–27). In several experiments, polyclonal HIVIG preparations were added to mixtures of MAbs with an added benefit (25–27). The structural properties of antibodies which are different for each subclass may influence their functional affinity for antigens (7, 21). The long hinge region of IgG3 permits enhanced flexibility of the Fab arms, thus permitting better divalent binding to multivalent epitopes spaced at a certain range of distances from each other (10, 21). Studies of murine and human antibodies have shown that epitope density can affect functional affinity (8). Fc regions also differ among subclasses, and it has been theorized that mouse IgG3 Fc regions tend to aggregate with one another, thereby facilitating the binding of more antibodies to a multivalent antigenic surface (19). In the present study, we sought to determine whether a particular IgG subclass, isolated from a polyclonal preparation of HIVIG, was more effective in in vitro virus neutralization assays. IgG3 had enhanced activity compared with IgG1 and IgG2 in HIV-1 envelope-based fusion assays that are commonly used as a surrogate for cell-to-cell viral transmission. In addition, more efficient neutralization of several HIV-1 strains (both X4 and R5), including the primary isolate DH123 (X4R5), by IgG3 was observed. Interestingly, solid-phase binding assays were not predictive of the higher neutralization efficiency of HIVIG IgG3. Important conformational epitopes may be lost when viral antigens are bound to a solid phase, such as ELISA plates, or on Western blots. In these assays, binding only indicates the overall presence of anti-HIV antibody and is not an indicator of function. However, in flow cytometric cell-binding assays (using 12E1 cells expressing several different HIV-1 envelopes), no differential binding was found with any of the IgG subclasses (data not shown). In summary, none of the binding assays used in our study were predictive of the superior neutralizing capacity of IgG3.
The increased anti-HIV-1 activity of IgG3 was lost when Fab fragments were generated, suggesting that the IgG1 and IgG3 polyclonal Fab fragments have similar affinities for neutralizing epitopes. This is not surprising, since HIVIG is made from the plasma of more than 100 infected individuals from different regions of the United States and therefore it is unlikely that fine specificities could account for the observed differences among subclasses. The functional affinity of the intact antibodies seems to differ among the subclasses, and this difference appears to depend upon the unique structure of the IgG3 molecule. This conclusion is in agreement with previous work by Cavacini et al., who prepared HIV-1-neutralizing MAbs with identical Fab regions fused to IgG1 and IgG3 constant regions. They showed that genetically engineered IgG3 MAb F105 (specific for a discontinuous epitope in the CD4-binding site) had superior neutralizing ability against some laboratory-adapted strains of HIV-1 compared to IgG1, despite their identical antigen-binding regions (5). The precise structural advantage of IgG3 could be due to enhanced Fab arm flexibility or to a hypothetical greater tendency to self-aggregate via Fc. F(ab′)2 fragments containing the hinge but not the Fc portion were generated to distinguish these possibilities. When IgG1 and IgG3 F(ab′)2 were compared, IgG3 maintained its superior neutralization activity (three- to fivefold lower ID50) that was similar to or better than that of the intact molecule (Tables 5 and 6). Thus, the presence of the hinge region, in the context of a bivalent molecule, was required and sufficient for the higher fusion inhibition activity of IgG3.
It is important to determine whether IgG3 antibodies also demonstrate enhanced anti-HIV-1 activity in vivo. Such a finding would have several implications. It is already apparent for vaccines and for prophylaxis that the breadth of neutralizing antibody specificities must be extensive and that no single MAb is likely to be fully effective. Improvement of functional affinity may further enhance the efficacy of antibodies. If IgG3 antibodies have better in vivo function, it may prove useful to engineer MAbs with the IgG3 heavy chain. In addition to passive immunization, prophylactic vaccines could be envisioned which utilize adjuvants that favor the generation of IgG3 antibodies. Normally, IgG3 accounts for only 5 to 10% of plasma IgG. Polyclonal HIVIG preparations could also be improved, since enrichment for IgG3 is simply achieved by the use of protein A columns. However, the use of IgG3 monoclonal and polyclonal preparations would also require certain considerations and concessions to this unique isotype. Because of its long hinge region, IgG3 is more susceptible to degradation by plasma proteases and it has a half-life of only 7 days (29). In contrast, the other subclasses exhibit a half-life of 23 days (29). For the same reasons, IgG3 preparations must be carefully manufactured so that trace amounts of contaminating serum proteases are removed. Avoidance of manufacturing steps which affect IgG3 structure, such as the use of β-propiolactone for viral inactivation, would be important (39, 40). None of the viral neutralization assays described here contained complement. However, IgG3 is the most effective complement-fixing antibody subclass (30). Complement-mediated lysis of virus-infected cells is also an important mechanism of defense against HIV-1, and this could confer an additional advantage in vivo (32, 44).
