Abstract
Background
It is unclear how perioperative hemoglobin decrease (ΔHb) influences the balance between risks and benefits of red blood cell transfusion after cardiac surgery.
Methods
We retrospectively analyzed data on 8186 adults who underwent valve surgery and/or coronary artery bypass grafting under cardiopulmonary bypass at two large cardiology centers. We explored the potential association of ΔHb, defined relative to the preoperative level and postoperative nadir, with a composite outcome of in-hospital mortality, myocardial infarction, stroke, and acute kidney injury using multivariable logistic regression, restricted cubic spline, and piecewise-linear models.
Results
Among 6316 patients without preoperative anemia, ΔHb ≥ 50 % was associated with an elevated risk of the composite outcome [adjusted odds ratio (aOR) 1.95, 95 % confidence interval (CI) 1.81–2.35]. Among 869 patients without preoperative anemia and with ΔHb ≥ 50 %, postoperative transfusion of no more than four units of red blood cell appeared to decrease the risk of the composite outcome, whereas transfusion of more than six units increased risk. Among 5447 patients without preoperative anemia and with ΔHb < 50 %, postoperative transfusion appeared not to decrease the risk of the composite outcome. Among 1870 patients with preoperative anemia, ΔHb ≥ 30 % significantly increased the risk of the composite outcome (aOR 1.61, 95 % CI 1.23–2.10), and this risk might be moderated by postoperative transfusion of no more than four units of red blood cell, but increased by transfusion of more than six units.
Conclusions
ΔHb may influence the balance between risks and benefits of red blood cell transfusion after cardiac surgery.
Keywords: Perioperative hemoglobin decrease, Transfusion, Cardiac surgery, Observational study
1. Introduction
Cardiopulmonary bypass (CPB) and related factors during cardiac surgery often result in significant perioperative blood loss and hemodilution, potentially leading to acute anemia and elevated risk of mortality and morbidity [[1], [2], [3], [4], [5]]. While red blood cell (RBC) transfusion during hospitalization is a common remedy for acute anemia, it may raise the risk of acute kidney injury, infection, or even death [[6], [7], [8], [9], [10]]. Thus, deciding whether to perform transfusion means balancing the risks of acute anemia against the potential benefits of transfusion.
Several randomized clinical trials have investigated which absolute hemoglobin level may be appropriate as a threshold or trigger for performing perioperative RBC transfusion, but results have been inconsistent [[11], [12], [13], [14]]. Since a patient's ability to tolerate acute anemia may depend strongly on his or her baseline hemoglobin level, which varies widely in the general population, researchers have recently explored a relative transfusion trigger based on the difference between pre-and intraoperative nadir hemoglobin levels [15,16]. Notably, two cohort studies found that patients with normal preoperative hemoglobin level were at higher risk of anemia-related complications after cardiac surgery if the intraoperative hemoglobin level was at least 50 % smaller than the preoperative one, even if intraoperative hemoglobin level remained at least 7 g/dL, which has often been used as an absolute transfusion trigger.
While these cohort studies demonstrate the potential superiority of a relative transfusion trigger over an absolute one, they did not take into account that the difference between preoperative hemoglobin level and postoperative nadir hemoglobin level may also affect the risk of adverse outcomes after cardiac surgery. In addition, they examined only patients with normal preoperative hemoglobin level, leaving open whether a relative transfusion trigger is appropriate for patients with preoperative anemia. Finally, they did not examine in detail how the volume of RBC transfusion may affect outcomes. The present retrospective observational study addressed these questions by analyzing over 8000 patients who underwent cardiac surgery at two large heart centers in China.
2. Materials and methods
2.1. Study design and population
This retrospective observational study included patients 18 years or older who underwent valve surgery and/or coronary artery bypass grafting (CABG) involving CPB either at West China Hospital of Sichuan University between January 1, 2011 and June 30, 2017, or at the Second Affiliated Hospital in the School of Medicine of Zhejiang University between September 1, 2013 and June 30, 2017. Exclusion criteria were as follows: patients whose surgeries also involved ascending aortic replacement or cardiac tumor resection, those who underwent emergency surgeries, patients who died on the operating table, those who could not be weaned off CPB in the operating room, or patients lacking available perioperative hemoglobin values.
This study was approved by the Ethics Committees at West China Hospital (approval no. 256/2017) and the Second Affiliated Hospital (approval no. 096/2017). Both committees waived the requirement for individual informed consent because of the retrospective study design. The study was registered with ClinicalTrials.gov under the identifier NCT04476134.
2.2. Data collection
We retrospectively collected data on demographic details, comorbidities, laboratory tests, perioperative examinations, surgical variables, transfusion, and postoperative variables from the Hospital Information System, Laboratory Information System, and Transfusion System at West China Hospital, or from the Electronic Medical Record System and “Do Care” System at the Second Affiliated Hospital. Data collection lasted from October 15, 2017, to December 20, 2017, at West China Hospital, and from November 4, 2017, to December 15, 2017, at the Second Affiliated Hospital. All medical data were handled using EpiData version 3.0 (EpiData Association, Odense, Denmark). To ensure accuracy and reliability, two investigators independently verified all collected data.
2.3. Perioperative management
Anesthesia and CPB were performed according to standard protocols [17,18]. Briefly, anesthesia was induced with midazolam, sufentanil, etomidate, and a muscle relaxant. Anesthesia was maintained with infusion of remifentanil, inhalation of sevoflurane, and intermittent injection of sufentanil and muscle relaxant. CPB management was similar across both centers. The membrane oxygenator (Medtronic, Minneapolis, MN, USA) was primed with 500 ml crystalline liquid and 1000 ml colloid solution. All patients initially received heparin (375 U/kg), which was also administered intermittently to maintain activated clotting time longer than 480 s. Blood flow rate was 2.0–2.5 L/m2/min to maintain mean arterial pressure between 50 and 80 mmHg during CPB. The heart was arrested by cold 4:1 blood cardioplegia. Body temperature was moderately cooled to 30–32 °C, and hematocrit was maintained above 20 %. After weaning from CPB, heparin was neutralized by infusing protamine in a 1:1 ratio to the initial dose of heparin.
All patients received standard RBC transfusion management. Both study sites treated anemia using erythropoietin or ferrous sulfate when appropriate, cell salvage was performed and tranexamic acid was administered perioperatively, and the following hemoglobin thresholds were applied when deciding on perioperative RBC transfusion: (1) if absolute hemoglobin level fell below 7 g/dL during CPB, 8 g/dL during surgery before or after CPB, or 9 g/dL during recovery in the intensive care unit (ICU) or general ward; or (2) if the attending physician ordered transfusion based on the patient's condition [17,[19], [20], [21]].
2.4. Assessment of perioperative hemoglobin and the composite outcome
Perioperative hemoglobin concentration was measured in all patients as follows: (1) at least once between admission and the day before surgery; (2) at least four times in the operating room, i.e. after intubation, at the beginning of CPB, before weaning off CPB, and after CPB; (3) once upon arrival in the ICU, then at least three times per day, or anytime when ICU clinicians determined it necessary; (4) at least once every two days after return to the recovery ward and until discharge; and (5) before and after RBC transfusion in the case of patients who received such transfusion. Hemoglobin concentration was determined by blood gas analysis using a Cobas b 123 device (Roche, Basel, Switzerland) at West China Hospital, or using a Cobas b 221 device (Roche) at the Second Affiliated Hospital of Zhejiang University.
Preoperative hemoglobin was defined as the last available measurement before surgery. Preoperative anemia was defined as preoperative hemoglobin level lower than 12.0 g/dL in women or lower than 13.0 g/dL in men [22]. Postoperative nadir hemoglobin was defined as the lowest level measured from ICU admission until day 3 after cardiac surgery [23,24]. Perioperative hemoglobin decrease (ΔHb) was calculated as ΔHb = (Preoperative hemoglobin – Postoperative nadir hemoglobin)/Preoperative hemoglobin × 100.
