Abstract
This study investigated the possible mechanisms of microRNA-124a on the differentiation of bone marrow mesenchymal stem cells (BMSCs) and its underlying mechanism. β-Thiol ethanol induced Notch1 mRNA expression, microRNA-124a inhibitor reduced the effects of β-thiol ethanol on Notch1 mRNA expression in BMSCs. Baicalin induced Hes1 mRNA expression, and microRNA-124a inhibitor reduced the effects of baicalin on Hes1 mRNA expression in BMSCs. Si-Notch1 suppressed Hes1 mRNA expression in BMSCs. Baicalin increased the effects of Notch1 on Hes1 mRNA expression in BMSCs. Si-Notch1 increased cell growth of BMSCs. Baicalin reduced the effects of si-Notch1 on cell growth and the differentiation of BMSCs. We demonstrated that microRNA-124a promoted the differentiation of BMSCs into neurons through Notch/Hes1 signal pathway.
Keywords: MicroRNA-124a, BMSCs, Notch, Hes1
Introduction
Ischemic stroke is a serious threat to human health because of its high incidence rate, high disability rate, and high mortality [1]. Therefore, the treatment of ischemic stroke is widely concerned by society [2]. In clinic, the treatment of stroke is mainly drug therapy, while interventional therapy and stem cell therapy are relatively few [3].
Biological characteristics of bone marrow mesenchymal stem cells (BMSCs): bone marrow mesenchymal stem cells (BMSCs) are seed cells commonly used in tissue engineering research [4]. Through in vitro culture, it is found that progenitor cells with multidirectional differentiation ability can be isolated from bone marrow. The bone marrow was isolated, expanded in vitro, and some of the cells adhered to the surface of the culture dish could be highly expanded in vitro [5]. The multidirectional differentiated cell group was named bone marrow mesenchymal stem cells. Until 1999, Pittinger uniformly named bone marrow mesenchymal stem cells (BMSCs) in Science magazine [6]. At present, bone marrow mesenchymal stem cells can be summarized as a subset of cells derived from non-hematopoietic cells, which originate from mesenchymal tissue and mainly exist in the connective tissue and organ interstitium of the whole body, and are the most abundant in bone marrow and have the ability of self-renewal and multidirectional differentiation [7]. They can be expanded in vitro, and can differentiate into chondrocytes, adipocytes, neuroid cells, matrix supporting hematopoietic stem cells and other cells after induction in vitro [8].
MicroRNA (miRNA) is a kind of small endogenous non-coding single-stranded RNA, which can negatively regulate the stability of mRNA and/or inhibit the translation of mRNA by combining with the complementary sequence in the 3′-untranslated region of the target gene, and most miRNAs play a role in ischemic stroke in a tissue-specific manner [9–11]. Its imbalance is closely related to the occurrence and progression of ischemic stroke [12].
Neural stem cells have a wide range of therapeutic potential in stroke, spinal cord injury and other neurological diseases [13]. There are neural stem cells with the ability of self-proliferation and differentiation in adult hippocampal dentate gyrus and subventricular area [14]. After cerebral ischemia, neural stem cells in these regions are activated to participate in the nerve regeneration after cerebral ischemia, thus improving the nerve function [15]. However, due to the influence of the local environment after ischemia, neural stem cells are not enough to fully repair the brain tissue damage, so improving the survival rate of neural stem cells after cerebral ischemia, regulating their proliferation and differentiation has become an urgent problem to be solved [16, 17].
Materials and methods
Isolation and identification of BMSCs
Bone marrow MSCs were harvested from Sprague–Dawley rats, aged 10 weeks and weighing 180–250 g, housed in a controlled environment (24 ± 1 °C, 12 h light/12 h dark cycle) and allowed food and water ad libitum [18, 19]. All procedures were conducted according to the guidelines of the Animal Care Committee. Bone marrow cells were flushed out and collected from the femur and tibia of rats, plated in T25 flasks, and cultured overnight in a 37 °C incubator with 5% CO2. BMSCs were incubated with alpha minimum essential medium (α-MEM, Gibco, CA, USA) containing 20% fetal bovine serum (FBS), 2 mM Glutamax, and 1% penicillin and streptomycin (PS) in a 5% CO2 atmosphere at 37 °C. BMSCs were incubated with PE-conjugated anti-CD29 (BD Biosciences, USA), anti-CD34 (BD Biosciences, USA), PE-conjugated anti-CD90 (BD Biosciences, USA), and then analyzed via flow cytometry (BD Biosciences, USA).
