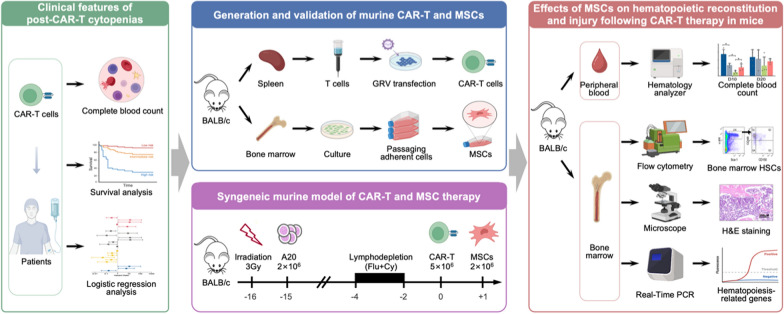
Abstract
Background
Chimeric antigen receptor (CAR)-T therapy has emerged as a promising treatment for hematologic malignancies. However, cytopenia remains one of the most frequent and challenging adverse effects of this therapy.
Methods
We conducted a retrospective analysis of 26 patients with relapsed/refractory aggressive B-cell lymphoma who received CAR-T therapy at our center. Subsequently, to investigate measures to address cytopenias following CAR-T therapy, we isolated and generated murine CAR-T cells and bone marrow-derived mesenchymal stem cells (MSCs), establishing a murine syngeneic CAR-T therapy model. We assessed the impact of MSC infusion on hematopoietic recovery post-CAR-T therapy by evaluating complete blood count, bone marrow hematopoietic stem cells and their subpopulations, bone marrow histomorphology, and hematopoiesis-related genes.
Results
All patients experienced cytopenias to varying degrees, with complete lineage involvement in half of the patients. Grade ≥ 3 cytopenias were observed in 88.46% of the patients. CAR-T therapy was associated with a higher incidence of biphasic, late-onset, or prolonged cytopenias. Survival analysis indicated that neutropenia and lymphopenia tended to be associated with better prognosis, whereas thrombocytopenia tended to be related to poorer outcomes. Through animal experiments, we discovered that MSCs infusion boosted HSCs and their long-term subpopulations, enhancing hematopoietic recovery, particularly in the megakaryocyte lineage, and mitigating bone marrow damage. Importantly, both in vitro and in vivo experiments demonstrated that MSCs did not compromise the activity or antitumor efficacy of CAR-T cells.
Conclusions
Our findings propose MSCs infusion as a promising strategy to address cytopenias, particularly thrombocytopenia, after CAR-T therapy. This approach could help overcome certain limitations of cellular immunotherapy by enhancing hematopoietic recovery without compromising the efficacy of CAR-T cells.
Highlights
Cytopenia is a frequently observed adverse effect following CAR-T therapy, and it is often characterized by biphasic and prolonged patterns.
MSCs play a critical role in promoting hematopoietic recovery and mitigating bone marrow damage in a murine model of CAR-T therapy
The activity and antitumor efficacy of CAR-T cells were not impaired by MSCs.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-024-03941-8.
Keywords: Chimeric antigen receptor T-cell therapy, Cytopenia, Mesenchymal stem cells, Thrombocytopenia, Cellular immunotherapy
Introduction
T cells genetically engineered to express chimeric antigen receptor (CAR) comprising of antigen recognition region and T cell signaling domains, known as CAR-T cells, could identify and eliminate tumor cells expressing specific antigens [1]. The efficacy of CAR-T therapy in hematological malignancies, especially in B-cell and plasma-cell malignancies, has been validated in a great number of clinical trials and real-world practices in recent years [2, 3]. Cytopenia is one of the most frequent adverse effects after CAR-T therapy. According to our previous clinical studies, cytopenias were observed in 100% and 82.4% of patients in anti-CD19 [4] and anti-BCMA [5] CAR-T therapies, respectively. In our subsequent systematic analysis involving 68 studies (n = 2950) of anti-CD19 CAR-T therapies, the overall incidence of anemia, thrombocytopenia, neutropenia, and lymphopenia and were 65%, 55%, 78%, and 70%, respectively [6]. Post-CAR-T cytopenias, compared to those caused by conventional therapies such as chemotherapy and radiotherapy, present with a longer duration and a biphasic pattern, characterized by the reappearance of cytopenias after an initial recovery [7, 8]. The mechanism of cytopenias following CAR-T therapy has not been fully elucidated. Prior therapy such as chemotherapy, immunotherapy, and hematopoietic stem cell transplantation (HSCT), along with lymphodepletion chemotherapy, could impair the bone marrow microenvironment, especially in heavily pre-treated patients [9]. Moreover, the robust immune response triggered by CAR-T cell infusion, involving the activation of monocytes, macrophages, and CAR-T cells themselves, may further disrupt bone marrow homeostasis [8, 10, 11]. Nevertheless, the incidence and pattern of post-CAR-T cytopenias vary across studies [12–15], warranting further research for a better understanding of this phenomenon.
Despite the use of blood transfusion and hematopoietic growth factors or agonists, which can provide some relief for cytopenia and promote hematopoiesis to some extent, post-CAR-T cytopenia remains an intractable issue globally, significantly affecting patients’ quality of life [16], indicating a need for effective therapeutic interventions. Mesenchymal stem cells (MSC), a population of pluripotent stem cells with multilineage differentiation capacity, possess remarkable qualities such as immunomodulatory and regenerative properties, low immunogenicity, extensive sources, and simple preparation [17]. Notably, MSCs play a pivotal role in the formation of bone marrow (BM) microenvironment through their capacity to differentiate into non-hematopoietic components and exert extensive immunoregulatory effects [18]. Studies have demonstrated that MSCs contribute to the maintenance and expansion of hematopoietic stem cells (HSCs) within the BM microenvironment [19, 20]. Moreover, MSCs have shown efficacy in facilitating HSCs engraftment and promoting hematopoietic recovery in HSCT, as well as alleviating BM injury and graft-versus-host disease (GvHD) [21, 22]. Given the potential detrimental effects of CAR-T therapy on the hematopoietic niche, we hypothesize that MSCs could serve as a promising therapeutic strategy to ameliorate BM impairment and enhance hematopoietic recovery following CAR-T therapy.
Hence, this study aims to to analyze single-center data to depict the characteristics of post-CAR-T cytopenias and identify the factors that influence their occurrence, thereby enabling a better understanding and management of CAR-T therapy-related cytopenia. Additionally, we intend to investigate the potential effects of MSC on ameliorating bone marrow injury and facilitating hematopoietic reconstruction in a murine CAR-T therapy model. This investigation holds the potential to deepen our comprehension of post-CAR-T cytopenias and offer valuable insights for the development of novel therapeutic interventions.
Materials and methods
Study population and criteria for cytopenia grading
A total of 26 patients with relapsed/refractory (R/R) aggressive B-cell lymphoma who received CAR-T therapy at the First Affiliated Hospital of Nanjing Medical University from April 2017 to May 2023 were retrospectively enrolled. All patients were followed up until June 2024, with a median follow-up time of 41 months (range, 1–67 months). This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Boards of the First Affiliated Hospital of Nanjing Medical University Ethics Committee (No. 2019-SR-335 and 2019-MD-512). Informed consents were obtained before enrollment.