We defined a composite outcome that included all-cause mortality during hospitalization, myocardial infarction, stroke, or acute kidney injury, as detailed in Table S1. If more than one adverse event occurred after cardiac surgery, only the first one was included in the analyses.
2.5. Statistical analyses
Continuous data were reported as mean ± standard deviation (SD) or median (interquartile range, IQR), while categorical data were reported as frequencies (percentages). Inter-group differences in continuous variables were assessed for significance using Student's t-test or Mann-Whitney U test, while differences in categorical variables were assessed using the chi-squared or Fisher's exact test. Sporadic missing values were addressed by imputing the median for continuous data or the mode for categorical data, as detailed in Table S2.
Potential confounders were identified based on biological plausibility, documented risk factors from the literature, and results of logistic regression analysis. Based on previous studies [15,16,25], we systematically searched the Medline, EMBASE, and Cochrane databases using the search terms risk factor, predictor, mortality, morbidity, adverse outcome, complication, and cardiac surgery to identify as many potential perioperative risk factors as possible. Additionally, logistic regression was applied to clinicodemographic and intraoperative variables within our cohort to pinpoint further risk factors. Variables associated with P < 0.1 in univariate logistic regression were entered into multivariable logistic regression. Those achieving a P < 0.05 in the multivariable analysis were considered potential confounders.
Crude associations between ΔHb and the composite outcome in patients with or without preoperative anemia were investigated using locally weighted scatterplot smoothing (lowess method). Patients were categorized into quartiles based on ΔHb for some analyses. Logistic regression models were developed and results were reported as unadjusted or adjusted odds ratios (ORs) with 95 % confidence intervals (CIs). Model 1, we tested the bivariate relationship between ΔHb and the composite outcome. Model 2, age, sex, and body mass index (BMI) were introduced into the model. Model 3, other potential confounders, including current smoking, preoperative comorbidities, left ventricular function, and other risk factors related to surgery and perioperative RBC transfusion were added. We performed tests for linear trends by entering the median value of each category of ΔHb as a continuous variable and re-running the models as stated above. To verify the robustness of the associations observed, sensitivity analyses were conducted. These analyses divided patients into quartiles and quintiles based on ΔHb and adjusted for additional factors including pulmonary hypertension, New York Heart Association (NYHA) classification, and perioperative transfusions of platelets (PLT) and fresh frozen plasma (FFP).
When there was evidence of non-linearity, restricted cubic spline models were applied to examine the quantitative association between ΔHb and the composite outcome in patients with or without preoperative anemia [26,27]. When fitting ΔHb as a restricted cubic spline, the number of knots was determined by fitting models with 3–5 knots and selecting the optimal model based on the minimized Akaike information criterion [28]. In spline models, covariates were fully adjusted and potential non-linearity was investigated using the Wald test. To identify appropriate cut-off values of ΔHb, a two-line piecewise linear model with a single “change point” was estimated by testing all possible values for the change point and choosing the value with the highest likelihood. Restricted cubic spline models were also created to assess the relationship between ΔHb and the composite outcome in prespecified subgroups stratified by sex, age, or perioperative RBC transfusion.
Logistic regression analyses were conducted separately on patients with or without preoperative anemia to determine the impact of preoperative risk factors on the cut-off values of ΔHb and their associations with the composite outcome. Results from these analyses were reported as odds ratios (ORs) and 95 % confidence intervals (CIs). We also used multivariable linear models to explore associations between ΔHb values that were above or below certain cut-offs and the composite outcome.
To further assess the role of cut-off values of ΔHb for deciding when and how many units of RBC to transfuse postoperatively, we ran multivariable-adjusted logistic regression models to examine the effects of postoperative RBC transfusion on the composite outcome.
All statistical tests were two-sided, and differences associated with P < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS 25.0 (IBM, Chicago, IL, USA) and R 3.6.2 (https://www.r-project.org).
3. Results
3.1. Demographic and clinical characteristics of the study population
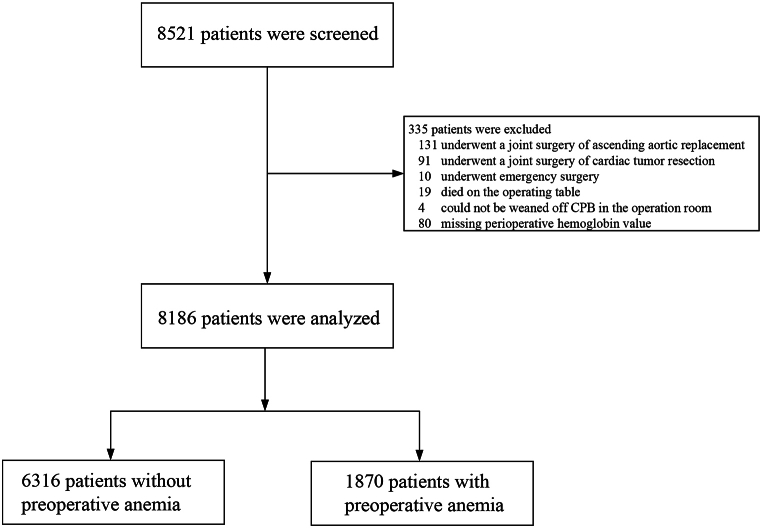
A total of 8521 patients were screened for enrollment, of whom 335 were excluded for the following reasons: undergoing surgery involving ascending aortic replacement (n = 131) or cardiac tumor resection (91), emergency surgery (10), death on the operating table (19), inability to be weaned off CPB (4), or missing perioperative hemoglobin values (80). Consequently, 8186 patients were included in the final analysis, with 1870 (22.8 %) identified as having preoperative anemia (Fig. 1).
Fig. 1.
Flowchart of the study. CPB, cardiopulmonary bypass.
The study cohort had a median age of 52.0 years (IQR 45.0–61.0 years), with 57.7 % being female. The predominant procedure was valve replacement alone, performed in 88.5 % (7241) of the cases. Nearly half of the patients (3,915, 47.8 %) received at least one unit of RBC during their hospital stay, with a median transfusion volume of 3.0 units. The median preoperative hemoglobin was 13.5 g/dL (IQR 12.3–14.6 g/dL), while the median postoperative nadir hemoglobin was 8.2 g/dL (IQR 7.3–9.0 g/dL), resulting in a median ΔHb of 38.5 % (IQR 32.0%–44.8 %) (Table 1).
Table 1.
Characteristics of patients undergoing cardiac surgery.
