Abstract
Background
Myceliophthora heterothallica belonging to Myceliophthora is considered as an environmental fungus and has not been reported to be pathogenic or colonizing in recent literature. The present case firstly reports a ventilation-associated pneumonia caused by Myceliophthora heterothallica among the aged adult.
Case presentation
A 67-years-old Asian female patient suffering from a sudden disturbance of consciousness for 3 h was admitted to our hospital. Cardiac arrest occurred during emergency transport, and sinus rhythm was restored after cardiopulmonary resuscitation. Invasive mechanical ventilation was given to this patient for respiratory failure. After mechanical ventilation, the lung CT images showed multiple cuneiform nodules arranging subpleural accompanying with ground-glass opacity. On the 5th day of mechanical ventilation, Myceliophthora heterothallica was cultured from endotracheal aspirates. Two methods, namely automatic microbial identification system and internal transcribed spacer sequencing were employed to identify this fungus. The present case firstly uncovered the colonization ability and pathogenicity of Myceliophthora heterothallica in the respiratory tract. After 28d of treatment with piperacillin-tazobactam, this patient weaned from the ventilator and recovered from consciousness with lung infection disappearance.
Conclusions
This is the first case report of ventilation-associated pneumonia in the aged patient caused by Myceliophthora heterothallica. This current case is worth for the clinical diagnosis and treatment of Myceliophthora heterothallica infection, and also enriches new pathogenic species found of thermothelomyces species.
Keywords: Myceliophthora Heterothallica, VAP, Aged, Fungus, Case report
Introduction
Myceliophthora heterothallica (M. heterothallica) belongs to the genus Myceliophthora [1], a group of Gram-negative, aerobic bacillus species pertaining to thermophilic fungi, namely melanized fungi, which usually occurred in the population living in particular climatic conditions and environmental conditions and always accompanying with immunocompromise (such as neutropenia and leukopenia) and stress reactions (such as surgery and cardiac arrest) [2, 3]. This genus is widely distributed, and most of them have been isolated from a variety of soils such as river bed sludge, compost, and plant roots [2, 4]. This genus is adaptable to the environment and is widely used in medicine, textile, and industrial production of functional enzymes [2, 4]. It is generally considered as an environmental fungus and has rarely been found to be pathogenic or colonizing [1]. In clinical infection, the colonization ability and pathogenicity which are manifested in respiratory tract by M. heterothallica have not been reported in existing literature.
Mechanical ventilation patients, especially the aged with underlying diseases, have a high risk of bacterial colonization and are very likely to occur ventilator-associated pneumonia (VAP) [5, 6]. The most common Gram-negative species participated in VAP are Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumanii, and meanwhile the usual Gram-positive specie is Staphylococcus aureus [7–9]. Although fungal infections rarely occur in VAP, the aged patients with VAP often suffer from fungal invasion. Mould, such as Aspergillus spp. might be participated in a few late-onset VAP (occurring after at least 5 days of hospitalization) occurrence, which is more likely to be triggered by multidrug resistant (MDR) pathogens (including carbapenem-resistant Acinetobacter baumannii) [5, 8, 9]. Factors such as diabetes mellitus, hypoproteinemia and mechanical ventilation after cardiac arrest might significantly increase the incidence of respiratory opportunistic fungal infections in the aged with VAP, which in turn increase the mortality and prolong the length of hospital stay [10, 11]. Therefore, it is necessary more timely and accurately to identify the clinical characteristics of rare opportunistic fungal pathogens in aged patients with VAP, for purpose of the reasonably application for antifungal drugs, which is more beneficial to this population.
In April 2023, M. heterothallica was firstly cultured from the endotracheal aspirates (ETA) of aged patient treated with invasive ventilator after cardiopulmonary resuscitation (CPR) owing to cardiac arrest in our hospital. The automatic microbial identification system and the internal transcribed spacer (ITS) of ribosomal DNA (rDNA) regions sequencing were employed for comprehensive identification of M. heterothallica combined with the fungal morphology observation, physiological and biochemical indicator levels, gene and protein characteristics. To date, this is the first case of an agedy patient with VAP caused by M. heterothallica. However, the clinic and prognostic characteristics of infections caused by M. heterothallica are significantly different with Thermothelomyces thermophila. The clinical characteristics of VAP in the aged caused by M. heterothallica are reported in this case detailly, as well as the biological characteristics and pathogenicity of this strain, so as to evaluate the clinical value of M. heterothallica as an opportunistic pathogenic fungus in aged patients with VAP.
