Abstract
Background
Noncommunicable diseases (NCDs) predominantly affect adults, but pathophysiological changes begin decades earlier, as a continuum, with initial events apparent in adolescence. Hence, early identification and intervention are crucial for the prevention and management of NCDs. We investigated the complex network of socioeconomic, behavioral, and metabolic factors associated with the presence of NCD in Brazilian adolescents.
Methods
We conducted a cross-sectional study nested within the São Luís segment of the Ribeirão Preto, Pelotas, and São Luís (RPS) cohort’s consortium, focusing on 18–19-year-olds (n = 2515). Data were collected prospectively, from which we constructed a complex network with NCD-related factors/indicators as nodes and their co-occurrences as edges. General and sex-based models analyzed: socioeconomic status, behavioral (smoking, alcohol, and other drugs use, unhealthy diet, poor sleep, physical inactivity), and metabolic factors (overweight/obesity, elevated blood pressure, poor lipid profile). We also looked for NCDs in adolescence like asthma, abnormal spirometry, depression, suicide risk, and poor oral health. The network was characterized by degree, betweenness, eigenvector, local transitivity, Shannon entropy, and cluster coefficient.
Results
The adolescents had an average age of 18.3 years, 52.3% were female and 47.7% male. 99.8% of them have a diet rich in free sugars, 15% are overweight/obese and 72.3% had an elevated TyG index. High free sugar emerged as the central hub, followed by high TyG index (an early marker of insulin resistance) and low socioeconomic class. In males, low fiber intake and a high triglycerides/HDL ratio highlighted cardiometabolic concerns; in females, sedentary behavior and poor sleep marked metabolic and psychological challenges, along with caries in both sexes.
Conclusions
Our findings provide insights into central health challenges during adolescence, such as high free sugars, insulin resistance, and low socioeconomic indicators, suggesting that interventions targeted at these central hubs could have a significant impact on their NCD network.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-024-01469-8.
Keywords: Adolescent, Noncommunicable diseases, Dietary sugar, Insulin resistance, Socioeconomic status, Complex network
Background
Non-communicable diseases (NCDs) are the leading cause of premature deaths globally, affecting individuals under 70 years old, especially in low and middle-income countries [1]. Addressing NCDs is critical to achieving the Agenda for Sustainable Development goals, which include a target to reduce premature mortality from NCDs by one-third by 2030 through prevention and treatment. Key to this effort are high-impact NCD interventions alongside the early identification of risk factors [2].
By the age of 40, many individuals will develop at least one NCD as a lifetime risk, and a third of them will be subsequently diagnosed with multiple NCDs due to shared risk factors [3]. Although NCDs predominantly affect adults, their pathophysiological continuum traces back to much earlier in life, with initial events detectable in youth [4]. Detecting early NCD signs, recognizing their determinants and risk factors, and understanding how these conditions cluster are essential for developing effective prevention and control strategies [5]. Adolescence is a sensitive period of human development, during which exposure to risk factors can profoundly impact biological and cognitive systems [6]. Behavioral factors are often established during this life period, potentially leading to metabolic dysfunctions and elevating the risk of developing NCDs later in life [7].
Traditional analyses of behavioral and metabolic conditions often fail to capture the complexity of their relationships at the onset of NCDs [8]. Identifying how and which related factors co-occur can improve diagnostic and intervention strategies at both clinical and population levels [9]. Moreover, public policies developed without considering the interconnected nature of these factors may lead to suboptimal resource allocation and less effective intervention programs [10]. Complex network analysis offers a holistic and comprehensive method to examine these intricate systems, especially in challenging environments with great social and healthcare disparities, allowing for the identification of subtle interactions and hidden patterns [11].
We hypothesize that non-linear relationships among contributing factors form a complex network, leading to NCD clusters already in adolescence. Accordingly, we investigated the complex connections between socioeconomic determinants, behavioral and metabolic factors with NCDs in Brazilian adolescents. This knowledge would shed light on the interconnected nature of these factors and the NCD signs, ultimately informing more effective prevention and intervention strategies.
Methods
Study design
A population-based cross-sectional study nested within the São Luís, segment of the Ribeirão Preto, Pelotas, and São Luís (RPS) cohort’s consortium in Brazil, focusing on 18–19 year-olds. All individuals who participated at the inception of the original cohort were invited to join the second follow-up (n = 687). To enhance the statistical power of the sample and minimize potential dropouts, the cohort follow-up also included 1133 individuals born in São Luís in 1997, identified through the Brazilian Live Birth Information System (SINASC). Additionally, the sample encompassed 695 volunteers born in 1997 and identified through schools and universities registered in SINASC [12]. Thus, the study's sample comprised 2515 adolescents, with 52.3% female and 47.7% male participants.
Ethical statement
This study was approved by the Ethics and Research Committee of the Federal University of Maranhão University Hospital (IRB #1302489). All participants signed the informed consent.
Data collection
To capture and analyze co-occurrence patterns in complex networks, we transformed variables into dichotomous forms in order to simplify the representation and interpretation of connections between health conditions and contributing factors.
Socioeconomic determinants
Socioeconomic information was collected using an assessment questionnaire. The socioeconomic variables included low educational attainment of the adolescent and the family head (up to elementary school), low family income (≤ USD 270.76, based on the Brazilian national minimum wage in 2016), and low socioeconomic class (C–E classes) based on the Brazilian Economic Classification Criteria (A representing the highest and E the lowest) [13].
Behavioral factors
Current tobacco use and current or former use of other drugs (marijuana, cocaine, heroin, ecstasy, crack, or LSD) were collected through a confidential self-reported questionnaire. High alcohol consumption was assessed using the Alcohol Use Disorders Identification Test (AUDIT).
Dietary information was collected using a quality–quantity Food Frequency Questionnaire (FFQ), administered by trained nutritionists, which asked about the frequency, portion size, and quantity of food consumed over the past 12 months. The final measures were reported as daily consumption. Dietary variables included high intake of saturated fats (≥ 10% of total calories), lipids (≥ 30% of total calories), sodium (≥ 5 g), and free sugars (≥ 5% of total calories, ideal limit according by the World Health Organization-WHO) [14]. Low fiber intake was defined as < 25 g for females and 38 g for males. Low protein intake was < 0.8 g/kg of body weight, while high protein intake was > 2 g/kg. Sensitivity analysis evaluated high free sugar consumption as 10% (maximum WHO guidelines limits) [14] and 25 g (maximum American Heart Association recommendation) [15].