| Characteristic | All patients (N = 8186) | Preoperative anemiaa |
|||||
|---|---|---|---|---|---|---|---|
| No |
Yes |
||||||
| ΔHbb < 50 % (n = 5447) | ΔHb ≥50 % (n = 869) | P | ΔHb <30 % (n = 851) | ΔHb ≥30 % (n = 1019) | P | ||
| Preoperative factors | |||||||
| Age, year | 52.0 (45.0–61.0) | 51.0 (44.0–60.0) | 56.0 (48.0–63.0) | <0.001 | 49.0 (42.0–60.0) | 57.0 (48.0–65.0) | <0.001 |
| Female | 4720 (57.7) | 3174 (58.3) | 435 (50.1) | <0.001 | 576 (67.7) | 535 (52.5) | <0.001 |
| BMI, kg/m2 | 22.7 (20.8–24.7) | 22.7 (21.1–25.0) | 22.7 (21.0–24.5) | 0.034 | 22.3 (20.2–23.7) | 22.5 (20.3–24.0) | 0.446 |
| Current smoking | 2291 (28.0) | 1498 (27.5) | 289 (33.3) | <0.001 | 188 (22.1) | 316 (31.0) | <0.001 |
| ASA classification | 0.027 | 0.502 | |||||
| II-III | 7696 (94.0) | 5165 (94.8) | 808 (93.0) | 788 (92.6) | 935 (91.8) | ||
| IV-V | 490 (6.0) | 282 (5.2) | 61 (7.0) | 63 (7.4) | 84 (8.2) | ||
| NYHA classification | 0.264 | 0.807 | |||||
| I | 144 (1.8) | 91 (1.7) | 17 (2.0) | 18 (2.1) | 18 (1.8) | ||
| II | 2006 (24.5) | 1348 (24.7) | 214 (24.6) | 202 (23.7) | 242 (23.7) | ||
| III | 5765 (70.4) | 3879 (71.2) | 608 (70.0) | 576 (67.7) | 702 (68.9) | ||
| IV | 271 (3.3) | 129 (2.4) | 30 (3.5) | 55 (6.5) | 57 (5.6) | ||
| Comorbidities | |||||||
| Chronic obstructive pulmonary disease | 67 (0.8) | 38 (0.7) | 10 (1.2) | 0.158 | 7 (0.8) | 12 (1.2) | 0.448 |
| Hypertension | 1341 (16.4) | 788 (14.5) | 179 (20.6) | <0.001 | 148 (17.4) | 226 (22.2) | 0.010 |
| Coronary heart disease | 1060 (12.9) | 567 (10.4) | 151 (17.4) | <0.001 | 117 (13.7) | 225 (22.1) | <0.001 |
| One diseased vessel | 231 (2.8) | 138 (2.5) | 30 (3.5) | 31 (3.6) | 32 (3.1) | ||
| Two diseased vessels | 193 (2.4) | 99 (1.8) | 29 (3.3) | 19 (2.2) | 46 (4.5) | ||
| Three diseased vessels | 519 (6.3) | 273 (5.0) | 82 (9.4) | 56 (6.6) | 108 (10.6) | ||
| Not investigated | 117 (1.4) | 57 (1.0) | 10 (1.2) | 11 (1.3) | 39 (3.8) | ||
| Previous myocardial infarction | 229 (2.8) | 119 (2.2) | 30 (3.5) | 0.023 | 27 (3.2) | 53 (5.2) | 0.033 |
| Infective endocarditis | 190 (2.3) | 44 (0.8) | 8 (0.9) | 0.733 | 76 (8.9) | 62 (6.1) | 0.020 |
| Atrial fibrillation | 3723 (45.5) | 2567 (47.1) | 462 (53.2) | 0.001 | 306 (36.0) | 388 (38.1) | 0.345 |
| Previous congestive heart failure | 1511 (18.5) | 993 (18.2) | 171 (19.7) | 0.307 | 174 (20.4) | 173 (17.0) | 0.055 |
| Peripheral arterial disease | 112 (1.4) | 62 (1.1) | 12 (1.4) | 0.538 | 13 (1.5) | 25 (2.5) | 0.162 |
| Previous cardiovascular surgery | 1825 (22.3) | 1081 (19.8) | 237 (27.3) | <0.001 | 186 (21.9) | 321 (31.5) | <0.001 |
| Previous transient ischemic attack | 36 (0.4) | 28 (0.5) | 3 (0.3) | 0.511 | 3 (0.4) | 2 (0.2) | 0.521 |
| Previous stroke | 336 (4.1) | 203 (3.7) | 36 (4.1) | 0.551 | 50 (5.9) | 47 (4.6) | 0.221 |
| Liver injury | 412 (5.0) | 263 (4.8) | 53 (6.1) | 0.111 | 46 (5.4) | 50 (4.9) | 0.627 |
| Renal dysfunction | 364 (4.4) | 161 (3.0) | 44 (5.1) | 0.001 | 67 (7.9) | 92 (9.0) | 0.373 |
| Diabetes mellitus | 528 (6.5) | 290 (5.3) | 67 (7.7) | 0.005 | 61 (7.2) | 110 (10.8) | 0.007 |
| Echocardiography | |||||||
| Left ventricular functionc, % | 0.514 | 0.091 | |||||
| Good | 7109 (86.8) | 4704 (86.4) | 755 (86.9) | 766 (90.0) | 884 (86.8) | ||
| Moderate | 1041 (12.7) | 719 (13.2) | 108 (12.4) | 83 (9.8) | 131 (12.9) | ||
| Poor | 36 (0.4) | 24 (0.4) | 6 (0.7) | 2 (0.2) | 4 (0.4) | ||
| Pulmonary hypertensiond, mmHg | 0.288 | 0.010 | |||||
| Normal | 6728 (82.2) | 4526 (83.1) | 725 (83.4) | 646 (75.9) | 831 (81.6) | ||
| Mild | 427 (5.2) | 259 (4.8) | 51 (5.9) | 54 (6.3) | 63 (6.2) | ||
| Moderate | 705 (8.6) | 465 (8.5) | 69 (7.9) | 92 (10.8) | 79 (7.8) | ||
| Severe | 326 (4.0) | 197 (3.6) | 24 (2.8) | 59 (6.9) | 46 (4.5) | ||
| Intraoperative factors | |||||||
| Type of surgery | <0.001 | <0.001 | |||||
| Valve | 7241 (88.5) | 4692 (91.1) | 721 (83.0) | 750 (88.1) | 808 (79.3) | ||
| Coronary artery bypass grafting | 794 (9.7) | 401 (7.4) | 116 (13.3) | 91 (10.7) | 186 (18.3) | ||
| Both | 151 (1.8) | 84 (1.5) | 32 (3.7) | 10 (1.2) | 25 (2.5) | ||
| Time on bypass, min | 114.0 (89.0–142.0) | 112.0 (88.0–138.0) | 126.0 (100.0–158.0) | <0.001 | 108.0 (85.0–137.0) | 121.0 (94.0–149.0) | <0.001 |
| Cross-clamp time, min | 75.0 (57.0–100.0) | 73.0 (56.0–98.0) | 82.0 (63.0–110.0) | <0.001 | 71.0 (53.0–95.0) | 81.0 (60.0–106.0) | <0.001 |
| Duration of surgery, h | 4.8 (4.0–5.5) | 4.7 (4.0–5.4) | 5.3 (4.3–6.1) | <0.001 | 4.6 (4.0–5.3) | 5.0 (4.2–5.9) | <0.001 |
| Perioperative hemoglobin | |||||||
| Preoperative hemoglobine, g/dL | 13.5 (12.3–14.6) | 13.9 (13.1–14.8) | 14.5 (13.7–15.4) | <0.001 | 10.7 (9.6–11.5) | 11.7 (11.1–12.1) | <0.001 |
| Postoperative nadir hemoglobinf g/dL | 8.2 (7.3–9.0) | 8.5 (7.8–9.4) | 6.8 (6.1–7.1) | <0.001 | 8.3 (7.7–8.8) | 7.1 (6.6–7.7) | <0.001 |
| ΔHbb, % | 38.5 (32.0–44.8) | 38.7 (33.5–43.4) | 53.5 (51.4–56.7) | <0.001 | 22.4 (15.9–26.4) | 37.4 (33.6–42.5) | <0.001 |
| Blood transfusion | |||||||
| Perioperative RBC transfusion | 3915 (47.8) | 1872 (34.4) | 639 (73.5) | <0.001 | 633 (74.4) | 771 (75.7) | 0.524 |
| Total RBC, units | 3.0 (2.0–5.0) | 2.0 (2.0–4.0) | 4.0 (2.0–7.5) | <0.001 | 4.0 (2.0–5.0) | 4.0 (2.0–6.0) | 0.130 |
| Intraoperative RBC transfusion | 1957 (23.9) | 891 (16.4) | 196 (22.6) | <0.001 | 427 (50.2) | 443 (43.5) | 0.004 |
| Volume of intraoperative RBC transfusion, units | 2.0 (2.0–4.0) | 2.0 (2.0–3.0) | 3.0 (2.0–6.0) | <0.001 | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 0.041 |
| Postoperative RBC transfusion | 2584 (31.6) | 1222 (22.4) | 501 (57.7) | <0.001 | 384 (45.1) | 477 (46.8) | 0.466 |
| Volume of postoperative RBC transfusion, units | 3.0 (2.0–4.0) | 2.0 (2.0–3.5) | 4.0 (2.0–7.0) | <0.001 | 3.0 (2.0–4.0) | 3.5 (2.0–5.0) | 0.047 |
| Perioperative PLT transfusion | 1078 (13.2) | 601 (11.0) | 156 (18.0) | <0.001 | 130 (15.3) | 191 (18.7) | 0.048 |
| Perioperative FFP transfusion | 2462 (30.1) | 1288 (23.6) | 481 (55.4) | <0.001 | 228 (26.8) | 465 (45.6) | <0.001 |
Values are presented as n (%) or median (interquartile range) unless otherwise noted. Continuous variables were compared using Student's t-test or Mann-Whitney U test, and categorical variables were compared using the chi-squared or Fisher's exact test.