Cell culture and transfection
BMSCs were incubated in a 5% CO2 atmosphere at 37 °C. Si-Notch1 plasmid and microRNA-124a inhibitor plasmid was transfected into BMSCs cell lines, respectively, by using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA). Transfection was performed according to the manufacturer’s instructions. Specifically, the plasmid was mixed with Lipofectamine 2000 and allowed to rest for 30 min. Subsequently, it was added to the cells, and 24 h after transfection, the cells were cultured using a fresh medium. Transfection was completed 48 h later and cultured for subsequent experiments. The experiment was performed in triplicate. The cells used in this study were within 10 passages.
Quantitative polymerase chain reaction (qPCR)
Total RNAs were isolated with RNA isolator total RNA extraction reagent (Takara, Tokyo, Japan), and cDNA was synthesized using PrimeScipt RT Master Mix(Takara, Tokyo, Japan). qPCR were performed with the ABI Prism 7500 sequence detection system according to the Prime-ScriptTM RT detection kit. The reaction mixtures were incubated at 50 °C for 15 min, followed by 95 °C for 5 min; then, 35 PCR cycles were performed with the following temperature profiles: 95 °C for 15 s, 60 °C for 30 s and 72 °C for 1 min. Relative levels of the sample mRNA expression were calculated and expressed as 2-DDCt. The experiment was performed in triplicate.
Proliferation assay
For Cell Counting Kit-8 (CCK-8), after 48 h of transfection, a total of approximately 5 × 103 cells/well was seeded in 96-well plate. After culturing at indicated time (0, 1, 2, 3 and 4 day), the cellular proliferation was detected using CellTiter-GloR Luminescent Cell Viability Assay (Promega, Madison, WI, USA) according to manufacturer’s instructions. The experiment was performed in triplicate.
Cellular induction and treatment
For cellular induction and treatment, 5 × 105 cells/well was seeded in 6-well plate. Cells which the cellular density reaches 70% were treated by si-Notch1, miRNA-124a inhibit or miRNA-124a mimc. After 48 h of transfection, a total of approximately, cells were induced by β-thiol ethanol and baicalin for 6 h. The experiment was performed in triplicate.
Immunofluorescence detection
Cells treated after crawling tablets, were fixed with 4% paraformaldehyde for 30 min, permeated with 0.5% Triton X-100 for 20 min, and sealed with 5% BSA at 37 °C for 30 min. They were incubated with NSE (1:200), MAP-2 (1:200) and GFAP (1:200) at 4 °C overnight. After washing, fluorescent secondary antibody was added, the film was sealed with DAPI and observed under fluorescence microscope (CKX53, Olympus).
Western blot
Tissue or cells samples were lysed with ice-cold RIPA buffer with complete protease and phosphatase inhibitors. The protein concentrations were measured using BCA protein assay kit. Total proteins were separated by SDS–PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were incubated with primary antibodies after blocking with 5% BSA in TBS, followed by incubation with peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology). The signals were detected with the ECL system and exposed by the ChemiDoc XRS system with Image Labsoftware (Bio-rad).
Statistical analyses
Graphad Prism 6 was used for the statistical analysis. p < 0.05 was considered statistically significant. Comparisons of data between groups were followed using Student’s t test or one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test.