Sedentary behavior was defined as < 150 min of physical activity per week by the Self-Administered Physical Activity Checklist (SAPAC) questionnaire. Poor sleep quality was based on scores > 5 in the Pittsburgh Sleep Quality Index (PSQI), and excessive daytime sleepiness by scores ≥ 9 on the Epworth Sleepiness Scale (ESS).
Metabolic conditions
We considered overweight/obesity as a body mass index > 25 kg/m2 and high body fat percentage ≥ 30% in females and ≥ 25% in males, using a BOD POD and a stadiometer (Altura Exata®). Hypertension was defined by blood pressure > 130/80 mmHg and high pulse wave velocity ≥ 90th percentile of the sample distribution [16]. Additional markers included high levels of total cholesterol (≥ 200 mg/dL), LDL-c (≥ 130 mg/dL), VLDL-c (≥ 30 mg/dL), triglycerides (≥ 150 mg/dL), and low HDL-c (< 40 mg/dL). The triglycerides/HDL-c ratio ≥ 1.75 [17] and the TyG index ≥ 7.94 [18] were utilized as markers of insulin resistance.
NCD in adolescence
Asthma was self-reported based on a previous medical diagnosis. Lung capacity was evaluated using the KoKo PFT Spirometer (Koko® Spirometer), with Forced Expiratory Volume in 1 s (FEV1) values < 80% of the predicted value considered abnormal [19].
Depression and suicide risk were assessed using the Mini-International Neuropsychiatric Interview (MINI), diagnosing major and recurrent major depressive episodes. High suicide risk was indicated by a score ≥ 6.
Oral health was assessed using the number of decayed teeth (DMFT index) and recording severe caries (characterized by visible pulpal involvement and ulceration caused by dislocated tooth fragments, fistula, or abscess). Periodontitis was classified following the American Academy of Periodontology/European Federation of Periodontology (AAP/EFP) guidelines and dichotomized as none or any periodontitis. The presence of bleeding on probing (BoP) was set if > 15% of surfaces were affected [20].
Data analysis
We created an undirected graph structure based on a co-occurrence matrix, where nodes represented contributing factors/health conditions and edges represented their co-occurrences. The edge was weighted according to the proportion of co-occurrences between conditions. We built general and sex-based models for analysis.
To describe the network's structure, we calculated four centrality measures specific to each node: degree, betweenness, eigenvector centrality, and local transitivity. The degree of a node (condition) represents its number of connections (edges), indicating its involvement in the network. Betweenness measures how often a node serves as a bridge on the shortest paths between other nodes; a higher betweenness value suggests the node (condition) acts as a mediator or bridge between other conditions, implying shared pathophysiological mechanisms or risk factors. Eigenvector centrality assigns a value to each node based on the centrality of the nodes to which it is connected, thus identifying influential nodes and assigning higher values to conditions connected to many other highly connected diseases. Local transitivity is the ratio of the number of triangles connected to a node to the number of triples centered on that node, indicating the probability that two diseases will co-occur if they are both linked to a third disease [21].
The Shannon entropy specific to each node was measured to evaluate the diversity of each node's connections, with higher values indicating more diverse connections [22]. Shannon entropy for the entire network was also computed to reflect the overall complexity of the network structure [23]. The clustering coefficient was also calculated to assess the likelihood that a node forms a triangle with two other nodes, thus providing insights into the network’s clustering characteristics.
Results
Population characteristics are presented in Table 1. Adolescents had an average age of 18.3 years, 52.3% were female and 47.7% male. 99.8% consumed more than 5% of their daily calories from free sugars, regardless of sex (p = 0.178). Additionally, 72.3% had a TyG index above 7.94. Approximately 70% belonged to socioeconomic classes C, D, or E, with 54.4% being females (p = 0.018). Furthermore, the education level of the family head, smoking, alcohol and drug use, high consumption of saturated fats and lipids, low fiber intake, metabolic conditions, poorer mental and respiratory health indicators, sedentary behavior, and periodontitis were all associated with the sex of the participant.
Table 1.