Preoperative anemia was defined as preoperative hemoglobin <12 g/dL for women or <13 g/dL for men.
ΔHb (%) was defined as (Preoperative hemoglobin – Postoperative nadir hemoglobin)/Preoperative hemoglobin × 100.
Categorized as good when the left ventricular ejection fraction ≥51 %, as moderately reduced when the fraction was 31–50 %, or as poor when the fraction ≤30 %.
Classified as mild when pulmonary arterial pressure was 25–34 mmHg, as moderate when pressure was 35–44 mmHg, or as severe when ≥45 mmHg.
Preoperative hemoglobin was defined as the last available measurement before surgery.
Postoperative nadir hemoglobin was defined as the lowest level measured from ICU admission until day 3 after cardiac surgery.BMI, body mass index; ASA, American Society of Anesthesiologists; NYHA, New York Heart Association; RBC, red blood cell; PLT, platelet; FFP, fresh frozen plasma.
3.2. The composite outcome and its risk factors
The composite outcome was observed in 1633 (19.9 %) patients, including 12 (0.1 %) patients with myocardial infarction, 26 (0.3 %) with stroke, 1605 (19.6 %) with acute kidney injury, and 109 (1.3 %) who died (Table 2). Patients experiencing the composite outcome were more likely to be aged 65 or older (30.1 % vs. 10.9 %), male (54.2 % vs. 39.4 %), have preoperative anemia (31.4 % vs. 20.7 %), and receive perioperative RBC (65.2 % vs. 43.5 %) or PLT transfusions (20.3 % vs. 11.4 %), with all comparisons reaching statistical significance (P < 0.001).
Table 2.
Adverse events after cardiac surgery.
| Adverse event | All patients (n = 8186) | Preoperative anemiaa |
|||||
|---|---|---|---|---|---|---|---|
| No |
Yes |
||||||
| ΔHbb<50 % (n = 5447) | ΔHb≥50 % (n = 869) | P | ΔHb<30 % (n = 851) | ΔHb≥30 % (n = 1019) | P | ||
| Ischemic event | |||||||
| Myocardial infarction | 12 (0.1) | 9 (0.2) | 1 (0.1) | 0.731 | 1 (0.1) | 1 (0.1) | 0.899 |
| Stroke | 26 (0.3) | 9 (0.2) | 7 (0.8) | 0.002 | 3 (0.4) | 7 (0.7) | 0.332 |
| Acute kidney injury | 1605 (19.6) | 813 (14.9) | 290 (33.4) | <0.001 | 172 (20.2) | 330 (32.4) | <0.001 |
| Stage 1 | 1180 (14.4) | 662 (12.2) | 182 (20.9) | 123 (14.5) | 213 (20.9) | ||
| Stage 2 | 259 (3.2) | 109 (2.0) | 53 (6.1) | 29 (3.4) | 68 (6.7) | ||
| Stage 3 | 166 (2.0) | 42 (0.8) | 55 (6.3) | 20 (2.4) | 49 (4.8) | ||
| In-hospital mortality | 109 (1.3) | 35 (0.6) | 30 (3.5) | <0.001 | 17 (2.0) | 27 (2.6) | 0.356 |
| The composite outcomec | 1633 (19.9) | 828 (15.2) | 293 (33.7) | <0.001 | 179 (21.0) | 333 (32.7) | <0.001 |
Values are n (%) unless otherwise noted. Categorical variables were compared with the chi-squared or Fisher's exact test.
Preoperative anemia was defined as preoperative hemoglobin <12 g/dL for women or <13 g/dL for men.
ΔHb (%) was defined as (Preoperative hemoglobin – Postoperative nadir hemoglobin)/Preoperative hemoglobin × 100.
The composite outcome was defined as any of the following: postoperative new-onset myocardial infarction, stroke, acute kidney injury, death, or a combination.
Multivariable logistic regression and literature review identified several potential confounders, including sex, age, BMI, current smoking, preoperative hemoglobin, American Society of Anesthesiologists (ASA) classification, comorbidities, left ventricular function, type of surgery, CPB duration, and perioperative RBC transfusion (Table S3). Comorbidities included the following conditions: chronic obstructive pulmonary disease, hypertension, previous myocardial infarction, infective endocarditis, atrial fibrillation, previous congestive heart failure, peripheral arterial disease, previous cardiovascular surgery, previous transient ischemic attack, previous stroke, liver injury, renal dysfunction, and diabetes mellitus.
3.3. Associations between ΔHb and risk of the composite outcome
ΔHb showed a non-linear relationship to the composite outcome in patients with or without preoperative anemia. After a certain ΔHb was reached, the risk of the composite outcome increased dramatically (Fig. S1, Table 3), leading us to search for potentially clinically valuable cut-off values. Specifically, in patients without preoperative anemia, the results of the multivariable logistic regression showed that ΔHb was positively associated with the composite outcome (P for trend <0.001). Patients in the fourth ΔHb quartile (ΔHb > 46.0 %) were at 67 % higher risk of the composite outcome than those in the first quartile (ΔHb < 34.4 %; adjusted OR 1.67, 95 % CI 1.35–2.07). Among those with preoperative anemia, ΔHb was still positively associated with the composite outcome (P for trend <0.001), and patients in the fourth quartile (ΔHb > 38.1 %) were at twice the risk of the composite outcome as those in the first quartile (ΔHb < 23.4 %; adjusted OR 2.10, 95 % CI 1.42–3.12). These associations remained robust after additionally adjusting for NYHA classification, pulmonary hypertension, perioperative transfusion with PLT or FFP; and after stratifying patients into ΔHb quintiles (Tables S4–5).
Table 3.
Risk of the composite outcome in patients in different quartiles of perioperative hemoglobin decrease (ΔHb).
| Patients without preoperative anemia | |||||
|---|---|---|---|---|---|
|
ΔHb quartile |
ΔHb range (%) |
Composite outcome, n/N (%) |
Odds ratio (95 % confidence interval) |
||
| Model 1a | Model 2b | Model 3c | |||
| 1st | <34.4 | 201/1579 (12.7) | 1 (reference) | 1 (reference) | 1 (reference) |
| 2nd | 34.4–40.2 | 221/1579 (14.0) | 1.12 (0.91–1.37) | 1.12 (0.90–1.37) | 1.07 (0.87–1.33) |
| 3rd | 40.3–46.0 | 266/1579 (16.8) | 1.39 (1.14–1.69) | 1.32 (1.08–1.61) | 1.16 (0.94–1.43) |
| 4th | >46.0 | 433/1579 (27.4) | 2.59 (2.15–3.12) | 2.37 (1.96–2.86) | 1.67 (1.35–2.07) |
| P for trend |
<0.001 |
<0.001 |
<0.001 |
||
| Patients with preoperative anemia | |||||
| 1st |
<23.4 |
105/468 (22.4) |
1 (reference) |
1 (reference) |
1 (reference) |
| 2nd | 23.4–31.2 | 92/467 (19.7) | 0.85 (0.62–1.16) | 0.81 (0.58–1.12) | 1.00 (0.69–1.45) |
| 3rd | 31.3–38.1 | 125/468 (26.7) | 1.26 (0.94–1.70) | 1.07 (0.79–1.47) | 1.36 (0.93–1.98) |
| 4th | >38.1 | 190/467 (40.7) | 2.37 (1.78–3.15) | 1.84 (1.36–2.49) | 2.10 (1.42–3.12) |
| P for trend | <0.001 | <0.001 | <0.001 | ||
Model l was unadjusted.