Results
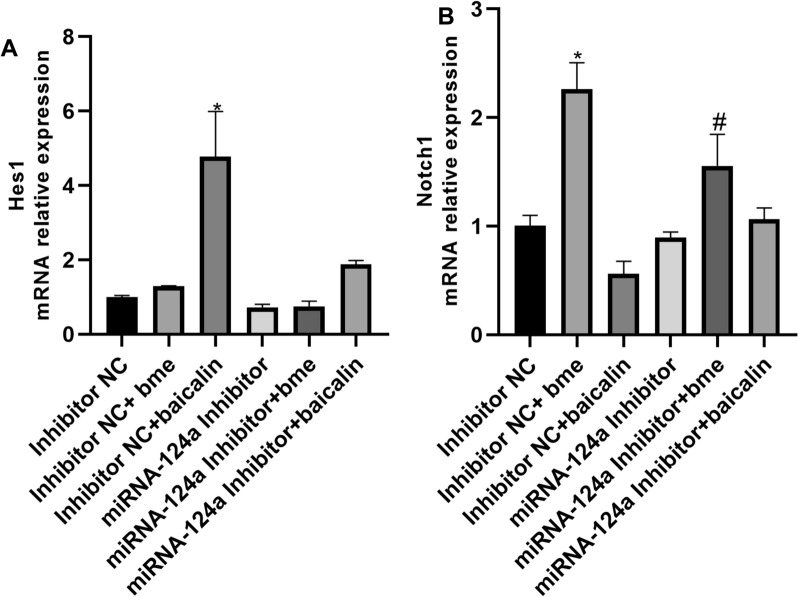
Baicalin induced Hes1 mRNA expression and β-thiol ethanol induced Notch1 mRNA expression in the differentiation of BMSCs into neurons by microRNA-124a
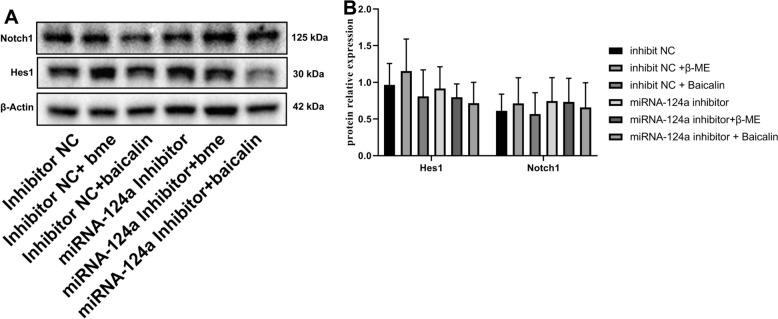
This study explored that the mechanism of microRNA-124a on the differentiation of BMSCs into neurons. Baicalin induced Hes1 mRNA expression, and microRNA-124a inhibitor reduced the effects of baicalin on Hes1 mRNA expression in BMSCs (Fig. 1A). β-Thiol ethanol induced Notch1 mRNA expression, microRNA-124a inhibitor reduced the effects of β-thiol ethanol on Notch1 mRNA expression in BMSCs (Fig. 1B). However, baicalin or β-thiol ethanol did not affect Hes1 and Notch1 protein expressions in BMSCs (Fig. 2).
Fig. 1.
Baicalin induced Hes1 mRNA expression and β-thiol ethanol induced Notch1 mRNA expression in the differentiation of BMSCs into neurons by microRNA-124a. Hes1 (A) and Notch1 (B) mRNA expression. *p < 0.01 compared with inhibitor NC, #p < 0.01 compared with microRNA-124a inhibitor
Fig. 2.
Hes1 and Notch1 protein expression. Hes1 and Notch1 protein expression (Western blot, A) and (statistical analyses, B). *p < 0.01 compared with inhibitor NC, #p < 0.01 compared with microRNA-124a inhibitor
Notch1 is one target for the baicalin on the differentiation of BMSCs into neurons
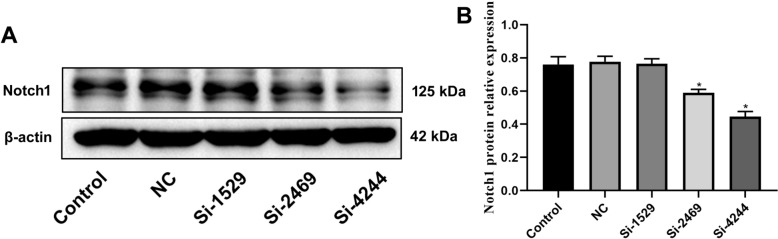
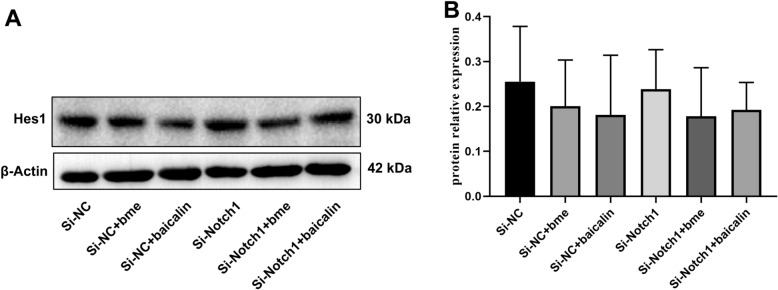
Next, Si-Notch1 plasmid reduced the Notch1 mRNA and protein expression in BMSCs (Figs. 3, 4). Si-Notch1 suppressed Hes1 mRNA expression in BMSCs (Fig. 5). Baicalin increased the effects of Notch1 on Hes1 mRNA expression in BMSCs (Fig. 5). However, baicalin did not affect Hes1 protein expression in BMSCs (Fig. 6). We found that si-Notch1 increased cell growth of BMSCs (Fig. 7). Baicalin reduced the effects of si-Notch1 on cell growth of BMSCs (Fig. 7).