Population characteristics. RPS São Luís (N = 2515)
| Overall | Male | Female | p-value | |
|---|---|---|---|---|
| Adolescent education | 0.160 | |||
| High school or more | 2486 (98.9) | 1177 (47.5) | 1301 (52.5) | |
| Elementary school or less | 27 (1.1) | 17 (63.0) | 10 (37.0) | |
| Family head education | 0.011 | |||
| High school or more | 1919 (76.5) | 942 (49.1) | 977 (50.9) | |
| Elementary school or less | 588 (23.5) | 253 (43.0) | 335 (57.0) | |
| Household income | 1.00 | |||
| > USD 270.76 | 1214 (54.2) | 575 (47.5) | 635 (52.5) | |
| ≤ USD 270.76 | 1026 (45.8) | 486 (47.5) | 537 (52.5) | |
| Socioeconomic class | 0.018 | |||
| A/B | 654 (29.5) | 334 (51.2) | 318 (48.8) | |
| C/D/E | 1560 (70.5) | 709 (45.6) | 845 (54.4) | |
| Smoking | < 0.001 | |||
| No | 2407 (96.5) | 1127 (46.8) | 1280 (53.2) | |
| Yes | 88 (3.5) | 61 (69.3) | 27 (30.7) | |
| Alcohol use | < 0.001 | |||
| No | 2026 (80.7) | 905 (44.8) | 1116 (55.2) | |
| Yes | 486 (19.3) | 290 (59.7) | 196 (40.3) | |
| Drug use | < 0.001 | |||
| No | 2030 (82.1) | 913 (45.0) | 1117 (55.0) | |
| Yes | 443 (17.9) | 264 (59.6) | 179 (40.4) | |
| Sutured fat consumptiona | < 0.001 | |||
| < 10% | 1819 (72.8) | 935 (51.4) | 884 (48.6) | |
| ≥ 10% | 680 (27.2) | 254 (37.4) | 426 (62.6) | |
| Lipids consumptiona | < 0.001 | |||
| < 30% | 2249 (90.0) | 1102 (49.0) | 1147 (51.0) | |
| ≥ 30% | 250 (10.0) | 87 (34.8) | 163 (65.2) | |
| Free sugar consumption | ||||
| < 5%a | 6 (0.2) | 5 (83.3) | 1 (16.7) | 0.178 |
| ≥ 5% | 2493 (99.8) | 1184 (47.5) | 1309 (52.5) | |
| < 10%a | 56 (2.2) | 29 (51.8) | 27 (48.2) | 0.616 |
| ≥ 10% | 2443 (97.8) | 1160 (47.5) | 1283 (52.5) | |
| < 25 gb | 25 (1.0) | 14 (56.0) | 11 (44.0) | 0.524 |
| ≥ 25 g | 2482 (99.0) | 1181 (47.6) | 1301 (52.4) | |
| Sodium consumption | 0.230 | |||
| < 5 g | 2415 (96.3) | 1145 (47.4) | 1270 (52.6) | |
| ≥ 5 g | 92 (3.7) | 50 (54.3) | 42 (45.7) | |
| Protein consumptionc | 0.950 | |||
| ≥ 0.8 g/kg | 1734 (70.5) | 833 (48.0) | 901 (52.0) | |
| < 0.8 g/kg | 725 (29.5) | 350 (48.3) | 375 (51.7) | |
| 0.690 | ||||
| ≤ 2.0 g/kg | 2227 (90.6) | 1068 (48.0) | 1159 (52.0) | |
| > 2.0 g/kg | 232 (9.4) | 115 (49.6) | 117 (50.4) | |
| Fiber consumption | < 0.001 | |||
| ≥ 25 g for women; ≥ 38 g for men | 1446 (58.0) | 558 (38.6) | 886 (61.4) | |
| < 25 g for women; < 38 g for men | 1049 (42.0) | 634 (60.4) | 415 (39.6) | |
| Body mass index | 0.530 | |||
| Eutrophic | 2002 (81.0) | 962 (48.1) | 1040 (51.9) | |
| Overweight/Obesity | 370 (15.0) | 169 (45.7) | 201 (54.3) | |
| Blood pressure | < 0.001 | |||
| < 130/80 mgHg | 2055 (83.3) | 882 (43.0) | 1168 (57.0) | |
| ≥ 130/80 mgHg | 413 (16.7) | 302 (73.1) | 111 (26.9) | |
| PWVd | < 0.001 | |||
| < 90th percentile | 1983 (89.0) | 920 (46.5) | 1058 (53.5) | |
| ≥ 90th percentile | 245 (11.0) | 153 (62.4) | 92 (37.6) | |
| Body fat | < 0.001 | |||
| < 30% for women; < 25% for men | 1810 (73.5) | 1059 (58.7) | 745 (41.3) | |