Overall, these studies indicate that IgG3 from polyclonal HIVIG has enhanced anti-HIV-1 activity compared to that of IgG1 and IgG2. Future studies will focus upon determining whether this advantage is retained in vivo. If in vivo studies confirm these results, strategies which favor IgG3 may provide a new approach for improving HIV vaccines and passive immunotherapy with MAbs or polyclonal antibodies.
ACKNOWLEDGMENTS
Orit Scharf and Hana Golding contributed equally to this work.
This research was supported by a Center for Biologics HIV Collaborative Research Award which was funded by the Center Director. O.S. was supported by the Research Participation Program at CBER administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. FDA.
We are grateful to Suzanne Epstein and Andrew Dayton for review of the manuscript.
Footnotes
Dedicated to Donald Tankersley, who contributed much helpful advice in the early stages of this project and who is deeply missed by the Laboratory of Plasma Derivatives.
REFERENCES
- 1.Baba T W, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini L A, Posner M R, Katinger H, Stiegler G, Bernacky B J, Rizvi T A, Schmidt R H L R, Keeling M E, Lu Y, Wright J E, Chou T-C, Ruprecht R M. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 2.Berman P W, Gregory T J, Riddle L, Nakamura G R, Champe M A, Porter J P, Wurm F M, Hershberg R D, Cobb E K, Eichberg J W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990;345:622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 3.Broder C C, Berger E. Fusogenic selectivity of the envelope glycoproteins is a major determinant of human immunodeficiency virus type 1 tropism for CD4+ T-cell lines vs. primary macrophages. Proc Natl Acad Sci USA. 1995;92:9004–9008. doi: 10.1073/pnas.92.19.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruck C, Thiriart C, Fabry L, Francotte M, Pala P, Van Opstal O, Culp J, Rosenberg M, De Wilde M, Heidt P. HIV-1 envelope-elicited neutralizing antibody titres correlate with protection and virus load in chimpanzees. Vaccine. 1994;12:1141–1148. doi: 10.1016/0264-410x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 5.Cavacini L A, Emes C L, Power J, Desharnais F D, Duval M, Montefiori D, Posner M R. Influence of heavy chain constant regions on antigen binding and HIV-1 neutralization by a human monoclonal antibody. J Immunol. 1995;155:3638–3644. [PubMed] [Google Scholar]
- 6.Collman R, Balliet J W, Gregory A A, Friedman H, Kolson D L, Nathenson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper L J N, Robertson D, Granzow R, Greenspan N S. Variable domain-identical antibodies exhibit IgG subclass-related differences in affinity and kinetic constants as determined by surface plasmon resonance. Mol Immunol. 1994;31:577–584. doi: 10.1016/0161-5890(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 8.Cooper L J N, Shikhman A R, Glass D D, Kangisser D, Cunningham M W, Greenspan N S. Role of specificity differences among streptococcal IgG antibodies expressing identical variable domains. J Immunol. 1993;150:2231–2242. [PubMed] [Google Scholar]
- 9.Cummins L M, Weinhold K J, Matthews T J, Langlois A J, Perno C F, Condie R M, Allain J-P. Preparation and characterization of an intravenous solution of IgG from human immunodeficiency virus-seropositive donors. Blood. 1991;77:1111–1117. [PubMed] [Google Scholar]
- 10.Dangl J L, Wensel T G, Morrison S L, Stryer L, Herzenberg L A, Oi V T. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 1988;7:1989–1994. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duhamel R C, Schur P H, Brendel K, Meezan E. pH gradient elution of human IgG1, IgG2, and IgG4 from protein A-Sepharose. J Immunol Methods. 1979;31:211–217. doi: 10.1016/0022-1759(79)90133-9. [DOI] [PubMed] [Google Scholar]
- 12.Earl P L, Koenig S, Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991;65:31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eichberg J W, Murthy K K, Ward R H R, Prince A M. Prevention of HIV infection by passive immunization with HIVIG or CD4-IgG. AIDS Res Hum Retrovir. 1992;8:1515. doi: 10.1089/aid.1992.8.1515. [DOI] [PubMed] [Google Scholar]