Fig. 3.
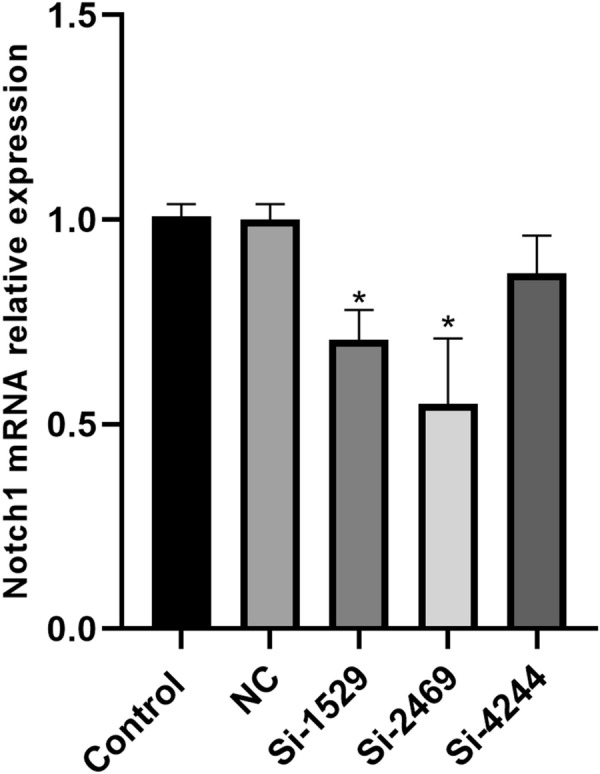
Notch1 mRNA expression. *p < 0.01 compared with NC
Fig. 4.
Notch1 protein expression. Notch1 protein expression (Western blot, A) and (statistical analyses, B). *p < 0.01 compared with NC
Fig. 5.
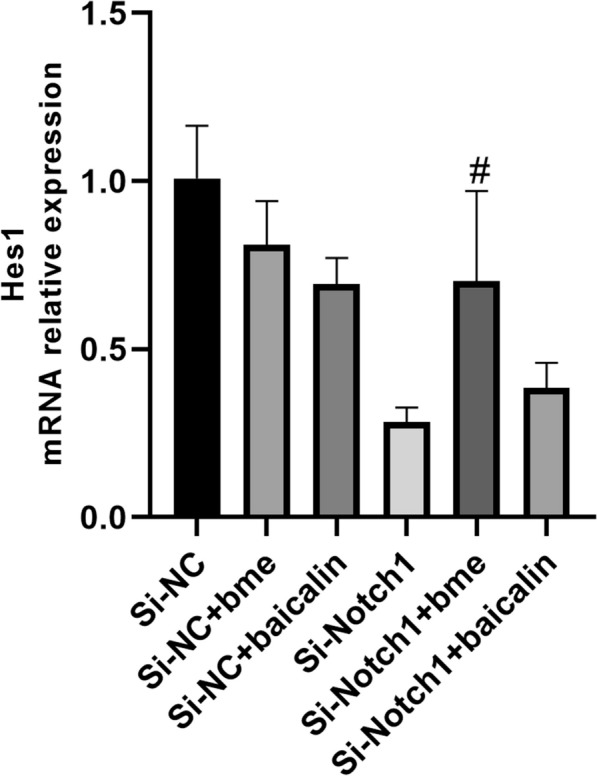
Notch1 is one target for the baicalin on Hes1 mRNA expression. #p < 0.01 compared with si-Notch1 group
Fig. 6.
Notch1 is one target for the baicalin on Hes1 protein expression
Fig. 7.