Model 2 was adjusted for sex, age (<65 or ≥ 65 year), and body mass index (<18.5, 18.5–24.9, 25.0–29.9 or ≥ 30.0 kg/m2).
Model 3 was adjusted for sex, age (<65 or ≥65 year), body mass index (<18.5, 18.5–24.9, 25.0–29.9 or ≥ 30.0 kg/m2), preoperative hemoglobin, current smoking, ASA classification, comorbidities (see section 3.2 in main text), left ventricular function, type of surgery, cardiopulmonary bypass duration, and perioperative red blood cell transfusion.
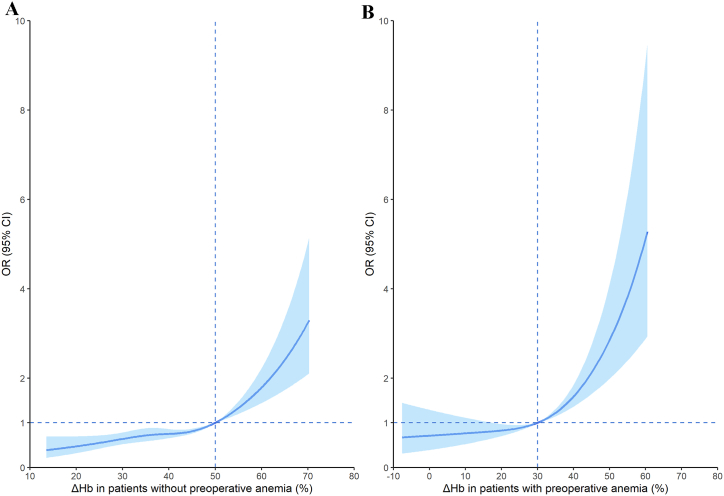
A flexible model comprising a restricted cubic spline component and a two-line piecewise linear component identified a change point of ΔHb of 49.1 % (standard error, 0.26) for patients without preoperative anemia and 29.9 % (standard error, 0.12) for patients with preoperative anemia. Based on these findings, we established proposed cut-off values for ΔHb at approximately 50 % (Fig. 2A) and 30 % (Fig. 2B), respectively. In subgroup analyses, restricted cubic spline indicated that the relationship remained consistent between ΔHb and the composite outcome when patients were stratified by sex, age, or perioperative RBC transfusion; interactions of these covariates with ΔHb were not statistically significant (Figs. S2–3).
Fig. 2.
Spline function plots of the association of perioperative hemoglobin decrease (ΔHb) with the composite outcome including in-hospital mortality, myocardial infarction, stroke, and acute kidney injury. The association was assessed in cardiac surgery patients (A) without or (B) with preoperative anemia. Solid lines indicate odds ratios (ORs), while blue-shaded areas indicate 95 % confidence intervals. Dashed lines indicate the reference condition. All models were adjusted for sex, age (<65 or ≥ 65 year), body mass index (<18.5, 18.5–24.9, 25.0–29.9 or ≥ 30.0 kg/m2), current smoking, ASA classification, comorbidities (see section 3.2 of the main text), preoperative hemoglobin level, left ventricular function, type of surgery, cardiopulmonary bypass duration, and perioperative red blood cell transfusion.
In patients without preoperative anemia, adjusted multivariable logistic regression indicated a significantly higher risk of the composite outcome for those with ΔHb ≥ 50 %, compared to those with ΔHb < 50 % (adjusted OR 1.95, 95 % CI 1.61–2.35; P < 0.001). For patients with a ΔHb below 50 %, each 10 % increase in ΔHb was associated with only a marginal increase in risk (adjusted OR 1.11, 95 % CI 1.00–1.24; P = 0.052). Conversely, for those with a ΔHb of 50 % or higher, further increases in ΔHb did not significantly affect the risk (adjusted OR 1.02 for each 10 % increase in ΔHb, 95 % CI 0.67–1.55; P = 0.924).
Among patients with preoperative anemia, those with ΔHb ≥ 30 % were at significantly higher risk of the composite outcome than those with ΔHb < 30 % (adjusted OR 1.61, 95 % CI 1.23–2.10; P = 0.001). Furthermore, increases in ΔHb were associated with a more pronounced increase in risk if ΔHb ≥ 30 % (adjusted OR 1.82 for each 10 % of ΔHb increase; 95 % CI 1.46–2.26; P < 0.001), but not if ΔHb < 30 % (adjusted OR 1.10 for each 10 % of ΔHb increase; 95 % CI 0.87–1.40; P = 0.415).
3.4. Associations between preoperative factors and risk of suffering a cut-off ΔHb value linked to the composite outcome
For patients without preoperative anemia, univariable analysis identified the following as associated with greater risk of suffering ΔHb ≥50 %: age 65 years or older (OR 1.83, 95 % CI 1.52–2.20; P < 0.001), male (OR 1.39, 95 % CI 1.21–1.61; P < 0.001), ASA classification 4–5 (OR 1.38, 95 % CI 1.04–1.84; P = 0.027), preoperative hemoglobin (OR 1.44, 95 % CI 1.36–1.52; P < 0.001), multiple comorbidities, undergoing CABG alone (OR 1.99, 95 % CI 1.60–2.48; P < 0.001), or undergoing both valve replacement and CABG (OR 2.62, 95 % CI 1.73–3.97; P < 0.001). Adjusted multivariable analysis confirmed the following as independently associated with higher risk of ΔHb ≥50 %: age 65 years or older (adjusted OR 1.71, 95 % CI 1.38–2.11; P < 0.001), preoperative hemoglobin (adjusted OR 1.56, 95 % CI 1.46–1.67; P < 0.001), atrial fibrillation as comorbidity (adjusted OR 1.38, 95 % CI 1.16–1.64, P < 0.001), undergoing CABG alone (adjusted OR, 2.53; 95 % CI, 1.76–3.63; P < 0.001), and undergoing both valve replacement and CABG (adjusted OR 2.76, 95 % CI 1.69–4.53; P < 0.001).
Conversely, among patients without preoperative anemia, the following factors appeared to protect against suffering ΔHb ≥50 %: male (adjusted OR 0.71, 95 % CI 0.56–0.89; P = 0.003), BMI between 18.5 and 24.9 kg/m2 (adjusted OR 0.72, 95 % CI 0.53–0.97; P = 0.029), BMI between 25.0 and 29.9 kg/m2 (adjusted OR 0.52, 95 % CI 0.37–0.72; P < 0.001), and BMI ≥30.0 kg/m2 (adjusted OR 0.48, 95 % CI 0.26–0.89; P < 0.020) (Table S6).
Among patients with preoperative anemia, adjusted multivariable analysis identified the following preoperative factors as associated with greater risk of suffering ΔHb ≥30 % (Table S7): age 65 years or older (adjusted OR 1.75, 95 % CI 1.28–2.40; P < 0.001), ASA classification 4–5 (adjusted OR 1.61, 95 % CI 1.06–2.43; P = 0.025), and preoperative hemoglobin (adjusted OR 2.90, 95 % CI 2.56–3.30; P < 0.001).