Case presentation
The patient’s medical process
A 67-years-old Asian female patient suffered from a sudden disturbance of consciousness for 3 h accompanying with hypertension and hypokalemia. After detailed examination, we found that the patient’s underlying diseases and the possible causes of a sudden disturbance of consciousness include hypokalemia, ventricular bigeminy, and central nervous system diseases caused by long-term hypertension, including hypoxic-ischemic encephalopathy, acute cerebral infarction, and microbleeds in thalamus, sub-clinical hyperthyroidism and emphysema. Cardiac arrest occurred during emergency transport, and sinus rhythm was restored after CPR. The patient was admitted to the intensive care unit (ICU) due to cardiac arrest. This patient was treated by invasive mechanical ventilation, placing central venous catheter, parenteral nutrition and potassium supplementation. On the second day of mechanical ventilation, the patient developed fever, yellow sputum was observed from ETA (the ETA pathogen specimen, with a neutrophil count exceeding 25 under low magnification and the pathogen concentration in quantitative culture is greater than 105 cfu/ml), and the breath sounds of both lungs were coarse. At the same time, the clinicians inferred from clinical experiences that the causative pathogens might be mainly Gram-negative bacteria, therefore the patient were treated by piperacillin sodium and tazobactam via intravenous injection. Then, on the fourth day of mechanical ventilation, the MDR bacteria, carbapenem-resistant Acinetobacter baumannii was cultured in the ETA, and the original regimen was continued. On the 5th day of mechanical ventilation, M. heterothallica was also cultured in the ETA. On the 6th day of mechanical ventilation, the patient developed persistent fever, with increased body temperature and yellow sputum. After 1 week of mechanical ventilation, all the same the patient was successfully weaned from the ventilator, she still has not restored consciousness. After ventilator withdrawal, the patient was treated continuously by deep sedation, potassium supplementation via central venous catheter, parenteral nutrition and other treatment regiments. On the 8th and 15th days after admission, the carbapenem-resistant Acinetobacter baumannii could still be cultured in sputum. Therefore, piperacillin sodium and tazobactam were continued, and elimination of sputum and bronchial dilatation were encouraged at the same time. On the 15th day after admission, the patient’s fever disappeared, the sputum decreased significantly, and the reexamination of lung computed tomography (CT) scan showed that the lung infection was remitted. On the 28th day after admission, the patient recovered from consciousness disturbance and was discharged from the hospital.