Cytopenias following CAR-T therapy was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 establish by National Cancer Institute (NCI). Cytopenias that persisted for ≥ 28 days were defined as prolonged cytopenia, and those that emerged on or after day 28 after infusion were defined as late-onset cytopenia. The reappearance of cytopenias subsequent to the recovery from early cytopenias was defined as biphasic cytopenia.
Animals
BALB/c mice, aged 6 weeks and weighing 19–21 g, were obtained from Vital River Laboratory Animal Technology (Zhejiang, China). All animal care and experimental procedures complied with the standards of Animal Welfare for the Care and Use of Laboratory Animals of Nanjing Medical University, and were approved by Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University (No. 2201014). The work has been reported in line with the ARRIVE guidelines 2.0. The mice in this study were anesthetized using an intravenous injection of pentobarbital sodium. For specimen collection, mice were euthanized by cervical dislocation following intravenous injection of pentobarbital sodium anesthesia. At the experimental endpoint, mice were humanely euthanized using carbon dioxide inhalation.
Generation of anti-mouse CD19 CAR-T cells
The anti-mouse CD19 CAR vector was kindly provided by Dr. Guang Hu from Iaso Biotherapeutics Co. Ltd. (Nanjing, China). The CAR was composed of the anti-mCD19 scFv linked to the murine CD8a hinge/transmembrane region, along with intracellular murine CD28 and CD3ζ signaling domains. The CAR construct was cloned into the pMYs-GFP-retroviral vector backbone. To generate the γ-retrovirus, Platinum-E cells (Cell Biolabs, Inc, CA, USA), serving as retroviral packaging cell, were transient transfected with the anti-mCD19 CAR vector using Lipofectamine 3000 (Invitrogen, CA, USA). The viral supernatants were collected at 48 h after transfection.
Splenocytes from BALB/c mice were isolated by passing through a 70 μm-pore strainer, and mononuclear splenocytes were obtained by the density gradient centrifugation over Mouse Lymphocyte Separation Medium (Dakewe Biotech, Shenzhen, China). CD3+ T cells from mononuclear splenocytes were purified using Mouse CD3ε MicroBead Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. T cells were cultured in X-VIVO 15 medium (Lonza, MD, USA) with 10% Fetal Bovine Serum (FBS) (Gibco, NY, USA), 100 U/mL interleukin (IL)-2 (SL Pharm, Beijing, China), 2 mM L-Glutamine (Gibco, NY, USA), and subsequently activated by Dynabeads Mouse T-Activator CD3/CD28 (Gibco, NY, USA). After 24 h of activation, mouse T cells were transduced with γ-retrovirus. Transduction efficiency was analyzed by green fluorescent protein (GFP) detection using flow cytometry.
Isolation, culture and identification of bone marrow MSCs
Mouse bone marrow MSCs were isolated and cultured as previously described [23]. In brief, the femurs of BALB/c mice were dissected by cutting at the joints following disassociation of muscles and ligaments. The bone marrow in the femur was flushed out using a 23-gauge needle attached to a syringe containing MEMα (Gibco, NY, USA) and purified by a 70 μm-pore strainer (Corning, NY, USA). Bone marrow cells were seeded on 95 mm culture dishes at a density of 2.5 × 105 cells per square centimeter with complete MEMα medium at 37℃ in 5% CO2. Nonadherent cells were removed after 24 h, and when 70–90% confluence was reached, cells were digested with trypsin and passaged at a ratio of 1:3. Phenotypic characteristics of MSCs were identified by flow cytometry. For all following experiments, MSCs were used at passage 4.
Flow cytometry
To detect the phenotypic characteristics of MSCs, cells were labeled with the following antibodies: PE-conjugated anti-mouse CD34 (clone: HM34), FITC‐conjugated anti-mouse CD45 (clone: 30-F11), PE/Cyanine7‐conjugated anti-mouse Sca-1 (clone: D7) and APC‐conjugated anti-mouse/human CD44 antibodies (clone: IM7). CAR expression was determined by the positive rate of GFP. To detect the degranulation of CAR-T cells, cells were labeled with PE/Cyanine7‐conjugated anti-mouse CD107a (clone: 1D4B). For the detection of HSCs, bone marrow cells are labeled with the following antibodies: FITC‐conjugated anti-mouse CD3ε (clone: 145-2C11), FITC‐conjugated anti-mouse/human B220 (clone: RA3-6B2), FITC‐conjugated anti-mouse/human CD11b (clone: M1/70), FITC‐conjugated anti-mouse Gr-1 (clone: RB6-8C5), FITC‐conjugated anti-mouse TER-119 (clone: TER-119), PE/Cyanine7‐conjugated anti-mouse Sca-1 (clone: D7), Brilliant Violet 421‐conjugated anti-mouse c-Kit (clone: 2B8), PE‐conjugated anti-mouse CD150 (clone: TC15-12F12.2), APC‐conjugated anti-mouse CD48 (clone: HM48-1). All antibodies were obtained from Biolegend (CA, USA) and used according to the manufacturer’s instructions. To assess the proliferation of CAR-T cells, cells are prelabeled with CellTrace Violet Dye (Invitrogen, CA, USA).
Data were collected using MACS Quant Analyzer 10 (Miltenyi Biotec, Bergisch Gladbach, Germany), and were analyzed with the FlowJo Software (Version 10.0) (TreeStar).
Degranulation and proliferation assay
MSCs were seeded into 96-well plates, and the degranulation assay was performed once the cell confluence reached 80–90%. Subsequently, 1 × 105 CAR-T cells ± 1 × 105 A20 cells (Cobioer Biosciences, Nanjing, China) were seeded in the wells with or without MSCs. The cells were co-incubated in the presence of 1:50 anti-mouse CD107a antibody and Monensin (Biolegend, CA, USA) for 6 h, and the expression of CD107a was detected by flow cytometry.
MSCs were seeded into 6-well plates, and proliferation assays were performed when 60–70% confluence was reached. CAR-T cells were labeled with 5 mM CellTrace Violet Dye, followed by the incubation with X-VIVO 15 medium (containing 5% FBS) to remove free dye remaining in the solution. Pelleted cells were resuspend in complete X-VIVO 15 medium. 1 × 106 CAR-T cells were co-cultured with 1 × 106 A20 cells in the presence or absence of MSCs. The CellTrace Violet fluorescence intensity of CAR-T cells was detected by flow cytometry with 405 nm excitation.
Cytolysis assay
CAR-T cells were co-cultured with adherent MSC until the MSCs reached 60–70% confluence. After 72 h, the co-cultured cells were harvested. Subsequently, 1 × 104 luciferase-expressing A20 cells (A20-Luc) (referred to as target cell, T for short) were plated in triplicate in U-bottom 96-well plates and co-incubated with CAR-T cells (referred to as effector cell, E for short) at different effector: target cell (E: T) ratios for 12 h. The luciferase assay was performed using the VivoGlo Luciferase Assay system (Promega, Madison, WI, USA) as per the manufacturer’s instructions.