- 14.Finlayson J. Immune globulins. Semin Thromb Hemostasis. 1979;6:44–74. doi: 10.1055/s-2007-1005095. [DOI] [PubMed] [Google Scholar]
- 15.Finlayson J. Immune globulins with special reference to their role in bacterial and viral infections. In: Easmon C S F, Jeljaszewicz J, editors. Medical microbiology. Vol. 1. London, England: Academic Press; 1982. pp. 129–182. [Google Scholar]
- 16.Frankel, S. S., R. M. Steinman, N. L. Michael, S. Ratto Kim, N. Bhardwaj, M. Pope, M. K. Louder, P. K. Ehrenberg, P. W. H. I. Parren, D. R. Burton, H. Katinger, T. C. VanCott, M. L. Robb, D. L. Birx, and J. R. Mascola. 1998. Neutralizing monoclonal antibodies block human immunodeficiency virus type 1 infection of dendritic cells and transmission to T cells. J. Virol. 9788–9794. [DOI] [PMC free article] [PubMed]
- 17.Gorny M K, Conley A J, Karwowska S, Buchbinder A, Xu J-Y, Emini E A, Koenig S, Zolla-Pazner S. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992;66:7538–7542. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorny M K, Xu J-Y, Karwowska S, Buchbinder A, Zolla-Pazner S. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol. 1993;150:635–643. [PubMed] [Google Scholar]
- 19.Greenspan N S, Cooper L J N. Intermolecular cooperativity: a clue to why mice have IgG3? Immunol Today. 1992;13:164–168. doi: 10.1016/0167-5699(92)90120-V. [DOI] [PubMed] [Google Scholar]
- 20.Heeney J L, Teeuwsen V J P, Van Gils M, Bogers W J M, DeGiuli Morghen C, Radaelli A, Barnett S, Morein B, Akerblom L, Wang Y, Lehner T, Davis D. Beta-chemokines and neutralizing antibody titers correlate with sterilizing immunity generated in HIV-1 vaccinated macaques. Proc Natl Acad Sci USA. 1998;95:10803–10808. doi: 10.1073/pnas.95.18.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber R, Bennett W S. Antibody-antigen flexibility. Nature. 1987;326:334–335. doi: 10.1038/326334a0. [DOI] [PubMed] [Google Scholar]
- 22.Jonak Z L, Clark R K, Matour D, Trulli S, Craig R, Henri E, Lee E V, Greig R, Debouck C. A human lymphoid recombinant cell line with functional human immunodeficiency virus type 1 envelope. AIDS Res Hum Retrovir. 1993;9:23–32. doi: 10.1089/aid.1993.9.23. [DOI] [PubMed] [Google Scholar]
- 23.Lambert J S, Mofenson L M, Fletcher C V, Moye J J, Stiehm E R, Meyer III W A, Nemo G J, Mathieson B J, Hirsch G, Sapan C V, Cummins L M, Jimenez E, O'Neill E, Kovacs A, Stek A. Safety and pharmacokinetics of hyperimmune anti-human immunodeficiency virus (HIV) immunoglobulin administered to HIV-infected pregnant women and their newborns. J Infect Dis. 1997;175:283–291. doi: 10.1093/infdis/175.2.283. [DOI] [PubMed] [Google Scholar]
- 24.Lusso P, Cocchi F, Balota C, Markham P D, Louie A, Farci P, Pal R, Gallo R C, Reitz M S., Jr Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J Virol. 1995;69:3712–3720. doi: 10.1128/jvi.69.6.3712-3720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C R, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascola J R, Louder M K, VanCott T C, Sapan C V, Lambert J S, Muenz L R, Bunow B, Birx D L, Robb M L. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J Virol. 1997;71:7198–7206. doi: 10.1128/jvi.71.10.7198-7206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascola J R, Stiegler G, VanCott T C, Katinger H, Carpenter C B, Hanson C E, Beary H, Hayes D, Frankel S S, Birx D L, Lewis M G. Protection of macaques against vaginal transmission of pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 28.Michaelsan T E, Frangione B, Franklin E C. Primary structure of the “hinge” region of human IgG3. J Biol Chem. 1977;252:883–889. [PubMed] [Google Scholar]
- 29.Morell A, Terry W D, Waldmann T A. Metabolic properties of IgG subclasses in man. J Clin Investig. 1970;49:673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oi V T, Vuong T M, Hardy R, Reidler J, Dangl J, Hezenberg L A, Stryer L. Correlation between segmental flexibility and effector function of antibodies. Nature. 1984;307:136–140. doi: 10.1038/307136a0. [DOI] [PubMed] [Google Scholar]