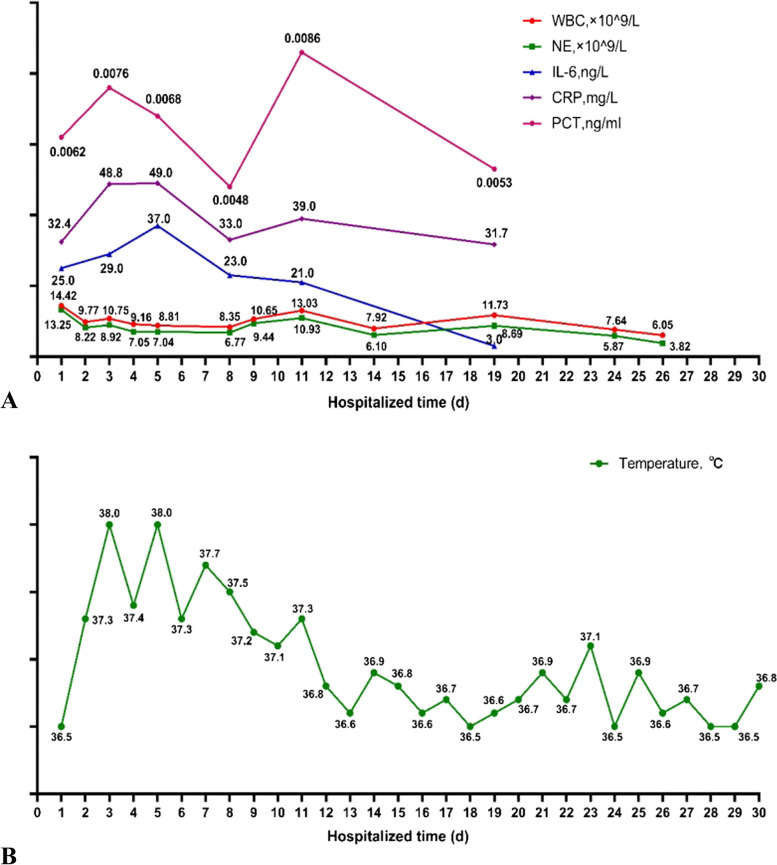
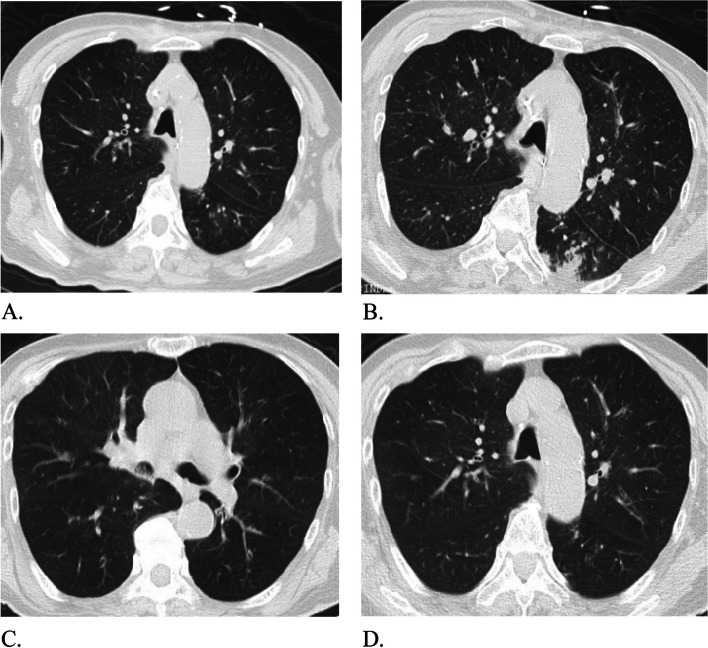
During admission, the patient developed persistent low-grade fever with the highest temperature of 38.5℃. The white blood cell count was increased (White blood cell count: 14.42*10^9/L, neutrophil percentage: 91.9%), and the blood C-reactive protein and interleukin-6 levels were significantly increased (CRP: 32.4U/L, IL-6:25 U/L), which were consistent with the indication of infection. The changes of various indicators of infection were shown in Fig. 1. A lung CT scan on the third day of mechanical ventilation showed bronchiolitis changes, which could not be excluded the possibility of viral inflammation. A repeat lung CT scan performed on the 6th day of mechanical ventilation showed the inflammation in the left lower lobe was worse. This lung CT scan showed micro-nodules in both lungs, thick walls with patchy fuzzy shadow, and multiple cuneiform nodules images arranging subpleural with ground-glass opacity. Therefore, according to these lung CT scan findings, the patient had signs of respiratory mycotic infection [12]. The changes of lung CT images were presented in Fig. 2. The results of antimicrobial susceptibility test (AST) by the Kirby-Bauer method from sputum culture and ETA were shown in Table 1.
Fig. 1.
The changes in various indicators of infection. A The trend of hematological markers of infection. The values of each indicator are presented next to each scatter point. WBC: White blood count, NE: Neutrophil absolute value, IL-6: Interleukin 6, PCT: Procalcitonin, CRP: C-reactive protein. B The fluctuation of patient's temperature
Fig. 2.
The changes of lung CT images. A The CT images on the 3rd day of mechanical ventilation. These images showed that there were micro-nodules distributed along the bronchovascular bundle in both lungs, a number of buds like changes, multiple signet ring signs and double track signs were seen locally, and the walls were thickened with patchy fuzzy shadow around. B The lung CT images on the 6th day of mechanical ventilation. Compared with the CT images on the 3rd day, the inflammation in the left lower lobe of the lung was aggravated. C and D The lung CT images after the withdraw of mechanical ventilation. Compared with the 6th day lung CT images, the inflammation of the left lower lung lobe was alleviated. At the same time, patchy high-density shadows were found in the middle bronchus and lower lobe bronchus of the right lung, which was considered to be sputum retention in the cavity
Table 1.