Syngeneic mouse model of CAR-T therapy and MSCs infusion
BALB/c mice were subjected to 3 Gy total body irradiation followed by the engraftment of 2 × 106 A20 cells at left flank via subcutaneously injection in a volume of 200 µL. CAR-T therapy was performed when tumor size reached approximately 300–350 mm3. The mice were randomly grouped and received daily intraperitoneal injections of 100 mg/kg cyclophosphamide and 30 mg/m2 fludarabine for 3 consecutive days (day − 4 to day − 2). One day after chemotherapy, 5 × 106 CAR-T cells were administered intravenously by tail vein injection (day 0). At the same time, an equal volume of PBS was injected into mice to serve as preconditioning control (Pre-Control). On day 1 post CAR-T infusion, 2 × 106 MSCs were intravenously injected via the tail vein (CAR-T + MSC), while an equal volume of PBS was injected to mice to serve a control (CAR-T). Normal health mice without any treatment served as normal control (NC).
Analysis of complete blood count and bone marrow HSCs in mice
On day 10 (D10) and day 20 (D20) following CAR-T therapy, peripheral blood samples were collected from to perform a complete blood count test. At the same time, the left femur was isolated, and all bone marrow cells in it were flushed out as described above. Subsequently, the number of nucleated cells in the bone marrow of a single femur was analyzed under a microscope. The remaining bone marrow cells were incubated with red blood cell lysis buffer (Solarbio, Beijing, China), followed by precise antibody staining against specific lineage markers (CD3ε, B220, CD11b, Gr-1 and TER-119) and progenitor markers (Sca-1, c-Kit, CD150 and CD48). HSCs and their subpopulations were detected by flow cytometry [24].
10 H&E staining of bone marrow
On days 10 and 20 after CAR-T therapy, the right femur of mice was isolated and fixed with a formaldehyde solution. Subsequently, the samples underwent decalcification using an ethylene diamine tetraacetic acid (EDTA) solution and were then dehydrated, paraffin embedded and sectioned at a thickness of 3 μm. After that, the tissue slides were dewaxed, rehydrated, and stained with hematoxylin and eosin. The histological and morphological changes were evaluated using an optical microscope.
11 quantitative real-time polymerase chain reaction (PCR)
Total RNA was extracted from BM cells with Trizol (Invitrogen, CA, USA) and cDNA was synthesized with HiScript III RT SuperMix reverse transcription kit (Vazyme, Nanjing, China). ChamQ SYBR qPCR Master Mix kit (Vazyme, Nanjing, China) was used for quantitative PCR to detect the expression of target genes, which was performed on StepOne Real-Time PCR System (Applied Biosystems, CA, USA). The specificity of the PCR product was verified by analyzing the melting curve. β-actin was used as an internal control, and the relative mRNA expression of target genes was analyzed using the 2–ΔΔCt method. Primers for target genes were listed in Supplementary Table 1.
12 statistical analysis
Variables that conformed to normal distribution were analyzed by Student’s t test or ANOVA, and Mann-Whitney U or Kruskal-Wallis tests were used for analyzing variables that did not comply with normal distribution. Progression-free survival (PFS) and overall survival (OS) were plotted as Kaplan-Meier curves and were compared using the log-rank test. The factors influencing the occurrence of various types of cytopenias were analyzed using a logistic regression model. Statistical analysis was performed by SPSS (Version 23.0) or GraphPad Prism (Version 8.0). All tests were two-sided, and P < 0.05 was considered statistically different.
Results
Clinical characteristics of patients underwent CAR-T therapy
A total of 26 patients, including 17 male and 9 female, with a median age of 52.5 years (range: 22–71), were enrolled in this study. All patients were diagnosed with aggressive B-cell lymphoma, with 20 cases of diffuse large B-cell lymphoma (DLBCL), 1 case of high-grade B-cell lymphoma, 1 case of primary mediastinal large B-cell lymphoma (PMLBL), and 4 cases of Richter transformation (3 cases were previously follicular lymphoma, and 2 cases were previously small lymphocytic lymphoma). Five cases (19.2%) were classified as stage III according to the Lugano staging system, while 17 cases (65.4%) were stage IV. Six patients (23.1%) presented with B symptoms. The median International Prognostic Index (IPI) was 2 (range: 0–5), and the median Eastern Cooperative Oncology Group (ECOG) performance status was 0 (range: 0–4). Bone marrow involvement was observed in 8 cases (30.8%), and the median Ki-67 index was 72.5 (range: 30–90). Patients had received a median of 3 prior lines of treatment (range: 1–8). Six patients (23.1%) had undergone autologous stem cell transplantation (ASCT), while 12 patients (46.2%) received bridging therapy. The pre-conditioning regimens consisted of 21 cases (80.8%) using the FC regimen and 5 cases (19.2%) using the BEAM regimen.
Eighteen patients (69.2%) received anti-CD19 CAR-T therapy, while eight patients (30.8%) received anti-CD20 CAR-T therapy. The median time from disease diagnosis to CAR-T therapy was 12.5 months (range: 3-104). Twenty patients experienced cytokine release syndrome (CRS), with a median CRS grade of 1 (range: 0–2), and there were no cases with grade ≥ 3 CRS. One patient (3.8%) developed immune effector cell-associated neurotoxicity syndrome (ICANS). After treatment, 16 patients (61.5%) achieved a best response of partial response (PR) or better, while 8 patients (30.8%) achieved a best response of complete response (CR) or better. The median PFS was 3.5 months and the median OS was13.5 months.
Features of cytopenias following CAR-T therapy
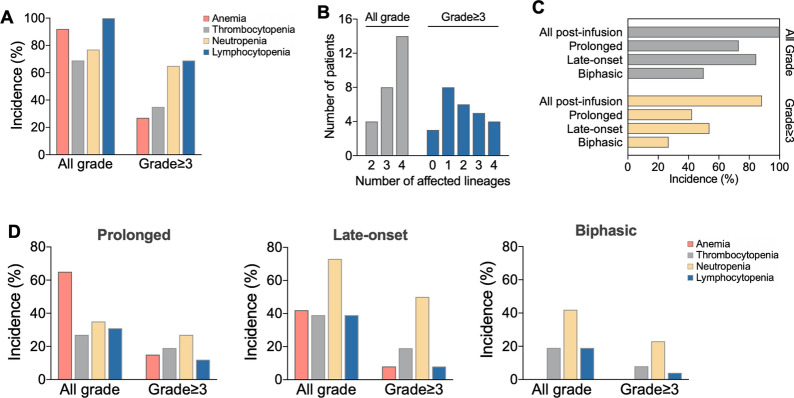
The incidence of all grades of anemia, thrombocytopenia, neutropenia, and lymphopenia after CAR-T infusion were 92.3%, 69.2%, 76.9%, and 100%, respectively, and the corresponding incidence of grade ≥ 3 cytopenia was 26.9%, 34.6%, 65.4%, and 69.2% (Fig. 1A). All patients experienced cytopenias to different extent, with complete lineage involvement in half of the patients. Grade ≥ 3 cytopenias were observed in 88.46% of the patients, with most cases exhibiting cytopenias involving only two or fewer lineages (Fig. 1B).