| ≥ 30% for women; ≥ 25% for men | 652 (26.5) | 121 (18.6) | 531 (81.4) | |
| Total cholesterol | < 0.001 | |||
| < 200 mg/dL | 2071 (90.1) | 1034 (50.1) | 1029 (49.9) | |
| ≥ 200 mg/dL | 228 (9.9) | 73 (32.0) | 155 (68.0) | |
| LDL-c | 0.001 | |||
| < 130 mg/dL | 2107 (92.0) | 1041 (49.4) | 1066 (50.6) | |
| ≥ 130 mg/dL | 184 (8.0) | 67 (36.4) | 117 (63.6) | |
| VLDL-c | 0.002 | |||
| < 30 mg/dL | 2089 (90.9) | 984 (47.3) | 1097 (52.7) | |
| ≥ 30 mg/dL | 209 (9.1) | 123 (58.9) | 86 (41.1) | |
| Triglycerides | < 0.001 | |||
| < 150 mg/dL | 2088 (91.1) | 985 (47.2) | 1103 (52.8) | |
| ≥ 150 mg/dL | 203 (8.9) | 123 (60.6) | 80 (39.4) | |
| HDL-c | < 0.001 | |||
| ≥ 40 mg/dL | 1834 (80.0) | 782 (42.6) | 1052 (57.4) | |
| < 40 mg/dL | 458 (20.0) | 326 (71.2) | 132 (28.8) | |
| Triglycerides/ HDL-c | < 0.001 | |||
| < 1.75 | 1471 (59.2) | 584 (39.8) | 882 (60.2) | |
| ≥ 1.75 | 1014 (40.8) | 599 (59.2) | 412 (40.8) | |
| TyG index | 0.688 | |||
| < 7.94 | 634 (27.7) | 307 (48.7) | 324 (51.3) | |
| ≥ 7.94 | 1653 (72.3) | 785 (47.6) | 864 (52.4) | |
| Asthma | 0.040 | |||
| No | 2209 (88.7) | 1035 (46.9) | 1174 (53.1) | |
| Yes | 282 (11.3) | 151 (53.5) | 131 (46.5) | |
| FEV1e | < 0.001 | |||
| > 80% | 1485 (66.1) | 758 (51.0) | 727 (49.0) | |
| ≤ 80% | 762 (33.9) | 316 (41.5) | 446 (58.5) | |
| Depression | < 0.001 | |||
| No | 2135 (85.7) | 1089 (51.0) | 1046 (49.0) | |
| Yes | 356 (14.3) | 97 (27.2) | 259 (72.8) | |
| Suicide risk | < 0.001 | |||
| 1–5 score | 2368 (94.5) | 1155 (48.8) | 1213 (51.2) | |
| ≥ 6 score | 139 (5.5) | 40 (28.8) | 99 (71.2) | |
| Physical activity | < 0.001 | |||
| ≥ 150 min | 1377 (55.2) | 892 (64.8) | 485 (35.2) | |
| < 150 min | 1117 (44.8) | 295 (26.4) | 822 (73.6) | |
| Sleep quality | < 0.001 | |||
| Good | 977 (46.3) | 527 (53.9) | 450 (46.1) | |
| Poor | 1133 (53.7) | 471 (41.6) | 662 (58.4) | |
| Sleepiness | < 0.001 | |||
| No | 1582 (63.2) | 808 (51.1) | 774 (48.9) | |
| Yes | 920 (36.8) | 383 (41.6) | 537 (58.4) | |
| Caries | 0.565 | |||
| No | 1054 (44.1) | 492 (46.8) | 559 (53.2) | |
| Yes | 1336 (55.9) | 640 (48.1) | 691 (51.9) | |
| Severe caries | 0.220 | |||
| No | 1459 (73.5) | 688 (47.3) | 766 (52.7) | |
| Yes | 527 (26.5) | 265 (50.6) | 259 (49.4) | |
| Periodontitis | 0.013 | |||
| No | 1548 (65.0) | 710 (46.0) | 835 (54.0) | |
| Yes | 832 (35.0) | 426 (51.4) | 403 (48.6) | |
| Bleeding on probingf | 0.706 | |||
| < 15% | 1352 (56.8) | 640 (47.5) | 708 (52.5) | |
| ≥ 15% | 1028 (43.2) | 496 (48.3) | 530 (51.7) |
The p-value was obtained from the Chi-squared test to observe differences between the sexes at a significance level of 5%. Totals may vary due to missing data for some variables
a% of total daily calories
bgrams per day
cConsumption in grams in relation to body weight
dPWV: Pulse wave velocity
eFEV1: Forced expiratory volume in the first second
f% of sites affected in comparison to the total evaluated
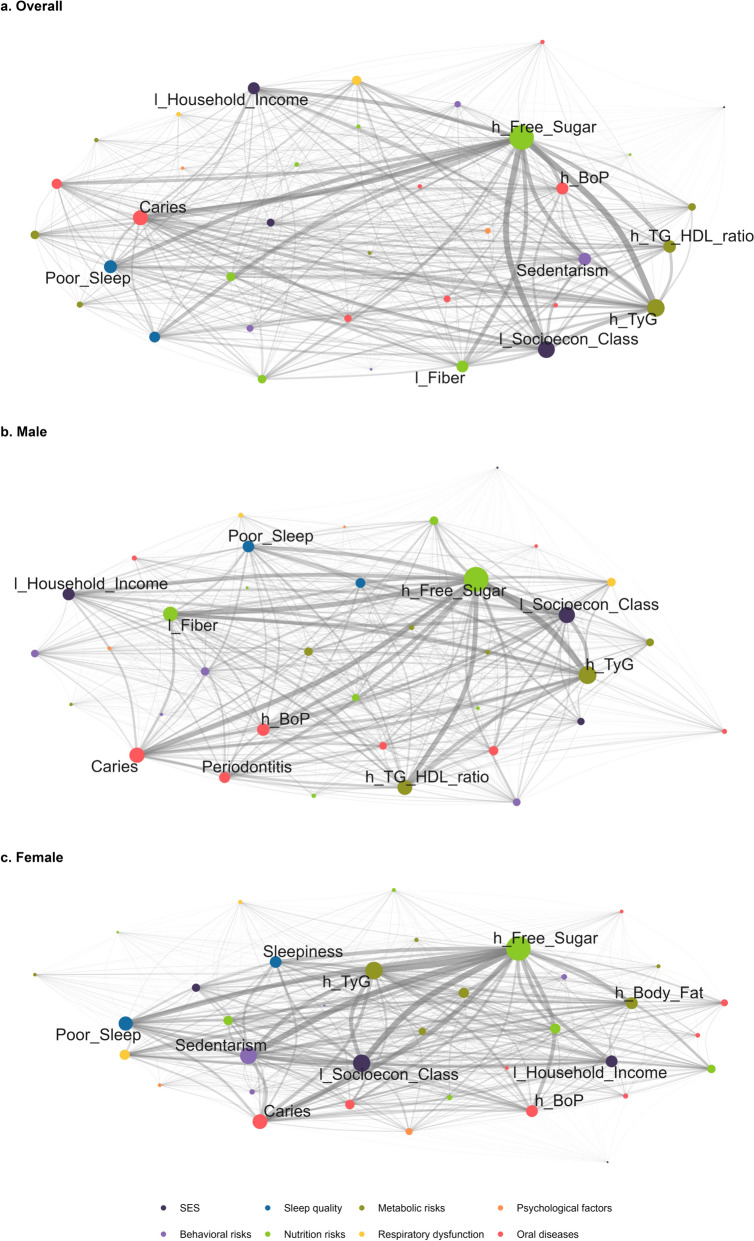
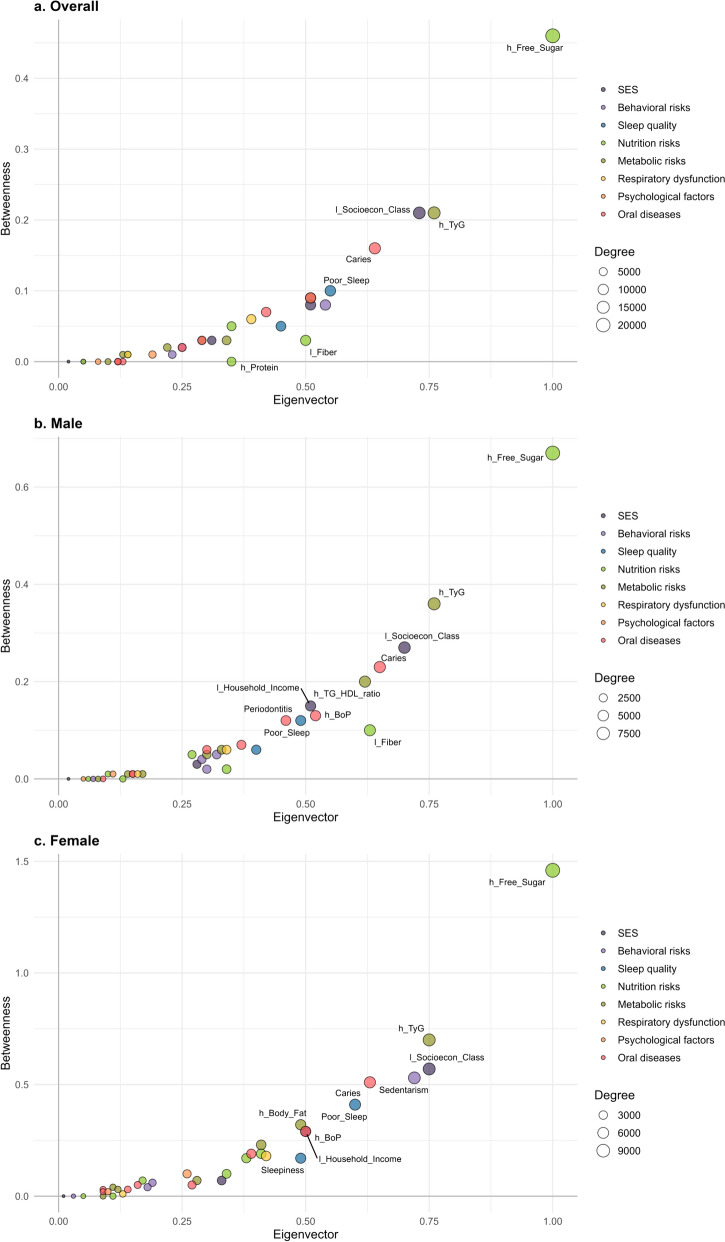