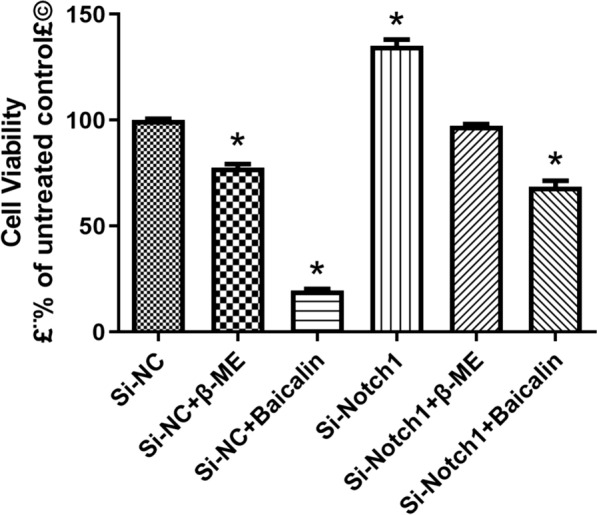
Notch1 is one target for the baicalin on cell growth of BMSCs into neurons. *p < 0.01 compared with si-NC
MicroRNA-124a regulated the cell growth and neuronal differentiation of BMSCs
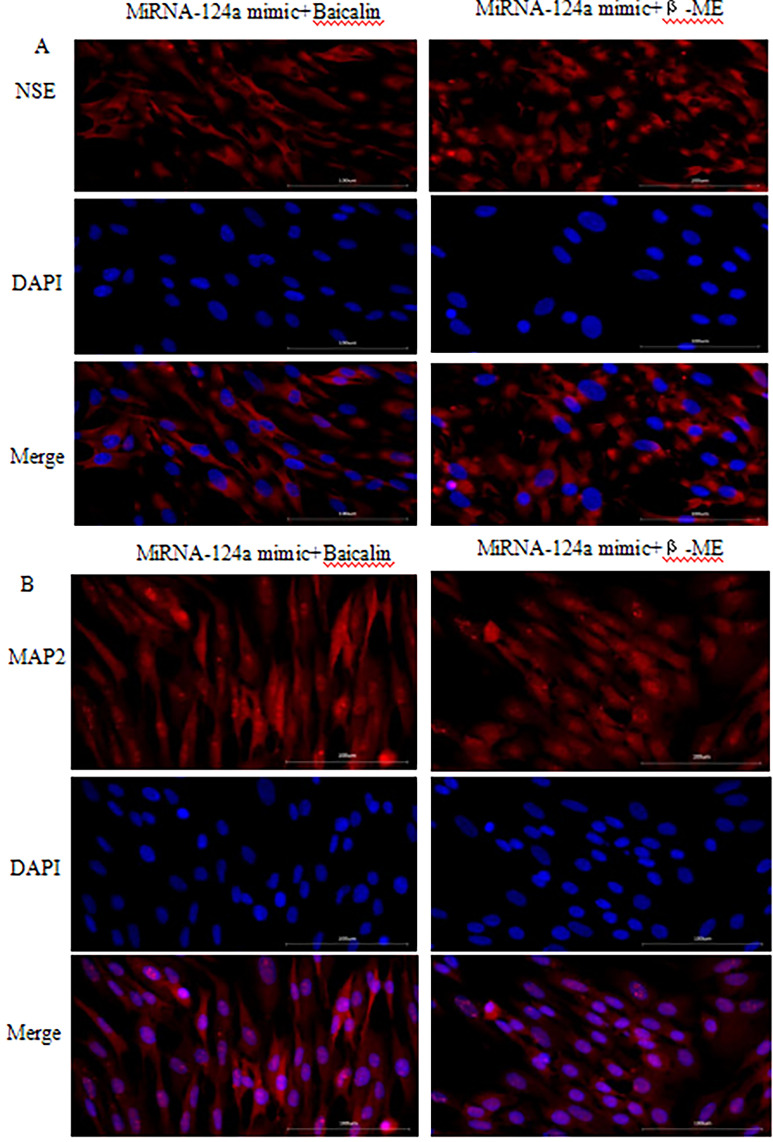
Lastly, the results of qPCR showed that microRNA-124a mimic treatment could increase microRNA-124a expression in BMSCs (Fig. 8). MicroRNA-124a increased cell growth of BMSCs (Fig. 9). Baicalin and β-thiol ethanol reduced the effects of microRNA-124a on cell growth of BMSCs (Fig. 9). MiRNA-124a inhibitor reduced the effects of β-ME and baicalin induced neuronal differentiation of BMSCs, and decreased expression of neuronal markers NSE and MAP-2. Conversely, MiRNA-124a mimic increased the effects of β-ME and baicalin induced neuronal differentiation of BMSCs, and promoted expression of neuronal markers NSE (Fig. 10A) and MAP-2 (Fig. 10B).
Fig. 8.