Fig. 1.
Clinical characteristics of cytopenias in patients with aggressive B-cell lymphoma underwent CAR-T therapy. (A) Occurrence of cytopenias in different lineages. (B) Number of lineages affected by cytopenias. (C) Incidence of different patterns of cytopenias. (D) Comparison of cytopenias in different lineages among three patterns of cytopenia
Three distinct patterns of cytopenias, including prolonged cytopenia, late-onset cytopenia and biphasic cytopenia, were evaluated to gain a more detailed understanding of post-CAR-T cytopenias (Fig. 1C, D). The incidence rates of grade ≥ 3 prolonged cytopenia, late-onset cytopenia, and biphasic cytopenia were 42.3%, 53.8%, and 26.9%, respectively. Anemia is most frequent in all grades of prolonged cytopenia, whereas the most common type of grade ≥ 3 prolonged cytopenia was neutropenia. Late-onset neutropenia had the highest incidence rate at 73.1%, with 50% of cases reaching grade ≥ 3 severity. In contrast, late-onset cytopenias in the erythroid lineage, megakaryocytic lineage, and lymphoid lineage had relatively lower and similar incidence rates. Similarly, neutropenia was also the most common type of late-onset cytopenia, with incidence rates of 42.3% for all grades and 23.1% for grade ≥ 3. However, no cases of biphasic anemia were observed in any of the patients.
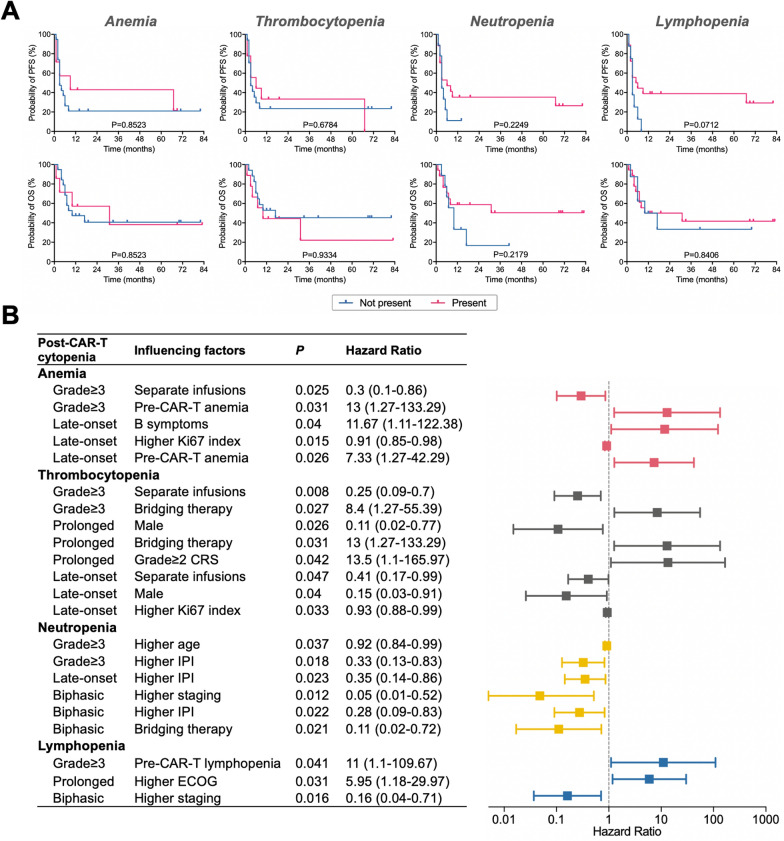
Survival analysis
To further elucidate the impact of cytopenias on patient prognosis following CAR-T therapy, and considering the high occurrence rate of all grade cytopenias, we analyzed the prognostic significance of grade ≥ 3 cytopenias following CAR-T infusion. Survival analysis revealed that post-CAR-T therapy anemia had minimal impact on overall survival. However, it appears that thrombocytopenia tends to indicate a poorer prognosis. Interestingly, in contrast, neutropenia and lymphopenia following CAR-T therapy appeared to be associated with a favorable prognosis (Fig. 2A).
Fig. 2.
Prognostic significance and influencing factors of cytopenias in patients with aggressive B-cell lymphoma underwent CAR-T therapy. (A) Prognostic significance of grade ≥ 3 post-CAR-T cytopenias. (B) Significant factors influencing cytopenias identified through logistic regression analysis
Analysis of factors influencing the occurrence of post-CAR-T cytopenia
To further analyze the clinical parameters influencing the occurrence of cytopenias after CAR-T therapy, logistic regression models were utilized to identify the significant factors. It was revealed that pre-infusion cytopenias, grade ≥ 2 CRS, bridging therapy, higher ECOG performance status, and B symptoms were statistically significant risk factors for post-CAR-T cytopenias. Conversely, separate infusions, male gender, higher IPI, higher staging, and higher Ki67 index were identified as statistically significant protective factors (Fig. 2B, Supplementary Table 2).
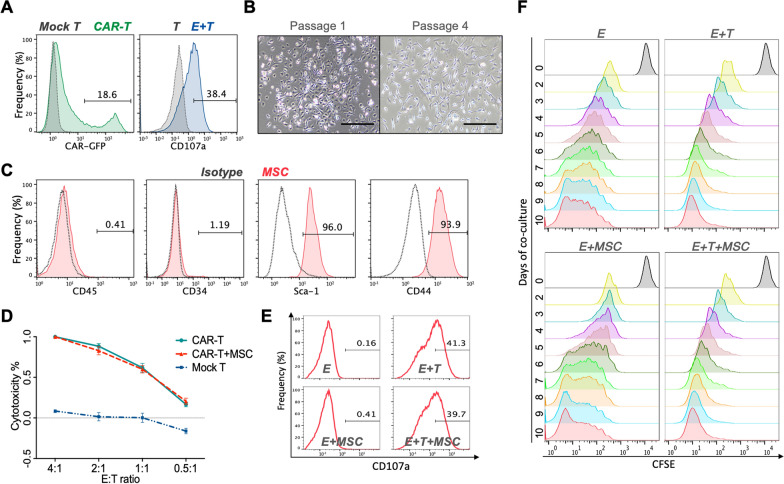
Identification of murine anti-mCD19 CAR-T cells and murine bone marrow MSCs
Murine anti-mCD19 CAR-T (mCAR-T) cells were generated by γ-retroviral transduction and the transduction efficiency was verified to be 18.6% by flow cytometry (Fig. 3A, left). Degranulation assay was subsequently performed and it was validated that mCAR-T cells were specifically activated by CD19+ A20 cells for degranulation (Fig. 3A, right). Murine MSCs were isolated from bone marrow, and a number of spindle-shaped and colony-like adherent cell were observed on day 3 of culture, while cells were heterogeneous in shape. The number and size of colonies increased gradually and the adherent cells were passaged. By Passage 4, the cells exhibited a uniform spindle-shaped morphology (Fig. 3B), and flow cytometry analysis revealed that MSC-specific antigen including Sca-1 (96%) and CD44 (93.9%) were highly expressed on these cells, whereas hematopoietic marker including CD45 (0.41%) were CD34 (1.19%) were not expressed (Fig. 3C).