3.5. Impact of postoperative RBC transfusion on risk of the composite outcome in patients who achieve the cut-off ΔHb value or not
Among patients without preoperative anemia and with ΔHb < 50 %, postoperative RBC transfusion did not significantly alter the risk of the composite outcome, regardless of whether the transfused volume was 0.1–2.0 units (adjusted OR 0.77, 95 % CI 0.59–1.01) or 2.1–4.0 units (adjusted OR 1.21, 95 % CI 0.87–1.67). However, transfusion of 4.1–6.0 units significantly increased the risk of the composite outcome (adjusted OR 2.49, 95 % CI 1.56–3.96), as did transfusion of >6.0 units (adjusted OR 4.17, 95 % CI 2.49–6.98) (Table 4). Among patients without preoperative anemia and with ΔHb ≥ 50 %, transfusion of 0.1–2.0 units significantly decreased the risk of the composite outcome (adjusted OR 0.42, 95 % CI 0.24–0.74), as did transfusion of 2.1–4.0 units (adjusted OR 0.58, 95 % CI 0.34–0.99). In contrast, transfusion of 4.1–6.0 units did not decrease risk (adjusted OR 1.21, 95 % CI 0.62–2.38), while transfusion of >6.0 units significantly increased it (adjusted OR 3.25, 95 % CI 1.99–5.30).
Table 4.
Association of postoperative red blood cell transfusion volume with risk of the composite outcome in patients without preoperative anemia, stratified by perioperative hemoglobin decrease (ΔHb).
| Patients with ΔHb <50 % | |||
|---|---|---|---|
| Units transfused (no. patients) | Composite outcome n (%) | Unadjusted odds ratio (95 % confidence interval) | Adjusted odds ratioa (95 % confidence interval) |
| 0.0 (4224) | 613 (14.5) | 1 (reference) | 1 (reference) |
| 0.1–2.0 (704) | 80 (11.4) | 0.76 (0.59–0.97) | 0.77 (0.59–1.01) |
| 2.1–4.0 (340) | 58 (17.1) | 1.21 (0.90–1.63) | 1.21 (0.87–1.67) |
| 4.1–6.0 (101) | 35 (34.7) | 3.12 (2.06–4.75) | 2.49 (1.56–3.96) |
| >6.0 (78) | 42 (53.8) | 6.87 (4.37–10.81) | 4.17 (2.49–6.98) |
| P for trend |
<0.001 |
<0.001 |
|
| Patients with ΔHb ≥ 50 % | |||
| 0.0 (368) |
139 (37.8) |
1 (reference) |
1 (reference) |
| 0.1–2.0 (156) | 22 (14.1) | 0.27 (0.16–0.45) | 0.42 (0.24–0.74) |
| 2.1–4.0 (149) | 28 (18.8) | 0.38 (0.24–0.61) | 0.58 (0.34–0.99) |
| 4.1–6.0 (56) | 19 (33.9) | 0.85 (0.47–1.53) | 1.21 (0.62–2.38) |
| >6.0 (140) | 85 (60.7) | 2.55 (1.71–3.80) | 3.25 (1.99–5.30) |
| P for trend | <0.001 | <0.001 | |
Adjusted for sex, age (<65, ≥65 year), body mass index (<18.5, 18.5–24.9, 25.0–29.9 and ≥ 30.0 kg/m2), current smoking, ASA classification, comorbidities (see section 3.2 in main text), preoperative hemoglobin, postoperative nadir hemoglobin (<7.0, ≥7.0 g/dL), left ventricular function, type of surgery, cardiopulmonary bypass duration, and intraoperative red blood cell transfusion.
Among patients with preoperative anemia and ΔHb < 30 %, transfusion of 0.1–2.0 units of RBC significantly decreased the risk of the composite outcome (adjusted OR 0.47, 95 % CI 0.27–0.82), as did transfusion of 2.1–4.0 units (adjusted OR 0.41, 95 % CI 0.20–0.84; Table 5). In contrast, the risk was not altered by transfusion of 4.1–6.0 units (adjusted OR 0.91, 95 % CI 0.43–1.95) or > 6.0 units (adjusted OR 1.99, 95 % CI 0.91–4.35). Among patients with preoperative anemia and ΔHb ≥ 30 %, transfusion of up to 6.0 units did not significantly change the risk of the composite outcome, while transfusion of >6.0 units significantly increased it (adjusted OR 1.94, 95 % CI 1.08–3.50).
Table 5.
Association of postoperative red blood cell transfusion volume with risk of the composite outcome in patients with preoperative anemia, stratified by perioperative hemoglobin decrease (ΔHb).
| Patients with ΔHb <30 % | |||
|---|---|---|---|
| Units transfused (no. patients) | Composite outcome n (%) | Unadjusted odds ratio (95 % confidence interval) | Adjusted odds ratioa (95 % confidence interval) |
| 0.0 (467) | 113 (24.2) | 1 (reference) | 1 (reference) |
| 0.1–2.0 (187) | 22 (11.8) | 0.42 (0.26–0.68) | 0.47 (0.27–0.82) |
| 2.1–4.0 (104) | 11 (10.6) | 0.37 (0.19–0.72) | 0.41 (0.20–0.84) |
| 4.1–6.0 (52) | 14 (26.9) | 1.15 (0.60–2.21) | 0.91 (0.43–1.95) |
| >6.0 (41) | 19 (46.3) | 2.71 (1.41–5.18) | 1.99 (0.91–4.35) |
| P for trend |
0.314 |
0.947 |
|
| Patients with ΔHb ≥ 30 % | |||
| 0.0 (n = 542) |
191 (35.2) |
1 (reference) |
1 (reference) |
| 0.1–2.0 (n = 174) | 40 (23.0) | 0.55 (0.37–0.81) | 0.91 (0.58–1.41) |
| 2.1–4.0 (n = 149) | 35 (23.5) | 0.56 (0.37–0.86) | 0.71 (0.44–1.15) |
| 4.1–6.0 (n = 83) | 30 (36.1) | 1.04 (0.64–1.68) | 1.10 (0.64–1.91) |
| >6.0 (n = 71) | 37 (52.1) | 2.00 (1.22–3.29) | 1.94 (1.08–3.50) |
| P for trend | 0.137 | 0.102 | |
Adjusted for sex, age (<65, ≥65 year), body mass index (<18.5, 18.5–24.9, 25.0–29.9 and ≥ 30.0 kg/m2), current smoking, ASA classification, comorbidities (see section 3.2 in main text), preoperative hemoglobin, postoperative nadir hemoglobin (<7.0, ≥7.0 g/dL), left ventricular function, type of surgery, cardiopulmonary bypass duration, and intraoperative red blood cell transfusion.
4. Discussion
It is unclear how the perioperative decrease in hemoglobin influences the balance between the risks and benefits of postoperative RBC transfusion. Here we provide evidence from a large sample at two major cardiology centers that the relative index of hemoglobin decrease (ΔHb), which takes into account preoperative hemoglobin level as well as the postoperative nadir, is positively associated with the risk of a composite outcome including in-hospital mortality, myocardial infarction, stroke, and acute kidney injury. In our study, the increased risk of the composite outcome is associated with a 30 % or higher drop in hemoglobin level (ΔHb) for patients with pre-operative anemia, whereas it is associated with a 50 % or higher drop for patients without pre-operative anemia. As long as transfusion volume was not too large, postoperative RBC transfusion protected against the composite outcome in patients with preoperative anemia whose ΔHb less than 30 % and in patients without preoperative anemia who have a ΔHb of 50 % or more. Our study underscores the potential of ΔHb as a valuable indicator for guiding decisions on the necessity and volume of RBC transfusion following cardiac surgery.