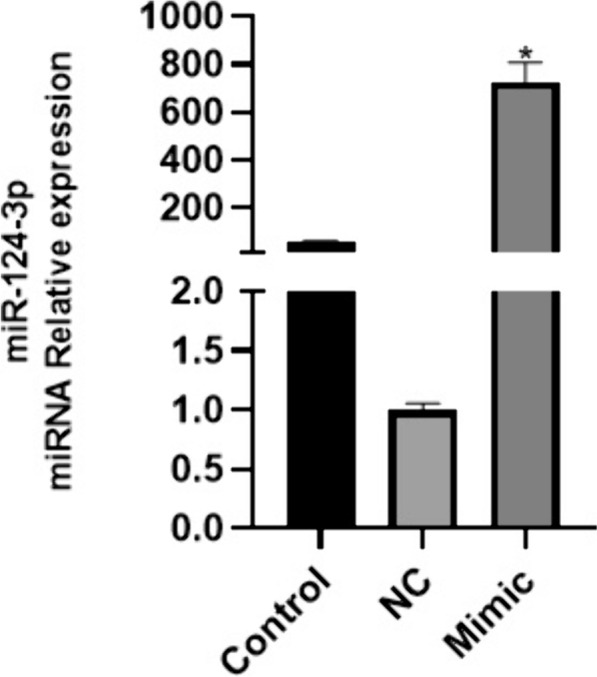
MicroRNA-124a mimic increased microRNA-124a expression in BMSCs. The qPCR results of microRNA-124a expression. *p < 0.05 compared with NC group
Fig. 9.
MicroRNA-124a regulated the cell growth of BMSCs into neurons. *p < 0.01 compared with Control group
Fig. 10.
Overexpression of MiRNA-124a promoted expression of neuronal markers NSE and MAP-2 of BMSCs into neurons
Discussion
BMSCs, also known as pluripotent stem cells, are stem cells derived from the mesoderm and have the ability of multidirectional differentiation and self-renewal to osteoblasts, chondrocytes, adipocytes and other multidirectional differentiation, and can differentiate into neural cells and glial cells in vivo and in vitro under specific conditions [20, 21]. Studies have confirmed that compounds, growth factors, gene modification, co-culture and in vivo transplantation can induce bone marrow mesenchymal stem cells to differentiate into neurons, which indicates that the neural differentiation process of BMSCs may involve multiple signal pathways, which require various signal pathway conduction and information integration to promote the reconstruction of cell structure [22–24]. In this study, baicalin induced Hes1 mRNA expression, and microRNA-124a inhibitor reduced the effects of baicalin on Hes1 mRNA expression in BMSCs. β-Thiol ethanol induced Notch1 mRNA expression, microRNA-124a inhibitor reduced the effects of β-thiol ethanol on Notch1 mRNA expression in BMSCs. Both of these show that microRNA-124a regulates Notch signaling pathway during MSCs differentiated into neurons. As in the case with these, Xu et al. showed that microRNA-124a protected against ischemia reperfusion injury through Notch signaling pathway [25]. However, the mechanism of the effects of baicalin on the promoting Hes1 mRNA expression in BMSCs was not clear.
NOTCH signal transduction pathway is one of the most important pathways that determine the fate of cells and plays an important role in cell differentiation, proliferation and apoptosis [26]. At present, it has been reported that cerebrovascular disease are sensitive to ferroptosis inducers, and its occurrence and development are closely related to ferroptosis [27, 28]. Notch1/Hes1 signal transduction plays an important role in cell differentiation, proliferation and apoptosis, and is also an important regulatory signal of ferroptosis. In the study of human small cell lung cancer, it was found that cMyc activated NOTCH signaling pathway can promote the sensitivity of small cell lung cancer cells to ferroptosis, suggesting that NOTCH signaling pathway is a potential regulatory molecular mechanism of ferroptosis [29–31]. In the present study, si-Notch1 increased cell growth of BMSCs. Baicalin reduced the effects of si-Notch1 on cell growth of BMSCs.
MicroRNAs, a short non-coding single-stranded RNA with a length of about 20–24 nucleotides, combine with mRNA through the principle of base complementary pairing to directly target the cleavage of mRNA or inhibit the translation of target genes to regulate the expression of post-transcriptional genes [32–34]. MicroRNAs can regulate the development and function of the nervous system by combining with mRNA [35]. Brain-specific microRNA-124 plays a regulatory role in the development stages of neurite generation [36]. MiR-124 affects the cell fate of glial cells, and induces their differentiation into neurons [37]. We found that microRNA-124a increased cell growth of BMSCs. Baicalin and β-thiol ethanol reduced the effects of microRNA-124a on cell growth of BMSCs. Underexpression of MiRNA-124a reduced the effects of β-ME and baicalin induced neuronal differentiation of BMSCs, and decreased expression of neuronal markers NSE and MAP-2. Conversely, overexpression of MiRNA-124a increased the effects of β-ME and baicalin induced neuronal differentiation of BMSCs, and promoted expression of neuronal markers NSE and MAP-2.