Results of AST by the Kirby-Bauer method
| Diameter of inhibition zone (mm) | The identified strains | TMP-SMZ | Tigecycline | Tobramycin | Polymyxin E | Meropenem | Piperacillin/Sulbactam |
|---|---|---|---|---|---|---|---|
| Sputum culture (March 10th) | Normal flora | - | - | - | - | - | - |
| ETA culture (March 14th) | Acinetobacter baumannii | ≤20 | 4 | ≤1 | 1 | ≥16 | ≥64 |
| ETA culture (March 15th) | M.heterothallica | - | - | - | - | - | - |
| Sputum culture (March 24th) | Acinetobacter baumannii | ≤20 | 2 | ≤1 | 2 | ≥16 | ≥128 |
| Sputum culture (March 31th) | Acinetobacter baumannii | ≤20 | 4 | ≤1 | ≤0.5 | ≥16 | ≥64 |
| Sputum culture (April 1st) | Acinetobacter baumannii | ≤20 | 4 | ≤1 | ≤0.5 | ≥16 | ≥64 |
TMP-SMZ Trimethoprim-sulfamethoxazole, AST Antimicrobial Susceptibility Test, ETA Endotracheal aspirates
According to the diagnostic criteria of VAP in the 2018 Chinese VAP diagnosis and treatment guidelines [13], this patient is consistent with the clinical diagnosis of fungal VAP and the pathogenic microbe is M. heterothallica.
Identification of pathogenic microorganism
The samples of ETA on day 5 of mechanical ventilation, acquired from this patient’s airway via sputum suctioning, were inoculated on potato dextrose agar (PDA; Becton Dickinson and Company Sparks, MD, USA), and incubated at 37℃ and 25℃ for at least 14 days respectively under daily examination. The fungal isolate was tentatively identified according to the macro- and micro-morphology.
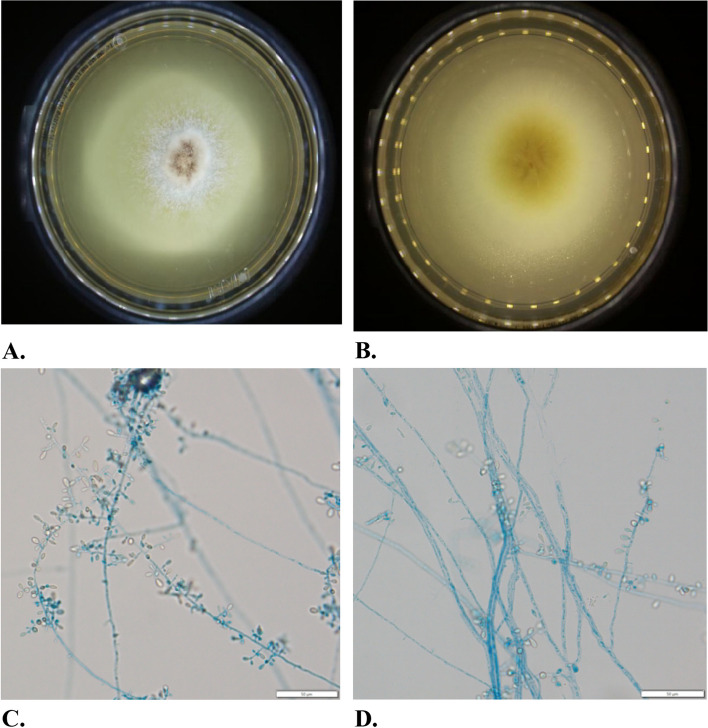
Mycological culture of the ETA grew rapidly pale pinkish grey and velvety fungal colonies (Fig. 3A). The hyphae were found to be hyaline, smooth, thin walled, septated, and frequently developed acute angle branching (Fig. 3B). The conidia were ovoidal to pyriform, hyaline, smooth-walled, terminal or lateral, singly or clustered, produced by ampulliform swellings from hyphae. Figure 3 shows the colony and microscopic morphology of M. heterothallica detailly.
Fig. 3.
The colony morphology of Myceliophthora heterothallica. A and B We observed fungi under microscope after 7 days at 25℃ on PDA. Macro-morphology of fungal isolate after 7 days at 25℃ on PDA. The surface of the colony appeared pale pinkish grey and velvety. The reverse of the colony presented light-yellow. C and D Micro-morphology of fungal isolate examined with lactophenol cotton blue stain. Hyaline hyphae were smooth, thin, septate, with conidia produced from ampulliform swellings. Ovoidal to pyriform conidia were hyaline, smooth, and singly or in small groups
For accurate molecular identification, extraction of fungal genomic DNA was performed as described previously [14]. ITS of rDNA regions of the isolate was amplified using ITS1 and ITS4 primers [15]. Then the amplified sequences were determined (Sangon Biotech Co., Ltd. (Shanghai, China), and identified by comparison with sequences deposited in the GenBank using a basic local alignment search tool (https://blast.ncbi.nlm.nih.gov/)). Blast search showed the acquired sequences of the isolate similarity of 100% to ITS regions of Thermothelomyces heterothallicus (formerly, M. heterothallica) CBS 202.75 (NCBI: txid1149848). On the basis of morphological and genomic characteristic, the isolate was identified as Thermothelomyces heterothallicus.