Figure 1 illustrates the complex networks, with node-specific measures detailed in Table S1. In all models, the highest degrees were observed for high free sugar consumption, high TyG index, and low socioeconomic class (Fig. 1 and Fig. 2). These were followed by caries, poor sleep, sedentary behavior, and high triglycerides/HDL-c ratio in the overall model (Fig. 2a). This pattern persisted in supplementary analysis defining high sugar consumption as ≥ 10% of daily calories and as ≥ 25 g (see Additional file, Fig. S2). Among males, caries, high triglycerides/HDL-c ratio, low fiber consumption, and high bleeding on probing were the next highest degrees of centrality (Fig. 2b), while among females, sedentarism, caries, poor sleep, and high body fat were observed (Fig. 2c). Similar patterns were observed for betweenness and eigenvector centrality (Fig. 2). Node-related Shannon entropy values ranged between 0.90 and 0.95, and local transitivity values ranged between 0.99 and 1.00. The cluster coefficient was 0.99, and Shannon entropy was − 5.3 across all models.
Fig. 1.
Complex networks of non-communicable diseases and risks by sex. RPS São Luís (N = 2515). The edges represent the co-occurrences between any two conditions, with thicker edges indicating a higher number of connections. The size of each node reflects its degree, meaning the number of connections it has with other nodes. Larger nodes indicate greater centrality. The figure with all the nodes identified can be found in the Additional file (Fig. S1). l_: Low; h_: High; PWV: pulse wave velocity; BoP: Gingival bleeding on probing
Fig. 2.
Complex networks node-specific measurements of non-communicable diseases and risks. RPS São Luís (N = 2515). The size of each node reflects its degree, meaning the number of connections it has with other nodes. Betweenness is a measure that quantifies the importance of a node in a network based on the number of shortest paths that pass through it, as a mediation value. Eigenvector measures a node's influence by considering the importance of the nodes it is connected to. The detailed values of each measurement are detailed in the Additional file (Table S1)
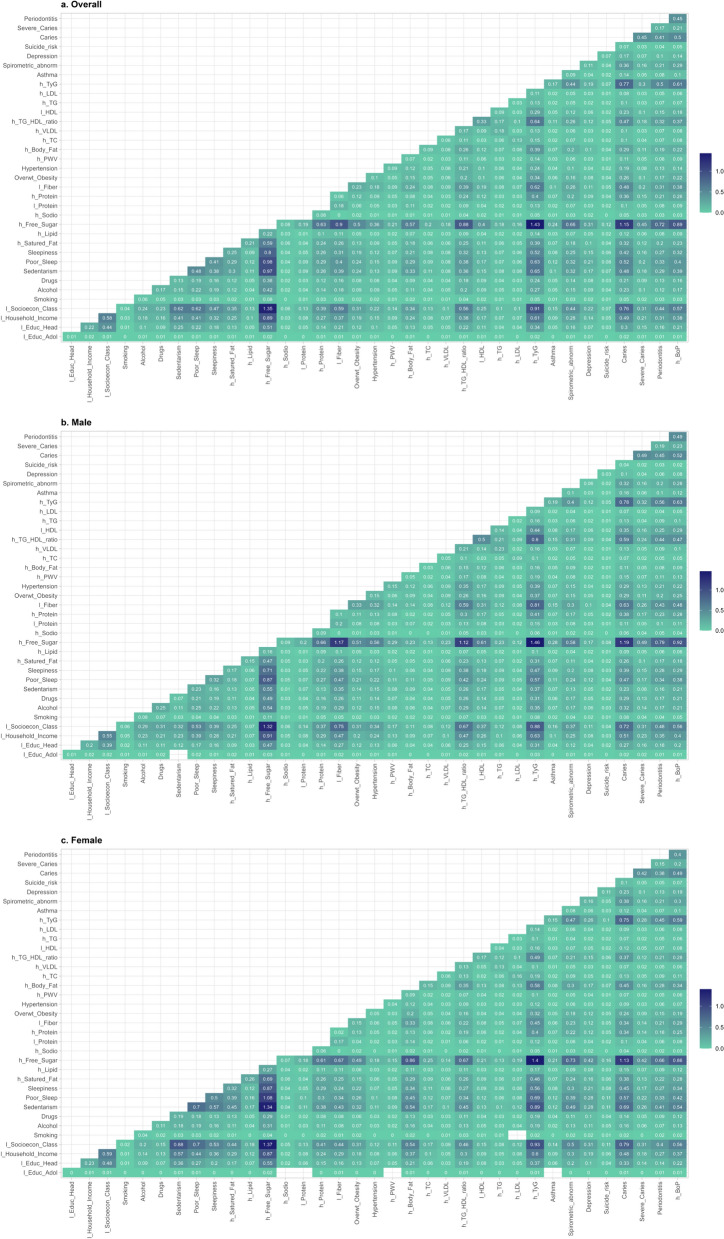
Figure 3 depicts the frequency of connections between each condition. In the overall model (Fig. 3a), the most frequent pairs were those linked to high sugar consumption: high TyG index (1.4%), low socioeconomic class (1.3%), and caries (1.2%), and poor sleep (1.0%). Among males (Fig. 3b), the most frequent pairs were those associated with high sugar consumption: high TyG index (1.5%), low socioeconomic class (1.3%), caries (1.2%), low fiber intake (1.2%), and high triglycerides/HDL-c ratio (1.1%). Among females (Fig. 3c), the most frequent pairs were those linked to high sugar consumption: high TyG index (1.4%), low socioeconomic class (1.4%), sedentary behavior (1.3%), caries (1.1%), and poor sleep (1.1%).