This study has some limitations. First, the molecular mechanism of how baicalin impacts on Hes1 mRNA expression in BMSCs requires in-depth investigation. Second, it needs to be explored more deeply how MiRNA-124a regulates Notch signaling pathway. Third, the experimental conclusion will need to be confirmed in vivo.
On the basis of these findings, we conclude that microRNA-124a promoted the differentiation of BMSCs into neurons through Notch signal pathway. The observations that microRNA-124a plays a broad role in the differentiation of BMSCs, and microRNA-124a might benefit the treatment of the differentiation of BMSCs into neurons. Also, baicalin might induce neuronal differentiation of BMSCs by regulating Notch signal pathway.
Acknowledgements
Not applicable.
Author contributions
DMW designed the experiments. LJJ, ZYZ and SXH performed the experiments. LX and SJH collected and analyzed the data. EYZ, HL and YQC drafted manuscript. All authors read and approved the final manuscript.
Funding
This study is supported by Hainan Province Science and Technology Special Fund (No. ZDYF2021SHFZ112), Hainan Province Clinical Medical Center.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the Hainan General Hospital. Mice were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The study is in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.do Kleyton Palmeira Ó, da Silva Freire AK, de Nóbrega DN, et al. Polymorphisms and gene expression of metalloproteinases and their inhibitors associated with cerebral ischemic stroke in young patients with sickle cell anemia. Mol Biol Rep. 2023;50(4):3341–53. [DOI] [PubMed] [Google Scholar]
- 2.Hu W, Li P, Zeng N, et al. Exploring the hub mechanisms of ischemic stroke based on protein–protein interaction networks related to ischemic stroke and inflammatory bowel disease. Sci Rep. 2023;13:1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding K, Chen H, Wang Y, et al. Emergency medical service utilization and timely treatment among acute ischemic stroke patients in Beijing from 2018 to 2021. Eur J Emerg Med. 2023. 10.1097/MEJ.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 4.Hu H, Hu X, Li L, et al. Exosomes derived from bone marrow mesenchymal stem cells promote angiogenesis in ischemic stroke mice via upregulation of MiR-21-5p. Biomolecules. 2022;12:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang CN, Song T, Tao X, et al. Bone marrow stromal cells attenuate oxygen and glucose deprivation followed by re-oxygenation-induced brain microvascular endothelial cell injury. Acta Neurobiol Exp. 2022;82:398–407. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Yang Y, Feng Y, et al. The association between liver fibrosis and depression in patients after ischemic stroke. BMC Neurol. 2023;23:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi G, Ke D, Gong P, et al. Serum YKL-40 levels and white matter hyperintensities in patients with acute ischemic stroke. J Inflamm Res. 2023;16:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Lin X, Yao C, et al. Transplantation of roxadustat-preconditioned bone marrow stromal cells improves neurological function recovery through enhancing grafted cell survival in ischemic stroke rats. CNS Neurosci Ther. 2022;28:1519–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Can U, Marzioglu E, Akdu S. Some miRNA expressions and their targets in ischemic stroke. Nucleosides Nucleotides Nucleic Acids. 2022;41:1224–62. [DOI] [PubMed] [Google Scholar]
- 10.Sohrabji F, Selvamani A. Sex differences in miRNA as therapies for ischemic stroke. Neurochem Int. 2019;127:56–63. [DOI] [PubMed] [Google Scholar]
- 11.Gugliandolo A, Silvestro S, Sindona C, et al. MiRNA: involvement of the MAPK pathway in ischemic stroke. A promising therapeutic target. Medicina. 2021;57:1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijayan M, Alamri FF, Al Shoyaib A, et al. Novel miRNA PC-5P-12969 in ischemic stroke. Mol Neurobiol. 2019;56:6976–85. [DOI] [PubMed] [Google Scholar]
- 13.Li F, Liu WC, Wang Q, et al. NG2-glia cell proliferation and differentiation by glial growth factor 2 (GGF2), a strategy to promote functional recovery after ischemic stroke. Biochem Pharmacol. 2020;171: 113720. [DOI] [PubMed] [Google Scholar]
- 14.Kim T, Jeong HY, Suh GJ. Clinical differences between stroke and stroke mimics in code stroke patients. J Korean Med Sci. 2022;37: e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiedt S, Brandmaier S, Kollmeier H, et al. Circulating metabolites differentiate acute ischemic stroke from stroke mimics. Ann Neurol. 2020;88:736–46. [DOI] [PubMed] [Google Scholar]