Discussion
This case is the first report of VAP in the aged caused by M. heterothallica. According to the published literature, M. heterothallica belongs to the branch of Myceliophthora [1, 16]. It was first discovered in China in 2016, which morphological characteristic is similar to Thermothelomyces thermophiles (formely, Myceliophthora thermophila) [17]. According to parsimonious consensusu tree of ITS1 region of this genus. M. heterothallica belongs to thermophilic species. There are four strains in thermophilic species, including M. thermophila, M. heterothallica, M. fergusii and M. hinnulea [5]. No clinical cases have been reported, which caused by M. fergusii and M. hinnulea. Only a few clinical cases of M. thermophiles have been reported [18, 19]. Although M. thermophiles is widely distributed in nature and is stronger to be adaptive to the environment [2], the susceptible populations infected by this species are often accompanied by underlying diseases that impaired the system of the immunity of body [20–23]. And the clinical features of infected cases caused by M. heterothallica were always critical, disseminated and fatal [23]. This case suggests that M. heterothallica not only could be detected in the respiratory tract, but also has respiratory pathogenicity.
Myceliophthora represents one genus of the myriad of saprobic, always opportunistic, generating mycotic disease. The histological lesions of Myceliophthora are similar in spite of etiology and localisation, which are identical to the tissue lesions of phaeohyphomycosis. In histology, chronic abscesses were heterogenous necrosis as characteristic lesions in abscesses center surrounded by the infiltration of macrophages, which comprise granulomatous reaction [3, 19]. The tissue lesions are frequently surrounded by a dense collagenous capsule [3, 18]. These chronic abscesses always occur in lung, including bronchial tissue, frequently accompanying by infarction casued by thrombogenesis in blood vessel. These infarction might lead to ischemic necrosis. Intravascular thrombi are frequently composed of filamentous material. Up to date, majorities of the pathogenic characteristics of Myceliophthora were derived from the researches of M. thermophila infection. M. thermophila has the thermophilic nature, and secrets melanin in cell walls associating with the fungal virulence [3]. Some studies have suggested that M. thermophila is more likely to reproduce, spread and survive in higher temperature environment, and also has stronger invasion to blood vessels. The hypha of M. thermophila invariably invade vessels, always resulting in local chronic giant cell granuloma, which is associated with phaeohyphomycosis [19]. Therefore the immunocompromised patients might be very likely to aspirate these fungi spores via respiratory tract, causing fatal disseminated mycosis through blood vessels invasion and the blood stream dissemination to the entire body [18, 19]. M. heterothallica reported in this case together with M. thermophila both belong to the genus Myceliophthora, thermophila species, and the radiological ground-glass opacity in this case corresponds to the M. thermophila characteristic infarcts centred by ischemic necrosis and surrounded by a parenchymal haemorrhagic edge [3]. Therefore we speculate that M. heterothallica has the identical pathogenic agents and pathological features with M. thermophila. However, the clinical manifestations of fungal infection caused by M. heterothallica are also different from M. heterothallica.
This patient in present report, who suffered from cardiac arrest and was given CPR, was forced to receive invasive ventilator due to secondary respiratory failure, and also had a long history of hypokalemia. On the 5th day after mechanical ventilation, M. heterothallica was found in ETA culture, and carbapenem-resistant Acinetobacter baumannii was found in ETA culture on the 4th day. We speculate that out-of-hospital cardiac arrest, hypokalemia and infections caused by MDR pathogens may be the main causes of M. heterothallica infection in this case. Out-of-hospital cardiac arrest is a major risk factor for VAP [6]. Out-of-hospital cardiac arrest often increases the incidence of VAP among ICU patients, and pathogens are often a variety of opportunistic pathogens [6]. Early hypothermia management and antibiotic prophylaxis could contribute to alleviate these above conditions [10, 24]. In addition, hypokalemia is often accompanied by immunodeficiency and respiratory muscle strength weakening, which increase the risk of opportunistic pathogens infection after mechanical ventilation [25, 26]. MDR pathogens might be likely isolated from late-onset VAP patients always accompanying with rare opportunistic fungus infection [5], therefore carbapenem-resistant Acinetobacter baumannii obtained from this late-onset VAP patient caused by M. heterothallica.