Current guidelines for managing patients undergoing cardiac surgery usually recommend a restrictive transfusion strategy based on an absolute transfusion trigger of 7–8 g/dL [[11], [12], [13],29,30]. However, the minimum hemoglobin level that patients with normal preoperative hemoglobin level can safely tolerate appears not to be a fixed value, but instead to depend on preoperative hemoglobin level [15,16]. Consistent with those studies, we found that patients without preoperative anemia experiencing a ΔHb of 50 % or more were at greater risk of the composite outcome, even though the previous studies relied on intraoperative nadir hemoglobin level relative to preoperative level. We focused instead on postoperative nadir hemoglobin level relative to the preoperative level because studies from our group and others have found that patients after cardiac surgery, especially those in the ICU, are more likely to require transfusion of substantial volumes of blood resources [7,31,32]. Our work suggests that defining ΔHb as the difference between preoperative and postoperative nadir can inform decisions about when to perform postoperative RBC transfusion and the number of units to transfuse.
Anemia is a common condition among patients undergoing cardiac surgery [1,33,34]. Notably, earlier studies focusing on relative hemoglobin indices excluded patients with preexisting anemia [15,16]. We found that such anemia reduced, from 50 % to 30 %, the cut-off ΔHb at which the risk of the composite outcome was elevated. These results indicate that preoperative anemia should be taken into account when deciding whether and how many units of RBC to administer. Moreover, our analysis revealed that patients with preoperative anemia who experienced a ΔHb of 30 % maintained higher hemoglobin level compared to those without preoperative anemia at a ΔHb of 50 %. These findings reinforce the idea that the lowest hemoglobin level that cardiac patients can safely tolerate depends on their preoperative level.
We examined whether certain preoperative factors might be associated with whether a patient suffered or not a cut-off value of ΔHb linked to an elevated risk of the composite outcome. If we could identify preoperative risk factors for dangerous ΔHb, it might help clinicians discover patients at high risk of hemoglobin decrease and implement perioperative blood transfusion management as early as possible, which may help reduce the need for RBC transfusion and improve prognosis. For patients without preoperative anemia, we identified the following preoperative risk factors: age 65 years or older, preoperative hemoglobin, comorbidity of atrial fibrillation, undergoing CABG alone or undergoing valve replacement, and CABG. Protective factors were male and higher BMI. Among patients with preoperative anemia, preoperative risk factors were age 65 years or older, ASA classification 4–5, and preoperative hemoglobin. These results should be verified and extended in other patient populations, which may lead to revision of guidelines on transfusion and management of cardiac surgery patients.
The impact of postoperative RBC transfusion on the composite outcome in our study depended on the volume transfused: up to a certain number of units, transfusion reduced the risk of the composite outcome, but transfusion of too many units increased the risk, consistent with the dose-dependent risk of mortality and severe morbidity in other studies of cardiac surgery patients receiving more than four units of RBC [4,[35], [36], [37]]. In the absence of preoperative anemia, the impact of postoperative RBC transfusion on the composite outcome also depended on ΔHb. Our findings suggest that the risks of postoperative RBC transfusion outweigh the benefits for cardiac surgery patients without preexisting anemia whose ΔHb is below 50 %. On the other hand, transfusion of up to four units of RBC may benefit patients without preexisting anemia whose ΔHb is 50 % or higher.
In the case of patients with preoperative anemia, our results indicate that postoperative RBC transfusion of up to four units may reduce the risk of the composite outcome, while transfusion of beyond six units increases the risk, regardless of ΔHb. This finding suggests that the influence of transfusion on outcomes in anemic patients may not vary with ΔHb. Before integrating these observations into clinical practice, further validation in diverse patient cohorts is essential.
Our study has several key strengths, including its large, multi-site sample, and the use of data detailed enough to take into account an extensive array of potential confounders. Importantly, this study is pioneering in its analysis of the association between ΔHb and composite adverse outcomes specifically in patients with preoperative anemia undergoing cardiac surgery, filling a critical gap in the existing literature.
At the same time, our study presents several limitations. We defined preoperative hemoglobin as the baseline hemoglobin level, which might have biased our assessment of the association between ΔHb and the composite outcome. Analogously, we cannot be certain that the postoperative nadir hemoglobin that we measured was the true nadir. Nevertheless, our nadir values should be relatively reliable because nadir levels occur in most patients within a few days after cardiac surgery [23,24] and the two heart centers applied the same hemoglobin measurement protocol to best capture the “correct” postoperative nadir Hb. Furthermore, despite our efforts to adjust for multiple confounders, the potential for residual confounding cannot be completely ruled out. Also, given the retrospective observational nature of our study, we cannot establish causality. Consequently, the findings should be interpreted with caution and ideally confirmed through future prospective studies to further explore these relationships.
5. Conclusions
In summary, this large retrospective, observational study suggests that a ΔHb of 30 % or greater in the presence of preoperative anemia or 50 % or greater in its absence is associated with a significantly higher risk of a composite of anemia-related outcomes. These findings indicate that the magnitude of ΔHb may potentially influence the balance between benefits and risks of postoperative RBC transfusion. It is recommended that future randomized trials explore the efficacy of absolute transfusion triggers versus relative ones such as ΔHb to better guide RBC transfusion in cardiac surgery patients.
Ethics statement
This study was approved by the Ethics Committees at West China Hospital (approval no. 256/2017) and the Second Affiliated Hospital (approval no. 096/2017). Both committees waived the requirement for individual informed consent because of the retrospective study design. The study design adheres to the requirements of the Medical Research Involving Human Subject Act and of the Declaration of Helsinki.
Consent for publication
Not applicable.
Funding statement
This work was supported by grant 81570374 from the National Natural Science Foundation of China (to LD), grant 2018272998 from the National and Zhejiang Health and Family Planning Commission (to MY), and grant 2020HXBH024 from the Postdoctoral Research Fund (to XHL).
Data availability statement
The data generated from electronic medical records during the current study are not publicly available for reasons of patient privacy.