Fig. 3.
Effect of mCAR-T cells on the function of MSCs in vitro. (A) Transduction efficiency (left) and degranulation assay (right) for murine anti-mCD19 CAR-T cells. (B) Morphology of murine MSCs at Passage 1 and Passage 4. (C) Phenotypic characteristics of MSCs detected by flow cytometry. (D) Cytolysis of CAR-T cells in the presence of MSCs. (E) Degranulation of CAR-T cells in the presence of MSCs. (F) Proliferation of CAR-T cells in the presence of MSCs
Function and proliferation of mCAR-T cells were not impaired by MSCs in vitro
Given that MSCs are significant immune regulatory cells, the function and proliferation of mCAR-T cells were detected in the presence of MSC to determine whether the activity of CAR-T cells would be influenced by MSCs.
Cytolysis assay of CAR-T cells was based the quantification of luminescence generated by the reporter genes Luciferase in tumor cells, and the analysis of the cytolysis assay showed that MSCs did not affect the in vitro killing function of mCAR-T cells (Fig. 3D). Consistently, degranulation assays performed by detecting CD107a expression indicated that MSCs did not impact the degranulation of CAR-T cells (Fig. 3E). In addition, the proliferation of CAR-T cells was not influenced by the presence of MSCs (Fig. 3F).
Mouse model of CAR-T therapy and MSCs infusion
Immunocompetent syngeneic murine models were established on BALB/c mice by infusing tumor cells (A20 cells), CAR-T cells and MSCs with common BALB/c backgrounds. Prior irradiation was conducted to induce the bone marrow injury, in addition to allowing a higher tumorigenesis rate. A flowchart of the mouse model was presented in Fig. 4A. CAR-T therapy elicited reduced activity, general presentation of malaise, and weight loss in mice, which gradually alleviate 7 to 10 days after CAR-T infusion. However, the introduction of MSCs had no impact on the activity or weight of the mice (Fig. 4B) or on the in vivo antitumor effects of the CAR-T cells (Fig. 4C).
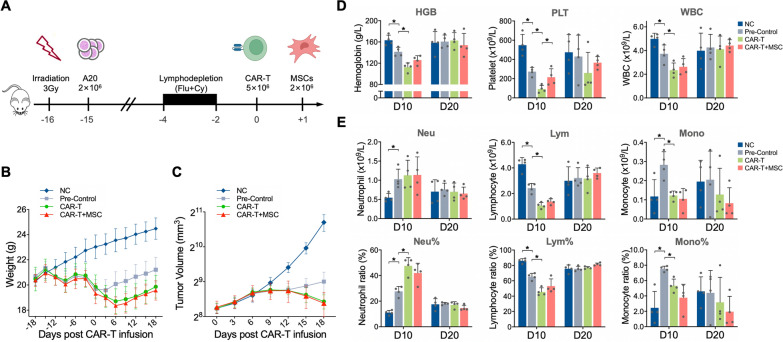
Fig. 4.
General condition and blood cell count of mice undergoing CAR-T therapy in combination with MSC infusion. (A) Flowchart illustrating the syngeneic mouse model of CAR-T therapy and MSC infusion. (B) Trend of mouse body weight. (C) Trend of tumor volumes. (D) Comparison of hemoglobin levels, platelet counts, and white blood cell counts on D10 and D20. (E) Comparison of proportions and counts of different leukocyte subpopulations on D10 and D20. (*P < 0.05)
Elevated platelet count and hemoglobin post MSC infusion
Complete blood count tests were performed on D10 and D20 following CAR-T infusion. The pre-conditioning, including irradiation and lymphodepletion, resulted in pancytopenias on D10, which were further exacerbated by CAR-T infusion. The addition of MSCs infusion to CAR-T therapy gave rise to a significantly higher platelet count (P < 0.05) and a slightly elevated hemoglobin level (P = 0.058) on D10, while no differences were observed on D20. However, the addition of MSC had no effect on the total white blood cell (WBC) count or the counts and proportions of WBC subgroups on both D10 and D20 (Fig. 4D, E).
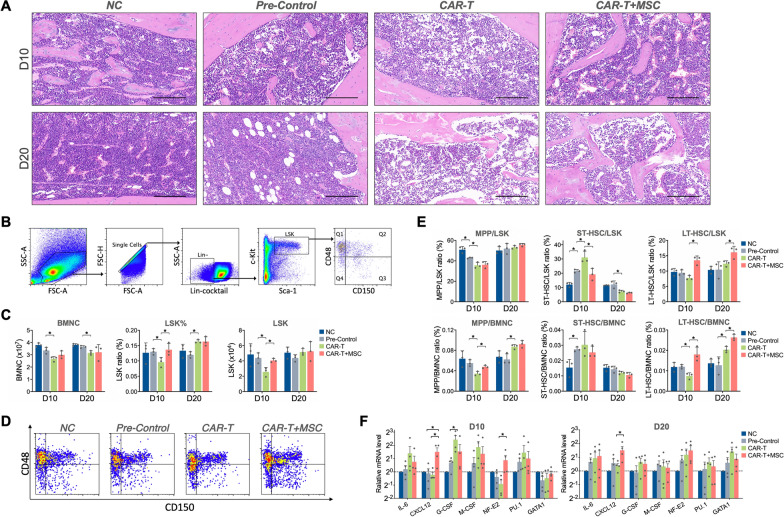
Alleviated bone marrow injury after MSCs infusion
In order to evaluate the pathologic changes of bone marrow after CAR-T therapy, H&E staining of bone marrow was performed following CAR-T infusion (Fig. 5A). On D10, the CAR-T group showed hemorrhagic bone marrow with more cavity and fewer nucleated cells, as well as reduced and morphologically abnormal megakaryocytes. Hemorrhage and cavity were also observed in the CAR-T + MSC group on D10, whereas present with a less severe injury. By D20, the number of bone marrow cells appeared to be increased in all the intervention groups. The impairment was alleviative on D20 in the CAR-T group compared with D10, while the hematopoietic tissues were loose and less recognized. The number of nucleated cells in the CAR-T + MSC group was significantly greater than that in the CAR-T group on D20.
Fig. 5.