The minority lung lesions caused by M. thermophila could be treated with sensitive antifungal agents alone [20, 21, 23]. Amphotericin B is often recommended as the first choice [20]. However, there is no study about the drug sensitivity of anti-M. heterothallica in vitro. Therefore, empirical treatment could be carried out according to anti-M. thermophila. This patient has not been treated with any antifungal drugs. According to the results of AST, this patient received piperacillin sulbactam, a broad-spectrum and anti-gram-negative bacteria sensitive drug controlling MDR pathogens infection. This patient was cured and discharged from hospital without any anti-fungi drugs, relating to a number of reasons as followed. Firstly, although M. heterothallica belongs to Myceliophthora thermophila species, there are quite differences from M. thermophila on the aspects of pathogenicity and virulence. The onset of pulmonary infection caused by M. heterothallica is more insidious, the pulmonary symptoms and signs are mild and no characteristic, the development trend of this disease is slow, and the prognosis of the disease is favorable. Secondly, there was no found on the pathogenicity of M. heterothallica among the previous clinic researches, the clinicians lacked relevant clinical experiences. Thirdly, the infections caused by this species are always accompanying with MDR bacterial invasion. Anti-MDR bacterial treatments might contribute the remission of the infection caused by this fungus species. Fourthly, With well-timed correction of hypokalemia, the respiratory muscle strength of patient had gradually increased and the expectoration becomes easier, ultimately contributing to pneumonia cure without anti-fungal treatment. This present case reports that M. heterothallica is virulence and pathogenic, and it is also speculated that it might have the same pathogenic mechanism with M. thermophila. However, the onset of pulmonary infection caused by M. heterothallica is insidious, and the symptoms and signs of infection are mild, which is different from the fatal disseminated mycosis caused by M. thermophila. Therefore, clinicians always overlook the possibility of fungal infection caused by this strain, which might increase the risk that patients may develop invasive fungal disease. M. heterothallica, belonging to Myceliophthora thermophila species are likely to be considered as contaminant when cultured from clinical specimen. However, considering thermophilic fungi are easy to adapted the internal environment of the host, and the critical patients are more likely to develop invasive fungal infection, we should pay more attention for rarely isolated fungi.
There are some limitations in this paper. Firstly, as to this woman in our case, bronchoalveolar lavage (BAL) was considered to be contraindicated because of persistent coagulation abnormalities, namely that the prothrombin time on routine coagulation tests was persistently prolonged for more than 3 s before weaning from the ventilator. In the future, if the patient’s status permits bronchoalveolar lavage fluids (BALF) need to be applied as the superior pathogenic specimens to identify the pathogenicity of M. heterothallica. Secondly, although the 2018 Chinese Guidelines for Diagnosis of Adult VAP did not provide clear criteria for the types and quantities of specimens collected for rare fungal pathogens. But if we could obtain multi site and multi pathway pathogenic specimens, it will not only contribute to identify rare fungi, but also provide more substantial evidences for the pathogenic characteristics of rare fungi.
Conclusion
This article reports aged VAP patient caused by M. heterothallica, including its clinical features, laboratory tests and disease outcome. In this study, a variety of identification methods, such as the automatic microbial identification system and ITS of rDNA regions sequencing were employed to accurately identify the growth of M. heterothallica in respiratory tract. This study is worth for the clinical diagnosis and treatment of M. heterothallica infection, and also enriches new discoveries of pathogenic species of Myceliophthora species.
Acknowledgements
The authors would like to thank the patients involved in the First Hospital of Jilin University and all the people in the department of laboratory medicine from the First Hospital of Jilin University as well as people involved in the laboratory work at the mycology research center of Jilin University.
Abbreviations
- VAP
Ventilator-associated Pneumonia
- MDR
Multidrug Resistant
- ETA
Endotracheal Aspirates
- CPR
Cardiopulmonary Resuscitation
- ITS
Internal Transcribed Spacer
- rDNA
Ribosomal DNA
- ICU
Intensive Care Unit
- CT
Computed Tomography
- AST
Antimicrobial Susceptibility Test
- PDA
Potato Dextrose Agar
- BAL
Bronchoalveolar Lavage
- BALF
Bronchoalveolar Lavage Fluid
Authors’ contributions
All authors contributed to the study conception and design. Case collection and review of the literature were performed by C. W.. C. Y. supervised the study and took the photos of fungi together with J. W.. The identification of fungi and data analysis were performed by D. W.. The first draft of the manuscript was written by C. W., D. H. and J. X. both proposed the framework of this case report, and also commented on previous versions of this manuscript. All authors read and approved the final manuscript.