Fig. 3.
Percentage of connections between each condition by sex. RPS São Luís (N = 2515). The values correspond to the percentage of co-occurrence between two conditions, representing the thickness of the edges in the networks. The darker, the greater the co-occurrence
Discussion
We identified a pattern of interconnection among NCDs in adolescents and their related factors. High free sugar consumption emerged as the central hub, followed by other significant elements such as the high TyG index (an early event in the diabetes continuum) and low socioeconomic status. These elements were highly interconnected, regardless of the adolescent's sex. To our knowledge, this is the first study analyzing the non-linear connections between related factos (socioeconomic, behavioral, and metabolic conditions) and NCDs in adolescents.
The prominence of high free sugar consumption consistently emerged across all analyses, highlighting its significant impact on adolescent health. Notably, the pattern persisted even when defining high sugar consumption as ≥ 10% of daily calories or ≥ 25 g. As the primary source of discretionary calories in the Western diet, high sugar intake has substantial implications for metabolic health. Excessive sugar consumption leads to de novo lipogenesis, resulting in increased fat storage, especially in the liver, and contributes to the development of insulin resistance [24]. Furthermore, sugar intake is associated with chronic low-grade inflammation and oxidative stress, which are key mechanisms in the pathogenesis of NCDs [25]. These processes can lead to a range of metabolic disturbances, including dyslipidemia, hypertension, and endothelial dysfunction, setting the stage for early onset of conditions such as type 2 diabetes and cardiovascular diseases [26]. We observed frequent pairings, such as high sugar consumption with elevated TyG index, a marker of insulin resistance [18], reinforces the metabolic impact of sugar intake on diabetes. Furthermore, the link between low socioeconomic status and high sugar consumption may be due to the greater availability and lower cost of sugary foods, which are more accessible to vulnerable families [27]. Moreover, the connection between sugar consumption and caries corroborates the harmful effects of excess sugar on oral health [28].
The TyG index had a high centrality in the analyzed models, underscoring dyslipidemia and insulin resistance [18], an early event of the diabetes continuum. Insulin resistance fosters a lipogenic state, increasing hepatic triglyceride synthesis and reducing lipoprotein lipase activity, resulting in elevated serum triglyceride levels and decreased HDL cholesterol [25]. In adipose tissue, several early steps in insulin signaling to lipolysis and lipogenesis in subcutaneous fat cells occur independently of the liver, contributing to variations in circulating triglycerides and HDL cholesterol [29]. This dyslipidemic state is a well-established risk factor for atherosclerosis, an early sign of the cardiovascular continuum [30]. The significance of dyslipidemia is further emphasized by the high eigenvector and betweenness values observed for the triglycerides/HDL ratio in our network analysis, indicating that this relationship is central and highly interconnected with other factors and conditions within the NCD network.
The centrality of low socioeconomic position in the complex networks of NCDs among adolescents highlights the importance of social determinants on health. Adolescents from lower socioeconomic classes were found to have a higher prevalence of adverse health outcomes, underscoring the association between socioeconomic disadvantage and NCDs [20, 31]. In 2022, approximately 31.6% of the Brazilian population lived in poverty, equating to about 67.8 million people [32]. Notably, the poverty rate is not uniform across Brazil; the Northeast region, where the study was conducted, has nearly 43.5% of its population living in poverty [32]. Theories of inequalities in health, such as the social determinants of health framework, explain how lower socioeconomic status often correlates with reduced access to healthcare, poorer nutrition, higher exposure to environmental hazards, and increased levels of stress, contributing to the development and progression of NCDs [33]. The chronic stress associated with socioeconomic hardship can lead to physiological changes, including increased inflammation and hormonal imbalances, which further exacerbate the risk of NCDs [34]. These findings may be particularly relevant for other settings with similar socioeconomic disparities, but contextual differences such as healthcare infrastructure, social safety nets, and dietary patterns may influence the extent to which these findings are applicable elsewhere.
Our results revealed similarities in the complex networks of males and females, which shared high sugar consumption, high TyG index, and low socioeconomic class as central nodes, underscoring the pervasive impact of these factors on adolescents’ health, irrespective of their sex. However, differences were observed beyond these shared elements. In the males’ network, the main factors were caries, high triglycerides/HDL-c ratio, and low fiber consumption. Low fiber intake and high triglycerides/HDL-c ratio in males suggest a dietary pattern predisposing them to higher cardiometabolic risk [35]. For females, sedentary behavior and poor sleep emerged alongside caries. Sedentary behavior increases the risk of obesity and metabolic syndrome, while poor sleep quality leads to hormonal imbalances and increased stress levels, both of which are risk factors for NCDs [36, 37].
Poor sleep quality and daytime sleepiness were not strongly related in this study as expected. Adolescents exhibit significant variability in their sleep needs and responses to sleep deprivation [38]. Some adolescents may have a higher tolerance for sleep loss, meaning they can function adequately during the day despite not getting enough sleep [39, 40]. The same was observed for socioeconomic class and household income. Household income reflects immediate financial resources, while socioeconomic class encompasses a wider range of factors, such as parents’ level of education, number of bathrooms, ownership of certain household utensils and access to safe water, among others [13].