Effects of MSCs on histopathology, HSC population and hematopoiesis-related genes of bone marrow in mice undergoing CAR-T therapy. (A) Hematoxylin-eosin (H&E) staining graph depicting bone marrow histopathology in each experimental group. (B) Flow cytometry gating strategy for hematopoietic stem cells and their subpopulations. (C) Comparison of bone marrow nucleated cell (BMNC) and Lin–Sca-1+c-Kit+ (LSK) cell populations. (D) Representative flow cytometry diagram illustrating subpopulations on D10. (E) Proportions of different subpopulations within the hematopoietic stem cell (HSC) compartment. (F) Relative expression levels of hematopoiesis-related genes in bone marrow cells. (*P < 0.05)
3.10 MSC infusion augmented the HSC and long-term HSC subpopulation.
The number of bone marrow nucleated cells (BMNC) were analyzed by counting nucleated cells in the unilateral femur and the HSCs were detected by flow cytometry using the gating strategy presented in Fig. 5B. We found that CAR-T infusion did not affect the BMNC count, yet boosted the number and proportion of LSK (Lin– Sca-1+ c-Kit+), which is the HSCs in mice (Fig. 5C). Following analysis of LSK subpopulations were performed by flow cytometry to identify long-term HSC (LT-HSC) (CD150+ CD48– LSK), short-term HSC (ST-HSC) (CD150– CD48– LSK) and multipotent progenitor cells (MPP) (CD150– CD48+ LSK) as described in previous study [24]. It was shown that CAR-T infusion contribute to the elevation of the long-term HSC subpopulation on both D10 and D20 (Fig. 5D-E). Taken together, these data demonstrated that MSCs induce the boost of HSCs and the long-term subpopulation therein directly and effectively.
11 effect of MSCs on mRNA expression of hematopoiesis-related genes
Considering the molecular regulation of hematopoiesis by the growth factors and transcription factors [25], relative mRNA expression of hematopoiesis-relevant genes including IL-6, CXCL12, G-CSF, M-CSF, NF-E2, PU.1, and GATA1 of bone marrow cells were determined by quantitative real-time PCR. As seen in Fig. 5F, mRNA level of CXCL12 and NF-E2 in bone marrow cells were significantly increased after MSC treatment (P < 0.05) on D10 post-CAR-T infusion. By D20 after CAR-T infusion, the expression of CXCL12 was still higher in the MSC + CAR-T group than that in the CAR-T group.
Discussion
Cytopenia is one of the most common adverse events in CAR-T therapy [26]. In this retrospective study on CAR-T therapy for aggressive B-cell lymphoma, all patients experienced cytopenia following CAR-T infusion, with 80.5% developing grade ≥ 3 cytopenia. The most frequently observed grade ≥ 3 cytopenias were lymphopenia and neutropenia, accounting for approximately two-thirds of cases. Notably, these cytopenias predominantly exhibited recovery over time, suggesting that lymphopenia and neutropenia may be primarily associated with the conditioning chemotherapy regimen. It is interesting to note that post-CAR-T neutropenia and lymphopenia tend to indicate a better prognosis, aligning with previous studies that a more effective lymphodepletion regimen predicts better CAR-T engraftment and stronger anti-tumor effects [27]. Fortunately, neutropenia following CAR-T therapy can be effectively managed with G-CSF, GM-CSF, and other interventions, thereby reducing the risk of complications such as infections. However, sustained immune suppression caused by lymphopenia and its potential consequences, especially under more potent lymphodepletion regimens, require close attention.
Anemia is common following CAR-T infusion, but it is mostly mild (Hb > 90 g/L), and tends to recover over time. Thrombocytopenia, particularly grade ≥ 3 thrombocytopenia, occurs less frequently compared to neutropenia and lymphopenia. However, late-onset and prolonged thrombocytopenia have higher incidence rates, with some patients experiencing long durations of thrombocytopenia, significantly increasing the risk of bleeding and impacting their quality of life. Previous studies have shown that 44% of patients still had thrombocytopenia 42 days after CAR-T therapy [8]. Another long-term follow-up study on anti-CD19 CAR-T therapy revealed that on the 360th day post-CAR-T infusion, 67.7% of patients still exhibited varying degrees of cytopenia, with a thrombocytopenia incidence of 38.7% [28]. In our study, patients with thrombocytopenia tended to have a poorer overall survival. Larger studies have confirmed that patients with prolonged thrombocytopenia following CAR-T infusion have worse outcomes [7, 29]. Hence, the extended duration of thrombocytopenia and its impact on patient prognosis pose urgent challenges that need to be addressed following CAR-T therapy.
Logistic regression analysis identified pre-infusion cytopenias, grade ≥ 2 CRS, higher ECOG performance status, and B symptoms as significant risk factors for post-CAR-T cytopenias. Furthermore, our previous systematic analysis indicated that young age, extensive prior treatments, and the use of CAR-T products with the CD28 costimulatory domain or dual-targeting were risk factors for post-CAR-T cytopenia [6]. This highlights the significant challenge of cytopenia in patients undergoing CAR-T therapy, particularly for those with high-risk factors such as female gender, pre-existing cytopenias, poor physical condition, multiple lines of therapy, and the presence of CRS. Therefore, effective strategies to address cytopenia following CAR-T therapy are crucial.
Given the critical role of MSC in the constitution and reconstruction of BM microenvironment [18, 30], we explored whether MSCs infusion could ameliorate hematopoietic injury following CAR-T therapy. We were excited to find that MSC infusion could accelerate the reconstruction of platelet and hemoglobin, ameliorate bone marrow injury, and boost HSCs and their long-term subpopulations. The long-term HSCs represent a population with a higher capacity for self-renewal, proliferation, and differentiation, suggesting a positive contribution of MSC to hematopoiesis.
As a significant immunomodulatory component [17], the concern that MSC infusion might impair CAR-T cell activity and efficacy needs to be addressed. Our findings indicate that MSCs do not impair the function and proliferation of CAR-T cells in vitro and do not compromise the anti-tumor efficacy of CAR-T cells in mice. Significantly, considering the observed correlation between post-CAR-T neutropenia and lymphopenia and favorable patient outcomes in our study, we conducted subsequent analyses on animal experiment data. It was revealed that MSC infusion primarily facilitated the reconstitution of the erythroid and megakaryocytic lineages in CAR-T treated mice, without affecting the recovery of neutrophil and lymphocyte. In light of these properties, MSCs infusion is unlikely to affect the efficacy of CAR-T therapy, demonstrating the feasibility of MSCs application in CAR-T therapy.
Hematopoiesis is a long and intricate journey regulated by various factors [25]. In an effort to identify the mechanisms by which MSCs facilitate the hematopoiesis after CAR-T therapy, we analyzed the expression of hematopoiesis-related genes and found elevated expression of CXCL12 and NF-E2 after MSC treatment. C-X-C motif chemokine ligand 12 (CXCL12), also known as stromal cell-derived factor 1 (SDF-1), is mainly secreted by bone marrow stromal cells and mediates the homing of HSCs to the BM via the CXCL12/CXCR4 axis, maintenaning HSCs in the BM microenvironment [31, 32]. Nuclear factor erythroid derived 2 (NF-E2) is a transcription factor regulating megakaryopoiesis and erythropoiesis [33, 34], and mice lacking NF-E2 exhibit profound thrombocytopenia and anemia [35]. It is thus speculated that MSCs may promote hematopoietic reconstitution after CAR-T therapy by upregulating CXCL12 and NF-E2. Continued efforts are warranted to verify this hypothesis through innovative techniques such as single-cell sequencing, thus paving the way for better understanding and clinical practice of MSCs in CAR-T therapy.