- 16.Kenmuir CL, Wechsler LR. Update on cell therapy for stroke. Stroke Vasc Neurol. 2017;2:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misra S, Kumar A, Kumar P, et al. Blood-based protein biomarkers for stroke differentiation: a systematic review. Proteomics Clin Appl. 2017;11:1700007. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Li S, Xu W, et al. Interleukin-17A promotes the differentiation of bone marrow mesenchymal stem cells into neuronal cells. Tissue Cell. 2021;69: 101482. [DOI] [PubMed] [Google Scholar]
- 19.Zhang P, Zhang H, Lin J, et al. Insulin impedes osteogenesis of BMSCs by inhibiting autophagy and promoting premature senescence via the TGF-β1 pathway. Aging. 2020;12:2084–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan S, Wang F, Cao J, et al. Exosomes derived from microRNA-146a-5p-enriched bone marrow mesenchymal stem cells alleviate intracerebral hemorrhage by inhibiting neuronal apoptosis and microglial M1 polarization. Drug Des Dev Ther. 2020;14:3143–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Wang C, He T, et al. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord injury rats via gap junction. Theranostics. 2019;9:2017–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan M, Xu Y, Wang W, et al. Differentiation into neurons of rat bone marrow-derived mesenchymal stem cells. Eur Cytokine Netw. 2014;25:58–63. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Zhang J, Chen A, et al. Combined transplantation of neural stem cells and bone marrow mesenchymal stem cells promotes neuronal cell survival to alleviate brain damage after cardiac arrest via microRNA-133b incorporated in extracellular vesicles. Aging. 2021;13:262–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Li H, Cai S, et al. CD157 in bone marrow mesenchymal stem cells mediates mitochondrial production and transfer to improve neuronal apoptosis and functional recovery after spinal cord injury. Stem Cell Res Ther. 2021;12:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu W, Jiang S, Liu Q. MicroRNA-124a protects the myocardium against ischemia reperfusion injury through regulation of the notch signaling pathway. Braz J Cardiovasc Surg. 2022;37:447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapoor A, Nation DA. Role of notch signaling in neurovascular aging and Alzheimer’s disease. Semin Cell Dev Biol. 2021;116:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louvi A, Arboleda-Velasquez JF, Artavanis-Tsakonas S. CADASIL: a critical look at a notch disease. Dev Neurosci. 2006;28:5–12. [DOI] [PubMed] [Google Scholar]
- 28.Zhao M, Li M, Zheng Y, et al. Identification and analysis of a prognostic ferroptosis and iron-metabolism signature for esophageal squamous cell carcinoma. J Cancer. 2022;13:1611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai Z, Zhao B, Deng Y, et al. Notch signaling in cerebrovascular diseases (review). Mol Med Rep. 2016;14:2883–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenough MA, Lane DJR, Balez R, et al. Selective ferroptosis vulnerability due to familial Alzheimer’s disease presenilin mutations. Cell Death Differ. 2022;29:2123–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Wang S, Xin Y, et al. Hydrogen sulfide alleviates the anxiety-like and depressive-like behaviors of type 1 diabetic mice via inhibiting inflammation and ferroptosis. Life Sci. 2021;278: 119551. [DOI] [PubMed] [Google Scholar]
- 32.Du K, Zhao C, Wang L, et al. MiR-191 inhibit angiogenesis after acute ischemic stroke targeting VEZF1. Aging. 2019;11:2762–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Song Y, He T, et al. M2 microglial small extracellular vesicles reduce glial scar formation via the miR-124/STAT3 pathway after ischemic stroke in mice. Theranostics. 2021;11:1232–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pu Z, Xu M, Yuan X, et al. Circular RNA circCUL3 accelerates the Warburg effect progression of gastric cancer through regulating the STAT3/HK2 axis. Mol Ther Nucleic Acids. 2020;22:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian Y, Chopp M, Chen J. Emerging role of microRNAs in ischemic stroke with comorbidities. Exp Neurol. 2020;331: 113382. [DOI] [PubMed] [Google Scholar]
- 36.Su X, Gu X, Zhang Z, et al. Retinoic acid receptor gamma is targeted by microRNA-124 and inhibits neurite outgrowth. Neuropharmacology. 2020;163: 107657. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki F, Okuno M, Tanaka T, et al. Overexpression of neural miRNAs miR-9/9* and miR-124 suppresses differentiation to Müller glia and promotes differentiation to neurons in mouse retina in vivo. Genes Cells. 2020;25(11):741–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.