As a limitation, we do not intend to infer temporality or causality between risk factors and health outcomes due to the cross-sectional nature of this study. Instead, we assume a complex, intricate network of non-linear relationships between multiple factors and NCDs in adolescence. Socioeconomic indicators and drug use were self-reported, which could introduce reporting bias. To mitigate this, we previously tested the instruments and provided comprehensive training for data collectors to ensure consistent administration and accurate responses. Furthermore, drug assessment was done through a confidential self-reported questionnaire. Dietary information was elicited using food frequency questionnaires, which may be prone to recall bias and measurement errors; to minimize bias, we used a quality–quantity instrument with photographic records and administered the questionnaires by trained nutritionists.
Our study has several strengths that enhance its significance and potential impact. We used validated instruments and standardized procedures, e.g., air displacement plethysmography (BOD POD) to evaluate body composition. Calibrated dentists conducted full-mouth examinations using international indices for caries and periodontitis, ensuring high data quality and reliability. Additionally, a population-based sample provides robust analysis and allows for the generalization of findings to similar urban populations. The novelty of this study lies in the complex network approach to investigating the interconnections among related factors and NCDs in adolescence, offering a broad perspective on the multifaceted nature of NCD onset.
Conclusions
In sum, our findings emphasize the importance of understanding the intricate interconnections among contributing factors and NCDs in adolescents. This study highlights the central roles of high free sugar consumption, high TyG index, and low socioeconomic status in these complex networks. By identifying these dietary patterns and socioeconomic disparities, we emphasize the need for early, multifaceted interventions to effectively mitigate the burden of NCDs that adolescents face.
Supplementary Information
Acknowledgements
Authors thanks all those who participated in this study.
Author contributions
SAC: Conception, data analysis, data interpretation, and drafting of the work. BSF: Data analysis, data interpretation, and critical review. FAR: Design of the work, data analysis, data interpretation, and critical review. AAF: Conception, data interpretation, critical review. GGN: Data interpretation, critical review. FRML: Data interpretation, critical review. LLCL: Data acquisition, data interpretation, critical review. RFLB: Data acquisition, data interpretation, critical review. EBAFT: Data acquisition, data interpretation, critical review. CMCA: Data acquisition, data interpretation, critical review. CCCR: Conception, data interpretation, critical review. All authors contributed to the final manuscript.
Funding
This study was supported by the Department of Science and Technology (DECIT/Brazilian Ministry of Health), the National Council for Scientific and Technological Development (CNPq), the São Paulo Research Foundation (FAPESP), the Maranhão State Research Foundation for Scientific and Technological Development (FAPEMA), the Foundation of Support to Teaching, Research and Assistance of Clinics Hospital of Ribeirão Preto Medical School, and the University Hospital of the Federal University of Maranhão, University of São Paulo (FAEPA), Coordination for the Improvement of Higher Education Personnel (CAPES): Finance Code 001, the PROCAD Amazônia (88881.719704/2022-01) and Amazônia Legal (0810/2020/88881.510244/2020-01).
Availability of data and materials
The data analyzed during this study is not publicly available, but can be obtained from the RPS São Luis cohort committee upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Ethics and Research Committee of the Federal University of Maranhão University Hospital (IRB #1302489). Written informed consent was obtained from all subjects/patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Naghavi M, Ong KL, Aali A, Ababneh HS, Abate YH, Abbafati C, et al. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet. 2024;403:2100–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations. Health—United Nations sustainable development. Sustainable development goals. https://www.un.org/sustainabledevelopment/health/. Accessed 15 May 2024
- 3.Licher S, Heshmatollah A, van der Willik KD, Stricker BHCh, Ruiter R, de Roos EW, et al. Lifetime risk and multimorbidity of non-communicable diseases and disease-free life expectancy in the general population: a population-based cohort study. PLoS Med. 2019;16: e1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes. Circulation. 2006;114:2850–70. 10.1161/CIRCULATIONAHA.106.655688. [DOI] [PubMed] [Google Scholar]
- 5.Faienza MF, Urbano F, Lassandro G, Valente F, D’Amato G, Portincasa P, et al. The cardiovascular disease (CVD) risk continuum from prenatal life to adulthood: a literature review. Int J Environ Res Public Health. 2022;19:8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11:42. 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National academies of sciences e and m, health and medicine division, Division of behavioral and social sciences and education, Board on children Y and F, Committee on applying lessons of optimal adolescent health to improve behavioral outcomes for youth. Promoting positive adolescent health behaviors and outcomes: Thriving in the 21st Century. Graham R, Kahn NF (Eds.). Washington, D.C.: National Academies Press; 2019. https://www.ncbi.nlm.nih.gov/books/NBK554988/. Accessed 15 May 2024 [PubMed]
- 8.Padrón-Monedero A. A pathological convergence theory for non-communicable diseases. Aging Med. 2023;6:328–37. 10.1002/agm2.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budreviciute A, Damiati S, Sabir DK, Onder K, Schuller-Goetzburg P, Plakys G, et al. Management and prevention strategies for non-communicable diseases (NCDs) and their risk factors. Front Public Health. 2020. 10.3389/fpubh.2020.574111/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banatvala N, Akselrod S, Bovet P, Mendis S. The WHO global action plan for the prevention and control of NCDs 2013–2030. In: Banatvala N, Akselrod S, Bovet P, Mendis S, editors. Noncommunicable diseases. London: Routledge; 2023. p. 234–9. [Google Scholar]
- 11.Vulliard L, Menche J. Complex networks in health and disease. Syst Med. Elsevier. 2021. pp. 26–33. https://linkinghub.elsevier.com/retrieve/pii/B978012801238311640X
- 12.Confortin SC, Ribeiro MRC, Barros AJD, Menezes AMB, Horta BL, Victora CG, et al. RPS Brazilian Birth Cohorts Consortium. In: R Preto, Pelotas, S Luís (Eds.) History, objectives and methods. Cad Saude Publica: Rio de Janeiro. 2021; 37. [DOI] [PubMed]
- 13.Association of Research Companies. Brazilian economic classification criteria. ABEP. 2008. pp. 1–3. http://www.abep.org/criterio-brasil. Accessed 26 May 2024.