Currently, clinical management of post-CAR-T cytopenias includes transfusions for anemia, G-CSF and GM-CSF for neutropenia, and TPO and TPO receptor agonists for thrombocytopenia. While MSCs have certain limitations compared to existing therapies, they also offer distinct advantages. Firstly, although MSCs can be derived from various tissues, their finite replicative capacity and lack of immortality require reliance on human tissue sources, restricting large-scale, standardized industrial production and introducing heterogeneity due to tissue source differences and processing methods. However, recent research indicates limited heterogeneity among MSCs from different sources by single-cell transcriptomic analysis [36], and as manufacturing processes mature, scalable and standardized production issue may be resolved in the future. Secondly, compared to the current clinical methods for addressing cytopenias, MSCs pose challenges of complex procedures and higher costs but possess advantages that current treatments lack. Existing growth factors or agonists sometimes only provide short-term promotion of hematopoiesis, and discontinuation of these therapies results in recurrent cytopenia. As stem cells, MSCs can promote hematopoietic reconstruction and immune regulation, potentially offering a curative approach for post-CAR-T cytopenias, reducing the inconvenience, high cost, and uncertainty of long-term drug use. Thirdly, although there are concerns about the safety of MSC applications in terms of immunogenicity, such as potential foreign-body responses triggered by the host immune system [37] and the risks of mutations or abnormal changes during in vitro expansion and culture, large-scale data do not currently indicate clear tumorigenicity. Moreover, extensive clinical research has validated the safety of MSCs in many clinical studies [38, 39]. Existing therapies, in fact, carry their own risks, including the potential for undetermined pathogen transmission through blood transfusions and adverse effects such as thrombosis, organ dysfunction, and pain associated with the use of growth factors or stimulants. Therefore, to comprehensively evaluate the safety of MSCs in comparison to existing treatments, further large-scale clinical studies are warranted. Finally, it is important to acknowledge that there is still a considerable gap between the findings obtained from animal experiments and their translation into clinical practice. Fortunately, there is a wealth of clinical research on the application of MSCs in GVHD, which provides valuable references for the preparation of bone marrow-derived MSCs and the administration of MSC infusions in patients with hematological malignancies.
Conclusion
To conclude, while CAR-T therapy has emerged as a novel and effective treatment for hematologic malignancies, its adverse effects remain unavoidable. Cytopenia is the most common adverse event following CAR-T therapy, with thrombocytopenia being particularly significant due to its prolonged duration, increased bleeding risk, and association with poor prognosis. Our research shows that MSC infusion could enhance hematopoietic recovery, particularly in the megakaryocyte lineage, mitigate bone marrow damage, and does not impair the activity or antitumor efficacy of CAR-T cells. This provides a potentially new and effective solution to ameliorate cytopenia following CAR-T therapy, thereby maximizing the advantages of cellular immunotherapy.
Supplementary Information
Acknowledgements
We would like to express our gratitude to Prof. Guang Hu and Dr. Wenjing Gao from Iaso Biotherapeutics Co. Ltd., China for their invaluable technical assistance in this study. We also thank the patients and their families for their participation. The authors declare that they have not used Artificial Intelligence in this study.
Author contributions
Yuan Xia: Conceptualization, Methodology, Investigation, Data curation, Validation, Visualization, Formal analysis, Writing - Original draft preparation; Li Wang: Methodology, Investigation, Data curation; Xuxing Shen and Ying Xu: Methodology, Investigation; Wei Xu and Jianyong Li: Resources, Supervision; Lei Fan: Conceptualization, Resources, Supervision; Lijuan Chen: Conceptualization, Resources, Validation, Supervision, Writing - Reviewing and Editing, Funding acquisition.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82370205), the Social Development Project of Jiangsu Science and Technology Plan (No. BE2022810), and the Key Program of Taizhou School of Clinical Medicine of Nanjing Medical University (No. TZKY20220310).
Data availability
All datasets of this article are included within the article.
Declarations
Ethics approval and consent to participate
The retrospective clinical study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Boards of the First Affiliated Hospital of Nanjing Medical University Ethics Committee: (1) No. 2019-MD-512; Title: Safety and efficacy of anti-CD19 CAR-T treatment in DLBCL Subjects; Date of approval: January 15, 2020; (2) No. 2019-SR-335; Title: A study on the efficacy and safety of CD20 CAR-T for the treatment of B-cell lymphoma; Date of approval: August 28, 2019. Written informed consents were obtained from all the patients before enrollment. All animal care and experimental procedures were complied with the standards of Animal Welfare for the Care and Use of Laboratory Animals of Nanjing Medical University, and were approved by Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University (No. 2201014. Title: Effect of mesenchymal stem cells on hematopoietic injury after CAR-T therapy in mice. Date of approval: January 11, 2022).
Consent for publication
All authors confirm their consent for publication.
Competing interests
The authors declare no competing interests in this work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dr. Yuan Xia and Li Wang contributed equally to this work.
Contributor Information
Lei Fan, Email: fanlei3014@126.com.
Lijuan Chen, Email: chenljb@126.com.