Funding
This case report receives no external funding.
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the GenBase under accession number C_AA084016.1 and are available at the following URL: https://ngdc.cncb.ac.cn/genbase/review/fc725c6994f9.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patient before the procedure.
Consent for publication
Written informed consent was obtained from the patient to publish this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dan He, Email: hedan@jlu.edu.cn.
Jiancheng Xu, Email: xjc@jlu.edu.cn.
References
- 1.Han YF, Wang C, Wang YR, Chen WH, Liang JD, Liang ZQ. A new thermophilic species of Myceliophthora from China. Mycol Prog. 2014;13(1):165–70. 10.13344/j.microbiol.china.150902. [Google Scholar]
- 2.van den Brink J, van Muiswinkel GC, Theelen B, Hinz SW, de Vries RP. Efficient plant biomass degradation by thermophilic fungus myceliophthora heterothallica. Appl Environ Microbiol. 2013;79(4):1316–24. 10.1128/aem.02865-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nourrisson C, Garcia-Hermoso D, Morio F, Kauffmann-Lacroix C, Berrette N, Bonhomme J, Poirier P, Lortholary O. Thermothelomyces thermophila human infections. Clin Microbiol Infect. 2017;23(5):338–41. 10.1016/j.cmi.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 4.van den Brink J, Samson RA, Hagen F, Boekhout T, De Vries RP. Phylogeny of the industrial relevant, thermophilic genera Myceliophthora and Corynascus. Fungal Divers. 2012;52(1):197–207. [Google Scholar]
- 5.Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46(Suppl 2). 10.1007/s00134-020-05980-0. [DOI] [PMC free article] [PubMed]
- 6.Hasslacher J, Steinkohl F, Ulmer H, Lehner G, Klein S, Mayerhoefer T, Joannidis M. Increased risk of ventilator-associated pneumonia in patients after cardiac arrest treated with mild therapeutic hypothermia. Acta Anaesthesiol Scand. 2022;66(6):704–12. 10.1111/aas.14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chanderraj R, Baker JM, Kay SG, Brown CA, Hinkle KJ, Fergle DJ, McDonald RA, Falkowski NR, Metcalf JD, Kaye KS, Woods RJ, Prescott HC, Sjoding MW, Dickson RP. In critically ill patients, anti-anaerobic antibiotics increase risk of adverse clinical outcomes. Eur Respir J. 2023;61(2). 10.1183/13993003.00910-2022. [DOI] [PMC free article] [PubMed]
- 8.Bassetti M, Mularoni A, Giacobbe DR, Castaldo N, Vena A. New antibiotics for hospital-acquired pneumonia and ventilator-associated pneumonia. Semin Respir Crit Care Med. 2022;43(2):280–94. 10.1055/s-0041-1740605. [DOI] [PubMed] [Google Scholar]
- 9.Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia. Semin Respir Crit Care Med. 2020;35(4):469–81. 10.1055/s-0034-1384752. [DOI] [PubMed] [Google Scholar]
- 10.François B, Cariou A, Clere-Jehl R, Dequin PF, Renon-Carron F, Daix T, Guitton C, Deye N, Legriel S, Plantefève G, Quenot JP, Desachy A, Kamel T, Bedon-Carte S, Diehl JL, Chudeau N, Karam E, Durand-Zaleski I, Giraudeau B, Vignon P, Le Gouge A. Prevention of early ventilator-associated pneumonia after cardiac arrest. N Engl J Med. 2019;381(19):1831–42. 10.1056/NEJMoa1812379. [DOI] [PubMed] [Google Scholar]
- 11.Shuili W, Hua WU, Xiaofeng L. Retrospective clinical analysis on pathogen spectrum distribution and antimicrobid resistance in VAP. J Clin Intern Med. 2005;(02):109–11.