- 14.Ruanpeng D, Thongprayoon C, Cheungpasitporn W, Harindhanavudhi T. Sugar and artificially sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM Int J Med. 2017;110:513–20. [DOI] [PubMed] [Google Scholar]
- 15.Vos MB, Kaar JL, Welsh JA, Van Horn LV, Feig DI, Anderson CAM, et al. Added sugars and cardiovascular disease risk in children: a scientific statement from the American heart association. Circulation. 2017;135:e1017-34. 10.1161/CIR.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao M, Mill JG, Yan W-L, Hong YM, Skidmore P, Stoner L, et al. Static cut-points of hypertension and increased arterial stiffness in children and adolescents: the international childhood vascular function evaluation consortium. J Clin Hypertens. 2019;21:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shim YS, Baek JW, Kang MJ, Oh YJ, Yang S, Hwang IT. Reference values for the triglyceride to high-density lipoprotein cholesterol ratio and non-high-density lipoprotein cholesterol in Korean children and adolescents: The Korean national health and nutrition examination surveys 2007–2013. J Atheroscler Thromb. 2016;23:1334–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reckziegel MB, Nepomuceno P, Machado T, Renner JDP, Pohl HH, Nogueira-de-Almeida CA, et al. The triglyceride-glucose index as an indicator of insulin resistance and cardiometabolic risk in Brazilian adolescents. Arch Endocrinol Metab. 2023. 10.20945/2359-3997000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global initiative for chronic obstructive lung disease. Gold spirometry guide. Global initiative for chronic obstructive lung disease (GOLD). 2010. https://goldcopd.org/gold-spirometry-guide/. Accessed 16 May 2024.
- 20.Ladeira LLC, Nascimento GG, Leite FRM, Alves-Costa S, Barbosa JMA, Alves CMC, et al. Obesity, insulin resistance, caries, and periodontitis: syndemic framework. Nutrients. 2023. 15. https://pubmed.ncbi.nlm.nih.gov/37630703/. Accessed 29 Jan 2024. [DOI] [PMC free article] [PubMed]
- 21.da Costa L F, Rodrigues FA, Travieso G, Villas Boas PR. Characterization of complex networks: a survey of measurements. Adv Phys. 2007;56:167–242. 10.1080/00018730601170527. [Google Scholar]
- 22.Eagle N, Macy M, Claxton R. Network diversity and economic development. Science. 2010;328:1029–31. 10.1126/science.1186605. [DOI] [PubMed] [Google Scholar]
- 23.Wiesner K, Ladyman J. Measuring complexity. 2019. http://arxiv.org/abs/1909.13243
- 24.Softic S, Gupta MK, Wang G-X, Fujisaka S, O’Neill BT, Rao TN, et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Investig. 2017;127:4059–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Zhu J, Kim JH, Sumerlin TS, Feng Q, Yu J. Metabolic health and adiposity transitions and risks of type 2 diabetes and cardiovascular diseases: a systematic review and meta-analysis. Diabetol Metab Syndr. 2023;15:60. 10.1186/s13098-023-01025-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayén A-L, Marques-Vidal P, Paccaud F, Bovet P, Stringhini S. Socioeconomic determinants of dietary patterns in low- and middle-income countries: a systematic review. Am J Clin Nutr. 2014;100:1520–31. [DOI] [PubMed] [Google Scholar]
- 28.Ladeira LLC, Nascimento GG, Leite FRM, Alves-Costa S, Thomaz EBAF, Alves CMC, et al. Sugar intake above international recommendations and oral disease burden: a population-based study. Oral Dis. 2022. 10.1111/odi.14464. [DOI] [PubMed] [Google Scholar]
- 29.Kerr AG, Andersson DP, Dahlman I, Rydén M, Arner P. Adipose insulin resistance associates with dyslipidemia independent of liver resistance and involves early hormone signaling. Arterioscler Thromb Vasc Biol. 2023;43:1054–65. [DOI] [PubMed] [Google Scholar]
- 30.Che B, Zhong C, Zhang R, Pu L, Zhao T, Zhang Y, et al. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: an analysis of UK biobank data. Cardiovasc Diabetol. 2023;22:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbosa JMA, Ribeiro CCC, Batista RFL, Brondani MA, Simões VMF, Bettiol H, et al. Behavioral risk factors for noncommunicable diseases associated with depression and suicide risk in adolescence. Rio de Janeiro: Cad Saude Publica; 2022. p. 38. [DOI] [PubMed] [Google Scholar]
- 32.Irene Gomes. Poverty drops to 31.6% of the population in 2022, after reaching 36.7% in 2021. IBGE. 2023. https://agenciadenoticias.ibge.gov.br/en/agencia-news/2184-news-agency/news/38574-poverty-drops-to-31-6-of-the-population-in-2022-after-reaching-36-7-in-2021. Accessed 2 Sep 2024.
- 33.Di Cesare M, Khang Y-H, Asaria P, Blakely T, Cowan MJ, Farzadfar F, et al. Inequalities in non-communicable diseases and effective responses. Lancet. 2013;381:585–97. [DOI] [PubMed] [Google Scholar]
- 34.Parker HW, Abreu AM, Sullivan MC, Vadiveloo MK. Allostatic load and mortality: a systematic review and meta-analysis. Am J Prev Med. 2022;63:131–40. [DOI] [PubMed] [Google Scholar]
- 35.Isasi CR, Parrinello CM, Ayala GX, Delamater AM, Perreira KM, Daviglus ML, et al. Sex differences in cardiometabolic risk factors among hispanic/latino youth. J Pediatr. 2016;176:121-127.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fatima Y, Doi SAR, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17:1154–66. [DOI] [PubMed] [Google Scholar]
- 37.Pearson N, Braithwaite RE, Biddle SJH, van Sluijs EMF, Atkin AJ. Associations between sedentary behaviour and physical activity in children and adolescents: a meta-analysis. Obes Rev. 2014;15:666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agostini A, Centofanti S. Normal sleep in children and adolescence. Child Adolesc Psychiatr Clin N Am. 2021;30:1–14. [DOI] [PubMed] [Google Scholar]
- 39.Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. J Sleep Res. 2005;14:239–44. [DOI] [PubMed] [Google Scholar]
- 40.Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed during this study is not publicly available, but can be obtained from the RPS São Luis cohort committee upon reasonable request.