References
- 1.Huang R, Li X, He Y, Zhu W, Gao L, Liu Y, Gao L, Wen Q, Zhong JF, Zhang C, et al. Recent advances in CAR-T cell engineering. J Hematol Oncol. 2020;13(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu J, Jiang G. The journey of CAR-T therapy in hematological malignancies. Mol Cancer. 2022;21(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Khazragy N, Ghozy S, Emad P, Mourad M, Razza D, Farouk YK, Mohamed NA, Ahmed MK, Youssef T, Bahnasawy YM, et al. Chimeric antigen receptor T cells immunotherapy: challenges and opportunities in hematological malignancies. Immunotherapy. 2020;12(18):1341–57. [DOI] [PubMed] [Google Scholar]
- 4.Fan L, Wang L, Cao L, Zhu H, Xu W, Li J. Phase I study of CBM.CD19 chimeric antigen receptor T cell in the treatment of refractory diffuse large B-cell lymphoma in Chinese patients. Front Med. 2022;16(2):285–94. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Chen LJ, Yang SS, Sun Y, Wu W, Liu YF, Xu J, Zhuang Y, Zhang W, Weng XQ, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci U S A. 2019;116(19):9543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia Y, Zhang J, Li J, Zhang L, Li J, Fan L, Chen L. Cytopenias following anti-CD19 chimeric antigen receptor (CAR) T cell therapy: a systematic analysis for contributing factors. Ann Med. 2022;54(1):2951–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagle SJ, Murphree C, Raess PW, Schachter L, Chen A, Hayes-Lattin B, Nemecek E, Maziarz RT. Prolonged hematologic toxicity following treatment with chimeric antigen receptor T cells in patients with hematologic malignancies. Am J Hematol. 2021;96(4):455–61. [DOI] [PubMed] [Google Scholar]
- 8.Fried S, Avigdor A, Bielorai B, Meir A, Besser MJ, Schachter J, Shimoni A, Nagler A, Toren A, Jacoby E. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transpl. 2019;54(10):1643–50. [DOI] [PubMed] [Google Scholar]
- 9.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaefer A, Huang Y, Kittai A, Maakaron JE, Saygin C, Brammer J, Penza S, Saad A, Jaglowski SM, William BM. Cytopenias after CD19 chimeric Antigen receptor T-Cells (CAR-T) therapy for diffuse large B-Cell lymphomas or transformed follicular lymphoma: a single Institution experience. Cancer Manag Res. 2021;13:8901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinhardt B, Lee P, Sasine JP. Chimeric Antigen receptor T-Cell therapy and Hematopoiesis. Cells. 2023;12(4):531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. Tisagenlecleucel in Children and Young adults with B-Cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, Riddell SR, Maloney DG, Boeckh M, Turtle CJ. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131(1):121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordeiro A, Bezerra ED, Hirayama AV, Hill JA, Wu QV, Voutsinas J, Sorror ML, Turtle CJ, Maloney DG, Bar M. Late events after treatment with CD19-Targeted Chimeric Antigen Receptor Modified T Cells. Biol Blood Marrow Transpl. 2020;26(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain T, Olson TS, Locke FL. How I treat cytopenias after CAR T-cell therapy. Blood. 2023;141(20):2460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzsimmons REB, Mazurek MS, Soos A, Simmons CA. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int 2018, 2018:8031718. [DOI] [PMC free article] [PubMed]
- 18.Pontikoglou C, Deschaseaux F, Sensebe L, Papadaki HA. Bone marrow mesenchymal stem cells: biological properties and their role in hematopoiesis and hematopoietic stem cell transplantation. Stem cell Reviews Rep. 2011;7(3):569–89. [DOI] [PubMed] [Google Scholar]
- 19.Li N, Feugier P, Serrurrier B, Latger-Cannard V, Lesesve JF, Stoltz JF, Eljaafari A. Human mesenchymal stem cells improve ex vivo expansion of adult human CD34 + peripheral blood progenitor cells and decrease their allostimulatory capacity. Exp Hematol. 2007;35(3):507–15. [DOI] [PubMed] [Google Scholar]
- 20.Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, Frenette PS. PDGFRalpha and CD51 mark human nestin + sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210(7):1351–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernardo ME, Fibbe WE. Mesenchymal stromal cells and hematopoietic stem cell transplantation. Immunol Lett. 2015;168(2):215–21. [DOI] [PubMed] [Google Scholar]
- 22.Wu KH, Wu HP, Chan CK, Hwang SM, Peng CT, Chao YH. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: from bench to bedsides. Cell Transpl. 2013;22(4):723–9. [DOI] [PubMed] [Google Scholar]
- 23.Huang S, Xu L, Sun Y, Wu T, Wang K, Li G. An improved protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. J Orthop Translat. 2015;3(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sommerkamp P, Romero-Mulero MC, Narr A, Ladel L, Hustin L, Schonberger K, Renders S, Altamura S, Zeisberger P, Jacklein K, et al. Mouse multipotent progenitor 5 cells are located at the interphase between hematopoietic stem and progenitor cells. Blood. 2021;137(23):3218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comazzetto S, Shen B, Morrison SJ. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev Cell. 2021;56(13):1848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Si X, Gu T, Liu L, Huang Y, Han Y, Qian P, Huang H. Hematologic cytopenia post CAR T cell therapy: etiology, potential mechanisms and perspective. Cancer Lett. 2022;550:215920. [DOI] [PubMed] [Google Scholar]
- 27.Amini L, Silbert SK, Maude SL, Nastoupil LJ, Ramos CA, Brentjens RJ, Sauter CS, Shah NN, Abou-El-Enein M. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat Rev Clin Oncol. 2022;19(5):342–55. [DOI] [PubMed] [Google Scholar]
- 28.Logue JM, Zucchetti E, Bachmeier CA, Krivenko GS, Larson V, Ninh D, Grillo G, Cao B, Kim J, Chavez JC, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2021;106(4):978–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabbara N, Sharp J, Gaut D, Pham TTD, Tang K, Oliai C, Sim MS, Schiller G, Young P, Sasine JP. Diminished durability of chimeric antigen receptor T-cell efficacy with severe or prolonged postinfusion cytopenias. Am J Hematol. 2022;97(7):E249–55. [DOI] [PubMed] [Google Scholar]
- 30.Andrzejewska A, Lukomska B, Janowski M. Concise Review: mesenchymal stem cells: from roots to Boost. Stem Cells. 2019;37(7):855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–88. [DOI] [PubMed] [Google Scholar]
- 32.Miao R, Lim VY, Kothapalli N, Ma Y, Fossati J, Zehentmeier S, Sun R, Pereira JP. Hematopoietic stem cell niches and signals Controlling Immune Cell Development and maintenance of immunological memory. Front Immunol. 2020;11:600127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mutschler M, Magin AS, Buerge M, Roelz R, Schanne DH, Will B, Pilz IH, Migliaccio AR, Pahl HL. NF-E2 overexpression delays erythroid maturation and increases erythrocyte production. Br J Haematol. 2009;146(2):203–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fock EL, Yan F, Pan S, Chong BH. NF-E2-mediated enhancement of megakaryocytic differentiation and platelet production in vitro and in vivo. Exp Hematol. 2008;36(1):78–92. [DOI] [PubMed] [Google Scholar]
- 35.Levin J, Peng JP, Baker GR, Villeval JL, Lecine P, Burstein SA, Shivdasani RA. Pathophysiology of thrombocytopenia and anemia in mice lacking transcription factor NF-E2. Blood. 1999;94(9):3037–47. [PubMed] [Google Scholar]
- 36.Huang Y, Li Q, Zhang K, Hu M, Wang Y, Du L, Lin L, Li S, Sorokin L, Melino G, et al. Single cell transcriptomic analysis of human mesenchymal stem cells reveals limited heterogeneity. Cell Death Dis. 2019;10(5):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gopalarethinam J, Nair AP, Iyer M, Vellingiri B, Subramaniam MD. Advantages of mesenchymal stem cell over the other stem cells. Acta Histochem. 2023;125(4):152041. [DOI] [PubMed] [Google Scholar]
- 38.Kadri N, Amu S, Iacobaeus E, Boberg E, Le Blanc K. Current perspectives on mesenchymal stromal cell therapy for graft versus host disease. Cell Mol Immunol. 2023;20(6):613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y, Huang C, Zheng L, Li Q, Ge J, Geng S, Zhai M, Chen X, Yuan H, Li Y, et al. Safety and efficacy of umbilical cord tissue-derived mesenchymal stem cells in the treatment of patients with aging frailty: a phase I/II randomized, double-blind, placebo-controlled study. Stem Cell Res Ther. 2024;15(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets of this article are included within the article.