- 31.Orenstein W A, Heymann D L, Ellis R J, Rosenberg R L, Nakano J, Halsey N A, Overturf G D, Hayden G F, Witte J J. Prophylaxis of varicella in high-risk children: dose-response effect of zoster immune globulin. J Pediatr. 1981;98:368–373. doi: 10.1016/s0022-3476(81)80697-x. [DOI] [PubMed] [Google Scholar]
- 32.Posner M R, Elboim H S, Cannon T, Cavacini L, Hideshima T. Functional activity of an HIV-1 neutralizing IgG human monoclonal antibody: ADCC and complement-mediated lysis. AIDS Res Hum Retrovir. 1992;8:553–558. doi: 10.1089/aid.1992.8.553. [DOI] [PubMed] [Google Scholar]
- 33.Prince A M, Reesink H, Pascual D, Gorowitz B, Hewlett I, Murthy K K, Cobb K E, Eichberg J W. Prevention of HIV infection by passive immunization with HIV immunoglobulin. AIDS Res Hum Retrovir. 1991;7:971–973. doi: 10.1089/aid.1991.7.971. [DOI] [PubMed] [Google Scholar]
- 34.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 35.Shibata R, Hoggan M D, Broscius H C, Englund G, Theodore T S, Bucklewhite A, Arthur L O, Israel Z, Schultz A, Lane H C, Martin M A. Isolation and characterization of a syncytium-inducing, macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J Virol. 1995;69:4453–4462. doi: 10.1128/jvi.69.7.4453-4462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M A. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–216. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 37.Shibata R, Siemon C, Czajak S C, Desrosiers R C, Martin M A. Live, attenuated simian immunodeficiency virus vaccines elicit potent resistance against a challenge with a human immunodeficiency virus type 1 chimeric virus. J Virol. 1997;71:8141–8148. doi: 10.1128/jvi.71.11.8141-8148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skvaril F, Morell A. The G4 subclass in IgG fractions prepared by ion-exchange chromatography. J Immunol. 1970;104:1310–1312. [PubMed] [Google Scholar]
- 39.Skvaril F, Gardi A. Differences among available immunoglobulin preparations for intravenous use. Pediatr Infect Dis J. 1988;7(Suppl. 5):S43–S48. [PubMed] [Google Scholar]
- 40.Skvaril F, Roth-Wicky B, Barandun S. IgG subclasses in human gamma-globulin preparations for intravenous use and their reactivity with staphylococcus protein A. Vox Sang. 1980;38:147–155. doi: 10.1111/j.1423-0410.1980.tb02342.x. [DOI] [PubMed] [Google Scholar]
- 41.Stiehm E R, Fletcher C V, Mofenson L M, Palumbo P E, Kang M, Fenton T, Sapan C V, Meyer III W A, Shearer W T, Hawkins E, Lowler M G, Bouquin P, Purdue L, Sloand E M, Nemo G J, Wara D, Bryson Y J, Starr S E, Petru A, Burchett S. Use of human immunodeficiency virus (HIV) human hyperimmune immunoglobulin in HIV type 1-infected children (Pediatric AIDS Clinical Trials Group protocol 273) J Infect Dis. 2000;181:548–554. doi: 10.1086/315224. [DOI] [PubMed] [Google Scholar]
- 42.Stiehm E R, Lambert J S, Mofenson L M, Bethel J, Whitehouse J, Nugent R, Moye J, Jr, Fowler M G, Mathieson B J, Reichelderfer P, Nemo G J, Korelitz J, Meyer III W A, Sapan C V, Jimenez E, Gandia J, Scott G, O'Sullivan M J, Kovacs A, Stek A, Shearer W T, Hammill H. Efficacy of zidovudine and human immunodeficiency virus (HIV) hyperimmune immunoglobulin for reducing perinatal HIV transmission from HIV-infected women with advanced disease: results of pediatric AIDS Clinical Trials Group protocol 185. J Infect Dis. 1999;179:567–575. doi: 10.1086/314637. [DOI] [PubMed] [Google Scholar]
- 43.Stiehm R E, Ashida E, Kim K S, Winston D J, Haas A, Gale R. Intravenous immunoglobulins as therapeutic agents. Ann Intern Med. 1987;107:367–382. doi: 10.7326/0003-4819-107-2-367. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan B L, Knopoff E J, Saifuddin M, Takefman D M, Saarloos M N, Sha B E, Spear G T. Susceptibility of HIV-1 plasma virus to complement-mediated lysis. Evidence for a role in clearance of virus in vivo. J Immunol. 1996;157:1791–1798. [PubMed] [Google Scholar]
- 45.Virella G P, Parkhouse R M. Papain sensitivity of heavy chain sub-classes on normal human IgG and localization of antigenic determinants for the sub-classes. Immunochemistry. 1971;8:243–250. doi: 10.1016/0019-2791(71)90478-2. [DOI] [PubMed] [Google Scholar]