- 12.Maesaki S. Mycotic pneumonia. Nihon Naika Gakkai Zasshi. 2005;94(11):2301–6. 10.2169/naika.94.2301. [DOI] [PubMed] [Google Scholar]
- 13.Zhai JM, Shi Y. Chinese guidelines for the diagnosis and treatment of hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Tuberc Respir J. 2018;41(4):255–80. 10.3760/cma.j.issn.1001-0939.2018.04.006. [Google Scholar]
- 14.He D, Hao J, Zhang B, Yang Y, Song W, Zhang Y, Yokoyama K, Wang L. Pathogenic spectrum of fungal keratitis and specific identification of Fusarium solani. Invest Ophthalmol Vis Sci. 2011;52(5):2804–8. 10.1167/iovs.10-5977. [DOI] [PubMed] [Google Scholar]
- 15.Korabecná M, Liska V, Fajfrlík K. Primers ITS1, ITS2 and ITS4 detect the intraspecies variability in the internal transcribed spacers and 5.8S rRNA gene region in clinical isolates of fungi. Folia Microbiol (Praha). 2003;48(2):233–8. 10.1007/bf02930961. [DOI] [PubMed] [Google Scholar]
- 16.Hutchinson MI, Powell AJ, Tsang A, O’Toole N, Berka RM, Barry K, Grigoriev IV, Natvig DO. Genetics of mating in members of the Chaetomiaceae as revealed by experimental and genomic characterization of reproduction in Myceliophthora Heterothallica. Fungal Genet Biol. 2016;86:9–19. 10.1016/j.fgb.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Yan-Feng H, Cheng W, Yu-Rong W, Wan-Hao C, Jian-Dong L, Zong-Qi L. Two new records of the genus Myceliophthora in China. Microbiol China. 2016;43(09):1960–5.
- 18.Yan-Feng H, Cheng W, Yu-Rong W, Wan-Hao C, Jian-Dong L, Zong-Qi L. A fatal case of pneumonia caused by Thermothelomycesthermophila. J Infect Chemother. 2023;29(1):87–9. 10.1016/j.jiac.2022.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Le Naourès C, Bonhomme J, Terzi N, Duhamel C, Galateau-Sallé F. A fatal case with disseminated Myceliophthora thermophila infection in a lymphoma patient. Diagn Microbiol Infect Dis. 2011;70(2):267–9. 10.1016/j.diagmicrobio.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Pundhir P, Tuda C, Vincentelli C, Morlote D, Rivera C. Scolecobasidium granulomatous pneumonia and abscess - an emerging opportunistic fungal pathogen: a case report. Int J STD AIDS. 2017;28(1):94–6. 10.1177/0956462416646688. [DOI] [PubMed] [Google Scholar]
- 21.Kralovic SM, Rhodes JC. Phaeohyphomycosis caused by Dactylaria (human dactylariosis): report of a case with review of the literature. J Infect. 1995;31(2):107–13. 10.1016/s0163-4453(95)92060-9. [DOI] [PubMed] [Google Scholar]
- 22.Dichtl K, Koc Ö, Forster J, Scharf C, Suerbaum S, Andrassy J, Wagener J, Schroeder I. An invasive infection caused by the thermophilic mold Talaromyces thermophilus. Infection. 2021;49(6):1347–53. 10.1007/s15010-021-01648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mareș M, Moroti-Constantinescu VR, Voroneanu L, Doroftei F, Covic A, Mederle OA. Invasive pulmonary infection due to Thermoascus crustaceus in a kidney transplant recipient. Infect Drug Resist. 2019;12:1929–34. 10.2147/idr.S209164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craven DE, Lei Y, Ruthazer R, Sarwar A, Hudcova J. Incidence and outcomes of ventilator-associated tracheobronchitis and pneumonia. Am J Med. 2013;126(6):542–9. 10.1016/j.amjmed.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Lugat A, Lasolle H, François M, Benhenda N, Bricaire L, Cornu E, Cristante J, Gitton A, Hadoux J, Kerlan V, Le Bras M, Mezzaroba V, Puerto M, Storey C, Ouzounian S, Donadille B, Raverot G, Drui D, Haissaguerre M. Pneumocystis pneumonia in patients with Cushing’s syndrome: a French multicenter retrospective study. Ann Endocrinol (Paris). 2023;84(1):37–44. 10.1016/j.ando.2022.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Kim MJ, Valerio C, Knobloch GK. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2023;107(1):59–70. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the GenBase under accession number C_AA084016.1 and are available at the following URL: https://ngdc.cncb.ac.cn/genbase/review/fc725c6994f9.