CRediT authorship contribution statement
Junhui He: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Data curation. Xinhao Liu: Writing – review & editing, Methodology, Funding acquisition, Formal analysis, Data curation. Li Zhou: Formal analysis. Changwei Chen: Data curation. Jing Liu: Data curation. Min Yan: Funding acquisition, Conceptualization. Yue Ming: Data curation. Zhong Wu: Conceptualization. Yingqiang Guo: Conceptualization. Jin Liu: Conceptualization. Lei Du: Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e37843.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Oprea A.D., Del Rio J.M., Cooter M., et al. Pre- and postoperative anemia, acute kidney injury, and mortality after coronary artery bypass grafting surgery: a retrospective observational study. Can. J. Anaesth. 2018;65(1):46–59. doi: 10.1007/s12630-017-0991-0. [DOI] [PubMed] [Google Scholar]
- 2.Duque-Sosa P., Martinez-Urbistondo D., Echarri G., et al. Perioperative hemoglobin area under the curve is an independent predictor of renal failure after cardiac surgery. Results from a Spanish multicenter retrospective cohort study. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0172021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranucci M., Baryshnikova E., Castelvecchio S., et al. Major bleeding, transfusions, and anemia: the deadly t-riad of cardiac surgery. Ann. Thorac. Surg. 2013;96(2):478–485. doi: 10.1016/j.athoracsur.2013.03.015. https://do-i.org/10.1016/j.athoracsur.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 4.Oliver E., Carrio M.L., Rodriguez-Castro D., et al. Relationships among haemoglobin level, packed red cell transfusion and clinical outcomes in patients after cardiac surgery. Intensive Care Med. 2009;35(9):1548–1555. doi: 10.1007/s00134-009-1526-0. [DOI] [PubMed] [Google Scholar]
- 5.Hessel E.A. 2nd, what's new in cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 2019;33(8):2296–2326. doi: 10.1053/j.jvca.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 6.Remy K.E., Hall M.W., Cholette J., et al. Mechanisms of red blood cell transfusion-related immunomodulation. Transfusion. 2018;58(3):804–815. doi: 10.1111/trf.14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horvath K.A., Acker M.A., Chang H., et al. Blood transfusion and infection after cardiac surgery. Ann. Thorac. Surg. 2013;95(6):2194–2201. doi: 10.1016/j.athoracsur.2012.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karkouti K. Transfusion and risk of acute kidney injury in cardiac surgery. Br. J. Anaesth. 2012;109(Suppl 1):i29–i38. doi: 10.1093/bja/aes422. [DOI] [PubMed] [Google Scholar]
- 9.Vlaar A.P., Hofstra J.J., Determann R.M., et al. The incidence, risk factors, and outcome of transfusion-related acute lung injury in a cohort of cardiac surgery patients: a prospective nested case-control study. Blood. 2011;117(16):4218–4225. doi: 10.1182/blood-2010-10-313973. [DOI] [PubMed] [Google Scholar]
- 10.Murphy G.J., Reeves B.C., Rogers C.A., et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 11.Mazer C.D., Whitlock R.P., Fergusson D.A., et al. Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. N. Engl. J. Med. 2018;379(13):1224–1233. doi: 10.1056/NEJMoa180-8561. [DOI] [PubMed] [Google Scholar]
- 12.Mazer C.D., Whitlock R.P., Fergusson D.A., et al. Restrictive or liberal red-cell transfusion for cardiac surgery. N. Engl. J. Med. 2017;377(22):2133–2144. doi: 10.1056/NEJMoa1711818. [DOI] [PubMed] [Google Scholar]
- 13.Murphy G.J., Pike K., Rogers C.A., et al. Liberal or restrictive transfusion after cardiac surgery. N. Engl. J. Med. 2015;372(11):997–1008. doi: 10.1056/NEJMoa1403612. [DOI] [PubMed] [Google Scholar]
- 14.Hajjar L.A., Vincent J.L., Galas F.R., et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304(14):1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 15.Hogervorst E., Rosseel P., van der Bom J., et al. Tolerance of intraoperative hemoglobin decrease during cardiac surgery. Transfusion. 2014;54(10 Pt 2):2696–2704. doi: 10.1111/trf.12654. [DOI] [PubMed] [Google Scholar]
- 16.Karkouti K., Wijeysundera D.N., Yau T.M., et al. The influence of baseline hemoglobin concentration on tolerance of anemia in cardiac surgery. Transfusion. 2008;48(4):666–672. doi: 10.1111/j.1537-2995.2007.01590.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Z.F., Jia X.P., Sun K., et al. Mild volume acute normovolemic hemodilution is associated with lower intraoperative transfusion and postoperative pulmonary infection in patients undergoing cardiac surgery -- a retrospective, propensity matching study. BMC Anesthesiol. 2017;17(1):13. doi: 10.1186/s128-71-017-0305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu Y., Lin J., Yang Y., et al. Lack of efficacy of ulinastatin therapy during cardiopulmonary bypass surgery. Chin Med J (Engl) 2015;128(23):3138–3142. doi: 10.4103/0366-6999.170364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y., Tang J., Du L., et al. Protamine dosage based on two titrations reduces blood loss after valve replacement surgery: a prospective, double-blinded, randomized study. Can. J. Cardiol. 2012;28(5):547–552. doi: 10.1016/j.cjca.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Ming Y., Liu J., Zhang F., et al. Transfusion of red blood cells, fresh frozen plasma, or platelets is associated with mortality and infection after cardiac surgery in a dose-dependent manner. Anesth. Analg. 2020;130(2):488–497. doi: 10.1213/ane.0000000000004528. [DOI] [PubMed] [Google Scholar]
- 21.Huang D., Chen C.W., Ming Y., et al. Risk of massive blood product requirement in cardiac surgery A large retrospective study from 2 heart centers. Medicine. 2019;98(5) doi: 10.1097/md.0000000000-014219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLean E., Cogswell M., Egli I., et al. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information System, 1993-2005. Public Health Nutr. 2009;12(4):444–454. doi: 10.1017/S1-368980008002401. [DOI] [PubMed] [Google Scholar]
- 23.George T.J., Beaty C.A., Kilic A., et al. Hemoglobin drift after cardiac surgery. Ann. Thorac. Surg. 2012;94(3):703–709. doi: 10.1016/j.athoracsur.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L., Liu X., Yan M., et al. Postoperative nadir hemoglobin and adverse outcomes in patients undergoing on-pump cardiac operation. Ann. Thorac. Surg. 2021;112(3):708–716. doi: 10.1016/j.athoracsur.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Spolverato G., Kim Y., Ejaz A., et al. Effect of relative decrease in blood hemoglobin concentrations on postoperative morbidity in patients who undergo major gastrointestinal surgery. JAMA Surg. 2015;150(10):949–956. doi: 10.1001/jamasurg.2015.1704. [DOI] [PubMed] [Google Scholar]
- 26.Durrleman S., Simon R. Flexible regression models with cubic splines. Stat. Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 27.Smith P.L. Splines as a useful and convenient statistical tool. 1979;33(2):57–62. [Google Scholar]
- 28.Harrell Jr F.E. And Survival Analysis. Springer; 2015. Regression modeling strategies: with applications to linear models, logistic and ordinal regression. [Google Scholar]
- 29.Shehata N., Mistry N., da Costa B.R., et al. Restrictive compared with liberal red cell transfusion strategies in cardiac surgery: a meta-analysis. Eur. Heart J. 2019;40(13):1081–1088. doi: 10.1093/eurheartj/ehy435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller M.M., Van Remoortel H., Meybohm P., et al. Patient blood management: recommendations from the 2018 frankfurt consensus conference. JAMA. 2019;321(10):983–997. doi: 10.1001/jama.20-19.0554. [DOI] [PubMed] [Google Scholar]
- 31.Fischer M.O., Guinot P.G., Debroczi S., et al. Individualised or liberal red blood cell transfusion after cardiac surgery: a randomised controlled trial. Br. J. Anaesth. 2022;128(1):37–44. doi: 10.1016/j.bja.2-021.09.037. [DOI] [PubMed] [Google Scholar]
- 32.Pasrija C., Ghoreishi M., Whitman G., et al. Mitigating the risk: transfusion or reoperation for bleeding after cardiac surgery. Ann. Thorac. Surg. 2020;110(2):457–463. doi: 10.1016/j.athoracsur.2019.10.076. [DOI] [PubMed] [Google Scholar]
- 33.Padmanabhan H., Brookes M.J., Luckraz H. Association between anemia and blood transfusion with long-term mortality after cardiac surgery. Ann. Thorac. Surg. 2020;110(2):749–750. doi: 10.1016/j.athoracsur.2020.02.043. [DOI] [PubMed] [Google Scholar]
- 34.Klein A.A., Collier T.J., Brar M.S., et al. The incidence and importance of anaemia in patients undergoing cardiac surgery in the UK - the first Association of Cardiothoracic Anaesthetists national audit. Anaesthesia. 2016;71(6):627–635. doi: 10.1111/anae.13423. [DOI] [PubMed] [Google Scholar]
- 35.Delaney M., Stark P.C., Suh M., et al. Massive transfusion in cardiac surgery: the impact of blood component ratios on clinical outcomes and survival. Anesth. Analg. 2017;124(6):1777–1782. doi: 10.1213/ANE.0000000000001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch C.G., Li L., Duncan A.I., et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit. Care Med. 2006;34(6):1608–1616. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- 37.Chelemer S.B., Prato B.S., Cox P.M., Jr., et al. Association of bacterial infection and red blood cell transfusion after coronary artery bypass surgery. Ann. Thorac. Surg. 2002;73(1):138–142. doi: 10.1016/s0003-4975(01)03308-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated from electronic medical records during the current study are not publicly available for reasons of patient privacy.